Abstract
Cervical mucosal melanoma is a rare, aggressive malignancy with poor prognosis due to delayed diagnosis and limited treatment efficacy. Current therapies, including immune checkpoint inhibitors (e.g., anti-PD-1 agents), chemotherapy, and targeted therapies, yield suboptimal response rates (<30%) and frequent resistance. Here, we report the first case of HER2-positive cervical mucosal melanoma successfully treated with T-DXd combined with Toripalimab. This combination induced significant tumor shrinkage (partial response) and demonstrated safety, highlighting the potential of ADC-based cross-tumor therapies and immune-targeted synergy. These findings support further clinical trials to validate this strategy in mucosal melanoma, addressing unmet needs in this refractory subtype.
Introduction
Cervical mucosal melanoma is a highly aggressive and rare malignant tumor, accounting for 1%-2% of all melanomas (1). Its biological behavior significantly differs from that of skin or extremity melanomas, exhibiting more pronounced local invasiveness and poorer prognosis (2). Due to its unique anatomical location and lack of early symptoms, most patients are diagnosed at advanced or locally unresectable stages. Currently, standard treatments for unresectable mucosal melanoma include immune checkpoint inhibitors (e.g., anti-PD-1 monoclonal antibodies), chemotherapy, and targeted therapy; however, the overall efficacy remains limited, with an objective response rate of less than 30%, and rapid progression is common (3). In recent years, Toripalimab, a domestic PD-1 inhibitor, has shown promise in metastatic mucosal melanoma (4). Phase II clinical trials have demonstrated that Toripalimab combined with Axitinib as neoadjuvant therapy induces an objective response rate of 48.3%, significantly increasing tumor-infiltrating CD3+ and CD8+ T lymphocytes, suggesting that immune microenvironment reprogramming may enhance antitumor effects (5). Additionally, in the adjuvant setting, Toripalimab has been shown to significantly prolong relapse-free survival compared to high-dose interferon-α2b and has better tolerability (6). However, monotherapy still faces challenges of primary or secondary resistance, highlighting the need for new combined therapeutic strategies.
Trastuzumab Deruxtecan (T-DXd) is an antibody-drug conjugate (ADC) targeting HER2, which exerts a “bystander effect” via its topoisomerase I inhibitor payload and has demonstrated significant activity in HER2-low expressing tumors (7). Although T-DXd has been approved for use in breast cancer, non-small cell lung cancer, gastric cancer, and gynecologic tumors (8, 9), its application in cervical mucosal melanoma has not been reported. T-DXd selectively targets HER2-positive tumor cells and induces cell lysis, leading to the release of tumor-associated antigens and remodeling of the tumor microenvironment, thereby enhancing tumor immunogenicity and facilitating antigen presentation (10). Meanwhile, toripalimab, a humanized monoclonal antibody against PD-1, blocks the PD-1/PD-L1 axis, thereby restoring T-cell function and promoting antitumor immune responses (11). Preclinical and early-phase clinical studies have demonstrated that the combination of these agents exhibits a favorable safety profile and promising antitumor activity across various solid malignancies (12, 13). Their complementary mechanisms of action provide a strong rationale for use in patients with HER2-positive tumors and an immunologically active tumor microenvironment. Based on genetic profiling that identified HER2 overexpression and/or amplification/mutation in the tumor, this study reports the first successful case of treating HER2-positive cervical mucosal malignant melanoma with T-DXd in combination with Toripalimab. This approach offers a new treatment direction for this refractory subtype. The significant tumor shrinkage (partial response) and safety data not only validate the feasibility of applying ADCs across tumor types but also lay an important foundation for future clinical trials exploring targeted-immune combination therapies in mucosal melanoma.
Case report
The patient is a 54-year-old female who presented with a four-month history of a cervical mass, discovered on routine gynecological examination on September 14, 2023. Physical examination revealed a 4 × 3 × 2 cm red, papillary, lobulated mass located on the anterior lip of the cervix. The lesion had a smooth surface and exhibited slight hemorrhagic changes. On September 15, 2023, a hysteroscopic examination was performed, and pathology showed proliferative endometrium in the uterine cavity (Figure 1A). The immunohistochemical results show AE1/AE3 (−), CD20 (−), CD3 (−), CD56 (partially +), CD10 (partially +), ER (−), CEA (−), CgA (−), Desmin (−), P16 (−), Ki-67 (index 80%), PR (−), p53 (wild-type), S-100 (+), Syn (−), SMA (−), TTF-1 (−), with scattered positivity for Melan-A and HMB45, and diffuse positivity for SOX10. Based on the histopathological and immunohistochemical findings, a diagnosis of primary malignant melanoma of the cervical mucosa was rendered.
Figure 1
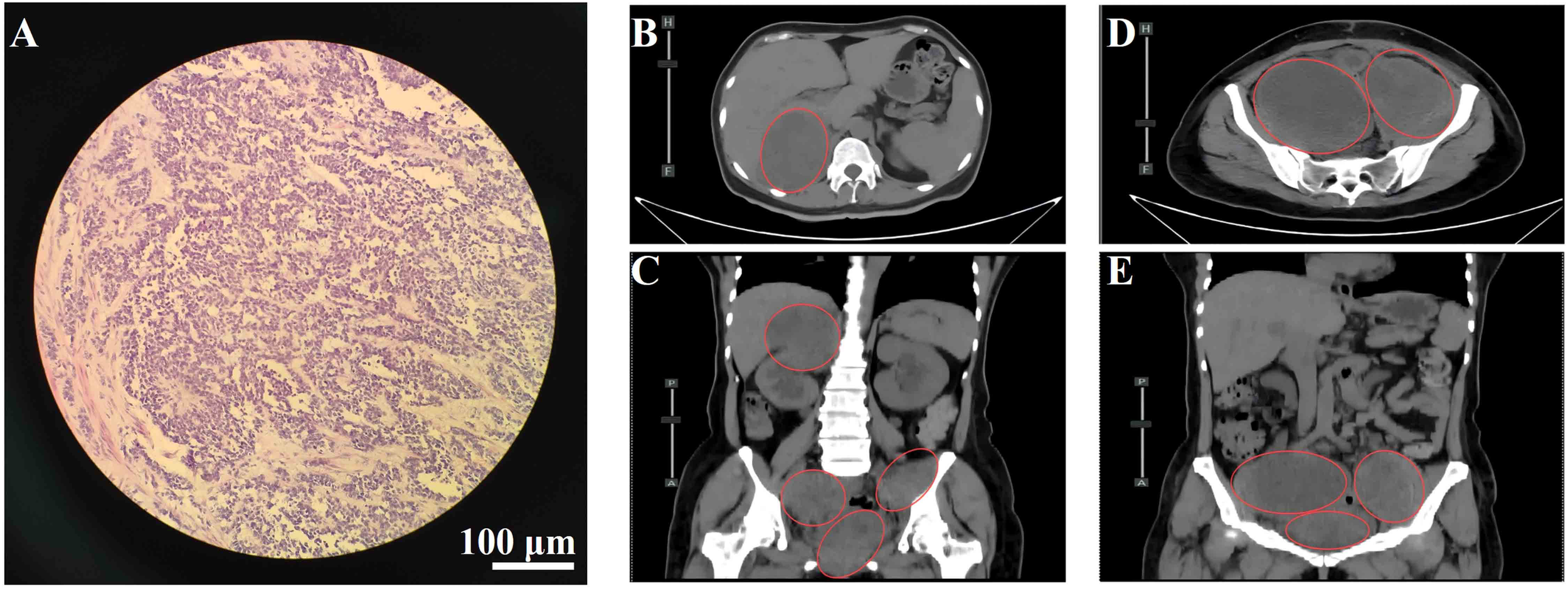
(A) Hematoxylin and eosin (H&E) staining of cervical tissue showing malignant melanoma. Scale bar = 100 μm; magnification, 200×. (B) Axial view of lesion in the hepatorenal space. (C) Coronal view of the hepatorenal space lesion. (D) Axial view of pelvic lesion. (E) Coronal view of the pelvic lesion. Red circles indicate metastatic lesions.
On January 8, 2024, a PET-CT scan was performed, revealing a cervical mass with soft tissue density and increased metabolic activity, consistent with high metabolic activity and suggestive of malignancy. A small lymph node along the right iliac vessel showed mild metabolic increase, indicating possible metastasis, and was recommended for follow-up. Additionally, a small ground-glass nodule in the left upper lobe of the lung exhibited no abnormal metabolic activity, likely representing a non-specific lesion, and was also recommended for follow-up. The scan also identified multiple small pulmonary nodules, likely inflammatory in nature, as well as liver cysts and multiple cysts in the ovaries. There was minimal pelvic fluid present, alongside a small area of softening in the left basal ganglia, and degenerative changes in the spine were noted.
On January 17, 2024, the patient underwent a radical hysterectomy, bilateral adnexectomy, and pelvic lymph node dissection under general anesthesia. Pathology confirmed cervical malignant melanoma (pT4aN0M0, stage IIB). The tumor was located in the cervical canal, measuring 3 × 3 cm, and invaded nearly the full thickness of the cervix. There were no signs of vaginal wall involvement, and the lymph nodes (left 8, right 6) were negative for metastasis. Immunohistochemistry was positive for Vimentin, S-100, SOX10, HMB45, Melan-A, and Ki-67 (80%+), with negative markers for CD45 and CK(P). No adjuvant therapy was administered postoperatively due to the patient’s refusal. The patient was readmitted on August 12, 2024, due to worsening bilateral leg pain for 3 weeks, which had intensified in the past week. On August 14, 2024, an abdominal CT revealed multiple metastatic lesions, including a low-density mass at the liver’s right lobe boundary measuring 3.3 × 3.5 cm, masses in the retroperitoneal space, right kidney, and pelvis with significant tumor invasion near the bladder and rectum, and bone destruction in the T10 vertebra, suspected to be metastatic (Figures 1B–E). These findings were consistent with disease recurrence, now classified as cT4aN0M1c, stage IV, with metastases to the bladder, rectum, liver, lungs, bones, abdomen, and pelvis.
The patient developed acute renal failure due to tumor progression and was recommended for nephrostomy to relieve obstruction, followed by antitumor treatment. On August 16, 2024, bilateral nephrostomy was performed under local anesthesia, and kidney function gradually improved thereafter. Next-generation sequencing revealed no detectable mutations in MET, KRAS, NRAS, ROS1, BRAF, or KIT. However, the tumor harbored both high-level ERBB2 (HER2) amplification, with an estimated copy number of 74.5, and a pathogenic ERBB2 point mutation (c.2264T>C, p.L755S, exon 19), detected at a high variant allele frequency of 98.36%. Given the patient’s acute renal injury and elevated creatinine levels, traditional chemotherapy was considered unsuitable due to compromised renal function. Genetic testing revealed the presence of HER2 co-alteration, including both HER2 amplification and a HER2 point mutation, suggesting potential sensitivity to HER2-targeted therapy. Therefore, the patient was administered an exploratory treatment regimen consisting of T-DXd (200 mg on Day 1) and toripalimab (240 mg on Day 1), for a total of nine treatment cycles. (Figure 2). Imaging assessments (chest and abdominal CT scans) were performed at approximately six-week intervals during the treatment period, specifically on October 2, 2024; November 12, 2024; December 24, 2024; and February 6, 2025. On September 11, 2024, following two cycles (approximately six weeks), a partial response (PR) was observed, with significant tumor shrinkage as assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. Subsequent evaluations on October 23, 2024, December 4, 2024, and January 16, 2025, showed stable disease (SD), with no progression but no further significant shrinkage of the tumors (Figure 3). As of February 7, 2025, the patient continues maintenance therapy with the same regimen and is being closely monitored for further disease progression. During the treatment period, complete blood count and liver function tests were monitored every three weeks. The patient tolerated the treatment well, with no significant adverse reactions observed.
Figure 2
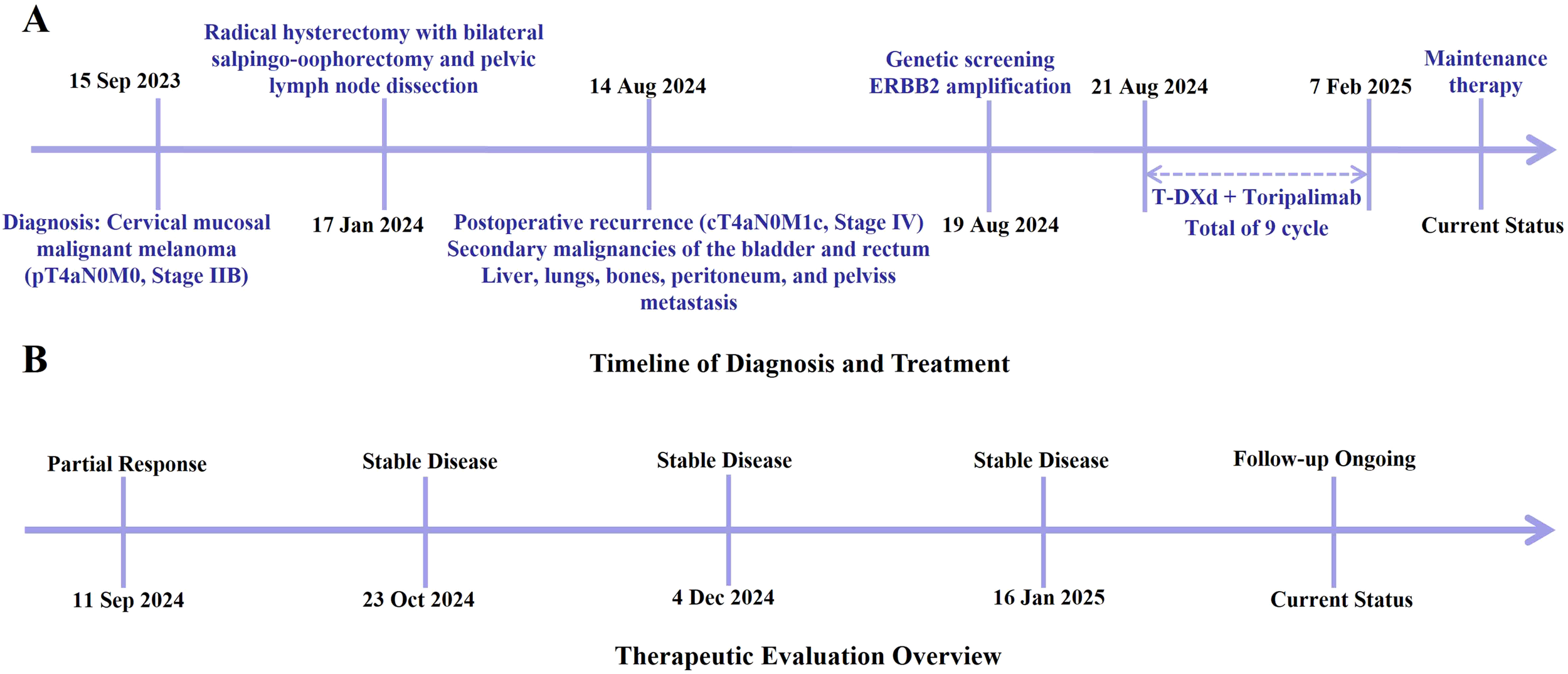
(A) Major clinical events from initial diagnosis through the treatment process of this case. (B) Overview of the therapeutic response and follow-up timeline.
Figure 3
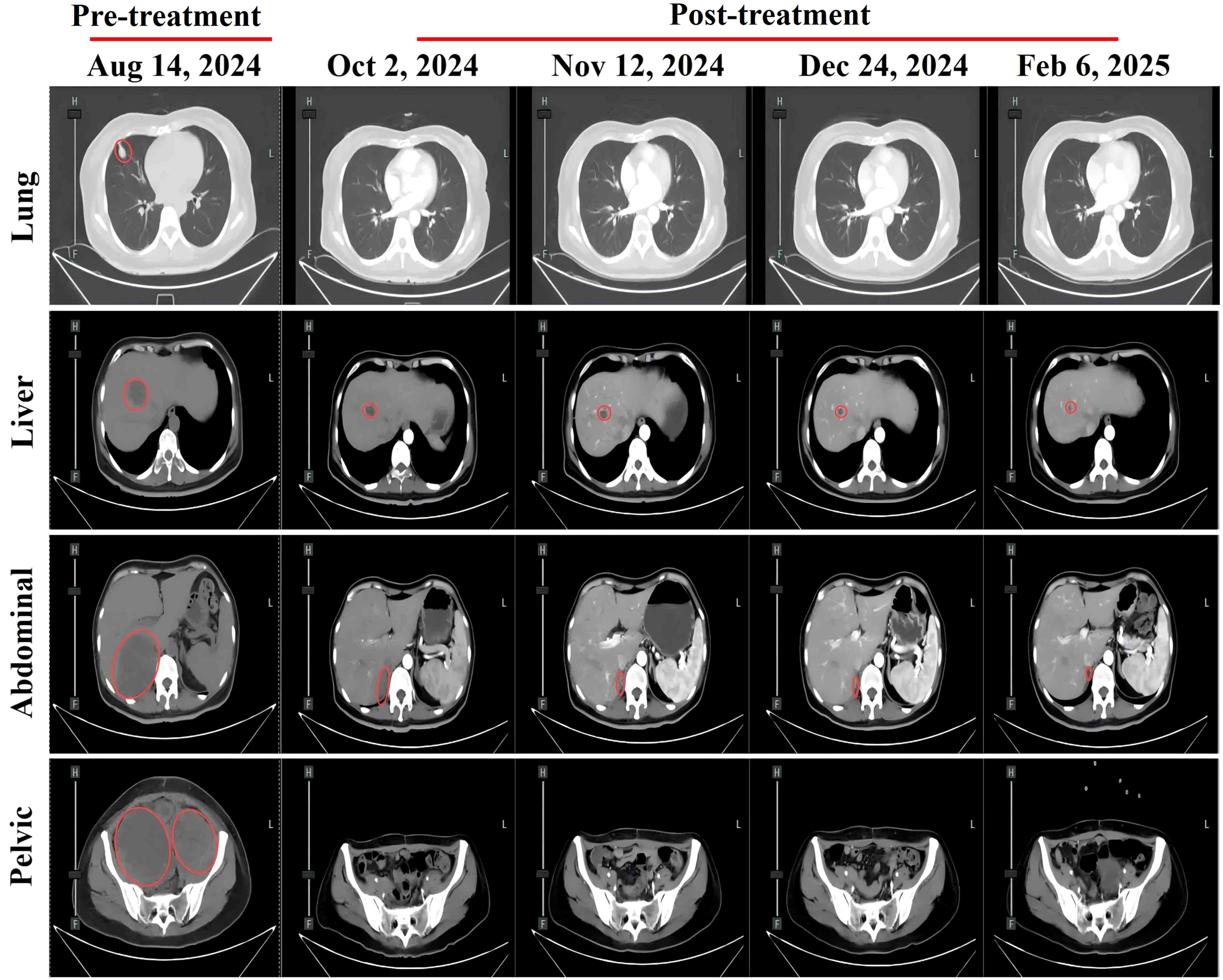
Abdominal CT scans at different treatment time points showing changes in metastatic lesions: pulmonary metastasis, hepatic metastasis, abdominal metastasis, and pelvic metastasis. Red circles indicate metastatic lesions.
Discussion
Current clinical management strategies for cervical mucosal melanoma primarily revolve around surgical intervention, supplemented with radiotherapy, chemotherapy, targeted therapy, and immunotherapy. However, the lack of standardized protocols due to disease rarity and insufficient large-scale clinical studies contributes to its universally poor prognosis. Despite aggressive surgery, postoperative recurrence rates remain alarmingly high, reaching 60%-80% (14). For advanced or recurrent cases, radiotherapy and chemotherapy (e.g., dacarbazine, temozolomide) are often used as adjuvant treatments, but their efficacy remains limited, providing only temporary local control. In recent years, immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors, CTLA-4 inhibitors) and targeted therapies (against BRAF or KIT mutations) have been introduced into clinical practice, with some cases showing prolonged survival. However, the overall response rate remains lower than that observed in cutaneous melanoma (15). Studies suggest that combining immunotherapy with radiotherapy may enhance local control, but large-scale clinical evidence is still lacking (16). Notably, mucosal melanoma exhibits distinct molecular characteristics compared to its cutaneous counterpart, such as a lower BRAF mutation rate and a higher prevalence of NF1 mutations, which restrict the applicability of targeted therapies (17). The 5-year survival rate remains below 15%, with early metastasis and high recurrence rates posing major challenges (18). Therefore, future efforts should focus on exploring comprehensive treatment models based on molecular subtyping and advancing exclusive clinical trials specifically for mucosal melanoma.
T-DXd is the new standard second-line treatment for HER2-positive metastatic breast cancer. In the DESTINY-Breast03 trial, compared to trastuzumab emtansine (T-DM1), T-DXd significantly extended progression-free survival (PFS) (median 28.8 months vs. 6.8 months) and overall survival (OS) (HR 0.33), with manageable safety (10). Additionally, T-DXd is the first ADC approved for HER2-low metastatic breast cancer (IHC 1+ or 2+/ISH negative), particularly effective in hormone receptor-positive or triple-negative breast cancer, and is recommended for use after chemotherapy (19, 20). In gastric cancer/gastroesophageal junction adenocarcinoma, T-DXd is approved for advanced patients previously treated with trastuzumab, with the DESTINY-Gastric01 trial showing significantly superior objective response rates compared to traditional chemotherapy (intravenous irinotecan or paclitaxel) (21). T-DXd is the first targeted therapy approved for HER2-mutant advanced non-small cell lung cancer (NSCLC), demonstrating durable antitumor activity in trials such as DESTINY-Lung02 (22). Although T-DXd has demonstrated significant efficacy in HER2-overexpressing gynecological malignancies such as cervical, endometrial, and ovarian cancers (23), there are currently no direct research reports on the use of T-DXd for the treatment of cervical mucosal melanoma.
This case report is the first to document significant clinical benefit achieved with the combination of toripalimab and T-DXd, in a patient with ERBB2-amplified cervical mucosal malignant melanoma. ERBB2 (the gene encoding HER2) amplification or overexpression drives tumor proliferation and metastasis through constitutive activation of downstream MAPK/PI3K signaling pathways (24). In mucosal melanomas, ERBB2 amplification occurs in 2%-5% of cases and correlates with aggressive behavior and poor prognosis (25). Studies confirms that high-level ERBB2 amplification reliably predicts HER2 protein overexpression, making it an ideal target for antibody-drug conjugates (26–28). As a next-generation HER2-targeted ADC, T-DXd demonstrates unique advantages through its deruxtecan payload, which exhibits potent membrane permeability and bystander effects - enabling sustained antitumor activity even in heterogeneous tumors with coexisting RAS/RAF mutations (29). The observed rapid and durable tumor regression aligns with preclinical evidence showing T-DXd’s robust efficacy in ERBB2-amplified xenograft models.
Mucosal melanomas are characterized by an immunosuppressive tumor microenvironment, where single-agent PD-1 inhibitors achieve response rates below 20% (30). Toripalimab may restore T-cell function by blocking PD-1/PD-L1 axis, while T-DXd potentially enhances antitumor immunity through immunogenic cell death and antigen release (31). This synergistic mechanism is supported by clinical data showing toripalimab combination with antiangiogenic agents (e.g., axitinib) elevates objective response rates to 48.3% in metastatic mucosal melanoma (5). Our findings further validate the feasibility of combining PD-1 inhibition with targeted therapy, potentially mediated by increased tumor-infiltrating CD8+ T cells observed post-treatment. Previous studies have demonstrated that tumors with ERBB2 amplification exhibit primary resistance to traditional anti-HER2 monoclonal antibodies (e.g., trastuzumab), while ADCs such as T-DXd can overcome therapeutic limitations posed by HER2 low expression or heterogeneous amplification through efficient delivery of topoisomerase I inhibitors (32, 33). In this case, the application of T-DXd aligns with literature reports. Literature indicates its significant improvement of CD3+-CD8+ T-cell ratios in neoadjuvant therapy for mucosal melanoma, positively correlating with pathological response rates (5). The combination therapy may create a synergistic “targeted killing + immune activation” effect, particularly beneficial for immunologically weak mucosal melanomas.
Primary cervical mucosal melanoma characterized by diagnostic challenges and lack of standardized treatment protocols (34). This case establishes a biomarker-driven treatment paradigm for this rare subtype through molecular confirmation of ERBB2 amplification, achieving a breakthrough beyond the limitations of traditional chemotherapy or single-agent immunotherapy. Though ERBB2 amplification is uncommon in melanoma, it may serve as a sensitivity marker for ADCs, enabling cross-cancer learning from targeted strategies in breast and lung cancers. While previous studies attributed HER2-targeted drug resistance to ERBB2 mutations or downstream pathway activation, this case suggests that in ERBB2 amplified tumors, ADC-immune checkpoint inhibitor combinations may reverse resistance through: (1) T-DXd-induced immunogenic cell death enhancing PD-1 inhibitor efficacy; (2) Toripalimab-mediated reversal of T-cell exhaustion and tumor microenvironment modulation. These mechanisms align with the cross-tumor synergistic effects observed in studies like JUPITER-02 (35).
This case underscores the necessity of systematic molecular profiling for rare malignancies. Although ERBB2 amplification may coexist with RAS/RAF co-mutations causing resistance to conventional anti-HER2 regimens, T-DXd maintains sensitivity (33). Therefore, routine ERBB2 IHC/FISH and NGS testing is recommended for cervical mucosal melanoma to identify potential beneficiaries. This case presents a promising novel therapeutic strategy for ERBB2-amplified cervical mucosal malignant melanoma, highlighting the value of molecular subtyping in guiding off-label therapies. Future multicenter collaborations should establish precision treatment systems for rare mucosal melanomas while exploring ADC-immunotherapy synergy mechanisms, ultimately improving survival outcomes for these highly aggressive malignancies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The ethics committee of Weihai Central Hospital Affiliated to Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
X-XW: Data curation, Investigation, Writing – original draft, Formal Analysis. M-XW: Software, Data curation, Writing – original draft. CS: Visualization, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ma Y Xia R Ma X Judson-Torres RL Zeng H . Mucosal melanoma: pathological evolution, pathway dependency and targeted therapy. Front Oncol. (2021) 11:702287. doi: 10.3389/fonc.2021.702287
2
Tacastacas JD Bray J Cohen YK Arbesman J Kim J Koon HB et al . Update on primary mucosal melanoma. J Am Acad Dermatol. (2014) 71:366–75. doi: 10.1016/j.jaad.2014.03.031
3
Nassar KW Tan AC . The mutational landscape of mucosal melanoma. Semin Cancer Biol. (2020) 61:139–48. doi: 10.1016/j.semcancer.2019.09.013
4
Sheng X Yan X Chi Z Si L Cui C Tang B et al . Axitinib in combination with toripalimab, a humanized immunoglobulin G(4) monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: an open-label phase IB trial. J Clin Oncol. (2019) 37:2987–99. doi: 10.1200/jco.19.00210
5
Lian B Li Z Wu N Li M Chen X Zheng H et al . Phase II clinical trial of neoadjuvant anti-PD-1 (toripalimab) combined with axitinib in resectable mucosal melanoma. Ann Oncol. (2024) 35:211–20. doi: 10.1016/j.annonc.2023.10.793
6
Lian B Si L Chi ZH Sheng XN Kong Y Wang X et al . Toripalimab (anti-PD-1) versus high-dose interferon-α2b as adjuvant therapy in resected mucosal melanoma: a phase II randomized trial. Ann Oncol. (2022) 33:1061–70. doi: 10.1016/j.annonc.2022.07.002
7
Modi S Jacot W Yamashita T Sohn J Vidal M Tokunaga E et al . Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
8
Li BT Smit EF Goto Y Nakagawa K Udagawa H Mazières J et al . Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
9
Doi T Shitara K Naito Y Shimomura A Fujiwara Y Yonemori K et al . Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. (2017) 18:1512–22. doi: 10.1016/s1470-2045(17)30604-6
10
Hurvitz SA Hegg R Chung WP Im SA Jacot W Ganju V et al . Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. (2023) 401:105–17. doi: 10.1016/s0140-6736(22)02420-5
11
Keam SJ . Toripalimab: first global approval. Drugs. (2019) . 79:573–8. doi: 10.1007/s40265-019-01076-2
12
Zhou L Yang KW Zhang S Yan XQ Li SM Xu HY et al . Disitamab vedotin plus toripalimab in patients with locally advanced or metastatic urothelial carcinoma (RC48-C014): a phase Ib/II dose-escalation and dose-expansion study. Ann Oncol. (2025) 36:331–9. doi: 10.1016/j.annonc.2024.12.002
13
Wang Y Gong J Wang A Wei J Peng Z Wang X et al . Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: a multicentre, open label, dose escalation and expansion phase 1 trial. EClinicalMedicine. (2024) 68:102415. doi: 10.1016/j.eclinm.2023.102415
14
Lopes Macêdo M Assunção Ribeiro da Costa RE Amaral Dos Reis C Da Silva Júnior RG Oliveira Pires A Vieira SC . Disease progression and death from cervical melanoma in a patient undergoing nivolumab therapy: A case report. Cureus. (2024) 16:e52811. doi: 10.7759/cureus.52811
15
Shan Z Liu F . Advances in immunotherapy for mucosal melanoma: harnessing immune checkpoint inhibitors for improved treatment outcomes. Front Immunol. (2024) 15:1441410. doi: 10.3389/fimmu.2024.1441410
16
Smart AC Giobbie-Hurder A Desai V Xing JL Lukens JN Taunk NK et al . Multicenter evaluation of radiation and immune checkpoint inhibitor therapy in mucosal melanoma and review of recent literature. Adv Radiat Oncol. (2024) 9:101310. doi: 10.1016/j.adro.2023.101310
17
Zhang S Zhang J Guo J Si L Bai X . Evolving treatment approaches to mucosal melanoma. Curr Oncol Rep. (2022) 24:1261–71. doi: 10.1007/s11912-022-01225-z
18
Wehbe J Jaikaransingh D Walker A . Immunotherapy as a treatment modality for mucosal melanoma of the head and neck: A systematic review. Med (Baltimore). (2022) 101:e29979. doi: 10.1097/md.0000000000029979
19
Cai ZL Yang HT Huang T Yu ZR Ren N Su JY et al . Efficacy and safety of trastuzumab deruxtecan in patients with solid tumors: a systematic review and meta-analysis of 3 randomized controlled trials. Am J Cancer Res. (2023) 13:3266–74.
20
Schlam I Tolaney SM Tarantino P . The efficacy of trastuzumab-deruxtecan for the treatment of patients with advanced HER2-low breast cancer. Expert Rev Anticancer Ther. (2024) 24:1059–66. doi: 10.1080/14737140.2023.2171993
21
Shitara K Bang YJ Iwasa S Sugimoto N Ryu MH Sakai D et al . Trastuzumab deruxtecan in HER2-positive advanced gastric cancer: exploratory biomarker analysis of the randomized, phase 2 DESTINY-Gastric01 trial. Nat Med. (2024) 30:1933–42. doi: 10.1038/s41591-024-02992-x
22
Li BT Meric-Bernstam F Bardia A Naito Y Siena S Aftimos P et al . Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol. (2024) 25:707–19. doi: 10.1016/s1470-2045(24)00140-2
23
Andrikopoulou A Zagouri F Goula K Haidopoulos D Thomakos N Svarna A et al . Real-world evidence of Trastuzumab Deruxtecan (T-DXd) Efficacy in HER2-expressing gynecological Malignancies. BMC Cancer. (2024) 24:1503. doi: 10.1186/s12885-024-13226-1
24
Zhang T Febres-Aldana CA Liu Z Dix JM Cheng R Dematteo RG et al . HER2 antibody-drug conjugates are active against desmoplastic small round cell tumor. Clin Cancer Res. (2024) 30:4701–13. doi: 10.1158/1078-0432.ccr-24-1835
25
Rolfo C Del Re M Russo A . Empower the potential of trastuzumab deruxtecan with novel combinations. Clin Cancer Res. (2023) 29:4317–9. doi: 10.1158/1078-0432.ccr-23-1700
26
Odintsov I Makarem M Nishino M Bachert SE Zhang T LoPiccolo J et al . Prevalence and therapeutic targeting of high-level ERBB2 amplification in NSCLC. J Thorac Oncol. (2024) 19:732–48. doi: 10.1016/j.jtho.2023.12.019
27
Marra A Chandarlapaty S Modi S . Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives. Nat Rev Clin Oncol. (2024) 21:185–202. doi: 10.1038/s41571-023-00849-9
28
Sukov WR Zhou J Geiersbach KB Keeney GL Carter JM Schoolmeester JK . Frequency of HER2 protein overexpression and HER2 gene amplification in endometrial clear cell carcinoma. Hum Pathol. (2023) 137:94–101. doi: 10.1016/j.humpath.2023.04.009
29
Martín M Pandiella A Vargas-Castrillón E Díaz-Rodríguez E Iglesias-Hernangómez T Martínez Cano C et al . Trastuzumab deruxtecan in breast cancer. Crit Rev Oncol Hematol. (2024) 198:104355. doi: 10.1016/j.critrevonc.2024.104355
30
Yue H Xu H Ma L Li X Yang B Wang X et al . A DXd/TLR7-agonist dual-conjugate anti-HER2 ADC exerts robust antitumor activity through tumor cell killing and immune activation. Mol Cancer Ther. (2024) 23:1639–51. doi: 10.1158/1535-7163.mct-24-0078
31
Oh KS Nam AR Bang JH Jeong Y Choo SY Kim HJ et al . Immunomodulatory effects of trastuzumab deruxtecan through the cGAS-STING pathway in gastric cancer cells. Cell Commun Signal: CCS. (2024) 22:518. doi: 10.1186/s12964-024-01893-3
32
Waliany S Wakelee H Ramchandran K Das M Huang J Myall N et al . Characterization of ERBB2 (HER2) alterations in metastatic non-small cell lung cancer and comparison of outcomes of different trastuzumab-based regimens. Clin Lung Cancer. (2022) 23:498–509. doi: 10.1016/j.cllc.2022.05.015
33
Singh H Sahgal P Kapner K Corsello SM Gupta H Gujrathi R et al . RAS/RAF comutation and ERBB2 copy number modulates HER2 heterogeneity and responsiveness to HER2-directed therapy in colorectal cancer. Clin Cancer Res. (2024) 30:1669–84. doi: 10.1158/1078-0432.ccr-23-2581
34
Dealberti D Bosoni D Spissu F Pisani C Pizio C Nappi L et al . Primary Malignant melanoma of the endocervix uteri and outpatient hysteroscopy as a diagnostic tool: case report and literature overview. Dis (Basel Switzerland). (2024) 12:126. doi: 10.3390/diseases12060126
35
Rajasekaran N Wang X Ravindranathan S Chin DJ Tseng SY Klakamp SL et al . Toripalimab, a therapeutic monoclonal anti-PD-1 antibody with high binding affinity to PD-1 and enhanced potency to activate human T cells. Cancer Immunol Immunother. (2024) 73:60. doi: 10.1007/s00262-024-03635-3
Summary
Keywords
cervical mucosal melanoma, trastuzumab deruxtecan (T-DXd), genomic profiling, toripalimab, HER2-positive tumors
Citation
Wang X-X, Wang M-X and Sui C (2025) Case Report: Trastuzumab deruxtecan plus toripalimab in ERBB2-amplified cervical mucosal melanoma. Front. Oncol. 15:1625521. doi: 10.3389/fonc.2025.1625521
Received
09 May 2025
Accepted
14 August 2025
Published
03 September 2025
Volume
15 - 2025
Edited by
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, Italy
Reviewed by
Alexandra Charalampopoulou, National Center of Oncological Hadrontherapy, Italy
Weiliang Hou, Naval Medical University, China
Updates
Copyright
© 2025 Wang, Wang and Sui.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Sui, sc789ok@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.