- 1Department of Thoracic Surgery, Hainan Hospital of Chinese People's Liberation Army (PLA) General Hospital, Sanya, Hainan, China
- 2Department of Traditional Chinese Medicine, Shanghai Tianyou Hospital, Shanghai, China
- 3Department of Intensive Care Unit, Hainan Hospital of Chinese People's Liberation Army (PLA) General Hospital, Sanya, Hainan, China
- 4Department of Oncology, Hainan Hospital of Chinese People's Liberation Army (PLA) General Hospital, Sanya, Hainan, China
Background: Carcinoembryonic antigen (CEA) is still the most valuable tumor marker in the diagnosis and prognosis of non-small cell lung cancer (NSCLC) patients; however, its application is largely limited by its low sensitivity in stage I cases. Research on reliable and highly cost-effective prognostic indicators in CEA normal stage I NSCLC is still needed.
Methods: A retrospective study was conducted in CEA normal stage I NSCLC patients. The prognostic value of peripheral blood cell fractions, including the absolute neutrophil count (ANC), was tested, and the differences in clinical features among the ANC-low or ANC-high subgroups were checked. The disease-free survival (DFS) and overall survival (OS) differences in these subgroups were run by Kaplan–Meier analysis, and the risk factors for survival were validated by a Cox proportional hazards model.
Results: Among the tested peripheral blood cell fractions, only ANC was found to be a significant factor in predicting DFS (P = 0.011) and OS (P=0.043). The ANC displayed a positive correlation with other fractions, including the absolute lymphocyte count (R=0.26, P<0.001), absolute monocyte count (R=0.56, P<0.001), and platelet count (R=0.29, P<0.001). With a cutoff at 3879/mm3, 83.72% (252/301) of patients were divided into ANC-low and 16.28% (49/301) into ANC-high. Patients in the ANC-low group also presented a superior DFS (log rank=8.64, P = 0.003) and OS (log rank=9.86, P = 0.002) than those in the ANC-high group; however, the ANC level was not validated as an independent prognostic factor for both DFS and OS.
Conclusion: Compared to other peripheral blood cell fractions, preoperative ANC was found to be a useful prognostic indicator in CEA normal stage I NSCLC; however, it was not validated as an independent prognostic factor and additional studies for its role in prognosis for these patients are still needed in future.
Introduction
Lung cancer is still a heavy health and economic burden in China, with over 80% being non-small cell lung cancer (NSCLC) (1, 2), which mainly comprises adenocarcinoma (ADC) and squamous carcinoma (SCC). In recent decades, although overall survival (OS) for locally advanced NSCLC and those with remote lesions has greatly improved due to the success of immunotherapy-based regimens and targeted therapies (3–5), radical resection is still the optimal choice for early-stage cases, specifically stage I. Fortunately, with the success of clinical trials, including ADAURA (6), Keynote091 (7), and IMpower 010 (8), the survival for stage IB cases has been further guaranteed. Nonetheless, the 5-year OS for stage IA patients ranges from 77-92%, declining up to 68% for stage IB patients (9). Although many innovative prognostic indicators, such as molecular residual disease (MRD), have been reported and are very helpful in subsequent treatment strategy decisions in these cases (10, 11), searching for easily accessible and highly cost-effective prognostic indicators is still needed at present in practice.
Carcinoembryonic antigen (CEA) is a classic tumor marker in NSCLC (12) that plays an important role not only in diagnosis (13) but also in prognosis (14). However, the usefulness of CEA in practice was also blocked due to its relatively low sensitivity, particularly in stage I cases, as previous studies suggested a positive rate ranging from 16.22% (145/894 in stage IA (15)) to 33.62% [274/815 in stage I (16)]. Some studies have tried to minimize its cutoff points to improve its prognostic efficacy in stage I patients (17, 18), but the results were not extensively validated. Thus, other reliable prognostic indicators are still needed for stage I cases, the majority of which present a normal CEA level. Interestingly, it was found that some inflammatory cells and cytokines played a key role in early tumorigenesis of lung cancer (19–21), which suggested that they may also have a role in prognosis. Previously, some studies found that indicators such as the neutrophil-lymphocyte ratio (NLR) (22, 23) and lymphocyte to monocyte ratio (LMR) (24) could have prognostic value in stage I NSCLC; however, these studies also included some patients with abnormal CEA. Neutrophils are the main fractions in peripheral blood and have broad functions in cancer development, such as promoting metastasis (25, 26) and progression (27), and enhancing cancer cell adhesion (28). The prognostic usefulness of the absolute neutrophil count (ANC) has been addressed in many cancers (29–33). In lung cancer, neutrophils were also found to be a promoter of disease progression (34), and they could also stimulate T-cell responses in early-stage disease (35). In line with the aforementioned studies (29–33), the prognostic value of ANC in lung cancer was also established in chemo-naïve stage IIIB or IV cases (36) or previously treated and subsequently received anlotinib patients (37). However, the value of ANC in CEA normal stage I cases is still largely unknown.
In this study, we aimed to detect the prognostic value of the ANC in CEA normal stage I NSCLC (ADC+SCC) patients.
Materials and methods
Data collection
From October 2012 to September 2022, patients who received radical resection for lung ADC and SCC at Hainan Hospital of Chinese PLA General Hospital were retrospectively enrolled. Clinical data, including age (>60 years vs. ≤60 years), sex (female vs. male), smoking or alcohol history (with vs. without), and comorbidity (hypertension or type 2 diabetes) (with vs. without), were collected. In addition, other parameters including the maximum tumor diameter (MTD), lymphovascular invasion/spread through air spaces (combined together due to the limited cases), surgical approach are also documented. Those with any of the following criteria were not included: 1. any period of neoadjuvant therapies; 2. absence of preoperative laboratory tests, in particular CEA; 3. infections presented with fever before surgery or with comorbidities long-term prednisone use; and 4. follow-up problems (refused or lost). The study was conducted according to the principles stated in the Declaration of Helsinki and was approved by the ethics committee of Hainan Hospital of Chinese PLA General Hospital (ID: S2023-12). Due to its retrospective nature, written informed consent was exempted.
Examination of ANC, other blood fractions and CEA
Laboratory data, including ANC (reference range: 1.75-7×109/L) and other peripheral blood cell fractions, including absolute lymphocyte count (ALC) (reference range: 0.70-4×109/L), absolute monocyte count (AMC), (reference range: 0.105-0.8×109/L) and platelet count (PLC) (reference range: 100-300×109/L), were obtained within 3 weeks before the surgery from routine blood tests by using an automatic blood cell analyzer (XN3000, Sysmex Corporation, Japan) as described previously (38). CEA (reference range: 0-5.0 ng/mL) was tested by the electrochemiluminescence method according to the manufacturer’s manual in an automatic analysis system (Cobas e 601, Roche, Switzerland) (38).
Definition of disease-free survival and OS
The follow-up is conducted by telephone, visiting the medical record and WeChat with an interval of every 3–6 months for the first 1–2 years and then annually for the next after the surgery as described previously (39). DFS was defined as the period from the day of surgery to the day of any recurrence, metastasis, or death from any cause, and OS was defined from the same point to the date of death from any cause. The latest follow-up point ended in December 2024.
Statistical analysis
The significance of ANC and other peripheral blood cell fractions in predicting DFS and OS was tested by receiver operating characteristic curve (ROC) analysis, and its capabilities in predicting the outcomes were further checked by time-dependent ROC curves and by estimating the area under the curve (AUC). Patients were then divided into ANC-low or ANC-high subgroups based on the optimal cutoff point. The differences in the clinical data among these subgroups were analyzed by the chi-square test. The correlations of ANC with ALC, AMC, and PLC were run by Pearson of Spearman tests if the Gaussian distribution (by Kolmogorov–Smirnov test) was not met for these factors and a linear regression analysis was also performed for quantitative describe the correlation between these markers. The DFS and OS differences in the ANC-low or ANC-high subgroups were checked by Kaplan–Meier analysis followed by log-rank tests. Risk factors for DFS and OS were tested by a Cox proportional hazards model with the factors entered into the model by the iterative forward LR method. Two-sided P<0.050 was considered statistically significant. All analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA), R (i386 4.1.1) and the correlation of ANC with other markers were determined using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
Results
General features of the cohort and the significance of ANC in predicting DFS and OS
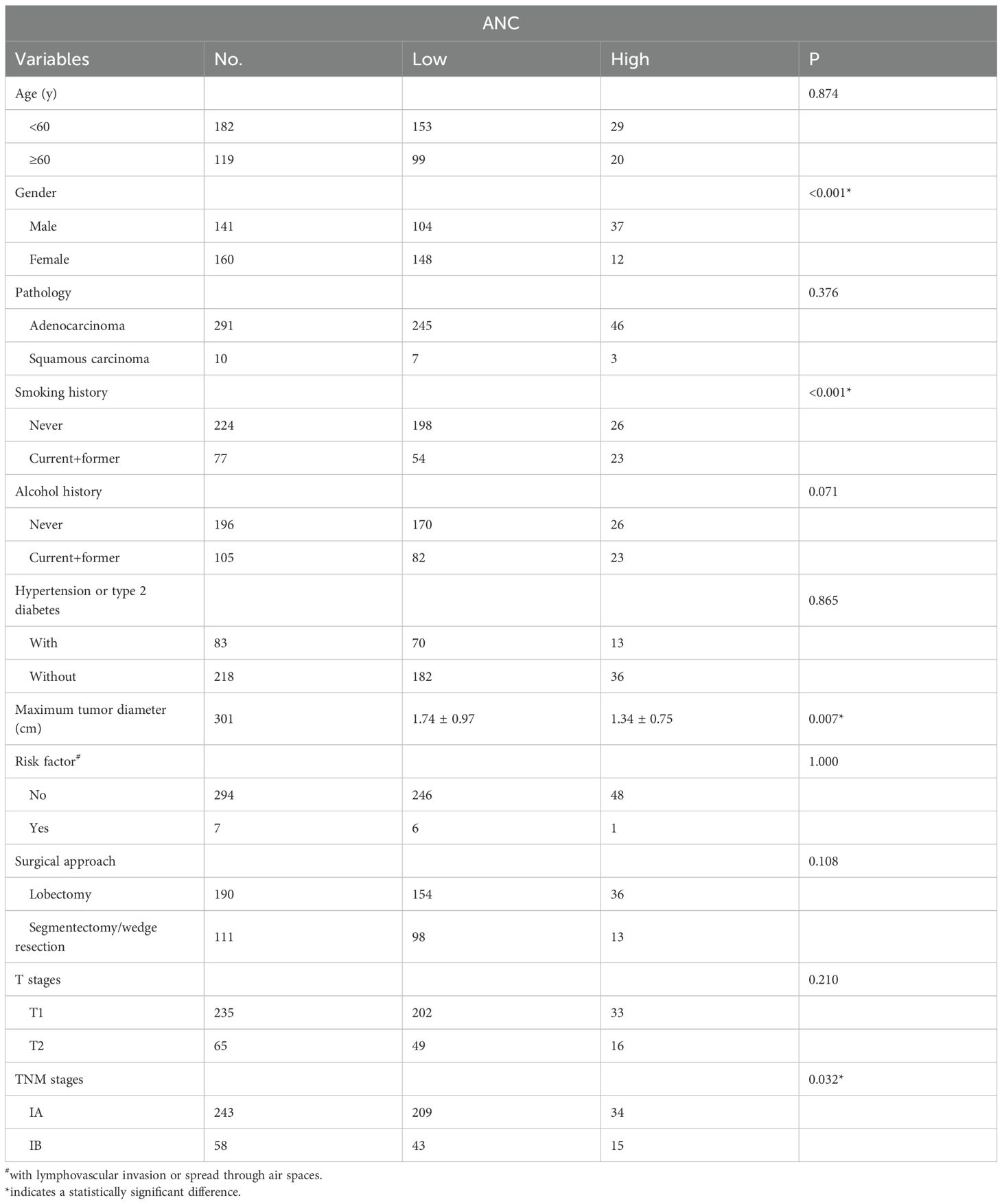
According to the exclusion criteria, a total of 301 patients were included in the cohort, with 160 females and 141 males. The median age of the patients was 57 years (y) (range: 23-79 y), and the median follow-up was 58 months (m) (range: 9-143 m). At the end of the follow-up, 9 deaths were registered, with 4 in stage IA and 5 in stage IB. By ROC analysis, only the ANC was found to be significant in predicting DFS (AUC=0.66, P = 0.011) and OS (AUC = 0.70, P = 0.043) (Figure 1) when compared with ALC (DFS: AUC = 0.53, P = 0.594, OS: AUC = 0.47, P = 0.777), AMC (DFS: AUC = 0.57, P = 0.252, OS: AUC = 0.57, P = 0.489) and PLC (DFS: AUC = 0.54, P = 0.571, OS: AUC = 0.67, P = 0.089). Further, time dependent ROC suggested ANC continuously keep satisfactory significance in predicting the DFS and OS (due to the limited events for OS in our study, its value only emerged 50 m after surgery). At last, patients were then divided into ANC low [83.72% (252/301)] or high [16.28% (49/301)] subgroups by the optimal cutoff point at 3879/mm3 with a sensitivity at 55.60% and a specificity at 82.90%.
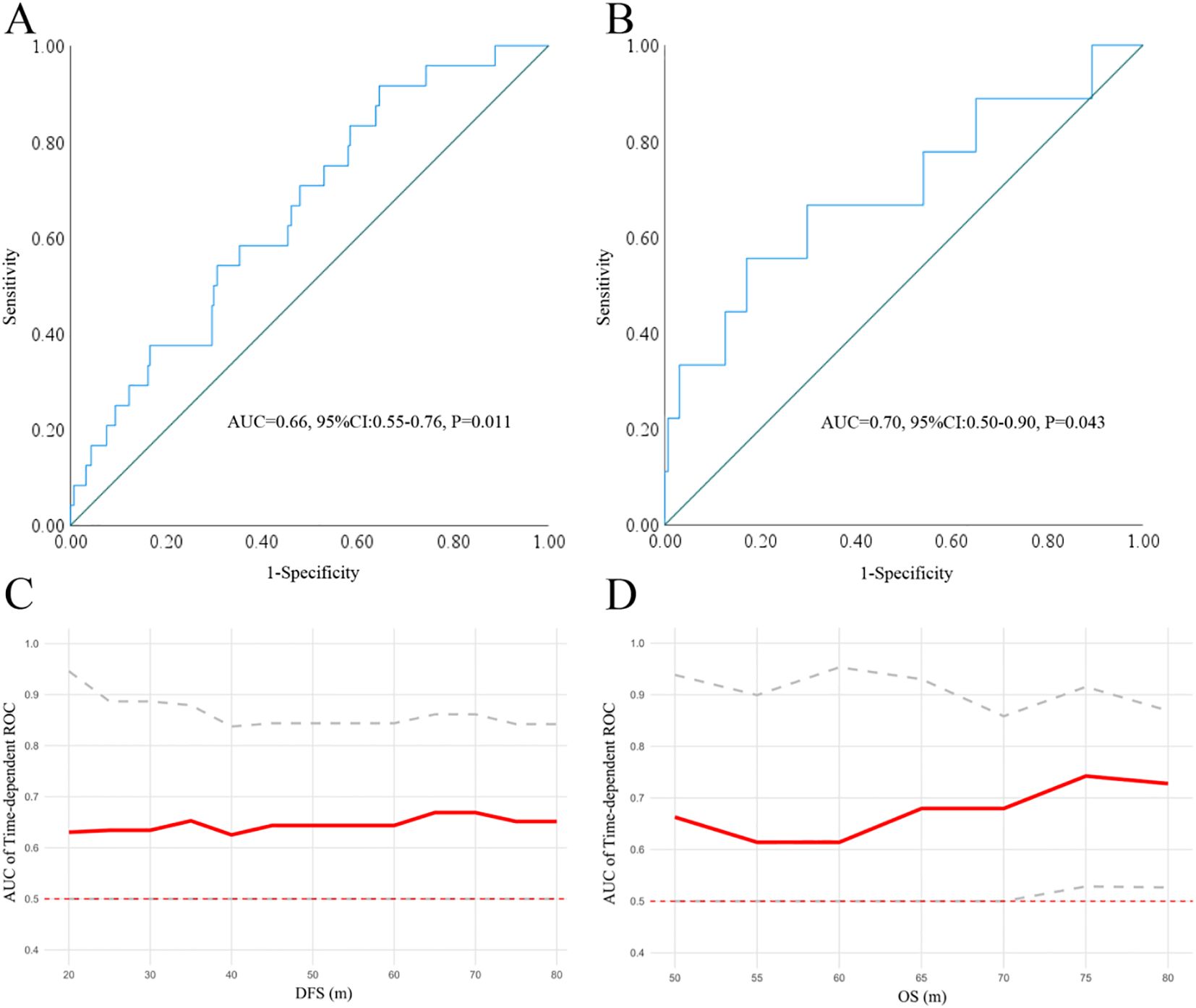
Figure 1. The significance of ANC in predicting DFS (A) and OS (B) by ROC analysis. Time dependent-ROC indicated that the ANC keep satisfactory significance in predicting DFS (C) and OS (D). ANC, absolute neutrophil count; ROC, receiver operating characteristic curve.
Differences in the clinical data among the ANC low or high subgroups
By the chi-square test, female patients, those without a smoking history, small MTD and IA stage were more likely to have a low ANC, and no significant differences were found in other data (Table 1).
Correlation of ANC with ALC, AMC and PLT
By the Kolmogorov–Smirnov test, the ANC was found to not meet the Gaussian distribution (Z=0.08, P<0.001), and significant correlations (Spearman correlation) were found for ANC with ALC (R = 0.26, P<0.001), ANC with AMC (R = 0.56, P<0.001) and ANC with PLC (R = 0.29, P<0.001). In addition, the linear regression analysis suggested a correlation for all these markers with the equation: ANC = 0.68-1.06×ALC+5.157×AMC+0.001×PLT (P[95%CI]=0.249 [-0.287-0.075], <0.001 [4.437-5.876] and 0.340 [-0.001-0.003] for ALC, AMC and PLT, respectively); among these, the correlation of ANC and AMC was the highest (Figure 2).
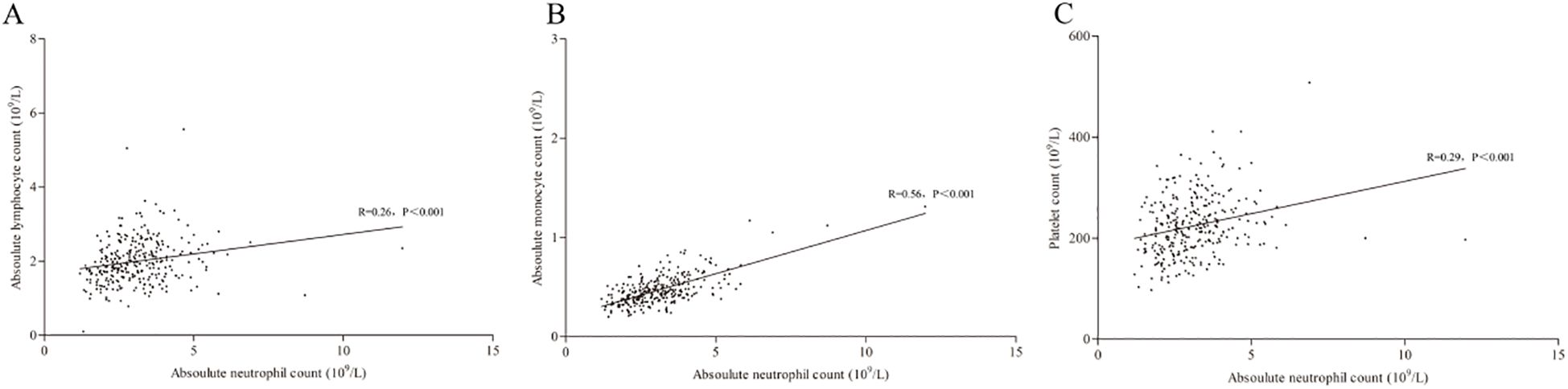
Figure 2. Correlation of ANC with ALC (A), AMC (B) and PLC (C). ANC, absolute lymphocyte count; AMC, absolute monocyte count; PLC, platelet count.
DFS and OS differences among ANC low or high subgroups
By Kaplan–Meier analysis, patients in the ANC low group displayed significantly better DFS (log rank=8.64, P = 0.003) and OS (log rank=9.86, P = 0.002) than those in the ANC high group (Figures 3A, B).
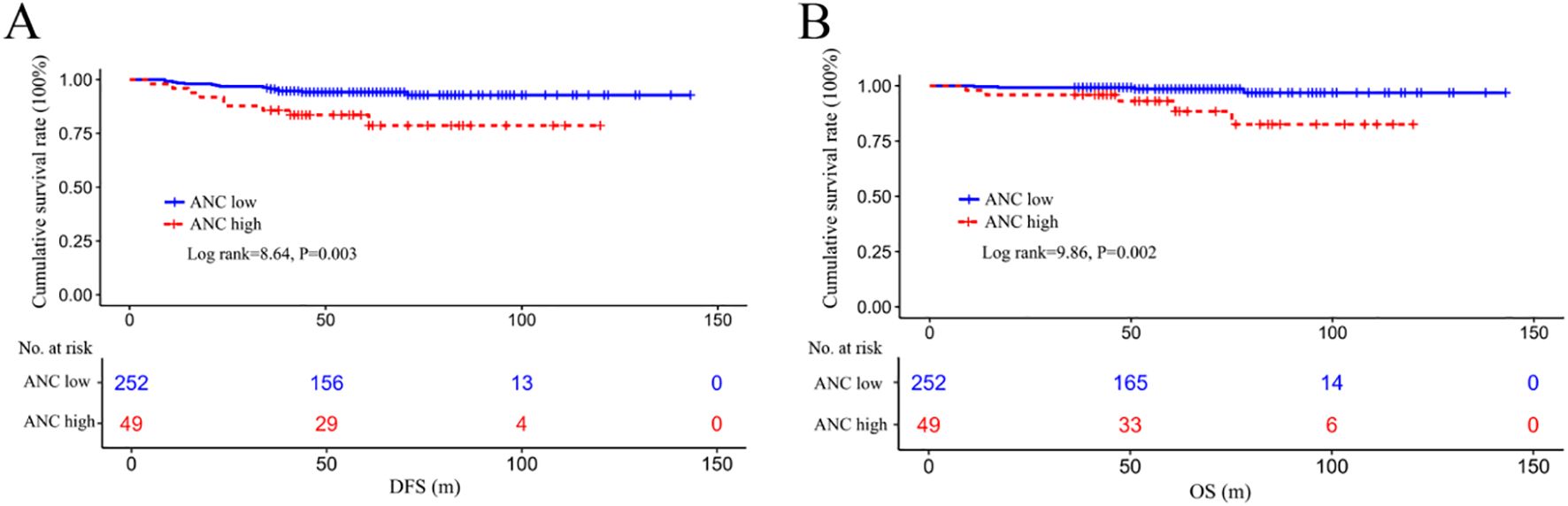
Figure 3. Survival differences in DFS (A) and OS (B) among the ANC-low or ANC-high subgroups. DFS, disease-free survival; OS, overall survival; ANC, absolute lymphocyte count.
Risk factors for outcomes determined by univariate and multivariate analyses
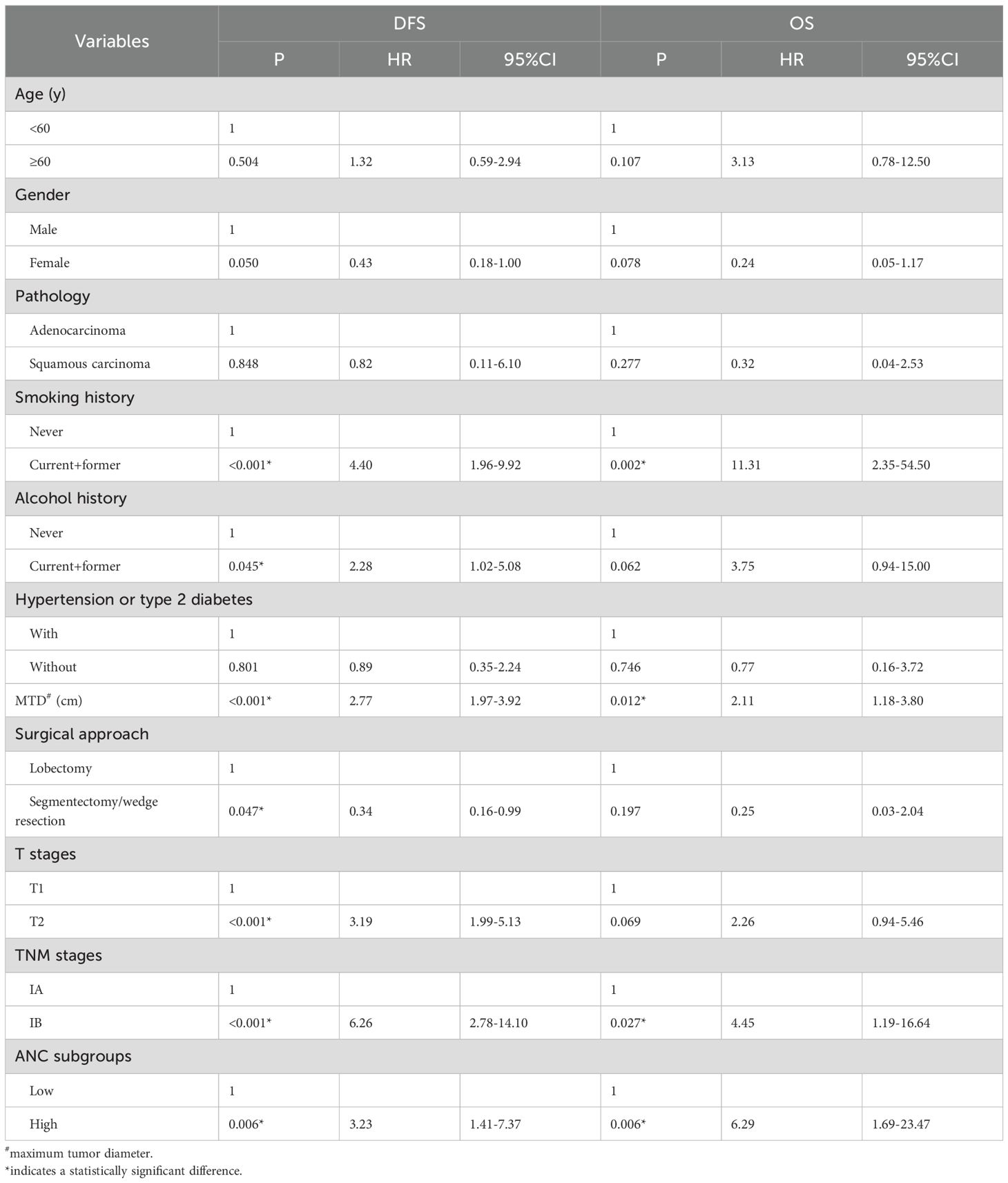
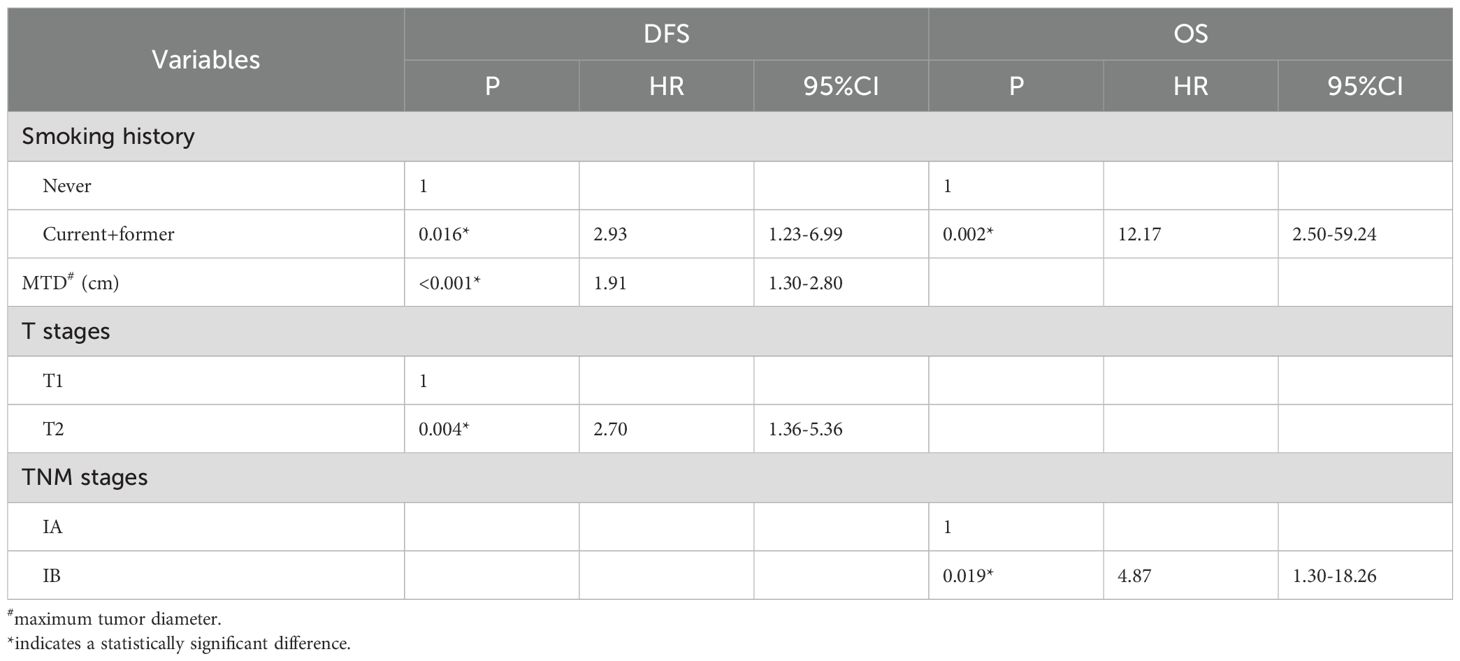
By the Cox hazard model, factors including smoking history, MTD, TNM stage, and ANC level were found to be significant risk factors for both DFS and OS in univariate analysis; whereas alcohol history, surgical approach and T stage were identified as additional risk factors for DFS (Table 2) (risk factor in Table 1 was not included due to the limited positive sample that cannot be included in the analysis). When the above factors (those additional risk factors for DFS were also included for OS) were entered into multivariate analysis, the ANC was not validated as an independent risk factor for both DFS and OS (Table 3).
Discussion
In this study, preoperative ANC was found to be the only significant prognostic indicator in predicting survival in CEA normal stage I NSCLC patients, in contrast to ALC, AMC, and PLC. Patients with a relatively low ANC before surgery had significantly better outcomes than those with a high ANC; however, it was not validated as an independent prognostic factor for both DFS and OS. To the best of our knowledge, this is the first report concerning the prognostic value of a specific peripheral blood fraction in CEA normal stage I NSCLC.
Previously, the prognostic role of ANC in cancer has been under extensive study, and the expansion of these cells in peripheral blood commonly predicts poor survival. Examples could be found in advanced gastric cancer, localized prostate cancer, metastatic colorectal cancer, and some head and neck malignancies (29–33). In NSCLC, Teramukai et al. studied 388 chemo-naïve stage IIIB or IV patients and found that in contrast to ALC and AMC, ANC was the only significant indicator for survival, and pretreatment high ANC (with a cutoff at 4500/mm3) was significantly correlated with poor OS and progression-free survival (PFS) (36). In addition, Zer et al., in a study with 88 advanced cases, received PD-1 inhibitor treatment and found a declined ANC during such therapy, indicating good disease control and therapy response (40); similarly, Murakami et al., reached a similar conclusion in a study with 213 patients receiving nivolumab treatment, (41). In addition, Chen et al. in a study with 71 advanced patients who received anlotinib treatment and found that compared to ALC, ANC was the only significant indicator correlated with both OS and PFS (37). Except for reports in advanced scenarios without surgery, there is also a report conducted in resected stage I-IIIA patients who found that ANC was positively associated with increased tumor burden and that surgical removal of the lesion resulted in a decrease in these cells in peripheral blood. The increase in these cells was independently correlated with poor OS (42). Nonetheless, few studies have explored the prognostic usefulness of ANC in stage I NSCLC, let alone in CEA normal background. Interestingly, two reports have indicated that a low preoperative NLR [cutoff points: 2.50 (22), 2.84 (23)] was significantly correlated with good DFS and OS in stage I NSCLC, including some cases with elevated CEA [16.73% (43/257) (22), 20.56% (37/180) (23)]. Although not conclusive, a low NLR in these studies may partially include some cases with a low ANC (accompanied by a normal ALC or high ALC), which may give some support to our results. In addition, we found that low ANC was more common in some features like females (92.50% (148/160)) and in those without a smoking history (88.39% (198/224)). As in previous studies in stage I NSCLC, the female sex and never-smokers were significant protective factors for good survival (43, 44). Moreover, we also found some significant correlations of ANC with other blood fractions, particularly AMC. Although these fractions did not show any prognostic value for either DFS or OS in our study, a study demonstrated the prognostic usefulness of AMC in stage I NSCLC (44). These results may contribute to the explanation of the positive role of ANC in survival in our study.
In recent years, cancer dissemination was found to be an early event (45), and these detached cells, also known as circulating tumor cells (CTCs), were found to act as precursors of metastasis and play a key role in recurrence and treatment failure in many cancers (46–48), including lung cancer (49, 50). It is also notable that these cells are found to be a powerful generator of CEA in lung cancer (51, 52). Taking into consideration the intrinsic degradation of CEA in the liver in patients, it was plausible that stage I cases would have a low frequency of CTCs when CEA is maintained in the normal range. Interestingly, neutrophils were found to play an important role in regulating lung cancer cells; for example, they could manipulate tumor angiogenesis and enhance the hypoxic microenvironment and Snail expression, which could then promote cell growth and disease progression (34, 53). In addition, they can also generate a unique structure, namely, neutrophil extracellular traps (NETs) (54), which can support metastasis (55). Furthermore, although not reported in lung cancer, neutrophils could interact with CTCs and escort these cells to enable cell cycle progression (56) and contribute to their survival by inhibiting peripheral leukocyte activation (57) or the formation of metastatic lesions (58, 59). Based on these facts, we speculate that the expansion of neutrophils, in particular a specific subset of these cells in peripheral blood, could increase the opportunity for their interaction with CTCs, although they presented with a low frequency in stage I cases, and promote the development of these cells as well as the formation of metastatic sites. All these biological processes would then result in poor survival in the patients; however, it was also notable that ANC level was not validated as an independent risk factor for both DFS and OS. In fact, the neutrophils are heterogeneous clusters with different functions in cancer development. For example, these cells in circulation can be divided into high- or low-density neutrophils, in which the low-density populations include mature and immature neutrophils (60). Although not reported in stage I cases, low-density neutrophils are likely to play a more important role in promoting resistance to immunotherapy in NSCLC (61). In recent years, increasing evidence has indicated that these cells are plastic during cancer development (62, 63). The dynamic change of different clusters of these cells can be an explanation for its failure as an independent risk factor in prognosis in present study. Additionally, it was long time established that smoking induced chronic inflammation can resulted in delayed neutrophil clearance (64); whereas quitting smoking after diagnosis can not only resulted in decreased white blood cells (65), but also improved survival in NSCLC patients irrespective of stage (66). It was notable that nearly half of the patients quitted smoking (33/77, data not shown) after surgery in our study, which may be the underlying reason for aforementioned change of ANC level in our study.
Our study also has some limitations except its retrospective nature and relatively small sample size. First, due to the relative short duration of follow up, the events for DFS and OS are rare, which could largely impair the statistical power and the reliability of the conclusions; second, although adjuvant chemotherapy was still under debate in stage IB cases (67), some patients did accept adjuvant treatment according to aforementioned clinical trials (6–8); however, the influence of such treatment cannot be further evaluated in our study due to the absent of these information. Building on these facts, our results should be further validated in future.
Conclusion
Overall, we found that preoperative ANC was the only significant prognostic indicator in stage I NSCLC compared to other peripheral blood cell fractions. Although ANC was found to be a useful prognostic indicator in CEA normal stage I NSCLC; however, it was not validated as an independent prognostic factor and additional studies for its role in prognosis for these patients are still needed in future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Hainan Hospital of Chinese PLA General Hospital (ID: S2023-12). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BW: Writing – original draft, Resources, Data curation, Formal analysis, Investigation. LL: Formal analysis, Writing – original draft, Supervision. MC: Investigation, Writing – original draft, Data curation. QY: Writing – original draft, Data curation, Investigation, Methodology. PL: Resources, Writing – original draft, Investigation. BY: Supervision, Conceptualization, Validation, Writing – review & editing, Methodology, Writing – original draft, Visualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hong QY, Wu GM, Qian GS, Hu CP, Zhou JY, Chen LA, et al. Prevention and management of lung cancer in China. Cancer. (2015) 121:3080–8. doi: 10.1002/cncr.29584
2. Chen P, Liu Y, Wen Y, and Zhou C. Non-small cell lung cancer in China. Cancer Commun. (2022) 42:937–70. doi: 10.1002/cac2.12359
3. Jaiyesimi IA, Leighl NB, Ismaila N, Alluri K, Florez N, Gadgeel S, et al. Therapy for stage IV non-small cell lung cancer without driver alterations: ASCO living guideline, version 2023.3. J Clin Oncol. (2024) 42:e23–43. doi: 10.1200/JCO.23.02746
4. Singh N, Temin S, Baker S Jr, Blanchard E, Brahmer JR, Celano P, et al. Therapy for stage IV non-small cell lung cancer with driver alterations: ASCO living guideline, version 2023.3. J Clin Oncol. (2024) 42:e1–e22. doi: 10.1200/JCO.22.00824
5. Tan AC and Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. (2022) 40:611–25. doi: 10.1200/JCO.21.01626
6. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
7. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
8. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
9. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
10. Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res. (2022) 28:3308–17. doi: 10.1158/1078-0432.CCR-21-3044
11. Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. (2022) 33:500–10. doi: 10.1016/j.annonc.2022.02.007
12. Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, and Nakanishi Y. Diagnostic value of CEA and CYFRA 21–1 tumor markers in primary lung cancer. Lung Cancer. (2013) 80:45–9. doi: 10.1016/j.lungcan.2013.01.002
13. Wang B, He YJ, Tian YX, Yang RN, Zhu YR, and Qiu H. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21–1 for diagnosis of lung cancer. Asian Pac J Cancer Prev. (2014) 15:9611–4. doi: 10.7314/APJCP.2014.15.22.9611
14. Cedrés S, Nuñez I, Longo M, Martinez P, Checa E, Torrejón D, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer. (2011) 12:172–9. doi: 10.1016/j.cllc.2011.03.019
15. Koike T, Yamato Y, Yoshiya K, and Toyabe S. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol. (2012) 7:1246–51. doi: 10.1097/JTO.0b013e31825871de
16. Kawachi R, Nakazato Y, Takei H, Koshi-ishi Y, and Goya T. Clinical significance of preoperative carcinoembryonic antigen level for clinical stage I non-small cell lung cancer: can preoperative carcinoembryonic antigen level predict pathological stage? Interact Cardiovasc Thorac Surg. (2009) 9:199–202. doi: 10.1510/icvts.2009.206698
17. Tomita M, Shimizu T, Hara M, Ayabe T, and Onitsuka T. Serum carcinoembryonic antigen level in non-small-cell lung cancer patients with preoperative normal serum level. Gen Thorac Cardiovasc Surg. (2009) 57:303–6. doi: 10.1007/s11748-008-0397-6
18. Kato T, Ishikawa K, Aragaki M, Sato M, Okamoto K, Ishibashi T, et al. Optimal predictive value of preoperative serum carcinoembryonic antigen for surgical outcomes in stage I non-small cell lung cancer: differences according to histology and smoking status. J Surg Oncol. (2013) 107:619–24. doi: 10.1002/jso.23293
19. Hill W, Lim EL, Weeden CE, Lee C, Augustine M, Chen K, et al. Lung adenocarcinoma promotion by air pollutants. Nature. (2023) 616:159–67. doi: 10.1038/s41586-023-05874-3
20. Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp Lung Res. (2005) 31:57–82. doi: 10.1080/01902140490495020
21. Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, et al. IL-1β-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. (2014) 74:4720–30. doi: 10.1158/0008-5472.CAN-14-0960
22. Takahashi Y, Horio H, Hato T, Harada M, Matsutani N, Morita S, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol. (2015) 22 Suppl 3:S1324–31. doi: 10.1245/s10434-015-4735-5
23. Liu W, Zhang T, Li L, Zou J, and Xu C. Assessing the prognostic value of the neutrophil-to-lymphocyte ratio in stage I non-small-cell lung cancer with complete resection. Can Respir J. (2022) 2022:6837872. doi: 10.1155/2022/6837872
24. Xia H, Sun Z, Deng L, Zhu D, and Wang D. Prognostic significance of the preoperative lymphocyte to monocyte ratio in patients with stage I non-small cell lung cancer undergoing complete resection. Cancer Invest. (2016) 34:378–4. doi: 10.1080/07357907.2016.1213276
25. Wculek SK and Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. (2015) 528:413–7. doi: 10.1038/nature16140
26. Tüting T and de Visser KE. Cancer. How neutrophils promote metastasis. Science. (2016) 352:145–6. doi: 10.1126/science.aaf7300
27. Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, and Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. (2005) 65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734
28. McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, and Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. (2009) 125:1298–305. doi: 10.1002/ijc.24409
29. Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. (2014) 15:945–50. doi: 10.7314/APJCP.2014.15.2.945
30. Bahig H, Taussky D, Delouya G, Nadiri A, Gagnon-Jacques A, Bodson-Clermont P, et al. Neutrophil count is associated with survival in localized prostate cancer. BMC Cancer. (2015) 15:594. doi: 10.1186/s12885-015-1599-9
31. Valero C, Pardo L, López M, García J, Camacho M, Quer M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. (2017) 39:219–26. doi: 10.1002/hed.24561
32. Grothey A, Yoshino T, Bodoky G, Ciuleanu T, Garcia-Carbonero R, García-Alfonso P, et al. Association of baseline absolute neutrophil counts and survival in patients with metastatic colorectal cancer treated with second-line antiangiogenic therapies: exploratory analyses of the RAISE trial and validation in an electronic medical record data set. ESMO Open. (2018) 3:e000347. doi: 10.1136/esmoopen-2018-000347
33. Diao P, Wu Y, Ge H, Li J, Zhang W, Huang R, et al. Preoperative circulating platelet, neutrophil, and lymphocyte counts predict survival in oral cancer. Oral Dis. (2019) 25:1057–66. doi: 10.1111/odi.13049
34. Zhang C, Tang B, Hu J, Fang X, Bian H, Han J, et al. Neutrophils correlate with hypoxia microenvironment and promote progression of non-small-cell lung cancer. Bioengineered. (2021) 12:8872–84. doi: 10.1080/21655979.2021.1987820
35. Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. (2014) 124:5466–80. doi: 10.1172/JCI77053
36. Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. (2009) 45:1950–8. doi: 10.1016/j.ejca.2009.01.023
37. Chen R, Lu FY, Liu B, Huang J, Zhou M, Dai R, et al. Absolute neutrophil count in the peripheral blood predicts prognosis in lung cancer patients treated with anlotinib. Cancer Manag Res. (2021) 13:3619–27. doi: 10.2147/CMAR.S307368
38. Huang X, Huan Y, Liu L, Ye Q, Guo J, and Yan B. Preoperative low absolute lymphocyte count to fibrinogen ratio correlated with poor survival in nonmetastatic colorectal cancer. World J Surg Oncol. (2022) 20:309. doi: 10.1186/s12957-022-02775-z
39. Xu HL, Zhao GQ, Lin JX, Ye QW, Xiang J, and Yan B. A combined preoperative red cell distribution width and carcinoembryonic antigen score contribute to prognosis prediction in stage I lung adenocarcinoma. World J Surg Oncol. (2023) 21:56. doi: 10.1186/s12957-023-02945-7
40. Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. (2018) 19:426–34. doi: 10.1016/j.cllc.2018.04.008
41. Murakami Y, Tamiya A, Taniguchi Y, Adachi Y, Enomoto T, Azuma K, et al. Retrospective analysis of long-term survival factors in patients with advanced non-small cell lung cancer treated with nivolumab. Thorac Cancer. (2022) 13:593–601. doi: 10.1111/1759-7714.14303
42. Mitchell KG, Diao L, Karpinets T, Negrao MV, Tran HT, Parra ER, et al. Neutrophil expansion defines an immunoinhibitory peripheral and intratumoral inflammatory milieu in resected non-small cell lung cancer: a descriptive analysis of a prospectively immunoprofiled cohort. J Immunother Cancer. (2020) 8:e000405. doi: 10.1136/jitc-2019-000405
43. Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, and Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non-small cell lung cancer after surgical resection. Lung Cancer. (2010) 68:398–402. doi: 10.1016/j.lungcan.2009.08.008
44. Christensen NL, Løkke A, Dalton SO, Christensen J, and Rasmussen TR. Smoking, alcohol, and nutritional status in relation to one-year mortality in Danish stage I lung cancer patients. Lung Cancer. (2018) 124:40–4. doi: 10.1016/j.lungcan.2018.07.025
45. Hu Z, Ding J, Ma Z, Sun R, Seoane JA, Scott Shaffer J, et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. (2019) 51:1113–22. doi: 10.1038/s41588-019-0423-x
46. Chu HY, Yang CY, Yeh PH, Hsu CJ, Chang LW, Chan WJ, et al. Highly correlated recurrence prognosis in patients with metastatic colorectal cancer by synergistic consideration of circulating tumor cells/microemboli and tumor markers CEA/CA19-9. Cells. (2021) 10:1149. doi: 10.3390/cells10051149
47. Li Z, Song M, Han S, Jin C, and Yang J. The prognostic role of circulating tumor cells in gastric cancer: A meta-analysis. Front Oncol. (2022) 12:963091. doi: 10.3389/fonc.2022.963091
48. Fabisiewicz A, Szostakowska-Rodzos M, Zaczek AJ, and Grzybowska EA. Circulating Tumor cells in early and advanced breast cancer; biology and prognostic value. Int J Mol Sci. (2020) 21:1671. doi: 10.3390/ijms21051671
49. Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. (2011) 178:989–96. doi: 10.1016/j.ajpath.2010.12.003
50. Bayarri-Lara C, Ortega FG, Cueto Ladrón de Guevara A, Puche JL, Ruiz Zafra J, de Miguel-Pérez D, et al. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PloS One. (2016) 11:e0148659. doi: 10.1371/journal.pone.0148659
51. Yamashita J, Matsuo A, Kurusu Y, Saishoji T, Hayashi N, and Ogawa M. Preoperative evidence of circulating tumor cells by means of reverse transcriptase-polymerase chain reaction for carcinoembryonic antigen messenger RNA is an independent predictor of survival in non-small cell lung cancer: a prospective study. J Thorac Cardiovasc Surg. (2002) 124:299–305. doi: 10.1067/mtc.2002.124370
52. Bao H, Bai T, Takata K, Yokobori T, Yokobori T, Ohnaga T, Hisada T, et al. High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells is associated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol Lett. (2018) 15:3061–7. doi: 10.3892/ol.2017.7671
53. Faget J, Groeneveld S, Boivin G, Sankar M, Zangger N, Garcia M, et al. Neutrophils and Snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep. (2017) 21:3190–204. doi: 10.1016/j.celrep.2017.11.052
54. Mauracher LM, Hell L, Moik F, Krall M, Englisch C, Roiß J, et al. Neutrophils in lung cancer patients: Activation potential and neutrophil extracellular trap formation. Res Pract Thromb Haemost. (2023) 7:100126. doi: 10.1016/j.rpth.2023.100126
55. Wang Y, Liu F, Chen L, Fang C, Li S, Yuan S, et al. Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway. Front Immunol. (2022) 13:867516. doi: 10.3389/fimmu.2022.867516
56. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566:553–7. doi: 10.1038/s41586-019-0915-y
57. Zhang J, Qiao X, Shi H, Han X, Liu W, Tian X, et al. Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumour Biol. (2016) 37:5397–404. doi: 10.1007/s13277-015-4349-3
58. Saini M, Szczerba BM, and Aceto N. Circulating tumor cell-neutrophil tango along the metastatic process. Cancer Res. (2019) 79:6067–73. doi: 10.1158/0008-5472.CAN-19-1972
59. Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. (2012) 72:3919–27. doi: 10.1158/0008-5472.CAN-11-2393
60. Shaul ME and Fridlender ZG. Cancer-related circulating and tumor-associated neutrophils-subtypes, sources and function. FEBS J. (2018) 285:4316–42. doi: 10.1111/febs.14524
61. Arasanz H, Bocanegra AI, Morilla I, Fernández-Irigoyen J, Martínez-Aguillo M, Teijeira L, et al. Circulating low density neutrophils are associated with resistance to first line anti-PD1/PDL1 immunotherapy in non-small cell lung cancer. Cancers. (2022) 14:3846. doi: 10.3390/cancers14163846
62. Shaul ME and Fridlender ZG. The dual role of neutrophils in cancer. Semin Immunol. (2021) 57:101582. doi: 10.1016/j.smim.2021.101582
63. Wu G, Pan B, Shi H, Yi Y, Zheng X, Ma H, et al. Neutrophils’ dual role in cancer: from tumor progression to immunotherapeutic potential. Int Immunopharmacol. (2024) 140:112788. doi: 10.1016/j.intimp.2024.112788
64. Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. (2008) 5:811–5. doi: 10.1513/pats.200809-100TH
65. Xu LM, Dai SP, and Zuo YX. Impacts of preoperative smoking and smoking cessation time on preoperative peripheral blood inflammatory indexes and postoperative hospitalization outcome in male patients with lung cancer and surgery treatment. Chin Med Sci J. (2020) 35:170–8. doi: 10.24920/003540
66. Gemine RE, Davies GR, Lanyon K, Rees SE, Campbell I, and Lewis KE. Quitting smoking improves two-year survival after a diagnosis of non-small cell lung cancer. Lung Cancer. (2023) 186:107388. doi: 10.1016/j.lungcan.2023.107388
Keywords: lung cancer, carcinoembryonic antigen, neutrophil count, disease-free survival, overall survival
Citation: Wang B, Liu L, Cui M, Ye Q, Li P and Yan B (2025) Preoperative absolute neutrophil count: a potential indicator for prognosis in carcinoembryonic antigen normal stage I non-small cell lung cancer. Front. Oncol. 15:1632597. doi: 10.3389/fonc.2025.1632597
Received: 29 May 2025; Accepted: 09 October 2025;
Published: 27 October 2025.
Edited by:
Nestor Villamizar, University of Miami Health System, United StatesReviewed by:
Slawomir Jakiela, Warsaw University of Life Sciences, PolandArjun Katailiha, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Wang, Liu, Cui, Ye, Li and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Yan, eV9iaW5nNDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Bailin Wang1†
Bailin Wang1† Bing Yan
Bing Yan