- 1Ganzhou Institute of Medical Imaging, Ganzhou Key Laboratory of Medical Imaging and Artificial Intelligence, Medical Imaging Center, Ganzhou People’s Hospital, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, China
- 2Department of pathology, Ganzhou People’s Hospital, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, China
- 3Department of Oncology, Ganzhou People’s Hospital, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, China
Primary intravenous synovial sarcoma (PISS) is an extremely rare disease originating from pluripotent mesenchymal stem cells, and has the potential to develop in nearly any part of the body. This report describes the case of a 54-year-old man who presented with a 20-day history of neck and facial swelling. Imaging findings on contrast-enhanced CT and 18F-FDG PET/CT revealed filling defects with markedly increased FDG uptake with involvement of multiple venous structures, including the superior vena cava, brachiocephalic vein, and left internal jugular vein, and enlarged lymph nodes near the aortic arch. A vascular interventional biopsy confirmed PISS. Here, we discuss the clinical characteristics and imaging findings of PISS and review 14 cases reported in previous studies.
Introduction
Synovial sarcoma often occurs in deep soft tissues adjacent to large joints. However, synovial sarcoma does not arise from the synovium, it can occur almost anywhere in the body, and multipotent mesenchymal stem cells are considered the origin of malignant cells (1, 2). Synovial sarcoma arising in large veins represents a rare clinical entity. Here, we report a case of primary intravenous synovial sarcoma (PISS) in the proximal great vein.
Case description
A 54-year-old man presented with a 20-day history of neck and facial swelling. Physical examination revealed jugular vein and chest wall venous distention, indicating superior vena cava (SVC) syndrome. No positive signs were found in the joints of the limbs. Laboratory tests revealed a D-dimer concentration of 1.31 mg/L (reference range 0–0.55 mg/L). No abnormalities in the levels of tumor markers were detected. Non-contrast CT reveals fusiform dilation of the superior vena cava (Figure 1A, *). Contrast-enhanced CT (CECT) (Figures 1B, C, white arrow) revealed heterogeneously enhanced soft tissue masses with filling defects in the SVC, brachiocephalic vein, and left internal jugular vein. 18F-flurodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) revealed intense FDG uptake (SUVmax 35.5; Figures 1D–G, red arrow) and enlarged lymph nodes near the aortic arch with intense FDG uptake (SUVmax 13.7; Figure 1, yellow arrow). No FDG uptake is observed in the filling defect of the right internal jugular vein. There was no evidence of distant metastasis. To alleviate symptoms, venous stenting was attempted via right femoral venipuncture; however, the surgery failed. Subsequently, an interventional biopsy of the lesion was performed. Histopathological examination of the lesion revealed synovial sarcoma (Figure 2A). Furthermore, the immunohistochemistry results revealed that the tumor cells were positive for CK, vimentin, TLE1 (Figures 2B–D), Fli-1 and β-catenin and negative for desmin, EMA, P63, S-100, SMA, CD31, and ERG, and the Ki-67 index was 80%. The patient was clinically diagnosed with primary intravenous synovial sarcoma (PISS) with mediastinal lymph node metastasis, leading to SVC obstruction syndrome. The patient subsequently underwent radiotherapy targeting the neck and upper mediastinum, which resulted in a reduction in neck and facial swelling. Imaging surveillance revealed no evidence of tumor progression during the 12-month follow-up. However, the patient subsequently committed suicide due to cancer-related depression.

Figure 1. Contrast-enhanced CT and 18F-FDG PET images for the PISS patient. (A–C) Non-contrast CT reveals fusiform dilation of the superior vena cava. Contrast-enhanced CT revealed filling defects in the superior vena cava, brachiocephalic vein, and bilateral internal jugular vein (white arrows). (D–G) 18F-FDG PET/CT revealed intense FDG uptake in the superior vena cava, brachiocephalic vein, and left internal jugular vein (SUVmax 35.5; red arrows) and enlarged lymph nodes near the aortic arch with intense FDG uptake (SUVmax 13.7; yellow arrows). No significant FDG uptake is observed in the filling defect of the right internal jugular vein. These imaging findings definitively demonstrate that the filling defect observed in the right internal jugular vein results from thrombosis, whereas the filling defects identified in other regions are attributable to tumor tissue infiltration.
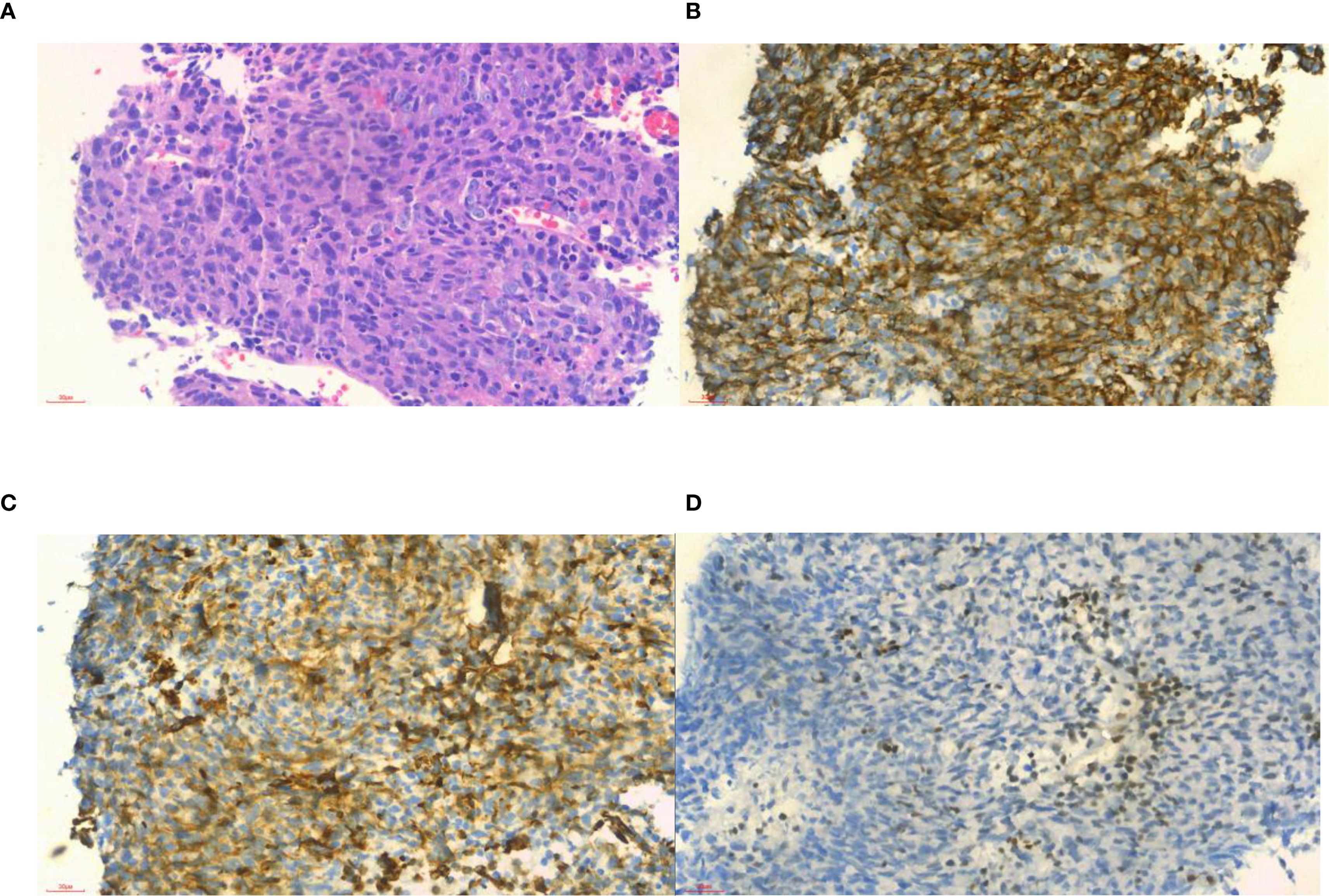
Figure 2. Histopathological and immunohistochemical examinations of samples from the patient. (A) Histologically, the tumor cells were composed of different proportions of epithelioid cells and spindle cells, suggesting a biphasic synovial sarcoma subtype (hematoxylin–eosin stain ×40); (B) positive in CK (×40); (C) positive in vimentin (× 40); and (D) focal positive in TLE1 (× 40).
Discussion
PISS is an extremely rare disease originating from pluripotent mesenchymal stem cells. PISS exhibits a characteristic chromosomal translocation t(X; 18) (p11; q11), resulting in the formation of the SS18-SSX fusion genes (1, 2). We collected all the case reports related to PISS from the PubMed database. To our knowledge, only 14 cases have been reported in the literature (2–15), with a mean age at diagnosis of 38 years and a female-to-male ratio of 5:2 (Table 1 in detail). Among these patients, eleven had lesions located in the inferior vena cava, iliac veins, femoral veins and lower extremity veins, whereas three had lesions located in the SVC, internal jugular vein and proximal subclavian vein. The clinical manifestations vary depending on the location of the disease. Lesions distal to the inferior vena cava typically manifest as lower extremity pain and swelling, whereas those involving the SVC primarily present with SVC obstruction syndrome. The prognosis of PISS is poor, with 1-year and 5-year survival rates of 45.3% and 11.5%, respectively (16). Furthermore, almost half of patients with synovial sarcoma experience local recurrence or metastasis after surgery (17).
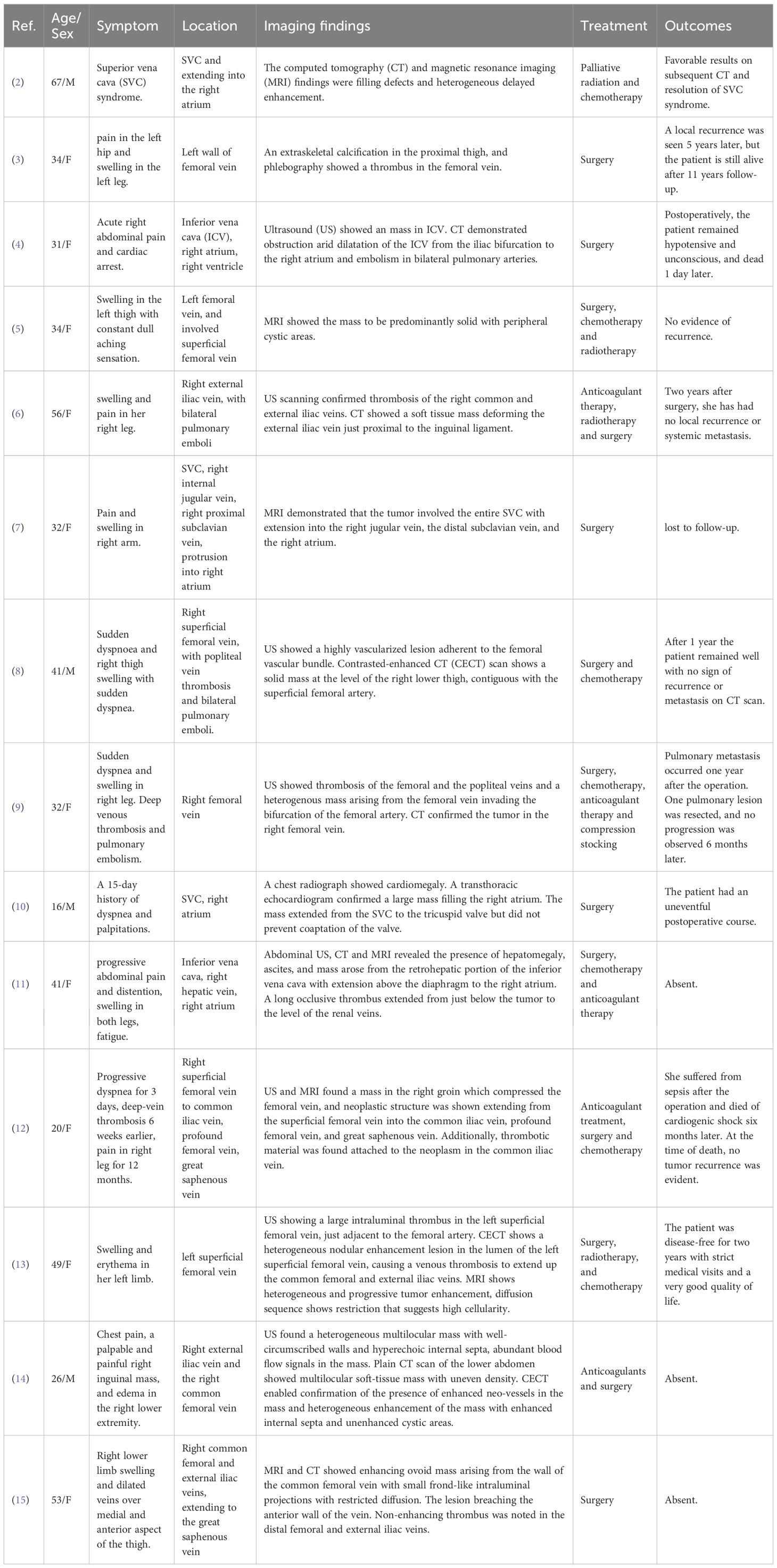
Table 1. Clinical and imaging findings of primary intravenous synovial sarcoma reported in previous literature.
To the best of our knowledge, this study represents the first report of ¹8F-FDG PET/CT imaging in PISS, which provides significant clinical value for early disease diagnosis. PISS is often complicated by venous thrombosis and pulmonary embolism (14). Although CECT cannot definitively differentiate thrombotic from neoplastic filling defects, ¹8F-FDG PET provides discriminatory power based on their distinct metabolic profiles: thrombi demonstrate absent FDG uptake, contrasting with avid uptake in tumor tissue. Furthermore, the presence of calcifications, venous dilation with intraluminal cysts, fluid–fluid levels, and septations within a thrombosed vein on CECT warrant the consideration of intravascular tumors mimicking thrombosis (15). The enhancement of intravascular masses on CECT serves as a reliable diagnostic marker for tumors (15), providing a key feature for differentiating PISS from venous thrombosis. Therefore, CECT and 18F-FDG PET/CT contribute to the early diagnosis of PISS by enabling identification of tumor-thrombus boundaries, detection of potential lymph node metastases, and exclusion of secondary cancerous thrombi originating from other locations.
PISS needs to be differentiated from benign intravascular tumors and tumor-like lesions, such as intravenous pyogenic granuloma, intravascular fasciitis, masson’s tumor, leiomyoma, and lipoma. Intravascular lipoma presents as a distinct low-density mass on CT (18). Intravenous pyogenic granuloma, masson’s tumor and leiomyoma show only slight FDG uptake on 18F-FDG PET/CT, which is markedly lower than that of PISS (19–21). Intravascular fasciitis may present high FDG uptake, mimicking sarcoma (22). However, intravascular fasciitis predominantly involves the microvasculature, and is characterized by focal and small lesions. The differential diagnosis between PISS and other intravenous malignancies, such as intravenous leiomyosarcomas, intimal sarcomas and fat-poor liposarcomas, depends on pathology.
There is currently no standardized treatment for PISS. The management of PISS typically involves a multimodal therapeutic strategy including surgical resection, systemic chemotherapy, and radiation therapy. In cases of PISS complicated by thrombotic events, thrombolytic therapy is often indicated as a therapeutic strategy. Since the prevention strategies for synovial sarcoma are not well-defined, early detection and treatment are crucial.
Conclusion
PISS is an exceptionally rare clinical entity. This study presents the first documented characterization of 18F-FDG PET/CT imaging in PISS, while emphasizing the indispensable utility of CECT and 18F-FDG PET/CT for both diagnosis and differential diagnosis. Notably, PISS frequently presents with concomitant thrombosis, and the detection of malignant tumor components within a thrombotic background can significantly facilitate early diagnosis of PISS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual and relative(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WT: Writing – original draft. ZW: Writing – review & editing. RH: Writing – review & editing. JW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Clinical Research Center for Medical Imaging in Jiangxi Province (No. 20223BCG7400199), the Science and Technology Program of the Health Commission of Jiangxi Province (No. 2025110045), the Ganzhou Science and Technology Planning Project (No. GZ2024YLJ041 and GZ2024ZSF064).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Naka N, Takenaka S, Araki N, Miwa T, Hashimoto N, Yoshioka K, et al. Synovial sarcoma is a stem cell Malignancy. Stem Cells. (2010) 28:1119–31. doi: 10.1002/stem.452
2. Costilla VC, Adams JC, and Mookadam F. Biphasic synovial sarcoma arising from superior vena cava. Eur Heart J Cardiovasc Imaging. (2012) 13:713. doi: 10.1093/ehjci/jes051
3. Miettinen M, Santavirta S, and Slätis P. Intravascular synovial sarcoma. Hum Pathol. (1987) 18:1075–7. doi: 10.1016/s0046-8177(87)80227-7
4. Shaw GR and Lais CJ. Fatal intravascular synovial sarcoma in a 31-year-old woman. Hum Pathol. (1993) 24:809–10. doi: 10.1016/0046-8177(93)90021-8
5. Robertson NJ, Halawa MH, and Smith ME. Intravascular synovial sarcoma. J Clin Pathol. (1998) 51:172–3. doi: 10.1136/jcp.51.2.172
6. White JS, Medlicott SAC, Brown H, Moore R, and Temple W. Intravascular synovial sarcoma of the external iliac vein and reconstruction with the superficial femoral vein. J Vasc Surg. (2005) 42:365–7. doi: 10.1016/j.jvs.2005.03.059
7. Tong GX, Goldblum JR, Qiu W, Skacel M, Downs-Kelly E, Contractor S, et al. Primary intravascular synovial sarcoma: A disease of young adult women? report of a case diagnosed by aspiration biopsy and review of the literature. Diagn Cytopathol. (2006) 34:834–8. doi: 10.1002/dc.20574
8. Coen M, Gagliano T, Tsolaki E, Marchetti G, Marchetti E, and Mascoli F. Primary intravascular synovial sarcoma of the femoral vein in a male patient, case report. Eur J Vasc Endovasc Surg. (2008) 36:224–6. doi: 10.1016/j.ejvs.2008.01.028
9. Schoneveld JM, Debing E, Verfaillie G, Geers C, and Van Den Brande P. Deep venous thrombosis and pulmonary embolism caused by an intravascular synovial sarcoma of the common femoral vein. Vasc Endovasc Surg. (2012) 46:693–5. doi: 10.1177/1538574412460770
10. Tuncer ON, Erbasan O, and Golbasi İ. Primary intravascular synovial sarcoma: case report. Heart Surg Forum. (2012) 15:297. doi: 10.1532/HSF98.20111159
11. Wise KB, Said SM, Clark CJ, Okuno SH, Shah SS, Park SJ, et al. Resection of a giant primary synovial sarcoma of the inferior vena cava extending into the right atrium with caval reconstruction under cardiopulmonary bypass and circulatory arrest. Perspect Vasc Surg Endovasc Ther. (2012) 24:95–101. doi: 10.1177/1531003512468035
12. Schreiner M, Sanad W, Pfitzner BM, Baumann G, and Knebel F. A primary intravascular synovial sarcoma causing deep-vein thrombosis and pulmonary embolism in a 20-year-old woman. Curr Oncol. (2015) 22:387–90. doi: 10.3747/co.22.2315
13. Chicas-Sett R, Farga-Albiol D, Collado E, Pacheco A, Zac C, Diaz R, et al. Intravascular biphasic synovial sarcoma: the beneficial role of adjuvant treatment approach in the pre-metastatic stage. Cureus. (2016) 8(4):e572. doi: 10.7759/cureus.572
14. Yan H, Zhou C, Yan F, Wen X, and Luo Y. Case 290: intravascular cystic synovial sarcoma. Radiology. (2021) 299:730–5. doi: 10.1148/radiol.2021192863
15. Jayakrishanan R, Valakkada J, Ayyappan A, Poyuran R, and Pitchai S. Femoral vein intravascular synovial sarcoma mimicking primary deep vein thrombosis—A rare cause of deep vein thrombosis. Indian J Radiol Imaging. (2024) 34:156–9. doi: 10.1055/s-0043-1771521
16. Sultan I, Bianco V, Habertheuer A, Kilic A, Gleason TG, Aranda-Michel E, et al. Long-term outcomes of primary cardiac Malignancies: multi-institutional results from the national cancer database. J Am Coll Cardiol. (2020) 75:2338–47. doi: 10.1016/j.jacc.2020.03.041
17. Xu J, Wang J, Cui L, and Wu X. Malignant inguinal monophasic synovial sarcoma: report of a case and review of the literature. World J Surg Onc. (2010) 8:102. doi: 10.1186/1477-7819-8-102
18. Bravi MC, Salvadei S, Scarponi P, Loforte A, Musumeci F, and Gasbarrone L. Intravascular lipoma of the superior vena cava. Intern Emerg Med. (2012) 7:79–81. doi: 10.1007/s11739-011-0584-9
19. Li Z, Su D, and Chen Y. 68Ga-FAPI and 18F-FDG PET/CT in intravenous leiomyomatosis. Clin Nucl Med. (2023) 48:994–6. doi: 10.1097/RLU.0000000000004835
20. Nguyen BD. Imaging of intravenous lobular capillary hemangioma of azygos vein. Clin Nucl Med. (2014) 39:e114–6. doi: 10.1097/RLU.0b013e318281657b
21. Van Houdt M, Fidlers T, Neven P, and Han S. (Intravascular) papillary endothelial hyperplasia or Masson’s tumour of the axilla in a patient with a history of breast cancer. BMJ Case Rep. (2024) 17:e261765. doi: 10.1136/bcr-2024-261765
Keywords: primary, intravenous synovial sarcoma, proximal great vein, 18F-FDG PET/CT, case report
Citation: Tang W, Wu Z, Huang R and Wei J (2025) Case Report: Primary intravenous synovial sarcoma in the proximal great vein: a case report and literature review. Front. Oncol. 15:1634106. doi: 10.3389/fonc.2025.1634106
Received: 23 May 2025; Accepted: 26 August 2025;
Published: 05 September 2025.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Massimo Baudo, Lankenau Institute for Medical Research, United StatesDeepanksha Datta, All India Institute of Medical Sciences Jodhpur, India
Copyright © 2025 Tang, Wu, Huang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawang Wei, d2Vpamlhd2FuZ0BtYWlsLmd6c3JteXkuY29t
†These authors have contributed equally to this work
 Wenjian Tang1†
Wenjian Tang1† Jiawang Wei
Jiawang Wei