- 1Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
Background: Postoperative complications significantly impact gastric cancer patients’ recovery and remain a major research focus. This study aimed to develop a machine learning model utilizing preoperative and intraoperative data to stratify the risk of early postoperative complications in patients undergoing radical gastrectomy.
Methods: Clinical data from gastric cancer patients who underwent radical gastrectomy at Peking Union Medical College Hospital between 2014 and 2024 were retrospectively collected. Using R software, ten machine learning algorithms—including eXtreme Gradient Boosting, Support Vector Machine, random forest, Neural Network, naive Bayes, logistic regression, Linear Discriminant Analysis, K-Nearest Neighbors, Generalized Linear Model with Elastic-Net Regularization and classification tree—were employed to construct predictive models for early postoperative complications. Nested cross-validation was applied for model validation, and performance was evaluated using receiver operating characteristic curves, decision curve analysis, and calibration curves.
Results: A total of 926 patients were included in this study, comprising 667 males (72%) and 259 females (28%), with 131 (14.13%) suffering postoperative complications. Predictive features included smoking, Nutritional Risk Screening 2002 score>3, reconstruction, clinical T-stage>1, operative time, neoadjuvant chemotherapy combined with immunotherapy or targeted therapy, and resection site. Among the ten models, eXtreme Gradient Boosting demonstrated the best predictive performance, achieving an area under the receiver operating characteristic curve (AUC) of 0.788, along with superior calibration and decision curve analysis results.
Conclusion: Based on preoperative and intraoperative data, the eXtreme Gradient Boosting model demonstrated the strongest predictive capability for postoperative complications following radical gastrectomy.These findings underscore the potential of machine learning-based models in stratifying the risk of early postoperative complications in patients undergoing radical gastrectomy, thereby enhancing clinical decision-making and improving patient outcomes in gastric cancer surgery.
Introduction
Gastric cancer (GC) is a common malignant tumor of the digestive system, ranking as the fifth most prevalent cancer globally and the third leading cause of cancer-related mortality (1, 2). Currently, surgical resection remains the most reliable and mainstream treatment for GC, with postoperative recovery playing a critical role in patient prognosis (3–6). Among the factors influencing patient recovery, short-term postoperative outcomes, particularly the occurrence of complications, hold significant clinical importance.
Machine learning (ML) has emerged as a cutting-edge tool in oncological research, offering substantial advantages over traditional statistical methods in constructing diagnostic and prognostic models (7, 8). In cancer studies, algorithms such as eXtreme Gradient Boosting (XGBoost), SVM (Support Vector Machine), random forest, Neural Network (NNET), naive Bayes, logistic regression, Linear Discriminant Analysis (LDA), K-Nearest Neighbors (KNN), Generalized Linear Model with Elastic-Net Regularization (GLMNet), and classification trees are widely employed for predictive modeling (9), with analysis typically conducted using R or Python.
Recent years have seen extensive research on postoperative complications in gastric cancer (10, 11).Several studies have applied ML to develop predictive models for such complication (6, 12, 13),however, many of these excluded patients who underwent neoadjuvant therapy or included only minimal data from this subgroup. To address this gap, our study using preoperative as well as intraoperative data incorporated a substantial cohort of neoadjuvant therapy recipients and developed ML-based predictive models to stratify the risk of early postoperative complications in GC. We anticipate that these models will facilitate clinical decision-making, assist in complication prevention, and ultimately promote accelerated postoperative recovery.
Materials and method
Patients selection
We retrospectively collected data from patients who underwent radical gastrectomy for GC at Peking Union Medical College Hospital between 2014 and 2024. Inclusion criteria were:(1) undergoing radical gastrectomy; (2) histopathological confirmation of gastric adenocarcinoma; (3) surgery performed by experienced gastrointestinal surgeons. Exclusion criteria included: (1) Age <18 or >80 years; (2) intraoperative detection of metastatic disease or other evidence of metastasis; (3) concurrent other malignant tumors; (4) severe cardiovascular, cerebrovascular, or other systemic comorbidities; (5) missing data exceeding 30% or loss to follow-up.
Definition of complications: Early postoperative complications were defined as any Clavien-Dindo grade ≥2 events occurring within 30 days after surgery.
Data collection
We collected demographic and clinical data from enrolled patients while controlling for potential confounding variables using the Directed Acyclic Graph (DGA) principle. Demographic characteristics included age and sex. Cinical data included preoperative data, intraoperative data and postoperative outcome. Preoperative clinical data encompassed length of preoperative hospitalization, smoking and alcohol consumption history, comorbidities (hypertension, diabetes mellitus, reflux esophagitis, pyloric obstruction), psychological disorders, previous abdominal surgery, H. pylori(HP) infection status, family history of malignant tumors, neoadjuvant treatment therapy, Nutritional Risk Screening-2002 (NRS-2002) score, and laboratory parameters including white blood cell(WBC) count, hemoglobin, glucose, albumin, albumin-to-globulin ratio(A/G), C-reactive protein(CRP), D-dimer, and tumor markers (CA242, AFP, CEA, CA19-9, CA724). Tumor location, clinical TNM stage (according to the AJCC 8th edition criteria), and Her-2 expression status were determined based on preoperative imaging and endoscopic biopsy findings. Intraoperative data consisted of resection site, anastomosis method, operative duration, intraoperative blood loss, endoscopy utilization, feeding tube placement, and blood transfusion. The primary postoperative outcome was the occurrence of complications.
Data analysis strategy
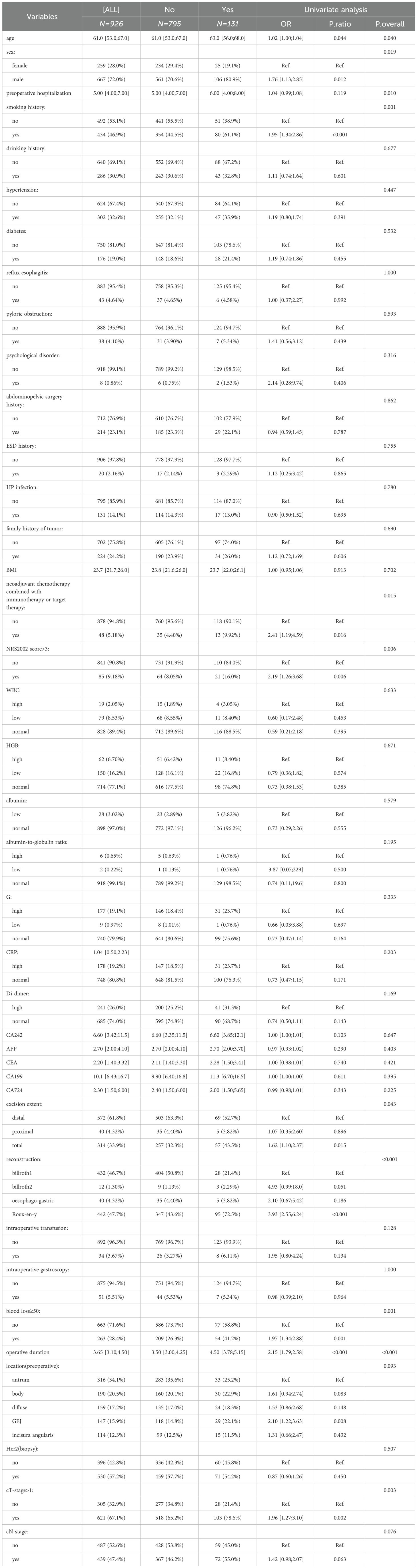
For variables with missing values less than 30%, multiple imputation was applied to the collected data. Univariate analysis and LASSO regression were performed on the processed data. In the univariate analysis, categorical data were assessed using the chi-square test or Fisher’s exact test, normally distributed numerical data were analyzed using the t-test (results presented as mean ± standard deviation), and non-normally distributed numerical data were evaluated using the rank-sum test (results expressed as median [25%;75%]. A p-value less than 0.05 was considered statistically significant. In the LASSO analysis, the optimal regularization parameter (λ) was selected, and all factors with non-zero coefficients were extracted. The intersection of these factors was used to construct ML models.
Based on the mlr3 system and relevant R packages, the following models were built and tuned: XGBoost, SVM, Random Forest, NNET, Naive Bayes, Logistic Regression, LDA, KNN, GLMNet, and Classification Tree. Nested cross-validation (Outer 5-fold CV +Inner 5-fold CV, resolution =3) was employed to evaluate these models, yielding AUC, accuracy, recall, and specificity. Receiver operating characteristic (ROC) curves, decision curve analysis (DCA) curves, and calibration curves were plotted to assess model performance, and the best-performing predictive model was selected.
Results
A total of 926 patients who underwent GC surgery were included in this study, comprising 667 males (72%) and 259 females (28%), with a median age of 61 years [53;67]. The baseline demographic and clinical characteristics are presented in Table 1. Postoperative complications occurred in 131 patients (14.13%), including anastomotic leakage, gastroparesis, hemorrhage, infection, obstruction, acute cardiovascular and cerebrovascular events.
The univariate analysis results are presented in Table 1, which shows significant differences between the complication and non-complication groups in the following variables: age(p=0.04), sex(p=0.019), preoperative hospitalization(p=0.01), smoking history(p=0.001), neoadjuvant chemotherapy combined with immunotherapy or targeted therapy(p=0.015), NRS2002 score>3(p=0.006), resection extent(p=0.043), anastomosis method(p<0.001), blood loss ≥50 mL(p=0.001), operative time(p<0.001), clinical T(cT)-stage>1(p=0.003).
The LASSO regression results are illustrated in Figure 1, revealing that factors significantly associated with postoperative complications included Roux-en-Y anastomosis, NRS2002 score >3, prolonged operative time, smoking, A/G, Billrouth II anastomosis, neoadjuvant therapy combined with immunotherapy or targeted therapy, cT-stage>1, G and excition extent. Weakly correlated factors included CA242.
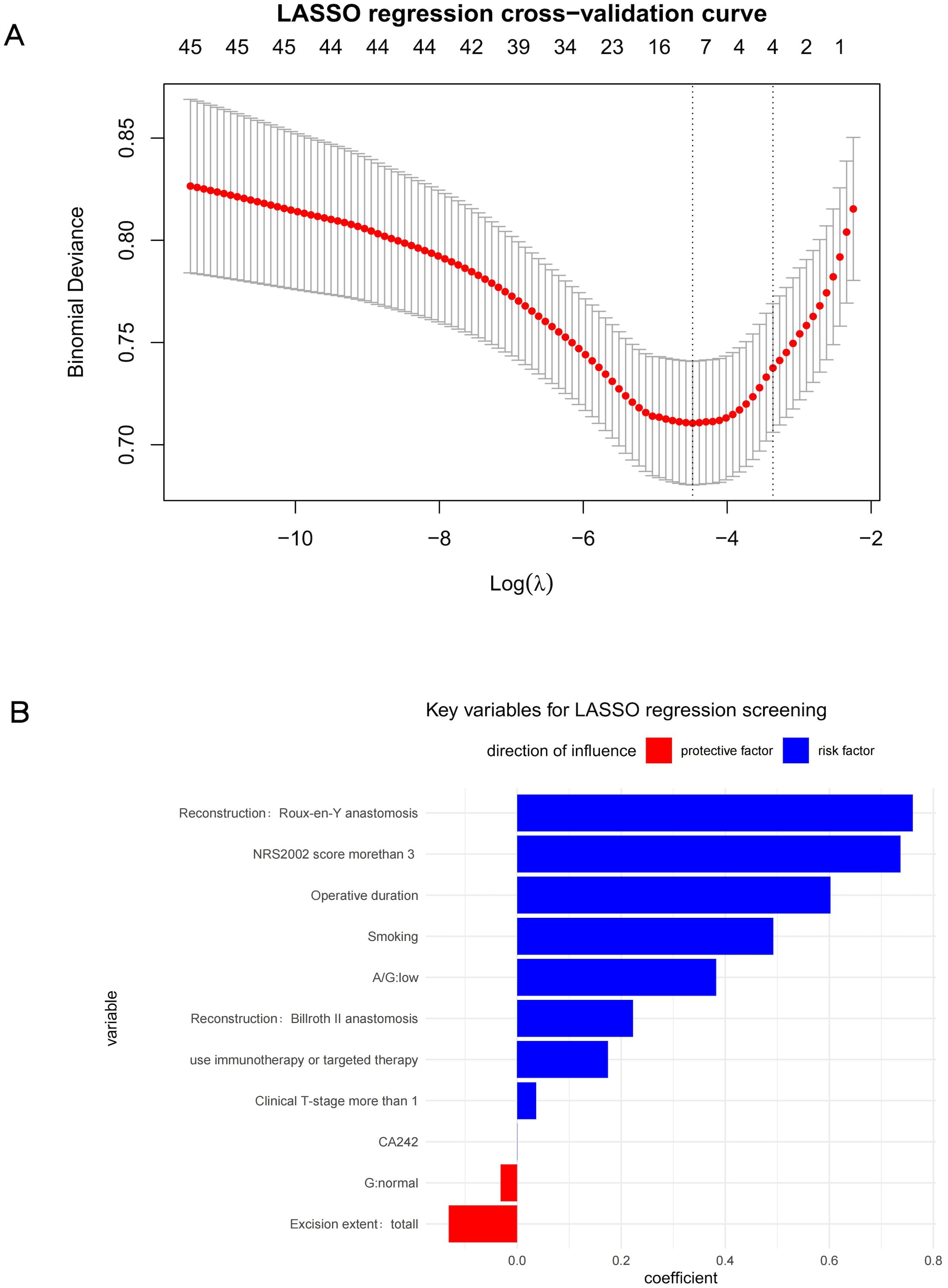
Figure 1. (A) LASSO regression cross−validation curve (B) Key variables for LASSO regression screening.
Based on the combined results of univariate and LASSO analyses, the following variables were selected for ML model construction: smoking, NRS2002 score >3, reconstruction method, cT-stage >1, operative time, neoadjuvant chemotherapy combined with immunotherapy or targeted therapy and resection extent.
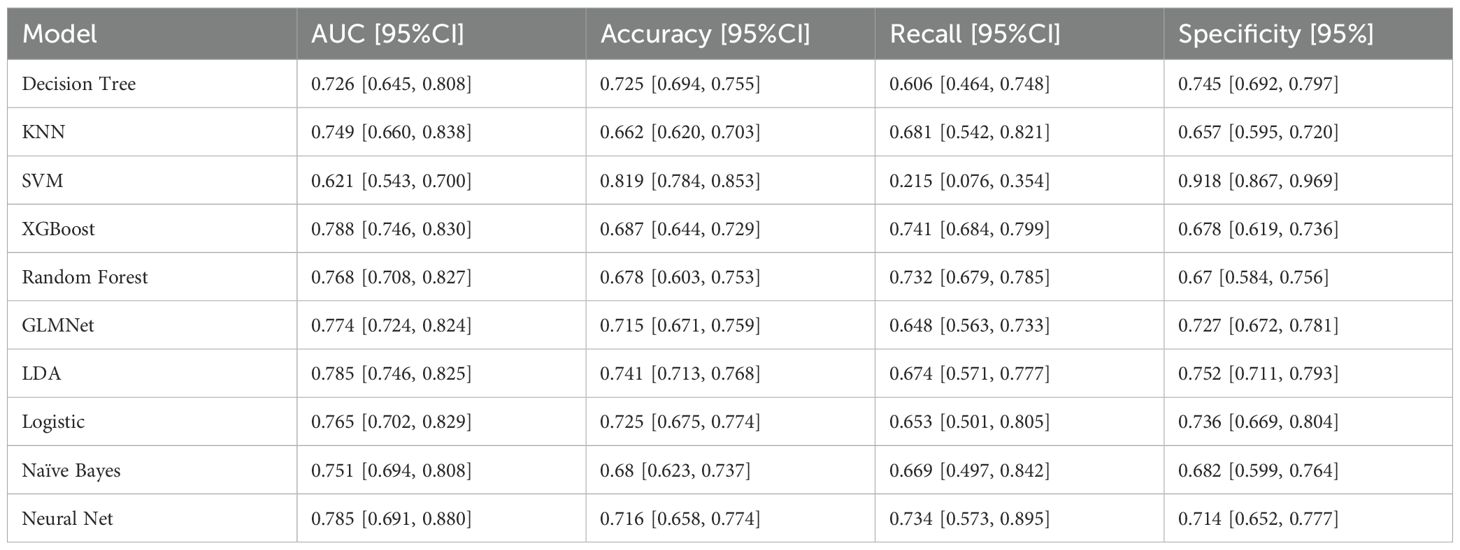
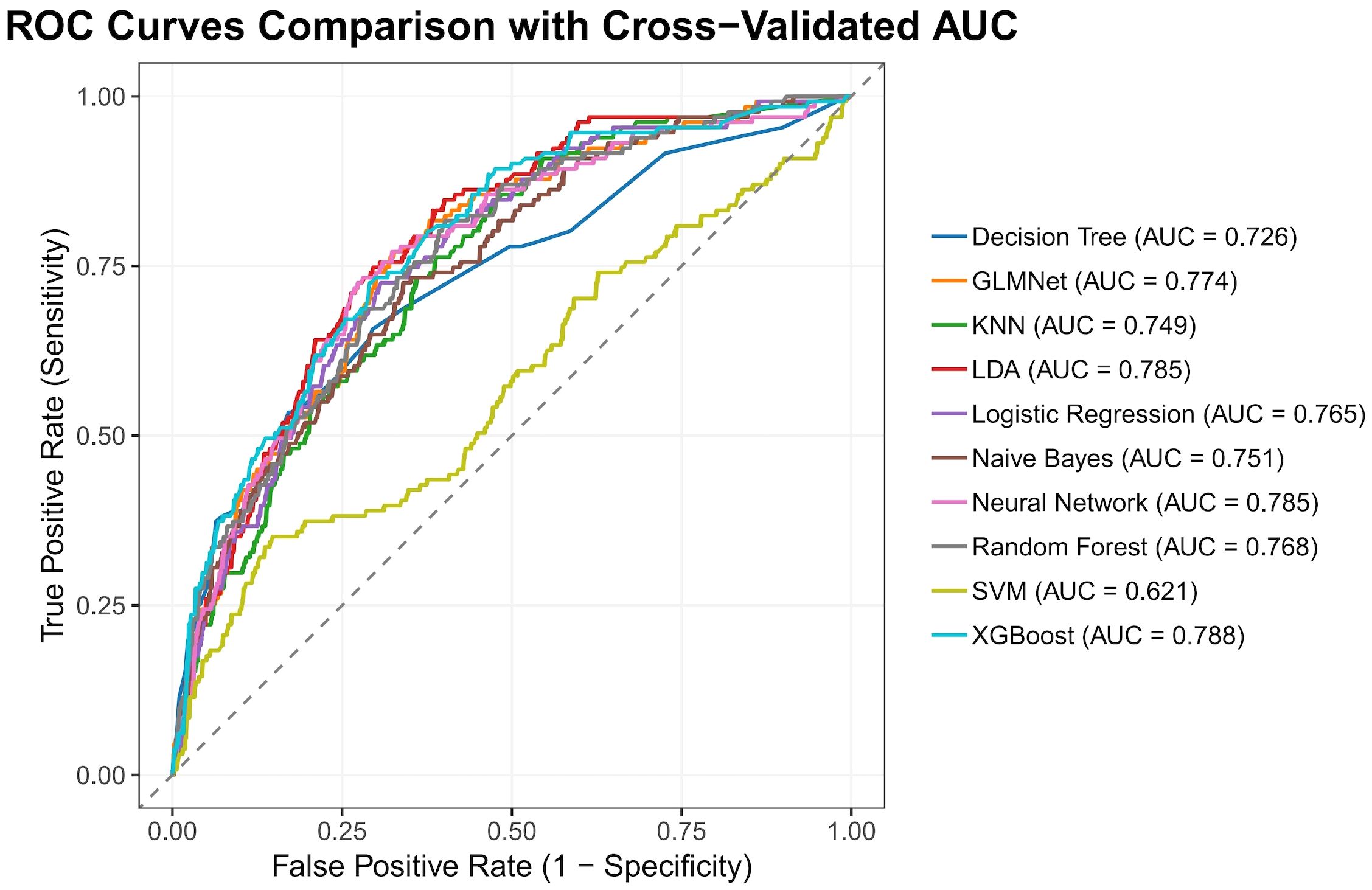
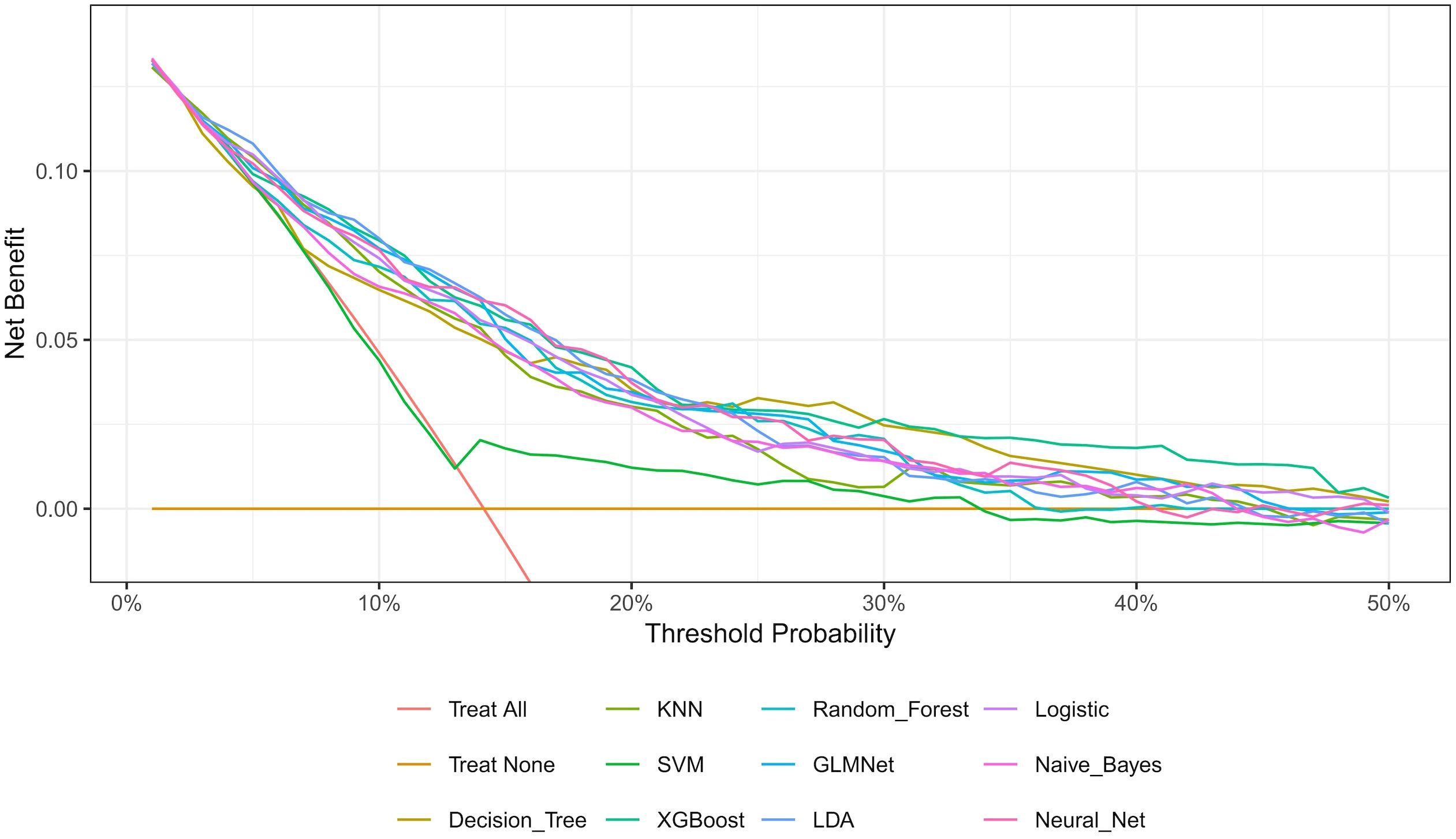
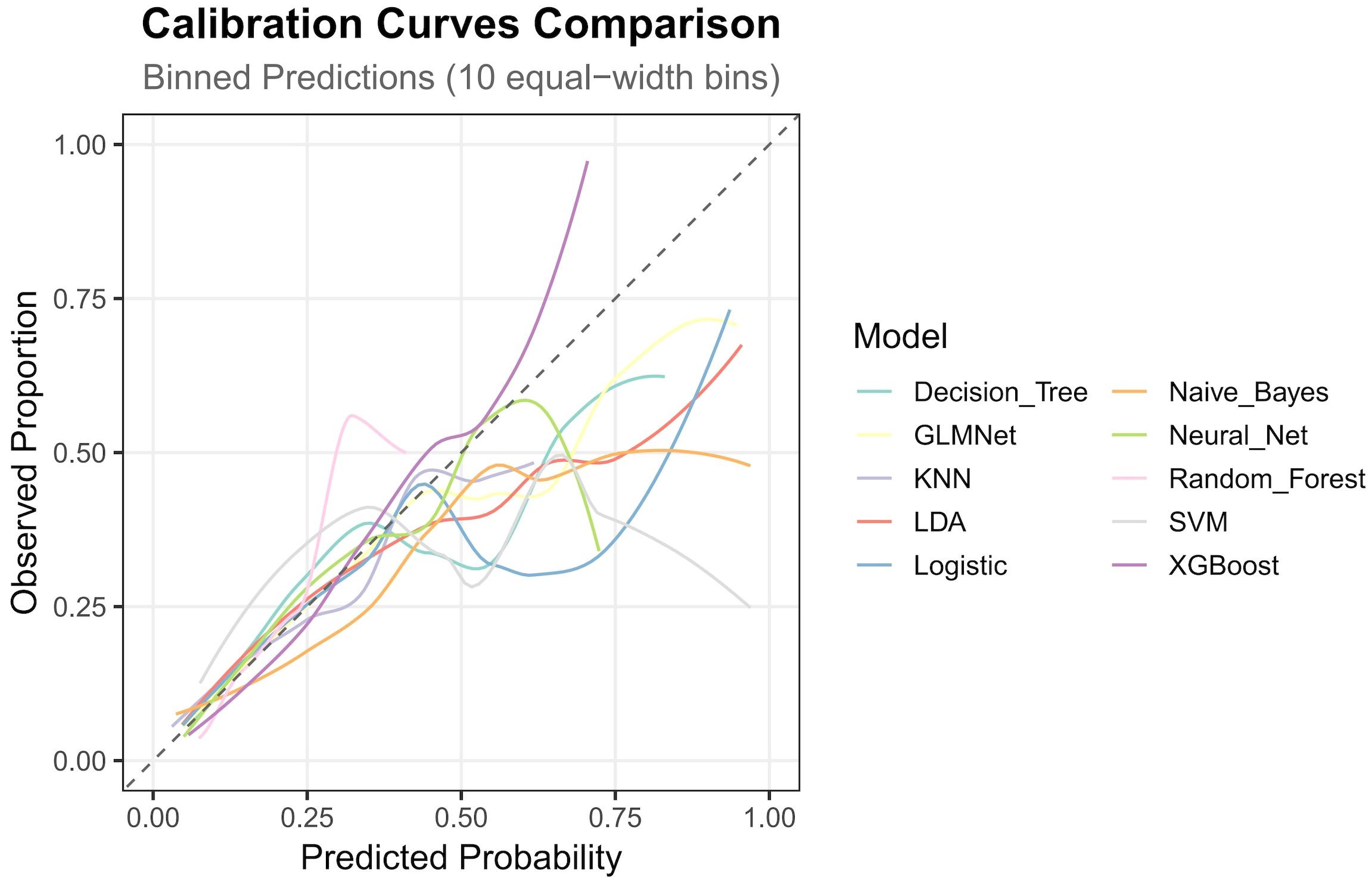
The nested cross-validation results for the XGBoost, SVM, Random Forest, NNET, Naive Bayes, Logistic Regression, LDA, KNN, GLMNet, and Classification Tree models are presented in Table 2. Among these models, XGBoost demonstrated the highest area under the receiver operating characteristic curve (AUC = 0.788) and a comparatively high recall value (0.741), indicating superior predictive performance. The ROC curves for each model are presented in Figure 2. The DCA and calibration curves for each model are shown in Figures 3 and 4. In the DCA, XGBoost provided the highest clinical net benefit, confirming its status as one of the top-performing models. Additionally, the calibration curve indicated that XGBoost had the best agreement between predicted and observed probabilities. In conclusion, the XGBoost model exhibited optimal performance in predicting postoperative complications in GC patients.
Discussion
Postoperative complications represent the most critical factor affecting recovery in GC patients and have long been a major concern for surgeons. While numerous studies have investigated predictors of various postoperative complications in GC (14, 15),and a limited number of ML-based models have been reported (12, 13),these studies typically excluded patients receiving neoadjuvant therapy. The present study addresses this research gap by including GC patients who underwent neoadjuvant treatment. Furthermore, we developed a machine learning-based predictive model for postoperative complications using preoperative and intraoperative data, which may ultimately guide clinical decision-making to prevent complications and facilitate postoperative recovery.
In terms of methodology, the predictive factors for model construction in this study were selected through a dual approach incorporating both univariate analysis and LASSO regression. This strategy not only preserves statistically significant variables but also accounts for feature interactions, thereby reducing the false-positive rate. Regarding ML algorithms, we employed ten distinct methods—XGBoost, SVM, Random Forest, NNET, Naive Bayes, Logistic Regression, LDA, KNN, GLMNet, and Classification Trees—selected for their suitability given our sample size and outcome characteristics. For model validation, we implemented nested cross-validation, which offers distinct advantages over conventional training-test splits. Specifically, this approach is more appropriate for smaller datasets, rigorously prevents data leakage, and enables phased validation, yielding more robust results (16). As this is a complication-prediction model, the AUC and recall metrics were prioritized in performance evaluation. Additionally, ROC, DCA, and calibration plots were utilized to visually assess the model’s predictive efficacy.
The LASSO and univariate analyses identified, Roux-en-Y anastomosis, NRS2002 score>3, prolonged operative time, smoking, and neoadjuvant chemotherapy combined with immunotherapy or targeted therapy, cT-stage >1 as significant risk factors for postoperative complications, while total gastric resection demonstrated protective effects. These findings suggest patients with smoking history, poor nutritional status, prolonged operative duration, complex surgical approaches, aggressive tumor biology face higher complication risks.
Surgery is the most critical factor contributing to postoperative complications. A multicenter retrospective study involving 2,508 patients (17) demonstrated that Roux-en-Y anastomosis and prolonged operative duration were risk factors for postoperative complications. Smoking and inflammatory status are associated with the development and poor prognosis of various tumors. Research by Kentaro Matsuo et al. (18)found that patients with a history of smoking and higher WBC levels at the gastroesophageal junction were more prone to anastomotic leakage after surgery. The study by Junbo Zuo’s team (19)indicated that malnutrition and sarcopenia were correlated with postoperative complications in GC patients. Luigi Marano (20)revealed that early immunonutrition support for postoperative GC patients significantly reduced the incidence of anastomotic leakage and infection events compared to those without such support. Conversely, Jingxia Lv (21) found that inadequate postoperative nutritional support was a risk factor for poor prognosis after radical gastrectomy. Tumor aggressiveness is also a key factor influencing postoperative complications. Studies have shown that GC patients with neural invasion and higher T-stage have worse prognoses (22, 23). These findings are consistent with our research results, further validating the reliability of this study.
In addition, the results of this study suggest that GC patients receiving neoadjuvant therapy combined with immunotherapy or targeted therapy may be at a higher risk of postoperative complications, which represents a novel finding. In recent years, although numerous studies on neoadjuvant therapy for GC have been published, the majority have focused on comparing the efficacy and safety of chemotherapy combined with targeted/immunotherapeutic agents versus conventional neoadjuvant chemotherapy regimens (24–26). However, there remains a scarcity of comparative analyses regarding postoperative complications between patients who underwent surgery following neoadjuvant therapy incorporating targeted or immunotherapeutic agents and those who did not receive any neoadjuvant treatment. A distinctive feature of this study is the inclusion of both patients who received neoadjuvant therapy and those who did not. It is noteworthy that patients receiving neoadjuvant chemotherapy combined with immunotherapy or targeted therapy often present with features inherently associated with a higher propensity for postoperative complications, such as more advanced tumor stages, broader invasive extent, and more complex surgical procedures.Furthermore, from a mechanistic perspective, previous studies have suggested that immunotherapy and targeted therapy may induce inflammatory responses or immune-related adverse events, thereby impairing tissue healing (27–29). These factors may explain the observed correlation between the combined neoadjuvant immunotherapeutic or targeted regimen and an increased risk of postoperative complications. Nevertheless, the precise mechanisms underlying the elevated risk of postoperative complications associated with neoadjuvant therapy combined with immunotherapy or targeted therapy warrant further investigation.
As shown in Figures 3 and 4 the XGBoost model demonstrated the best predictive performance among the ML models evaluated. It achieved optimal results across ROC, DCA and calibration curves. Furthermore, Table 2 shows that the XGBoost model also achieved a high recall, a performance metric that is often more critical than accuracy in predicting complications, especially when dealing with imbalanced class distributions. These findings collectively confirm the high efficiency, accuracy, and clinical utility of the XGBoost prediction model.
During the development of the XGBoost prediction model, both preoperative and intraoperative predictors were incorporated to enable immediate postoperative risk stratification for complications. This approach facilitates early intervention in high-risk patients. Clinically, factors such as smoking history, preoperative NRS2002 score, neoadjuvant therapy regimen, and clinical tumor stage are readily available before surgery. This study has identified smoking history, NRS2002 score >3, neoadjuvant therapy combined with targeted or immunotherapy, and cT-stage >1 as significant risk factors for postoperative complications. Therefore, preoperative interventions—including smoking cessation counseling, nutritional support, adequate tumor downstaging, and optimal selection of neoadjuvant therapy—may help mitigate these risks. Furthermore, for patients who are candidates for different surgical approaches, the model can be utilized preoperatively to calculate and compare the predicted risk stratification intervals for complications associated with various surgical techniques, thereby assisting in the selection of the most appropriate procedure. Concurrently, efforts should be made to shorten the operative duration during surgery. Finally, for patients predicted to be at high risk for complications immediately after surgery, more intensive monitoring of their postoperative condition is essential. Timely implementation of corresponding management measures is crucial to accelerate recovery and improve patient prognosis.
The highlight of this study lies in the application of ML methods to establish a predictive model for postoperative complications in all GC patients (including those who underwent neoadjuvant therapy), with the model evaluated using cross-nested validation. Additionally, it was found that chemotherapy combined with immunotherapy or targeted therapy may contribute to the occurrence of postoperative complications. This study also has limitations. It is a single-center retrospective study, which inherently limits the sample size. Furthermore, the inclusion of certain intraoperative variables in this predictive model may partially compromise its utility for preoperative clinical decision-making. In the future, our team will continue to expand the database and incorporate external datasets to further validate the model.
Conclusion
This study developed a ML-based predictive model using preoperative and intraoperative data to forecast postoperative complications in GC patients undergoing radical gastrectomy, including those receiving neoadjuvant therapy. Key predictive factors included smoking, NRS2002score>3, reconstruction method, extent of resection, T-stage >1, operative time and neoadjuvant therapy combining immunotherapy or targeted therapy. Among the ten evaluated models, the XGBoost model demonstrated the highest AUC (0.788), exhibiting superior reliability and greater clinical decision-making benefit in predicting postoperative complications for GC patients. These findings highlight the significant potential of artificial intelligence in improving complication prediction and facilitating faster postoperative recovery in GC patients.
Data availability statement
The datasets generated and analyzed during the current study are not publicly available due the policy of the institution but are available from the authors on reasonable request. Requests to access the datasets should be directed to bGlydXlpbnlpeHVlQDE2My5jb20=.
Ethics statement
The studies involving humans were approved by Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (Approval No. I-24PJ0626). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. ZZ: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. JY: Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National High Level Hospital Clinical Research Funding of China (grant number: 2022-PUMCH-B-005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used Deepseek in order to polish the language.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, and Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, and Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. (2018) 10:239–48. doi: 10.2147/CMAR.S149619
3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
4. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
5. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.004
6. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). (2021) 41:747–95. doi: 10.1002/cac2.12193
7. Frantzi M, Tymoszuk P, Salcher S, Gomez-Gomez E, Morillo AC, Melchior F, et al. Prediction of prostate cancer biochemical recurrence after radical prostatectomy by collagen models using multiomic profiles. Eur Urol Oncol. (2025). doi: 10.1016/j.euo.2025.03.016
8. Lavanya JMS and Subbulakshmi P. Innovative approach towards early prediction of ovarian cancer: machine learning- enabled xai techniques. Heliyon. (2024) 10:e29197. doi: 10.1016/j.heliyon.2024.e29197
9. Elsayid NN, Aydaross Adam EI, Yousif Mahmoud SM, Saadeldeen H, Nauman M, Ali Ahmed TA, et al. The role of machine learning approaches in pediatric oncology: A systematic review. Cureus. (2025) 17:e77524. doi: 10.7759/cureus.77524
10. GlobalSurg Collaborative and NIHR Global Health Unit on Global Surgery. Impact of malnutrition on early outcomes after cancer surgery: an international, multicentre, prospective cohort study. Lancet Glob Health. (2023) 11:e341–e9. doi: 10.1016/s2214-109x(22)00550-2
11. Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer: A randomized clinical trial. JAMA Surg. (2021) 156:954–63. doi: 10.1001/jamasurg.2021.3182
12. Hong QQ, Yan S, Zhao YL, Fan L, Yang L, Zhang WB, et al. Machine learning identifies the risk of complications after laparoscopic radical gastrectomy for gastric cancer. World J Gastroenterol. (2024) 30:79–90. doi: 10.3748/wjg.v30.i1.79
13. Ji K, Shi L, Feng Y, Wang L, Guo H, Li H, et al. Construction and interpretation of machine learning-based prognostic models for survival prediction among intestinal-type and diffuse-type gastric cancer patients. World J Surg Oncol. (2024) 22:275. doi: 10.1186/s12957-024-03550-y
14. Davis JL and Ripley RT. Postgastrectomy syndromes and nutritional considerations following gastric surgery. Surg Clin North Am. (2017) 97:277–93. doi: 10.1016/j.suc.2016.11.005
15. Meng C, Cao S, Li L, Xia L, Chu X, Jiang L, et al. Short-term outcomes of preoperative computed tomography angiography versus standard assessment in patients with BMI ≥ 25.0 kg/M(2) undergoing laparoscopic gastrectomy: the GISSG20–01 randomized clinical trial. Gastric Cancer. (2025) 28:283–93. doi: 10.1007/s10120-024-01580-9
16. Arlot S and Celisse A. A survey of cross-validation procedures for model selection. Stat Surveys. (2010) 4:40–79. doi: 10.1214/09-ss054
17. Yu Z, Liang C, Xu Q, Li R, Gao J, Gao Y, et al. Analysis of postoperative complications and long term survival following radical gastrectomy for patients with gastric cancer. Sci Rep. (2024) 14:23869. doi: 10.1038/s41598-024-74758-x
18. Matsuo K, Lee SW, Tanaka R, Imai Y, Honda K, Taniguchi K, et al. T stage and venous invasion are crucial prognostic factors for long-term survival of patients with remnant gastric cancer: A cohort study. World J Surg Oncol. (2021) 19:291. doi: 10.1186/s12957-021-02400-5
19. Palleiko BA, Dickson KM, Crawford A, Shafique S, Emmerick I, Uy K, et al. Preoperative risk factors for anastomotic leak after esophagectomy with gastric reconstruction: A 6-year national surgical quality improvement (NSQIP) database analysis. Surgery. (2024) 176:93–9. doi: 10.1016/j.surg.2024.03.029
20. Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: A prospective randomized study. Ann Surg Oncol. (2013) 20:3912–8. doi: 10.1245/s10434-013-3088-1
21. Lv J, Li X, Li X, Wang M, Zhang Z, Wang D, et al. Insufficient enteral nutrition is an independent risk factor for poor clinical outcomes in gastric cancer patients following radical gastrectomy. Am J Transl Res. (2025) 17:320–9. doi: 10.62347/htyy3971
22. Cozac-Szőke AR, Radu GN, Negovan A, Cozac DA, Turdean S, Tinca AC, et al. Prognostic impact of klintrup-mäkinen (Km) score in gastric cancer and its association with pathological parameters. Med (Kaunas). (2025) 61:715. doi: 10.3390/medicina61040715
23. He FQ, Xu R, Zhou D, Zhou X, and Chen XD. Disparities in overall survival of gastric cancer patients after radical gastrectomy: an age and rural-urban residence-based cohort study with propensity score matching analysis. Sci Rep. (2025) 15:8479. doi: 10.1038/s41598-025-93463-x
24. Li C, Duan Y, Zhou S, Tang T, Yang Y, and Zhou L. Evaluating the efficacy and safety of neoadjuvant immunochemotherapy versus chemotherapy in locally advanced gastric cancer undergoing radical gastrectomy: A retrospective study. World J Surg Oncol. (2025) 23:121. doi: 10.1186/s12957-025-03710-8
25. Kang YK, Kim HD, Cho H, Park YS, Lee JS, and Ryu MH. Phase 2 study of neoadjuvant durvalumab plus docetaxel, oxaliplatin, and S-1 with surgery and adjuvant durvalumab plus S-1 for resectable locally advanced gastric cancer. J Immunother Cancer. (2025) 13. doi: 10.1136/jitc-2024-010635
26. Zhang X, Wang J, Wang G, Zhang Y, Fan Q, Lu C, et al. First-line sugemalimab plus chemotherapy for advanced gastric cancer: the gemstone-303 randomized clinical trial. Jama. (2025) 333:1305–14. doi: 10.1001/jama.2024.28463
27. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-pd-1/pd-L1 treatment for Malignancies: A meta-analysis. Front Pharmacol. (2017) 8:730. doi: 10.3389/fphar.2017.00730
28. Kuo PJ, Lin PC, and Hsieh CH. Wound healing in cancer patients under immunotherapy. Int J Surg. (2025). doi: 10.1097/JS9.0000000000002819
Keywords: gastric cancer, complication, machine learning, predictive model, postoperative outcome
Citation: Li R, Zhao Z and Yu J (2025) Development and validation of a machine learning model for predicting early postoperative complications after radical gastrectomy. Front. Oncol. 15:1631260. doi: 10.3389/fonc.2025.1631260
Received: 19 May 2025; Accepted: 29 September 2025;
Published: 14 October 2025.
Edited by:
Arunkumar Krishnan, Levine Cancer Institute, United StatesReviewed by:
Alessandro Dario Mazzotta, Sapienza University of Rome, ItalyDaorong Wang, Yangzhou University, China
Copyright © 2025 Li, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianchun Yu, eXUtamNoQDE2My5jb20=
 Ruyin Li
Ruyin Li Zirui Zhao
Zirui Zhao Jianchun Yu
Jianchun Yu