- 1Thoracic Surgery Unit, Department of Cardiac, Thoracic, Vascular Sciences and Public Health (DSCTV), University of Padova, Padova, Italy
- 2Pathology Unit, Department of Cardiac, Thoracic, Vascular Sciences and Public Health (DSCTV), University of Padova, Padova, Italy
Introduction: Early-stage lung adenocarcinoma (ADC) is curable by surgical resection in most cases. However, unexpectedly, some patients experience distant disease relapse. Emerging evidence suggests that microscopic tumor characteristics may increase the risk of tumor relapse. Consequently, we aimed to test different microscopic variables to assess their association with distant recurrence (DR).
Materials and methods: We retrieved all cases of radically treated stage I-IIA ADCs from 2016 to 2020. Clinical and pathological variables were assessed for their association with DR using univariable and multivariable logistic regression. An EGFR-adjusted model was also provided.
Results: A total of 259 patients were treated (214 lobectomies and 45 segmentectomies). After resection, 54 patients relapsed, 28 of whom had distant recurrences (DR). Spread through air spaces (STAS) was detected in 48% of samples, while vascular invasion (VI) was present in 53%, occurring 17% more frequently in those with DR. Tumor size was larger in patients with recurrence, with the largest tumors observed in those with local recurrence (25.5 mm in local vs. 23.5 mm in DR; p=0.028). Dedifferentiated (G3) ADCs were more prevalent in DR cases, accounting for 48% of samples. In univariate regression, surgical margins, LVI, necrosis, G3 primary tumors, and STAS were significant factors. In multivariate analysis, STAS showed a trend towards significance (p=0.07) while G3 remained decisive (p<0.01). The EGFR-adjusted model for DR yielded slightly better results (p=0.05 and p<0.01 respectively).
Conclusions: Dedifferentiation and partially STAS are key pathological predictor of distant recurrence in resected stage I-IIA ADCs. The contribution of LVI and tumor necrosis in DR needs to be further clarified. Tumor aggressiveness goes beyond the simple size measurement, claiming for a reassessment of risk models for recurrence after surgery.
1 Introduction
Lung cancer remains one of the leading causes of cancer-related mortality worldwide, due to its aggressive nature and late-stage diagnosis in many patients (1). Despite this, the implementation of lung cancer screening programs, advances in neoadjuvant therapies, and the identification of novel targetable driver mutations offer promising avenues for improving the management of non-small cell lung cancer (NSCLC) (2, 3). However, cancer relapse remains a major clinical challenge, as even in localized cases, recurrence occurs in 20-50% of patients despite adequate and complete resection. Moreover, recurrence within one year is one of the most significant negative prognostic factors for survival, highlighting the need for better risk stratification and adjuvant treatment strategies (4). Evidence suggests that other factors beyond the tumor size and the achievement of an R0 resection must also be considered as important predictors of recurrence (5). Features such as histologic grade, vascular and lymphatic invasion (LVI), spread through air spaces (STAS), and tumor microenvironment characteristics have been increasingly recognized for their role in tumor aggressiveness and metastatic potential (6). This study aimed to evaluate the relationship between microscopic tumor features and distant recurrence after curative-intent resection of early-stage lung adenocarcinoma. The goal was to identify histopathological factors beyond tumor size and resection status that contribute to distant recurrence risk, thereby enhancing risk stratification and improving prognostic assessment in stage I-IIA ADCs.
2 Materials and methods
This monocentric retrospective study was conducted at the Thoracic Surgery Unit, University of Padova, Italy. Data were analyzed from a consecutive cohort of patients who underwent an upfront resection for ADC between January 1, 2016, and December 31, 2020. The study complied with the principles of the Declaration of Helsinki, and all patients provided written informed consent for participation in the department’s research activities before surgery. The study design received approval from the local Ethics Committee (PD n°0038657, 31/05/2024).
2.1 Inclusion criteria
The study included patients over 18 years of age with primary ADC (p-stage I-IIA, 8th AJCC TNM classification) who underwent lobectomy or segmentectomy with radical hilar and mediastinal lymphadenectomy. A preoperative diagnosis of ADC was confirmed by CT-guided or bronchoscopic biopsy, or through postoperative pathological assessment.
Patients with benign or secondary pulmonary lesions were excluded. To enhance sample comparability in line with previous studies, non-anatomical resections (e.g., wedge resections) were also excluded.
2.2 Data retrieval
Data were collected from the perioperative setting, including registry information such as age at surgery, gender, body mass index (BMI), comorbidities, and smoking history. Preoperative pulmonary function tests (Forced Vital Capacity - FVC, Forced Expiratory Volume in one second - FEV1, and alveolar carbon monoxide diffusion limit - DLCO/VA) were performed to evaluate operative tolerance and predicted postoperative risk. Additional data included operative time, type of resection, and intraoperative details. Postoperative follow-up data were gathered from outpatient visits, radiological assessment, and clinical evaluations. In cases of missing follow-up information, data was obtained via phone contact or censored at the last available visit.
2.3 Pathological examination
Pathological data were derived from resected specimens and included growth pattern, histologic grade (G1 to G3), necrosis (<10%, 10-30%, >30%), and tumor-infiltrating lymphocytes (TILs, <10%, 10-30%, >30%). The presence of Spread Through Air Spaces (STAS), lymphovascular invasion (LVI), and pleural invasion were also assessed. Surgical margins were assessed by pathologists based on the distance between the tumor surface and the nearest structure (parenchymal suture or closed bronchus, artery, or vein), with the mechanically closed 2 mm space from stapler sutures excluded. Margins were considered adequate if they measured greater than 20 mm or, if smaller than 20 mm, exceeded the tumor diameter. PD-L1 expression was quantified using the tumor proportion score (TPS, %), and pathological TNM staging was assigned according to the 8th edition of the AJCC TNM classification.
2.4 Surveillance
Follow-up was conducted through regular contrast-enhanced CT scans at 3, 6, or 12 months, in accordance with established guidelines (7). For patients with impaired renal function, non-contrast chest CT scans were approved on a case-by-case basis. After three years, surveillance was performed annually with chest and upper abdominal CT scans. Cerebral MRI was requested only in case of suspected encephalic recurrence. Any suspicion of local or distant recurrence was promptly reviewed by the oncological tumor board.
Locoregional recurrence was defined as any recurrence involving the parenchymal, vascular, or bronchial suture line, ipsilateral hilar and/or mediastinal lymph nodes, or the same lung. Distant recurrence included recurrence in ipsilateral or contralateral serous membranes (pleura, pericardium), the contralateral lung, contralateral hilar and/or mediastinal lymph nodes, the interscalene space, or other extra-thoracic organs. Simultaneous locoregional and distant recurrence was considered as distant recurrence. Upon confirmation, treatment plans and follow-up schedules were determined. Disease-Free Survival (DFS) was defined as the time from surgery to the confirmation of any radiological recurrence (locoregional or distant), as validated by the oncological tumor board.
2.5 Statistical analysis
Continuous variables were reported as median and interquartile range (IQR), while categorical variables were presented as absolute numbers and percentages. Group comparisons were performed using Fisher’s exact test, Pearson’s chi-squared test, or Wilcoxon’s test, as appropriate. Univariable logistic regression was used to analyze associations between clinical and pathological variables and distant recurrence. Significant variables were subsequently included in a multivariable logistic regression model. Stepwise elimination based on the Akaike Information Criterion was used to identify the most parsimonious model. Missing data were addressed using multiple imputation. Considering that the EGFR molecular status is a possible confounder due to its relevance in the clincal practice, we intentionally analyzed data introducing the EGFR status into a EGFR-adjusted multivariate model.
All statistical analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
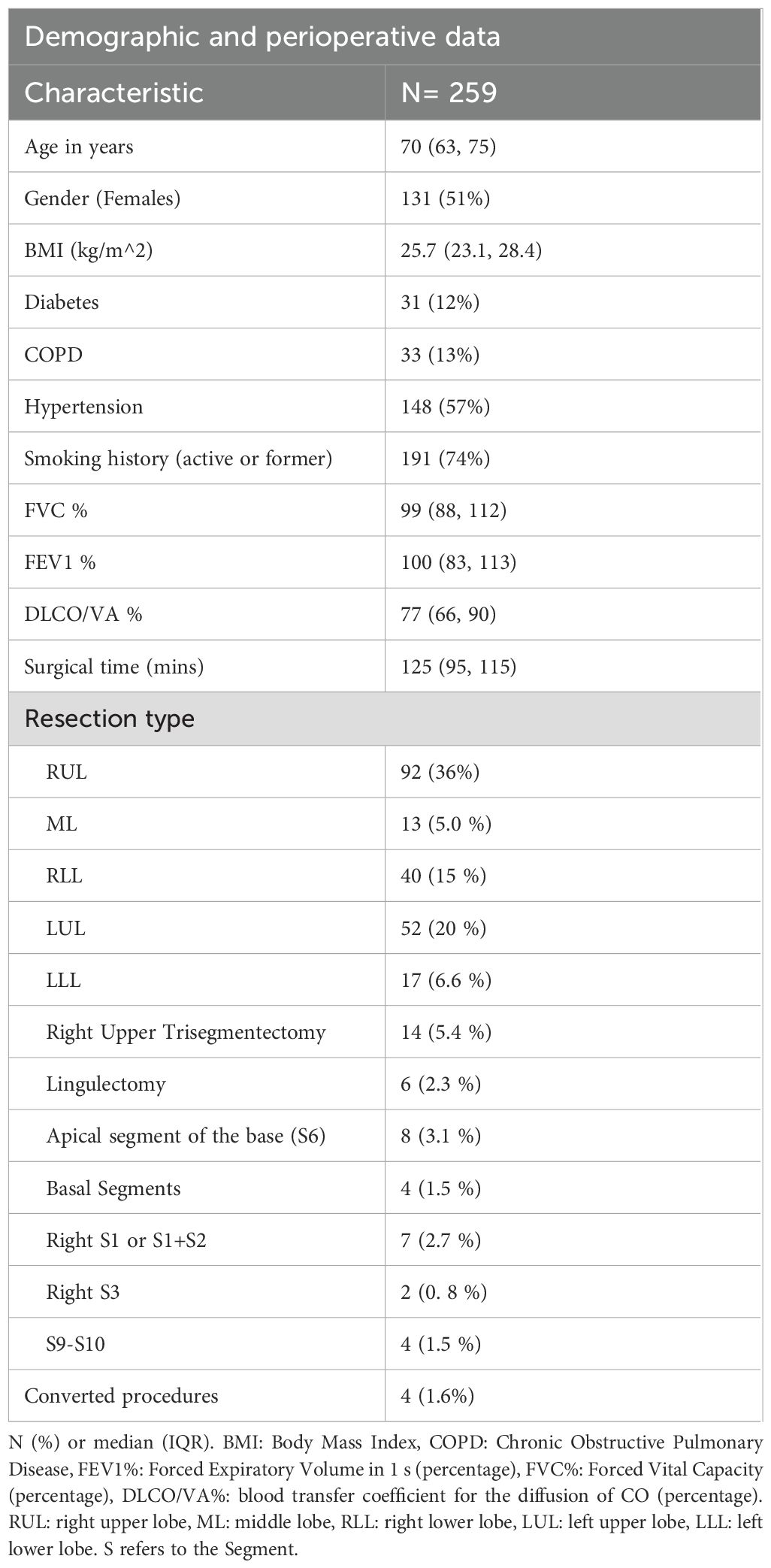
A total of 259 patients were included in the study, with a median age of 70 years (IQR 63-75). Of these, 131 (51%) were female, and 191 (74%) had a history of smoking. Hypertension was the most common comorbidity, affecting 148 patients (57%), followed by diabetes (31 patients, 12%) and chronic obstructive pulmonary disease (COPD) (33 patients, 13%). All patients had sufficient respiratory function to undergo lobectomy. Surgical procedures included 214 lobectomies, with a slight predominance of right upper lobectomies (n=92, 36%), and 45 segmentectomies, primarily involving the left upper lobe (14 upper trisegmentectomies, 5.4%) (Table 1).
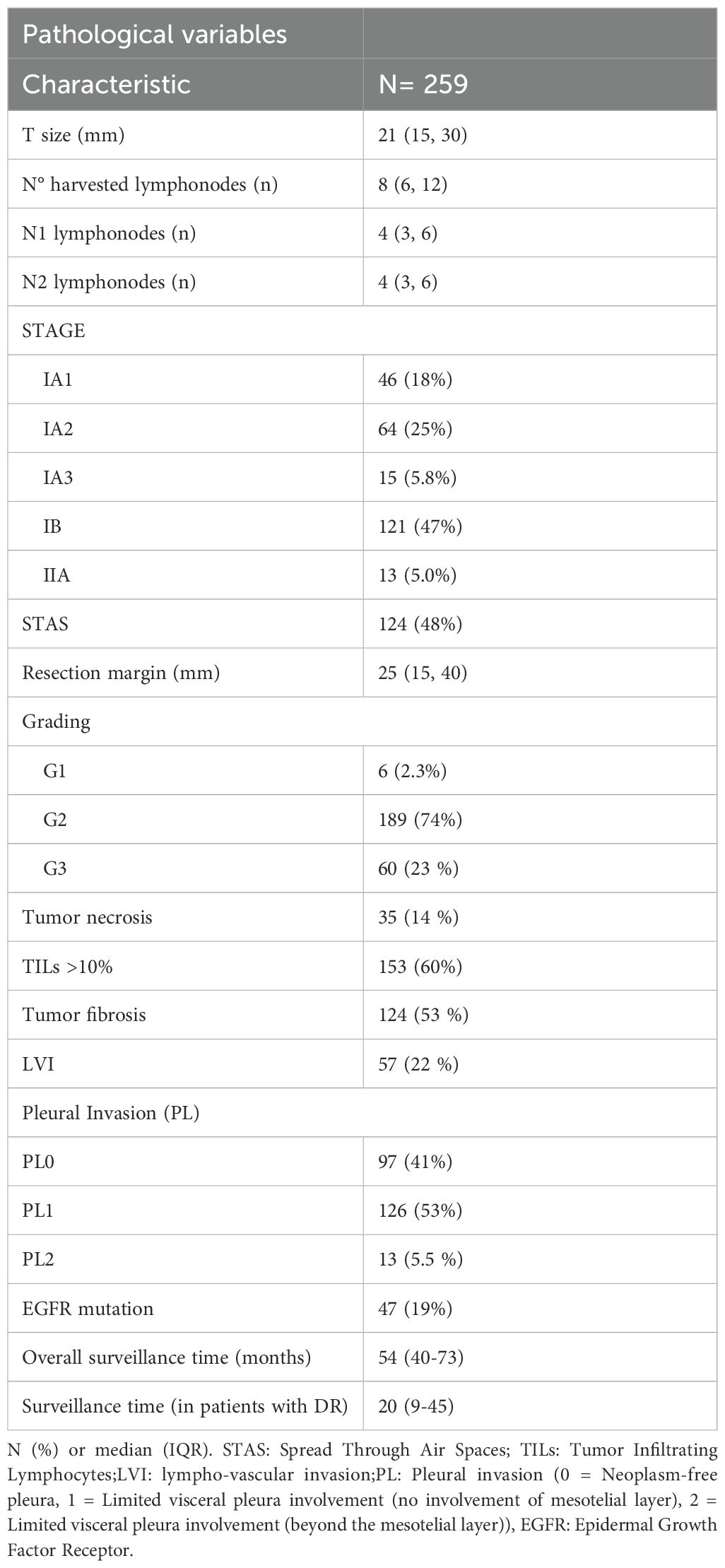
Pathological findings showed a median tumor diameter of 21 mm (IQR 15-30), with the most common pathological stages being IB (n=121, 47%) and IA2 (n=64, 25%). STAS was identified in 124 cases (48%), and the majority of tumors were moderately differentiated (G2, n=189, 74%). Lymphovascular invasion (LVI) was observed in 57 cases (22%), while microscopic pleural involvement as observed in 53% of patients. Only 13 cases (5.5%) exhibited cancer beyond the mesotelial pleural layer (PL2). The median surgical margin was 25 mm (IQR 15-40).
Details of all pathological data are summarized in Table 2. The distribution of perioperative and pathological data did not show any significant difference between patients who experienced recurrence and those who remained disease-free during the surveillance (Supplementary Materials – Supplementary Table S1 and Supplementary Table S2).
The median surveillance time was 60 months (IQR 46-75). During this period, 54 patients experienced disease recurrence, including 26 local and 28 distant relapse, respectively. Pathological analysis indicated that tumor size was larger in patients with recurrence, particularly in those with local recurrence (25.5 mm vs 23.5 mm in patients with distant recurrence or 20 mm in those who remained relapse-free; p=0.028). STAS was more frequently observed in patients with distant recurrence (67.9% vs 42.3% and 46.5% in distant, locoregional, and no recurrence, respectively). A higher proportion of G3 tumors was found in the distant recurrence group (44.4% vs 24% and 20.9%). TILs were less common in patients with local recurrence (38%), while nearly 60% of patients with distant recurrence exhibited more than 10% tumor lymphocytic infiltration (p=0.056). The disease stage distribution was comparable among patients with distant, local or no relapse. The EGFR mutation harbored in 47 cases, accounting for almost the 20% of the sample. In detail, only 6 of those patients had a distant failure (roughly one in five patients with cancer spreading far from the surgical edge) (Table 3).
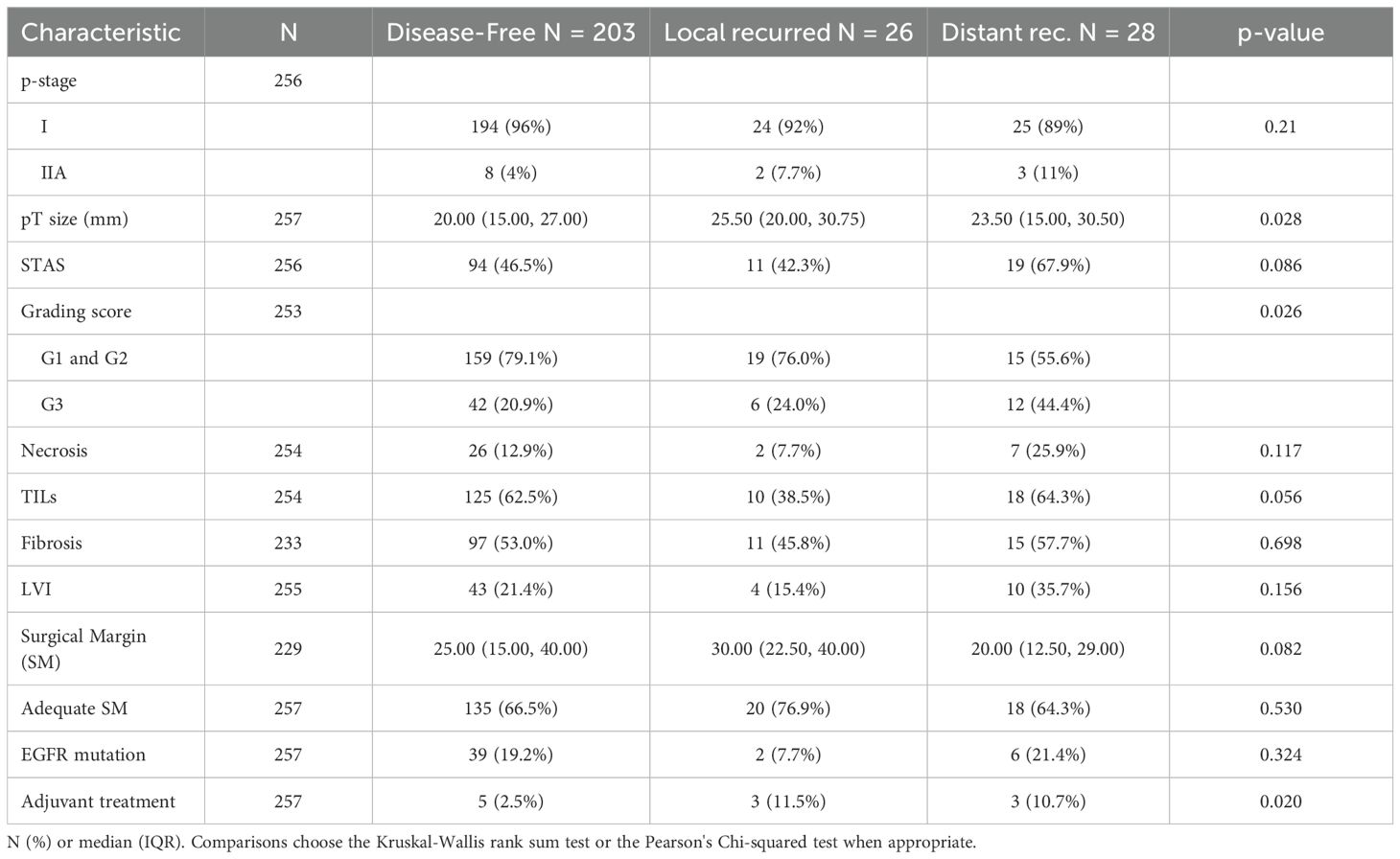
Table 3. Cross-table comparing pathological and microscopical data among those who had local or distant relapse and those who remained disease-free.
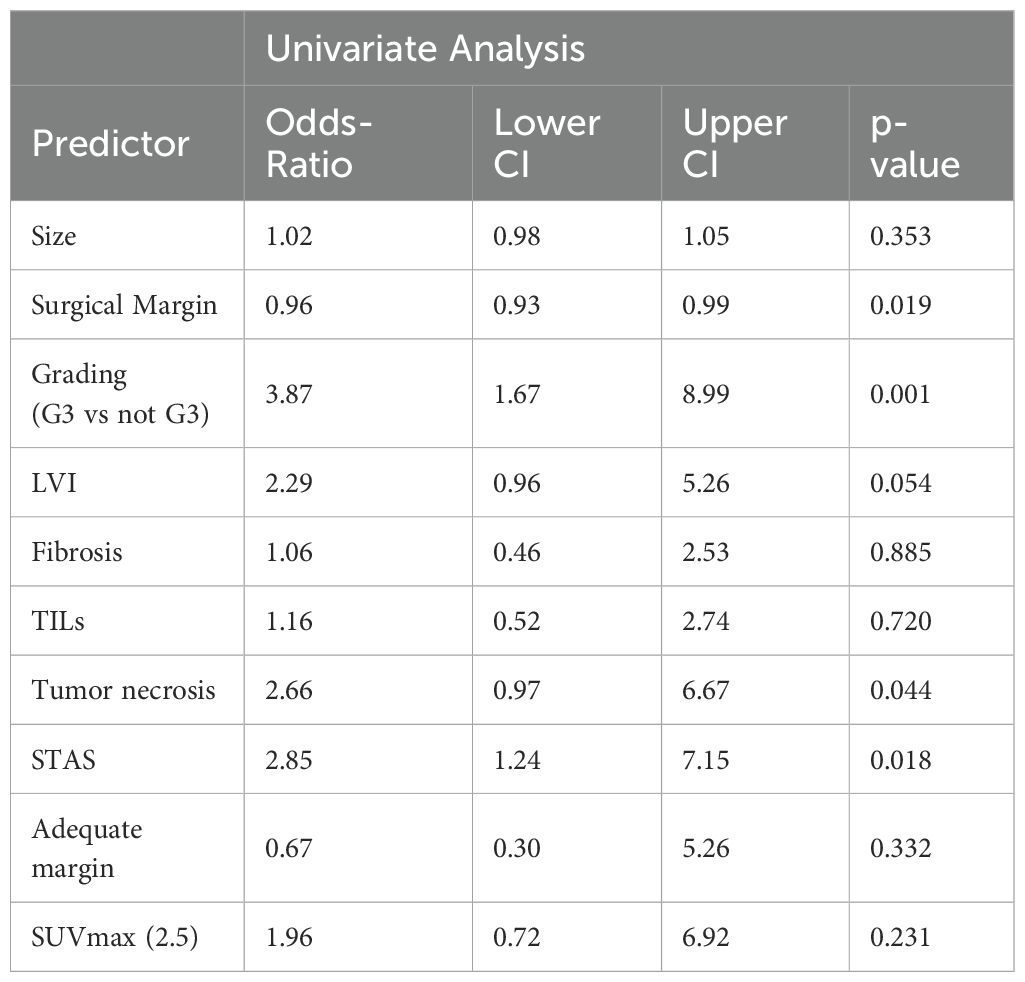
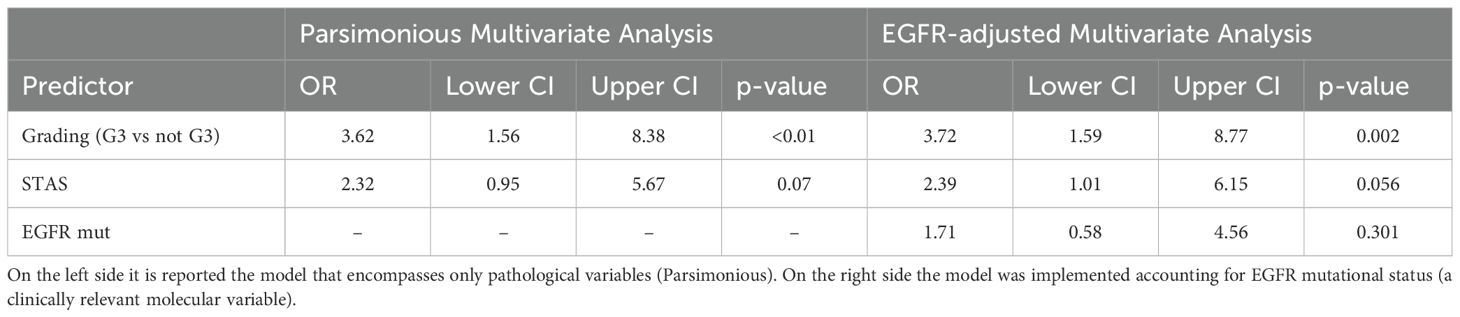
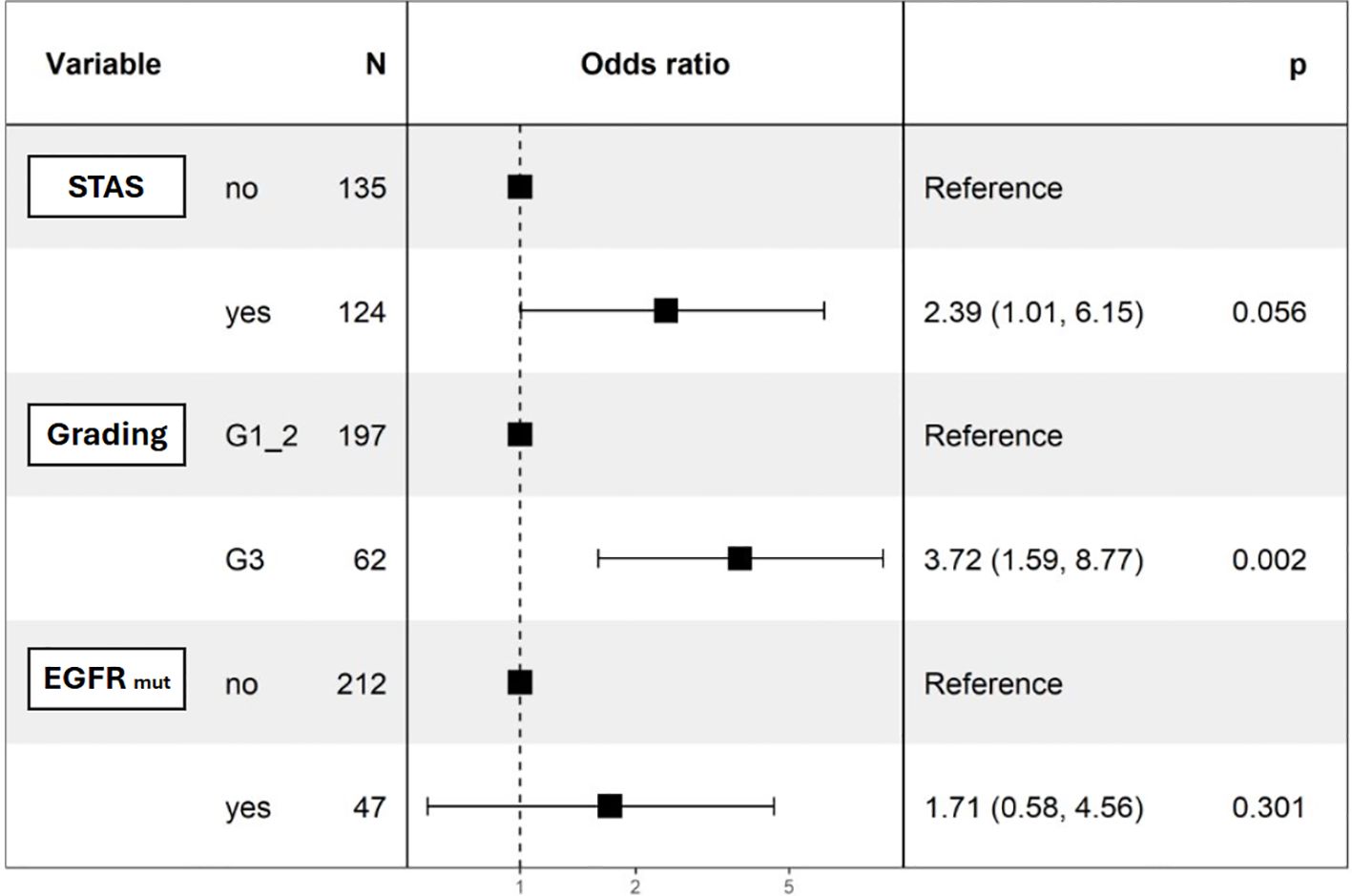
Univariable logistic regression revealed significant associations between distant recurrence and surgical margin (p=0.019), necrosis (p=0.001), G3 grading (p=0.044), and alveolar diffusion (p=0.018). LVI showed a trend toward significance (p=0.054) (Table 4). Multivariable logistic regression analysis was divided into two models. The first accounted for pathological predictors only (Parsimonious model - PM) wilst the second also considered the EGFR molecular feature according to the mutational status (EGFR-adjusted Model – EFGR-M). Both of them identified histologic grade as a significant predictor of distant relapse (OR 3.62, 95% CI 1.56-8.38, p<0.01 and OR 3.72, 95% CI 1.59-8.77, p<0.01 respectively). STAS showed a marginal association (OR 2.32, 95% CI 0.95-5.67, p=0.07) in the PM and almost a significance in the EGFR-M (OR 2.39, 95% CI 1.01-6.15, p=0.06). The EGFR status did not influence distant recurrence in our analysis (OR 1.71, 95% CI 0.58-4.56, p=0.301) (Table 5; Figure 1).
In a median surveillance period of 54 months (IQR 40-73), the 96%, 87% and 78% of patients remained overall disease-free at 1-, 3-, and 5-years after surgery. More in detail, taking into account only those who were diagnosed with distant failure during the surveillance, which lasted a median of 20 months (IQR 9-45), they showed a disease-free survival of 97%, 92% and 89% (Figure 2).
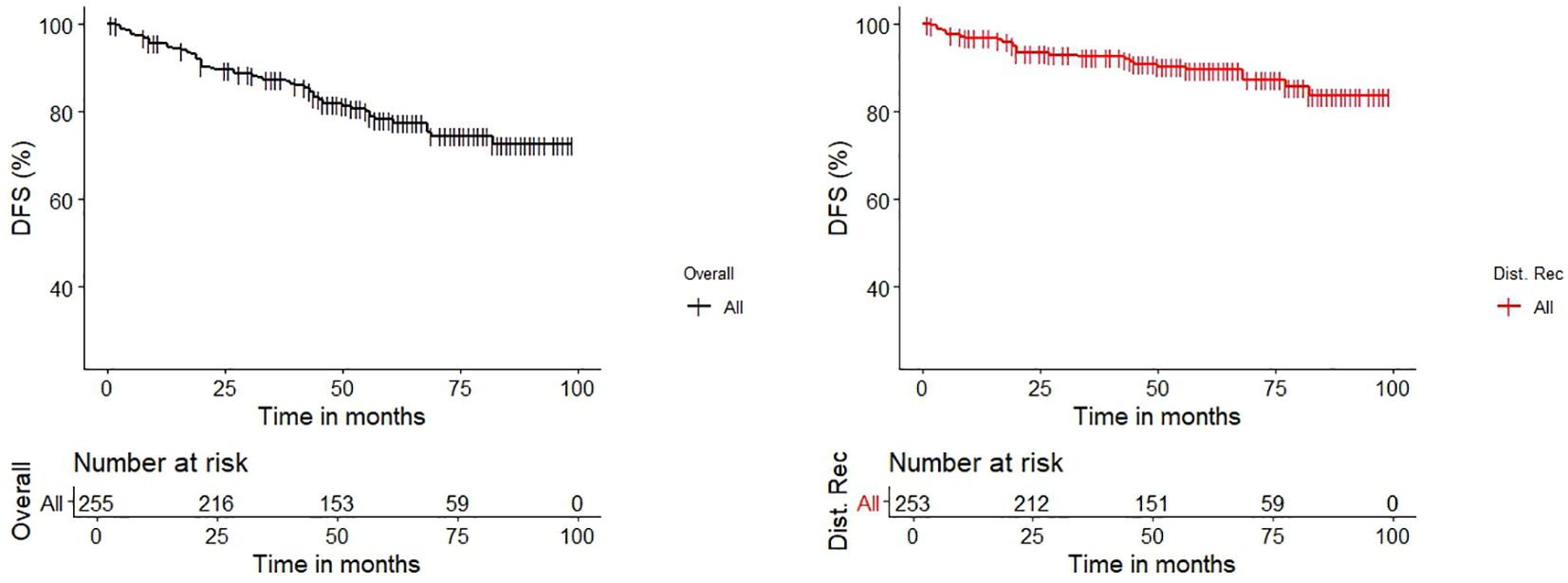
Figure 2. Kaplan-Meier Estimates graphs for Disease-Free Survival (DFS) of the entire cohort (black line) and for the cohort of patients which had distant recurrence after curative-intent surgery (red line).
4 Discussion
Despite complete surgical resection with clear margins, postoperative recurrence in stage I-IIA NSCLC remains a major clinical concern. According to cancer biology paradigms, a localized neoplasm should theoretically remain in an equilibrium phase and surgery should be considered a curative treatment (8). However, disease relapse after surgery in early-stage resected NSCLCs is widely reported which suggests that unforeseen microscopic tumor cells may already exist beyond the resection edge at the time of operation. This is why running a scheduled postoperative surveillance is fundamental, particularly within the first two years (9, 10).
In fact, the progressive decline of recurrence-free survival spanning from stage IA to stage III reinforces the correlation between tumor size and relapse rates (5). However, small adenocarcinomas (ADCs) can exhibit aggressive behavior as well, even in early stages, calling for a clearer and refined approach to recurrence risk stratification, going beyond the mere tumor size feature (10–12). While surgical margins have been widely studied, several findings suggest that recurrence risk may also be influenced by microscopic tumor characteristics, regardless of the anatomical resection technique (13–18).
It is plausible that additional, yet unidentified, risk factors of the tumor microenvironment should contribute to ensure the cancer spread beyond the adequate surgical margin. For example, peritumoral structures, such as vessels, lymphatics, and the alveolar tissue, are progressively invaded by growing lung cancer in certain manners that are still only partially understood (19), and this process may play an underestimate role in locoregional or distant failure after a curative-intent surgery (20, 21).
Our findings indicate that STAS, necrosis, histological grade, and surgical margins were significant predictors in the univariable analysis. Notably, our multivariable analysis identified histologic grade as the strongest predictor of distant relapse, with STAS demonstrating only a marginal association in the P-M (p=0.07) and a better fit in the EGFR-M (p=0.05). This further encourages reassessment of the relative importance of these factors in a preoperative setting. These results support previous studies that have identified STAS as a prognostic marker for recurrence (22), but at the same time our findings suggest that alveolar spread may be related to the tumor dedifferentiation (G3). This distinction is critical, as it may influence risk stratification and postoperative management, particularly in patients with STAS-positive, high-grade tumors. Furthermore, tumor necrosis and poorly differentiated histology are associated with increased metabolic and mitotic activity, indicative of aggressive disease with potential for a rapid completion of the epithelial-to-mesenchymal transition (23–25). These features suggest that, irrespective of tumor size and surgical margin, tumor microscopic aggressiveness can be the soil for a distant recurrence even when adequate curative-intent surgery has been performed.
Our results should be interpreted cautiously due to the relatively small sample size and low number of events (only 28 patients with distant recurrence), but similar findings have been reported in the literature. For instance, Fick et al. (26) identified PET-CT uptake, G3 differentiation, LVI, and visceral pleural invasion as predictors of recurrence in stage I ADCs. In another study, Fick et al. (27) found that late recurrence in stage I-III ADCs was associated with pathologic stage, LVI, pleural involvement, and dedifferentiated histology. Furthermore, Wang et al. demonstrated that histologic grade is a better predictor of distant metastasis than tumor stage in early-stage ADCs (28). Our predictors align with Wang’s study, as tumor grade was a more reliable predictor of distant metastasis than tumor size in our analysis.
Finally, LVI has long been considered an independent pathological prognostic factor for recurrence. In this study, LVI showed only a trend toward significance, while in a previous study from our group, LVI was associated with both overall recurrence and survival, with STAS lacking significance (22, 29–33). This discrepancy suggests that analysing recurrence as a single event (made of local and distant recurrence together) may obscure important differences in postoperative tumor spread process, reinforcing the need for stratified risk assessment.
Although molecular profiling was not the primary endpoint of this study, it is worth noting that our center was already performing EGFR mutational testing in early-stage resected lung adenocarcinoma during the study period (2016–2020), anticipating current precision medicine approaches. Among the 54 patients who experienced recurrence, 8 harbored EGFR mutations. While this represents a minority of recurrent cases, had these patients been treated today, they might have been eligible for adjuvant Osimertinib as per the ADAURA trial (34), potentially delaying or preventing recurrence. For this reason, a tailored analysis was performed, showing no influences of EGFR status in the multivariate model for distant recurrence in our study.
Our study aligns with the main literature, highlighting the evolving landscape of postoperative management and the importance of integrating histologic, clinical, and molecular risk factors to guide adjuvant strategies in early-stage disease.
This study has some limitations that should be outlined. As a single-center, retrospective analysis, the findings could be influenced by selection bias and may not be fully generalizable to other institutions or broader populations. Additionally, the relatively small sample size and the limited number of distant failures (only 28 cases) may have reduced the statistical power, potentially affecting the ability to confirm STAS, LVI, and tumor necrosis as independent predictors in multivariable analysis. The lack of data on adjuvant therapies is another potential limitation, as treatment decisions could have influenced recurrence patterns. Despite this, the small contribution of patients receiving adjuvant did not affect our results.
5 Conclusions
This study suggests that the G3 histologic grade in resected stage I-IIA ADCs is a key predictor of distant recurrence, calling for a closer postoperative surveillance in these patients. While STAS, LVI, and tumor necrosis were associated with recurrence, their independent prognostic value remains uncertain, likely due to sample size limitations. These findings reinforce the importance of tumor aggressiveness beyond traditional staging parameters and underscore the need for refined risk stratification models to optimize postoperative surveillance.
Data availability statement
The datasets presented in this article are not readily available because Italian privacy policy does not allow to share patients’ records in public. Requests to access the datasets should be directed to Prof. Andrea Dell’Amore, YW5kcmVhLmRlbGxhbW9yZUB1bmlwZC5pdA==.
Ethics statement
The studies involving humans were approved by Territorial Ethics Committee Central-Eastern Veneto Area. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Patients signed an informed consent to participate to our Department’s activity once admitted to our ward.
Author contributions
AB: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. GP: Data curation, Methodology, Resources, Writing – original draft. GC: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. MM: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. FP: Conceptualization, Investigation, Supervision, Writing – original draft. VV: Data curation, Investigation, Methodology, Writing – original draft. EP: Data curation, Investigation, Resources, Writing – original draft. AB: Data curation, Investigation, Resources, Writing – original draft. SS: Data curation, Investigation, Resources, Writing – original draft. GC: Data curation, Investigation, Resources, Writing – original draft. EF: Data curation, Supervision, Validation, Writing – original draft. AR: Resources, Supervision, Validation, Writing – original draft. MS: Methodology, Supervision, Validation, Writing – original draft. SN: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. AD: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. FC: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. FR: Methodology, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1626863/full#supplementary-material
References
1. Cancer (IARC) TIA for R on. Global cancer observatory . Available online at: https://gco.iarc.fr/ (Accessed June 16, 2022).
2. Thai AA, Solomon BJ, Sequist LV, Gainor JF, and Heist RS. Lung cancer. Lancet. (2021) 398:535–54. doi: 10.1016/S0140-6736(21)00312-3
3. Herbst RS, Morgensztern D, and Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
4. Wang C, Shao J, Song L, Ren P, Liu D, and Li W. Persistent increase and improved survival of stage I lung cancer based on a large-scale real-world sample of 26,226 cases. Chin Med J (Engl). (2023) 136:1937–48. doi: 10.1097/CM9.0000000000002729
5. Ganti AK, Klein AB, Cotarla I, Seal B, and Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. (2021) 7:1824–32. doi: 10.1001/jamaoncol.2021.4932
6. Jimenez Londoño GA, Pérez-Beteta J, Amo-Salas M, Honguero-Martinez AF, Pérez-García VM, Lucas Lucas C, et al. Clinicopathologic and metabolic variables from 18F-FDG PET/CT in the prediction of recurrence pattern in stage I-III non-small cell lung cancer after curative surgery. Ann Nucl Med. (2025). doi: 10.1007/s12149-025-02021-y
7. ESMO. ESMO clinical practice guidelines: lung and chest tumours . Available online at: https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-lung-and-chest-tumours (Accessed February 26, 2025).
8. Rajaram R, Huang Q, Li RZ, Chandran U, Zhang Y, Amos TB, et al. Recurrence-free survival in patients with surgically resected non-small cell lung cancer: A systematic literature review and meta-analysis. Chest. (2024) 165:1260–70. doi: 10.1016/j.chest.2023.11.042
9. Relli V, Trerotola M, Guerra E, and Alberti S. Abandoning the notion of non-small cell lung cancer. Trends Mol Med. (2019) 25:585–94. doi: 10.1016/j.molmed.2019.04.012
10. Warkentin MT, Tammemägi MC, Freedman MT, Ragard LR, Hocking WG, Kvale PA, et al. Factors associated with small aggressive non-small cell lung cancers in the national lung screening trial: A validation study. JNCI Cancer Spectr. (2018) 2:pkx010. doi: 10.1093/jncics/pkx010
11. Bianchi F, Nuciforo P, Vecchi M, Bernard L, Tizzoni L, Marchetti A, et al. Survival prediction of stage I lung adenocarcinomas by expression of 10 genes. J Clin Invest. (2007) 117:3436–44. doi: 10.1172/JCI32007
12. Zhang J, Gold KA, Lin HY, Swisher SG, Xing Y, Lee JJ, et al. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol. (2015) 10:682–90. doi: 10.1097/JTO.0000000000000456
13. Yano K, Yotsukura M, Watanabe H, Akamine T, Yoshida Y, Nakagawa K, et al. Oncological feasibility of segmentectomy for inner-located lung cancer. JTCVS Open. (2024) 18:261–75. doi: 10.1016/j.xjon.2024.02.015
14. Masai K, Sakurai H, Sukeda A, Suzuki S, Asakura K, Nakagawa K, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol. (2017) 12:1788–97. doi: 10.1016/j.jtho.2017.08.015
15. Liu W, Lai H, Wang Z, and Liu L. Does surgical margin affect recurrence and survival after sublobar pulmonary resection for lung cancer? Interact Cardiovasc Thorac Surg. (2022) 34:1089–94. doi: 10.1093/icvts/ivab328
16. West H, Hu X, Chirovsky D, Walker MS, Wang Y, Kaushiva A, et al. Clinical and economic impact of recurrence in early-stage non-small-cell lung cancer following complete resection. Future Oncol. (2023) 19:1415–27. doi: 10.2217/fon-2023-0024
17. Jeong WG, Choi H, Chae KJ, and Kim J. Prognosis and recurrence patterns in patients with early stage lung cancer: a multi-state model approach. Transl Lung Cancer Res. (2022) 11:1279–91. doi: 10.21037/tlcr-22-148
18. Detterbeck FC, Woodard GA, Bader AS, Dacic S, Grant MJ, Park HS, et al. The proposed ninth edition TNM classification of lung cancer. Chest. (2024) 166:882–95. doi: 10.1016/j.chest.2024.05.026
19. Mittal D, Gubin MM, Schreiber RD, and Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. (2014) 27:16–25. doi: 10.1016/j.coi.2014.01.004
20. Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. (2012) 7:723–30. doi: 10.1097/JTO.0b013e31824a9022
21. Yun JK, Lee GD, Choi S, Kim Y-H, Kim DK, Park S-I, et al. Various recurrence dynamics for non-small cell lung cancer depending on pathological stage and histology after surgical resection. Transl Lung Cancer Res. (2022) 11:1327–36. doi: 10.21037/tlcr-21-1028
22. Nicotra S, Melan L, Pezzuto F, Bonis A, Silvestrin S, Verzeletti V, et al. Significance of spread through air spaces and vascular invasion in early-stage adenocarcinoma survival: A comprehensive clinicopathologic study of 427 patients for precision management. Am J Surg Pathol. (2024) 48:605–14. doi: 10.1097/PAS.0000000000002199
23. Park SY, Lee H-S, Jang H-J, Lee GK, Chung KY, and Zo JI. Tumor necrosis as a prognostic factor for stage IA non-small cell lung cancer. Ann Thorac Surg. (2011) 91:1668–73. doi: 10.1016/j.athoracsur.2010.12.028
24. Moon SW, Kim JJ, Jeong SC, Kim YH, and Han JW. Clinical significance of tumor necrosis and viability in non-small cell lung cancer. J Thorac Dis. (2022) 14:892–904. doi: 10.21037/jtd-21-1597
25. Ito M, Miyata Y, Kushitani K, Kagimoto A, Ueda D, Tsutani Y, et al. Pathological high Malignant grade is higher risk of recurrence in pN0M0 invasive lung adenocarcinoma, even with small invasive size. Thorac Cancer. (2021) 12:3141–9. doi: 10.1111/1759-7714.14163
26. Fick CN, Dunne EG, Vanstraelen S, Toumbacaris N, Tan KS, Rocco G, et al. High-risk features associated with recurrence in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg. (2025) 169:436–444.e6. doi: 10.1016/j.jtcvs.2024.05.009
27. Fick CN, Dunne EG, Toumbacaris N, Tan KS, Mastrogiacomo B, Park BJ, et al. Late recurrence of completely resected stage I to IIIA lung adenocarcinoma. J Thorac Cardiovasc Surg. (2025) 169:445–453.e3. doi: 10.1016/j.jtcvs.2024.06.026
28. Wang Y, Smith MR, Dixon CB, D’Agostino R, Liu Y, Ruiz J, et al. IASLC grading system predicts distant metastases for resected lung adenocarcinoma. J Clin Pathol. (2024). doi: 10.1136/jcp-2024-209649
29. Park C, Lee IJ, Jang SH, and Lee JW. Factors affecting tumor recurrence after curative surgery for NSCLC: impacts of lymphovascular invasion on early tumor recurrence. J Thorac Dis. (2014) 6:1420–8. doi: 10.3978/j.issn.2072-1439.2014.09.31
30. Wang J, Chen J, Chen X, Wang B, Li K, and Bi J. Blood vessel invasion as a strong independent prognostic indicator in non-small cell lung cancer: a systematic review and meta-analysis. PloS One. (2011) 6:e28844. doi: 10.1371/journal.pone.0028844
31. Suaiti L, Sullivan TB, Rieger-Christ KM, Servais EL, Suzuki K, and Burks EJ. Vascular Invasion Predicts Recurrence in Stage IA2-IB Lung Adenocarcinoma but not Squamous Cell Carcinoma. Clin Lung Cancer. (2023) 24:e126–33. doi: 10.1016/j.cllc.2022.12.006
32. Gabor S, Renner H, Popper H, Anegg U, Sankin O, Matzi V, et al. Invasion of blood vessels as significant prognostic factor in radically resected T1-3N0M0 non-small-cell lung cancer. Eur J Cardiothorac Surg. (2004) 25:439–42. doi: 10.1016/j.ejcts.2003.11.033
33. Park S, Lee SM, Choe J, Choi S, Kim S, Do K-H, et al. Sublobar resection in non-small cell lung cancer: patient selection criteria and risk factors for recurrence. Br J Radiol. (2023) 96:20230143. doi: 10.1259/bjr.20230143
Keywords: distant recurrence, relapse, lung cancer, adenocarcinoma, pathological predictors
Citation: Bonis A, Pagliarini G, Comacchio GM, Mammana M, Pezzuto F, Verzeletti V, Pellizzer E, Berni A, Silvestrin S, Cannone G, Faccioli E, Rebusso A, Schiavon M, Nicotra S, Dell’Amore A, Calabrese F and Rea F (2025) Histologic grade and STAS as key predictors of distant recurrence in resected early-stage lung adenocarcinoma: a single-center study. Front. Oncol. 15:1626863. doi: 10.3389/fonc.2025.1626863
Received: 11 May 2025; Accepted: 12 August 2025;
Published: 15 September 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Eswari Dodagatta-Marri, University of California, San Francisco, United StatesMohammad Mahdi Fanaeian, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Bonis, Pagliarini, Comacchio, Mammana, Pezzuto, Verzeletti, Pellizzer, Berni, Silvestrin, Cannone, Faccioli, Rebusso, Schiavon, Nicotra, Dell’Amore, Calabrese and Rea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Dell’Amore, YW5kcmVhLmRlbGxhbW9yZUB1bmlwZC5pdA==; Marco Mammana, bWFyY28ubWFtbWFuYUBhb3BkLnZlbmV0by5pdA==
 Alessandro Bonis
Alessandro Bonis Giulia Pagliarini1
Giulia Pagliarini1 Marco Mammana
Marco Mammana Federica Pezzuto
Federica Pezzuto Vincenzo Verzeletti
Vincenzo Verzeletti Eleonora Faccioli
Eleonora Faccioli Marco Schiavon
Marco Schiavon Andrea Dell’Amore
Andrea Dell’Amore Fiorella Calabrese
Fiorella Calabrese Federico Rea
Federico Rea