- 1Department of Radiation Oncology (Maastro), GROW Research Institute for Oncology and Reproduction, Maastricht University Medical Center+, Maastricht, Netherlands
- 2Department of Medical Oncology, University Hospital of Udine, Udine, Italy
- 3Department of Pulmonary Diseases, GROW Research Institute for Oncology and Reproduction, Maastricht University Medical Centre+, Maastricht, Netherlands
Lung cancer is the leading cause of cancer-related death world-wide. Although the standard of care for patients with advanced stage lung cancer has significantly improved with the advent of immunotherapy and targeted agents, the overall prognosis remains poor. It highlights the need for improved patient selection utilizing prognostic and predictive biomarkers. Given the limited feasibility of serial lung tumor tissue biopsies, liquid biopsies have gained specific interest in achieving this aim. Radiotherapy, commonly used alongside systemic treatments, can induce the release of immuno-stimulatory and immuno-suppressive molecules, triggering the immune- and inflammatory responses and releasing associated molecules. This review specifically focusses on immune-related molecules that are measurable in the blood and which have potential prognostic and/or predictive value in patients with lung cancer treated with radiotherapy alone or in combination with systemic agents. Such immune-related molecules include cytokines and chemokines, damage-associated molecular patterns, soluble receptors and ligands, and proteins expressed on the immune cell surface of circulating immune cells. Classical cytokines IL-6, IL-8, and TGF-β1 were the most studied molecules in patients with lung cancer treated with radiotherapy and were associated with poor survival and increased risk of radiation-induced toxicity. To date, there are still some barriers before these promising findings can be implemented in regular clinical practice. Practical points to achieve this goal are also addressed in this review.
1 Introduction
Lung cancer remains one of the leading causes of cancer-related deaths worldwide, with a 5-year overall survival (OS) rate of only 25% (1). Depending on the type of lung cancer, the stage of the disease, and the overall health of the patient, treatment options can include surgery, radiotherapy (RT), chemotherapy, immunotherapy, targeted therapy or a combination of these (2–6). RT is a treatment modality that can be used in all stages of lung cancer. In recent years, both technological advancements (e.g., intensity-modulated RT) and the integration of immunotherapy have broadened the indication for RT and have improved patients’ outcomes by reducing RT-related toxicity and increasing OS, respectively (7, 8). However, in patients with lung cancer, radio-resistance and radiation-induced toxicity are both significant contributors to RT failure, resulting in cancer progression and deterioration of quality of life (QoL) (9).
Whereas research has mainly focused on the level and mechanisms of RT-induced DNA damage, and the repair capacity of irradiated cancer cells, the field has largely ignored the fact that the radiosensitivity of cancer cells in patients is greatly affected by the immunocompetence of the host (10). RT not only induces DNA double-strand breaks followed by some forms of cell death (i.e. apoptosis, necrosis, mitotic catastrophe, or replicative senescence) but it can also induce clinically relevant tumor-targeting immune responses, which critically rely on the host’s immune status and the antigenicity of cancer cells and their capacity to generate adjuvant signals (11, 12). Upon RT-induced DNA damage, DNA accumulation in the cytoplasm of irradiated cells can be sensed by cytoplasmic nucleic acid sensors, resulting in activation of the cyclic GMP-AMP synthase-simulator of interferon genes (cGAS-STING) pathways which in turn lead to a systematically interferon type I (IFN-I) driven immunity program (13, 14). Besides, RT can induce an immunogenic variant of tumor cell death (ICD), which is accompanied by the expression and release of damage-associated molecular patterns (DAMPs). These RT-induced immunogenic responses can result in the uptake of tumor-associated antigens (TAAs) by dendritic cells (DCs) that present them to cytotoxic CD8+ T-cells recruited from circulatory system, subsequently priming and activating the anti-tumor immunity response (15).
In contrast to these RT-induced immunostimulatory effects, RT can also induce immunosuppressive responses, such as the secretion of immune suppressive cytokines (i.e. granulocyte-macrophage colony-stimulating factor, GM-CSF; transforming growth factor-β, TGF-β) from tumor cells, which promote the migration of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Treg) from the circulation towards irradiated areas (16). Also, there is increasing evidence showing that irradiation can induce the expression of the immune checkpoint programmed death ligand 1 (PD-L1) on tumor cells. PD-L1 can induce T-cell anergy (i.e., absence of the normal immune response to a particular antigen or allergen) or apoptosis by binding to PD-1 on activated T-cells, and thereby prevent the killing of cancer cells (17, 18).
Besides the radiosensitivity and immunogenicity of tumor cells itself, the tumor microenvironment (TME) can shift from an immunosuppressive “cold” TME to an immunostimulatory “hot” TME and vice versa (19). An immune-desert TME is generally characterized by high numbers of MDSCs and Tregs and enrichment of immunosuppressive cytokines, such as TGF-β and interleukin-10 (IL-10). An immune-enriched TME is characterized by high PD-L1 expression on the tumor cell surface, high number of effector immune cells (e.g., CD8+ T-cells, natural killer cells), and immunostimulatory cytokines and chemokines (i.e. interferons). The switch from a “cold” towards a “hot” tumor highly depends on the local production of cytokines, chemokines and other soluble factors but also on the trafficking or modulation of immune cell subsets recruited from circulatory system into the TME (19). This transition may be impaired by intrinsic tumor cell radio-resistance mechanisms, tumor heterogeneity, resistance-promoting microenvironment and immunocompetence of the host, resulting in a proportion of patients that initially do or do not respond to RT (10).
Another key aspect limiting the success of RT for lung cancer treatment is RT-induced lung injury (RILI), which encompasses any lung toxicity induced by RT and can manifest acutely – in an early phase – as radiation pneumonitis and in a late phase as radiation pulmonary fibrosis resulting from chronic pulmonary tissue damage (8, 20). Acute radiation damage induced on endothelial and epithelial cells mainly includes DNA damage and release of reactive oxygen species (ROS), which cause cell death, release of pro-inflammatory proteins like CXC-chemokine ligand 12 (CXCL12), interleukin-1 (IL-1), recruiting immune cells to irradiated areas, edema of the alveolar walls and increased vascular disruption (21). Protracted fractions of irradiation can cause endothelial cell dysfunction, leading to increased membrane permeability, detachment and apoptosis, activation and release of inflammatory cytokines like TGF-β, eventually leading to chronic radiation-induced pulmonary fibrosis (22, 23). Almost one out of four patients (24%) with stage II-III non-small-cell lung cancer (NSCLC) treated with chemoradiotherapy experience RILI with grade 2 or more (24, 25), underlining the need for predictive and prognostic biomarkers.
Tissue biopsies are the golden standard procedure for diagnosis and molecular testing, however, a tissue biopsy is an invasive method that is not always feasible and repeatable. Also, the diagnostic information obtained from these tumor biopsies is only used for treatment selection, without providing any additional information regarding radio-resistance or the development of RILI (26). Moreover, tissue biopsies can only provide information limited to a single timepoint and a single tumor location. Tissue biopsies are also unable to capture the dynamic changes of the tissue during treatment. In recent years, liquid biopsies have gained interest due to their non-invasive, cost-effective nature and the ability to be repeatedly collected. Peripheral blood is the main source of circulating immune-related molecules and therefore may be a valuable surrogate for the actual systemic immune status of patients’ before, during, and after treatment. To date, there is limited understanding of whether the immunocompetence of the host and the RT-induced immunological changes can be captured systemically in the blood, and whether these features may serve as prognostic or predictive markers for treatment outcome. As such, there is an urgent need for liquid prognostic and predictive biomarkers that can aid in patient selection and monitoring treatment outcomes for patients with lung cancer treated with RT. This review aims to summarize and discuss the current literature regarding peripheral immune-related proteins that are associated with treatment responses and treatment outcome in patients with lung cancer treated with RT alone or in combination with other systemic anti-cancer medications. Clinically relevant immunological prognostic and predictive biomarkers can help clinicians to timely identify the patients with a poor prognosis or the patients who are at high risk to develop RT-induced toxicity.
2 Prognostic and predictive peripheral immune biomarkers
RT can exert immunostimulatory and immunosuppressive effects, both locally, within the irradiated TME, and systemically, outside the radiation field (27). Molecules involved in these RT-activated immunological signaling cascades include cytokines, chemokines, damage-associated molecular patterns (DAMPs), soluble proteins such as soluble receptors and ligands, and soluble forms of immune cell surface proteins. The circulating levels of these proteins and their fluctuations before, during and after treatment may represent the actual immunological status of patients. This information may aid clinicians to predict the patient’s prognosis and tailor treatments.
To date, numerous clinical trials have investigated the prognostic and predictive significance of these circulating immune-related proteins in the blood of patients with lung cancer treated with RT, as summarized in Tables 1, 2 and discussed in detail below. Prognostic biomarkers are defined as biomarkers that provide information about the oncological outcome, regardless of treatment, whereas predictive biomarkers indicate the probability of a therapeutic benefit from a specific therapy.
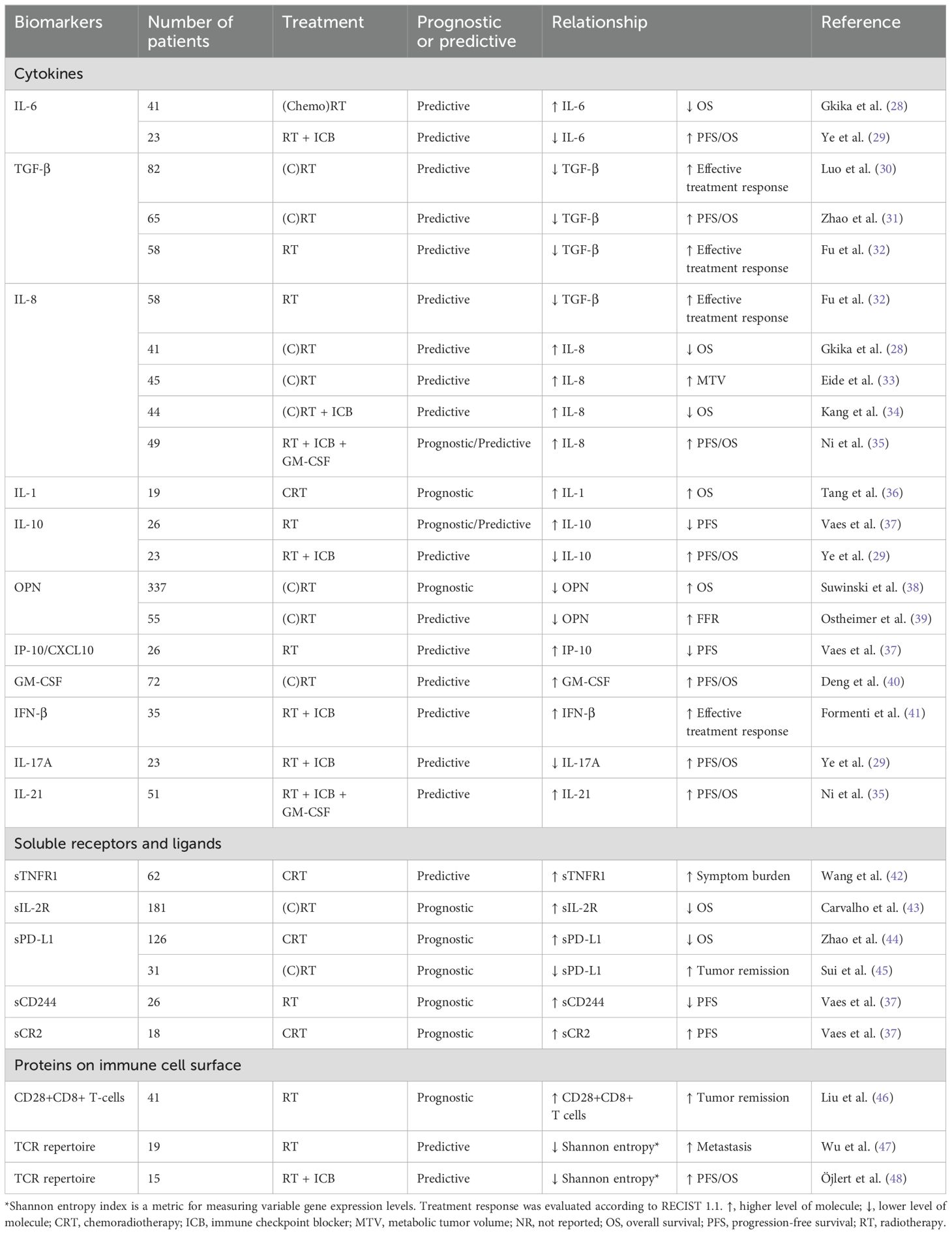
Table 1. The prognostic and predictive value of circulating immune-related biomarkers in relation to treatment outcome in patients with lung cancer treated with radiotherapy.
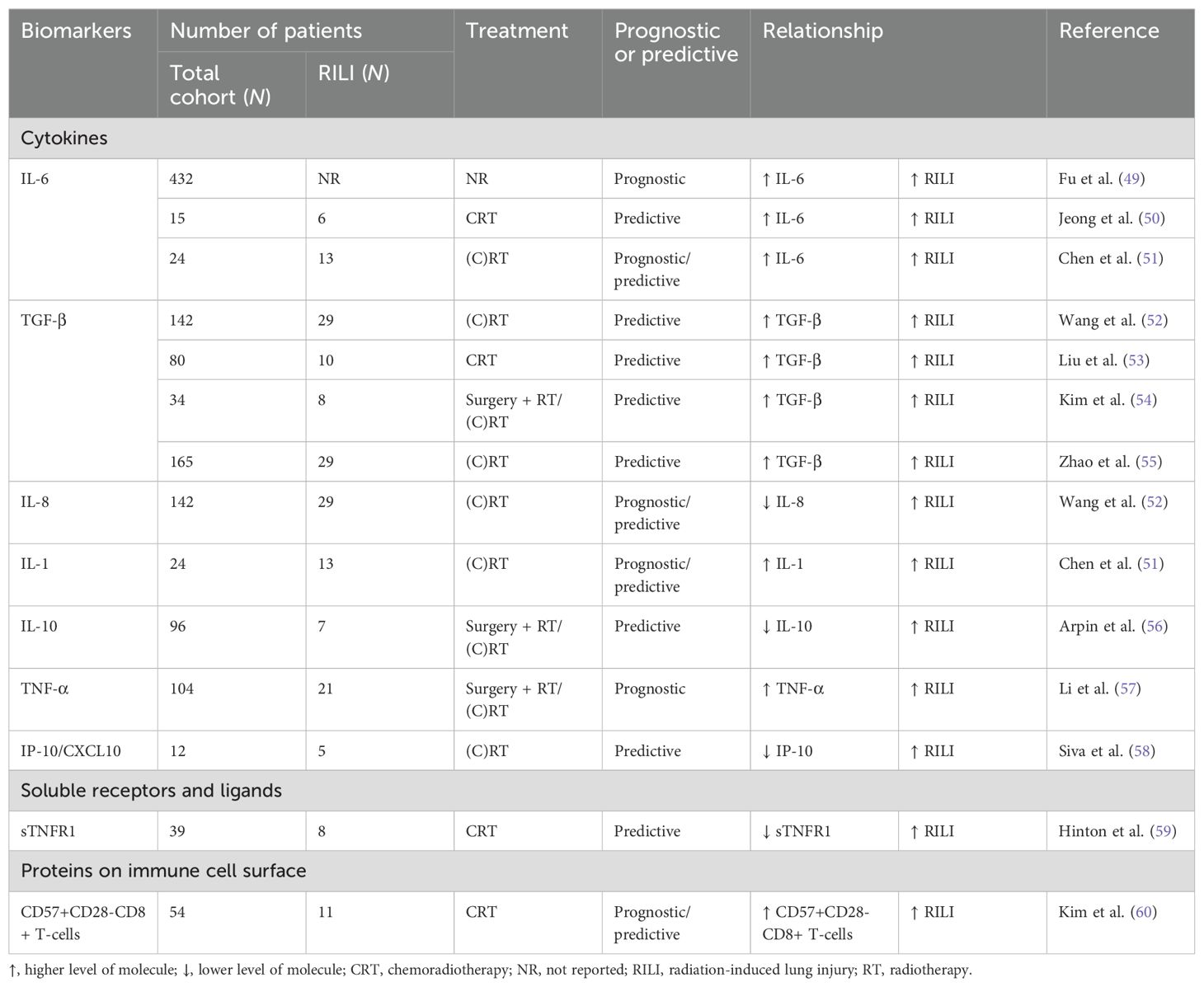
Table 2. The prognostic and predictive value of circulating immune-related biomarkers in relation to the development of RILI (GR≥2) in patients with lung cancer treated with radiotherapy.
2.1 Cytokines
Cytokines are soluble polypeptides that mediate cell-to-cell communication, functioning as chemical messengers in the human body. They are produced and secreted by many different cell types, including immune cells, epithelial cells, and endothelial cells that are present in the TME and healthy surrounding tissue. There are different subclasses of cytokines, including interleukins, chemokines, interferons, and tumor necrosis factors (TNF), which all play an important role in regulating inflammatory responses. These cytokines are often produced in a cascade as one cytokine stimulates its target cells to produce and secrete additional cytokines, and increases in hundreds fold in response to the injury (61).
Irradiation can induce acute responses in the irradiated tissue, resulting in the production of numerous inflammation-related cytokines such as IL-1, IL-6, IL-8, and TNF-α within minutes to hours (62). These pro-inflammatory cytokines are instrumental in generating free radicals and oxidative stress, leading to secondary DNA damage, inflammation, and potentially RILI (63, 64), whereas anti-inflammatory cytokines, such as IL-10 and TGF-β show anti-oxidative properties (65, 66). Also, RT-induced tissue damage results in the expression and/or release of DAMPs in a time-dependent manner. Whereas ATP is secreted rapidly after irradiation, others are secreted hours to days after RT mainly by dead cells (22, 67).
2.1.1 Interleukin-6
IL-6 is a multifunctional cytokine that is produced by many cell types like tumor cells, immune cells, and smooth muscles that play important roles during inflammation and immune responses (68). IL-6 is involved in the acute inflammation process by inducing the production of C-reactive proteins and serum amyloid A (SAA), but also by reducing serum iron levels resulting in anemia. Furthermore, IL-6 is recognized as a key regulator of immunosuppression in patients with advanced cancer (69, 70). Blockade of IL-6 in mice has been shown to significantly inhibit lung cancer progression, tumor cell–intrinsic STAT3 activation, tumor cell proliferation, and angiogenesis (71).
In pre-clinical studies, IL-6 levels have been shown to increase after irradiation and predominantly exert immunosuppressive functions. For example, Ao et al. showed that serum IL-6 levels were already increased 6 hours after irradiation in mice (72). Similarly, Xin et al. showed that IL-6 levels significantly increased within 24 hours after irradiation, mediating macrophage infiltration and promoting tumor metastasis in mice (73).
In the clinical setting, numerous studies have evaluated the role of circulating IL-6 in patients with lung cancer treated with RT. For example, in patients with histologically proven thoracic malignancies, Gkika et al. showed that circulating levels of IL-6 at the end of RT and during follow-up were inversely correlated with OS (28), as well as Ye and associates for both PFS and OS (29). A meta-analysis performed by Fu and colleagues revealed that patients with RILI had significantly higher serum IL-6 levels before RT than those without RILI (49). Similar findings have been reported by numerous others (42, 50, 51, 74). Furthermore, besides high IL-6 levels before RT, increased IL-6 levels during and after RT also appeared to be associated with the development of RILI. For instance, Jeong et al. showed that IL-6 levels peaked at week 3 after RT initiation in patients who developed RILI (50). Chen and associates showed that absolute levels of IL-6 were significantly higher before, during and after RT in patients who developed RILI (51). These findings suggest that IL-6 could be a promising biomarker for developing RILI.
2.1.2 Transforming growth factor-β
TGF-β is a master regulator of cellular proliferation and tissue homeostasis (75). There are three known isoforms of TGF-β, of which TGF-β1 is the most abundant and ubiquitously expressed (76). In cancer, TGF-β plays dual roles as it can exert tumor suppressor effects on normal healthy cells and early carcinogenesis by regulating cell growth and apoptosis. However, during tumor development, these tumor suppressor effects are often lost and then switches to promote cancer progression, invasion, and tumor metastasis (77). In the TME, TGF-β can promote suppression of the anti-tumor immunity by inducing polarization of macrophages towards the M2 anti-inflammatory phenotype, inhibiting the release of IL-2 by naïve T cells to prevent proliferation of cytotoxic T-lymphocytes (CTLs) and NK cells, and inducing cancer-associated fibroblasts to release interleukin-11 (IL-11) that can increase the metastatic capacity of cancer cells (78–81). Suppression of TGF-βs by antibody-mediated TGF-β neutralization in combination with RT has been shown to increase the numbers of CTLs and NK cells within the TME (82).
Numerous pre-clinical studies have investigated the role of TGF-β during irradiation. In vivo studies showed that RT-induced ROS activate TGF-β, which encompasses the release of TGF-β from the latency-associated protein (LAP) that is required for the binding of TGF-β to its receptor (83). Also, the clearance of RT-induced dead tumor cells by macrophages can trigger the release of TGF-β by macrophages (79). Furthermore, TGF-β has been shown to exert multiple functions within irradiated lung cancer cells. On one hand, TGF-β can promote the DNA damage response both in vitro and in vivo reducing the radiosensitivity of tumor cells (84). On the other hand, TGF-β signaling has been shown to be involved in ionizing radiation-induced fibrosis through both canonical and noncanonical TGF-β pathways (79), either by Smad 2/3 pathway or mir-21 (85), Rho/ROCK (86) and NADPH oxidase (87) in lung.
Given that TGF-β regulates a plethora of cellular responses, numerous clinical trials have investigated its clinical significance in patients with lung cancer. For example, Luo et al. investigated the potential predictive value of TGF-β1 in 82 patients with lung cancer treated with RT (30). Within one week after RT, TGF-β1 levels were significantly decreased in patients who achieved an effective response according to RECIST 1.1 compared to patients who did not. Also, TGF-β1 levels were negatively correlated with circulating CD4+, CD8+ and the CD4+/CD8+ ratio during and at the end of RT (30). In addition, patients who had significantly reduced TGF-β1 levels 2 weeks after initiation of RT compared to pre-RT seemed to have a better treatment response than those who had higher TGF-β1 levels (30). Zhao et al. collected data from patients with NSCLC treated with chemoradiotherapy and showed that 4 weeks after the first fraction of RT, decreased TGF-β levels were significantly associated with a prolonged OS and PFS compared to patients with increased TGF-β1 levels (31). Similarly, Fu et al. showed that in patients with unresectable NSCLC treated with three-dimensional conformal radiation therapy (3D-CRT), TGF-β1 levels were significantly decreased in patients who achieved a radiological response after 3D-CRT (32). Overall, decreased TGF-β1 levels during and after RT seem to be associated with better outcomes.
Besides the association of TGF-β1 with treatment response and outcome, TGF-β1 has also been associated with the development of RILI. Wang et al. showed that a higher mean lung dose and a higher TGF-β1 2w/pre ratio (i.e., TGF-β levels at 2 weeks after RT initiation divided by the TGF-β1 levels before the first fraction of RT) in combination with lower pre-treatment IL-8 levels were associated with a higher risk of developing RILI in patients with NSCLC (52). Similarly, Liu et al. demonstrated that in patients with stage III NSCLC who underwent 3D-CRT, circulating TGF-β1 levels increased during the first 2 weeks after the first fraction of RT and were significantly increased at week 6 in the patients who developed RILI (grade ≥1) (53). Similarly, Kim et al. observed significant associations between the changes of TGF-β1 during the time course of RT and the risk of developing RILI in patients with lung cancer (54). Zhao et al. reported similar results in patients with stage I-III NSCLC (55). They showed that patients with increased TGF-β1 levels 4 weeks after the first fraction of RT are more likely to develop RILI than those who do not. These results were validated by them in a larger cohort (88). In summary, alterations in TGF-β levels during and after RT are predominantly associated with a high risk of developing RILI.
2.1.3 Interleukin-8
Interleukin-8 (IL-8/CXCL8), is a pro-angiogenic and pro-inflammatory chemokine (89). The biological effects of IL-8 are mediated through the binding of IL-8 to two cell-surface G protein–coupled receptors, CXCR1 and CXCR2 (90). Different stimuli can induce the expression and release of IL-8 by various cell types, including inflammatory signals (i.e. TNF-α, IL-1β), chemical and environmental stresses (i.e. RT and hypoxia), and steroid hormones (89, 91). In tissue specimens of patients with lung cancer, high levels of IL-8 have been shown to correlate with tumor stage and prognosis (92, 93). Furthermore, IL-8 has been shown to stimulate tumor cell proliferation and promote angiogenesis by recruiting endothelial cells to the TME (94).
So far, only a limited number of pre-clinical studies have investigated the role of RT on the expression and release of IL-8 and its function in lung cancer models. In vitro, RT has been shown to induce IL-8 expression via the p38/MAPK and NF-κB signaling pathways in lung cancer cells (95). In vivo studies also showed that stereotactic ablative radiotherapy (SABR) could induce IL-8 secretion by lung cancer cells (96). Furthermore, Kühlmann et al. showed that increased IL-8 levels in the supernatant of irradiated lung epithelial cells could stimulate collagen synthesis and matrix production in lung fibroblasts (97). Accordingly, irradiation may stimulate the secretion of IL-8, which in turn can promote tumor development and lung fibrosis in lung cancer.
Numerous clinical studies have investigated the prognostic and predictive value of IL-8 in patients with lung cancer treated with RT (28, 33–35). In a recent study, Gkika et al. showed that IL-8 levels during and at the end of RT were negatively correlated with OS (28). Eide et al. revealed that IL-8 serum levels before, during and after treatment were all positively correlated with the metabolic tumor volume (i.e. FDG uptake) in patients with advanced NSCLC undergoing palliative RT (33). In 2023, Kang et al. investigated the predictive value of IL-8 in patients with advanced NSCLC who received hypo-fractionated RT combined with PD-1 blockade immunotherapy (34). In this study, high pre-treatment levels of circulating IL-8 were significantly associated with a poor prognosis and 3 months after treatment, a remarkable decrease of IL-8 was only observed in patients in the partial remission group compared to the non-responder group (34). Notwithstanding, Ni and colleagues showed that higher pre-SBRT and post-SBRT levels of circulating IL-8 were prognostic and predictive, respectively, for improved PFS and OS, although not significant (35).
Furthermore, IL-8 has been shown to be a good predictor for developing post-RT toxicities. Wang et al. collected blood samples from 142 patients with stage I-III NSCLC treated with RT and found that low circulating IL-8 levels before and 2–4 weeks during RT were significantly associated with a higher risk of developing RILI (52).
2.1.4 Interleukin-1
The interleukin-1 (IL-1) family, comprising 11 cytokines, plays a central role in innate and acquired immunity (98). Previous studies have mainly focused on interleukin-1α (IL-1α) and interleukin-1β (IL-1β) during irradiation. IL-1α, predominantly produced by mesenchymal cells, is a key cytokine involved in acute inflammatory responses. It can induce the production of other cytokines such as IL-2, IL-6, and TNF-α, and enhance their relative biologic effectivity (99, 100). The main producers of IL-1β are innate immune cells, such as monocytes and macrophages (101). In cancer, IL-1 has been shown to promote carcinogenesis, induce tumor growth, metastasis and exert immunosuppressive functions (102).
Numerous pre-clinical studies have investigated the effects of irradiation on IL-1 expression in lung cancer. In healthy mice lung tissues, RT induces a biphasic expression pattern of IL-1α. An initial rise was observed at 6 hours after irradiation followed by a drop to basal levels at 2 weeks, whereas these levels increased again at 8 weeks due to RT-induced inflammation (103). Interestingly, Johnston et al. showed that IL-1α and IL-1β remained elevated in healthy mouse lungs up to 6 months after irradiation and contributed to the radiation-induced pulmonary fibrosis (104). Kang et al. demonstrated that IL-1β enhances migration and invasion in the A549 NSCLC cells via the NF-κB–RIP1- IL-1β pathway (105).
There are only a limited number of clinical studies that have explored the dynamic changes and potential prognostic or predictive value of IL-1 in patients with lung cancer treated with RT. Trovò et al. reported decreased IL-1 levels in 13 patients with locally advanced NSCLC within 4 weeks following radical moderated hypo-fractionated RT (60 Gy/25 fractions) (106). However, it was not reported whether the decreased IL-1 levels were associated with treatment outcome as the purpose of the study was only to assess the kinetics of plasmatic cytokines during RT. In the NCT01725165 phase II trial, high baseline circulating IL-1α levels were significantly associated with improved outcomes in 19 patients with oligometastatic NSCLC (36). Furthermore, IL-1α has also been identified as a potential predictive biomarker. Chen et al. revealed that in patients with lung cancer (n=24), IL-1α blood levels were significantly higher before, during and after RT in patients who developed RILI (51). In addition, the authors showed that both IL-1α and IL-6 circulating levels gradually increased and were positively correlated with time, especially after RT, which may suggest that both IL-1α and IL-6 are involved in the response to radiation injury (51, 107).
2.1.5 Interleukin-10
IL-10 is a key anti-inflammatory cytokine that modulates inflammation and maintains cell homeostasis. It is mainly produced by monocytes, macrophages, and cytotoxic T-cells and can inhibit the synthesis of pro-inflammatory cytokines like IL-2 and TNF-α, while it also exerts immunostimulatory effects on B-cells, cytotoxic T cells and thymocytes (108–110). An in vitro study showed that low-dose irradiation of 4 Gy can induce IL-10 secretion by lung tumor cells 6–48 hours post-irradiation (111). In vivo, IL-10 significantly increased from 24 to 96 hours after irradiation. It showed that hypo-fractionated RT could induce the production of IL-10 by CD8+ T-cells, enhancing their proliferation, differentiation, activity, and function (18).
Only a few studies have investigated the clinical significance of IL-10 in patients with lung cancer undergoing RT. We demonstrated that high circulating levels of IL-10 at baseline, during, and end of stereotactic body radiotherapy (SBRT) were significantly associated with worse PFS in patients with stage I NSCLC (n=26) (37). Ye and his colleagues corroborated these results for increased plasma levels of IL-10 following treatment in Nivolumab responders (29). For RILI, Arpin et al. performed a multivariate analysis of serum cytokine levels on patients with NSCLC during the first two weeks of RT (56). Their results indicated that elevated IL-6 and decreased IL-10 levels were associated with a high likelihood of developing RILI .
2.1.6 Other cytokines
In addition to the cytokines mentioned above, several other circulating cytokines have been reported to have prognostic or predictive potential in patients with lung cancer treated with RT. However, the association of these cytokines with patient outcomes has only been reported in a very limited number of clinical studies.
One of these cytokines is osteopontin (OPN), a secreted phosphorylated glycoprotein that is involved in inflammation, tumor progression, and metastasis (112). OPN is activated under hypoxia and OPN concentrations are associated with both tumor hypoxia and outcomes after RT in patients with cancer (113, 114). In 337 patients with NSCLC, Suwinski et al. showed that low OPN concentrations before the start of (chemo)radiation were significantly associated with a favorable OS (38). Ostheimer et al. reported a higher risk of relapse in patients with inoperable NSCLC whose OPN were stable or increased 4 weeks after RT (n=55), indicating that OPN may be associated with a more aggressive cancer phenotype (39, 115). In 2016, Carvalho et al. improved a clinical prognostic model by incorporating OPN (43). The inclusion of OPN significantly improved the discrimination of the model to better predict the prognosis of patients with stage I-IIIB NSCLC treated with RT.
Other immune-related cytokines reported in patients with lung cancer treated with RT include TNF-α (57), IFN-β (41), IFN-γ (37, 116), interferon gamma-induced protein 10 (IP-10/CXCL10) (37), monocyte chemoattractant protein-1 (MCP-1) (58), vascular endothelial growth factor (VEGF) (38), erythropoietin (EPO) (38), GM-CSF (116), IL-17A (29), and IL-21 (35). TNF-α is a pro-inflammatory cytokine which can induce the synthesis and release of other cytokines, including IL-6 and IL-1 (117). TNF-α levels have been shown to increase after RT in patients with lung cancer (n=104), but its relationship with survival was not reported (57). Furthermore, the baseline TNF-α levels were higher in patients who developed RILI (57). Siva et al. showed that patients with stage I-III NSCLC who developed RILI have decreased circulating levels of IP-10/CXCL10, MCP-1 and eotaxin after the first fraction of RT compared to patients without RILI (n=12) (58). Also, Vaes et al. implied that after the first fraction of SBRT, increased IP-10/CXCL10 levels were significantly associated with a shorter PFS for patients with stage I NSCLC (n=26) (37). Deng et al. demonstrated that upregulated GM-CSF during RT correlated with longer OS and PFS in patients with unresectable lung cancer, and it was an independent predictive factor (40), which is in line with its antitumor immune function (118). Lastly, Formenti et al. analyzed the blood samples of patients with metastatic lung cancer that were treated with palliative RT and the anti-CTLA-4 antibody, ipilimumab (n=35) (41). They showed that IFN-β was significantly increased 22 days after completion of RT in patients with partial/complete response and stable disease but not in patients with progressive disease or death.
2.2 Damage-associated molecular patterns
Other interesting immune-related molecules that can act as predictive and prognostic biomarkers include DAMPs. Heat shock proteins (HSPs), particularly HSP70 and HSP90 (37, 119), are intracellular chaperones that can act as damage-associated molecular patterns (DAMPs) when exposed on the cell surface or released extracellularly during stress or cell death. In patients with NSCLC treated with immunotherapy and chemotherapy, increased plasma levels of HSP90 at diagnosis has shown to be prognostic for survival (119). In addition, high mobility group box 1 (HMGB1) and interferon-1 (IFN-I) were also investigated in patients with NSCLC whom have been treated with radiotherapy alone or in combination with immunotherapy (Ipilimumab), respectively. Whilst HMGB1 was not associated with survival rates, IFN-I showed to be predictive for treatment response (41).
2.3 Soluble receptors and ligands released from immune cell surface
Soluble receptors and ligands may also have potential predictive or prognostic value in patients with lung cancer treated with RT. Soluble receptors either are formed by alternative mRNA splicing, resulting in a polypeptide lacking a transmembrane region that is secreted by the cell, or it is a direct derivative from proteolytic cleavage of the membrane-bound receptor proteins from the cell surface (120). Receptors generally consist of a cytoplasmic domain, a transmembrane domain, and an extracellular domain. Soluble receptors generally comprise the extracellular domain and, therefore, retain the ability to bind the ligand (121). However, in contrast to membrane-bound receptors, soluble receptors cannot transmit signals to cells directly, but they can affect binding and activation of membrane receptors and co-receptors and, therefore, indirectly regulate cellular signaling (122). Recent studies have shown that circulating soluble receptors and ligands are potential cancer biomarkers, implicated in cancer progression, metastasis, immune evasion, and inflammation (44, 122, 123).
2.3.1 Soluble tumor necrosis factor receptor-1
As mentioned before, TNF-α is an important pro-inflammatory cytokine involved in many pathologies (124). It exerts its biological effects by binding to receptors like TNFR1, which is widely expressed and, upon activation, can promote the proliferation, apoptosis or metastasis of lung cancer cells (125, 126). Soluble TNFR1 (sTNFR1) is generated by proteolytic cleavage of membrane-bound receptors by TNF-α converting enzyme (TACE), leading to a transiently reduced cellular responsiveness (127, 128). Interestingly, Hinton et al. showed that patients with stage II-IV NSCLC have higher baseline sTNFR1 before chemoradiotherapy compared to healthy individuals. Also, a temporary decline (2–4 weeks during RT) of sTNFR1 and TACE levels were observed in patients with RILI (59). Furthermore, Wang et al. showed that increased sTNFR1 levels 8 weeks post-chemoradiation were positively associated with increased symptom burden (e.g., pain, fatigue, distress) in 62 patients with stage I-IV NSCLC (42).
2.3.2 Soluble interleukin-2 receptor
Interleukin-2 (IL-2) is a well-studied cytokine with pleiotropic effects (129). It can induce the activation of effector T cells and stimulate the growth of NK- and B-cells (130). IL-2R is expressed on various immune cells, varying from antigen-presenting cells to conventional T-cells and regulatory T-cells (Treg), releasing soluble IL-2 receptor (sIL-2R) upon immune activation (131). sIL-2R can modulate the biological function of IL-2 in serum (132). Carvalho et al. showed that higher concentrations of sIL-2R before start of treatment were associated with a worse OS in 181 patients with inoperable NSCLC who had undergone (chemo)-radiotherapy (43).
2.3.3 Soluble programmed cell death ligand 1
Soluble forms of immune checkpoints have also been identified as key modulators in cancer pathogenesis (133). The PD-1/PD-L1 pathway controls the induction and maintenance of immune tolerance within the TME, with PD-L1 binding to PD-1 on T-cells to inhibit the immune response (134). PD-L1 is mainly expressed on tumor cells and some immune cells under inflammatory conditions (135). The soluble form of PD-L1 (sPD-L1) is produced by shedding the transmembrane domain of PD-L1 (136). Zhao et al. showed that high baseline levels of sPD-L1 were correlated with worse OS in patients with inoperable NSCLC treated with RT (44). They also showed that sPD-L1 levels tended to decrease during RT and got back to baseline levels months after RT. Similarly, Sui et al. reported in 31 patients with unresectable NSCLC that low levels of sPD-L1 before treatment initiation were associated with an objective response to concurrent chemoradiotherapy (45).
2.3.4 Other soluble receptors and ligands
Other soluble receptors that were found to be related to treatment outcomes of patients with lung cancer treated with RT included CD244 and complement receptor 2 (CR2). CD244 (2B4) is a Signaling Lymphocyte Activation Molecule (SLAM) family immunomodulatory receptor that binds to high-affinity ligand CD48. CD244 is expressed by immune cells, such as monocytes, dendritic cells, NK cells, and T cells (137). Pre-clinical studies have shown that increased CD244 expression in the TME corresponds to increased immunosuppression via CD8+ T-cell exhaustion and increased production of immunosuppressors by MDSCs (138). Vaes et al. revealed that higher plasma CD244 levels before the first fraction of SBRT tended to be associated with a worse PFS in patients with stage I NSCLC (37). CR2 (Complement Receptor 2, or CD21) is a glycosylated transmembrane protein mainly expressed on B cells that binds to C3d, involved in linking the innate and adaptive immune system (139). Vaes et al. also showed that in patients with stage III NSCLC treated with concurrent chemoradiotherapy, higher levels of CR2 before the first fraction of RT were significantly associated with a better PFS (37).
2.4 Proteins on the immune cell surface of circulating immune cells
2.4.1 Cluster of differentiation molecules
Naive- and effector CD8+ (cytotoxic) T cells are crucial in immune surveillance and the adaptive immunity against infection and cancer (140). The predictive and prognostic value of various CD8+ T-cell subsets have already been shown. Kim et al. indicated that high levels of T-cells with a senescence phenotype (CD57+CD28−CD8+ T cells) before, during and after treatment are correlated with increased RILI in 54 patients with stage II-III NSCLC treated with concurrent chemoradiotherapy. Also, high levels of circulating CD57+CD28−CD8+ T-cells before treatment were an independent predictor of grade ≥2 RILI (60). Besides, Liu et al. indicated that high amounts of CD8+CD28+T cells before treatment are related to improved early response to SABR in patients with metastatic NSCLC (46). In addition, Zafra and associates investigated CD8+PD1+ and CD8+PDL1+ as predictive biomarkers after the first stereotactic ablation RT fraction (141). However, both were elevated in the responders and non-responders, making them not eligible as distinct biomarker.
2.4.2 T cell receptor repertoire
T-cell receptors (TCRs) are highly diverse heterodimeric surface receptors that mediate T-cell responses by recognizing specific antigens on major histocompatibility complex (MHC) molecules of antigen-presenting cells (APCs) (142). The spectrum of TCR epitopes responsible for tumor neoantigen recognition is diverse owing to the random formation of neoantigens, derived from numerous genetic alterations between patients (143). Each patient’s immune system must maintain a diversified TCR repertoire to recognize the variety of tumor neoantigens (144). Recent studies emphasize the role of TCR sequencing and repertoire analysis in understanding tumor biology, immune responses during treatment, and developing immunotherapies (145, 146). Increasing evidence indicated that the TCR repertoire changes after RT in patients with lung cancer (41, 147, 148). For example, Wu et al. indicated that the TCR repertoire diversity is reduced in patients with stage I NSCLC treated with SBRT (n=19). Moreover, diversity levels of TCR clones were lower after SBRT in the patients who developed distant metastases than in those who did not (47). Also, Öjlert et al. performed T-cell receptor sequencing in patients with stage IV NSCLC treated with SBRT and anti-PD-L1 (atezolizumab) immunotherapy (n=15) and showed decreased or stable diversity after RT in the best responders, and increased diversity at disease progression. Moreover, expansion of TCR clones was observed more often in responders (48). Similar results were reported by Formenti et al. in patients with metastatic lung cancer treated with CTLA-4 blockade combined with RT (41).
3 Discussion
The immune system’s diversity and the actual heterogeneous immune status before, during, and after treatment result in different treatment responses among patients. A reliable biomarker or preferably a panel of biomarkers to predict and monitor treatment responses is crucial for adjusting treatment protocols and personalizing interventions. This review summarized the current literature on peripheral immune-related molecules that may have prognostic and/or predictive value for outcomes and RT-induced toxicity in patients with lung cancer (Figure 1). Key molecules with a strong negative predictive value for treatment outcomes include IL-6, IL-8, and TGF-β1. Also, we observed that IL-6 and TGF-β1 are both prognostic and predictive for the development of RILI in patients with lung cancer. Other molecules, including IL-1, OPN, IP-10/CXCL10, IL-10, GM-CSF, IFN-β, IL-17A, IL-21, TNF-α, sTNFR1, sIL-2R, sPDL1, CD244, CR2, CD28+CD8+ T-cells, CD57+CD28-CD8 T-cells, and TCR repertoire have also been identified as potential prognostic or predictive biomarkers for patient outcomes. However, these molecules have only been assessed in a limited number of small-scale studies.
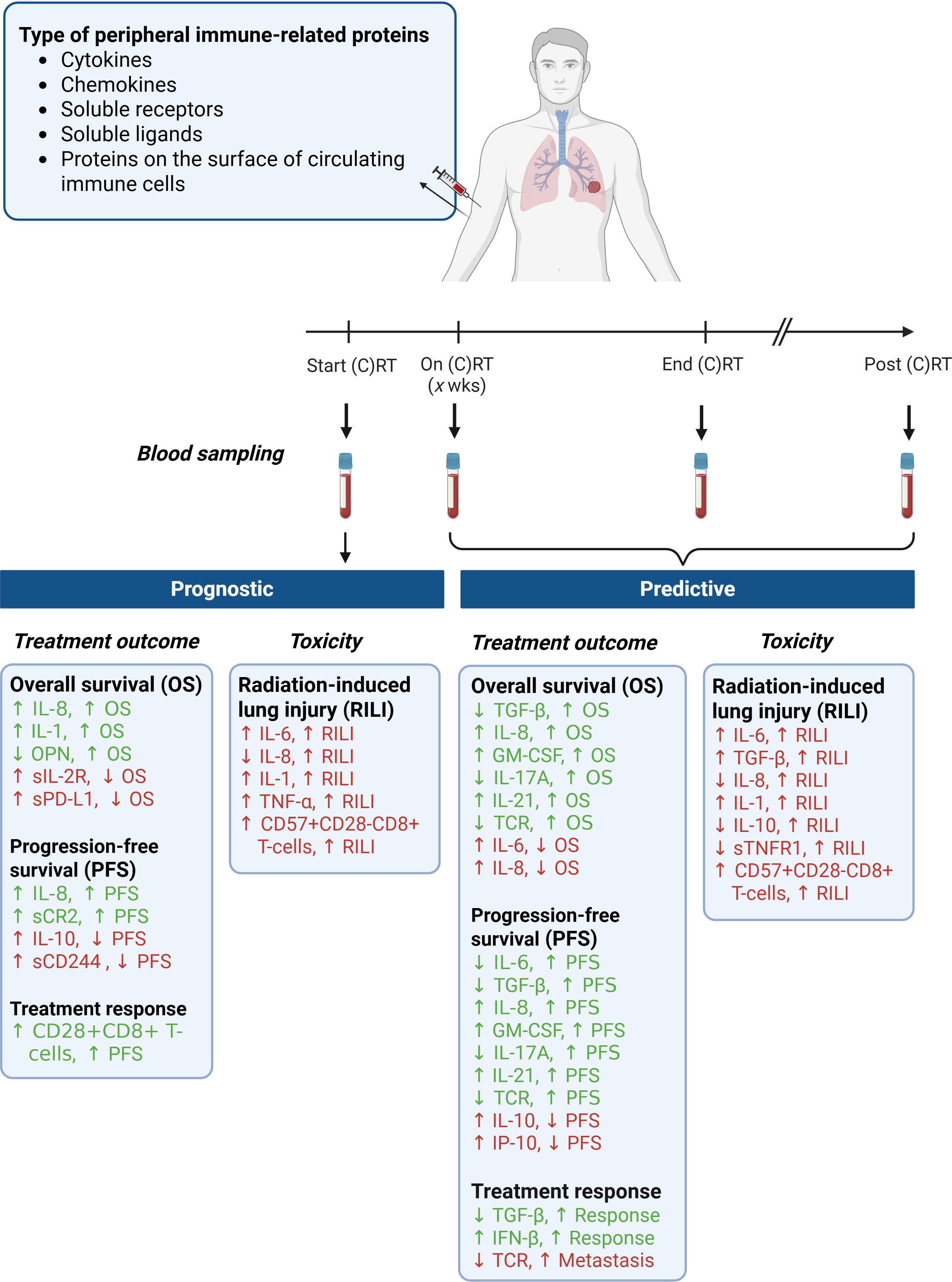
Figure 1. Prognostic and predictive biomarkers of peripheral immune-related proteins in patients with lung cancer treated with (chemo) radiotherapy. (C)RT, (chemo) radiotherapy; OS, overall survival; PFS, progression-free survival); RILI, radiation-induced lung injury. Created with BioRender.com.
In this review, we showed that circulating levels of IL-6, IL-8, and TGF-β1 in the blood of patients with lung cancer treated with RT were consistently negatively associated with survival and RT-related toxicity. Measuring these proteins and validating their usefulness in real-life datasets will lead to improved patient selection and tailored preventive strategies for RT-induced toxicity. Besides, there are several ongoing phase 1/2 clinical trials aiming to investigate the clinical benefit of including blockade of IL-6, IL-8 or TGF-β in the treatment of patients with advanced lung cancer (IL-6: NCT05704634; IL-8: NCT04123379, NCT04572451; TGF-β: NCT03732274, NCT05537051). As such, IL-6, IL-8, and TGF-β are potential biomarkers and targets that should be further explored.
Despite extensive knowledge about the biological roles of these molecules, their clinical relevance as prognostic or predictive biomarkers remains poorly understood since their local biological effect is not always consistent with the clinically assessed responses. For example, IL-1 can induce inflammatory responses and can play a role in cancer progression, and as such, it was expected that IL-1 would negatively correlate with patient outcome (149). However, Tang et al. showed that higher baseline IL-1α levels were associated with improved outcomes in patients with oligometastatic NSCLC, possibly due to a systemic anti-tumor inflammatory state exhibited by induction chemotherapy (36). Similarly, it has been shown that irradiation can induce the expression of PD-L1 on the tumor cell surface of lung cancer cells (150). However, Zhao et al. showed that circulating sPD-L1 levels tended to decrease during RT and normalized 3 months after RT (44). This might be a result of the tumor death occurred by treatment and the recovered level of sPD-L1 coming from the immune cell response to RT. Also, IL-8, which is a pro-inflammatory cytokine, can stimulate collagen synthesis and matrix production inducing lung fibroblasts (97). However, in contrast to these local biological functions, low circulating levels of IL-8 before and during RT were associated with a higher risk of RILI in patients with NSCLC (52). These inconsistent findings highlight the need for more research and prospective clinical trials to understand better the mechanisms and clinical implications of these markers in RT.
Peripheral immune-related biomarkers are promising in the field of RT and can provide information on the host’s actual immune status before, during, and after treatment. Also, blood-based biomarkers can be monitored longitudinally and multiple biomarkers can be assessed simultaneously without sample limitation. Nevertheless, to date, there are still no reliable prognostic or predictive blood-based biomarker(s) to predict treatment outcomes in patients with lung cancer treated with RT, despite the circulating levels of numerous potential biomarkers have been assessed in several clinical trials.
An emerging but still incompletely understood phenomenon in the context of radiotherapy combined with immunotherapy is the abscopal effect - a systemic antitumor response occurring beyond the irradiated field (151). This effect has attracted growing interest as a potential outcome measure in immuno-oncology studies (29, 35, 41, 141, 152). However, current literature on the abscopal effect remains limited and presents several challenges. To date, only Mathew and his colleagues reported that abscopal responses (9 out of 29 patients) at 4 weeks following treatment were associated with prolonged increase in dendritic cells subset DC1, T-helper 1-like CD4 T-cells and circulating IL-12 (152). Other studies only report indirect correlations between immune-related peripheral-blood markers and abscopal responses, using surrogate outcomes like survival outcomes or objective response rates – complete or partial responses evaluated in all sites of the disease (29, 35, 41, 141). Notably, even when considering all cancer types, the occurrence of the abscopal effect remains rare. A systematic review by Abuodeh et al. highlighted that only 46 cases of abscopal effects were reported between 1969 and 2014, amongst which only 3 patients with primary lung cancer (153). Aside from the limited population, there is also lack of standardized, quantifiable criteria for defining and assessing abscopal responses, making it hard to corroborate the results.
Even though it falls outside the scope of our review, we want to acknowledge the relevance of other molecular biomarkers with potential immunological effects that can be measured in blood, encompassing circulating tumor cells (154), ctDNA (155, 156), m(i)RNA (141) and exosomes (35). For future studies developing risk assessment models for RT in combination with immunotherapy, a broader spectrum of biomarkers in blood should be explored than solely immune-related molecules.
There are multiple challenges and limitations hampering both the identification and implementation of promising biomarkers in daily clinical practice (Figure 2). First of all, several types of biases causes failure in biomarker discovery and validation studies: for example, patient selection, specimen collection, specimen analysis, and patient evaluation. To date, numerous guidelines are available, providing researchers with an overview of practical considerations and potential pitfalls for their biomarker research (157, 158). Furthermore, recent technological advances have significantly enhanced the capacity for biomarker discovery, however, these innovations are often expensive. Consequently, many promising biomarkers remain confined to small-scale clinical studies, hampering the broader validation and clinical implementation. Also, the use of assays from different manufacturers further complicates the validation of promising biomarkers, while these vary in sensitivity and specificity. As a result, the outcomes from small-small scale studies cannot be compared. Also, most available tests are designated for research use only (RUO) and are therefore not validated for diagnostic applications. Lastly, the implementation of the ‘In vitro Diagnostic Medical Devices Regulation’ (IVDR), which replaces the IVD Directive 98/79/EC, have resulted in drastic changes for practically all stakeholders (i.e. manufacturers, notified bodies, medical laboratories) in the field of in vitro diagnostic medical devices. As a consequence, manufacturers have to make strong investments (i.e. time, resources, and budget) to meet these new regulatory requirements (159, 160). Also, such regulations hinder the innovative capacity of medical laboratories preventing the development of new IVDs that could improve patient care.
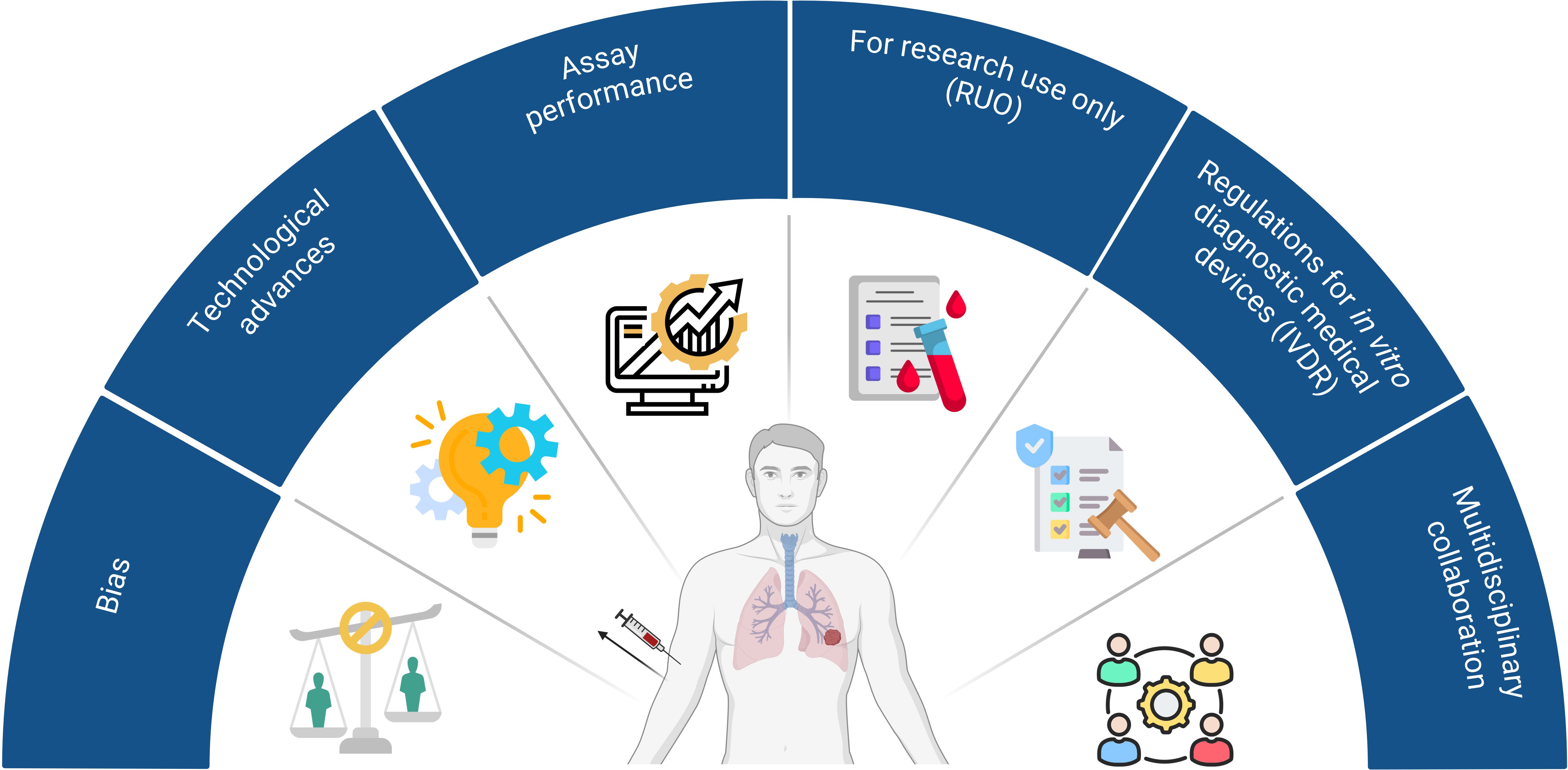
Figure 2. Current challenges and limitations to implement promising biomarkers in daily clinical care. 1) Bias is the main cause of failure in biomarker discovery and validation studies and can occur during patient selection, specimen collection, specimen analysis, and patient evaluation; 2) New technological advances have significantly enhanced the capacity for biomarker discovery, however, these innovations are expensive hampering the implementation thereof in large-scale studies; 3) Assays from different manufacturers vary in sensitivity and specificity. As a consequence, results from different trials cannot be compared. 4) Most assays are designated for research use only (RUO) and are not validated for diagnostic applications. 5) Manufacturers have to make strong investments (time, resources, and budget) to meet the new regulatory requirements to bring an in vitro diagnostic medical device (IVD) on the market (IVDR); 6) Lack of multidisciplinary collaboration among researchers, clinicians and industrial partners. Created with BioRender.com.
Despite all these efforts, only a small number of biomarkers have been successfully validated and implemented in daily clinical care. One key barrier to clinical translation is the lack of multidisciplinary collaboration among researchers, clinicians, and industry partners. Most trials included in our review only assessed the circulating levels of specific proteins in a small sample size. However, due to significant heterogeneity in study designs, patient populations, and methodologies, the findings are difficult to compare and cannot be reliably corroborated. First, blood samples have been collected at different timepoints before, during and after treatment. Second, the patient populations vary substantially between the studies, including variations in tumor stages and treatment regimen (e.g., types of systemic drugs and various fractionation schemes). For example, low-doses radiation therapy have shown promising immunomodulatory effects, potentially reversing tumor resistance to immunotherapy, compared to high-dose radiation schemes (161). However, in-vivo evidence is currently lacking for lung cancer on the effect of different radiation schemes. As such, to set the stage for widespread clinical implementation and acceptance of these new lung cancer biomarkers, efforts are needed to set up collaborative, multidisciplinary studies.
4 Conclusion
This review focused on peripheral blood immune-related biomarkers with potential prognostic or predictive value for patients with lung cancer treated with RT. These findings could help researchers and clinicians to further validate promising candidates in prospective trials and implement them in daily clinical practice.
Author contributions
SL: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. RV: Conceptualization, Data curation, Supervision, Visualization, Writing – original draft, Writing – review & editing. IL: Formal analysis, Visualization, Writing – review & editing. FC: Conceptualization, Writing – review & editing. LH: Conceptualization, Writing – review & editing. MV: Conceptualization, Supervision, Writing – review & editing. DD: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
FC reports outside of this manuscript personal fees as invited speaker from AstraZeneca, Roche, and Johnson & Johnson and as advisor to Regeneron and MSD; institutional funding as a local principal investigator PI from AstraZeneca and MSD; reports non-financial interests as member of the ESMO guideline committee on metastatic NSCLC. LH reports outside of this manuscript fees as an invited speaker from AstraZeneca, Bayer, Lilly, MSD, high5oncology, Takeda, Janssen, GSK, Sanofi, Pfizer, Medtalks, Benecke, VJOncology, Medimix; all payments were paid to the institution with the exception of Medtalks, Benecke, VJOncology, Medimix; fees paid to her institution for advisory board membership from Advisory boards: Abbvie, Amgen, Anhearth, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi, GSK, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, Summit Therapeutics, and Takeda; institutional research grants from Roche Genentech, AstraZeneca, Boehringer Ingelheim, Takeda, Merck, Pfizer, Novartis, and Gilead; institutional funding as a local principal investigator PI from AstraZeneca, GSK, Novartis, Merck, Roche, Takeda, Blueprint, Mirati, Abbvie, Gilead, MSD, Merck, Amgen, Boehringer Ingelheim, Pfizer, Daiichi, Amgen, and BMS. Member guideline committees: Dutch guidelines on NSCLC, brain metastases and leptomeningeal metastases, ESMO guidelines on metastatic NSCLC, non-metastatic NSCLC, and SCLC non-financial. Other non-financial: former secretary and current chair NVALT studies foundation, subchair of EORTC metastatic NSCLC systemic therapy, and vicechair scientific committee Dutch Thoracic Group. DR reports outside of this manuscript research grants from and support and advisor to: AstraZeneca, BMS, Beigene, Philips, and Olink institutional financial interests, no personal financial interests; Advisory board: Eli-Lily institutional financial interests, no personal financial interests.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. (2023) 18:181–93. doi: 10.1016/j.jtho.2022.10.003
3. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Domine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
4. Passaro A, Wang J, Wang Y, Lee SH, Melosky B, Shih JY, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study☆. Ann Oncol. (2024) 35:77–90. doi: 10.1016/j.annonc.2023.10.117
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines (NCCN Guidelines ®) in Oncology for Non-Small Cell Lung Cancer Version 7.2024. (Plymouth Meeting, PA, USA: National Comprehensive Cancer Network (NCCN)) (2024). Version 7.2024.
6. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines (NCCN Guidelines ®) in Oncology for Small Cell Lung Cancer Version 3.2024 (2024). (Plymouth Meeting, PA, USA: National Comprehensive Cancer Network (NCCN)).
7. Vinod SK and Hau E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology. (2020) 25:61–71. doi: 10.1111/resp.13870
8. De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, and Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. (2019) 5:13. doi: 10.1038/s41572-019-0064-5
9. Zhou T, Zhang LY, He JZ, Miao ZM, Li YY, Zhang YM, et al. Review: Mechanisms and perspective treatment of radioresistance in non-small cell lung cancer. Front Immunol. (2023) 14:1133899. doi: 10.3389/fimmu.2023.1133899
10. Busato F, Khouzai BE, and Mognato M. Biological mechanisms to reduce radioresistance and increase the efficacy of radiotherapy: state of the art. Int J Mol Sci. (2022) 23:10211. doi: 10.3390/ijms231810211
11. Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, and Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. (2012) 2:88. doi: 10.3389/fonc.2012.00088
12. Watanabe T, Sato GE, Yoshimura M, Suzuki M, and Mizowaki T. The mutual relationship between the host immune system and radiotherapy: stimulating the action of immune cells by irradiation. Int J Clin Oncol. (2023) 28:201–8. doi: 10.1007/s10147-022-02172-2
13. Chen Q, Sun L, and Chen ZJ. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat Immunol. (2016) 17:1142–9. doi: 10.1038/ni.3558
14. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. (2017) 8:15618. doi: 10.1038/ncomms15618
15. Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J immunother Cancer. (2020) 8:e000337. doi: 10.1136/jitc-2019-000337
16. Zhang C, Liang Z, Ma S, and Liu X. Radiotherapy and cytokine storm: risk and mechanism. Front Oncol. (2021) 11:670464. doi: 10.3389/fonc.2021.670464
17. Kim K-J, Kim J-H, Lee S, Lee E-J, Shin E-C, and Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. (2017) 8:41242–55. doi: 10.18632/oncotarget.17168
18. Zhao X, Li J, Zheng L, Yang Q, Chen X, Chen X, et al. Immune response on optimal timing and fractionation dose for hypofractionated radiotherapy in non–small-cell lung cancer. Front Mol Biosci. (2022) 9:786864. doi: 10.3389/fmolb.2022.786864
19. Portella L and Scala S. Ionizing radiation effects on the tumor microenvironment. Semin Oncol. (2019) 46:254–60. doi: 10.1053/j.seminoncol.2019.07.003
20. Giuranno L, Ient J, De Ruysscher D, and Vooijs MA. Radiation-induced lung injury (RILI). Front Oncol. (2019) 9:877. doi: 10.3389/fonc.2019.00877
21. Guipaud O, Jaillet C, Clément-Colmou K, François A, Supiot S, and Milliat F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br J Radiol. (1089) 2018:91. doi: 10.1259/bjr.20170762
22. Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, and Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. (2022) 22:124–38. doi: 10.1038/s41577-021-00568-1
23. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, and Ludwig M. Radiation-induced lung injury: assessment and management. Chest. (2019) 156:150–62. doi: 10.1016/j.chest.2019.03.033
24. Iyengar P, Zhang-Velten E, Court L, Westover K, Yan Y, Lin MH, et al. Accelerated hypofractionated image-guided vs conventional radiotherapy for patients with stage II/III non-small cell lung cancer and poor performance status: A randomized clinical trial. JAMA Oncol. (2021) 7:1497–505. doi: 10.1001/jamaoncol.2021.3186
25. Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, et al. Effect of postoperative radiotherapy for patients with pIIIA-N2 non–small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA Oncol. (2021) 7:1178–85. doi: 10.1001/jamaoncol.2021.1910
26. Mamdani H, Ahmed S, Armstrong S, Mok T, and Jalal SI. Blood-based tumor biomarkers in lung cancer for detection and treatment. Trans Lung Cancer Res. (2017) 6:648–60. doi: 10.21037/tlcr.2017.09.03
27. Lynch C, Pitroda SP, and Weichselbaum RR. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. (2024) 25:e352–e62. doi: 10.1016/S1470-2045(24)00075-5
28. Gkika E, Adebahr S, Brenner A, Schimek-Jasch T, Radicioni G, Exner JP, et al. Changes in blood biomarkers of angiogenesis and immune modulation after radiation therapy and their association with outcomes in thoracic Malignancies. Cancers. (2021) 13:5725. doi: 10.3390/cancers13225725
29. Ye H, Pang H, Shi X, Ren P, Huang S, Yu H, et al. Nivolumab and hypofractionated radiotherapy in patients with advanced lung cancer: ABSCOPAL-1 clinical trial. Front Oncol. (2021) 11:657024. doi: 10.3389/fonc.2021.657024
30. Luo J, Hu S, Wei T, Sun J, Liu N, and Wang J. TGF-beta 1 levels are associated with lymphocyte percentages in patients with lung cancer treated with radiation therapy. OncoTargets Ther. (2018) 11:8349–55. doi: 10.2147/ott.s175956
31. Zhao L, Ji W, Zhang L, Ou G, Feng Q, Zhou Z, et al. Changes of circulating transforming growth factor-²1 level during radiation therapy are correlated with the prognosis of locally advanced non-small cell lung cancer. J Thorac Oncol. (2010) 5:521–5. doi: 10.1097/JTO.0b013e3181cbf761
32. Fu ZZ, Gu T, Fu BH, Hua HX, Yang S, Zhang YQ, et al. Relationship of serum levels of VEGF and TGF-β1 with radiosensitivity of elderly patients with unresectable non-small cell lung cancer. Tumour Biol. (2014) 35:4785–9. doi: 10.1007/s13277-014-1628-3
33. Eide HA, Knudtsen IS, Sandhu V, Løndalen AM, Halvorsen AR, Abravan A, et al. Serum cytokine profiles and metabolic tumor burden in patients with non-small cell lung cancer undergoing palliative thoracic radiation therapy. Adv Radiat Oncol. (2018) 3:130–8. doi: 10.1016/j.adro.2017.12.007
34. Kang P, Liu D, Li L, Guo X, Ye Y, Li Y, et al. Interleukin 8 in plasma is an efficacy marker for advanced non-small cell lung cancer treated with hypofractionated radiotherapy and PD-1 blockade. Cytokine. (2023) 163:156133. doi: 10.1016/j.cyto.2023.156133
35. Ni J, Wang X, Wu L, Ai X, Chu Q, Han C, et al. Sintilimab in combination with stereotactic body radiotherapy and granulocyte-macrophage colony-stimulating factor in metastatic non-small cell lung cancer: The multicenter SWORD phase 2 trial. Nat Commun. (2024) 15:7242. doi: 10.1038/s41467-024-51807-7
36. Tang C, Lee W-C, Reuben A, Chang L, Tran H, Little L, et al. Immune and circulating tumor DNA profiling after radiation treatment for oligometastatic non-small cell lung cancer: translational correlatives from a mature randomized phase II trial. Int J Radiat OncologyBiologyPhysics. (2020) 106:349–57. doi: 10.1016/j.ijrobp.2019.10.038
37. Vaes RD, Reynders K, Sprooten J, Nevola KT, Rouschop KM, Vooijs M, et al. Identification of potential prognostic and predictive immunological biomarkers in patients with stage I and stage III non-small cell lung cancer (NSCLC): a prospective exploratory study. Cancers. (2021) 13:6259. doi: 10.3390/cancers13246259
38. Suwinski R, Giglok M, Galwas-Kliber K, Idasiak A, Jochymek B, Deja R, et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Cancer. (2019) 19:427. doi: 10.1186/s12885-019-5617-1
39. Ostheimer C, Bache M, Güttler A, Reese T, and Vordermark D. Prognostic information of serial plasma osteopontin measurement in radiotherapy of non-small-cell lung cancer. BMC Cancer. (2014) 14:858. doi: 10.1186/1471-2407-14-858
40. Deng G, Hu P, Zhang J, Liu Q, Liang N, Xie J, et al. Elevated serum granulocyte-macrophage colony-stimulating factor levels during radiotherapy predict favorable outcomes in lung and esophageal cancer. Oncotarget. (2016) 7:85142–50. doi: 10.18632/oncotarget.13202
41. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. (2018) 24:1845–51. doi: 10.1038/s41591-018-0232-2
42. Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behavior Immunity. (2010) 24:968–74. doi: 10.1016/j.bbi.2010.03.009
43. Carvalho S, Troost EG, Bons J, Menheere P, Lambin P, and Oberije C. Prognostic value of blood-biomarkers related to hypoxia, inflammation, immune response and tumour load in non-small cell lung cancer–A survival model with external validation. Radiother Oncol. (2016) 119:487–94. doi: 10.1016/j.radonc.2016.04.024
44. Zhao J, Zhang P, Wang J, Xi Q, Zhao X, Ji M, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Medicine. (2017) 96:e6102. doi: 10.1097/md.0000000000006102
45. Sui X, Jiang L, Teng H, Mi L, Li B, Shi A, et al. Prediction of clinical outcome in locally advanced non-small cell lung cancer patients treated with chemoradiotherapy by plasma markers. Front Oncol. (2021) 10:625911. doi: 10.3389/fonc.2020.625911
46. Liu C, Hu Q, Hu K, Su H, Shi F, Kong L, et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Trans Med. (2019) 17:120. doi: 10.1186/s12967-019-1872-9
47. Wu L, Zhu J, Rudqvist NP, Welsh J, Lee P, Liao Z, et al. T-cell receptor profiling and prognosis after stereotactic body radiation therapy for stage I non-small-cell lung cancer. Front Immunol. (2021) 12:719285. doi: 10.3389/fimmu.2021.719285
48. Öjlert ÅK, Nebdal D, Snapkov I, Olsen V, Kidman J, Greiff V, et al. Dynamic changes in the T cell receptor repertoire during treatment with radiotherapy combined with an immune checkpoint inhibitor. Mol Oncol. (2021) 15:2958–68. doi: 10.1002/1878-0261.13082
49. Fu ZZ, Peng Y, Cao LY, Chen YS, Li K, and Fu BH. Correlations between serum IL-6 levels and radiation pneumonitis in lung cancer patients: A meta-analysis. J Clin Lab analysis. (2016) 30:145–54. doi: 10.1002/jcla.21828
50. Jeong BK, Kim JH, Jung MH, Kang KM, and Lee YH. Cytokine profiles of non-small cell lung cancer patients treated with concurrent chemoradiotherapy with regards to radiation pneumonitis severity. J Clin Med. (2021) 10:699. doi: 10.3390/jcm10040699
51. Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. (2002) 12:26–33. doi: 10.1053/srao.2002.31360
52. Wang S, Campbell J, Stenmark MH, Zhao J, Stanton P, Matuszak MM, et al. Plasma levels of IL-8 and TGF-β1 predict radiation-induced lung toxicity in non-small cell lung cancer: A validation study. Int J Radiat OncologyBiologyPhysics. (2017) 98:615–21. doi: 10.1016/j.ijrobp.2017.03.011
53. Liu Y, Xia T, Zhang W, Zhong Y, Zhang L, Wang X, et al. Variations of circulating endothelial progenitor cells and transforming growth factor-beta-1 (TGF-β1) during thoracic radiotherapy are predictive for radiation pneumonitis. Radiat Oncol. (2013) 8:189. doi: 10.1186/1748-717X-8-189
54. Kim J-Y, Kim Y-S, Kim Y-K, Park H-J, Kim S-J, Kang J-H, et al. The TGF-β1 dynamics during radiation therapy and its correlation to symptomatic radiation pneumonitis in lung cancer patients. Radiat Oncol. (2009) 4:59. doi: 10.1186/1748-717X-4-59
55. Zhao L, Sheldon K, Chen M, Yin MS, Hayman JA, Kalemkerian GP, et al. The predictive role of plasma TGF-β1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung Cancer. (2008) 59:232–9. doi: 10.1016/j.lungcan.2007.08.010
56. Arpin D, Perol D, Blay J-Y, Falchero L, Claude L, Vuillermoz-Blas S, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol. (2005) 23:8748–56. doi: 10.1200/jco.2005.01.7145
57. Li B, Chen SH, Lu HJ, and Tan YH. Predictive values of TNF-α, IL-6, IL-10 for radiation pneumonitis. Iranian J Radiat Res. (2016) 14:173–9. doi: 10.1186/s12879-020-051852-2
58. Siva S, MacManus M, Kron T, Best N, Smith J, Lobachevsky P, et al. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PloS One. (2014) 9:e109560. doi: 10.1371/journal.pone.0109560
59. Hinton T, Karnak D, Tang M, Jiang R, Luo Y, Boonstra P, et al. Improved prediction of radiation pneumonitis by combining biological and radiobiological parameters using a data-driven Bayesian network analysis. Trans Oncol. (2022) 21:101428. doi: 10.1016/j.tranon.2022.101428
60. Kim KH, Pyo H, Lee H, Oh D, Noh JM, Ahn YC, et al. Association of T cell senescence with radiation pneumonitis in patients with non-small cell lung cancer. Int J Radiat OncologyBiologyPhysics. (2023) 115:464–75. doi: 10.1016/j.ijrobp.2022.07.018
61. Zhang JM and An J. Cytokines, inflammation, and pain. Int anesthesiol clinics. (2007) 45:27–37. doi: 10.1097/AIA.0b013e318034194e
62. Schaue D, Kachikwu EL, and McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. (2012) 178:505–23. doi: 10.1667/RR3031.1
63. Han D, Ybanez MD, Ahmadi S, Yeh K, and Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxidants Redox Signaling. (2009) 11:2245–63. doi: 10.1089/ars.2009.2611
64. Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways1. Radiat Res. (2003) 159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2
65. Liu RM and Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radical Biol Med. (2010) 48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026
66. Qian L, Hong JS, and Flood PM. Role of microglia in inflammation-mediated degeneration of dopaminergic neurons: neuroprotective effect of interleukin 10. J Neural Transm Supplementum. (2006) 70:367–71. doi: 10.1007/978-3-211-45295-0_56
67. Ohshima Y, Tsukimoto M, Takenouchi T, Harada H, Suzuki A, Sato M, et al. γ-Irradiation induces P2X7 receptor-dependent ATP release from B16 melanoma cells. Biochim Biophys Acta (BBA)-General Subjects. (2010) 1800:40–6. doi: 10.1016/j.bbagen.2009.10.008
68. Lierova A, Jelicova M, Nemcova M, Proksova M, Pejchal J, Zarybnicka L, et al. Cytokines and radiation-induced pulmonary injuries. J Radiat Res. (2018) 59:709–53. doi: 10.1093/jrr/rry067
69. Lee JJ, Kim HJ, Yang CS, Kyeong HH, Choi JM, Hwang DE, et al. A high-affinity protein binder that blocks the IL-6/STAT3 signaling pathway effectively suppresses non-small cell lung cancer. Mol Ther. (2014) 22:1254–65. doi: 10.1038/mt.2014.59
70. Rašková M, Lacina L, Kejík Z, Venhauerová A, Skaličková M, Kolář M, et al. The role of IL-6 in cancer cell invasiveness and metastasis–overview and therapeutic opportunities. Cells. (2022) 11:3698. doi: 10.3390/cells11223698
71. Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras–mutant lung cancer. Cancer Res. (2016) 76:3189–99. doi: 10.1158/0008-5472.CAN-15-2840
72. Ao X, Zhao L, Davis MA, Lubman DM, Lawrence TS, and Kong F-M. Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J Hematol Oncol. (2009) 2:6. doi: 10.1186/1756-8722-2-6
73. Wang X, Yang X, Tsai Y, Yang L, Chuang KH, Keng PC, et al. IL-6 Mediates Macrophage Infiltration after Irradiation via Up-regulation of CCL2/CCL5 in Non-small Cell Lung Cancer. Radiat Res. (2017) 187:50–9. doi: 10.1667/rr14503.1
74. Hartsell WF, Scott CB, Dundas GS, Mohiuddin M, Meredith RF, Rubin P, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer?: analysis of RTOG 91-03. Am J Clin Oncol. (2007) 30:368–76. doi: 10.1097/01.coc.0000260950.44761.74
75. Chaudhury A and Howe PH. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigné enigma. IUBMB Life. (2009) 61:929–39. doi: 10.1002/iub.239
76. Kubiczkova L, Sedlarikova L, Hajek R, and Sevcikova S. TGF-β – an excellent servant but a bad master. J Trans Med. (2012) 10:183. doi: 10.1186/1479-5876-10-183
77. Neel J-C, Humbert L, and Lebrun J-J. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. (2012) 2012:381428. doi: 10.5402/2012/381428
78. Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. (2012) 22:571–84. doi: 10.1016/j.ccr.2012.08.013
79. Farhood B, khodamoradi E, Hoseini-Ghahfarokhi M, Motevaseli E, Mirtavoos-Mahyari H, Eleojo Musa A, et al. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol Res. (2020) 155:104745. doi: 10.1016/j.phrs.2020.104745
80. Siegel PM and Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. (2003) 3:807–21. doi: 10.1038/nrc1208
81. Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. (2016) 7:52294–306. doi: 10.18632/oncotarget.10561
82. Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFβ Is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. (2015) 75:2232–42. doi: 10.1158/0008-5472.can-14-3511
83. Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, et al. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat Res. (2006) 166:839–48, 10. doi: 10.1667/RR0695.1
84. Du S, Bouquet S, Lo C-H, Pellicciotta I, Bolourchi S, Parry R, et al. Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non–small-cell lung cancer cells in vitro and in vivo. Int J Radiat OncologyBiologyPhysics. (2015) 91:91–9. doi: 10.1016/j.ijrobp.2014.09.026
85. Dai YH, Li XQ, Dong DP, Gu HB, Kong CY, and Xu Z. P27 promotes TGF-β-mediated pulmonary fibrosis via interacting with MTORC2. Can Respir J. (2019) 2019:7157861. doi: 10.1155/2019/7157861
86. Monceau V, Pasinetti N, Schupp C, Pouzoulet F, Opolon P, and Vozenin M-C. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug targets. (2010) 11:1395–404. doi: 10.2174/1389450111009011395
87. Biernacka A, Dobaczewski M, and Frangogiannis NG. TGF-β signaling in fibrosis. Growth factors. (2011) 29:196–202. doi: 10.3109/08977194.2011.595714
88. Zhao L, Wang L, Ji W, Wang X, Zhu X, Hayman JA, et al. Elevation of plasma TGF-β1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: A combined analysis from Beijing and Michigan. Int J Radiat OncologyBiologyPhysics. (2009) 74:1385–90. doi: 10.1016/j.ijrobp.2008.10.065
89. Brat DJ, Bellail AC, and Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncology. (2005) 7:122–33. doi: 10.1215/s1152851704001061
90. Holmes WE, Lee J, Kuang WJ, Rice GC, and Wood WI. Structure and functional expression of a human interleukin-8 receptor. Sci (New York NY). (1991) 253:1278–80. doi: 10.1126/science.1840701
91. Waugh DJJ and Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. (2008) 14:6735–41. doi: 10.1158/1078-0432.ccr-07-4843
92. Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martín-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across Malignancies of multiple tissue origins. Clin Cancer Res. (2014) 20:5697–707. doi: 10.1158/1078-0432.CCR-13-3203
93. Young RJ, Tin A, Brown N, Jitlal M, Lee S, and Woll P. Analysis of circulating angiogenic biomarkers from patients in two phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br J cancer. (2012) 106:1153–9. doi: 10.1038/bjc.2012.50
94. Luppi F, Longo AM, de Boer WI, Rabe KF, and Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. (2007) 56:25–33. doi: 10.1016/j.lungcan.2006.11.014
95. Song Y-H, Chai Q, Wang N-l, Yang F-F, Wang G-H, and Hu J-Y. X-rays induced IL-8 production in lung cancer cells via p38/MAPK and NF-κB pathway. Int J Radiat Biol. (2020) 96:1374–81. doi: 10.1080/09553002.2020.1683643
96. Oweida A, Sabri S, Al-Rabea A, Ebrahimi M, Ruo R, Fraser R, et al. Response to stereotactic ablative radiotherapy in a novel orthotopic model of non-small cell lung cancer. Oncotarget. (2018) 9:1630–40. doi: 10.18632/oncotarget.22727
97. Kühlmann UC, Chwieralski CE, Reinhold D, Welte T, and Bühling F. Radiation-induced matrix production of lung fibroblasts is regulated by interleukin-8. Int J Radiat Biol. (2009) 85:138–43. doi: 10.1080/09553000802641136
98. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. (2018) 281:8–27. doi: 10.1111/imr.12621
99. Mawatari M, Kohno K, Mizoguchi H, Matsuda T, Asoh K, Van Damme J, et al. Effects of tumor necrosis factor and epidermal growth factor on cell morphology, cell surface receptors, and the production of tissue inhibitor of metalloproteinases and IL-6 in human microvascular endothelial cells. J Immunol (Baltimore Md: 1950). (1989) 143:1619–27. doi: 10.4049/jimmunol.143.5.1619
100. Österreicher J, Škopek J, Jahns J, Hildebrandt G, Psutka J, Vilasová Z, et al. β1-Integrin and Il-1α expression as bystander effect of medium from irradiated cells: the pilot study. Acta Histochemica. (2003) 105:223–30. doi: 10.1078/0065-1281-00710
101. Schindler R, Ghezzi P, and Dinarello C. IL-1 induces IL-1. IV. IFN-gamma suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol (Baltimore Md: 1950). (1990) 144:2216–22. doi: 10.4049/jimmunol.144.6.2216
102. Garlanda C and Mantovani A. Interleukin-1 in tumor progression, therapy, and prevention. Cancer Cell. (2021) 39:1023–7. doi: 10.1016/j.ccell.2021.04.011
103. Rübe CE, Wilfert F, Palm J, König J, Burdak-Rothkamm S, Liu L, et al. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlentherapie und Onkologie. (2004) 180:442. doi: 10.1007/s00066-004-1265-7
104. Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, and Finkelstein JN. Early and Persistent Alterations in the Expression of Interleukin-1α, Interleukin-1β and Tumor Necrosis Factor α mRNA Levels in Fibrosis-Resistant and Sensitive Mice after Thoracic Irradiation. Radiat Res. (1996) 145:762–7. doi: 10.2307/3579368
105. Kang AR, Cho JH, Lee N-G, Kwon J-H, Song J-Y, Hwang S-G, et al. Radiation-induced IL-1β expression and secretion promote cancer cell migration/invasion via activation of the NF-κB–RIP1 pathway. Biochem Biophys Res Commun. (2021) 534:973–9. doi: 10.1016/j.bbrc.2020.10.057
106. Trovo M, Giaj-Levra N, Furlan C, Bortolin MT, Muraro E, Polesel J, et al. Stereotactic body radiation therapy and intensity modulated radiation therapy induce different plasmatic cytokine changes in non-small cell lung cancer patients: a pilot study. Clin Trans Oncol. (2016) 18:1003–10. doi: 10.1007/s12094-015-1473-x
107. Chen Y, Hyrien O, Williams J, Okunieff P, Smudzin T, and Rubin P. Interleukin (IL)-1A and IL-6: Applications to the predictive diagnostic testing of radiation pneumonitis. Int J Radiat OncologyBiologyPhysics. (2005) 62:260–6. doi: 10.1016/j.ijrobp.2005.01.041
108. Conti P, Kempuraj D, Kandere K, Gioacchino MD, Barbacane RC, Castellani ML, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Letter. (2003) 86:123–9. doi: 10.1016/S0165-2478(03)00002-6
109. Wilke CM, Wei S, Wang L, Kryczek I, Kao J, and Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer immunology immunother: CII. (2011) 60:1529–41. doi: 10.1007/s00262-011-1104-5
110. Standiford TJ and Deng JC. INTERLEUKINS | IL-10. In: Laurent GJ and Shapiro SD, editors. Encyclopedia of Respiratory Medicine. Academic Press, Oxford (2006). p. 373–7.
111. Ran J, Wang J, Dai Z, Miao Y, Gan J, Zhao C, et al. Irradiation-induced changes in the immunogenicity of lung cancer cell lines: based on comparison of X-rays and carbon ions. Front Public Health. (2021) 9:666282. doi: 10.3389/fpubh.2021.666282
112. Tan Y, Zhao L, Yang YG, and Liu W. The role of osteopontin in tumor progression through tumor-associated macrophages. Front Oncol. (2022) 12:953283. doi: 10.3389/fonc.2022.953283
113. Overgaard J, Eriksen JG, Nordsmark M, Alsner J, and Horsman MR. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. (2005) 6:757–64. doi: 10.1016/S1470-2045(05)70292-8
114. Smolska-Ciszewska B, Mrochem-Kwarciak J, Karczmarczyk K, Deja R, and Suwiński R. Pretreatment plasma osteopontin level as a marker of response to curative radiotherapy and hormonal treatment for prostate cancer. Radiother Oncol. (2024) 200:110518. doi: 10.1016/j.radonc.2024.110518
115. Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun W, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. (2005) 11:4646–52. doi: 10.1158/1078-0432.ccr-04-2013
116. Deniz ÇD, Gürbilek M, and Koç M. Prognostic value of interferon-gamma, interleukin-6, and tumor necrosis factor-alpha in the radiation response of patients diagnosed with locally advanced non-small-cell lung cancer and glioblastoma multiforme. Turkish J Med Sci. (2018) 48:117–23. doi: 10.3906/sag-1611-77
117. Wang ZY and Bjorling DE. Tumor necrosis factor-α induces expression and release of interleukin-6 by human urothelial cells. Inflammation Res. (2011) 60:525–32. doi: 10.1007/s00011-010-0298-x
118. van de Laar L, Coffer PJ, and Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. (2012) 119:3383–93. doi: 10.1182/blood-2011-11-370130
119. Chen S, Yu Q, and Zhou S. Plasmatic levels of HSP90alpha at diagnosis: A novel prognostic indicator of clinical outcome in advanced lung cancer patients treated with PD-1/PD-L1 inhibitors plus chemotherapy. Front Oncol. (2021) 11:765115. doi: 10.3389/fonc.2021.765115
120. Heaney ML and Golde DW. Soluble receptors in human disease. J leukocyte Biol. (1998) 64:135–46. doi: 10.1002/jlb.64.2.135
121. Park EJ and Lee CW. Soluble receptors in cancer: mechanisms, clinical significance, and therapeutic strategies. Exp Mol Med. (2024) 56:100–9. doi: 10.1038/s12276-023-01150-6
122. Kefaloyianni E. Soluble forms of cytokine and growth factor receptors: mechanisms of generation and modes of action in the regulation of local and systemic inflammation. FEBS Letter. (2022) 596:589–606. doi: 10.1002/1873-3468.14305
123. Wang Q, Zhang J, Tu H, Liang D, Chang DW, Ye Y, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J immunother cancer. (2019) 7:334. doi: 10.1186/s40425-019-0810-y
124. Idriss HT and Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microscopy Res technique. (2000) 50:184–95. doi: 10.1002/1097-0029(20000801)50:3<184::aid-jemt2>3.0.co;2-h
125. Waetzig GH, Rosenstiel P, Arlt A, Till A, Bräutigam K, Schäfer H, et al. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-β1. FASEB J. (2005) 19:91–3. doi: 10.1096/fj.04-2073fje
126. Shi G and Hu Y. TNFR1 and TNFR2, which link NF-κB activation, drive lung cancer progression, cell dedifferentiation, and metastasis. Cancers. (2023) 15:4299. doi: 10.3390/cancers15174299
127. Waetzig GH, Rosenstiel P, Arlt A, Till A, Bräutigam K, Schäfer H, et al. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-beta1. FASEB J. (2005) 19:91–3. doi: 10.1096/fj.04-2073fje
128. Porteu F and Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. (1990) 172:599–607. doi: 10.1084/jem.172.2.599
129. Skrombolas D and Frelinger JG. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev Clin Immunol. (2014) 10:207–17. doi: 10.1586/1744666x.2014.875856
130. Liao W, Lin JX, and Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. (2013) 38:13–25. doi: 10.1016/j.immuni.2013.01.004
131. Damoiseaux J. The IL-2 – IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin Immunol. (2020) 218:108515. doi: 10.1016/j.clim.2020.108515
132. Yang ZZ, Grote DM, Ziesmer SC, Manske MK, Witzig TE, Novak AJ, et al. Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. (2011) 118:2809–20. doi: 10.1182/blood-2011-03-340885
133. Chakrabarti R, Kapse B, and Mukherjee G. Soluble immune checkpoint molecules: Serum markers for cancer diagnosis and prognosis. Cancer Rep. (2019) 2:e1160. doi: 10.1002/cnr2.1160
134. Han Y, Liu D, and Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10:727–42.
135. Sharpe AH, Wherry EJ, Ahmed R, and Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. (2007) 8:239–45. doi: 10.1038/ni1443
136. Hassounah NB, Malladi VS, Huang Y, Freeman SS, Beauchamp EM, Koyama S, et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol Immunother. (2019) 68:407–20. doi: 10.1007/s00262-018-2284-z
137. Agresta L, Hoebe KHN, and Janssen EM. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front Immunol. (2018) 9:2809. doi: 10.3389/fimmu.2018.02809
138. Wherry EJ and Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. (2015) 15:486–99. doi: 10.1038/nri3862
139. Ram S. Complement. In: Schmidt TM, editor. Encyclopedia of Microbiology, 4th ed. Academic Press, Oxford (2019). p. 714–28.
140. Raskov H, Orhan A, Christensen JP, and Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. (2021) 124:359–67. doi: 10.1038/s41416-020-01048-4
141. Zafra J, Onieva JL, Oliver J, Garrido-Barros M, Gonzalez-Hernandez A, Martinez-Galvez B, et al. Novel blood biomarkers for response prediction and monitoring of stereotactic ablative radiotherapy and immunotherapy in metastatic oligoprogressive lung cancer. Int J Mol Sci. (2024) 25:4533. doi: 10.3390/ijms25084533
142. Frank ML, Lu K, Erdogan C, Han Y, Hu J, Wang T, et al. T-cell receptor repertoire sequencing in the era of cancer immunotherapy. Clin Cancer Res. (2023) 29:994–1008. doi: 10.1158/1078-0432.ccr-22-2469
143. Hebeisen M, Allard M, Gannon PO, Schmidt J, Speiser DE, and Rufer N. Identifying individual T cell receptors of optimal avidity for tumor antigens. Front Immunol. (2015) 6:582. doi: 10.3389/fimmu.2015.00582
144. Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. (2017) 171:934–49.e16. doi: 10.1016/j.cell.2017.09.028
145. Mazzotti L, Gaimari A, Bravaccini S, Maltoni R, Cerchione C, Juan M, et al. T-Cell receptor repertoire sequencing and its applications: focus on infectious diseases and cancer. Int J Mol Sci. (2022) 23:8590. doi: 10.3390/ijms23158590
146. Li N, Yuan J, Tian W, Meng L, and Liu Y. T-cell receptor repertoire analysis for the diagnosis and treatment of solid tumor: A methodology and clinical applications. Cancer Commun (London England). (2020) 40:473–83. doi: 10.1002/cac2.12074
147. Zhou P, Chen D, Zhu B, Chen W, Xie Q, Wang Y, et al. Stereotactic body radiotherapy is effective in modifying the tumor genome and tumor immune microenvironment in non-small cell lung cancer or lung metastatic carcinoma. Front Immunol. (2021) 11:594212. doi: 10.3389/fimmu.2020.594212
148. Baxevanis CN, Gritzapis AD, Voutsas IF, Batsaki P, Goulielmaki M, Adamaki M, et al. T-cell repertoire in tumor radiation: the emerging frontier as a radiotherapy biomarker. Cancers. (2022) 14:2674. doi: 10.3390/cancers14112674
149. Gelfo V, Romaniello D, Mazzeschi M, Sgarzi M, Grilli G, Morselli A, et al. Roles of IL-1 in cancer: from tumor progression to resistance to targeted therapies. Int J Mol Sci. (2020) 21:6009. doi: 10.3390/ijms21176009
150. Liu M, Wang X, Li W, yu X, Flores-Villanueva P, Xu-Monette Z, et al. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis. (2020) 9:72. doi: 10.1038/s41389-020-00257-z
151. Reynders K, Illidge T, Siva S, Chang JY, and De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. (2015) 41:503–10. doi: 10.1016/j.ctrv.2015.03.011
152. Mathew D, Ohri N, Ngiow SF, Halmos B, Wumesh KC, Garg M, et al. Revitalizing systemic immune responses in advanced NSCLC using FLT3L and SBRT. medRxiv. (2025) [preprint]. doi: 10.1101/2025.01.29.25321332
153. Abuodeh Y, Venkat P, and Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. (2016) 40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001
154. Xu Y, Ren X, Jiang T, Lv S, Gao K, Liu Y, et al. Circulating tumor cells (CTCs) and hTERT gene expression in CTCs for radiotherapy effect with lung cancer. BMC Cancer. (2023) 23:475. doi: 10.1186/s12885-023-10979-z
155. Lebow ES, Eichholz J, Boe L, Zhang Z, Kratochvil LB, Gelblum DY, et al. Cell-free circulating tumor DNA for nonInvasive molecular profiling among patients undergoing definitive chemoradiation for locally advanced lung cancer. Adv Radiat Oncol. (2025) 10:101727. doi: 10.1016/j.adro.2025.101727
156. Yang K, Noh JM, Kim YJ, and Pyo H. Early dynamics of circulating tumor DNA following curative hypofractionated radiotherapy related to disease control in lung cancer. Diagnostics (Basel). (2025) 15:1198. doi: 10.3390/diagnostics15101198
157. Ou FS, Michiels S, Shyr Y, Adjei AA, and Oberg AL. Biomarker discovery and validation: statistical considerations. J Thorac Oncol. (2021) 16:537–45. doi: 10.1016/j.jtho.2021.01.1616
158. Simon RM, Paik S, and Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Institute. (2009) 101:1446–52. doi: 10.1093/jnci/djp335
159. Lubbers BR, Schilhabel A, Cobbaert CM, Gonzalez D, Dombrink I, Brüggemann M, et al. The new EU regulation on in vitro diagnostic medical devices: implications and preparatory actions for diagnostic laboratories. HemaSphere. (2021) 5:e568. doi: 10.1097/HS9.0000000000000568
160. Spitzenberger F, Patel J, Gebuhr I, Kruttwig K, Safi A, and Meisel C. Laboratory-developed tests: design of a regulatory strategy in compliance with the international state-of-the-art and the regulation (EU) 2017/746 (EU IVDR [In vitro diagnostic medical device regulation]). Ther Innovation Regul Science. (2022) 56:47–64. doi: 10.1007/s43441-021-00323-7
Keywords: lung cancer, radiotherapy, biomarkers, prognostic, predictive, peripheral blood, immune-related proteins
Citation: Lyu S, Vaes RDW, Laven IEWG, Cortiula F, Hendriks LEL, Vooijs MA and De Ruysscher DKM (2025) The prognostic and predictive value of peripheral immune-related proteins in patients with lung cancer treated with radiotherapy. Front. Oncol. 15:1625212. doi: 10.3389/fonc.2025.1625212
Received: 08 May 2025; Accepted: 17 June 2025;
Published: 17 July 2025.
Edited by:
Udo S. Gaipl, University Hospital Erlangen, GermanyReviewed by:
Gabriele Multhoff, Technical University of Munich, GermanyYona Keisari, Tel Aviv University, Israel
Copyright © 2025 Lyu, Vaes, Laven, Cortiula, Hendriks, Vooijs and De Ruysscher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rianne D. W. Vaes, cmlhbm5lLnZhZXNAbWFhc3Ryby5ubA==
 Shaowen Lyu
Shaowen Lyu Rianne D. W. Vaes
Rianne D. W. Vaes Iris E. W. G. Laven1
Iris E. W. G. Laven1 Francesco Cortiula
Francesco Cortiula Lizza E. L. Hendriks
Lizza E. L. Hendriks Marc A. Vooijs
Marc A. Vooijs Dirk K. M. De Ruysscher
Dirk K. M. De Ruysscher