- 1Department of Nutrition and Dietetics, The University of Faisalabad, Faisalabad, Pakistan
- 2Department of Nutritional Sciences, Government College University Faisalabad, Faisalabad, Pakistan
- 3Institute of Home Sciences, Faculty of Food, Nutrition and Home Sciences, University of Agriculture, Faisalabad, Pakistan
- 4Department of Health Sciences, University of York, York, United Kingdom
- 5Department of Human Nutrition and Dietetics, NUST School of Health Sciences, National University of Sciences & Technology (NUST), Sector H-12, Islamabad, Pakistan
The co-occurrence of type 2 diabetes mellitus and breast cancer has received considerable attention due to their global prevalence and shared metabolic pathways, greatly affecting quality of life and life expectancy, especially in women. Epidemiological evidence indicates that women with type 2 diabetes mellitus have a 20-30% higher risk of developing breast cancer than women without type 2 diabetes mellitus. This review was conducted through a comprehensive and structured literature search to identify relevant peer-reviewed studies examining the relationship between type 2 diabetes mellitus and breast cancer. To ensure the quality and relevance of the included literature, only studies published in English were considered. The focus was on literature addressing pathological mechanisms, epidemiological data, and shared risk factors contributing to the coexistence of these conditions. Preference was given to recent publications, including systematic reviews, meta-analyses, and high-quality original research articles. The primary databases searched included PubMed, Scopus, Web of Science, and Google Scholar. The increased risk of breast cancer among type 2 diabetic patients is largely attributed to shared risk factors such as obesity, hyperglycemia, dietary patterns, physical inactivity, age, hormonal imbalances, and genetic predispositions, all of which contribute to the coexistence of these conditions. Chronic inflammation, hyperinsulinemia, and persistent hyperglycemia, together with dysregulation of adipokine and estrogen signaling, create a carcinogenic environment that facilitates the development of breast cancer in type 2 diabetic patients. This review emphasizes the urgent need for a multidisciplinary approach to prevention and treatment. Effective intervention strategies can reduce the dual burden of these diseases, resulting in better patient outcomes and improved quality of life.
1 Introduction
Non-communicable diseases (NCDs) represent the leading cause of death globally, more than all other causes. The impact of these diseases is growing faster in low-income countries than in other regions. Among NCDs, type 2 diabetes mellitus and cancer pose significant challenges to healthcare systems (1). Type 2 Diabetes mellitus is a prevalent (2), multifactorial, and heterogeneous disorder (3). It is characterized by a high insulin state resulting from insulin resistance in adipose and muscle tissues, which triggers an insufficient compensatory increase in insulin production. Over time, cellular decompensation and absolute insulin levels decrease, but this usually only occurs in the advanced stages of type 2 diabetes mellitus (4).
According to the International Diabetes Federation (IDF), type 2 diabetes mellitus is diagnosed when fasting blood glucose is ≥126 mg/dL or 2-hour plasma glucose during an oral glucose tolerance test is ≥200 mg/dL. While these criteria are essential for clinical identification, the broader concern lies in the resulting metabolic disturbances. Symptoms such as fatigue, excessive thirst, frequent urination, and numbness arise from sustained glucose imbalance. These disruptions contribute to serious long-term complications, leading to increased healthcare costs, reduced quality of life, and higher mortality rates (5).
Type 2 diabetes mellitus currently affects approximately 537 million adults aged 20 to 79 globally, representing 10.5% of all adults in this age range. By 2030, the number of people with type 2 diabetes mellitus is expected to increase to 643 million, reaching 783 million by 2045. According to the 10th edition of the International Diabetes Federation (IDF), the incidence of type 2 diabetes mellitus in Southeast Asia (SEA) countries has been increasing for at least 20 years, with current estimates exceeding previous predictions (6). Worldwide, 1 in 10 and over 3 in 4 adults with type 2 diabetes mellitus live in low- and middle-income countries. In Pakistan, a developing country in South Asia, the prevalence of type 2 diabetes mellitus has reached epidemic proportions. Pakistan ranks 3rd in prevalence, affecting 30.8% of adults, 26.9% undiagnosed. Type 2 Diabetes mellitus is the 8th leading cause of death worldwide, contributing to 17.5% of deaths in Pakistan (7). This trend is accompanied by increased rates of certain cancers, leading to speculation that there may be a possible direct link between type 2 diabetes mellitus and cancer. This trend is likely due to the increasing westernization of lifestyle, a trend probably shared by most Asian populations (8). The link between the two diseases was first suggested in 1934 and has been extensively researched now recognized type 2 diabetes mellitus is a risk factor for various types of cancer (9).
A substantial body of evidence now highlights a clear and consistent increase in cancer risk associated with type 2 diabetes mellitus. For type 2 diabetes mellitus, the strength of this association varies by cancer site, being stronger for pancreatic, liver, breast, bladder, endometrial, colorectal, non-Hodgkin’s lymphoma, and kidney cancers. Although the risk of stomach cancer is high in the Japanese population, this trend may not be universal. Men with type 2 diabetes mellitus typically have a 10–20% lower risk of prostate cancer, which is linked to lower circulating testosterone levels. There is limited data for other rarer cancers, hindering firm conclusions. Mortality risk is particularly high for pancreatic, colon, liver, and bladder cancers, yet data on rare cancer outcomes are scarce (10).
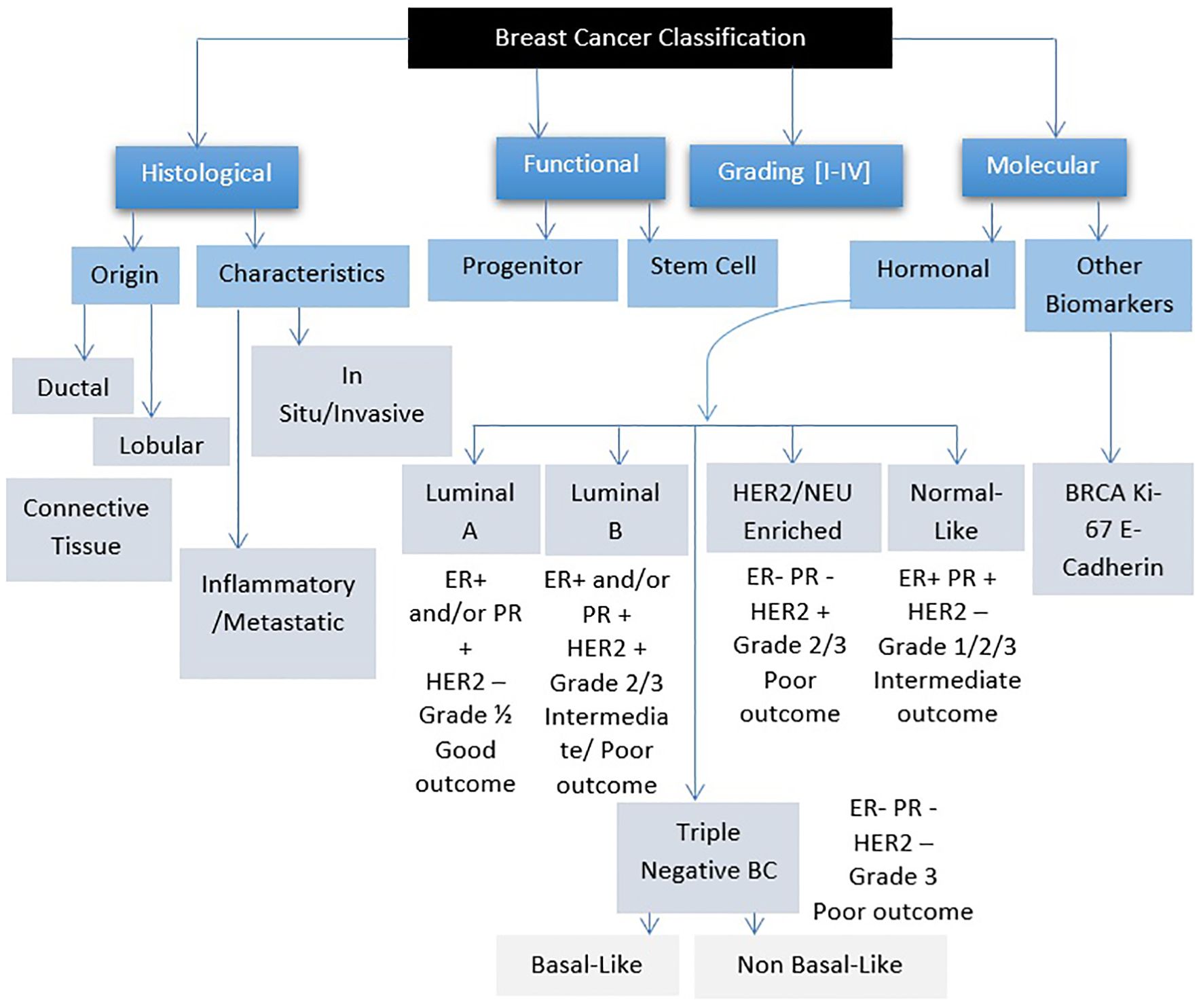
Breast cancer refers to a group of diseases where cells in breast tissue change and divide uncontrollably, often resulting in the formation of a lump or mass. It can be further classified based on hormone receptor profiles and other factors (Figure 1) (11). Most cases of breast cancer start in the milk glands (lobules) or the tubes (ducts) that connect the milk glands to the nipple. In the early stages, breast cancer usually has no symptoms, which is why breast screening plays an important role in early detection. The most common physical symptom is a painless lump, although in some cases, breast cancer can spread to the underarm lymph nodes, causing a lump or swelling to develop before the tumor becomes noticeable. Less common signs and symptoms include breast pain, heaviness, thickening, swelling, dimpling, or redness of the breast skin, changes in the nipple, sudden discharge (especially bloody), itching, or retraction. Any persistent breast changes should be evaluated by a physician (12).
Breast cancer is the most commonly diagnosed cancer in women and the fifth leading cause of cancer deaths worldwide. In 2018 alone, more than 2 million new cases were documented. Approximately 1 in 8 women (13%) and ~1 in 1000 males are diagnosed with invasive breast cancer during their lifetime, with 1 in 39 women (3%) dying of the disease. These figures represent the average risk and account for deaths from other causes that may preempt a breast cancer diagnosis. However, an individual woman’s risk is influenced by age, race or ethnicity, personal or family medical history, and reproductive history (11, 12). Pakistan ranks 1st in Asia and 2nd worldwide in breast cancer incidence, with 90,000 cases and 40,000 deaths reported annually. The report states that 1 out of every 9 Pakistani women is at risk of breast cancer at some point in her life (13).
Since the twentieth century, many hypotheses have proposed a link between type 2 diabetes mellitus and cancer, with increasing evidence, particularly associating type 2 diabetes mellitus with cancer risk, prognosis challenges, and treatment outcomes (14). A well-designed meta-analysis supports these findings, showing that type 2 diabetic women have a 23% higher risk of breast cancer than non-type 2 diabetic women. Similarly, another recent meta-analysis found that preexisting type 2 diabetes mellitus increases 37% all-cause mortality and a 17% increase in breast cancer mortality in women (15). The association between these diseases is due to shared risk factors (16), with various comorbidity confounders exacerbating the situation. Chronic obesity and an inactive lifestyle, which develops hyperinsulinemia, may be the underlying causes, and type 2 diabetes mellitus may be an innocent bystander (17). Additional potential confounding factors include gender, age, diet, alcohol consumption, smoking (18), and use of insulin therapy (19).
Type 2 diabetes mellitus can make the clinical course of cancer more aggressive, increase its metastatic potential, and potentially promote cancer growth by making the host less resistant to the disease. This may be due to a compromised immune system in type 2 diabetics. Furthermore, type 2 diabetes mellitus is also associated with physical frailty and reduced quality of life in cancer patients, which significantly compromises the outcome of their treatment (1).
2 Epidemiology links between type 2 diabetes mellitus and breast cancer
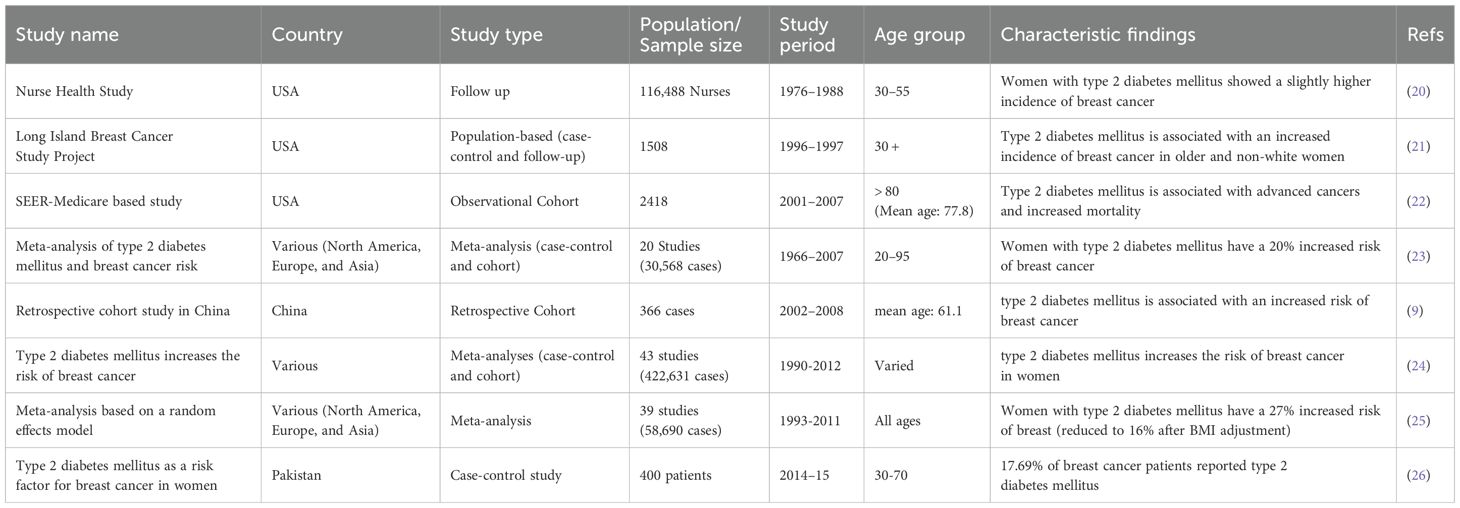
Breast cancer incidence rates are significantly higher in Western countries than in Asia, with half of all cases and 60% of associated deaths occurring in developing countries (Table 1). Although incidence and mortality rates have decreased in North America and parts of Europe, they continue to increase in Asian countries (27).
The link between type 2 diabetes mellitus and breast cancer was strongest in Europe (RR=1.88, 95% CI: 1.56-2.25), followed by the United States (RR=1.16, 95% CI: 1.12-1.20). In Asia, the correlation showed no significant difference (RR=1.01, 95% CI: 0.84-1.21), suggesting that ethnic and regional factors may influence breast cancer incidence. The difference in breast cancer mortality between type 2 diabetic individuals and non-type 2 diabetic individuals is greater in Asia (RR=2.05) than in the United States (RR=1.40). This disparity can be attributed to variations in access to health care, socioeconomic and educational status, lifestyle choices, and especially mammography utilization rates (28).
3 Potential confounding factors
3.1 Non-modifiable factors
3.1.1 Age
Age is a non-modifiable factor that plays a crucial role in the development of both type 2 diabetes mellitus and breast cancer. As individuals age, insulin sensitivity decreases, and pancreatic beta-cell function declines, increasing the risk of type 2 diabetes mellitus. Similarly, advancing age contributes to a higher incidence of breast cancer, particularly after menopause, due to cumulative hormonal exposure and age-associated genetic mutations. Breast cancer is most common in women aged 50 years and older, largely linked to prolonged estrogen exposure and decreased immune surveillance. Recent evidence highlights that approximately 80% of breast cancer cases among individuals with type 2 diabetes mellitus occur in those aged 60 and above, whereas only 20.5% arise in the 18–59 age group. However, younger individuals with type 2 diabetes mellitus exhibit nearly double the relative risk of developing breast cancer compared to older non-type 2 diabetics (29).
3.1.2 Gender
Gender significantly influences breast cancer risk in individuals with type 2 diabetes mellitus. Women with type 2 diabetes mellitus are observed to have a 20–30% higher risk of developing breast cancer compared to non-type 2 diabetic women, largely due to the interaction between hyperinsulinemia, insulin resistance, and estrogen-driven pathways. These mechanisms create a hormonal and inflammatory environment that fosters tumor growth. In contrast, breast cancer in type 2 diabetic men is rare but may be slightly more prevalent than in non-type 2 diabetic males, potentially due to obesity-induced aromatization of androgens to estrogens and altered testosterone levels. While literature on male breast cancer in type 2 diabetes mellitus remains limited, the existing data suggest the need to consider sex-specific hormonal dynamics in understanding risk patterns (30, 31).
3.1.3 Height
Height has emerged as a potential risk factor for breast cancer in various populations, including type 2 diabetics. Research suggests that being tall may slightly increase breast cancer risk, regardless of type 2 diabetes mellitus status. This link is thought to be due to elevated levels of growth factors and hormones, such as insulin-like growth factor 1 (IGF-1), which play a role in cancer development. Numerous studies have established a significant correlation between tall height and breast cancer in both type 2 diabetic and non-type 2 diabetic individuals. A large analysis including more than 5 million women found that every 10-centimeter (approximately 4 inches) increase in height was associated with a 17% higher risk of breast cancer. Although the underlying mechanisms are still unclear, they may involve variations in early developmental patterns, hormonal levels, and genetic predispositions. Furthermore, height has been linked to an increased risk of several other types of cancer (32, 33).
3.1.4 Genetic predisposition
Genetic predisposition is a critical determinant of breast cancer risk, especially when combined with metabolic disturbances from type 2 diabetes mellitus. Individuals with a family history of breast cancer are at significantly increased risk, and this risk may be compounded in type 2 diabetic patients. Mutations in high-penetrance genes such as BRCA1, BRCA2, TP53, PTEN, CDH1, STK11, and ATM are well-established contributors to hereditary breast cancer. Additionally, type 2 diabetes mellitus-related polymorphisms and single-nucleotide polymorphisms (SNPs) in genes regulating insulin signaling and inflammatory pathways may interact with these mutations, further elevating cancer susceptibility. The co-occurrence of type 2 diabetes mellitus and genetic mutations may enhance oxidative stress, insulin resistance, and estrogen synthesis—factors that synergistically promote carcinogenesis (34, 35).
3.1.5 Race and ethnicity
Race and ethnicity play an important role in influencing breast cancer risk among individuals with type 2 diabetes mellitus, highlighting significant disparities in both incidence and outcomes between different demographic groups. Extensive research consistently indicates that African American and Hispanic women with type 2 diabetes mellitus have a 20-30% higher risk of breast cancer than their non-type 2 diabetic counterparts and other racial or ethnic groups (36). These disparities are multifaceted, including socioeconomic status, disparities in healthcare access, genetic variations, and potentially complex interactions between type 2 diabetes mellitus and breast cancer risk factors within specific racial or ethnic populations (37).
3.1.6 Dense breast tissue
Dense breast tissue is a major challenge in breast cancer detection and risk assessment in type 2 diabetic patients. It is characterized by a higher proportion of fibrous and glandular tissue, which can obscure the tumors on mammograms, potentially delaying diagnoses and affecting treatment outcomes (38, 39). This issue is important for women with type 2 diabetes mellitus, as they already face an elevated risk of breast cancer due to metabolic factors associated with type 2 diabetes mellitus (40).
3.1.7 Menstrual periods
The timing of reproductive milestones—such as early menarche (before age 12) and late menopause (after age 55)—plays a pivotal role in breast cancer risk, especially in women with type 2 diabetes mellitus. Extended exposure to endogenous estrogen increases the likelihood of developing hormone-receptor-positive breast cancers. In women with type 2 diabetes mellitus, metabolic dysfunctions further disrupt hormonal balance, exacerbating this risk. Premenopausal breast cancers in type 2 diabetics are often aggressive and hormone-receptor-negative, whereas postmenopausal cases are commonly hormone-receptor-positive and influenced by adipose-derived estrogen. The combination of prolonged estrogen exposure and insulin resistance creates a permissive environment for tumor development (41).
3.2 Modifiable factors
3.2.1 Body mass index
Body Mass Index (BMI), a measure of body fat relative to height and weight, affects breast cancer risk in patients with type 2 diabetes mellitus. A high BMI level is linked to increased levels of insulin and estrogen, both of which contribute to the development of breast cancer. Research shows that every 5 kg/m² rise in Body Mass Index corresponds to a 12% higher risk of postmenopausal breast cancer (42). Women with type 2 diabetes mellitus and obesity are at higher risk, as excess weight increases metabolic disturbances and inflammation, which can lead to poorer cancer outcomes (43).
3.2.2 Specific fat accumulation areas
Specific areas of fat accumulation, such as visceral fat and waist circumference, affect breast cancer risk in type 2 diabetic patients. An increase in visceral fat, which surrounds the abdominal organs, is strongly associated with insulin resistance and high inflammation levels, which contribute to increased estrogen levels and breast cancer risk (44). Studies show that central obesity, characterized by excess fat around the abdomen, is particularly harmful in people with type 2 diabetes mellitus, as it exacerbates metabolic disorders and hormonal imbalances (45). Research has highlighted that every 5 cm increase in waist circumference is associated with a 7% higher breast cancer risk in postmenopausal women (46).
3.2.3 Dietary habits
Recent studies indicate an important role of dietary habits in influencing the risk of breast cancer in type 2 diabetic individuals. A cohort study involving 10,000 participants over ten years indicated that a higher intake of fish, eggs, leafy vegetables, and nuts was linked to a lower breast cancer risk (47). These nutritious foods, which are abundant in omega-3 fatty acids, antioxidants, and essential vitamins, provide a protective effect against breast cancer in type 2 diabetic individuals. Additionally, a meta-analysis supports these findings, suggesting that a diet emphasizing these food groups may significantly reduce breast cancer risk in type 2 diabetic women (48).
A population-based cohort study of 20,000 women aged 40–70 years, followed for 15 years, found an association between dietary patterns and risk of breast cancer. Participants completed a detailed dietary assessment using a food frequency questionnaire. During the study period, 1,500 cases of breast cancer were diagnosed. Analysis revealed that higher intakes of red meats (HR = 1.18, 95% CI 1.04-1.34), processed meats (HR = 1.25, 95% CI 1.10-1.42), and sugary drinks (HR = 1.32, 95% CI 1.16-1.50) were associated with elevated risk of breast cancer. In contrast, a diet rich in whole grains, vegetables, fruits, and lean protein was associated with a lower risk (HR = 0.85, 95% CI 0.76-0.95). These findings highlight the importance of dietary changes in lowering breast cancer risk within the studied population (49).
In a case-control study conducted in northern Alberta, Canada, researchers investigated the relationship between dietary factors and breast cancer risk in 577 women diagnosed with breast cancer in 1976-77, alongside 826 age-stratified female controls without the disease. Participants were queried about specific aspects of their diet. This study found significant trends in relative risks (RRs) across tertiles of consumption for several nutrients. Increasing frequency of beef consumption showed RRs of 1.0, 2.3, and 1.5 (test for trend, p < 0.001), indicating a significant trend towards increased risk with higher intake. Similarly, pork consumption demonstrated RRs of 1.0, 1.6, and 2.2 (test for trend, p < 0.001), indicating a significant link with breast cancer risk. Consumption of sweet desserts also showed a trend with RRs of 1.0, 1.3, and 1.5 (test for trend, p = 0.01), suggesting increased risk associated with higher intake levels. Additionally, greater risks were observed in using table butter and frying with butter or margarine than with vegetable oils (50).
3.2.4 Aluminum foil and utensils
Studies have suggested mechanisms by which aluminum exposure may contribute to cancer development. Aluminum salts, commonly consumed through cooked food or stored in aluminum foil, have estrogen-like effects in vitro, possibly affecting hormone-sensitive cancers such as breast cancer (51). Additionally, while aluminum foil is generally not used directly for cooking acidic or salty foods because of its reactive properties, evidence suggests that aluminum can leach into food, especially when heated (52). In contrast, comprehensive reviews and epidemiological studies have found no significant association between exposure to aluminum from foil or utensils and breast cancer risk. A systematic review concluded that current evidence does not establish a direct causal relationship between aluminum exposure from cookware or other sources and breast cancer (53).
3.2.5 Repeated use of same oil for cooking
Repeated use of the same oil, especially when exposed to high temperatures and repeated heating, leads to the formation of harmful substances like polycyclic aromatic hydrocarbons (PAHs) and advanced glycation end products (AGEs). PAHs are produced during the incomplete combustion of organic matter, including oil when heated to high temperatures. AGEs, on the other hand, are compounds formed by the reaction of sugars with proteins, lipids, or nucleic acids during the cooking processes at high temperatures. Both PAHs and AGEs have been implicated in oxidative stress, inflammation, and cancer development. For individuals with type 2 diabetes mellitus, who already face an elevated risk of breast cancer due to metabolic and hormonal factors, additional exposure to these carcinogenic compounds from reused oil may further increase their risk. Therefore, adopting cooking practices that include oil rotation, moderate heating, and choosing oils with high smoke points and healthy fatty acid profiles can help reduce these risks (54).
3.2.6 Physical activity
Research shows that a sedentary lifestyle contributes to insulin resistance and high levels of circulating insulin and insulin-like growth factors, both of which correlate with an increased breast cancer risk. Type 2 Diabetics with low physical activity levels often struggle to maintain a healthy weight, increasing their breast cancer risk. For instance, a meta-analysis found a positive association between low physical activity levels and breast cancer risk in type 2 diabetic individuals. Therefore, promoting regular physical activity in type 2 diabetic individuals is important not only for type 2 diabetes management but also for reducing breast cancer risk, highlighting the importance of lifestyle modification as a key component in health management strategies (25, 55).
3.2.7 Stress
Chronic stress triggers a cascade of hormonal and immune responses that can contribute to cancer progression. Chronic stress interferes with the hypothalamic-pituitary-adrenal (HPA) axis, resulting in increased production of stress hormones, particularly cortisol. These hormones, in turn, disrupt immune function and promote chronic inflammation, creating a favorable environment for tumor growth and metastasis. Psychologically, chronic stress can also contribute to unhealthy behaviors including poor dietary choices, alcohol consumption and, smoking, which are linked to an increased cancer risk. Moreover, stress can increase insulin resistance and glucose dysregulation, which are common features of type 2 diabetes mellitus, thereby increasing the breast cancer risk in type 2 diabetics (56, 57).
3.2.8 Disrupted sleep cycle
A recent meta-analysis reported that short-term night shift work was associated with a modest increase in the risk of breast cancer (58), with the highest risk found in individuals who worked shifts during early adulthood (59, 60). Exposure to light at night disrupts the production of melatonin, a hormone that regulates sleep. Experimental evidence suggests that melatonin can inhibit the growth of small, established tumors and prevent new tumors (61). A 2019 review of human and animal studies by the International Agency for Research on Cancer concluded that “night shift work,” as distinct from “shift work” (identified in 2007), is carcinogenic to humans due to its association with cancers such as breast, prostate, and colorectal (62).
3.2.9 Metabolic syndrome
Metabolic syndrome, a set of biological abnormalities including obesity, dyslipidemia, hypertension, and insulin resistance, often leading to type 2 diabetes mellitus, has been recognized as a contributing factor to Triple-negative breast cancer (TNBC). This association was highlighted in a case-control study of 555 West African women. Additionally, a retrospective study of 1416 type 2 diabetic breast cancer patients diagnosed between 2015 and 2020 showed a strong association between poor blood sugar management and an elevated risk of TNBC. Supporting this, an Indian clinical study found that TNBC patients who underwent seven cycles of neoadjuvant chemotherapy exhibited higher biomarkers of metabolic syndrome, including type 2 diabetes mellitus, than untreated patients (44).
3.2.10 Alcohol
About 16% of breast cancer cases in the United States are linked to alcohol (63). The risk of breast cancer in women increases by about 7%-10% for every 10 grams of alcohol consumed per day (about one drink). Women who drink 2 to 3 alcoholic drinks per day have a 20% greater breast cancer risk than women who do not drink alcohol (64). Although the exact mechanism is not fully understood, alcohol may increase risk by raising levels of estrogen and other hormone, or by increasing the density of breast tissue (65).
3.2.11 Tobacco
Growing evidence suggests that smoking may slightly increase the risk of breast cancer, particularly in women who have smoked for many years or started smoking at a young age (66). A family history of breast cancer can further increase this risk (67). Additionally, research shows that exposure to secondhand smoke, especially during childhood, may also contribute to the risk of breast cancer in the future (68).
3.2.12 Environmental chemicals and pollutants
Several occupational, environmental, and chemical exposures have been suggested as potential causes of breast cancer. However, epidemiological studies have generally found no clear association between environmental pollutants and breast cancer risk. Research has shown no link between high levels of organochlorines, such as DDT, in the blood or fat tissue of adults and breast cancer risk (69). However, exposure to DDT during critical stages of development, such as in utero, during infancy, or before puberty, has been linked to an increased breast cancer risk later in life (70). Animal studies show that long-term, high-dose exposure to certain chemicals can promote the development of mammary tumor, but it is not yet clear whether low levels of exposure in the general environment do the same. Many of these chemicals have not been well studied in humans, making this an active area of research (71).
3.2.13 Endocrine disruptors
Endocrine disruptors, often consisting of compounds such as phthalates, parabens, and triclosan, are known to mimic or interfere with hormones like androgen and estrogen, disrupting normal hormonal signaling pathways. Phthalates are a group of chemicals found in plastics, fragrances, and personal care products that have been linked to hormone disruption, specifically affecting estrogen and testosterone levels. They have been linked to reproductive and developmental problems and are being investigated for potential health effects (72). Parabens are widely used as preservatives in cosmetics and personal care products to inhibit microbial growth. They have been detected in human breast tissue samples and are suspected of having a role in breast cancer development due to their estrogenic properties and ability to penetrate the skin (73). Triclosan, an antimicrobial agent used in some deodorants and toothpaste, has also raised concerns about its role in endocrine disruptors and antibiotic resistance (74). Formaldehyde-releasing preservatives in hair dyes and other products release carcinogenic formaldehyde, while aromatic amines in hair dyes are classified as possible human carcinogens (75).
3.2.14 Breastfeeding
Most research shows that breastfeeding for a year or more slightly decreases the breast cancer risk in women, with greater reductions seen with longer durations. A review of 47 studies from 30 countries found that the risk of breast cancer decreases by 4% for every 12 months of breastfeeding. It works by suppressing ovulation, thereby reducing lifelong exposure to estrogen, the hormone associated with breast cancer development. It promotes breast tissue maturation, aids in postpartum weight loss, and improves insulin sensitivity (76).
3.2.15 Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is marked by hormonal imbalances, such as increased androgen levels and insulin resistance, which can lead to breast cancer risk. Insulin resistance, a common feature of PCOS, leads to high circulating insulin levels and increased production of insulin-like growth factor (IGF), both of which are associated with the development and progression of breast cancer. Additionally, the chronic inflammation and hormonal dysregulation seen in PCOS may create a favorable environment for cancer cell growth (77).
3.2.16 Oral contraceptives
Most research shows that current or recent use of oral contraceptives (combined estrogen and progesterone) is associated with a relatively small (about 20%) increased risk of breast cancer, especially among women who have had it before their first pregnancy. Studies of progestin-only intrauterine devices have had mixed results, but a large Denmark study linked its use to a 20% increased risk of breast cancer. On the other hand, the injectable progestin-only contraceptive, depot-medroxyprogesterone acetate (Depo-Provera), has not been linked to breast cancer, although the sample size may have been too small to detect a clear association (78). The risk is reduced when women stop using for at least 10 years, as are never users. Data on “ultra-low-dose” (20 micrograms) estrogen formulations are limited and less clear (79). Overall, it is estimated that one additional case of breast cancer is diagnosed for every 7,690 women who use hormonal contraception for one year (78).
4 Pathophysiology
Insulin signaling is important for maintaining metabolic homeostasis by finely regulating glucose uptake, glycogen synthesis, and lipid metabolism in response to varying blood glucose levels. When blood glucose rises, insulin binds its receptor on target cells, initiating autophosphorylation and subsequent recruitment of insulin receptor substrates (IRS) and Shc proteins. IRS activates the PI3K-AKT pathway, which is important for glucose transport, glycogen synthesis, and lipid metabolism, ensuring efficient utilization or storage of glucose and suppression of lipolysis, autophagy, proteasomal activity, and apoptosis under adequate insulin levels. In addition, Shc proteins activate the MAPK pathway, regulating cellular proliferation, differentiation, and gene transcription. Disruption of insulin signaling, as seen in insulin resistance and type 2 diabetes mellitus, leads to impaired glucose uptake and dysregulated lipid metabolism, which contributes to metabolic dysfunction and hyperglycemia. Beyond metabolic regulation, insulin and insulin-like growth factors affect cancer biology by activating the HIF1 signaling pathway. This pathway, critical for cellular adaptation to hypoxic conditions in solid tumors, regulates glycolysis, angiogenesis, and cell survival. Insulin-mediated activation of HIF1 signaling highlights its role in promoting cancer cell survival, proliferation, and adaptation to the anti-tumor microenvironments, highlighting the complex interplay between metabolic processes and cancer progression (Figure 2) (11) In the case of insulin resistance, it is important to note that different types of insulin-activated, signaling pathways identify an important critical distinction. The primary metabolic effects of insulin are mediated through the PI3K-Akt pathway; however, it is in this pathway that insulin resistance presents problems.
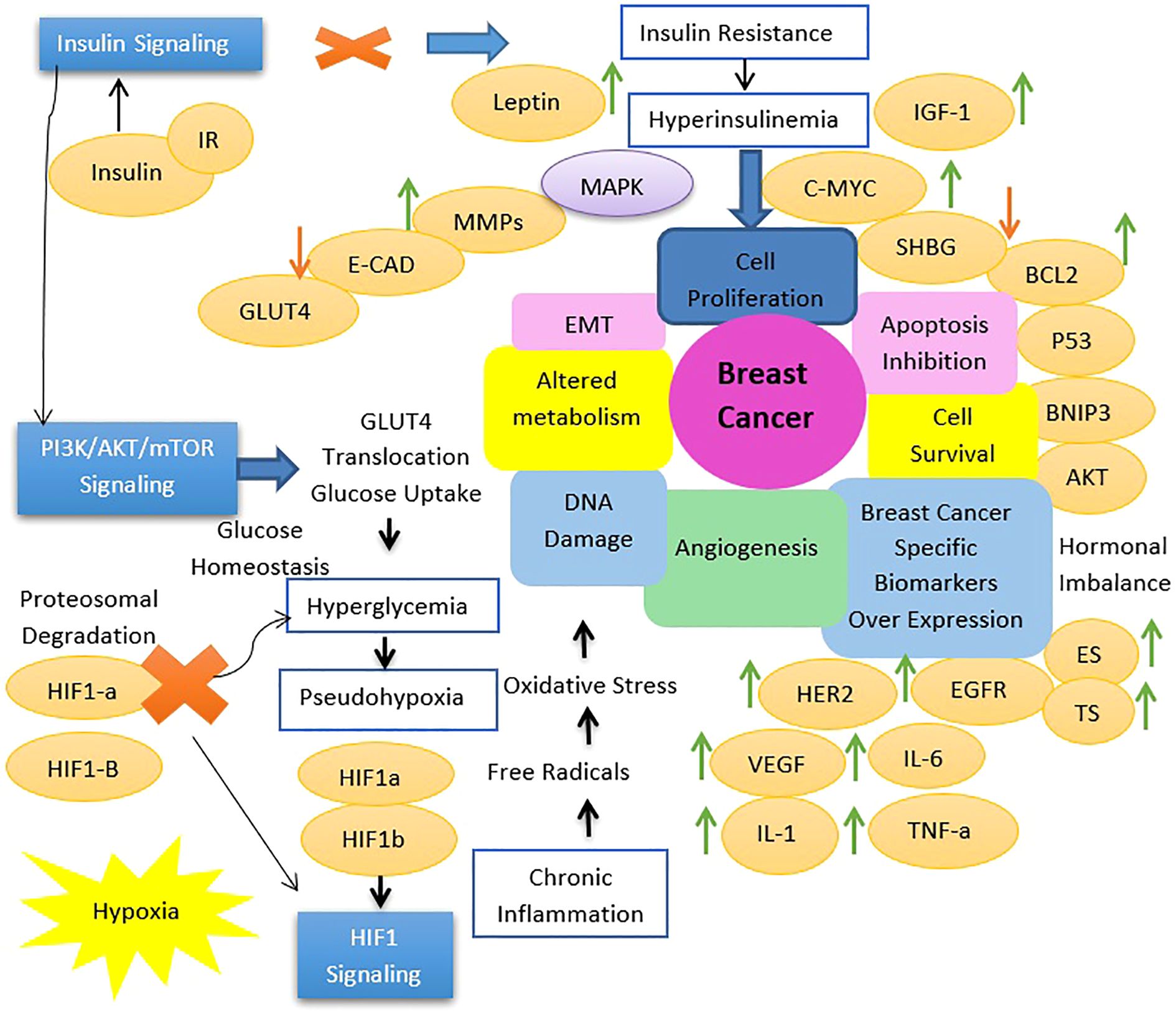
Figure 2. Transition in cell signaling associated with type 2 diabetes mellitus induced breast tumorigenesis.
As for the MAPK signaling pathway, it would be quite intact in such a situation. Quite likely, this demonstration of signaling still being conferred by insulin would indicate that there was a shift toward this pathway to preferentially activate it. Because the MAPK pathway is largely concerned with growth, proliferation, and differentiation of the cell, rather than any of the metabolic functions associated with insulin, this would concern what is endorsing the preferential activation of this pathway in insulin resistance (35, 80, 81). Thus, the most preferred activation of the MAPK pathway for insulin resistance has real physiological consequences at that level. This then means that, if elevated, MAPK signaling could be contributing a lot to increased cell proliferation and survival, thereby contributing to tumor growth and progression, and this phenomenon can be observed in a few insulin-resistant states (80, 82, 83).
Some key breast cancer genes, like BRCA1/2, TP53, PTEN, CDH1, and STK11, significantly correlate with Type 2 Diabetes (type 2 diabetes mellitus. BRCA1/2 mutations raise type 2 diabetes mellitus risk, with high BMI being one potential factor via metabolic misregulation. TP53, a tumor suppressor, also controls metabolism, and its high level in type 2 diabetes mellitus patients suggests a common stress response. PTEN acts directly on insulin signaling active in both breast cancer and type 2 diabetes mellitus via the PI3K/Akt pathway. Likewise, some early connections of CDH1 (cell adhesion) and STK11 (metabolic LKB1 regulator) with type 2 diabetes mellitus establish common pathways for both diseases. This profound genetic interplay suggests possible common molecular mechanisms between breast cancer and type 2 diabetes mellitus. Thus, such an integrated health management approach should be warranted (84–86).
5 Barriers to breast cancer diagnosis in people with type 2 diabetes mellitus
Major barriers to early diagnosis and treatment of breast cancer among rural Pakistani women are limited awareness, geographical difficulties, and financial limitations. A widespread lack of awareness of breast cancer symptoms and screening methods is associated with delays in diagnosis and treatment. This lack of knowledge is exacerbated by the geographical isolation of rural areas, where limited infrastructure and transportation difficulties prevent timely access to healthcare services. Women often have to travel long distances for diagnostic and treatment facilities, such as mammography and chemotherapy. Additionally, financial barriers pose considerable challenges, as diagnostic tests and treatments are often prohibitively expensive for economically disadvantaged families, forcing some women to forgo essential medical care or even sell assets to cover expenses, thereby perpetuating poverty. Furthermore, socio-cultural norms and economic dependence limit women’s decision-making power regarding their health. Addressing these barriers requires comprehensive strategies, including better health education, expanding healthcare infrastructure in rural areas, and providing financial support to ensure equitable access to breast cancer screening and treatment. Efforts should focus on empowering women with knowledge, improving accessibility, and reducing the financial burdens for early diagnosis and better outcomes of breast cancer in rural populations in Pakistan (87, 88).
6 Strategies to overcome type 2 diabetes mellitus and breast cancer
Overcoming breast cancer in type 2 diabetic patients requires tailored strategies that address the specific needs of the situation, taking into account the disparities between developed and developing countries (89). There is an urgent need to reduce modifiable risk factors and increase screening through comprehensive national policies, community initiatives, and individual behavioral interventions. Although this strategy is effective but underutilized, especially in low socioeconomic areas. Tobacco taxes have significantly reduced smoking among people of low socioeconomic status, but taxes in the U.S. are still below WHO recommendations. Improving access to healthy and affordable food in areas of low socioeconomic status is essential to address healthcare disparities. Barriers to health care services, targeted communication efforts are needed to reduce cultural and language barriers. Cancer prevention efforts focus on reducing tobacco use and obesity and increasing screening and vaccination. A systematic approach that includes low socioeconomic status and racial/ethnic minorities is essential, with greater emphasis on vulnerable groups (90, 91).
Additionally, ongoing efforts in cancer epidemiology, including occupational exposures, environmental factors, and genetics, are important components of public health programs to promote healthy interventions. However, more research is needed to understand changes in cancer-related attitudes and their impact on cancer incidence and survival rates. Recognizing the challenges of behavior change, policymakers and public health professionals should consider interventions at multiple levels, including the individual level, community, and systemic perspectives (92).
7 Conclusion
The interplay between type 2 diabetes mellitus and breast cancer highlights the urgent need for targeted interventions. Combined risk factors such as obesity, hyperglycemia, and hormonal imbalances create a cancer-prone environment in type 2 diabetic women. Addressing chronic inflammation and metabolic dysregulation is key to disrupting this connection. A multidisciplinary approach, combining lifestyle changes, early screening, and personalized treatment, holds promise for reducing the double burden of these diseases. Focusing on prevention and comprehensive care will not only save lives but also improve the quality of life for those affected worldwide.
Author contributions
RS: Conceptualization, Data curation, Methodology, Writing – original draft. NA: Conceptualization, Writing – review & editing. BI: Conceptualization, Data curation, Writing – review & editing. SA: Conceptualization, Data curation, Writing – review & editing. NI: Conceptualization, Methodology, Supervision, Writing – original draft. AA: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wolde HF, Molla MD, Aragie H, Adugna DG, Teferi ET, Melese EB, et al. High burden of diabetes and prediabetes among cancer patients at University of Gondar comprehensive specialized hospital, Northwest Ethiopia. Sci Rep. (2023) 13:9431. doi: 10.1038/s41598-023-36472-y
2. Tomic D, Shaw JE, and Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. (2022) 18:525–39. doi: 10.1038/s41574-022-00690-7
3. Cignarelli A, Genchi VA, Caruso I, Natalicchio A, Perrini S, Laviola L, et al. Diabetes and cancer: Pathophysiological fundamentals of a ‘dangerous affair’. Diabetes Res Clin Practice. (2018) :143:378–88. doi: 10.1016/j.diabres.2018.04.002
4. Wolf I, Sadetzki S, Catane R, Karasik A, and Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. (2005) 6:103–11. doi: 10.1016/S1470-2045(05)01736-5
5. Eketunde AO. Diabetes as a risk factor for breast cancer. Cureus. (2020) 12. doi: 10.7759/cureus.8010
6. Kumar A, Gangwar R, Ahmad Zargar A, Kumar R, and Sharma A. Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Curr Diabetes Rev. (2024) 20:105–14. doi: 10.2174/1573399819666230413094200
7. International Diabetes Federation. IDF Diabetes Atlas (2021). Available online at: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (Accessed July 17, 2025).
8. Noto H, Goto A, Tsujimoto T, Osame K, and Noda M. Latest insights into the risk of cancer in diabetes. J Diabetes Invest. (2013) 4:225–32. doi: 10.1111/jdi.12068
9. Zhang PH, Chen ZW, Lv D, Xu YY, Gu WL, Zhang XH, et al. Increased risk of cancer in patients with type 2 diabetes mellitus: a retrospective cohort study in China. BMC Public Health. (2012) 12:1–6. doi: 10.1186/1471-2458-12-567
10. Harding JL, Shaw JE, Peeters A, Cartensen B, and Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. (2015) 38:264–70. doi: 10.2337/dc14-1996
11. Durrani IA, Bhatti A, and John P. The prognostic outcome of ‘type 2 diabetes mellitus and breast cancer’association pivots on hypoxia-hyperglycemia axis. Cancer Cell Int. (2021) 21:351. doi: 10.1186/s12935-021-02040-5
12. American Cancer Society. Breast Cancer Facts & Figures 2022-2024. Atlanta: American Cancer Society, Inc (2022). Available online at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/2022-2024-breast-cancer-fact-figures-acs.pdf (Accessed July 17, 2025).
13. Munawwar A, Sajjad A, Faisal S, Rasul A, Zarbab A, Bibi A, et al. Basic findings of incidence of breast cancer in allied hospital faisalabad, Pakistan: A retrospective study. Iranian J Public Health. (2023) 52:1199. doi: 10.18502/ijph.v52i6.13000
14. Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. (2011) 47:1928–37. doi: 10.1016/j.ejca.2011.03.003
15. Zhao XB and Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Medicine. (2016) 95:e5602. doi: 10.1097/MD.0000000000005602
16. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, and Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. (2018) 6:e6–15. doi: 10.1016/S2213-8587(17)30366-2
17. Renehan AG, Tyson M, Egger M, Heller RF, and Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
18. Noto H and Raskin P. Hepatitis C infection and diabetes. J Diabetes Its Complications. (2006) 20:113–20. doi: 10.1016/j.jdiacomp.2006.01.001
19. McCall JL, Tuckey JA, and Parry BR. Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br J Surg. (1992) 79:1361–3. doi: 10.1002/bjs.1800791240
20. Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care. (2003) 26:1752–8. doi: 10.2337/diacare.26.6.1752
21. Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, and Gammon MD. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. (2012) 23:1193–203. doi: 10.1007/s10552-012-9989-7
22. Griffiths RI, Danese MD, Gleeson ML, and Valderas JM. Epidemiology and outcomes of previously undiagnosed diabetes in older women with breast cancer: an observational cohort study based on SEER-Medicare. BMC Cancer. (2012) 12:1–4. doi: 10.1186/1471-2407-12-613
23. García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, García-Martinez JM, Castaño A, and de la Vieja A. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer. (2016) 114:716–22. doi: 10.1038/bjc.2016.37
24. Hardefeldt PJ, Edirimanne S, and Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocrine Related Cancer. (2012) 19:793. doi: 10.1530/ERC-12-0242
25. Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. (2012) 107:1608–17. doi: 10.1038/bjc.2012.414
26. Tabassum I, Mahmood H, and Faheem M. Type 2 diabetes mellitus as a risk factor for female breast cancer in the population of Northern Pakistan. Asian Pacific J Cancer Prev. (2016) 17:3255–8. doi: 10.14456/apjcp.2016.84/APJCP.2016.17.7.3255
27. Zhou Y, Zhang X, Gu C, and Xia J. Influence of diabetes mellitus on mortality in breast cancer patients. ANZ J Surg. (2015) 85:972–8. doi: 10.1111/ans.12877
28. Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. (2011) 12:1061–5.
29. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
30. Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, and Hux JE. Diabetes mellitus and breast cancer: a retrospective population-based cohort study. Breast Cancer Res Treat. (2006) 98:349–56. doi: 10.1007/s10549-006-9172-5
31. Smith U and Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. (2009) :52:1699–708. doi: 10.1007/s00125-009-1441-5
32. Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. (2013) 14:665–78. doi: 10.1111/obr.12028
33. Kabat GC, Heo M, Kamensky V, Miller AB, and Rohan TE. Adult height in relation to risk of cancer in a cohort of Canadian women. Int J Cancer. (2013) 132:1125–32. doi: 10.1002/ijc.27704
34. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. (2003) 72:1117–30. doi: 10.1086/375033
35. Zhang Y, Ding Y, Zhu N, Mi M, Lu Y, Zheng J, et al. Emerging patterns and trends in global cancer burden attributable to metabolic factors, based on the Global Burden of Disease Study 2019. Front Oncol. (2023) 13:1032749. doi: 10.3389/fonc.2023.1032749
36. Newman LA and Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. (2017) 152:485–93. doi: 10.1001/jamasurg.2017.0005
37. Jones BA, Kasl SV, Howe CL, Lachman M, Dubrow R, Curnen MM, et al. African-American/White differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer: Interdiscip Int J Am Cancer Society. (2004) 101:1293–301. doi: 10.1002/cncr.20500
38. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. New Engl J Med. (2007) 356:227–36. doi: 10.1056/NEJMoa062790
39. Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. (2014) 106:dju255. doi: 10.1093/jnci/dju255
40. Ma H, Wang Y, Sullivan-Halley J, Weiss L, Burkman RT, Simon MS, et al. Breast cancer receptor status: do results from a centralized pathology laboratory agree with SEER registry reports? Cancer Epidemiol Biomarkers Prev. (2009) 18:2214–20. doi: 10.1158/1055-9965.EPI-09-0301
41. Colditz GA, Bohlke K, and Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. (2014) 145:567–79. doi: 10.1007/s10549-014-2993-8
42. Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. (2014) 25:1901–14. doi: 10.1093/annonc/mdu042
43. Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. (2015) 16:36–46. doi: 10.1016/S1470-2045(14)71123-4
44. Harvie M, Hooper L, and Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. (2003) 4:157–73. doi: 10.1046/j.1467-789X.2003.00108.x
45. Iyengar NM, Hudis CA, and Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. (2015) 66:297–309. doi: 10.1146/annurev-med-050913-022228
46. Pischon T, Lahmann PH, Boeing H, Tjønneland A, Halkjær J, Overvad K, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. (2006) 118:728–38. doi: 10.1002/ijc.21398
47. Zhang CX, Ho SC, Fu JH, Cheng SZ, Chen YM, and Lin FY. Dietary patterns and breast cancer risk among Chinese women. Cancer Causes Control. (2011) 22:115–24. doi: 10.1007/s10552-010-9681-8
48. Farvid MS, Chen WY, Michels KB, Cho E, Willett WC, and Eliassen AH. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ. (2016) 353:1–12. doi: 10.1136/bmj.i2343
49. Bessaoud F, Daures JP, and Gerber M. Dietary factors and breast cancer risk: a case control study among a population in Southern France. Nutr Cancer. (2008) 60:177–87. doi: 10.1080/01635580701649651
50. Lubin JH, Burns PE, Blot WJ, Ziegler RG, Lees AW, and Fraumeni JF Jr. Dietary factors and breast cancer risk. Int J Cancer. (1981) 28:685–9. doi: 10.1002/ijc.2910280605
51. Darbre PD. Aluminium, antiperspirants and breast cancer. J Inorg Biochem. (2005) 99:1912–9. doi: 10.1016/j.jinorgbio.2005.06.001
52. Sultan SA, Khan FA, Wahab A, Fatima B, Khalid H, Bahader A, et al. Assessing leaching of potentially hazardous elements from cookware during cooking: A serious public health concern. Toxics. (2023) 11:1–14. doi: 10.3390/toxics11070640
53. Exley C. Human exposure to aluminium. Environ Sci: Processes Impacts. (2013) 15:1807–16. doi: 10.1039/C3EM00374D
54. Choe E and Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Safety. (2006) 5:169–86. doi: 10.1111/j.1541-4337.2006.00009.x
55. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA: Cancer J Clin. (2010) 60:207–21. doi: 10.3322/caac.20078
56. Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci. (2012) 109:5995–9. doi: 10.1073/pnas.1118355109
57. Sephton SE, Sapolsky RM, Kraemer HC, and Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. (2000) 92:994–1000. doi: 10.1093/jnci/92.12.994
58. Manouchehri E, Taghipour A, Ghavami V, Ebadi A, Homaei F, and Latifnejad Roudsari R. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Women’s Health. (2021) 21:1–6. doi: 10.1186/s12905-021-01233-4
59. Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, et al. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am J Epidemiol. (2017) 186:532–40. doi: 10.1093/aje/kwx140
60. Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, et al. Night shift work and breast cancer: a pooled analysis of population-based case–control studies with complete work history. Eur J Epidemiol. (2018) 33:369–79. doi: 10.1007/s10654-018-0368-x
61. Stevens RG, Brainard GC, Blask DE, Lockley SW, and Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA: Cancer J Clin. (2014) 64:207–18. doi: 10.3322/caac.21218
62. Ward EM, Germolec D, Kogevinas M, McCormick D, Vermeulen R, Anisimov VN, et al. Carcinogenicity of night shift work. Lancet Oncol. (2019) 20:1058–9. doi: 10.1016/S1470-2045(19)30455-3
63. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
64. Liu Y, Nguyen N, and Colditz GA. Links between alcohol consumption and breast cancer: a look at the evidence. Women’s Health. (2015) 11:65–77. doi: 10.2217/WHE.14.62
65. Rustagi AS, Scott CG, Winham SJ, Brandt KR, Norman AD, Jensen MR, et al. Association of daily alcohol intake, volumetric breast density, and breast cancer risk. JNCI Cancer Spectrum. (2021) 5:pkaa124. doi: 10.1093/jncics/pkaa124
66. Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, and Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. (2013) 105:515–25. doi: 10.1093/jnci/djt023
67. Jones ME, Schoemaker MJ, Wright LB, Ashworth A, and Swerdlow AJ. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. (2017) 19:1–4. doi: 10.1186/s13058-017-0908-4
68. White AJ, D’Aloisio AA, Nichols HB, DeRoo LA, and Sandler DP. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control. (2017) 28:667–75. doi: 10.1007/s10552-017-0903-1
69. Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of lindane, DDT, and 2, 4-dichlorophenoxyacetic acid. Lancet Oncol. (2015) 16:891–2. doi: 10.1016/S1470-2045(15)00081-9
70. Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, et al. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. (2015) 100:2865–72. doi: 10.1210/jc.2015-1841
71. Rodgers KM, Udesky JO, Rudel RA, and Brody JG. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res. (2018) 160:152–82. doi: 10.1016/j.envres.2017.08.045
72. Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, and Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspectives. (2012) 120:935–43. doi: 10.1289/ehp.1104052
73. Barr L, Metaxas G, Harbach CA, Savoy LA, and Darbre PD. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. (2012) 32:219–32. doi: 10.1002/jat.1786
74. Sivamani RK, Jagdeo JR, Elsner P, and Maibach HI eds. Cosmeceuticals and active cosmetics. Florida, U.S.A: CRC Press (2015). doi: 10.1201/b18895
75. Swenberg JA, Moeller BC, Lu K, Rager JE, Fry RC, and Starr TB. Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol Pathol. (2013) 41:181–9. doi: 10.1177/0192623312466459
76. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50–302 women with breast cancer and 96–973 women without the disease. Lancet. (2002) 360:187–95. doi: 10.1016/S0140-6736(02)09454-0
77. Barry JA, Azizia MM, and Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. (2014) 20:748–58. doi: 10.1093/humupd/dmu012
78. Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, and Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. New Engl J Med. (2017) 377:2228–39. doi: 10.1056/NEJMoa1700732
79. Westhoff CL and Pike MC. Hormonal contraception and breast cancer. Contraception. (2018) 98:171–3. doi: 10.1016/j.contraception.2018.05.002
80. Bjornsdottir HH, Rawshani A, Rawshani A, Franzén S, Svensson A-M, Sattar N, et al. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci Rep. (2020) 10:17376. doi: 10.1038/s41598-020-73668-y
81. De Silva S, Tennekoon KH, and Karunanayake EH. Overview of the genetic basis toward early detection of breast cancer. Breast Cancer: Targets Ther. (2019) 11:71–80. doi: 10.2147/BCTT.S185870
82. Subaşıoğlu A, Güç ZG, Gür EÖ, Tekindal MA, and Atahan MK. Genetic, surgical and oncological approach to breast cancer, with BRCA1, BRCA2, CDH1, PALB2, PTEN and TP53 variants. Eur J Breast Health. (2023) 19:55. doi: 10.4274/ejbh.galenos.2022.2022-7-2
83. Abonyi-Tóth Z, Rokszin G, Fábián I, Kiss Z, Jermendy G, Kempler P, et al. Incident cancer risk in patients with incident type 2 diabetes mellitus in Hungary (Part 1). Cancers. (2024) 16:1745. doi: 10.3390/cancers16132414
84. Abonyi-Tóth Z, Rokszin G, Sütő G, Fábián I, Kiss Z, Jermendy G, et al. Incident Cancer risk of patients with prevalent type 2 diabetes mellitus in Hungary (Part 2). Cancers. (2024) 16:2414. doi: 10.3390/cancers16132414
85. Wang M, Yang Y, and Liao Z. Diabetes and cancer: Epidemiological and biological links. World J Diabetes. (2020) 11:227. doi: 10.4239/wjd.v11.i6.227
86. Shiovitz S and Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. (2015) 26:1291–9. doi: 10.1093/annonc/mdv022
87. Saeed S, Asim M, and Sohail MM. Fears and barriers: problems in breast cancer diagnosis and treatment in Pakistan. BMC Women’s Health. (2021) 21:1–0. doi: 10.1186/s12905-021-01293-6
88. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118–964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. (2012) 13:1141–51. doi: 10.1016/S1470-2045(12)70425-4
89. King GL, Park K, and Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award Lecture. Diabetes. (2016) 65:1462–71. doi: 10.2337/db16-0152
90. Lee H, Ghebre R, Le C, Jang YJ, Sharratt M, and Yee D. Mobile phone multilevel and multimedia messaging intervention for breast cancer screening: pilot randomized controlled trial. JMIR mHealth uHealth. (2017) 5:e7091. doi: 10.2196/mhealth.7091
91. Goding Sauer A, Siegel RL, Jemal A, and Fedewa SA. Current prevalence of major cancer risk factors and screening test use in the United States: disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev. (2019) 28:629–42. doi: 10.1158/1055-9965.EPI-18-1169
Keywords: epidemiological data, hyperglycemia, type 2 diabetes mellitus, breast cancer, adipokine, pro-carcinogenic environment, multidisciplinary approach
Citation: Saroosh R, Ahmad N, Israr B, Arif S, Itrat N and Ahmad AMR (2025) Navigating the nexus of type 2 diabetes mellitus and breast cancer: a comprehensive review of co-occurrence. Front. Oncol. 15:1624896. doi: 10.3389/fonc.2025.1624896
Received: 08 May 2025; Accepted: 12 August 2025;
Published: 08 September 2025.
Edited by:
Tengteng Wang, Rutgers Cancer Institute of New Jersey, United StatesReviewed by:
Armando Rojas, Catholic University of the Maule, ChileGergo A. Molnar, University of Pécs, Hungary
Copyright © 2025 Saroosh, Ahmad, Israr, Arif, Itrat and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Momin Rizwan Ahmad, YWJkdWwubW9taW5AeW9yay5hYy51aw==; Nizwa Itrat, bml6d2FxYW1hckBnbWFpbC5jb20=
 Rabiya Saroosh
Rabiya Saroosh Nazir Ahmad
Nazir Ahmad Beenish Israr3
Beenish Israr3 Nizwa Itrat
Nizwa Itrat Abdul Momin Rizwan Ahmad
Abdul Momin Rizwan Ahmad