- Department of Gastroenterology, Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Background: Small cell carcinoma of the esophagus (SCCE) is a rare and highly aggressive malignancy characterized by early metastatic propensity. Traditional chemotherapy has a poor curative effect and a short survival period Recent evidence demonstrates that combining immune checkpoint inhibitors (ICIs) with chemotherapy significantly improves therapeutic outcomes in both advanced esophageal carcinoma and small cell lung cancer. This study aims to evaluate the efficacy of first-line ICIs combined with chemotherapy in patients with advanced SCCE.
Patients and methods: This single-center retrospective study analyzed 31 patients with advanced SCCE who initiated first-line systemic therapy at our institution between January 2021 and August 2024. All patients received physician-determined treatment regimens. The study aimed to evaluate the efficacy of first-line immunotherapy combined with chemotherapy in advanced SCCE.
Results: Median progression-free survival (PFS) was significantly longer in the immunotherapy group (9.3 months; 95% CI 6.3-12.3) compared to the non-immunotherapy group (5.4 months; 95% CI 3.5-7.3; P = 0.046). Patients receiving chemotherapy alone demonstrated the shortest PFS (3.2 months; 95% CI 2.1-4.3), while those receiving combined chemotherapy and immunotherapy achieved the longest PFS (10.0 months; 95% CI 3.8-16.1). Median overall survival (OS) of patients with combined immunotherapy showed a trend of prolongation (17.0 months 95% CI 12.9-21.13 vs. 11.6 months 95% CI 4.7-18.6), but no statistically significant difference was observed (p = 0.055). Multivariate analyses suggested that the combination of immunotherapy, or its absence, may affect patient prognosis. Numerical improvements were observed in the immunotherapy group for both objective response rate (ORR: 31.3% vs. 21.4%) and disease control rate (DCR: 93.7% vs. 85.7%).
Conclusion: Esophageal small cell carcinoma remains a highly aggressive malignancy with poor prognosis in advanced stages. This retrospective real-world study suggests that first-line immunotherapy combined with chemotherapy may significantly improve progression-free survival in patients with advanced SCCE compared to chemotherapy alone.
1 Introduction
Small cell carcinoma of the esophagus (SCCE) is a lethal malignancy with a dismal prognosis, accounting for 0.8%-2.4% of all esophageal cancers. It exhibits high aggressiveness and an early propensity for metastasis. Although its biological behavior resembles that of small cell lung cancer (SCLC), its prognosis is notably worse. The 5-year survival rate for SCCE is less than 5%, and only 10% of patients survive longer than one year (1).
For advanced SCCE, multidrug chemotherapy (typically including cisplatin or carboplatin with etoposide) remains the primary treatment. While radiotherapy may enhance local control, recurrence and drug resistance rates are high (2). Recently, the combination of immune checkpoint inhibitors (ICIs) with chemotherapy has become the standard first-line therapy for esophageal squamous cell carcinoma (ESCC), offering potential synergistic benefits and significantly improved efficacy in the advanced setting (3). Similarly, in first-line treatment of SCLC, combining ICIs (such as atezolizumab or durvalumab) with chemotherapy has demonstrated significant survival advantages over chemotherapy alone (4, 5). Given this background, we conducted a retrospective analysis of SCCE patients at our center to evaluate whether combining chemotherapy with immunotherapy improves survival outcomes in SCCE.
2 Methods
2.1 Study design and participants
This retrospective study collected data from SCCE patients treated at the Fourth Hospital of Hebei Medical University between January 2021 and August 2024. Inclusion criteria were: (1) histologically confirmed, previously untreated SCCE;.Exclusion criteria included: (1) history of malignancy; (2) clinical stage T1N0M0 or earlier; and (3) incomplete information or loss to follow-up. The study protocol was approved by the institutional review board of our institution. Due to the retrospective nature of this study, the ethics committee waived informed consent.
All patients had a histologically confirmed SCCE diagnosis based on immunohistochemical staining for common neuroendocrine markers, including neuron-specific enolase (NSE), synaptophysin (Syn), chromogranin A (CgA), cytokeratin 56 (CK56), cytokeratin (CK), and lymphocyte antigen 56 (CD56). Staging was performed using both the 8th edition of the UICC/AJCC TNM Classification of Carcinoma of the Esophagus and Esophagogastric Junction and the Veterans Administration Lung Study Group (VALSG) staging system.
Routine baseline examinations included physical examination, endoscopy with biopsy, and contrast-enhanced computed tomography (CT).
2.2 Treatment
Due to the absence of standardized treatment guidelines, the therapeutic strategy was determined by physician discretion and patient preference. Chemotherapy regimens primarily consisted of platinum-based agents (e.g., cisplatin or carboplatin) combined with other cytotoxic drugs such as etoposide or paclitaxel. Administered immune checkpoint inhibitors (ICIs) comprised Sintilimab, Serplulimab, and Adebelimab. Radiation oncologists planned radiotherapy based on tumor location, size, and involvement of regional lymph nodes, delivering doses ranging from 45 Gy to 55 Gy.
2.3 Statistical analysis
Clinicopathologic characteristics of patients and progression-free survival (PFS) and overall survival (OS) were compared between treatment groups. Additionally, objective response rate (ORR) and disease control rate (DCR) were compared. Categorical variables are presented as frequencies and percentages, and comparisons between groups were performed using the χ² test. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models. P<0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics and treatment
A total of 39 patients were enrolled in the trial. Eight cases were excluded for not meeting the inclusion criteria: one patient staged as T1N0M0, two patients with concurrent other tumors, two patients who received neoadjuvant chemotherapy, two patients who did not receive chemotherapy, and one patient lost to follow-up. Consequently, 31 patients comprised the per-protocol population.
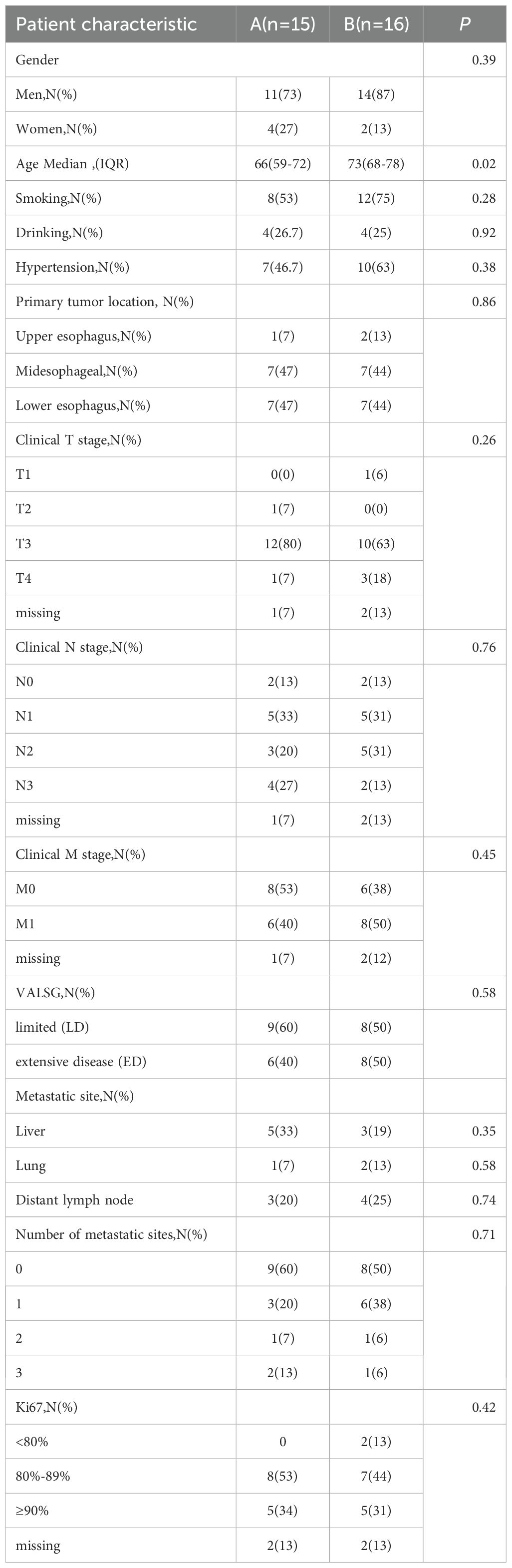
The cohort exhibited a male predominance. Tumors arose primarily in the mid-esophagus (45%) and lower esophagus (45%). The liver (26%) and distant lymph nodes (23%) were the most common metastatic sites.
Patients were divided into two treatment arms: Arm A (n=15) received chemotherapy and/or radiotherapy, while Arm B (n=16) received chemotherapy and/or radiotherapy plus immune checkpoint inhibitors. Demographic and clinical characteristics were generally comparable between the two arms, except for age (Table 1).
3.2 Progression-free survival
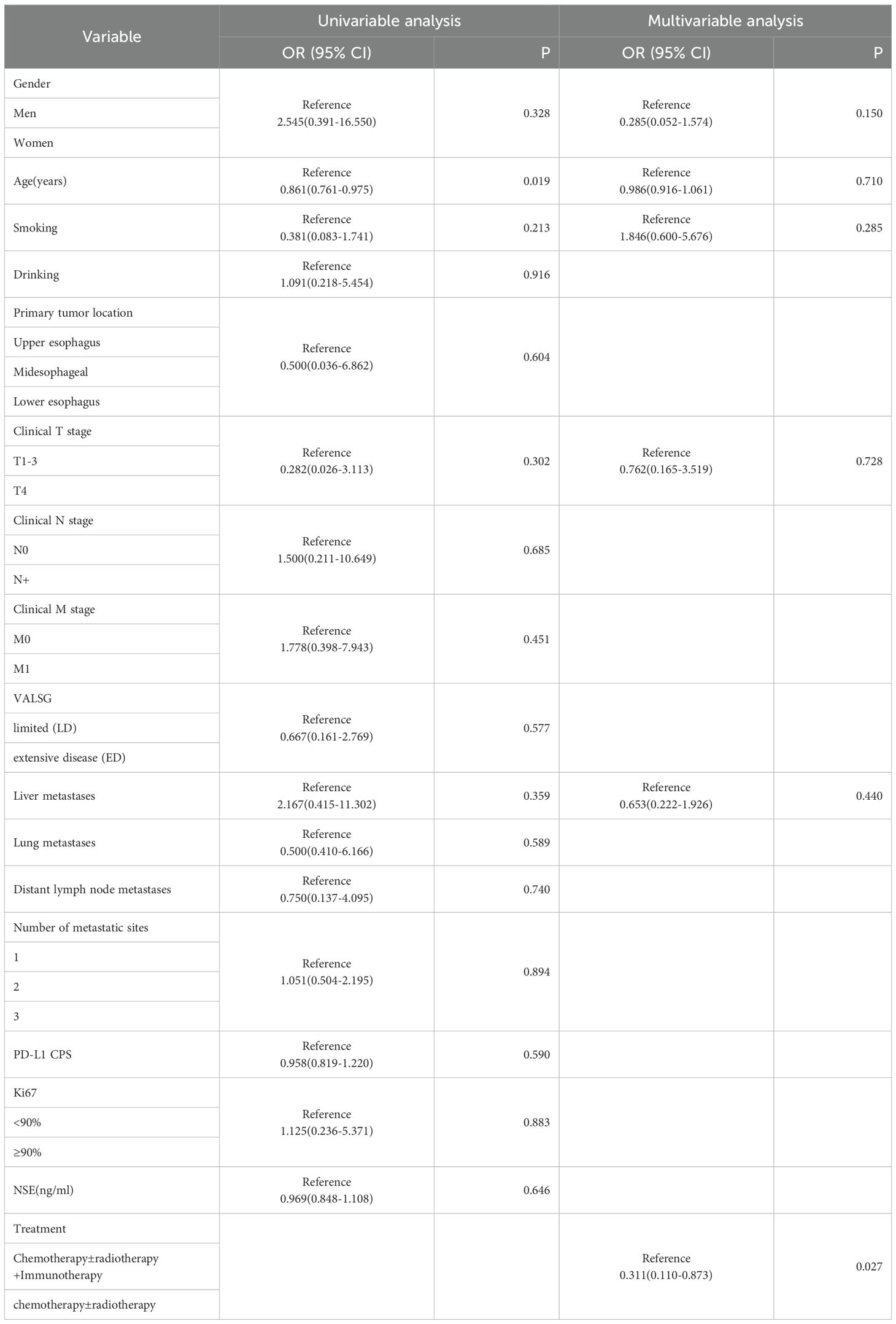
As of the follow-up cutoff in March 2025, one patient was lost to follow-up., PFS events were documented in 30 patients. The addition of ICIs to therapy significantly improved median PFS compared to the control group (9.3 months vs. 5.4 months, p = 0.046; Figure 1). Among the treatment subgroups, patients receiving chemotherapy alone exhibited the worst median PFS (3.2 months, 95% CI 2.1-4.3), while those receiving chemotherapy combined with ICIs demonstrated the best median PFS (10.0 months, 95% CI 3.8-16.1; Figure 2). Multifactorial analyses were performed to identify clinical features that might affect median PFS of patients (Table 2).
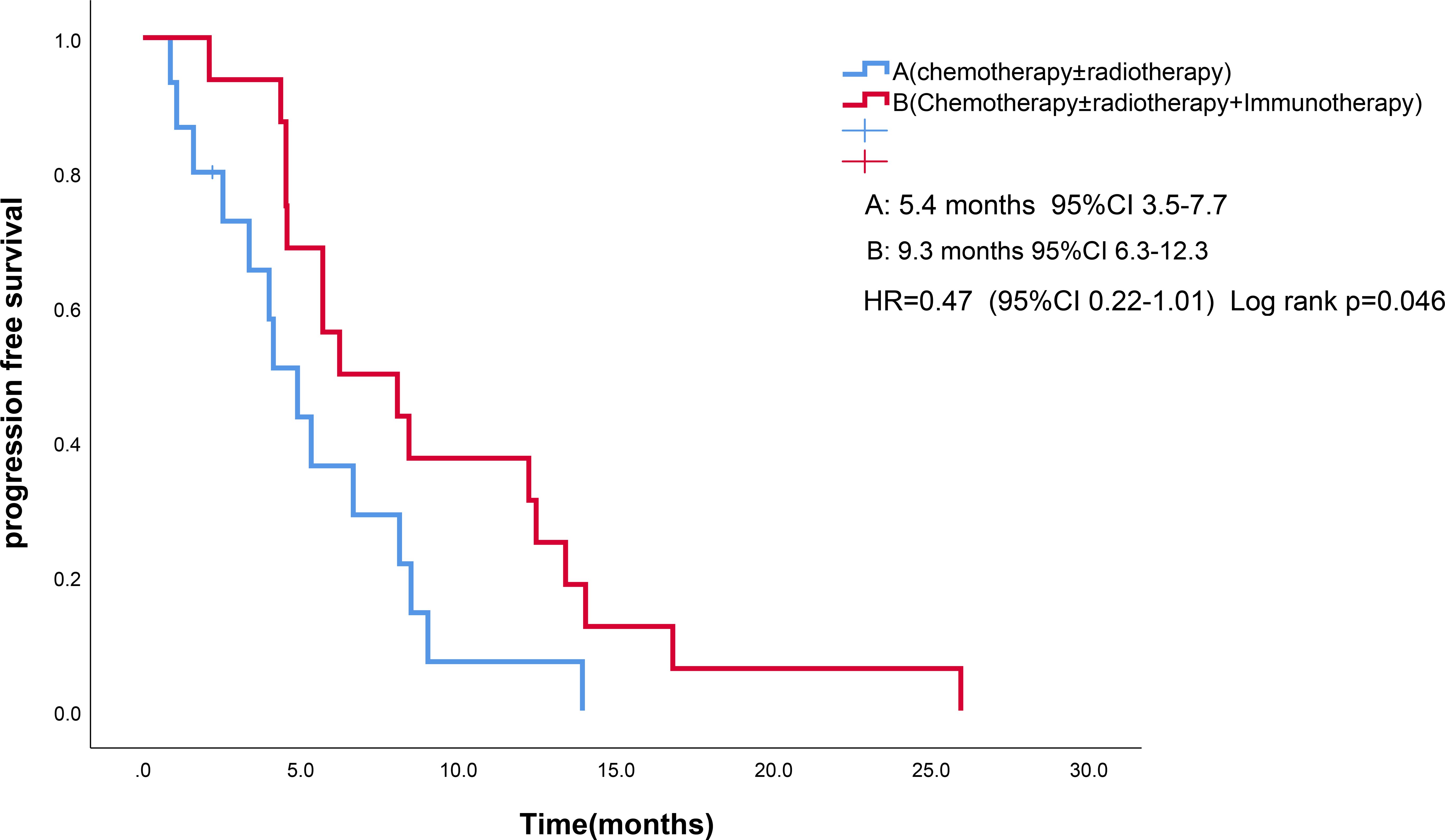
Figure 1. Kaplan-Meier survival estimates in PFS. (A), chemotherapy and/or radiotherapy ; (B), chemotherapy and/or radiotherapy plus ICIs; HR, hazard ratio; ICIs, Immune checkpoint inhibitors; PFS, Progression Free Survival.
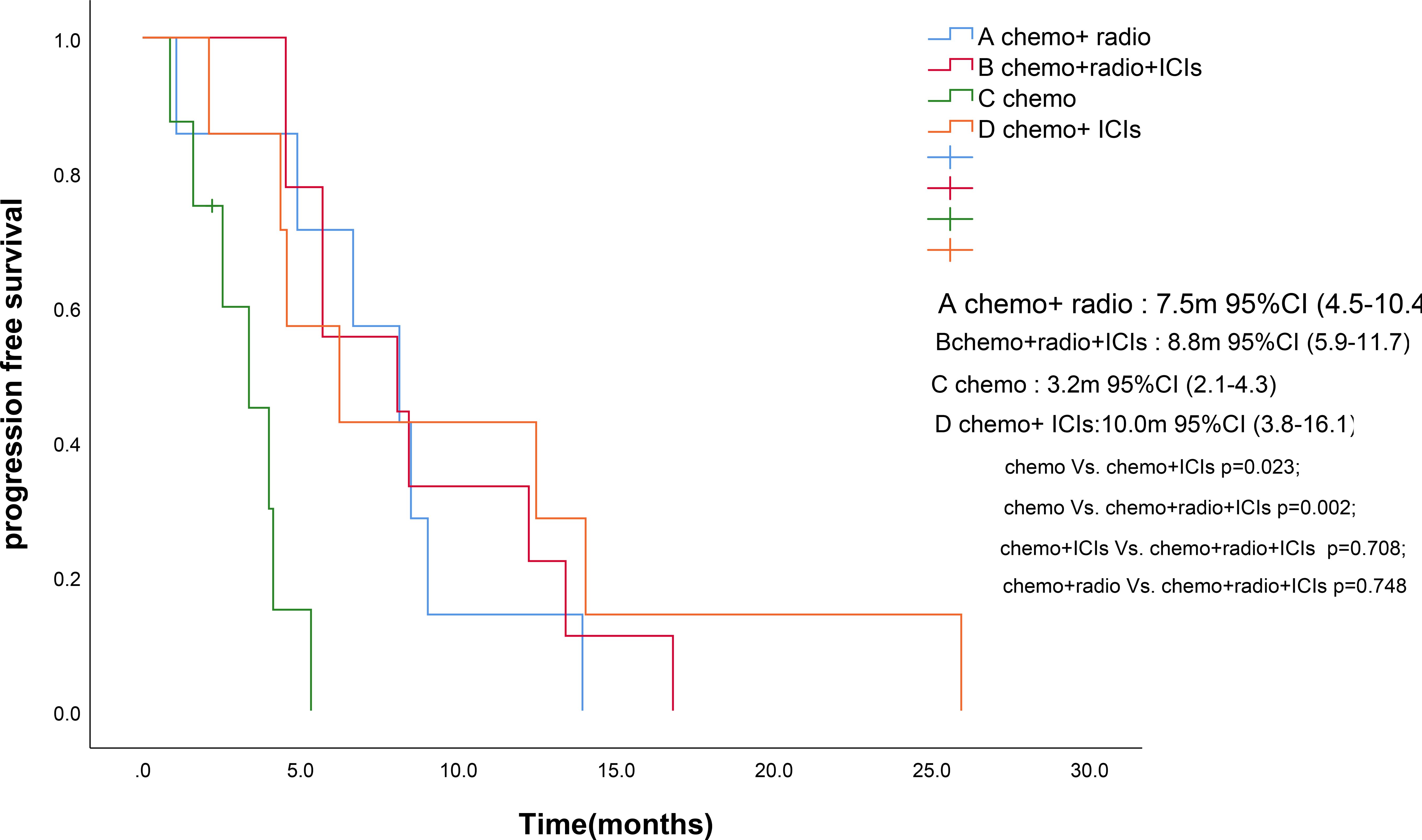
Figure 2. Kaplan-Meier survival estimates in PFS. (A), chemotherapy and radiotherapy ; (B), chemotherapy and radiotherapy and ICIs; (C), chemotherapy; (D), chemotherapy and ICIs. ICIs, Immune checkpoint inhibitors; PFS, Progression Free Survival. .
3.3 Overall survival
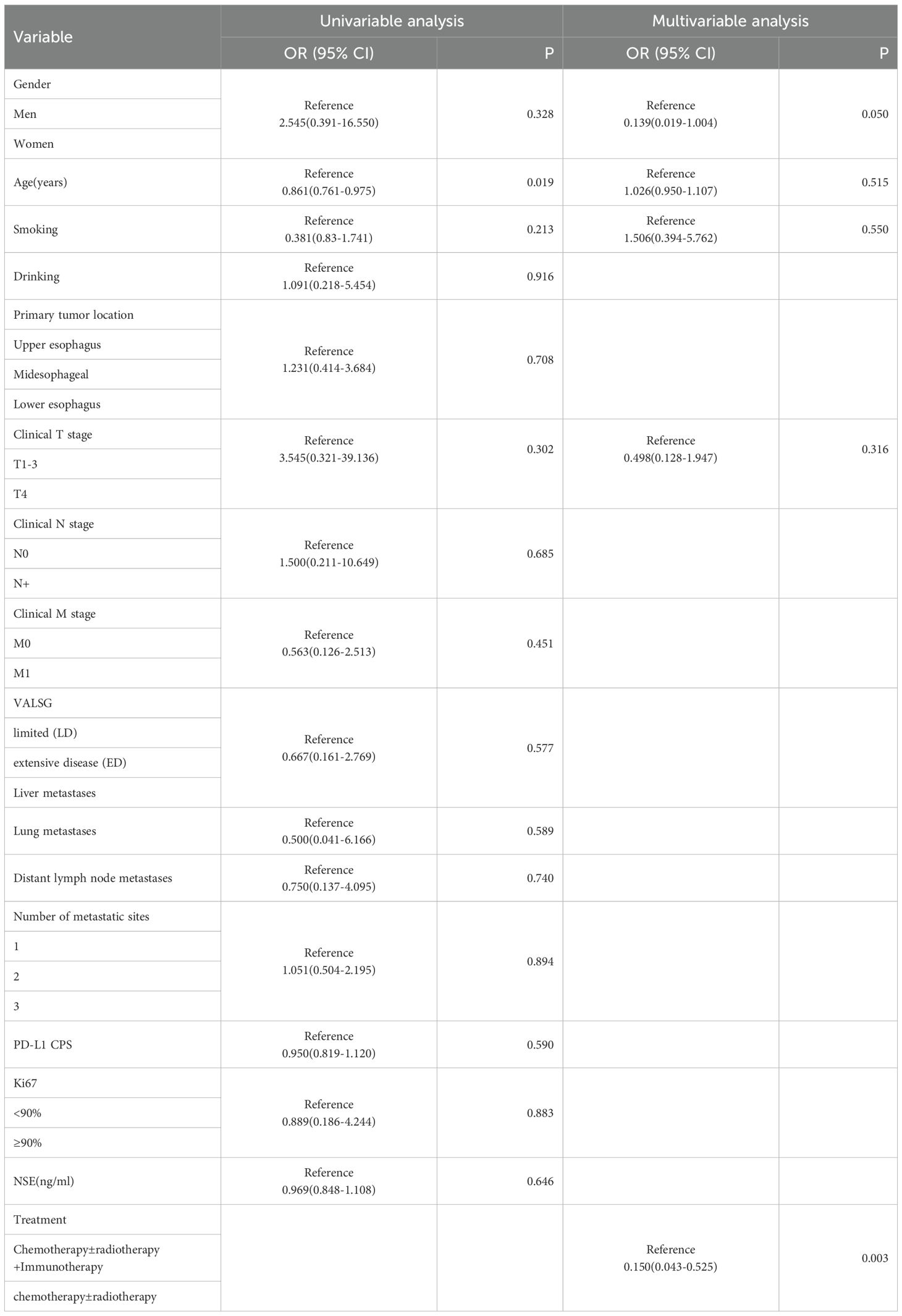
Four patients were lost to follow-up for OS. OS events were documented in 27 patients. Median OS for combined immunotherapy and noncombined immunotherapy were 17.0 months(95% CI 12.9-21.13) and 11.6 months (95% CI 4.7-18.6), respectively (p=0.055) (Figure 3). Multifactorial analyses were performed to identify clinical features that might affect OS of patients (Table 3).
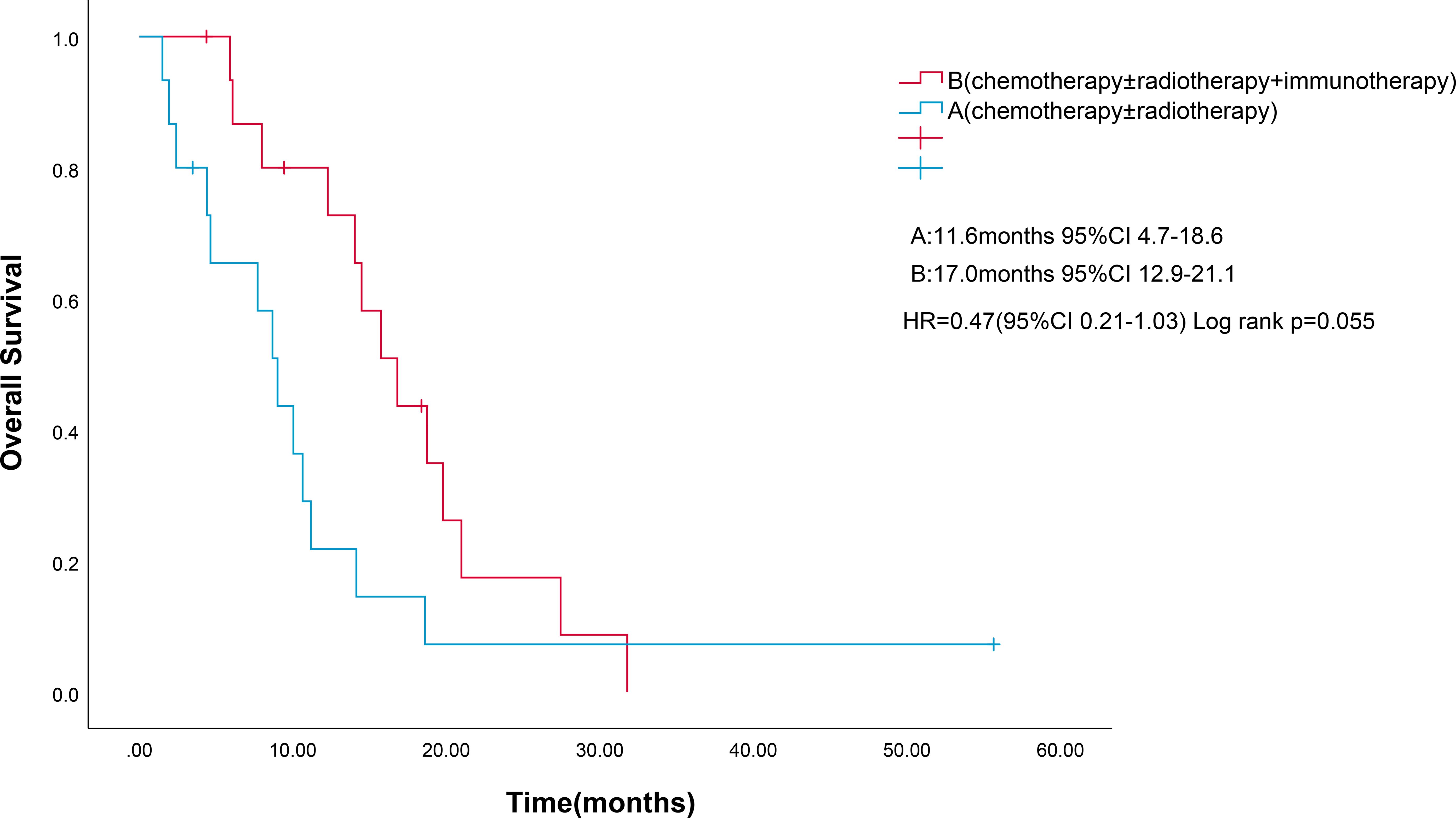
Figure 3. Kaplan-Meier survival estimates in OS. (A), chemotherapy and/or radiotherapy; (B), chemotherapy and/or radiotherapy plus ICIs; HR, hazard ratio; ICIs, Immune checkpoint inhibitors; OS, overall survival. .
3.4 Efficacy
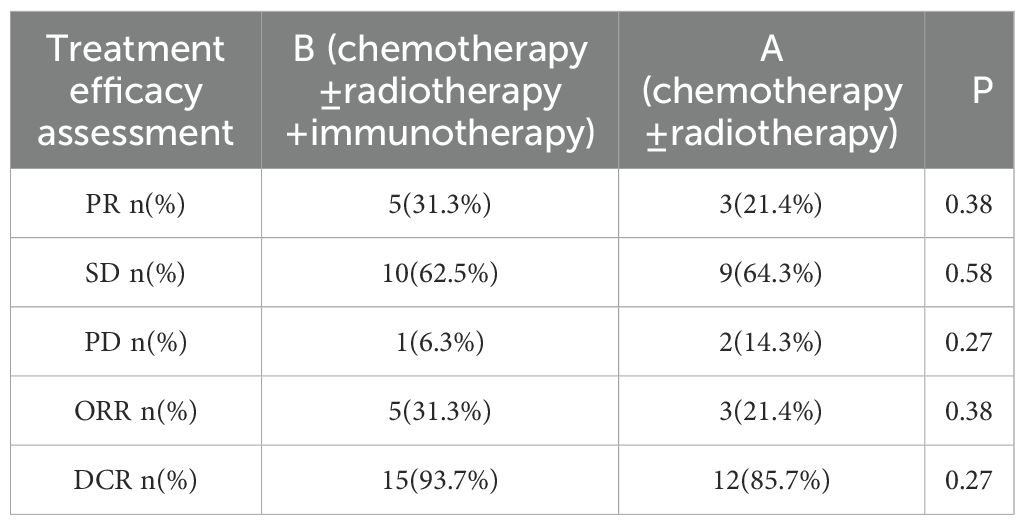
Treatment efficacy was evaluated in all patients. In Arm A, 3 patients (21.4%) achieved a partial response (PR), 9 patients (64.3%) had stable disease (SD), and 2 patients (14.3%) experienced progressive disease (PD). In Arm B, 5 patients (31.3%) achieved a PR, 10 patients (62.5%) had SD, and 1 patient (6.3%) experienced PD. The addition of ICIs was associated with a higher objective response rate (ORR: 31.3% vs. 21.4%) and disease control rate (DCR: 93.7% vs. 85.7%) compared to Arm A (Table 4).
3.5 Adverse events
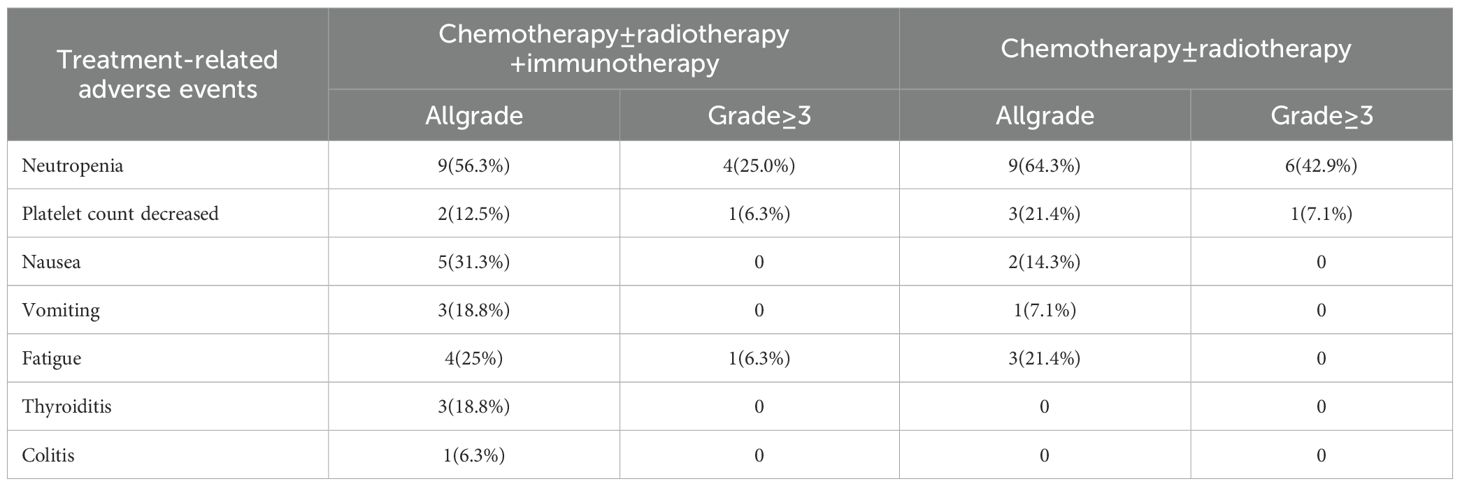
The most common adverse events (AEs) were neutropenia (Arm A: 64.3% vs Arm B: 56.3%) and nausea (Arm A: 14.3% vs Arm B: 31.3%). The rate of grade 3–4 neutropenia was 42.9% in Arm A compared to 25.0% in Arm B. Compared with Arm A, the addition of ICIs was associated with an increase in grade 1–2 immune-related adverse events (irAEs), with an incidence rate of 25.0% (including 3 cases of thyroiditis and 1 case of colitis) (Table 5).
4 Discussion
SCCE is a rare malignant tumor characterized by low incidence but high malignancy. Its biological behavior resembles that of small cell lung cancer, yet it carries a worse prognosis.
SCCE occurs predominantly in males (6), exhibiting high proliferative activity and rich neovascularization (7). Tumors are commonly located in the middle or lower third of the esophagus (8). In our study, the liver (n=8, 26%) was the most common metastatic site, followed by distant lymph nodes, consistent with previous research (9).
The staging of SCCE is primarily based on the UICC/AJCC TNM staging system and the Veterans Administration Lung Study Group (VALSG) criteria. For patients with stage cT1-2N0M0 SCCE, adjuvant therapy significantly improved survival compared to surgery alone (5-year overall survival [OS]: 32.8% vs. 19.2%; median survival time [MST]: 44.0 vs. 33.0 months; P = 0.035) (10). In contrast, for advanced SCCE, no standardized treatment guidelines currently exist. Consequently, most patients receive chemotherapy or chemoradiotherapy. While multiple studies have demonstrated that chemoradiotherapy can improve survival rates in advanced SCCE, further clinical trials are needed to establish the optimal treatment approach (11).
Recently, immune checkpoint inhibitors (ICIs) have emerged as a promising treatment strategy for esophageal cancer. Previous studies demonstrated that ICIs can alleviate immunosuppressive effects and enhance anti-tumor activity (12). ICIs have shown significant survival benefits in patients with esophageal squamous cell carcinoma, with superior responses observed in those expressing higher levels of PD-L1 (3).
Currently, ICIs combined with chemotherapy represent the standard first-line treatment for advanced esophageal carcinoma. Supporting evidence comes from trials in small cell lung cancer (SCLC), a disease with biological similarities to SCCE. The CASPIAN trial established the efficacy of durvalumab combined with chemotherapy in treatment-naïve extensive-stage SCLC, demonstrating a significant improvement in median overall survival (13.0 months vs. 10.3 months) (4). Similarly, the IMpower133 trial showed that atezolizumab plus chemotherapy significantly improved overall survival (OS) and progression-free survival (PFS) in extensive-stage SCLC (5, 13).
Consistent with this trend, our study found that the addition of ICIs was associated with significant improvements in median PFS (9.3 vs. 5.4 months, P = 0.046), objective response rate (ORR: 31.3% vs. 21.4%), and disease control rate (DCR: 93.7% vs. 85.7%). In our study, median OS of patients with combined immunotherapy showed a trend of prolongation (17.0 months vs. 11.6 months), but no statistically significant difference was observed (p = 0.055). This may be due to the fact that some patients added immunotherapy to second-line treatment. Multivariate analyses suggested that the combination of immunotherapy, or its absence, may affect patient prognosis.
However, due to the limited sample size of this study, PD-L1, TMB, and MSI data could not be obtained, and the study involved a variety of immune checkpoint inhibitors, which may have caused some deviation in the results. The small sample size (n=31) and the unbalanced group sizes (15 versus 16) in this study may cause some deviation in the results. Larger studies are warranted to confirm the efficacy of ICIs specifically in SCCE, perform subgroup analyses, and identify the patient populations most likely to benefit.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Fourth Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because due to the retrospective nature of this study, the ethics committee waived informed consent.
Author contributions
NL: Writing – original draft. RZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang C, Yu GM, Zhang M, Wu W, and Gong LB. S-1 plus apatinib followed by salvage esophagectomy for irinotecan-refractory small cell carcinoma of the esophagus: A case report and review of the literature. Med (Baltimore). (2020) 99:e18892. doi: 10.1097/MD.0000000000018892
2. Medgyesy CD, Wolff RA, Putnam JB Jr, and Ajani JA. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer. (2000) 88:262–7. doi: 10.1002/(SICI)1097-0142(20000115)88:2<262::AID-CNCR3>3.0.CO;2-K
3. Ren W, Zhang H, Li Y, Sun W, Peng H, Guo H, et al. Efficacy and safety of PD-1/PD-L1 inhibitors as first-line treatment for esophageal squamous cell carcinoma: a systematic review and meta-analysis. Front Immunol. (2025) 16:1563300. doi: 10.3389/fimmu.2025.1563300
4. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
5. Erciyestepe M, Ekinci ÖB, Doğan H, Öztürk AE, Aydın O, Büyükkuşcu A, et al. Factors affecting survival and prognosis in extensive stage small cell lung cancer. BMC Pulm Med. (2025) 25:160. doi: 10.1186/s12890-025-03625-w
6. Ku JW, Zhang DY, Song X, Li XM, Zhao XK, Lv S, et al. Characterization of tissue chromogranin A (CgA) immunostaining and clinicohistopathological changes for the 125 Chinese patients with primary small cell carcinoma of the esophagus. Dis Esophagus. (2017) 30:1–7. doi: 10.1093/dote/dox041
7. Koide N, Saito H, Suzuki A, Sato T, Koiwai K, Nakamura N, et al. Clinicopathologic features and histochemical analyses of proliferative activity and angiogenesis in small cell carcinoma of the esophagus. J Gastroenterol. (2007) 42:932–8. doi: 10.1007/s00535-007-2114-0
8. Vos B, Rozema T, Miller RC, Hendlisz A, Van Laethem JL, Khanfir K, et al. Small cell carcinoma of the esophagus: a multicentre Rare Cancer Network study. Dis Esophagus. (2011) 24:258–64. doi: 10.1111/j.1442-2050.2010.01133.x
9. Yang Y, Yu J, Chen S, Wang X, Wu F, Huang C, et al. A novel risk stratification system for primary small-cell carcinoma of the esophagus: indication for prognostication and staging. J Natl Cancer Cent. (2025) 5:212–20. doi: 10.1016/j.jncc.2025.02.003
10. Xu L, Yang YS, Li B, Cao YQ, Lin SY, Yu YK, et al. Multimodality therapy and survival outcomes in resectable primary small cell carcinoma of the esophagus: A multicenter retrospective study. Ann Surg Oncol. (2025) 32:848–59. doi: 10.1245/s10434-024-16532-x
11. Ji A, Jin R, Zhang R, and Li H. Primary small cell carcinoma of the esophagus: progression in the last decade. Ann Transl Med. (2020) 8:502. doi: 10.21037/atm.2020.03.214
12. He S, Xu J, Liu X, and Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. (2021) 11:3379–92. doi: 10.1016/j.apsb.2021.03.008
13. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. (2021) 39:619–30. doi: 10.1200/JCO.20.01055
Keywords: esophageal small cell carcinoma, immune checkpoint inhibitors, therapeutic efficacy, adverse event, prognostic analysis
Citation: Li N and Zhang Rx (2025) Treatment efficacy of first-line immunotherapy in advanced esophageal small cell carcinoma: A real-world retrospective study. Front. Oncol. 15:1630210. doi: 10.3389/fonc.2025.1630210
Received: 17 May 2025; Accepted: 12 September 2025;
Published: 02 October 2025.
Edited by:
Zhen Liu, Zhejiang University, ChinaReviewed by:
Jing Liu, Johns Hopkins University, United StatesHuazhen Xu, Johns Hopkins University, United States
Copyright © 2025 Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui xing Zhang, enJ4QG1lZG1haWwuY29tLmNu
 Nan Li
Nan Li Rui xing Zhang*
Rui xing Zhang*