- 1Cleveland Clinic, Cleveland, OH, United States
- 2Johnson & Johnson, Horsham, PA, United States
- 3Carenity, Paris, France
- 4Translational Genomics Research Institute, Phoenix, AZ, United States
Introduction: Multiple myeloma (MM) is a malignant plasma cell disorder characterized by the clonal expansion of abnormal plasma cells within the bone marrow. The management of relapsed/refractory multiple myeloma (RRMM) represents a significant challenge as the disease relapses or becomes refractory to previous treatments. Recent advances in therapy have expanded RRMM treatment options. This study aimed to gain a deeper understanding of patients' treatment preferences regarding available therapeutic options.
Methods: This study was designed as a non-interventional descriptive cross-sectional study based on an online discrete choice experiment (DCE) among adult RRMM patients living in the between USA November 2023 and March 2024. The survey included attributes and levels derived from an extensive literature review and guided interviews conducted with MM patients. Preference data were analyzed using a conditional logistic (CL) regression model and relative attribute importance (RAI) scores were calculated. Patients’ willingness to trade off overall response rate (ORR) was evaluated using the partworth utilities estimated from the CL model.
Results: 149 MM patients completed the survey; 66% had received 1–2 prior lines of therapy, 15% three prior lines, 19% four or more prior lines. Patients significantly preferred treatments with longer progression-free survival (PFS) and overall survival (OS) and higher ORR (RAI: 36.4% and 22.1%, respectively). With respect to adverse events assessed in this study, patients expressed concern for cytokine release syndrome (CRS) (RAI: 15.2%) and infections (RAI: 11.9%). In contrast, nail/skin disorders, duration of hospitalization, and taste disorder were less important to patients. Patients would be willing to accept a high risk of CRS (72% over no risk) to gain 29% increase in ORR.
Conclusions: Patients showed a clear preference for treatment efficacy (PFS/OS and ORR). This study confirmed patients’ valuation on treatment attributes in the new treatment landscape and highlighted the importance of shared treatment decision-making for optimal clinical outcomes.
1 Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder characterized by the clonal expansion of abnormal plasma cells within the bone marrow (1). As the second most common hematologic malignancy, MM accounts for approximately 10% of all blood cancers, with approximately 36,000 new cases diagnosed in 2024 in the United States alone (2, 3). MM predominantly affects older adults, with a median age of diagnosis around 70 years, and has a slightly higher incidence in males than females (1, 4). The incidence is also twice as high in those of African descent and is diagnosed at a young age in African American and Latino American patients. This hematologic disease manifests through various debilitating symptoms due to the accumulation of malignant plasma cells, impacting normal blood cell production, renal function and bone integrity, resulting in fatigue, extensive bony pain, infections and the need for dialysis in certain patients. Consequently, MM places a substantial cost and quality-of-life (QoL) burden on patients and the healthcare system.
MM is a progressive, incurable disease characterized by cycles of remission and relapse, especially in advanced stages, which are known as relapsed refractory multiple myeloma (RRMM). RRMM is marked by either the reappearance of disease symptoms after prior improvement or resistance to existing therapies, with disease progression observed during or within 60 days post-treatment (5). Patients frequently undergo multiple lines of therapy, with each subsequent regimen demonstrating reduced efficacy and shorter duration of remission (6). Patients who have been exposed to proteasome inhibitors (PIs), monoclonal antibodies (mAbs) targeting CD38, immunomodulatory agents (IMiDs, triple-class exposed [TCE]), have particularly poor outcomes and need new treatment options with different mechanisms of action (3). The recent introduction of chimeric antigen receptor T-cell (CAR-T) therapy has dramatically improved outcomes in myeloma with deeper and more durable remissions than prior therapies, but also with challenges in accessing this complex therapy (7).
Recently, bispecific antibody therapies have emerged as promising options for RRMM patients. The FDA has approved several of these therapies, including teclistamab, elranatamab and talquetamab, for TCE patients who have undergone at least four prior lines of therapy. Teclistamab and elranatamab, both targeting B-cell maturation antigen (BCMA) and CD3 receptors, have also shown promising efficacy, with teclistamab achieving an ORR of 65% (8) and elranatamab demonstrating an ORR of 61% (9). Talquetamab, an IgG4 antibody, targets G protein-coupled receptor family C group 5 member D (GPRC5D) and CD3 receptors and facilitates T-cell–mediated lysis of MM-specific cells, with a reported overall response rate (ORR) of 67-74% (10). These recent advances illustrate the growing range of options available for the treatment of RRMM but also highlight the complexity of decision making for patients affected by this disease.
In parallel with the approval of bispecific antibodies, additional T-cell–redirecting therapies are under development, including trispecific antibodies that may offer enhanced efficacy by targeting multiple tumor antigens and reduce the risk of immune escape. For example, early clinical results for JNJ-5322, a next-generation trispecific antibody that simultaneously targets BCMA and GPRC5D while engaging CD3, were presented at the 2025 European Hematology Association (EHA) Congress and demonstrated encouraging anti-myeloma activity in heavily pretreated patients (11).
While prior studies have examined RRMM treatment preferences, few have explored trade-offs specific to T-cell redirection therapies, particularly regarding side effects such as CRS, infections, and taste or skin symptoms. As novel agents with distinct benefit-risk profiles continue to emerge, understanding how patients prioritize efficacy, safety, and treatment convenience is essential to support informed, personalized treatment decisions. In this context, it is increasingly important for clinicians to understand patient preferences to select therapies that align with patient values, improve adherence and minimize the risk of premature discontinuation.
Discrete choice experiments (DCEs) are particularly useful for capturing patient priorities and trade-offs by presenting respondents with hypothetical scenarios that reflect realistic treatment attributes. DCEs, which are widely used in health economics and patient-centered research, provide insight into factors such as efficacy, side-effect profiles, and treatment administration that influence the decision-making process, ultimately supporting clinicians in offering personalized care (12, 13).
In this study, we applied DCE methodology to explore the factors influencing treatment preferences among RRMM patients who have received at least one prior line of therapy. We aim to highlight the specific considerations and trade-offs that shape patient decision making in light of recent therapeutic developments.
2 Materials and methods
2.1 Study design and data sources
This descriptive, cross-sectional, observational, non-interventional, online stated preference survey was conducted in the United States of America (USA) from November 2023 to March 2024.
Data were collected through the Carenity patient community platform, local partnerships and online social media campaigns. The Carenity platform is an online patient community, launched in 2011, where patients affected by chronic disease can share their experiences, find health-related information, and contribute to medical research by participating in online studies. Partnerships were developed with local organizations (patient organizations or market research agencies) who invited their own communities/members to participate in the survey.
2.2 Ethical considerations
This study was conducted in accordance with the study protocol, the Declaration of Helsinki, the International Society of Pharmacoepidemiology guidelines for Good Pharmacoepidemiology Practices, and the General Data Protection Regulation. The protocol and survey materials were submitted for ethical review, and written approval was obtained from the Institutional Review Board (WIRB-Copernicus) in the USA in November 2023. All patients completed the informed consent form before any patient data were collected.
2.3 Study overview
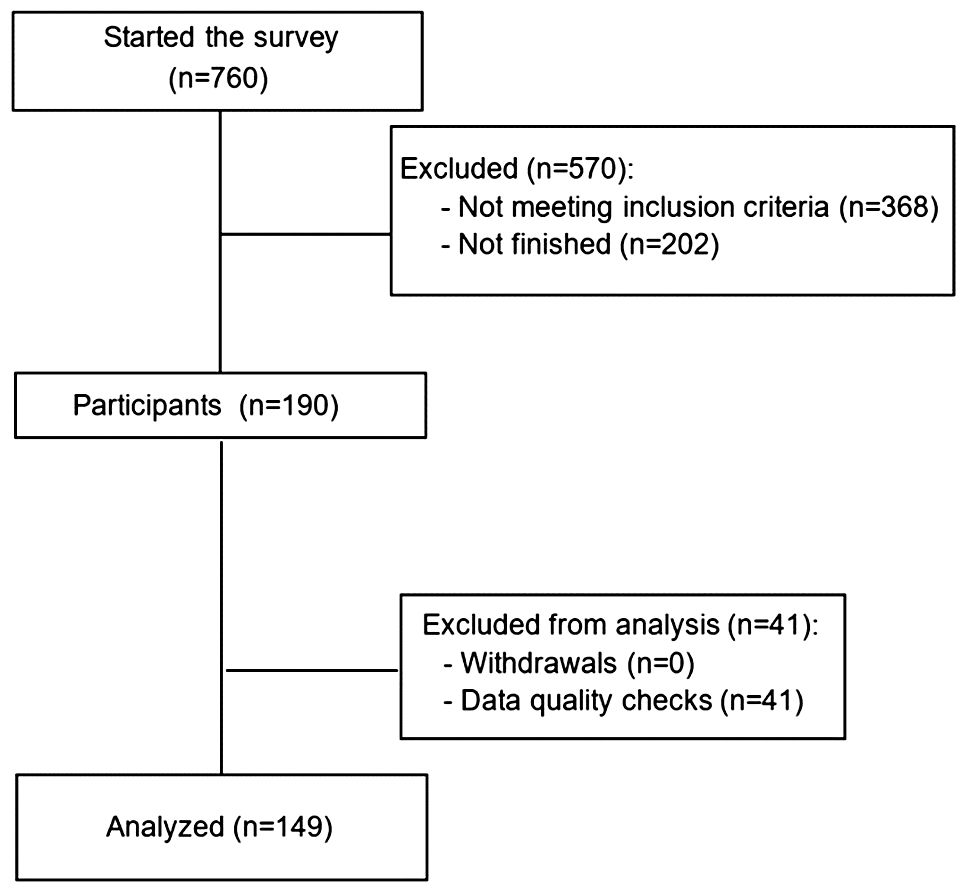
Eligible patients were consenting adults (at least 18 years of age), living in the USA with a self-reported diagnosis of RRMM who had received at least one prior line of treatment for MM. Respondents who did not meet these criteria or had incomplete data (i.e., those who had never started or completed the questionnaire) were excluded.
The questionnaire consisted of 32 questions (Appendix 1 in Supplementary Materials), divided into four parts: A) Screener, B) DCE to elicit patients’ preferences, C) Sociodemographic and medical profile, D) Impact of the disease on life and treatment burden.
2.4 Attributes and level development
A literature review was conducted with the objective of identifying published clinical evidence on product characteristics that were previously considered to be key in patients’ treatment decisions. Attributes related to treatment efficacy were identified, with two main categories of study endpoints: those measuring the efficacy in terms of time elapsed before the occurrence of a particular event (e.g., overall survival [OS], progression-free survival [PFS], time to next treatment [TTNT], event-free survival [EFS], duration of response [DoR]) and endpoints relating to response to treatment, including the overall response rate (ORR), very good partial response (VGPR) rate and partial response (PR) rate. In the context of DCE, the efficacy attributes most commonly reported in the literature were OS, PFS and ORR. These were also among the most readily comprehensible to patients.
A substantial number of adverse events (AEs) associated with RRMM treatments have been reported in the literature. Consequently, a number of attributes related to the safety profile of the treatments were identified. The most commonly reported AEs were pain, neuropathy, infections, digestive disorders, anemia, cytokine release syndrome (CRS), vision disturbances and skin disorders. Given the increasing clinical use of bispecific therapies in RRMM patients, attributes such as CRS, infection and GPRC5D-related symptoms were prioritized to better assess patient preferences regarding their distinct safety profiles.
A further crucial aspect of a treatment is its mode of administration. The literature review identified several characteristics associated with treatment, including its frequency, its method of administration, localization of administration and necessity for monitoring.
The selection of levels was based on the clinical profiles of three bispecific antibody therapies approved in the US, namely talquetamab (14), elranatamab (15) and teclistamab (16), as well as conventional treatments based on a real-world study (17–21).
A qualitative study was conducted to ensure that the attributes were comprehensive and relevant. Six MM patients were interviewed to explore their perspectives on the most important factors when selecting a treatment for MM, as well as their expectations of MM treatments.
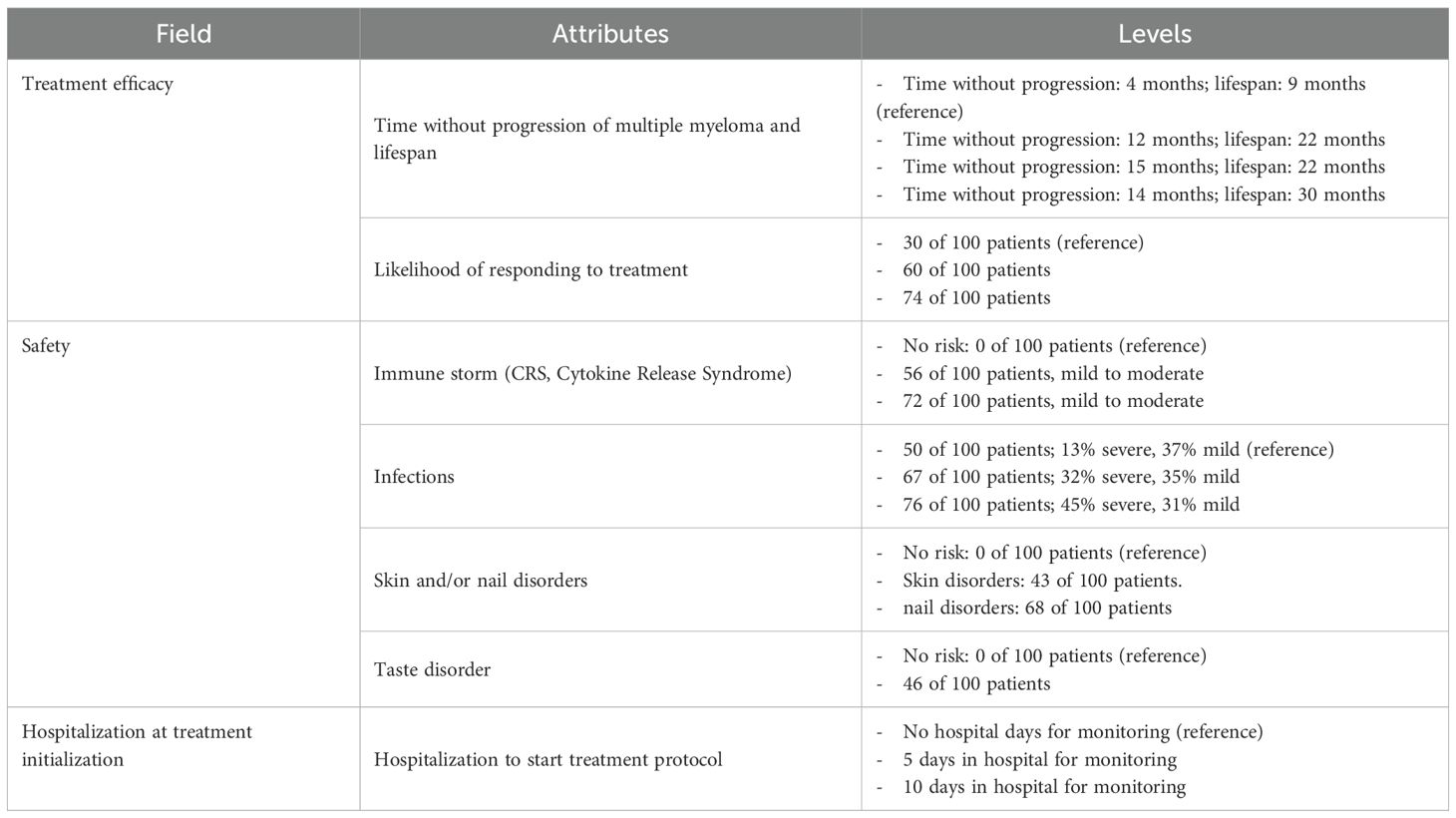
The final DCE design consisted of seven attributes, with the levels corresponding to each attribute described in Table 1.
2.5 DCE design
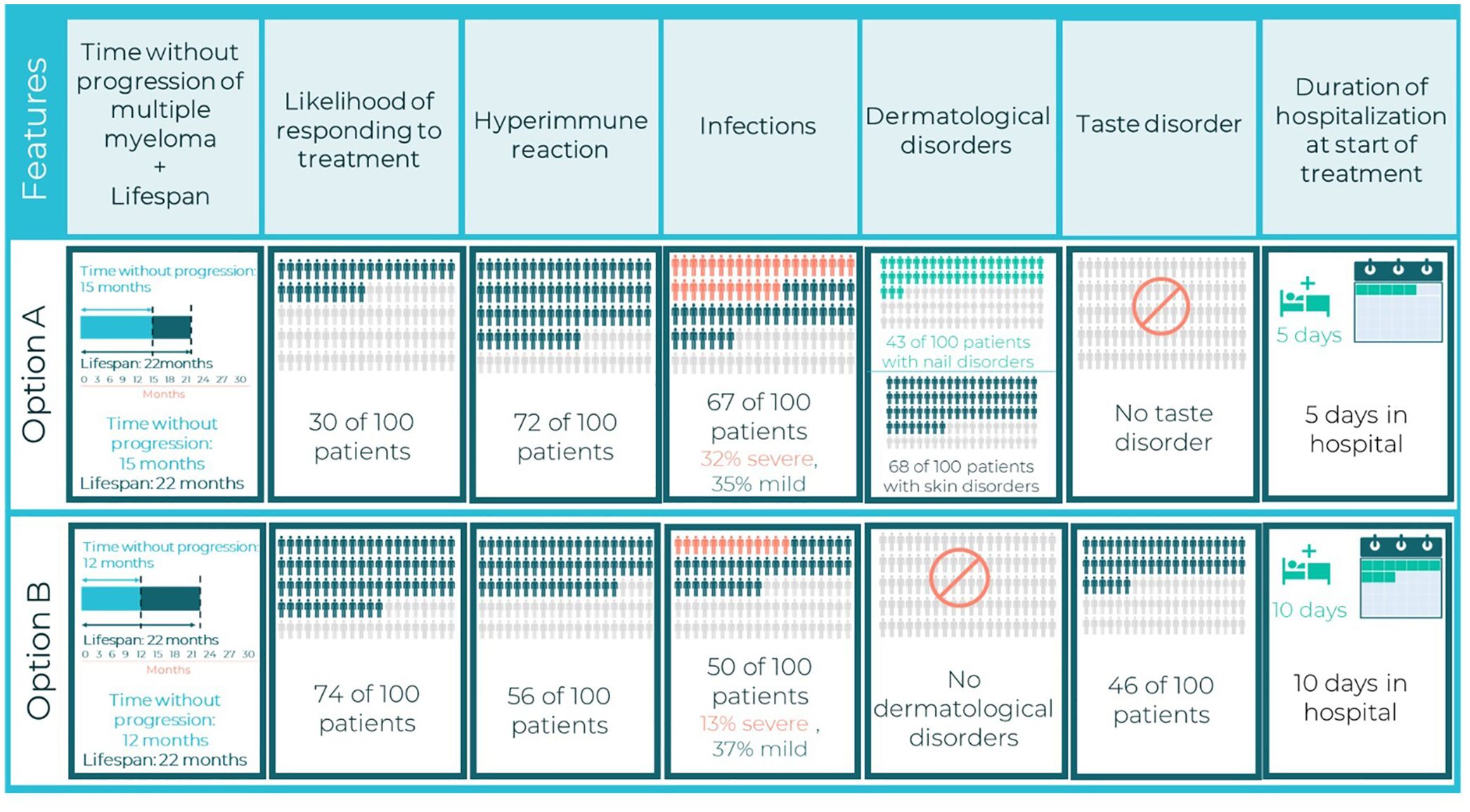
The combinations of attribute levels displayed for each hypothetical treatment option, within each choice task in a DCE, were generated with a D-efficient experimental design to ensure that the choice tasks collected the maximum amount of information about the trade-offs between the attributes (12, 22, 23). The experimental design was generated using the AlgDesign library of the R software (version 1.2.1) (24). DCE design comprised 24 choice tasks, which were grouped into three blocks of eight tasks each. To minimize the cognitive burden of the DCE survey, patients were randomized to one of the three blocks. Across the choice tasks, patients were repeatedly asked to choose between two mutually exclusive hypothetical treatment alternatives (Treatment A or Treatment B) with different levels of benefits/risks and modes of administration (Figure 1). In addition to these eight experimental choice tasks, patients also completed one internal validity choice task. One choice task was repeated to assess whether patients were consistent in their choices (whether patients chose the same option as they had selected previously).
2.6 Statistical analysis
Based on the number of attributes, choice sets and alternatives, a sample size of 125 patients was deemed sufficient for DCE (25).
Descriptive statistics were used to examine the sociodemographic characteristics and all variables that were not directly related to the DCE methodology. Categorical variables were reported descriptively using frequencies and percentages, while continuous variables were presented as mean ± SD, median, lower and upper quartile, minimum and maximum values.
A conditional logistic (CL) regression model was used to analyze the patients’ treatment preferences. This model estimates the patients’ sensitivities to changes in the treatment attributes, also referred to as parthworth utilities, relative to a reference level. The estimated parthworth utilities were then used to calculate scores of relative attribute importance (RAI). RAI scores are conditional on the range of attribute levels, with a sum of 100%. They serve to illustrate the contribution of each attribute to treatment preferences. No covariates were incorporated into the initial CL model as there are no a priori variables that are known to influence the DCE results. The CL model was constructed to include all the treatment attributes described previously and presented in the DCE choice cards. These treatment attributes were coded as categorical variables. The reference levels were defined as the least favorable treatment characteristics (Table 1). To identify the preferred levels within a specific treatment attribute, comparisons were made between the CL coefficient (i.e., partworth utility) of the level of interest and the reference level’s partworth utility, which was constrained at 0 (26).
Based on the partworth utilities evaluated in the main CL model, the patients’ willingness to trade off for ORR and the overall utilities of each treatment option were calculated using the random utility model (27).
To understand potential differences in patient preferences, subgroup analyses were conducted by key variables of interest including number of prior lines of treatment, age, gender, disease duration, living area (urban, suburban, rural), income, activity level, and treatment history.
All tests were bidirectional and a p-value <0.05 was considered statistically significant. Data management and statistical analyses were conducted using R software version R 4.0.5.
3 Results
3.1 Patient demographics and clinical characteristics at study inclusion
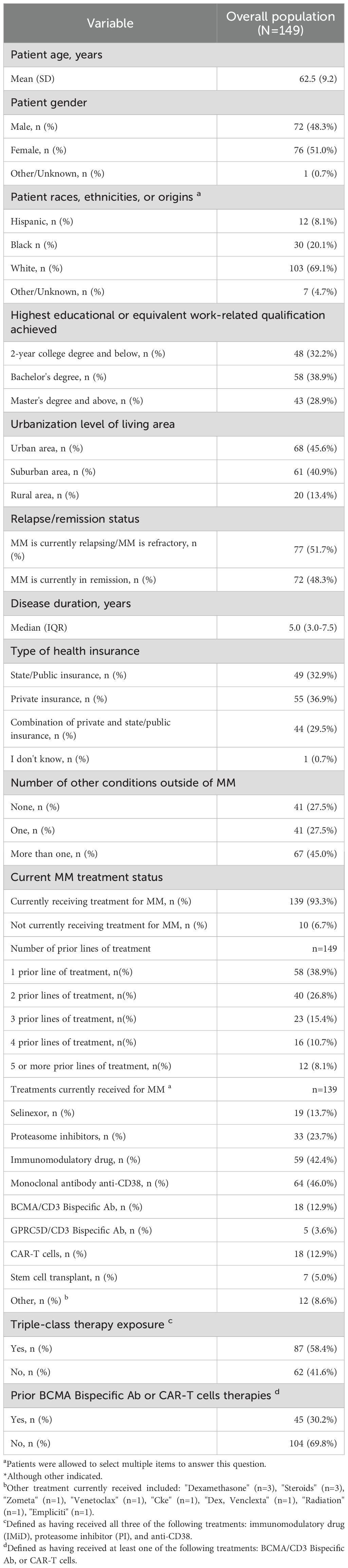
Overall, 149 patients with MM met the inclusion criteria and completed the survey (Figure 2). Patient sociodemographic and clinical characteristics are presented in Table 2. The mean age of patients was 63 years and 51% were women. Among them, 69% were White, 68% had a bachelor’s degree or higher and 46% lived in an urban area. The median disease duration was five years and 18.8% of patients had at least 4 prior lines of treatments. Most patients (93.3%) were receiving treatment for MM at the time of the survey, the most frequently received therapeutic agents being monoclonal antibodies (46.0%). A total of 12.9% of patients were undergoing CAR-T therapies, 12.9% BCMA-targeted bispecific antibody and 3.6% GPRC5D-targeted bispecific antibody. Overall, 58.4% of patients were exposed to triple-class therapy, and 30.2% had prior bispecific or CAR-T therapy.
3.2 Patient preference for treatment attributes
The consistency rate for the holdout task was 76.5%, indicating a good level of quality of responses (25, 28).
Based on the CL model results, patients ranked the importance of the different attributes for their treatment choice (Figure 3). Patients showed a clear preference for attributes related to treatment efficacy, with the combination of PFS and OS ranking first (RAI: 36.4%), followed by ORR (RAI, 22.1%). The two efficacy attributes accounted for more than half of the decision making. With the exception of taste disorders (RAI: 0%), which were considered the least important factor, attributes representing adverse events were ranked third (RAI of CRS, 15.2%), fourth (RAI of infections, 11.9%) and fifth (RAI of nail/skin disorders, 7.9%) in patients' treatment decision-making processes. The length of hospitalization at the start of treatment, which was ranked sixth in terms of importance, was not a significant factor influencing patient preferences (RAI, 6.5%).
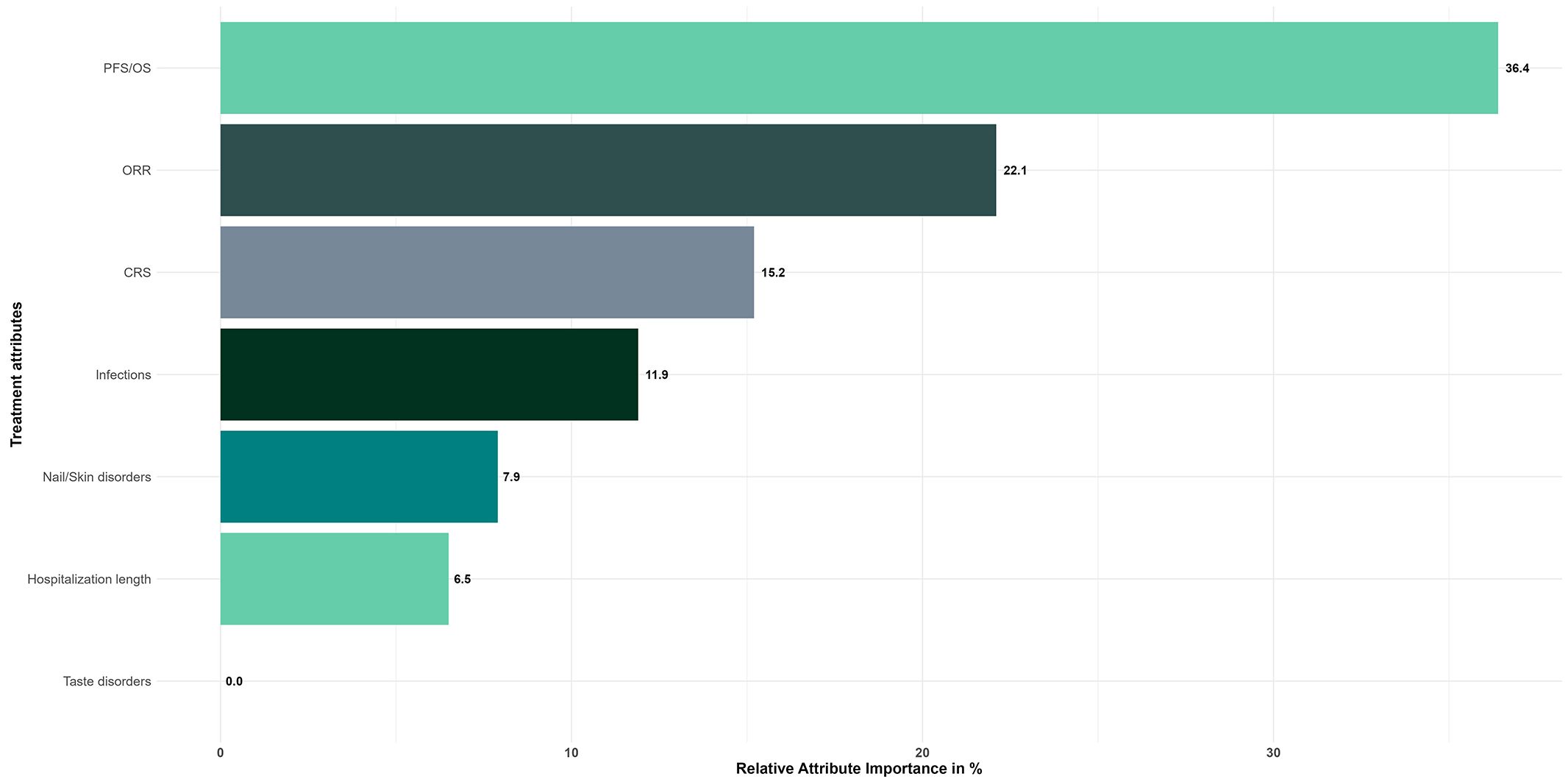
Figure 3. Relative attribute importance scores for treatment attributes - Overall study population (N=149). Reference levels of each attribute were defined as follows: PFS/OS, 4/9 months; ORR, 30%; CRS, 0%; Infections, 50%; Nail/Skin disorders, 0%; Taste disorders, 0%; Hospitalization length, 0 days.
The probability of selecting a treatment was found to increase significantly (p<0.001) by 109.7% when the PFS and OS were observed to rise from the reference level (4/9 months) to 15 and 22 months, respectively (Figure 4). The probability also increased by 66.7% when the ORR rose from the reference level (30%) to 74% (p<0.001). Conversely, the probability of selecting a treatment decreased significantly (p<0.001) by 45.9% when the risk of CRS increased from the reference level (0%) to 72%. It also decreased by 35.8% when the risk of infections increased from the reference level (50%) to 76% (p=0.009), and by 23.9% when the risk of nail and skin disorders increased from the reference level (0%) to 43% and 58% respectively (p=0.004). The probability of choosing a treatment was not significantly reduced when the risk of taste disorders increased, nor when the length of hospital stay was lengthened (p-values>5%).
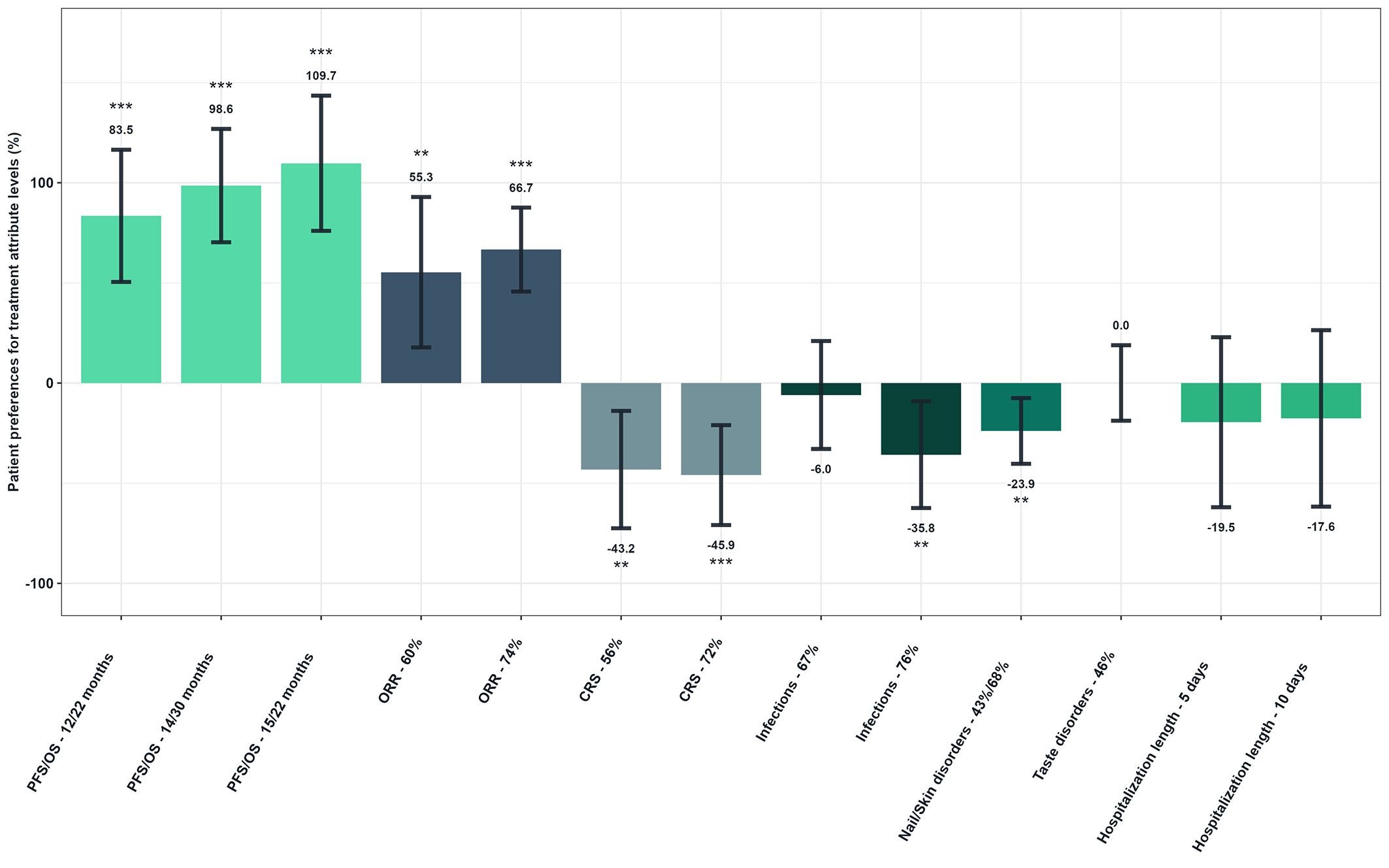
Figure 4. Patient preferences for treatment attribute levels - Overall study population (N=149). Reference levels of each attribute were defined as follows: PFS/OS, 4/9 months; ORR, 30; CRS, 0%; Infections, 50%; Nail/Skin disorders, 0%; Taste disorders, 0%; Hospitalization length, 0 days. As examples to illustrate the interpretation of the results, the probability of choosing a treatment was increased by 83.5% when PFS/OS increased from reference level to 12/22 months. Conversely, it decreased by 43.2% when the risk of CRS was increasing from reference level to 56%.
Figure 5 shows patients’ willingness to trade off for ORR (Figure 5). Patients would be willing to accept a high risk of CRS (72% over no risk) if the hypothetical treatment provided a 28.9% increase in ORR. Similarly, patients would tolerate a 76% risk of infections (over 50%) in exchange for an additional 25.8% ORR. Patients would also be willing to accept a 43% and 68% risk of nail and skin disorders to gain 17.4% of ORR.
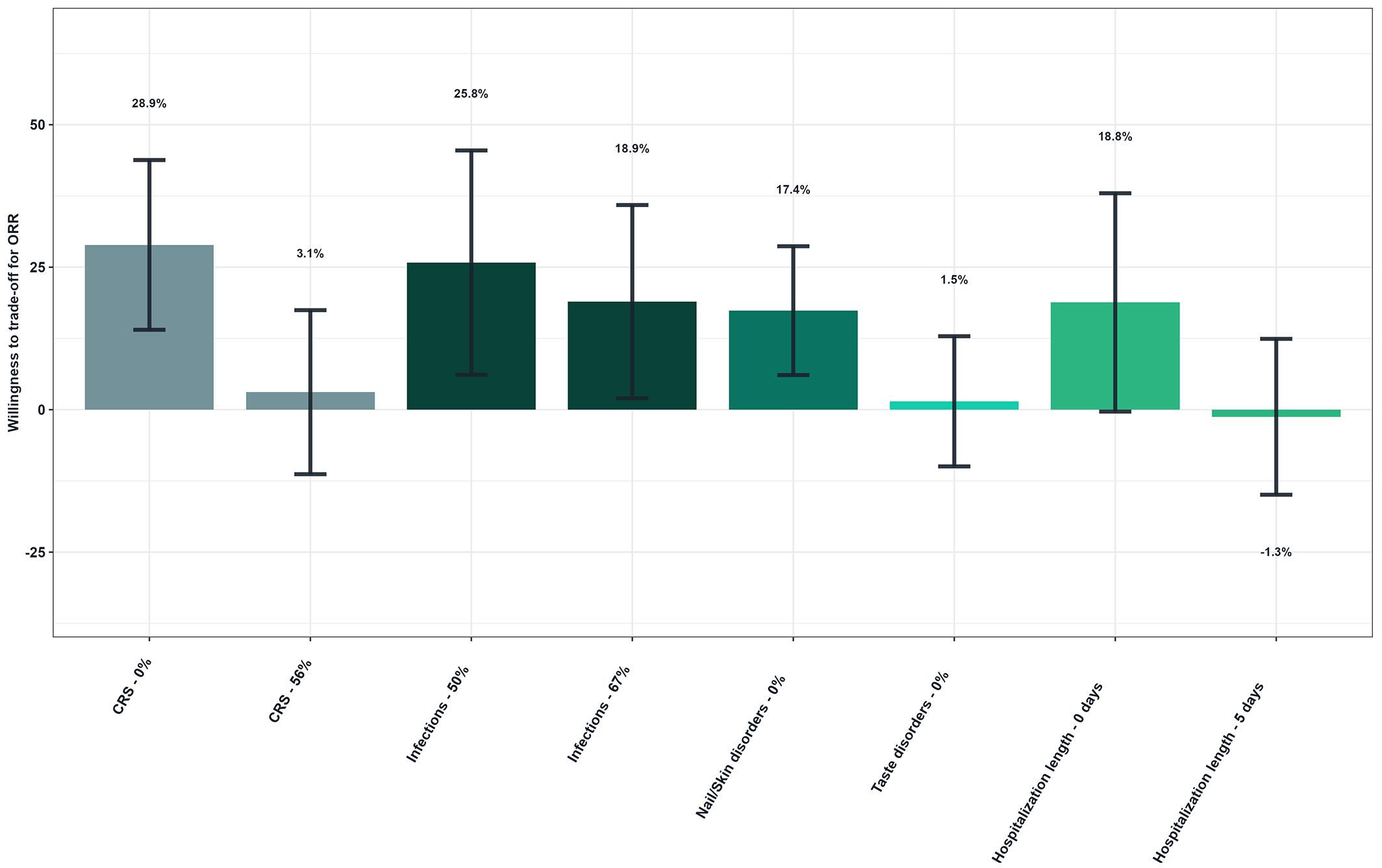
Figure 5. Patients’ willingness to trade off for overall response rate - Overall study population (N=149). Reference levels of each attribute were defined as follows: CRS, 72%; Infections, 76%; Nail/Skin disorders, 43%/68%; Taste disorders, 46%; Hospitalization length, 10 days. As an illustrative example, patients would be willing to accept a higher risk of CRS (72% over no risk) if the hypothetical treatment provided a 28.9% increase in ORR.
In all subgroup analyses, efficacy attributes were always preferred (Appendix 2 in the Supplementary Materials). While no significant differences in patient preferences were observed between earlier and later-line treatment settings, PFS and OS combination was consistently ranked as the top priority. Patients receiving later-line treatments ranked CRS risk as their second priority, whereas those in earlier-line settings prioritized treatments with the highest ORR. Additionally, patients previously exposed to BCMA bispecific antibodies or CAR-T therapies tended to place greater emphasis on ORR, whereas non-exposed patients prioritized PFS/OS. However, the interpretation of these findings is limited by the reduced sample sizes in subgroup analyses, which may preclude the identification of statistically significant differences.
3.3 Impact of the disease on life and treatment burden
In terms of the impact of the disease on life and treatment burden, most patients were unable to engage in strenuous activity but were nevertheless capable of undertaking light work (42.3%). A total of 38.9% were ambulatory and capable of self-care, yet unable to carry out any work. Only 12.8% were fully active without restriction. The primary factors influencing the selection of a treatment were the potential long-term impact on health and well-being (58.4%), the recommendations from HCPs (53.0%), and the ability to maintain usual daily activities during treatment (31.5%). The most bothersome consequence of changing MM treatment, as ranked by 28.2% of patients, was the risk of severe side effects, while 11.4% of patients identified adapting to a new form of treatment as their primary concern. Regarding treatment cost, 50% of patients reported that arranging insurance coverage for a new treatment was or would be a bothersome consequence of changing their MM therapy, with 10% identifying it as the most bothersome. Additionally, 24% of patients indicated that insurance coverage or financial considerations influenced their choice of oncologist for MM management. A total of 24.8% of patients indicated that they were not bothered by treatment changes.
4 Discussion
As novel therapeutic modalities enhance the prognosis of MM, evaluating their efficacy and safety profiles becomes crucial, particularly given new benefit/risk profile with the emerging therapies. A recent network meta-analysis comparing 34 treatment options for RRMM has demonstrated the complexity of treatment decisions due to the potential toxicities associated with these therapies (29). In addition, another multinational study involving patients with MM revealed concerns about severe side effects, including permanent organ damage, bone fractures, and neuropathic complications. This further emphasizes the importance of considering the balance between toxicity and efficacy when making treatment decisions (30). The significant side effects commonly associated with RRMM treatments, including neuropathy, infections, digestive problems, anemia, CRS, and vision problems, underscore the need for comprehensive patient-provider discussions regarding treatment options. This enables the provision of comprehensive information to patients regarding both severe and milder adverse events that may impact their daily lives and independence.
In 2021, the International Myeloma Working Group (IMWG) updated their treatment guidelines for RRMM, recommending a personalized approach based on patient’s history and treatment responses (31). The intention of these guidelines is to assist healthcare providers in making complex treatment choices. The objective of these recommendations is to facilitate the process of shared decision-making process for both HCPs and patients who are confronted with complex choices. This involves providing patients with comprehensive information about the latest therapeutic options, thereby facilitating their ability to make well-informed decisions regarding their care. Consequently, patients can evaluate the benefits of novel therapies in comparison to the potential adverse effects and other pertinent factors, considering their individual preferences and overall QoL. Therefore, understanding patient preferences is crucial for the optimal treatment decision for RRMM. In parallel, the IMWG emphasizes that healthcare providers consider not only clinical efficacy and safety but also patient-specific factors such as frailty, comorbidities, and treatment goals, highlighting the need for alignment between medical judgment and patient values in shared decision-making.
This study used a DCE to assess the preferences of 149 patients with RRMM to gain insights into the influence of various treatment attributes on their decision-making processes. The results demonstrated that patients assigned the greatest importance to efficacy attributes, particularly PFS and OS, which were rated as the most important (RAI, 36.4%), followed by ORR. Patients were willing to tolerate a higher risk of adverse events such as cytokine release syndrome (CRS) and infections to gain in exchange for an increase in ORR. Notably, the model estimated that patients would accept up to a 72% risk of CRS for a 29% absolute increase in ORR. While this finding reflects the strong preference for efficacy observed across the sample, it should be interpreted with caution. These estimates are derived from a hypothetical, controlled choice experiment and may not fully capture the complexities of real-world decision-making, which occurs under clinical uncertainty and physician guidance. In practice, patients’ actual choices are influenced by factors such as physician recommendations, emotional responses, trust, health literacy, and the way risks and benefits are communicated. Furthermore, although the CRS and ORR levels used in the DCE were grounded in clinical trial data to reflect plausible ranges seen with emerging therapies, individual tolerance for side effects in a real clinical context may be more conservative. Therefore, while these trade-off values provide useful directional insights into patient preferences, they are not intended to predict exact behavior in clinical settings. This perspective also helps contextualize why patients in this study placed relatively less weight on milder, less life-threatening complications, such as taste, skin, and nail disorders, which had a relatively low impact on patient choices, compared to survival outcomes and severe side effects.
Prior studies using DCEs in the context of MM have consistently demonstrated that patients prioritize treatment efficacy while also considering the route of administration, toxicity, survival, remission period, and costs (32, 33). For example, a DCE was conducted in 2022, involving 296 RRMM patients across the USA, United Kingdom (UK), and several European countries. The findings indicated that the most influential attributes in treatment decision-making were a 25 to 85% increase in ORR and a six-month to two-year OS increase, together accounting for approximately 50% of the decision weight. This study revealed that although patients place a high value on treatment efficacy, many are willing to accept side effects such as neuropathy, fatigue, or cognitive impairment in exchange for improved survival rates (32). Similarly, another study reported that patients placed a high valued on increased life expectancy and time to relapse, with pain and fatigue being identified as significant considerations (34). A notable finding is that patients' current health state exerts a greater influence on treatment preferences than their disease status, suggesting that individual health conditions play a pivotal role in decision-making processes. In addition, the findings of Fifer et al.'s research suggested that patients attribute considerable importance to treatment efficacy, particularly in terms of OS, while also taking into account factors such as mode of administration and side effects (35). The findings indicated the necessity of incorporating patient preferences into treatment decision-making for MM and reflects the recurring concern reported by patients about extending survival, despite the potential negative impact of certain adverse effects. Patients demonstrated a propensity to make trade-offs between efficacy, adverse effects, and administration procedures in pursuit of enhanced health outcomes (32).
Although this study primarily evaluated attributes relevant to bispecific therapies, the findings also align with patient preferences observed in studies of CAR T-cell therapy (36, 37), further underscoring the importance of efficacy and the willingness of patients to tolerate certain risks for improved survival outcomes.
This is the first DCE study that included GPRC5D-related treatment attributes. We found that GRPC5D related symptoms such as taste, skin, and nail AEs had relatively low importance in patients’ preference. The lower prioritization of taste, nail, and skin disorders in our study suggests that these issues are either less familiar or less significant to RRMM patients, or they may not be perceived as major barriers to treatment adherence, despite their potential impact on QoL. One plausible explanation is that very few patients in our sample had direct experience with these symptoms —due to the novelty of the GPRC5D target—leading to reduced salience in their preference formation. Indeed, unfamiliarity with these AEs may have limited patients’ ability to fully assess their potential burden, thereby affecting the weight given to these attributes in the DCE. To overcome patients’ unfamiliarity, educational context was provided during the survey, and detailed attribute definitions were given to participants (Appendix 1, Section B in Supplementary Materials). Nevertheless, this pattern may also reflect a broader prioritization of survival and treatment efficacy over QoL-related concerns, particularly in the context of advanced disease. This trend is consistent with findings from other studies, such as that published by Thomas et al., which showed that patients with RRMM rather prioritize treatment efficacy and survival outcomes over potential side effects, even when the latter ones may significantly impact their QoL (32).
Moreover, the relatively low awareness of these milder adverse effects may be attributed to a predominant focus on more life-threatening complications, such as CRS and infections, in clinical discussions. Fifer et al. pointed out the tendency for severe adverse events to dominate patient-provider conversations, thereby overshadowing discussions about milder adverse events such as taste changes and skin reactions (35). This highlights the necessity for improvements in the fields of patient education and shared decision making. Although severe complications naturally capture more attention, healthcare providers should also address the importance of less severe adverse events to ensure that patients are fully informed about all potential treatment-related effects.
This study has several limitations. First, the reliance on self-reported data for diagnostic purposes, the assessment of disease severity, and the characterization of clinical features introduce the potential for recall bias or inaccuracies in reporting, as these details were not verified in medical records or by physicians. This lack of objective corroboration may impact the reliability of the findings on patient characteristics. Second, the sampling method may introduce a selection bias. Participants were sourced from the Carenity platform and local partners, which may limit the generalizability of the results. Patients with more advanced disease or severe symptoms may be underrepresented, given that they are often less likely to engage with online surveys. Third, the sample skewed toward highly educated, White patients, which further limits the generalizability of the findings—particularly in the context of MM, a disease that disproportionately affects individuals of African descent. This underrepresentation of racially and ethnically diverse populations, along with the overrepresentation of highly educated respondents, may affect the applicability of the stated preferences observed in this study. These sampling imbalances highlight the need for broader and more inclusive recruitment strategies (e.g., in-clinic or telephone-based approaches) that ensure adequate representation of underserved groups in future preference research.
Additionally, this study focused primarily on attributes associated with bispecific therapies, which may limit generalizability to other treatment modalities in RRMM. The exclusion of cost-related attributes, which frequently influence patient decision-making in real-world settings, suggests that future studies should consider integrating these factors. Furthermore, while this study assessed patient preferences regarding adverse events such as taste, skin, and nail disorders, only 16% of the surveyed patients had received a bispecific antibody, of which only five patients had been treated with talquetamab. Consequently, most respondents may not have had direct experience with these side effects, potentially impacting the accuracy of their risk perceptions and preferences. However, this proportion is consistent with real-world treatment patterns: approximately 45% of patients with ≥4 prior lines of therapy received a BCMA bispecific antibody in 2023 (38) and in our sample, 30% of heavily pretreated patients had received bispecifics. Given the challenges of reaching late-line patients in online surveys, this reflects a reasonably representative distribution within the current clinical landscape.
Additionally, the study is subject to the inherent methodological limitations common to DCEs. Despite an accuracy of 76.5% in this study, it is possible that stated preferences may differ from actual treatment decisions in a real-life context. Desjeux et al. suggest that answers of patients to choice tasks may potentially be different from what they would actually choose if faced with the alternative in real life (39). Moreover, the authors highlight a learning effect of the patients who possibly tend to set their choice according to the first profiles or attributes which are proposed to them. This bias is mitigated by the fact that not all respondents will see the same sequence of attributes. Cognitive fatigue resulting from repeated choice tasks may also impact on the accuracy of responses, thereby complicating the interpretation of results. It is notable that the DCE did not account for other potentially influential factors, such as out-of-pocket costs, deductibles, or the presence of comorbidities. The exclusion of these elements restricts the scope of the findings and suggests that future studies should integrate these factors to provide a more comprehensive understanding of patient preferences.
Finally, emerging molecular insights in RRMM—such as alterations in the MAPK signaling pathway, including BRAF mutations and dysregulation of the Capicua transcriptional repressor—are increasingly relevant to disease progression, drug resistance, and extramedullary disease (40). As precision therapies targeting these molecular features gain clinical traction, patient preferences may evolve accordingly. Incorporating biomarker-driven attributes into future DCEs may be critical for accurately capturing preferences in the context of personalized medicine. Doing so would help align patient-centered care with advances in genomic oncology.
Despite these limitations, the study's key strengths include the rigorous application of DCE methodology, which effectively captures patient preferences by mimicking real-world trade-offs. Additionally, the sample size exceeded the minimum recommended for this type of analysis, enhancing the reliability of the results.
In view of these findings, it is of utmost importance to gain a deeper understanding of patient preferences in the management of RRMM. It would be beneficial for future studies to aim for the incorporation of a more diverse patient population and explore the impact of a broader range of factors influencing treatment decisions. By enhancing our understanding of patient preferences and incorporating additional variables, research can facilitate the development of more personalized treatment strategies that align with the needs and values of patients and optimize treatment decisions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving humans was approved by Institutional Review Board: WIRB-Copernicus. The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BF: Conceptualization, Validation, Visualization, Writing – review & editing. HL: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. JL: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. SP: Conceptualization, Validation, Writing – review & editing. AP-S: Conceptualization, Validation, Writing – review & editing. XZ: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. JM: Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by Johnson & Johnson.
Acknowledgments
The authors are extremely thankful to the patients participating in the survey, for sharing their experiences and valuable contributions. We also thank Sahar Haidar, an ELSE CARE employee and Florence Boulmé on behalf of ELSE CARE for the medical writing assistance.
Conflict of interest
BF is a consultant for Janssen, GSK, and Sanofi. HL is an employee of Johnson & Johnson and holds stock options. JL reports current employment with Carenity. SP is a current employee of Johnson & Johnson and holds stock options. AP-S is a current employee of Johnson & Johnson and may hold stock options. XZ is a current employee of Johnson & Johnson and holds stock options. JM reports consultancy with Amgen, BMS, Janssen, Menarini and Sanofi.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1628121/full#supplementary-material
References
1. Albagoush SA, Shumway C, and Azevedo AM. Multiple myeloma. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2024).
2. Cancer stat facts: myeloma - surveillance, epidemiology, and end results program. Available online at: https://seer.cancer.gov/statfacts/html/mulmy.html (Accessed March 9, 2025).
3. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematology. (2020) 95:548–67. doi: 10.1002/ajh.25791
4. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
5. Rajkumar SV, Harousseau J-L, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. (2011) 117:4691–5. doi: 10.1182/blood-2010-10-299487
6. Sonneveld P. Management of multiple myeloma in the relapsed/refractory patient. Hematol Am Soc Hematol Educ Program. (2017) 2017:508–17. doi: 10.1182/asheducation-2017.1.508
7. Swan D, Madduri D, and Hocking J. CAR-T cell therapy in Multiple Myeloma: current status and future challenges. Blood Cancer J. (2024) 14:206. doi: 10.1038/s41408-024-01191-8
8. Usmani SZ, Garfall AL, van de Donk NWCJ, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. (2021) 398:665–74. doi: 10.1016/S0140-6736(21)01338-6
9. Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. (2023) 29:2259–67. doi: 10.1038/s41591-023-02528-9
10. Rasche L, Schinke C, Touzeau C, Minnema MC, van de Donk NWCJ, Rodríguez-Otero P, et al. Long-term efficacy and safety results from the phase 1/2 MonumenTAL-1 study of talquetamab, a GPRC5D×CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Eur Hematol Assoc (EHA). (2024). doi: 10.1016/S2152-2650(24)00976-5
11. van de Donk NWCJ, Vega G, Perrot A, Anguille S, Auriol A, Minnema M, et al. First-in-human study of JNJ-79635322 (JNJ-5322), a novel, next-generation trispecific antibody (TsAb), in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Initial phase 1 results. JCO. (2025) 43(16):7505. doi: 10.1200/JCO.2025.43.16_suppl.7505
12. de Bekker-Grob EW, Ryan M, and Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. (2012) 21:145–72. doi: 10.1002/hec.1697
13. Ryan M and Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy. (2003) 2:55–64.
14. Chari A, Touzeau C, Schinke C, Minnema MC, Berdeja J, Oriol A, et al. 157 Talquetamab, a G protein-coupled receptor family C group 5 member D ×CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma: phase 1/2 results from MonumenTAL-1. American Society of Hematology Annual Meeting, Abstract 157. Blood. (2022) 140:384–7. doi: 10.1182/blood-2022-159707
15. Bahlis NJ, Tomasson MH, Mohty M, Niesvizky R, Nooka AK, Manier S, et al. Efficacy and safety of elranatamabin patients with relapsed/refractory multiple myeloma naïve to B-cell maturation antigen (BCMA)-directed therapies: results from cohort A of the magnetismm-3 study. Blood. (2022) 140:391–3. doi: 10.1182/blood-2022-162440
16. Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. (2022) 387:495–505. doi: 10.1056/NEJMoa2203478
17. Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. (2019) 33:2266–75. doi: 10.1038/s41375-019-0435-7
18. Hájek R, Minařík J, Straub J, Pour L, Jungova A, Berdeja JG, et al. Ixazomib-lenalidomide-dexamethasone in routine clinical practice: effectiveness in relapsed/refractory multiple myeloma. Future Oncol. (2021) 17:2499–512. doi: 10.2217/fon-2020-1225
19. Mateos MV, Weisel K, De Stefano V, Goldschmidt H, Delforge M, Mohty M, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. (2022) 36:1371–6. doi: 10.1038/s41375-022-01531-2
20. Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:781–94. doi: 10.1016/S1470-2045(19)30152-4
21. Rubinstein SM and Derman BA. Infection rates are high across the multiple myeloma continuum, not just with bispecific antibodies. Eur J Cancer. (2023) 189:112926. doi: 10.1016/j.ejca.2023.05.014
22. Lancsar E and Louviere J. Conducting discrete choice experiments to inform healthcare decision making. PharmacoEconomics. (2008) 26:661–77. doi: 10.2165/00019053-200826080-00004
23. Ryan M. Using conjoint analysis to elicit preferences for health care. BMJ. (2000) 320:1530–3. doi: 10.1136/bmj.320.7248.1530
24. Wheeler RE. The R project for statistical computing: optFederov. Vienna, Austria: AlgDesign (2004). Available online at: https://www.r-project.org/ (Accessed September 8, 2023).
25. Johnson RM and Orme BK. Getting the most from CBC. Sawtooth Software Research Paper Series. Sequim, WA: Sawtooth Software (2003).
26. Orme BK. Getting started with conjoint analysis: strategies for product design and pricing research. Madison: Research Publishers LLC (2006).
27. McFadden D. Conditional logit analysis of qualitative choice behavior. In: Frontiers in econometrics New York: Academic Press (1973). p. 105–42.
28. Orme BK. Getting started with conjoint analysis: Strategies for product design and pricing research. Madison, WI: Research Publishers LLC (2006).
29. Minakata D, Fujiwara S-I, Yokoyama D, Noguchi A, Aoe S, Oyama T, et al. Relapsed and refractory multiple myeloma: A systematic review and network meta-analysis of the efficacy of novel therapies. Br J Haematol. (2023) 200:694–703. doi: 10.1111/bjh.18654
30. Janssens R, Lang T, Vallejo A, Galinsky J, Plate A, Morgan K, et al. Patient preferences for multiple myeloma treatments: A multinational qualitative study. Front Med. (2021) 8. doi: 10.3389/fmed.2021.686165
31. Moreau P, Kumar SK, San Miguel J, Davies F, Zamagni E, Bahlis N, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. (2021) 22:e105–e18. doi: 10.1016/S1470-2045(20)30756-7
32. Thomas C, Ailawadhi S, Popat R, Kleinman D, Ross MM, Gorsh B, et al. Treatment preferences of patients with relapsed or refractory multiple myeloma in the United States, United Kingdom, Italy, Germany, France, and Spain: results from a discrete choice experiment. Front Med. (2023) 10. doi: 10.3389/fmed.2023.1271657
33. Leleu X, Mateos M-V, Delforge M, Lewis P, Schindler T, Gibson C, et al. Assessment of multiple myeloma patient preferences on treatment choices: an international discrete choice study. Blood. (2015) 126:2086. doi: 10.1182/blood.V126.23.2086.2086
34. Tervonen T, Duenas A, Collacott H, Lam A, Gries KS, Carson R, et al. Current health state affected patient preferences more than disease status: A discrete choice experiment in multiple myeloma. Value Health. (2023) 26:909–17. doi: 10.1016/j.jval.2023.01.016
35. Fifer SJ, Ho K-A, Lybrand S, Axford LJ, and Roach S. Alignment of preferences in the treatment of multiple myeloma – a discrete choice experiment of patient, carer, physician, and nurse preferences. BMC Cancer. (2020) 20:546. doi: 10.1186/s12885-020-07018-6
36. Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. (2016) 128:1688–700. doi: 10.1182/blood-2016-04-711903
37. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. New Engl J Med. (2019) 380:1726–37. doi: 10.1056/NEJMoa1817226
38. Herms L, Su Z, Paulus J, and Zackon I. Real-world utilization of bispecific antibodies for treatment of relapsed/refractory multiple myeloma in the US community oncology setting. Blood. (2024) 144:2410. doi: 10.1182/blood-2024-208825
39. Desjeux G, Colin C, and Launois R. La mesure de la disposition à payer : La méthode des choix discrets. J d’économie médicale. (2005) 23:364−70.
40. Da Vià MC, Solimando AG, Garitano-Trojaola A, Barrio S, Munawar U, Strifler S, et al. CIC mutation as a molecular mechanism of acquired resistance to combined BRAF-MEK inhibition in extramedullary multiple myeloma with central nervous system involvement. Oncologist. (2020) 25:112–8. doi: 10.1634/theoncologist.2019-0356
Keywords: discrete choice experiment, multiple myeloma, patient preferences, trade-offs, treatment attributes
Citation: Faiman B, Le HH, Laurent J, Patel S, Paner-Straseviciute A, Zhang X and Mikhael J (2025) A discrete choice experiment analysis to understand patient preferences for multiple myeloma treatments. Front. Oncol. 15:1628121. doi: 10.3389/fonc.2025.1628121
Received: 13 May 2025; Accepted: 29 August 2025;
Published: 16 September 2025.
Edited by:
Segundo Mariz, European Medicines Agency, NetherlandsReviewed by:
Antonio Giovanni Solimando, University of Bari Aldo Moro, ItalyJoselle Cook, Mayo Clinic, United States
Copyright © 2025 Faiman, Le, Laurent, Patel, Paner-Straseviciute, Zhang and Mikhael. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beth Faiman, ZmFpbWFuYkBjY2Yub3Jn
 Beth Faiman
Beth Faiman Hoa H. Le2
Hoa H. Le2 Julie Laurent
Julie Laurent