- 1Department of Radiation Therapy, Affiliated Zhongshan Hospital of Dalian University, Dalian, China
- 2Department of Breast Surgery, Dalian Women and Children's Medical Group, Dalian, China
- 3Department of Intervention, The Second Hospital Affiliated to Dalian Medical University, Dalian, China
Tall cell carcinoma with reversed polarity (TCCRP) is a primary invasive breast cancer that is clinicopathologically characterized by reversed polarity of the nucleus of the tumor cells near the cell edge. This pathologic feature is so rare that only 82 cases of TCCRP have been reported worldwide since it was first reported in 2003. Herein, we present the 83rd case of TCCRP. Going one step further than the previous reports, our case includes genetic testing. We sought to explore whether there are new mutation sites, with a view to identifying more therapeutic options for patients in future, to find PIK3CA p.H1047R mutation and IDH2 R172T mutation.We have found the macrophase makers CD68/CD163 double positive in this patient, which may indicate a poor prognosis in the early time. Therefore, the patient was treated with postoperative radiotherapy. The patient continues to be followed up for prognostic evaluation, and no recurrence or metastasis occurred until now. The case has represented a full view to gaining an improved understanding of TCCRP.
Introduction
Tall cell carcinoma with reversed polarity (TCCRP) was first reported in 2003 and included as a histological subtype in the WHO breast cancer classification in 2019 (1). Morphologically, TCCRP resembles papillary carcinoma of the thyroid gland, with eosinophilic or hyaline cytoplasm of tumor cells, low-density ground-glass chromatin, as well as nuclear grooves and intranuclear pseudo-inclusions in some tumor cells. However, in immunohistochemistry studies, TCCRP exhibits no concordance with thyroid-specific markers Thyroid Transcription Factor-1(TTF-1), Thyroglobulin(TG), which proves that TCCRP lesions do not occur as a result of breast metastasis of papillary thyroid carcinoma. In addition, an interesting finding was “reversed polarity,” which refers to the arrangement of the tumor cell nuclei at the cell margins (2). In addition to the specific morphological features of TCCRP, the RET gene rearrangements and BRAF gene mutations typical of papillary thyroid carcinoma are not found in TCCRP (3, 4). Instead, IDH2 R172S or R172T locus mutations are detected frequently (4), and, in a few cases, inactivating TET2 mutations are detected, usually accompanied by gene mutations in the PI3K signaling pathway (2–5).
Because of the small number of cases worldwide, the mild clinical course, and the absence of specific signs and symptoms, the diagnosis of TCCRP is established on the basis of the histopathological feature of polarity reversal and is supplemented by genetic testing (6, 7), which is not mandatory. However, when the differential diagnosis between TCCRP and other types of papillary lesions of breast origin, such as solid papillary carcinoma, is not difficult or when the patient presents with a concurrent papillary thyroid carcinoma of indistinguishable origin, it would be necessary to depend on the specific molecular biology of hotspot mutations in the PI3K and IDH2 genes for diagnosis.
Due to the rarity of the condition and good postoperative prognosis of most patients, only some patients are treated with adjuvant chemotherapy (7), with very few instances of lymph node metastases, distant metastases, and recurrences. Therefore, TRRCP is currently considered to be a unique (1, 2, 8), inert subtype of breast cancer, with low metastatic potential (3, 9). However, in the current case, we detected PIK3CA p.H1047R mutation as well as IDH2 R172T mutation (5), which further confirms the significance of the PIK3CA mutation site corresponding to the PIK3 and mTOR pathways for immunotherapy. Although the condition is rare and the prognosis is generally good in most cases, there have been some cases of distant and brain metastasis (10, 11). With this report, we sought to provide evidence for the therapeutic benefit of radiotherapy and targeted drugs in this condition.
Case report
We report the 83rd case of TCCRP, Han Chinese, in a 68-year-old female patient with triple-negative breast cancer, with last menstrual period at the age 45 years, and no family history of cancer. Moreover, she denied any history of smoking or alcohol consumption, any history of infectious diseases, any history of hormone replacement therapy or prolonged radiation exposure. The patient’s occupation was retired, with no specific occupational hazards identified. Physical examination upon admission revealed bilaterally symmetrical breasts without obvious masses or skin changes. No peau d’orange appearance, skin dimpling, nipple retraction, or deviation was observed. There was no nipple discharge or bleeding. Palpation identified no nodules, tenderness, or fixation to the skin or underlying tissues, with normal mobility. No obvious abnormalities were detected in either breast. The patient had undergone surgical removal of a fibroadenoma in the left breast in 2014. In 2022, a right breast mass was detected, and vacuum-assisted biopsy of the right breast mass was performed. The intraoperative frozen section showed a intraductal papilloma with atypical ductal hyperplasia in a portion of the right breast mass. Paraffin-based pathology following the biopsy showed a tall cell breast carcinoma, with reverse polarity of the right breast. Immunohistochemistry of the frozen section revealed that the lesion was ER (-), PR (-), Her2(-), with Ki-67 (+15%).
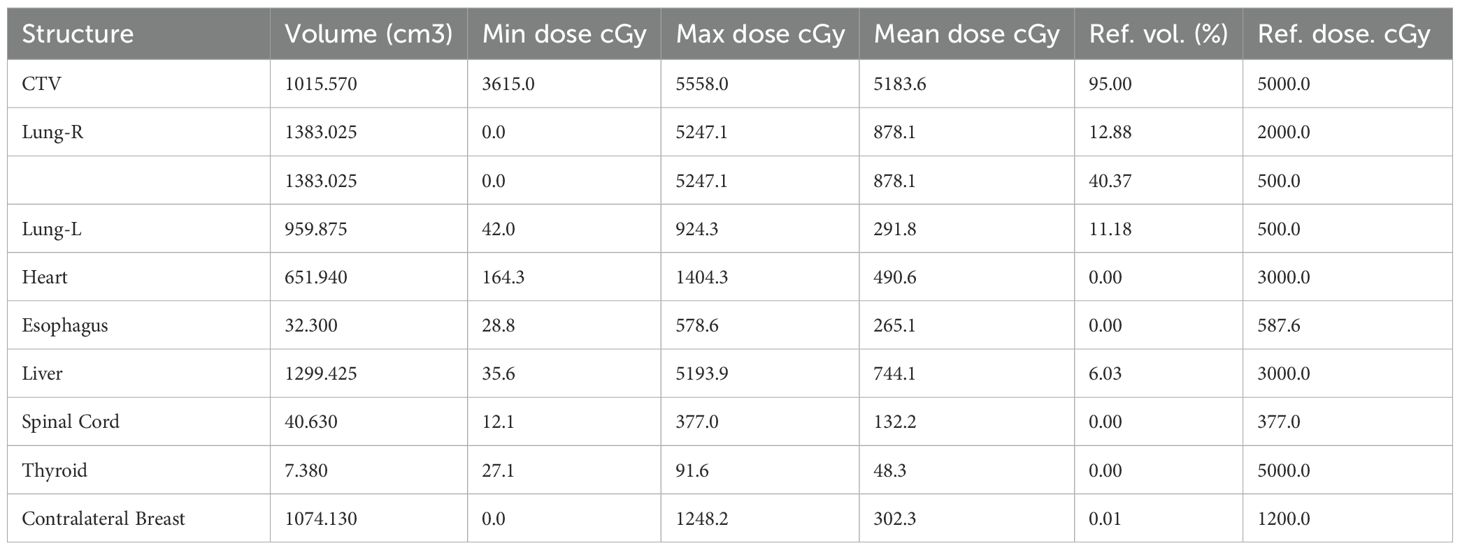
The patient underwent a secondary procedure to ensure negative margins. Preoperative ultrasound of the right breast showed slightly enhanced localized echogenicity at 7–9 o’clock, with a size of 3.5×3.2×2.4 cm, unclear borders, irregular morphology, no obvious blood flow signal on color Doppler flow imaging, and no obvious enlargement of lymph nodes in both axillae (Figures 1A, B). Contrast-enhanced magnetic resonance imaging (MRI) scan of the breast showed multiple scattered small nodular foci of significant enhancement in the right breast, with most of the lesions having a diameter of 2–3 mm and clear and smooth borders. One of the lesions was larger (diameter about 6mm) and located in the outer and lower quadrant, having a smooth border, with time-signal intensity curve (TIC) of the plateau pattern and significant enhancement of the ductal region seen beside it. The upper edge of the skin of the right breast was not smooth, and the reconstruction image showed a rounded area of slightly high signal intensity (Figure 1C). The right breast mass was then excised, and the upper, lower, inner, and outer margins of the excised tissue were collected and sent for examination. During the operation, and a bulky piece of breast tissue was seen, with size 7.5 × 6 × 1.7cm, and a grayish-yellow area was seen at the marking place, 0.8 × 0.6 × 0.5cm, with medium texture. The remaining breast tissue appeared grayish white, interspersed with areas of grayish yellow and was soft in texture. No cancerous tissue was seen at the margins of the excised breast tissue (upper, lower, inner, or outer). The tumor cells were arranged in a multinodular, solid, papillary arrangement, while the tumor cells themselves were hypercolumnar cytoplasmic, eosinophilic, and rich in mitochondria. Dispersed in the nodules were single round to oval mesonuclear grade cells. The oval nucleus showed invaginations of the nuclear membrane parallel to the long axis of the nucleus or a nuclear furrow with folded nuclear membrane, and the nuclear furrow invagination was encapsulated into the cytoplasm as intranuclear pseudo-inclusion bodies (Figures 2A1-3). The results of immunohistochemistry were as follows: ER (-), PR (-), HER-2 (-), Ki-67 (+ about 10%), AR (-), CK5/6 (+), CK7 (+), P63 (-), Calponin (-), SIA (-), SMMHC (-), GCDFP-15 (-), GATA-3 (-), TTF1 (-), CD68 (histiocyte+), CD163 (histiocyte+) (Figures 2B–E). On the basis of the investigative results, the patient was treated with local radiotherapy of the right breast after surgery, with planning target volume of 50Gy/25Fx (Figures 3A–D, Table 1). Dose Volume Histogram (DVH) shows, for any given point on a curve, the corresponding dose (X-value) and volume (Y-value) can be determined. For example, the CTV (Clinical Target Volume) curve shows that 100% of the volume received at least 36 Gy, and 95% of the volume (as per our planning goal) received the prescribed dose of 50 Gy. Steep curves (e.g., CTV) are ideal for target volumes, indicating a homogeneous dose with a sharp fall-off outside the target. Gradual curves (e.g., Lung-R) are typical for OARs(Off-Axis Ratio), showing that a gradient of doses is delivered across the organ. No acute adverse events of grade 2 or higher (according to CTCAE v5.0 criteria) during the radiotherapy was found. The patient was scheduled for periodic follow-up. This comprehensive surveillance strategy encompassed serial tumor marker evaluation (CEA, CA 125, CA 15-3), breast and abdominal ultrasonography, with the former including detailed inspection of the axillary, supraclavicular, and infraclavicular lymph nodes. Cross-sectional imaging via CT was utilized for the chest and head, supplemented by whole-body bone scintigraphy to screen for osseous metastases. Every 3 months for the first year, every 6 months for the second year and then annually thereafter. At the most recent follow-up visit in Aug, 2025 (approximately 24 months post-treatment), the patient remained asymptomatic with no clinical or radiological evidence of local recurrence or distant metastasis. The detailed timeline of key events related to the disease has been showed in Table 2. The resected cancer tissue was subjected to next-generation sequencing (NGS) using a GENESEEQPRIME® pan-cancer panel covering approximately 1.5 Mb of the coding regions of over 400 genes (including exons, fusion-associated introns, variable shear regions, and specific microsatellite (MS) locus regions of more than 400 genes related to tumor targeting, diagnosis, prognosis, and tumor development). The TMB was calculated as 2.1 mutations/Mb, which is classified as TMB-Low based on the established cut-off of ≥ft mut/Mb for panels of this size. The results of NGS revealed that our patient had a missense mutation in exon 20 of the p.H1047R (PIK3CA), with a mutation abundance of 25.00%, and p.R172T exon 4 missense mutation in IDH2, with a mutation abundance of 21.91%. Additionally, 2.1 mutations/Mb, indicating a low tumor mutational burden (TMB), sorted in the top 84.59% were noted (Figures 4A–C). The mutational sites of enzymes related to polymorphisms in drug metabolism are as follows: ERCC2 gene polymorphic mutation, XRCC1 Q399R pure-sum mutation, UGT1A1 polymorphic mutation, TYMS-6bp/-6bo polymorphic mutation, CYP2B6 gene pure-sum polymorphic mutation, and NQO1 gene polymorphic mutationality (Table 3). Mineralocorticoid receptor (MR)-related genes and MS-high (MSI-H) were not detected, and HLA-I typing was determined to be partially pure-sum (Table 4).
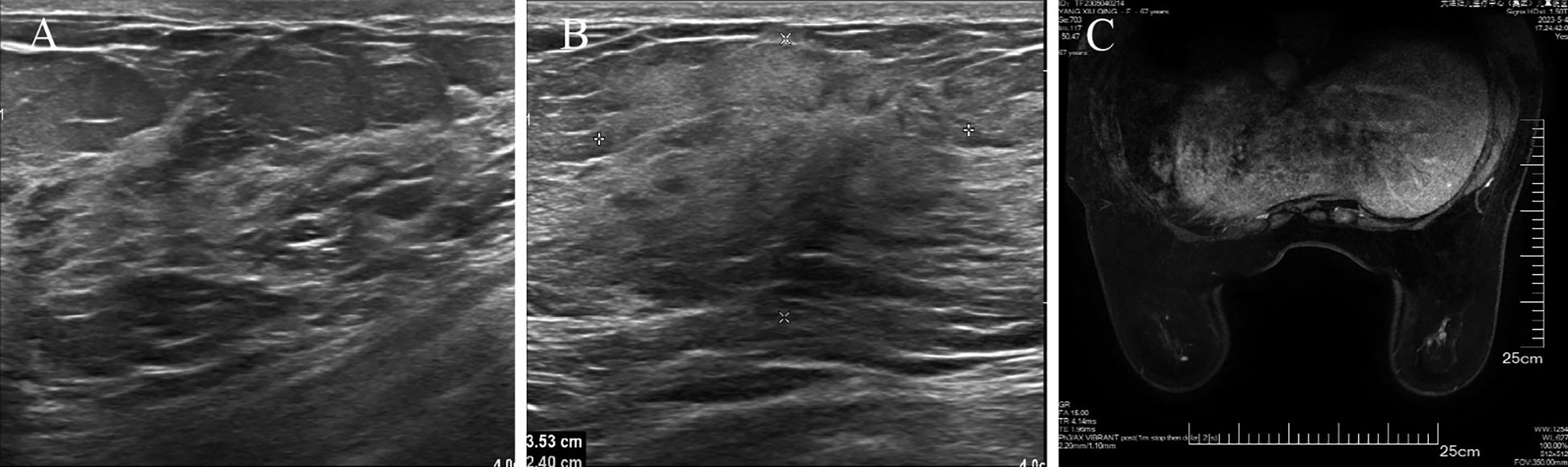
Figure 1. Preoperative imaging results of TCCRP. (A, B) Ultrasound imaging before the patient underwent a secondary procedure. (C) MR enhancement scan before the patient underwent a secondary procedure. Axial view of a T1-weighted contrast-enhanced fat-suppressed MR image obtained before the patient underwent a secondary procedure.
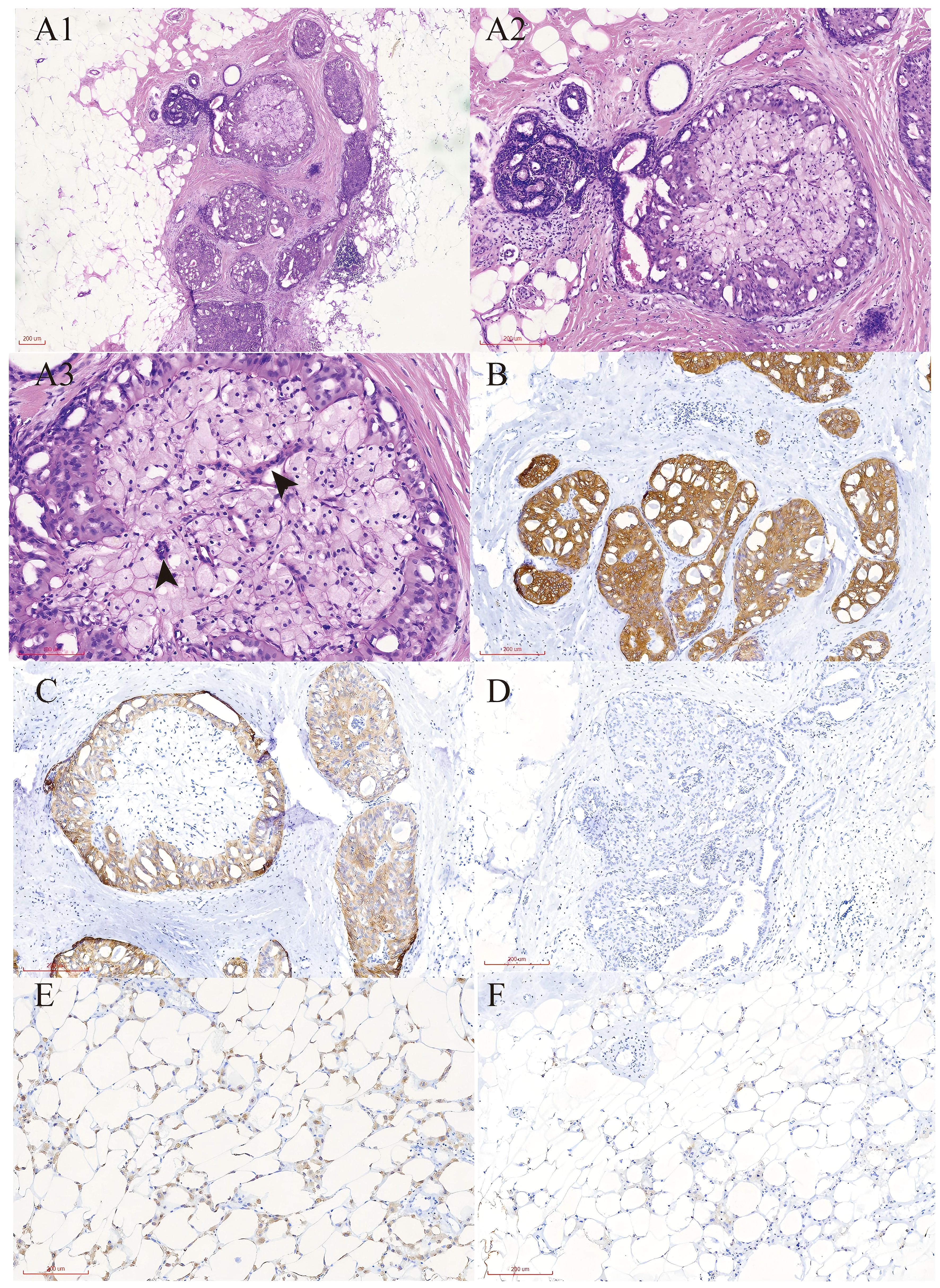
Figure 2. Pathological report of TCCRP. (A) Representative H&E stained sections show the histological features of tall cell carcinoma with reversed polarity. Magnification;A1×100,A2×200,A3×400. (B) Immunohistochemistry results show the cells were cytoplasmic positive for CK7.Magnification; ×100. (C) Immunohistochemistry results show the cells were cytoplasmic positive for CK5/6.Magnification; ×100. (D) Immunohistochemistry results show the cells were cytoplasmic positive for Ki67.Magnification; ×100. (E) Immunohistochemistry results show the cells were cytoplasmic positive for CD68.Magnification; ×100. (F) Immunohistochemistry results show the cells were cytoplasmic positive for CD163. Magnification; ×100.
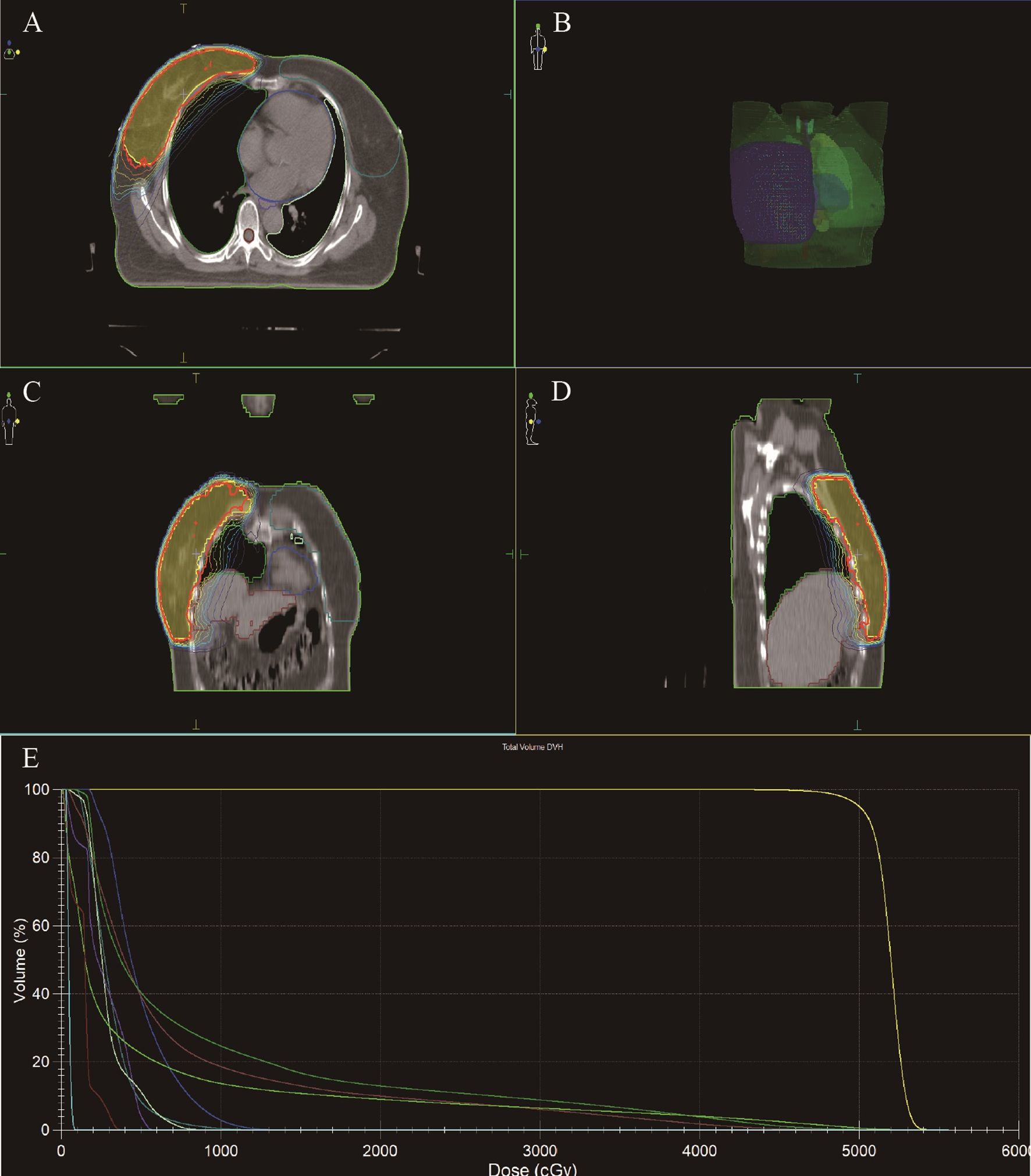
Figure 3. Radiotherapy target area outlining in TCCRP. (A-D) Radiation Treatment Target Delineation Image shows the target areas of tumor and normal tissues. (E) Total Volume DVH (DVH, Dose Volume Histogram) shows the distribution of radiation doses received by target areas of tumor and normal tissues. The DVH is a graphical representation of the radiation dose distribution delivered to the target volumes and critical organs at risk (OARs). The X-axis represents the radiation dose (in Gy). The Y-axis represents the volume of the structure (as a percentage) that receives at least the corresponding dose on the X-axis.
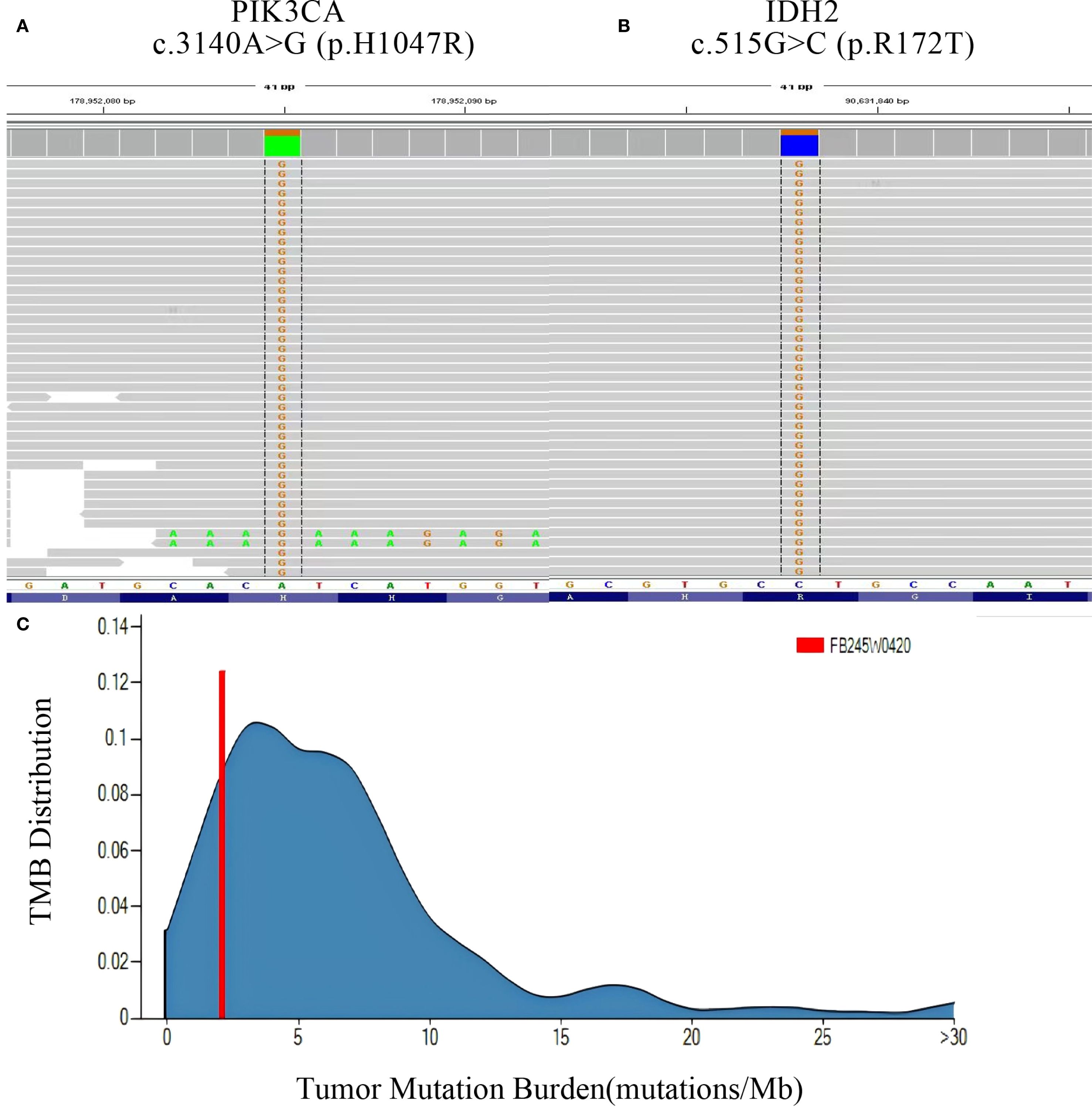
Figure 4. Panoramic genetic testing results of TCCRP. (A, B) NGS reads showing the mutation sites and abundance of PI3KCA and IDH2 genes. (C) TMB(Tumor Mutation Burden) reads showing the distribution range of TMB, respectively.
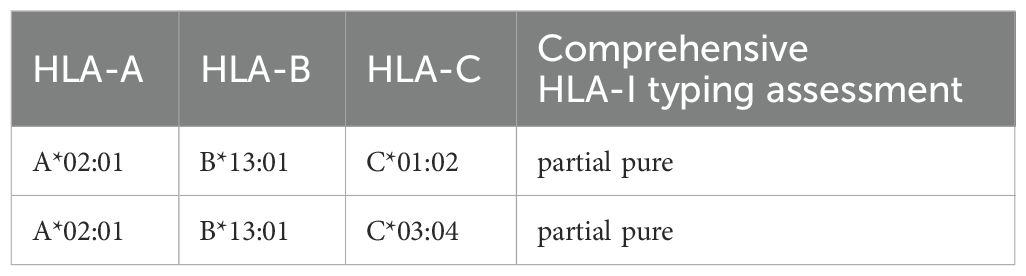
Table 4. Comprehensive HLA-I typing assessment results predict efficacy against immune checkpoint inhibitors.
Discussion
Worldwide, breast cancer ranks first in incidence rate (23.8%) and mortality rate (15.4%), surpassing lung cancer. According to the latest report (cancer) released by The National Cancer Center of China (NCC), there were 357,200 new cases of breast cancer in 2022, accounting for 7.4% of the 4,824,700 new cancer cases. This shows that the incidence of breast cancer among the Chinese female population with cancer (2,290,800, cases) is the second highest, after lung cancer. Additionally, breast cancer is one of the top five causes of cancer-related mortality (12, 13).
In terms of molecular typing, the types of breast cancer recognized are as follows: luminal A (ER+/PR+,HER-2-), Luminal B (ER+/PR+,HER-2+), HER-2 overexpression (ER-/PR-/HER-2+), and Basal-like (ER-/PR-/HER-2-). Our patient had the basal-like (ER-/PR-/HER-2-) type. Although most of the molecular subtypes of TCCRP patients are triple-negative (5, 8), some patients present with tumors that are ER/PR weakly positive. Among the 83 patients reported thus far, TCCRP has shown no significant correlation with ethnicity and geography, and the age of onset of the disease ranges from 40 to 85 years.
The results of immunohistochemistry studies in this case were as follows: showed ER(-), PR(-), HER-2 (-), Ki-67(+ about 10%), AR(-), CK5/6(+), CK7(+), P63(-), Calponin(-), SIA(-), SMMHC(-), GCDFP-15(-), GATA-3(-), TTF1(-), CD68 (histiocyte+), and CD163 (histiocyte+). The diagnosis of TCCRP is determined not only by its distinctive morphology and molecular genotype, the variable expression of conventional breast markers. Among these markers, CD68 and CD163 are two important indicators that are related to the tumor microenvironment (TME). TME is a general term that refers to the environment in which tumor cells are located, and it is mainly composed of mesenchymal cells, neighboring cells, blood vessels, and immune cells, in addition to tumor cells. The TME can be divided into a non-immune microenvironment, which is dominated by fibroblasts and vascular endothelial cells, and an immune microenvironment, which is dominated by macrophages. The components of the TME, especially, the tumor-associated macrophages (TAMs), are the occurrence of breast cancer and tumorigenesis (14). This makes these important targets in the diagnosis and treatment of breast cancer. Leukocyte differentiation antigen molecule 68 (CD68) is considered to be one of the main markers involved in the activation and infiltration of TAMs, while CD68 is a characteristic marker of M1-type macrophages, which exert a tumor-killing effect caused by DNA damage, resulting from the production of oxygen free radicals, and inhibit tumor cell growth. CD163 is known to be a characteristic marker of M2-type macrophages, which are considered to be immune cells involved in tumor growth and metastasis, and its activities include the induction of angiogenesis, inhibition of T-cell activity, and release of chemokines such as inflammatory growth factors, for the promotion of tumor cell growth. Some studies have shown that the five-year progression-free survival (PFS) is higher in non-metastatic breast cancer patients with low expression of CD68-positive macrophages and that the prognosis of patients is worse when CD68 positivity is highly expressed (15). TAMs with double positivity for CD68 and CD163 are more likely to be found in lymph node metastatic tumors in the tumor microenvironment (5, 6). Macrophages with CD68/CD163 double positivity are associated with poor prognosis of early-stage breast cancer, high-level histological grading, and high Ki67 expression. Positivity of macrophages only for CD163 has been found to be associated with tumor stage and lymph node status (16), whereas macrophage positivity for only CD68 has been associated with the molecular subtype of breast cancer, independent of other clinicopathological parameters. In this patient with TCCRP, we found the presence of TAM M1-type and M2-type markers for the first time. During the short term of the postoperative follow-up, this patient has had good overall prognosis; however, continued follow-up for 10–15 years is still needed to assess the efficacy of the treatment as a whole. Most of the current evidence has shown that the presence of CD68/CD163 double-positive macrophages in the early stage represents a poor prognosis (13); therefore, after discussing the risks of the condition adequately with the patient, we opted to administer total breast radiation therapy to further minimize the risk of future recurrence (8, 12, 13, 17). Although the prognostic significance of TAMs in TCCRP remains undefined, the presence of a CD68+/CD163+ macrophage infiltrate, a signature associated with adverse features in broader breast cancer cohorts, was considered a potential high-risk factor in this individual case. Moreover, combined with the patient’s willingness for maximal local control after breast-conserving surgery, the patient was scheduled for adjuvant radiotherapy postoperatively. This highlights the necessity for larger studies to validate prognostic markers and define optimal adjuvant therapy in TCCRP.
In addition, the results of NGS showed that our patient had 2.1 mutations/Mb, which indicates a low TMB and for which immunotherapy was considered to be of little benefit. In addition, the patient had a tumor-specific mutation of the PIK3CA gene at exon 20, a missense mutation with mutation type c.3140A>G (p.H1047R), and mutation abundance of 25.00% as well as IDH2 gene mutation P.R172T at exon 4, missense mutation with mutation type c.515G>C (p.R172T), and mutation abundance of 21.91%; this is a non-embryonic mutation that is not hereditary and is consistent with the existing findings. PIK3CA p.H1047R mutation is located in the structural domain of phosphatidylinositol 3/4 kinase and is a common activating mutation in the PIK3CA gene. PIK3CA has been implicated in various cancers, including gastric cancer, hepatocellular cancer, non-small-cell lung cancer, and breast cancer and it can activate the PI3K/AKT/mTOR signaling pathway by enhancing the activity of PI3K lipid kinase, thus promoting the invasive and metastasizing ability of cancer cells and contributing to tumor development. PIK3CA-activating mutations can increase the sensitivity of PI3K, mTOR inhibitors such as everolimus (18). In 2019, the FDA approved a regimen comprising the inhibitor apelalisib in combination with fulvestrant, targeting the PI3Kα isoforms, as well as the regimen of capivaserti in combination with fulvestrant, targeting three AKT isoforms to be used in patients with HR-positive, HER2-negative breast cancer harboring PIK3CA mutations. Fulvestrant has no estrogenic effect per se, but it can bind ER to competitively antagonize the estrogenic effect; when fulvestrant binds to ER, it can block ER function by inducing ER degradation. IDH2 p.R172T mutation has been reported in osteoblastoma, chondrosarcoma, and hemolymphoma, and R172 is a hotspot mutation site of IDH2 (12). Studies have shown that mutation in the R172 site can be used to treat patients with HR-positive HER2-negative breast cancer carrying PIK3CA mutation. The R172 locus mutation can influence IDH2-encoded protein isocitrate dehydrogenase activity by decreasing the levels of intracellular α-ketoglutarate, which may promote the conversion of isocitrate to 2-hydroxyglutarate (2-HG); The resultant overaccumulation of 2-HG causes excessive cellular proliferation, which, in turn, contributes to oncogenic changes and tumor growth and promotes tumor development (2). Some studies have shown that the IDH2 inhibitor endesipine can be used to treat relapsed or refractory acute myelogenous leukemia carrying IDH2 mutations. Considering these observations, we believe that it may be worthwhile to examine whether relapsed and metastatic TCCRP patients with PIK3CA-activating mutations and IDH2 mutations would benefit from the drugs everolimus and endesipine. In future, we also intend to explore the effects of these drugs and their molecular mechanisms through more case studies and in vitro and in vivo investigations, so as to obtain more evidence regarding the targeted therapy of TCCRP (8, 10, 11, 19).
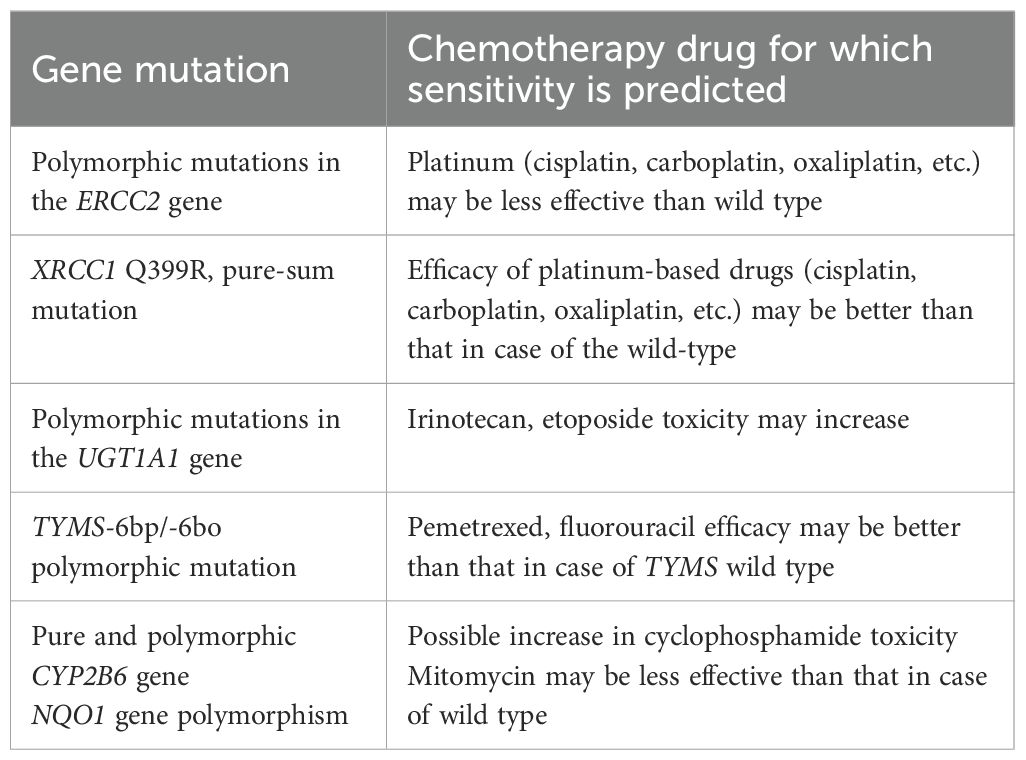
Furthermore, in this case, the results of NGS for the sensitivity of the tumor to common chemotherapeutic drugs revealed CYP2B6*6 polymorphism, i.e., the simultaneous occurrence of p.Q172H and p.K262R heterozygous mutations in both exons 4 and 5 of the CYP2B6 gene; this, in turn, reduces the in vivo activity of the 2B6 enzyme and decreases its effects on drug metabolism, leading to increased toxic adverse effects of cyclophosphamide and isocyclophosphamide (9). The p.K751Q heterozygous mutation in the ERCC2 gene is a single-nucleotide polymorphism (rs13181) missense mutation, which is associated with the risk of various tumors such as lung, bladder, breast, and skin cancers, and low risk indicators. Lung cancer patients carrying the K751Q mutation have been shown to be resistant to platinum drugs. The p.P187S pure mutation (i.e., the c.559C>T mutation) in the NQO1 gene is a missense mutation caused by the single-nucleotide polymorphism rs1800566, with a risk allele of T. This mutation can lead to a loss of activity by affecting the enzyme’s stability, which has a protective function against benzene toxicity, is therefore disruptive. The mutation can increase the risk of breast cancer as well as lung cancer in non-smokers as well as the risk of hematotoxicity and leukemia in individuals with environmental exposure to benzene. In addition, this polymorphism may be associated with a poorer prognosis in breast cancer patients who have received doxorubicin and cyclophosphamide chemotherapy. The TYMS gene 6-bp pure deletion polymorphism (rs151264360) is a deletion mutation of a 6-bp (TTAAAG) nucleotide fragment in the 3-UTR region. This genotype reduces the level of expression of thymidylate synthase compared to the wild-type and enhances the expression of fluoride and the chemotherapeutic agent pemetrexed (20). The UGT1A1*28 polymorphism (rs34983651) is an A(TA)(6)TAA>A(TA)(7)TAA mutation triggered by TA insertion upstream of the gene. This heterozygous polymorphism significantly reduces the expression and activity of the UGT1A1 enzyme, which increases the toxicity and adverse effects of irinotecan and etoposide (21). The XRCC1 gene p.Q399R pure mutation is a missense mutation caused by single-nucleotide polymorphism rs25487, which is associated with the risk of non-small-cell lung cancer, breast cancer, colorectal cancer, gastric cancer, and other types of tumors. GG pure mutation is associated with the efficacy of platinum-based chemotherapeutic drugs and can enhance the response rate of cells to platinum-based drugs. Therefore, in this case of TCCRP, the patient had increased sensitivity to the drugs pemetrexed and fluorouracil, with increased toxicity to the drugs irinotecan, etoposide, and cyclophosphamide. These chemotherapeutic drug choices form the basis of the treatment for possible subsequent recurrence and metastasis. HLA-I typing was determined to be partially pure-sum, which hints that overall survival after immune checkpoint inhibitor treatment was lower for pure and partially pure HLA1 compared to total pure cases.
TCCRP is rare worldwide, has an inert pathological type, and progresses to advanced stages only in a few cases. However, it is important patients with TCCRP are monitored with long-term follow-up, to rule out the possibility of recurrence and metastasis. Currently, there is inadequate evidence to show that postoperative adjuvant radiotherapy is beneficial for patients with TCCRP. However, since our patient had positivity for both CD68 and CD163, which is considered to indicate poor prognosis and since the patient requested postoperative adjuvant radiotherapy, we irradiated the whole breast on the affected side, without subsequent dosing in the tumor bed area. The patient continues to be followed up, and until the time of preparation of this manuscript, she has not shown any signs of recurrence or metastasis. We believe that publication of further reports on similar cases and long-term follow-up of the patients will help us determine whether or not adjuvant radiotherapy can be beneficial.
In conclusion, our report not only adds to the evidence regarding the diagnosis of a rare pathotype, but also identifies, for the first time, the presence of CD68/CD163 in TCCRP. We have also discussed the chemosensitivity of different genetic loci involved in this condition as well as feasibility of using targeted drugs against the PI3K and the IDH2 mutation loci in TCCRP. We have also employed postoperative adjuvant radiotherapy in this breast-conserving patient and followed up its effect on prognosis in this case, with a view to gain insight into the individualized and precise treatment of TCCRP. Our experience, in this case, may bring more prospective and cautionary evidence about TCCRP, and TAM, as an important target of immune checkpoint inhibitors, may trigger more new lines of investigation regarding immunotherapy for TCCRP.
Data availability statement
The data presented in the study are deposited in the NGDC repository, accession number HRA014091.
Ethics statement
The studies involving humans were approved by the Bioethics Committee of Zhongshan Hospital, Dalian University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WD: Writing – original draft. LZ: Conceptualization, Writing – review & editing. JG: Investigation, Writing – review & editing. ZW: Writing – review & editing, Data curation. MR: Writing – review & editing, Methodology. HW: Writing – review & editing, Formal analysis. YD: Validation, Writing – review & editing. CG: Resources, Writing – review & editing. JJ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Science Foundation of China (#81803109) and the Doctor Study-up Foundation of Liaoning Province (2019-BS-010) and the Innovation Support Program for High-level Talents (Young Science and Technology Star Project) of Dalian Science and Technology Bureau (2021RQ018) and the Scientific Medical Research Program Project of Dalian National Health Commission (2211033).
Acknowledgments
We sincerely thank the patient and her family, and written informed consent was obtained from the patient for this case report and any accompanying images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ER: Estrogen receptor
PR: Progesterone receptor
HER2: Human epidermal growth factor receptor 2
AR: Androgen receptor
TMB: Tumor mutational burden
TAM: Tumor-associated macrophages
CK5/6: Cytokeratin 5/6
CK7: Cytokeratin 7
P63: Tumor protein 63
SIA: Sialic acid
SMMHC: Smooth Muscle Myosin Heavy Chain
GCDFP-15: Gross Cystic Disease Fluid Protein-15
GATA-3: GATA Binding Protein 3
TG: Thyroglobulin
TTF1: Thyroid Transcription Factor-1
CD68: Cluster of Differentiation 68
CD163: Cluster of Differentiation 163
DVH: Dose Volume Histogram
CTV: Clinical Target Volume
OAR: Organs at risk
References
1. Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. (2020) 77:181–5. doi: 10.1111/his.14091
2. Chiang S, Weigelt B, Wen HC, Pareja F, Raghavendra A, Luciano G, et al. IDH2 mutations define a unique subtype of breast cancer with altered nuclear polarity. Cancer Res. (2016) 76:7118–29. doi: 10.1158/0008-5472.CAN-16-0298
3. Wei Y, Ding L, Song X, Tian X, Min N, Guan Q, et al. Tall cell carcinoma with reversed polarity: case report with gene sequencing and literature review. Gland Surg. (2021) 10:3147–54. doi: 10.21037/gs-21-591
4. Bhargava R, Florea AV, Pelmus M, Jones MW, Bonaventura M, Wald A, et al. Breast tumor resembling tall cell variant of papillary thyroid carcinoma. Am J Clin Pathol. (2017) 147:399–410. doi: 10.1093/ajcp/aqx016
5. Lozada JR, Basili T, Pareja F, Alemar B, Paula AC, Gularte-Merida R, et al. Solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms (solid papillary carcinomas with reverse polarity) harbour recurrent mutations affecting IDH 2 and PIK 3 CA : a validation cohort. Histopathology. (2018) 73:339–44. doi: 10.1111/his.13522
6. Pareja F, da Silva EM, Frosina D, Geyer FC, Lozada JR, Basili T, et al. Immunohistochemical analysis of IDH2 R172 hotspot mutations in breast papillary neoplasms: applications in the diagnosis of tall cell carcinoma with reverse polarity. Modern Pathol. (2020) 33:1056–64. doi: 10.1038/s41379-019-0442-2
7. Lei Z, Wang YX, Wang ZY, Yang CG, and Pan GQ. Case report: Tall cell carcinoma with reversed polarity of the breast: an additional case and review of the literature. Front Oncol. (2024) 14:1302196. doi: 10.3389/fonc.2024.1302196
8. Jassim M, Premalata CS, Okaly GVP, and Srinivas C. Tall cell carcinoma with reverse polarity of breast: report of a case with unique morphologic and molecular features. Turkish J Pathol. (2020). doi: 10.5146/tjpath.2020.01511
9. Helsby NA, Yong M, van Kan M, de Zoysa JR, and Burns KE. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br J Clin Pharmacol. (2019) 85:1925–34. doi: 10.1111/bcp.14031
10. Shulman Z, O’neill A, Kouneli S, Shaaban A, and Jenkins S. EP-276 Tall Cell Carcinoma with Reverse Polarity (TCCRP) of the breast; a rare form of breast cancer. Br J Surg. (2022) 109:znac245.073. doi: 10.1093/bjs/znac245.073
11. Elghobashy M, Jenkins S, Shulman Z, O’Neil A, Kouneli S, Shaaban AM, et al. Tall cell carcinoma with reversed polarity: case report of a rare special type of breast cancer and review of the literature. Biomedicines. (2023) 11:2376. doi: 10.3390/biomedicines11092376
12. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
13. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
14. Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, et al. Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer. (2018) 9:2308–16. doi: 10.7150/jca.25155
15. Ni C, Yang L, Xu Q, Yuan H, Wang W, Xia W, et al. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer. (2019) 10:4463–72. doi: 10.7150/jca.33914
16. Jamiyan T, Kuroda H, Yamaguchi R, Abe A, and Hayashi M. CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Archiv. (2020) 477:767–75. doi: 10.1007/s00428-020-02855-z
17. Zhang X, Wu H, Wang Z, Zhou Y, Mao F, Lin Y, et al. Tall cell carcinoma of the breast with reverse polarity: case report with gene sequencing and literature review. Gland Surg. (2021) 10:837–43. doi: 10.21037/gs-20-695
18. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. New Engl J Med. (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
19. Haefliger S, Muenst S, Went P, Bihl M, Dellas S, Weber WP, et al. Tall cell carcinoma of the breast with reversed polarity (TCCRP) with mutations in the IDH2 and PIK3CA genes: a case report. Mol Biol Rep. (2020) 47:4917–21. doi: 10.1007/s11033-020-05553-w
20. Khushman M, Patel GK, Maharjan AS, McMillin GA, Nelson C, Hosein P, et al. The prevalence and clinical relevance of 2R/2R TYMS genotype in patients with gastrointestinal Malignancies treated with fluoropyrimidine-based chemotherapy regimens. pharmacogenomics J. (2021) 21:308–17. doi: 10.1038/s41397-021-00210-2
Keywords: tall cell carcinoma with reversed polarity, TCCRP, TAM, PI3K and IDH2, precise treatment
Citation: Duan W, Zhang L, Gao J, Wang Z, Ren M, Wu H, Deng Y, Guo C and Jiang J (2025) Tall cell carcinoma with reversed polarity of breast cancer: a rare case report and review of the literature. Front. Oncol. 15:1635976. doi: 10.3389/fonc.2025.1635976
Received: 27 May 2025; Accepted: 29 September 2025;
Published: 03 November 2025.
Edited by:
Nektarios I. Koufopoulos, University General Hospital Attikon, GreeceReviewed by:
Menelaos Samaras, National and Kapodistrian University of Athens, GreeceMarta Eguía Larrea, Neurosurgery Service, University Hospital of Salamanca, Spain
Copyright © 2025 Duan, Zhang, Gao, Wang, Ren, Wu, Deng, Guo and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianing Jiang, amluZ19qaWFuaW5nQDEyNi5jb20=; Chunlong Guo, MTMzMjIyNjYzNjZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wenqi Duan1†
Wenqi Duan1† Zhe Wang
Zhe Wang Jianing Jiang
Jianing Jiang