- 1Department of Imaging and Functional Medicine, Skåne University Hospital, Lund/Malmö, Sweden
- 2Diagnostic Radiology, Department of Translational Medicine, Lund University, Malmö, Sweden
- 3Department of Radiology, Helsingborg Hospital, Helsingborg, Sweden
- 4Diagnostic Radiology, Department of Clinical Sciences, Lund University, Lund, Sweden
- 5Division of Urological Cancers, Department of Translational Medicine, Lund University, Malmö, Sweden
- 6Department of Urology, Skåne University Hospital, Malmö, Sweden
Objective: The aim of this study is to describe how the use of diagnostic imaging for prostate cancer (PCa) has evolved over time and to determine whether there are any differences in access to diagnostic imaging, type of cancers detected, and mortality based on the education level of patients.
Methods: 11,063 men were recruited between 1991 and 1996 and then prospectively followed until 2020. All new cases of PCa were recorded. At baseline, data on education level, heredity for cancer, and health status were collected. Incident PCa diagnoses during the study period were ascertained through record matching with national healthcare registers. The registers provided more detailed data on the cancer type and imaging performed.
Results: 1,816 men with diagnosed were PCa during the study period were included. No differences were seen between education levels in regard to access to diagnostic methods or tumour aggressiveness at diagnosis. Furthermore, no differences were seen in PCa-specific mortality, but there was higher overall mortality among individuals with a lower education level. During the study period, the use of plain radiographic examinations decreased, while the use of computed tomography (CT), prostate magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT) increased.
Conclusion: Early detection and diagnostic methods for PCa have evolved over the last 30 years. In a healthcare system where men diagnosed with PCa had equal access to diagnostic pathways, no differences are seen in PCa specific mortality. Nevertheless, men with lower education level still had higher overall mortality.
Introduction
Prostate cancer (PCa) is one of the most common cancers in the world, but while its incidence is increasing, mortality has decreased over the last 30 years (1, 2). Prior to the introduction of prostate-specific antigen (PSA) testing in the 1980s, patients were generally diagnosed after presenting with clinical symptoms or due to incidental findings at transurethral resection of the prostate (TURP). As PSA testing was performed more regularly and more asymptomatic cases of PCa were detected, an increase in incidence was seen (3). In parallel, improved imaging techniques using computed tomography (CT), bone scintigraphy, magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT) with increasingly advanced tracers have been introduced (4–6).
Historically, elevated PSA led to a physical examination, and in cases of remaining suspicion, core biopsies were performed (3). MRI was not routinely included in the diagnostic workup but was occasionally performed after repeated negative standard biopsies, for local staging, or as part of active surveillance. In recent years however, there has been a shift towards an "MRI first" approach, meaning that MRI examinations are conducted before patients undergo biopsies. This shift has led to an increase in targeted image-guided biopsies rather than standard systematic transrectal biopsies. This development arose in accordance with the conclusions of studies such as the PRECISION study, which demonstrated the benefits of MRI-targeted biopsies (7, 8).
Currently, there are no countries with ongoing population-based screening programs for PCa. Instead, a large proportion of PSA testing has historically been opportunistic, i.e. done on the initiative of the individual patient or physician (9). Socioeconomic factors are known to have an impact on participation in PSA testing, which could impact the outcome of the disease (10). High socioeconomic status correlates with better cancer outcomes, and for patients with PCa, this affects the chance of cancer detection and the time until curative treatment is received (11). Education level is also an important factor that is associated with socioeconomic status as it is determined early in life and deeply influences future employment, income, and usage of the healthcare system (12). Furthermore, education level is relatively easy to measure and tends to be stable over time.
Several studies have examined the advantages and disadvantages of national population-based screening programs for PCa (13–15). In 2018, the Swedish National Board of Health and Welfare decided not to implement nationwide screening but instead recommended organized testing, which in 2020 prompted several Swedish healthcare regions to initiate a digitally based organized PCa testing program (OPT). In this program, men between the ages of 50 and 74 years are offered a PSA test at prespecified intervals, and individuals with elevated PSA levels are then further examined with MRI (16, 17). As PSA-testing is offered free of charge to all male individuals in the eligible age group, the OPT project could in theory lead to more equal diagnostic and testing pathways. This is a well-founded assumption, but there is very limited research on how factors such as education level influence access to diagnostic examinations and whether it is relevant for PCa outcomes. In 2013, a Swedish group studied whether inappropriate use of diagnostic examinations had decreased over time and confirmed that such a trend was present (18). However, the study could not analyze which modalities were used or where the changes were found. The OPT project has since expanded and now covers almost the entire country.
In summary, little is known about how the use of diagnostic modalities has changed over time, or how education level influences access and outcomes. The aim of this study is therefore to describe the evolution of PCa diagnostics over 30 years in a prospective, population-based cohort, and to assess potential differences by education level as a proxy for socioeconomical status in order to later analyze the impact of the OPT project.
Methods
This prospective population-based study included 11,063 men who were recruited between 1991 and 1996. No new individuals were added after 1996. Thus, the entire cohort was closed and followed until 2020. Data were collected from various diagnose specific and high-coverage healthcare registers. A flowchart of the data-collection process is presented in Figure 1.
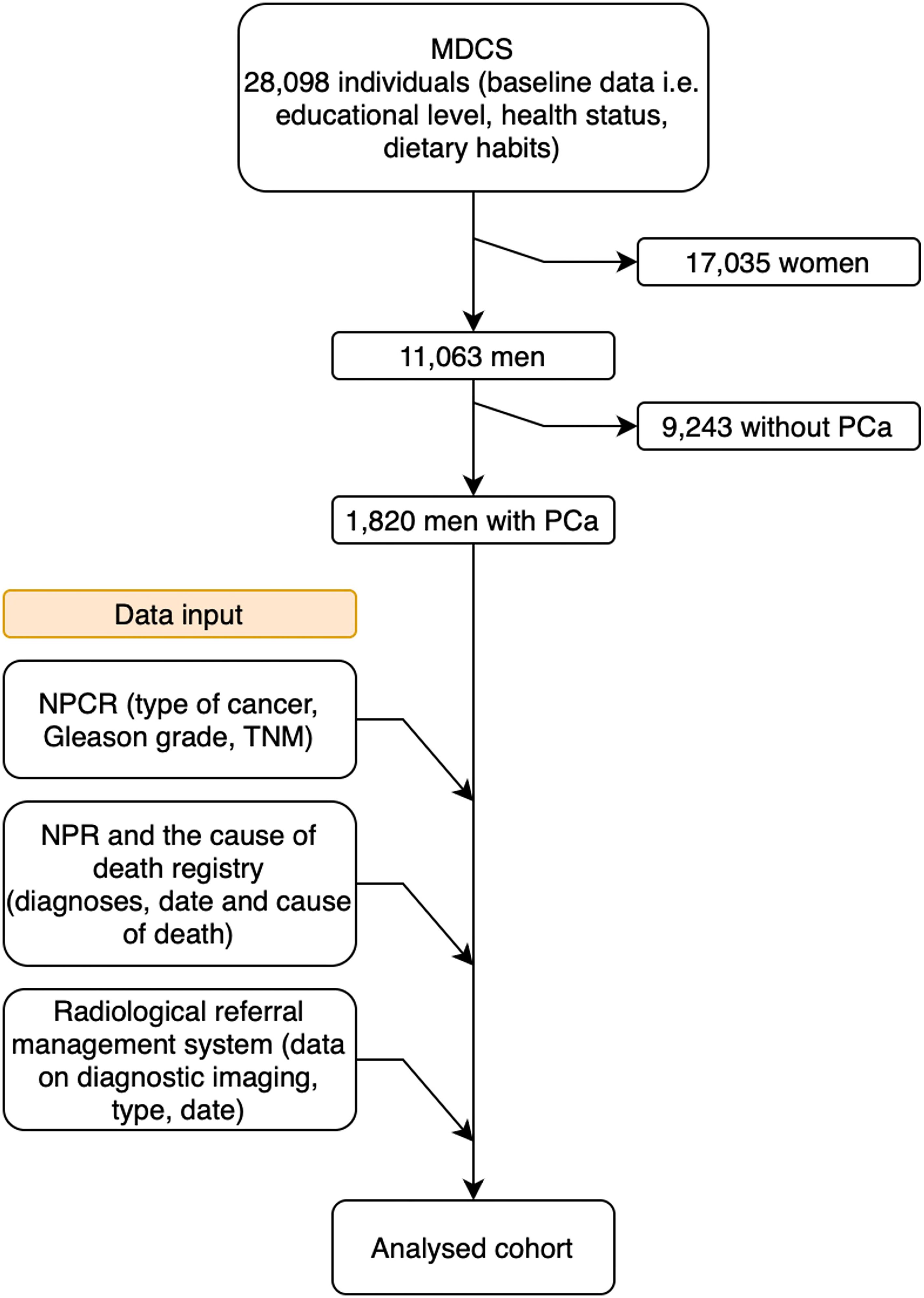
Figure 1. Flowchart of the data collection process. MDCS, Malmö Diet and Cancer Study; PCa. Prostate cancer; NPCR, National Prostate Cancer Register; TNM, Tumor, node, metastasis; NPR, National Patient Register.
Cohort
Between 1991 and 1996, 28,098 individuals from the general population were recruited to the Malmö Diet and Cancer Study (MDCS) cohort. The aim was to study the relationship between diet and subsequent cancer risk. Participants answered extensive questionnaires about their educational background, health status, and dietary habits. They also underwent comprehensive health examinations. Details regarding the recruitment process of the cohort have been described previously (19).
The MDCS cohort included a total of 11,063 men. These men have been prospectively followed up, and data on any new diagnoses or deaths have been continuously collected from national mandatory health registers (mainly the National Patient Register (NPR) and the Swedish Cancer Register). The NPR has been shown to be highly valid, and it is mandatory for all healthcare providers to report to the registers (20). Within the NPR, all hospital-discharge diagnoses are documented according to International Classification of Diseases (ICD-9 and ICD-10) (21). Data on cancer diagnosis was retrieved from the Swedish cancer register, which is also a mandatory healthcare register with high validity and coverage of around 99% with documentation of all cancer diagnoses (22, 23).
Specific data concerning each patient's prostate cancer and how the patient entered the healthcare pathway, were collected from the Swedish NPCR: Gleason score and the tumor characteristics, locoregional lymph-node, and distant metastases (TNM) stage. The register was founded in 1998 and has been utilized increasingly. Since its creation, 235,000 PCa cases have been documented in the register (21, 24). The NPCR has been found to have high validity for PCa diagnoses and Gleason scores (25). The Gleason score was not available for patients diagnosed prior to 1997.
Imaging modalities and ethical considerations
Data regarding imaging used were retrieved from the radiological referral management system. Data on the type of modality and number of examinations the individuals had undergone 6 months before and after the diagnosis of PCa were retrieved. All participants in the MDCS provided informed consent upon inclusion in the primary cohort, and ethical approval was given by the Ethics Committee of Lund University (LU-51-90, approved 1990). Ethical approval for the present study was obtained from the Swedish National Ethical Review Authority (approval number: 2021-05037, approved 2022).
Statistical analysis
Descriptive statistics were used for the type of diagnostic imaging examination and stratified by education level. The cohort is historical, and the educational system changed between the time of the participants' education and the time of recruitment, so we sought to adjust their educational backgrounds to align with the modern system and make the education levels understandable in the current context. Furthermore, this prevents any group from being disproportionately different in size compared to the others. Therefore, education levels were divided into four categories based on the total years of education completed: primary education (up to 8 years), lower secondary education (9–10 years), upper secondary education (11–12 years), and at least one year beyond secondary education.
Cox regression analyses were used to study relative risks for overall mortality and cause-specific mortality (PCa). The models were adjusted for the year and age at diagnosis and country of birth. The cause-specific mortality analysis was verified by performing a competing-risk regression (Fine–Gray). Mortality data were also analyzed using Kaplan–Meier curves. All analyses were performed using R version 4.3.3 except for the competing-risk regression, which was performed using STATA SE version 18.
Results
Cohort
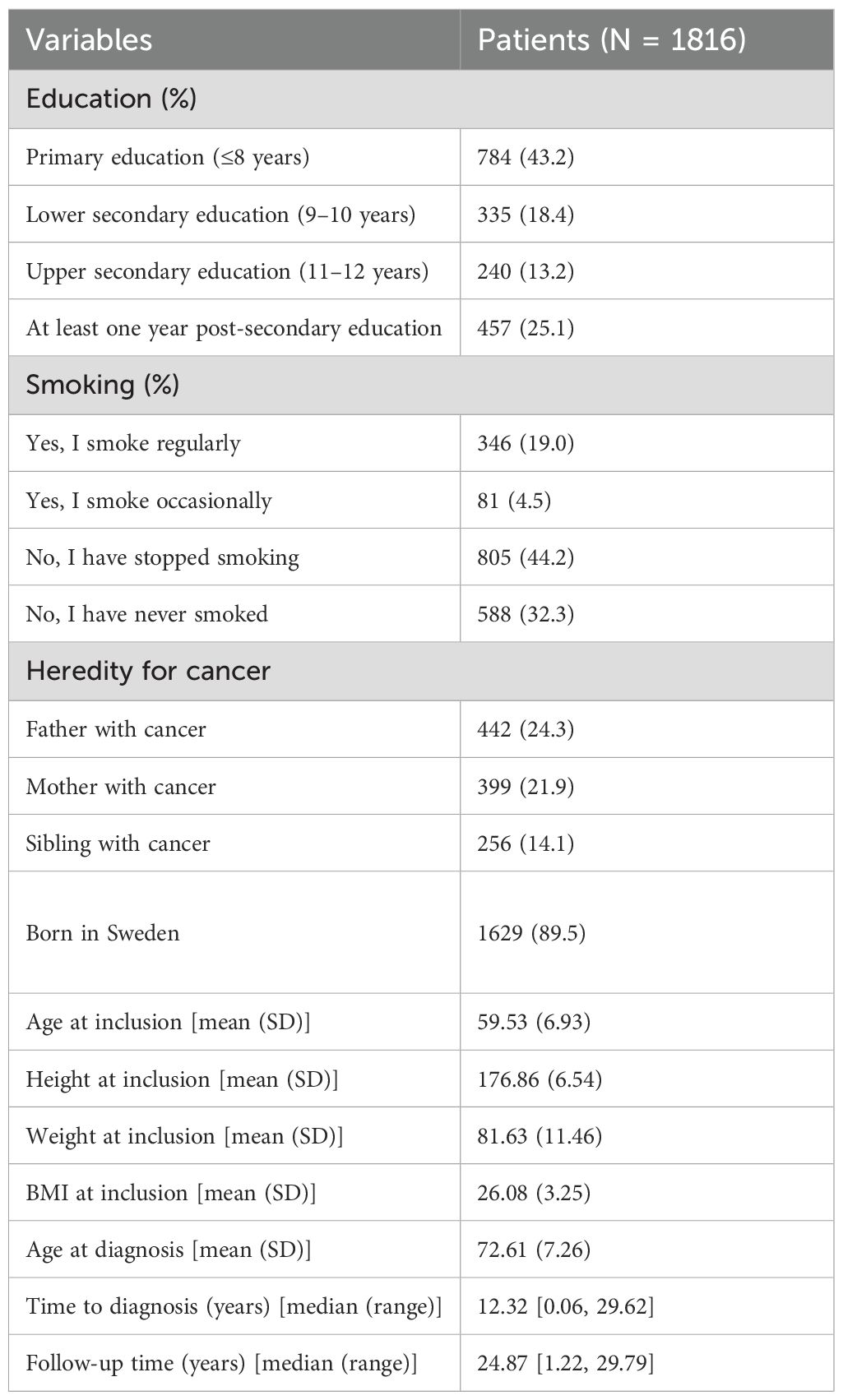
During the study period (1991–2020), 1,820 (16.7%) of the 11,063 men in the MDCS cohort were diagnosed with PCa. Full data on educational level was missing for 4 patients (0.2%) and the studied cohort therefore consisted of 1816 patients. The median follow-up time was 24.9 years, and the median age at diagnosis was 72.3 years (range 50–96 years). The largest study group (43.2%) had a primary level of education, while 18.4% had lower secondary education, 13.2% had upper secondary education and had graduated from high school, and 25.2% of the patients with PCa had some form of post-secondary education. The mean body mass index (BMI) was 26.1, and a majority (76.5%) either did not smoke or had quit smoking (Table 1).
Imaging
In total, the patients with PCa underwent 73,343 diagnostic imaging examinations, of which 2,279 were associated with their primary cancer diagnosis. Throughout the follow-up period, the use of plain radiographic examinations declined, whereas the use of CT, prostate MRI, and PET/CT increased. The proportion of bone scintigraphy remained constant during the study period (Figure 2). The use of PET/CT increased later in the study period, and in total, 115 PET/CT examinations were performed. A smaller proportion of these were prostate-specific membrane antigen [¹8F]PSMA-1007 PET/CT (10 examinations (8.7%), all performed in 2019 and 2020), but the clear majority were [¹¹C]choline PET/CT. For a minority of the cases before 2019, no exact information on the used tracer is available and it cannot be excluded that other radiopharmaceuticals such as [¹8F]fluorocholine, [¹8F]NaF, [¹8F]FDG) were used.
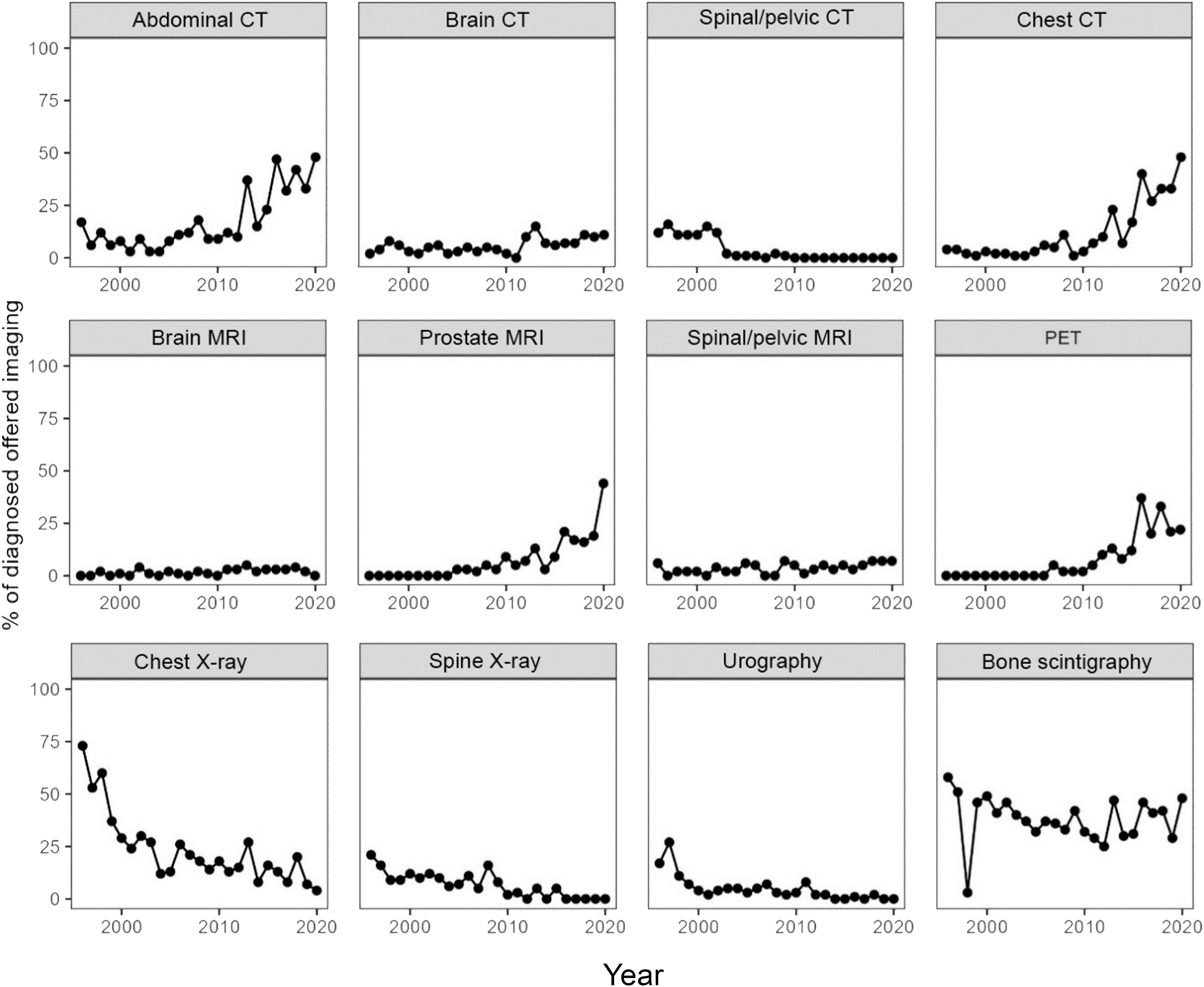
Figure 2. The proportion of diagnosed patients per year who received each type of imaging in conjunction with their diagnosis.
Impacts of education level
The proportion of men diagnosed with a PCa during the study period was equally distributed between different education levels. More patients with higher education entered the healthcare pathway through a healthcare examination (48.9% in the group with highest education vs. 40.6% in the group with the lowest education level). Correspondingly, the group with the highest education entered less frequently with lower urinary tract symptoms (19.6% for the highest education group vs. 27.3% for the group with lowest education) (Table 2). Furthermore, there were no differences in the type of diagnostic examinations used for the PCa diagnosis (Figures 3, 4). No differences were seen in the severity of the Gleason score between the education groups. A majority were found to have a Gleason score of 7 or higher. No Gleason score was available for 355 patients (19.5%) (Table 3).
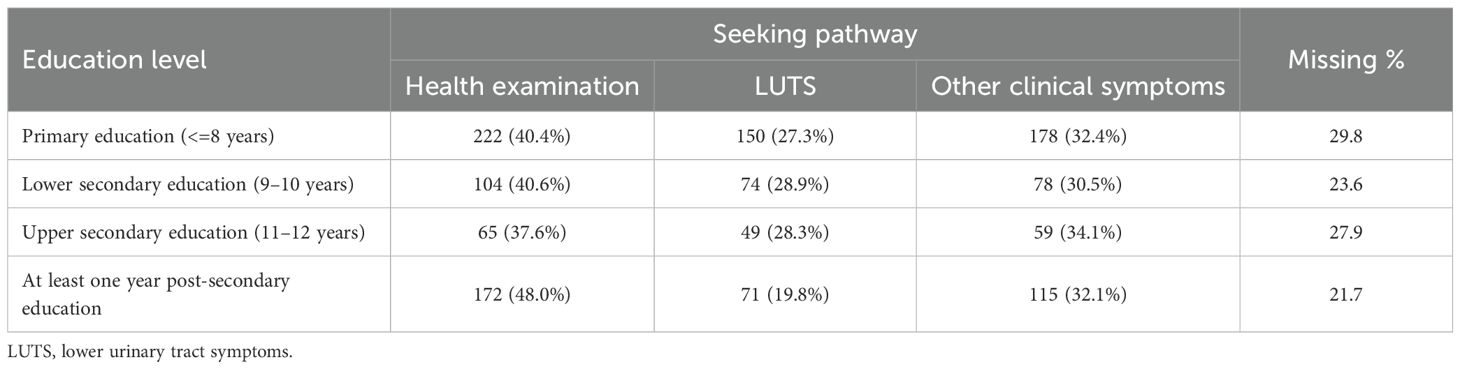
Table 2. The number (and proportion) of men within each educational level and the corresponding seeking pathway which led to the prostate cancer diagnosis.
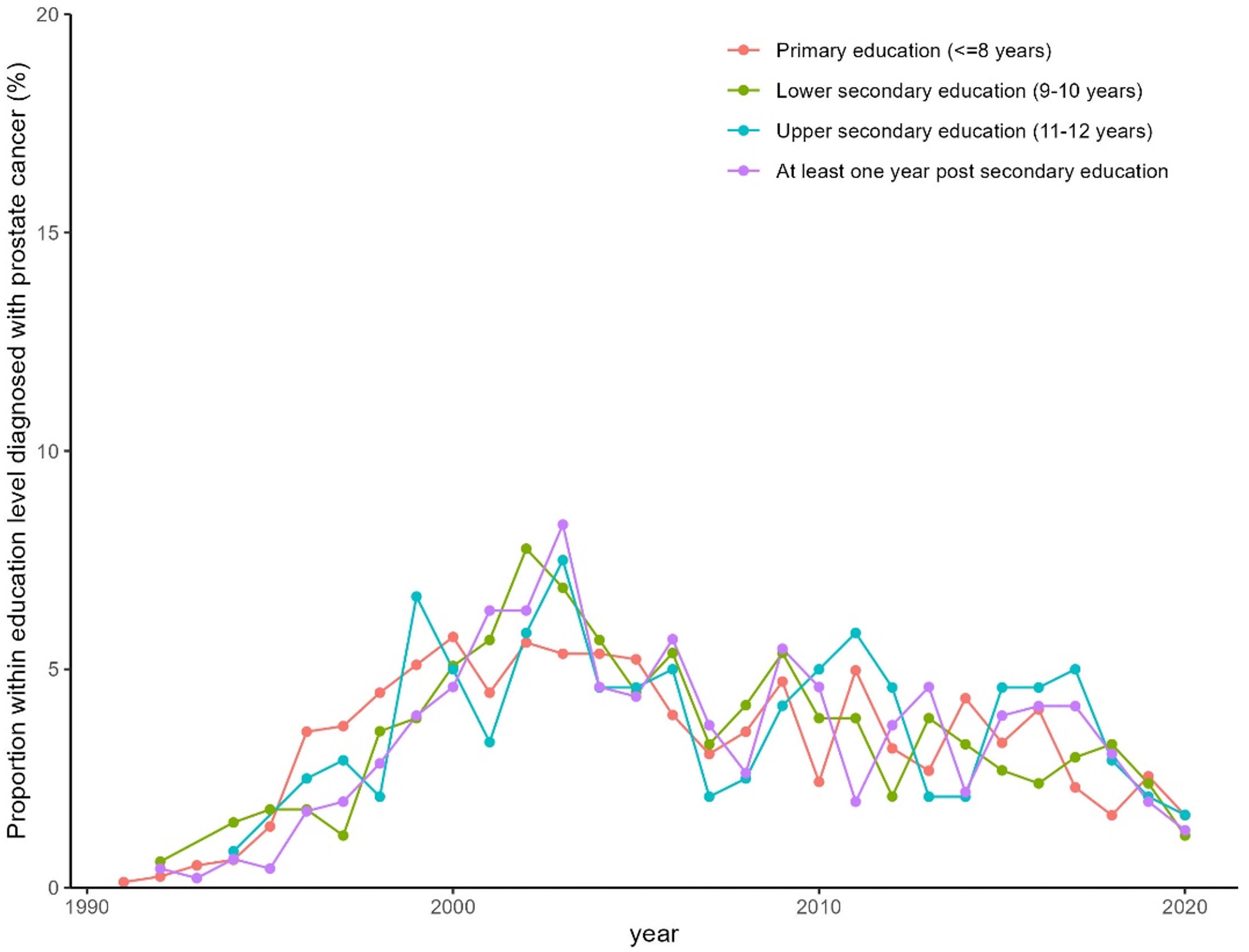
Figure 3. Proportion of men in the MCDC diagnosed with prostate cancer per year stratified by education level.
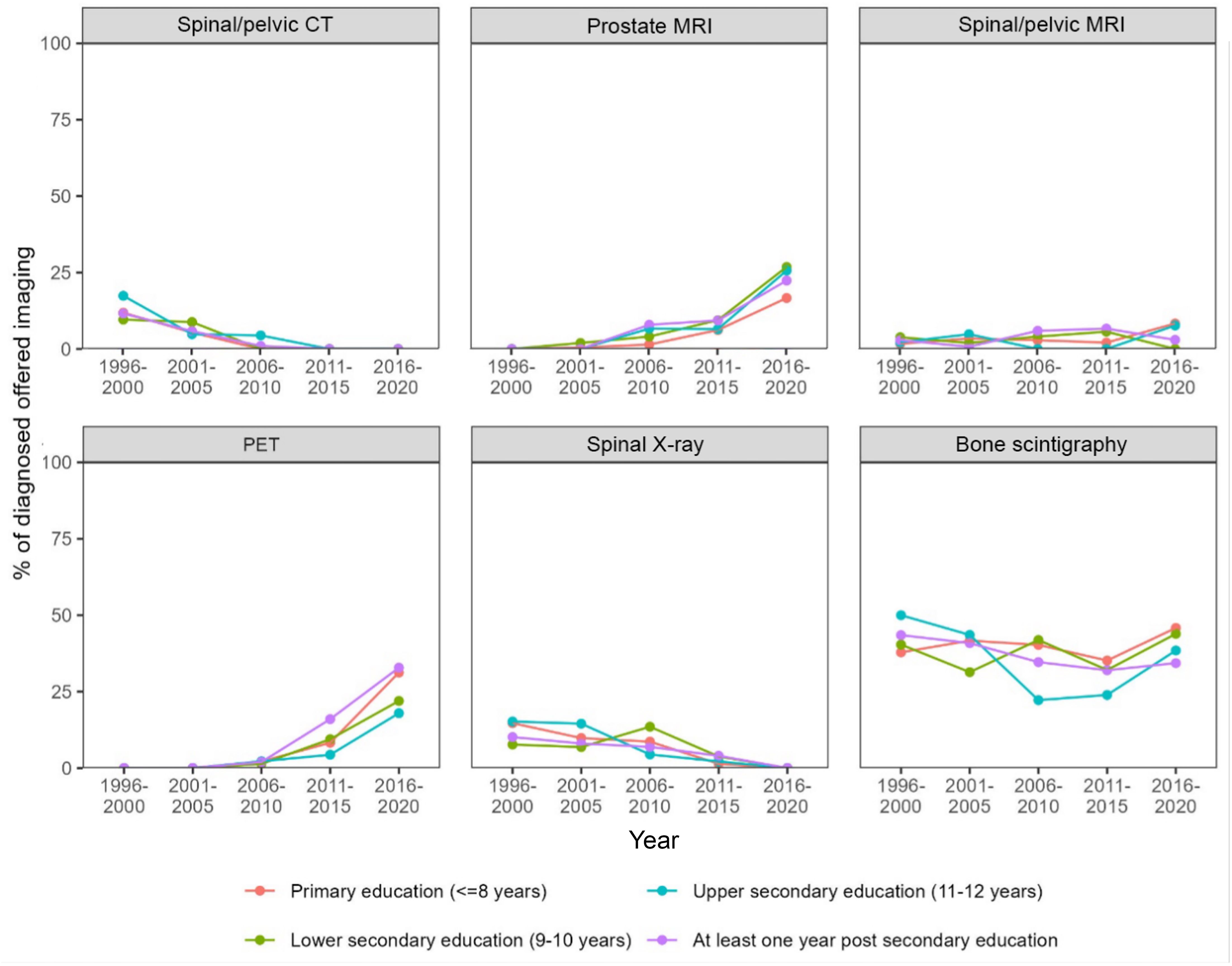
Figure 4. Proportion of diagnosed patients per year who received each type of imaging diagnostic at the time of diagnosis stratified by education level.
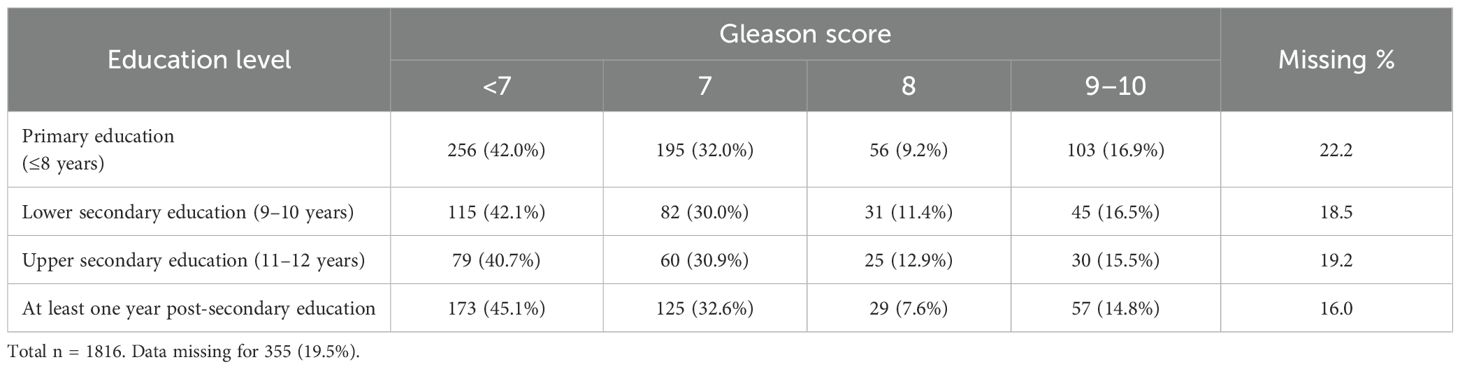
Table 3. The number (and proportion) of men within each education level with the corresponding Gleason score.
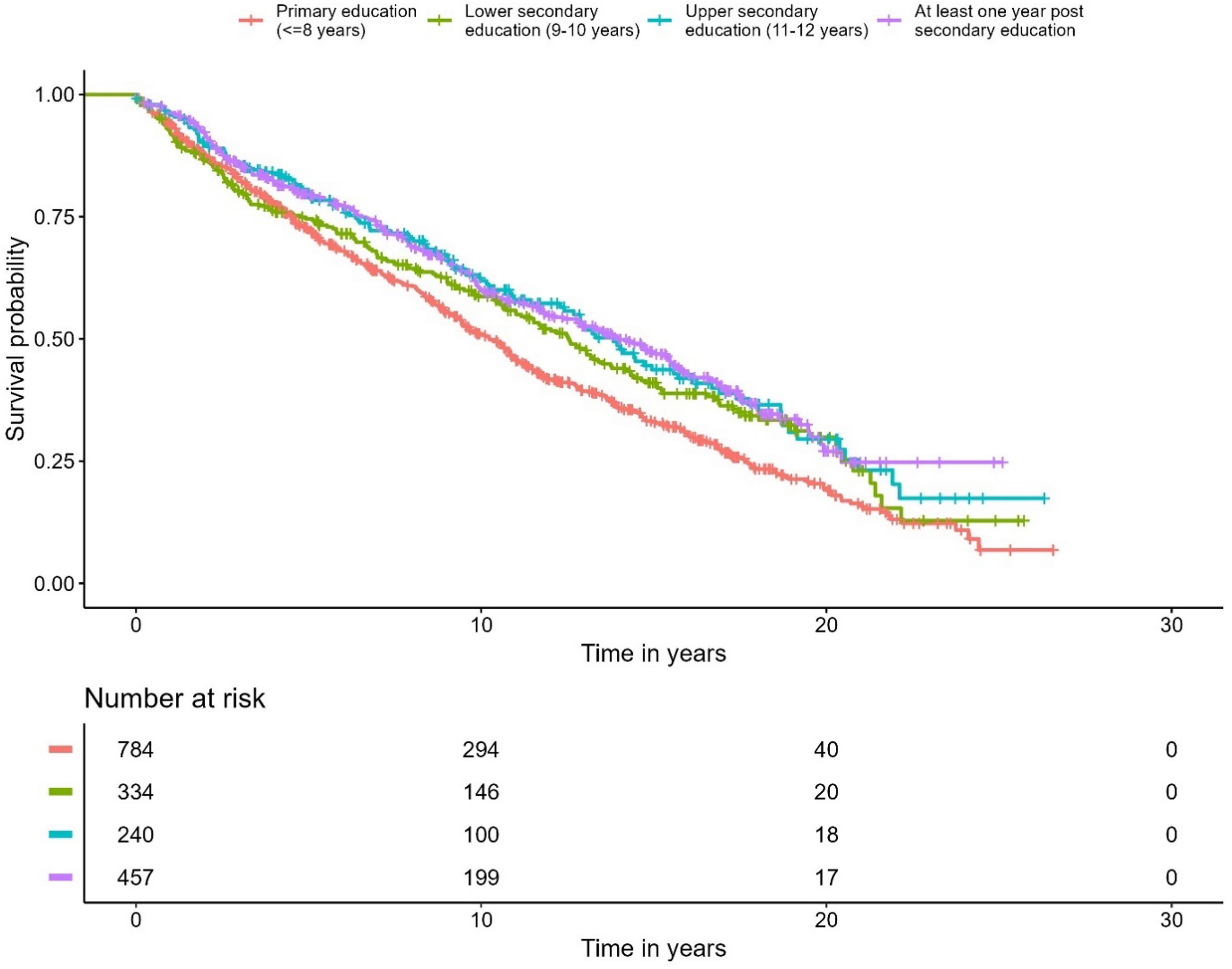
A correlation was observed between education level and overall survival. In the Cox regression model, men with the highest education level had 28% lower overall mortality compared to the group with the lowest education level (p < 0.001) (Figure 5, Table 4). No differences were seen in the PCa-specific survival analysis between education levels (Figure 6, Table 5). Further, a competing risk regression was performed to rule out impacts of competing risks on the result, but the analysis did not show any significant differences.
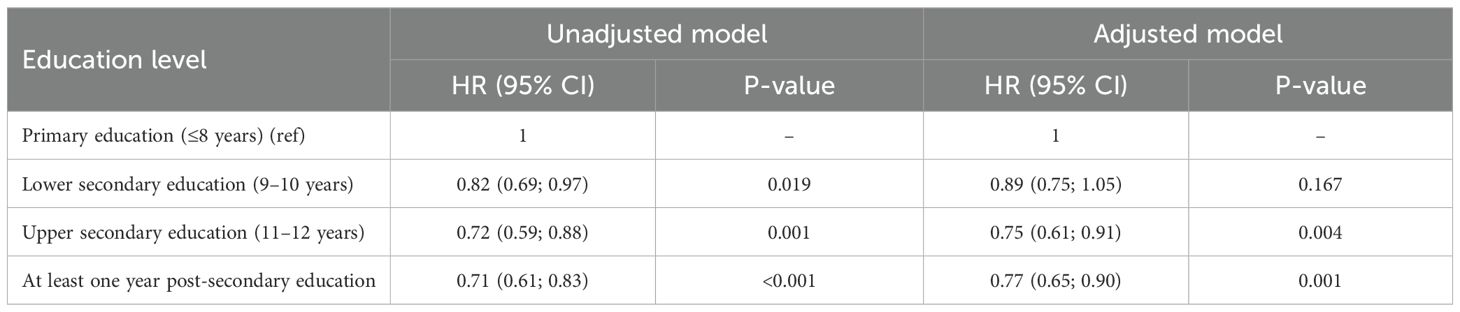
Table 4. Hazard ratios for unadjusted and adjusted Cox regression (unadjusted and adjusted for age at diagnosis, year of diagnosis and country of birth).
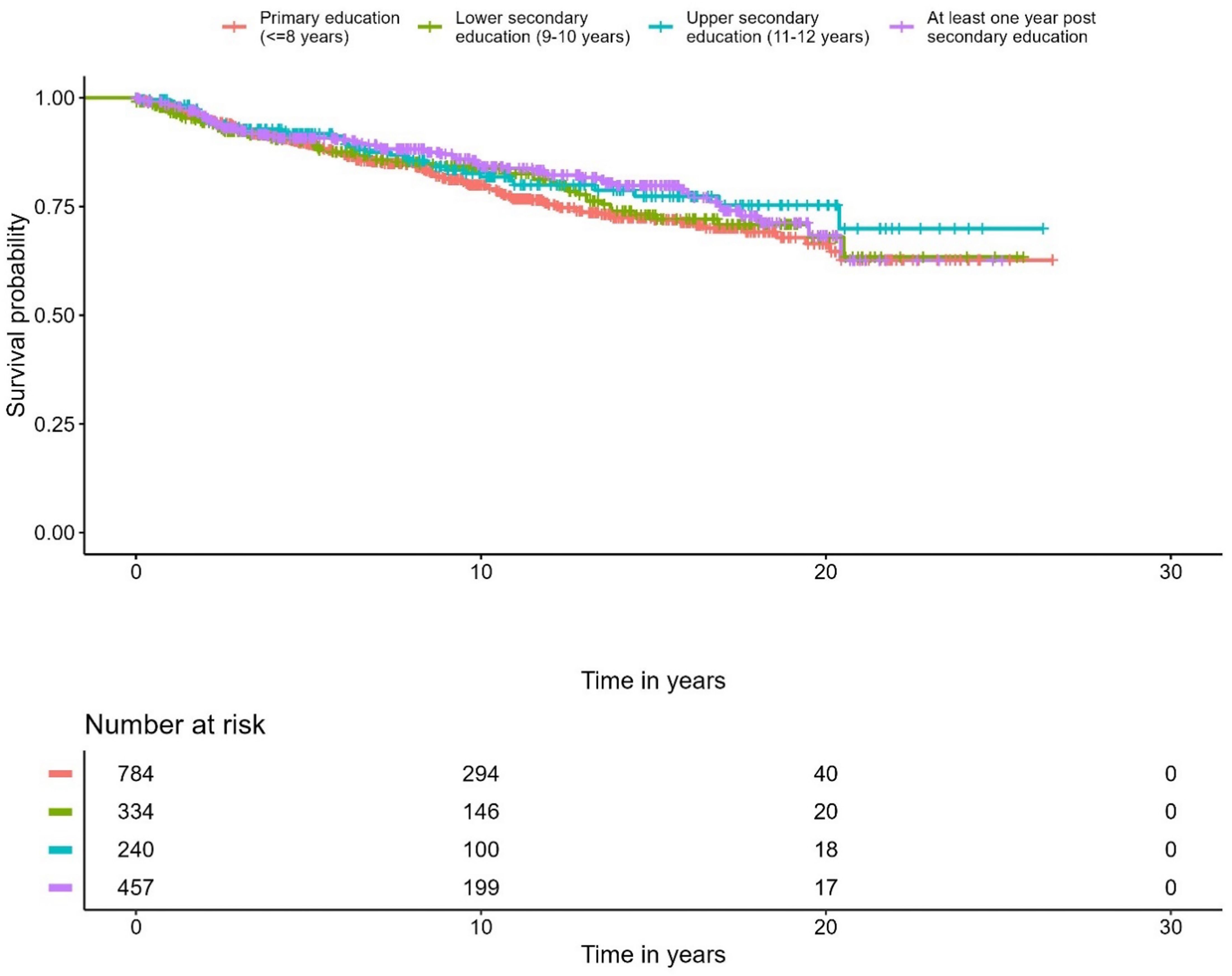
Figure 6. Kaplan-Meier curve of cause specific mortality for prostate cancer, stratified by educational level.
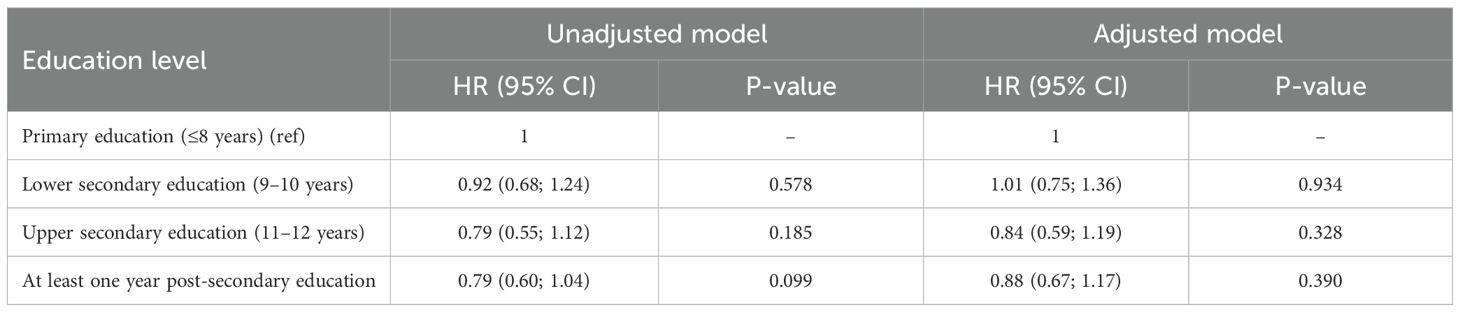
Table 5. Hazard ratios for unadjusted and adjusted Cox regression for cause specific mortality (unadjusted and adjusted for age at diagnosis, year of diagnosis and country of birth).
Discussion
There are three main findings in this study. First, no differences were seen between education levels in regard to access to diagnostic methods, tumor aggressiveness, or PCa-specific mortality. Second, despite the first finding, there was still higher overall mortality among individuals with a lower level of education. Lastly, unsurprisingly, the use of plain radiographic examinations decreased, while the use of CT, MRI, and PET/CT increased between 1991 and 2020.
The Swedish healthcare system is almost exclusively publicly funded with patients paying a low fee, while the remainder is financed through taxes. But even in such a system, the patient fees and education level can influence whether individuals seek medical care and their access to it (26, 27). PCa among individuals in this study were not detected through organized testing but in clinical routine, including a variety of unorganized detection pathways, such as opportunistic PSA testing, clinical symptoms, or incidental examination findings (i.e. after TURP). We observed modest differences in healthcare seeking pathways across educational levels, with health examinations being a more common route of detection among patients with higher education. Our study did not identify any differences in access to diagnostic methods, severity of detected PCa or cause specific mortality. One of the primary goals of implementing the OPT in Sweden was to ensure more equal access to PCa diagnostics (16).
An early study that analyzed the influence of socioeconomic factors on participation in OPT showed that socioeconomic factors may have an impact (28). These findings are consistent with those of other studies analyzing the influence of socioeconomic factors on participation in other cancer screening programs (29). These results are not surprising as we know that socioeconomic disparities in accessing healthcare are determined not only by financial means to obtain care, but also by how individuals process information and the resources they possess to translate that knowledge into concrete actions. There are also studies showing the opposite, where little or no associations were seen between socioeconomics and access to PCa diagnostics or care (30). One interpretation of the results could be that there are greater barriers to overcome in encouraging men to seek healthcare rather than differences in the care they receive once they are within the healthcare system. Therefore, these somewhat contrasting results are interesting in the context of the new OPT system and how to evaluate its results.
In recent years, there has been a significant shift in the diagnostics of PCa towards an "MRI first" approach. This shift gained momentum in the years preceding 2020, partly due to the findings from the PRECISION and Gothenburg studies (7, 13, 14).
Additionally, the PI-RADS diagnostic algorithm, which uses MRI for assessment, has been modified and increasingly adopted in prostate diagnostics (31). Recent evidence further supports this diagnostic approach. PI-RADS v2 combined with PSA density has shown high predictive value for clinically significant prostate cancer in biopsy−naïve patients (32). A potential benefit of this shift is a reduction in the numbers of biopsies of indolent tumors as combining systematic with MRI−targeted biopsy achieves the highest detection rates overall (33). MRI remains reliable even in men with prior negative biopsies, delivering detection rates comparable to biopsy−naïve populations (34). While this "MRI first" approach was partially evident towards the end of our study period, it had not yet been fully implemented. Nevertheless, a tendency towards more MRI in the later study years can be observed in our results.
Another tendency that was noticeable in later years is the increasing use of [¹8F]PSMA-1007 PET/CT in in more advanced disease or cases of PSA persistence after prostatectomy, which may impact treatment and survival (35, 36). The [¹8F]PSMA-1007 PET/CT examinations performed in our cohort were all carried out in the two last years (2019 and 2020). The use of conventional radiographic methods also declined which reflects the developments in diagnostic practice.
Interestingly, while we noted a difference in overall survival based on education level, there was no difference in PCa-specific mortality. This indicates that equal access to healthcare can eliminate survival differences for specific disorders. At the same time, socioeconomic status is multifactorial, which is represented in the survival differences between the observed education-level groups. The higher all-cause mortality in lower education groups likely reflects differences in health behaviors (e.g. smoking, adiposity), comorbidity burden, and social determinants of health. This interpretation is consistent with prior Swedish and international findings (11, 37, 38). Although the results are specific to a sub-population in Sweden, they are of interest to many other healthcare systems as a large number of the world's systems are either publicly funded or have a broad public health-insurance network (39). Detailed exploration of these factors was beyond the scope of our study, which focused on diagnostic access, but we emphasize this gradient as an important public health concern.
Study limitations and strengths
There are several limitations to this study. First, the cohort was closed. Although the included individuals had a spread in age, no new individuals were recruited after 1996. Consequently, the cohort as a whole aged over time, and since increased age is a risk factor for cancer development, there has been a gradual increase in risk within the cohort. On the other hand, the age distribution of the cohort has contributed to a relatively even spread of incident cancer cases over the follow-up period, which can be seen as a strength, given the study's aim to analyze the development of PCa over time. Second, Gleason scores were not recorded in the Swedish National Prostate Cancer Register prior to 1997, which limits the ability to assess tumor aggressiveness for the earliest cases. However, the majority of prostate cancer diagnoses in this cohort occurred after 1997, meaning Gleason scores were available for most patients. Third, the study cohort differed slightly from the general population of Malmö in that the education level was somewhat higher and the proportion of foreign-born men lower (19). These differences may bias our findings toward underestimating disparities, since both higher education and native birth are associated with greater healthcare access and improved outcomes and the results of the present study may therefore not fully reflect inequalities present in the broader population. However, this probably influences the outcome to only a limited extent since the results were internally compared within the cohort and stratified by education level.
Moreover, while we report differences in overall mortality by education level, our analyses were not designed to assess the contributions of lifestyle, comorbidity, or other social determinants. This question has been addressed in prior studies but remains relevant for interpreting our results.
For the performed PET/CT examinations, tracer metadata were incompletely recorded for early examinations. Where available, [¹¹C]choline predominated. [¹8F]PSMA-1007 PET/CT was introduced in 2019 and dominated from that point onward. The main strengths of this study lie in the prospective nature of the cohort. The study population was recruited as a cross-section of the population approximately 30 years ago. Information on lifestyle habits, which is often difficult to obtain retrospectively, was collected at the start of the study. Therefore, the data on these variables can be considered particularly reliable. Other important variables for the study, such as data on imaging diagnostic methods, were obtained from the healthcare digital medical-record system and can therefore be considered complete.
Conclusion
Early detection and diagnostic methods for PCa have evolved over the last 30 years. In a healthcare system where men diagnosed with PCa had equal access to diagnostic pathways, no differences are seen in PCa specific mortality. Nevertheless, men with lower education level still had higher overall mortality.
Data availability statement
The cohort comes from a prospective study which followed individuals over 30 years. Since data contains personal identification it can't be made publicly available but will be available upon formal request with legal and ethical review. Requests to access these datasets should be directed to https://www.malmo-cohorts.lu.se.
Ethics statement
The studies involving humans were approved by Ethics Committee of Lund University (number LU-51-90) and Swedish National Ethical Review Authority (approval number: 2021-05037). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LW: Project administration, Writing – review & editing, Formal analysis, Data curation, Methodology, Writing – original draft. ET: Writing – review & editing, Conceptualization, Methodology, Writing – original draft. JB: Methodology, Writing – original draft, Conceptualization, Writing – review & editing. AB: Writing – review & editing, Writing – original draft. SZ: Writing – review & editing, Methodology, Writing – original draft, Conceptualization. EB: Software, Supervision, Resources, Investigation, Visualization, Conceptualization, Funding acquisition, Validation, Formal analysis, Writing – review & editing, Project administration, Data curation, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was financed by MAS Cancer Foundation (Allmänna Sjukhusets i Malmö Stiftelse för bekämpande av cancer) and Swedish governmental funding of clinical research (ALF).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Welch HG and Albertsen PC. Reconsidering prostate cancer mortality - the future of PSA screening. N Engl J Med. (2020) 382:1557–63. doi: 10.1056/NEJMms1914228
2. Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. (2021) 4:877–92. doi: 10.1016/j.euo.2021.09.006
3. Litwin MS and Tan HJ. The diagnosis and treatment of prostate cancer: A review. Jama. (2017) 317:2532–42. doi: 10.1001/jama.2017.7248
4. Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, and Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. (1987) 317:909–16. doi: 10.1056/nejm198710083171501
5. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. (1991) 324:1156–61. doi: 10.1056/nejm199104253241702
6. Ravizzini G, Turkbey B, Kurdziel K, and Choyke PL. New horizons in prostate cancer imaging. Eur J Radiol. (2009) 70:212–26. doi: 10.1016/j.ejrad.2008.09.019
7. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. (2018) 378:1767–77. doi: 10.1056/NEJMoa1801993
8. Drost FH, Osses D, Nieboer D, Bangma CH, Steyerberg EW, Roobol MJ, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: A cochrane systematic review and meta-analysis. Eur Urol. (2020) 77:78–94. doi: 10.1016/j.eururo.2019.06.023
9. Albertsen PC, Bjerner LJ, Pasovic L, Müller S, Fosså S, Carlsson SV, et al. Opportunistic prostate-specific antigen testing in Norwegian men: a public health challenge. BJU Int. (2024) 133:104–11. doi: 10.1111/bju.16211
10. Moses KA, Zhao Z, Bi Y, Acquaye J, Holmes A, Blot WJ, et al. The impact of sociodemographic factors and PSA screening among low-income Black and White men: data from the Southern Community Cohort Study. Prostate Cancer Prostatic Dis. (2017) 20:424–9. doi: 10.1038/pcan.2017.32
11. Tomic K, Ventimiglia E, Robinson D, Häggström C, Lambe M, and Stattin P. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population-based study. Int J Cancer. (2018) 142:2478–84. doi: 10.1002/ijc.31272
12. Galobardes B, Shaw M, Lawlor DA, Lynch JW, and Davey Smith G. Indicators of socioeconomic position (part 1). J Epidemiol Community Health. (2006) 60:7–12. doi: 10.1136/jech.2004.023531
13. Kohestani K, Månsson M, Arnsrud Godtman R, Stranne J, Wallström J, Carlsson S, et al. The GÖTEBORG prostate cancer screening 2 trial: a prospective, randomised, population-based prostate cancer screening trial with prostate-specific antigen testing followed by magnetic resonance imaging of the prostate. Scand J Urol. (2021) 55:116–24. doi: 10.1080/21681805.2021.1881612
14. Hugosson J, Godtman RA, Carlsson SV, Aus G, Grenabo Bergdahl A, Lodding P, et al. Eighteen-year follow-up of the Göteborg Randomized Population-based Prostate Cancer Screening Trial: effect of sociodemographic variables on participation, prostate cancer incidence and mortality. Scand J Urol. (2018) 52:27–37. doi: 10.1080/21681805.2017.1411392
15. Pinsky PF and Parnes H. Screening for prostate cancer. N Engl J Med. (2023) 388:1405–14. doi: 10.1056/NEJMcp2209151
16. Alterbeck M, Järbur E, Thimansson E, Wallström J, Bengtsson J, Björk-Eriksson T, et al. Designing and implementing a population-based organised prostate cancer testing programme. Eur Urol Focus. (2022) 8:1568–74. doi: 10.1016/j.euf.2022.06.008
17. Alterbeck M, Thimansson E, Bengtsson J, Baubeta E, Zackrisson S, Bolejko A, et al. A pilot study of an organised population-based testing programme for prostate cancer. BJU Int. (2024) 133:87–95. doi: 10.1111/bju.16143
18. Makarov DV, Loeb S, Ulmert D, Drevin L, Lambe M, and Stattin P. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst. (2013) 105:1306–13. doi: 10.1093/jnci/djt175
19. Manjer J, Carlsson S, Elmståhl S, Gullberg B, Janzon L, Lindström M, et al. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. (2001) 10:489–99. doi: 10.1097/00008469-200112000-00003
20. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. (2011) 11:450. doi: 10.1186/1471-2458-11-450
21. Van Hemelrijck M, Wigertz A, Sandin F, Garmo H, Hellström K, Fransson P, et al. Cohort profile: the national prostate cancer register of Sweden and prostate cancer data base Sweden 2. 0. Int J Epidemiol. (2013) 42:956–67. doi: 10.1093/ije/dys068
22. Sakakibara S, Pazzagli L, and Linder M. Consistency between the national patient register and the swedish cancer register. Pharmacoepidemiol Drug Saf. (2024) 33:e5780. doi: 10.1002/pds.5780
23. Barlow L, Westergren K, Holmberg L, and Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. (2009) 48:27–33. doi: 10.1080/02841860802247664
24. Crump C, Stattin P, Brooks JD, Sundquist J, Sieh W, and Sundquist K. Mortality risks associated with depression in men with prostate cancer. Eur Urol Oncol. (2024) 7:1411–9. doi: 10.1016/j.euo.2024.03.012
25. Tomic K, Sandin F, Wigertz A, Robinson D, Lambe M, and Stattin P. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. (2015) 51:101–11. doi: 10.1016/j.ejca.2014.10.025
26. Lagarde M and Palmer N. The impact of user fees on access to health services in low- and middle-income countries. Cochrane Database systematic Rev. (2011) 2011:Cd009094. doi: 10.1002/14651858.Cd009094
27. Ehsan AN, Wu CA, Minasian A, Singh T, Bass M, Pace L, et al. Financial toxicity among patients with breast cancer worldwide: A systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e2255388. doi: 10.1001/jamanetworkopen.2022.55388
28. Emil J, Erik H, Thomas B-E, Ola B, and Rebecka Arnsrud G. Associations between socioeconomic factors and PSA testing in a population-based organised testing programme and routine healthcare: a register-based study of 50-year-old men. BMJ Oncol. (2024) 3:e000400. doi: 10.1136/bmjonc-2024-000400
29. Bygrave A, Whittaker K, and Sanchia AA. The impact of interventions addressing socioeconomic inequalities in cancer-related outcomes in high-income countries: A systematic review. J Public Health Res. (2020) 9:jphr.2020.1711. doi: 10.4081/jphr.2020.1711
30. Strömberg U, Berglund A, Carlsson S, Thellenberg Karlsson C, Lambe M, Lissbrant IF, et al. Socioeconomic inequality in prostate cancer diagnostics, primary treatment, rehabilitation, and mortality in Sweden. Int J Cancer. (2024) 155:637–45. doi: 10.1002/ijc.34932
31. Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. (2019) 76:340–51. doi: 10.1016/j.eururo.2019.02.033
32. Massanova M, Vere R, Robertson S, Crocetto F, Barone B, Dutto L, et al. Clinical and prostate multiparametric magnetic resonance imaging findings as predictors of general and clinically significant prostate cancer risk: A retrospective single-center study. Curr Urol. (2023) 17:147–52. doi: 10.1097/cu9.0000000000000173
33. Massanova M, Barone B, Caputo VF, Napolitano L, Ponsiglione A, Del Giudice F, et al. The detection rate for prostate cancer in systematic and targeted prostate biopsy in biopsy-naive patients, according to the localization of the lesion at the mpMRI: A single-center retrospective observational study. Prostate. (2024) 84:1234–43. doi: 10.1002/pros.24761
34. Barone B, Napolitano L, Calace FP, Del Biondo D, Napodano G, Grillo M, et al. Reliability of multiparametric magnetic resonance imaging in patients with a previous negative biopsy: comparison with biopsy-naïve patients in the detection of clinically significant prostate cancer. Diagnostics (Basel). (2023) 13. doi: 10.3390/diagnostics13111939
35. Combes AD, Palma CA, Calopedos R, Wen L, Woo H, Fulham M, et al. PSMA PET-CT in the diagnosis and staging of prostate cancer. Diagnostics (Basel). (2022) 12. doi: 10.3390/diagnostics12112594
36. Weiner AB, Agrawal R, Valle LF, Sonni I, Kishan AU, Rettig MB, et al. Impact of PSMA PET on prostate cancer management. Curr Treat Options Oncol. (2024) 25:191–205. doi: 10.1007/s11864-024-01181-9
37. Xu L, Wang X, Pan X, Wang X, Wang Q, Wu B, et al. Education level as a predictor of survival in patients with multiple myeloma. BMC Cancer. (2020) 20:737. doi: 10.1186/s12885-020-07178-5
38. Vincerževskiene I, Jasilionis D, Austys D, Stukas R, Kaceniene A, and Smailyte G. Education predicts cervical cancer survival: a Lithuanian cohort study. Eur J Public Health. (2017) 27:421–4. doi: 10.1093/eurpub/ckw261
39. Ortiz-Ospina ER M. Healthcare spending ourworldindata.org (2017). Available online at: https://ourworldindata.org/financing-healthcare (Accessed May 26, 2025).
Keywords: prostate cancer screening, diagnostic radiology, epidemiology, prostate cancer, mortality
Citation: Will L, Thimansson E, Bengtsson J, Bjartell A, Zackrisson S and Baubeta E (2025) Prostate cancer diagnostics: evolution over 30 years and the impact of education level – a prospective population-based study. Front. Oncol. 15:1636292. doi: 10.3389/fonc.2025.1636292
Received: 27 May 2025; Accepted: 15 September 2025;
Published: 03 October 2025.
Edited by:
Le Lu, Alibaba DAMO Academy, United StatesReviewed by:
Biagio Barone, ASL Napoli 1 Centro, ItalyManuela Andrea Hoffmann, Johannes Gutenberg University Mainz, Germany
Copyright © 2025 Will, Thimansson, Bengtsson, Bjartell, Zackrisson and Baubeta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erik Baubeta, ZXJpay5iYXViZXRhX2ZyaWRoQG1lZC5sdS5zZQ==
 Leon Will
Leon Will Erik Thimansson
Erik Thimansson Johan Bengtsson
Johan Bengtsson Anders Bjartell
Anders Bjartell Sophia Zackrisson
Sophia Zackrisson Erik Baubeta
Erik Baubeta