- The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
HIV-induced gut microbiota dysbiosis perpetuates mucosal barrier disruption and systemic inflammation despite antiretroviral therapy (ART), creating a tumor-permissive microenvironment. This review synthesizes evidence linking HIV-associated microbial alterations to oncogenesis through three convergent metabolic axes: (1) butyrate deficiency impairing epithelial energy metabolism and anti-tumor immunity; (2) tryptophan metabolism dysregulation compromising gut barrier integrity via Akkermansia muciniphila depletion and Enterococcus-mediated phenylethylamine overproduction; and (3) vitamin B biosynthesis defects disrupting DNA repair and Th1/Th2 balance. Comparative profiling across HIV-associated malignancies—non-Hodgkin lymphoma, cervical cancer, hepatocellular carcinoma, and lung cancer—reveals conserved dysbiotic signatures: depletion of anti-inflammatory taxa (Bacteroidetes, Bifidobacterium) and expansion of pro-inflammatory genera (Proteobacteria, Shigella). These alterations activate NF-κB/STAT3 signaling, fostering IL-6/TNF-α-driven chronic inflammation. Emerging interventions, including Bifidobacterium-derived metabolites and butyrate supplementation, demonstrate potential to enhance immunotherapy efficacy and reverse chemoresistance. However, causal microbiota-tumor relationships remain unproven, and key AIDS-defining cancers (Kaposi sarcoma, anal carcinoma) lack microbial association studies. Prioritizing longitudinal multi-omics analyses, organoid models, and LMIC-focused clinical trials may advance microbiota-directed strategies for HIV-associated cancer prevention and treatment.
1 Introduction
Acquired immunodeficiency syndrome (AIDS) is a chronic disease caused by the human immunodeficiency virus (HIV). As of 2023, global HIV/AIDS statistics reveal two critical challenges: an estimated 39.9 million people living with HIV/AIDS worldwide, and approximately 1.3 million new infections occurring in that year alone, underscoring its persistent status as a major global health crisis (1). Geographic disparities in disease burden are particularly striking, with sub-Saharan Africa accounting for nearly two-thirds of global HIV infections. Notably, over 90% of cases are concentrated in low- and middle-income countries (LMICs) (2), where vulnerable populations including women and children under 15 face heightened infection risks due to lower social demographic indices (3).
The health challenges in LMICs extend beyond HIV prevalence. These regions frequently experience synergistic health burdens including childhood stunting, malnutrition, environmental pollution, and enteric pathogen infections. Such conditions create a biological milieu where gut microbiota dysbiosis manifests more severe consequences than in high-income nations, predisposing populations to immune dysregulation, recurrent infections, and chronic inflammation (4). Compounding these challenges, LMICs often lack the advanced diagnostic technologies and healthcare infrastructure required for effective cancer management, resulting in disproportionate cancer-related morbidity (5). Emerging evidence suggests that gut microbiota dysbiosis may contribute to HIV-associated oncogenesis, but its causal role remains controversial. The key issues include bidirectional causality, the difficulty of predicting functional impacts through diversity metrics, and the direct alteration of the microbiome by HIV treatment regimens (6).
As the body’s central hub for digestive, immune, and metabolic functions, the gastrointestinal tract plays a pivotal role in maintaining physiological homeostasis. A healthy human gut hosts a complex ecosystem of over 100 trillion microorganisms, predominantly bacterial species (7). This microbiota fulfills essential physiological roles through: 1) establishing a biological barrier against pathogens; 2) enhancing intestinal development and metabolic capacity; 3) regulating nutrient absorption via interactions with intestinal epithelial and Paneth cells; 4) modulating immune cell proliferation and systemic immune maturation (8); 5) communicating with the brain via the gut-brain axis; and 6) aiding in the biotransformation and detoxification of harmful substances (6). Molecular signaling through bacterial components (lipopolysaccharides, peptidoglycans) and microbial metabolites (short-chain fatty acids [SCFAs]) further enables cross-regulation of immune cells including epithelial cells, macrophages, dendritic cells, and neutrophils (9–11).
Notably, the gastrointestinal tract serves as the primary site for HIV replication and pathogenesis. Viral infection triggers a cascade of pathological events: lymphocyte destruction, profound CD4+ T-cell depletion, pro-inflammatory cytokine storm, immune barrier disruption, and microbiota dysbiosis - all contributing to disease progression acceleration (12, 13). Crucially, even with successful viral suppression through highly active antiretroviral therapy (HAART), persistent defects remain in gut mucosal repair, microbial community structure restoration, and inflammatory resolution (14). These unresolved alterations constitute key barriers to immune reconstitution in treated patients (15).
Emerging therapeutic strategies targeting gut microbiota modulation show promise for addressing these challenges. Interventions including prebiotics, probiotics, fecal microbiota transplantation, and phage therapy demonstrate multifaceted benefits: 1) restoring microbial ecological balance and SCFA production (16); 2) enhancing gut barrier integrity (“leaky gut” repair); and 3) improving systemic immunity through increased CXCR3+ CD4+ T-cell populations and anti-inflammatory responses (17). Although some studies have reported that probiotics can reduce levels of inflammation markers such as D-dimer, clinical evidence supporting their role in promoting immune recovery in HIV-infected individuals remains limited. The heterogeneity in study design, including strain selection and intervention duration, may affect the evaluation of their efficacy (18).
HIV/AIDS-induced depletion of CD4+ T lymphocytes leads to progressive immunosuppression, creating a permissive microenvironment for tumorigenesis and metastatic progression (19). HIV-associated malignancies are clinically categorized into two distinct groups: AIDS-defining cancers (ADCs) and non-AIDS-defining cancers (NADCs) (20). The ADC classification encompasses Kaposi’s sarcoma, non-Hodgkin lymphoma, and invasive cervical carcinoma, while NADCs include anal carcinoma, hepatocellular carcinoma, and lung cancer (21, 22). Notably, emerging evidence implicates gut microbial dysbiosis in the pathogenesis of several HIV-associated malignancies, including non-Hodgkin lymphoma, cervical cancer, hepatocellular carcinoma, and lung cancer. For instance, in non-Hodgkin’s lymphoma, the microbiota metabolite phenylethylamine promotes abnormal B-cell proliferation and EBV activation (23). In HPV-related cervical and anal cancers, the reduction of Lactobacillus weakens local mucosal immunity, facilitating persistent HPV infection (24). In colorectal cancer, toxins from Fusobacterium nucleatum and Escherichia coli, such as colibactin, directly damage DNA and suppress the immune system (25). This growing body of research positions gut microbiota modulation as a promising therapeutic frontier for HIV-related oncogenesis.
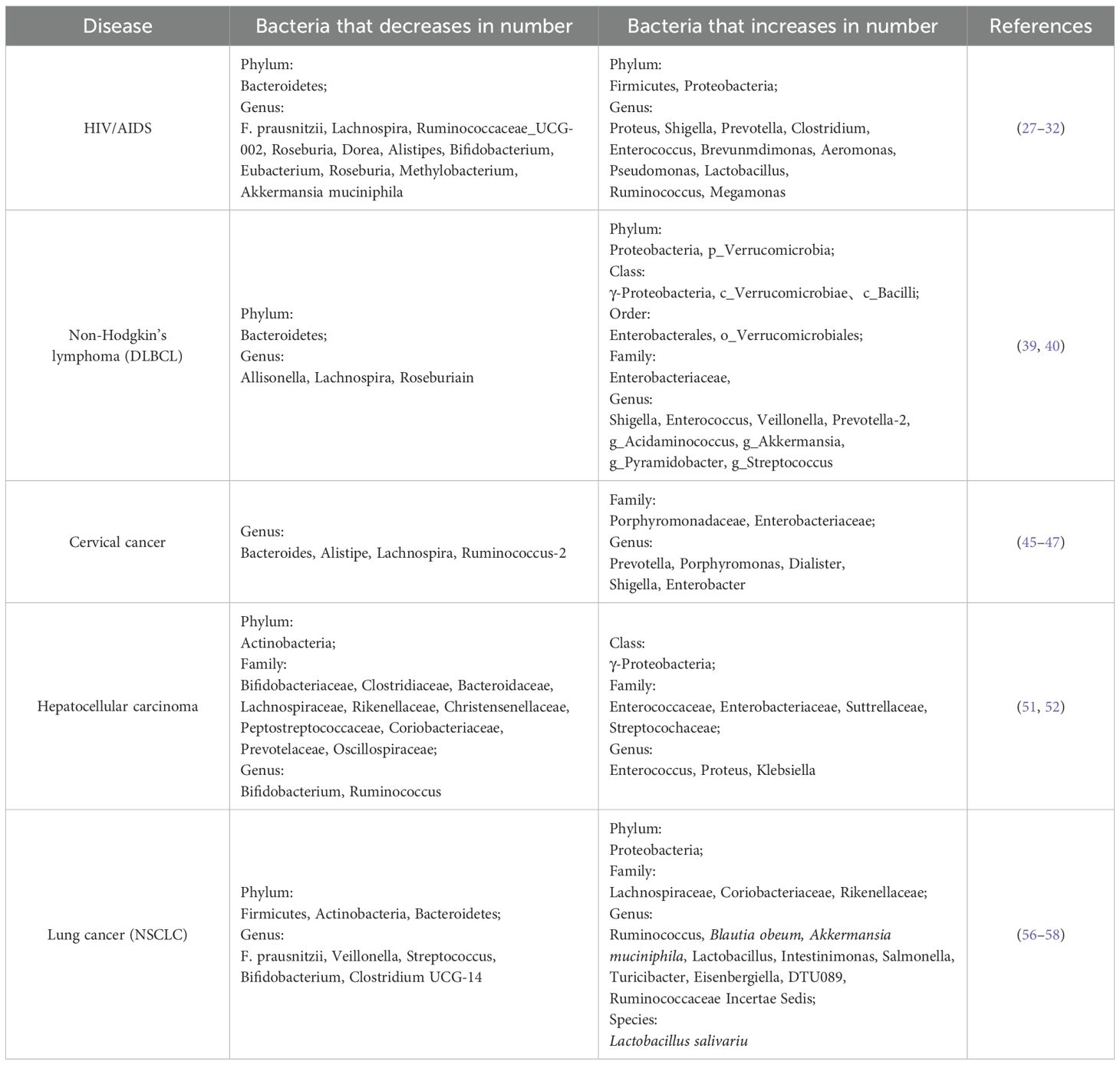
Through systematic analysis of gut microbial alterations in HIV-associated malignancies (diffuse large B-cell lymphoma, cervical cancer, hepatocellular carcinoma, and lung cancer) integrated with metabolic network profiling (26), we identified three convergent pathological mechanisms: 1) Depletion of butyrate-producing bacteria disrupts epithelial energy metabolism and anti-tumor surveillance; 2) Microbial community shifts impair vitamin B biosynthesis, compromising DNA repair mechanisms; 3) Intestinal barrier dysfunction facilitates microbial translocation, triggering chronic systemic inflammation. These microbial-metabolic perturbations synergistically activate NF-κB and STAT3 signaling pathways, generating a pro-inflammatory cytokine milieu (IL-6, TNF-α, IL-1β) that establishes a tumor-promoting microenvironment through autocrine and paracrine cascades. Our tripartite framework - analyzing butyrate metabolism, intestinal barrier integrity, and vitamin B biosynthesis - elucidates critical intersections between HIV-induced gut dysbiosis and oncogenesis. This mechanistic stratification provides: (i) clinical biomarkers for monitoring malignancy risk in HIV patients, and (ii) therapeutic targets for developing microbiota-directed adjuvant therapies.
2 The HIV/AIDS gut microbiota
The gastrointestinal tract is the primary target for HIV pathogenesis, and is associated with alterations in the gut microbiota related to HIV/AIDS. Following HIV infection, characteristic microbiota dysbiosis manifests through three key alterations: 1) Quantitative reduction in microbial biomass and α-diversity; 2) Ecological shift marked by increased pathobionts (e.g., Prevotella, Proteus) and decreased beneficial taxa (e.g., Lachnospira, Bifidobacterium); 3) Functional niche displacement facilitating opportunistic pathogen expansion. Phylum-level analyses reveal significant depletion of Bacteroidetes concomitant with overrepresentation of Firmicutes, Proteobacteria, and Clostridium clusters, effectively compromising microbial antagonism and barrier protection (27, 28). Notably, specific microbial signatures correlate with disease progression. Li et al. identified Shigella enrichment and Methylobacterium depletion as biomarkers for severe CD4+ T-cell depletion (<200 cells/µL) (29). Longitudinal analysis by Zhang et al. demonstrated stage-dependent taxonomic shifts: Early HIV infection exhibits elevated Enterococcus, Brevundimonas, and Aeromonas, while advanced stages correlate with progressive loss of butyrogenic genera (Faecalibacterium prausnitzii, Roseburia) and expansion of pro-inflammatory taxa (Enterococcus, Lactobacillus) (30). Antiretroviral therapy (ART) introduces additional microbial perturbations distinct from HIV-induced changes. Emerging evidence suggests certain ART regimens may exacerbate dysbiosis through microbiota composition alteration and enhanced microbial translocation (28). Post-ART stratification reveals persistent dysbiosis in both immune responders (IR) and non-responders (INR), characterized by depletion of immunomodulatory taxa (Bifidobacterium, Eubacterium) and enrichment of Clostridium clusters compared to healthy controls (31). The gut microbiota-mucosal barrier axis plays pivotal roles in HIV pathogenesis. Dysbiosis-induced mucolytic activity reduction, particularly the depletion of Akkermansia muciniphila (“gut sentinel”), disrupts critical homeostatic mechanisms: 1) Impaired mucus layer maintenance through diminished epithelial mucin secretion (32); 2) Compromised cross-feeding networks for butyrate producers (F. prausnitzii, Roseburia) that lack autonomous mucin degradation capacity (33). The microbiota influences the progression of HIV and HPV infections through immune regulation, metabolic intervention, and mucosal barrier modulation (show in Table 1).
3 Gut microbiota profiling in HIV-associated malignancies
3.1 HIV-associated non-Hodgkin lymphoma
HIV-associated lymphomagenesis arises from multifactorial interactions involving virus-specific mechanisms and microbial dysregulation. The pathogenic triad of HIV-induced immunosuppression, direct viral oncogenesis, and gamma herpesvirus co-infections (EBV/HHV-8) creates a permissive microenvironment for lymphoma development (34). Clinical epidemiology demonstrates striking correlations between immunosuppression severity and lymphoma risk, with CD4+ T-cell counts below 200 cells/µL and elevated HIV viral load serving as independent risk predictors. Notably, HIV-positive non-Hodgkin lymphoma (NHL) patients exhibit doubled mortality rates compared to HIV-negative counterparts within two years post-diagnosis (59% vs. 30%) (35). Emerging evidence positions gut microbiota dysbiosis as a fourth pathogenic dimension through dual mechanisms of chronic inflammation potentiation and immune checkpoint dysregulation, directly associating microbial imbalance with increased NHL risk (36). Among NHL subtypes, diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) predominate in HIV-infected populations, with DLBCL showing particularly strong gut microbiota associations (37, 38).
Comparative microbiota analyses reveal distinct ecological shifts in untreated DLBCL patients. Yuan et al. demonstrated through 16S rRNA sequencing of 25 DLBCL patients and 26 controls a characteristic dysbiosis pattern featuring Proteobacteria expansion (particularly γ-Proteobacteria/Enterobacteriaceae) and Bacteroidetes depletion at phylum levels. Genus-level alterations included pathogenic enrichment (Shigella, Enterococcus) alongside commensal depletion (Allisonella, Lachnospira). Mechanistically, Escherichia coli-derived genotoxins (colibactin/CDT) induce epithelial DNA damage through double-strand break formation, while Proteobacteria overgrowth correlates with therapy resistance and immune checkpoint inhibition (39). Lin et al. further identified progressive dysbiosis in advanced DLBCL through 16S rDNA analysis of 35 patients, showing stage-dependent increases in Verrucomicrobia, Bacilli, and Streptococcus despite preserved α/β diversity. Notably, Bacteroidetes abundance positively correlated with butyrate production capacity, suggesting metabolic consequences of phylum-level shifts (40).
The conserved microbial signature shared between HIV infection and DLBCL pathogenesis encompasses Proteobacteria/Prevotella expansion coupled with Bacteroidetes/Lachnospira depletion. This overlapping dysbiosis profile suggests a continuum of microbial-immune interactions contributing to lymphomagenesis, where enterococcal overgrowth and butyrogenic deficit may synergistically drive inflammation-mediated oncogenesis.
3.2 HIV-associated cervical cancer microbiota
Cervical cancer (CC) represents the most prevalent AIDS-defining malignancy, with HIV-infected women exhibiting six-fold higher incidence compared to the general population (41). This elevated risk stems from synergistic interactions between HIV and human papillomavirus (HPV), particularly pronounced in sub-Saharan Africa where HIV contributes to 20% of regional CC burden (42). The co-pathogenesis mechanism involves: 1) HIV-mediated impairment of HPV clearance; 2) Increased susceptibility to high-risk HPV subtypes (43); and 3) Accelerated progression from cervical intraepithelial neoplasia (CIN3) to invasive carcinoma, even under antiretroviral therapy (44). Emerging evidence implicates gut microbiota dysbiosis as a co-factor through estrogen metabolism modulation and systemic immunosuppression, facilitating HPV persistence and carcinogenesis (45).
Comparative microbiota profiling of 42 CC patients versus 46 controls revealed three consistent alterations: 1) Enrichment of pro-inflammatory genera (Prevotella, Porphyromonas); 2) Depletion of immunoregulatory taxa (Bacteroides, Alistipes); and 3) Lachnospira deficiency impairing mucosal homeostasis. Mechanistic studies demonstrate Legionella pneumophila activates TLR2-mediated IL-17/Th17 axis via dendritic cell stimulation (IL-1β/IL-6/IL-23), establishing chronic pro-carcinogenic inflammation (46).
Radiotherapy-induced microbiota changes show temporal dynamics: Early-stage chemoradiotherapy (CRT) patients exhibit Porphyromonas dominance, while long-term survivors develop Shigella/Enterobacteriaceae enrichment (47). Crucially, enhanced microbiota diversity correlates with improved survival outcomes, mediated through activated CD4+ T-cell infiltration and optimized anti-tumor immunity. Clinical parameter correlations further validate: 1) Inverse associations between patient age and Bacteroides abundance; 2) Disease stage progression with Shigella overgrowth; and 3) Protective roles of Ruminococcus 2 in neoplasia suppression (45). The shared microbial signature between HIV and CC pathogenesis features Prevotella/Shigella expansion coupled with Bacteroides/Lachnospira depletion, suggesting conserved pathways of dysbiosis-driven carcinogenesis in AIDS-related malignancies.
3.3 HIV-associated hepatocellular carcinoma microbiota
The epidemiological landscape of HIV-associated malignancies has shifted with antiretroviral therapy (ART) success: Reduced AIDS-defining tumor incidence contrasts with rising non-AIDS-defining cancers, particularly hepatocellular carcinoma (HCC). This transition reflects ART-mediated viral suppression, immune reconstitution, and prolonged survival, unmasking HCC as a growing oncological burden in aging HIV populations (48). Coinfection dynamics play pivotal roles, as shared transmission routes between HIV and hepatotropic viruses (HBV/HCV) amplify HCC risk. HIV-infected individuals exhibit a higher HBV/HCV prevalence and an elevated HCC incidence versus HIV-negative counterparts, with a lower CD4+ T-cell counts cells/µL further enhancing risk (49). Gut-liver axis dysregulation emerges as a central pathogenic mechanism. Microbial metabolites translocate via the portal vein, impairing hepatic immunosurveillance through: 1) Disrupted tight junction integrity from short-chain fatty acid (SCFA) depletion; 2) Enterococcus-driven lipopolysaccharide (LPS) leakage activating proinflammatory cascades; and 3) Treg cell dysregulation via butyrate deficit (50). Zhang et al. conducted longitudinal analysis of 74 male HCC patients, revealing progressive microbiota alterations: Early-stage disease showed enrichment of Lachnospiraceae and Peptostreptococcaceae, while terminal stages exhibited marked Enterococcaceae/Enterobacteriaceae expansion alongside depletion of Bifidobacteriaceae and SCFA-producing taxa (51). Yan et al. extended these findings through comparative analysis of 90 subjects (HBV-HCC, HBV-liver cirrhosis, healthy controls), identifying conserved dysbiosis patterns in chronic liver disease: Proinflammatory γ-Proteobacteria and Streptococcus enrichment coupled with reduced butyrate producers (Ruminococcus, Oscillospira) (52). This microbial configuration suggests gut-derived immunomodulation significantly influences hepatocarcinogenesis. The convergent microbiota signature in HIV and HCC patients features: pathobiont predominance (Enterococcus/Proteobacteria), commensal depletion (Bifidobacterium/Lachnospiraceae) and bacteroidaceae family reduction. These alterations establish a pathogenic cycle of barrier dysfunction, microbial translocation, and hepatic inflammation, underscoring the gut microbiota’s role in HIV-associated HCC progression.
3.4 HIV-associated lung cancer microbiota
Lung cancer (LC) constitutes a predominant non-AIDS-defining malignancy and leading cause of mortality among HIV-associated tumors. While elevated smoking rates in HIV-infected populations contribute significantly to LC etiology, emerging evidence identifies independent HIV-specific risk factors including uncontrolled viral load, CD4+ T-lymphocyte depletion, recurrent bacterial pneumonia, and accelerated aging processes (53). Notably, HIV infection demonstrates an independent association with LC development beyond conventional risk factors (54). The compromised gut mucosal barrier in HIV infection facilitates microbial translocation, perpetuating systemic inflammation and immunosuppression even under antiretroviral therapy (ART). This dysbiosis-driven pathophysiology may mediate pulmonary carcinogenesis through bacterial dissemination, inflammatory cascade activation, and metabolic perturbation (54, 55). Zheng et al. conducted 16S rRNA sequencing in 42 early-stage LC patients and 65 healthy controls, revealing phylum-level dysbiosis characterized by Bacteroidetes/Proteobacteria expansion and Firmicutes/Actinomycetota depletion, potentially impairing short-chain fatty acid (SCFA) production. Genus-level analysis identified Ruminococcus enrichment alongside Faecalibacterium prausnitzii and Bifidobacterium reduction in early LC. Metastatic cases uniquely exhibited Akkermansia muciniphila and Blautia obeum predominance. Concurrent metabolic pathway alterations (steroid biosynthesis, bile secretion) in LC-associated microbiota suggest systemic pathophysiological modulation (56). Non-small cell lung cancer (NSCLC), representing the majority of LC cases, demonstrates distinct microbial signatures. Tesolato et al. reported decreased Bacteroidetes and increased Lactobacillus/Salmonella in 19 NSCLC patients versus 20 controls, with DTU089 and Ruminococcaceae Incertae Sedis emerging as NSCLC-specific biomarkers (57). Therapeutic implications surface from Grenda et al., analyzing 214 advanced NSCLC patients receiving immune checkpoint inhibitors (ICIs): Antibiotic pretreatment correlated with Bifidobacteriaceae depletion and reduced ICI efficacy, while Butyricicoccaceae abundance predicted first-line therapy response. Akkermansia muciniphila enrichment associated with favorable treatment outcomes, and Firmicutes predominance correlated with prolonged progression-free survival (58). The overlapping gut microbiota profile between HIV infection and LC progression features Proteobacteria/Ruminococcus expansion and Bacteroidetes/Bifidobacterium depletion, with post-treatment Akkermansia resurgence suggesting conserved microbial remodeling patterns. However, mechanistic elucidation of gut-lung axis interactions in HIV-associated pulmonary carcinogenesis requires further investigation.
4 Metabolic regulatory networks of dysbiosis in HIV/AIDS gut microbiota
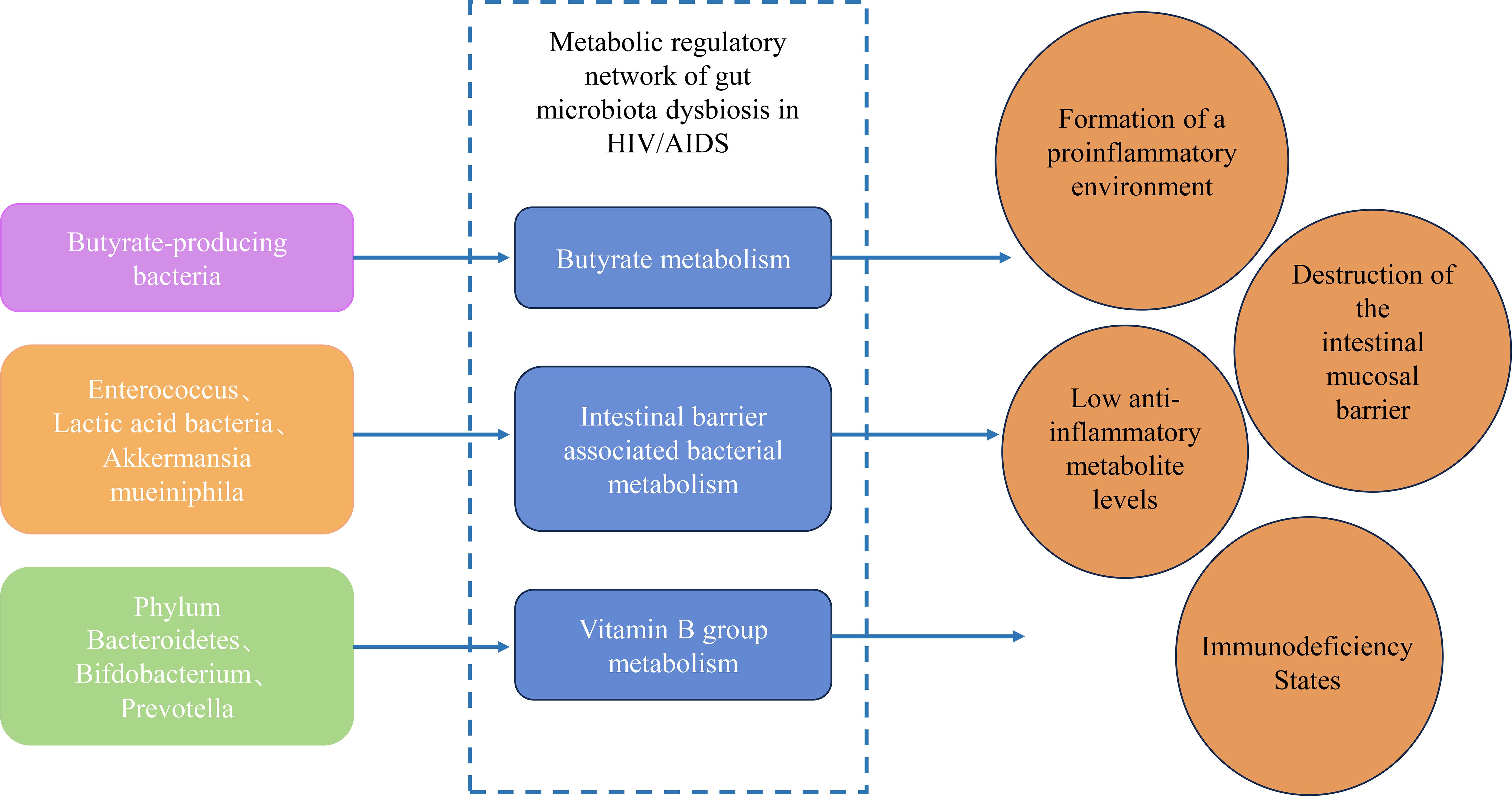
The gut microbiota in HIV/AIDS undergoes profound compositional and functional shifts, disrupting key metabolic pathways that govern immune homeostasis, barrier integrity, and anti-tumor surveillance. These perturbations arise from the depletion of symbiotic taxa critical for nutrient synthesis and the expansion of pathobionts that exacerbate inflammation. Central to this dysregulation are three interconnected metabolic axes: butyrate biosynthesis, gut barrier-associated bacterial metabolism, and vitamin B-dependent immunomodulation. Each axis contributes to a vicious cycle of microbial translocation, chronic inflammation, and immune exhaustion, creating a permissive milieu for oncogenesis (Figure 1).
4.1 Butyrate metabolism
HIV-associated gut dysbiosis significantly reduces butyrate-producing strains including Faecalibacterium prausnostzii, Lachnospira, and Roseburia, impairing butyrate biosynthesis (30). Butyrate originates primarily from microbial fermentation of undigested carbohydrates via glycolysis (EMP), pentose phosphate (HMP), and Enter-Doudoroff (ED) pathways, with additional production through acetate/lactate cross-feeding (59). Intestinal absorption occurs via H+-coupled (MCT1) and Na+-coupled (SMCT1) monocarboxylate transporters (49) (Supplementary Figure S1). This microbial metabolite exerts pleiotropic effects through three principal mechanisms:
Butyrate has immunomodulatory, anti-inflammatory and anti-tumor effects (60). Butyrate enhances regulatory B cell (Breg) immunosuppression by upregulating 5-hydroxyindole-3-acetic acid (5-HIAA) via Bifidobacterium-mediated tryptophan metabolism, activating aryl hydrocarbon receptor (AhR) and IL-10 signaling (61). Butyrate can also promote the proliferation and activation of regulatory T cells (Treg cells) and plays an important role in immune regulation (62). Additionally, butyrate can modulate the activity of inflammation-related pathways such as G protein-coupled receptors (GPRs), NF-κB, and JAK/STAT, thereby reducing the release of pro-inflammatory cytokines, inhibiting intestinal inflammatory responses, and maintaining intestinal immune balance (63). Concurrently, it induces dendritic cell (DC) expression of IDO1 and Aldh1A2 through histone deacetylase inhibition (HDACi), promoting FoxP3+ Treg differentiation while suppressing IFN-γ+ proinflammatory T cells (64). Butyrate stabilizes intestinal barrier function via HIF-1 activation in epithelial cells (65) and modulates NF-κB signaling to suppress TNF-α/IL-6 production (66). G-protein coupled receptor activation (GPR41/FFAR3, GPR109A/HCAR2) further enhances mucosal immunity through IgA secretion during inflammation (67).
In terms of anti-tumor activity, normal gut epithelial cells derive energy primarily from butyrate β-oxidation, whereas tumor cells rely on glycolytic metabolism—a metabolic shift termed the “Warburg effect” that supports rapid proliferation in hypoxic microenvironments (68, 69). Exploiting the Warburg effect, butyrate accumulates in tumor cells due to their preferential glycolysis over oxidative metabolism. This HDAC inhibition induces chromatin remodeling, cell cycle arrest, and apoptosis - mechanisms demonstrated to overcome sorafenib resistance in hepatocellular carcinoma (70). It is hypothesized that HIV-induced butyrate deficiency establishes a pathogenic triad of intestinal barrier disruption, chronic inflammation, and immune dysfunction, creating a permissive microenvironment for tumorigenesis and metastasis.
4.2 Gut barrier-associated bacterial metabolism
HIV-associated gut dysbiosis is characterized by enrichment of Enterococcus and lactic acid bacteria (Lactobacillus) alongside depletion of Akkermansia muciniphila (Akk), collectively contributing to mucosal barrier compromise and microbial translocation. This altered microbial consortium demonstrates enhanced L-tryptophan metabolic activity, with Enterococcus and Lactobacillus abundance positively correlating with intestinal tryptophan levels (71) (Supplementary Figure S2). Tryptophan catabolism proceeds through three primary routes: (i) IDO1-mediated conversion to kynurenine (KP) in immune/gut epithelial cells, suppressing TH17 differentiation and reducing IL-17/IL-22 production to impair mucosal repair (72); (ii) bacterial tryptophanase-dependent generation of AhR/PXR-activating indole derivatives modulating barrier integrity (73) and (iii) chromaffin cell serotonin synthesis via tryptophan hydroxylase 1 (TPH1), the rate-limiting enzyme catalyzing tryptophan hydroxylation to 5-hydroxytryptophan (5-HTP), which is subsequently decarboxylated to form 5-HT (74).
Enterococcus expansion in HIV infection drives phenylethylamine overproduction, directly inducing epithelial shedding (30) and potentiating microbial translocation (75). Concurrent akkermansia depletion may potentially disrupt critical protective mechanisms. One possible mechanism involves TLR2-mediated AMPK activation, which could enhance tight junction assembly (ZO-1/claudin-2) through the regulation of CDX2. Additionally, inhibition of NF-κB might lead to a reduction in pro-inflammatory cytokines (such as TNF-α and IL-6), while potentially elevating anti-inflammatory mediators like TGF-β and IL-10 (76) (Supplementary Figure S3). Furthermore, it is hypothesized that Akkermansia’s metabolic symbiosis with butyrate producers may be compromised by the loss of mucin-derived acetate/propionate and nutrient supply (30, 77). These alterations—potential overgrowth of pathobionts and depletion of sentinel species—may contribute to a microenvironment conducive to tumorigenesis, potentially through sustained barrier dysfunction and dysregulation of inflammatory-immune responses.
4.3 Vitamin B metabolism
HIV-associated gut dysbiosis disrupts vitamin B metabolism, critically impairing anti-inflammatory and immunoregulatory functions. Vitamin B1 (thiamine) serves as an essential cofactor in glycolysis, TCA cycle, and pentose phosphate pathways (78). Bacteroides species synthesize thiamine pyrophosphate (TPP) through dedicated transporters (79), with HIV-induced Bacteroides depletion correlating with reduced Peyer’s patch B-cell follicle development and compromised intestinal immunity (80). Vitamin B3 (niacin/nicotinamide) modulates redox balance via NAD metabolism (81), where deficiency associates with decreased gut microbiota α-diversity and Bacteroidetes depletion (82). Niacin exerts anti-inflammatory effects through PGD2/DP1-mediated vascular stabilization and IL-8 suppression (83), while restoring LPS/IL-1β-induced metabolic disturbances in isoleucine and glutamine pathways (84). Vitamin B6 exists as six vitamers, with pyridoxal phosphate (PLP) constituting the bioactive form. Gut Bacteroides fragilis and Bifidobacterium longum mediate its synthesis, while deficiency promotes Prevotella expansion (79). B6 deficiency disrupts Th1/Th2 balance, reducing IL-2 while elevating IL-4/IL-5/IL-10, impairing T-cell immunity (85). PLP additionally functions as a kynurenine pathway cofactor with tumor-suppressive potential (86). Clinical evidence from 44 HCC and 28 decompensated cirrhosis patients demonstrates impaired hepatic B6 metabolism correlating with disease progression (87). Murine models reveal vitamin B6 supplementation alleviates CCl4-induced hepatocyte damage and inflammation, while pyridoxine (PN) preserves intestinal barrier integrity by inhibiting advanced glycation end products (88). In non-HIV systems, depletion of Bacteroidetes/Bifidobacterium and Prevotella enrichment correlate with vitamin B deficiency states that foster pro-inflammatory microenvironments. In the context of HIV, synergistic dysbiosis may potentially amplify such deficiencies, creating a hypothesized metabolic-immune milieu conducive to tumorigenesis. However, direct evidence linking HIV-specific vitamin B deficiency to oncogenesis remains limited and warrants mechanistic validation.
5 Discussion
The gastrointestinal tract constitutes HIV’s primary reservoir, sustaining viral replication and progressive mucosal damage despite antiretroviral therapy (ART). Persistent gut microbiota dysbiosis and unresolved epithelial barrier defects perpetuate systemic immune activation and chronic inflammation, establishing a tumor-permissive microenvironment characterized by immunosuppression and metabolic dysregulation. Among HIV-associated malignancies, non-Hodgkin lymphoma (DLBCL), cervical carcinoma, hepatocellular carcinoma (HCC), and non-small cell lung cancer (NSCLC) exhibit conserved gut microbial signatures: depletion of anti-inflammatory taxa (Bacteroidetes, Bifidobacterium, butyrate producers) alongside expansion of pathogenic genera (Proteobacteria, Shigella, Enterococcus). These alterations disrupt microbial antagonism and niche protection, mirroring dysbiosis patterns observed in HIV infection.
Metabolic network analysis reveals three interconnected pathways linking HIV-induced dysbiosis to oncogenesis: (i) butyrate deficiency impairing epithelial energy metabolism and anti-tumor surveillance; (ii) tryptophan metabolism dysregulation compromising gut barrier integrity; (iii) vitamin B biosynthesis defects disrupting DNA repair and immune homeostasis. Synergistically, these perturbations activate NF-κB/STAT3 signaling, generating a pro-inflammatory cytokine milieu (IL-6, TNF-α, IL-1β) that promotes tumor progression through autocrine-paracrine cascades.
Emerging microbiota-directed interventions demonstrate therapeutic potential. Bifidobacterium pseudolongum, through its metabolite acetate, activates the GPR43 receptor and inhibits the activity of the IL-6/STAT3 signaling pathway via this mechanism. The IL-6/STAT3 pathway plays a role in promoting tumor growth and metastasis in various cancers, and thus its inhibition may slow the progression of HCC (89). PD-1/PD-L1 inhibitors are important tools in current cancer immunotherapy, capable of restoring the immune system’s attack on cancer cells by preventing tumor immune evasion. The co-administration of probiotics can modulate the gut microbiota, thereby enhancing the efficacy of PD-1/PD-L1 inhibitors and boosting the function of immune cells within the gut, significantly prolonging the survival of NSCLC patients (90). Although HCC and NSCLC share certain commonalities in tumorigenesis and immune regulation mechanisms, such as inflammation-driven tumorigenesis and immune evasion, they also exhibit distinct differences in specific molecular mechanisms. Further research is needed to elucidate the specific roles of these mechanisms. Butyrate supplementation reverses cisplatin resistance in cervical cancer by inhibiting epithelial-mesenchymal transition (91). Despite these advances, clinical translation requires rigorous validation through multicenter trials.
Collectively, HIV-associated gut dysbiosis fosters tumorigenesis through tripartite mechanisms: immunodeficiency potentiation, mucosal barrier disruption, and metabolic-immune crosstalk dysregulation. The conserved microbial and metabolic signatures across malignancies provide actionable biomarkers for cancer risk stratification and therapeutic targeting in HIV-associated oncogenesis.
6 Future perspective
This review delineates conserved microbial signatures across HIV infection and associated malignancies, revealing fundamental interconnections between gut dysbiosis and oncogenesis. Our analysis discusses three cardinal microbial-metabolic disturbances driving tumor progression in immunocompromised hosts: butyrate biosynthesis collapse, intestinal barrier disintegration, and vitamin B metabolism disruption. These pathological alterations synergistically activate NF-κB/STAT3 signaling cascades, generating a self-perpetuating inflammatory milieu characterized by IL-6/TNF-α/IL-1β overproduction that facilitates tumor immune evasion and metastatic progression. The convergent depletion of immunomodulatory taxa (Bacteroidetes, Bifidobacterium) alongside expansion of pro-inflammatory genera (Proteobacteria, Enterococcus) establishes a microbial profile predictive of cancer risk in HIV patients, transcending specific malignancy types.
Emerging therapeutic strategies targeting microbial metabolites demonstrate clinical potential yet require rigorous validation. Probiotic interventions and metabolite supplementation show capacity to restore gut-liver axis homeostasis in HCC, enhance checkpoint inhibitor efficacy in NSCLC, and reverse chemotherapy resistance in cervical carcinoma. However, critical knowledge gaps persist regarding causal microbiota-tumor relationships and temporal dynamics of dysbiosis progression. Current limitations include insufficient longitudinal cohort data tracking microbial changes pre-/post-malignancy development, and inadequate mechanistic models validating metabolite-mediated oncogenic pathways. Notably, two AIDS-defining cancers (Kaposi sarcoma, anal carcinoma) lack established microbiota associations, warranting dedicated investigation to determine potential gut-virome interactions.
Future research directions should prioritize: 1) Multi-omics longitudinal studies correlating microbial shifts with tumorigenesis timelines; 2) Organoid models elucidating metabolite-mediated epigenetic regulation; 3) Clinical trials evaluating microbiota modulation as adjuvant therapy. Particular emphasis should address LMIC-specific challenges including enteric pathogen co-infections and nutritional deficiencies that exacerbate HIV-related dysbiosis. By resolving these scientific and translational barriers, we may develop microbiota-based biomarkers for early cancer detection and personalized therapeutic regimens tailored to HIV-associated oncogenesis mechanisms.
Author contributions
QM: Conceptualization, Writing – original draft, Writing – review & editing. LX: Investigation, Writing – original draft, Writing – review & editing. FX: Writing – original draft, Writing – review & editing. XS: Writing – original draft, Writing – review & editing. JY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation Project (U1604287), Key Research and Development Promotion Special Project of Henan Province(232102311222), and Special Research Project on Traditional Chinese Medicine Science in Henan Province(2023ZXZX1057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1634388/full#supplementary-material
Supplementary Figure 1 | Butyrate metabolism.
Supplementary Figure 2 | Tryptophan metabolism.
Supplementary Figure 3 | Akkermansia muciniphila biological function.
References
1. Kirk SE, Young C, Berry H, Hanson R, Moreland A, Fonner V, et al. Comparison of at-home versus in-clinic receipt of long-acting injectable cabotegravir/rilpivirine. Clin Infect Dis. (2025) 80:613–7. doi: 10.1093/cid/ciae472
2. Fauci AS and Folkers GK. HIV/AIDS and COVID-19: shared lessons from 2 pandemics. Clin Infect Dis. (2025) 80:1074–9. doi: 10.1093/cid/ciae585
3. Gao TY, Zhao LK, Liu X, Li HY, Ma YT, Fang W, et al. Disease burden of AIDS in last 30-year period and its predicted level in next 25-years based on the global burden disease 2019. BMC Public Health. (2024) 24:2384. doi: 10.1186/s12889-024-19934-4
4. Njunge JM and Walson JL. Microbiota and growth among infants and children in low-income and middle-income settings. Curr Opin Clin Nutr Metab Care. (2023) 26:245–52. doi: 10.1097/mco.0000000000000927
5. Radich JP, Briercheck E, Chiu DT, Menon MP, Sala Torra O, Yeung CCS, et al. Precision medicine in low- and middle-income countries. Annu Rev pathol. (2022) 17:387–402. doi: 10.1146/annurev-pathol-042320-034052
6. Walker AW and Hoyles L. Human microbiome myths and misconceptions. Nat Microbiol. (2023) 8:1392–6. doi: 10.1038/s41564-023-01426-7
7. Ley RE, Peterson DA, and Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. (2006) 124:837–48. doi: 10.1016/j.cell.2006.02.017
8. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U States A. (2004) 101:15718–23. doi: 10.1073/pnas.0407076101
9. Zhan Z, Liu W, Pan L, Bao Y, Yan Z, and Hong L. Overabundance of Veillonella parvula promotes intestinal inflammation by activating macrophages via LPS-TLR4 pathway. Cell Death discov. (2022) 8:251. doi: 10.1038/s41420-022-01015-3
10. Turner S, Raisley B, Roach K, Bajaña S, Munroe ME, James JA, et al. Gram-positive bacteria cell wall peptidoglycan polymers activate human dendritic cells to produce IL-23 and IL-1β and promote T(H)17 cell differentiation. Microorganisms. (2023) 11(1):173. doi: 10.3390/microorganisms11010173
11. Gül E, Enz U, Maurer L, Abi Younes A, Fattinger SA, Nguyen BD, et al. Intraluminal neutrophils limit epithelium damage by reducing pathogen assault on intestinal epithelial cells during Salmonella gut infection. PloS pathogens. (2023) 19:e1011235. doi: 10.1371/journal.ppat.1011235
12. Goedert JJ. The microbiota and HIV: shedding light on dark matters. AIDS (London England). (2017) 31:863–5. doi: 10.1097/qad.0000000000001410
13. McGinty T, Mirmonsef P, Mallon PW, and Landay AL. Does systemic inflammation and immune activation contribute to fracture risk in HIV? Curr Opin HIV AIDS. (2016) 11:253–60. doi: 10.1097/coh.0000000000000275
14. Sereti I, Verburgh ML, Gifford J, Lo A, Boyd A, Verheij E, et al. Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. (2023) 42:113336. doi: 10.1016/j.celrep.2023.113336
15. Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS (London England). (2015) 29:2409–18. doi: 10.1097/qad.0000000000000869
16. Liu P, Liu Z, Wang J, Wang J, Gao M, Zhang Y, et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
17. Gebrayel P, Nicco C, Al Khodor S, Bilinski J, Caselli E, Comelli EM, et al. Microbiota medicine: towards clinical revolution. J Trans Med. (2022) 20:111. doi: 10.1186/s12967-022-03296-9
18. Moreno E, Ron R, and Serrano-Villar S. The microbiota as a modulator of mucosal inflammation and HIV/HPV pathogenesis: From association to causation. Front Immunol. (2023) 14:1072655. doi: 10.3389/fimmu.2023.1072655
19. Hatano Y, Ideta T, Hirata A, Hatano K, Tomita H, Okada H, et al. Virus-driven carcinogenesis. Cancers. (2021) 13(11):2625. doi: 10.3390/cancers13112625
20. Szychowiak P, Boulain T, Timsit JF, Elabbadi A, Argaud L, Ehrmann S, et al. Clinical spectrum and prognostic impact of cancer in critically ill patients with HIV: a multicentre cohort study. Ann Intensive Care. (2023) 13:74. doi: 10.1186/s13613-023-01171-4
21. Shiels MS and Engels EA. Evolving epidemiology of HIV-associated Malignancies. Curr Opin HIV AIDS. (2017) 12:6–11. doi: 10.1097/coh.0000000000000327
22. Engels EA. Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am J Transplant. (2019) 19:3223–32. doi: 10.1111/ajt.15495
23. Tursiella ML, Bowman ER, Wanzeck KC, Throm RE, Liao J, Zhu J, et al. Epstein-Barr virus nuclear antigen 3A promotes cellular proliferation by repression of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. PloS pathogens. (2014) 10:e1004415. doi: 10.1371/journal.ppat.1004415
24. Yang X, Da M, Zhang W, Qi Q, Zhang C, and Han S. Role of Lactobacillus in cervical cancer. Cancer Manage Res. (2018) 10:1219–29. doi: 10.2147/cmar.S165228
25. Galasso L, Termite F, Mignini I, Esposto G, Borriello R, Vitale F, et al. Unraveling the role of fusobacterium nucleatum in colorectal cancer: molecular mechanisms and pathogenic insights. Cancers. (2025) 17(3):368. doi: 10.3390/cancers17030368
26. Niekamp P and Kim CH. Microbial metabolite dysbiosis and colorectal cancer. Gut liver. (2023) 17:190–203. doi: 10.5009/gnl220260
27. Yang X, Su B, Zhang X, Liu Y, Wu H, and Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J leukocyte Biol. (2020) 107:597–612. doi: 10.1002/jlb.4mr1019-189r
28. Geng ST, Zhang ZY, Wang YX, Lu D, Yu J, Zhang JB, et al. Regulation of gut microbiota on immune reconstitution in patients with acquired immunodeficiency syndrome. Front Microbiol. (2020) 11:594820. doi: 10.3389/fmicb.2020.594820
29. Li S, Su B, Wu H, He Q, and Zhang T. Integrated analysis of gut and oral microbiome in men who have sex with men with HIV Infection. Microbiol spectrum. (2023) 11:e0106423. doi: 10.1128/spectrum.01064-23
30. Zhang Y, Xie Z, Zhou J, Li Y, Ning C, Su Q, et al. The altered metabolites contributed by dysbiosis of gut microbiota are associated with microbial translocation and immune activation during HIV infection. Front Immunol. (2022) 13:1020822. doi: 10.3389/fimmu.2022.1020822
31. Xie Y, Sun J, Wei L, Jiang H, Hu C, Yang J, et al. Altered gut microbiota correlate with different immune responses to HAART in HIV-infected individuals. BMC Microbiol. (2021) 21:11. doi: 10.1186/s12866-020-02074-1
32. Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, et al. The bacterium akkermansia muciniphila: A sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol. (2020) 11:645. doi: 10.3389/fimmu.2020.00645
33. Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. (2013) 7:949–61. doi: 10.1038/ismej.2012.158
34. Noy A. HIV lymphoma and burkitts lymphoma. Cancer J (Sudbury Mass). (2020) 26:260–8. doi: 10.1097/ppo.0000000000000448
35. Omar A, Marques N, and Crawford N. Cancer and HIV: the molecular mechanisms of the deadly duo. Cancers. (2024) 16(3):546. doi: 10.3390/cancers16030546
36. Al-Khazaleh AK, Chang D, Münch GW, and Bhuyan DJ. The gut connection: exploring the possibility of implementing gut microbial metabolites in lymphoma treatment. Cancers. (2024) 16(8):1464. doi: 10.3390/cancers16081464
37. Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN guidelines insights: B-cell lymphomas, version 3.2019. J Natl Compr Cancer Network: JNCCN. (2019) 17:650–61. doi: 10.6004/jnccn.2019.0029
38. Masnikosa R, Cvetković Z, and Pirić D. Tumor biology hides novel therapeutic approaches to diffuse large B-cell lymphoma: A narrative review. Int J Mol Sci. (2024) 25(21):11384. doi: 10.3390/ijms252111384
39. Yuan L, Wang W, Zhang W, Zhang Y, Wei C, Li J, et al. Gut microbiota in untreated diffuse large B cell lymphoma patients. Front Microbiol. (2021) 12:646361. doi: 10.3389/fmicb.2021.646361
40. Lin Z, Mao D, Jin C, Wang J, Lai Y, Zhang Y, et al. The gut microbiota correlate with the disease characteristics and immune status of patients with untreated diffuse large B-cell lymphoma. Front Immunol. (2023) 14:1105293. doi: 10.3389/fimmu.2023.1105293
41. Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Global Health. (2021) 9:e161–e9. doi: 10.1016/s2214-109x(20)30459-9
42. Ibrahim Khalil A, Mpunga T, Wei F, Baussano I, de Martel C, Bray F, et al. Age-specific burden of cervical cancer associated with HIV: A global analysis with a focus on sub-Saharan Africa. Int J cancer. (2022) 150:761–72. doi: 10.1002/ijc.33841
43. Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res (Amsterdam Netherlands). (2019) 8:100174. doi: 10.1016/j.pvr.2019.100174
44. Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet (London England). (2021) 398:2084–92. doi: 10.1016/s0140-6736(21)02178-4
45. Chang L, Qiu L, Lei N, Zhou J, Guo R, Gao F, et al. Characterization of fecal microbiota in cervical cancer patients associated with tumor stage and prognosis. Front Cell infection Microbiol. (2023) 13:1145950. doi: 10.3389/fcimb.2023.1145950
46. Sims TT, Colbert LE, Zheng J, Delgado Medrano AY, Hoffman KL, Ramondetta L, et al. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecologic Oncol. (2019) 155:237–44. doi: 10.1016/j.ygyno.2019.09.002
47. Sims TT, El Alam MB, Karpinets TV, Dorta-Estremera S, Hegde VL, Nookala S, et al. Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation. Commun Biol. (2021) 4:237. doi: 10.1038/s42003-021-01741-x
48. Goyal G, Enogela EM, Burkholder GA, Kitahata MM, Crane HM, Eulo V, et al. Burden of chronic health conditions among people with HIV and common non-AIDS-defining cancers. J Natl Compr Cancer Network: JNCCN. (2025) 23(7):e257018. doi: 10.6004/jnccn.2025.7018
49. Chiao EY, Coghill A, Kizub D, Fink V, Ndlovu N, Mazul A, et al. The effect of non-AIDS-defining cancers on people living with HIV. Lancet Oncol. (2021) 22:e240–e53. doi: 10.1016/s1470-2045(21)00137-6
50. Rajapakse J, Khatiwada S, Akon AC, Yu KL, Shen S, and Zekry A. Unveiling the complex relationship between gut microbiota and liver cancer: opportunities for novel therapeutic interventions. Gut Microbes. (2023) 15:2240031. doi: 10.1080/19490976.2023.2240031
51. Zhang N, Gou Y, Liang S, Chen N, Liu Y, He Q, et al. Dysbiosis of gut microbiota promotes hepatocellular carcinoma progression by regulating the immune response. J Immunol Res. (2021) 2021:4973589. doi: 10.1155/2021/4973589
52. Yan F, Zhang Q, Shi K, Zhang Y, Zhu B, Bi Y, et al. Gut microbiota dysbiosis with hepatitis B virus liver disease and association with immune response. Front Cell infection Microbiol. (2023) 13:1152987. doi: 10.3389/fcimb.2023.1152987
53. Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS (London England). (2012) 26:1017–25. doi: 10.1097/QAD.0b013e328352d1ad
54. Konstantinidis I, Crothers K, Kunisaki KM, Drummond MB, Benfield T, Zar HJ, et al. HIV-associated lung disease. Nat Rev Dis primers. (2023) 9:39. doi: 10.1038/s41572-023-00450-5
55. Cribbs SK, Crothers K, and Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. (2020) 100:603–32. doi: 10.1152/physrev.00039.2018
56. Zheng Y, Fang Z, Xue Y, Zhang J, Zhu J, Gao R, et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. (2020) 11:1030–42. doi: 10.1080/19490976.2020.1737487
57. Tesolato S, Vicente-Valor J, Paz-Cabezas M, Gómez-Garre D, Sánchez-González S, Ortega-Hernández A, et al. Gut microbiota signatures with potential clinical usefulness in colorectal and non-small cell lung cancers. Biomedicines. (2024) 12(3):703. doi: 10.3390/biomedicines12030703
58. Grenda A, Iwan E, Krawczyk P, Frąk M, Chmielewska I, Bomba A, et al. Attempting to identify bacterial allies in immunotherapy of NSCLC patients. Cancers. (2022) 14(24):6250. doi: 10.3390/cancers14246250
59. Bridgeman SC, Northrop W, Melton PE, Ellison GC, Newsholme P, and Mamotte CDS. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol Res. (2020) 160:105174. doi: 10.1016/j.phrs.2020.105174
60. Kang J, Sun M, Chang Y, Chen H, Zhang J, Liang X, et al. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anti-cancer Drugs. (2023) 34:227–37. doi: 10.1097/cad.0000000000001413
61. Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. (2020) 31:837–51.e10. doi: 10.1016/j.cmet.2020.03.003
62. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
63. Mathew OP, Ranganna K, and Yatsu FM. Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells. Biomed pharmacother = Biomed pharmacotherapie. (2010) 64:733–40. doi: 10.1016/j.biopha.2010.09.017
64. Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, and Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. (2002) 130:245–55. doi: 10.1046/j.0009-9104.2002.01977.x
65. Fachi JL, Felipe JS, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. (2019) 27:750–61.e7. doi: 10.1016/j.celrep.2019.03.054
66. Recharla N, Geesala R, and Shi XZ. Gut microbial metabolite butyrate and its therapeutic role in inflammatory bowel disease: A literature review. Nutrients. (2023) 15(10):2275. doi: 10.3390/nu15102275
67. Isobe J, Maeda S, Obata Y, Iizuka K, Nakamura Y, Fujimura Y, et al. Commensal-bacteria-derived butyrate promotes the T-cell-independent IgA response in the colon. Int Immunol. (2020) 32:243–58. doi: 10.1093/intimm/dxz078
68. Schwartz L, Supuran CT, and Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anti-cancer Agents medicinal Chem. (2017) 17:164–70. doi: 10.2174/1871520616666161031143301
69. Manches O, Frleta D, and Bhardwaj N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. (2014) 35:114–22. doi: 10.1016/j.it.2013.10.003
70. Kumar M, Kaur R, Kanthaje S, Dhiman RK, and Chakraborti A. Bacterial metabolite butyrate in modulating sorafenib-targeted microRNAs to curtail its resistance in hepatocellular carcinoma. J Cancer Res Clin Oncol. (2023) 149:5823–39. doi: 10.1007/s00432-022-04544-7
71. Iacob S and Iacob DG. Infectious threats, the intestinal barrier, and its trojan horse: dysbiosis. Front Microbiol. (2019) 10:1676. doi: 10.3389/fmicb.2019.01676
72. Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. (2019) 10:2113. doi: 10.3389/fimmu.2019.02113
73. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell infection Microbiol. (2018) 8:13. doi: 10.3389/fcimb.2018.00013
74. Tabolacci C, Caruso A, Micai M, Galati G, Lintas C, Pisanu ME, et al. Biogenic amine metabolism and its genetic variations in autism spectrum disorder: A comprehensive overview. Biomolecules. (2025) 15(4):539. doi: 10.3390/biom15040539
75. Li Y, Yan T, Yin L, Cheng Y, and Jia X. Isolation and identification of tyramine-producing bacteria and their biogenic amines formation during fermentation of sufu. Cell Mol Biol (Noisy-le-Grand France). (2022) 68:75–88. doi: 10.14715/cmb/2022.68.1.11
76. Shi M, Yue Y, Ma C, Dong L, and Chen F. Pasteurized Akkermansia muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients. (2022) 14(4):764. doi: 10.3390/nu14040764
77. Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B(12) production by intestinal symbionts. mBio. (2017) 8(5):e00770-17. doi: 10.1128/mBio.00770-17
78. Manzetti S, Zhang J, and van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry. (2014) 53:821–35. doi: 10.1021/bi401618y
79. Hossain KS, Amarasena S, and Mayengbam S. B vitamins and their roles in gut health. Microorganisms. (2022) 10:1168. doi: 10.3390/microorganisms10061168
80. Kunisawa J, Sugiura Y, Wake T, Nagatake T, Suzuki H, Nagasawa R, et al. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. (2015) 13(6):122–31. doi: 10.1016/j.celrep.2015.08.063
81. Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, et al. Vitamin C and B(3) as new biomaterials to alter intestinal stem cells. J Biomed materials Res Part A. (2019) 107:1886–97. doi: 10.1002/jbm.a.36715
82. Fangmann D, Theismann EM, Türk K, Schulte DM, Relling I, Hartmann K, et al. Targeted microbiome intervention by microencapsulated delayed-release niacin beneficially affects insulin sensitivity in humans. Diabetes Care. (2018) 41:398–405. doi: 10.2337/dc17-1967
83. Li J, Kong D, Wang Q, Wu W, Tang Y, Bai T, et al. Niacin ameliorates ulcerative colitis via prostaglandin D(2)-mediated D prostanoid receptor 1 activation. EMBO Mol Med. (2017) 9:571–88. doi: 10.15252/emmm.201606987
84. Santoru ML, Piras C, Murgia F, Spada M, Tronci L, Leoni VP, et al. Modulatory effect of nicotinic acid on the metabolism of caco-2 cells exposed to IL-1β and LPS. Metabolites. (2020) 10(5):204. doi: 10.3390/metabo10050204
85. Elmadfa I and Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocrine Metab Immune Disord Drug targets. (2019) 19:1100–15. doi: 10.2174/1871530319666190529101816
86. Stach K, Stach W, and Augoff K. Vitamin B6 in health and disease. Nutrients. (2021) 13(9):3229. doi: 10.3390/nu13093229
87. Mei M, Liu D, Tang X, You Y, Peng B, He X, et al. Vitamin B6 metabolic pathway is involved in the pathogenesis of liver diseases via multi-omics analysis. J hepatocellular carcinoma. (2022) 9:729–50. doi: 10.2147/jhc.S370255
88. Kamphuis JBJ, Reber L, Eutamène H, and Theodorou V. Increased fermentable carbohydrate intake alters colonic mucus barrier function through glycation processes and increased mast cell counts. FASEB J. (2022) 36:e22297. doi: 10.1096/fj.202100494RRR
89. Song Q, Zhang X, Liu W, Wei H, Liang W, Zhou Y, et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J hepatol. (2023) 79:1352–65. doi: 10.1016/j.jhep.2023.07.005
90. Shah H and Ng TL. A narrative review from gut to lungs: non-small cell lung cancer and the gastrointestinal microbiome. Trans Lung Cancer Res. (2023) 12:909–26. doi: 10.21037/tlcr-22-595
Keywords: HIV, gut microbiota, metabolite, tumor, immune
Citation: Meng Q, Xu L, Xu F, Shen X and Yue J (2025) HIV-associated gut dysbiosis drives oncogenesis through metabolic-immune crosstalk: mechanisms and therapeutic implications. Front. Oncol. 15:1634388. doi: 10.3389/fonc.2025.1634388
Received: 24 May 2025; Accepted: 04 August 2025;
Published: 21 August 2025.
Edited by:
Haobin Zhao, Weifang People’s Hospital, ChinaReviewed by:
Elena Moreno, Ramón y Cajal University Hospital, SpainCopyright © 2025 Meng, Xu, Xu, Shen and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyu Yue, eXVlamluZ3l1QGFsaXl1bi5jb20=
 Qingquan Meng
Qingquan Meng Liran Xu
Liran Xu Furong Xu
Furong Xu Xiaohan Shen
Xiaohan Shen Jingyu Yue
Jingyu Yue