Abstract
Background:
The aim of this study was to assess the real-world effectiveness and safety of first-line treatment with cadonilimab plus paclitaxel-platinum ± bevacizumab for persistent, recurrent, or metastatic cervical cancer (p/r/m CC).
Methods:
In this retrospective real-world study from Jiangsu Cancer Hospital (January 2021–February 2025), patients with p/r/m CC received first-line cadonilimab plus paclitaxel-platinum ± bevacizumab or paclitaxel-platinum ± bevacizumab. Co-primary endpoints were progression-free survival (PFS) and safety; overall survival (OS), objective response rate (ORR), and disease control rate (DCR) were secondary. Kaplan–Meier and log-rank methods were applied, with prognostic factors analyzed using Cox models.
Results:
Among 169 eligible patients (50 cadonilimab plus TP; 119 TP), median follow-up was 33.2 months [interquartile range (IQR): 12.2–35.2]. Cadonilimab addition significantly prolonged mPFS [20.2 vs. 12.2 months; hazard ratio (HR): 0.531, p = 0.019], with 12- and 24-month PFS rates of 65.83% and 48.62% versus 50.71% and 29.57%, respectively. ORR improved from 40.3% to 54.0%, while DCR remained high in both cohorts (92.0% vs. 90.8%). mOS was not reached in the cadonilimab plus TP group and was 37.5 months with TP alone. Cadonilimab increased low-grade immune-related or gastrointestinal adverse events, with the most common being rash or itching (38.0%), pyrexia (32.0%), constipation (58.0%), and diarrhea (50.0%). However, events in grades 3–5 were infrequent. Subgroup analyses showed a generally consistent PFS benefit with cadonilimab across most predefined patient subsets.
Conclusions:
In real-world clinical settings, cadonilimab plus TP ± bevacizumab provides a durable PFS benefit with acceptable safety and supports first−line use for p/r/m CC; additional follow-up is essential to determine its impact on OS.
1 Introduction
Female-specific cancers such as breast, cervical, ovarian, and uterine cancer collectively account for approximately 40% of the overall cancer burden in women (1). Cervical cancer (CC) is one of the most common malignancies of the female reproductive system and ranks fourth globally in both incidence and mortality among women (2). Early-stage CC generally has a favorable prognosis; however, the 5-year survival rate for recurrent or metastatic cervical cancer (r/mCC) is less than 17% (3). Apart from platinum-based palliative chemotherapy, treatment options for patients with r/mCC remain limited and are primarily palliative in nature (4).
Based on the GOG-204 trial, cisplatin–paclitaxel has come to be recognized as the standard first-line regimen for r/m CC (5). The JCOG-0505 study further demonstrated that carboplatin plus paclitaxel may serve as an alternative for patients previously treated with or intolerant to cisplatin (6). Additionally, the GOG-240 study showed that adding bevacizumab to first-line chemotherapy confers a survival advantage (7). Consequently, the current standard first-line regimens typically consist of paclitaxel combined with either cisplatin or carboplatin ± bevacizumab (8). With the growing application of immunotherapy in CC, its indications have expanded from later-line to first-line settings. To date, three major phase III randomized controlled trials (RCTs) have reported results on first-line immunotherapy for r/m CC: KEYNOTE-826 (9), BEATcc (10), and AK104-303 (11).
Bispecific antibodies, engineered to concurrently engage dual immune checkpoints and/or signaling pathways within a single molecule, have emerged as a promising strategy to enhance synergistic antitumor activity. Beyond PD−1/CTLA−4 constructs, ivonescimab (AK112) co−targets PD−1 and VEGF−A, illustrating the rationale for cross−pathway bispecific antibodies (12). Similarly, Yi et al. (13) demonstrated that a PD−L1/TGF−β bispecific antibody can concurrently potentiate antitumor immunity and reprogram an immunosuppressive tumor microenvironment.
Cadonilimab is a first-in-class bispecific that simultaneously engages PD-1 and CTLA-4 and has been authorized in China for r/m CC after progression on platinum-based therapy (14). AK104-303, a phase III, randomized, double-blind, multicenter study conducted in China, assessed cadonilimab plus paclitaxel-platinum ± bevacizumab as initial systemic treatment for p/r/m CC. Adding cadonilimab to chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS), with acceptable tolerability: mPFS, 12.7 vs. 8.1 months [hazard ratio (HR) = 0.62; 95% confidence interval (CI): 0.49–0.80; p < 0.0001], and mOS, not reached vs. 22.8 months (HR = 0.64; 95% CI: 0.48–0.86; p = 0.0011).
While RCTs are designed with strict inclusion criteria and aim to minimize confounding by limiting non-study medications, they may not fully reflect real-world clinical practice (15, 16). Evidence regarding the real-world effectiveness, safety, maintenance strategies, and integration with radiotherapy of front-line immunotherapy remains limited. Recent reviews (17, 18) have underscored the need to complement randomized data with real-world outcomes to optimize patient selection and therapeutic decision-making. Moreover, real-world evidence regarding the use of cadonilimab in CC remains scarce, and its effectiveness and safety require validation in real-world clinical settings.
Therefore, we retrospectively assessed real-world effectiveness and safety outcomes of first-line treatment with cadonilimab plus paclitaxel-platinum ± bevacizumab for p/r/m CC.
2 Materials and methods
2.1 Patients
From January 2021 to February 2025, patients with p/r/m CC who received first-line cadonilimab plus paclitaxel-platinum ± bevacizumab or paclitaxel-platinum ± bevacizumab alone at the Department of Gynecologic Radiation Oncology, Jiangsu Cancer Hospital, were enrolled in the study.
The inclusion and exclusion criteria are summarized in Figure 1. Notably, cadonilimab was first approved for use in mainland China on 29 June 2022; therefore, all patients in the cadonilimab plus TP group were enrolled after this date.
Figure 1
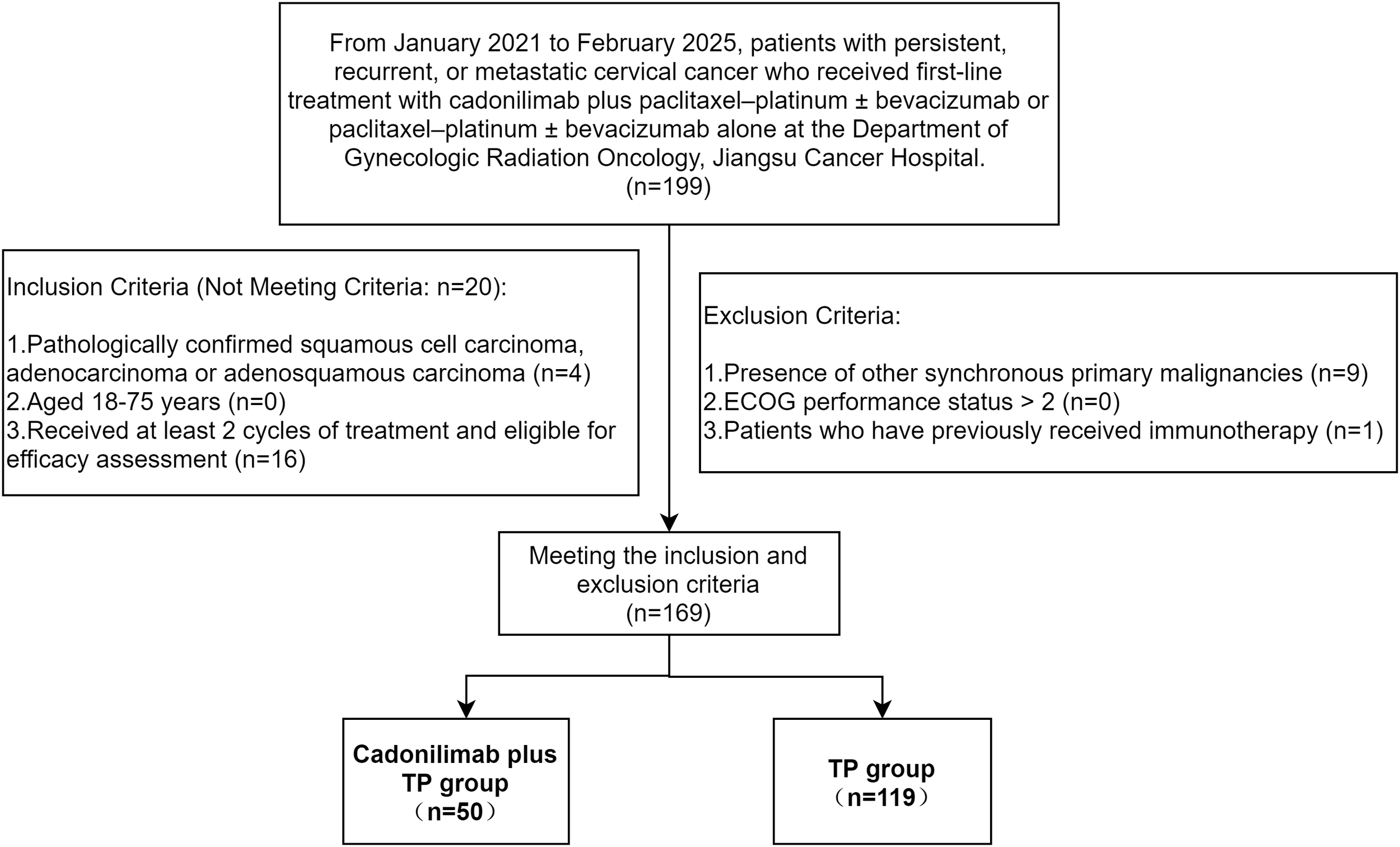
Flowchart of patient inclusion and exclusion criteria.
Following exclusion of 30 patients, 169 were analyzed. The Ethics Committee of Jiangsu Cancer Hospital approved the protocol (KY-2025-067) and waived informed consent due to the retrospective design.
2.2 Treatment protocol
One of the following formulations was administered: paclitaxel (135–175 mg/m²), paclitaxel liposome (135–175 mg/m²), or albumin-bound paclitaxel (260 mg/m²). When appropriate, docetaxel (60–75 mg/m²) was administered as a substitute. The taxane was combined with cisplatin (50 mg/m²) or, alternatively, with carboplatin (AUC 4–5), both given via intravenous infusion, q3w. This combination constituted the TP regimen, which was administered with or without concurrent bevacizumab (15 mg/kg, intravenous infusion, q3w). Chemotherapy dosages were adjusted as needed according to the patient’s general condition and treatment tolerance.
Cadonilimab (AK104; manufactured by Akeso Biopharma Inc., Zhongshan, China) was administered intravenously at a dose of 10 mg/kg, q3w in the cadonilimab plus TP group.
Radiotherapy was administered at the discretion of the treating radiation oncologist, with treatment parameters tailored based on lesion characteristics, including metastatic or recurrent site, tumor volume, and surrounding organ constraints.
Maintenance therapy strategies were guided by multidisciplinary consensus, considering radiographic response assessments and patient preference. These strategies encompassed a range of approaches, including immunotherapy monotherapy (either a PD-1 inhibitor or cadonilimab q3w), targeted therapy maintenance (bevacizumab q3w or oral anlotinib 8–12 mg on days 1–14 of each q3w cycle), and combination regimens involving both immunotherapy and targeted agents. The duration of maintenance therapy was individualized based on multiple factors, including disease stability, patient tolerance, clinician judgment, and patient preference.
2.3 Outcome assessment and safety evaluation
After treatment completion, patients underwent surveillance every 3 months during the initial 2 years, with final follow-up data censored in June 2025.
Response after two cycles was determined by pelvic magnetic resonance imaging (MRI; non-contrast- and contrast-enhanced). Chest and abdominal computed tomography (CT) scans with identical contrast enhancement protocols were also performed. Responses were CR (complete response)/PR (partial response)/SD (stable disease)/PD (progressive disease); objective response rate (ORR) = CR + PR and disease control rate (DCR) = CR + PR + SD (RECIST v1.1).
Additionally, in the cadonilimab plus TP group, the best overall confirmed response was evaluated. We generated a waterfall plot displaying each patient’s maximal percent change from baseline in the aggregate diameter of target lesions, with the corresponding confirmed best overall response (BOR) annotated. A swim-lane plot was constructed to illustrate the follow-up duration for each patient, including the time to first CR/PR/SD/PD, death, and last follow-up date. The background color of each bar denoted the confirmed BOR.
PFS was defined as the time from treatment initiation to either documented disease progression or death from any cause. OS was defined as the time from treatment initiation to death from any cause. Treatment-related adverse events (TRAEs) were graded by NCI-CTCAE v5.0 (19).
2.4 Statistical analysis
Analyses and figure generation were performed in SPSS (v29.0) and R (v4.5.1). Categorical data are presented as counts and percentages (n, %), while continuous measures are summarized as mean (SD). Between-group differences for categorical variables were assessed using Pearson’s χ² or Fisher’s exact test, as appropriate. Kaplan–Meier methods were used to plot survival, and groups were compared with the log-rank test. Cox proportional-hazards models were applied to explore prognostic factors for PFS and OS; covariates with p < 0.05 in univariable analyses were entered into multivariable models. All hypothesis tests were two-tailed, with p < 0.05 indicating statistical significance.
3 Results
3.1 Baseline characteristics
A total of 169 patients were included in the study, with 119 receiving first-line treatment with paclitaxel-platinum ± bevacizumab (TP group) and 50 receiving cadonilimab combined with paclitaxel-platinum ± bevacizumab (cadonilimab plus TP group). Baseline characteristics were comparable across groups (Table 1).
Table 1
| Characteristic | TP group (n = 119), N (%) | Cadonilimab plus TP group (n = 50), N (%) | χ 2 | p-value |
|---|---|---|---|---|
| Age | 0.002 | 0.961 | ||
| <65 | 102 (85.7) | 43 (86.0) | ||
| ≥65 | 17 (14.3) | 7 (14.0) | ||
| Diabetes | 1.529 | 0.216 | ||
| Yes | 21 (17.6) | 13 (26.0) | ||
| No | 98 (82.4) | 37 (74.0) | ||
| Hypertension | 2.840 | 0.092 | ||
| Yes | 32 (26.9) | 20 (40.0) | ||
| No | 87 (73.1) | 30 (60.0) | ||
| Pathological diagnosis | 0.017 | 0.898 | ||
| Squamous cell carcinoma | 99 (83.2) | 42 (84.0) | ||
| Adenocarcinoma | 20 (16.8) | 8 (16.0) | ||
| Histologic grade | 2.443 | 0.118 | ||
| 1, 2, x | 68 (57.1) | 22 (44.0) | ||
| 3 | 51 (42.9) | 28 (56.0) | ||
| ECOG PS | 1.253 | 0.534 | ||
| 0 | 28 (23.5) | 11 (22.0) | ||
| 1 | 67 (56.3) | 25 (50.0) | ||
| 2 | 24 (20.0) | 14 (28.0) | ||
| Disease status | 6.820 | 0.033 | ||
| Metastatic (FIGO 2018: IVB) | 38 (31.9) | 14 (28.0) | ||
| Persistent or recurrent with distant metastases | 56 (47.1) | 16 (32.0) | ||
| Persistent or recurrent without distant metastases | 25 (21.0) | 20 (40.0) | ||
| Recurrence-free interval | 0.016 | 0.900 | ||
| ≤1 year | 63 (52.9) | 27 (54.0) | ||
| >1 year | 56 (47.1) | 23 (46.0) | ||
| Lymph node metastasis | 0.061 | 0.805 | ||
| Yes | 81 (68.1) | 35 (70.0) | ||
| No | 38 (31.8) | 15 (30.0) | ||
| Lung metastasis | 3.046 | 0.081 | ||
| Yes | 37 (31.1) | 9 (18.0) | ||
| No | 82 (68.9) | 41 (82.0) | ||
| Pelvic cavity metastasis | 9.318 | 0.002 | ||
| Yes | 39 (32.8) | 29 (58.0) | ||
| No | 80 (67.2) | 21 (42.0) | ||
| Bone metastasis | 2.216 | 0.137 | ||
| Yes | 23 (19.3) | 5 (10.0) | ||
| No | 96 (80.7) | 45 (90.0) | ||
| Liver metastasis | —— | 1.000 | ||
| Yes | 7 (5.9) | 2 (4.0) | ||
| No | 112 (94.1) | 48 (96.0) | ||
| Metastatic region count | 0.512 | 0.474 | ||
| Single | 50 (42.0) | 24 (48.0) | ||
| Multiple | 69 (58.0) | 26 (52.0) | ||
| Bevacizumab use during the treatment | 0.981 | 0.322 | ||
| Yes | 45 (37.8) | 23 (46.0) | ||
| No | 74 (62.2) | 27 (54.0) | ||
| Radiotherapy for recurrence | 0.920 | 0.337 | ||
| Yes | 76 (63.9) | 28 (56.0) | ||
| No | 43 (36.1) | 22 (44.0) | ||
| Maintenance therapy | 23.129 | <0.001 | ||
| Yes | 26 (21.8) | 30 (60.0) | ||
| No | 93 (78.2) | 20 (40.0) |
Patient characteristics (N = 169).
For 2×2 contingency table variables with expected cell counts less than 5 (e.g., liver metastasis), Fisher’s exact test was used instead of the chi-square test, and the chi-square value was not reported.
Bold values are shown solely for visual clarity and do not carry any statistical implication.
A majority were younger than 65 years: 102 patients (85.7%) in the TP group and 43 (86.0%) in the cadonilimab plus TP group. ECOG PS 0–1 was documented in 95 (79.8%) patients in the TP group and 36 (72.0%) patients in the cadonilimab plus TP group. Squamous cell carcinoma was the predominant pathological subtype in both groups (83.2% vs. 84.0%).
A total of 38 patients in the TP group and 14 patients in the cadonilimab plus TP group were initially diagnosed with metastatic disease (FIGO 2018: IVB). Bevacizumab was administered to 45 patients (37.8%) in the TP group and 23 patients (46.0%) in the cadonilimab plus TP group. Additionally, 76 patients (63.9%) in the TP group and 28 patients (56.0%) in the cadonilimab plus TP group received regional radiotherapy targeting recurrent or metastatic lesions.
Relative to the TP group, the cadonilimab plus TP group showed a greater proportion of pelvic cavity metastasis (58.0% vs. 32.8%, p = 0.002) and was more likely to receive maintenance therapy (60.0% vs. 21.8%, p < 0.001).
3.2 Best overall response in the cadonilimab plus TP group
Within the cadonilimab plus TP group, patients received a median of 5.2 treatment cycles (range: 3–8). We conducted a dedicated analysis of the confirmed BOR.
Among the 50 evaluable patients, BOR is summarized in Figure 2. Six patients (12.0%) achieved a CR and 22 (44.0%) achieved a PR, yielding an ORR of 56.0%. A further 19 patients (38.0%) experienced SD, resulting in a DCR of 94.0%. PD as the best response was documented in three patients (6.0%).
Figure 2
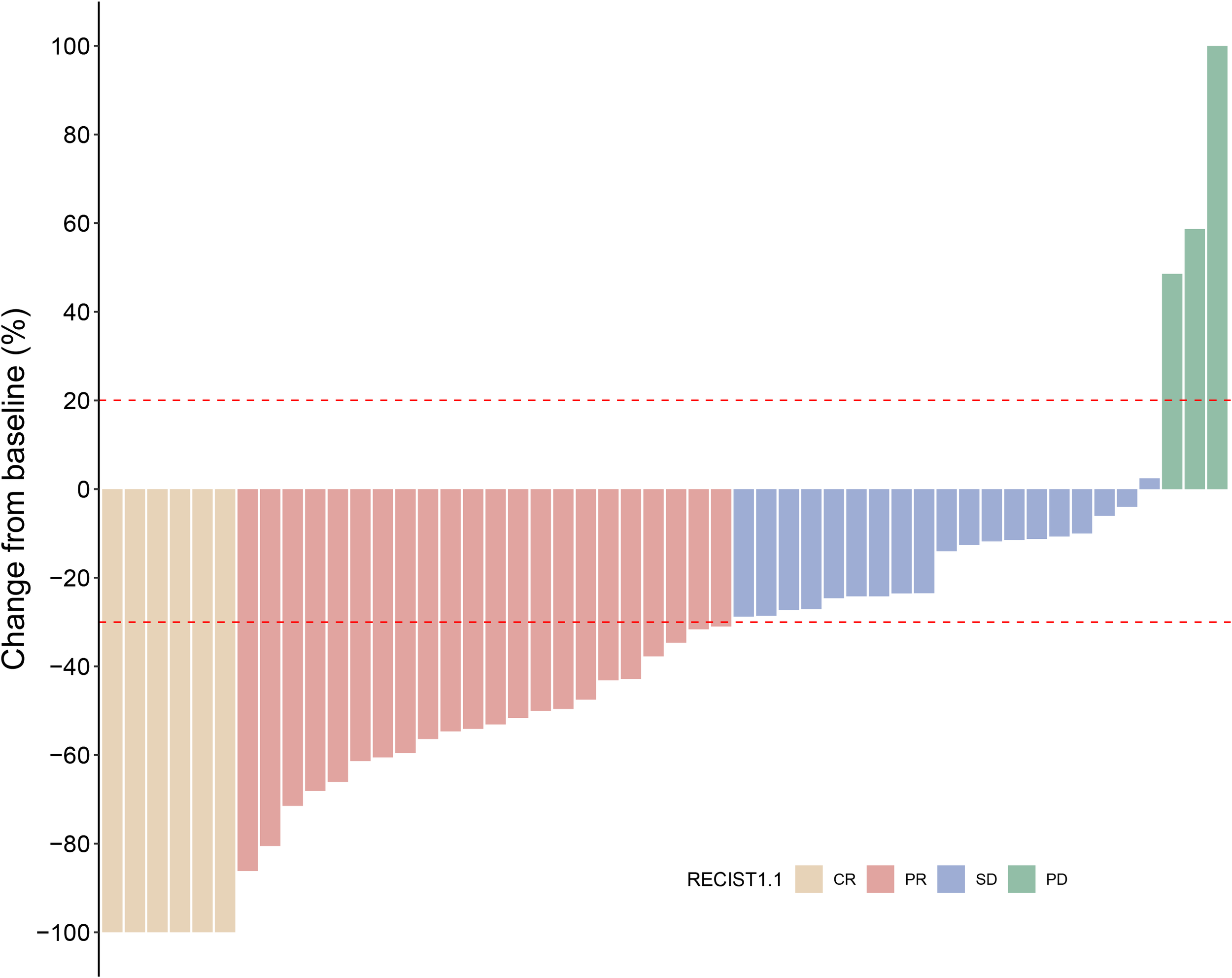
Waterfall plot of the best percentage of change from baseline in sum of diameters with best overall confirmed response (N = 50).
The duration of treatment and timing of response for each evaluable patient in the cadonilimab plus TP group are illustrated in the swim-lane plot (Figure 3). Each horizontal bar represents an individual patient, with bar length corresponding to the time from treatment initiation to the last follow-up or death. The bar color indicates the confirmed BOR as assessed per RECIST v1.1. Markers overlaid on each bar depict the timing of the first objective responses (CR or PR), the first SD, the first PD, and death. The first objective responses occurred rapidly: the median time to initial CR or PR was 2.45 months [interquartile range (IQR): 1.58–3.25]. At the data cutoff, 22 of 28 responders (78.6%) had not experienced disease progression and remained under follow-up.
Figure 3
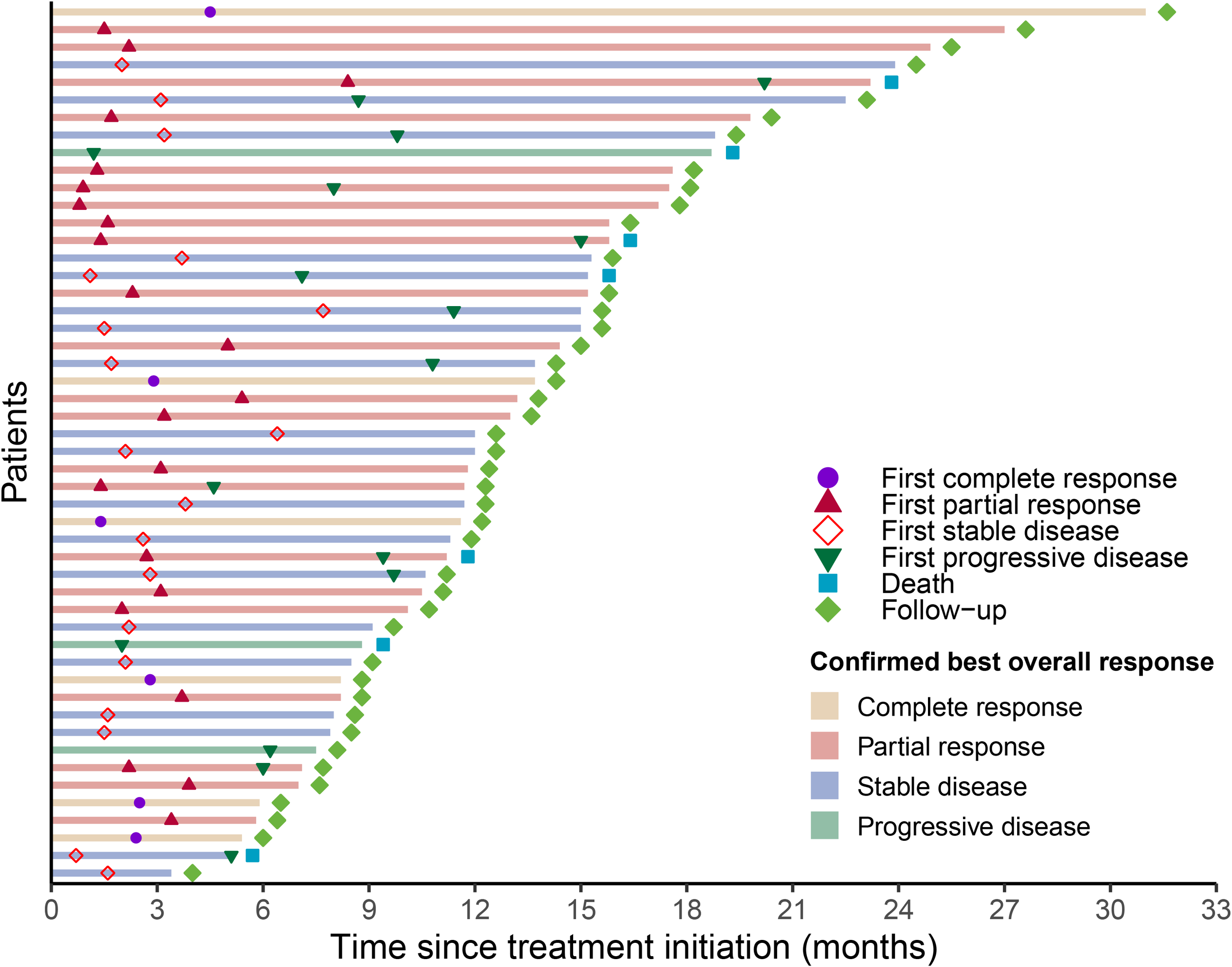
Progression events and duration of response according to RECIST 1.1.
Representative imaging findings are presented to visually illustrate treatment response. Figure 4 shows the pretreatment and posttreatment radiologic images of paratracheal and para-aortic lymph node metastases in a patient who achieved a CR.
Figure 4
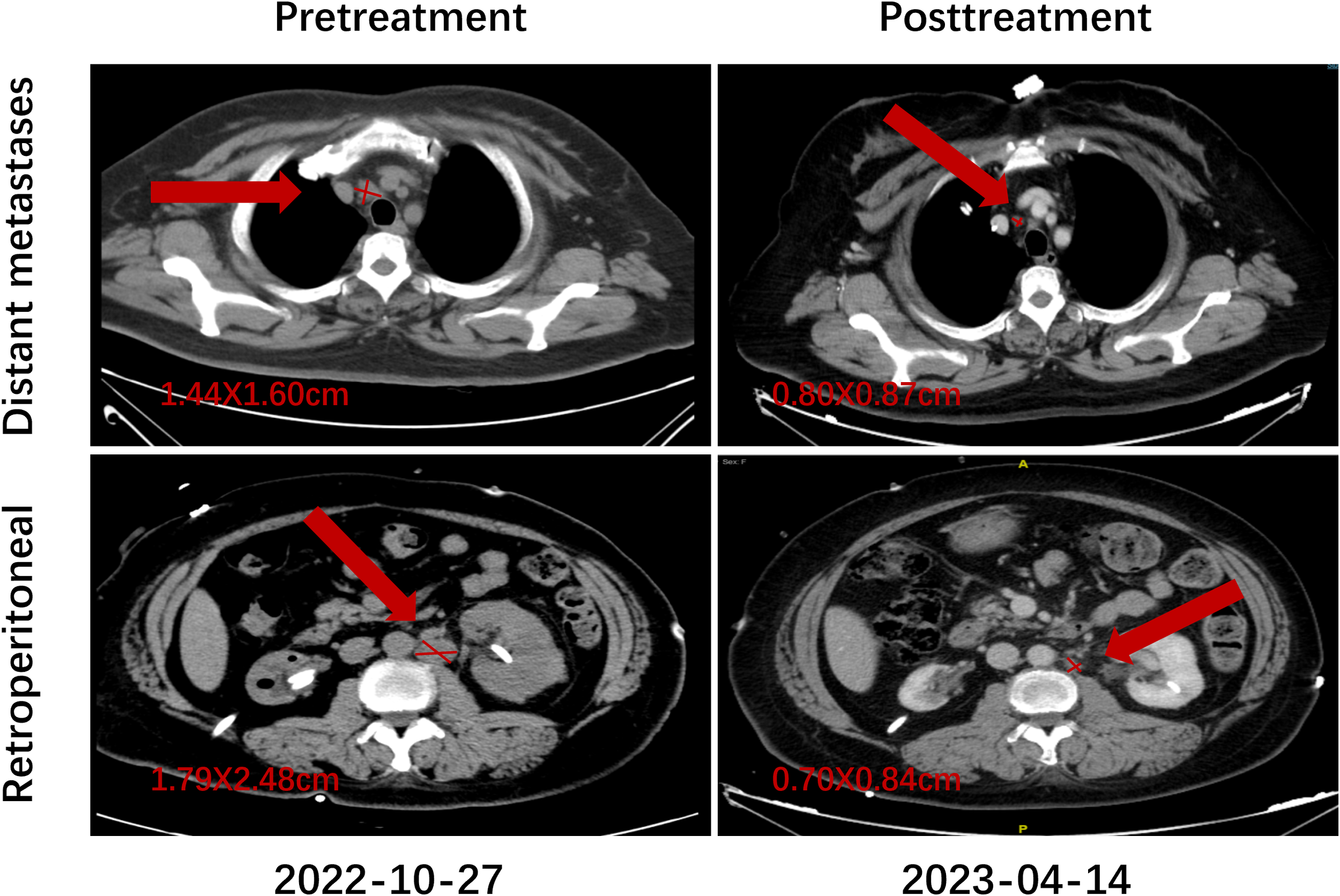
Representative imaging of treatment response in a patient who achieved CR.
3.3 Efficacy
At the June 2025 data cutoff, follow-up duration estimated by the reverse Kaplan–Meier approach was 33.2 months overall (IQR: 12.2–35.2). By treatment group, median follow-up was 40.7 months (IQR: 17.6–40.4) in the TP group and 13.0 months (IQR: 8.6–15.8) in the cadonilimab plus TP group.
After two treatment cycles, a short-term efficacy assessment was conducted, and a numerically higher proportion of patients receiving cadonilimab plus TP achieved a CR (12.0% vs. 5.9%) and a PR (42.0% vs. 34.5%), whereas rates of SD (38.0% vs. 50.4%) and PD (8.0% vs. 9.2%) were correspondingly lower relative to TP alone (Table 2).
Table 2
| Response | TP group (n = 119), N (%) | Cadonilimab plus TP group (n = 50), N (%) | p-value |
|---|---|---|---|
| CR | 7 (5.9) | 6(12.0) | 0.208 |
| PR | 41 (34.5) | 21 (42.0) | 0.353 |
| SD | 60 (50.4) | 19 (38.0) | 0.140 |
| PD | 11 (9.2) | 4(8.0) | 0.795 |
| ORR | 48 (40.3) | 27(54.0) | 0.103 |
| DCR | 108 (90.8) | 46(92.0) | 0.795 |
Response evaluation after two cycles of treatment (N = 169).
Among the 169 evaluable patients, the addition of cadonilimab produced a pronounced and statistically significant delay in tumor progression: mPFS was 20.2 months (95% CI: 15.0–NR) versus 12.2 months (95% CI: 9.7–17.1) with chemotherapy alone (log-rank p = 0.019). By contrast, a clear OS advantage had not yet emerged at the data cutoff; mOS was not reached in the cadonilimab plus TP group (95% CI: 23.2–NR) and was 37.5 months (95% CI: 32.4–NR) in the TP group, with overlapping Kaplan–Meier curves and a non-significant log-rank comparison (p = 0.732). These findings indicate that cadonilimab confers an early and durable PFS benefit, whereas a definitive OS benefit awaits longer follow-up (Figure 5).
Figure 5
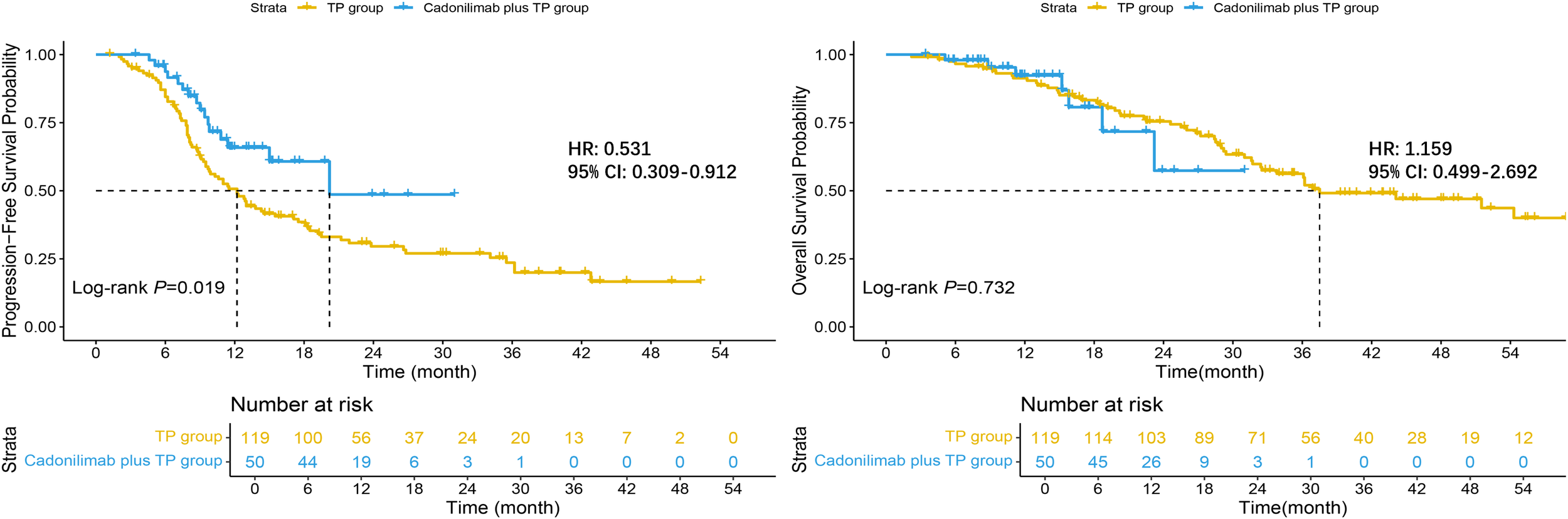
Kaplan–Meier curves for survival outcomes.
At the landmark analysis, the cadonilimab plus TP yielded higher PFS rates than TP alone: 65.8% (95% CI: 52.6–82.5) versus 50.7% (95% CI: 42.3–60.8) at 12 months and 48.6% (95% CI: 29.0–81.5) versus 29.6% (95% CI: 21.9–39.9) at 24 months. OS rates were similarly high at 12 months—92.3% (95% CI: 84.3–100.0) with cadonilimab plus TP and 91.4% (95% CI: 86.4–96.6) with TP—but diverged at 24 months, with the investigational arm at 57.4% (95% CI: 33.5–98.2) versus 75.5% (95% CI: 67.8–84.0) for TP alone; the wide confidence interval in the cadonilimab cohort indicates limited events and underscores the need for longer follow-up to confirm any definitive OS benefit.
3.4 Treatment-related adverse events
TRAEs were common yet largely low−grade, indicating a manageable toxicity profile for both regimens (Table 3). The cadonilimab plus TP group showed higher incidences of hypoalbuminemia (46.0% vs. 27.7%), hypothyroidism (28.0% vs. 7.6%), pyrexia (32.0% vs. 11.8%), rash/itching (38.0% vs. 8.4%), constipation (58.0% vs. 31.9%), diarrhea (50.0% vs. 27.7%), and fatigue (74.0% vs. 45.4%), each reaching statistical significance (p < 0.05). Conversely, no significant between−group differences were observed for hematologic abnormalities such as anemia, leukopenia, neutropenia, or thrombocytopenia, or for elevations in alanine or aspartate aminotransferase (p > 0.05). Grade 3–5 TRAEs were infrequent across both cohorts; only severe leukopenia occurred less often with cadonilimab plus TP (8.0% vs. 21.0%, p = 0.041).
Table 3
| Adverse events | TP group (n = 119), N (%) | Cadonilimab plus TP group (n = 50), N (%) | p-value | |||
|---|---|---|---|---|---|---|
| All grades | Grades 3–5 | All Grades | Grades 3–5 | All grades | Grades 3–5 | |
| Anemia | 88 (73.9) | 7 (5.9) | 41 (82.0) | 7 (14.0) | 0.261 | 0.122 |
| White blood cell count decreased | 73 (61.3) | 25 (21.0) | 29 (58.0) | 4 (8.0) | 0.685 | 0.041 |
| Neutrophil count decreased | 55 (46.2) | 22 (18.5) | 21 (42.0) | 4 (8.0) | 0.615 | 0.085 |
| Platelet count decreased | 64 (53.8) | 1 (0.8) | 23 (46.0) | 0 | 0.356 | 1.000 |
| Hypoalbuminemia | 33 (27.7) | 0 | 23 (46.0) | 1 (2.0) | 0.021 | 0.296 |
| Alanine aminotransferase increased | 29 (24.4) | 1 (0.8) | 15 (30.0) | 0 | 0.446 | 1.000 |
| Aspartate aminotransferase increased | 18 (15.1) | 1 (0.8) | 14 (28.0) | 0 | 0.051 | 1.000 |
| Hypokalemia | 27 (22.7) | 3 (2.5) | 13 (26.0) | 2 (4.0) | 0.644 | 0.633 |
| Hypothyroidism | 9 (7.6) | 0 | 14 (28.0) | 1 (2.0) | <0.001 | 0.296 |
| Pyrexia | 14 (11.8) | 0 | 16 (32.0) | 1 (2.0) | 0.002 | 0.296 |
| Immune-related myocarditis | 0 | 0 | 1 (2.0) | 1 (2.0) | 0.296 | 0.296 |
| Nausea/Vomiting | 76 (63.9) | 1 (0.8) | 39 (78.0) | 2 (4.0) | 0.072 | 0.209 |
| Decreased appetite | 72 (60.5) | 0 | 36 (72.0) | 0 | 0.156 | —— |
| Rash/Itching | 10 (8.4) | 1 (0.8) | 19 (38.0) | 1 (2.0) | <0.001 | 0.505 |
| Constipation | 38 (31.9) | 0 | 29 (58.0) | 0 | 0.002 | —— |
| Diarrhea | 33 (27.7) | 0 | 25 (50.0) | 0 | 0.005 | —— |
| Fatigue | 54 (45.4) | 1 (0.8) | 37 (74.0) | 1 (2.0) | <0.001 | 0.505 |
Treatment-related adverse events (N = 169).
Among the 50 patients who received cadonilimab plus TP, two permanently discontinued cadonilimab due to grade ≥3 immune-related adverse events (irAEs). One patient developed grade 3 hypothyroidism, presenting with severe fatigue and bradycardia. Levothyroxine replacement therapy was initiated, resulting in gradual symptom improvement. Another patient experienced immune-related myocarditis, manifesting as anterior chest discomfort and significant fatigue. Laboratory evaluation revealed elevated creatine kinase (352 U/L), myoglobin (93.7 ng/mL), N-terminal pro-B-type natriuretic peptide (NT-proBNP: 318.8 pg/mL), and cardiac troponin T (77.95 pg/mL). Immunotherapy was discontinued, and the patient was referred to the cardiology department of a general hospital for further evaluation and management. Cardiac biomarkers were closely monitored during follow-up. Additionally, one patient temporarily interrupted cadonilimab in combination with chemotherapy due to the development of a rectovaginal fistula, which was surgically treated, allowing for subsequent resumption of treatment.
Overall, the addition of cadonilimab to paclitaxel-platinum chemotherapy increased several low−grade immune−related and gastrointestinal toxicities without exacerbating the burden of serious adverse events, supporting the regimen’s acceptable safety and tolerability.
3.5 Subgroup analysis
In prespecified subgroup analyses (Figure 6), cadonilimab plus TP prolonged PFS versus TP alone (HR = 0.53, 95% CI: 0.31–0.91; p = 0.022). The treatment effect consistently favored cadonilimab plus TP across most strata.
Figure 6
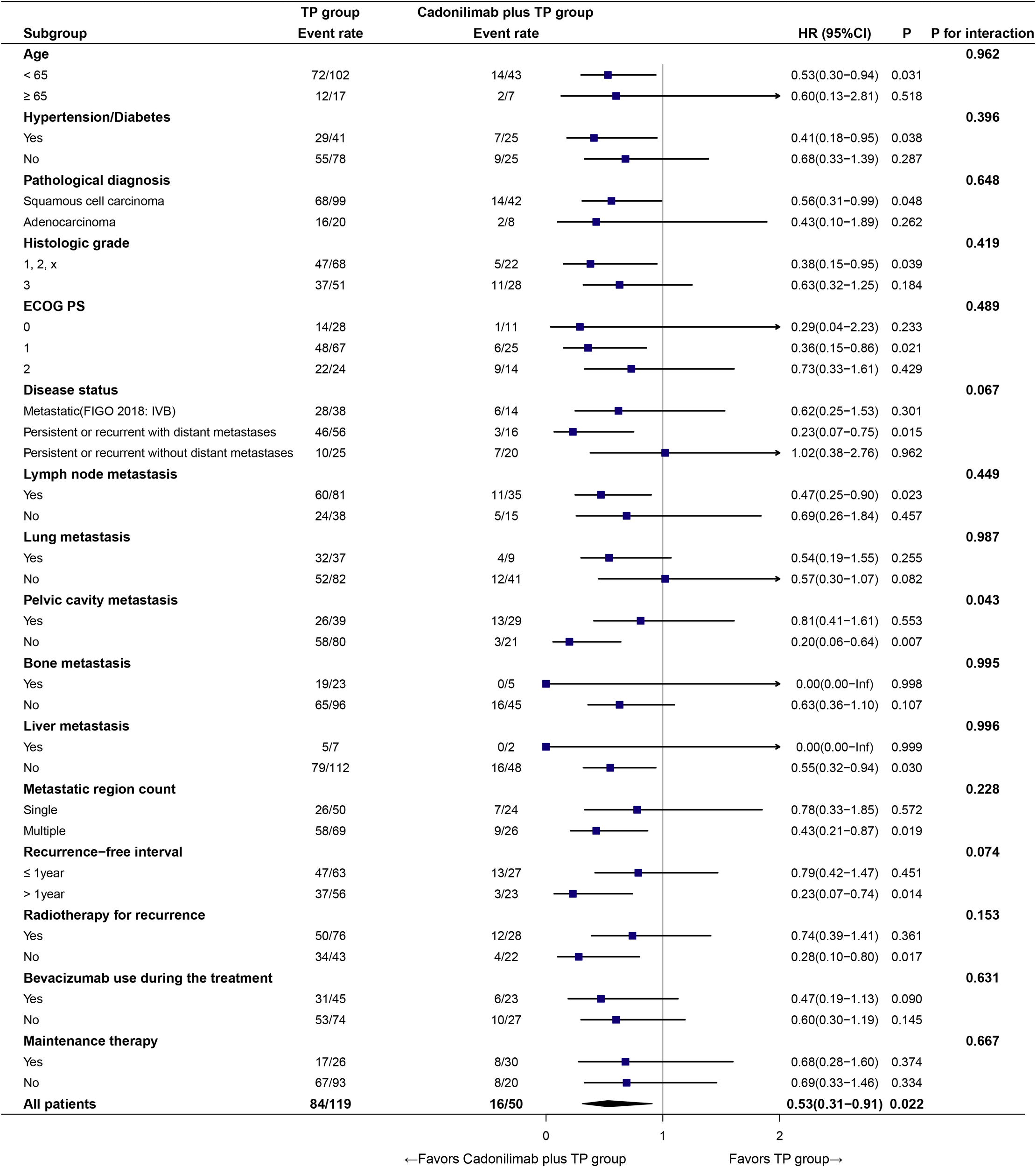
Subgroup analysis of PFS.
No significant treatment–subgroup interactions were detected for age, hypertension/diabetes, pathological diagnosis, histologic grade, ECOG PS, disease status, lymph node or lung/bone/liver metastasis, number of metastatic regions, recurrence−free interval, radiotherapy for recurrence, bevacizumab use during the treatment, or maintenance therapy (all p for interaction >0.05), except for pelvic cavity metastasis (p for interaction = 0.043). In that subgroup, patients without pelvic involvement derived a marked benefit (HR = 0.20, 95% CI: 0.06–0.64).
These findings suggest a broadly consistent benefit across diverse clinical subpopulations, with a potentially greater effect in the absence of pelvic cavity metastasis.
3.6 Prognostic factor analysis
In the univariate analysis (Table 4), an ECOG PS of 2 was significantly associated with inferior PFS (HR = 2.367, 95% CI: 1.535–3.651; p < 0.001). Higher tumor burden, including multiple metastatic regions (HR = 1.894, 95% CI: 1.244–2.884; p = 0.003) and lung metastasis (HR = 1.688, 95% CI: 1.120–2.544; p = 0.012), was also associated with poorer outcomes. Maintenance therapy was significantly associated with prolonged PFS (HR = 0.512, 95% CI: 0.325–0.806; p = 0.004), particularly among patients who received maintenance therapy for at least 1 year (HR = 0.217, 95% CI: 0.068–0.688; p = 0.009). A recurrence-free interval longer than 1 year was also associated with better PFS (HR = 0.659, 95% CI: 0.441–0.986; p = 0.043). Response evaluation after two cycles of treatment was a prognostic factor: patients achieving CR or PR after two cycles showed significantly improved PFS compared to those with SD (HR = 1.953, 95% CI: 1.270–3.004; p = 0.002). Additionally, first-line cadonilimab plus paclitaxel-platinum (± bevacizumab) reduced the hazard of progression by approximately 47% versus chemotherapy alone (HR = 0.531, 95% CI: 0.309–0.912; p = 0.022).
Table 4
| Variable | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (≥65 vs. <65) | 1.045 (0.592–1.843) | 0.879 | 0.993 (0.471–2.097) | 0.986 | ||||
| Diabetes (Yes vs. No) | 1.062 (0.650–1.736) | 0.810 | 0.831 (0.421–1.639) | 0.593 | ||||
| Hypertension (Yes vs. No) | 0.944 (0.613–1.454) | 0.794 | 0.846 (0.477–1.499) | 0.566 | ||||
| ECOG PS (2 vs. ≤1) | 2.367 (1.535–3.651) | <0.001 | 2.284 (1.423–3.667) | <0.001 | 3.021 (1.783–5.120) | <0.001 | 3.592 (2.005–6.436) | <0.001 |
| Disease status | ||||||||
| Persistent or recurrent with distant metastases vs. Metastatic (FIGO 2018: IVB) | 0.985 (0.635–1.528) | 0.947 | 0.827 (0.466–1.467) | 0.515 | ||||
| Persistent or recurrent without distant metastases vs. Metastatic (FIGO 2018: IVB) | 0.635 (0.354–1.137) | 0.126 | 0.526 (0.238–1.166) | 0.114 | ||||
| Pathological diagnosis (Adenocarcinoma vs. Squamous cell carcinoma) | 1.090 (0.654–1.818) | 0.741 | 1.139 (0.592–2.195) | 0.696 | ||||
| Histologic grade (3 vs. 1,2,x) | 1.183 (0.798–1.755) | 0.403 | 1.198 (0.722–1.990) | 0.484 | ||||
| Metastatic region count (Multiple vs. Single) | 1.894 (1.244–2.884) | 0.003 | 1.562 (0.941–2.592) | 0.085 | 2.101 (1.195–3.695) | 0.010 | 2.020 (1.035–3.942) | 0.039 |
| Lymph node metastasis (Yes vs. No) | 1.315 (0.853–2.028) | 0.215 | 1.486 (0.844–2.618) | 0.170 | ||||
| Lung metastasis (Yes vs. No) | 1.688 (1.120–2.544) | 0.012 | 1.784 (1.071–2.973) | 0.026 | 1.551 (0.925–2.603) | 0.096 | ||
| Pelvic cavity metastasis (Yes vs. No) | 1.193 (0.796–1.789) | 0.393 | 1.039 (0.609–1.773) | 0.889 | ||||
| Bone metastasis (Yes vs. No) | 1.104 (0.669–1.821) | 0.699 | 2.106 (1.148–3.863) | 0.016 | 1.426 (0.715–2.843) | 0.314 | ||
| Liver metastasis (Yes vs. No) | 0.894 (0.363–2.204) | 0.808 | 2.159 (0.858–5.431) | 0.102 | ||||
| Radiotherapy for recurrence (Yes vs. No) | 0.924 (0.616–1.385) | 0.702 | 1.061 (0.629–1.789) | 0.825 | ||||
| Bevacizumab use during the treatment (Yes vs. No) | 0.867 (0.577–1.302) | 0.492 | 1.049 (0.611–1.802) | 0.862 | ||||
| Maintenance therapy (Yes vs. No) | 0.512 (0.325–0.806) | 0.004 | 0.746 (0.443–1.254) | 0.269 | 0.737 (0.397–1.367) | 0.332 | ||
| 1 year of maintenance therapy (Yes vs. No) | 0.217 (0.068–0.688) | 0.009 | 0.330 (0.096–1.132) | 0.078 | 0.042 (0.001–1.753) | 0.096 | ||
| Recurrence-free interval (>1 year vs. ≤1 year) | 0.659 (0.441–0.986) | 0.043 | 0.662 (0.400–1.094) | 0.107 | 0.556 (0.328–0.944) | 0.030 | 0.736 (0.398–1.363) | 0.330 |
| Response evaluation after 2 cycles of treatment (SD vs. CR+PR) | 1.953 (1.270–3.004) | 0.002 | 1.691 (1.089–2.628) | 0.019 | 2.066 (1.137–3.756) | 0.017 | 1.622 (0.875–3.004) | 0.124 |
| Number of treatment cycles (≥6 cycles vs. <6 cycles) | 0.759 (0.506–1.138) | 0.182 | 1.023 (0.612–1.711) | 0.931 | ||||
| Therapy (Cadonilimab plus TP vs. TP) | 0.531 (0.309–0.912) | 0.022 | 0.599 (0.328–1.094) | 0.095 | 1.159 (0.499–2.692) | 0.732 | ||
Univariate and multivariate Cox regression analysis of PFS and OS in all patients (N = 169).
Bold values are shown solely for visual clarity and do not carry any statistical implication
In multivariate analysis, ECOG PS = 2 (HR = 2.28, 95% CI: 1.42–3.67; p < 0.001), lung metastasis (HR = 1.78, 95% CI: 1.07–2.97; p = 0.026), and lack of early objective response (SD vs. CR/PR; HR = 1.69, 95% CI: 1.09–2.63; p = 0.019) were identified as independent adverse prognostic factors.
OS analyses yielded a pattern similar to that observed for PFS. On univariate assessment, ECOG PS = 2 remained the strongest adverse factor (HR = 3.021, 95% CI: 1.783–5.120; p < 0.001), while multiple metastatic sites (HR = 2.101, 95% CI: 1.195–3.695; p = 0.010), bone metastasis (HR = 2.106, 95% CI: 1.148–3.863; p = 0.016), recurrence−free interval ≤ 1 year (HR = 0.556, 95% CI: 0.328–0.944; p = 0.030), and early SD versus objective response after two cycles (HR = 2.066, 95% CI: 1.137–3.756; p = 0.017) were also significant. Multivariate modeling confirmed ECOG PS = 2 (HR = 3.592, 95% CI: 2.005–6.436; p < 0.001) and multiple metastatic regions (HR = 2.020, 95% CI: 1.035–3.942; p = 0.039) as independent predictors of poorer OS.
4 Discussion
Clinical deployment of bispecific antibodies in solid malignancies has accelerated. Moreover, an ongoing phase III study (NCT05446883) is evaluating QL1706, a PD-1/CTLA-4 bispecific with functional similarity to cadonilimab, as first-line treatment with paclitaxel-platinum ± bevacizumab for patients with r/m CC.
In our real-world study, cadonilimab combined with standard chemotherapy conferred survival benefits with an acceptable safety profile in patients with p/r/m CC. The key efficacy endpoints: PFS and the observed pattern of adverse events were largely consistent with findings from the AK104–303 trial.
Cadonilimab plus TP produced a clear PFS advantage over TP alone (mPFS: 20.2 vs. 12.2 months; log-rank p = 0.019), with higher 12- and 24-month PFS rates (65.83% and 48.62% vs. 50.71% and 29.57%, respectively). The direction of benefit is consistent with the AK104–303 trial, in which cadonilimab combined with chemotherapy also outperformed chemotherapy alone (mPFS: 12.7 vs. 8.1 months). However, the OS data remain immature at the current follow−up, and no statistically significant difference has been observed between groups to date. Notably, the mPFS in both groups of our real−world cohort exceeded that reported in AK104−303. This difference may reflect the frequent use of individualized local radiotherapy for metastatic lesions (63.9% in the TP group and 56.0% in the cadonilimab plus TP group) and the adoption of more diverse maintenance strategies in routine practice. By contrast, maintenance in AK104−303 was limited to cadonilimab with or without bevacizumab in the investigational arm, and the trial report did not specify whether local radiotherapy to metastatic sites was permitted or utilized (11). Extensive data (20–24) support integrating local radiotherapy (including metastasis−directed SBRT) with systemic therapy as a reasonable strategy when overall tumor burden has been cytoreduced or is confined to a limited number of lesions.
In terms of safety, the most common adverse event was anemia (82.0%), followed by other known chemotherapy-related toxicities, such as nausea/vomiting, fatigue, decreased appetite, white blood cell count decreased, and constipation, all of which were manageable. Notably, irAEs such as rash/itching (38.0%), pyrexia (32.0%), and hypothyroidism (28.0%) occurred at relatively high frequencies, highlighting the importance of early recognition and timely management of irAEs to ensure treatment continuity.
We further identified that an ECOG PS of 2 consistently emerged as the strongest adverse prognostic factor for both PFS and OS in univariate and multivariate analyses. A high tumor burden, particularly the presence of lung metastasis and involvement of multiple metastatic sites, was also independently associated with poorer outcomes. Consistent with prior evidence, a greater baseline tumor burden portends inferior outcomes in advanced CC (25). Across multicenter cohorts (26), multi−organ or multisite metastases confer significantly shorter survival than single−site disease, whereas “low−burden” presentations, such as ≤5 lesions or nodal−only involvement, are independently associated with more favorable outcomes. In contrast, achieving an early objective response (CR/PR vs. SD) after two cycles of treatment was significantly associated with improved survival, emphasizing the prognostic relevance of early treatment response.
Moreover, maintenance therapy, particularly when administered for ≥1 year, was significantly linked to prolonged PFS, suggesting its potential role in sustaining clinical benefit. A multicenter real−world study (27) of first−line maintenance therapy, including PD−1 inhibitors, targeted agents, or both, reported significant gains in both PFS and OS; notably, >8 cycles correlated with additional PFS/OS extension, supporting a duration–response relationship that aligns with a ≥1-year strategy. A recurrence-free interval longer than 1 year prior to treatment initiation was also correlated with more favorable PFS.
Mechanistically, cadonilimab leverages concurrent PD−1 and CTLA−4 blockade to reinvigorate effector T−cell activity and augment T−cell priming while mitigating Treg−mediated suppression (28, 29); its tetravalent, Fc−silent architecture confers preferential avidity in dual−high PD−1/CTLA−4 tumor milieus and minimizes Fc−mediated toxicities (30), thereby supporting rational combinations with chemotherapy, anti−angiogenic agents, and local modalities (31–33).
Building on dual-pathway immune activation and tumor microenvironment remodeling, combination strategies with chemotherapy, anti-angiogenic therapy, and local modalities (e.g., radiotherapy or interventional procedures) show promise for further clinical development. Mechanistic and translational studies are needed to clarify how these regimens enhance efficacy and overcome resistance through pathway blockade, vascular normalization, and immune-contexture reshaping, thereby informing treatment sequencing and biomarker-guided patient selection.
Nonetheless, this research has a few limitations, such as a limited sample size, a relatively brief follow-up period, and being conducted at a single center. Moreover, PD−L1 tumor expression status was missing in a subset of patients and therefore was not incorporated into subgroup analyses or related exploratory evaluations. Future investigations with larger, multicenter real−world cohorts and complete biomarker profiling are warranted to corroborate these findings.
5 Conclusions
In real-world single-center practice, first-line cadonilimab plus paclitaxel-platinum ± bevacizumab improved PFS and increased ORR versus chemotherapy alone, with manageable toxicity and consistent subgroup effects, supporting frontline use and prompting further study of maintenance therapy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangsu Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Investigation, Writing – original draft, Data curation, Methodology, Visualization. CW: Conceptualization, Writing – review & editing. DZ: Supervision, Writing – review & editing. BZ: Validation, Writing – review & editing. XH: Writing – review & editing, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This paper was supported by the National Natural Science Foundation of China (No. 82172804 and No. 82103552), Key Project of Jiangsu Provincial Health Commission (No.K2019028), Nanjing Science and Technology Plan Project (No.2022SX00001663).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Li T Zhang H Lian M He Q Lv M Zhai L et al . Global status and attributable risk factors of breast, cervical, ovarian, and uterine cancers from 1990 to 2021. J Hematol Oncol. (2025) 18:5. doi: 10.1186/s13045-025-01660-y
2
Siegel RL Giaquinto AN Jemal A . Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3
Li H Wu X Cheng X . Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. (2016) 27:e43. doi: 10.3802/jgo.2016.27.e43
4
O’Malley DM Oaknin A Monk BJ Selle F Rojas C Gladieff L et al . Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol Oncol. (2021) 163:274–80. doi: 10.1016/j.ygyno.2021.08.018
5
Monk BJ Sill MW McMeekin DS Cohn DE Ramondetta LM Boardman CH et al . Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J Clin Oncol: Off J Am Soc Clin Oncol. (2009) 27:4649–55. doi: 10.1200/JCO.2009.21.8909
6
Kitagawa R Katsumata N Shibata T Kamura T Kasamatsu T Nakanishi T et al . Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol: Off J Am Soc Clin Oncol. (2015) 33:2129–35. doi: 10.1200/JCO.2014.58.4391
7
Tewari KS Sill MW Penson RT Huang H Ramondetta LM Landrum LM et al . Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240). Lancet (lond Engl). (2017) 390:1654–63. doi: 10.1016/S0140-6736(17)31607-0
8
Nishio S Yonemori K Usami T Minobe S Yunokawa M Iwata T et al . Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: results from KEYNOTE-826. Cancer Sci. (2022) 113:3877–87. doi: 10.1111/cas.15479
9
Monk BJ Colombo N Tewari KS Dubot C Caceres MV Hasegawa K et al . First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol: Off J Am Soc Clin Oncol. (2023) 41:5505–11. doi: 10.1200/JCO.23.00914
10
Oaknin A Gladieff L Martínez-García J Villacampa G Takekuma M De Giorgi U et al . Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Lancet (lond Engl). (2024) 403:31–43. doi: 10.1016/S0140-6736(23)02405-4
11
Wu X Sun Y Yang H Wang J Lou H Li D et al . Cadonilimab plus platinum-based chemotherapy with or without bevacizumab as first-line treatment for persistent, recurrent, or metastatic cervical cancer (COMPASSION-16): a randomised, double-blind, placebo-controlled phase 3 trial in China. Lancet. (2024) 404:1668–76. doi: 10.1016/S0140-6736(24)02135-4
12
Xiong A Wang L Chen J Wu L Liu B Yao J et al . Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China. Lancet (lond Engl). (2025) 405:839–49. doi: 10.1016/S0140-6736(24)02722-3
13
Yi M Li T Niu M Wu Y Zhao B Shen Z et al . Blockade of CCR5+ T cell accumulation in the tumor microenvironment optimizes anti-TGF-β/PD-L1 bispecific antibody. Advanced Sci. (2024) 11:e2408598. doi: 10.1002/advs.202408598
14
Keam SJ . Cadonilimab: first approval. Drugs. (2022) 82:1333–9. doi: 10.1007/s40265-022-01761-9
15
Bini M Quesada S Meeus P Rodrigues M Leblanc E Floquet A et al . Real-world data on newly diagnosed BRCA-mutated high-grade epithelial ovarian cancers: the french national multicenter ESME database. Cancers. (2022) 14:4040. doi: 10.3390/cancers14164040
16
Krasenbaum LJ Pedarla VL Thompson SF Tangirala K Cohen JM Driessen MT . A real-world study of acute and preventive medication use, adherence, and persistence in patients prescribed fremanezumab in the United States. J Headache Pain. (2022) 23:54. doi: 10.1186/s10194-022-01413-z
17
Ogasawara A Hasegawa K . Recent advances in immunotherapy for cervical cancer. Int J Clin Oncol. (2025) 30:434–48. doi: 10.1007/s10147-025-02699-0
18
Lee SJ Yoo JG Kim JH Park J-Y Lee J-Y Lee Y-Y et al . Gynecologic oncology in 2024: breakthrough trials and evolving treatment strategies for cervical, uterine corpus, and ovarian cancers. J Gynecol Oncol. (2025) 36:e72. doi: 10.3802/jgo.2025.36.e72
19
Freites-Martinez A Santana N Arias-Santiago S Viera A . CTCAE versión 5.0. Evaluación de la gravedad de los eventos adversos dermatológicos de las terapias antineoplásicas. Actas Dermo-Sifiliográficas. (2021) 112:90–2. doi: 10.1016/j.ad.2019.05.009
20
Li J Wang Y Huo L Huang X Shi L Huang L et al . Definitive irradiation as a first treatment strategy for primary and metastatic sites of newly diagnosed IVB cervical cancer that presented with synchronous oligometastases. Radiat Oncol. (2023) 18:159. doi: 10.1186/s13014-023-02320-6
21
Sherwani Z Parikh S Yegya-Raman N McKenna K Deek M Jabbour S et al . Stereotactic body radiation therapy in gynecologic oligometastases: an effective but underutilized approach. Cancers. (2023) 15:3526. doi: 10.3390/cancers15133526
22
Macchia G Nardangeli A Laliscia C Fodor A Draghini L Gentile PC et al . Stereotactic body radiotherapy in oligometastatic cervical cancer (MITO-RT2/RAD study): a collaboration of MITO, AIRO GYN, and MaNGO groups. Int J Gynecological Cancer. (2022) 32:732–9. doi: 10.1136/ijgc-2021-003237
23
Wang Y Farmer M Izaguirre EW Schwartz DL Somer B Tillmanns T et al . Association of definitive pelvic radiation therapy with survival among patients with newly diagnosed metastatic cervical cancer. JAMA Oncol. (2018) 4:1288. doi: 10.1001/jamaoncol.2018.2677
24
Onal C Gultekin M Oymak E Guler OC Yilmaz MT Yuce Sari S et al . Stereotactic radiotherapy in patients with oligometastatic or oligoprogressive gynecological Malignancies: a multi-institutional analysis. Int J Gynecological Cancer. (2020) 30:865–72. doi: 10.1136/ijgc-2019-001115
25
Park S Park W Park SH Kim J-Y Kim JH Kim H et al . Oncologic outcomes according to the level of disease burden in patients with metachronous distant metastases from uterine cervical cancer: a Korean Radiation Oncology Group study (KROG 18-10). J Gynecol Oncol. (2022) 33:e32. doi: 10.3802/jgo.2022.33.e32
26
Mailankody S Dhanushkodi M Ganesan TS Radhakrishnan V Christopher V Ganesharajah S et al . Recurrent cervical cancer treated with palliative chemotherapy: real-world outcome. ecancer. (2020) 14:1122. doi: 10.3332/ecancer.2020.1122
27
Peng C Li X Tang W Zhu W Yan P Chen J et al . Real-world outcomes of first-line maintenance therapy for recurrent or metastatic cervical cancer: a multi-center retrospective study. Int Immunopharmacol. (2024) 129:111578. doi: 10.1016/j.intimp.2024.111578
28
Wei SC Duffy CR Allison JP . Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. (2018) 8:1069–86. doi: 10.1158/2159-8290.CD-18-0367
29
Wei SC Anang N-AAS Sharma R Andrews MC Reuben A Levine JH et al . Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci USA. (2019) 116:22699–709. doi: 10.1073/pnas.1821218116
30
Pang X Huang Z Zhong T Zhang P Wang ZM Xia M et al . Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs. (2023) 15:2180794. doi: 10.1080/19420862.2023.2180794
31
Xiao Y Wang Y Li J Cheng C Song C Wang X et al . Stereotactic body radiotherapy plus cadonilimab (PD-1/CTLA-4 bispecific antibody) as third-line or beyond therapy for refractory solid tumors: A phase 1b study. Cancer Commun. (2025) 1–12. doi: 10.1002/cac2.70051
32
Gao X Xu N Li Z Shen L Ji K Zheng Z et al . Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. (2023) 24:1134–46. doi: 10.1016/S1470-2045(23)00411-4
33
Qiao Q Han C Ye S Li J Shao G Bai Y et al . The efficacy and safety of cadonilimab combined with lenvatinib for first-line treatment of advanced hepatocellular carcinoma (COMPASSION-08): a phase Ib/II single-arm clinical trial. Front Immunol. (2023) 14:1238667. doi: 10.3389/fimmu.2023.1238667
Summary
Keywords
cadonilimab, immunotherapy, cervical cancer, progression-free survival, adverse events
Citation
Wang X, Wang C, Zong D, Zhu B and He X (2025) First-line treatment with cadonilimab plus paclitaxel-platinum ± bevacizumab for persistent, recurrent, or metastatic cervical cancer: a retrospective real-world study. Front. Oncol. 15:1634779. doi: 10.3389/fonc.2025.1634779
Received
25 May 2025
Accepted
06 August 2025
Published
27 August 2025
Volume
15 - 2025
Edited by
Alberto Farolfi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Reviewed by
Ming Yi, Zhejiang University, China
Tianye Li, Zhejiang University School of Medicine, China
He Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Updates
Copyright
© 2025 Wang, Wang, Zong, Zhu and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia He, hexiabm@163.com; Biqing Zhu, zhubiqing81@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.