Abstract
Objective:
Both hematopoietic stem cell transplantation (HSCT) and chemotherapy combined with tyrosine kinase inhibitors (TKIs) have shown therapeutic efficacy in patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). This study aimed to compare the clinical outcomes of HSCT and TKI-combined chemotherapy regimens in Ph+ ALL through a meta-analysis.
Methods:
We systematically searched PubMed (from 1966), Embase (from 1974), and the Cochrane Library (from 1993) up to April 30, 2025, for eligible studies. Overall survival (OS) and disease-free survival (DFS) were evaluated using hazard ratios (HRs) with 95% confidence intervals (CIs), while relapse risk was assessed using odds ratios (ORs) with 95%CIs. A random-effects model was applied for all analyses.
Results:
The meta-analysis included 35 studies involving 3,827 patients with Ph+ ALL. Allogeneic HSCT (allo-HSCT) was associated with significantly better OS (HR: 0.60; 95% CI: 0.45–0.81; P = 0.001) and DFS (HR: 0.40; 95% CI: 0.30–0.54; P < 0.001) compared to TKI-based chemotherapy. No significant differences in OS (HR: 0.97; 95% CI: 0.70–1.34; P = 0.845) or DFS (HR: 0.92; 95% CI: 0.67–1.26; P = 0.605) were observed between allo-HSCT and autologous HSCT (auto-HSCT). Moreover, allo-HSCT was associated with a significantly lower relapse risk than both TKI-based chemotherapy (OR: 0.28; 95% CI: 0.16–0.51; P < 0.001) and auto-HSCT (OR: 0.39; 95% CI: 0.27–0.54; P < 0.001).
Conclusion:
This meta-analysis demonstrates that allo-HSCT provides superior survival outcomes compared to TKI-based chemotherapy in patients with Ph+ ALL. Although survival outcomes are similar between allo-HSCT and auto-HSCT, allo-HSCT is associated with a significantly reduced risk of relapse.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/, identifier INPLASY202550012.
1 Introduction
Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is a distinct subtype of ALL characterized by the presence of the BCR::ABL1 fusion gene, which encodes a constitutively active BCR-ABL1 tyrosine kinase oncoprotein (1). In adults with ALL, Ph+ ALL accounts for 20%–25% of cases, whereas its incidence in pediatric patients ranges from 3%–5% (2, 3). This genetic aberration serves not only as a critical diagnostic marker but also informs risk stratification and guides targeted treatment strategies.
Before the introduction tyrosine kinase inhibitors (TKIs), the standard management of Ph+ ALL relied on intensive chemotherapy, with overall survival (OS) rates remaining below 40% (4). The integration of TKIs into chemotherapy regimens has significantly improved clinical outcomes, achieving complete hematologic remission in 94%–100% of patients and reducing induction-related mortality to less than 5% (5, 6). As a result, TKI-based chemotherapy has become the first-line treatment for newly diagnosed Ph+ ALL, leading to substantial improvements in both remission rates and long-term survival (7).
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) continues to be the standard consolidation therapy for eligible patients with suitable donors, supported by robust evidence of its efficacy (8–10). Both HLA-matched related and unrelated donor transplants have demonstrated favorable outcomes. A multicenter study conducted in Southwest China further indicated that haploidentical HSCT (haplo-HSCT) offers survival benefits comparable to those of matched sibling transplantation in Ph+ ALL patients (11).
In the TKI era, autologous HSCT (auto-HSCT) has emerged as a context-dependent alternative, particularly for patients without access to an allo-HSCT donor or those deemed medically unsuitable for allo-HSCT. TKIs can effectively reduce tumor burden to achieve minimal residual disease (MRD)-negative or low-MRD status prior to auto-HSCT, thereby mitigating key limitations of this approach—such as the absence of a graft-versus-leukemia effect and the risk of graft contamination. In TKI-pretreated patients, relapse due to graft contamination is now reported in less than 5% of cases (12). Importantly, data from the European Society for Blood and Marrow Transplantation Acute Leukemia Working Group indicate that myeloablative auto-HSCT can provide leukemia-free survival comparable to that of allo-HSCT in Ph+ ALL patients who maintain complete molecular remission (CMR) for more than three months following TKI therapy (13).
Despite these therapeutic advances, the optimal treatment strategy for Ph+ ALL remains a subject of debate. To address this uncertainty, we conducted a systematic review and meta-analysis to compare the therapeutic efficacy of HSCT and TKI-combined chemotherapy regimens in patients with Ph+ ALL.
2 Methods
2.1 Search strategy and selection criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (14). The study protocol was registered on the INPLASY platform (No: INPLASY202550012). Eligible studies were those that directly compared clinical outcomes between HSCT and TKI-based chemotherapy in patients with Ph+ ALL. No restrictions were placed on publication language. We searched PubMed (from 1966), Embase (from 1974), and the Cochrane Library (from 1993) from their earliest available dates up to April 30, 2025. The search strategy incorporated Boolean operators and the Medical Subject Heading (MeSH) term: “Philadelphia chromosome-positive acute lymphoblastic leukemia”. Additional searches were performed in ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), conference proceedings from major hematology meetings, and reference lists of included studies and relevant systematic reviews published in the past five years. Studies were excluded if they involved upfront immunotherapy (e.g., blinatumomab, inotuzumab ozogamicin) as part of the initial induction or consolidation therapy in either the intervention or control group. This criterion was applied to ensure a consistent comparison between the core therapeutic strategies of interest—HSCT and TKI-based chemotherapy. Studies in which immunotherapy was used solely as salvage treatment for relapsed disease were retained.
To minimize selection bias, two reviewers independently screened studies in a blinded manner. Initial screening was based on titles and abstracts to exclude clearly irrelevant studies. Subsequently, full-text articles were reviewed to determine final eligibility. Any disagreements were resolved through discussion or by consultation with a third senior reviewer. The inclusion criteria were structured using the PICOS framework: (1) Population: patients with Ph+ ALL confirmed by cytogenetics or molecular biology; (2) Intervention: HSCT (allogeneic or autologous); (3) Comparison: TKI combined with intensive chemotherapy; (4) Outcomes: OS, disease-free survival (DFS), and relapse incidence; and (5) Study design: prospective or retrospective comparative studies.
2.2 Data collection and quality assessment
Data extraction covered the following information: first author, publication year, study design, country, sample size, mean age, type of TKI, disease status, details of the intervention and control groups, and reported outcomes. Methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) for observational studies (15). The NOS assigns a maximum of 9 points across three domains: (1) Selection (4 points): representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that the outcome was not present at baseline; (2) Comparability (2 points): control for confounding factors; and (3) Outcome (3 points): assessment of outcome, adequacy of follow-up, and completeness of follow-up. Studies scoring ≥7, 5–6, and <5 were considered high, moderate, and low quality, respectively. A three-step quality control process was implemented: (1) Two researchers independently performed quality assessments using a standardized electronic form; (2) Discrepancies of ≥ 2 points were resolved by a senior methodologist according to the NOS manual; and (3) All extracted data were double-entered and cross-verified. Logical inconsistencies were corrected by referring to the original source documents. A final check for missing data was conducted before database lock.
2.3 Statistical analysis
Treatment effects for OS and DFS were summarized as hazard ratios (HRs) with 95% confidence intervals (CIs). Relapse incidence was analyzed using odds ratios (ORs) with 95%CIs. All meta-analyses were performed using the DerSimonian–Laird random-effects model to incorporate potential clinical heterogeneity (16, 17). Heterogeneity was assessed using Cochran’s Q test (significance threshold: P < 0.10) and the I² statistic, with I2 ≥ 50% indicating substantial heterogeneity (18, 19). Sensitivity analyses were conducted using the leave-one-out method, supported by Baujat plots to identify influential studies (20). Prespecified subgroup analyses were performed based on study design, country, intervention type, TKI type, and study quality. Between-subgroup differences were evaluated using mixed-effects meta-regression with restricted maximum likelihood estimation. Permutation tests were applied to avoid normality assumptions (21). Publication bias was evaluated using contour-enhanced funnel plots, Egger’s regression test, and Begg’s rank correlation test (22, 23). If significant asymmetry was detected (P < 0.10), the trim-and-fill method was used to estimate adjusted effect sizes (24). All statistical tests were two-sided, with a significance level of α = 0.05. Analyses were performed using STATA version 12.0 (StataCorp, College Station, TX, USA) with the “metan” package for meta-analysis.
3 Results
3.1 Literature search
A total of 1,947 articles were initially identified through electronic database searches. After removing duplicates, 1,275 articles remained. Screening of titles and abstracts led to the exclusion of 1,134 articles. The remaining 141 studies underwent full-text review, of which 35 met the inclusion criteria and were included in the meta-analysis (13, 25–58). Manual searches of the reference lists of these studies identified three additional potentially eligible articles; however, further verification confirmed that these had already been captured in the electronic search and were therefore excluded. The study selection process is summarized in Figure 1.
Figure 1

PRISMA flow diagram illustrating the literature search and study selection process.
3.2 Study characteristics
The baseline characteristics of the included studies and patients are presented in Table 1. The 35 eligible studies, published between 2010 and 2024, involved a total of 3,827 patients with Ph+ ALL, with individual study sample sizes ranging from 18 to 569. Eleven studies were prospective in design, while 24 were retrospective. Geographically, 16 studies were conducted in Western countries and 19 in Eastern countries. Among the chemotherapy arms of the included studies, the initial TKI agents used were distributed as follows: imatinib (first-generation) in 17 studies, dasatinib (second-generation) in 3 studies, nilotinib (second-generation) in 2 studies, ponatinib (third-generation) in 2 studies, and mixed TKI cohorts in 10 studies. One study did not specify the TKI type used. Quality assessment using the NOS yielded the following scores: 10 studies scored 9 points, 7 scored 8 points, 13 scored 7 points, 2 scored 6 points, and 3 scored 5 points.
Table 1
| Study | Study design | Country | Sample size | Median age (years) | TKI type | Disease status | Intervention and control | Study quality |
|---|---|---|---|---|---|---|---|---|
| Bassan 2010 (25) | Prospective | Italy | 54 | 47.1 | Imatinib | BCR-ABL1 P190 (69.0%); BCR-ABL1 P210 (28.0%); t (9:22): 70.2%; additional aberrations: 33.0%; CD10+: 94.0%; CD20+: 37.0% | HSCT (Allo-HSCT; auto-HSCT); NILG protocol 09/00 | 9 |
| Li 2010 (26) | Retrospective | China | 63 | 34.6 | Imatinib | BCR-ABL1 P190 (61.9%); BCR-ABL1 P210 (19.0%) | Allo-HSCT; Vincristine, daunorubicin, cyclophosphamide, prednisone and L-asparagine | 6 |
| Thyagu 2012 (27) | Retrospective | Canada | 28 | 46.0 | Imatinib | CD10+: 96.4%; CD20+: 53.6% | Allo-HSCT; Doxorubicin, vincristine, asparaginase, dexamethasone plus mercaptopurine plus methotrexate | 7 |
| Pfeifer 2012 (28) | Retrospective | Germany | 115 | NA | Imatinib | NA | Allo-HSCT; standard chemotherapy regimen | 5 |
| Tanguy-Schmidt 2013 (29) | Prospective | France | 43 | 43.0 | Imatinib | NA | HSCT (Allo-HSCT; auto-HSCT); Etoposide, HD-ara, and mitoxantrone | 7 |
| Konopacki 2013 (30) | Retrospective | France | 18 | 53.6 | Imatinib or dasatinib | NA | Allo-HSCT; Hyper-CVAD or GRAALL protocol | 7 |
| Wetzler 2014 (31) | Prospective | USA | 34 | 45.0 | Imatinib | t (9:22): 11.8%; t (9:22)+additional aberrations: 44.1% | Allo-HSCT; Auto-HSCT | 7 |
| Fielding 2014 (32) | Prospective | UK | 130 | NA | Imatinib | NA | HSCT (Allo-HSCT; auto-HSCT); standard chemotherapy regimen | 9 |
| Chalandon 2015 (33) | Prospective | France | 196 | NA | Imatinib | t (9:22): 92.2% | Allo-HSCT; Auto-HSCT | 9 |
| Ravandi 2015 (34) | Prospective | USA | 72 | 55.0 | Dasatinib | BCR-ABL ela2: 72.2%; b2a2: 18.1%; b2a2+b3a2: 2.8%; b3a2/e1a3: 5.6% | Allo-HSCT; Hyper-CVAD | 9 |
| Daver 2015 (35) | Prospective | USA | 39 | 51.0 | Imatinib | BCR-ABL1 P190 (67.0%); BCR-ABL1 P210 (33.0%) | Allo-HSCT; Hyper-CVAD | 7 |
| Sun 2015 (36) | Retrospective | China | 62 | NA | Imatinib | NA | Allo-HSCT; vincristine, daunorubicin, cyclophosphamide and prednisone | 8 |
| Togasaki 2015 (37) | Retrospective | Japan | 22 | 53.0 | Imatinib | NA | Allo-HSCT; Hyper-CVAD or Ph-positive ALL 202 protocol | 7 |
| Kim 2015 (38) | Prospective | Korea | 82 | 47.0 | Nilotinib | BCR-ABL1 transcript, major: 28.0%; minor: 65.0% | Allo-HSCT; daunorubicin, vincristine, prednisone, HD-Ara, and etoposide | 9 |
| Tan 2015 (39) | Prospective | China | 36 | NA | Imatinib | NA | Allo-HSCT; Auto-HSCT | 5 |
| Kuang 2016 (40) | Retrospective | China | 49 | NA | Imatinib | BCR-ABL1 P190 (70.6%); BCR-ABL1 P210 (25.5%) | Allo-HSCT; vincristing and dexamethasone | 9 |
| Ravandi 2016 (41) | Prospective | USA | 78 | 44.0 | Dasatinib | NA | Allo-HSCT; Hyper-CVAD | 8 |
| Kanfar 2016 (42) | Retrospective | Saudi Arabia | 133 | NA | Imatinib | NA | Allo-HSCT; DFCI pediatric ALL protocol | 7 |
| Kozlowski 2017 (43) | Retrospective | Sweden | 42 | 64.8 | Imatinib or dasatinib | BCR-ABL: 35.0% | Allo-HSCT; EWALL-backbone therapy, ABCDV protocol, hyper-CVAD, or daunorubicin/cytarabine | 7 |
| Fujisawa 2017 (44) | Retrospective | Japan | 65 | 47.8 | Imatinib | t (9:22): 35.3%; additional aberrations: 64.7%; CD20+: 27.9%; CD13+: 52.9%; CD33+: 35.3%; CD34+: 94.1%; BCR-ABL1 transcript, major: 25.0%; minor: 70.6% | Allo-HSCT; daunorubicin, cyclophosphamide, vincristine, and prednisolone | 9 |
| Liu 2017 (45) | Retrospective | China | 86 | NA | Imatinib, nilotinib or dasatinib | NA | Allo-HSCT; Auto-HSCT | 5 |
| Hatta 2018 (46) | Retrospective | Japan | 96 | NA | Imatinib | t (9:22): 51.0%; additional aberrations: 49.0%; CD13/CD33+: 43.0% | Allo-HSCT; Ph-positive ALL 202 protocol | 9 |
| Jabbour 2018 (47) | Prospective | USA | 62 | NA | Ponatinib | BCR-ABL1 P190 (74.0%); BCR-ABL1 P210 (25.0%) | Allo-HSCT; Hyper-CVAD | 9 |
| Giebel 2018 (13) | Retrospective | Europe | 569 | 40.7 | NA | NA | Allo-HSCT; Auto-HSCT | 8 |
| Wang 2018 (48) | Retrospective | China | 133 | 37.0 | Imatinib | BCR-ABL1 P190 (71.4%); BCR-ABL1 P210 (27.1%); t (9:22): 40.6% | Allo-HSCT; Hyper-CVAD | 7 |
| Agrawal 2019 (49) | Retrospective | India | 41 | 35.0 | Imatinib or dasatinib | NA | Allo-HSCT; Hyper-CVAD | 7 |
| Chang 2019 (50) | Retrospective | China | 70 | 45.0 | Dasatinib | BCR-ABL1 P190 (65.7%); BCR-ABL1 P210 (34.3%) | Allo-HSCT; Hyper-CVAD, BFM-like, or pediatric-inspired ALL regimen | 8 |
| Liu 2019 (51) | Retrospective | China | 27 | 40.0 | Nilotinib | BCR-ABL1 P190 (80.0%); BCR-ABL1 P210 (20.0%) | Allo-HSCT; cyclophosphamide, vincristine, cytarabine, teniposide, dexamethasone | 7 |
| Wang 2020 (52) | Retrospective | China | 134 | 38.5 | Imatinib or dasatinib | BCR-ABL ela2: 73.1%; e13a2 or e14a2: 26.9% | Allo-HSCT; Hyper-CVAD, or daunorubicin, vincristine, and prednisolone | 8 |
| Ghobadi 2020 (53) | Retrospective | USA | 186 | 52.1 | Imatinib, dasatinib, or ponatinib | NA | Allo-HSCT; Hyper-CVAD | 9 |
| Lyu 2021 (54) | Retrospective | China | 119 | NA | Imatinib, dasatinib, or nilotinib | BCR-ABL1 P190 (73.1%); BCR-ABL1 P210 (22.7%); t (9:22): 52.9%; additional aberrations: 29.4% | Allo-HSCT; Auto-HSCT | 7 |
| Wu 2022 (55) | Retrospective | China | 198 | 38.9 | Imatinib, dasatinib, or nilotinib | BCR-ABL1 P190 (39.9%); BCR-ABL1 P210 (24.7%) | Allo-HSCT; Hyper-CVAD or CALLG2008 | 8 |
| Othman 2022 (56) | Retrospective | USA | 22 | 53.4 | Ponatinib | CD20+: 50.0%; BCR-ABL1 P190 (27.2%); BCR-ABL1 P210 (13.6%) | Allo-HSCT; Hyper-CVAD | 6 |
| Badar 2024 (57) | Retrospective | USA | 431 | 52.0 | Imatinib, dasatinib, nilotinib, or ponatinib | BCR-ABL1 P190 (75.0%); BCR-ABL1 P210 (25.0%) | Allo-HSCT; intensive or non-intensive chemotherapy | 7 |
| Hu 2024 (58) | Retrospective | China | 292 | 38.0 | Imatinib or dasatinib | BCR-ABL1 P190 (70.0%); BCR-ABL1 P210 (30.0%) | Allo-HSCT; Hyper-CVAD | 8 |
Baseline characteristics of identified studies and involved patients.
NA indicated “not available.”
3.3 Overall survival
A total of 27 studies compared OS between allo-HSCT and TKI-based chemotherapy, and 9 studies compared allo-HSCT with auto-HSCT (Figure 2). Pooled analysis showed that allo-HSCT was associated with significantly improved OS compared to TKI-based chemotherapy (HR: 0.60; 95% CI: 0.45–0.81; P = 0.001). In contrast, no significant difference in OS was observed between allo-HSCT and auto-HSCT (HR: 0.97; 95% CI: 0.70–1.34; P = 0.845). Substantial heterogeneity was detected in the allo-HSCT vs. TKI-based chemotherapy comparison (I2 = 60.5; P < 0.001), whereas no heterogeneity was observed for allo-HSCT vs. auto-HSCT (I2 = 0.0; P = 0.459). Sensitivity analyses confirmed the stability of the pooled OS estimates in both comparisons (Supplementary Figure S1). Subgroup analyses indicated that the OS benefit of allo-HSCT over TKI-based chemotherapy was consistent in retrospective studies, studies conducted in Eastern countries, studies using imatinib, and high-quality studies. No significant OS differences were observed between allo-HSCT and auto-HSCT across any subgroups (Table 2). No evidence of publication bias was detected for either comparison (allo-HSCT vs. TKI-based chemotherapy: Egger’s P = 0.861; Begg’s P = 0.428; allo-HSCT vs. auto-HSCT: Egger’s P = 0.072; Begg’s P = 0.118) (Supplementary Figure S2).
Figure 2
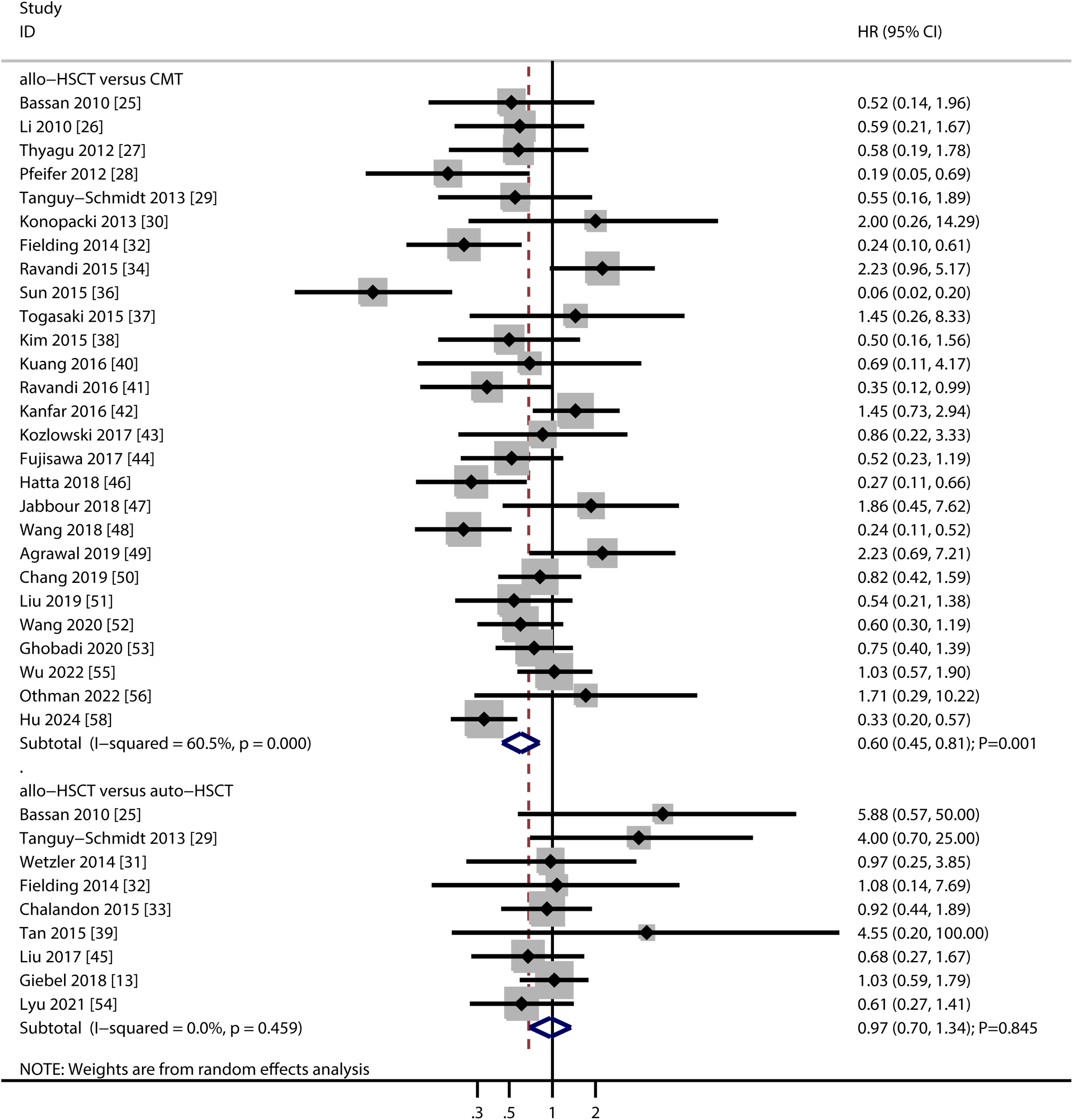
Forest plot summarizing the results for overall survival.
Table 2
| Outcomes | Factors | Subgroups | HR or OR and 95%CI | P value | I2 (%) | P value for heterogeneity | P value between subgroups |
|---|---|---|---|---|---|---|---|
| OS (allo-HSCT versus CMT) | Study design | Prospective | 0.65 (0.32-1.29) | 0.216 | 63.5 | 0.012 | 0.620 |
| Retrospective | 0.59 (0.42-0.83) | 0.002 | 61.3 | < 0.001 | |||
| Country | Eastern | 0.56 (0.38-0.82) | 0.003 | 67.1 | < 0.001 | 0.313 | |
| Western | 0.68 (0.43-1.10) | 0.114 | 50.6 | 0.022 | |||
| TKI type | Dasatinib | 0.90 (0.35-2.31) | 0.819 | 73.6 | 0.023 | 0.008 | |
| Imatinib | 0.41 (0.26-0.66) | < 0.001 | 61.5 | 0.002 | |||
| Imatinib or dasatinib | 0.76 (0.37-1.57) | 0.457 | 63.2 | 0.028 | |||
| Imatinib, dasatinib, or ponatinib | 0.88 (0.57-1.36) | 0.564 | 0.0 | 0.464 | |||
| Nilotinib | 0.52 (0.25-1.08) | 0.080 | 0.0 | 0.919 | |||
| Ponatinib | 1.80 (0.59-5.46) | 0.299 | 0.0 | 0.942 | |||
| Study quality | High | 0.61 (0.45-0.84) | 0.002 | 62.6 | < 0.001 | 0.581 | |
| Moderate | 0.52 (0.17-1.56) | 0.242 | 50.8 | 0.131 | |||
| OS (allo-HSCT versus auto-HSCT) | Study design | Prospective | 1.27 (0.73-2.20) | 0.396 | 0.0 | 0.418 | 0.229 |
| Retrospective | 0.83 (0.55-1.25) | 0.381 | 0.0 | 0.518 | |||
| Country | Eastern | 0.69 (0.38-1.26) | 0.223 | 0.0 | 0.473 | 0.185 | |
| Western | 1.12 (0.76-1.66) | 0.574 | 0.0 | 0.482 | |||
| Study quality | High | 1.00 (0.70-1.45) | 0.979 | 2.5 | 0.406 | 0.610 | |
| Moderate | 0.96 (0.23-4.06) | 0.953 | 24.8 | 0.249 | |||
| DFS (allo-HSCT versus CMT) | Study design | Prospective | 0.35 (0.18-0.69) | 0.002 | 61.5 | 0.024 | 0.629 |
| Retrospective | 0.42 (0.30-0.58) | < 0.001 | 68.4 | < 0.001 | |||
| Country | Eastern | 0.35 (0.24-0.51) | < 0.001 | 65.4 | < 0.001 | 0.008 | |
| Western | 0.50 (0.32-0.79) | 0.002 | 58.2 | 0.014 | |||
| TKI type | Dasatinib | 0.56 (0.28-1.14) | 0.112 | 10.6 | 0.290 | < 0.001 | |
| Imatinib | 0.33 (0.21-0.52) | < 0.001 | 58.7 | 0.005 | |||
| Imatinib or dasatinib | 0.31 (0.12-0.76) | 0.011 | 81.2 | 0.005 | |||
| Imatinib, dasatinib, nilotinib, or ponatinib | 0.52 (0.37-0.73) | < 0.001 | – | – | |||
| Imatinib, dasatinib, or ponatinib | 0.71 (0.49-1.04) | 0.076 | 0.0 | 0.574 | |||
| Nilotinib | 0.30 (0.14-0.60) | 0.001 | 0.0 | 0.516 | |||
| Ponatinib | 1.59 (0.28-8.94) | 0.597 | – | – | |||
| Study quality | High | 0.39 (0.29-0.53) | < 0.001 | 67.2 | < 0.001 | 0.781 | |
| Moderate | 0.59 (0.11-3.16) | 0.536 | 63.5 | 0.098 | |||
| DFS (allo-HSCT versus auto-HSCT) | Study design | Prospective | 0.97 (0.56-1.67) | 0.904 | 0.0 | 0.692 | 0.831 |
| Retrospective | 0.90 (0.61-1.32) | 0.585 | 0.0 | 0.748 | |||
| Country | Eastern | 1.02 (0.57-1.83) | 0.936 | 0.0 | 0.626 | 0.669 | |
| Western | 0.88 (0.61-1.28) | 0.505 | 0.0 | 0.785 | |||
| Study quality | High | 0.89 (0.63-1.24) | 0.479 | 0.0 | 0.874 | 0.519 | |
| Moderate | 1.22 (0.49-3.04) | 0.669 | – | – | |||
| Relapse (allo-HSCT versus CMT) | Study design | Prospective | 0.63 (0.13-2.99) | 0.556 | – | – | 0.456 |
| Retrospective | 0.26 (0.14-0.50) | < 0.001 | 70.6 | < 0.001 | |||
| Country | Eastern | 0.15 (0.06-0.36) | < 0.001 | 64.3 | 0.016 | 0.003 | |
| Western | 0.54 (0.28-1.05) | 0.070 | 51.5 | 0.083 | |||
| TKI type | Imatinib | 0.16 (0.06-0.40) | < 0.001 | 58.5 | 0.034 | 0.008 | |
| Imatinib or dasatinib | 0.53 (0.07-3.83) | 0.532 | 72.8 | 0.025 | |||
| Imatinib, dasatinib, nilotinib, or ponatinib | 0.39 (0.27-0.58) | < 0.001 | – | – | |||
| Imatinib, dasatinib, or ponatinib | 0.68 (0.33-1.39) | 0.290 | – | – | |||
| Study quality | High | 0.27 (0.14-0.52) | < 0.001 | 70.9 | < 0.001 | 0.676 | |
| Moderate | 0.44 (0.15-1.29) | 0.133 | – | – | |||
| Relapse (allo-HSCT versus auto-HSCT) | Study design | Prospective | 0.36 (0.19-0.67) | 0.001 | 0.0 | 0.724 | 0.772 |
| Retrospective | 0.40 (0.27-0.59) | < 0.001 | 0.0 | 0.820 | |||
| Country | Eastern | 0.46 (0.25-0.86) | 0.015 | 0.0 | 0.964 | 0.489 | |
| Western | 0.36 (0.24-0.53) | < 0.001 | 0.0 | 0.886 | |||
| Study quality | High | 0.38 (0.26-0.54) | < 0.001 | 0.0 | 0.909 | 0.726 | |
| Moderate | 0.45 (0.17-1.22) | 0.118 | – | – |
Subgroup analyses for OS, DFS, and relapse.
3.4 Disease-free survival
Twenty-three studies reported DFS comparisons between allo-HSCT and TKI-based chemotherapy, and eight studies compared allo-HSCT with auto-HSCT (Figure 3). Allo-HSCT was associated with significantly better DFS than TKI-based chemotherapy (HR: 0.40; 95% CI: 0.30–0.54; P < 0.001). No significant DFS difference was found between allo-HSCT and auto-HSCT (HR: 0.92; 95% CI: 0.67–1.26; P = 0.605). Significant heterogeneity was present in the allo-HSCT vs. TKI-based chemotherapy analysis (I2 = 65.5; P < 0.001), but not in the allo-HSCT vs. auto-HSCT comparison (I2 = 0.0; P = 0.897). Sensitivity analyses supported the robustness of the DFS results (Supplementary Figure S1). Subgroup analyses showed that allo-HSCT was associated with improved DFS compared to TKI-based chemotherapy in most subgroups, except in those using dasatinib, imatinib/dasatinib/ponatinib, or ponatinib as TKI therapy, and in studies of moderate quality. No significant DFS differences were observed between allo-HSCT and auto-HSCT in any subgroup (Table 2). Publication bias was not detected for DFS outcomes (allo-HSCT vs. TKI-based chemotherapy: Egger’s P = 0.654; Begg’s P = 0.267; allo-HSCT vs. auto-HSCT: Egger’s P = 0.260; Begg’s P = 0.266) (Supplementary Figure S2).
Figure 3

Forest plot summarizing the results for disease-free survival.
3.5 Relapse
Eleven studies compared relapse risk between allo-HSCT and TKI-based chemotherapy, and six studies compared allo-HSCT with auto-HSCT (Figure 4). Allo-HSCT was associated with a significantly lower relapse risk compared to both TKI-based chemotherapy (OR: 0.28; 95% CI: 0.16–0.51; P < 0.001) and auto-HSCT (OR: 0.39; 95% CI: 0.27–0.54; P < 0.001). Heterogeneity was substantial in the allo-HSCT vs. TKI-based chemotherapy comparison (I² = 67.9; P = 0.001) but absent in the allo-HSCT vs. auto-HSCT (I2 = 0.0%; P = 0.952). Sensitivity analyses confirmed the stability of these results (Supplementary Figure S1). Subgroup analyses indicated that allo-HSCT was associated with a lower relapse risk compared to TKI-based chemotherapy in retrospective studies, studies from Eastern countries, those using imatinib, or mixed TKIs (imatinib/dasatinib/nilotinib/ponatinib), and high-quality studies. A consistent reduction in relapse risk was also observed for allo-HSCT over auto-HSCT across nearly all subgroups, except in studies of moderate quality (Table 2). No significant publication bias was detected for relapse outcomes (allo-HSCT vs. TKI-based chemotherapy: Egger’s P = 0.431; Begg’s P = 0.640; allo-HSCT vs. auto-HSCT: Egger’s P = 0.924; Begg’s P = 1.000) (Supplementary Figure S2).
Figure 4

Forest plot summarizing the results for relapse risk.
4 Discussion
Our meta-analysis, which included 35 studies involving 3,827 patients with Ph+ ALL, offers comprehensive evidence supporting the therapeutic advantages of allo-HSCT. The key findings indicate that allo-HSCT significantly improves both OS and DFS compared to TKI-based chemotherapy, while yielding survival outcomes comparable to those of auto-HSCT. Importantly, allo-HSCT was associated with a significantly lower risk of relapse than both TKI-based chemotherapy and auto-HSCT, with a particularly pronounced risk reduction observed in comparison with TKI-based regimens. These results remained consistent across multiple sensitivity and subgroup analyses, underscoring the robustness of our conclusions.
Previous meta-analyses have also evaluated the efficacy of allo-HSCT in patients with Ph+ ALL. Ponvilawan et al. (59), who included 26 studies, reported that HSCT led to superior outcomes compared with chemotherapy alone. They also found that auto-HSCT provided survival outcomes similar to those of allo-HSCT in patients without suitable donors or when haploidentical transplantation was not feasible. Similarly, Zeng et al. (60), in a meta-analysis of 15 studies, identified TKI-combined chemotherapy as a viable post-remission treatment option for adult Ph+ ALL patients ineligible for allo-HSCT. However, these earlier studies had notable limitations, such as potential omissions in literature coverage and insufficient exploratory analyses comparing auto-HSCT and allo-HSCT. Our study not only updates prior evidence systematically but also provides in-depth comparative and subgroup analyses to address these gaps.
In the treatment of adult patients with Ph+ ALL, comparative analyses have demonstrated superior survival benefits with allo-HSCT over TKI-based regimens. This advantage can be attributed to two primary mechanisms. First, the myeloablative conditioning regimens used in allo-HSCT allow more comprehensive eradication of leukemic cells, including quiescent leukemic stem cells that are often resistant to conventional therapies. Second, donor-derived hematopoietic stem cells reconstitute normal hematopoietic and immune functions, thereby fundamentally correcting the patient’s dysregulated hematopoiesis (61). Following transplantation, donor immune cells mediate graft-versus-leukemia (GVL) effects by targeting residual malignant cells—an immune-mediated antitumor response that effectively eliminates minimal residual disease. This dual mechanism, combining direct cytoreduction and sustained immune surveillance, significantly reduces relapse rates and prolongs survival (62). Notably, TKI maintenance therapy following allo-HSCT has become a standard of care in recent years, with evidence indicating that it further reduces relapse risk by 30–40% in patients with Ph+ ALL (63). This introduces a potential confounding factor: the favorable outcomes associated with allo-HSCT in our meta-analysis may reflect not only the GVL effect but also the impact of post-transplant TKI maintenance, rather than allo-HSCT alone. Unfortunately, due to limited reporting, we were unable to disentangle this confounding effect. Similarly, among the TKI-based chemotherapy studies, 24 out of 35 (68%) did not provide details on maintenance duration, precluding a fair comparison between “induction plus maintenance” and “allo-HSCT with or without maintenance.”
Notably, allo-HSCT can overcome TKI resistance, which develops in approximately 30–40% of patients receiving long-term TKI therapy. Even in TKI-resistance cases, allo-HSCT exerts antileukemic effects through alternative mechanisms—such as GVL cytotoxicity and the elimination of chemotherapy-insensitive cells—thereby offering a viable salvage treatment option. The substantial heterogeneity observed in comparisons between allo-HSCT and TKI-based therapy can be largely explained by the type of TKI used. For first-generation imatinib, allo-HSCT provides clear benefits in both OS and DFS, as imatinib’s limited efficacy against resistant clones (e.g., T315I mutation) increases reliance on the GVL effect conferred by allo-HSCT. In contrast, second-generation dasatinib and third-generation ponatinib—with their broader mutation coverage and deeper MRD)clearance—attenuate this benefit, to the extent that allo-HSCT no longer significantly improves OS or DFS compared to these potent TKIs. This observation aligns with the notion that “not all TKIs are equal” and helps explain why earlier studies demonstrated a stronger superiority of allo-HSCT, whereas more recent trials have questioned its necessity in the era of advanced TKIs. Subgroup analyses further revealed that the survival advantage of allo-HSCT over TKI-based therapy was more pronounced in retrospective studies and Eastern populations, possibly reflecting regional variations in TKI accessibility and transplant expertise. The absence of significant heterogeneity in comparisons between allo-HSCT and auto-HSCT suggests greater standardization of clinical practices within transplantation settings.
The superior relapse control achieved with allo-HSCT may be attributed to a dual mechanism: the synergistic interaction between the GVL effect and the intensified conditioning regimen (64). The GVL effect represents the core immunological advantage of allo-HSCT, wherein immune cells—such as donor-derived T cells and natural killer cells—recognize and target leukemia-specific antigens as non-self. The intensified conditioning regimen establishes a foundation for this response: high-dose chemotherapy, often combined with radiotherapy, effectively eliminates leukemia cells—including drug-resistant and quiescent leukemia stem cells—thereby reducing tumor burden. This not only facilitates the engraftment of donor hematopoietic stem cells but also minimizes the resistance of residual leukemia cells to the GVL effect. This immunological benefit is particularly critical in Ph+ ALL, where persistent MRD following chemotherapy remains a major clinical challenge. Notably, despite the superior relapse control observed with allo-HSCT, no statistically significant difference in OS was detected between allo-HSCT and auto-HSCT. This finding merits further investigation. Potential explanations include transplantation-related mortality offsetting the survival benefit from relapse reduction, or differences in the efficacy of salvage therapies following disease recurrence.
A transformative trend in the management of adult Ph+ ALL is the integration of immunotherapies, particularly bispecific T-cell engagers such as blinatumomab (anti-CD19/CD3). In contrast to intensive chemotherapy or transplantation, blinatumomab targets malignant B-cell precursors with high specificity, thereby reducing off-target toxicity and treatment-related mortality—key limitations of conventional regimens (65, 66). Notably, MD Anderson Cancer Center has pioneered efforts to optimize transplant utilization in the immunotherapy era, proposing a “transplant-deferral” strategy in which frontline therapy combines blinatumomab with TKIs to achieve deep and sustained molecular remission, while reserving allo-HSCT exclusively for patients with disease progression, persistent MRD, or TKI resistance (67). Our findings should be interpreted within this evolving paradigm: although allo-HSCT remains superior to TKI-based chemotherapy in preventing relapse, blinatumomab may narrow this gap by enabling improved long-term disease control with reduced toxicity. For patients ineligible for allo-HSCT, blinatumomab may represent a viable alternative to auto-HSCT, although long-term data on relapse rates are still limited. Future meta-analyses should incorporate direct comparative data between blinatumomab-containing regimens and HSCT to further refine treatment algorithms.
An important consideration for patients ineligible for allo-HSCT is whether auto-HSCT combined with TKI maintenance offers advantages over second- or third-generation TKI monotherapy. Currently, direct comparative evidence is lacking, as no randomized trials or large cohort studies have addressed this question. However, indirect data can inform clinical decision-making. For patients with access to second- or third-generation TKIs, monotherapy maintenance is generally preferred (67), as it yields 3-year leukemia-free survival rates comparable to those of auto-HSCT plus TKI, while avoiding transplant-related risks. Auto-HSCT with TKI maintenance remains a valuable option in two scenarios: (1) low- and middle-income countries or resource-limited settings where advanced-generation TKIs are unavailable; and (2) patients with persistent MRD positivity despite first- or second-generation TKI therapy. Future head-to-head trials are warranted to clarify the optimal strategy in this population.
Several limitations of this study should be acknowledged. First, the predominance of retrospective studies (24/35) introduces potential selection bias. Second, geographic imbalance may limit the generalizability of our findings, particularly given regional disparities in healthcare resource allocation. Third, substantial heterogeneity in TKI-based chemotherapy and HSCT protocols could not be fully explored due to insufficient reporting of relevant data, which limits our ability to identify subgroups that may derive greater benefit from specific therapies. Fourth, variability in TKI treatment duration and transplant conditioning regimens precludes precise protocol-specific recommendations. Fifth, we were unable to account for the role of TKI maintenance therapy—now a standard of care following both allo-HSCT and chemotherapy—which represents a critical unmeasured confounder. Finally, this meta-analysis shares the inherent limitations of all studies based on published literature, including potential publication bias and restricted granularity for subgroup analyses.
5 Conclusions
This comprehensive meta-analysis establishes allo-HSCT as the optimal consolidative therapy for Ph+ ALL, demonstrating superior survival outcomes and relapse control compared to TKI-based regimens. Importantly, while allo-HSCT and auto-HSCT showed comparable survival benefits, allo-HSCT maintained superior antileukemic efficacy, reducing the risk of relapse by 61% compared to auto-HSCT. Based on the synthesized evidence, we propose the following therapeutic algorithm: (1) Allo-HSCT for eligible patients with suitable donors should be prioritized for patients receiving first-generation imatinib, those exhibiting early TKI resistance, or those with high-risk genetic features. For patients on second- or third-generation TKIs (e.g., dasatinib, ponatinib) who achieve durable MRD negativity, allo-HSCT may be deferred in favor of continued TKI maintenance; (2) Auto-HSCT combined with at least 12 months of post-transplant TKI maintenance may be considered for a select group of patients ineligible for allo-HSCT, specifically those who sustain durable complete molecular remission on TKI therapy, lack access to second- or third-generation TKI monotherapy, or have a history of persistent MRD positivity. It is important to note that when available, second- or third-generation TKI monotherapy is generally preferred over auto-HSCT plus TKI, as current evidence does not demonstrate the superiority of the auto-HSCT strategy; and (3) In regions with limited transplant access or for patients unable to tolerate transplantation, prolonged TKI/chemotherapy combinations or blinatumomab-based immunotherapy may serve as a bridge to delayed transplantation or as long-term maintenance for patients who achieve MRD negativity.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XG: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. HZ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. FS: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. XZ: Data curation, Investigation, Methodology, Writing – review & editing. HW: Investigation, Methodology, Writing – review & editing. MY: Investigation, Methodology, Writing – review & editing. YL: Investigation, Methodology, Writing – review & editing. QF: Investigation, Methodology, Writing – review & editing. GZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Technology Plan Project of Zhejiang Province (2021434504), the Jiaxing Key Discipline Fund (2019-ZC-10, 2023-FC-005), Jiaxing Science and Technology Program (2021AD30147), the Medical and Health Research Project of Zhejiang Province (2024KY1676) and Scientific Research Fund of Zhejiang Provincial Education Department (Y202455563).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1627825/full#supplementary-material
References
1
Foà R Chiaretti S . Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. (2022) 386:2399–411. doi: 10.1056/NEJMra2113347
2
Bernt KM Hunger SP . Current concepts in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia. Front Oncol. (2014) 4:54. doi: 10.3389/fonc.2014.00054
3
Fielding AK . Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: a broader range of options, improved outcomes, and more therapeutic dilemmas. Am Soc Clin Oncol Educ Book. (2015) 35:e352–9. doi: 10.14694/EdBook_AM.2015.35.e352
4
Jabbour E Short NJ Jain N Haddad FG Welch MA Ravandi F et al . The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J Hematol Oncol. (2023) 16:22. doi: 10.1186/s13045-023-01409-5
5
Gökbuget N Boissel N Chiaretti S Dombret H Doubek M Fielding A et al . Diagnosis, prognostic factors, and assessment of ALL in adults: 2024 ELN recommendations from a European expert panel. Blood. (2024) 143:1891–902. doi: 10.1182/blood.2023020794
6
Thomas DA Faderl S Cortes J O’Brien S Giles FJ Kornblau SM et al . Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. (2004) 103:4396–407. doi: 10.1182/blood-2003-08-2958
7
Hoelzer D Bassan R Boissel N Roddie C Ribera JM Jerkeman M . ESMO Clinical Practice Guideline interim update on the use of targeted therapy in acute lymphoblastic leukaemia. Ann Oncol. (2024) 35:15–28. doi: 10.1016/j.annonc.2023.09.3112
8
Ribera JM . Optimal approach to treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: how to best use all the available tools. Leuk Lymphoma. (2013) 54:21–7. doi: 10.3109/10428194.2012.708753
9
Ravandi F . Managing Philadelphia chromosome-positive acute lymphoblastic leukemia: role of tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. (2011) 11:198–203. doi: 10.1016/j.clml.2011.03.002
10
Brissot E Labopin M Beckers MM Socié G Rambaldi A Volin L et al . Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. (2015) 100:392–9. doi: 10.3324/haematol.2014.116954
11
Gao L Zhang C Gao L Liu Y Su Y Wang S et al . Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicenter study in Southwest China. J Hematol Oncol. (2015) 8:90. doi: 10.1186/s13045-015-0186-5
12
Giebel S Labopin M Gorin NC Caillot D Leguay T Schaap N et al . Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur J Cancer. (2014) 50:411–7. doi: 10.1016/j.ejca.2013.08.027
13
Giebel S Labopin M Potter M Poiré X Sengeloev H Socié G et al . Comparable results of autologous and allogeneic haematopoietic stem cell transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia in first complete molecular remission: An analysis by the Acute Leukemia Working Party of the EBMT. Eur J Cancer. (2018) 96:73–81. doi: 10.1016/j.ejca.2018.03.018
14
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15
Wells G Shea B O’Connell D . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON: Ottawa Hospital Research Institute (2009). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed May 10, 2025).
16
DerSimonian R Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
17
Ades AE Lu G Higgins JP . The interpretation of random-effects meta-analysis in decision models. Med Decis Making. (2005) 25:646–54. doi: 10.1177/0272989X05282643
18
Deeks JJ Higgins JPT Altman DG . Analyzing data and undertaking meta-analyses. In: HigginsJGreenS, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. The Cochrane Collaboration, Oxford, UK (2008). chap 9.
19
Higgins JP Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20
Tobias A . Assessing the influence of a single study in meta-analysis. Stata Tech Bull. (1999) 47:15–7.
21
Altman DG Bland JM . Interaction revisited: the difference between two estimates. BMJ. (2003) 326:219. doi: 10.1136/bmj.326.7382.219
22
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
24
Duvall S Tweedie R . A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc. (2000) 95:89–98.
25
Bassan R Rossi G Pogliani EM Di Bona E Angelucci E Cavattoni I et al . Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. (2010) 28:3644–52. doi: 10.1200/JCO.2010.28.1287
26
Li Y Zou D Zhao Y Mi Y Wang J Qiu L . Clinical characteristics and outcomes of adults with Philadelphia chromosome positive and/or bcr-abl positive acute lymphoblastic leukemia: a single center study from China. Leuk Lymphoma. (2010) 51:488–96. doi: 10.3109/10428190903370361
27
Thyagu S Minden MD Gupta V Yee KW Schimmer AD Schuh AC et al . Treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia with imatinib combined with a paediatric-based protocol. Br J Haematol. (2012) 158:506–14. doi: 10.1111/j.1365-2141.2012.09182.x
28
Pfeifer H Wettner C Wassmann B Giagounidis A Stelljes M Duhrsen U et al . Long term follow up of elderly patients with philadelphiapositive acute lymphoblastic leukaemia (PH+ALL): Updated results of the GMALL elderly trials. Haematologica. (2012) 97:463.
29
Tanguy-Schmidt A Rousselot P Chalandon Y Cayuela JM Hayette S Vekemans MC et al . Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. (2013) 19:150–5. doi: 10.1016/j.bbmt.2012.08.021
30
Konopacki J Arnautou P Kerbout M Segot A Souleau B Bories D et al . Post induction treatment of philadelphie positive chromosome all: TKI based-intensive chemotherapy or allohsct? Haematologica. (2013) 98:266–7.
31
Wetzler M Watson D Stock W Koval G Mulkey FA Hoke EE et al . Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. (2014) 99:111–5. doi: 10.3324/haematol.2013.085811
32
Fielding AK Rowe JM Buck G Foroni L Gerrard G Litzow MR et al . UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. (2014) 123:843–50. doi: 10.1182/blood-2013-09-529008
33
Chalandon Y Thomas X Hayette S Cayuela JM Abbal C Huguet F et al . Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. (2015) 125:3711–9. doi: 10.1182/blood-2015-02-627935
34
Ravandi F O’Brien SM Cortes JE Thomas DM Garris R Faderl S et al . Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. (2015) 121:4158–64. doi: 10.1002/cncr.29646
35
Daver N Thomas D Ravandi F Cortes J Garris R Jabbour E et al . Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. (2015) 100:653–61. doi: 10.3324/haematol.2014.118588
36
Sun YQ Wang J Jiang Q Xu LP Liu DH Zhang XH et al . Haploidentical hematopoietic SCT may be superior to conventional consolidation/maintenance chemotherapy as post-remission therapy for high-risk adult ALL. Bone Marrow Transplant. (2015) 50:20–5. doi: 10.1038/bmt.2014.195
37
Togasaki E Shono K Onoda M Yokota A . Retrospective analysis of Philadelphia chromosome-positive acute lymphoblastic leukemia treated with allogeneic hematopoietic stem cell transplant versus chemotherapy combined with tyrosine kinase inhibitor. Leuk Lymphoma. (2015) 56:244–7. doi: 10.3109/10428194.2014.914198
38
Kim DY Joo YD Lim SN Kim SD Lee JH Lee JH et al . Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. (2015) 126:746–56. doi: 10.1182/blood-2015-03-636548
39
Tan SM Ong TC Zakaria MZ Liew PK Syed Abd Kadir SS Tan J et al . Single Asian centre experience in haematopoietic stem cell transplantation for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplantation. (2015) 50:S479.
40
Kuang P Liu T Pan L Zhu H Wu Y Ye Y et al . Sustaining integrating imatinib and interferon-α into maintenance therapy improves survival of patients with Philadelphia positive acute lymphoblastic leukemia ineligible for allogeneic stem cell transplantation. Leuk Lymphoma. (2016) 57:2321–9. doi: 10.3109/10428194.2016.1144882
41
Ravandi F Othus M O’Brien SM Forman SJ Ha CS Wong JYC et al . US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in philadelphia chromosome positive ALL. Blood Adv. (2016) 1:250–9. doi: 10.1182/bloodadvances.2016001495
42
Kanfar SS Chan SM Gupta V Schimmer AD Schuh AC Sibai H et al . Outcomes of adult philadelphia positive acute lymphoblastic leukemia patients treated with pediatric multi-agent chemotherapy and imatinib and the impact of residual disease monitoring on survival. Blood. (2016) 128:3976–. doi: 10.1182/blood.V128.22.3976.3976
43
Kozlowski P Lennmyr E Ahlberg L Bernell P Hulegårdh E Karbach H et al . Age but not Philadelphia positivity impairs outcome in older/elderly patients with acute lymphoblastic leukemia in Sweden. Eur J Haematol. (2017) 99:141–9. doi: 10.1111/ejh.12896
44
Fujisawa S Mizuta S Akiyama H Ueda Y Aoyama Y Hatta Y et al . Phase II study of imatinib-based chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Am J Hematol. (2017) 92:367–74. doi: 10.1002/ajh.24653
45
Liu X Jiang E Huang Y Qiu L Feng S Han M . Which patients with ph+ Acute lymphoblastic leukemia should undergo autologous hematopoietic stem cell transplantation in TKI era? Blood. (2017) 130:3286–.
46
Hatta Y Mizuta S Matsuo K Ohtake S Iwanaga M Sugiura I et al . Final analysis of the JALSG Ph+ALL202 study: tyrosine kinase inhibitor-combined chemotherapy for Ph+ALL. Ann Hematol. (2018) 97:1535–45. doi: 10.1007/s00277-018-3323-8
47
Jabbour E Short NJ Ravandi F Huang X Daver N DiNardo CD et al . Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. (2018) 5:e618–27. doi: 10.1016/S2352-3026(18)30176-5
48
Wang J Jiang Q Xu LP Zhang XH Chen H Qin YZ et al . Allogeneic stem cell transplantation versus tyrosine kinase inhibitors combined with chemotherapy in patients with philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2018) 24:741–50. doi: 10.1016/j.bbmt.2017.12.777
49
Agrawal N Verma P Yadav N Ahmed R Mehta P Soni P et al . Outcome of philadelphia positive acute lymphoblastic leukemia with or without allogeneic stem cell transplantation in a retrospective study. Indian J Hematol Blood Transfus. (2019) 35:240–7. doi: 10.1007/s12288-018-1005-2
50
Chang J Douer D Aldoss I Vahdani G Jeong AR Ghaznavi Z et al . Combination chemotherapy plus dasatinib leads to comparable overall survival and relapse-free survival rates as allogeneic hematopoietic stem cell transplantation in Philadelphia positive acute lymphoblastic leukemia. Cancer Med. (2019) 8:2832–9. doi: 10.1002/cam4.2153
51
Liu B Wang Y Zhou C Wei H Lin D Li W et al . Nilotinib combined with multi-agent chemotherapy in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia: a single-center prospective study with long-term follow-up. Ann Hematol. (2019) 98:633–45. doi: 10.1007/s00277-019-03594-1
52
Wang L Du J Huang A Tang G Gong S Cheng H et al . Chemotherapy vs. allogeneic transplantation as post molecular remission therapy in patients aged less than 60 years with Philadelphia-positive ALL. Bone Marrow Transplant. (2020) 55:245–8. doi: 10.1038/s41409-019-0514-4
53
Ghobadi A Mohammed KA Faramand R Kantarjian HM Jabbour E Short NJ et al . Allogeneic hematopoietic stem cell transplant versus notransplant in adult patients with philadelphia chromosome positiveacute lymphoblastic leukemia in first complete remission and complete molecular remission. Blood. (2020) 136:46–8. doi: 10.1182/blood-2020-141143
54
Lyu M Jiang E He Y Yang D Ma Q Pang A et al . Comparison of autologous and allogeneic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. (2021) 26:65–74. doi: 10.1080/16078454.2020.1868783
55
Wu C Zeng M Chen Y Wu Y . Tyrosine kinase inhibitors and reduced-dose chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. (2022) 27:1032–40. doi: 10.1080/16078454.2022.2119344
56
Othman T Moskoff BN Ho G Tenold ME Azenkot T Krackeler ML et al . Clinical experience with frontline Hyper-CVAD-based regimens, including Hyper-CVAD plus ponatinib, in patients with acute lymphoblastic leukemia treated at a comprehensive cancer center. Leuk Res. (2022) 119:106885. doi: 10.1016/j.leukres.2022.106885
57
Badar T Narra R Mims AS Heckman MG Shallis RM Fahad S et al . Impact of induction regimens intensity and allogeneic stem cell transplantation on survival of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A multi-institutional study. Am J Hematol. (2024) 99:2388–91. doi: 10.1002/ajh.27475
58
Hu L Li Z Yang S Zhao T Duan W Qin Y et al . Allogeneic stem cell transplantation is still a highly curative therapy in adults with philadelphia chromosome-positive acute lymphoblastic leukaemia. Ann Hematol. (2024) 103:3745–54. doi: 10.1007/s00277-024-05682-3
59
Ponvilawan B Kungwankiattichai S Charoenngam N Owattanapanich W . Is stem cell transplantation still needed for adult Philadelphia chromosome-positive acute lymphoblastic leukemia receiving tyrosine kinase inhibitors therapy?: A systematic review and meta-analysis. PloS One. (2021) 16:e0253896. doi: 10.1371/journal.pone.0253896
60
Zeng Q Xiang B Liu Z . Comparison of allogeneic hematopoietic stem cell transplantation and TKI combined with chemotherapy for adult philadelphia chromosome positive acute lymphoblastic leukemia: a systematic review and meta-analysis. Cancer Med. (2021) 10:8741–53. doi: 10.1002/cam4.4413
61
Fielding AK Rowe JM Richards SM Buck G Moorman AV Durrant IJ et al . Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. (2009) 113:4489–96. doi: 10.1182/blood-2009-01-199380
62
Chiaretti S Vitale A Vignetti M Piciocchi A Fazi P Elia L et al . A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. (2016) 101:1544–52. doi: 10.3324/haematol.2016.144535
63
Warraich Z Tenneti P Thai T Hubben A Amin H McBride A et al . Relapse prevention with tyrosine kinase inhibitors after allogeneic transplantation for philadelphia chromosome-positive acute lymphoblast leukemia: A systematic review. Biol Blood Marrow Transplant. (2020) 26:e55–64. doi: 10.1016/j.bbmt.2019.09.022
64
Mammadli M Huang W Harris R Sultana A Cheng Y Tong W et al . Targeting interleukin-2-inducible T-cell kinase (ITK) differentiates GVL and GVHD in allo-HSCT. Front Immunol. (2020) 11:593863. doi: 10.3389/fimmu.2020.593863
65
Canichella M Trawinska MM Mazzone C de Fabritiis P Abruzzese E . Treatment-free remission in ph+ ALL without allogeneic stem cell transplantation: current evidence and future directions. Cancers (Basel). (2025) 17:2457. doi: 10.3390/cancers17152457
66
King AC Pappacena JJ Tallman MS Park JH Geyer MB . Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia. Leuk Res. (2019) 79:27–33. doi: 10.1016/j.leukres.2019.02.009
67
Jabbour E Short NJ Jain N Huang X Montalban-Bravo G Banerjee P et al . Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. (2023) 10:e24–34. doi: 10.1016/S2352-3026(22)00319-2
Summary
Keywords
Philadelphia chromosome-positive, acute lymphoblastic leukemia, tyrosine kinase inhibitor, hematopoietic stem cell transplantation, meta-analysis
Citation
Gao X, Zeng H, Sun F, Zhao X, Wu H, Yan M, Li Y, Fu Q and Zhang G (2025) Comparing therapeutic effects of hematopoietic stem cell transplantation, tyrosine kinase inhibitors and chemotherapy in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a systematic review and meta-analysis. Front. Oncol. 15:1627825. doi: 10.3389/fonc.2025.1627825
Received
13 May 2025
Accepted
29 September 2025
Published
15 October 2025
Volume
15 - 2025
Edited by
Mario Tiribelli, University of Udine, Italy
Reviewed by
William Slayton, University of Florida, United States
Zaid Abdel Rahman, King Hussein Cancer Center, Jordan
Updates
Copyright
© 2025 Gao, Zeng, Sun, Zhao, Wu, Yan, Li, Fu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Zhang, 921950782@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.