- 1Pediatrics Hospital, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 2School of Pediatrics, Henan University of Chinese Medicine, Zhengzhou, Henan, China
- 3Oncology Department, Henan Province Hospital of Chinese Medicine (The Second Affiliated Hospital of Henan University of Chinese Medicine), Zhengzhou, Henan, China
Background: Derived neutrophil-to-lymphocyte ratio (dNLR) is an emerging blood-based inflammatory biomarker previously reported to have prognostic value in various malignancies. This study aimed to investigate the prognostic significance of dNLR in patients with distal cholangiocarcinoma (dCCA) after curative resection.
Methods: Clinicopathological data of patients with dCCA in our hospital from Jan.2014 to Jun.2024 was analyzed retrospectively. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive value of dNLR and to identify the optimal cutoff. Survival differences between groups stratified by dNLR were compared using Kaplan-Meier analysis. Candidate variables were screened through univariate analysis using Kaplan-Meier, random forest, Recursive Feature Elimination (RFE) and least absolute shrinkage and selection operator (LASSO) regression models. Multivariate Cox regression analysis identified independent prognostic factors, which were subsequently integrated into a predictive model visualized via a nomogram. Model performance was assessed using ROC curves, calibration curves, and decision curve analysis (DCA).
Results: A total of 177 patients were enrolled in this study. ROC analysis revealed an area under the curve (AUC) of 0.707 for dNLR in predicting postoperative survival, with an optimal cutoff value of 1.60. Patients stratified into a low-dNLR group (≤ 1.60) demonstrated significantly improved recurrence-free survival (41 months) and overall survival (17 months) compared to those in the high-dNLR group (> 1.60) (p < 0.05). Univariate and multivariate combined with 3 machine learning analyses identified preoperative dNLR > 1.60 as an independent adverse prognostic factor for postoperative outcomes, incorporating with other independent predictors (preoperative total bilirubin, carbohydrate antigen 19–9 levels, T-stage, portal venous system invasion, and lymph node metastasis) further enhanced the predictive accuracy of the prognostic model.
Conclusion: A preoperative dNLR > 1.60 is an independent risk factor associated with poor prognosis in patients with dCCA. The clinical prediction model based on machine learning incorporating dNLR effectively predicts postoperative outcomes in this patient population.
Introduction
Cholangiocarcinoma (CCA) represents a malignant tumor arising from biliary epithelial cells and constitutes one of the less common malignancies. Distal cholangiocarcinoma (dCCA), originating from the mid-to-lower portion of the common bile duct, accounts for approximately 30%–40% of all extrahepatic cholangiocarcinomas (1). Risk factors for dCCA notably include bile duct stones, cholestasis, chronic biliary inflammation, and anatomical abnormalities of the biliary tree (2). Due to poor responsiveness to chemotherapy, surgical resection remains the mainstay of treatment; however, early-stage dCCA is often clinically occult, leading to a generally poor prognosis (3–5). Currently, most cholangiocarcinoma research predominantly focuses on intrahepatic cholangiocarcinoma (iCCA), whereas dCCA lacks novel biomarkers for accurately predicting recurrence risk and clinical outcomes. Effective prognostication of dCCA patient survival can significantly aid clinicians in optimizing clinical decisions, individualizing follow-up intervals, and enabling earlier detection of tumor recurrence or metastasis, thereby potentially improving long-term patient outcomes.
Inflammation is now recognized as one of the critical driving factors in carcinogenesis and tumor progression, with chronic inflammatory states believed to accelerate tumor growth and adversely impact patient prognosis (6). Consequently, accurately assessing inflammatory status in cancer patients could effectively predict clinical outcomes. Blood-based inflammatory markers, routinely derived from complete blood counts, offer distinct advantages due to their easy availability and simple computation. Various indices, including the lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI), and systemic immune-inflammation index (SII), have demonstrated prognostic value in patients with dCCA (7–10). The derived neutrophil-to-lymphocyte ratio (dNLR), which integrates counts of peripheral leukocytes and neutrophils, was initially introduced by Proctor et al. in 2012, demonstrating prognostic efficacy comparable to the conventional neutrophil-to-lymphocyte ratio (NLR) across multiple cancer types (11). Subsequently, dNLR has been widely adopted as a prognostic indicator in various malignancies, including gastric, pancreatic, and breast cancers, where an elevated dNLR is consistently associated with poor outcomes (12–14).
Within biliary malignancies, dNLR has also demonstrated considerable prognostic utility. Grenader et al. reported, in a cohort of 462 advanced cholangiocarcinoma patients, that low dNLR was significantly associated with improved overall survival and better responsiveness to gemcitabine-cisplatin chemotherapy compared with high dNLR (15). This finding was further supported by Buyuksimsek et al., who validated prognostic significance of dNLR in predicting outcomes for advanced CCA patients treated with gemcitabine plus oxaliplatin (GEMOX) (16). Additionally, Zhang et al. demonstrated, in a retrospective analysis of 231 surgically treated iCCA patients, that elevated preoperative dNLR strongly correlated with reduced recurrence-free survival and overall survival (17). However, despite these advances, no prior studies have assessed the prognostic relevance of dNLR specifically in dCCA patients following curative resection.
Therefore, the present study aimed to retrospectively evaluate patients with dCCA who underwent curative surgery at our center, focusing specifically on elucidating the prognostic value of preoperative dNLR. Through this investigation, we seek not only to expand the clinical utility of dNLR as a biomarker but also to identify novel, potentially effective predictors of postoperative outcomes for dCCA patients.
Methods
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of The First Affiliated Hospital of Henan University (No. 2024-412). Due to the retrospective nature of the study, participant informed consent was waived, and the study design was approved by the appropriate ethics review board
Patients and clinicopathological factors
This retrospective study analyzed data from patients with dCCA who underwent surgical treatment at our institution between Jan.2014 and Jun.2024. Patients were screened based on the following inclusion and exclusion criteria. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Inclusion and exclusion criteria
Inclusion criteria (1): Underwent surgical resection for biliary lesions at our department between January 2014 and June 2024 (2); Preoperative assessments confirmed no contraindications for surgery (3); Postoperative pathological diagnosis of dCCA (adenocarcinoma) (4); Availability of clinical data and complete follow-up information.
Exclusion criteria (1): Evidence of concurrent bacterial or viral infection preoperatively (2); Coexisting autoimmune diseases (3); History or coexistence of other malignancies (4); Receiving neoadjuvant chemotherapy before surgery (5); Perioperative mortality.
Patient stratification and definitions
Preoperative complete blood count (CBC) data, specifically total leukocyte and neutrophil counts within one week before surgery, were extracted for each patient. The dNLR was calculated as follows: Neutrophil count/(White blood cell count - Neutrophil count). Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive performance of dNLR regarding one-year postoperative survival, and the area under the ROC curve (AUC) was calculated to identify the optimal dNLR cutoff value. Subsequently, patients were stratified into distinct groups based on this optimal cutoff, and comparisons were performed between these groups.
Data collection and follow-up protocol
Baseline demographic data, intraoperative data, and postoperative recovery data were extracted from electronic medical records at our institution, and comparisons of perioperative characteristics were conducted between patient groups. Follow-up assessments were scheduled at 1 and 3 months post-discharge, every 3 months up to 2 years, and subsequently at 6-month intervals beyond the 2-year mark. Follow-ups were performed via outpatient clinic visits or telephone calls conducted by trained personnel. The primary endpoint of follow-up was patient death, and the secondary endpoint was tumor recurrence. Follow-up evaluations included blood tests (CBC, serum biochemistry, carbohydrate antiten 19-9) and radiological imaging (contrast-enhanced abdominal CT, chest CT), documentation of adjuvant treatments, and assessment of recurrence and survival status.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation (SD), while those with non-normal distribution were presented as median and interquartile range (IQR). Missing data were handled by following methods depending on the type of data. Patients with missing survival and tumor recurrence data and other categorized data were excluded to avoid the potential impact of data imputation. Normally distributed indexes with missing data were imputed with mean value, while non-normally distributed indexes with missing data were imputed with median value. Comparisons between groups for normally distributed continuous variables were performed using Student’s t-test, whereas non-normally distributed variables were analyzed using the Mann-Whitney U test. Categorical variables were compared using the chi-square (χ²) test or Fisher’s exact test when the sample size was ≤40 or expected frequency was <1. The “randomForestSRC”, “Recursive feature elimination” and “LASSO regression” were used to screen candidate variables and rank the importance for all factors. Survival curves were constructed using Kaplan-Meier analysis, with differences between groups assessed via the log-rank test. Independent prognostic factors were identified by multivariate analysis employing Cox proportional hazards regression. Statistical analyses were conducted with SPSS software version 26.0 and R Studio (version R 4.4.3), with a p <0.05 considered statistically significant. Figures were generated using R Studio (version R 4.4.3) and GraphPad Prism (version 9.0).
Construction and validation of the prognostic nomogram
Variables identified in the multivariate analysis were cross-referenced with the top-ranked features from the random forest analysis, recursive feature elimination and the LASSO coefficients. Multiple methods were employed for variable selection in this study. Random survival forests were used to evaluate the importance of each feature. Variable importance was quantified by the increase in cumulative out-of-bag (OOB) prediction error after permutation, and node splitting was based on the log-rank statistic. The Random survival forest (RSF) model was constructed with 2000 trees. Additionally, a Cox proportional hazards model with L1 regularization (LASSO-Cox) was implemented to perform variable selection while controlling model complexity. The optimal regularization parameter (λ) was determined using 10-fold cross-validation, and variables with non-zero coefficients at the λ_min value were retained for further analysis. Recursive feature elimination was also applied to iteratively remove the least informative features. Cross-validation and hyperparameter tuning were applied to improve the model’s robustness and interpretability and avoid overfitting. SHapley Additive exPlanations (SHAP) were utilized to interpret of machine-learning models and visualize their variable importance. The overlapping factors of machine-learning models were then integrated to develop a robust survival prediction nomogram model. The predictive performance of the nomogram was evaluated using ROC curves (constructed using the “pROC” package in R). Model discrimination was assessed using the bootstrap-corrected concordance index (c-index) (“rms” package in R). Model calibration was visualized through calibration plots (“rms” package in R). The clinical utility of the nomogram was analyzed using decision curve analysis (DCA) (“ggDCA” package in R).
Results
Patient demographics and clinical characteristics
A total of 177 patients were included in this study, comprising 89 males and 88 females, with a median age of 66.0 (IQR: 57.0–72.0) years. Among the included patients, 90 had a history of hypertension and 46 had a history of diabetes. Jaundice was the initial symptom in 148 patients, among whom 87 received preoperative biliary drainage procedures: 19 underwent endoscopic retrograde cholangiopancreatography (ERCP) with biliary stent placement, and 68 underwent percutaneous transhepatic cholangial drainage (PTCD). Other presenting symptoms included abdominal pain (n=17) and gastrointestinal symptoms (n=5). The detailed baseline clinical characteristics are summarized in Table 1.
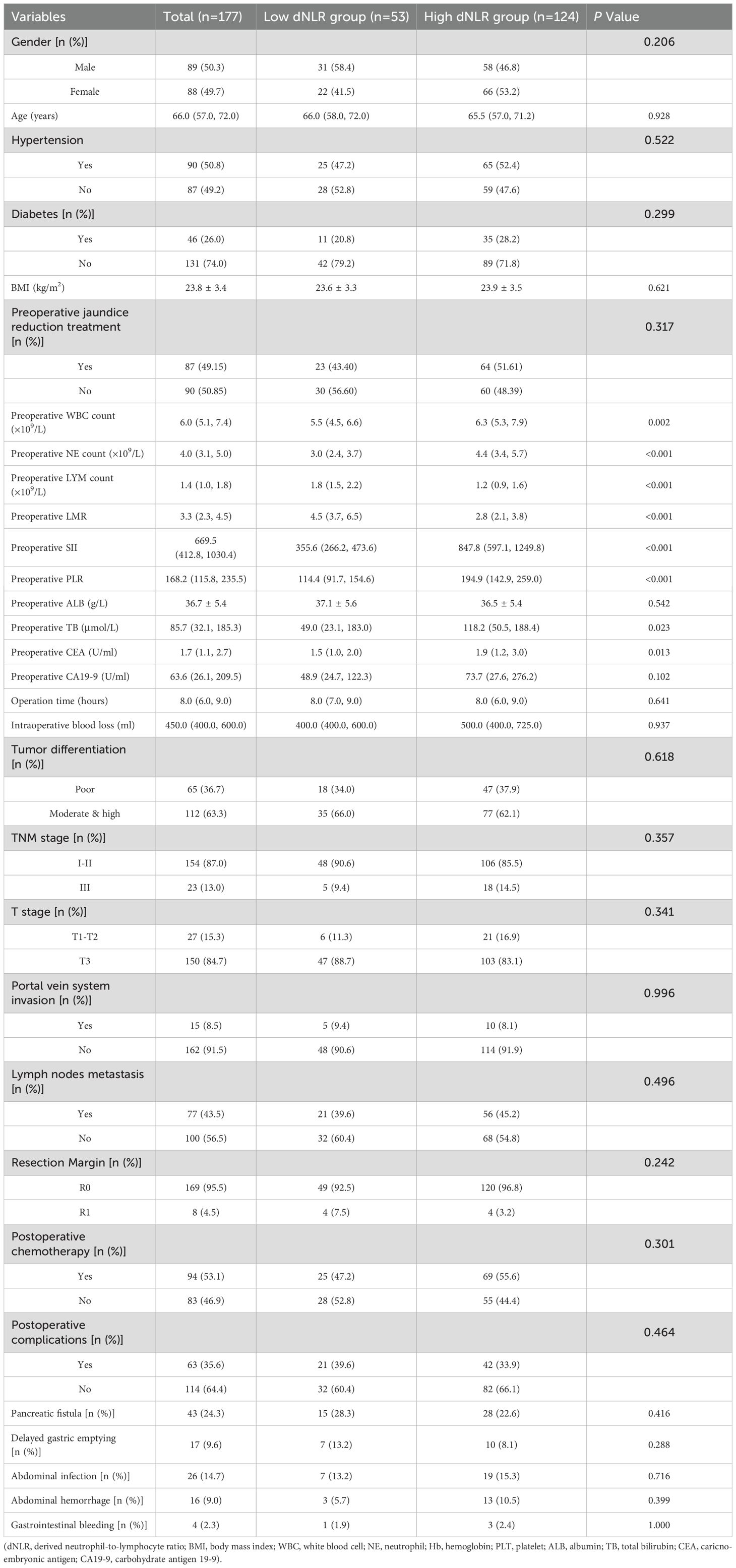
Table 1. Comparison of perioperative condition and major postoperative complications between low and high dNLR group.
Following preoperative evaluation to exclude surgical contraindications, all patients underwent curative pancreaticoduodenectomy at our center. Patients identified intraoperatively as having portal venous invasion underwent concurrent vascular resection with end-to-end anastomosis reconstruction. Median operative time was 8.0 hours, and median intraoperative blood loss was 450.0 mL. Postoperative pathology confirmed dCCA (adenocarcinoma) in all patients. Portal vein invasion was present in 15 patients (8.5%), and lymph node metastasis was identified in 77 patients (43.5%). Postoperative complications occurred in 63 patients, yielding an overall complication rate of 35.6%. Details of major complications are presented in Table 1.
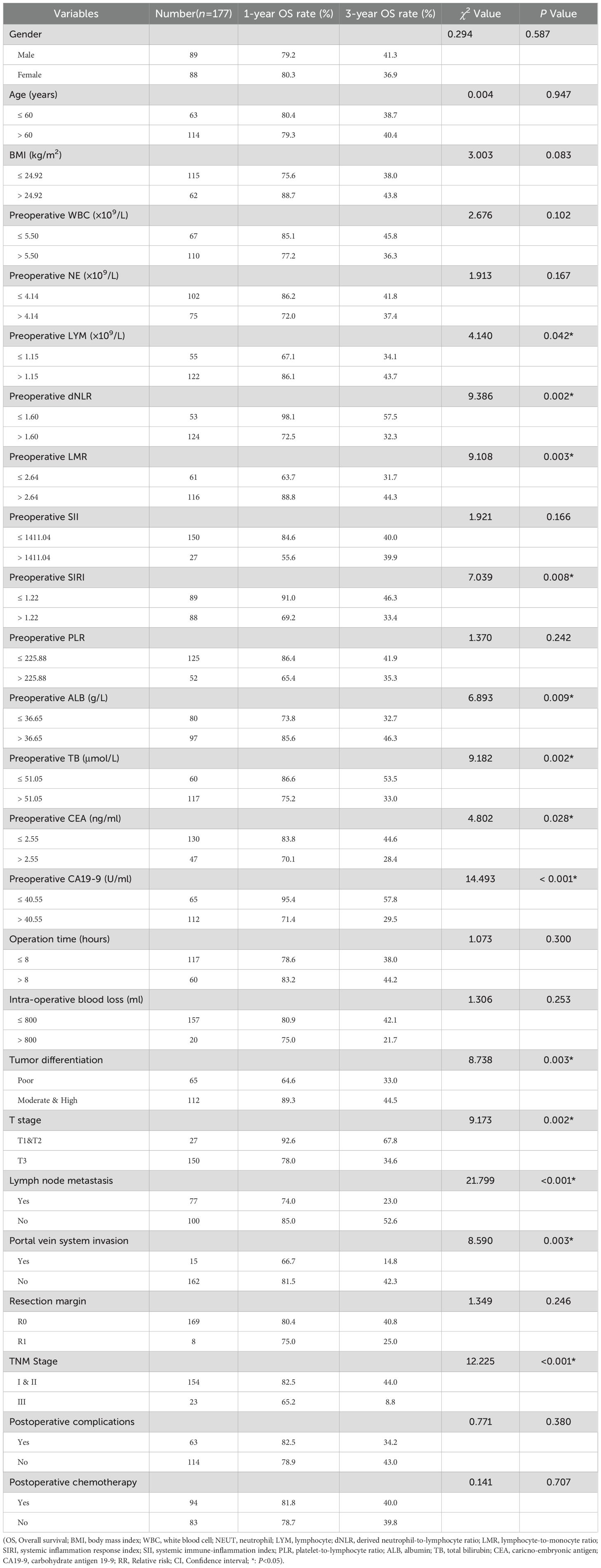
Patient follow-up continued until January 2025, with a median overall survival (OS) of 30 months. The 1-, 3-, and 5-year OS rates were 80.2%, 39.9%, and 30.1%, respectively. Median recurrence-free survival (RFS) was 26 months, with 1-, 3-, and 5-year RFS rates of 70.5%, 44.8%, and 36.1%, respectively.
Patient stratification by preoperative dNLR and prognostic evaluation
To evaluate the prognostic value of preoperative dNLR in dCCA, we generated a ROC curve correlating dNLR with 1-year postoperative survival. The AUC for dNLR was 0.707 (95% CI: 0.618–0.796), surpassing other previously reported inflammatory indices for dCCA prognosis, including the lymphocyte-to-monocyte ratio (LMR; 0.688, 95% CI: 0.591–0.785), platelet-to-lymphocyte ratio (PLR; 0.603, 95% CI: 0.492–0.714), systemic inflammatory response index (SIRI; 0.690, 95% CI: 0.595–0.786), and systemic immune-inflammation index (SII; 0.621, 95% CI: 0.514–0.728; Figure 1A). Based on the ROC analysis, the optimal dNLR cutoff value was determined to be 1.60, yielding a sensitivity of 97.1% and specificity of 37.4%.
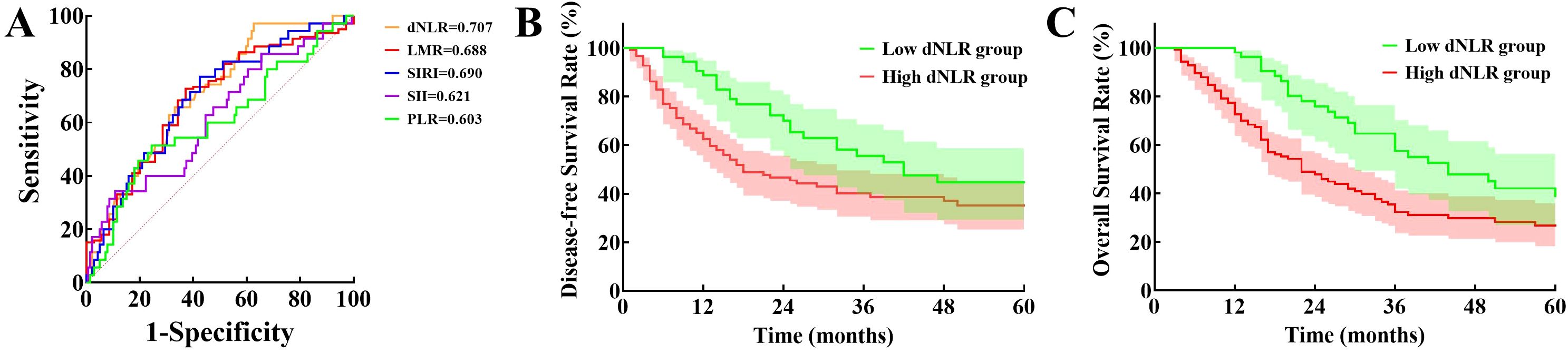
Figure 1. Evaluation of prognostic value of dNLR: (A) Comparison of receiver operator characteristic curves for derived neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, systemic inflammatory response index, systemic immune-inflammation index, and platelet-to-lymphocyte ratio in predicting postoperative prognosis. (B) Disease-free survival between low and high derived neutrophil-to-lymphocyte ratio groups. (C) Overall survival between the two groups. (dNLR, Derived neutrophil-to-lymphocyte ratio; LMR, Lymphocyte-to-monocyte ratio; SIRI, Systemic inflammatory response index; SII, Systemic immune-inflammation index; PLR, Platelet-to-lymphocyte ratio).
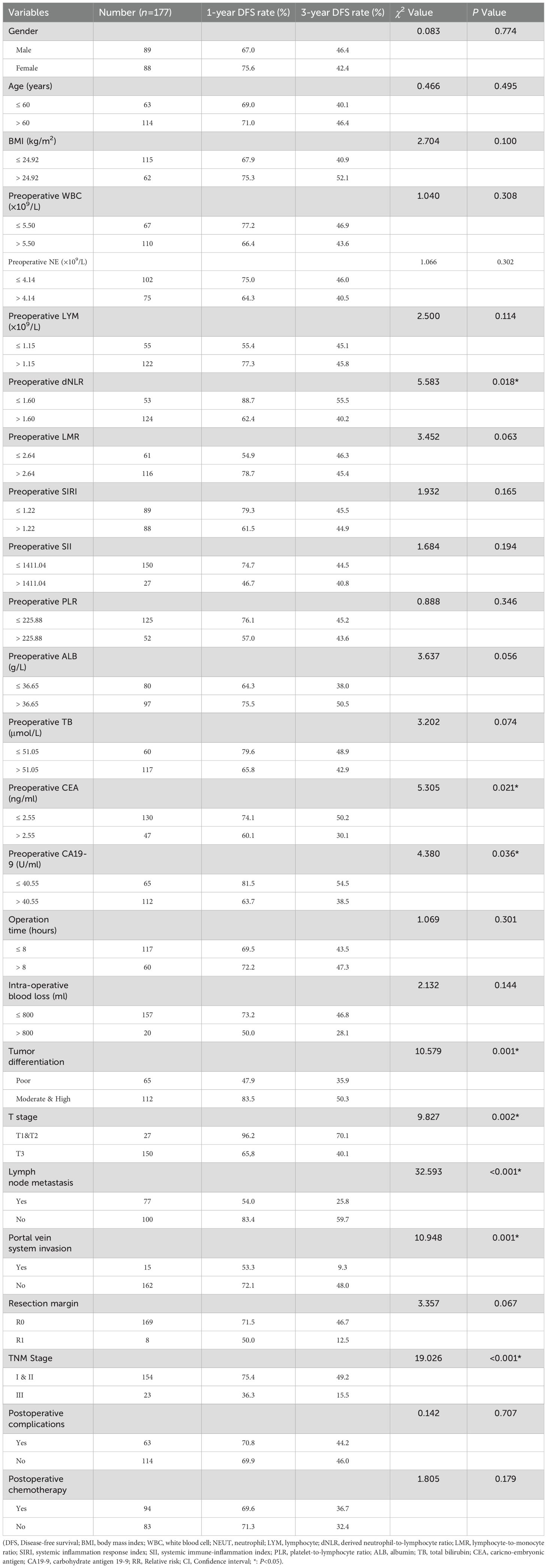
Patients were subsequently categorized into low-dNLR (≤ 1.60, n = 53) and high-dNLR (> 1.60, n=124) groups. Comparison of perioperative characteristics and postoperative complications between groups is shown in Table 1. Preoperative total leukocyte counts, neutrophil counts, PLR, SIRI, SII, total bilirubin, and carcinoembryonic antigen (CEA) levels were significantly lower, whereas preoperative lymphocyte counts and LMR were significantly higher in the low-dNLR group compared to the high-dNLR group (p < 0.05). There were no significant differences between the groups regarding other baseline parameters, intraoperative characteristics, or postoperative complications (p > 0.05).
Median RFS time was significantly longer in the low-dNLR group (41 months) compared to the high-dNLR group (17 months), with 1-, 3-, and 5-year RFS rates of 88.7%, 55.5%, and 44.6% versus 62.4%, 40.2%, and 35.1%, respectively (χ² = 5.583, p = 0.018; Figure 1B). Median OS was also significantly greater in the low-dNLR group (44 months) than in the high-dNLR group (22 months), with corresponding 1-, 3-, and 5-year OS rates of 98.1%, 57.5%, and 38.8% compared to 72.5%, 32.3%, and 26.7% (χ² = 15.257, p < 0.001; Figure 1C).
Independent risk factors for tumor recurrence after surgery for dCCA
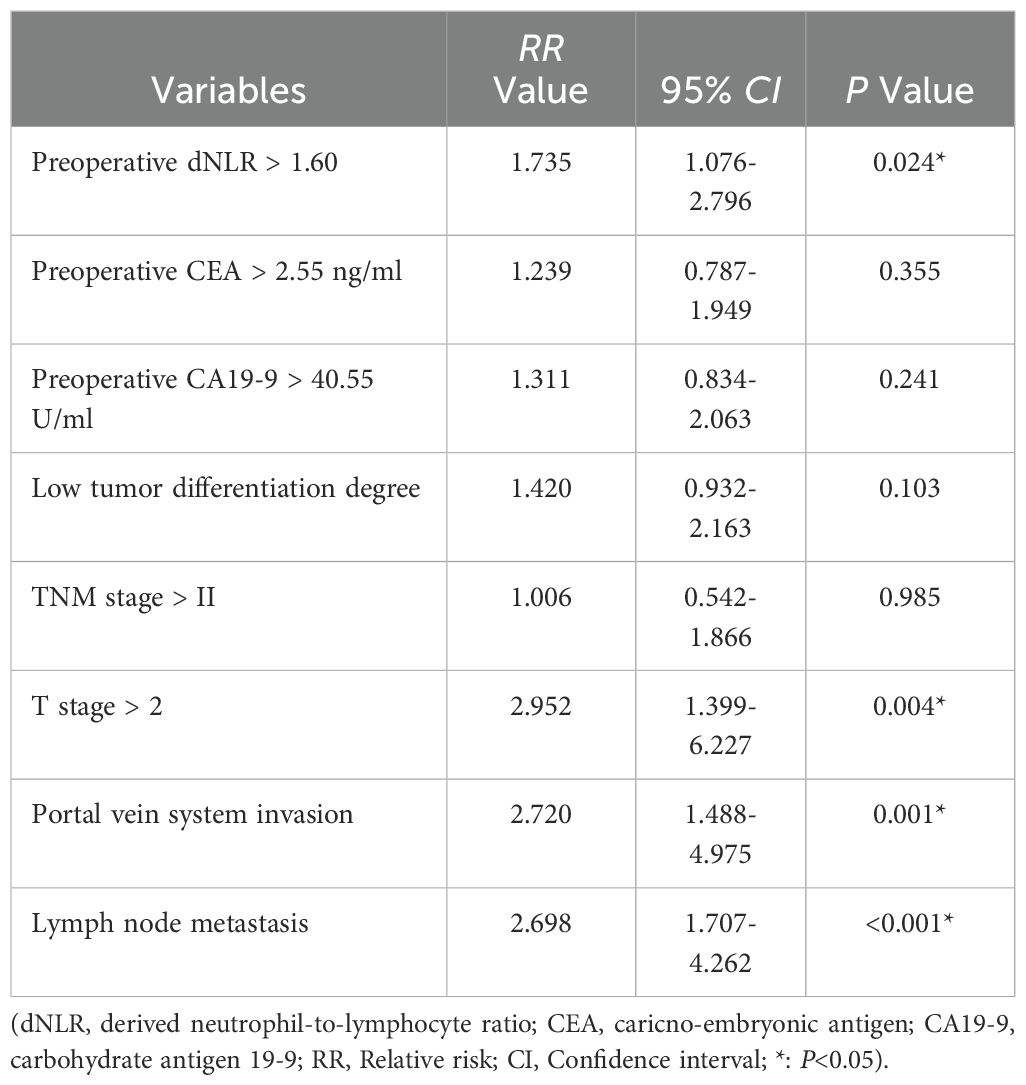
Univariate analysis was conducted using preoperative clinical data, intraoperative data, postoperative pathology findings, and recovery data as independent variables, with RFS as the dependent variable. Results indicated that preoperative dNLR, CEA, CA19–9 levels, tumor differentiation, TNM stage, T stage, portal vein invasion, and lymph node metastasis were potential risk factors significantly associated with recurrence (p < 0.05; Table 2). Multivariate Cox regression analysis confirmed that a preoperative dNLR > 1.60 independently predicted postoperative tumor recurrence (RR = 1.735, 95% CI: 1.076 – 2.796). Additionally, T stage > T2, portal vein invasion, and lymph node metastasis were identified as independent predictors of postoperative recurrence in dCCA patients (Table 3).
Independent risk factors for long-term overall survival in dCCA
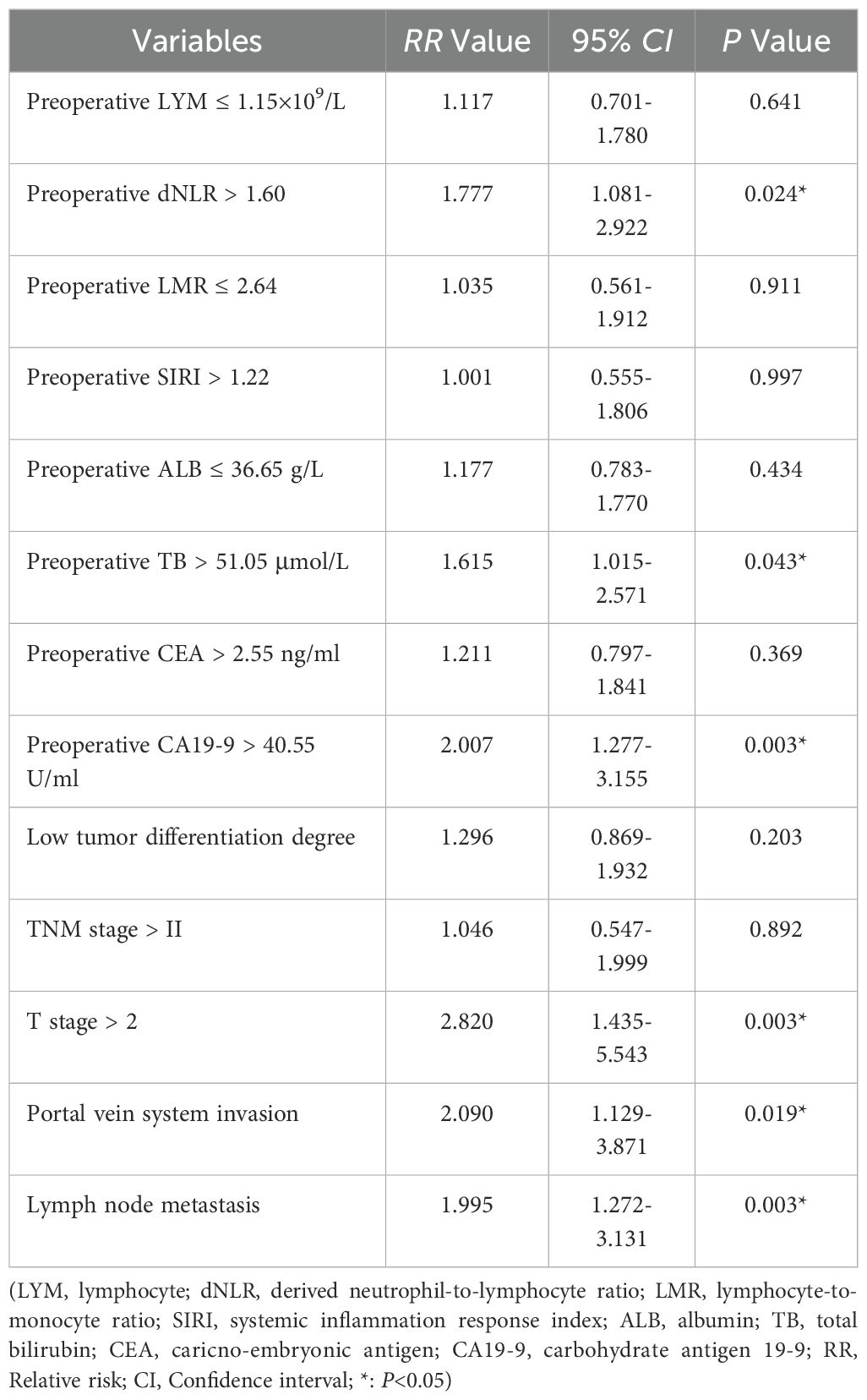
Univariate analysis using preoperative clinical data, intraoperative data, postoperative pathological findings, and postoperative recovery data identified preoperative lymphocyte count, dNLR, LMR, SIRI, albumin, total bilirubin, CEA, CA19-9, tumor differentiation, TNM stage, T stage, portal vein invasion, and lymph node metastasis as significant risk factors associated with overall survival (p < 0.05; Table 4). Multivariate Cox regression confirmed that a preoperative dNLR > 1.60 was an independent predictor of decreased overall survival (RR = 1.777, 95% CI: 1.081 – 2.922). Additionally, preoperative total bilirubin > 51.05 µmol/L, CA19-9 > 40.55 mmol/L, T stage > T2, portal vein invasion, and lymph node metastasis emerged as significant independent risk factors for poor overall survival in dCCA (Table 5).
Machine learning–based prognostic feature selection for overall survival
Machine learning methods were applied to jointly identify prognostic risk factors associated with OS. RSF was first used to assess the importance of all candidate variables. Variables were ranked according to their importance scores, and features showing the highest relevance to prognosis were preliminarily selected (Figure 2).
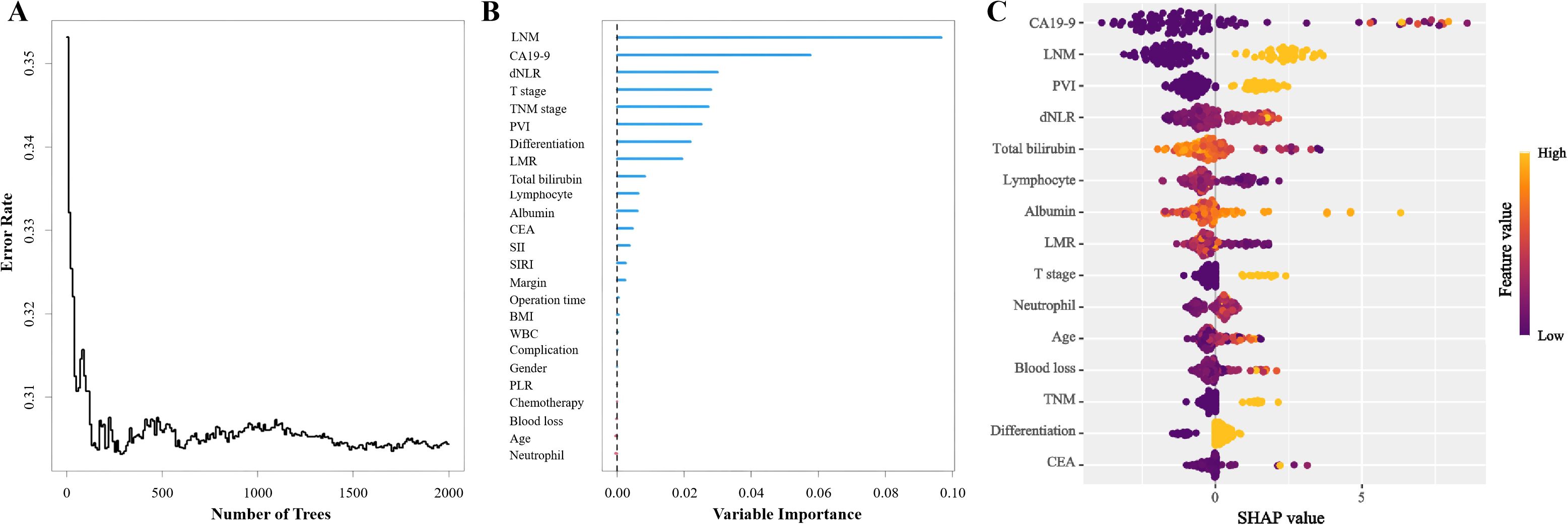
Figure 2. Random Survival Forest model for patients with distal cholangiocarcinoma: (A) Change of error rate with number of trees. (B) Variable importance of Random Survival Forest model. (C) SHapley Additive exPlanations of Random Survival Forest model. (LNM, Lymph node metastasis; CA19-9, Carbohydrate antigen 19-9; dNLR, Derived neutrophil-to-lymphocyte ratio; PVI, Portal vein invasion; LMR, Lymphocyte-to-monocyte ratio; CEA, Carcino-embryonic antigen; SII, Systemic immune-inflammation index; SIRI, Systemic inflammatory response index; BMI, Body mass index; WBC, White blood cell; PLR, Platelet-to-lymphocyte ratio).
Subsequently, least absolute shrinkage and selection operator–penalized Cox regression (LASSO-Cox) was employed for regularized regression analysis. Among the variables, dNLR, T stage, and lymph node metastasis exhibited the highest absolute coefficients (Figure 3).
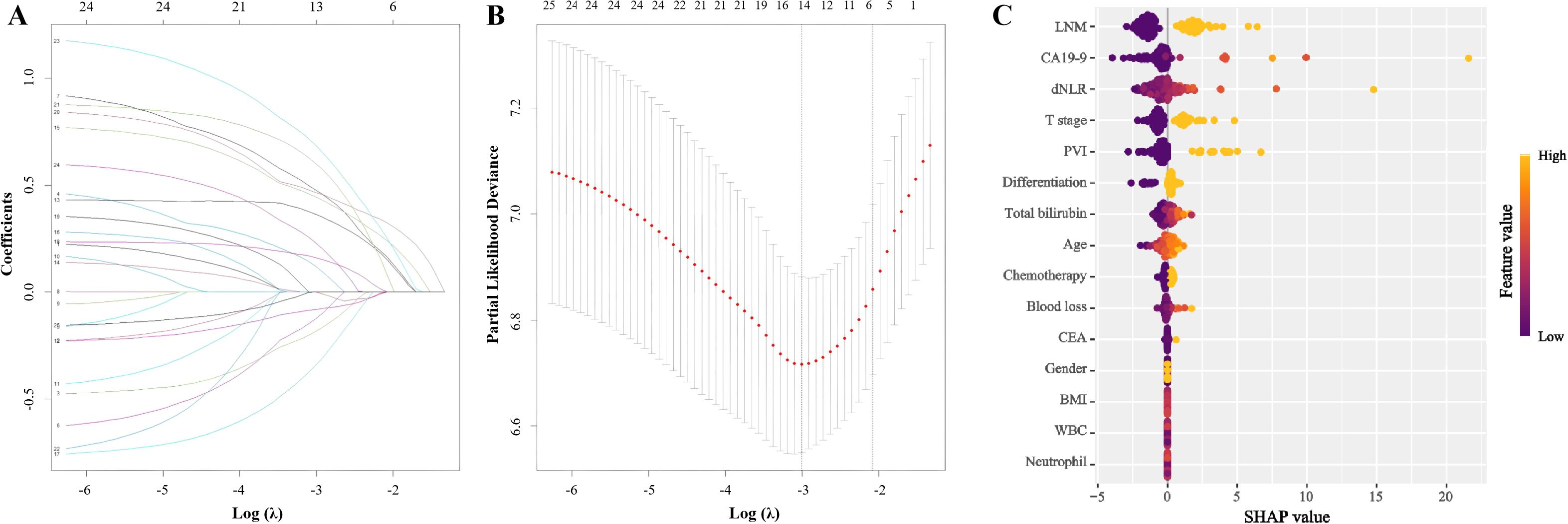
Figure 3. Lasso-Cox regression for variable selection. (A) Lasso coefficient profiles of the 25 variables. The x-axis represents the log-transformed lambda (λ), and the y-axis shows the coefficients. (B) Ten-fold cross-validation for tuning parameter selection in the Lasso-Cox model. The x-axis represents log(λ), and the y-axis shows the partial likelihood deviance. (C) SHapley Additive exPlanations of Lasso-Cox regression model. (LNM, Lymph node metastasis; CA19-9, Carbohydrate antigen 19-9; dNLR, Derived neutrophil-to-lymphocyte ratio; PVI, Portal vein invasion; CEA, Carcino-embryonic antigen; BMI, Body mass index; WBC, White blood cell).
Recursive feature elimination was then conducted to evaluate the predictive performance of different variable subsets. The optimal subset was determined based on cross-validation, and the top six variables were identified as the most suitable: dNLR, total bilirubin, CA19-9, lymph node metastasis, portal vein invasion, and T stage (Figure 4). These six factors were further incorporated into a multivariate Cox regression model. Integrating the results from all three selection strategies, the final model was built and validated based on this selected feature set.
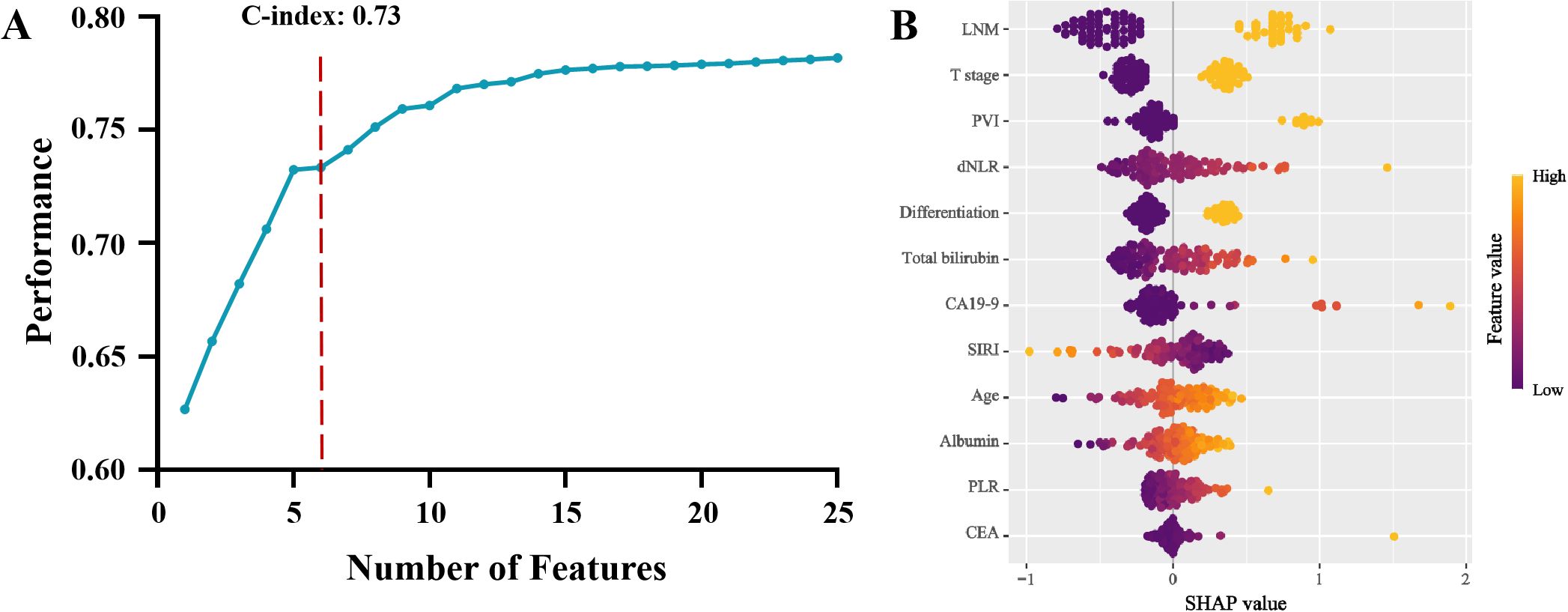
Figure 4. Recursive feature elimination algorithm model and its variable importance: (A) Predictive performance of different feature subsets. (B) SHapley Additive exPlanations of Recursive feature elimination algorithm model (LNM, Lymph node metastasis; PVI, Portal vein invasion; dNLR, Derived neutrophil-to-lymphocyte ratio; CA19-9, Carbohydrate antigen 19-9; SIRI, Systemic inflammatory response index; PLR, Platelet-to-lymphocyte ratio; CEA, Carcino-embryonic antigen).
Establishment and validation of a prognostic model for dCCA based on dNLR
To further enhance the prognostic utility of dNLR for dCCA outcomes, enrolled patients were randomly divided into training (70%) and validation (30%) sets. A predictive nomogram incorporating independent risk factors identified through multivariate Cox regression was constructed (Figure 5A). ROC analyses of this nomogram showed favorable performance in predicting 2- and 3-year postoperative survival in the training set (AUC: 0.833, 0.756) and validation set (AUC: 0.827, 0.813; Figures 5B, C). As observed in the calibration curve (Figures 5D, E), the points generally followed the diagonal line across most of the probability range in both training set and validation set, demonstrating a reasonably good calibration performance in postoperative 2- and 3-year prognosis prediction in both training and validation set. DCA curves demonstrated this model provided net benefits in predicting postoperative 2- and 3-year survival outcomes in training and validation set under the threshold probability of 5-96%, 9-87% and 5-79%, 9-95%, respectively, further demonstrating the clinical applicability of this model in predicting postoperative prognosis of dCCA patients (Figures 5F–I).
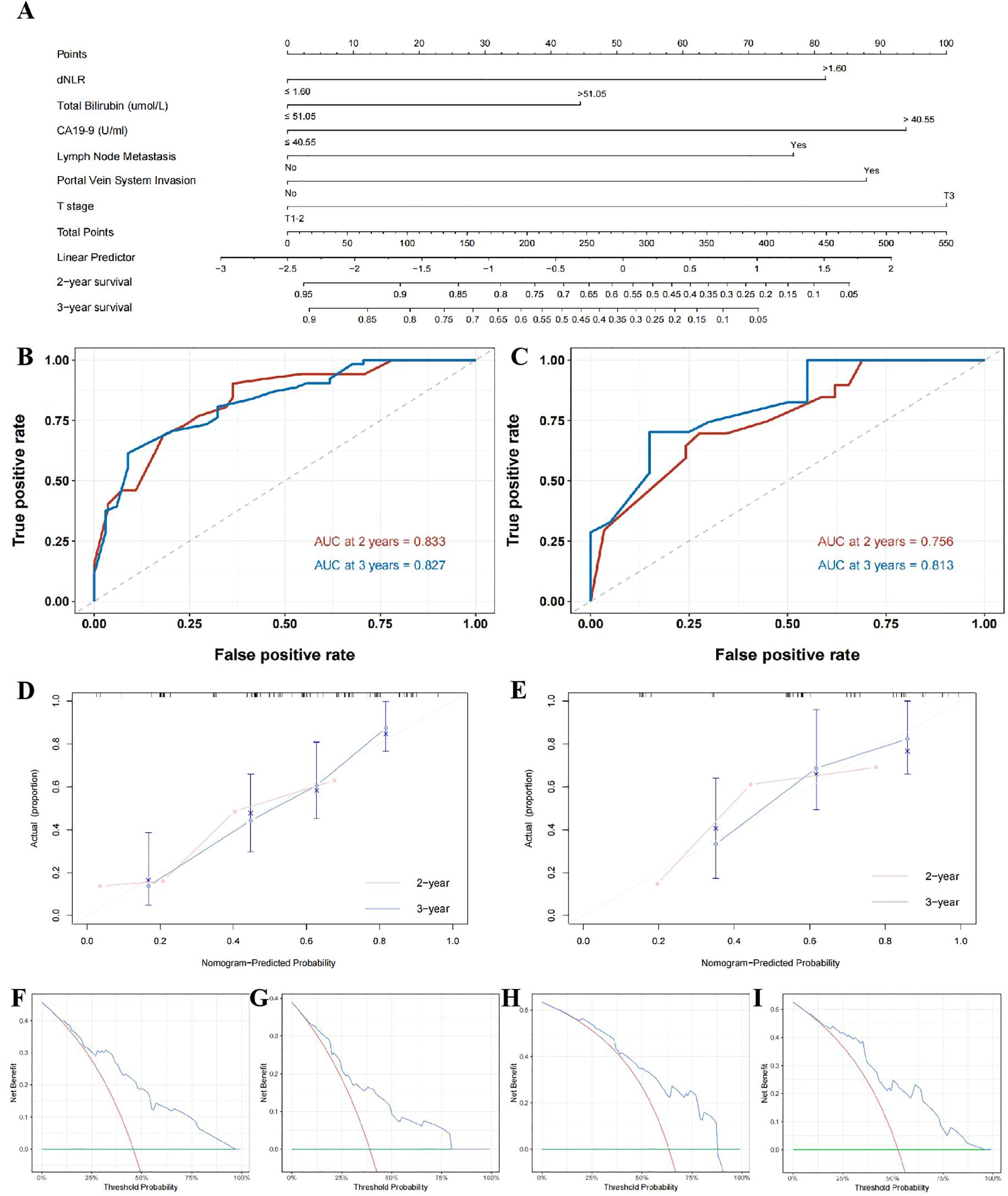
Figure 5. Nomogram based on dNLR for prognostic prediction in distal cholangiocarcinoma. (A); time-dependent ROC curves for predicting 2- and 3-year postoperative survival in the training cohort (B) and validation cohort (C); calibration curves for the nomogram in the training cohort (D) and validation cohort (E); decision curve analysis (DCA) for predicting 2-year survival in the training cohort (F) and validation cohort (G), and 3-year survival in the training cohort (H) and the validation cohort (I).
Discussion
dCCA is a relatively uncommon malignancy associated with poor long-term survival outcomes. Early prediction of postoperative survival in dCCA can guide clinical decision-making, optimize follow-up intervals, and facilitate earlier detection of recurrence or metastasis, thus potentially improving patient prognosis. In this study, we first reported the predictive value of the dNLR for postoperative prognosis in dCCA, confirming that an elevated dNLR is an independent adverse prognostic factor. Moreover, due to the unsatisfying specificity of dNLR in predicting postoperative prognosis, we further integrated dNLR with other independent risk factors into a Cox regression model to enhance its predictive accuracy, providing a robust and clinically applicable model to more precisely estimate prognosis in patients with dCCA. In clinical practice, both dNLR and proposed prediction model may better guide clinical decision making. Patients with low dNLR and risk points may benefit from surgical treatment and achieve long-term survival, and should consider upfront surgery firstly. For those with high dNLR and risk points, clinicians should arrange more frequent follow-up schedule and earlier implementation of adjuvant therapy if necessary to prolong their postoperative survival time.
As an emerging inflammatory biomarker, dNLR includes only peripheral leukocyte and neutrophil counts, rendering it convenient for clinical computation and application. Previously, dNLR was primarily identified as a valuable predictor of response to immune checkpoint inhibitors in multiple malignancies (18, 19). Additionally, several studies have validated high preoperative dNLR as an independent risk factor for poor postoperative prognosis in cancer patients. Deng et al., retrospectively analyzing 389 gastric cancer patients who underwent radical resection, reported an AUC of 0.683 for dNLR predicting postoperative survival, identifying dNLR >1.85 as significantly associated with poor OS (20). Similarly, Absenger et al. observed prolonged DFS (132 months) and OS time (147 months) in colorectal cancer patients with low dNLR compared to those with elevated levels (21). Zhang et al. further demonstrated in 231 iCCA patients that dNLR ≥1.5 was significantly correlated with adverse postoperative outcomes (17). Consistent with these findings, our study extended the clinical applicability of dNLR, establishing it as an independent predictor of postoperative prognosis in dCCA patients. Furthermore, to comprehensively assess the predictive performance of dNLR, we compared its prognostic AUC (0.707) with other previously reported inflammatory biomarkers including LMR, SII, PLR, and SIRI, revealing a superior prognostic value of dNLR. It was also identified as an important risk factor for postoperative prognosis according to machine-learning models and Cox regression models, underscoring its efficacy and clinical utility in predicting postoperative outcomes in dCCA patients.
Current evidence suggests that the poor prognosis associated with high dNLR primarily results from elevated peripheral neutrophil counts. Neutrophils are central components of inflammatory responses and have been reported to promote tumor progression through multiple pathways. First, neutrophils generate reactive oxygen species (ROS) inducing tissue and DNA damage, thus facilitating carcinogenesis, and further enhancing tumor metastasis through modulation of cytokines such as IL-1β (22, 23). Additionally, neutrophils secrete cytokines including TGF-β, VEGF, OSM, IL-10, and HGF, which directly promote tumor angiogenesis and tumor progression (24). Moreover, under the influence of tumor cells, neutrophils can form neutrophil extracellular traps (NETs), contributing further to tumor progression (25). Recent studies have specifically explored neutrophil-driven progression in cholangiocarcinoma. Zhou et al. reported that tumor-associated neutrophils interacting with macrophages promote the secretion of OSM in intrahepatic cholangiocarcinoma, subsequently activating STAT3 signaling and facilitating tumor growth (26). Yoshimoto et al. observed neutrophil extracellular traps enhancing metastatic potential in intrahepatic cholangiocarcinoma cells, with Zhang et al. further elucidating that NET-DNA activation of ITGAV/NFκB signaling increased tumor proliferation, activation, metastasis, and adversely influenced prognosis (27, 28). These findings collectively suggest that neutrophils infiltrating cholangiocarcinoma predominantly exert pro-tumorigenic effects. In our cohort, elevated neutrophil counts observed in high-dNLR patients align with previously reported correlations between peripheral neutrophilia and local neutrophil infiltration in cholangiocarcinoma. Hence, we speculate that elevated dNLR could reflect increased local neutrophil infiltration, thereby accelerating tumor progression and metastasis and ultimately adversely affecting patient outcomes (29).
In addition to elevated neutrophil counts, our cohort demonstrated significantly reduced lymphocyte counts in patients with high dNLR. Lymphocytes are the primary mediators of antitumor immunity, and lower lymphocyte counts or proportions directly reflect impaired antitumor immune responses, correlating with poor prognosis across numerous malignancies (30–32). In cholangiocarcinoma, lymphocytes—particularly CD8+ T cells—play a critical prognostic role. Kitano et al. reported that reduced local CD8+ T-cell infiltration was associated with significantly poorer overall survival in extrahepatic CCA patients; similarly, Kim et al. demonstrated that patients with higher CD8+ T-cell infiltration (≥100 cells/high-power field) exhibited improved OS time and DFS time (49.7 and 23.3 months, respectively) compared to controls (33, 34). Beyond CD8+ T cells, atypical T-cell subsets also exhibit potent antitumor activity. Zimmer et al. identified mucosal-associated invariant T (MAIT) cells in iCCA, correlating increased local MAIT cell infiltration with substantially improved survival (median: 59.5 months) (35). Likewise, increased counts of natural killer (NK) cells with recognized antitumor properties have been positively correlated with improved survival in CCA patients (36). These data reinforce that lymphocyte counts and proportions significantly influence recurrence and prognosis in CCA. Consequently, the combination of increased neutrophils and decreased lymphocytes, characteristic of high-dNLR patients, likely contributes significantly to their inferior prognosis.
Currently, adjuvant chemotherapy constitutes an essential component in CCA management, significantly influencing patient prognosis. Previous studies have demonstrated the utility of dNLR in predicting chemotherapy responsiveness, with high-dNLR patients exhibiting superior sensitivity to gemcitabine-cisplatin/oxaliplatin regimens compared to those with low dNLR (15, 16). Given that gemcitabine and platinum-based regimens remain standard adjuvant treatments for CCA, we hypothesize that high-dNLR patients may particularly benefit from postoperative chemotherapy, potentially improving survival outcomes, and propose dNLR as a predictive marker for chemotherapy response. However, due to incomplete chemotherapy records and regimen heterogeneity in our cohort, we could not definitively analyze the predictive value of dNLR for chemotherapy response—an area warranting future research.
Our study also identified elevated total bilirubin levels and the classical tumor marker CA19–9 as independent risk factors associated with poor long-term prognosis in patients with dCCA. CA19–9 is widely utilized as a serological biomarker for both the clinical diagnosis and prognostic evaluation of CCA. Tella et al. (37) reviewed data from the United States National Cancer Database (NCDB), encompassing 2,100 patients with extrahepatic CCA, and found that CA19–9 levels were elevated in nearly 1,500 patients, accounting for over 70% of the cohort. Notably, patients with elevated CA19–9 exhibited a significantly shorter median survival compared to those with normal CA19–9 levels (8.5 months vs. 16.0 months, p < 0.01). Furthermore, CA19–9 was identified as an independent prognostic factor for long-term survival (RR = 1.72, 95% CI: 1.46–2.02). Interestingly, subsequent studies have highlighted that in cases of dCCA and pancreatic head carcinoma, obstructive jaundice caused by tumor-induced biliary obstruction can lead to an abnormal elevation of both bilirubin and CA19–9 levels. Such elevations may not accurately reflect the true tumor burden or predict long-term outcomes, thereby confounding prognostic assessments. In response to this challenge, innovative approaches have been proposed, such as employing the ratio of CA19–9 to bilirubin to mitigate the influence of inflammation and biliary obstruction on biomarker interpretation (38–40).
Consistent with these findings, our study also demonstrated that bilirubin and CA19–9 exert independent, non-collinear effects on the postoperative prognosis of dCCA patients. This reinforces the concept that both markers should be carefully interpreted as distinct prognostic indicators. In future clinical applications, these findings may enable clinicians to more precisely evaluate and intervene in the postoperative management of dCCA patients, ultimately contributing to improved long-term outcomes in this challenging population.
This study has several limitations. First, as a single-center retrospective study, selection bias, including sample selection and data recording, was unavoidable, limiting the reliability and promotion value of our result. Prospective multicenter studies with larger sample sizes are necessary to confirm our conclusions. Second, although our predictive model incorporating dNLR underwent rigorous internal validation, external validation remains essential for broader applicability. Third, while we discussed possible reasons underlying dNLR’s predictive utility, our study did not conclusively elucidate underlying biological mechanisms, which warrant further investigation. Lastly, we did not assess the predictive role of dNLR for adjuvant treatment efficacy, thus restricting clinical applicability; future studies should further explore this aspect to comprehensively validate dNLR’s clinical utility.
Conclusion
As a composite blood-based inflammatory markers, dNLR serves as a novel prognostic indicator for dCCA. CA19–9 levels, lymph node metastasis, portal vein invasion, and tumor differentiation were identified as independent prognostic factors affecting survival in dCCA patients as well. By employing our machine-learning-driven prognostic prediction model, clinicians can facilitate early risk stratification and targeted interventions, ultimately contributing to improved clinical outcomes in patients with dCCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of The First Affiliated Hospital of Henan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Writing – original draft, Formal Analysis, Methodology, Conceptualization, Resources. LB: Conceptualization, Resources, Writing – original draft, Formal Analysis, Methodology. XM: Resources, Writing – original draft, Formal Analysis. SZ: Investigation, Writing – original draft, Data curation. PZ: Writing – review & editing, Funding acquisition, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China Youth Project (82405482), Special Project on Traditional Chinese Medicine Research in China (2022JDZX113); Excellent Teaching Case Project for Professional Degree Graduate Students at the First Affiliated Hospital of Henan University of Traditional Chinese Medicine (YJSJPAL-202213).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qurashi M, Vithayathil M, and Khan SA. Epidemiology of cholangiocarcinoma. Eur J Surg Oncol. (2025) 51:107064. doi: 10.1016/j.ejso.2023.107064
2. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. (2021) 7:65. doi: 10.1038/s41572-021-00300-2
3. Valle JW, Kelley RK, Nervi B, Oh DY, and Zhu AX. Biliary tract cancer. LANCET. (2021) 397:428–44. doi: 10.1016/S0140-6736(21)00153-7
4. Kidanemariam S, Gu J, Yoon JH, Challapalli JV, Fruh V, and Sax AJ. Cholangiocarcinoma: epidemiology and imaging-based review. R I Med J (2013). (2024) 107:43–8.
5. Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, et al. Invernizzi P et al: Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. (2022) 27:100737. doi: 10.1016/j.aohep.2022.100737
6. Coussens LM and Werb Z. Inflammation and cancer. NATURE. (2002) 420:860–7. doi: 10.1038/nature01322
7. Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, and Ogata Y. Association of preoperative platelet-to-lymphocyte ratio with poor outcome in patients with distal cholangiocarcinoma. Oncology. (2019) 96:290–8. doi: 10.1159/000499050
8. Zeng D, Wang Y, Wen N, Lu J, Li B, and Cheng N. The prognostic value of preoperative peripheral blood inflammatory biomarkers in extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Front Oncol. (2024) 14:1437978. doi: 10.3389/fonc.2024.1437978
9. Zhang WH, Zhao Y, Zhang CR, Huang JC, Lyu SC, and Lang R. Preoperative systemic inflammatory response index as a prognostic marker for distal cholangiocarcinoma after pancreatoduodenectomy. World J Gastrointest Surg. (2024) 16:2910–24. doi: 10.4240/wjgs.v16.i9.2910
10. Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, et al. Systemic immune-inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today. (2021) 51:1602–9. doi: 10.1007/s00595-021-02312-7
11. Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, and Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. (2012) 107:695–9. doi: 10.1038/bjc.2012.292
12. Ren K, Yin Y, He F, Shao Y, and Wang S. Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer MANAG Res. (2018) 10:4891–8. doi: 10.2147/CMAR.S180695
13. Song S, Li C, Li S, Gao H, Lan X, and Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. (2017) 10:3145–54. doi: 10.2147/OTT.S138039
14. Szkandera J, Stotz M, Eisner F, Absenger G, Stojakovic T, Samonigg H, et al. Ress AL et al: External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PloS One. (2013) 8:e78225. doi: 10.1371/journal.pone.0078225
15. Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol. (2015) 26:1910–6. doi: 10.1093/annonc/mdv253
16. Buyuksimsek M, Kidi MM, Ogul A, Mirili C, and Paydas S. The effect of inflammatory markers on survival in advanced biliary tract carcinoma treated with gemcitabine/oxaliplatin regimen. J Gastrointest Cancer. (2021) 52:249–55. doi: 10.1007/s12029-020-00396-x
17. Zhang ZF, Wang T, Kong JJ, Yang LP, Yang XW, and Wang WT. The predictive value of perioperative inflammatory indicatorsin prognosis of the intrahepatic cholangiocarcinoma patients after hepatectomy. Sichuan Da Xue Xue Bao Yi Xue Ban. (2020) 51:403–10. doi: 10.12182/20200560207
18. Alessi JV, Ricciuti B, Alden SL, Bertram AA, Lin JJ, Sakhi M, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. (2021) 9:e003536. doi: 10.1136/jitc-2021-003536
19. Kim CG, Kim MH, Kim JH, Kim SG, Kim GM, Kim TY, et al. Park S et al: On-treatment derived neutrophil-to-lymphocyte ratio and survival with palbociclib and endocrine treatment: analysis of a multicenter retrospective cohort and the PALOMA-2/3 study with immune correlates. Breast Cancer Res. (2023) 25:4. doi: 10.1186/s13058-022-01601-4
20. Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, et al. Gao T et al: Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. (2015) 13:66. doi: 10.1186/s12967-015-0409-0
21. Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. (2013) 109:395–400. doi: 10.1038/bjc.2013.346
22. Wculek SK, Bridgeman VL, Peakman F, and Malanchi I. Early neutrophil responses to chemical carcinogenesis shape long-term lung cancer susceptibility. iScience. (2020) 23:101277. doi: 10.1016/j.isci.2020.101277
23. Zhong J, Li Q, Luo H, and Holmdahl R. Neutrophil-derived reactive oxygen species promote tumor colonization. Commun Biol. (2021) 4:865. doi: 10.1038/s42003-021-02376-8
24. Tecchio C, Scapini P, Pizzolo G, and Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. (2013) 23:159–70. doi: 10.1016/j.semcancer.2013.02.004
25. Adrover JM, McDowell S, He XY, Quail DF, and Egeblad M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. (2023) 41:505–26. doi: 10.1016/j.ccell.2023.02.001
26. Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. (2021) 9:e001946. doi: 10.1136/jitc-2020-001946
27. Yoshimoto M, Kagawa S, Kajioka H, Taniguchi A, Kuroda S, Kikuchi S, et al. Teraishi F et al: Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. (2023) 567:216260. doi: 10.1016/j.canlet.2023.216260
28. Zhang C, Wu D, Dong B, Liao G, Yu Y, Huang S, et al. Li T et al: The scaffold of neutrophil extracellular traps promotes CCA progression and modulates angiogenesis via ITGAV/NFkappaB. Cell Commun Signal. (2024) 22:103. doi: 10.1186/s12964-024-01500-5
29. Watanabe A, Harimoto N, Araki K, Kubo N, Igarashi T, Tsukagoshi M, et al. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma. Anticancer Res. (2019) 39:941–7. doi: 10.21873/anticanres.13197
30. Yin LX, Routman DM, Day CN, Harmsen WS, Haller T, Bartemes K, et al. Neben-Wittich M et al: Low postoperative lymphocyte count increases risk of progression in human papillomavirus associated oropharyngeal cancer. Head Neck. (2022) 44:2760–8. doi: 10.1002/hed.v44.12
31. Miyamoto N, Inoue H, Inui T, Sasa S, Aoyama M, Okumura K, et al. Yukishige S et al: Absolute Lymphocyte Count Changes During Neoadjuvant Chemotherapy are Associated With Prognosis of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Patients. Clin Breast Cancer. (2023) 23:e68–76. doi: 10.1016/j.clbc.2023.01.005
32. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18:148. doi: 10.1186/s12876-018-0877-9
33. Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Arima K et al: Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. (2018) 118:171–80. doi: 10.1038/bjc.2017.401
34. Kim R, Coppola D, Wang E, Chang YD, Kim Y, Anaya D, et al. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget. (2018) 9:23366–72. doi: 10.18632/oncotarget.25163
35. Zimmer CL, Filipovic I, Cornillet M, O’Rourke CJ, Berglin L, Jansson H, et al. Johansson H et al: Mucosal-associated invariant T-cell tumor infiltration predicts long-term survival in cholangiocarcinoma. HEPATOLOGY. (2022) 75:1154–68. doi: 10.1002/hep.32222
36. Shi H, Li Z, and Zhu M. Circulating immune cells predict prognosis and clinical response to chemotherapy in cholangiocarcinoma. Curr Med Chem. (2025) 32:595–607. doi: 10.2174/0109298673296618240424095548
37. Tella SH, Kommalapati A, Yadav S, Bergquist JR, Goyal G, Durgin L, et al. Novel staging system using carbohydrate antigen (CA) 19–9 in extra-hepatic cholangiocarcinomaand its implications on overall survival. Eur J Surg Oncol. (2020) 46:789–95. doi: 10.1016/j.ejso.2020.01.016
38. Wang J, Lyu SC, Zhou L, Wang H, Pan F, Jiang T, et al. Prognostic analysis of pancreatic carcinoma with portal system invasion following curative resection. Gland Surg. (2021) 10:35–49. doi: 10.21037/gs-20-495
39. Lyu SC, Wang J, Huang M, Wang HX, Zhou L, He Q, et al. CA19–9 level to serum γ-glutamyltransferase as a potential prognostic biomarker in patients with pancreatic head carcinoma. Cancer Manag Res. (2021) 13:4887–98. doi: 10.2147/CMAR.S313517
Keywords: machine learning, pancreaticoduodenectomy, distal cholangiocarcinoma, derived neutrophil-to-lymphocyte ratio (dNLR), prognosis
Citation: Yin Y, Bai L, Mu X, Zhang S and Zhai P (2025) A multi-algorithm prognostic model combining inflammatory indices and surgical features in distal cholangiocarcinoma. Front. Oncol. 15:1625703. doi: 10.3389/fonc.2025.1625703
Received: 09 May 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Shaocheng Lyu, Capital Medical University, ChinaReviewed by:
Hengcheng Zhang, Brigham and Women’s Hospital and Harvard Medical School, United StatesFulei Wuchu, University of Michigan, United States
Pan Bochen, Nanjing Medical University, China
Xiaolong Tian, Yale University, United States
Copyright © 2025 Yin, Bai, Mu, Zhang and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panpan Zhai, MTg3MzgxMTQxMzlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yi Yin
Yi Yin Luyuan Bai3†
Luyuan Bai3† Panpan Zhai
Panpan Zhai