- 1Department of General Surgery, Zhongshan Hospital, Xiamen University, Xiamen, China
- 2The School of Clinical Medicine, Fujian Medical University, Fuzhou, China
- 3Department of Vascular Surgery, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan, China
- 4Department of Ultrasound Medicine, Zhongshan Hospital, Xiamen University, Xiamen, China
Backgrounds: Lateral lymph node metastasis (cN1b) is a major factor affecting the prognosis and recurrence of papillary thyroid cancer (PTC). Currently, there is some controversy regarding whether to dissect the contralateral central lymph nodes in patients with cT1-T2N1b unilateral PTC. The purpose of this study was to investigate the risk factors for contralateral central lymph node metastasis (CCLNM) and to summarize the significance of prophylactic contralateral central lymph node dissection (CCLND), to provide reference information for clinical intervention.
Methods: The data of 99 patients with cT1-T2N1b unilateral PTC from August 2021 to October 2024 were retrospectively analyzed. Multifactorial analysis was performed using logistic regression to analyze the risk factors for CCLNM in patients with cT1-T2N1b unilateral PTC. The analysis of the CCLNM rate and metastasis mode summarized the clinical significance of prophylactic CCLND.
Results: CCLNM occurred in 55 cases (55/99,55.6%), and the total number of lymph nodes cleared from the contralateral central lymph node was 6.1 ± 4.9, of which the number of metastatic lymph nodes was 1.5 ± 1.9; There was no statistically significant difference between the CCLNM and non-metastasis groups in terms of the rate of lymph node metastasis in the ipsilateral lateral cervical region (zones II, III, IV and V) and the ipsilateral central zone (P>0.05). There was no statistically significant difference between the metastatic group and the non-metastatic group in terms of the number of lymph nodes cleared in the ipsilateral lateral cervical region (zones II, III, IV and V) (P > 0.05). Compared with the non-metastatic group, the metastatic group had more positive lymph nodes and fewer negative lymph nodes in the ipsilateral central region, and the difference was statistically significant (P < 0.05). Logistic regression analysis showed that microcalcification and Hashimoto’s thyroiditis in the metastasis group were independent factors for the occurrence of CCLNM, and the difference was statistically significant (P<0.05).
Conclusion: The occurrence of CCLNM in cT1-T2N1b unilateral PTC is related to several factors. Lymph node dissection can help reduce the risk of recurrence and reoperation due to CCLNM; therefore CCLND cannot be ignored.
1 Introduction
In the United States, approximately 1.2% of people will be diagnosed with thyroid cancer at some point in their lives, with an estimated 44,020 new cases of thyroid cancer expected in 2025 (1). Over the past 40 years, with the widespread use of diagnostic imaging technology and the popularity of fine needle aspiration biopsy, the incidence of thyroid cancer has risen sharply, with an astonishing increase of 313% (2). Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, accounting for approximately 84% of thyroid cancers (2). Although PTC progresses slowly and has a good overall prognosis, it is often accompanied by cervical lymph node metastasis in the early stages, and lymph node metastasis has been proven to be an important risk factor affecting the survival rate of PTC patients. At the same time, lymph node metastasis is closely related to a higher risk of local recurrence, distant metastasis and death (3).
The first stop for cervical lymph node metastases is usually the central lymph nodes, followed by the ipsilateral lateral cervical lymph nodes, then the contralateral cervical lymph nodes, and mediastinal lymph nodes. However, an increasing number of patients are clinically found to have direct involvement of lateral lymph node metastasis (LLNM) without central lymph node metastasis (CLNM), that is, ‘jumping lymph node metastasis’. The 2022 National Comprehensive Cancer Network (NCCN) guidelines and the 2015 American Thyroid Association (ATA) guidelines both support total thyroidectomy (TT) for patients with LLNM (zones IIzo) metastasis (N1b) (4, 5). For patients with cN1b unilateral PTC, TT + ipsilateral cervical lymph node dissection and ipsilateral central lymph node dissection are typically performed. However, a common problem encountered by thyroid surgeons in clinical practice is whether to perform prophylactic contralateral central lymph node dissection (CCLND) should be in patients with cT1-T2N1b unilateral PTC without malignant foci in the contralateral glandular lobe. In this regard, no uniform guidelines have been issued by domestic and international guidelines. However, according to domestic and international literature, the incidence of contralateral central lymph node metastasis (CCLNM) is approximately 20%-50% (6–10), and neglecting to clear the contralateral central lymph node greatly increases the probability of postoperative recurrence and distant metastasis and increases the likelihood of secondary surgery. Therefore, CCLND in the initial surgery can reduce the probability of lymph node recurrence, make the TNM staging more accurate, and help the choice of subsequent treatment options. However, at the same time, this also increases the difficulty of surgery and the probability of postoperative complications for surgeons to a certain extent. This study aimed to further analyze the clinical significance of preventive CCLND in patients with cT1-T2N1b unilateral PTC, explore high-risk factors for CCLNM, and provide reference materials for clinical intervention.
2 Material and methods
2.1 General information
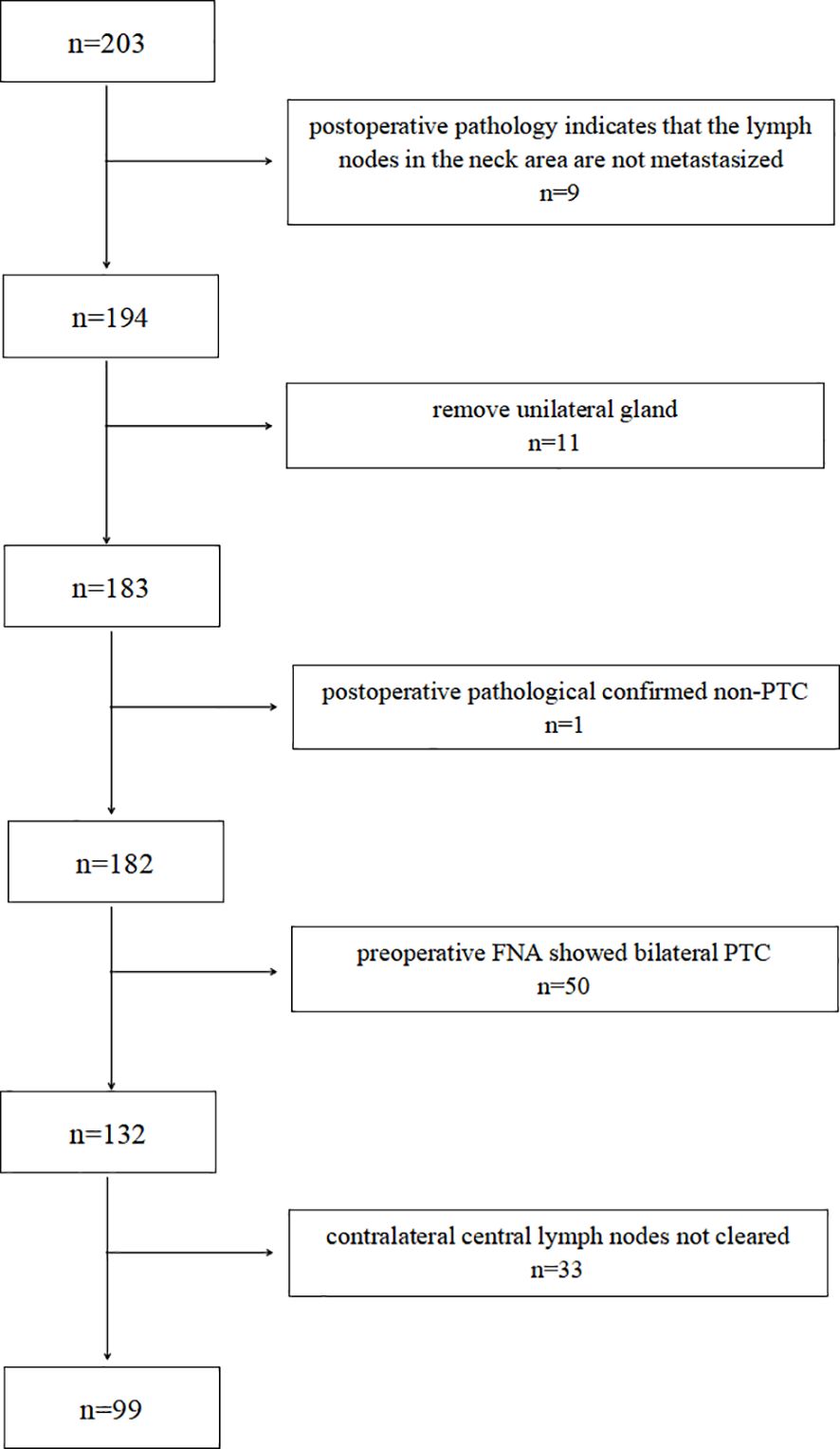
A retrospective analysis was performed on 400 N1b PTC patients admitted to the Department of General Surgery of Zhongshan Hospital, Xiamen University, from August 2021 to October 2024. Based on the inclusion and exclusion criteria, 99 patients with cT1-T2N1b unilateral PTC were included in the study. A patient screening flowchart is presented in Figure 1.
The inclusion criteria: ①unilateral PTC with LLNM confirmed by postoperative pathology; ②TT and ipsilateral cervical lymph node dissection combined with bilateral central lymph node dissection; ③lesions were located in unilateral lobes only; ④patients were examined by imaging examination and ultrasound-guided fine-needle puncture of cervical lymph nodes prior to the operation, which fulfilled the criteria of cervical lymph node positivity; ⑤ Preoperative imaging studies did not reveal any suspicious metastatic lymph nodes in the contralateral central lymph node region. ⑥complete clinical and pathological data. The exclusion criteria. ①history of previous neck surgery or radiotherapy; ②postoperative pathology of non-PTC; ③history of other malignant tumors causing cervical lymph node metastasis; ④those severe organic diseases that cannot tolerate surgery.
This study was approved by the Medical Ethics Committee of Zhongshan Hospital, Xiamen University, china. The study design and writing followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
2.2 Surgical procedures
A tracheal tube with neuromonitoring was placed through the mouth and general anesthesia was administered. The patient was placed in a natural supine position, with a filler placed under the shoulder and the neck slightly stretched. All the patients underwent total thyroidectomy and ipsilateral cervical lymph node dissection combined with bilateral central lymph node dissection. The subcutaneous operating space was established according to the surgical approach selected by the patient prior to surgery. The white line of the neck was incised to expose the thyroid gland and the thyroid glands on the affected and contralateral sides were removed. During the fine dissection during the operation, attention was paid to protecting important tissues, such as the parathyroid gland and the recurrent laryngeal nerve. The ipsilateral cervical lymph nodes (zones IIon), the ipsilateral central lymph nodes (zones VI), and the contralateral central lymph nodes (zones VI) were dissected in turn. The scope of cervical lymph node dissection was along the medial edge of the sternocleidomastoid muscle, dissected upward to the digastric plane; along the internal jugular vein, dissected to the vagus nerve; along the subclavian free, dissected downward to the subclavian vein, and continued to be freed outward to the anterior edge of the trapezius muscle. The scope of lymph node dissection in the central neck area was upward to the hyoid plane, outward to the lateral edge of the common carotid artery on both sides, and downward to the level of the innominate artery. All lymph nodes and fat tissues in front of the trachea, in front of the larynx, and beside the tracheoesophagus, that is, above the innominate artery behind the manubrium of the sternum, are cleared. When removing the lower pole and pretracheal lymph nodes, the lymph nodes and fat tissues were pulled upward to the thymus. When cleaning, the differences in anatomical structures on the left and right sides should be noted to reduce the possibility of omission. Finally, the white line of the neck was sutured and a drainage tube was placed.
2.3 Observation indicators
All patients had preoperative primary thyroid lesions and suspicious cervical lymph nodes, as confirmed by ultrasound-guided thyroid fine-needle aspiration pathology. Preoperative neck or thyroid ultrasonography, enhanced CT, laryngoscopy, and other relevant examinations were performed. Postoperative pathological data were recorded, including extraglandular infiltration, ipsilateral LLNM metastasis, ipsilateral CLNM metastasis, and CCLNM.
The patients were discharged from the hospital without any obvious discomfort, in good general condition, with drains removed, and wounds healing well without obvious redness, swelling, hardness, hematoma, or effusion. All patients received thyroid-stimulating hormone suppression therapy postoperatively. According to the guidelines and based on the postoperative pathological staging, it is recommended that patients undergo radioactive iodine therapy in the oncology department 1–3 months after surgery.
2.4 Statistics
SPSS software (version 26.0) was used to process data. Continuous variable data are expressed as mean ± standard deviation (x ± s), and the independent sample t-test was used. Categorical variable data are expressed as n (%) and the X2 test was used. Logistic regression analysis was used to analyze the risk factors of CCLNM in patients with unilateral PTC and LLNM. Statistical significance was set at P < 0.05.
3 Results
3.1 Comparison of CCLNM and clinical related factors
As shown in Table 1, the proportion of microcalcification and Hashimoto thyroiditis (HT) in the CCLNM group was significantly lower than that in the non-metastatic group (P<0.05).
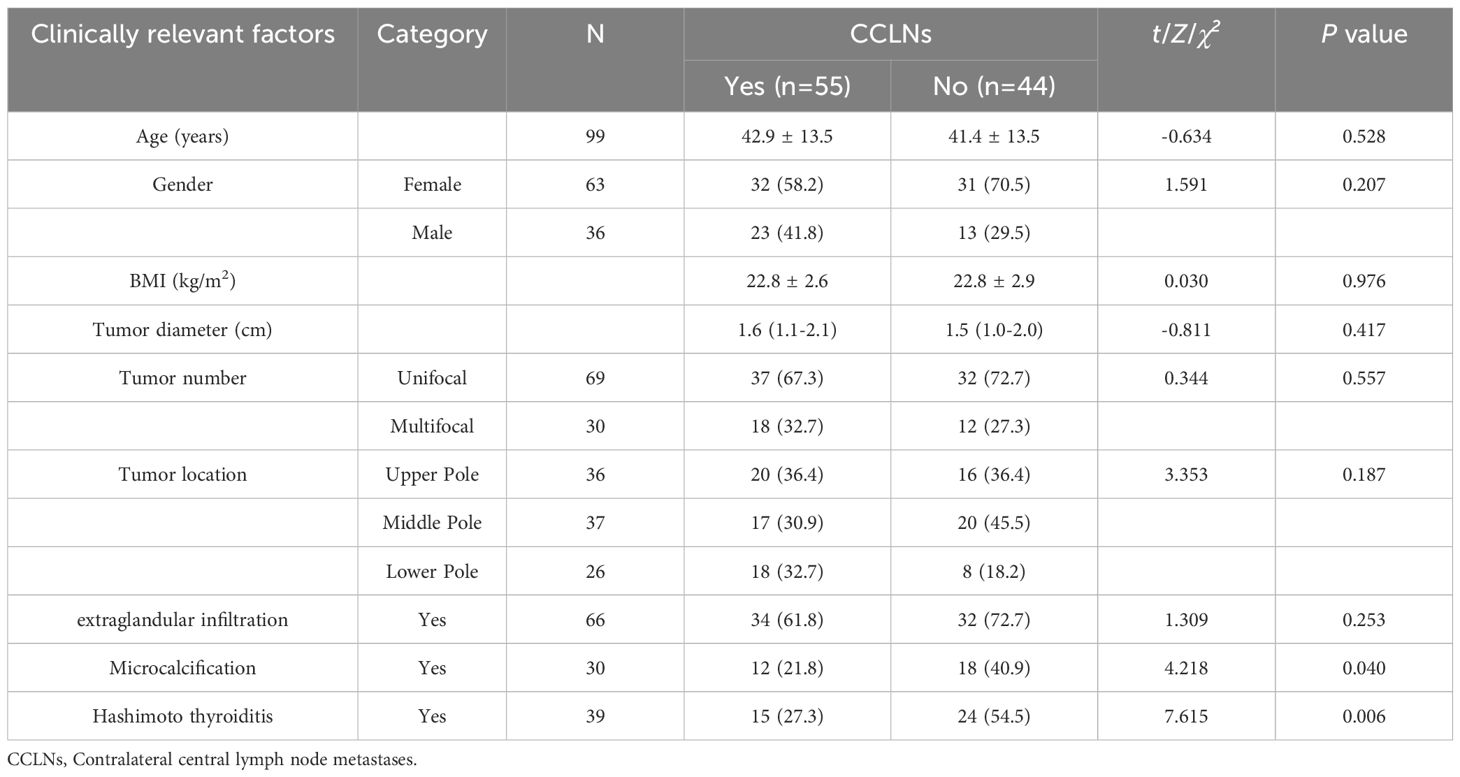
Table 1. Analysis of the correlation between contralateral central lymph node metastasis and clinical factors.
3.2 Comparison in terms of lymph node metastasis rate and number of cleared lymph nodes
CCLNM occurred in 55 cases (55/99,55.6%), and the total number of lymph nodes cleared from the contralateral central lymph node was 6.1 ± 4.9, of which the number of non-metastatic lymph nodes was 4.7 ± 4.6, and the number of metastatic lymph nodes was 1.5 ± 1.9; ipsilateral CLNM occurred in 91 cases (91/99,91.9%), bilateral CLNM occurred in 53 cases (53/99,53.5%), and ipsilateral CLNM without CCLNM occurred in 38 cases (38/99,38.4%).
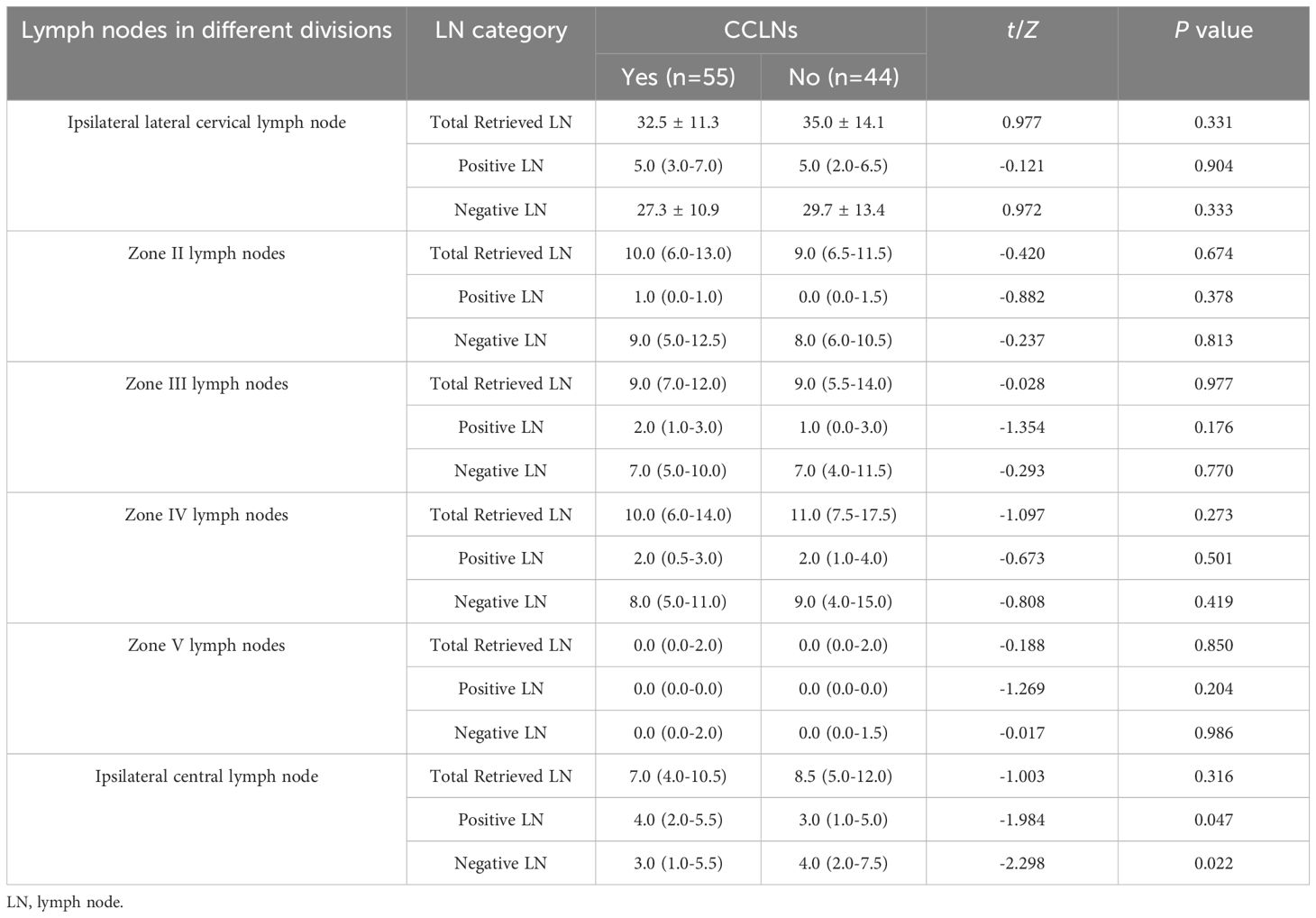
There was no statistically significant difference between the CCLNM and the non-metastasis groups in terms of the rates of ipsilateral lateral cervical zone (zones II, III, IV and V) and ipsilateral CLNM (P>0.05), as shown in Table 2.
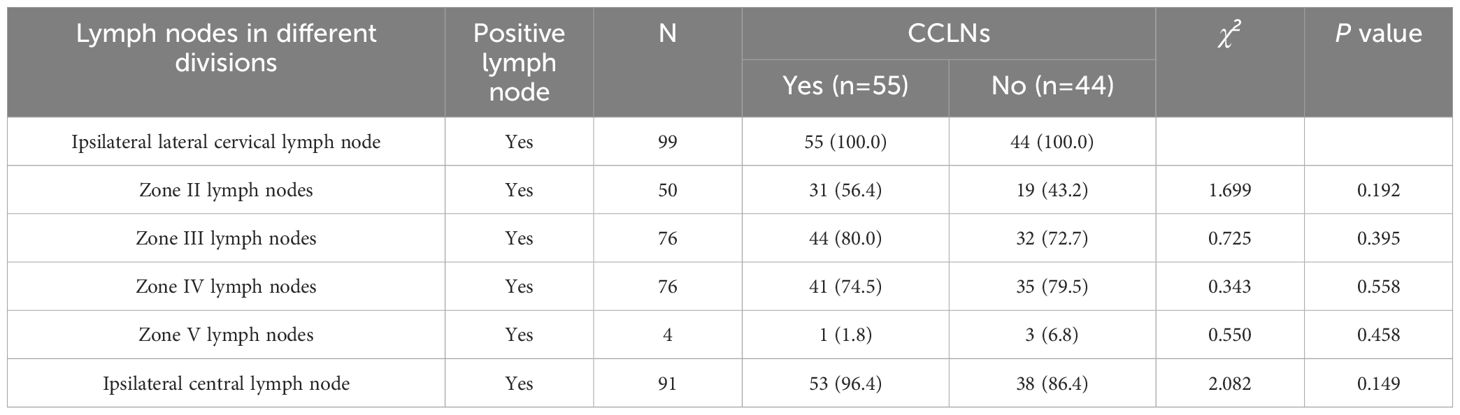
Table 2. Analysis of the correlation between lymph node metastasis in different zones and contralateral central lymph node metastasis.
Comparing the metastasis group with the non-metastasis group in terms of the total number of lymph nodes, the total number of positive lymph nodes, and the total number of negative lymph nodes in the ipsilateral cervical zone clearance (zones II, III, IV and V), the difference was not statistically significant (P>0.05); the metastasis group, compared with the non-metastasis group, had more total positive and fewer total negative lymph nodes in the ipsilateral central zone clearance, and the difference was statistically significant (P<0.05). For more details, see Table 3.
3.3 Logistic regression analysis of cT1-T2N1b unilateral PTC patients who developed contralateral central regional lymph node metastasis
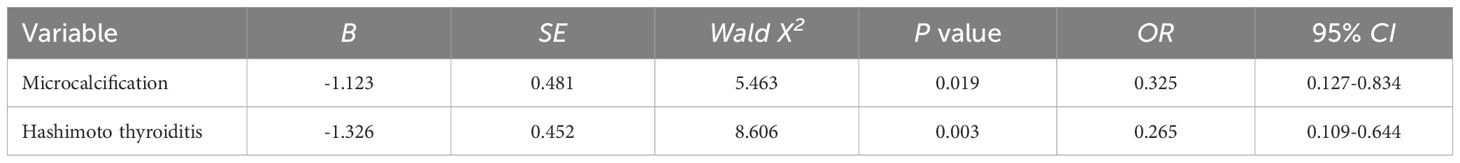
The statistically significant indicators in Tables 1, 2 were included in the logistic multifactorial regression analysis. The results showed that microcalcification and HT were independent factors for the occurrence of CCLNM, and the difference was statistically significant (P<0.05) (Table 4).
4 Discussion
Lymph node metastasis in the lateral cervical region is a major factor affecting the prognosis and recurrence of PTC as well as a factor that determines the extent of resection of the primary lesion. In the past, when performing thyroid cancer resection, selective lymph node dissection was usually adopted to reduce the occurrence of secondary surgery. According to the ATA and NCCN guidelines, prophylactic central lymph node dissection should be considered for PTC patients with advanced tumor stage cN0-1b (4, 5). However, it has also been suggested that prophylactic central zone lymph node dissection is not necessary if the tumor is small, no peritumoral invasion is seen and the central lymph nodes are negative during preoperative imaging assessment (11). Immediate ultrasound is an accurate tool for identifying metastatic lymph nodes (12), but suspicious ultrasound signs (round, cystic changes, hyperechoic lesions or microcalcifications, irregular chaotic vascular formations, and loss of lymphatic gates) are not highly specific (13). At the same time, the diagnostic performance of computed tomography scans is influenced by the subjective judgment of the radiologist, which leads to inaccurate staging assessment or understaging or over-staging in a significant proportion of patients. Studies have shown that occult metastases can be detected in the central region in 40%-70% of cases, and because of the high rate of occult metastases and even clinicopathological findings of micrometastases in about 90% of patients, prophylactic central lymph node dissection can help detect them, and consequently, to further change the staging of PTC and the adjuvant therapeutic regimen in the postoperative period (14, 15). The present study focused on patients with cT1-T2N1b unilateral PTC, aiming to investigate the feasibility of contralateral central lymph node dissection and to analyze the high-risk factors for CCLNM in this group of patients, with the aim of providing a strong basis for controlling the recurrence rate of the disease and improving the prognosis.
The central regions of the neck (pre-laryngeal, pre-tracheal, and paratracheal tissues) have a high number of interconnected branches through which tumor cells can metastasize from one central region to the contralateral central region, thus causing lymph node metastasis in the contralateral central region. Although PTC cell multiplication occurs in a predictable stepwise manner through the orderly arranged lymphatic system, jump metastases can also occur when uninvolved contiguous areas are interspersed with involved tumors (16). The possibility of involvement of the contralateral central lymph nodes in patients with jump metastases in the unilateral lateral cervical zone has not been fully clarified. In this study, we conducted an in-depth analysis of the clinical factors affecting the occurrence of metastases in the contralateral central zone, and the results showed a correlation between CCLNM and microcalcifications and HT. Although previous studies have explored the risk factors for cervical lymph node metastasis in patients with PTC, the findings remain controversial. The presence of microcalcifications on ultrasound is usually indicative of a significant proliferation of fibrous tissue and tumor vasculature within the lesion, accompanied by calcification and deposition of cancer cells, which is a specific indicator for the diagnosis of PTC and an important factor contributing to the increased risk of metastasis (17, 18). Previous studies have also confirmed a correlation between CLNM and microcalcifications, with microcalcifications increasing the risk of CLNM. This finding is inconsistent with the results of this study. However, the primary reason for this discrepancy is the relatively small sample size included in this study. Additionally, the cases included in this study were limited to patients with cT1-T2N1b unilateral PTC. These factors remind us that when exploring the impact of microcalcification differences, it is essential to carefully consider the limitations that may exist in research methods and data analysis, while also being mindful of potential biases in patient selection. Future studies should further expand the sample size and conduct a more in-depth exploration of the relationship between cN0 PTC and microcalcifications. A retrospective study in Korea that in patients with PTC accompanied by HT, although the number of lymph nodes cleared by central lymph node dissection was increased, it did not increase the detection rate of positive lymph nodes, and at the same time, it showed that HT was independently correlated with good prognosis in patients with PTC (19). This finding is consistent with the results of the present study and supported by other studies (20, 21). This may be due to the characteristic high concomitant nature of HT in PTC as well as the fact that HT causes cervical lymph node enlargement through a chronic inflammatory immune response, resulting in reactive lymph node hyperplasia frequently observed in the central region (19, 22). In actual surgery, the number of central lymph node dissections inevitably increases and the percentage of positive lymph nodes is somewhat reduced.
In addition, the prevailing view is that patients with PTC with larger tumor size, located at the upper pole, and multifocal PTC have more aggressive tumors, which may also be risk factors for the occurrence of lymph node metastasis (20, 23, 24). However, this conclusion was not reached in this study. Tumor diameter is critical for postoperative TNM pathological staging and prognosis risk assessment in PTC patients. It is positively correlated with growth rate and tissue invasion extent and is more likely to result in central lymph node metastasis (25). The ATA guidelines recommend routine prophylactic central lymph node dissection for patients with T3 or T4 tumors, as the risk of lymph node metastasis increases in patients with tumor diameters of 4 cm or larger (5). Based on the Surveillance, Epidemiology, and End Results (SEER) database, a multivariable analysis was conducted on thyroid cancer patients who underwent surgical treatment between 2002 and 2012 (n=80,565), and the results confirmed a correlation between tumor diameter ≥i cm and central region metastasis (25). This study showed no statistically significant association between tumor diameter and CCLNM. The primary reasons for this outcome include: first, cases were strictly limited to T1–2 stages (tumor diameter ≤4 cm), and a stratified risk analysis was conducted based on tumor diameter; Second, sample size limitations. These findings suggest that tumor diameter may not be a necessary indicator for CCLND in the management of early-stage PTC (T1–2 stages).
In this study, the ipsilateral CLNM rate was as high as 91.9% (91/99), CCLNM rate was 55.6% (55/99), and bilateral CLNM rate was 53.5% (53/99). Among the patients without ipsilateral CLNM, 25.0% (2/8) had positive contralateral central lymph nodes. Some studies have shown that the high or low rate of cervical lymph node metastasis in patients with PTC is closely related to their specific metastasis pattern, and this metastasis follows a certain zoning rule (26). Lymph node metastasis usually first occurs in the central lymph nodes (anterior laryngeal, anterior tracheal, and paratracheal tissues), and lymphatic vessels communicate with each other between the bilateral central zones. Metastasis of the central lymph nodes on one side can cause tumor cells to metastasize to the contralateral central zone through lymphatic vessel branches (16). In this study, the lymph node metastasis rates of patients in the cervical zones II, III, IV and V were 50.5% (50/99), 76.8% (76/99), 76.8% (76/99), and 5.1% (5/99), respectively, with the main metastasis occurring in zones III and IV. Therefore, from the perspective of the lateral cervical lymph node metastasis rate or the overall law of cervical lymph node metastasis, when lateral cervical lymph node metastasis occurs, the risk of metastasis in the contralateral central zone cannot be ignored. Therefore, it is necessary to perform preventive CCLND in patients with PTC in clinical practice. The ATA and NCCN guidelines also believe that patients with clinically positive lymph nodes (cN1) are suitable candidates for preventive central lymph node dissection (4, 5). However, the main argument against prophylactic central lymph node dissection is its possible increased risk of surgical complications (27, 28). Among these complications, parathyroid injury and recurrent laryngeal nerve injury are the most common and may result in temporary or permanent hypocalcaemia and hoarseness. The incidence of temporary and permanent hypocalcaemia has been reported to be as high as 44% and 4%, respectively, and temporary and permanent recurrent laryngeal nerve injuries are as high as 7.3% and 3.6%, respectively (29). However, Chae et al. found that unilateral versus bilateral central lymph node dissection resulted in no significant difference in the incidence of laryngeal recurrent nerve injury and temporary hypocalcaemia (8). The results of a meta-analysis also showed that total thyroidectomy alone and total thyroidectomy combined with prophylactic central lymph node dissection did not differ in the incidence of permanent hypoparathyroidism and temporary or permanent recurrent laryngeal nerve injury (30). Other researchers have reached similar conclusions and found no statistically significant difference between the incidence of permanent hypoparathyroidism and permanent recurrent laryngeal nerve injury in these two groups (31–33). Therefore, it is recommended that the operation be performed by an experienced surgeon familiar with surgical anatomy, with precise preoperative localization using tracers and intraoperative dissection using advanced stripping equipment to preserve the parathyroid glands and the recurrent laryngeal nerve, thus effectively avoiding postoperative complications. If prophylactic dissection can be performed at the time of the initial surgery, it can avoid the short-term metastatic spread of cancer cells due to missed lymph node dissection, thus reducing the probability of metastasis and recurrence (31, 32, 34). In addition, this can avoid the possibility of having to undergo a secondary operation because of the occurrence of metastasis and recurrence. Secondary surgery will not only increase the overall difficulty of surgery, such as scar adhesion and anatomical structure changes, but also increase the incidence of surgical complications, which will cause great physical and psychological burden to the patients (32, 35). In summary, prophylactic clearance of contralateral central lymph nodes not only helps to remove undetected involved lymph nodes and reduces the probability of postoperative recurrence and metastasis but also more accurately determines the pathological stage of postoperative TNM, which provides more accurate information for patients’ postoperative treatment options, follow-up protocols, and assessment of the risk of postoperative recurrence, and thus greatly improves the patient’s prognosis.
The limitations of this study are as follows: Firstly, as a retrospective study, its inherent design makes it difficult to completely avoid the problem of potential bias. Second, this study was a single-center study with a relatively small sample size, which limited our ability to comprehensively assess the impact of prophylactic central lymph node dissection on patients’ long-term prognosis and quality of life. To overcome these limitations and obtain more accurate and comprehensive findings, we plan to conduct multicenter, large-sample, randomized controlled clinical studies in the future, with a view to providing more reliable and robust evidence support in this area.
5 Conclusion
Based on the above analysis of the metastasis rate, metastasis pattern, and clinical risk factors of the contralateral central lymph nodes in patients with cT1-T2N1b unilateral PTC, this study suggests that the CCLND should not be ignored in patients with unilateral cN1b. Metastasis of the contralateral central lymph nodes in cT1-T2N1b unilateral PTC is closely association with many factors. CCLND can help reduce the risk of recurrence and reoperation due to contralateral lymph node metastasis, thereby providing patients with a more comprehensive and effective treatment strategy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Zhongshan Hospital, Affiliated with Xiamen University, Xiamen, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. RQ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YT: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XH: Investigation, Methodology, Supervision, Writing – review & editing. QD: Software, Supervision, Validation, Writing – review & editing. KL: Methodology, Supervision, Writing – review & editing. EL: Investigation, Software, Writing – review & editing. PK: Methodology, Supervision, Writing – review & editing. JF: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. GW: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Boucai L, Zafereo M, and Cabanillas ME. Thyroid cancer: A review. JAMA. (2024) 331:425–35. doi: 10.1001/jama.2023.26348
3. Chéreau N, Buffet C, Trésallet C, Tissier F, Leenhardt L, and Menegaux F. Recurrence of papillary thyroid carcinoma with lateral cervical node metastases: Predictive factors and operative management. Surgery. (2016) 159:755–62. doi: 10.1016/j.surg.2015.08.033
4. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:925–51. doi: 10.6004/jnccn.2022.0040
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
6. Kang SK, Kim DI, Im DW, Lee S, Choi JB, Jung YJ, et al. A retrospective study of factors affecting contralateral central-neck lymph node metastasis in unilateral papillary thyroid carcinoma. Asian J Surg. (2023) 46:3485–90. doi: 10.1016/j.asjsur.2022.10.081
7. Tan HL, Huang BQ, Li GY, Wei B, Chen P, Hu HY, et al. A prediction model for contralateral central neck lymph node metastases in unilateral papillary thyroid cancer. Int J Endocrinol. (2021) 2021:6621067. doi: 10.1155/2021/6621067
8. Chae BJ, Jung CK, Lim DJ, Song BJ, Kim JS, Jung SS, et al. Performing contralateral central lymph node dissection in papillary thyroid carcinoma: a decision approach. Thyroid. (2011) 21:873–7. doi: 10.1089/thy.2010.0214
9. Koo BS, Choi EC, Park YH, Kim EH, and Lim YC. Occult contralateral central lymph node metastases in papillary thyroid carcinoma with unilateral lymph node metastasis in the lateral neck. J Am Coll Surg. (2010) 210:895–900. doi: 10.1016/j.jamcollsurg.2010.01.037
10. Moo TA, Umunna B, Kato M, Butriago D, Kundel A, Lee JA, et al. Ipsilateral versus bilateral central neck lymph node dissection in papillary thyroid carcinoma. Ann Surg. (2009) 250:403–8. doi: 10.1097/SLA.0b013e3181b3adab
11. Raffaelli M, Sessa L, De Crea C, Fadda G, Princi P, Rossi ED, et al. Is it possible to intraoperatively modulate the extent of thyroidectomy in small papillary thyroid carcinoma? Surgery. (2021) 169:77–81. doi: 10.1016/j.surg.2020.04.043
12. Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:122–37. doi: 10.3322/caac.21389
13. Holoubek SA and Sippel RS. Lymph node imaging for thyroid cancer. Clin Endocrinol (Oxf). (2024) 100:96–101. doi: 10.1111/cen.14993
14. De Napoli L, Matrone A, Favilla K, Piaggi P, Galleri D, Ambrosini CE, et al. Role of prophylactic central compartment lymph node dissection on the outcome of patients with papillary thyroid carcinoma and synchronous ipsilateral cervical lymph node metastases. Endocr Pract. (2020) 26:807–17. doi: 10.4158/EP-2019-0532
15. Roh JL, Kim JM, and Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. (2011) 18:2245–50. doi: 10.1245/s10434-011-1600-z
16. Feng JW, Qin AC, Ye J, Pan H, Jiang Y, and Qu Z. Predictive factors for lateral lymph node metastasis and skip metastasis in papillary thyroid carcinoma. Endocr Pathol. (2020) 31:67–76. doi: 10.1007/s12022-019-09599-w
17. Zhou L, Yao J, Ou D, Li M, Lei Z, Wang L, et al. A multi-institutional study of association of sonographic characteristics with cervical lymph node metastasis in unifocal papillary thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:965241. doi: 10.3389/fendo.2022.965241
18. Luo X, Wang J, Xu M, Zou X, Lin Q, Zheng W, et al. Risk model and risk stratification to preoperatively predict central lymph node metastasis in papillary thyroid carcinoma. Gland Surg. (2020) 9:300–10. doi: 10.21037/gs.2020.03.02
19. Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of coexistent Hashimoto’s thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf). (2018) 88:123–8. doi: 10.1111/cen.13475
20. Wang Y, Zheng J, Hu X, Chang Q, Qiao Y, Yao X, et al. A retrospective study of papillary thyroid carcinoma: Hashimoto’s thyroiditis as a protective biomarker for lymph node metastasis. Eur J Surg Oncol. (2023) 49:560–7. doi: 10.1016/j.ejso.2022.11.014
21. Marotta V, Sciammarella C, Chiofalo MG, Gambardella C, Bellevicine C, Grasso M, et al. Hashimoto’s thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. (2017) 24:485–93. doi: 10.1530/ERC-17-0085
22. Vita R, Ieni A, Tuccari G, and Benvenga S. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma. Rev Endocr Metab Disord. (2018) 19:301–9. doi: 10.1007/s11154-018-9474-z
23. Zhuo X, Yu J, Chen Z, Lin Z, Huang X, Chen Q, et al. Dynamic nomogram for predicting lateral cervical lymph node metastasis in papillary thyroid carcinoma. Otolaryngol Head Neck Surg. (2022) 166:444–53. doi: 10.1177/01945998211009858
24. Zhou B and Qin J. High-risk factors for lymph node metastasis in contralateral central compartment in unilateral papillary thyroid carcinoma(cT1N0). Eur J Surg Oncol. (2021) 47:882–7. doi: 10.1016/j.ejso.2020.10.018
25. Shi RL, Qu N, Yang SW, Ma B, Lu ZW, Wen D, et al. Tumor size interpretation for predicting cervical lymph node metastasis using a differentiated thyroid cancer risk model. Onco Targets Ther. (2016) 9:5015–22. doi: 10.2147/OTT.S107187
26. Rotstein L. The role of lymphadenectomy in the management of papillary carcinoma of the thyroid. J Surg Oncol. (2009) 99:186–8. doi: 10.1002/jso.21234
27. Sippel RS, Robbins SE, Poehls JL, Pitt SC, Chen H, Leverson G, et al. A randomized controlled clinical trial: no clear benefit to prophylactic central neck dissection in patients with clinically node negative papillary thyroid cancer. Ann Surg. (2020) 272:496–503. doi: 10.1097/SLA.0000000000004345
28. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Prophylactic central neck dissection might not be necessary in papillary thyroid carcinoma: analysis of 11,569 cases from a single institution. J Am Coll Surg. (2016) 222:853–64. doi: 10.1016/j.jamcollsurg.2016.02.001
29. Yan XQ, Zhang ZZ, Yu WJ, Ma ZS, Chen ML, and Xie BJ. Prophylactic central neck dissection for cN1b papillary thyroid carcinoma: A systematic review and meta-analysis. Front Oncol. (2021) 11:803986. doi: 10.3389/fonc.2021.803986
30. Harries V, McGill M, Wang LY, Tuttle RM, Wong RJ, Shaha AR, et al. Is a prophylactic central compartment neck dissection required in papillary thyroid carcinoma patients with clinically involved lateral compartment lymph nodes? Ann Surg Oncol. (2021) 28:512–8. doi: 10.1245/s10434-020-08861-4
31. Wang Y, Xiao Y, Pan Y, Yang S, Li K, Zhao W, et al. The effectiveness and safety of prophylactic central neck dissection in clinically node-negative papillary thyroid carcinoma patients: A meta-analysis. Front Endocrinol (Lausanne). (2022) 13:1094012. doi: 10.3389/fendo.2022.1094012
32. Yazıcı D, Çolakoğlu B, Sağlam B, Sezer H, Kapran Y, Aydın Ö, et al. Effect of prophylactic central neck dissection on the surgical outcomes in papillary thyroid cancer: experience in a single center. Eur Arch Otorhinolaryngol. (2020) 277:1491–7. doi: 10.1007/s00405-020-05830-1
33. Dobrinja C, Troian M, Cipolat Mis T, Rebez G, Bernardi S, Fabris B, et al. Rationality in prophylactic central neck dissection in clinically node-negative (cN0) papillary thyroid carcinoma: Is there anything more to say? A decade experience in a single-center. Int J Surg. (2017) 41 Suppl 1:S40–s47. doi: 10.1016/j.ijsu.2017.01.113
34. Barczyński M, Konturek A, Stopa M, and Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. (2013) 100:410–8. doi: 10.1002/bjs.8985
35. Medas F, Canu GL, Cappellacci F, Anedda G, Conzo G, Erdas E, et al. Prophylactic central lymph node dissection improves disease-free survival in patients with intermediate and high risk differentiated thyroid carcinoma: A retrospective analysis on 399 patients. Cancers (Basel). (2020) 12(6):1658. doi: 10.3390/cancers12061658
Keywords: papillary thyroid carcinoma, lateral lymph node metastasis, contralateral central lymph node metastasis, preventive central lymph node dissection, surgical treatment
Citation: Lin S, Qiu R, Tang Y, Hong X, Ding Q, Li K, Lin E, Kuang P, Fu J and Wu G (2025) A study on the clinical value of prophylactic contralateral central lymph node dissection in patients with cT1-T2N1b unilateral papillary thyroid cancer. Front. Oncol. 15:1629656. doi: 10.3389/fonc.2025.1629656
Received: 16 May 2025; Accepted: 30 June 2025;
Published: 16 July 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Haggi Mazeh, Hadassah Medical Center, IsraelChunlei Nie, Harbin Medical University Cancer Hospital, China
Copyright © 2025 Lin, Qiu, Tang, Hong, Ding, Li, Lin, Kuang, Fu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinbo Fu, amltYm8yMDA2QDE2My5jb20=; Guoyang Wu, d3VndW95YW5nbWFpbEBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Suqiong Lin1†
Suqiong Lin1† Rongliang Qiu
Rongliang Qiu Qiangbin Ding
Qiangbin Ding Ke Li
Ke Li Penghao Kuang
Penghao Kuang Jinbo Fu
Jinbo Fu Guoyang Wu
Guoyang Wu