- Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of Dalian Medical University, Dalian, China
Background: Primary pulmonary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) localized in the trachea and bronchus is rare and diagnostically challenging due to nonspecific clinical and radiological features.
Case description: We present two cases of primary endobronchial MALT lymphoma incidentally diagnosed via bronchoscopy. We assessed the characteristic bronchoscopic finding of endobronchial MALT lymphoma, radiological examinations and overall treatment strategies.
Conclusion: This study highlights the importance of considering endobronchial MALT lymphoma as a potential diagnosis. A thorough bronchoscopic evaluation of the central airways, along with appropriate biopsy of suspicious lesions, is crucial. Endobronchial MALT lymphoma should be suspected when targeting multiple widely stalked and smooth submucosal nodules or protrusions, mainly located in the central airways.
1 Introduction
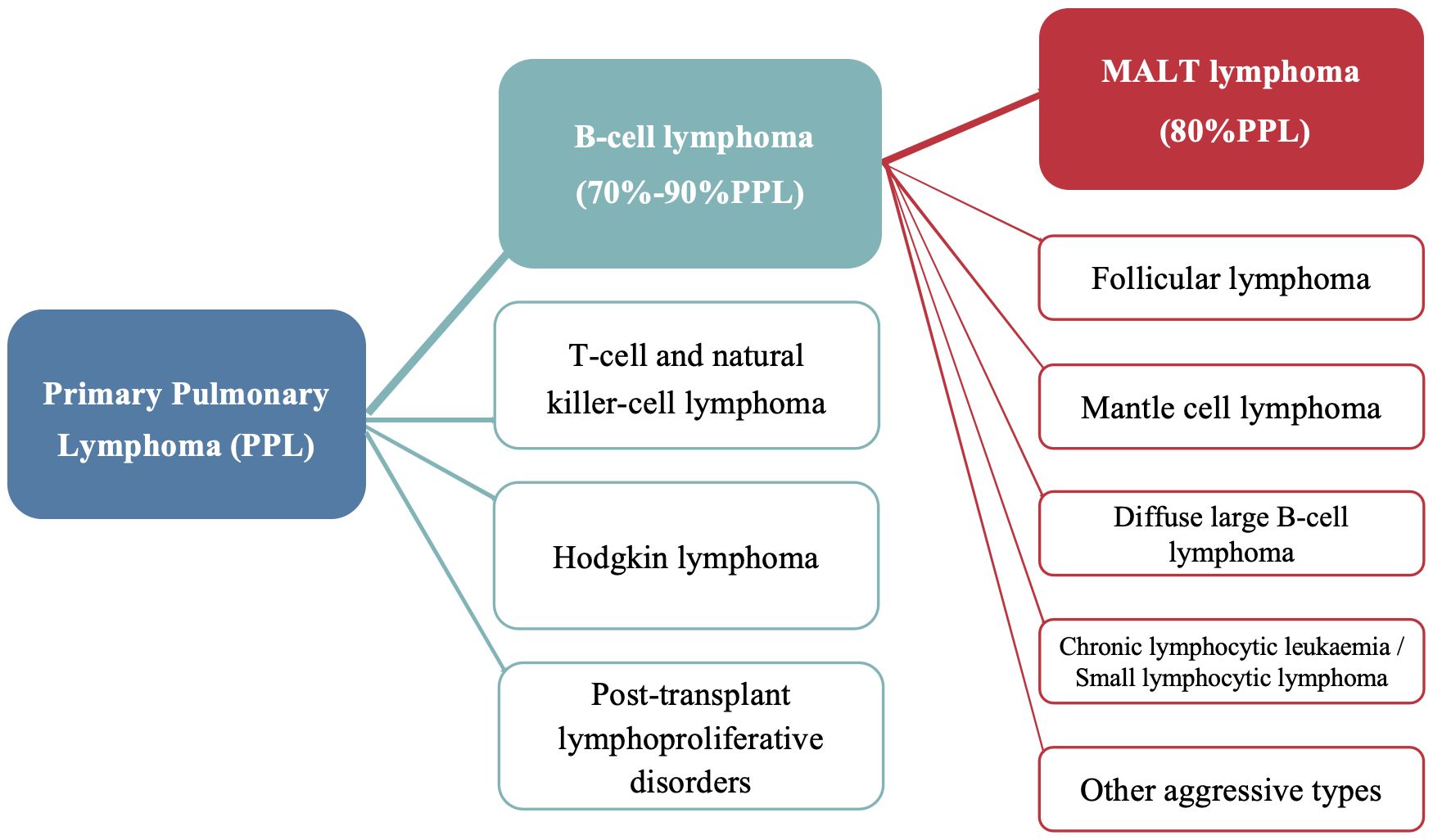
Primary pulmonary lymphoma (PPL) refers to a clonal proliferation of lymphoid cells localized to the lung parenchyma and/or bronchi, with no evidence of extrapulmonary involvement at initial diagnosis or within the following three months (1). It accounts for only 0.5%–1% of primary pulmonary malignancies and <1% of non-Hodgkin’s lymphoma (2). Primary pulmonary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma is a type of primary pulmonary B-cell non-Hodgkin’s lymphoma, accounting for nearly 90% of such cases and 80% PPL (Figure 1) (3). However, primary endobronchial MALT lymphoma that is confined to the tracheobronchial tree remains very rare with only a few cases reported (4–6). Approximately half of patients with pulmonary MALT lymphoma are asymptomatic (7), and nonspecific computed tomography (CT) findings often lead to misdiagnosis, particularly in cases with isolated endobronchial involvement. Here, we report two cases of endobronchial MALT lymphoma incidentally diagnosed via bronchoscopy. The first case presented with pneumonia, and bronchoscopy examination revealed multiple nodular protrusions in the trachea and right middle lobar bronchus, with biopsy confirming MALT lymphoma. The second case presented with asymptomatic cavitary lung lesions, and MALT lymphoma with only tracheal involvement was diagnosed via transbronchial biopsy.
2 Diagnostic assessment
2.1 Case 1
A 65-year-old man with diabetes and a history of smoking was admitted for a 10-day fever and 3-day dyspnea. The clinical information and laboratory results of the patient on admission were unremarkable and summarized in Table 1. Pulmonary function tests indicated that ventilation function was normal and diffusion function was slightly decreased. Chest CT revealed bilateral inflammatory infiltrates and mildly enlarged mediastinal lymph nodes. Bronchoscopy identified yellow-white secretions in the carina and bilateral main bronchus, and the testing of bronchoalveolar lavage fluid (BALF) for Mycobacterium tuberculosis, general bacteria and fungi smear, galactomannan tests and cryptococcus smear were all negative. Further BALF metagenomic next-generation sequencing revealed a positive result for Klebsiella aerogenes, with 100 uniquely mapped reads, which was consistent with the initial diagnosis of community acquired pneumonia. Unexpectedly, multiple broad-based, smooth nodular lesions were observed in the trachea and right middle lobar bronchus. Mucosal biopsy demonstrated a large number of lymphoid cells infiltration, and immunohistochemistry staining revealed CD20 (+), CD19 (+), Bcl-2 (+), Bcl-6 (partial+), CD21 (FDC net+), CD23 (FDC net+), CD3 (–), CD5 (-), CD10 (-), CD43 (-), Cyclin D1 (-), Ki67 (+20%) (Figure 2). The pathology was consisitent with MALT lymphoma. Positron emission tomography (PET)-CT showed mild 18 F-fluorodeoxyglucose (FDG) uptake with an average maximum standardized uptake value (SUVmax) 2.8-4.6 in hilar and mediastinal nodes.
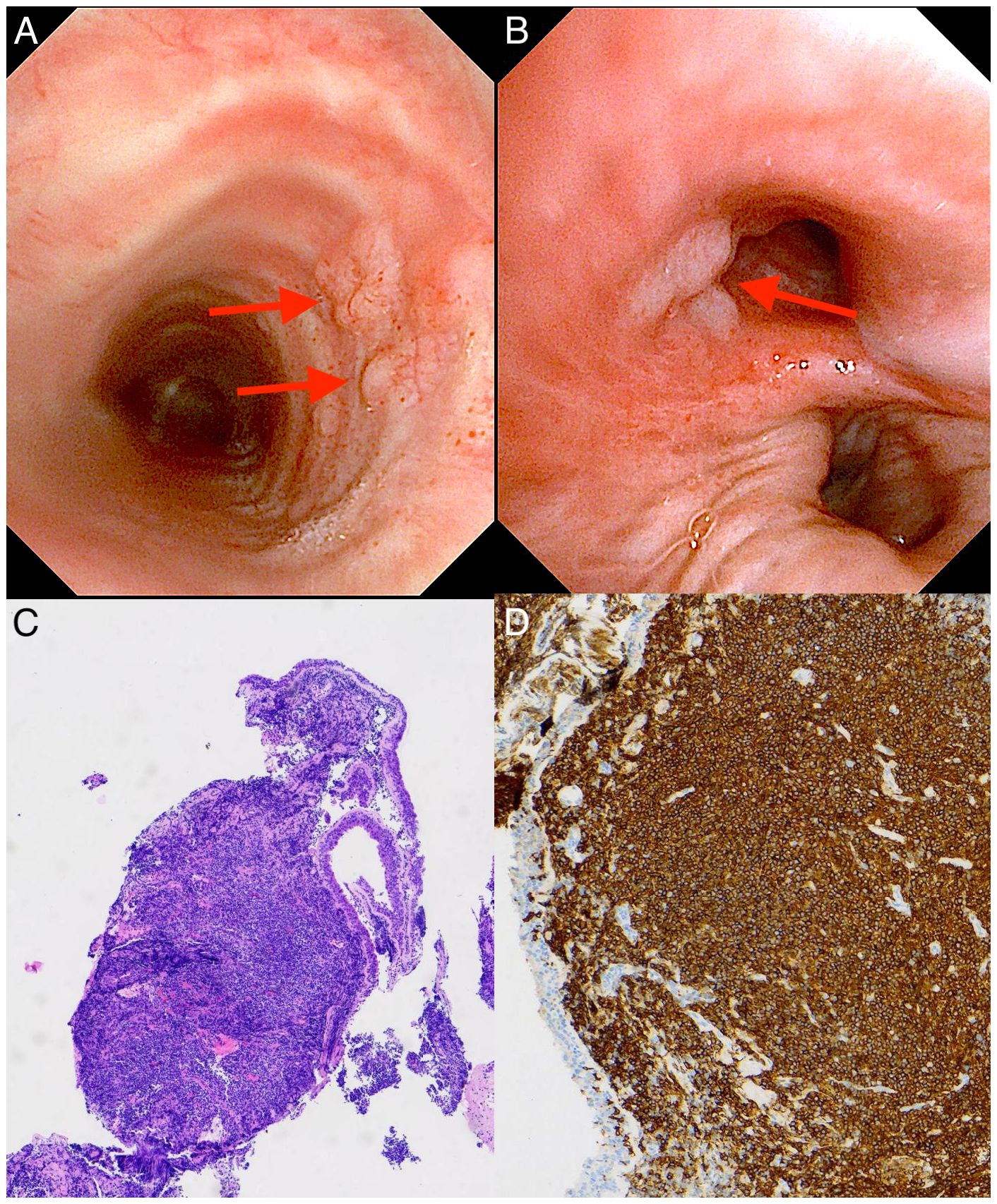
Figure 2. Bronchoscopic findings and pathological biopsy of case 1 with primary endobronchial MALT lymphoma. (A, B) Bronchoscopy showing multiple broad-based, smooth nodular lesions localized in the trachea [red arrows in (A)] and right middle lobar bronchus [red arrow in (B)]. (C) Light microscopic image showing diffuse diffuse infiltration of lymphoma cells, mostly centrocyte-like lymphocytes with deep blue nuclei. (Hematoxylin-Eosin stain; 40×magnification). (D) Immunohistochemical examination showing the cells were diffusely positive for B cell markers of CD20, manifested as dense brown staining of the cell membrane and pale blue staining of the nucleus (with hematoxylin counterstaining) (×100).
2.2 Case 2
A 62-year-old asymptomatic woman was referred for a solitary cavitary lung lesion detected 18 months prior. Her clinical information and laboratory results were insignificant and shown in Table 1. Pulmonary function suggested partial reversible mild obstructive ventilation dysfunction and normal diffusion function, which supported the diagnosis of asthma. Further, bronchoscopy revealed several nodular protrusions along the trachea; Biopsy demonstrated lymphoid hyperplasia, with immunohistochemical examination showing lymphoma cells were positive for CD20, PAX-5, Bcl-2, CD21 (FDC net), CD23 (FDC net), CK (epithelium), Bcl-6 (partial), CD5 (T cell), Ki67 (+15%), but negative for CD10, CD30, Cyclin D1 and SOX11 (Figure 3). The pathogen detection in the BALF was all negative. Finally, we made a diagnosis of MALT lymphoma. After discharge, the patient was admitted to the hematology department for further evaluation. The bone marrow biopsy and gastrointestinal endoscopy indicated no lymphoma involvement. PET-CT detected bilateral lung nodules without FDG avidity.
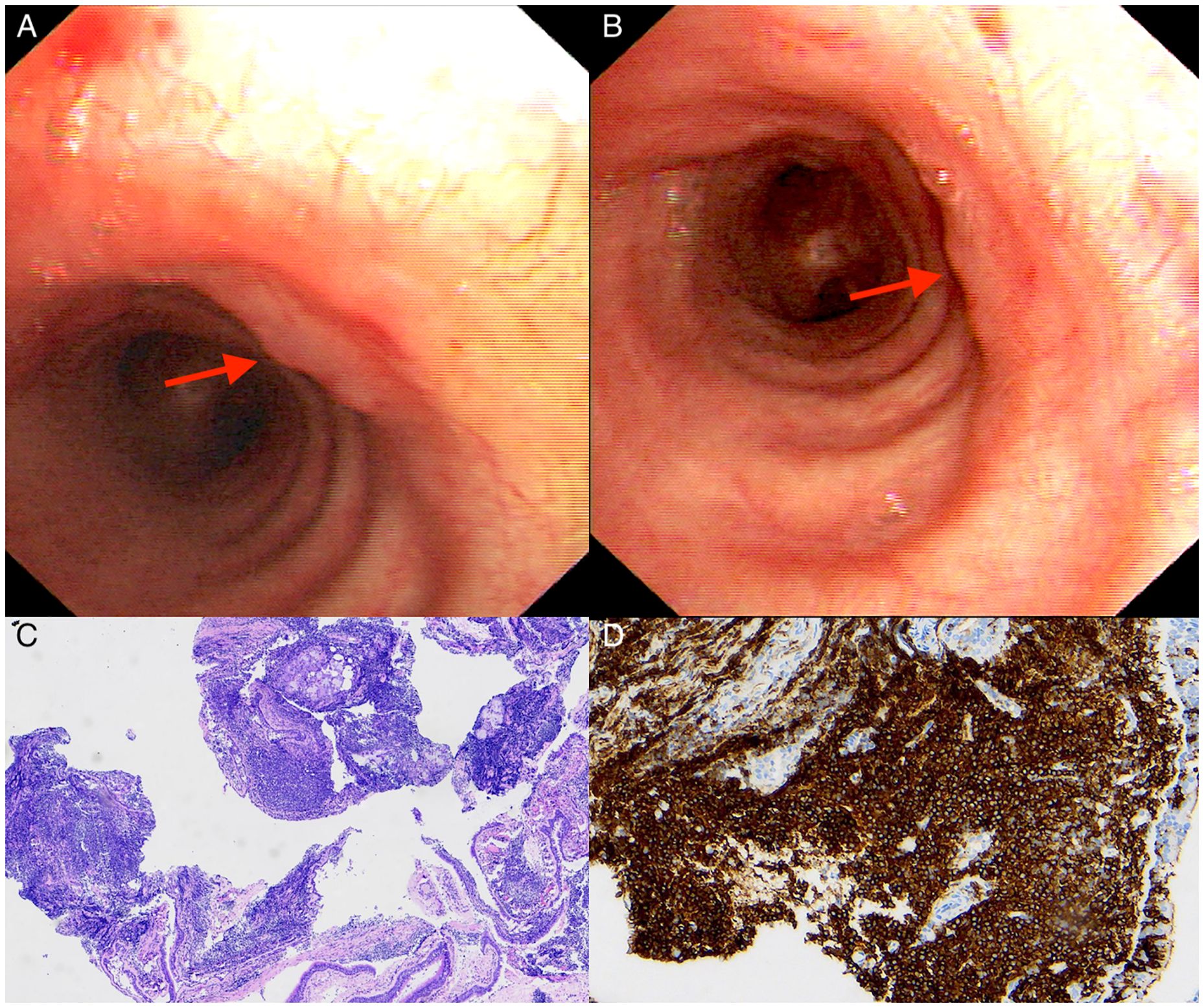
Figure 3. Bronchoscopic and pathological findings of case 2 with primary endobronchial MALT lymphoma. (A, B) Bronchoscopy showing several nodular protrusions covered with smooth bronchial mucosa along the trachea (red arrows). (C) Microscopically showing diffuse infiltration of lymphoma cells. (Hematoxylin-Eosin stain; 40×magnification). (D) The lymphoma cells were diffusely positive for CD20 (×100).
3 Intervention, follow-up and outcomes
Taking into account the patient’s preference, case 1 opted for no special therapeutic intervention for MALT lymphoma and close observation. During the 9-month follow-up after pneumonia treatment, the patient showed no clinical symptoms, and the re-examination of chest CT indicated that the pneumonia had totally absorbed and the MALT lymphoma remained progression-free. Case 2 also received watchful waiting therapy due to she was asymptomatic and her MALT lymphoma was confined to the trachea. Case 2 remained stable at 7-month follow-up.
4 Discussion
The diagnosis of pulmonary MALT lymphoma relies on histological examination. Morphologically, it is characterized by small lymphoid cells resembling centrocyte-like cells or monocytoid cells, along with lymphoepithelial lesions and follicular colonization (8). Immunohistochemical analysis aids in differential diagnosis and confirmation. In our cases, CD20 (+), CD19 (+), and PAX-5 (+) confirmed B-cell lineage, while a Ki67 proliferation index of 15%-20% indicated its indolent nature. CD21 (FDC net+), CD23 (FDC net+) and CK (+) suggested the lymphoma invading follicular structures and the bronchial epithelium. The absence of CD10, CD30, SOX11 and Cyclin D1 help rule out other B-cell lymphoma. Additionally, PET-CT, bone marrow biopsy, gastrointestinal endoscopy, and 7-9 months of follow-up revealed no extrapulmonary involvement, infections, or other malignancies. Therefore, both cases were accurately diagnosed as primary pulmonary MALT lymphoma.
The clinical and radiological presentation of primary pulmonary MALT lymphoma is highly variable. When parenchymal involvement occurs, typical manifestations include nodules, consolidations, irregular airway wall thickening, luminal stenosis, and/or atelectasis. Approximately 70% of cases show bilateral or multifocal lesions, while 30% exhibit hilar or mediastinal lymphadenopathy (9, 10). Besides endobronchial MALT lymphoma, differential diagnosiss for endobronchial nodular lesions should also include primary and metastatic airway malignancies, benign tumors (e.g., endobronchial chondromas), infectious processes (e.g., tuberculosis), inflammatory conditions (e.g., sarcoidosis), other rare disorders (e.g., tracheobronchial ossification). It’s worth noting that Dieulafoy’s lesions can also be found in the submucosa of the bronchus, improper transbronchial biopsy may lead to massive bleeding (11). Accurate diagnosis requires comprehensive evaluation incorporating clinical presentation, imaging findings, and pathological examination.
The diagnostic efficacy of bronchoscopy is higher when it targets visible endobronchial lesions or radiographically apparent abnormalities. Yoon et al. classified CT findings of endobronchial MALT lymphoma into three patterns: isolated intraluminal nodules, multiple nodular protrusions, and diffuse wall thickening (12). Notably, 79.6% of lesions localized to central airways, with multiple nodules (60.4%) being most common, while solitary nodules and diffuse wall thickening accounted for 25% and 10.4%, respectively (13). Therefore, careful observation of trachea under bronchoscopy is crucial for diagnosing endobronchial MALT lymphoma (14). In 2018, Kawaguchi et al. reviewed 20 cases of endobronchial MALT lymphomaand highlighted that several widely stalked nodular protrusions covered with smooth bronchial mucosa were a characteristic bronchoscopic finding of endobronchial MALT lymphoma (15). Our two cases exhibited similar features: multiple widely stalked and smooth submucosal nodules or protrusions, mainly located at the central airways (trachea or bronchus). PET-CT is not routinely recommended for MALT lymphoma due to absent FDG avidity in up to 50% of cases. Low FDG uptake may be associated with small tumor size and low Ki-67 index, with typical SUVmax values ranging from 2.2 to 6.3 (16, 17), reflecting the indolent nature of MALT lymphoma.
There is no standard treatment for primary pulmonary MALT lymphoma. Options include observation, radiotherapy, immunotherapy, chemotherapy, or surgery, with excellent 5-year survival rates (87.5%–93.6%) (18). Given its indolent nature, asymptomatic or early-stage patients may benefit from watchful waiting or localized radiotherapy (19, 20). Kunye Kwak et al. reported 10-year event-free (EFS) and overall survival (OS) rates of 78.7% and 100% for localized therapies, outperforming systemic chemotherapy, which was 56.9% and 71.7%, respectively (21). Similarly, Wei Yan et al. found no OS difference between observation and rituximab-based therapy in early-stage disease (22). Besides, surgical resection may benefit patients with high tumor burden, in-situ progression or suspected malignant transformation. A study of 123 patients with primary pulmonary MALT lymphoma compared three first-line treatments. Results showed that OS was high in all groups (93% at 6 years), though surgery had the best results (100%), followed by active monitoring (91%) and systemic therapy (76%). Complete surgical resection had better long-term disease control (6-year EFS: 74% vs. 65% vs. 62%; P=0.013) (23). For inoperable patients, anti-CD20 monoclonal antibody with or without chemotherapy was effective, providing a 50% 5-year EFS and 90% OS, regardless of disease location (24).
Our study has several limitations. First, the small sample size of only two cases limits the generalizability of the findings. With low incidence, larger and multicenter studies are needed to better characterize the bronchoscopic features of primary endobronchial MALT lymphoma. Second, the follow-up period (7-9 months) was relatively short, which may not fully capture the long-term progression or outcomes of the “watch and wait” approach in such patients. Future research should focus on advanced molecular techniques assisting the early and noninvasive diagnosis. Clinical trials comparing conservative observation with localized therapies (e.g., radiotherapy) or systemic treatments (e.g., rituximab-based regimens) are also needed to establish standardized guidelines for asymptomatic or early-stage patients.
5 Conclusion
Primary endobronchial MALT lymphoma with isolated trachea and bronchus involvement is rare and easily misdiagnosed. Thorough bronchoscopy examination of the central airways and appropriate biopsy of characteristic lesions are important. Endobronchial MALT lymphoma should be considered when targeting multiple widely stalked and smooth submucosal nodules or protrusions, mainly located at the central airways.
6 Patient perspective
Because of both patients were asymptomatic and remained stable, they refused re-examination of bronchoscopy. But both patients have agreed to undergo long-term outpatient follow-up.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of First Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LS: Writing – original draft, Writing – review & editing. M-YC: Writing – original draft, Writing – review & editing. MF: Writing – original draft, Writing – review & editing, Supervision, Validation. CD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cadranel J, Wislez M, and Antoine M. Primary pulmonary lymphoma. Eur Respir J. (2002) 20:750–62. doi: 10.1183/09031936.02.00404102
2. Borie R, Wislez M, Antoine M, Copie-Bergman C, Thieblemont C, and Cadranel J. Pulmonary mucosa-associated lymphoid tissue lymphoma revisited. Eur Respir J. (2016) 47:1244–60. doi: 10.1183/13993003.01701-2015
3. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Edn. Geneva: World Health Organization (2008).
4. Minami D, Ando C, Sato K, Moriwaki K, Sugahara F, Nakasuka T, et al. Multiple mucosa-associated lymphoid tissue lymphoma of the trachea. Intern Med. (2017) 56:2907–11. doi: 10.2169/internalmedicine.8269-16
5. Liao TY, Lin CC, Yuan CT, Lin CK, and Ho CC. Mucosa-associated lymphoid tissue lymphoma with isolated endobronchial involvement. Respirol Case Rep. (2020) 8:e00672. doi: 10.1002/rcr2.672
6. Ikejima K, Matsusako M, Yamada D, Murakami M, Ito R, Kanomata N, et al. A case of primary endobronchial mucosa-associated lymphoid tissue lymphoma stable on 16-year observation. J Thorac Imaging. (2022) 37:W109–11. doi: 10.1097/RTI.0000000000000674
7. Cheah CY and Seymour JF. Marginal zone lymphoma: 2023 update on diagnosis and management. Am J Hematol. (2023) 98:1645–57. doi: 10.1002/ajh.27058
8. Sanguedolce F, Zanelli M, Zizzo M, Bisagni A, Soriano A, Cocco G, et al. Primary pulmonary B-cell lymphoma: A review and update. Cancers (Basel). (2021) 13:415. doi: 10.3390/cancers13030415
9. Hare SS, Souza CA, Bain G, Seely JM, Frcpc, Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. (2012) 85:848–64. doi: 10.1259/bjr/16420165
10. Wang Y, He Z, Zhu Z, and Luo R. Primary pulmonary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type: a case report and literature review. Am J Transl Res. (2022) 14:9072–7.
11. Xing X, Liu J, Xu S, Deng Y, and Yang J. Research advances in Dieulafoy's disease of the bronchus (Review). Exp Ther Med. (2022) 23:100. doi: 10.3892/etm.2021.11023
12. Yoon RG, Kim MY, Song JW, Chae EJ, Choi CM, and Jang S. Primary endobronchial marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: CT findings in 7 patients. Korean J Radiol. (2013) 14:366–74. doi: 10.3348/kjr.2013.14.2.366
13. Arai R, Tanifuji M, Nagai A, Ebihara A, Iwamoto T, Onaka S, et al. A case of endobronchial mucosa-associated lymphoid tissue (MALT) lymphoma successfully treated with radiotherapy and a review of the literature. Respirol Case Rep. (2024) 12:e01369. doi: 10.1002/rcr2.1369
14. Lat T, Sanchez JF, McGraw MK, Hodjat P, White HD, and Boethel CD. Decision-making in diagnosis of bronchus-associated lymphoid tissue lymphoma. Proc (Bayl Univ Med Cent). (2021) 34:451–5. doi: 10.1080/08998280.2021.1889275
15. Kawaguchi T, Himeji D, Kawano N, Shimao Y, and Marutsuka K. Endobronchial mucosa-associated lymphoid tissue lymphoma: A report of two cases and a review of the literature. Intern Med. (2018) 57:2233–6. doi: 10.2169/internalmedicine.0150-17
16. Kiesewetter B and Raderer M. How can we assess and measure prognosis for MALT lymphoma? A review of current findings and strategies. Expert Rev Hematol. (2021) 14:391–9. doi: 10.1080/17474086.2021.1909468
17. Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest. (2008) 133:433–40. doi: 10.1378/chest.07-1956
18. Zhen CJ, Zhang P, Bai WW, Song YZ, Liang JL, Qiao XY, et al. Mucosa-associated lymphoid tissue lymphoma of the trachea treated with radiotherapy: A case report. World J Clin Cases. (2023) 11:1607–14. doi: 10.12998/wjcc.v11.i7.1607
19. Girinsky T, Paumier A, Ferme C, Hanna C, Ribrag V, Leroy-Ladurie F, et al. Low-dose radiation treatment in pulmonary mucosa-associated lymphoid tissue lymphoma: a plausible approach? A single-institution experience in 10 patients. Int J Radiat Oncol Biol Phys. (2012) 83:e385–9. doi: 10.1016/j.ijrobp.2012.01.005
20. Santopietro M, Kovalchuk S, Battistini R, Puccini B, Annibali O, Romano I, et al. Treatment and prognosis of primary pulmonary lymphoma: A long-term follow-up study. Eur J Haematol. (2021) 106:49–57. doi: 10.1111/ejh.13507
21. Kwak K, Yoo KH, Choi YS, Park Y, Kim BS, Yoon SE, et al. Long-term survival outcomes of 'watch and wait' in patients with bronchus-associated lymphoid tissue lymphoma: a multicenter real-world data analysis in Korea. Ann Hematol. (2024) 103:4193–202. doi: 10.1007/s00277-024-05902-w
22. Yan W, Wu B, Liao AJ, Yang W, and Wang HH. Watch-and-wait or immediate immunotherapy/immunochemotherapy in patients with phase IE primary pulmonary MALT lymphoma? A multicenter retrospective study. Ann Hematol. (2021) 100:709–14. doi: 10.1007/s00277-021-04396-0
23. Joffe E, Leyfman Y, Drill E, Rajeeve S, Zelenetz AD, Palomba ML, et al. Active surveillance of primary extranodal marginal zone lymphoma of bronchus-associated lymphoid tissue. Blood Adv. (2021) 5:345–51. doi: 10.1182/bloodadvances.2020003213
24. Zucca E, Conconi A, Martinelli G, Bouabdallah R, Tucci A, Vitolo U, et al. Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy [. J Clin Oncol. (2017) 35:1905–12. doi: 10.1200/JCO.2016.70.6994
Keywords: primary pulmonary lymphoma (PPL), MALT lymphoma, wait and watch approach, PET-CT, transbronchial biopsy (TBB)
Citation: Sha L, Cai M-Y, Feng M and Dong C (2025) Case Report: Two cases of primary pulmonary endobronchial extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue localized in the trachea and bronchus. Front. Oncol. 15:1626336. doi: 10.3389/fonc.2025.1626336
Received: 10 May 2025; Accepted: 29 July 2025;
Published: 14 August 2025.
Edited by:
Swarna Kanchan, Marshall University, United StatesReviewed by:
Rita Assi, Indiana University Melvin and Bren Simon Comprehensive Cancer Center, United StatesMinu Kesheri, Boise State University, United States
Bhagaban Mallik, The University of Iowa, United States
Chandandeep Kaur, Indian Institute of Horticultural Research (ICAR), India
Poonam Kaithal, Babasaheb Bhimrao Ambedkar University, India
Copyright © 2025 Sha, Cai, Feng and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Dong, ZG9uZ2NoYW5nODc5M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Lei Sha†
Lei Sha† Meng-Yao Cai
Meng-Yao Cai Chang Dong
Chang Dong