- Department of Cancer Epidemiology and Prevention, Affiliated Cancer Hospital of Zhengzhou University/Henan Cancer Hospital, Henan Engineering Research Center of Cancer Prevention and Control, Henan International Joint Laboratory of Cancer Prevention, Zhengzhou, China
Introduction: Cervical cancer, predominantly caused by high-risk human papillomavirus (HR-HPV) infections, is a critical public health issue. Effective screening is essential. This study compares the SureX® HPV genotyping test, which detects 25 HPV types, with the widely used Digene Hybrid Capture® 2 (HC2) test, which targets 13 HR-HPV types, to determine their effectiveness in cervical cancer screening among Chinese women.
Methods: From September to December 2016, women aged 21–64 years in Shanxi Province were screened for cervical cancer using both SureX® HPV and HC2 tests. Women with abnormal cytology test would be referred for colposcopy and biopsied if necessary. Concordance rates and kappa coefficients were calculated to analyze the concordance between the two tests. Sensitivity and specificity for detecting cervical intraepithelial neoplasia grade 2 and higher (CIN2+) cases of the two tests were calculated.
Results: Among 3028 subjects analyzed in this study, the positive rate of the common set of 13 HR-HPV types for the SureX® HPV test was 15.0%, slightly higher than HC2 test’s 13.5%. The overall concordance rate was 93.9% (95% CI: 92.95%-94.66%), with a kappa coefficient of 0.749 (0.715-0.783). Both tests demonstrated a sensitivity of 80.00% (54.81%-92.95%) for detecting CIN2+, with specificities of 85.30% (83.99%-86.52%) for SureX® HPV and 86.82% (85.57%-87.98%) for HC2. For CIN3+ detection, both tests had 100% (67.56%-100%) sensitivity, with specificities of 85.20% (83.89%-86.42%) for SureX® HPV and 86.72% (85.46%-87.89%) for HC2.
Discussion: The SureX® HPV test exhibited excellent concordance with HC2 in detecting the common 13 HR-HPV types and similar sensitivity for identifying CIN2+ cases. Its broader capability to detect 25 HPV genotypes positions it as a promising option for cervical cancer screening.
1 Introduction
Cervical cancer is a major public health problem. With an estimated 661,021 new cases and 348,189 deaths in 2022 worldwide, this disease ranks as the fourth most frequently diagnosed cancer and the fourth cause of cancer deaths among women (1). Experimental as well as epidemiological studies have identified that more than 90% of cervical cancers are caused by infection with human papillomavirus (HPV) (2–5). At present, more than 450 HPV genotypes have been identified including 13 high-risk human papillomavirus (HR-HPV), which are responsible for cervical neoplasia and other anogenital and oropharyngeal cancers (6, 7).
Cytology-based and HPV-based cervical cancer screening, colposcopy and histological diagnosis followed by the treatment of cervical intraepithelial neoplasms (CINs) have been widely used worldwide and reduced population-level cervical cancer incidence and mortality (4, 8–10). Compared with cytology-based test, HPV-based test demonstrated better sensitivity against the detection of cervical cancer and precursors, and could extend the screening interval (11, 12). In 2021, the World Health Organization (WHO) recommended the use of DNA-based HPV testing as a first-choice screening method for cervical cancer (13).
Up to date, there are a variety of HR-HPV testing assays. The digene Hybrid Capture® 2 (HC2) HR-HPV DNA Test is the first HR-HPV testing assay that was approved by the U.S. Food and Drug Administration (FDA) for cervical cancer screening and management. It’s an in vitro nucleic acid hybridization assay designed for the qualitative detection of 13 HR-HPV types in cervical specimens. HC2 has been extensively validated and remains widely used in clinical practice due to its robust performance. However, it does not provide individual genotyping information, particularly for HPV 16 and 18, which are known to confer the highest risk of cervical carcinogenesis. Without specific genotype information, risk-based triage becomes less accurate, potentially leading to over-referral, unnecessary colposcopies, and increased patient anxiety. As genotype-specific management has become increasingly emphasized in screening guidelines (14, 15), assays capable of identifying individual HPV types may offer additional clinical value in risk stratification and patient management.
The SureX® HPV genotyping test (SureX® HPV test) is a novel HPV DNA detection method to detect and genotype 25 HPV types (HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82 and 83). In this study, we evaluated the performance of the SureX® HPV test in comparison with the HC2 test for cervical cancer screening among Chinese women, focusing on the detection of a common set of 13 HR-HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). The results may provide evidence for the clinical applicability of the SureX® HPV test as an alternative screening tool, offering comparable performance to HC2 while enabling broader genotype detection.
2 Methods
2.1 Study population
From September 2016 to December 2016, eligible women were recruited for cervical cancer screening program in Yangcheng County maternal and child health hospital, Shanxi, China. Women would be included if they: (1) were aged 21–64 years old with intact cervix; (2) had a quite well health condition and an expected good adherence to accept routine screening (including colposcopy and biopsy) for cervical cancer; (3) gave a signed informed consent. Women would be excluded if they: (1) were pregnant or within 8 weeks postpartum; (2) had a history of cervical surgery or pelvic radiotherapy; (3) had a history of cervical cancer or precancerous lesions. This study was approved by the Institutional Review Board of Affiliated Cancer hospital of Zhengzhou University.
2.2 Screening procedures
Eligible women were enrolled after they signed informed consent. The gynecological examination was conducted by a physician, and two samples of cervical exfoliated cells were collected for cytology test and HPV test by HC2 test. To evaluate the performance of SureX® HPV test, the remaining samples of cervical swab specimens previously examined by HC2 test were retrieved and subjected to SureX® HPV test. Women with abnormal cytology test would be referred for colposcopy. The histological result obtained after colposcopy was regarded as the final diagnosis (Figure 1).
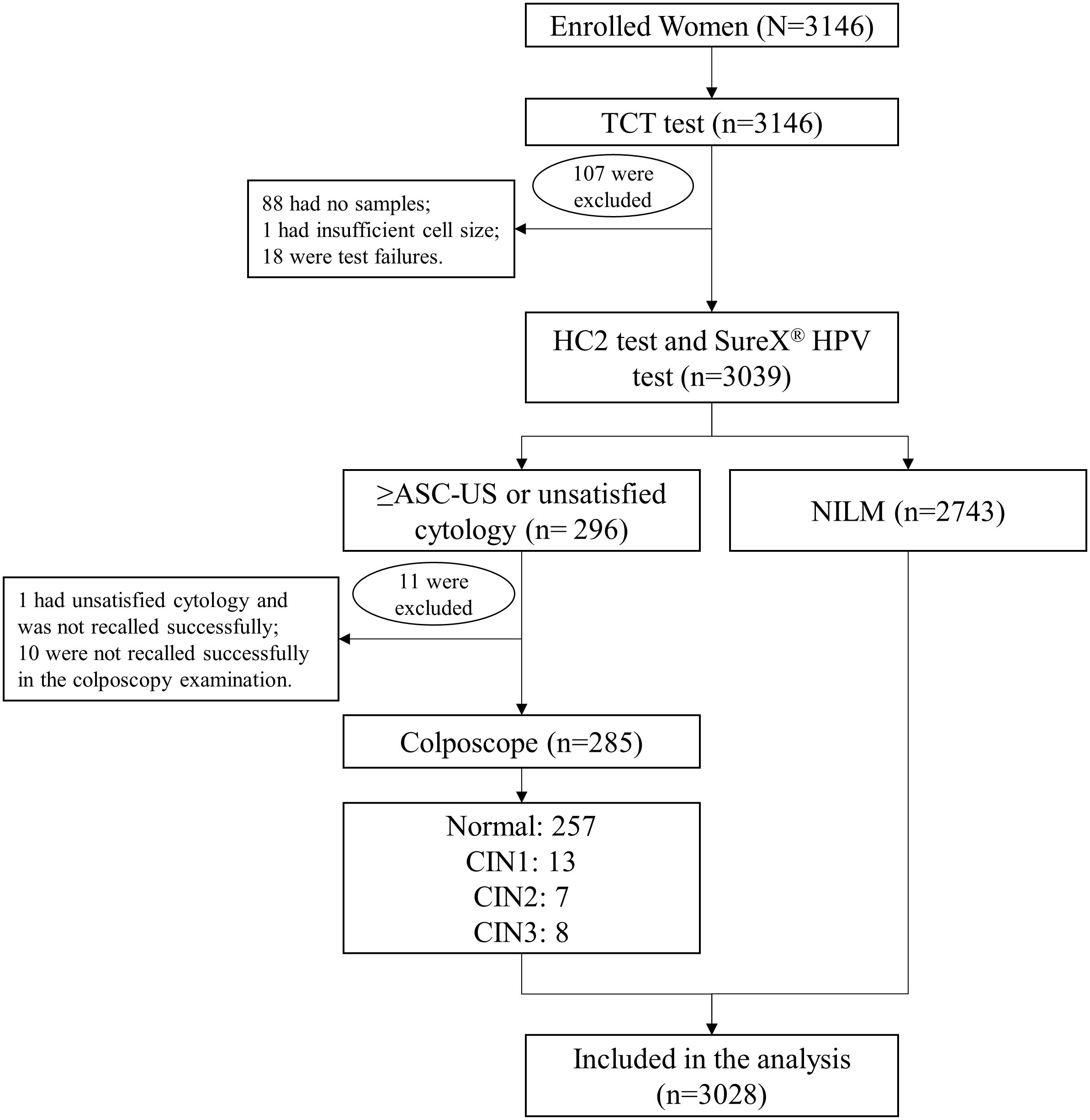
Figure 1. Flowchart of the cervical cancer screening procedure. Abbreviations: TCT, Thinprep liquid-based cytologic test; ASC-US, atypical squamous cells undetermined significance; NILM, negative for intraepithelial lesion or malignancy; CIN, cervical intraepithelial neoplasia.
2.3 Cytology test
The Thinprep liquid-based cytologic test (TCT) was adopted to detect the cytology. All cytological samples were evaluated according to the Bethesda system (TBS) with following results: negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells undetermined significance (ASC-US), atypical squamous cells‐cannot exclude HSIL (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous of cervical carcinoma (SCC), atypical glandular cells (AGC).
2.4 HC2 test
The HC2 test (Qiagen, Gaithersburg, MD, USA) is a sandwich capture molecular hybridization assay, combining chemiluminescence-based signal amplification with nucleic acid hybridization principles. It specifically detects 13 HR-HPV types HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). The process involves the capture of RNA-DNA hybrids on an antibody-coated microtiter plate surface. Following immobilization, these hybrids are detected by introducing an alkaline phosphatase-conjugated antibody to the RNA-DNA complexes. Subsequent addition of a chemiluminescent substrate, cleaved by alkaline phosphatase, generates luminescence. A luminometer is employed to semiquantitatively measure the emitted light in relative light units (RLU). Samples registering measurements below the 1.0 RLU cut-off are interpreted as negative.
2.5 SureX® HPV test
The SureX® HPV test (Ningbo HEALTH Gene Technologies Co., Ltd., China) uses multiplex polymerase chain reaction (PCR) and capillary electrophoresis to amplify target HPV DNA. A total of 25 HPV types (HPV 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82 and 83) were detected and genotyped. According to the length of specific amplified fragments, DNA fragments of oncogenes E6/E7 of 25 HPV types were cloned, PCR-amplified, and recovered via gel electrophoresis in the laboratory. In addition, the concentration of each DNA fragment was measured by Agilent 2100, and the copy number was calculated. Finally, each DNA fragment was mixed to obtain a high concentration HPV positive control (10,000,000 copies/μL). Therefore, through PCR amplification of the target DNA based on the length of the PCR product, the SureX® HPV test achieves accurate and comprehensive genotyping results.
2.6 Colposcopy and histological diagnosis
Women with abnormal cytology test (≥ASC-US or unsatisfied cytology test) would be referred for colposcopy. Based on colposcopy results, samples may be taken for histopathological examination if necessary. The histopathological diagnosis was classified according to the WHO histological criteria for cervical tumors and was used as the gold standard, with cervical intraepithelial neoplasia grade 2 and higher (CIN2+) considered positive.
2.7 Statistical analysis
All statistical analyses were conducted using SAS 9.4 and OpenEpi (available at http://www.openepi.com/). Concordance rates and kappa coefficients with 95% confidence intervals (CIs) were calculated to evaluate the agreement between HC2 test and SureX® HPV test for detecting the 13 common HR-HPV types. Taking colposcopy and pathological diagnosis as the gold standard, the sensitivity, specificity, positive predictive value, negative predictive value of HC2 test and SureX® HPV test in detecting CIN2 + and CIN3 + lesions were calculated using the “Screening” module in OpenEpi, with the Wilson Score method applied to derive the 95% CIs. McNemar’s test was used to compare paired proportions between the two HPV tests. All differences with P values of <0.05 (two-tailed) were considered statistically significant.
3 Results
3.1 Characteristics of the study population
A total of 3146 women were enrolled in the cervical cancer screening program. In this study, a total of 118 subjects were excluded, of which 88 were “no samples”, 1 was “insufficient sample volume”, 18 were “test failures”, 1 had unsatisfied cytology and was not recalled successfully, and 10 were not recalled successfully in the colposcopy examination. Finally, data of 3028 subjects were analyzed in this study, with the mean age of 45.5 ± 7.83 years old.
3.2 Comparison of positive rate for SureX® HPV test and HC2 test
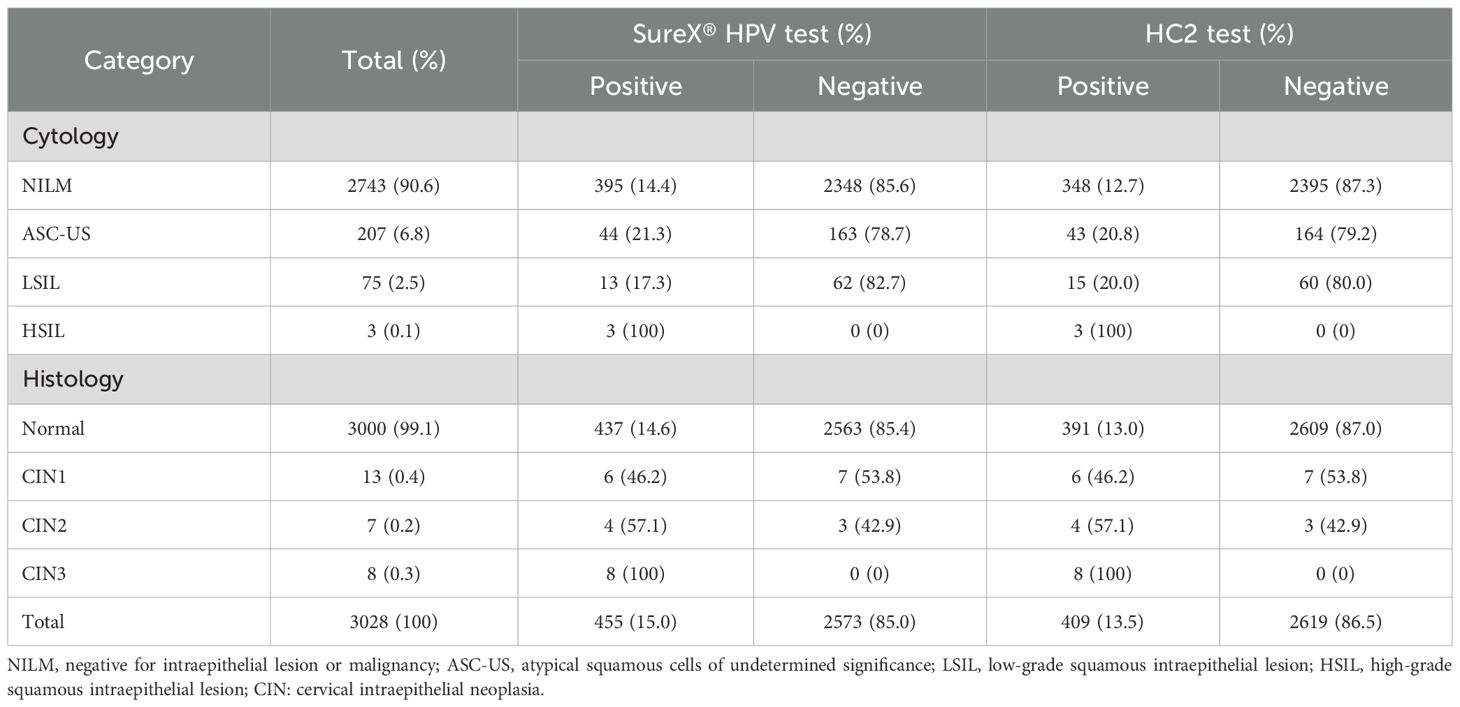
Of all the 3028 women, 2743 (90.6%) were diagnosed as NILM, 285 (9.4%) were ASC‐US or worse. The overall positive rates of the common set of 13 HR-HPV types for the SureX® HPV test and HC2 test were 15.0% and 13.5%, respectively. In total, a greater percentage of specimens were positive using the SureX® HPV test (P<0.05) (Table 1).
The positive rate of the SureX® HPV test and HC2 test were completely consistent in women with pathological diagnoses of CIN1, CIN2 and CIN3. However, in women with normal pathological diagnoses, the HPV positivity rate of SureX® HPV test is higher than that of HC2 test. More details can be found in Table 1.
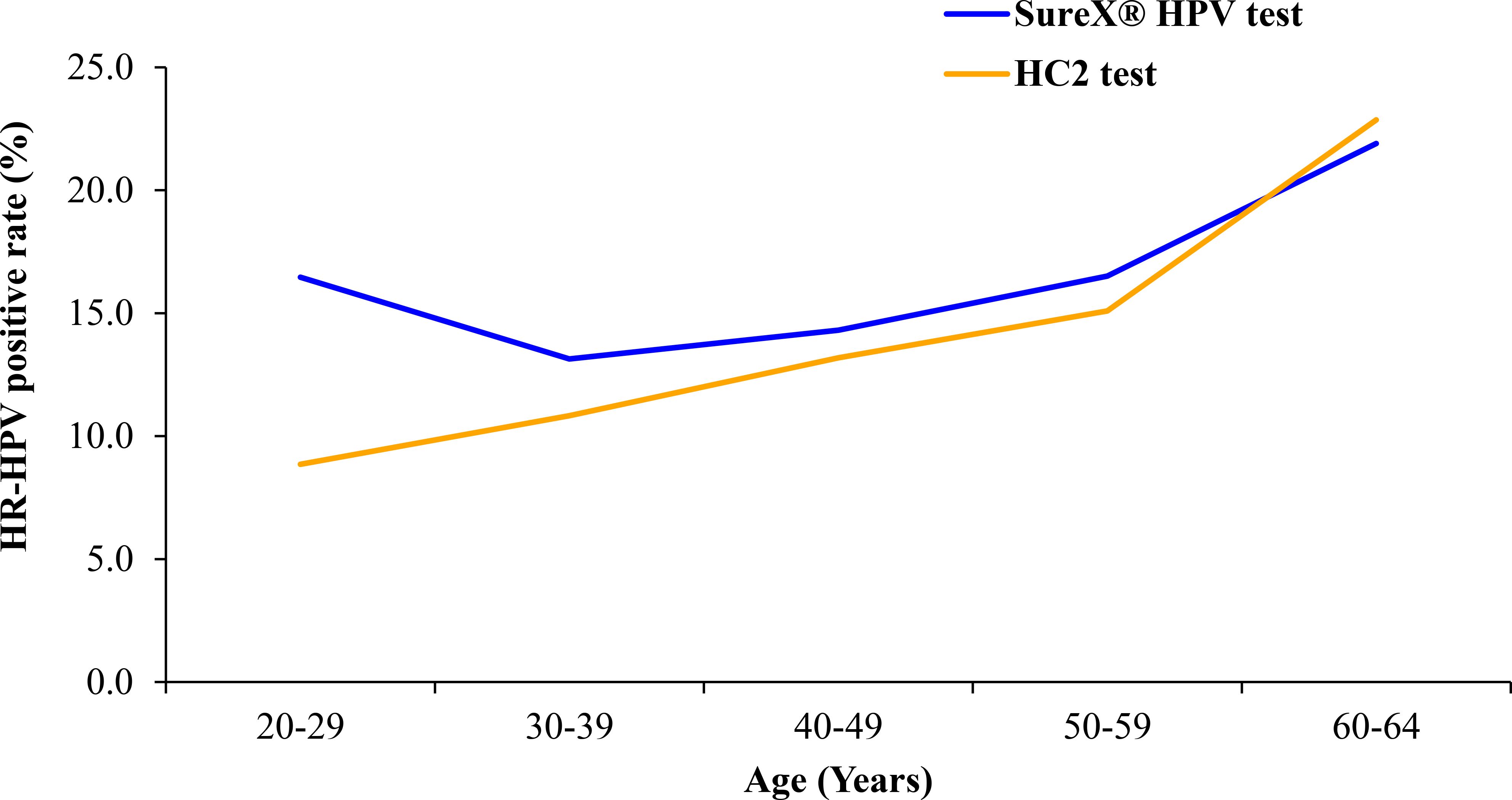
The HR-HPV prevalence for both the SureX® HPV test and HC2 test was assessed across different age groups, as illustrated in Figure 2. The prevalence of HR-HPV exhibited an upward trend corresponding to the increasing age of the women in the study. Notably, in the 20–29 age group, the HR-HPV positive rate was higher for the SureX® HPV test compared to the HC2 test.
Furthermore, the SureX® HPV test provided detection of 25 HPV genotypes in total, with an overall HPV positivity rate of 22.0%. The genotype-specific positivity rates for all 25 types are summarized in Supplementary Table 1.
3.3 Concordance between the SureX® HPV test and the HC2 test
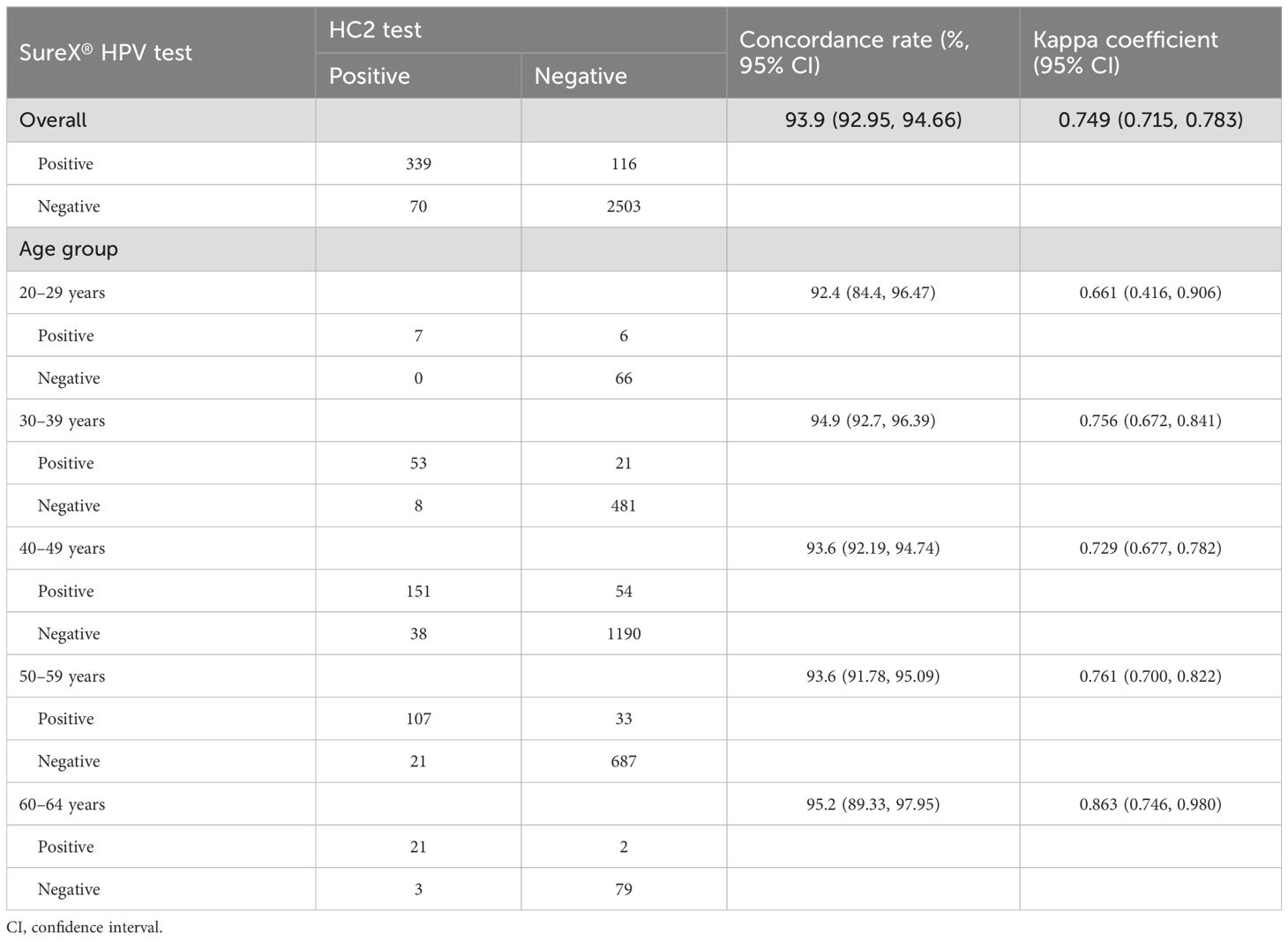
The Table 2 presents the overall concordance rate and age-specific rates for the SureX® HPV test and HC2 test. The overall concordance rate was 93.9% (95% CI: 92.95%-94.66%), with a kappa coefficient of 0.749 (95% CI: 0.715-0.783). Variations in concordance rates and kappa coefficients were observed across different age groups, with the most optimal performance observed in the 60–64 age group.
3.4 The sensitivity and specificity of SureX® HPV test and HC2 test for the detection of CIN2+ and CIN3+ cases
For the detection of CIN2+, the sensitivity of both SureX® HPV test and HC2 test was 80.00% (95% CI: 54.81%-92.95%); the specificity of SureX® HPV test and HC2 test were 85.30% (95% CI, 83.99%-86.52%) and 86.82% (95% CI, 85.57%-87.98%), respectively. Regarding to the detection of CIN3+, the sensitivity of both tests was 100% (95% CI: 67.56%-100%); the specificity of SureX® HPV test and HC2 test were 85.20% (95% CI, 83.89%-86.42%) and 86.72% (95% CI, 85.46%-87.89%), respectively (Table 3).
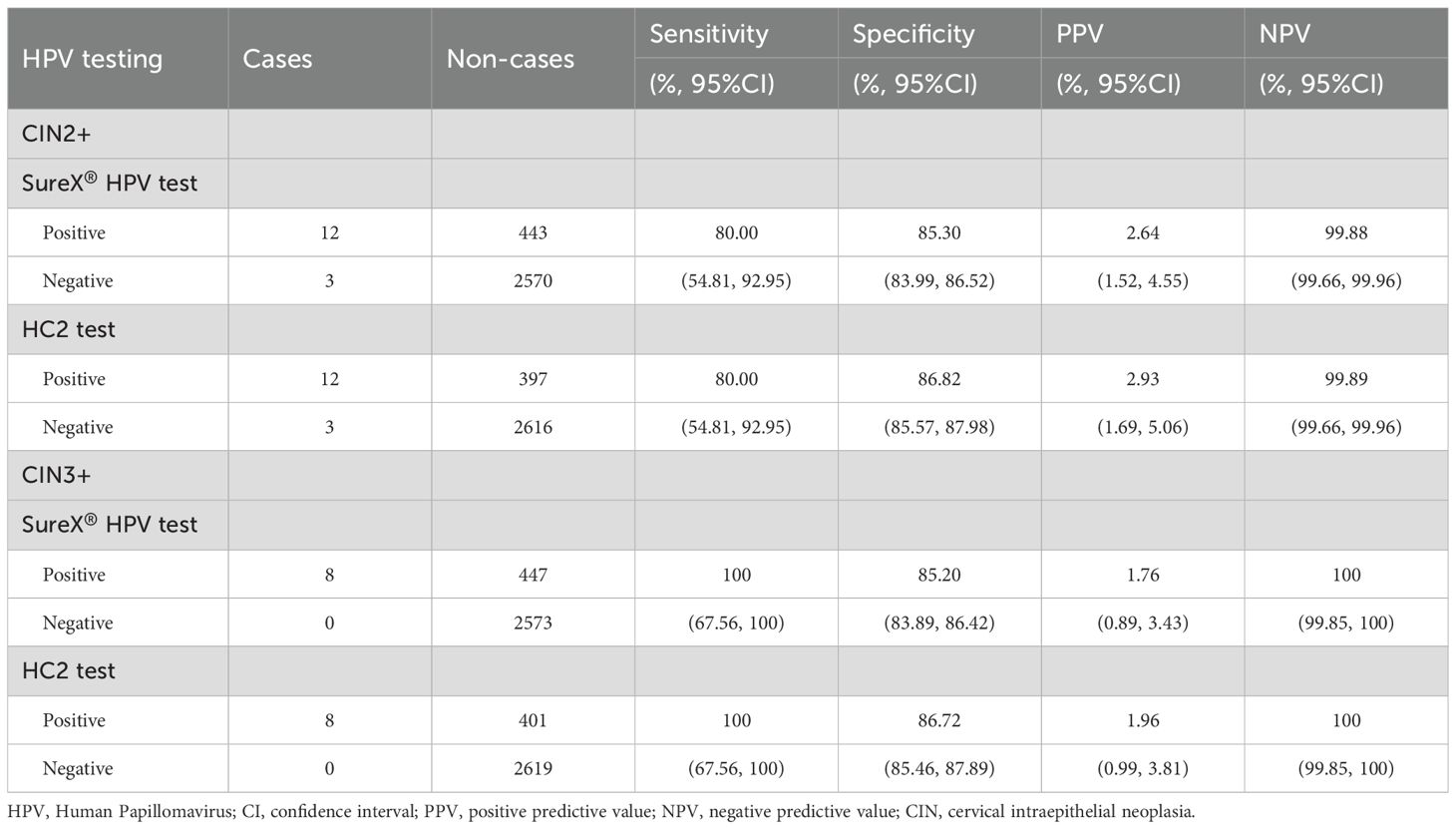
Table 3. The sensitivity and specificity of SureX® HPV test and HC2 test for the detection of CIN2+ cases.
4 Discussion
The DNA-based HPV testing has been recommended by WHO for a first-choice screening method for cervical cancer and been widely used worldwide (13). The HC2 test, approved by FDA, was the pioneering HR-HPV screening method designated to qualitatively detect 13 specific HR-HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). Despite its initial prominence, the limitations of the HC2 test hinder its application in cervical cancer screening in China. Addressing this, the SureX® HPV test has been introduced as a novel method capable of detecting and genotyping 25 HPV types. In response to the limitations posed by existing technologies, the SureX® HPV test introduces a distinctive approach by utilizing a multiplex PCR-capillary electrophoresis method. This innovative technique enables the simultaneous detection and identification of 25 HPV genotypes in a single tube, with a specific focus on the oncogenic E6/E7 regions. With the aim of enhancing screening efficiency, the SureX® HPV test emerges as a promising alternative to established methods. In this study, we compared the performance of the SureX® HPV test and the HC2 test in screening cervical cancer among Chinese women, with a specific focus on detecting the common set of 13 HR-HPV types. The results revealed an outstanding concordance rate between the SureX® HPV test and the HC2 test.
The overall agreement rate was found to be 93.9% between the SureX® HPV and the HC2 test. Notably, the robust concordance rates were observed across all age groups, affirming the reliability of the SureX® HPV test in comparison to the HC2 test for detecting HR-HPV types. In addition, the overall agreement demonstrated comparability with values reported in other studies that compared HC2 with various PCR-based methods (16, 17). While our findings align with the results of several published studies comparing HC2 with other HR-HPV screening tests (18, 19), it is essential to acknowledge a potential underestimation of agreement between the two tests. This discrepancy may arise from the alkali condition of denatured HC2 samples, leading to DNA degradation over time. Consequently, this could reduce the level of remaining HR-HPV DNA available for detection by the SureX® HPV test. In addition to the comparative analysis, our study identified a correlation between HR-HPV prevalence and the age of the women involved. The prevalence of HR-HPV in our study population exhibited an upward trend with increasing age, a pattern consistent with previous research findings (20, 21).
The method of DNA-based HPV test for the screening of cervical cancer and precancerous lesions was characterized by high sensitivity, while specificity being closely associated with the positive rate of HPV (22, 23). The specificity differed greatly among different populations. In the study, whether considering CIN2+ or CIN3+, both the SureX® HPV test and HC2 test exhibited the same sensitivity, and their specificity values were approximately equal. Notably, certain studies have reported that the specificity of HPV DNA detection can fall below 50% (24, 25). Furthermore, following an extensive analysis of clinical samples, the HC2 test has emerged as the “gold standard” for HR-HPV detection (26–28). Consequently, there exists a reasonable consistency between the results obtained from the SureX® HPV test and those from the HC2 test.
Furthermore, in contrast to previously developed and widely used HPV detection methods, such as real-time PCR, the SureX® HPV test, utilizing a multiplex PCR-capillary electrophoresis method, demonstrates the capability to detect and identify 25 HPV genotypes in a single tube, with a focus on specific oncogenic E6/E7. The efficiency is notable, as approximately 96 specimens can be detected and reported within approximately 5 hours when utilizing a 24-channel-equipped capillary electrophoresis platform.
In summary, our study compared a novel HPV genotyping test, the SureX® HPV test, with the HC2 test, revealing a robust consistency in HR-HPV detection. And the sensitivity and specificity for CIN2+ lesions and CIN3+ lesions of the SureX® HPV test were equivalent to that of the HC2 test. Therefore, SureX® HPV test, as a novel method for detecting and genotyping 25 HPV types in a single analysis, is an accurate, safe, and cost-effective HPV detection method, which may become a feasible alternative to HC2 test in cervical cancer screening.
Data availability statement
The datasets presented in this article are not readily available because individual-level study data cannot be shared publicly due to its confidentiality. Aggregated data, from which participants are not identifiable, can be shared upon request. Requests to access the datasets should be directed to Shaokai Zhang, c2hhb2thaXpoYW5nQDEyNi5jb20=
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Affiliated Cancer hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HS: Investigation, Methodology, Writing – original draft. HW: Data curation, Formal Analysis, Writing – original draft. YL: Writing – original draft, Conceptualization, Data curation. HX: Project administration, Writing – original draft. PC: Writing – review & editing, Supervision. XS: Writing – review & editing, Data curation. ML: Writing – review & editing, Investigation, Project administration. PL: Writing – review & editing, Visualization. KL: Writing – review & editing, Project administration, Supervision. LZ: Writing – review & editing, Formal Analysis. SZ: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This is a provisional file, not the final typeset article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1627935/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Xu M, Cao C, Wu P, Huang X, and Ma D. Advances in cervical cancer: current insights and future directions. Cancer Commun (London England). (2025) 45:77–109. doi: 10.1002/cac2.12629
3. Olusola P, Banerjee HN, Philley JV, and Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. (2019) 8:622. doi: 10.3390/cells8060622
4. Okunade KS. Human papillomavirus and cervical cancer. J obstetrics gynaecology: J Institute Obstetrics Gynaecology. (2020) 40:602–8. doi: 10.1080/01443615.2019.1634030
5. Zhang S, Xu H, Zhang L, and Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chin J Cancer Res = Chung-kuo yen cheng yen chiu. (2020) 32:720–8. doi: 10.21147/j.issn.1000-9604.2020.06.05
6. Burd EM. Human papillomavirus laboratory testing: the changing paradigm. Clin Microbiol Rev. (2016) 29:291–319. doi: 10.1128/CMR.00013-15
7. Crosbie EJ, Einstein MH, Franceschi S, and Kitchener HC. Human papillomavirus and cervical cancer. Lancet (London England). (2013) 382:889–99. doi: 10.1016/S0140-6736(13)60022-7
8. Kaufman HW, Alagia DP, Chen Z, Onisko A, and Austin RM. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin pathology. (2020) 154:510–6. doi: 10.1093/ajcp/aqaa074
9. Learman LA and Garcia FAR. Screening for cervical cancer: new tools and new opportunities. Jama. (2018) 320:647–9. doi: 10.1001/jama.2018.11004
10. Perkins RB, Wentzensen N, Guido RS, and Schiffman M. Cervical cancer screening: A review. Jama. (2023) 330:547–58. doi: 10.1001/jama.2023.13174
11. Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA: Cancer J Clin. (2020) 70:321–46. doi: 10.3322/caac.21628
12. Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. (2017) 8:CD008587. doi: 10.1002/14651858.CD008587.pub2
13. WHO. WHO guideline for screening and treatment of cervical pre−cancer lesions for cervical cancer prevention[EB/OL] (2021). Available online at: https://www.who.int/publications-detail-redirect/9789240030824 (Accessed April 17, 2025).
14. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J lower genital tract Dis. (2019) 24:102–31. doi: 10.1097/lgt.0000000000000525
15. Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecologic Oncol. (2015) 136:178–82. doi: 10.1016/j.ygyno.2014.12.022
16. Cook DA, Smith LW, Law J, Mei W, van Niekerk DJ, Ceballos K, et al. Aptima HPV Assay versus Hybrid Capture(®) 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clin virology: Off Publ Pan Am Soc Clin Virology. (2017) 87:23–9. doi: 10.1016/j.jcv.2016.12.004
17. Huijsmans CJ, Geurts-Giele WR, Leeijen C, Hazenberg HL, van Beek J, de Wild C, et al. HPV Prevalence in the Dutch cervical cancer screening population (DuSC study): HPV testing using automated HC2, cobas and Aptima workflows. BMC cancer. (2016) 16:922. doi: 10.1186/s12885-016-2961-2
18. Rao A, Sandri MT, Sideri M, Young S, Sharma A, and Behrens C. Comparison of hybrid capture 2 High-Risk HPV results in the low positive range with cobas® HPV Test results from the ATHENA study. J Clin virology: Off Publ Pan Am Soc Clin Virology. (2013) 58:161–7. doi: 10.1016/j.jcv.2013.06.041
19. Zhao G, Tian Y, Du Y, Sun J, Wang Z, Ma Y, et al. Comparison of CerviHPV and Hybrid Capture 2 HPV tests for detection of high-risk HPV infection in cervical swab specimens. Diagn cytopathology. (2019) 47:439–44. doi: 10.1002/dc.24134
20. Li M, Du X, Lu M, Zhang W, Sun Z, Li L, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med virology. (2019) 91:473–81. doi: 10.1002/jmv.25331
21. Wu C, Zhu X, Kang Y, Cao Y, Lu P, Zhou W, et al. Epidemiology of Humanpapilloma virus infection among women in Fujian, China. BMC Public Health. (2017) 18:95. doi: 10.1186/s12889-017-4651-7
22. Vargas-Hernández VM, Vargas-Aguilar VM, and Tovar-Rodríguez JM. Primary cervical cancer screening. Cirugia y cirujanos. (2015) 83:448–53. doi: 10.1016/j.circir.2014.09.001
23. Chatzistamatiou K, Moysiadis T, Angelis E, Kaufmann A, Skenderi A, Jansen-Duerr P, et al. Diagnostic accuracy of high-risk HPV DNA genotyping for primary cervical cancer screening and triage of HPV-positive women, compared to cytology: preliminary results of the PIPAVIR study. Arch gynecology obstetrics. (2017) 295:1247–57. doi: 10.1007/s00404-017-4324-x
24. Szarewski A, Mesher D, Cadman L, Austin J, Ashdown-Barr L, Ho L, et al. Comparison of seven tests for high-grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 study. J Clin Microbiol. (2012) 50:1867–73. doi: 10.1128/jcm.00181-12
25. Nakamura M, Nakade K, Orisaka S, Iwadare J, Mizumoto Y, and Fujiwara H. Comparison study of BD onclarity HPV with digene HC2 high-risk HPV DNA test and roche cobas 4800 HPV for detecting high-risk human papillomavirus in Japan. Am J Clin pathology. (2019) 151:263–9. doi: 10.1093/ajcp/aqy124
26. Chung HS, Hahm C, and Lee M. Comparison of the clinical performances of the AdvanSure HPV Screening Real-Time PCR, the Abbott Real-Time High-Risk HPV Test, and the Hybrid Capture High-Risk HPV DNA Test for Cervical Cancer Screening. J virological Methods. (2014) 205:57–60. doi: 10.1016/j.jviromet.2014.04.021
27. Burroni E, Sani C, Bisanzi S, and Ocello C. HPV primary test in the cervical cancer screening: reproducibility assessment and investigation on cytological outcome of Hybrid Capture 2 borderline samples. Epidemiologia e prevenzione. (2016) 40:164–70. doi: 10.19191/ep16.3-4.Ad03.077
Keywords: human papillomavirus, SureX ® HPV test, HC2 test, genotypes, cervical cancer screening
Citation: Sun H, Wang H, Liu Y, Xu H, Chen P, Sun X, Li M, Li P, Li K, Zheng L and Zhang S (2025) Comparison of the SureX® HPV genotyping test with the Digene Hybrid Capture® 2 test in cervical cancer screening. Front. Oncol. 15:1627935. doi: 10.3389/fonc.2025.1627935
Received: 13 May 2025; Accepted: 10 July 2025;
Published: 24 July 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2025 Sun, Wang, Liu, Xu, Chen, Sun, Li, Li, Li, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaokai Zhang, c2hhb2thaXpoYW5nQDEyNi5jb20=
 Hehao Sun
Hehao Sun Hong Wang
Hong Wang Yin Liu
Yin Liu Huifang Xu
Huifang Xu Peipei Chen
Peipei Chen Shaokai Zhang
Shaokai Zhang