- 1Section of Oncology, Department of Engineering for Innovation Medicine (DIMI), University of Verona School of Medicine and Verona University Hospital Trust, Verona, Italy
- 2Dietetic Service, University and Hospital Trust (AOUI) of Verona, Verona, Italy
- 3Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
- 4Department of Medicine and Surgery, Medical Oncology Unit, University Hospital of Parma, Parma, Italy
- 5Comprehensive Cancer Center, Medical Oncology Department, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 6Department of Translational Medicine and Surgery, Clinical Nutrition Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS & Research and Training Center in Human Nutrition, Università Cattolica del Sacro Cuore, Rome, Italy
- 7Biostatistics, IRCCS Regina Elena National Cancer Institute, Rome, Italy
- 83trees Healthcare, Viterbo, Italy
- 9Medical Oncology Unit, Ospedale Isola Tiberina - Gemelli Isola, Rome, Italy
Background: Loss of skeletal muscle mass may serve as a valuable indicator of treatment efficacy and survival in individuals with lung cancer undergoing immunotherapy. This investigation sought to pinpoint accessible markers that could reflect the presence of muscle degradation.
Methods: A retrospective study was conducted on patients with advanced non-small cell lung cancer (NSCLC) who received first-line pembrolizumab therapy from June 2018 to September 2021. Data collected included computed tomography (CT)-based body composition, clinical and radiological characteristics, along with thyroid function tests (free triiodothyronine [fT3] and free thyroxine [fT4]). Predictive factors were evaluated using multivariate logistic regression models.
Results: Among 31 patients, muscle wasting was observed in 58.1%. Performance Status (PS) emerged as a strong predictor (p=0.005), and a significant link was also found between muscle depletion and fT3/fT4 ratio (p=0.0296). After adjusting for PS, the association with the hormone ratio remained suggestive though not statistically definitive (p=0.091). ROC curve analysis identified a threshold value of 2.84 for fT3/fT4 ratio, which best differentiated patients at higher versus lower risk of muscle loss. Notably, 77.3% of individuals with muscle wasting had a ratio below this cut-off, compared to only 14.3% of those with higher ratios (p=0.006). While no significant correlation was found between the hormone ratio and progression-free survival (PFS), a meaningful association with overall survival (OS) was observed (p=0.032).
Conclusions: Despite the limited sample size, fT3/fT4 ratio appears to be a promising and accessible biomarker for identifying muscle wasting, which may be linked to diminished treatment response and shorter survival in patients with NSCLC.
Introduction
Immune checkpoint inhibitors (ICIs) have transformed the therapeutic paradigm for advanced non-small cell lung cancer (NSCLC), yielding substantial clinical benefits. However, durable responses are observed in only a minority of patients, underscoring the critical need for predictive biomarkers to optimize patient selection and therapeutic outcomes (1).
Skeletal muscle wasting (SMW) is a multifactorial condition influenced by aberrant interactions among cytokines, endocrine signaling pathways, metabolic alterations, nutritional deficits, and reduced physical activity (2). SMW, related to inflammation and metabolic dysfunction, may impair immune response and reduce immunotherapy efficacy in NSCLC (2). Moreover, in NSCLC and other malignancies, SMW has emerged as a negative prognostic factor and a potential negative biomarker of immunotherapy response (3–5).
Interestingly, type 2 and 3 iodothyronine deiodinases (DIO2 and DIO3) are present in skeletal muscle, where thyroid hormone signaling plays a crucial role in muscle development, function, and regeneration (6). In this light, the ratio of free triiodothyronine (fT3) to free thyroxine (fT4) is an easily assessable and reliable indicator of deiodinases regulating active thyroid hormones. Exploratory studies have linked a low fT3/fT4 ratio with reduced muscle mass and SMW in several non-cancer conditions (7, 8). Moreover, the fT3/fT4 ratio has been found to correlate with survival outcomes in patients with solid tumors (colorectal cancer and renal cell carcinoma) treated with tyrosine kinase inhibitors (9, 10) and in patients with urothelial carcinoma treated with immunotherapy (11). fT3/fT4 ratio, strictly related to muscle metabolism and systemic inflammation (6, 12), may reflect metabolic status and immune system function and thus serve as a useful predictor of immunotherapy response in NSCLC. Based on this evidence, we hypothesized that the fT3/fT4 ratio could serve as an easily assessable surrogate marker of skeletal muscle status and an indirect predictor for response to immunotherapy in patients with advanced NSCLC. We therefore conducted a study to explore the relationship between fT3/fT4 ratio, SMW and clinical outcome in NSCLC patients treated with upfront pembrolizumab.
Materials and methods
We retrospectively analyzed a cohort of consecutive patients with advanced NSCLC treated with first-line pembrolizumab monotherapy between June 2018 and September 2021, at three Italian oncology centers (University of Verona, Gemelli University Hospital and Parma University Hospital). Inclusion criteria were: age >18 years; histologically confirmed diagnosis of advanced and measurable disease according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria; PD-L1 expression ≥ 50% assessed by immunohistochemistry (IHC); prior administration of more than one dose of pembrolizumab as first-line treatment, based on clinical judgment, aligned with established guidelines and best clinical practices; availability of pre-treatment abdominal computed tomography (CT) scans, performed within 3 months before starting immunotherapy. Patients with incomplete clinical data or lacking adequate imaging were excluded.
The study was approved by the local Ethical Committee (Prot. 2193CESC, 2022) and it was conducted according to the ethical standards of Declaration of Helsinki.
The following clinical-radiological and laboratory features were collected: age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking history, comorbidities, disease stage, baseline thyroid hormones levels (fT3 and fT4), weight, height, Body Mass Index (BMI) and data on routine pre-immunotherapy CT based body composition.
Body composition assessment was performed by measuring the cross-sectional areas of skeletal muscle and fat tissue using a single axial CT slice taken at the level of the third lumbar vertebra (L3), a region known to reliably reflect total body tissue distribution (3).
Image analysis at the L3 level was carried out using a freehand segmentation approach, which closely aligned with the principles of the Alberta protocol (an established method based on tissue attenuation thresholds) utilizing built-in functionalities of SliceOmatic software version 5.0 (TomoVision, 3280 Chemin Milletta, Magog, J1X 0R4, Canada), distinguishing muscle from visceral, subcutaneous and intermuscular adipose tissues using anatomic knowledge and tissue-specific Hounsfield unit (HU) ranges. Skeletal muscle area (SMA) was identified within an attenuation range of −29 to +150 HU, visceral adipose tissue (VAT) was identified between -150 and -50 HU, subcutaneous adipose tissue (SAT) and intermuscular adipose tissue (IMAT) between -190 and -30 HU. The evaluated muscle groups included the psoas, erector spinae, quadratus lumborum, transversus abdominis, internal and external obliques, and rectus abdominis. To account for individual body size, the SMA was normalized to body surface area, resulting in the skeletal muscle index (SMI), expressed in cm²/m². This step was essential to precisely quantify muscle volume, which can vary significantly depending on an individual’s body composition. To categorize patients exhibiting skeletal muscle wasting (SMW), we employed sex-specific and BMI-adjusted SMI thresholds as outlined by Martin et al (13).
SMW was defined as SMI of <43 cm2/m2 for men with BMI < 25 kg/m2, SMI < 53 cm2/m2 for men with BMI ≥ 25 kg/m2, and SMI < 41 cm2/m2 for women. The main objective of the study was the association of SMW with predictive factors (e.g., ECOG PS, fT3/fT4 ratio).
Continuous variables were expressed as medians and range, categorical variables as frequencies and percentages. Progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan-Meier methods and compared using the log-rank test. A p-value < 0.05 was considered statistically significant. A multivariate logistic regression, including ECOG PS and fT3/fT4 ratio, was developed using stepwise regression (forward selection, enter limit and remove limit, p = 0.10 and p = 0.15, respectively), to identify independent predictors of outcome. Receiver operating characteristic (ROC) analysis was conducted to determine the optimal cut-off for the fT3/fT4 ratio in predicting SMW. Statistical evaluation was performed by use of the SPSS 29.0 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
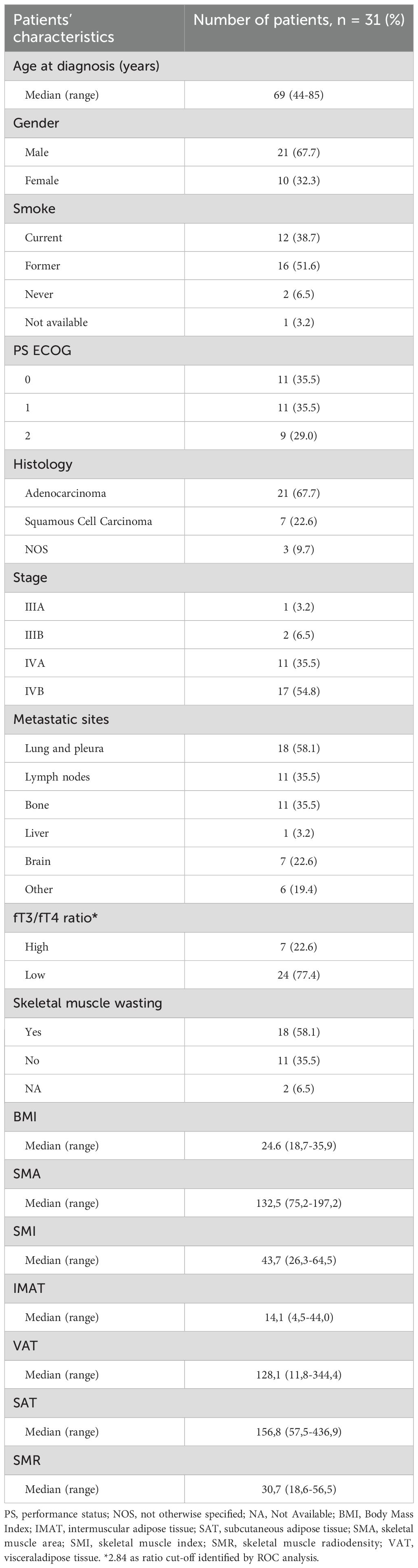
Patients’ characteristics
Thirty-one patients with advanced NSCLC who received first-line pembrolizumab were evaluated for clinical and metabolic parameters, as described above. Of the patients analyzed, 21 (67.7%) were male, and the median age was 69 years (range: 44–85 years). A substantial proportion (90%) had a history of smoking, and 38.7% were active smokers at the initiation of treatment. Adenocarcinoma was the predominant histological subtype, observed in 67.7% of cases. The prevalence of muscle wasting was 58.1%. Values of basal fT3, fT4 and their ratio, were collected and reported in Table 1.
Association between SMW and clinical variables
To investigate potential associations between SMW and other clinical variables, a regression model was applied. The analysis identified a statistically significant association between SMW and ECOG PS (p = 0.005). Interestingly, a significant association was observed between SMW and the fT3/fT4 ratio (p = 0.0296). When ECOG PS was accounted for as a confounding variable, the association between the fT3/fT4 ratio and muscle wasting demonstrated a trend toward significance (p = 0.091).
fT3/fT4 ratio threshold, subgroup and survival analyses
To further explore this relationship, ROC analysis was performed, which identified 2.84 as the optimal fT3/fT4 ratio threshold for distinguishing between low- and high-risk classifications for muscle depletion with the highest predictive accuracy. The ROC curve is a graphical plot that illustrates the diagnostic ability of a binary classifier system by plotting the true positive rate (sensitivity) against the false positive rate (1-specificity) at various threshold settings. In this context, the ROC curve was used to determine how well the fT3/fT4 ratio could discriminate between patients with and without muscle wasting. At the threshold of 2.84, the fT3/fT4 ratio demonstrated the highest predictive performance for identifying patients at risk of muscle depletion. This cut-off was then used to stratify patients into two groups: those with a “low” ratio (<2.84) and those with a “high” ratio (≥2.84). A subgroup analysis of patients with SMW revealed a marked disparity in the distribution of the fT3/fT4 ratio. Among these patients, 77.3% exhibited a low fT3/fT4 ratio, whereas only 14.3% had a high ratio, a statistically significant difference (p = 0.006) (Figure 1A). While no significant association was found between the fT3/fT4 ratio and PFS (p = 0.567), a significant correlation was observed with OS (p = 0.032) (Figure 1B).
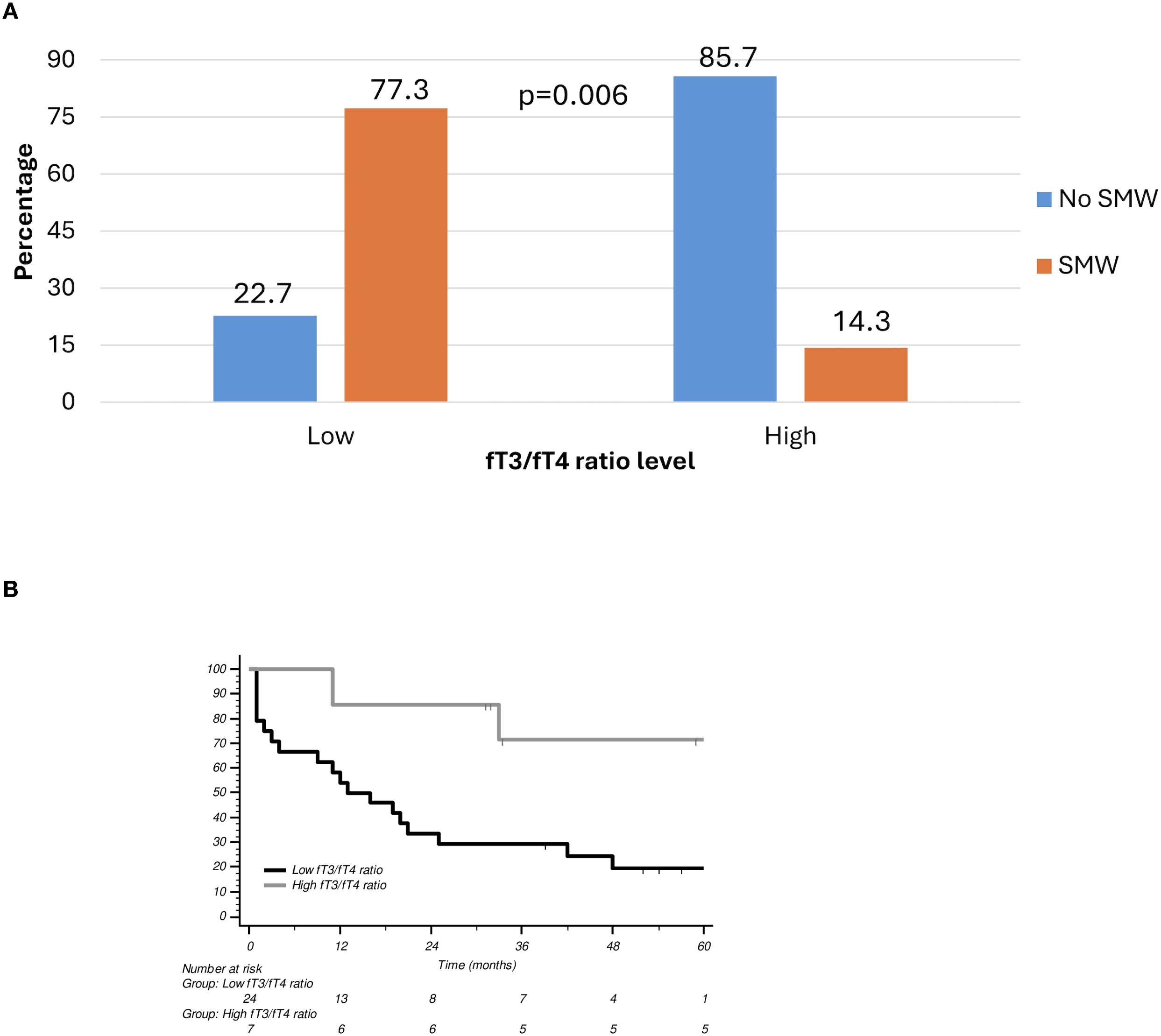
Figure 1. (A) Skeletal muscle wasting according to fT3/fT4 ratio level. (B) Overall survival (OS) curves according to fT3/fT4 ratio. SMW, Skeletal Muscle Wasting.
Discussion
Early identification, through clinical (and biological) biomarkers, of long-term responders to immunotherapy is essential for optimizing treatment strategies and improving clinical outcomes.
In our study, low fT3/fT4 ratio was significantly associated with a higher prevalence of SMW, even after adjusting for ECOG PS, preliminarily suggesting that fT3/fT4 ratio may serve as a proxy identifying SMW in patients with advanced NSCLC. Accordingly, recent findings in euthyroid individuals with type 2 diabetes mellitus demonstrated that a low fT3/fT4 ratio was significantly associated with sarcopenia, muscle mass, strength, and physical performance (7). Moreover, a significant inverse association between fT3/fT4 ratio and SMI (even after adjusting for age, metabolic factors, and comorbidities) was evaluated in a large retrospective study across Chinese subjects over 45 years old without cancer (8). In this light, fT3/fT4 ratio may reflect not only metabolic resilience but also systemic alterations associated with disease burden and catabolism, highlighting its potential role in identifying patients at increased risk of SMW and enabling earlier intervention and tailored supportive strategies in lung cancer.
Early detection of SMW is particularly relevant, representing one of the few ideally modifiable prognostic factors in several malignancies, including NSCLC (3). The baseline identification of these patients could facilitate their prompt referral to dedicated, multidisciplinary therapeutic interventions, such as tailored nutritional counselling, personalized exercise programs, and pharmacological approach, aimed at optimizing body composition and thus improving overall prognosis. Furthermore, while no statistically significant correlation was observed between the fT3/fT4 ratio and PFS (probably due to the small sample size), a significant association was identified with OS, suggesting a potential prognostic role for this parameter in immunotherapy-treated NSCLC.
However, our study has some limitations. Firstly, the small sample size may limit the statistical power and generalizability of our findings. Secondly, the exclusive inclusion of patients treated with pembrolizumab monotherapy may restrict the applicability of the results to broader NSCLC populations receiving other immune checkpoint inhibitors or chemoimmunotherapy combinations. Lastly, we assessed only baseline fT3/fT4 ratio values, without evaluating their longitudinal changes over the course of treatment, which could provide further insight into the dynamic relationship between thyroid function, treatment response and clinical outcomes. These limitations, along with our preliminary findings, have prompted us to design a prospective observational study to further investigate the role of the fT3/fT4 ratio in relation to body composition, as well as muscle strength and physical performance, and treatment response in advanced NSCLC. The study will specifically include patients undergoing immunotherapy, either alone or in combination with chemotherapy, to strengthen the clinical applicability of fT3/fT4 ratio. Furthermore, we will monitor longitudinal changes in fT3/fT4 ratio during treatment to evaluate its dynamic association with disease progression, therapeutic response, and muscle mass changes.
Conclusions
fT3/fT4 ratio may represent a simple but reliable proxy for early detection of SMW in NSCLC patients receiving upfront mono-immunotherapy. Given the prognostic relevance of SMW, this parameter could support baseline clinical risk stratification. Further prospective studies are warranted to validate its utility and explore its association with immunotherapy response and long-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study was approved by the local ethical committee (prot. 2193CESC, 2022) and it was conducted according to the ethical standards of Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Author contributions
SE: Writing – original draft, Writing – review & editing, Resources, Data curation. MS: Resources, Data curation, Writing – original draft, Writing – review & editing. IT: Writing – original draft, Writing – review & editing, Resources. IMS: Writing – review & editing, Resources, Writing – original draft. LP: Resources, Writing – original draft, Writing – review & editing. DT: Resources, Writing – review & editing, Writing – original draft. AA: Resources, Writing – review & editing, Writing – original draft. JI: Writing – review & editing, Resources, Writing – original draft. LC: Writing – review & editing, Writing – original draft, Resources. AD: Writing – original draft, Resources, Writing – review & editing. AS: Resources, Writing – review & editing, Writing – original draft. MC: Writing – review & editing, Resources, Writing – original draft. IS: Methodology, Formal analysis, Writing – review & editing, Resources, Writing – original draft. MCM: Validation, Supervision, Writing – original draft, Visualization, Writing – review & editing. FL: Supervision, Writing – review & editing, Writing – original draft, Validation, Visualization. MT: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision. EB: Supervision, Writing – review & editing, Writing – original draft, Validation, Visualization. MM: Writing – review & editing, Supervision, Visualization, Writing – original draft, Validation. SP: Writing – review & editing, Supervision, Writing – original draft, Visualization, Validation. LB: Supervision, Writing – review & editing, Writing – original draft, Visualization, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
LB and AA are supported by grants from Associazione Pietro Casagrande ONLUS. E.B. is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Investigator Grant No. IG20583), by Institutional funds of Università Cattolica del Sacro Cuore (UCSC-project D1), and funds of Ministero della Salute (Ricerca Corrente 2023). SP is supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Next Gen Clinician Scientist 2023 n° 30204). These funding are unrelated to this study.
Conflict of interest
Author MT received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Takeda, Amgen, Merck, Sanofi, Johnson & Johnson, Daiichi Sankyo. Author MT received institutional research grants from Astra-Zeneca, Boehringer Ingelheim and Roche. Author MT received travel support from Amgen and Takeda. Author EB has received grants or contracts from Astra-Zeneca, Roche and honoraria for lectures from Merck-Sharp & Dome, Astra-Zeneca, Pfizer, Eli-Lilly, Bristol-Myers Squibb, Novartis, Takeda and Roche. Author EB has been member of Data Safety Monitoring Board or Advisory Board of Merck-Sharp & Dome, Pfizer, Novartis, Bristol-Myers Squibb, Astra-Zeneca, and Roche. SP received honoraria or speakers’ fees from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Merck, Takeda, Amgen, Novartis, and Roche, outside the submitted manuscript. Author LB received speakers’ fees from AstraZeneca, Merck Sharp & Dohme, and Roche, outside the submitted manuscript; travel fees from Takeda. Author FL was employed by 3trees Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AC declared a past co-authorship with the author EB to the handling editor.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang Y, Safi M, Hirsch FR, Lu S, Peters S, Govindan R, et al. Immunotherapy for advanced-stage squamous cell lung cancer: the state of the art and outstanding questions. Nat Rev Clin Oncol. (2025) 22(3):200–14. doi: 10.1038/s41571-024-00979-8
2. Trestini I, Caldart A, Dodi A, Avancini A, Tregnago D, Sartori G, et al. Body composition as a modulator of response to immunotherapy in lung cancer: time to deal with it. ESMO Open. (2021) 6:100095. doi: 10.1016/j.esmoop.2021.100095
3. Trestini I, Belluomini L, Dodi A, Sposito M, Caldart A, Kadrija D, et al. Body composition derangements in lung cancer patients treated with first-line pembrolizumab: A multicentre observational study. J Cachexia Sarcopenia Muscle. (2024) 15:2349–60. doi: 10.1002/jcsm.13568
4. Wang J, Cao L, and Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis. Int Immunopharmacol. (2020) 88:106907. doi: 10.1016/j.intimp.2020.106907
5. Trestini I, Gkountakos A, Carbognin L, Avancini A, Lanza M, Molfino A, et al. Muscle derangement and alteration of the nutritional machinery in NSCLC. Crit Rev Oncol Hematol. (2019) 141:43–53. doi: 10.1016/j.critrevonc.2019.06.007
6. Salvatore D, Simonides WS, Dentice M, Zavacki AM, and Larsen PR. Thyroid hormones and skeletal muscle–new insights and potential implications. Nat Rev Endocrinol. (2014) 10:206–14. doi: 10.1038/nrendo.2013.238
7. Wang K, Zhang D, Cao G, Wang C, Wang L, Zhao R, et al. A low free T3 to free T4 ratio is associated with sarcopenia in euthyroid patients with type 2 diabetes mellitus. J Diabetes Res. (2022) 2022:2305156. doi: 10.1155/2022/2305156
8. Zhu Z, Qian Y, Ding P, Jin K, Chen J, Fu J, et al. Exploring the association between muscle mass and thyroid function in Chinese community subjects over 45 years old with normal thyroid function: a cross-sectional analysis. Front Endocrinol (Lausanne). (2024) 15:1411805. doi: 10.3389/fendo.2024.1411805
9. Pasqualetti G, Schirripa M, Dochy E, Fassan M, Ziranu P, Puzzoni M, et al. Thyroid hormones ratio is a major prognostic marker in advanced metastatic colorectal cancer: Results from the phase III randomised CORRECT trial. Eur J Cancer. (2020) 133:66–73. doi: 10.1016/j.ejca.2020.04.023
10. Maruzzo M, Verzoni E, Vitale MG, Dionese M, Buti S, Galli L, et al. Prognostic value of thyroid hormone ratio in patients with advanced metastatic renal cell carcinoma: results from the threefour study (Meet-URO 14). Front Oncol. (2021) 11:787835. doi: 10.3389/fonc.2021.787835
11. Pierantoni F, Dionese M, Basso U, Lai E, Cavasin N, Erbetta E, et al. The prognostic value of thyroid hormone levels in immunotherapy-treated patients with metastatic urothelial carcinoma. Clin Genitourin Cancer. (2023) 21:e378–e85. doi: 10.1016/j.clgc.2023.04.006
12. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflammation. (2016) 2016:6757154. doi: 10.1155/2016/6757154
Keywords: non-small cell lung cancer (NSCLC), muscle wasting, immune checkpoint inhibitors (ICIs), thyroid hormones, sarcopenia, body composition
Citation: Eccher S, Sposito M, Trestini I, Scaglione IM, Pasqualin L, Tregnago D, Avancini A, Insolda J, Confortini L, Dodi A, Stefani A, Cintoni M, Sperduti I, Mele MC, Loupakis F, Tiseo M, Bria E, Milella M, Pilotto S and Belluomini L (2025) Low fT3/fT4 ratio as a proxy for muscle wasting in patients with advanced non-small cell lung cancer treated with pembrolizumab. Front. Oncol. 15:1635321. doi: 10.3389/fonc.2025.1635321
Received: 26 May 2025; Accepted: 28 July 2025;
Published: 27 August 2025.
Edited by:
Vanesa Gregorc, IRCCS Candiolo Cancer Institute, ItalyReviewed by:
Angelo Castello, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyKecheng Huang, Huazhong University of Science and Technology, China
Copyright © 2025 Eccher, Sposito, Trestini, Scaglione, Pasqualin, Tregnago, Avancini, Insolda, Confortini, Dodi, Stefani, Cintoni, Sperduti, Mele, Loupakis, Tiseo, Bria, Milella, Pilotto and Belluomini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Pilotto, c2FyYS5waWxvdHRvQHVuaXZyLml0
†These authors share first authorship
‡These authors share last authorship
 Serena Eccher
Serena Eccher Marco Sposito
Marco Sposito Ilaria Trestini
Ilaria Trestini Ilaria Mariangela Scaglione1
Ilaria Mariangela Scaglione1 Alice Avancini
Alice Avancini Alessandra Dodi
Alessandra Dodi Alessio Stefani
Alessio Stefani Marco Cintoni
Marco Cintoni Isabella Sperduti
Isabella Sperduti Emilio Bria
Emilio Bria Michele Milella
Michele Milella Sara Pilotto
Sara Pilotto Lorenzo Belluomini
Lorenzo Belluomini