- 1Inner Mongolia Medical University, Hohhot, China
- 2Inner Mongolia Medical University Affiliated Cancer Hospital, Hohhot, China
Gastric cancer (GC) remains the foremost contributor to global cancer mortality, largely attributable to metastatic dissemination and therapeutic refractoriness. Emerging data implicate the Wnt/β-catenin signaling cascade as a pivotal regulator of epithelial-mesenchymal plasticity, stemness acquisition, and multidrug tolerance in GC. This review delineates the molecular landscape of Wnt/β-catenin aberrations, encompassing genomic perturbations (NAT10, SMC4), non-coding RNA circuitry (LINC00665, circ0000670), and (epigenetic reprogramming (e.g., miR-33b hypermethylation). Mechanistically, these alterations cooperate with EMT drivers to potentiate metastatic outgrowth and therapeutic evasion. Of particular translational significance are emerging interventions targeting this axis: phytochemicals (Rutin, ginsenoside Rg3) with dual Wnt-CSC inhibitory activity, CRISPR-edited epigenetic modulators (TET1/FOXO4), and immune checkpoint blockade-Wnt inhibitor synergism. Notwithstanding preclinical success, clinical implementation faces two critical bottlenecks—pathway pleiotropy and biomarker paucity. To bridge this gap, we propose a precision oncology framework leveraging multi-omics-guided patient stratification, potentially reshaping GC therapeutic paradigms.
1 Introduction
The global burden of gastric cancer (GC) poses a substantial challenge to global health. Despite the documented decrease in observed incidence and mortality rates at the global level, it continues to be the third leading cause of cancer-related deaths (1). In Asia, the incidence and mortality rates of gastric cancer continue to be elevated, despite a trend of decline. There is an urgent need for enhanced efforts aimed at achieving early diagnosis and providing accurate treatment (2).
The Wnt/β-catenin signaling pathway has garnered significant attention as a crucial mechanism governing tissue growth, development, and tumorigenesis (3). The Wnt/β-catenin cascade emerges as a pivotal regulator of gastric oncogenesis, with its dysregulation constituting a therapeutic priority. Pathologically sustained activation of this pathway within the GC tumor microenvironment drives invasive and metastatic phenotypes, positioning it among the most consequential molecular drivers of disease progression.
The epithelial-mesenchymal transition (EMT) represents a dynamic cellular reprogramming process enabling epithelial cells to transiently acquire mesenchymal-like properties (4). conferring malignant traits such as: Clonogenic plasticity (tumor cell totipotency); Immune evasion mechanisms; Metabolic adaptability; Therapeutic resistance (5). This phenotypic shift is molecularly characterized by upregulation of mesenchymal markers (e.g., N-cadherin, vimentin) (6).
Critically, Wnt/β-catenin signaling stabilizes cytoplasmic β-catenin, enabling its nuclear translocation to activate oncogenic effectors (c-Myc, cyclin D1) that coordinate proliferative bursts, stemness maintenance, and EMT initiation (7). Recent advancements in single-cell sequencing have uncovered substantial intratumoral heterogeneity regarding Wnt/β-catenin activation across various gastric cancer subtypes. This variability may elucidate the differing responses to treatment observed in these patients (8). In addition, the interaction between the Wnt signaling pathway and components of the tumor microenvironment—specifically, cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs)—has been identified as a crucial factor that affects epithelial-mesenchymal transition (EMT) plasticity and drug resistance (9). This review synthesizes mechanistic advances in Wnt/β-catenin-driven GC pathogenesis and critically evaluates emerging therapeutic paradigms.
2 Molecular mechanisms of Wnt dysregulation
2.1 Genetic drivers
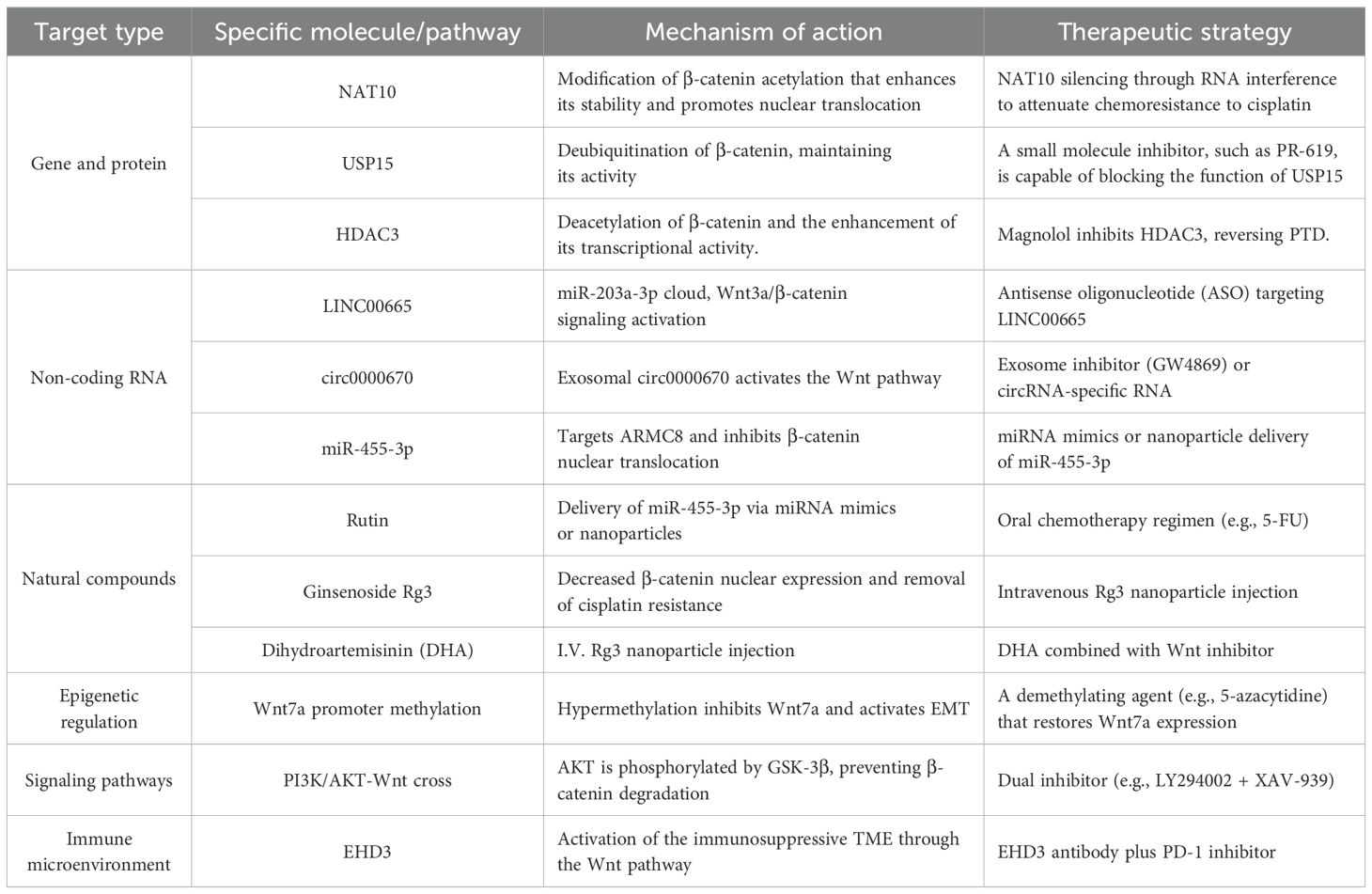
NAT10/USP15(post-translational modification), NAT10 (N-acetyltransferase 10) serves as a crucial member of the GNAT family (10), exhibiting RNA acetyltransferase activity (11). By catalyzing the AC4C modification (12), NAT10 improves the stability of mRNA and enhances its translation efficiency (12–14). NAT10 is implicated in tumor metabolic reprogramming and plays a critical role in the progression, metastasis, and drug resistance of various malignant tumors, including gastric cancer (15–17). Beyond its RNA epitranscriptomic function, NAT10 acetylates β-catenin at specific lysine residues (K49/K312), bolstering its cytoplasmic stability and nuclear translocation, thereby triggering EMT via E-cadherin suppression and N-cadherin/Snail induction. Clinically, NAT10 overexpression correlates with reduced overall survival in advanced GC and confers cisplatin resistance (IC50 reduction by 60% upon knockdown) (18);USP15 (Ubiquitin-specific protease 15), a pivotal member of the ubiquitin-specific protease (USP) family (19, 20). The N-terminal region of the protein under consideration contains the DUSP domain in conjunction with two UBL domains. These domains play a crucial role in the stabilization of β-catenin by removing ubiquitin tags, thereby preventing its proteasomal degradation. Furthermore, pharmacological inhibition of this mechanism effectively suppresses hepatic metastasis in vivo (21).
SMC4/AGGF1(nuclear transport), SMC4 (Structural Maintenance of Chromosomes Protein 4) is located at chromosome band 3q25.33 (22). Similarly, it promotes the nuclear import of β-catenin by interacting with importin-α, thereby enhancing the expression of oncogenic targets such as cyclin D1 and Bcl-2. Preclinical studies indicate that knockdown of SMC4 via RNA interference (RNAi) significantly inhibits tumor growth, resulting in a 70% reduction in xenograft volume (23); AGGF1(Angiogenic factor) has unequivocally been shown to enhance the accumulation of β-catenin by phosphorylating GSK-3β at Ser9, thereby inhibiting its kinases’ activity. The blockade of AGGF1 reverses ascites formation in models of peritoneal dissemination (24, 25).
BZW1 (secretory regulation), BZW1 (basic leucine zipper protein 1) is classified as a member of the bZIP superfamily (26). As an oncogene, BZW1 significantly influences the prognosis of patients with gastric cancer by facilitating the migration and invasion of tumor cells. It promotes Wnt3a secretion through enhanced ER-Golgi trafficking, thereby amplifying paracrine β-catenin signaling. Importantly, overexpression of BZW1 is strongly correlated with resistance to 5-fluorouracil (5-FU), exhibiting a 4.2-fold increase in expression levels (9).
2.2 Epigenetic & non-coding RNA networks
The dysregulation of noncoding RNAs (ncRNAs) represents a crucial layer of epigenetic regulation over Wnt/β-catenin signaling. Distinct subtypes of ncRNAs exert either oncogenic or tumor-suppressive effects through various mechanisms, including transcriptional, post-transcriptional, and chromatin-modifying processes.
1. LncRNAs play a pivotal role in orchestrating Wnt activation through diverse and multifaceted interactions:Transcriptional regulation: LINC00665 interacts with YBX1 to enhance the activity of the Wnt3a promoter, thereby promoting the nuclear accumulation of β-catenin. Clinically, elevated serum levels of LINC00665 serve as a diagnostic biomarker for metastatic breast cancer (27). H19 similarly recruits the histone acetyltransferase p300 to β-catenin, thereby enhancing TCF/LEF-dependent transcription. The application of antisense oligonucleotides targeting H19 effectively suppresses lung metastasis in patient-derived xenograft (PDX) models (28); Epigenetic modulation: TP73-AS1: Recruits PRC2 to deposit H3K27me3 marks at the WIF1 promoter, epigenetically silencing this Wnt antagonist in EBV-associated GC. 5-azacytidine treatment restored WIF1 expression and curtailed lymph node metastasis (29). Critically, H3K27me3-mediated silencing represents histone modification rather than direct DNA methylation; its functional synergy with DNA methyltransferases (DNMTs) in GC requires further validation; miRNA sponging: LINC01225 sequesters miR-483-3p, thereby derepressing Wnt1. This mechanism has been validated through rescue experiments (30). ZEB2-AS1 plays a crucial role in stabilizing ZEB2 mRNA, thereby suppressing E-cadherin expression and activating Wnt5a signaling. Notably, the knockdown of ZEB2-AS1 leads to a significant reduction of peritoneal metastases by 50% (31).
2. CircRNAs play a significant role in the crosstalk of the Wnt pathway and the remodeling of the microenvironment:circ_0006646 upregulates HMGB1 by sponging miR-665, facilitating β-catenin nuclear translocation. High circ_0006646 expression correlates with shorter progression-free survival (32). Exosomal circ0000670, which is induced by cigarette smoke, activates Wnt signaling in precancerous gastric epithelium, thereby promoting dysplasia. This exosome-mediated pathway highlights the role of environmental carcinogens in epigenetic reprogramming (33). However, the receptor-mediated uptake mechanism of exosomal circ0000670 in gastric cells is unknown.
3. miRNAs modulate Wnt activity with precision by directly targeting components of the pathway:Oncogenic miRNAs, specifically miR-20b and miR-324-5p, collaboratively inhibit SUFU, a formidable suppressor of the Wnt signaling cascade. This dual inhibition synergizes with the porcupine inhibitor LGK974, resulting in an 80% reduction in cancer cell viability (34–36); Tumor-suppressive miRNAs: miR-455-3p targets ARMC8 to inhibit β-catenin nuclear transport, with mimics demonstrating a suppression of liver metastasis in vivo (37, 38). miR-497 directly interacts in conjunction with the 3’ untranslated region (UTR) of β-catenin. Furthermore, the nanoparticle-mediated delivery of miR-497 analogs results in a 65% inhibition of tumor growth (30).
2.3 Exosome-mediated regulation
Environmental reprogramming:Cigarette smoke promotes the exosomal packaging of circ0000670, which subsequently activates the Wnt/β-catenin signaling pathway in gastric precancerous cells (E-cadherin↓, N-cadherin↑). The levels of serum exosomal circ0000670 are correlated with the progression of dysplasia. GW4869 (exosome inhibitor) reduces hepatic metastases in murine models (33, 39). Critically, GW4869’s non-specific blockade of all exosome secretion may disrupt physiological intercellular communication.
Pathogen-driven dysregulation: Toxins produced by Helicobacter pylori activate Wnt signaling, leading to the induction of epithelial-mesenchymal transition (EMT), marked by the upregulation of Snail expression and the concomitant downregulation of E-cadherin levels. This process also promotes cancer stem cell (CSC) features, evidenced by elevated CD44 and Nanog expression. Notably, this phenotype can be reversed through the application of Wnt inhibitors such as XAV939 (40). Furthermore, H. pylori upregulates FRA-1, which collaborates with β-catenin to enhance c-Myc transcription while simultaneously recruiting DNMT3A to silence miR-200b, thereby amplifying the crosstalk between Wnt and NF-κB pathways (41).
DNA Methylation and Histone Modification: Promoter Hypermethylation Silences the Tumor-Suppressive Gene Wnt7a, a Defect That Can Be Reversed by 5-Azacytidine (42). HDAC3-mediated deacetylation enhances the stability of β-catenin/TCF4 complexes, while the HDAC3 inhibitor Honokiol effectively inhibits peritoneal dissemination (43). Concurrently, the suppression of miR-33b through promoter methylation and CUL4B-mediated repression exacerbates the activation of Wnt/NF-κB pathways. Furthermore, low levels of miR-33b are predictive of resistance to platinum-based therapies (44) Figure 1:
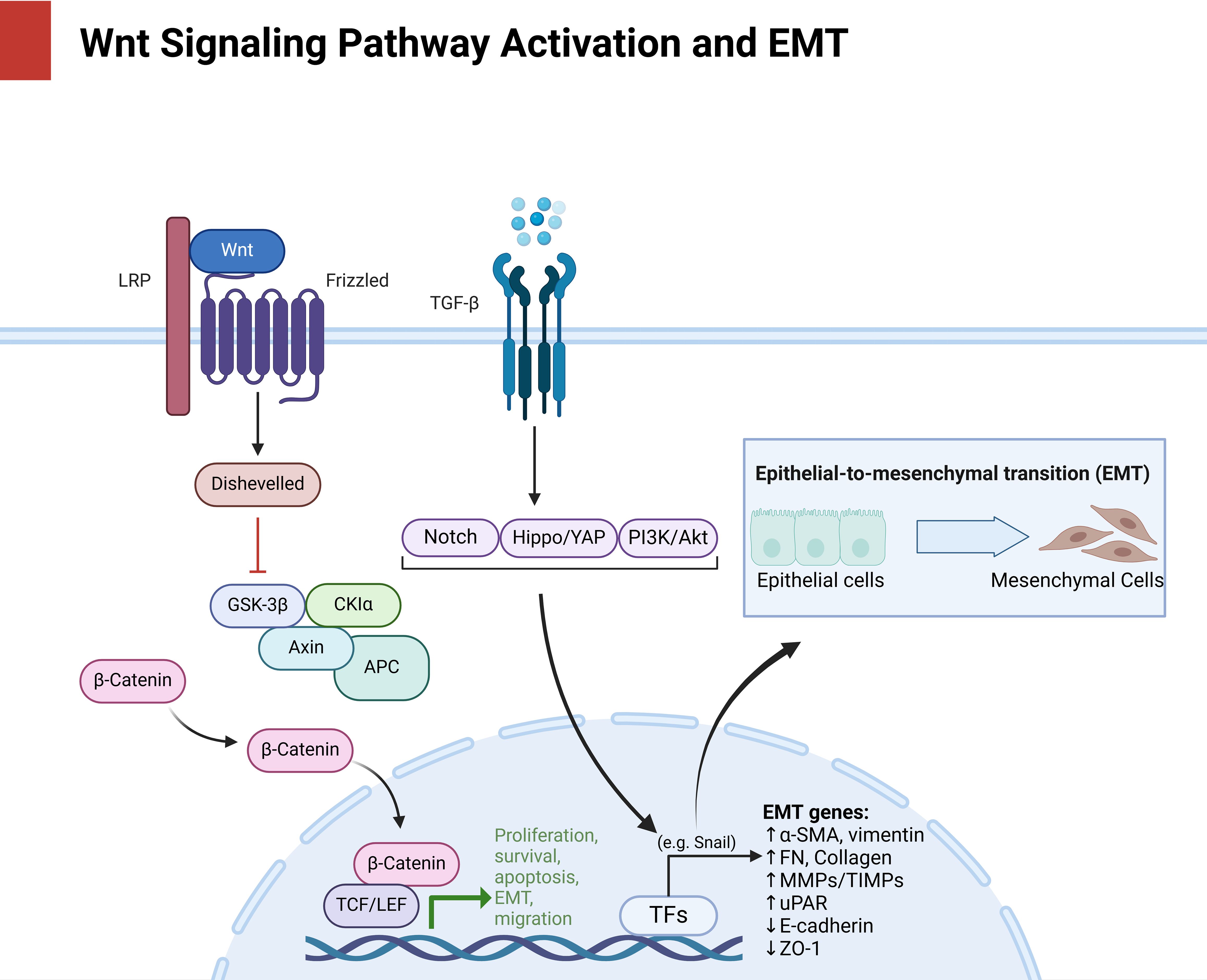
Figure 1. Delineates the multilevel activation of Wnt/β-catenin signaling (LRP/Frizzled/Dishevelled axis) and its convergence with EMT drivers (Snail, vimentin, E-cadherin loss), positioning this axis as a nexus for metastatic adaptation and therapy evasion, as mechanistically dissected in subsequent sections.
3 Therapeutic resistance mechanism
Mechanistic studies reveal Wnt/β-catenin signaling as a central orchestrator of therapeutic resistance in GC through two predominant axes:
3.1 Cisplatin resistance
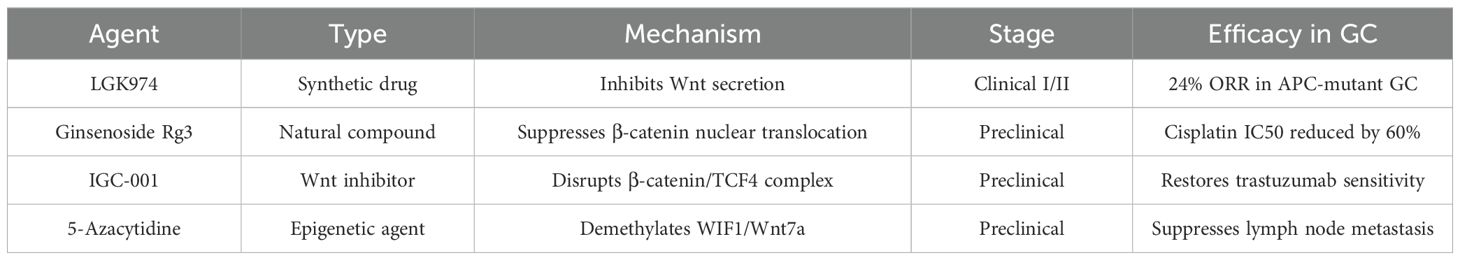
NAT10/TMEM10 Axis: Drives EMT via β-catenin activation, culminating in cisplatin evasion (45). Epigenetic Silencing: miR-33b promoter hypermethylation exacerbates multidrug resistance by sustaining β-catenin stability (44). Therapeutic Countermeasure: Ginsenoside Rg3 synergistically suppresses β-catenin, achieving 45% tumor regression in preclinical models (46).
3.2 Targeted therapy resistance
Wnt3a/FZD6 Hyperactivation: Induces EMT (E-cadherin↓, vimentin↑) and trastuzumab resistance (47, 48). Rescue Strategy: Wnt inhibitor ICG-001 restores drug sensitivity and triggers apoptosis via TCF/β-catenin complex disruption (48).
Collectively, these findings establish Wnt/β-catenin as a multidimensional resistance hub integrating: Epigenetic reprogramming; Transcriptional rewiring; TME crosstalk. This mechanistic convergence provides a rationale for β-catenin-targeted combinatorial regimens.
4 EMT-driven metastasis
4.1 Pro-metastatic regulators
Claudin-18-Deficient GC: Upregulates Snail2 (core EMT transcription factor) with concurrent Wnt/β-catenin pathway mutations, propelling mesenchymal transition (49). LINC01225 (Oncogenic lncRNA): Activates Wnt/β-catenin signaling to drive EMT, enhancing proliferative, migratory, and invasive capacities; LINC01225 silencing ablates EMT phenotypes and suppresses Wnt activity (30). FOXC1-β-catenin Axis: FOXC1 transcriptionally activates β-catenin via promoter binding, triggering EMT (E-cadherin↓, N-cadherin↑, vimentin↑) and metastatic dissemination.β-catenin inhibition reverses FOXC1-driven invasiveness (50). RSPO2 has been found to enhance WNT/β-catenin signaling, thereby promoting the invasion and migration of gastric cancer (GC) cells. Although the dynamic regulatory mechanism of the FOXC1-β-catenin axis in metastatic lesions has not yet been elucidated This finding lends further credence to the hypothesis that components within the Wnt signaling pathway play a crucial role in the regulation of these processes (51).
4.2 Metastasis suppressors
The antitumorigenic effects of Mist1 in gastric cancer (GC) have been demonstrated to occur through the modulation of the epithelial-to-mesenchymal transition (EMT) and metastasis. Specifically, Mist1: Inhibits EMT and GC metastasis by suppressing β-catenin transcriptional activity and attenuating Wnt signaling. Overexpression reduces tumor growth and distant metastasis in preclinical models (52). Zic1: Disrupts β-catenin/TCF4 complex formation, ablating Wnt target gene expression (c-Myc, cyclin D1) and impairing cell adhesion/invasion.Zic1 activation correlates with improved clinical outcomes The aforementioned effects have been demonstrated to be associated with an improved patient prognosis (53).
4.3 Multi-pathway integration
The present study investigates the role of microRNA-33b, a tumor suppressor microRNA, in the context of gastric cancer (GC). It is observed that microRNA-33b is downregulated in GC, a phenomenon attributed to promoter hypermethylation and the presence of long non-coding RNA (lncRNA)-mediated competing endogenous RNA (ceRNA) networks. It has been shown to inhibit epithelial-mesenchymal transition (EMT) and metastasis by concurrently targeting NF-κB, MAP8, and the Notch signaling pathway (44). CUL4B-mediated transcriptional repression and DNA hypermethylation have been shown to work together to suppress microRNA-33b (miR-33b). This, in turn, has been demonstrated to enhance pro-endometriosis signaling through multiple pathways, including NF-κB and Notch1. The aforementioned interplay has been demonstrated to promote aggressive tumor behavior (44). In the context of biological processes, miR-33b has been identified as a critical regulator that integrates epigenetic, transcriptional, and post-transcriptional signals. This integrated regulation functions to impede a range of processes, including epithelial-mesenchymal transition (EMT) and metastasis. The loss of miR-33b initiates a cascade of oncogenic pathway activations.
The following table summarizes the main therapeutic strategies and their mechanisms of action for the Wnt/β - catenin signaling pathway (Table 1):
5 Immune evasion orchestrated by Wnt/EMT axis in gastric cancer
The Wnt/β-catenin-EMT axis drives immunosuppression in gastric cancer through three synergistic mechanisms:
1. T-cell Exclusion: EMT-transformed cells secrete CXCL12 to establish physical barriers that block CD8+ T-cell infiltration, particularly in diffuse-type GC, correlating with “immune desert” phenotypes (54, 55). Concurrently, hsa_circ_0001479 (upregulated in GC) inhibits CD8+ T-cell recruitment via the miR-133a-5p/DEK/c-Myc axis while activating Wnt signaling, forming a feedforward loop to sustain immune evasion (56).
2. Myeloid Reprogramming: Wnt activation induces IL-6/G-CSF secretion, expanding polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) that express ARG1/iNOS to suppress T-cell function in peritoneal metastases (54, 57). The orphan GPCR GPR176 (overexpressed in GC) further polarizes macrophages toward M2 immunosuppressive states via Wnt signaling (57).
3. Checkpoint Dysregulation: Nuclear β-catenin/TCF4 complexes directly transcribe PD-L1 in intestinal-type GC with APC mutations, elevating PD-L1 expression 3.2-fold versus β-catenin-negative tumors (58, 59). This is exacerbated by EHD3 (a Wnt/EMT activator) which associates with poor prognosis and reduced CD8+ T-cell infiltration (58). Therapeutic synergy emerges from co-targeting: Disrupting Wnt signaling (e.g., β-catenin knockdown) enhances PD-1 antibody efficacy by increasing tumor cell apoptosis and CD8+ T-cell activity by 47% in co-culture models (59).
Figure 2: These mechanisms establish Wnt/EMT-immune evasion as a therapeutic vulnerability, warranting combinatorial strategies discussed in Section 6.
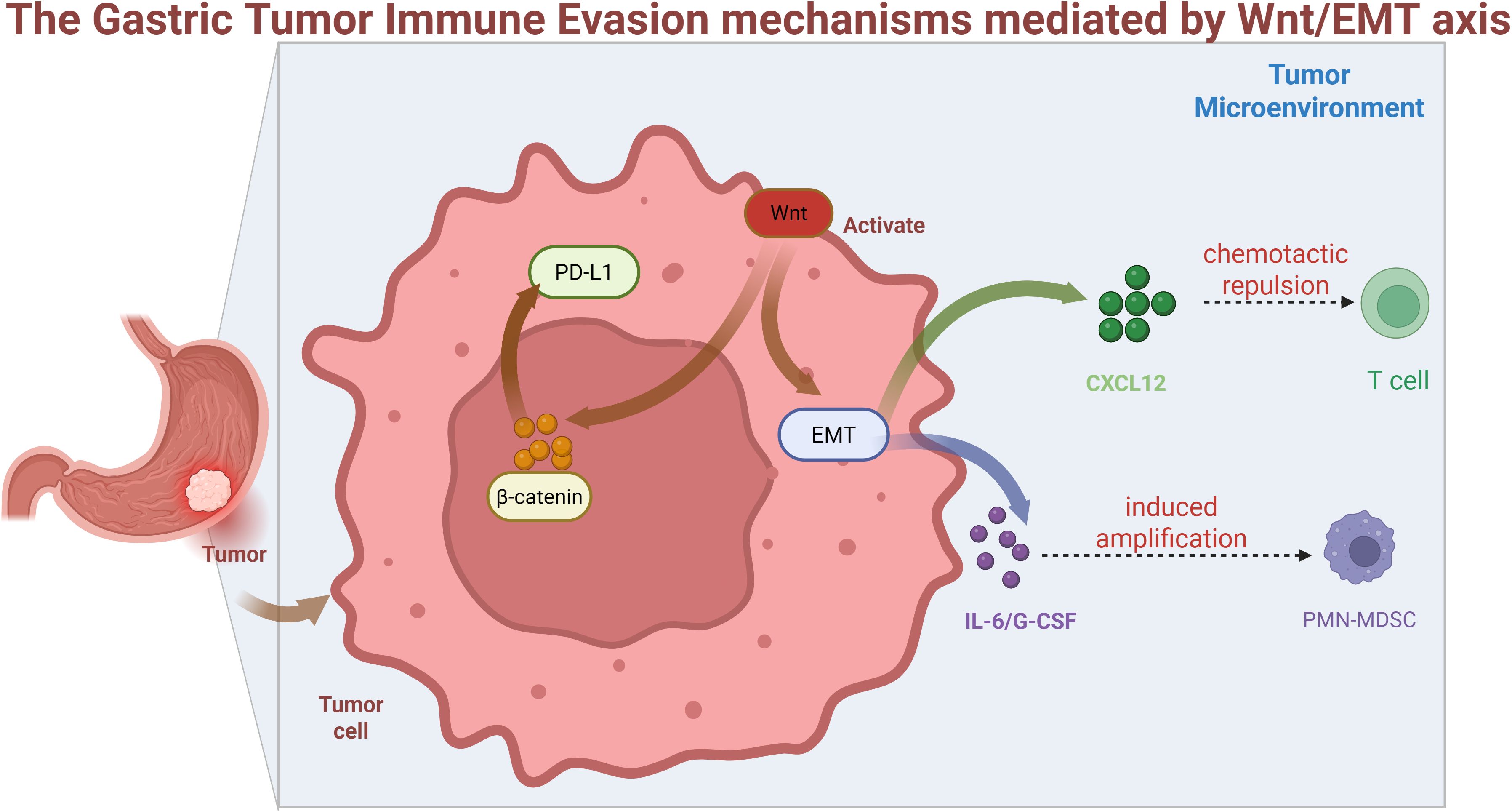
Figure 2. Wnt activation in tumor cells drives β-catenin nuclear translocation, inducing EMT and PD-L1 expression. EMT-derived CXCL12 secretion causes T-cell chemotactic repulsion, while IL-6/G-CSF release promotes PMN-MDSC expansion, collectively establishing an immunosuppressive tumor microenvironment.
6 Targeted therapy strategies and clinical potential
6.1 Natural compounds and synthetic drugs
Multiple phytochemicals demonstrate anti-gastric cancer activity through modulation of critical signaling pathways. Rutin, a bioactive flavonoid, exerts dose-dependent antitumor effects by suppressing Wnt/β-catenin signaling-mediated cellular proliferation, migration, and epithelial-to-mesenchymal transition (EMT) in gastric malignancies (60). The compound in question has been demonstrated to exhibit a dose-dependent antitumor effect. Dihydroartemisinin (DHA) has also been shown to impede the Wnt pathway, thereby hindering tumor progression. Additionally, honokiol has been shown to target HDAC3, inhibit epithelial plasticity (EP), and induce endoplasmic reticulum stress (61). Dihydroartemisinin (DHA) has also been shown to impede the Wnt pathway, thereby hindering tumor progression. Furthermore, honokiol has been observed to target HDAC3, inhibit epithelial plasticity (EP), and induce endoplasmic reticulum stress. In addition, gallic acid (GA) has been demonstrated to mitigate gastric precancerous lesions (GPL) by inhibiting Wnt/β-catenin signaling, thereby reversing EMT markers such as E-cadherin upregulation and N-cadherin/vimentin downregulation. Moreover, it has been demonstrated to block the transition from inflammation to cancer in both cellular and murine models (43, 62) Bidens pilosa-derived polyacetylene analogues (compounds 1-2) exhibit dual-pathway inhibition targeting both Wnt/β-catenin and Hippo/YAP axes, effectively suppressing metastasis through EMT marker modulation - specifically reducing vimentin/snail expression while enhancing E-cadherin levels (63). It has been demonstrated that the addition of berberine (BBR) to a patient’s treatment regimen has the capability of reversing the phenotypic transition of gastric cancer (GC). This outcome is attributed to the drug’s capacity to target the TGF-β/Smad, PI3K/Akt, and Wnt/β-catenin signaling pathways. This alkaloid restores epithelial characteristics through upregulation of E-cadherin/ZO-1 coupled with downregulation of mesenchymal markers including N-cadherin and TGF-β1 (64).
6.2 Gene and RNA-targeted therapy
Emerging genetic interventions demonstrate significant potential in gastric cancer (GC) management through modulation of Wnt/β-catenin signaling and associated pathways: Circ_0003789: This oncogenic circular RNA drives GC progression and EMT via Wnt/β-catenin activation. Its suppression markedly inhibits tumor growth and metastasis in preclinical models (65). lncRNA PCAT6/miR-15a Axis: PCAT6 functions as a competitive endogenous RNA (ceRNA) to promote proliferation through Wnt/β-catenin and RB/E2F pathway activation. Targeted PCAT6 inhibition reverses these oncogenic effects (66). The study’s findings suggest that the inhibition of metastasis and proliferation of gastric cancer (GC) is achieved by suppressing Wnt/β-catenin signaling through the downregulation of WISP2. This observation provides further evidence that this pathway could serve as a viable therapeutic target (67). The TTY15/let-7a-5p/Wnt1 axis has emerged as a pivotal regulatory factor in this process. onco-protective long non-coding RNA TTTY15, which is silenced by CRISPR/Cas9, has been demonstrated to disrupt the regulatory loop between let-7a-5p and Wnt1. This disruption, in turn, has been shown to attenuate Wnt/β-catenin-driven PTD and progression (68). TET1/FOXO4 Axis: CRISPR-induced TET1 overexpression sequesters β-catenin in the cytoplasm, inhibiting Wnt signaling while stabilizing FOXO4 to regulate cancer stem cell (CSC) properties and EMT processes (69). NCAPG Targeting: NCAPG knockdown reverses Wnt/β-catenin activation, reduces EMT markers (e.g., Snail suppression with E-cadherin upregulation), and induces apoptosis in gastric adenocarcinoma (70). FNDC1 Silencing: CRISPR-mediated FNDC1 suppression inhibits Wnt/β-catenin signaling and EMT, effectively blocking peritoneal metastasis while offering dual diagnostic-therapeutic utility (71). The following investigation will address the CXXC5/KANK1 regulation. The restoration of CXXC5 or KANK1 expression through CRISPR/Cas9 technology has been demonstrated to suppress Wnt/β-catenin/Axin2 signaling, reverse EMT, and promote apoptosis in germ cells (GCs). It has been demonstrated that synergistic effects emerge when combinatorial targeting strategies are employed (72). CRISPR/Cas9 has been demonstrated to facilitate the precise modulation of Wnt/β-catenin regulators, including TET1 and NCAPG. This modulation has been shown to suppress ETP and metastasis (69, 70). The utilization of CRISPR/Cas9 technology in conjunction with non-coding RNAs (TTTY15) or epigenetic modifiers (TET1) results in the modification of oncogenic signaling networks, thereby enhancing the specificity of therapeutic interventions (68, 69).
6.3 Prognostic markers
Exosomal circ0000670 and plasma LINC01225: Potential non-invasive diagnostic markers (23, 30, 33): The utilization of CRISPR/Cas9 technology in conjunction with non-coding RNAs (TTTY15) or epigenetic modifiers (TET1) results in the modification of oncogenic signaling networks, thereby enhancing the specificity of therapeutic interventions (61, 73). Elevated levels of APOD in gastric cancer (GC) have been shown to promote proliferation and metastasis via the Wnt/β-catenin/EMT axis. In addition, APOD has been linked to unfavorable prognoses, functioning as a prognostic biomarker associated with the remodeling of the tumor microenvironment (74). TET1/FOXO4: Reduced expression levels of TET1 and FOXO4 have been identified as a prognostic indicator for colorectal cancer, indicating a negative correlation with patient outcomes. The repression of these genes has been demonstrated to activate Wnt/β-catenin signaling, thereby enhancing the characteristics of the disease (69). The present study investigates the role of the long non-coding RNA (lncRNA) SNHG11 in colorectal cancer (CRC). The elevated expression of SNHG11 in CRC is associated with adverse outcomes by fostering EMT and autophagy through the modulation of microRNA (miRNA) correspondence, as also demonstrated by the activation of Wnt/β-catenin. This finding suggests that SNHG11 has the potential to serve as a therapeutic and prognostic indicator (75). Fosl1: The elevated expression of Fosl1 in colorectal cancer (CRC) and colon cancer has been demonstrated to be associated with a poor prognosis. This association is attributed to the promotion of metastasis that occurs through Smurf1-mediated activation of the Wnt/β-catenin signaling pathway and induction of epithelial-mesenchymal transition (EMT) (76). Asymmetric dimethylarginine (ADMA) is a serum marker that has been the focus of research in the context of gastric cancer. Elevated levels of ADMA have been demonstrated to be concomitant with diminished mortality rates in patients diagnosed with this condition. The role of ADMA in enhancing epithelial-to-mesenchymal transition (EMT) and metastasis through Wnt/β-catenin signaling pathways has been attributed to this phenomenon. It has been determined that this attribute establishes ADMA as a non-invasive prognostic biomarker (65). SERPINH1: The demonstration that SERPINH1 expression in gastric cancer is associated with lymph node metastasis and poor survival outcomes has been well-documented. It is hypothesized that the association is mediated through the activation of the Wnt/β-catenin signaling and epithelial-mesenchymal transition (EMT) pathways (77), which has been established as a validated therapeutic and prognostic target (78). LDLRAD2 upregulation in gastric cancer has been shown to promote metastasis through the Axin1/β-catenin axis-mediated Wnt activation and epithelial-mesenchymal transition (EMT). This upregulation functions as an independent prognostic indicator for advanced disease (79). These markers have been observed to interact with Wnt/β-catenin and epithelial-mesenchymal transition (EMT) signaling pathways, thereby facilitating metastasis. The dysregulation of these genes, whether through the process of over-expression or under-expression, has been demonstrated to be a significant factor in the progression of tumor stages to more advanced stages, the development of therapeutic resistance, and the subsequent reduction in survival outcomes (69, 74, 76, 79). It has been posited that the targeting of specific markers, including SNHG11 antisense oligonucleotides and ADMA inhibitors. The aforementioned findings have the potential to result in a reversal of the Wnt/β-catenin-mediated pathway associated with PTD (75, 77).
7 Challenges and future perspectives
7.1 Pathway complexity
The Wnt/β-catenin signaling pathway exhibits substantial cross-talk with Hedgehog, Notch, and TGF-β pathways, thereby establishing a dynamic network that contributes to tumor heterogeneity and therapy resistance in gastric cancer (GC) (80, 81). The complexity of therapeutic targeting is further compounded by context-dependent interactions between the Wnt pathway and other signaling pathways. These additional pathways include, but are not limited to, PI3K/AKT and Hippo/YAP. Inhibition of one pathway may result in compensatory activation of alternative oncogenic signals (7, 82). The complex interaction among the Wnt signal pathway and other signal pathways (e.g., PI3K/AKT and Hippo/YAP) presents substantial difficulties in the development of effective therapeutic interventions. It is imperative to note that the inhibition of these pathways has the potential to result in unintended consequences (8).
7.2 Delay in clinical translation
Despite the initial optimism fueled by the preliminary clinical data, the translation of Wnt-targeted therapies, such as PORCN inhibitors and β-catenin degraders, into clinical practice has proven to be fraught with substantial challenges. These challenges stem from issues related to off-target toxicity, inadequate bioavailability, and the absence of predictive biomarkers (83, 84). A mere 5% of Wnt-related gastric cancer studies progress to clinical trials, underscoring the necessity for patient-derived organoid models and three-dimensional bioprinting systems. Addressing the discrepancy between preclinical and clinical data is imperative (85, 86). The following essay will address the challenges associated with drug delivery. In the context of future research studies, the utilization of nanoparticle-based Wnt inhibitors and proteolysis-targeted chimeras (PROTACs) holds promise for enhancing specificity and reducing systemic toxicity (87).
The representative agents targeting Wnt/β-catenin in GC, spanning clinical and preclinical stages, are summarized in Table 2.
7.3 Individualized treatment
The UPS (ubiquitin-proteasome system) scoring system is a method of evaluating and stratifying gastric cancer patients according to the activity of the canonical Wnt/β-catenin pathway. The system under discussion has been demonstrated to facilitate the formulation of personalized therapy options, such as LGK974, for tumors that exhibit high levels of UPS activity (55). Combination strategies: The combination of Wnt/β-catenin targeting and immune Checkpoint Inhibitor (ICI) therapy (e.g., anti-PD-1) or Epigenetic Agent (EA) therapy (e.g., HDAC inhibitors) has demonstrated synergistic efficacy in preclinical models. This finding supports the commencement of phase II clinical trials (59, 88) Liquid biopsy-based monitoring: The analysis of circulating tumor DNA (ctDNA) for mutations in the Wnt pathway, including CTNNB1 and APC, enables real-time modifications to therapeutic regimens, thereby addressing resistance (89). Clinical trials enriched with priority biomarkers (e.g., Fosl1 increased, TET1 decreased) aim to evaluate the efficacy of Wnt-targeted therapy in molecularly defined subtypes of gastric cancer (90).
8 Discussion
In this review, a thorough investigation of the molecular mechanisms underlying the Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition (EMT) in gastric cancer (GC) is presented, along with a discussion of the possible implications for therapy. In addition, we examine the essential roles of genetic, epigenetic, and non-coding RNA (ncRNA) regulatory networks in these processes. The ensuing analysis is structured into three primary domains: research advancements, obstacles in clinical application, and potential avenues for future investigation.
8.1 Discovery of core mechanism
An exhaustive investigation was undertaken to elucidate the molecular mechanisms through which genetic regulators, including NAT10 and SMC4, modulate the Wnt/β-catenin signaling pathway via post-translational modifications (e.g., acetylation) or nuclear transportation processes. For instance, NAT10 has been demonstrated to be significantly correlated with cisplatin resistance, a phenomenon that can be attributed, at least in part, to its capacity to stabilize β-catenin and to initiate a process known as epithelial-mesenchymal transition (EMT) (18), It has been demonstrated that the strategic targeting of NAT10 could represent a promising approach to enhance chemotherapeutic sensitivity. Furthermore, non-coding RNAs (e.g., LINC00665 and circ0000670) contribute significantly to tumor progression by interacting with critical molecules within the Wnt pathway (such as Wnt3a and HMGB1). It is imperative to note that exosomal particles, induced by the presence of cigarette smoke, have been observed to promote the development of precancerous lesions by stimulating the Wnt signaling pathway (33), This study provides novel insights into the regulatory mechanisms of tumorigenesis by environmental carcinogens through epigenetic pathways.
The findings of the present study offer a more exhaustive explanation of the mechanism through which epigenetic deregulation, for instance the hypermethylation of the miR-33b promoter, considerably exacerbates the epithelial-mesenchymal transition (EMT) and chemoresistance. This process involves interactions among multiple pathways, including NF-κB and Notch (44). The restoration of microRNA-33b expression has been identified as a potential strategy for impeding the Wnt/β-catenin signaling pathway, thereby effectively disrupting signals associated with metastasis promotion. The findings of the present study underscore the substantial potential of this receptor as a therapeutic target for combination treatments.
8.2 Exploration and limitations of therapeutic strategies
A thorough review of the existing literature was conducted to investigate the various approaches designed to target the Wnt/β-catenin-EMT pathway. The present analysis encompassed a comprehensive range of substances, including, but not limited to, natural substances such as rutin and ginsenoside Rg3. Furthermore, the review also incorporated state-of-the-art genetic engineering methods, including CRISPR/Cas9 targeting TET1/FOXO4. For instance, ginsenoside Rg3 was found to decrease the IC50 value of cisplatin by 60% through the suppression of β-catenin nuclear localization (46). Concurrently, the knockout of the FNDC1 gene exerted a substantial inhibitory effect on peritoneal metastasis (71). Nonetheless, the implementation of these strategies in clinical practice remains challenging due to issues such as off-target effects associated with CRISPR technology and inadequate bioavailability of natural compounds, which underscore the necessity for further refinement (83, 84).
The integrated treatment regimens that were proposed, including the concurrent administration of the Wnt inhibitor ICG-001 and PD-1 inhibitors, have demonstrated a synergistic effect in preclinical studies (59). The effectiveness of this method in patients with gastric cancer, however, remains to be validated through further studies. Moreover, the observation of alterations within the Wnt signaling pathway (e.g., CTNNB1 and APC) through liquid biopsy possesses the capability to enable the real-time adjustment of therapeutic regimens (89).
8.3 Challenges and future directions
It has been established that there exists a complex relationship between the Wnt pathway and an array of other signaling networks, including the PI3K/AKT and Hippo/YAP networks (7, 82). It is conceivable that this may result in compensatory activation subsequent to the inhibition of a singular pathway. For instance, the suppression of Wnt signaling may lead to the compensatory activation of the Hedgehog or Notch pathways (80, 81). It is conceivable that this may result in compensatory activation subsequent to the inhibition of a singular pathway. For instance, the suppression of Wnt signaling may result in the compensatory activation of the Hedgehog or Notch pathways.
With regard to biomarkers, exosomal circ0000670 and plasma LINC01225 have been shown to possess promising diagnostic potential (30, 33), their prognostic relevance in subtypes of gastric cancer (GC) requires validation through large-scale cohort studies. We propose combining multi-omics data, including scores from the ubiquitin-proteasome system (55), with patient-derived organoid models (85, 86) to promote accurate classification and the creation of personalized treatment approaches.
9 Conclusion
Recent studies have identified the Wnt/β-catenin signaling pathway as a critical factor in the development of gastric cancer, as well as in the promotion of tumors and chemoresistance. The targeting of key molecules within this pathway—such as NAT10, circadian proteins, and HDAC3—or the employment of combinations of natural products may provide solutions to the current therapeutic challenges. In order to move forward, it is essential to enhance mechanistic research, foster interdisciplinary strategies, and facilitate the clinical translation of precision treatments for gastric cancer.
Author contributions
RS: Writing – review & editing, Writing – original draft. ZC: Writing – review & editing. JL: Writing – review & editing. RJ: Writing – review & editing, Resources, Funding acquisition. ZG: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the following grants: High-Level Clinical Specialty Construction Project of Peking University Cancer Hospital Inner Mongolia Hospital (Grant No. 2024YNYB003): “Role of CKIP-1 in Regulating M2 Macrophage Polarization via the PI3K-Akt Signaling Pathway in KRAS-Mutant Colorectal Cancer.” Inner Mongolia Autonomous Region Public Hospital High-Level Clinical Specialty Development Project (Grant No. 2023SGGZ072): “Study on the Relationship Between the Immunosuppressive Microenvironment and Neoadjuvant Therapy in Gastrointestinal Tumors.” Public Hospital Scientific Research Joint Fund Project (Grant No. 2024GLLH0392): “Mechanism of ADORA2B in Regulating the Tumor Microenvironment of Gastric Cancer by Targeting CMTM6.”
Acknowledgments
We want to express our gratitude to the corresponding authors (Ru Ji and Zhijuan Guo) for their continuous guidance throughout the process of topic selection, framework design and academic depth of this review; to the co-first author (Zhenwen Cao) for his core contribution in literature integration and theoretical analysis; and to the second author (Jie Li) for his technical support in data organization and chart optimization. At the same time, we are grateful for the resource assistance provided by the Affiliated Tumor Hospital of Inner Mongolia Medical University. The authors sincerely acknowledge the technical support provided by the Inner Mongolia Medical University Affiliated Cancer Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thrift AP and El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. (2020) 18:534–42. doi: 10.1016/j.cgh.2019.07.045
2. Shin WS, Xie F, Chen B, Yu P, Yu J, To KF, et al. Updated epidemiology of gastric cancer in asia: decreased incidence but still a big challenge. Cancers. (2023) 15. doi: 10.3390/cancers15092639
3. Wen X, Wu Y, Awadasseid A, Tanaka Y, and Zhang W. New advances in canonical wnt/B-catenin signaling in cancer. Cancer Manage Res. (2020) 12:6987–98. doi: 10.2147/CMAR.S258645
4. Dongre A and Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. (2019) 20:69–84. doi: 10.1038/s41580-018-0080-4
5. Brabletz S, Schuhwerk H, Brabletz T, and Stemmler MP. Dynamic emt: A multi-tool for tumor progression. EMBO J. (2021) 40:e108647. doi: 10.15252/embj.2021108647
6. Peng Z, Wang C-X, Fang E-H, Wang G-B, and Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. (2014) 20:5403–10. doi: 10.3748/wjg.v20.i18.5403
7. Song Y, Li Z-X, Liu X, Wang R, Li L-W, and Zhang Q. The wnt/B-catenin and pi3k/akt signaling pathways promote emt in gastric cancer by epigenetic regulation via H3 lysine 27 acetylation. Tumour Biology: J Int Soc For Oncodevelopmental Biol Med. (2017) 39:1010428317712617. doi: 10.1177/1010428317712617
8. Bian S, Wang Y, Zhou Y, Wang W, Guo L, Wen L, et al. Integrative single-cell multiomics analyses dissect molecular signatures of intratumoral heterogeneities and differentiation states of human gastric cancer. Natl Sci Rev. (2023) 10:nwad094. doi: 10.1093/nsr/nwad094
9. Zhang W, Zhang N, Yang Z, Zhang X, Sun A, Wang L, et al. Overexpression of bzw1 promotes invasion and metastasis of gastric cancer cells by regulating wnt/B-catenin signaling and promoting epithelial-mesenchymal transition. Nan Fang Yi Ke Da Xue Xue Bao. (2024) 44:354–62. doi: 10.12122/j.issn.1673-4254.2024.02.18
10. Xie L, Zhong X, Cao W, Liu J, Zu X, and Chen L. Mechanisms of nat10 as ac4c writer in diseases. Mol Ther Nucleic Acids. (2023) 32:359–68. doi: 10.1016/j.omtn.2023.03.023
11. Chimnaronk S, Suzuki T, Manita T, Ikeuchi Y, Yao M, Suzuki T, et al. Rna helicase module in an acetyltransferase that modifies a specific trna anticodon. EMBO J. (2009) 28:1362–73. doi: 10.1038/emboj.2009.69
12. Ito S, Horikawa S, Suzuki T, Kawauchi H, Tanaka Y, Suzuki T, et al. Human nat10 is an atp-dependent rna acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal rna (Rrna). J Biol Chem. (2014) 289:35724–30. doi: 10.1074/jbc.C114.602698
13. Sleiman S and Dragon F. Recent advances on the structure and function of rna acetyltransferase kre33/nat10. Cells. (2019) 8. doi: 10.3390/cells8091035
14. Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, et al. Acetylation of cytidine in mrna promotes translation efficiency. Cell. (2018) 175. doi: 10.1016/j.cell.2018.10.030
15. Zhang Y, Jing Y, Wang Y, Tang J, Zhu X, Jin W-L, et al. Nat10 promotes gastric cancer metastasis via N4-acetylated col5a1. Signal Transduct Target Ther. (2021) 6:173. doi: 10.1038/s41392-021-00489-4
16. Deng M, Zhang L, Zheng W, Chen J, Du N, Li M, et al. Helicobacter pylori-induced nat10 stabilizes mdm2 mrna via rna acetylation to facilitate gastric cancer progression. J Exp Clin Cancer Research: CR. (2023) 42:9. doi: 10.1186/s13046-022-02586-w
17. Yang Q, Lei X, He J, Peng Y, Zhang Y, Ling R, et al. N4-acetylcytidine drives glycolysis addiction in gastric cancer via nat10/sept9/hif-1α Positive feedback loop. Adv Sci (Weinh). (2023) 10:e2300898. doi: 10.1002/advs.202300898
18. Chen Y, Yang J, Du Y, Yan Z, Gao J, Zhang H, et al. Acetyltransferase nat10 promotes gastric cancer progression by regulating the wnt/B-catenin signaling pathway and enhances chemotherapy resistance. Discov Oncol. (2025) 16:173. doi: 10.1007/s12672-025-01917-5
19. Teyra J, Singer AU, Schmitges FW, Jaynes P, Kit Leng Lui S, Polyak MJ, et al. Structural and functional characterization of ubiquitin variant inhibitors of usp15. Structure. (2019) 27:1. doi: 10.1016/j.str.2019.01.002
20. Peng Y, Liao Q, Tan W, Peng C, Hu Z, Chen Y, et al. The deubiquitylating enzyme usp15 regulates homologous recombination repair and cancer cell response to parp inhibitors. Nat Commun. (2019) 10:1224. doi: 10.1038/s41467-019-09232-8
21. Zhong M, Zhou L, Fang Z, Yao YY, Zou JP, Xiong JP, et al. Ubiquitin-specific protease 15 contributes to gastric cancer progression by regulating the wnt/B-catenin signaling pathway. World J Gastroenterol. (2021) 27:4221–35. doi: 10.3748/wjg.v27.i26.4221
22. Ma R-M, Yang F, Huang D-P, Zheng M, and Wang Y-L. The prognostic value of the expression of smc4 mrna in breast cancer. Dis Markers. (2019) 2019:2183057. doi: 10.1155/2019/2183057
23. Zhu M, Zhang X, Gao K, Zhang L, Feng X, Wang H, et al. Structural maintenance of chromosome protein 4 promotes the progression of cardia adenocarcinoma via regulation of the wnt/B-catenin signaling pathway. Comb Chem High Throughput Screen. (2024) 27:611–20. doi: 10.2174/1386207326666230426112941
24. Tian X-L, Kadaba R, You S-A, Liu M, Timur AA, Yang L, et al. Identification of an angiogenic factor that when mutated causes susceptibility to klippel-trenaunay syndrome. Nature. (2004) 427:640–5. doi: 10.1038/nature02320
25. Yao HH, Zhao YJ, He YF, Huang DB, and Wang W. Knockdown of aggf1 inhibits the invasion and migration of gastric cancer via epithelial-mesenchymal transition through wnt/B-catenin pathway. Cancer Cell Int. (2019) 19:41. doi: 10.1186/s12935-019-0765-6
26. Li S, Chai Z, Li Y, Liu D, Bai Z, Li Y, et al. Bzw1, a novel proliferation regulator that promotes growth of salivary muocepodermoid carcinoma. Cancer Lett. (2009) 284:86–94. doi: 10.1016/j.canlet.2009.04.019
27. Wang J, Shen D, Li S, Li Q, Zuo Q, Lu J, et al. Linc00665 activating wnt3a/B-catenin signaling by bond with ybx1 promotes gastric cancer proliferation and metastasis. Cancer Gene Ther. (2023) 30:1530–42. doi: 10.1038/s41417-023-00657-4
28. Liu J, Wang G, Zhao J, Liu X, Zhang K, Gong G, et al. Lncrna H19 promoted the epithelial to mesenchymal transition and metastasis in gastric cancer via activating wnt/B-catenin signaling. Dig Dis. (2022) 40:436–47. doi: 10.1159/000518627
29. He ZC, Yang F, Guo LL, Wei Z, and Dong X. Lncrna tp73-as1 promotes the development of epstein-barr virus associated gastric cancer by recruiting prc2 complex to regulate wif1 methylation. Cell Signal. (2021) 110094. doi: 10.1016/j.cellsig.2021.110094
30. Xu Y, Zhang G, Zou C, Qi W, Gong Z, Zhang G, et al. Long non-coding rna linc01225 promotes proliferation, invasion and migration of gastric cancer via wnt/B-catenin signalling pathway. J Cell Mol Med. (2019) 23:7581–91. doi: 10.1111/jcmm.14627
31. Wang F, Zhu W, Yang R, Xie W, and Wang D. Lncrna zeb2-as1 contributes to the tumorigenesis of gastric cancer via activating the wnt/B-catenin pathway. Mol Cell Biochem. (2019) 456:73–83. doi: 10.1007/s11010-018-03491-7
32. Qin J, Zhen S, Wang J, Lv W, Zhao Y, Duan Y, et al. Function of hsa_Circ_0006646 as a competing endogenous rna to promote progression in gastric cancer by regulating the mir-665-hmgb1 axis. J Gastrointest Oncol. (2023) 14:1259–78. doi: 10.21037/jgo-23-240
33. Liang Z, Fang S, Zhang Y, Zhang X, Xu Y, Qian H, et al. Cigarette smoke-induced gastric cancer cell exosomes affected the fate of surrounding normal cells via the circ0000670/wnt/B-catenin axis. Toxics. (2023) 11. doi: 10.3390/toxics11050465
34. Peng Y, Qin Y, Zhang X, Deng S, Yuan Y, Feng X, et al. Mirna-20b/sufu/wnt axis accelerates gastric cancer cell proliferation, migration and emt. Heliyon. (2021) 7:e06695. doi: 10.1016/j.heliyon.2021.e06695
35. Peng Y, Zhang X, Lin H, Deng S, Qin Y, He J, et al. Dual activation of hedgehog and wnt/B-catenin signaling pathway caused by downregulation of sufu targeted by mirna-150 in human gastric cancer. Aging (Albany NY). (2021) 13:10749–69. doi: 10.18632/aging.202895
36. Peng Y, Zhang X, Lin H, Deng S, Qin Y, Yuan Y, et al. Sufu mediates emt and wnt/B-catenin signaling pathway activation promoted by mirna-324-5p in human gastric cancer. Cell Cycle. (2020) 19:2720–33. doi: 10.1080/15384101.2020.1826632
37. Zhan T, Chen M, Liu W, Han Z, Zhu Q, Liu M, et al. Mir-455-3p inhibits gastric cancer progression by repressing wnt/B-catenin signaling through binding to armc8. BMC Med Genomics. (2023) 16:155. doi: 10.1186/s12920-023-01583-y
38. Yu L, Xu Y, Yang J, Gao L, Li H, Wang Z, et al. Mir-497 inhibits the growth and metastasis of sgc-7901 human gastric cancer anoikis resistant cells via blocking wnt/B-catenin signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2023) 39:617–25.
39. Zhang D, Luo Q, Xiao L, Chen X, Yang S, and Zhang S. Exosomes derived from gastric cancer cells promote phenotypic transformation of hepatic stellate cells and affect the Malignant behavior of gastric cancer cells. J Cancer Res Ther. (2024) 20:1157–64. doi: 10.4103/jcrt.jcrt_749_23
40. Guo K, Duan J, Lu J, Xiao L, Han L, Zeng S, et al. Tumor necrosis factor-A-inducing protein of helicobacter pylori promotes epithelial-mesenchymal transition and cancer stem-like cells properties via activation of wnt/B-catenin signaling pathway in gastric cancer cells. Pathog Dis. (2022) 80:5-8. doi: 10.1093/femspd/ftac025
41. Yang Y, Dong K, and Shao S. The effect of helicobacter pylori on the expression of fra-1 in gastric epithelial cells and its mechanism. Microb Pathog. (2019) 129:257–65. doi: 10.1016/j.micpath.2019.02.022
42. Liu Y, Qiao Y, Zhang H, Li W, and Zheng J. Wnt7a, frequently silenced by cpg methylation, inhibits tumor growth and metastasis via suppressing epithelial-mesenchymal transition in gastric cancer. J Cell Biochem. (2019) 120:18142–51. doi: 10.1002/jcb.29118
43. Wu SM, Jan YJ, Tsai SC, Pan HC, Shen CC, Yang CN, et al. Targeting histone deacetylase-3 blocked epithelial-mesenchymal plasticity and metastatic dissemination in gastric cancer. Cell Biol Toxicol. (2023) 39:1873–96. doi: 10.1007/s10565-021-09673-2
44. Zhang W, Jiang B, Zhu H, Cheng A, Li C, Huang H, et al. Mir-33b in human cancer: mechanistic and clinical perspectives. BioMed Pharmacother. (2023) 161:114432. doi: 10.1016/j.biopha.2023.114432
45. Fu Q, Wu X, Lu Z, Chang Y, Jin Q, Jin T, et al. Tmem205 induces tam/M2 polarization to promote cisplatin resistance in gastric cancer. Gastric Cancer. (2024) 27:998–1015. doi: 10.1007/s10120-024-01517-2
46. Meng ZQ, Zhang R, Wu XW, Jin TF, and Zhang MH. Ginsenoside rg3 regulates cisplatin resistance in gastric cancer by wnt/B-catenin signaling pathway. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. (2022) 44:366–76. doi: 10.3881/j.issn.1000-503X.14775
47. Kim Y, Bae YJ, Kim JH, Kim H, Shin SJ, Jung DH, et al. Wnt/B-catenin pathway is a key signaling pathway to trastuzumab resistance in gastric cancer cells. BMC Cancer. (2023) 23:922. doi: 10.1186/s12885-023-11447-4
48. Liu W, Yuan J, Liu Z, Zhang J, and Chang J. Label-free quantitative proteomics combined with biological validation reveals activation of wnt/B-catenin pathway contributing to trastuzumab resistance in gastric cancer. Int J Mol Sci. (2018) 19:1. doi: 10.3390/ijms19071981
49. Voutsadakis IA. Molecular alterations in claudin 18 suppressed and non-suppressed gastric adenocarcinomas to guide targeted therapies. Tissue Barriers. (2024) 13:2348852. doi: 10.1080/21688370.2024.2348852
50. Sun Y, Lin C, Ding Q, and Dai Y. Overexpression of foxc1 promotes tumor metastasis by activating the wnt/B-catenin signaling pathway in gastric cancer. Dig Dis Sci. (2022) 67:3742–52. doi: 10.1007/s10620-021-07226-5
51. Zhang H, Han X, Wei B, Fang J, Hou X, Lan T, et al. Rspo2 enhances cell invasion and migration via the wnt/B-catenin pathway in human gastric cancer. J Cell Biochem. (2019) 120:5813–24. doi: 10.1002/jcb.27867
52. Xie X, Zhou Z, Song Y, Zhang X, Dang C, and Zhang H. Mist1 inhibits epithelial-mesenchymal transition in gastric adenocarcinoma via downregulating the wnt/B-catenin pathway. J Cancer. (2021) 12:4574–84. doi: 10.7150/jca.59138
53. Ge Q, Hu Y, He J, Chen F, Wu L, Tu X, et al. Zic1 suppresses gastric cancer metastasis by regulating wnt/B-catenin signaling and epithelial-mesenchymal transition. FASEB J. (2020) 34:2161–72. doi: 10.1096/fj.201901372RR
54. Zhang G, Zhang X, Pan W, Chen X, Wan L, Liu C, et al. Dissecting the spatial and single-cell transcriptomic architecture of cancer stem cell niche driving tumor progression in gastric cancer. Adv Sci (Weinh). (2025) 12:e2413019. doi: 10.1002/advs.202413019
55. Wang H, Lin Q, Wu X, and Wang B. Ubiquitin-proteasome system reveals clinical subtypes with distinct molecular characteristics and prognoses in gastric cancer. Transl Oncol. (2023) 32:101660. doi: 10.1016/j.tranon.2023.101660
56. Zang J, Xiao L, Shi X, Liu S, Wang Y, Sun B, et al. Hsa_Circ_0001479 accelerates tumorigenesis of gastric cancer and mediates immune escape. Int Immunopharmacol. (2023) 124:110887. doi: 10.1016/j.intimp.2023.110887
57. Lin J, Ke L, Cheng S, Lu W, Hu Y, He X, et al. Orphan class a gpcrs signature predicts prognosis and immune microenvironment in gastric cancer: gpr176 drives tumor progression through wnt signaling and macrophage polarization. Mediators Inflammation. (2025) 2025:7977933. doi: 10.1155/mi/7977933
58. Yu J, Yan Y, Hua C, and Song H. Ehd3 promotes gastric cancer progression via wnt/B-catenin/emt pathway and associates with clinical prognosis and immune infiltration. Am J Cancer Res. (2023) 13:4401–17.
59. Li J, Zhang H, Bei S, Zhang X, Li H, Ye L, et al. Disruption of wnt/B-catenin pathway elevates the sensitivity of gastric cancer cells to pd-1 antibody. Curr Mol Pharmacol. (2022) 15:557–69. doi: 10.2174/1874467214666210617163821
60. Huang H, Shi J, Chen W, and Liu L. Rutin suppresses the Malignant biological behavior of gastric cancer cells through the wnt/B-catenin pathway. Discov Oncol. (2024) 15:407. doi: 10.1007/s12672-024-01281-w
61. Ma Y, Zhang P, Zhang Q, Wang X, Miao Q, Lyu X, et al. Dihydroartemisinin suppresses proliferation, migration, the wnt/B-catenin pathway and emt via tnks in gastric cancer. Oncol Lett. (2021) 22:688. doi: 10.3892/ol.2021.12949
62. Liao W, Wen Y, Wang J, Zhao M, Lv S, Chen N, et al. Gallic acid alleviates gastric precancerous lesions through inhibition of epithelial mesenchymal transition via wnt/B-catenin signaling pathway. J Ethnopharmacol. (2023) 302:115885. doi: 10.1016/j.jep.2022.115885
63. Cai J, Shi SY, Cheng F, Wei M, Zou K, Yu XQ, et al. Polyacetylene isomers isolated from bidens pilosa L. Suppress the metastasis of gastric cancer cells by inhibiting wnt/B-catenin and hippo/yap signaling pathways. Molecules. (2023) 28:2. doi: 10.3390/molecules28041837
64. Du H, Gu J, Peng Q, Wang X, Liu L, Shu X, et al. Berberine suppresses emt in liver and gastric carcinoma cells through combination with tgfβr regulating tgf-B/smad pathway. Oxid Med Cell Longev. (2021) 2021:2337818. doi: 10.1155/2021/2337818
65. Shao Z, Yuan C, Hu J, Wu Y, and Zeng C. Circ_0003789 facilitates gastric cancer progression by inducing the epithelial-mesenchymal transition through the wnt/B-catenin signaling pathway. Cancer Biother Radiopharm. (2023) 38:102–10. doi: 10.1089/cbr.2020.4044
66. Dong D, Lun Y, Sun B, Sun H, Wang Q, Yuan G, et al. Silencing of long non-coding rna pcat6 restrains gastric cancer cell proliferation and epithelial-mesenchymal transition by targeting microrna-15a. Gen Physiol Biophys. (2020) 39:1–12. doi: 10.4149/gpb_2019044
67. Jiang C, Xu D, Feng H, Ren Z, Li X, Chen Y, et al. Hnrnpa1 promotes the metastasis and proliferation of gastric cancer cells through wisp2-guided wnt/B-catenin signaling pathway. Discov Oncol. (2024) 15:465. doi: 10.1007/s12672-024-01354-w
68. Zheng X, Peng B, Wu X, Ye J, Zhao H, Li Y, et al. Male-specific long non-coding rna testis-specific transcript, Y-linked 15 promotes gastric cancer cell growth by regulating wnt family member 1/B-catenin signaling by sponging microrna let-7a-5p. Bioengineered. (2022) 13:8605–16. doi: 10.1080/21655979.2022.2053814
69. Qi J, Cui D, Wu QN, Zhao Q, Chen ZH, Li L, et al. Targeting wnt/B-catenin signaling by tet1/foxo4 inhibits metastatic spreading and self-renewal of cancer stem cells in gastric cancer. Cancers (Basel). (2022) 14:10–12. doi: 10.3390/cancers14133232
70. Zhang X, Zhu M, Wang H, Song Z, Zhan D, Cao W, et al. Overexpression of ncapg inhibits cardia adenocarcinoma apoptosis and promotes epithelial-mesenchymal transition through the wnt/B-catenin signaling pathway. Gene. (2021) 766:145163. doi: 10.1016/j.gene.2020.145163
71. Jiang T, Gao W, Lin S, Chen H, Du B, Liu Q, et al. Fndc1 promotes the invasiveness of gastric cancer via wnt/B-catenin signaling pathway and correlates with peritoneal metastasis and prognosis. Front Oncol. (2020) 10:590492. doi: 10.3389/fonc.2020.590492
72. Chen X, Wang X, Yi L, and Song Y. The kn motif and ankyrin repeat domains 1/cxxc finger protein 5 axis regulates epithelial-mesenchymal transformation, metastasis and apoptosis of gastric cancer via wnt signaling. Onco Targets Ther. (2020) 13:7343–52. doi: 10.2147/ott.S240991
73. Wen H, Mi Y, Li F, Xue X, Sun X, Zheng P, et al. Identifying the signature of nad+ Metabolism-related genes for immunotherapy of gastric cancer. Heliyon. (2024) 10:e38823. doi: 10.1016/j.heliyon.2024.e38823
74. Shah NN, Dave BP, Shah KC, Shah DD, Maheshwari KG, Chorawala MR, et al. Disabled-2, a versatile tissue matrix multifunctional scaffold protein with multifaceted signaling: unveiling its potential in the cancer battle. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397:5533–57. doi: 10.1007/s00210-024-03037-3
75. Wu Q, Ma J, Wei J, Meng W, Wang Y, and Shi M. Lncrna snhg11 promotes gastric cancer progression by activating the wnt/B-catenin pathway and oncogenic autophagy. Mol Ther. (2021) 29:1258–78. doi: 10.1016/j.ymthe.2020.10.011
76. Liu Y, Yue M, and Li Z. Fosl1 promotes tumorigenesis in colorectal carcinoma by mediating the fbxl2/wnt/B-catenin axis via smurf1. Pharmacol Res. (2021) 165:105405. doi: 10.1016/j.phrs.2020.105405
77. Guo Q, Xu J, Huang Z, Yao Q, Chen F, Liu H, et al. Adma mediates gastric cancer cell migration and invasion via wnt/B-catenin signaling pathway. Clin Transl Oncol. (2021) 23:325–34. doi: 10.1007/s12094-020-02422-7
78. Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, et al. Serpinh1 regulates emt and gastric cancer metastasis via the wnt/B-catenin signaling pathway. Aging (Albany NY). (2020) 12:3574–93. doi: 10.18632/aging.102831
79. Wei Y, Zhang F, Zhang T, Zhang Y, Chen H, Wang F, et al. Ldlrad2 overexpression predicts poor prognosis and promotes metastasis by activating wnt/B-catenin/emt signaling cascade in gastric cancer. Aging (Albany NY). (2019) 11:8951–68. doi: 10.18632/aging.102359
80. Kim J-H, Shin HS, Lee SH, Lee I, Lee YS, Park JC, et al. Contrasting activity of hedgehog and wnt pathways according to gastric cancer cell differentiation: relevance of crosstalk mechanisms. Cancer Sci. (2010) 101:328–35. doi: 10.1111/j.1349-7006.2009.01395.x
81. Yao Y, Ni Y, Zhang J, Wang H, and Shao S. The role of notch signaling in gastric carcinoma: molecular pathogenesis and novel therapeutic targets. Oncotarget. (2017) 8:53839–53. doi: 10.18632/oncotarget.17809
82. Melucci E, Casini B, Ronchetti L, Pizzuti L, Sperati F, Pallocca M, et al. Expression of the hippo transducer taz in association with wnt pathway mutations impacts survival outcomes in advanced gastric cancer patients treated with first-line chemotherapy. J Transl Med. (2018) 16:22. doi: 10.1186/s12967-018-1385-y
83. Park W-J and Kim MJ. A new wave of targeting 'Undruggable' Wnt signaling for cancer therapy: challenges and opportunities. Cells. (2023) 12. doi: 10.3390/cells12081110
84. Matsuoka T and Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol. (2018) 24:2818–32. doi: 10.3748/wjg.v24.i26.2818
85. Vistoso Monreal A, Zhao H, Sedghizadeh PP, and Lin D-C. Patient-derived tumor organoids to model drug response in gastric cancer. Cell Rep Med. (2024) 5:101650. doi: 10.1016/j.xcrm.2024.101650
86. Wang X, Yang X, Liu Z, Shen Z, Li M, Cheng R, et al. 3d bioprinting of an in vitro hepatoma microenvironment model: establishment, evaluation, and anticancer drug testing. Acta Biomater. (2024) 185:173–89. doi: 10.1016/j.actbio.2024.07.019
87. Chen J-J, Jin J-M, Gu W-J, Zhao Z, Yuan H, Zhou Y-D, et al. Crizotinib-based proteolysis targeting chimera suppresses gastric cancer by promoting met degradation. Cancer Sci. (2023) 114:1958–71. doi: 10.1111/cas.15733
88. Badie A, Gaiddon C, and Mellitzer G. Histone deacetylase functions in gastric cancer: therapeutic target? Cancers. (2022) 14:2. doi: 10.3390/cancers14215472
89. Uchôa Guimarães CT, Ferreira Martins NN, Cristina da Silva Oliveira K, Almeida CM, Pinheiro TM, Gigek CO, et al. Liquid biopsy provides new insights into gastric cancer. Oncotarget. (2018) 9:15144–56. doi: 10.18632/oncotarget.24540
Keywords: gastric cancer, Wnt/β-catenin signaling pathway, epithelial-mesenchymal transition, chemoresistance, targeted therapy
Citation: Shi R, Cao Z, Li J, Ji R and Guo Z (2025) Targeting the Wnt/β-catenin pathway and epithelial-mesenchymal transition in gastric cancer: mechanisms, therapeutic strategies, and clinical challenges. Front. Oncol. 15:1633699. doi: 10.3389/fonc.2025.1633699
Received: 26 May 2025; Accepted: 11 August 2025;
Published: 29 August 2025.
Edited by:
Chun Ju Chang, University at Buffalo, United StatesReviewed by:
Sivapar V. Mathan, All India Institute of Medical Sciences, IndiaJer-yen Yang, China Medical University, Taiwan
Copyright © 2025 Shi, Cao, Li, Ji and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ru Ji, MTAwOTE2NDM1NkBxcS5jb20=; Zhijuan Guo, OTA4NzY2MzYxQHFxLmNvbQ==
†These authors have contributed equally to this work
 Ruixin Shi
Ruixin Shi Zhenwen Cao1,2†
Zhenwen Cao1,2† Jie Li
Jie Li Ru Ji
Ru Ji