- 1Department of Psychological Sciences and Health, University of Strathclyde, Glasgow, United Kingdom
- 2Glasgow Head and Neck Cancer (GLAHNC) Research Group, Glasgow, United Kingdom
- 3School of Medicine, Dentistry and Nursing, University of Glasgow, Glasgow, United Kingdom
- 4Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 5Department of Computer and Information Sciences, University of Strathclyde, Glasgow, United Kingdom
- 6Beatson West of Scotland Cancer Centre, Glasgow, United Kingdom
- 7Department of Otolaryngology – Head and Neck Surgery, Glasgow Royal Infirmary/Queen Elizabeth University Hospital, Glasgow, United Kingdom
Introduction: Patient-initiated follow-up (PIFU) after treatment for head and neck cancer (HNC) relies on the signs and symptoms of recurrence being detectable by patients. We examine the evidence for patient-reported symptoms as an indicator of recurrence.
Methods: A search was conducted via OvidMEDLINE and Embase (2010 to January 2024) plus sources of grey literature for studies which describe patient-reported symptoms and recurrent disease. Findings are reported as per PRISMA guidelines.
Results: Twenty studies were included which were highly heterogenous. The median sensitivity of patient-reported symptoms to detect recurrence is 47.3%. Median specificity, positive-predictive value (PPV) and negative-predictive value (NPV) were 79.3%, 9.3% and 98.0% respectively. New symptoms were generally reported at routine follow-up rather than expedited appointments.
Conclusion: The high specificity and NPV of patient-reported symptoms means recurrence is unlikely in the absence of symptoms. Patient education and collection of prospective data through digital health technologies may increase the effectiveness of PIFU.
1 Introduction
Head and neck cancer (HNC) is the 8th most common cancer in the UK (1). Following treatment, over a third of patients experience recurrence depending on the tumour stage and primary site. Treatment options for recurrent disease include salvage surgery, re-irradiation, palliative chemotherapy and/or immunotherapy. A recent meta-analysis reported 5-year overall survival following salvage surgery between 26-67% (2). Salvage treatments generally carry significant morbidity but are more likely to be successful if recurrence is detected at an earlier stage (3) and therefore the emphasis remains on early identification.
The rationale for follow-up after HNC treatment is the detection of recurrent disease and the management of post-treatment toxicity. Current UK recommendations are for patients to be seen at least every 2 months for the first 2 years, followed by every 3–6 months for a minimum of 5 years in total. It is recommended that patients should have clinical examination at every follow-up including, when appropriate, nasopharyngolaryngoscopy (4). Patient-initiated follow-up (PIFU) is not currently routine practice, and many patients prefer a scheduled follow-up approach for reassurance and reliable access to information (5). PIFU has been mooted in HNC since there is limited evidence that regular follow-up impacts survival outcomes and outpatient capacity can struggle to meet the demand of recommended appointment frequency. A recent systematic review of PIFU following treatment for other cancer types found similar rates of recurrence, survival, quality of life, fear of recurrence and patient satisfaction in breast and colorectal cancer compared to conventional follow-up. However, it is noted that all breast cancer PIFU programmes included regular mammograms and colorectal PIFU programmes included either regular testing for faecal occult blood or CT scans (6).
For PIFU to replace the function of routine surveillance in HNC, it should be a reliable tool to identify recurrence; this has not yet been demonstrated but is the subject of on-going research (7). In this systematic review, we aim to examine the value of patient-reported symptoms in the detection of recurrence and second primary (SP) in HNC and therefore the potential role of symptoms in PIFU.
2 Methods
2.1 Eligibility criteria
2.1.1 Inclusion
● Studies describing patient-reported signs or symptoms after curative treatment for Head and Neck squamous-cell carcinoma (HNSCC),
● Some or all the patients in the cohort received primary cancer treatment after January 2010,
● Studies report rates of recurrence and/or SP detection in relation to patient symptoms,
● Full text available in English.
2.1.2 Exclusion
● Study subjects have known recurrent or metastatic HNSCC, non-SCC histology, cutaneous, upper oesophageal cancer or thyroid cancer,
● Patients receiving palliative or non-curative treatment,
● Case reports,
● No original data presented e.g. review articles.
2.2 Information sources
The search was conducted on OvidMEDLINE (1974 to January 26 2024) and Embase (1946 to January 26 2024). Sources of grey literature were searched via four channels: the online repository Open Access Thesis and Dissertations (oatd.org), ClinicalTrials.gov, MedRxiv, and a Google search where the first 100 hits were screened for relevance. The references and citations of included studies were also subject to screening followed by full-text review if deemed relevant.
2.3 Search strategy
This review was registered on PROSPERO (CRD42024510566) and reported according to PRISMA guidelines (8). OvidMEDLINE and Embase were searched separately using the following terms: (head and neck cancer) AND (patient reported or patient-reported or symptom*) AND (recurrent or recurrence or second primary). “Head and neck cancer” as subject/keyword and “recurrence” were used to search the oatd.org database with a filter for English-language. The search terms are described in detail and for other sources in Appendix 1.
2.4 Selection process
Duplicates were manually removed by screening of the title, first author name and year of publication. Abstracts were screened for inclusion or exclusion by two authors (KH and RH) according to the criteria above and full-text review was performed with over-sight by CD who made a final decision on inclusion in cases of disagreement.
A cut-off of treatment prior to 2010 was applied since the wide-spread adoption of intensity-modulated radiotherapy (IMRT) around this time reduced treatment-related long-term sequelae (9). Similarly, the role of human-papilloma virus (HPV) in oropharyngeal cancer was recognised and changed the understanding of risk and recurrence related to these cancers (10).
2.5 Data collection
Data was extracted by KH. Data items retrieved were the first author, country of study, year of publication, number of patients included in study, period of patient treatment, demographic and clinical characteristics of patients, the patient-reported outcome measure used, and key findings related to recurrence of disease or SP.
2.6 Effect measures
Data was collected on the number of true positives (patients with reported symptoms and confirmed recurrence), true negatives (asymptomatic patients without recurrence), false positives (symptomatic without recurrence) and false negatives (asymptomatic with recurrence). Where sufficient data was reported a calculation of sensitivity, specificity, positive-predictive value (PPV) and negative predictive value (NPV) was performed. Confidence intervals of 95% were calculated using RStudio 2024.12.0.
2.7 Synthesis methods
Variables as described above were tabulated. A narrative synthesis of results was performed. Studies were almost exclusively observational in nature with heterogenous study populations and study design therefore meta-analysis could not be meaningfully performed.
2.8 Quality assessment and risk of bias
Most of the studies were a cohort study involving longitudinal observation of a group of patients following treatment and assessment of their outcomes. There are no recommended risk-of-bias tools specific to this study design. The following sources of potential bias were assessed, derived from the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies (11): 1) representativeness of the population, 2) method of assessing symptoms, 3) adequacy of follow-up and 4) identification of and control for potential confounding factors.
3 Results
3.1 Study selection
The search of OvidMEDLINE and Embase yielded a total of 1192 results. After de-duplication there were 887 records remaining. Six articles were removed as the publication was not available in English language and a further 213 were removed as the full text was not available e.g. conference abstract only. The remaining 668 records were reviewed by KH and RH for eligibility. Twenty-two theses were also screened for inclusion and one dissertation was sought for full-text review. ClinicalTrials.gov search yielded 123 records which were screened for relevance, but none sought for full-text review. MedRxiv found 83 records, 1 sought for full-text review. A Google search did not yield any previously unfound records. The cut-off for treatment time prior to 2010 was applied to the final set of records since it is not possible to search or filter for this in the conventional way. The process of study selection is shown in the PRISMA flow-diagram (Figure 1).
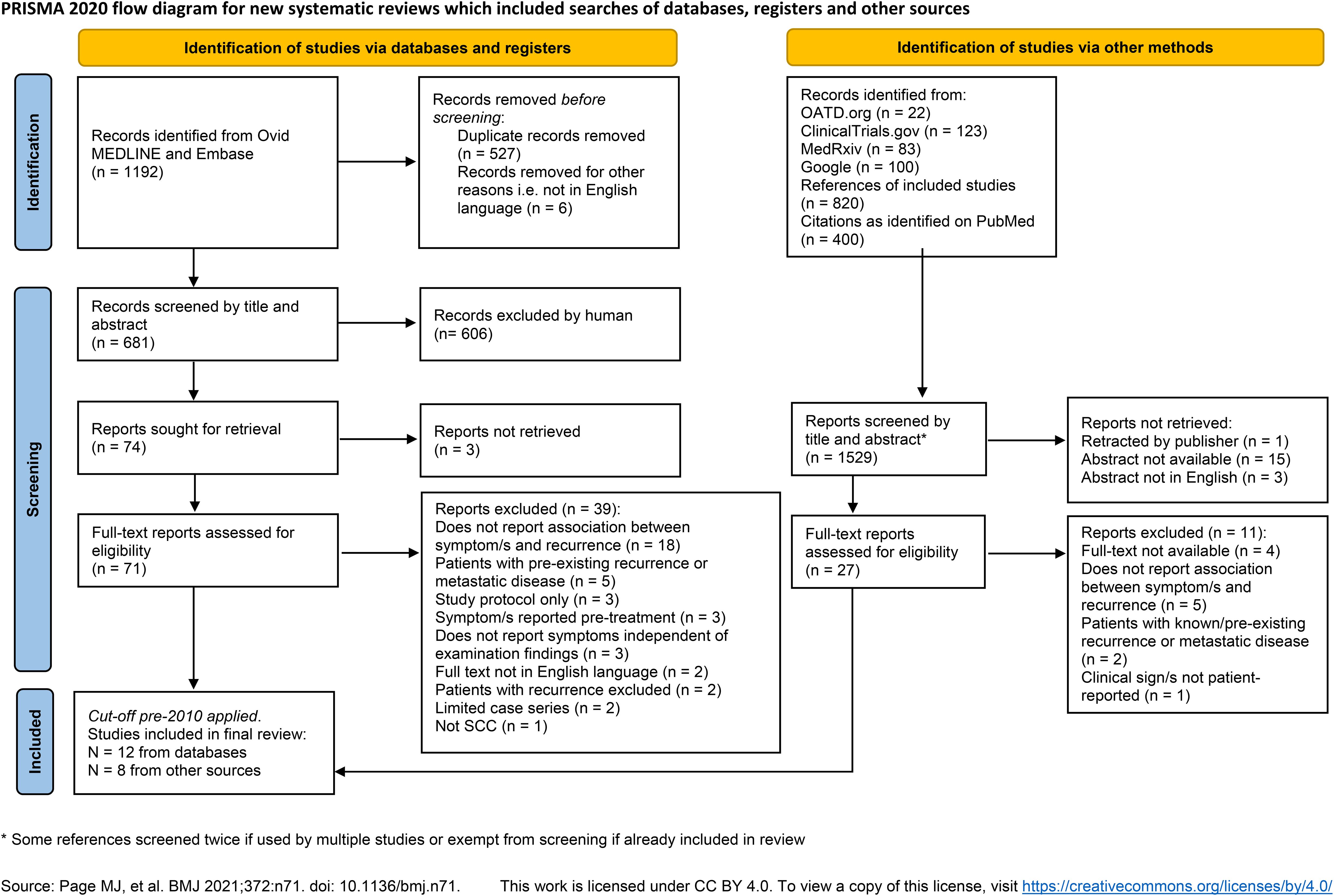
Figure 1. PRISMA flow diagram shows the data sources, excluded records and reasons for exclusion. *Some references screened twice if used by multiple studies or exempt from screening if already included in review. Source: Page MJ, et al. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. This work is licensed under CC BY 4.0. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
3.2 Study characteristics
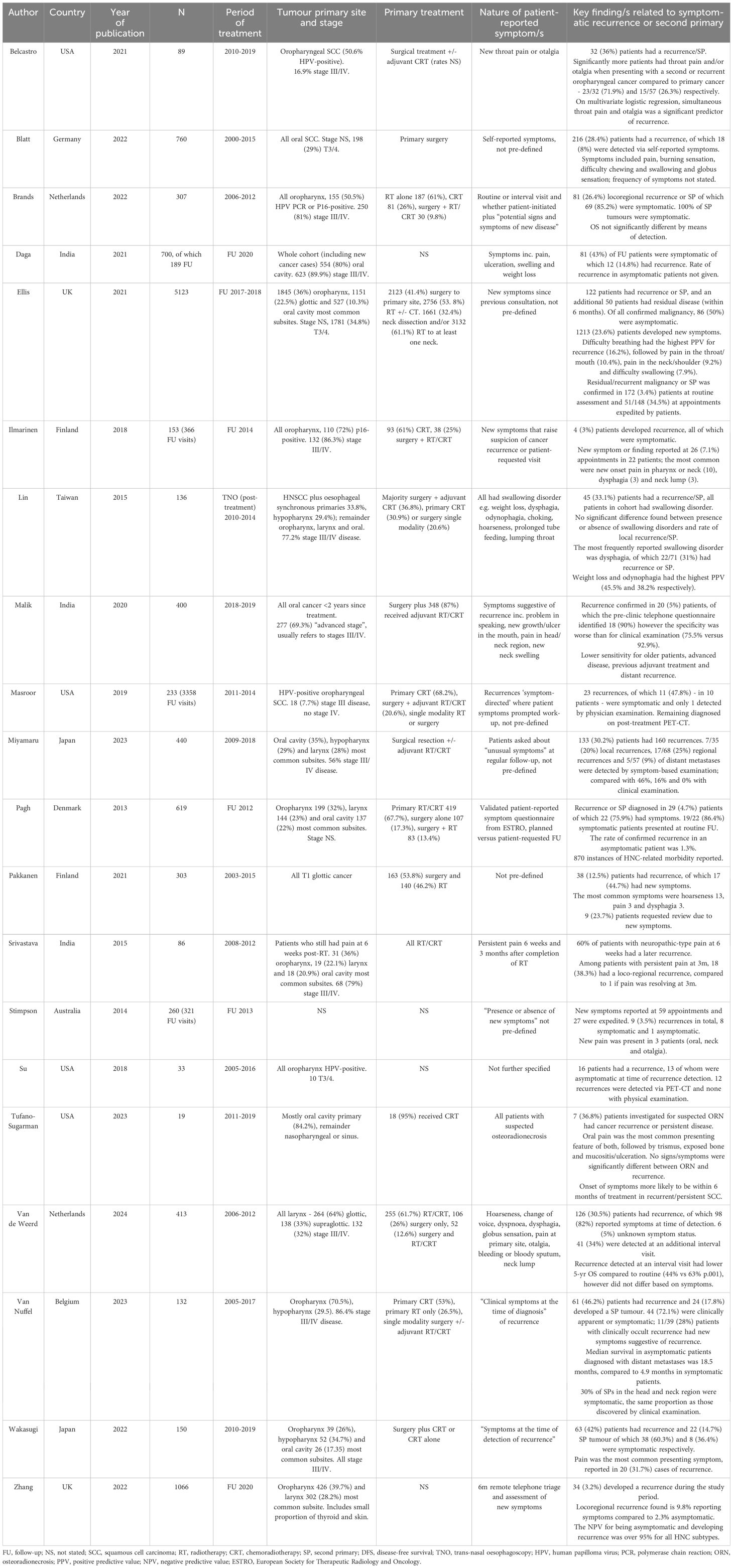
A total of 20 studies are included in this review, published between 2013-2024. Key features and results of individual studies can be found in Table 1 (12–31). The study populations represent a heterogeneous group of patients with different primary disease site and stages and varying treatment modalities. There are 17 cohort studies, of which 4 are retrospective. In these studies, the rate of symptomatic and asymptomatic recurrences or SP are generally reported however few state the rate of patient-reported symptoms in the whole cohort. There were 3 case series, of which 2 assessed a patient group which were all symptomatic.
3.3 Quality and risk of bias
The focus of the studies varied greatly, and in some cases, represent a narrow subset of the HNC patient population. For example, Pakkanen et al. only include T1 laryngeal cancer and Wakasugi et al. only include patients with locally advanced (T3/4) disease. The studies by Lin and Tufano-Sugarman, whose patients all had swallowing problems and symptoms in-keeping with osteoradionecrosis respectively, are not typical of the HNC post-treatment population. One study of patients with oropharyngeal tumours included only those who received surgical treatment, whereas the usual treatment modality for many of these patients would be primary chemoradiotherapy and therefore this is also an atypical cohort.
In most studies, the nature of patient-reported symptoms was either entirely undefined or loosely described, such as symptoms “suggestive of recurrence”. One paper used a validated questionnaire to capture patient-reported symptoms (22). We cannot be confident of the completeness of symptom data when collected retrospectively from patients’ notes although this is most pertinent for the absence of symptom data as symptoms are unlikely to have been recorded as present in error.
Seventeen studies followed patients up for an adequate length of time for recurrence to become apparent after the onset of recorded symptoms. The study by Daga et al. presented a retrospective audit of patients with HNC presenting to the hospital during a period of COVID lockdown in India for 2 months. Stimpson and colleagues invited patients attending follow-up to complete a questionnaire which included questions about the presence or absence of new symptoms. This was compared with findings at the clinic appointment, such as suspicious of recurrence. Therefore these two studies only represented a snapshot which could potentially miss recurrence associated with the reported symptoms. Since most studies were retrospective, there was not an issue with loss to follow-up or patient attrition and generally reporting bias was not a concern as all cases or consecutive cases within the period were reported.
Since most of the cohort studies were not primarily designed to evaluate symptomatic recurrence, few identified and attempted to account for associated confounding factors which could have influenced the rate of symptomatic recurrence, such as stage or treatment modality. Ten studies identified and controlled for one or more other factors which might influence the prevalence of post-treatment symptoms.
3.4 Results of synthesis
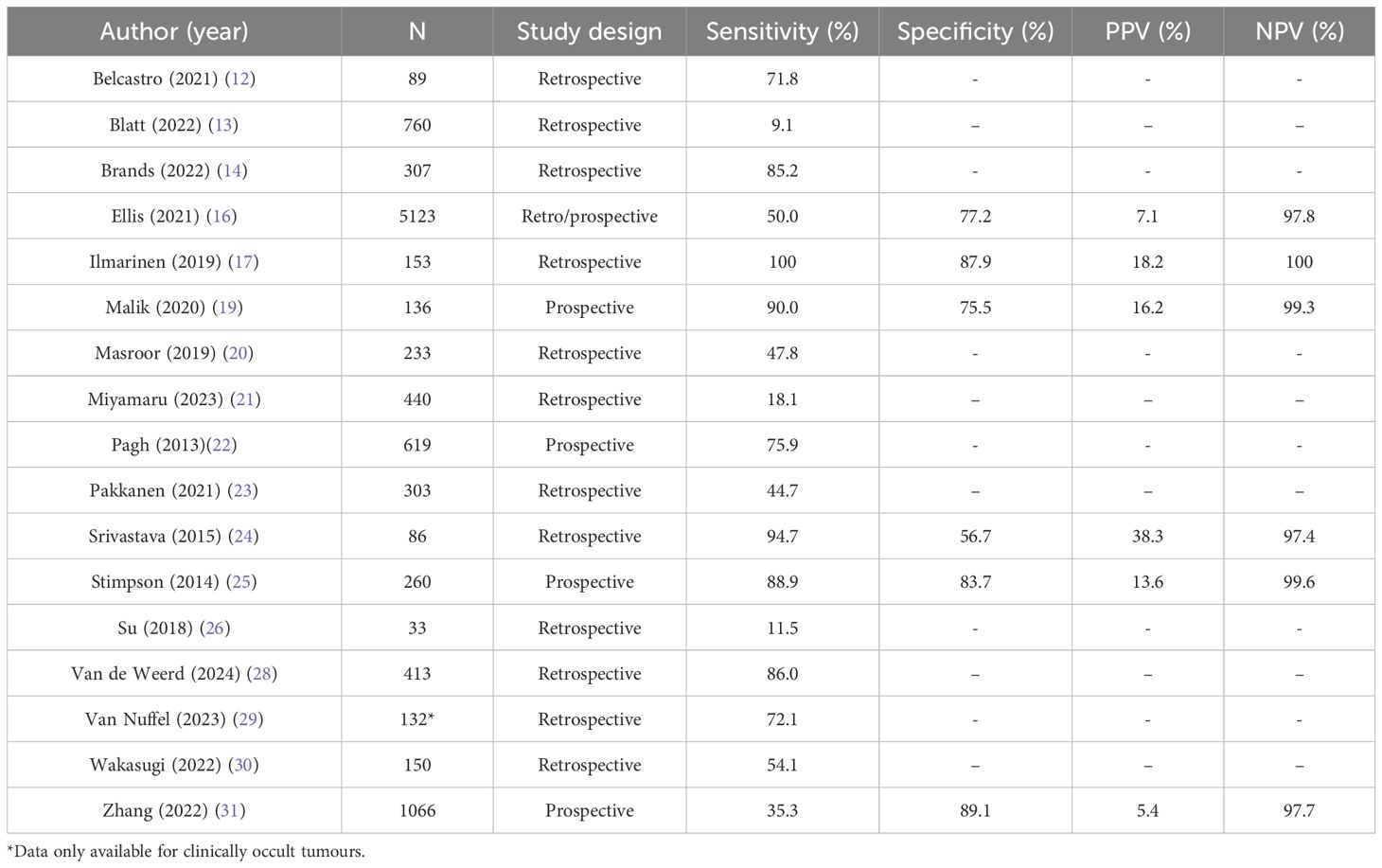
The diagnostic power of patient-reported symptoms to detect recurrence as reported in the 17 cohort studies are shown in Table 2. The median sensitivity is 47.3% [CI 44.3, 50.2], so fewer than half of patients with a recurrence will have recognisable symptoms. The reported sensitivity ranged widely from 9.1% to 100%. There was no obvious association between the predominant cancer subsites represented in the study and the sensitivity of patient-reported symptoms. Indeed, the worst reported sensitivity was in a cohort of patients all with oral cancer (13), but another study of entirely oral cancer patients by Malik et al. reported 90.0% sensitivity (19). The second worst reported sensitivity was in a study of patients with HPV-positive oropharyngeal cancer by Su et al, however the highest reported sensitivity was in the study by Ilmarinen which was also all patients with oropharyngeal cancer, of which 72% were HPV-positive. A much greater proportion of patients in the latter study were advanced stage, but again this is not a consistent pattern across the studies.
Sufficient data was reported in 6 cohort studies to calculate specificity, PPV and NPV of which the median is 79.3% [CI 78.3, 80.2], 9.3% [CI 7.9, 10.8] and 98.0% [CI 97.6, 98.3] respectively. This does not include case-controlled studies where either all patients were symptomatic, or all had recurrent disease. Data was able to be retrieved from studies representing all cancer subsites but only the studies by Ilmarinen and Malik included a single subsite. The specificity ranges from 56.7 - 89.1%, meaning that most patients who are asymptomatic will not have a recurrence (true negative), however symptoms are not a highly specific indicator of disease. Both PPV and NPV are determined by the prevalence of disease in the population. Five out of the six studies reported a very low recurrence rate (≤5%) and therefore NPV is expected to be high since it is inversely related to prevalence.
The most frequent patient-reported signs and symptoms varied slightly between studies but were predictable ‘red flags’ such as throat pain, hoarseness and difficulty swallowing. There is limited evidence for the relationship between the timing of patients’ symptoms and recurrence e.g. whether new onset is more pertinent. Of note, several studies report the number of patients expediting their appointments due to symptoms but there appears to be a significant cohort of patients across the studies who experienced symptoms but were seen at routine follow-up intervals.
4 Discussion
4.1 Results in context
Morbidity following treatment for HNC is common and, in the included studies, the recording of symptom rate in individuals without recurrence is poor. A European study of all cancer types (mostly breast cancer) following radiotherapy found a symptom rate of 55% and a quarter of these symptomatic patients had a recurrence (32). In comparison, the PPV of symptoms in HNC is consistently poor. This may be because the overall rate of symptoms in HNC is higher and therefore it is less discriminating for disease. A PIFU service for HNC may therefore be less efficient at detecting recurrent disease than in other cancers and have less impact in reducing follow-up demand. It also bears repeating that in PIFU programmes for other cancer types, additional testing including imaging is performed routinely (6). Since UK guidance is currently only to perform additional post-treatment imaging if clinically indicated, any such PIFU programme in HNC would need to determine on what basis imaging is requested. If it is based on symptoms then this could drive a significant increase in the demand for radiological tests.
Multiple studies found that patients with symptoms, which should have raised concern for recurrence, did not have expedited clinical review. This could be because patients did not inform their clinical team of new symptoms. If so, this is somewhat at odds with the findings from Lorenc et al. of high levels of confidence amongst HNC survivors in contacting the clinic upon identifying symptoms (33). This perhaps reflects the self-selection of patients with high levels of understanding and engagement who participated in the interview study. Patients who were interviewed also had higher rates of post-graduate education than the pool of HNC survivors they were drawn from (37.9% versus 29.5%) possibly meaning they were generally better informed. The issue of patient awareness highlights the need for patient education about red flag symptoms and self-examination. INTEGRATE audited patient education about red flag symptoms, as part of UK guidance for HNC follow-up consultations, and found documentation of verbal information-giving in 20.2% of appointments and written in 0.2% (16). This indicates significant room for improvement but could also reflect incomplete documentation.
4.2 Limitations of evidence
Very few studies pre-defined the symptoms which patients were asked about during follow-up. Despite this, expected symptoms of HNC recurrence such as pain and dysphagia arose repeatedly. Presumably these symptoms were elicited or volunteered by patients and recorded because they are known to be associated with HNC presentations. However, it is unclear whether this represents a complete picture of patients’ symptoms for example dry mouth, sore mouth and dental issues are very common patient-reported concerns, but these were not well represented. Most studies in this review were retrospective in nature. It is probable that there is missing data where symptoms deemed to be less important have not been recorded. This along with the lack of pre-defined symptoms means that it is not possible to draw any strong conclusions.
The patient populations in the included studies are very heterogenous; reflected in the wide range of sensitivity (9.1-100%) for patient reported symptoms. The overall prevalence of symptoms in patients after treatment for HNC is poorly described and therefore it is not possible to determine the extent to which patient-reported measures could have a meaningful role in identifying the presence of recurrence. The rate of recurrence reported in the included studies is hugely variable but particularly low for the studies which reported sufficient data to calculate PPV and NPV. Given these values are both dependent on the population prevalence, these results should be interpreted with caution.
4.3 Limitations of the review
Despite the terms employed in the literature search being broad, many papers were found via references and citations rather than the initial search. Regardless, the authors are confident that this approach including grey literature sources has yielded a complete picture of the available evidence on this subject. As mentioned earlier there are no valid risk-of-bias tools for observational cohort studies of this nature however we have based reporting on an existing tool and have captured the common concerns about these studies.
This review includes a heterogenous group of patients in terms of primary site, stage, treatment modality and tumour HPV-status. Tests of sensitivity and specificity are specific to the population and the population prevalence of the outcome of interest, both of which are highly variable in these studies. The tests of diagnostic power should therefore be interpreted with a significant degree of caution. This is reflected in the wide range of sensitivity and specificity values amongst the studies. This warrants further investigation with data segregated by disease subsite and with prospective collection of symptom data.
4.4 Implications of the results
This review suggests that symptoms in isolation are not a reliable method of detection of HNC recurrence. As patients are very unlikely to have a recurrence in the absence of symptoms, we should consider whether follow-up based primarily around recurrence detection is in patients’ best interest. Patients can have life-long morbidity after HNC diagnosis and treatment (34). For some patients, psychological morbidity including fear of cancer recurrence and body image disturbance are more pronounced than physical concerns. These aspects might be better managed in a different setting by alternative healthcare professionals e.g. Cancer Nurse Specialists (CNS) and clinical psychologists. There is a concern that PIFU models of surveillance after treatment could delay the identification of some recurrence presentations. We may question whether reliance on PIFU is justified when the outcomes of salvage treatments are generally more favourable in early stage, small volume disease.
Some studies reported that patients were experiencing symptoms at their consultation, but they were seen at a routine visit, i.e. they had not expedited their follow-up appointment. We must ensure patients are equipped with the knowledge and skills required to maximise symptom detection by educating them about red flag symptoms, empowering them to highlight to clinicians when they might be experiencing them and provide a route for urgent review.
To more accurately address the question of whether patient-reported signs and symptoms could be used to detect recurrence, patients should be asked to report all symptoms in a reliable and repeatable manner. Use of validated patient-reported outcome measures would be ideal but there should also be consensus on the measures used to compare outcomes across treatment centres. Digital tools such as smartphone applications may be useful to encourage patients to record and report signs and symptoms on a regular basis to identify symptom trends. International collaberators have already embarked on creating such as system in HNC (35). In the future, artificial intelligence tools may be trained to handle large datasets and identify common patterns which may predict recurrence however for this to be possible, accurate and more granular prospective data must be available.
5 Conclusion
The specificity of patient-reported symptoms is good meaning if patients do not report new or worsening symptoms, clinical teams can be reassured that the chance of recurrent disease is low. However, sensitivity is very poor in some studies therefore patient-reported signs and symptoms in isolation are not a reliable means of recurrence or SP detection. This question needs further investigation using prospective, pre-defined symptom data to build a complete picture of the prevalence of symptoms in the HNC follow-up population. Patient education, collection of data via digital symptom-tracking and the use of validated PROMs may optimise the diagnostic yield of patient-reported signs and symptoms.
Author contributions
KH: Writing – review & editing, Formal analysis, Conceptualization, Data curation, Methodology, Writing – original draft. RH: Writing – review & editing, Data curation. AL: Methodology, Supervision, Writing – review & editing. RM: Supervision, Writing – review & editing, Methodology. CP: Methodology, Writing – review & editing. CD: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Chief Scientist Office (CSO), part of the Scottish Government Health Directorate, via an Innovation Academic Fellowship (IAF/23/01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1632592/full#supplementary-material
References
1. Cancer Research UK. Head and neck cancer incidence statistics . Available online at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers (Accessed 9/7/2024).
2. Bulbul MG, Genovese TJ, Hagan K, Rege S, Qureshi A, and Varvares MA. Salvage surgery for recurrent squamous cell carcinoma of the head and neck: Systematic review and meta-analysis. Head Neck. (2022) 44:275–85. doi: 10.1002/hed.26898
3. Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. (2000) 110:1–18. doi: 10.1097/00005537-200003001-00001
4. Homer JJ and Winter SC. Head and neck cancer: United Kingdom national multidisciplinary guidelines, sixth edition. J Laryngol Otol. (2024) 138:S31. doi: 10.1017/S0022215123001615
5. McLaren O, Perkins C, Zhu Y, Smith M, and Williams R. Patient perspectives on surveillance after head and neck cancer treatment: A systematic review. Clin Otolaryngol. (2021) 46:1345–53. doi: 10.1111/coa.13846
6. Dretzke J, Chaudri T, Balaji R, Mehanna H, Nankivell P, and Moore DJ. PETNECK2 Research Team. A systematic review of the effectiveness of patient-initiated follow-up after cancer. Cancer Med. (2023) 12:19057–71. doi: 10.1002/cam4.v12.18
7. Nankivell P, Gaunt P, Gaunt C, Sissons J, Liaskou E, Jefferson Y, et al. PETNECK2 Research Team. PET-CT-guided, symptom-based, patient-initiated surveillance versus clinical follow-up in head neck cancer patients (PETNECK2): study protocol for a multicentre feasibility study and non-inferiority, randomised, phase III trial. BMC Cancer. (2024) 24:823. doi: 10.1186/s12885-024-12470-9
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
9. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhinde SA, Clark C, et al. PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2011) 12:127–36. doi: 10.1016/S1470-2045(10)70290-4
10. Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, Ulmer KM, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. (2009) 119:1542–9. doi: 10.1002/lary.v119:8
11. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et alThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. University of Ottawa, Canada: The Ottawa Hospital Research Institute. Available at: https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf.
12. Belcastro A, Smith BD, Heidel RE, and Hechler BL. Incidence of pain complaints in oropharyngeal squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 132:626–32. doi: 10.1016/j.oooo.2021.03.005
13. Blatt S, Krüger M, Sagheb K, Barth M, Kämmerer PW, and Al-Nawas B. Tumor recurrence and follow-up intervals in oral squamous cell carcinoma. J Clin Med. (2022) 11(23):7061. doi: 10.3390/jcm11237061
14. Brands MT, Swinkels IJ, Aarts AM, Verbeek AL, Merkx MA, Marres HA, et al. Value of routine follow-up in oropharyngeal squamous cell cancer patients treated with curative intent. Head Neck. (2023) 45:586–94. doi: 10.1002/hed.27269
15. Daga D, Mishra A, Sharma SS, Rai AK, Valsareddy SK, and Singh U. Safe delivery of surgical care in head and neck cancer patients during COVID-19-an audit of pattern of presentation and treatment strategies in an oncology centre in the northern India. Indian J Surg Oncol. (2021) 12:250–6. doi: 10.1007/s13193-021-01399-1
16. INTEGRATE. Post-treatment head and neck cancer care: national audit and analysis of current practice in the United Kingdom. Clin Otol. (2021) 46:284–94. doi: 10.1111/coa.13616
17. Ilmarinen T, Keski-Säntti H, Markkanen-Leppänen M, Haapaniemi A, Tapiovaara L, Atula T, et al. De-escalation of post-treatment surveillance in oropharyngeal cancer. Head Neck. (2019) 40:1457–62. doi: 10.1002/hed.25593
18. Lin PH, Wang CP, Lou PJ, Ko JY, Hsiao TY, and Chen TC. Evaluation of swallowing disorders by use of transnasal esophagoscopy in patients treated for head and neck cancer. Head Neck. (2016) 38(Suppl 1):E1137–43. doi: 10.1002/hed.24174
19. Malik A, Nair S, Sonawane K, Lamba K, Ghosh-Laskar S, Prabhash K, et al. Outcomes of a telephone-based questionnaire for follow-up of patients who have completed curative-intent treatment for oral cancers. JAMA Otolaryngol Head Neck Surg. (2020) 146:1102–8. doi: 10.1001/jamaoto.2020.2404
20. Masroor F, Corpman D, Carpenter DM, Ritterman WM, Cheung KHN, and Wang KH. Association of NCCN-recommended posttreatment surveillance with outcomes in patients with HPV-associated oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. (2019) 145:903–8. doi: 10.1001/jamaoto.2019.1934
21. Miyamaru S, Nishimoto K, Murakami D, Kuraoka K, Saito H, and Orita Y. The timing and methods for detection of recurrence in patients with head and neck cancer. Acta Oto-Laryngologica. (2023) 143:617–22. doi: 10.1080/00016489.2023.2237520
22. Pagh A, Vedtofte T, Lynggaard CD, Rubek N, Lonka M, Johansen J, et al. The value of routine follow-up after treatment for head and neck cancer. A national survey from DAHANCA. Acta Oncol. (2013) 52:277–84. doi: 10.3109/0284186X.2012.741324
23. Pakkanen P, Ilmarinen T, Halme E, Irjala H, Koivunen P, Pukkila M, et al. T1 glottic laryngeal cancer: the role of routine follow-up visits in detecting local recurrence. Eur Arch Otorhinolaryngol. (2021) 278:4863–9. doi: 10.1007/s00405-021-06983-3
24. Srivastava P, Kingsley PA, Srivastava H, Sachdeva J, and Kaur P. Persistent post-radiotherapy pain and locoregional recurrence in head and neck cancer-is there a hidden link? Korean J Pain. (2015) 28:116–21. doi: 10.3344/kjp.2015.28.2.116
25. Stimpson P, Batt M, and Vallance N. Head and neck cancer recurrence: a prospective analysis of 401 follow-up visits to an Australian cancer centre. Clin Otolaryngol. (2014) 39:292–6. doi: 10.1111/coa.2014.39.issue-5
26. Su W, Miles BA, Posner M, Som P, Kostakoglu L, Gupta V, et al. Surveillance imaging in HPV-related oropharyngeal cancer. Anticancer Res. (2018) 38:1525–9. doi: 10.21873/anticanres.12380
27. Tufano-Sugarman AM, Wang KY, Kohn N, Ghaly M, Parashar B, Frank D, et al. Osteoradionecrosis versus cancer recurrence: an unresolved clinical dilemma. ORL J Otorhinolaryngol Relat Spec. (2023) 85:28–35. doi: 10.1159/000527261
28. van de Weerd C, Geurts SME, Vercoulen RJMT, van Veggel IHF, Brands MT, Marres HAM, et al. Value of routine follow-up for recurrence detection after treatment with curative intent for laryngeal squamous cell carcinoma. Eur J Surg Oncol. (2024) 50:107304. doi: 10.1016/j.ejso.2023.107304
29. Van Nuffel J, Verbruggen K, Voordeckers M, De Brucker Y, Platteaux N, Foulon I, et al. A retrospective analysis: follow up with 18F-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography in oro- and hypopharyngeal squamous cell carcinoma patients. J Laryngol Otol. (2023) 138(7):747–54. doi: 10.1017/S0022215123001214
30. Wakasugi T, Nguyen TN, Takeuchi S, Ohkubo JI, and Suzuki H. Pattern of recurrence after platinum-containing definitive therapy and efficacy of salvage treatment for recurrence in patients with squamous cell carcinoma of the head and neck. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.876193
31. Zhang H, Hardman JC, Tikka T, Nankivell P, Mehanna H, Paleri V, et al. Symptom-based remote assessment in post-treatment head and neck cancer surveillance: A prospective national study. Clin Otolaryngol. (2022) 47:561–7. doi: 10.1111/coa.13948
32. Ataman OU, Barrett A, Davidson S, De Haas-Kock D, Dische S, Dubray B, et al. (REACT working group of ESTRO). Audit of effectiveness of routine follow-up clinics after radiotherapy for cancer: a report of the REACT working group of ESTRO. Radiother Oncol. (2004) 73:237–49. doi: 10.1016/j.radonc.2004.05.001
33. Lorenc A, Greaves C, Duda J, Brett J, Matheson L, Fulton-Lieuw T, et al. (PETNECK2 Research Team). Exploring the views of patients’ and their family about patient-initiated follow-up in head and neck cancer: A mixed methods study. Eur J Cancer Care. (2022) 31(6):e13641. doi: 10.1111/ecc.13641
34. Nilsen ML, Belsky MA, Scheff N, Johnson JT, Zandberg DP, Skinner H, et al. Late and long-term treatment-related effects and survivorship for head and neck cancer patients. Curr Treat Options Oncol. (2020) 21:92. doi: 10.1007/s11864-020-00797-x
35. Cavalieri S, Vener C, LeBlanc M, Lopez-Perez L, Fico G, Resteghini C, et al. BD4QoL Consortium. A multicenter randomized trial for quality of life evaluation by non-invasive intelligent tools during post-curative treatment follow-up for head and neck cancer: Clinical study protocol. Front Oncol. (2023) 31:1048593. doi: 10.3389/fonc.2023.1048593
Keywords: head and neck cancer, cancer morbidity, cancer recurrence, patient reported outcome (PRO), symptomatic recurrence
Citation: Hulse K, Hurley R, Lowit A, Maguire R, Paterson C and Douglas CM (2025) Patient-reported symptoms in the detection of head and neck cancer recurrence: a systematic review. Front. Oncol. 15:1632592. doi: 10.3389/fonc.2025.1632592
Received: 21 May 2025; Accepted: 30 May 2025;
Published: 18 June 2025.
Edited by:
Carlo Resteghini, Humanitas University, ItalyReviewed by:
Paula T. Bradley, Newcastle University, United KingdomCasey Fazer-Posorske, Mayo Clinic, United States
Copyright © 2025 Hulse, Hurley, Lowit, Maguire, Paterson and Douglas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Hulse, a2F0ZS5odWxzZUBzdHJhdGguYWMudWs=
 Kate Hulse
Kate Hulse Rhona Hurley
Rhona Hurley Anja Lowit
Anja Lowit Roma Maguire5
Roma Maguire5 Catriona M. Douglas
Catriona M. Douglas