- Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Guilin Medical University, Guilin, Guangxi, China
Primary Pulmonary Lymphoepithelial Carcinoma (PLEC) is a rare subtype of non-small cell lung cancer (NSCLC) that exhibits a strong association with Epstein–Barr virus (EBV) infection and shows distinctive geographic and ethnic predilections. Over the past decades, significant efforts have been made to elucidate the pathogenic mechanisms of PLEC, and progress in diagnosis, treatment, and disease monitoring has been achieved. This review focuses on EBV-driven oncogenic mechanisms in PLEC and explores the relationship between EBV infection, tumor progression, and clinical prognosis. We further summarize the molecular pathology, tumor immune microenvironment, and clinicopathological characteristics of PLEC. These insights may offer a theoretical foundation for EBV-targeted and immunotherapeutic strategies in PLEC.
1 Introduction
Lung cancer remains the second most commonly diagnosed malignancy and the leading cause of cancer-related deaths worldwide, accounting for an estimation of 2.2 million new cases and 1.8 million deaths annually (1–4). The majority (approximately 85%) of cases of lung cancers are classified as NSCLC according to the 2015 World Health Organization (WHO) classification of lung tumors (5). PLEC is a rare lung cancer subtype in NSCLC, characterized by undifferentiated carcinoma cells, ultrastructural features reminiscent of squamous cell carcinoma and abundant lymphoid stroma (6–9). The disease was first reported by Louis R. Berger and colleagues in 1987. Its prevalence shows a marked geographic bias and pertains to sex and age. Most of the cases were reported in Asia (mainly in Hong Kong and Guangdong, China), where young non-smokers were primarily affected, with female patients being more than male patients and concentrated ages between 51 and 55 years (10–15). The median age of onset of European patients is greater than that of the patients from Asia. The median age at diagnosis was 65 years (range: 15 years to 86 years) for European patients, in whom male patients and Caucasian patients accounting for 58.1% and 64.4%, respectively. Hence, the epidemiological characteristics of European patients with PLEC may be different from that of patients from Asia (16). In addition, over 90% of cases of PLEC from Asian are related to EBV infection. However, in the European and American population, the association of PLEC with EBV is low (8, 9). In the 2015 WHO classification, PLEC was categorized as other NSCLC or undifferentiated carcinoma, accounting for less than 1% of all NSCLC cases. In the 2021 WHO classification, PLEC has been reclassified as squamous cell carcinoma. Although PLEC has a more favorable prognosis compared to lung adenocarcinoma, squamous cell carcinoma, and large cell lung cancer (16–18), the five-year survival rate for PLEC remains around 74%, and effective improvement remains challenging (19, 20). This is attributed to a lack of diagnosis and treatment guidelines, clinical trials, and experience in targeted therapy and immunotherapy for PLEC. Therefore, we review the roles of EBV infection, molecular pathological changes, and immune features in PLEC, providing valuable insights for clinical research on targeted therapy and immunotherapy.
2 The pathogenicity of EBV in PLEC
2.1 EBV infection
EBV is among the most prevalent and persistent infections in humans. Approximately 95% of the world’s population experiences persistent, asymptomatic EBV infection throughout their lifetime, primarily affecting lymphocytes and oropharyngeal epithelial cells (21). There is evidence that the respiratory tract serves as the primary site of EBV hosting. The detection of EBV in bronchoalveolar fluid further suggests that lung tissue may serve as a primary reservoir for EBV (22). The life cycle of EBV encompasses two distinct phases: the latency period and the lytic phase (alternatively described as cleavage followed by reactivation). Following the initial infection which is typically asymptomatic, EBV establishes a lifelong persistent infection within the host. During latency, the EBV genome is replicated as an episome in the S phase of the cell cycle. Subsequently, these replicated episomes are distributed to daughter cells during cell division. The latent EBV expresses gene products that may possess carcinogenic properties, including Epstein-Barr virus nuclear antigen 1 (EBNA1), latent membrane protein-1 (LMP1) and microRNAs (miRNAs) (23–25). Notably, these gene products exhibit high expression levels in PLEC (15), indicating that the latent infection of EBV plays a significant role in the initiation and progression of PLEC. Osorio et al. addressed the epidemiological and experimental evidence of a potential role of EBV (26). However, the precise carcinogenic mechanisms underlying these associations remain to be elucidated through further investigation.
While EBV positivity has been considered a defining feature of PLEC, emerging studies have reported rare cases of EBV-negative PLEC. These EBV-negative cases challenge the current understanding of PLEC pathogenesis and suggest the existence of alternative oncogenic pathways. Studies from independent cohorts yielded similar results: patients with high EBV DNA before pretreatment or positive EBV DNA after treatment had significantly poorer progress free survival (PFS). Circulating EBV DNA levels provide prognostic value for survival and treatment response in patients with PLEC (27). Hence, elucidating the precise role of EBV in PLEC development is essential not only for understanding its typical viral-driven mechanism but also for distinguishing it from phenotypically similar but etiologically distinct tumors.
2.2 EBV-encoded gene products in PLEC
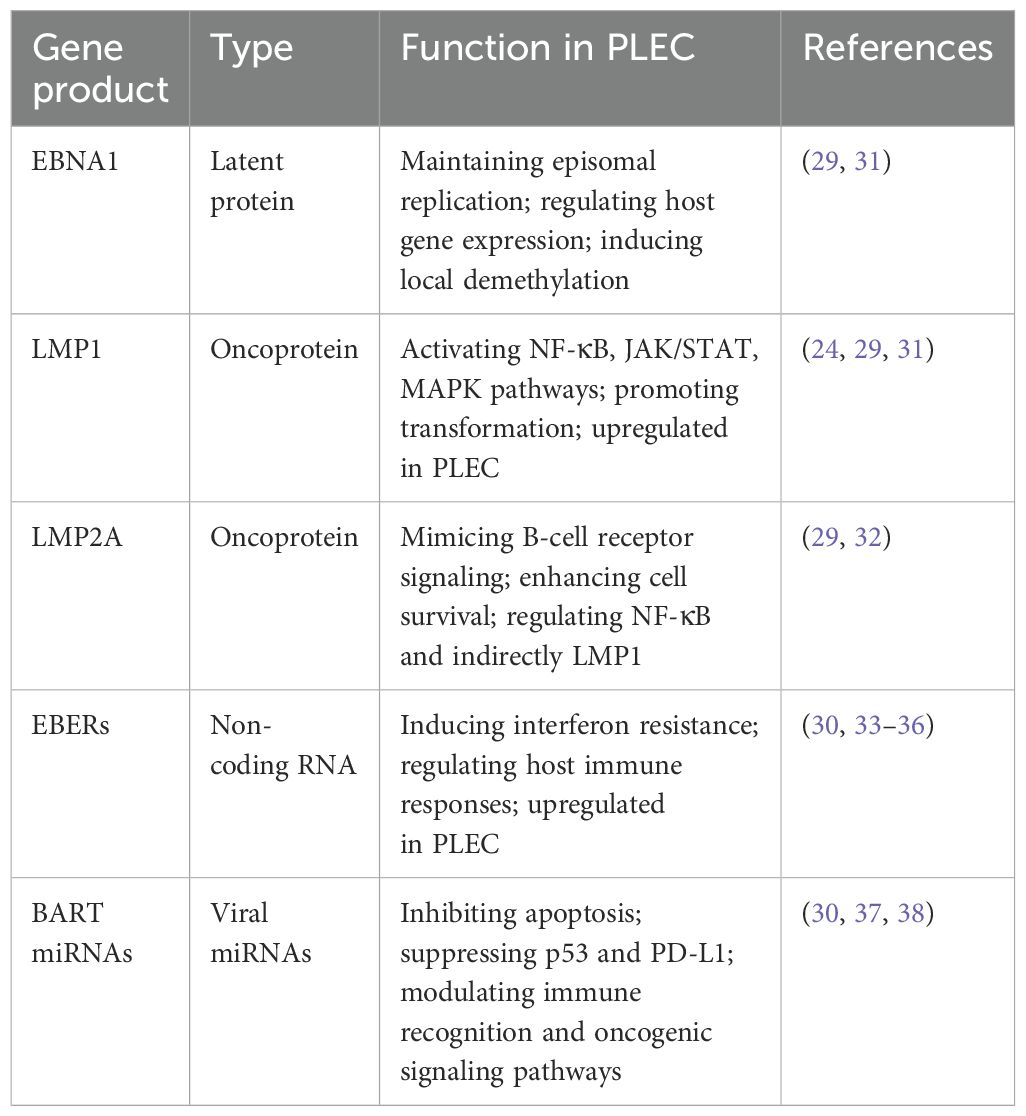
The role of EBV in tumorigenesis correlates with the expression levels of the latent genes and the extent of genomic aberrations in the host (28). EBV contributes to PLEC pathogenesis through the expression of multiple gene products, including latent proteins, non-coding RNAs, and virus-encoded miRNAs. In PLEC, EBV is primarily found in a latency type II infection pattern, with consistent expression of key latent genes such as LMP1, latent membrane protein 2A (LMP2A), and EBNA1, whereas EBNA2 is usually absent (29). In addition, EBV-encoded small RNAs (EBERs) and miRNAs are upregulated in tumor tissues. These products cooperatively regulate host signaling, immune evasion, and epigenetic modifications (30). The key viral gene products and their functional classifications are summarized in Table 1.
LMP1 is a constitutively active oncoprotein that mimics a tumor necrosis factor receptor (24). LMP1 plays a crucial role in the pathogenesis of EBV-associated tumors, such as nasopharyngeal carcinoma (NPC) and Hodgkin lymphoma (39, 40). Multiple studies have consistently reported that LMP1 expression is upregulated in PLEC tissues (15, 29, 41), reinforcing its oncogenic potential in a ‘pulmonary’ context. Mechanistically, LMP1 activates the tumor necrosis factor receptor-associated factor (TRAF)-mediated Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway (42, 43). In line with this, Hong et al. observed frequent deletions of TRAF3 and NFKBIA in PLEC samples, suggesting that disruption of the LMP1–TRAF3 axis may represent a key molecular mechanism driving PLEC tumorigenesis (44). Unlike in NPC, where LMP1 activates both canonical and non-canonical NF-κB pathways primarily via TRAF2, recent findings suggest that in PLEC, TRAF3 downregulation may drive more sustained non-canonical NF-κB signaling, which may contribute to a more immunosuppressive tumor environment and resistance to apoptosis in PLEC.
LMP2A has been identified as the typical latency type II transcript encoded by EBV, and it is expressed in PLEC (29). Preliminary genetic studies suggested that LMP2A was not essential for in vitro growth transformation of B cells (32). However, recent studies have demonstrated that LMP2A can function as a simulated receptor, thereby promoting the malignant transformation of B cells. Nevertheless, the precise oncogenic mechanism of LMP2A remains to be elucidated. Studies have shown that LMP2A can indirectly regulate the expression of LMP1 by modulating the activity of NF-kB in epithelial cells (45). Consequently, the interaction of LMP2A with LMP1 in PLEC may enhance its oncogenic potential.
EBNA1 is a pivotal factor in the establishment of latent infection of EBV in proliferating B cells. EBNA1 has been demonstrated to stimulate replication of DNA, regulate transcription of both virus and host genes, and tether the virus to cell chromosomes (31). It has been demonstrated that EBNA1 exhibits a high degree of affinity for the recognition site in the human genome. The binding of EBNA1 to its target sequence has been shown to cause local demethylation, thereby promoting the activation of silent cell promoters (46). In a study by Wu et al., the expression of EBNA1 was found to be up-regulated based on genome sequencing of EBV isolated from 78 PLEC patients and 37 healthy controls (29). Despite the established role of EBNA1 as an oncoprotein expressed in all EBV-associated tumors (47), further investigation is required to elucidate its potential carcinogenic effect in PLEC.
Non-coding RNAs are transcripts that are not translated into proteins. EBER1 and EBER2 are EBERs that possess high abundance (typically reaching up to 107 copies per cell) in latent infection (33). Their expression is considered the gold standard for identifying EBV-positive tumors via in situ hybridization (34). Chen et al. examined tumor tissues from 42 patients with PLEC by EBER detection, and 78.6% (33/42) showed positive results (48). Functionally, EBERs contribute to immune modulation and may promote cell survival. Recent findings indicate that EBER2 enhances B-cell growth by upregulating the deubiquitinase UCHL1, a process that may also be relevant in EBV-positive epithelial tumors (36).
EBV encodes two major clusters of miRNAs: miR-BHRF1 and miR-BART. The BamHI A rightward transcript (BART) cluster is preferentially expressed in epithelial tumors such as PLEC and NPC (37). Chen et al. performed RNA sequencing on fresh frozen tissues from eight patients with PLEC, and found that BART5-3P and BART20–3 were upregulated in PLEC, but not BART. The region between BART5-3P and BART20-3P is the dominant integration site of EBV. Functionally, miR-BART5-3p suppresses TP53-mediated apoptosis by directly targeting the 3′UTR of p53 mRNA, while miR-BART20-3p downregulates MICB, an NKG2D ligand, thereby reducing NK cell-mediated tumor surveillance. Of these eight patients, five had low expression of p53 and programmed death-ligand 1(PD-L1), and the prognosis was poor (30). It has been reported that the high expression of miR-BART in NPC promotes cancer development by targeting various cell and viral genes. Although primarily studied in NPC, the upregulation of miR-BART in PLEC suggests shared mechanisms of EBV-driven tumorigenesis (38).
2.3 EBV integration sites contribute to the initiation of PLEC
Chen et al. identified 288 integration breakpoints of EBV on chromosomes after whole exome sequencing in 128 PLEC patients. They found that the intergenic regions were the preferred integration sites of EBV in PLEC. EBV was easily integrated into the intergenic and intronic regions of two up-regulated miR-BARTs, namely BART5-3P and BART20-3P. These fragile regions were susceptible to DNA damage, which increases the possibility of EBV DNA insertion into the host genome and contributes to the occurrence of PLEC (30). Wu et al. identified 179 EBV host integration sites by bioinformatics methods, only 7 sites were validated by targeted PCR amplification and Sanger sequencing (29). These validated integration events occurred in three patients in advanced stages of PLEC (stages III and IV), suggesting to us that EBV integration may be somehow associated with the stage of tumor progression. The investigators identified an integration hotspot on chromosome 4q28.3 subband, a finding that is particularly striking because it may point to a key oncogenic mechanism. The hotspot was integrated twice in the same patient, further highlighting its potential importance in tumourigenesis. In addition, another integration breakpoint was located in the adjacent subband 4q31.21, which further narrowed the scope of the study and allowed scientists to more precisely investigate the effects of these integration events on gene expression and function (29). In summary, the above studies not only revealed the integration sites of EBV in host cells, but also preliminarily explored the effects of these integration events on gene expression and function. These findings provide new clues for a comprehensive insight of the mechanism of EBV-associated tumourigenesis, as well as potential targets for future therapeutic strategies.
2.4 Immune deficiency and EBV infection
Although the immune system can largely control EBV infection, the virus cannot be eliminated. To produce new viral progeny, EBV is reactivated from the latently infected cells. After analyzing the copy number variations of 46 cases of PLEC, Hong et al. found that the narrow region of 9p21.3 (chr9: 22028316-22041442) showed a focal and significant deletion, and the nearby region within 9p21.3 also showed a high-frequency deletion involving the type I interferon (IFN) gene. Type I IFN is a frontline defense against viral infection and a key component of host-virus confrontation. The level of CD8+ tumor-infiltrating lymphocytes (TILs) in tumors with the 9p21.3 deletion was lower than that in tumors without the 9p21.3 deletion. This suggests that frequent loss of type I IFN gene may lead to a lack of host immune response to the virus and persistent EBV infection in PLEC (44). These findings suggest that deletions in immune-regulatory loci, such as type I IFN genes on 9p21.3, may contribute to persistent EBV infection and immune evasion in PLEC (49).
2.5 Epigenetic susceptibility of the host genome in PLEC
EBV-encoded oncoproteins regulate cellular epigenetic mechanisms to reprogram viral and host epigenetic genomes, particularly in the early stages of infection (50). Abnormal epigenetic modifications mainly include CpG methylation and histone modification. In epithelial cells, LMP1 can upregulate DNA methyltransferase (46). Although LMP1 is highly expressed in PLEC (15, 41, 44), its carcinogenic effect through the regulation of epigenetic mechanisms needs further investigation.
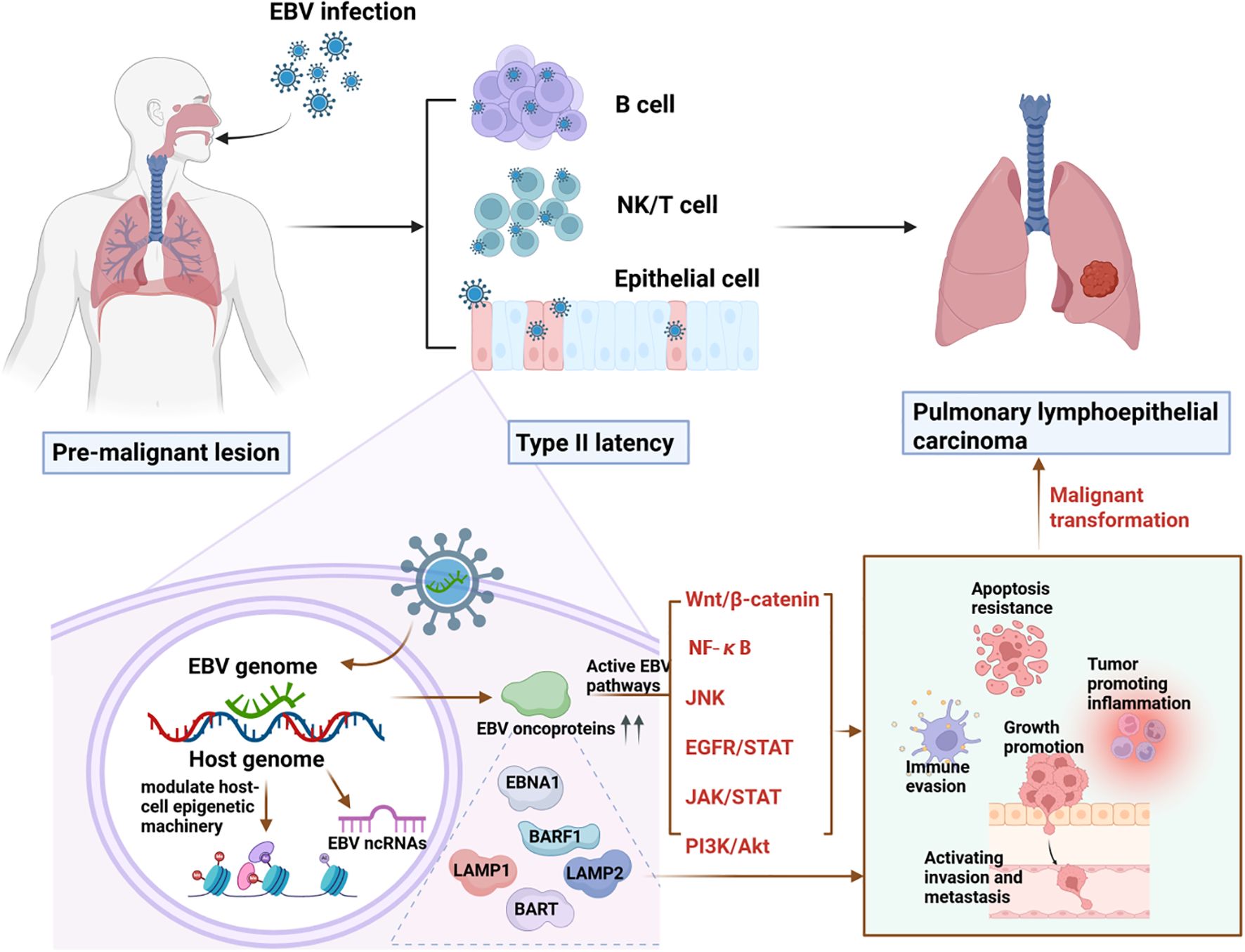
2.6 A proposed model for EBV-driven PLEC
EBV contributes to PLEC tumorigenesis through a sequential cascade involving epigenetic reprogramming, oncogenic signaling activation, and dysregulation of host gene expression. Thus, we propose a model for EBV-driven PLEC as shown in Figure 1. Initially, EBV infection alters epigenetic marks including DNA methylation and histone modifications in bronchial or alveolar epithelial cells, thereby disrupting normal gene expression to promote uncontrolled proliferation, and impair apoptosis. These epigenetic changes facilitate the activation of multiple oncogenic pathways, such as NF-κB, PI3K/AKT, and JAK/STAT, mediated by viral proteins like LMP1 and LMP2A. Furthermore, EBV-encoded gene products—including EBNA1, BARF1, and BART miRNAs—enhance tumor progression by fostering immune evasion, chronic inflammation, and resistance to apoptosis. Together, these mechanisms form an integrated network driving the initiation and advancement of PLEC.
3 Association of EBV with the progression and prognosis of PLEC
Serum EBV DNA not only holds significant value in diagnosis, but also provide valuable information for disease monitoring. Ngan et al. detected free EBV DNA in the serum of PLEC patients using quantitative real-time polymerase chain reaction (q-PCR) and found that the level of serum EBV DNA in patients with primary PLEC usually changes after treatment (51). A rapid decline in EBV DNA in the serum may be associated with a favorable response to treatment, while the rising level of EBV DNA after treatment may indicate drug resistance or recurrence of the tumor. Xie et al. determined the EBV DNA titer in 429 PLEC patients, further confirming the feasibility of monitoring treatment response in late-stage cases (52). They showed that serum EBV-DNA can reflect tumor burden and can be used as a tumor marker to assess treatment response. By tracking the EBV DNA levels before and after treatment, researchers can determine the duration of an effective treatment for PLEC patients.
EBV DNA positivity, particularly values exceeding 10,000 copies/mL, has been associated with poorer survival outcomes in PLEC. Several studies have demonstrated that cancer staging in patients with PLEC is closely correlated with changes in EBV DNA copy number. The results of a recent study showed that the median value of EBV DNA was as high as 29,500 copies/mL (ranging from 0 to 28,400,000 copies/mL), a value that is much higher than those of previous studies (13). This may be related to the fact that most of the PLEC patients selected in the study were in advanced stages. The study also found a significant association between high EBV DNA subgroups (i.e., ≥41,900 copies/mL) at baseline and poorer survival prognosis (13). Hence, EBV DNA may become an important biomarker for predicting patient prognosis.
4 Genetic landscape of PLEC
4.1 Copy number variations and altered signaling pathways in PLEC
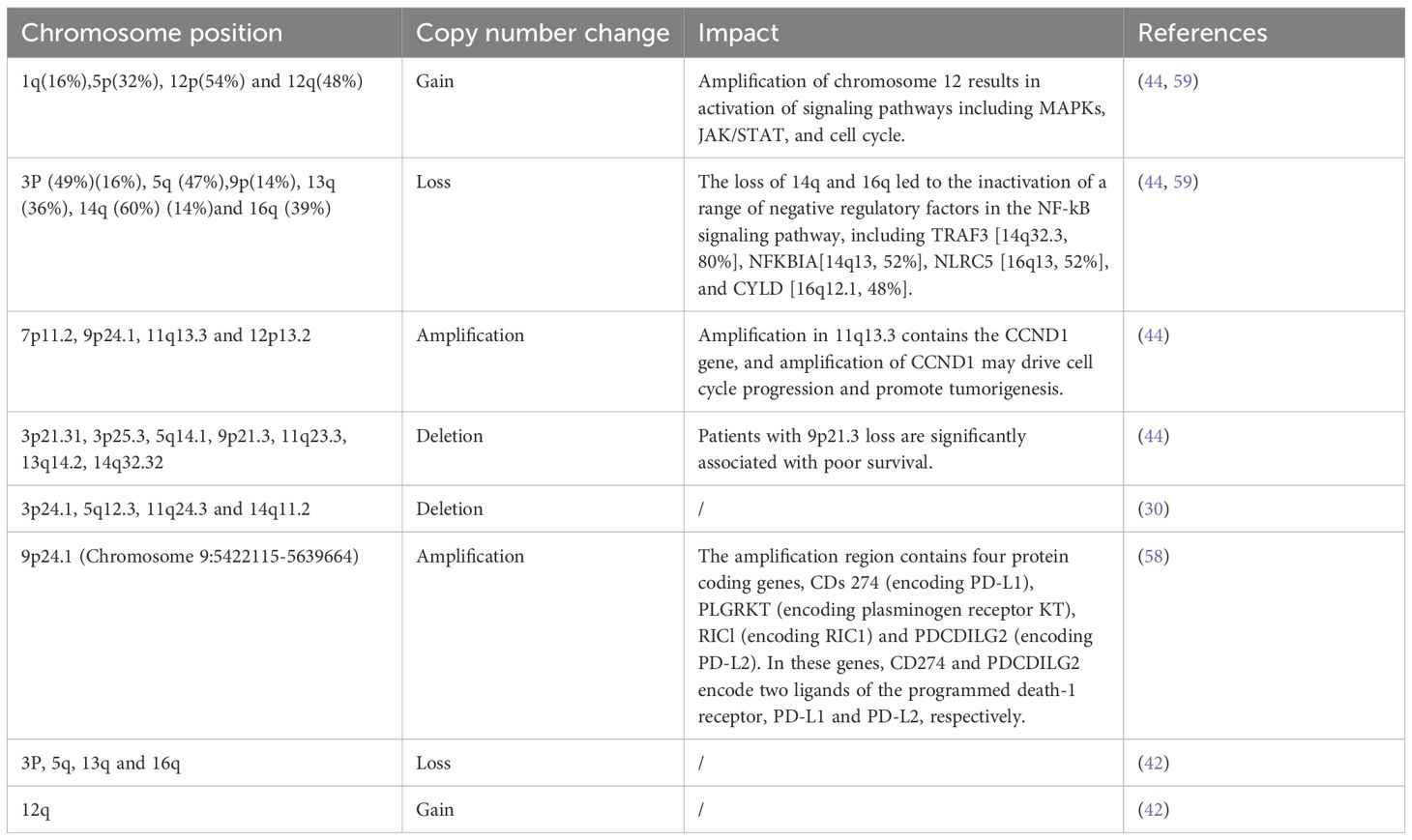
Numerous copy number variations (CNVs) have been identified in PLEC, and their biological and clinical impacts are summarized in Table 2. The CNVs, characterized by frequent gains in oncogenic regions and losses in tumor suppressor loci, play a crucial role in shaping the molecular landscape and immunogenicity of PLEC.
Based on the mutation profile, key signaling pathways associated with PLEC have been suggested in PLEC, such as cell cycle, JAK/STAT and NF-κB pathways. Mutations or deletions of TP53, amplification of MDM2 and CCND1, deletions of CDKN2A/B and RB1 are implicated in the dysregulation of cell cycle in PLEC (42, 44, 53, 54). The frequent dysregulation of the JAK/STAT pathway in PLEC is primarily due to the deletion of CISH, followed by mutations or deletions of PTPRD, and mutations or amplification of JAK2 (44). CISH encodes a cytokine-induced SH2-containing protein from the SOCS family, which serves as a key negative regulator of the JAK/STAT pathway (55). PTPRD encodes a tumor suppressor that negatively regulates JAK/STAT pathway by dephosphorylation and inactivation of STAT3 (56). In addition to deletions of key negative regulators of NF-κB signaling pathway (including TRAF3, CYLD, NFKBIA, and NLRC5), somatic mutations or amplification of several components of the canonical NF-κB pathway, including FADD, TRAF2, TRAF6 and CARD11, have been identified in PLEC. FADD is an apoptosis adaptor protein that activates the NF-κB signaling pathway through recruitment of caspase-8. TRAF2 and TRAF6 are members of the TRAF protein family that mediate NF-κB signaling pathway activation and participate in the regulation of inflammation, antiviral response and apoptosis (57).
Interestingly, studies have identified mutual exclusivity between LMP1 overexpression and the three key pathway (cell cycle, JAK/STAT and NF-κB) aberrations (44). Furthermore, several regulatory genes involved in immune escape, such as CD274 (PD-L1) and PDCD1LG2 (PD-L2), were found to be amplified in PLEC (44, 58). These findings suggest that both somatic alterations and viral factors may collaborate in the tumorigenesis and progression of PLEC.
4.2 Drive mutations in PLEC
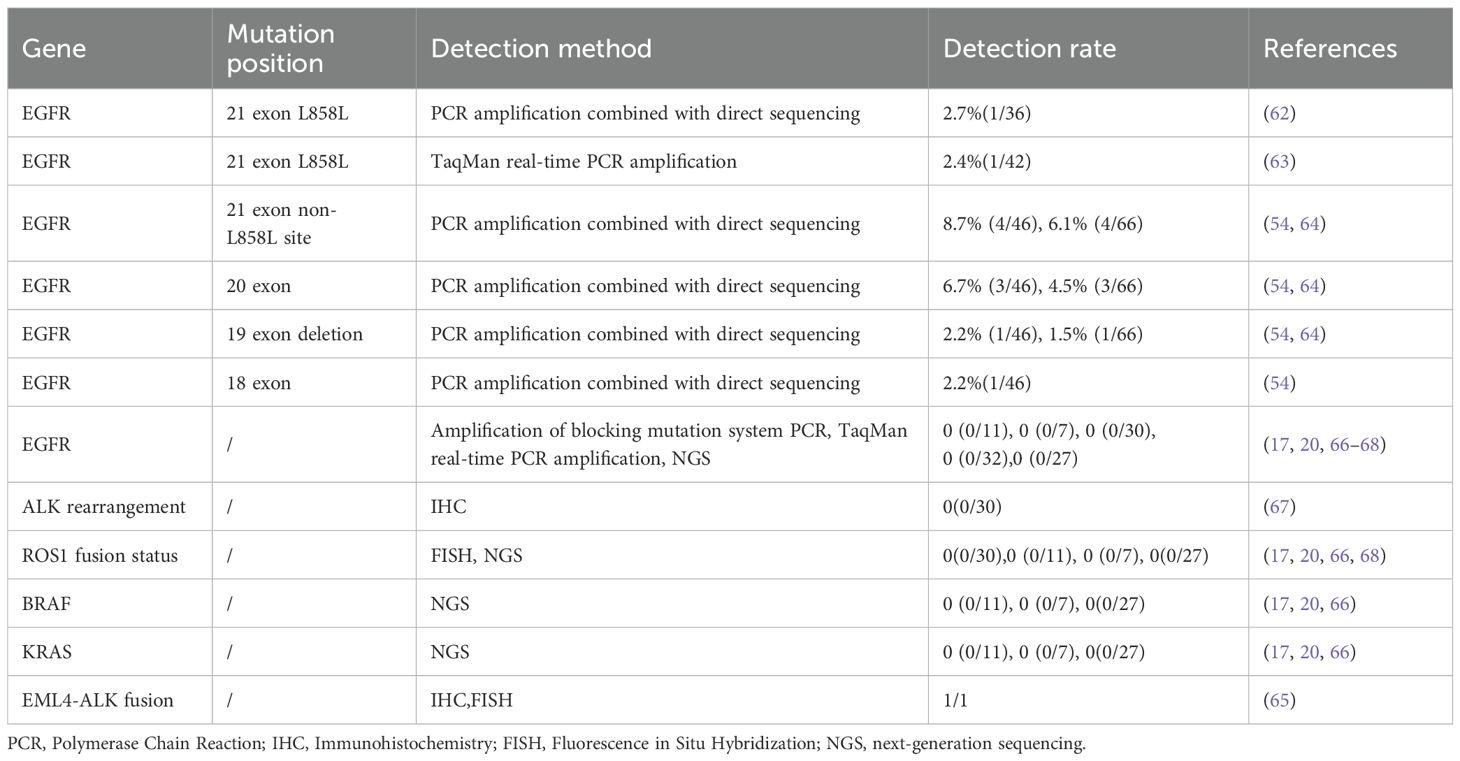
Studies have shown that approximately 73.9% of Chinese patients with NSCLC harbor at least one actionable mutation, as defined by the National Comprehensive Cancer Network guidelines, including mutations in the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma viral oncogene (KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and repressor of silencing 1 (ROS1) (60). EGFR mutations, including point mutations and small insertions/deletions, were the first identified pharmaceutically targetable mutations in NSCLC and remain the most widely used predictive biomarker for EGFR tyrosine kinase inhibitors. The most common EGFR mutations associated with sensitivity to tyrosine kinase inhibitors include the deletion of exon 19 (approximately 45% of patients with EGFR mutations) and the L858R mutation in exon 21 (approximately 40%) in NSCLC (61). In PLEC, the identified EGFR mutations include the L858R mutation in exon 21, non-L858R mutation in exon 21, deletions in exon 19, mutations in exon 20, and mutations in exon 18 (Table 3) (54, 62–64). Overall, alterations in EGFR and ALK genes are relatively rare in primary PLEC However, there may still be some special cases with EGFR or ALK gene alterations that interact with other factors or are related to individual differences of patients, tumor heterogeneity, or detective methods (65). The current data tentatively suggests that EGFR-targeted therapy is not suitable for patients with advanced PLEC due to lack of the typical driver mutations as observed in NSCLC. The low prevalence of classic driver mutations in PLEC implies that PLEC may be driven by a distinct tumorigenic pathway, rather than by conventional oncogenic mutations found in NSCLC.
5 Immune landscape of PLEC
5.1 Adaptive immune cells in PLEC microenvironment
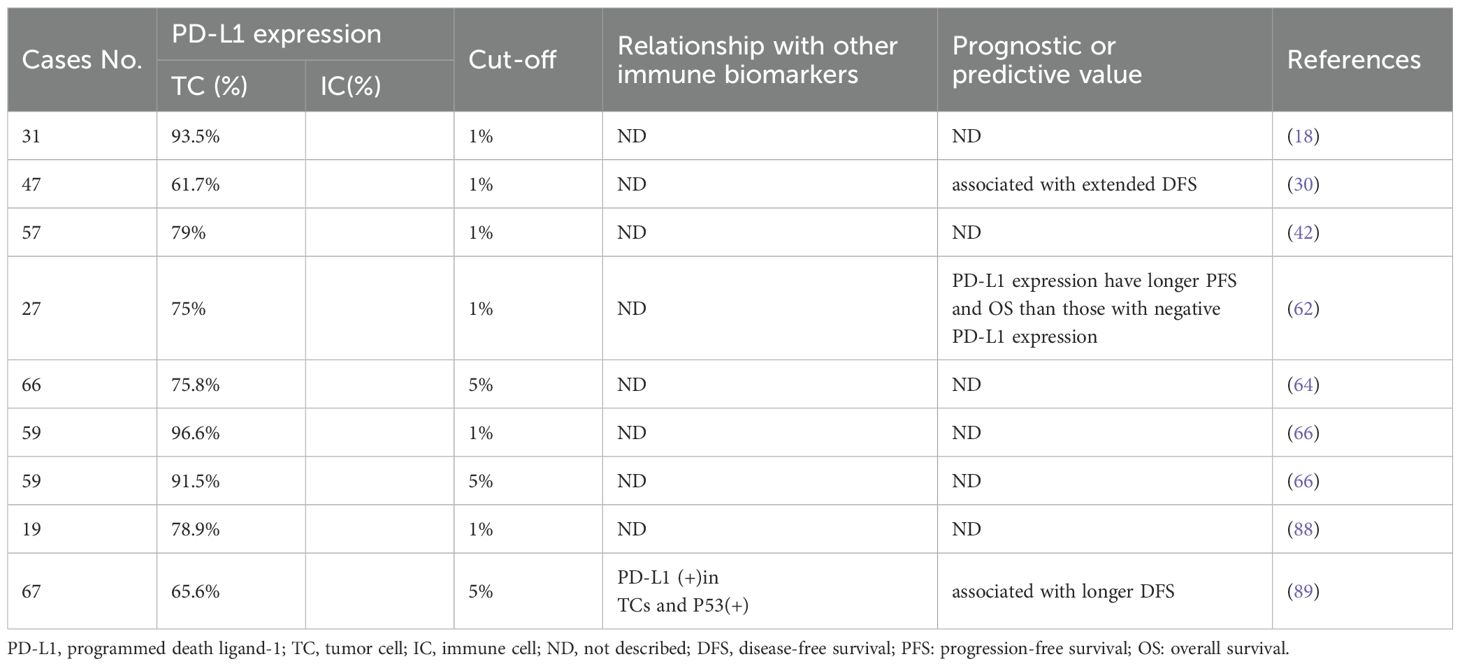
The tumor microenvironment (TME) refers to the complex milieu surrounding a tumor, encompassing cellular components, extracellular matrix (ECM), and vascular networks, which collectively influence tumor initiation, growth, and metastasis (Figure 2). TME usually includes immune cells, stromal cells, ECM and other secreting molecules, blood and lymphovascular network. The immune cell population within the TME includes T cells, B cells, tumor-associated macrophages (TAMs), dendritic cells (DC), natural killer cells (NK), neutrophils and myeloid suppressor cells (MDSCs), and others. The various subsets of immune cells in the TME exhibit distinct functions, which influence tumor progression through multiple mechanisms (69). Research has found that the recurrence risk of stage I NSCLC may be attributed to alterations in the immune and metabolic microenvironment (70). PLEC is a malignant epithelial tumor characterized by pronounced lymphocytic infiltrates. Kasai et al. observed TILs in EBV-positive PLEC. Most CD3-positive T cells were labeled as CD8 and TIA-1 positive but were negative for granzyme-B, indicating that TILs are resting cytotoxic T-lymphocyte (CTLs) (71). Chang et al. analyzed the lymphocyte composition in the stroma surrounding PLEC tumor cells by immunohistochemistry, and they found that CD8+ cells and B cells were present in all cases. In total, the number of stained cells of CD8 positive cells exceeded that of B cells (72). The results of the study by Kobayashi et al. showed that in PLEC, infiltrated T cells were more than B cells, and CD8 positive cells were more than CD4 positive cells (73). Yo Kawaguchi reported a case about a 70-year-old male with PLEC, in which tumor regression occurred during treatment with selective serotonin reuptake inhibitors (SSRIs). Subsequently, histological examination revealed infiltration of CD3+, CD4+ and CD8+ lymphocytes around the tumor. It has been hypothesized that SSRIs may activate these lymphocytes, potentially leading to spontaneous tumors regression (74). There are also studies reporting the impact of neoadjuvant therapy on PD-L1 expression and CD8+ lymphocyte density in NSCLC (75).Studies have demonstrated that EBV-specific CD8+ TILs in EBV-driven PLEC exhibit heterogeneity and partial deficiency in PD-1 expression (76). Furthermore, other researchers have identified polyclonal plasma cells, polymorphonuclear granulocytes, and CD56+ NK cells, alongside CD3+ T-lymphocytes and CD79a+ B-lymphocytes among the infiltrating cells in PLEC (77, 78). To date, the relationship between lymphocyte infiltration and patient prognosis remains debated in PLEC. The distribution of various lymphatic subpopulations in PLEC requires further elucidation through single-cell sequencing and large multicenter studies (79).
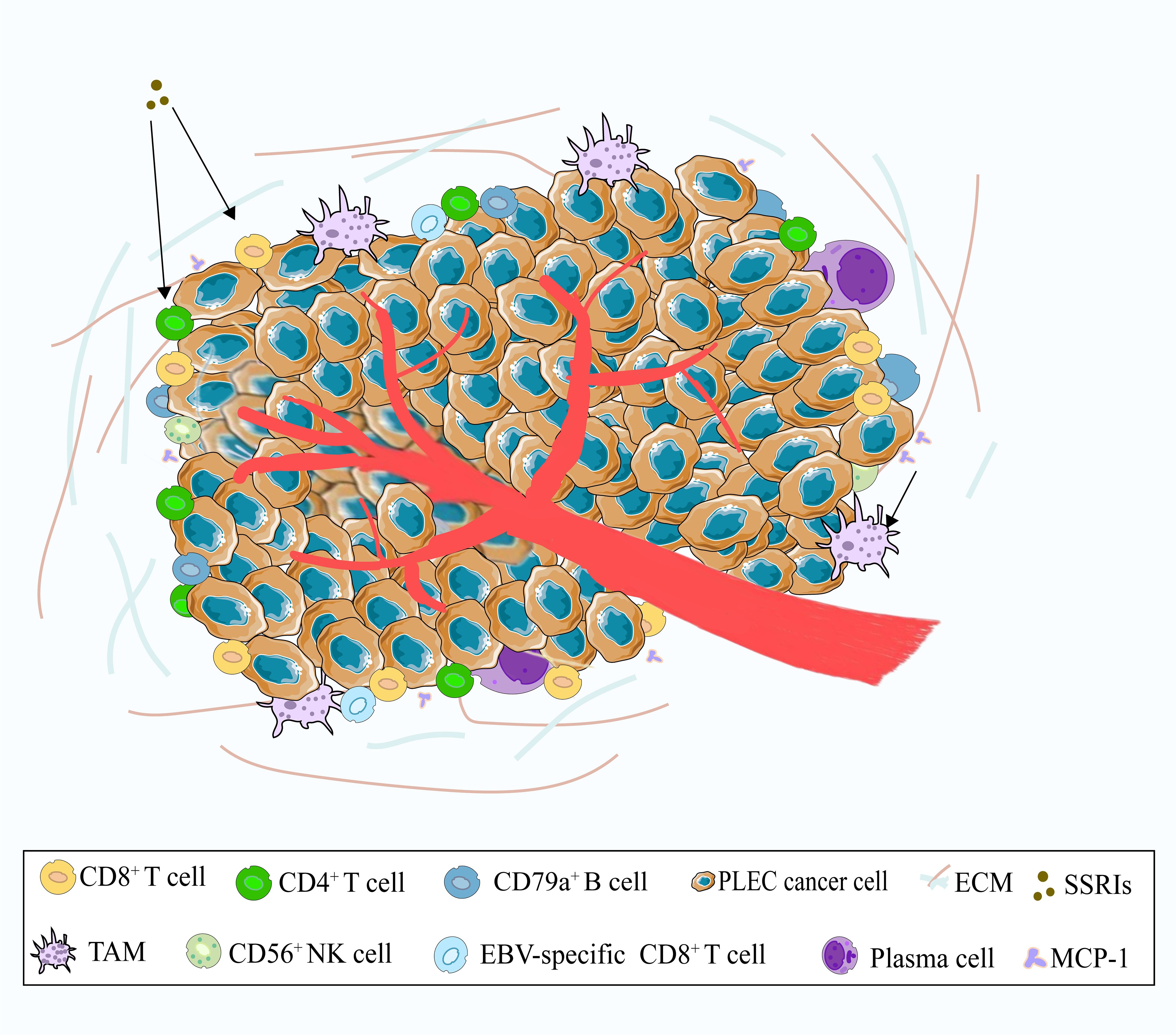
Figure 2. A schematic illustration of the tumor microenvironment of PLEC. The main immune cells include CD8+ T cells, CD4+ T cells, CD79a+ B cells, and TAMs. Other cells such as EBV-specific CD8+ T cells, CD56+ NK cells, and plasma cells are relatively rare. In addition, SSRIs may activate CD8+ T cells and CD4+ T cells, while MCP-1 may recruit TAMs.
5.2 TAMs in PLEC microenvironment
TAMs are among the tumor-infiltrating immune cells (TIIC) within tumor microenvironment. TAMs are derived from MDSCs or monocytes and play pivotal roles in promoting tumor growth, metastasis, angiogenesis, and immunosuppressive functions (80). PLEC is closely associated to a variety of mononuclear inflammatory cells, including a substantial population of TAMs, which contribute to its pathophysiology. The study of Wong et al. demonstrates that the expression of monocyte chemoattractant protein-1 (MCP-1) in PLEC tumor cells plays a critical role in the recruitment of TAMs in PLEC (81). Wang et al. show that pre-treatment monocyte-to-lymphocyte ratios (MLR) may serve as an independent prognostic marker in patients with PLEC and could guide the optimization of treatment strategies (82). TAMs in PLEC tumors are thought to contribute to the inhibition of anti-tumor immune response. However, given the limited sample sizes in previous studies, this hypothesis requires further validation in larger cohort studies.
5.3 Expression and clinical significance of PD-L1 in PLEC
The programmed cell death protein 1 (PD1) - PD-L1 axis presents a critical immune checkpoint pathway, which can be hijacked by cancer cells to evade immune surveillance (83, 84). PD-L1 expression is a biomarker for improving the survival of advanced PLEC and the potential effectiveness of immunotherapy (85). Blockade of PD1-PD-L1 axis in NSCLC has led to durable objective response and significantly improved survival compared to conventional therapies (86). The combination of PD-1/PD-L1 and lymphocyte activation gene 3 (LAG-3) is associated with the clinical activity of immune checkpoint inhibitors (ICIs) in metastatic PLEC (87). Studies have shown that PD-L1 expression in PLEC is elevated compared to the average levels observed in NSCLC, suggesting that immunotherapy may be a promising treatment strategy for PLEC (18, 64). However, the prognostic value of PD-L1 expression in PLEC tumor cells remains controversial (88, 89). The discrepancies in findings across studies may be attributable to several factors, including the types of PD-L1 antibodies used, varying thresholds for defining positive expression, and differences in specimen collection methods (Table 4). These differences highlight the urgent need for standardized PD-L1 assessment protocols in PLEC, Multi-center, large-sample clinical studies aiming to address the consistency of efficacy of different PD-L1 expression cut-offs and PD-L1 antibodies have become important for the immunotherapy of PLEC.
5.4 Therapeutic prospects
Currently, there are no standardized treatment protocols for PLEC due to its rarity. However, its association with EBV infection, high PD-L1 expression, and activation of oncogenic signaling pathways such as NF-κB suggests several potential therapeutic strategies (Figure 3).
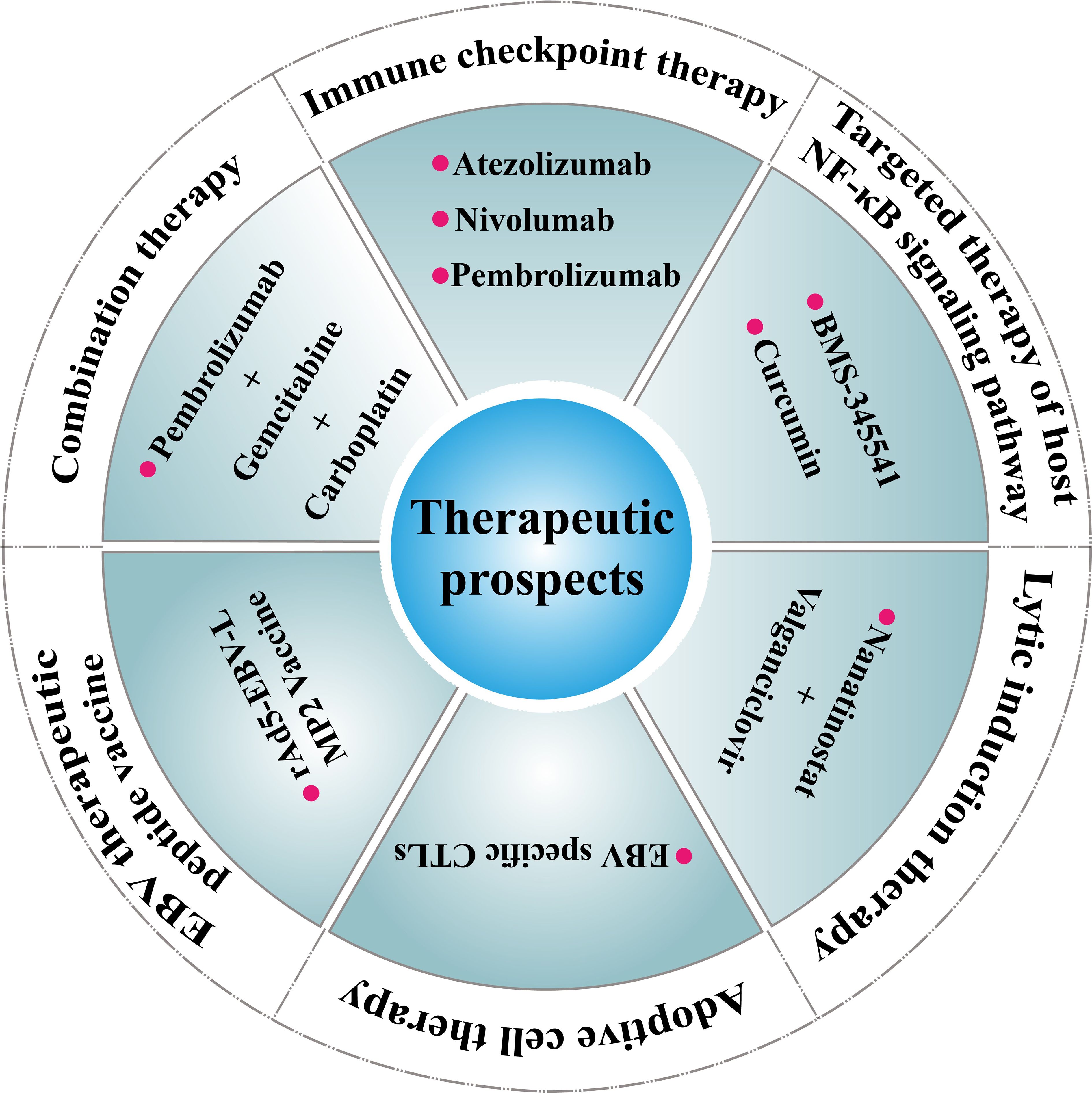
Figure 3. Therapeutic prospects for PLEC. Immune checkpoint therapy (e.g., Atezolizumab, Nivolumab, Pembrolizumab) Ref (90); Targeted therapy of host NF-κB signaling pathway (e.g., BMS-345541, Curcumin Ref) (93); Adoptive cell therapy (e.g., EBV specific CTLs Ref (96); Lytic induction therapy (e.g., Nanatinostat + Valganciclovir) Ref (97); EBV therapeutic peptide vaccine (e.g., rAd5-EBV-LMP2 Vaccine) Ref (98); Combination therapy (e.g., Pembrolizumab+Gemcitabine+Carboplatin) Ref (99).
ICIs have shown promise in EBV-associated tumors and may offer clinical benefit in PLEC (90, 91). Case series and retrospective analyses have reported durable responses to PD-1/PD-L1 blockade in patients with advanced or relapsed disease. Nonetheless, variability in PD-L1 detection methods, cut-off thresholds, and tumor heterogeneity complicates patient selection. Refinement of biomarker assessment—including co-expression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Lymphocyte Activation Gene 3 (LAG3), or tumor mutational burden (TMB) status—may help identify responders more accurately (92).
Given that LMP1-mediated activation of the NF-κB pathway is a hallmark of EBV-driven oncogenesis, this axis represents another promising target. Preclinical studies in related EBV-positive malignancies have explored the use of NF-κB pathway inhibitors, such as proteasome inhibitors or natural compounds (93), though their clinical efficacy in PLEC remains untested (94, 95).
In addition, EBV-directed therapies are under development. Approaches such as lytic induction therapy combined with antiviral agents (e.g., ganciclovir), adoptive transfer of EBV-specific CTLs, and EBV peptide vaccines have shown encouraging results in NPC and post-transplant lymphoproliferative disorders (96–98). These modalities could be translated to PLEC with further validation (18, 95).
Combination therapies, such as ICIs plus chemotherapy, radiotherapy, or anti-angiogenic agents, may also enhance antitumor responses by modulating both tumor and immune compartments (99). Given the immunologically active microenvironment of PLEC, multimodal regimens may offer a rational approach, though prospective clinical trials are needed (92).
Briefly, these emerging strategies highlight the importance of tailoring treatment based on both viral and immune profiles in PLEC. Future research should focus on validating these approaches in clinical settings and establishing disease-specific therapeutic guidelines.
6 Histopathological diagnosis and immunohistochemical features of PLEC
Although the molecular and immunological features of PLEC have been well characterized, histopathological evaluation remains essential for accurate diagnosis, particularly in differentiating PLEC from poorly differentiated squamous cell carcinoma. This section summarizes the key histological and imaging features of PLEC, including its lymphoepithelioma-like morphology and immunohistochemical profile, which complement molecular findings (Figure 4). Studies have shown that PLEC primarily occurs in the right middle lobe and left lower lobe of the lung. This conclusion has been corroborated by retrospective studies, and further investigations conducted in Macao suggest that the distribution of PLEC in the lungs follows a specific pattern (100–102). PLEC typically presents as isolated, solitary, round or oval lesions, lacking a capsule and well-defined borders upon gross examination. The cross-sectional appearance is characterized by a pale white or brown color, with good elasticity and a fish-like shape, rarely exhibiting necrosis or cavitation (72, 103, 104). These features are helpful for the initial identification of PLEC during surgery. Microscopically, PLEC is characterized by small, nest-like or patchy hyperplasia of epithelial tumor cells, which were separated by abundant lymphoid stroma or lymphocyte aggregates. The tumor cell nuclei are large, round or oval with prominent nucleoli (11, 105–107). Although PLEC may be histologically confused with Undifferentiated Nasopharyngeal carcinoma (UNPC), it exhibits features such as granulomatous inflammation, focal keratosis, alveolar space diffusion and squamous differentiation pattern, which distinguish it from UNPC (79). In addition, PLEC and lung squamous cell carcinoma (LUSC) exhibit similar biomarkers expression patterns in immunohistochemical analysis, indicating that PLEC displays the characteristics of squamous cell differentiation. However, the abundant lymphocyte infiltration and large tumor cell nuclei in PLEC distinguish it morphologically from LUSC (48). Recent studies have identified a spectrum of morphological characteristics in PLEC. At one end of this spectrum, classic PLEC is characterized by stroma rich in lymphocyte infiltration, while at the other end, it resembles poorly differentiated squamous cell carcinoma with minimal interstitial lymphocyte infiltration. This spectrum change suggests that PLEC with absent interstitial lymphocyte infiltration is frequently misdiagnosed as squamous cell carcinoma in clinical practice (79). Therefore, comprehensive pathological and immunohistochemical analysis should be conducted for suspected PLEC cases to prevent misdiagnosis. The clinicopathological features, especially the spectrum of lymphoid infiltration and histological overlapping with squamous cell carcinoma, underscore the diagnostic challenge of PLEC and the importance of combined morphological and molecular evaluation.
Figure 4. Histopathological and diagnostic characteristics of PLEC. This schematic summarizes key features of PLEC including favored anatomical locations, gross morphology, microscopic features, tumor lineage, and differential diagnosis.
The immunohistochemical pattern is characterized by reduced p53, deletion of c-erbB-2 and high expression of PD-L1. The combination of these features not only helps us understand its tumor biological mechanism, but also provides important clues for clinical treatment. The reduced expression of p53 may be related to the uncontrolled proliferation of tumor cells and the increased tolerance to DNA damage. Specifically, the absence of p53 enables tumor cells to evade normal cell cycle checkpoints and apoptotic procedures, further promoting the proliferation of tumor cells and the malignant transformation of tumors. The absence of c-erbB-2 is a common feature. This absence may indicate that the dependence of tumor cells on external growth signals is weakened, and the growth of tumors no longer depends on the excessive activation of c-erbB-2. The deletion of c-erbB-2 may also lead to tumor cells activating proliferation signals through other alternative mechanisms, thereby maintaining their proliferation and survival abilities. PLEC often shows high expression of PD-L1, which indicates that the tumor may adopt an immune escape strategy to avoid recognition and clearance by the host’s immune system. This immunohistochemical pattern provides us with a more comprehensive biological perspective of PLEC and important information for developing treatment strategies.
7 Unresolved questions and future challenges
Despite growing insights into the molecular and immune features of PLEC, several key questions remain unanswered. First, the prognostic significance of PD-L1 expression in PLEC is inconsistent. While some studies link PD-L1 positivity to favorable outcomes, others report no correlation (17, 108). These discrepancies may reflect differences in cut-off thresholds (e.g., 1% vs. 5%), antibody clones, detection platforms, and limited sample sizes. Standardized protocols and larger, prospective studies are needed to clarify its clinical relevance. Second, although EBV latency type II is shared with other EBV-driven tumors such as NPC and EBV-associated gastric cancer (EBVaGC), its downstream effects in PLEC remain poorly defined (18, 109, 110). In NPC, latency II activates LMP1/NF-κB and LMP2A/PI3K-Akt pathways, promoting immune escape and tumor progression. PLEC, however, appears to exhibit distinct immune infiltration patterns and unique EBV-encoded miRNAs expression profiles (e.g., BART5-3p, BART20-3p), possibly targeting lung-specific pathways such as interferon signaling (30). The tissue-specific consequences of latency II in the pulmonary context require further investigation. Third, the immune microenvironment of PLEC is still poorly characterized (109). Single-cell or spatial transcriptomic analyses have yet to be applied, limiting understanding of immune cell composition, heterogeneity, and interactions with EBV-infected cells. In addition, EBV strain diversity in lung tumors and its clinical impact remain unexplored. Finally, the lack of in vivo models or lung organoids for EBV infection hinders functional validation of proposed mechanisms (109). Addressing these gaps will require integrative genomic and immunologic studies, improved model systems, and cross-disciplinary collaboration. A deeper understanding of these unresolved issues is essential for advancing diagnosis and therapy in PLEC.
8 Conclusion and future perspectives
PLEC is a rare subtype of NSCLC, and its development and progression are influenced by genetic alterations, immune activity, and EBV infection. Although some progress has been made in understanding the molecular pathobiology of PLEC, large-scale clinical studies are still needed to clarify its molecular mechanisms, clinical characteristics, and to inform standardized treatment strategies. In the future, it will be interesting to compare the transcriptomic profiles between infected cells and adjacent non-infected cells using single-cell multi-omics technologies to identify virus-induced early carcinogenic events in PLEC. Additionally, patient-derived organoids from PLEC may be constructed, and used in combination with efficient gene-editing tools such as CRISPR-associated protein 9 (CRISPR-Cas9) to evaluate the impact of genetic biomarkers on drug response. This approach will facilitate drug development and advance precision medicine for PLEC.
Author contributions
MX: Writing – original draft. DY: Writing – review & editing. LL: Writing – review & editing. CT: Writing – review & editing. BM: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No.82060006, 82160002, 82160014), Guangxi Key Research and Development Plan (No. GuiKe AB24010096), Specific Research Project of Guangxi for Research Bases and Talents (No. GuiKe AD24999032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leiter A, Veluswamy RR, and Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
2. Oliver AL. Lung cancer: epidemiology and screening. Surg Clin North Am. (2022) 102:335–44. doi: 10.1016/j.suc.2021.12.001
3. Bade BC and Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. (2020) 41:1–24. doi: 10.1016/j.ccm.2019.10.001
4. Barta JA, Powell CA, and Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. (2019) 85. doi: 10.5334/aogh.2419
5. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
6. Priya A, Spalgais S, Kulshrestha R, and Kumar R. Primary pulmonary lymphoepithelial-like carcinoma: A rare childhood Malignancy. Med J Armed Forces India. (2023) 79:220–4. doi: 10.1016/j.mjafi.2021.08.006
7. Samaras MG, Koufopoulos N, Mitsos S, Dylja E, Monokrousou A, Tomos P, et al. Lymphoepithelial carcinoma of the lung: A case report and review of the literature. Cureus. (2024) 16:e70309. doi: 10.7759/cureus.70309
8. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
9. Giesen C, Del Aguila Mejia J, Armon S, Cierco Jimenez R, Myles N, Goldman-Levy G, et al. Exploratory evidence maps for the WHO Classification of Tumours 5th edition for lung and thymus tumors. Virchows Arch. (2024) 485:869–78. doi: 10.1007/s00428-024-03886-6
10. Begin LR, Eskandari J, Joncas J, and Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. (1987) 36:280–3. doi: 10.1002/jso.2930360413
11. Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao H, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer. (2012) 118:4748–58. doi: 10.1002/cncr.27452
12. Castro CY, Ostrowski ML, Barrios R, Green LK, Popper HH, Powell S, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol. (2001) 32:863–72. doi: 10.1053/hupa.2001.26457
13. Zou Q, Luo K, Kang L, Huang C, Mai J, Lin Y, et al. Clinical significance of baseline Epstein-Barr virus DNA for recurrent or metastatic primary pulmonary lymphoepithelioma-like carcinoma. Future Oncol. (2023) 19:2481–92. doi: 10.2217/fon-2023-0722
14. Zhong YM, Chen J, Jiang J, Zhou WB, Gao LL, Zhang SL, et al. Plasma EBV quantification is associated with the efficacy of immune checkpoint blockade and disease monitoring in patients with primary pulmonary lymphoepithelioma-like carcinoma. Clin Transl Immunol. (2024) 13:e1515. doi: 10.1002/cti2.1515
15. Han AJ, Xiong M, and Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol. (2000) 114:220–6. doi: 10.1309/148K-ND54-6NJX-NA61
16. He J, Shen J, Pan H, Huang J, Liang W, and He J. Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Dis. (2015) 7:2330–8. doi: 10.3978/j.issn.2072-1439.2015.12.62
17. Zhang Q, Dai Y, Jin L, Shi S, Liu C, Rong R, et al. Clinicopathological characteristics and cancer-specific prognosis of primary pulmonary lymphoepithelioma-like carcinoma: a population study of the US SEER database and a Chinese hospital. Front Oncol. (2023) 13:1103169. doi: 10.3389/fonc.2023.1103169
18. Zhou Y, Huang J, Lan J, Hu H, Yuan Z, Dong L, et al. Comparison of first-line immunotherapy efficacy between advanced lung squamous cell carcinoma and pulmonary lymphoepithelioma-like carcinoma: A propensity score matching multicenter study. J Cancer Res Ther. (2023) 19:1011–8. doi: 10.4103/jcrt.jcrt_2711_22
19. Jiang WY, Wang R, Pan XF, Shen YZ, Chen TX, Yang YH, et al. Clinicopathological features and prognosis of primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis. (2016) 8:2610–6. doi: 10.21037/jtd.2016.08.40
20. Fan Y, Shan Q, Gong J, Qin J, and Lu H. Molecular and clinical characteristics of primary pulmonary lymphoepithelioma-like carcinoma. Front Mol Biosci. (2021) 8:736940. doi: 10.3389/fmolb.2021.736940
21. Yin H, Qu J, Peng Q, and Gan R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med Microbiol Immunol. (2019) 208:573–83. doi: 10.1007/s00430-018-0570-1
22. Lung ML, Lam WK, So SY, Lam WP, Chan KH, and Ng MH. Evidence that respiratory tract is major reservoir for Epstein-Barr virus. Lancet. (1985) 1:889–92. doi: 10.1016/s0140-6736(85)91671-x
23. Wilson JB, Manet E, Gruffat H, Busson P, Blondel M, and Fahraeus R. EBNA1: oncogenic activity, immune evasion and biochemical functions provide targets for novel therapeutic strategies against epstein-barr virus- associated cancers. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10040109
24. Wang L and Ning S. New look of EBV LMP1 signaling landscape. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13215451
25. Park MC, Kim H, Choi H, Chang MS, and Lee SK. Epstein-Barr Virus miR-BART1-3p Regulates the miR-17–92 Cluster by Targeting E2F3. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222010936
26. Osorio JC, Blanco R, Corvalan AH, Munoz JP, Calaf GM, and Aguayo F. Epstein-barr virus infection in lung cancer: insights and perspectives. Pathogens. (2022) 11. doi: 10.3390/pathogens11020132
27. Wei X, Wei Z, Zheng G, Xie T, Huo Z, Huang Y, et al. Prognostic significance of circulating Epstein-Barr virus DNA in pulmonary lymphoepithelioma-like carcinoma: A meta-analysis and validation study. J Med Virol. (2023) 95:e28349. doi: 10.1002/jmv.28349
28. Murata T, Sato Y, and Kimura H. Modes of infection and oncogenesis by the Epstein-Barr virus. Rev Med Virol. (2014) 24:242–53. doi: 10.1002/rmv.1786
29. Wu YX, Zhang WL, Wang TM, Liao Y, Zhang YJ, Xiao RW, et al. Genomic landscapes of epstein-barr virus in pulmonary lymphoepithelioma-like carcinoma. J Virol. (2022) 96:e0169321. doi: 10.1128/JVI.01693-21
30. Chen B, Zhang Y, Dai S, Zhou P, Luo W, Wang Z, et al. Molecular characteristics of primary pulmonary lymphoepithelioma-like carcinoma based on integrated genomic analyses. Signal Transduct Target Ther. (2021) 6:6. doi: 10.1038/s41392-020-00382-6
31. Lu F, Wikramasinghe P, Norseen J, Tsai K, Wang P, Showe L, et al. Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1). Virol J. (2010) 7:262. doi: 10.1186/1743-422X-7-262
32. Longnecker R, Miller CL, Miao XQ, Tomkinson B, and Kieff E. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 (LMP2) are dispensable for lymphocyte infection and growth transformation in vitro. J Virol. (1993) 67:2006–13. doi: 10.1128/JVI.67.4.2006-2013.1993
33. Howe JG and Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci U S A. (1986) 83:9006–10. doi: 10.1073/pnas.83.23.9006
34. Notarte KI, Senanayake S, Macaranas I, Albano PM, Mundo L, Fennell E, et al. MicroRNA and other non-coding RNAs in epstein-barr virus-associated cancers. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13153909
35. Gregorovic G, Boulden EA, Bosshard R, Elgueta Karstegl C, Skalsky R, Cullen BR, et al. Epstein-barr viruses (EBVs) deficient in EBV-encoded RNAs have higher levels of latent membrane protein 2 RNA expression in lymphoblastoid cell lines and efficiently establish persistent infections in humanized mice. J Virol. (2015) 89:11711–4. doi: 10.1128/JVI.01873-15
36. Li Z, Baccianti F, Delecluse S, Tsai MH, Shumilov A, Cheng X, et al. The Epstein-Barr virus noncoding RNA EBER2 transactivates the UCHL1 deubiquitinase to accelerate cell growth. Proc Natl Acad Sci U S A. (2021) 118. doi: 10.1073/pnas.2115508118
37. Lin S and Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. (2015) 15:321–33. doi: 10.1038/nrc3932
38. Lung RW, Hau PM, Yu KH, Yip KY, Tong JH, Chak WP, et al. EBV-encoded miRNAs target ATM-mediated response in nasopharyngeal carcinoma. J Pathol. (2018) 244:394–407. doi: 10.1002/path.5018
39. Lo AK, Lo KW, Ko CW, Young LS, and Dawson CW. Inhibition of the LKB1-AMPK pathway by the Epstein-Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol. (2013) 230:336–46. doi: 10.1002/path.4201
40. Lin HC, Chang Y, Chen RY, Hung LY, Chen PC, Chen YP, et al. Epstein-Barr virus latent membrane protein-1 upregulates autophagy and promotes viability in Hodgkin lymphoma: Implications for targeted therapy. Cancer Sci. (2021) 112:1589–602. doi: 10.1111/cas.14833
41. Wong MP, Chung LP, Yuen ST, Leung SY, Chan SY, Wang E, et al. In situ detection of Epstein-Barr virus in non-small cell lung carcinomas. J Pathol. (1995) 177:233–40. doi: 10.1002/path.1711770304
42. Chau SL, Tong JH, Chow C, Kwan JS, Lung RW, Chung LY, et al. Distinct molecular landscape of epstein-barr virus associated pulmonary lymphoepithelioma-like carcinoma revealed by genomic sequencing. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12082065
43. Soni V, Cahir-McFarland E, and Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol. (2007) 597:173–87. doi: 10.1007/978-0-387-70630-6_14
44. Hong S, Liu D, Luo S, Fang W, Zhan J, Fu S, et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun. (2019) 10:3108. doi: 10.1038/s41467-019-10902-w
45. Nan J, Wang Y, Yang J, and Stark GR. IRF9 and unphosphorylated STAT2 cooperate with NF-kappaB to drive IL6 expression. Proc Natl Acad Sci U S A. (2018) 115:3906–11. doi: 10.1073/pnas.1714102115
46. Takacs M, Segesdi J, Banati F, Koroknai A, Wolf H, Niller HH, et al. The importance of epigenetic alterations in the development of epstein-barr virus-related lymphomas. Mediterr J Hematol Infect Dis. (2009) 1:e2009012. doi: 10.4084/MJHID.2009.012
47. El-Sharkawy A, Al Zaidan L, and Malki A. Epstein-barr virus-associated Malignancies: roles of viral oncoproteins in carcinogenesis. Front Oncol. (2018) 8:265. doi: 10.3389/fonc.2018.00265
48. Chen B, Chen X, Zhou P, Yang L, Ren J, Yang X, et al. Primary pulmonary lymphoepithelioma-like carcinoma: a rare type of lung cancer with a favorable outcome in comparison to squamous carcinoma. Respir Res. (2019) 20:262. doi: 10.1186/s12931-019-1236-2
49. Chen Y, Ouyang D, Wang Y, Pan Q, Zhao J, Chen H, et al. EBV promotes TCR-T-cell therapy resistance by inducing CD163+M2 macrophage polarization and MMP9 secretion. J Immunother Cancer. (2024) 12. doi: 10.1136/jitc-2023-008375
50. Li L, Ma BBY, Chan ATC, Chan FKL, Murray P, and Tao Q. Epstein-barr virus-induced epigenetic pathogenesis of viral-associated lymphoepithelioma-like carcinomas and natural killer/T-cell lymphomas. Pathogens. (2018) 7. doi: 10.3390/pathogens7030063
51. Ngan RK, Yip TT, Cheng WW, Chan JK, Cho WC, Ma VW, et al. Circulating Epstein-Barr virus DNA in serum of patients with lymphoepithelioma-like carcinoma of the lung: a potential surrogate marker for monitoring disease. Clin Cancer Res. (2002) 8:986–94.
52. Xie M, Wu X, Wang F, Zhang J, Ben X, Zhang J, et al. Clinical significance of plasma epstein-barr virus DNA in pulmonary lymphoepithelioma-like carcinoma (LELC) patients. J Thorac Oncol. (2018) 13:218–27. doi: 10.1016/j.jtho.2017.10.031
53. Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, et al. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod Pathol. (2020) 33:626–38. doi: 10.1038/s41379-019-0391-9
54. Chang YL, Wu CT, Shih JY, and Lee YC. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci. (2011) 102:282–7. doi: 10.1111/j.1349-7006.2010.01768.x
55. Chikuma S, Kanamori M, Mise-Omata S, and Yoshimura A. Suppressors of cytokine signaling: Potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. (2017) 108:574–80. doi: 10.1111/cas.13194
56. Veeriah S, Brennan C, Meng S, Singh B, Fagin JA, Solit DB, et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci U S A. (2009) 106:9435–40. doi: 10.1073/pnas.0900571106
57. Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, et al. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res. (2000) 254:14–24. doi: 10.1006/excr.1999.4733
58. Yeh YC, Ho HL, Lin CI, Chou TY, and Wang YC. Whole-exome sequencing of epstein-barr virus-associated pulmonary carcinoma with low lymphocytic infiltration shows molecular features similar to those of classic pulmonary lymphoepithelioma-like carcinoma: evidence to support grouping together as one disease entity. Am J Surg Pathol. (2021) 45:1476–86. doi: 10.1097/PAS.0000000000001722
59. Yin W, Jin J, Bao H, Chen H, Wang C, Cheng G, et al. Tumor-infiltrating lymphocytes-based subtypes and genomic characteristics of EBV-associated lymphoepithelioma-like carcinoma. J Pathol. (2022) 257:650–62. doi: 10.1002/path.5916
60. Wen S, Dai L, Wang L, Wang W, Wu D, Wang K, et al. Genomic signature of driver genes identified by target next-generation sequencing in chinese non-small cell lung cancer. Oncologist. (2019) 24:e1070–81. doi: 10.1634/theoncologist.2018-0572
61. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. (2014) 9:154–62. doi: 10.1097/JTO.0000000000000033
62. Sha Z, Wei Y, Gao T, Luo Y, Chen J, Li T, et al. Clinical observation of pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis. (2021) 13:5683–90. doi: 10.21037/jtd-21-1369
63. Wang L, Lin Y, Cai Q, Long H, Zhang Y, Rong T, et al. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis. (2015) 7:1556–62. doi: 10.3978/j.issn.2072-1439.2015.05.11
64. Chang YL, Yang CY, Lin MW, Wu CT, and Yang PC. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer. (2015) 88:254–9. doi: 10.1016/j.lungcan.2015.03.017
65. Ose N, Kawai T, Ishida D, Kobori Y, Takeuchi Y, and Senba H. Pulmonary lymphoepithelioma-like carcinoma with echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene. Respirol Case Rep. (2016) 4:e00200. doi: 10.1002/rcr2.200
66. Wu Q, Wang W, Zhou P, Fu Y, Zhang Y, Shao YW, et al. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol Res Pract. (2020) 216:153043. doi: 10.1016/j.prp.2020.153043
67. Shen Y, Hu F, Zhang B, Li C, Zhang X, and Han B. Clinicopathological characteristics with EGFR, ALK, ROS1 genetic alternation and prognostic analysis of primary lymphoepithelioma-like carcinoma. Transl Cancer Res. (2019) 8:2350–6. doi: 10.21037/tcr.2019.09.51
68. Liu Q, Ma G, Yang H, Wen J, Li M, Yang H, et al. Lack of epidermal growth factor receptor gene mutations in exons 19 and 21 in primary lymphoepithelioma-like carcinoma of the lung. Thorac Cancer. (2014) 5:63–7. doi: 10.1111/1759-7714.12060
69. Bejarano L, Jordao MJC, and Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. (2021) 11:933–59. doi: 10.1158/2159-8290.CD-20-1808
70. Zhang S, Xiao X, Zhu X, Chen X, Zhang X, Xiang J, et al. Dysregulated immune and metabolic microenvironment is associated with the post-operative relapse in stage I non-small cell lung cancer. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14133061
71. Kasai K, Kon S, Sato N, Muraishi K, Yoshida H, Nakai N, et al. Case report of lymphoepithelioma-like carcinoma of the lung–lymphoid population consisting of cytotoxic T cells in resting state. Pathol Res Pract. (1999) 195:773–9. doi: 10.1016/S0344-0338(99)80120-4
72. Chang YL, Wu CT, Shih JY, and Lee YC. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol. (2002) 26:715–23. doi: 10.1097/00000478-200206000-00004
73. Kobayashi M, Ito M, Sano K, Honda T, and Nakayama J. Pulmonary lymphoepithelioma-like carcinoma: predominant infiltration of tumor-associated cytotoxic T lymphocytes might represent the enhanced tumor immunity. Intern Med. (2004) 43:323–6. doi: 10.2169/internalmedicine.43.323
74. Kawaguchi Y, Fujita T, and Hanaoka J. Spontaneous regression of pulmonary lymphoepithelioma-like carcinoma. Ann Thorac Surg. (2015) 99:2197–9. doi: 10.1016/j.athoracsur.2014.07.088
75. Zens P, Bello C, Scherz A, von Gunten M, Ochsenbein A, Schmid RA, et al. The effect of neoadjuvant therapy on PD-L1 expression and CD8+lymphocyte density in non-small cell lung cancer. Mod Pathol. (2022) 35:1848–59. doi: 10.1038/s41379-022-01139-y
76. Simoni Y, Becht E, Li S, Loh CY, Yeong JPS, Lim TKH, et al. Partial absence of PD-1 expression by tumor-infiltrating EBV-specific CD8(+) T cells in EBV-driven lymphoepithelioma-like carcinoma. Clin Transl Immunol. (2020) 9:e1175. doi: 10.1002/cti2.1175
77. Yang L, Liang H, Liu L, Guo L, Ying JM, Shi SS, et al. CD56+ lymphoepithelioma-like carcinoma of the lung: A case report and literature review. World J Clin cases. (2020) 8:1257–64. doi: 10.12998/wjcc.v8.i7.1257
78. Morbini P, Riboni R, Tomaselli S, Rossi A, and Magrini U. Eber- and LMP-1-expressing pulmonary lymphoepithelioma-like carcinoma in a Caucasian patient. Hum Pathol. (2003) 34:623–5. doi: 10.1016/s0046-8177(03)00081-9
79. Yeh YC, Kao HL, Lee KL, Wu MH, Ho HL, and Chou TY. Epstein-barr virus-associated pulmonary carcinoma: proposing an alternative term and expanding the histologic spectrum of lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol. (2019) 43:211–9. doi: 10.1097/PAS.0000000000001173
80. Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. (2010) 207:2439–53. doi: 10.1084/jem.20100587
81. Wong MP, Cheung KN, Yuen ST, Fu KH, Chan AS, Leung SY, et al. Monocyte chemoattractant protein-1 (MCP-1) expression in primary lymphoepithelioma-like carcinomas (LELCs) of the lung. J Pathol. (1998) 186:372–7. doi: 10.1002/(SICI)1096-9896(199812)186:4<372::AID-PATH204>3.0.CO;2-8
82. Wang L, Long W, Li PF, Lin YB, and Liang Y. An elevated peripheral blood monocyte-to-lymphocyte ratio predicts poor prognosis in patients with primary pulmonary lymphoepithelioma-like carcinoma. PLoS One. (2015) 10:e0126269. doi: 10.1371/journal.pone.0126269
83. Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol. (2015) 42:523–38. doi: 10.1053/j.seminoncol.2015.05.003
84. O’Donnell JS, Massi D, Teng MWL, and Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. (2018) 48:91–103. doi: 10.1016/j.semcancer.2017.04.015
85. Archwamety A, Ruangchira-Urai R, Akewanlop C, and Korphaisarn K. Primary pulmonary lymphoepithelioma-like carcinoma treated with immunotherapy: A case report and literature review. Thorac Cancer. (2022) 13:2539–41. doi: 10.1111/1759-7714.14580
86. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. (2015) 372:2018–28. doi: 10.1056/NEJMoa1501824
87. Zhong YM, Yin K, Chen Y, Xie Z, Lv ZY, Yang JJ, et al. PD-1/PD-L1 combined with LAG3 is associated with clinical activity of immune checkpoint inhibitors in metastatic primary pulmonary lymphoepithelioma-like carcinoma. Front Immunol. (2022) 13:951817. doi: 10.3389/fimmu.2022.951817
88. Chen J, Gu C, Chen X, Dai C, Zhao S, Xie H, et al. Clinicopathological and prognostic analyses of 86 resected pulmonary lymphoepithelioma-like carcinomas. J Surg Oncol. (2021) 123:544–52. doi: 10.1002/jso.26276
89. Yu XY, Zhang XW, Wang F, Lin YB, Wang WD, Chen YQ, et al. Correlation and prognostic significance of PD-L1 and P53 expression in resected primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis. (2018) 10:1891–902. doi: 10.21037/jtd.2018.03.14
90. Wu Z, Xian X, Wang K, Cheng D, Li W, and Chen B. Immune checkpoint blockade therapy may be a feasible option for primary pulmonary lymphoepithelioma-like carcinoma. Front Oncol. (2021) 11:626566. doi: 10.3389/fonc.2021.626566
91. Zhou N, Tang H, Yu S, Lin Y, Wang Y, and Wang Y. Anti-PD-1 antibodies, a novel treatment option for advanced chemoresistant pulmonary lymphoepithelioma carcinoma. Front Immunol. (2022) 13:1001414. doi: 10.3389/fimmu.2022.1001414
92. Zhang X, Zhou Y, Chen H, Chen C, Lin Z, He LN, et al. PD-1 inhibition plus platinum-based chemotherapy (PBC) or PBC alone in the first-line treatment of locally advanced or metastatic pulmonary lymphoepithelioma-like carcinoma. Front Immunol. (2022) 13:1015444. doi: 10.3389/fimmu.2022.1015444
93. Wong TS, Chan WS, Li CH, Liu RW, Tang WW, Tsao SW, et al. Curcumin alters the migratory phenotype of nasopharyngeal carcinoma cells through up-regulation of E-cadherin. Anticancer Res. (2010) 30:2851–6.
94. Giehler F, Ostertag MS, Sommermann T, Weidl D, Sterz KR, Kutz H, et al. Epstein-Barr virus-driven B cell lymphoma mediated by a direct LMP1-TRAF6 complex. Nat Commun. (2024) 15:414. doi: 10.1038/s41467-023-44455-w
95. Yarza R, Bover M, Agullo-Ortuno MT, and Iglesias-Docampo LC. Current approach and novel perspectives in nasopharyngeal carcinoma: the role of targeting proteasome dysregulation as a molecular landmark in nasopharyngeal cancer. J Exp Clin Cancer Res. (2021) 40:202. doi: 10.1186/s13046-021-02010-9
96. Canichella M and de Fabritiis P. Adoptive cell immunotherapy in relapse/refractory epstein-barr virus-driven post-transplant lymphoproliferative disorders. Antibodies (Basel). (2025) 14. doi: 10.3390/antib14020047
97. Haverkos B, Alpdogan O, Baiocchi R, Brammer JE, Feldman TA, Capra M, et al. Targeted therapy with nanatinostat and valganciclovir in recurrent EBV-positive lymphoid Malignancies: a phase 1b/2 study. Blood Adv. (2023) 7:6339–50. doi: 10.1182/bloodadvances.2023010330
98. Si Y, Deng Z, Lan G, Du H, Wang Y, Si J, et al. The safety and immunological effects of rAd5-EBV-LMP2 vaccine in nasopharyngeal carcinoma patients: A phase I clinical trial and two-year follow-up. Chem Pharm Bull (Tokyo). (2016) 64:1118–23. doi: 10.1248/cpb.c16-00114
99. Hung HY, Lai WA, Chuang CH, and Yang CJ. Combination of chemotherapy and immunotherapy may overcome the resistance to immunotherapy alone in pulmonary lymphoepithelial carcinoma. Kaohsiung J Med Sci. (2024) 40:601–2. doi: 10.1002/kjm2.12827
100. Ma H, Wu Y, Lin Y, Cai Q, Ma G, and Liang Y. Computed tomography characteristics of primary pulmonary lymphoepithelioma-like carcinoma in 41 patients. Eur J Radiol. (2013) 82:1343–6. doi: 10.1016/j.ejrad.2013.02.006
101. Zhou N, Lin Y, Peng X, Wang Y, and Wang Y. Thorough survey and analysis of pulmonary lymphoepithelioma-like carcinoma in Macau and multimodality treatment for advanced disease. Lung Cancer. (2019) 138:116–23. doi: 10.1016/j.lungcan.2019.10.004
102. Lei Y, Zhou J, Liu J, Xia X, Wang P, Peng Y, et al. The CT and PET/CT findings in primary pulmonary lymphoepithelioma-like carcinoma with pathological correlation: a study of 215 cases. Clin Radiol. (2022) 77:e201–7. doi: 10.1016/j.crad.2021.10.010
103. Han AJ, Xiong M, Gu YY, Lin SX, and Xiong M. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol. (2001) 115:841–50. doi: 10.1309/BUAN-BGFW-69U9-C3H8
104. Sathirareuangchai S and Hirata K. Pulmonary lymphoepithelioma-like carcinoma. Arch Pathol Lab Med. (2019) 143:1027–30. doi: 10.5858/arpa.2018-0149-RS
105. Qin Y, Gao G, Xie X, Zhu Z, Guan W, Lin X, et al. Clinical features and prognosis of pulmonary lymphoepithelioma-like carcinoma: summary of eighty-five cases. Clin Lung Cancer. (2019) 20:e329–37. doi: 10.1016/j.cllc.2018.12.014
106. Tay CK, Chua YC, Takano A, Min Chee MY, Lim WT, Lim C, et al. Primary pulmonary lymphoepithelioma-like carcinoma in Singapore. Ann Thorac Med. (2018) 13:30–5. doi: 10.4103/atm.ATM_304_17
107. Hayashi T, Haba R, Tanizawa J, Katsuki N, Kadota K, Miyai Y, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagn Cytopathol. (2012) 40:820–5. doi: 10.1002/dc.21670
108. Tan B, Xu K, Lyu Y, Liang Y, Liang R, Lei K, et al. Single-cell analysis reveals transcriptomic features and therapeutic targets in primary pulmonary lymphoepithelioma-like carcinoma. Commun Biol. (2025) 8:394. doi: 10.1038/s42003-025-07819-0
109. Torne AS and Robertson ES. Epigenetic mechanisms in latent epstein-barr virus infection and associated cancers. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16050991
Keywords: pulmonary lymphoepithelial carcinoma, Epstein-Barr virus, genomic landscape, tumor immune microenvironment, immunotherapy
Citation: Xie M, Yao D, Lei L, Tang C and Mo B (2025) Epstein–Barr virus–driven molecular pathogenesis of primary pulmonary lymphoepithelial carcinoma. Front. Oncol. 15:1630415. doi: 10.3389/fonc.2025.1630415
Received: 03 June 2025; Accepted: 25 July 2025;
Published: 13 August 2025.
Edited by:
Wenbo Ma, Southern Medical University, ChinaReviewed by:
Zebo Jiang, Zhuhai Hospital of Integrated Traditional Chinese & Western Medicine, ChinaPengyu Yao, Jinan Maternity And Child Care Hospital, China
Copyright © 2025 Xie, Yao, Lei, Tang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biwen Mo, bW9iaXdlbjIwMDJAc29odS5jb20=
 Mingyuan Xie
Mingyuan Xie Dong Yao
Dong Yao Biwen Mo
Biwen Mo