- 1Yuncheng Central Hospital affiliated to Shanxi Medical University, Department of Gastrointestinal Surgery, Yuncheng, Shanxi, China
- 2Department of General Surgery, Tianji Hospital, Changzhi, Shanxi, China
- 3Department of General Surgery, Heji Hospital, Changzhi, Shanxi, China
- 4The First Affiliated Hospital of the Air Force Medical University 986 Hospital, Department of Gastrointestinal Surgery, Xi’an, China
- 5The First Affiliated Hospital of the Air Force Medical University, Department of Gastrointestinal Surgery, Xi’an, China
Background: While reduced-port laparoscopic gastrectomy(RPLG) has emerged as a minimally invasive alternative, its standardization and long-term efficacy remain underexplored. This study evaluates the comparative outcomes of three-port (TPLDG) versus five-port laparoscopic distal gastrectomy (FPLDG).
Methods: This prospective multicenter study enrolled 355 gastric cancer patients meeting selection criteria. Surgical procedures adhered to D2 lymphadenectomy guidelines, with TPLDG utilizing a left-sided approach without auxiliary ports. Primary endpoints included inflammatory markers, recovery parameters, and 3-year survival outcomes.
Results: The operative outcomes showed comparable results between groups, with similar operative times [140(125,160) vs. 135(120,150) minutes, p=0.068)] and total lymph node retrieved [(22(19,27) vs. 22(18,27) nodes, p=0.696)]. Notably, the TPLDG group demonstrated significant recovery advantages, including earlier flatus [(2(2,3) vs.3(2,3) days, p<0.001)], shorter hospital stays [4(3,5) vs. 5.2(4.2,6.3) days, p<0.001)], and reduced inflammatory responses as evidenced by lower postoperative CRP [(48.2 ± 21.4) vs. (68.5 ± 25.6) mg/L, p<0.01)] and IL-6 levels [(82.3 ± 31.2) vs. (115.4 ± 38.5)pg/mL, p<0.01)]. Importantly, oncological outcomes remained equivalent between groups, with comparable 3-year disease-free survival (85.4% vs 85.8%, p=0.85) and overall survival rates (89.4% vs. 89.2%, p=0.70), which were consistently maintained across stage-stratified analyses.
Conclusion: TPLDG achieves comparable oncological outcomes to conventional FPLDG while offering significant advantages in postoperative recovery and inflammatory response reduction. The left-sided three-port technique represents a viable standardized approach for RPLG, particularly suited for D2 lymphadenectomy in Asian populations.
1 Introduction
Gastric cancer (GC) ranks as the fifth most common malignancy worldwide and the third leading cause of cancer-related deaths (1). Currently, surgical resection remains the cornerstone of treatment, with continuous advancements aimed at enhancing postoperative recovery and oncological outcomes (2). Since its introduction in 1994, laparoscopic gastrectomy has gained widespread acceptance and become a mainstream surgical option for GC (3). In China, several randomized controlled trials have confirmed the safety and efficacy of laparoscopic D2 lymphadenectomy for locally advanced GC, demonstrating its advantages in promoting faster recovery (4, 5). Moreover, studies indicate that laparoscopic gastrectomy offers multiple benefits over open surgery, even in advanced GC following neoadjuvant therapy, with its safety and effectiveness well validated (6).
With the growing emphasis on precision medicine, surgeons are increasingly focused on tailoring surgical strategies to individual patients to achieve optimal outcomes. In recent years, efforts have been made to minimize surgical trauma. Unlike conventional multiport laparoscopic surgery, reduced-port laparoscopic gastrectomy (RPLG)-a less invasive approach with fewer incisions-has emerged (7, 8). Multiple studies suggest that RPLG yields comparable postoperative and oncological outcomes, including 5-year overall survival rates, to traditional multiport laparoscopic gastrectomy, while offering superior cosmetic satisfaction and improved oral intake (9, 10). However, RPLG presents a steeper learning curve, limiting its widespread adoption, and the techniques remain non-standardized. Most current RPLG procedures require additional ports or auxiliary instruments, which may exacerbate the “chopstick effect” and “tailing effect,” prolonging the learning curve and increasing surgical costs (11–13).
Given that most laparoscopic gastrectomies are performed from the left-sided approach and D2 lymphadenectomy remains the standard for advanced GC, our preliminary study validated the feasibility and short-term safety of a three-port laparoscopic gastrectomy with left-sided positioning (14). This approach eliminates the need for extra instruments while enabling both D1+ and D2 lymphadenectomy. To further evaluate its safety and long-term efficacy, we analyzed data from a prospective multicenter observational study, aiming to advance the development of reduced-port laparoscopic gastrectomy.
2 Materials and methods
2.1 Inclusion criteria
Patients meeting the following criteria were enrolled:
1. Aged 18–75 years; 2. Body mass index (BMI) between 17–24 kg/m²; 3. Preoperative pathological confirmation of primary gastric adenocarcinoma located in the middle or lower third of the stomach; 4. Preoperative assessment indicating a tumor diameter ≤5 cm; 5. No evidence of distant metastasis (e.g., liver, lung) on contrast-enhanced CT; 6. Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; 7. Clinical stage cT1-4aN0-3M0 (AJCC 8th edition TNM staging) with planned R0 resection via totally laparoscopic radical distal gastrectomy; 8. Early GC deemed unsuitable for endoscopic resection upon preoperative evaluation, or patients/families opting against endoscopic treatment.
2.2 Exclusion criteria
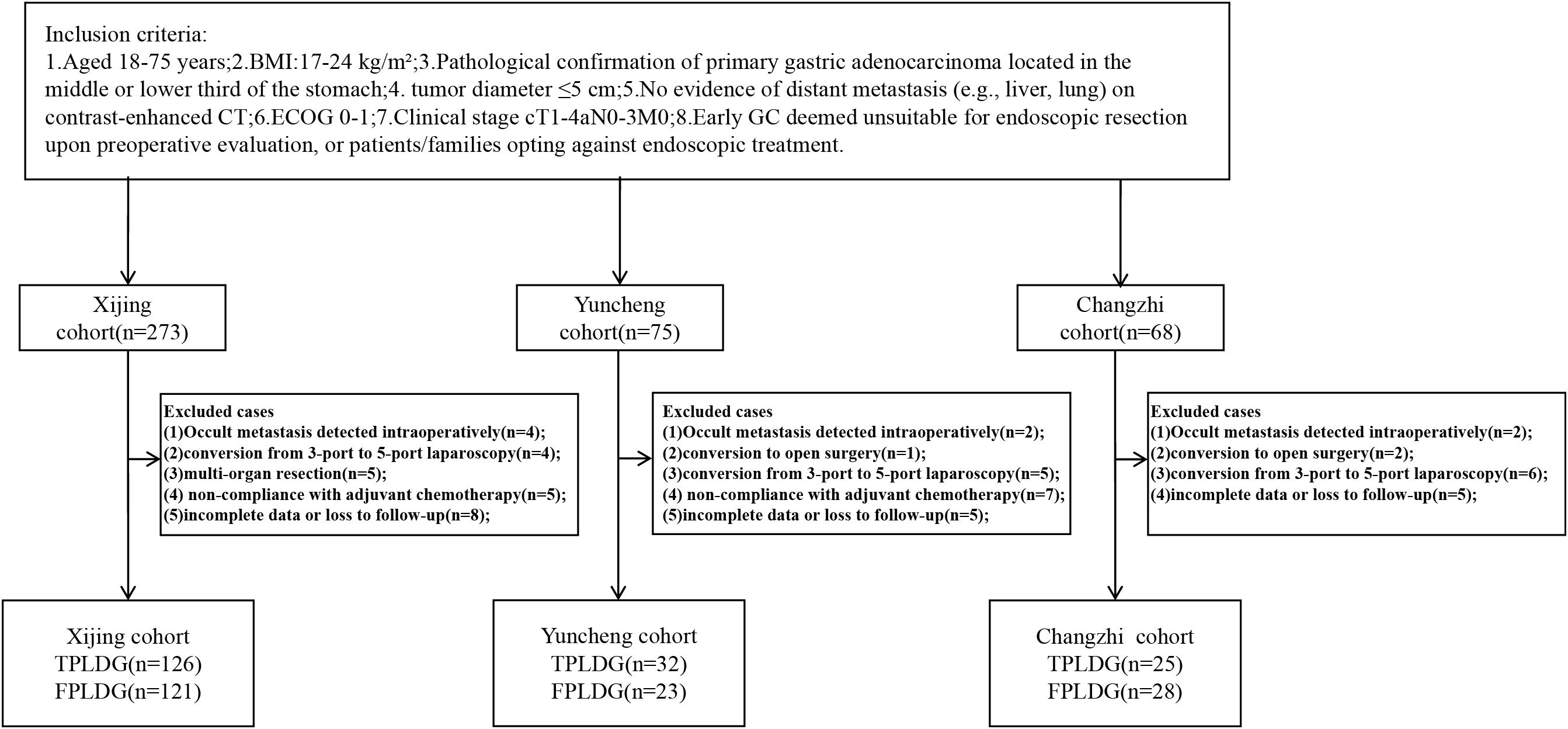
Patients were excluded if they met any of the following: 1. Previous upper abdominal surgery (except cholecystectomy) or requirement for multi-organ resection; 2. Conversion to five-port laparoscopic distal gastrectomy (FPLDG) or open surgery during three-port totally laparoscopic distal gastrectomy (TPLDG); 3. Emergency surgery due to complications (bleeding, perforation, obstruction); 4. Non-compliance with postoperative adjuvant chemotherapy; 5. Severe systemic comorbidities contraindicating laparoscopic surgery (additional criteria detailed in the clinical trial registry). This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Air Force Medical University (Approval No. KY20212225-C-1). All participants provided written informed consent. The trial was registered at www.chictr.org.cn (ChiCTR2200060322). By April 2024, 416 patients were initially enrolled. Exclusions included: 8 cases: occult metastasis detected intraoperatively; 3 cases: conversion to open surgery; 15 cases: conversion from 3-port to 5-port laparoscopy; 5 cases: multi-organ resection; 12 cases: non-compliance with adjuvant chemotherapy; 18 cases: incomplete data or loss to follow-up; Final cohort: 355 patients (see Figure 1 for recruitment flowchart).
2.3 Trocar placement
For patients in the FPLDG group, they are placed in a supine position with legs abducted. A 10 mm Trocar is inserted through a small incision below the umbilicus as the observation port. After exploring the abdominal and pelvic organs to confirm the absence of distant metastasis, a 12 mm Trocar is inserted below the costal margin at the left anterior axillary line as the main operation port, and a 5 mm Trocar is inserted at the umbilical level on the left midclavicular line as the auxiliary port. The placement on the right side is the opposite of that on the left side. The chief surgeon stands on the left side of the patient, the assistant stands on the right side of the patient, and the scope holder stands between the patient’s legs. For the TPTLDG group, on the basis of the control group, the two Trocar ports on the right side (assistant’s position) are removed, and the other layout is consistent with that of the FPLDG group (14).
2.4 Surgical management
The technical details of the three-port laparoscopic gastrectomy for GC with the left-side standing position have been described in previous reports (14). Briefly, the patient is placed in a supine position with the head elevated and the feet lowered. An observation port and two operation ports are created at the umbilicus and on the left and right sides of the patient respectively. The operation is performed without the assistance of an assistant. The surgical operation techniques, including gastric resection and lymph node dissection, follow the principles of the guidelines for the treatment of GC, which are consistent with those of traditional laparoscopic gastrectomy for GC. D2 lymph node dissection is performed for all tumors. After the gastric body is transected by a laparoscopic stapler, the 10 mm Trocar port below the umbilicus is enlarged to 3–4 cm to remove the specimen. Depending on the location of the tumor, Billroth-I anastomosis or Billroth-II posterior gastric wall anastomosis is selected, and Braun anastomosis is completed at the umbilical incision. The surgeon evaluates whether to place a drainage tube according to the completion of the operation and records the placement situation. No nasogastric tube is placed in all patients. The urinary catheter is removed immediately after the patient wakes up from anesthesia. For other management, multimodal analgesia, early mobilization, and early oral feeding are carried out with reference to the consensus guidelines of enhanced recovery after surgery for GC.
2.5 Main observation indicators
Postoperative inflammatory and nutritional indicators: preoperative and postoperative levels of high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), white blood cell count (WBC), and albumin (Alb).
Postoperative complication rate and mortality: defined as any complication or death occurring within 30 days postoperatively. The severity of complications was classified according to the Clavien-Dindo grading system (15).
Long-term survival outcomes: follow-up was conducted via hospitalization records, outpatient visits, WeChat, or telephone to assess complications within 30 days postoperatively, as well as 30-day and 90-day readmission rates. Follow-up continued until March 2025.Overall survival (OS): Defined as the time from surgery to death from any cause. Disease-free survival (DFS): Defined as the time from surgery to death or disease recurrence. Postoperatively, patients were followed up every 6 or 12 months for 5 years, including chest and abdominal computed tomography (CT) and endoscopic examinations. Patients with pathological stage II-III received guideline-recommended chemotherapy regimens.
2.6 Statistical methods
Normally distributed continuous variables were expressed as mean ± standard deviation ( ± s) and compared using Student’s t-test. Categorical variables were compared using the χ² test or Fisher’s exact test, as appropriate. Non-normally distributed continuous variables were expressed as median (Q1, Q3) and compared using the Mann-Whitney U test. Count data were presented as absolute numbers. Kaplan-Meier analysis was used to assess survival rates. All statistical analyses were performed using SPSS 22.0, and a P-value < 0.05 was considered statistically significant.
3 Results
3.1 Pathological characteristics and operative outcomes
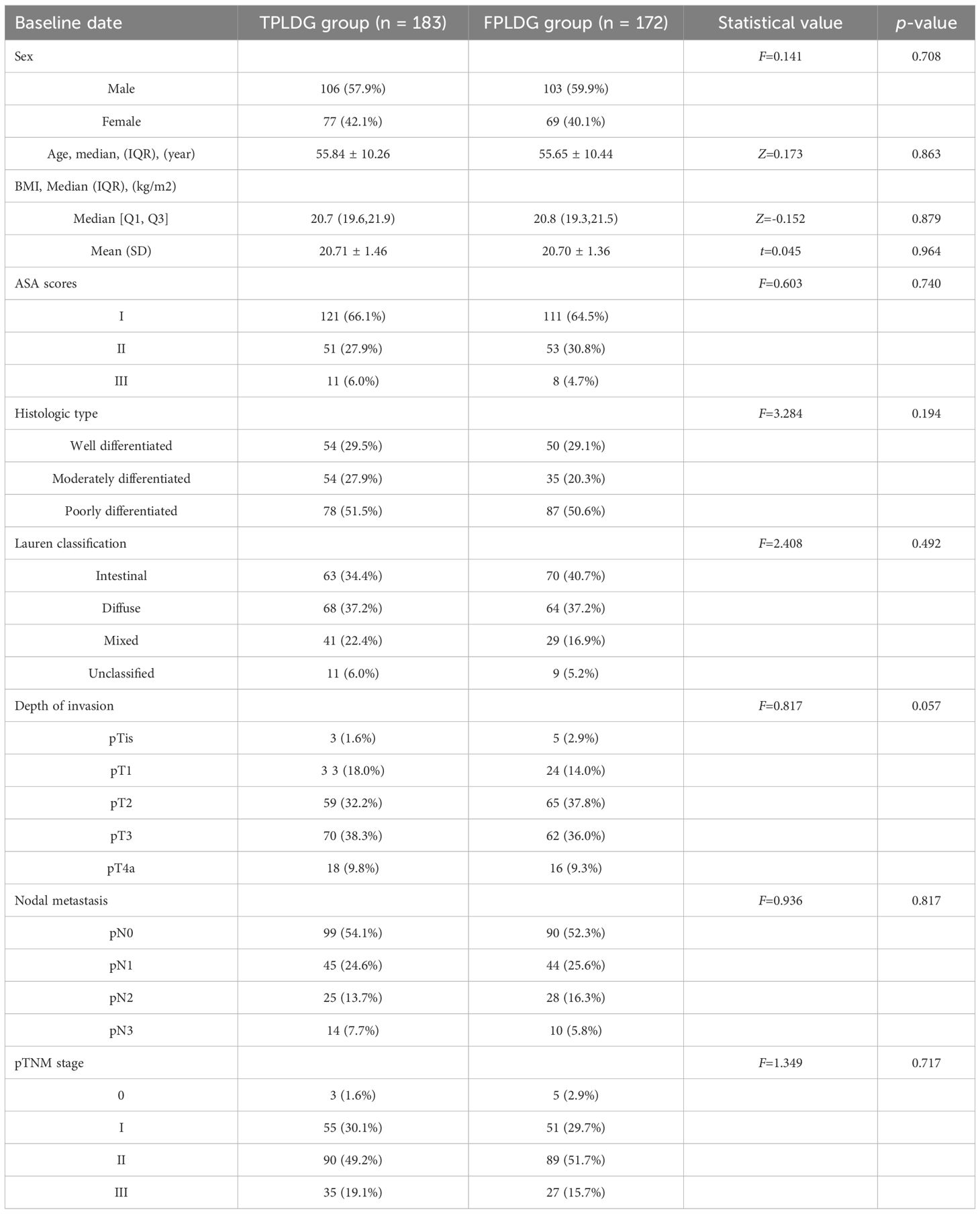
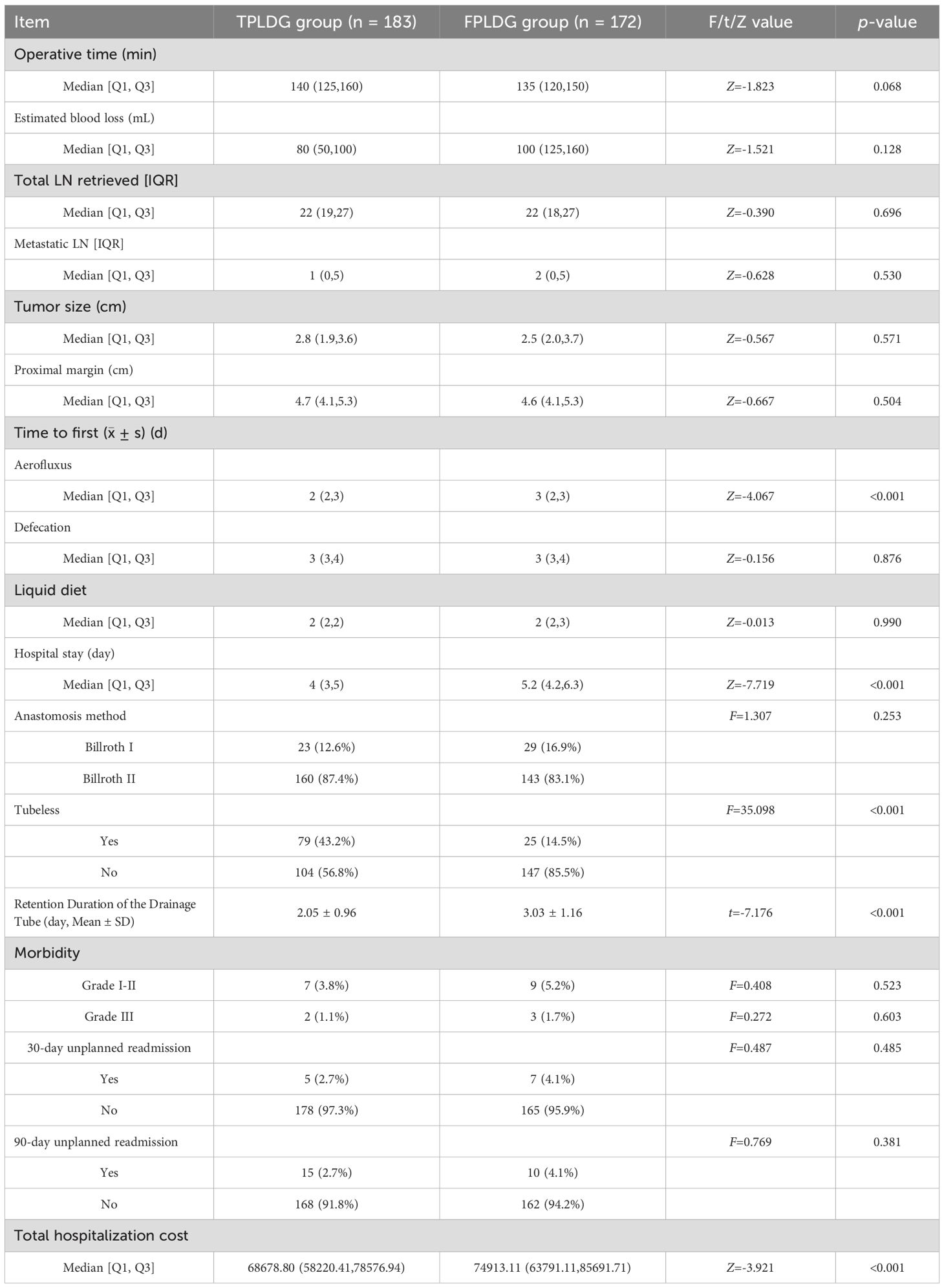
No significant differences were observed between the two groups regarding age, BMI, sex, ASA classification, tumor stage, histological type, or Lauren classification (Table 1). The operative time was 140 (125, 160) minutes in the TPLDG group versus 135 (120, 150) minutes in the FPLDG group (p=0.068). Estimated blood loss was 80 (50, 100) mL and 100 (125, 160) mL, respectively(p=0.128). The numbers of total retrieved lymph nodes were 22(19,27) in the TPLDG group and 22(18,27)in the FPLDG group (p=0.696).The two groups showed comparable results in postoperative complication rates, mortality, and 30-/90-day readmission rates. (Table 2).
3.2 Recovery outcomes
The TPLDG group showed earlier time to first flatus[2(2,3) vs. 3(2,3) day, Z=-4.067, p<0.001], shorter hospital stay [4(3,5) vs. 5.2(4.2,6.3) day, Z=-7.719, p<0.001]), higher tubeless rate, and lower total hospitalization costs in terms of postoperative recovery.(Table 2)To further compare the differences in postoperative recovery between the two groups, we analyzed inflammatory markers on postoperative day 3. The results demonstrated that the TPLDG group had significantly lower levels of C-reactive protein (CRP) and interleukin-6 (IL-6) compared to the FPLDG group (Table 3).
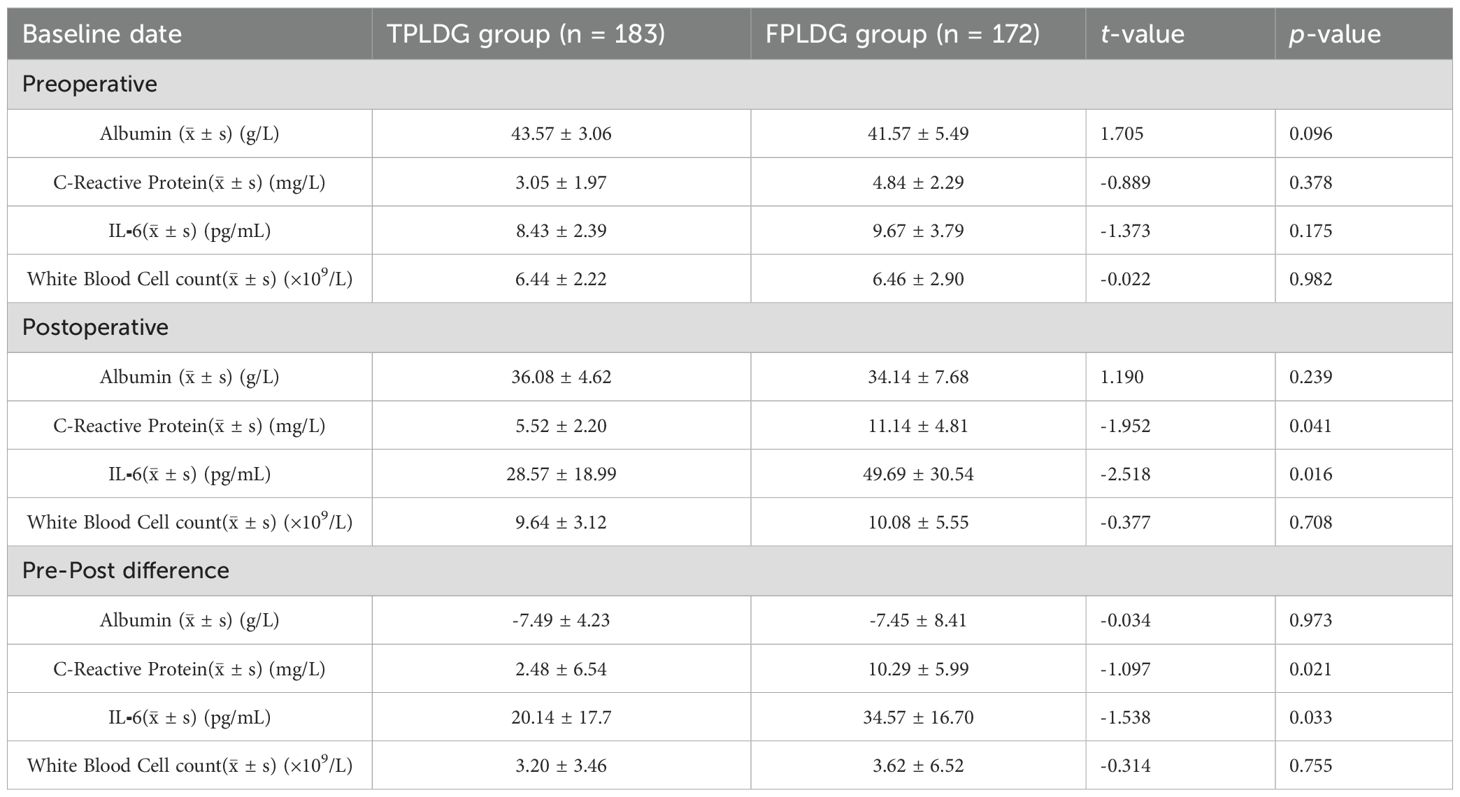
Table 3. Comparison of inflammatory response markers and nutritional indicators between the two groups.
3.3 Survival outcomes
The 3-year DFS rates were 85.4% for the TPLDG group and 85.8% for the FPLDG group (p=0.85, Figure 2A), while the 3-year OS rates were 89.4% and 89.2%, respectively (p=0.70, Figure 2D). Given the significant influence of disease stage on prognosis, we performed a stratified survival analysis. As shown in Figures 2B, C, E, F, the survival curves were compared between the two groups across different pathological stages. No significant differences in DFS were observed in either stage I (p=0.31, Figure 2B) or stages II–III (p=0.62, Figure 2C). Similarly, OS did not differ significantly between the groups for stage I (p=0.79, Figure 2E) or stages II–III (p = 0.55, Figure 2F).
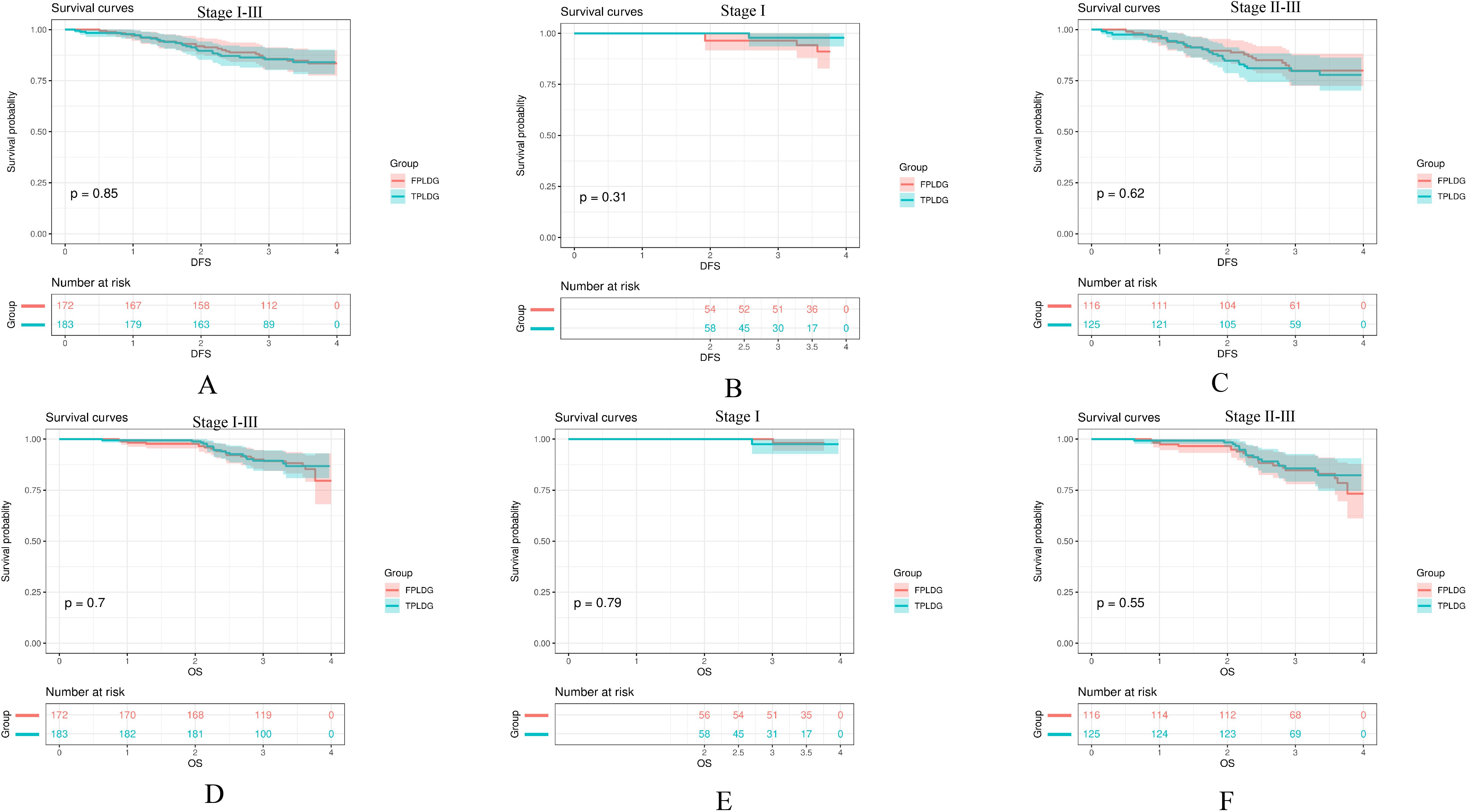
Figure 2. Kaplan-Meier survival curves comparing the TPLDG and FPLDG groups: (A) Disease-free survival (DFS), (B) DFS in stage I, (C) DFS in stages II–III, (D) Overall survival (OS), (E) OS in stage I, and (F) OS in stages II–III.
4 Discussion
With the continuous development of surgical techniques and instruments, reduced-port laparoscopic GC surgery has gradually received attention in clinical practice (8, 10, 13). This study focuses on left-side standing three-port laparoscopic radical distal gastrectomy, exploring its long-term safety and efficacy, and has certain significance in the treatment of early and some advanced GC.
Regarding the application status of reduced-port techniques, the single-port method has gradually been replaced by the single-port +1 technique (16–18). However, it requires an additional port, increasing costs, and also poses problems such as interference between instruments and limited visual fields (19, 20). The long-term safety of the right-side standing three-port method has been verified, but it is limited to early GC, and there are certain difficulties in D2 lymph node dissection (8). In comparison, the TPTLDG in this study shows unique advantages. It does not require special equipment. The design of the two ports effectively avoids the problems of instrument conflicts and visual field limitations in single-port +1 surgery. The standing position of the surgeon and the surgical operation are similar to those of traditional laparoscopic surgery, making it easier for gastrointestinal surgeons to master (14). In terms of effectiveness and safety, this study has been verified by prospective data. In patients with early and advanced GC, the long-term survival rate of TPLDG is equivalent to that of FPLDG, and it does not increase the risk of surgical complications. This result is consistent with other studies. The main reason is that the cases are mainly early and mid-stage GC, and there is no significant difference in the number of lymph nodes dissected.
The results of our previous studies have shown that patients in the TPTLDG group have shorter incision lengths and smaller umbilical incisions, reducing abdominal wall trauma and improving the aesthetic effect of the abdominal wall (14). In terms of postoperative recovery-related indicators, the overall levels of CRP and IL-6 on the third day after surgery in the TPTLDG group are lower, indicating that patients have a smaller stress response to reduced-port surgery (20). This also leads to a shorter time for the first postoperative anal exhaust and a shorter hospital stay (7). All these indicate that TPTLDG is helpful for patients’ postoperative recovery. We believes that this is because reduced-port surgery requires more precise and accurate operation, and it is mostly carried out by experienced surgeons, reducing repeated grasping of tissues (7, 21). Compared with FPLDG, the TPLDG group has no repeated grasping of tissues by assistants, which may reduce tissue damage to a certain extent. From the perspective of health economics, TPTLDG reduces the instruments used by assistants, does not require additional puncture devices and professional multi-channel puncture devices, reduces the cost of instruments, and has a shorter hospital stay, so the hospitalization cost is lower (22).
In addition, this study also summarizes the operation key points of TPTLDG: an independent main surgeon operation port, the left-side standing position is more in line with the traditional standing position of the surgeon, and in special cases, Trocar can be added to convert to FPLDG; through the left-side approach, it is safe and feasible to perform D2 lymph node dissection on some patients with advanced gastric cancer and those who have received neoadjuvant chemotherapy; during gastroenteric anastomosis, the surgeon needs to skillfully cooperate with both hands and use a laparoscopic linear cutting and closing device to complete the operation; after the surgeon learns the reduced-port surgical method, the concept of simplifying complexity established can help guide conventional laparoscopic surgery.
However, this study also has certain limitations. In terms of the research design, although it is a prospective study, it uses an observational design, making it difficult to completely avoid selection bias. The surgeons participating in the study are all experienced gastric surgery experts, but the factor of the learning curve has not been fully considered, which makes it necessary to be cautious when promoting the research conclusions. In addition, the clinical trial only included patients with distal gastric cancer and those with a BMI ≤ 24 kg/m², and did not involve total gastrectomy and proximal gastrectomy, because the lymph node dissection and digestive tract reconstruction of the latter two are more difficult, and it is more prudent to carry out relevant trials while ensuring surgical safety.
In conclusion, this study confirms that for patients with early and some advanced GC, TPTLDG surgery has equivalent long-term oncological efficacy to traditional five-port surgery, and has the advantages of short abdominal wall incisions, small surgical trauma, fast postoperative recovery, and low hospitalization costs. Under the condition of ensuring patient safety, it can be used as an effective solution to the shortage of surgical assistants. However, due to the limitations of the study, large-scale randomized clinical trials are still needed in the future to further clarify its clinical advantages in patients with different stages of gastric cancer and different BMIs, and to promote the development and application of reduced-port laparoscopic gastric cancer surgery.
Data availability statement
The datasets presented in this article are not readily available because of the need to protect patient privacy data. Requests to access the datasets should be directed to JW, d2VpamlhbmdwZW5nMjAxNUAxNjMuY29t.
Ethics statement
This study was approved by the Medical Ethics Committee of Yuncheng Central Hospital in Shanxi Province (YXLL2023068), the Medical Ethics Committee of the First Affiliated Hospital of Air Force Medical University (KY20212225-C-1), and the Medical Ethics Committee of Changzhi Medical College Affiliated Heji Hospital (202307). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XF: Conceptualization, Data curation, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Data curation, Formal Analysis, Resources, Software, Writing – review & editing, Supervision. XPG: Conceptualization, Data curation, Formal Analysis, Resources, Software, Writing – review & editing. XG: Conceptualization, Formal Analysis, Project administration, Resources, Software, Supervision, Visualization, Writing – review & editing. JG: Data curation, Formal Analysis, Investigation, Resources, Supervision, Visualization, Writing – review & editing. YS: Conceptualization, Data curation, Project administration, Resources, Supervision, Visualization, Writing – review & editing. JW: Conceptualization, Data curation, Formal Analysis, Resources, Software, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Shanxi Province Key Medical Research Projects (Grant No. 2023XM054), with Yangyang Song as the fund holder.
Acknowledgments
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, and Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5, PMID: 32861308
2. Ong CT, Schwarz JL, and Roggin KK. Surgical considerations and outcomes of minimally invasive approaches for gastric cancer resection. Cancer. (2022) 128:3910–8. doi: 10.1002/cncr.34440, PMID: 36191278
3. Son T and Hyung WJ. Laparoscopic gastric cancer surgery: Current evidence and future perspectives. World J Gastroenterol. (2016) 22:727–35. doi: 10.3748/wjg.v22.i2.727, PMID: 26811620
4. Liu F, Huang C, Xu Z, Su XQ, Zhao G, Ye JX, et al. Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol. (2020) 6:1590–7. doi: 10.1001/jamaoncol.2020.3152, PMID: 32815991
5. Huang C, Liu H, Hu Y, Sun YH, Su XQ, Cao H, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. (2022) 157:9–17. doi: 10.1001/jamasurg.2021.5104, PMID: 34668963
6. Li Z, Shan F, Ying X, Zhang Y, Jian E Y, Wang YK, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: A randomized clinical trial. JAMA Surg. (2019) 154:1093–101. doi: 10.1001/jamasurg.2019.3473, PMID: 31553463
7. Alarcón I, Yang T, Balla A, and Morales-Conde S. Single/reduced port surgery vs. conventional laparoscopic gastrectomy: systematic review and meta-analysis. Minim Invasive Ther Allied Technol. (2022) 31:515–24. doi: 10.1080/13645706.2021.1884571, PMID: 33600291
8. Hwang D, Yoo M, Min GH, Lee E, Kang SH, Park YS, et al. Comparison of reduced port gastrectomy and multiport gastrectomy in korea: ad hoc analysis and nationwide survey on gastric cancer 2019. J Gastric Cancer. (2025) 25:330–42. doi: 10.5230/jgc.2025.25.e15, PMID: 40200876
9. Kim JW. Current issues in reduced-port gastrectomy: A comprehensive review. J Gastric Cancer. (2024) 24:57–68. doi: 10.5230/jgc.2024.24.e9, PMID: 38225766
10. Teng W, Liu J, Liu W, Jiang J, Chen M, and Zang W. Short-term outcomes of reduced-port laparoscopic surgery versus conventional laparoscopic surgery for total gastrectomy: a single-institute experience. BMC Surg. (2023) 23:75. doi: 10.1186/s12893-023-01972-1, PMID: 36997904
11. Chung JH, Hwang J, Park SH, Kim KY, Cho M, Kim YM, et al. Identifying the best candidates for reduced port gastrectomy. Gastric Cancer. (2024) 27:176–86. doi: 10.1007/s10120-023-01438-6, PMID: 37872358
12. Wang L, Chen X, Ma X, Miao W, Wang C, and Yan S. Comparison of clinical safety and feasibility between reduced-port laparoscopic radical gastrectomy and conventional laparoscopic radical gastrectomy: A retrospective study. Front Surg. (2022) 9:995194. doi: 10.3389/fsurg.2022.995194, PMID: 36248360
13. Wang CY, Chen YH, and Huang TS. Reduced-port robotic radical gastrectomy for gastric cancer: a single-institute experience. BMC Surg. (2022) 22:198. doi: 10.1186/s12893-022-01645-5, PMID: 35590316
14. Wei J, Yang X, Gao R, Wang W, Li X, and Ji G. Initial experience with triple port laparoscopic distal gastrectomy. Front Oncol. (2022) 12:1042314. doi: 10.3389/fonc.2022.1042314, PMID: 36776381
15. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick DR, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2, PMID: 19638912
16. Omori T, Yamamoto K, Hara H, Shinno N, Yamamoto Sugimura K, et al. A randomized controlled trial of single-port versus multi-port laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. (2021) 35:4485–93. doi: 10.1007/s00464-020-07955-0, PMID: 32886237
17. Omori T, Fujiwara Y, Yamamoto K, et al, Yanagimoto Y, and Sugimura KMasuzawa T. The safety and feasibility of single-port laparoscopic gastrectomy for advanced gastric cancer. J Gastrointest Surg. (2019) 23:1329–39. doi: 10.1007/s11605-018-3937-0, PMID: 30187335
18. Zhu G, Lang X, Zhou S, Li BW, Sun QH, Yu L, et al. Short- and long-term outcomes of single-port versus multiport laparoscopic radical gastrectomy for gastric cancer: a meta-analysis of propensity score-matched studies and randomized controlled trials. BMC Surg. (2023) 23:223. doi: 10.1186/s12893-023-02134-z, PMID: 37559035
19. Fujita K, Omori T, Hara H, Shinno N, Yasui M, Wada H, et al. Three-year follow-up outcomes of postoperative quality of life from a randomized controlled trial comparing multi-port versus single-port laparoscopic distal gastrectomy. Surg Endosc. (2025) 39:269–79. doi: 10.1007/s00464-024-11213-y, PMID: 39528658
20. Cao C, Tian X, Wang XZ, and Wang Q. Comparative analysis of conventional laparoscopic surgery and single-incision laparoscopic surgery in gastric cancer treatment: Outcomes and prognosis. World J Gastrointest Surg. (2024) 16:3786–93. doi: 10.4240/wjgs.v16.i12.3786, PMID: 39734434
21. Oh SE, Seo JE, An JY, Choi MG, Sohn TS, Bae JM, et al. Compliance with D2 lymph node dissection in reduced-port totally laparoscopic distal gastrectomy in patients with gastric cancer. Sci Rep. (2021) 11:3658. doi: 10.1038/s41598-021-83386-8, PMID: 33574571
22. Lai H, Yi Z, Long D, Liu JG, Qin HQ, Mo XW, et al. Is the 5-port approach necessary in laparoscopic gastrectomy? Comparison of surgical effects of reduced-port laparoscopic gastrectomy and conventional laparoscopic-assisted gastrectomy: A meta-analysis. Med (Baltimore). (2020) 99:e22525. doi: 10.1097/MD.0000000000022525, PMID: 33080686
Keywords: gastrectomy, laparoscopy, reduced port surgery, stomach neoplasm, survival
Citation: Fang X, Li Z, Gao X, Guo X, Ji G, Song Y and Wei J (2025) Safety and efficacy of left-sided three-port laparoscopic gastric cancer surgery: a prospective observational study. Front. Oncol. 15:1627001. doi: 10.3389/fonc.2025.1627001
Received: 12 May 2025; Accepted: 14 August 2025;
Published: 08 September 2025.
Edited by:
Zhen Liu, Zhejiang University, ChinaReviewed by:
Natale Calomino, University of Siena, ItalyErik Brecelj, Institute of Oncology Ljubljana, Slovenia
Copyright © 2025 Fang, Li, Gao, Guo, Ji, Song and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangpeng Wei, d2VpamlhbmdwZW5nMjAxNUAxNjMuY29t; Yanyang Song, MTM2NTM1OTk0MjZAMTYzLmNvbQ==
†These authors share first authorship
 Xuan Fang1†
Xuan Fang1† Jiangpeng Wei
Jiangpeng Wei