- 1Department of Digestive Oncology, Beijing Chaoyang Integrative Medicine Rescue and First Aid Hospital, Beijing, China
- 2General Surgery Department, Friendship Hospital, Capital Medical University, Beijing, China
Gastric cancer remains a global health burden due to its late diagnosis and poor prognosis. Conversion therapy aims to make the initially unresectable tumor resectable through systemic treatment, providing the opportunity for long-term survival. The rise of immunotherapy has brought new potential to this field. Immunotherapy combined with chemotherapy, anti-angiogenic drugs or chemoradiotherapy has shown good efficacy in specific patients. This review summarizes the current evidence of conversion strategies based on immunotherapy, emphasizes key biomarkers, and explores the future direction of precise, multi-modal treatment.
1 Introduction
Gastric cancer remains one of the most prevalent and lethal malignancies globally, with high incidence, low early detection rates, and poor survival outcomes. According to GLOBOCAN 2020, gastric cancer ranks fifth in incidence and fourth in cancer-related mortality worldwide, with approximately 1.09 million new cases and 769,000 deaths annually (1, 2). Nearly half of these cases occur in China, where the early detection rate remains below 30%, resulting in 60%-70% of patients being diagnosed at a locally advanced (stage III) or metastatic (stage IV) stage. Correspondingly, five-year survival drops to ~30% for stage III and below 10% for stage IV disease. Due to the lack of early symptoms and effective screening programs, most patients are diagnosed with advanced-stage disease (3, 4).Traditional surgical resection and monotherapy chemotherapy are insufficient to meet the clinical needs of patients with advanced disease. As such, there is growing interest in multidisciplinary strategies aimed at converting initially unresectable tumors into resectable ones.
The emergence of immune checkpoint inhibitors (ICIs) has brought significant advances in the treatment of advanced gastric cancer and opened new avenues for conversion therapy. This review outlines recent progress in immunotherapy-based conversion strategies, summarizes key clinical evidence, and discusses ongoing challenges and future directions in this evolving field (5, 6).
2 Concept, indications, and significance of conversion therapy
Although neoadjuvant therapy, systemic therapy, and conversion therapy may utilize similar pharmacological agents, there are fundamental differences in their clinical objectives and the populations to which they apply. Neoadjuvant therapy is primarily administered to patients who are technically resectable, with the aim of reducing tumor volume, enhancing surgical resection rates, and improving long-term survival outcomes. In contrast, systemic therapy is indicated for patients with distant metastases, focusing on prolonging survival and enhancing quality of life.
Conversion therapy occupies a position between these two approaches; it is appropriate for patients initially deemed either technically or oncologically unresectable or marginally resectable (6, 7). The primary objective of conversion therapy is to achieve R0 resection following systemic treatment—such as chemotherapy, targeted therapies, or immunotherapy—thereby improving prognosis (8). Clinical studies have demonstrated that the median overall survival (OS) for patients who successfully undergo R0 resection can reach 24–36 months, significantly surpassing the 8–12 months observed in patients receiving palliative care (9–13).
Candidates for conversion therapy can generally be categorized into two groups: (i)patients with locally advanced disease (e.g., T4b or N2-N3), where invasion of adjacent structures or bulky nodal metastases renders upfront resection unfeasible; and (ii)Patients with favorable tumor biology (such as her2 positive or MSI-H/dMMR) or limited metastasis (single-organ metastasis such as liver metastasis, ovarian metastasis, retroperitoneal lymph node metastasis, supraclavicular lymph node metastasis, etc.) may be resectable after systemic treatment (10, 14).
To optimize patient selection, a biologically oriented classification system for stage IV gastric cancer has been proposed, integrating tumor burden, resectability, and peritoneal dissemination status. This framework divides patients into four categories:
Category 1: Technically resectable metastases without macroscopic peritoneal dissemination, such as solitary liver lesions, isolated para-aortic lymph node metastasis, or positive peritoneal cytology. These patients are typically treated with neoadjuvant chemotherapy rather than conversion therapy.
Category 2: Marginally resectable metastases, including multiple liver lesions, major vascular involvement, or extensive nodal disease. These cases may benefit from systemic therapy to enable potential resection.
Category 3: Includes patients with peritoneal metastasis, which is traditionally associated with poor prognosis and limited treatment options. However, in highly selected cases, systemic therapy may induce a good peritoneal response, making it possible to consider cytoreductive surgery in specialized centers.
Category 4: Non-curable metastases, such as peritoneal spread with distant organ involvement (e.g., lung, bone), where conversion therapy is only considered in highly responsive tumors (15).
This classification framework helps distinguish patients eligible for surgery with or without induction therapy and supports individualized treatment planning. As an integrated, multidisciplinary strategy, conversion therapy represents a major paradigm shift in the management of advanced gastric cancer—from empirical, stage-based approaches toward personalized, biology-guided treatment. Future studies are needed to enhance patient stratification, refine systemic regimens, and improve the R0 resection rate.
3 Application and progress of immunotherapy in conversion therapy
3.1 Immune checkpoint inhibitors combined with chemotherapy
Building upon prior progress in perioperative chemotherapy, immune checkpoint blockade has emerged as a promising strategy to further enhance resectability and long-term survival in gastric cancer. Pivotal trials such as MAGIC and FLOT4-AIO established the value of neoadjuvant chemotherapy followed by surgery, demonstrating improved tumor downstaging (e.g., tripling ypT0 rates), a 15%-20% increase in R0 resection rates, and prolonged 5-year survival, without increasing perioperative morbidity (16–18).These findings established neoadjuvant chemotherapy as the standard backbone for locally advanced, resectable gastric cancer, and laid the foundation for subsequent integration of immunotherapy in the conversion setting.
Multiple phase III trials have since confirmed that chemo-immunotherapy confers a survival advantage as first-line treatment for advanced gastric cancer. These findings have not only expanded the therapeutic scope of immune checkpoint inhibitors but also laid the foundation for their earlier incorporation into the management of locally advanced gastric cancer(LAGC). In Asian populations, the phase III ORIENT-16 trial demonstrated that sintilimab plus chemotherapy significantly prolonged OS compared with chemotherapy alone—in the overall population (15.2 vs. 12.3 months; HR = 0.77, P = 0.009) and particularly in patients with PD-L1 CPS ≥5 (19.2 vs. 12.9 months; HR = 0.66, P < 0.001)—supporting its use as a first-line treatment for advanced gastric or gastroesophageal junction adenocarcinoma in Chinese patients (19).Similarly, in the Chinese subgroup of CheckMate-649, 5-year OS reached 24% versus 8% with chemotherapy alone in CPS≥5 patients (20, 21). KEYNOTE-062 has showed that pembrolizumab monotherapy has achieved an mOS of 17.4 months in patients with CPS ≥10, outperforming chemotherapy (10.8 months) (22).More broadly, KEYNOTE-859 confirmed the survival benefit of pembrolizumab plus chemotherapy in an unselected advanced GC/GEJC population (mOS: 13.0 vs 11.3 months, HR = 0.78, P < 0.001). In the Chinese subgroup, mOS improved to 15.9 months vs 12.2 months (HR = 0.68), and reached 21.4 months in patients with CPS≥10 (HR = 0.51), with an ORR of 80%. These findings suggest that higher PD-L1 expression may be associated with greater clinical benefit. Collectively, these findings indicate that a subset of PD-L1-high patients may already have entered a “long-term survival plateau”, and that immune therapy is moving beyond palliative intent toward earlier-stage disease (23).
Building on these advances, the PD-1/CTLA-4 bispecific antibody cadonilimab exhibited broad and sustained efficacy in the COMPASSION-15 trial (24). Notably, nearly half of the enrolled patients had a PD-L1 CPS <5, and 23% had CPS <1. Interim analysis showed that cadonilimab combined with chemotherapy significantly improved OS compared to chemotherapy alone (15.0 vs 10.8 months; HR = 0.62; P < 0.001). Even among patients with CPS <5, OS was extended to 14.8 months vs 11.1 months (HR = 0.70), challenging the assumption that PD-L1-low tumors are unresponsive to immunotherapy. These findings suggest that dual checkpoint inhibition may achieve consistent efficacy across a broader biomarker spectrum.
Against the backdrop of survival benefits achieved with immunotherapy in advanced gastric cancer, several pivotal phase III trials have explored its application in earlier stages, particularly in patients with borderline resectable or locally advanced disease. Although these studies are largely designed as perioperative trials, many enrolled populations that reflect typical conversion therapy scenarios—namely, tumors initially deemed unresectable or marginally operable. As such, these findings have important implications for immunotherapy-driven conversion strategies aiming to enhance resectability and long-term outcomes.
The global phase III MATTERHORN trial (NCT04592913) is a randomized, double-blind, placebo-controlled study assessing durvalumab in combination with FLOT chemotherapy in patients with resectable GC/GEJC (25). A total of 474 patients with clinical stage T2-T4 and N0-3M0 disease were randomized to receive perioperative FLOT with or without durvalumab, followed by 10 cycles of adjuvant immunotherapy. Interim results showed a significantly higher pCR rate in the durvalumab arm (19% vs 7%; odds ratio = 3.08; P < 0.00001), along with superior tumor downstaging (pT0: 21% vs 10%; pN0: 47% vs 33%). R0 resection and surgical completion rates were comparable between groups. These findings support the potential of integrating immunotherapy into perioperative regimens to enhance pathological response without compromising surgical safety. Long-term survival data are pending.KEYNOTE-585 (NCT03221426) is the first global phase III trial to evaluate perioperative PD-1 blockade in combination with chemotherapy for resectable GC/GEJC. Patients with cT3-T4/N+M0 disease received three preoperative and eleven postoperative cycles of chemotherapy, along with pembrolizumab or placebo. The pembrolizumab group achieved a significantly higher pCR rate (13.4% vs 2.0%; P < 0.001) and extended median event-free survival (EFS: 44.4 vs 25.7 months; HR = 0.81). Although the difference in OS was not statistically significant (71.8 vs 55.7 months; HR = 0.86; P = 0.057), a stronger benefit was observed in patients with PD-L1 CPS ≥1 (HR = 0.73). Treatment-related adverse events (TRAEs) were comparable between groups (64% vs 63%). These results support the feasibility of perioperative chemo-immunotherapy and highlight the potential role of extended adjuvant immunotherapy in high-risk populations (26, 27).
Acknowledging regional variations in tumor biology and treatment response, two pivotal Chinese phase II studies-NEOSUMMIT (28) and PERSIST (29)-have provided key evidence supporting immunotherapy-based conversion strategies in East Asian populations. The NEOSUMMIT trial (NCT04354662) enrolled 108 patients with locally advanced GC/GEJC and randomized them to receive toripalimab plus SOX/XELOX or chemotherapy alone. The immunotherapy arm demonstrated significantly higher rates of tumor regression grade (TRG) 0/1 (44.4% vs 20.4%; P = 0.009) and pCR: 22.2% vs 7.4%; P = 0.030). Notably, all six patients in the dMMR subgroup achieved pCR, compared to none in the control group. Tumor downstaging (ypT0-2) occurred more frequently with toripalimab (46.3% vs 22.2%; P = 0.008). Patients with intestinal or mixed histological types exhibited better responses than those with diffuse-type tumors (2.3-fold higher, P < 0.01). Adverse event rates were comparable (TRAEs: 37.0% vs 33.3%), and immune-related toxicities remained manageable. The PERSIST trial (29) (NCT04982939) adopted a “sandwich” strategy-neoadjuvant immunotherapy, surgery, and adjuvant immunotherapy-using sintilimab combined with SOX in 240 patients with locally advanced GC/GEJC. The combination group achieved a pCR rate of 27.9%, significantly higher than 4.8% in the control arm (P < 0.001), and a major pathological response (MPR,≤10% residual tumor) rate of 65.2% versus 20.4%. Tumor downstaging was observed in 79.7% of patients, and the R0 resection rate exceeded 91% (91.8% vs 89.3%). Grade 3–4 TRAEs occurred in only 4.1% of cases, and no perioperative mortality was reported. As the first phase II trial to validate a domestically developed PD-1 inhibitor in this context, PERSIST strongly supports the feasibility and safety of conversion immunotherapy in Chinese patients. Together with NEOSUMMIT, it highlights the potential for enhanced immunotherapeutic sensitivity in East Asian populations, possibly due to higher prevalence of proximal tumors, Epstein-Barr virus (EBV) positivity, and favorable molecular subtypes such as dMMR/MSI-H and intestinal histology. Table 1 lists an overview of key clinical trials investigating chemoimmunotherapy in conversion therapy.
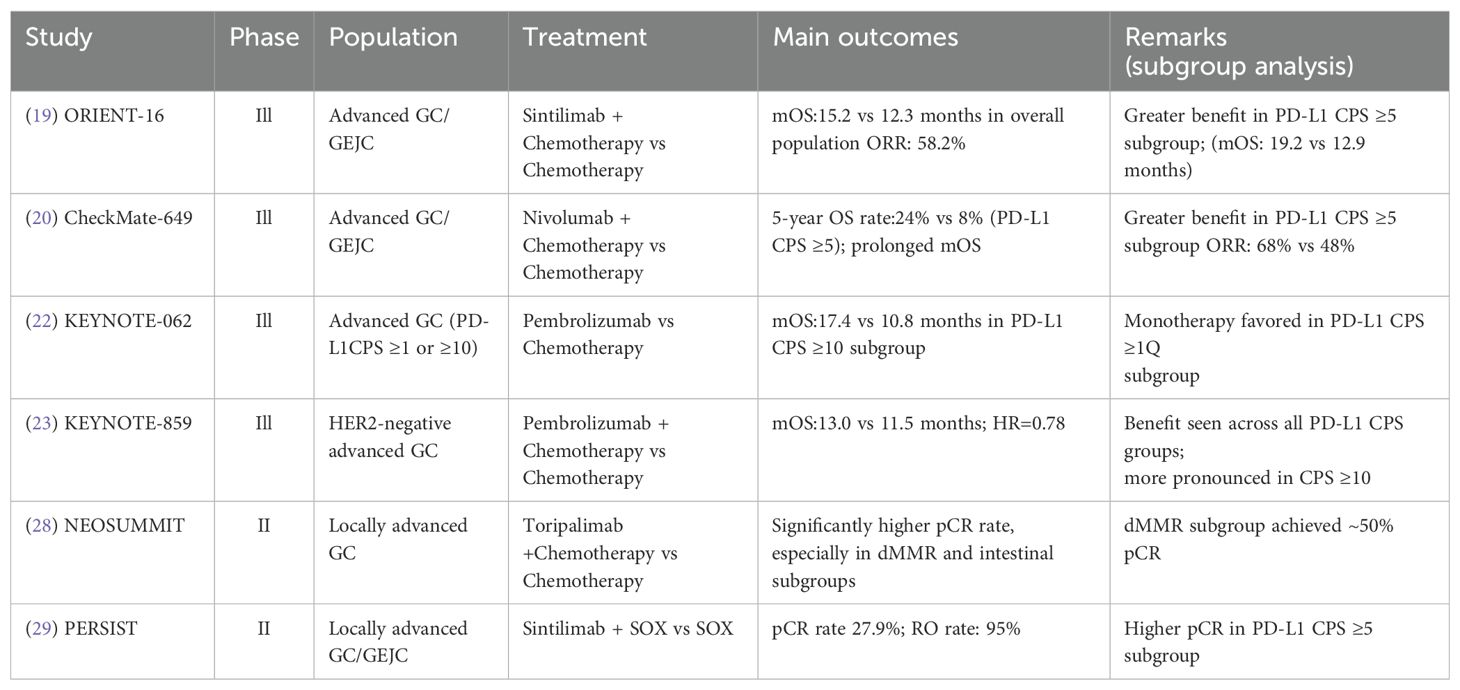
Table 1. Key clinical trials of immunotherapy combined with chemotherapy in conversion therapy for gastric cancer.
3.2 Multimodal combination therapy strategy
Beyond chemical immunotherapy, multimodal strategies combining immune checkpoint inhibitors, anti-angiogenic drugs, and chemotherapy have shown encouraging potential in increasing conversion rates. Antiangiogenic therapy enhances this synergy by normalizing the tumor vascular system, alleviating hypoxia, and reprogramming the tumor microenvironment (TME). These changes promote CD8+ T cell infiltration and effector function, while immune checkpoint blockade restores T cell activity (30, 31). These mechanisms jointly drive the synergistic anti-tumor immune response, as shown in Figure 1.
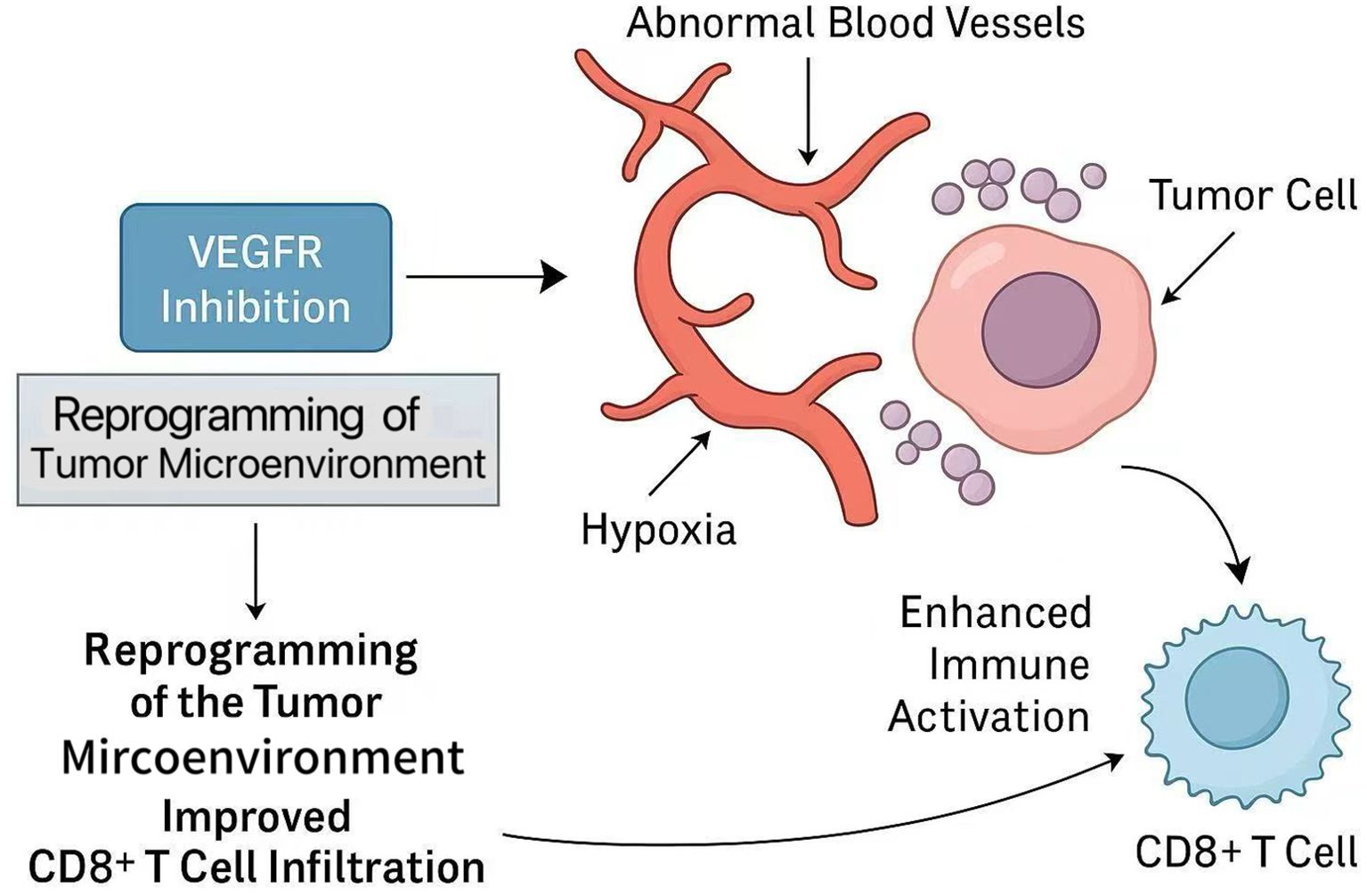
Figure 1. Mechanism by which VEGFR inhibition enhances anti-tumor immunity and synergizes with immune checkpoint blockade.
The DRAGON-IV/AHEAD-G208 (32)trial, a phase III multicenter randomized controlled study, evaluated the efficacy of triple therapy with camrelizumab, apatinib, and SOX chemotherapy in patients with resectable gastric cancer or GEJC. The combination arm achieved a significantly higher pCR rate (18.3% vs 5.0%) compared to chemotherapy alone. While surgical completion and R0 resection rates were similar between groups, the triple regimen induced deeper pathological responses. Biomarker analysis showed a 68% reduction in VEGF pathway activity, supporting the hypothesis that anti-angiogenic therapy facilitates TME remodeling and immunotherapy synergy. In terms of safety, grade ≥ 3 TRAEs-primarily neutropenia and thrombocytopenia-were manageable and did not impair surgical feasibility. Importantly, biomarker analyses indicated that patients with PD-L1 CPS ≥ 5 and EBV-positive tumors achieved higher pCR rates, underscoring the value of biomarker-guided patient selection in optimizing treatment outcomes.
Collectively, these findings support multimodal immunotherapy as a viable strategy for conversion therapy, particularly in biomarker-enriched subgroups.
3.3 Cutting-edge exploration of dual immunotherapy
While bispecific antibodies like cadonilimab have broadened immunotherapy applicability in PD-L1-low populations, dual immune checkpoint blockade offers a chemotherapy-free alternative for molecularly defined subgroups-particularly patients with MSI-H or dMMR gastric cancer. This approach marks a paradigm shift from histopathologic staging to molecular-driven treatment selection, offering a strong rationale for conversion therapy in patients with high tumor immunogenicity but initially unresectable or borderline-resectable disease.
The INFINITY trial (NCT04817826), a phase II study, evaluated neoadjuvant durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) in patients with resectable MSI-H gastric or gastroesophageal junction adenocarcinoma. The study reported a pCR rate of 60% and a 2-year relapse-free survival (RFS) rate of 85% (33). Subgroup analysis revealed that patients with T2-T3 tumors achieved a pCR rate of 88.9%, while those with T4 disease showed a markedly lower rate of 16.7%, suggesting an inverse correlation between tumor invasiveness and immunotherapy responsiveness. The NEONIPIGA trial (NCT04006262) investigated neoadjuvant nivolumab plus ipilimumab followed by adjuvant nivolumab in patients with localized MSI-H/dMMR gastric or gastroesophageal junction adenocarcinoma. The study achieved a pCR rate of 58.6% and a major pathological response (MPR, ≤10% residual tumor) rate of 79% (34). Immune-related adverse events (irAEs) primarily colitis, pneumonitis, and hepatitis were manageable and did not impair surgical feasibility or R0 resection outcomes.
Collectively, these studies suggest that dual checkpoint inhibition can induce profound tumor regression in MSI-H/dMMR gastric cancer, enabling curative resection without chemotherapy. This chemo-free strategy may be particularly valuable for patients who are medically unfit for cytotoxic agents or exhibit high immunogenicity. However, broader clinical adoption requires confirmation in randomized phase III trials with stratification by T stage, baseline resectability, and molecular features.
3.4 New attempts at immuno-combination radiotherapy
Radiotherapy has historically played a limited role in gastric cancer management. However, emerging studies combining radiotherapy with immunotherapy have introduced new strategies for improving local tumor control and resectability in patients with locally advanced, borderline resectable, or initially unresectable disease. The synergistic effect arises from radiotherapy’s capacity to induce immunogenic cell death, enhance antigen presentation, and remodel TME, thereby increasing T cell infiltration and potentiating immune checkpoint blockade.
The SHARED study (35) (ChiCTR1900024428) evaluated neoadjuvant chemoradiotherapy combined with sintilimab in 34 patients with cT3-4N+ or T4b gastric/gastroesophageal junction adenocarcinoma. Patients received concurrent radiotherapy (45-50.4 Gy), chemotherapy, and PD-1 blockade. The trial reported a pCR rate of 38.2% (13/34; 95% CI, 22.2%-56.4%), substantially higher than historical pCR rates for conventional chemoradiotherapy (10%-15%) (36–38). The R0 resection rate reached 94.7%, and median EFS was 21.1 months. These results suggest that immuno-radiotherapy may enhance pathological response and improve curative resection rates in patients with high-risk locoregional disease.
The Neo-PLANET trial (39) enrolled 36 patients with cT3-4N+ proximal gastric cancer and assessed camrelizumab combined with concurrent chemoradiotherapy (45 Gy). The pCR rate reached 33.3% (12/36; 95% CI, 18.6%-51.0%), slightly lower than in the SHARED study. However, nodal downstaging (ypN0) was achieved in 77.8% of patients, and the 2-year OS rate reached 76.1%. These findings suggest that, in addition to enhancing pCR, radioimmunotherapy may offer substantial regional disease.
Safety profiles varied between the two regimens. In SHARED, grade 3–4 TRAEs occurred in 39.3%, primarily hematologic toxicities. In contrast, the Neo-PLANET trial reported a higher TRAE rate of 80.6%, largely attributable to lymphopenia, though without compromising surgical feasibility. Both studies achieved high R0 resection rates (91.7%-94.7%), exceeding the typical 85%-89% observed with conventional chemoradiotherapy (40).
Overall, early-phase data support the feasibility of combining immunotherapy with chemoradiotherapy in locally advanced gastric cancer. Nevertheless, validation in larger randomized trials is warranted to confirm survival benefits and optimize treatment protocols. Table 2 summarizes representative multimodal and emerging conversion therapy strategies in combination with immunotherapy.
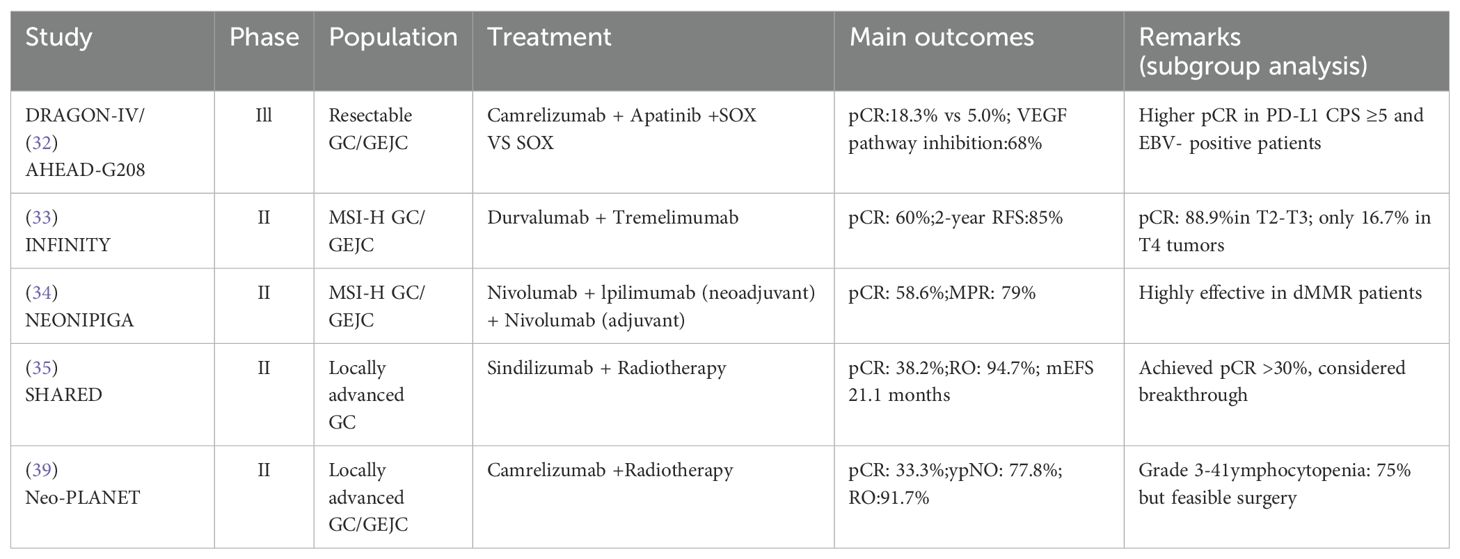
Table 2. Key clinical trials of multimodal and emerging immunotherapy strategies in conversion therapy for gastric cancer.
4 Discussion
Despite encouraging progress, the application of immunotherapy in the conversion therapy of LAGC is still in its infancy and is developing rapidly. Many key challenges still need to be addressed before the immune-based treatment strategies are widely incorporated into clinical practice.
First, there is a disconnect between pathological response and long-term survival. For example, although the KEYNOTE-585 trial has shown significant improvements in pCR and EFS, it has not yet translated into statistically significant OS benefits, highlighting the urgent need for reliable alternative endpoints and long-term follow-up.
Secondly, there is a high degree of heterogeneity in immune responses between patients. Patients with diffuse histological subtypes or low PD-L1 expression levels often have limited benefits; in addition, compared with advanced diseases, the predictive value of traditional biomarkers (such as PD-L1 combined positive score, CPS) in perioperative period is relatively weak.
Third, irAEs-such as pneumonia, hepatitis, and colitis-although mostly manageable, still cause about 10%-15% of patients to terminate treatment early, potentially affecting the integrity of treatment options in the real world (41–43).
In addition, the biomarker system is not yet uniform, which continues to limit the accuracy of patient screening. Although PD-L1 CPS, microsatellite instability (MSI) and tumor mutation burden (TMB) have predictive value in specific populations, their roles in perioperative treatment decisions are still inconsistent and lack prospective verification (44). In recent years, the predictive value of inflammation-related indicators has gradually attracted attention. Systemic inflammatory response indicators such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have not only been shown to be associated with pathological remission rate and OS in a number of studies, but also been found to be significantly associated with R0 resection rate in a retrospective cohort study in Slovakia, suggesting that they have potential application value in efficacy prediction and risk stratification in immune combined conversion therapy (45).
5 Conclusion
Immunotherapy has opened up a new way for the conversion therapy of LAGC, but its clinical integration is still limited by biological complexity, regional variability and lack of effective predictors. In the future, the implementation of dynamic monitoring tools such as ct DNA and radiomics, as well as multi-dimensional biomarker analysis that integrates genomic, immunological and microbiome data, is expected to improve patient selection. Moreover, next-generation immunotherapy platforms - including bispecific antibodies, antibody-drug conjugates (ADCs), neoantigen vaccines, and oncolytic viruses -may improve efficacy while minimizing systemic toxicity (46). These innovations will help realize the full potential of immunotherapy-based transformation strategies in clinical practice.
Author contributions
YS: Writing – review & editing, Writing – original draft. XZ: Data curation, Writing – review & editing. WD: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fonc.2025.1730862.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
GC: Gastric cancer
R0 resection: Curative resection with negative margins
pCR: Pathological complete response
ICI: Immune checkpoint inhibitor
OS: Overall survival
MSI-H: Microsatellite instability–high
dMMR: Deficient mismatch repair
LAGC: Locally advanced gastric cancer
CPS: Combined positive score
PD-1: Programmed cell death protein 1
PD-L1: Programmed death-ligand 1
GEJC: Gastroesophageal junction cancer
CTLA-4: Cytotoxic T-lymphocyte–associated protein 4
EFS: Event-free survival
TRAEs: Treatment-related adverse events
MPR: Major pathological response
EBV: Epstein–Barr virus
TME: Tumor microenvironment
CTLA-4: Cytotoxic T-lymphocyte–associated protein 4
RFS: Relapse-free survival
ctDNA: Circulating tumor DNA
ADC: Antibody–drug conjugate.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660, PMID: 33538338
2. Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond). (2024) 44:127–72. doi: 10.1002/cac2.12516, PMID: 38160327
3. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108, PMID: 35143424
4. Yang K, Choi YY, Zhang WH, Chen XZ, Song MK, Lee J, et al. Strategies to improve treatment outcome in gastric cancer: a retrospective analysis of patients from two high-volume hospitals in Korea and China. Oncotarget. (2016) 7:44660–75. doi: 10.18632/oncotarget.9378, PMID: 27191995
5. Zhang X, Liu B, Wang R, Li X, and Zhou W. Current status of neoadjuvant immunotherapy for the treatment of gastric cancer. Clin Transl Oncol. (2024) 26:2097–108. doi: 10.1007/s12094-024-03437-0, PMID: 38504071
6. Mokdad AA, Yopp AC, Polanco PM, Mansour JC, Reznik SI, Heitjan DF, et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: A propensity score-matched analysis. JAMA Oncol. (2018) 4:31–8. doi: 10.1001/jamaoncol.2017.2805, PMID: 28975352
7. Kim TH, Uyama I, Rha SY, Bencivenga M, An J, Wyrwicz L, et al. Conversion therapy for stage IV gastric cancer: report from the expert consensus meeting at KINGCA WEEK 2024. J Gastric Cancer. (2025) 25:133–52. doi: 10.5230/jgc.2025.25.e9, PMID: 39822172
8. Yang H, Ji K, and Ji J. Current status and perspectives of conversion therapy for advanced gastric cancer. Chin J Cancer Res. (2022) 34:109–14. doi: 10.21147/j.issn.1000-9604.2022.02.05, PMID: 35685991
9. Chevallay M, Wassmer CH, Iranmanesh P, Jung MK, and Mönig SP. Multimodal treatment in oligometastatic gastric cancer. World J Gastrointest Oncol. (2022) 14:434–49. doi: 10.4251/wjgo.v14.i2.434, PMID: 35317315
10. Kerkar SP, Kemp CD, Duffy A, Kammula US, Schrump DS, Kwong KF, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials. (2009) 10:121. doi: 10.1186/1745-6215-10-121, PMID: 20030854
11. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17:309–18. doi: 10.1016/S1470-2045(15)00553-7, PMID: 26822397
12. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, and Martens UM. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. (2017) 3:1237–44. doi: 10.1001/jamaoncol.2017.0515, PMID: 28448662
13. Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, et al. International retrospective cohort study of conversion therapy for stage IV gastric cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. (2021) 6:227–40. doi: 10.1002/ags3.12515, PMID: 35261948
14. Shin MK, Choi MG, Kim ST, Kang WK, Sohn TS, An JY, et al. The clinical implication of conversion surgery in patients with stage IV gastric cancer who received systemic chemotherapy. Biomedicines. (2023) 11:3097. doi: 10.3390/biomedicines11113097, PMID: 38002099
15. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, and Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z, PMID: 26643880
16. Cunningham D, Allum WH, Stenning SP, Thompson JN, and Van de Velde CJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. (2006) 355:11–20. doi: 10.1056/NEJMoa055531, PMID: 16822992
17. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. (2019) 393:1948–57. doi: 10.1016/S0140-6736(18)32557-1, PMID: 30982686
18. Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. (2015) 15:532. doi: 10.1186/s12885-015-1529-x, PMID: 26194186
19. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918, PMID: 38051328
20. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2, PMID: 34102137
21. Liu T, Bai Y, Lin X, Li W, Wang J, Zhang X, et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Cancer. (2023) 152:749–60. doi: 10.1002/ijc.34296, PMID: 36121651
22. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370, PMID: 32880601
23. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6, PMID: 37875143
24. Shen L, Zhang Y, Li Z, Zhang X, Gao X, Liu B, et al. First-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma: a randomized, double-blind, phase 3 trial. Nat Med. (2025) 31(4):1163–70. doi: 10.1038/s41591-024-03450-4, PMID: 39843940
25. Janjigian YY, Al-Batran SE, Wainberg ZA, Van Cutsem E, Molena D, and Muro K. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. Ann Oncol. (2023) 34:S1315–6. doi: 10.1016/j.annonc.2023.10.074
26. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, and Tabernero J. Phase III KEYNOTE-585 study of chemotherapy (Chemo)+ pembrolizumab (Pembro) vs chemo+ placebo as neoadjuvant/adjuvant treatment for patients (Pts) with gastric or gastroesophageal junction (G/GEJ) cancer. Ann Oncol. (2018) 29:viii268. doi: 10.1093/annonc/mdy151.095
27. Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. (2024) 25:212–24. doi: 10.1016/S1470-2045(23)00541-7, PMID: 38134948
28. Yuan SQ, Nie RC, Jin Y, Liang CC, Li YF, Jian R, et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat Med. (2024) 30:552–9. doi: 10.1038/s41591-023-02721-w, PMID: 38167937
29. Ding X, Wang X, Li B, Wang L, Guo H, Shang L, et al. PERSIST: A multicenter, randomized phase II trial of perioperative oxaliplatin and S-1 (SOX) with or without sintilimab in resectable locally advanced gastric/gastroesophageal junction cancer (GC/GEJC). ascopubs.org. (2023), 364–4. doi: 10.1200/JCO.2023.41.4_suppl.364
30. Liu ZL, Chen HH, Zheng LL, Sun LP, and Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. (2023) 8:198. doi: 10.1038/s41392-023-01460-1, PMID: 37169756
31. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, and Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29, PMID: 29508855
32. Li S, Yu W, Liu Z, Lv W, Shi D, Yu D, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib,S-1 ± oxaliplatin for locally advanced cT4a/bN+ gastric cancer. ascopubs.org. (2021), 4061–1. doi: 10.1200/JCO.2021.39.15_suppl.4061
33. Pietrantonio F, Raimondi A, Lonardi S, Murgioni S, Cardellino GG, Tamberi S, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). ascopubs.org. (2023), 358–8. doi: 10.1200/JCO.2023.41.4_suppl.358
34. André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. (2023) 41:255–65. doi: 10.1200/JCO.22.00686, PMID: 35969830
35. Wei J, Lu X, Liu Q, Fu Y, Liu S, Zhao Y, et al. Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm phase 2 trial. Nat Commun. (2023) 14:4904. doi: 10.1038/s41467-023-40480-x, PMID: 37580320
36. Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer. (2017) 81:183–90. doi: 10.1016/j.ejca.2017.04.027, PMID: 28628843
37. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6, PMID: 26254683
38. Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol Off J Am Soc Clin Oncol. (2006) 24:3953–8. doi: 10.1200/JCO.2006.06.4840, PMID: 16921048
39. Tang Z, Wang Y, Liu D, Wang X, Xu C, Yu Y, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat Commun. (2022) 13:6807. doi: 10.1038/s41467-022-34403-5, PMID: 36357415
40. Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, et al. TOPGEAR: A randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. (2017) 24:2252–8. doi: 10.1245/s10434-017-5830-6, PMID: 28337660
41. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481, PMID: 29320654
42. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923, PMID: 30242316
43. Thompson JA, Schneider BJ, Brahmer J, Zaid MA, Achufusi A, Armand P, et al. NCCN guidelines® Insights: management of immunotherapy-related toxicities, version 2. 2024 J Natl Compr Canc Netw. (2024) 22:582–92. doi: 10.6004/jnccn.2024.0057, PMID: 39536465
44. Konishi T, Kosuga T, Ichikawa D, Komatsu S, Okamoto K, Konishi H, et al. Conversion surgery for stage IV gastric cancer. Front Oncol. (2019) 9:1158. doi: 10.3389/fonc.2019.01158, PMID: 29394583
45. Palaj J, Kečkéš Š, Marek V, Dyttert D, Sabol M, Durdík Š, et al. Single centre experience with conversion surgery for advanced and metastatic gastric cancer in Slovakia. Sci Rep. (2025) 15:13381. doi: 10.1038/s41598-025-98656-y, PMID: 40251306
Keywords: gastric cancer, immunotherapy, conversion therapy, R0 resection rate, multimodal therapy
Citation: Su Y, Zhang X and Deng W (2025) Immunotherapy in conversion therapy for gastric cancer: current status, progress, and challenges. Front. Oncol. 15:1637657. doi: 10.3389/fonc.2025.1637657
Received: 29 May 2025; Accepted: 05 September 2025;
Published: 22 September 2025; Corrected: 20 November 2025.
Edited by:
Zhen Liu, Zhejiang University, ChinaReviewed by:
Zhifang Zhang, City of Hope National Medical Center, United StatesÖtrs Péter Horváth, Pécs University, Hungary
Wei Jiangpeng, Air Force Medical University, China
Copyright © 2025 Su, Zhang and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Deng, ZGVuZ3dlaXdlaUAxMjYuY29t
 Yi Su
Yi Su Xin Zhang
Xin Zhang Wei Deng
Wei Deng