- Department of Radiation Oncology, Chongqing University Cancer Hospital, Chongqing, China
Glioblastoma (GBM) is a highly aggressive brain tumor with a poor prognosis, characterized by rapid progression and limited treatment options. This review explores the emerging role of physical activity as a complementary therapy in GBM management, focusing on its multifaceted effects on tumor biology, immune modulation, and patient quality of life. Exercise has been shown to influence key molecular pathways involved in GBM progression, including the RTK/PI3K/Akt/mTOR signaling cascade, angiogenesis, and metabolic reprogramming. Additionally, physical activity enhances immune surveillance by mobilizing cytotoxic T cells and natural killer (NK) cells, while reducing immunosuppressive cells like Tregs and MDSCs. Clinical and preclinical evidence suggests that exercise may improve cognitive function, reduce treatment-related toxicity, and prolong survival in GBM patients. Despite these promising findings, significant gaps remain in understanding the optimal exercise regimens and their mechanistic underpinnings. Future research should prioritize personalized approaches, integration with novel therapies, and multi-omics analyses to elucidate exercise-induced changes in the tumor microenvironment (TME). This review underscores the potential of physical activity to revolutionize neuro-oncology therapy, offering a paradigm shift in GBM treatment strategies.
1 Introduction
Glioblastoma multiforme (GBM) accounts for 54% of all glioma cases and 16% of all primary brain neoplasms, making it the most common malignant primary brain tumor in adults (1, 2). Gliomas are a diverse group of primary tumors that originate from glial cells and the supportive cells of the central nervous system (CNS), which includes the brain and spinal cord. These tumors arise when glial cells undergo abnormal growth, leading to the formation of masses that can disrupt normal neurological function (3). Gliomas are among the most common types of brain tumors, encompassing various subtypes such as astrocytomas, oligodendrogliomas, and glioblastomas. The behavior and prognosis of gliomas can vary widely depending on factors like tumor grade, location, and molecular characteristics (4).
GBM is classified as a grade IV glioblastoma by the World Health Organization (WHO) and is the most malignant diffuse astrocytic glioma (5). With median survival spans of around 16 months after first diagnosis and reported 5-year survival rates close to 5%, GBM has an exceedingly bad prognosis (6). Different molecular and genetic factors give birth to the primary and secondary types of this cancer (7). Approximately 90% of identified cases are primary GBM, which primarily affects older people and develops de novo without prior tumor lesions (6). Secondary GBM is primarily seen in younger individuals and results from the advancement of low-grade diffuse astrocytoma or anaplastic astrocytoma (6). Isocitrate dehydrogenase (IDH) mutation status was included in the WHO 2021 classification of diffuse gliomas, allowing for the differentiation of IDH wild-type glioblastoma from IDH-mutant glioblastoma (8). Apart from the higher death rate, patients with GBM have a far lower health-related quality of life (QOL) than those with other tumor types (8).
In addition to neurological symptoms like headaches, seizures, and cognitive decline, including memory loss and speech problems, patients with brain cancer frequently have neurological impairments like balance, motor function, and visual problems at the time of diagnosis (9). A range of toxicities accompany treatment measures in addition to the tumor-related symptoms. Treatment options include radiation, chemotherapy, corticosteroid injections, and surgical excision, which can be used singly or in combination (10). Fatigue, myopathy, decreased physical functioning, sleeplessness, greater cognitive decline, mood problems, and psychological anguish are some of the most crippling side effects associated with these therapy techniques (11–20). Anticonvulsant drugs that are often provided to patients may make their feelings of sleepiness and exhaustion worse (21). As a result, patients are often unable to continue working and are subject to legal limitations on their capacity to drive, both of which significantly lower their quality of life (22). There are now few pharmacological treatments available to successfully lessen the incapacitating symptoms connected to brain cancer and its management. To manage the often-crippling symptoms of brain cancer including GBM clinicians commonly employ supportive pharmacological therapies (23). Corticosteroids, most notably dexamethasone, are widely used to rapidly reduce peritumoral edema and intracranial pressure, offering prompt relief from headaches, nausea, and focal neurological impairments (24). Anticonvulsants, such as levetiracetam, valproic acid, phenytoin, and carbamazepine, are also standard for preventing and controlling seizure activity linked to tumor presence or treatment. Additionally, temozolomide, used in combination with radiotherapy for GBM, can stabilize symptoms by targeting rapidly dividing tumor cells and improving overall neurological function (25). Because there are so few proven therapy choices, patients suffer from a significant symptom load that is typically untreated and recalcitrant to treatment. The substantial unmet supportive care requirements that patients and their caregivers report highlight this difficulty (26–28). An expanding body of research supports the link between physical activity and enhanced outcomes for cancer patients, including better symptom control, improvements in both physical and psychological health, enhanced quality of life (QOL), and extended survival (29–31). While adverse effects may occasionally arise, as is the case with any form of physical intervention, clinical evaluations have confirmed that individualized exercise programs, which are carefully adjusted according to the patient’s diagnosis, ongoing treatment, and specific needs, are generally safe to implement both during and after cancer therapy (29, 30). This robust evidence base has driven the establishment of exercise guidelines tailored to oncology, which emphasize that all individuals undergoing cancer treatment should avoid a sedentary lifestyle (31, 32). Nonetheless, it is important to recognize that most current studies have concentrated on the most frequently diagnosed cancers and primarily involve patients with early-stage disease, thereby limiting the generalizability of findings to those with rarer or more advanced forms of cancer (31). Moreover, mechanistic investigations indicate that exercise may bolster anti-tumor immunity, activating natural killer and T cells, modulating inflammation, and improving blood-brain barrier permeability, thereby potentially increasing the efficacy of chemotherapy and immunotherapy (33).
In people with high-grade glioma, respondents report that maintaining physical activity improved well-being and helped delay functional decline (34, 35). A growing number of clinical trials are underway or in protocol, including studies on circuit-based resistance training aimed at preserving muscle function and independence in glioblastoma patients (36). While these investigations are still limited in scale, they mark a growing recognition that exercise interventions can be tailored even for individuals with aggressive brain tumors. Despite these encouraging signs, most current evidence stems from small, often preliminary studies, and there is limited research incorporating glioma patients into mainstream cancer exercise guidelines. This has resulted in a lack of consensus on optimal exercise type, intensity, timing, and safety precautions specific to glioma management. Further rigorous, glioma-specific trials, ideally randomized and multicenter, are needed to establish evidence-based exercise protocols aimed at improving clinical outcomes, maintaining function, and enhancing quality of life in this patient population.
Therefore, this review aims to comprehensively examine the current evidence on how physical activity influences GBM progression, focusing on its effects at the molecular, immunological, metabolic, and neurological levels. By synthesizing findings from both preclinical and clinical studies, this review seeks to highlight the therapeutic potential of exercise as an adjunct strategy in GBM treatment. We also aim to identify key gaps in the literature and propose future research directions to better understand the mechanistic pathways through which exercise may improve patient outcomes in the context of this aggressive brain tumor.
2 Molecular pathogenesis of GBM
Many molecular changes in GBM have been identified in the past two decades, which have allowed for a more thorough description of the tumor and improved our knowledge of the molecular landscape of gliomas and the oncogenic pathways disrupted in this cancer (1). It is believed that different genetic pathways give birth to the main and secondary GBM subtypes, which probably account for variations in clinical prognosis and response to treatment (6, 7). Overexpression of the EGFR gene, loss of heterozygosity (LOH) on chromosome 10q that affects the phosphatase and tensin homolog (PTEN) gene, mutations in the TERT promoter, deletion of CDKN2A (p16), and, less frequently, amplification of mouse double minute 2 (MDM2) are the most common characteristics of primary GBM (6, 7, 37). Mutations in the retinoblastoma (RB) pathway, amplification of platelet-derived growth factor A (PDGFA) and its receptor PDGFR-α, LOH on chromosome 19q, and mutations in IDH1/2, TP53, and the alpha-thalassemia/mental retardation syndrome X-linked gene (ATRX) are among the characteristics of secondary GBMs (37). The three main signaling cascades that cause these genetic changes are the RB signaling system, the p53 tumor suppressor pathway, and the receptor tyrosine kinase (RTK)/RAS/phosphatidylinositol 3-kinase (PI3K) pathway (38). Other pathways include anaerobic metabolism, AMP-activated protein kinase (AMPK) activity, hypoxia-inducible factor (HIF) signaling, and neuroinflammatory reactions in the brain (37–40). The pathophysiology and development of GBM are greatly influenced by epigenetic processes, such as promoter CpG island DNA hypermethylation, dysregulated production of microRNAs (miRNAs/miRs), and post-translational histone changes (41).
It is commonly known that one of the most common genetic changes in malignant gliomas is the receptor tyrosine kinase (RTK) signaling pathway (38). The most often altered and amplified sites in these tumors are the EGFR and PDGFRA genes (38). In GBM, EGFR-mediated downstream signaling promotes improved cell division, greater tumor invasiveness, and chemoresistance via controlling a variety of cellular functions, including migration, proliferation, and survival (42). Amplification of EGFR protein expression, deletion of downstream inhibitory regulators, and constitutively active EGFR variants like EGFRvIII, the most common mutation among amplified EGFR alleles in GBM, are some of the mechanisms that enhance EGFR signaling (38). The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway is one of the several downstream signaling pathways that are later activated as a result of this cascade (43). Developing novel therapy approaches, like as targeted therapies with monoclonal antibodies, requires an understanding of the complex biochemical processes underlying GBM etiology and progression.
In the tumor microenvironment (TME), which is made up of immune cells, endothelial cells, and cancer-associated fibroblasts, hypoxia-inducible factors (HIFs) and other metabolic intermediates are essential for creating a favorable environment that promotes tumor growth and metastasis (44). By improving the flow of blood and nutrients to cancerous cells, HIFs promote angiogenesis. The increased development of cancer caused by HIF-1, which increases the production of vascular endothelial growth factor (VEGF), is intimately associated with the angiogenic switch seen throughout tumor progression (44). The TME, which metabolically supports the tumor, aids in its integration into surrounding tissues, and eventually permits intravasation into the systemic circulation, thereby promoting metastatic dissemination, has been shown to exhibit increased VEGF expression in previous studies (45). The previous study by Cao et al. showed that amplification of the pro-apoptotic 14-3-3ζ gene stimulated the PI3K/Akt signaling pathway, which is connected to the elevation of VEGF and HIF-1α expression in gliomas (45). In individuals with GBM, this mechanism corresponds with a worse prognosis and increases tumor malignancy.
A key modulator of cellular development, metabolic activities, autophagy, and cell polarity under normal physiological settings, AMPK is an essential regulator of cellular energy balance (46). Clarifying the regulatory processes controlling AMPK and its functional change toward aiding carcinogenesis in cancer is therefore crucial. Previous studies have demonstrated that increased AMPK expression plays a crucial role in bioenergetic pathways in GBM by increasing tumor growth through interactions with the HIF-VEGF axis (47). In the TME, AMPK also controls the expression of glucose transporter proteins on tumor cells, which promotes improved glucose absorption to support bioenergetic pathways and ATP generation (47). One important mediator that makes it easier for AMPK signaling to engage with the control of HIF and glucose transporter protein function is cyclic AMP response element-binding protein 1 (CREB1) (47). Previous research has examined the functions of these three elements as well as the fundamental processes via which altering this pathway may slow the growth of tumors. Research indicates that inhibiting CREB1 and AMPK lowers levels of GA-binding protein alpha chain, a transcription factor essential for controlling mitochondrial activity, as well as the production of glucose transporter proteins and HIF (47).
The development of GBM is linked to metabolic byproducts produced by changed energy pathways, where the cancer cells experience a metabolic shift toward glycolysis, leading to increased lactate generation (48). One of the main causes of tumor development and progression is lactate buildup and its interaction with the hydroxycarboxylic acid receptor 1 (HCAR1) (49). Anaerobic metabolic pathways are promoted by the TME, which results in a substantial buildup of lactate and an increase in the expression of the HCAR1 (49). VEGF overexpression and increased angiogenic signaling are correlated with this upregulation, especially in the cerebral milieu, which is a feature specific to GBM. According to the research currently accessible, GBM patients’ peripheral tissues do not exhibit a comparable increase in HCAR1 receptor activation (49). A glycolytic molecule called aldehyde dehydrogenase (ALDH) has been linked to the metabolic dysregulation of GBM, and increased expression of ALDH has been demonstrated to increase tumor aggressiveness (50). Because ALDH promotes glioma stem cells (GSCs), which increases the aggressiveness of GBM cells, there is a correlation between greater expression of ALDH and increased malignancy (50). Because of their resistance to temozolomide, GSCs can survive therapy and play a role in tumor recurrence (51). Further aggravating edema in GBM, these cells overexpress vascular endothelial growth factor receptor 2 (VEGFR2), which promotes endothelial cell migration, proliferation, and vascular permeability (51). Targeting GSCs may effectively inhibit tumor progression and overcome resistance to current anti-angiogenic treatments, as evidenced by recent studies showing that selective deletion of stromal cell-derived factor 1 (SDF-1), which interacts with CXCR4 expressed on GSCs, suppresses tumor growth and extends survival in GBM models (52).
Through mechanisms such as inducing apoptosis in damaged cells, maintaining genomic stability, inhibiting angiogenesis, and regulating cellular metabolism and the TME, p53, a transcription factor and tumor suppressor, plays a crucial role in tumor prevention (53). In addition, p53 is an essential modulator of the TME, immunological responses, invasion, metastasis, autophagy, cellular metabolism, and stem cell maintenance (54). The p53 pathway is often dysregulated in GBM; according to The Cancer Genome Atlas (2013), investigations have shown changes in the ARF-MDM2-p53 axis in up to 94.1% of GBM-derived cell lines and about 84% of cases (55). The co-occurrence of p53 mutations and IDH1 mutations in secondary GBM contributes to the intricacy and continuous discussion of p53’s function in GBM pathogenesis (56). The tumor suppressor gene PTEN can negatively regulate the vital PI3K/Akt signaling pathway, which controls the development and survival of cells (57). At least 60% of GBM cases include deregulation of the PI3K signaling pathway as a result of chromosomal 10q23 mutations in the PTEN gene and the resulting LOH (58). PTEN genetic mutation is associated with the poor survival of patients with GBM (59). The development of more potent treatment approaches depends on the advancement of our understanding of the molecular processes behind the p53 and PTEN signaling pathways, which are important therapeutic targets in GBM (60).
GBM-related inflammatory processes can cause abnormal reactions in the brain microenvironment, which can aid in the growth of tumors and the dysregulation of the microenvironment (61). The effectiveness of cancer treatments, especially targeted therapy like anti-VEGF medicines, might be jeopardized by elevated inflammatory activity. The delivery and efficacy of therapy are hampered by persistent neuroinflammation, which alters the TME and triggers adaptive inflammatory responses (61). Tie2-expressing monocytes have been shown in earlier research to be a pro-angiogenic cell type that expresses important gene transcripts, such as VEGF (62). By expressing endothelial markers like CD31 and VEGF receptors and by morphologically resembling endothelial cells, myeloid-derived suppressor cells (MDSCs) may support the structural integrity of tumor-associated neo-endothelium (63). p53 mutations lead to persistent chronic inflammation in GBM, a disease associated with a poor prognosis and high death rates (64). The primary effect of p53 gene mutation is the increase of tumor necrosis factor-alpha (TNF-α) and C-C motif chemokine ligand-2 (CCL2). This increase is positively associated with increased infiltration of monocyte-derived immune cells and microglia, which may worsen inflammatory processes in GBM while also obstructing the effectiveness and delivery of treatment (64).
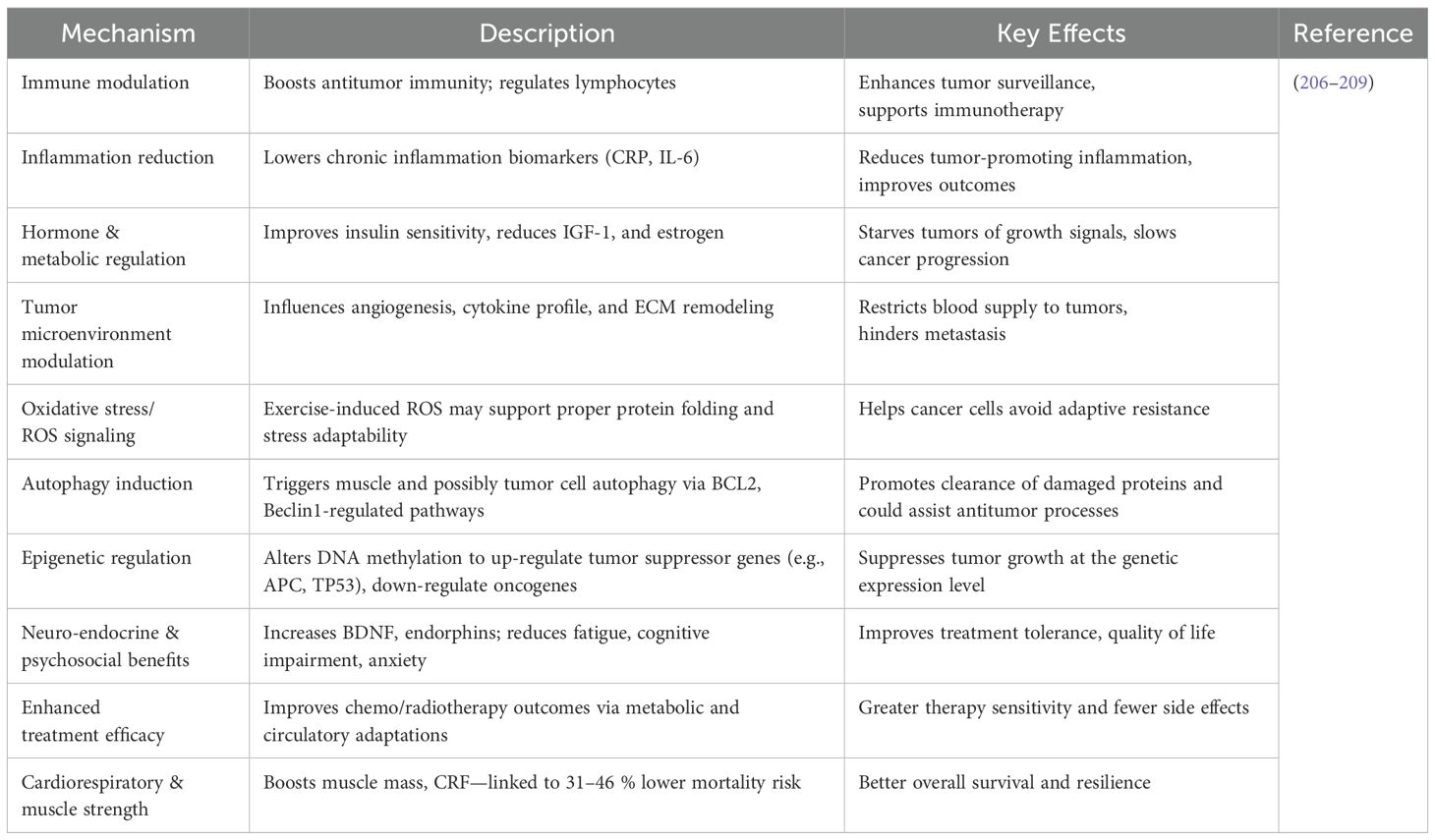
Hypoxic circumstances that inhibit the expression of cylindromatosis (CYLD), a tumor suppressor that acts as a deubiquitinating enzyme to control important signaling pathways, may be the cause of the decreased effectiveness of targeted treatments in the context of inflammation (65). Guo et al. demonstrated that the inhibition of CYLD is a key factor driving inflammatory responses within the GBM microenvironment (65). Therefore, in the setting of GBM, CYLD may operate as a tumor suppressor whose expression is inhibited and its function is lost. Furthermore, through paracrine processes, CYLD downregulation may promote GBM-associated tissue inflammation by amplifying downstream TNF-α and NF-κB signaling (65). This cascade increases the tumor’s nutritional supply and exacerbates its malignant phenotype by promoting angiogenesis and decreasing the effectiveness of anti-angiogenic drugs (1, 65). The comprehensive information about molecular pathogenesis is shown in Table 1. Exercise-mediated modulation of tumor growth through metabolic and immune pathways is shown in Figure 1.
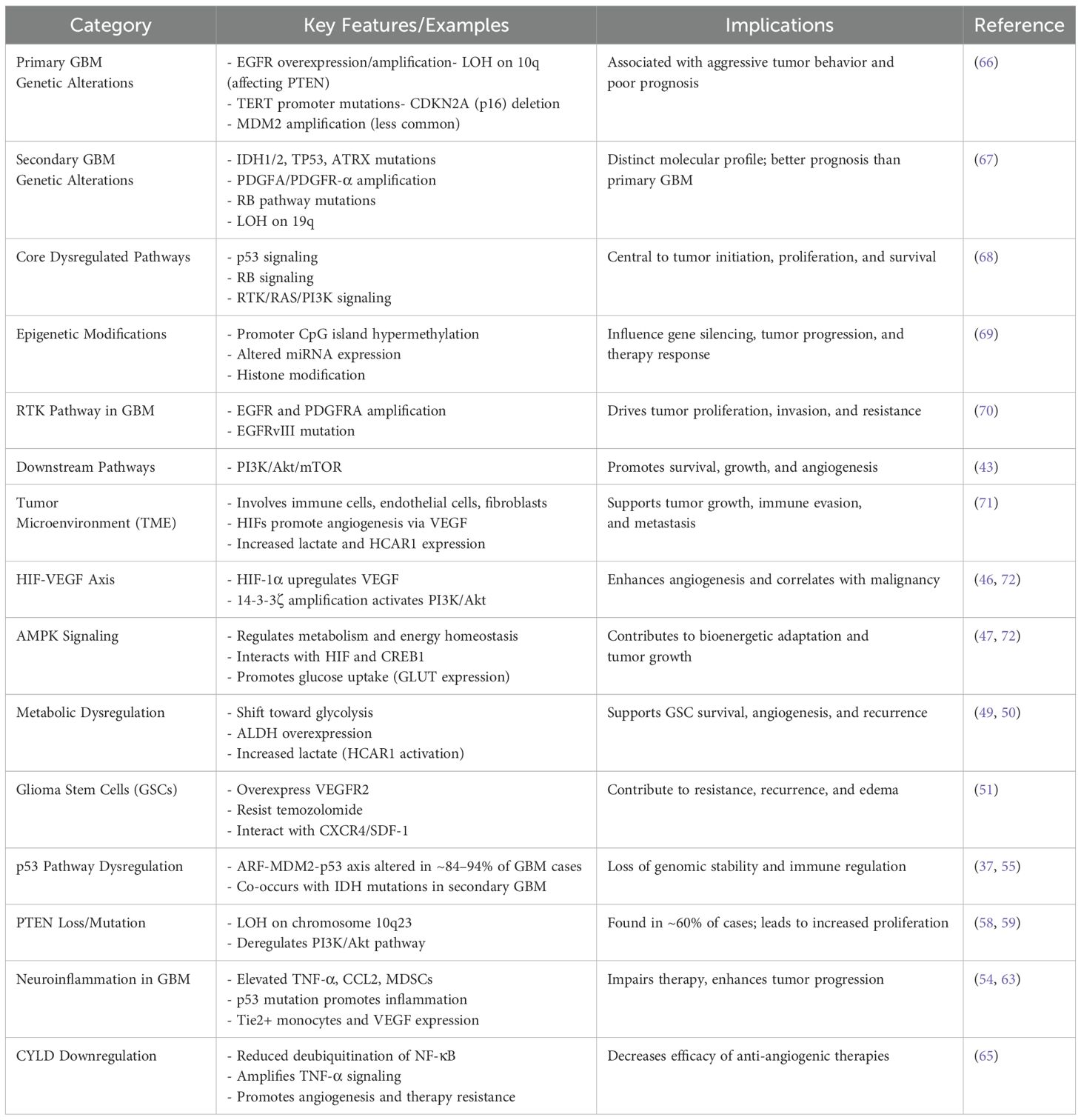
Table 1. Key molecular and pathological features of GBM (1).
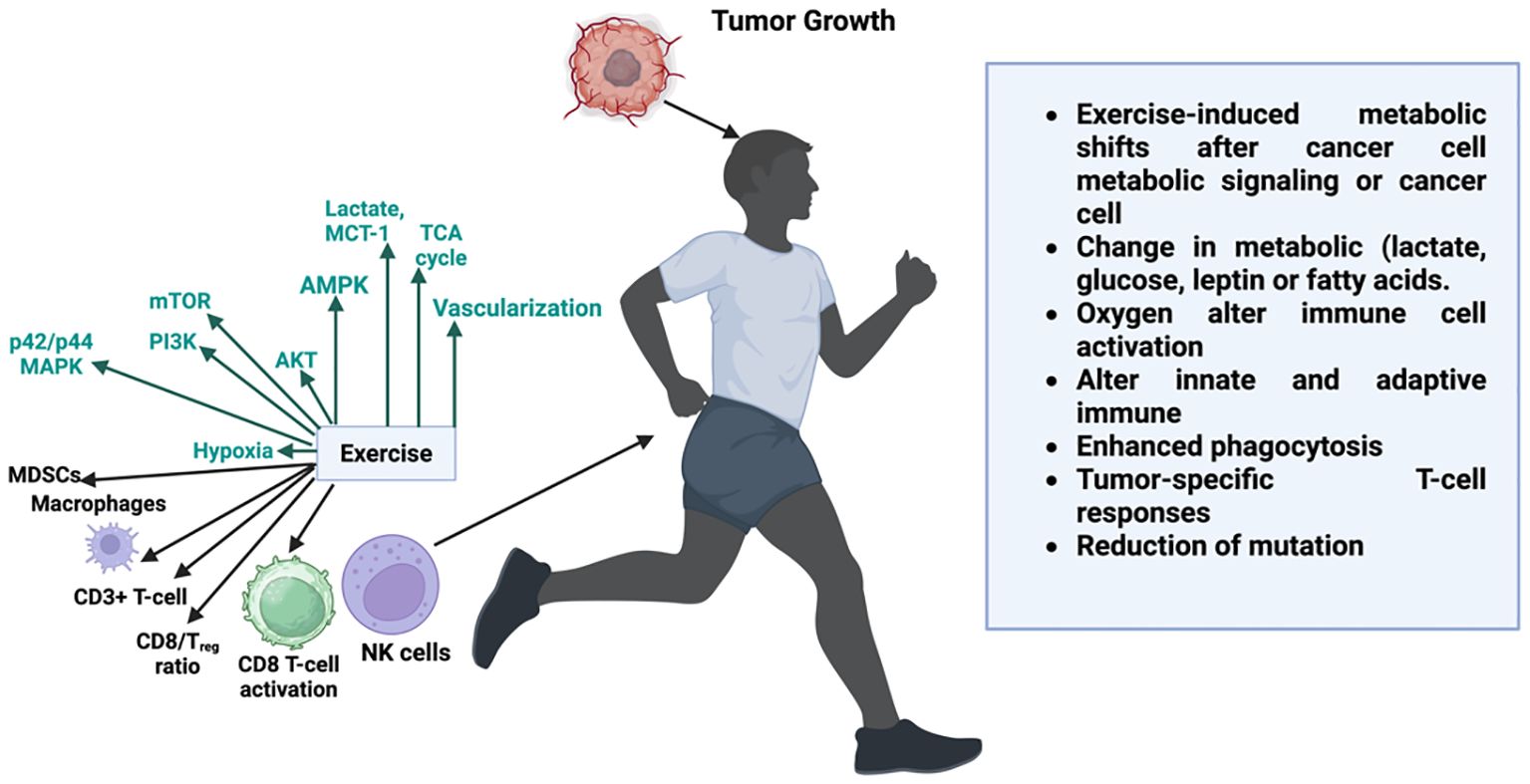
Figure 1. Exercise-mediated modulation of tumor growth through metabolic and immune pathways. Physical activity influences both systemic metabolism and tumor microenvironment by altering key signaling pathways (e.g., AMPK, AKT, PI3K, mTOR, p42/p44 MAPK), improving oxygenation, and enhancing vascularization. These shifts impact immune cell dynamics boosting CD8+ T-cell activation, increasing the CD8+/Treg ratio, activating NK cells, and reducing immunosuppressive cells such as MDSCs and TAMs. Exercise-induced changes in metabolites (lactate, glucose, fatty acids) and improved oxygen delivery enhance both innate and adaptive immune responses, augment phagocytosis, and promote tumor-specific T-cell responses, collectively contributing to reduced tumor growth and mutation burden.
3 Mechanisms of exercise impacting GBM progression
Exercise may influence GBM progression through multiple integrated mechanisms that align closely with GBM’s unique biology. First, physical activity enhances antitumor immunity within the typically immunosuppressed GBM microenvironment. It increases infiltration and activation of natural killer (NK) cells and cytotoxic T lymphocytes and reduces suppressive cells such as regulatory T cells and M2-like microglia, thereby improving local immune surveillance (33, 73). Simultaneously, exercise contributes to normalization of the blood–brain barrier (BBB) via improved endothelial and vascular function, enhancing the penetration of chemotherapeutic agents and immunotherapies into GBM tumors (74).
By improving perfusion and reducing hypoxia, exercise also reshapes the GBM TME, mitigating treatment resistance driven by hypoxic niches (33, 75). Metabolically, exercise induces systemic release of myokines and exerkines such as IGF-1, adiponectin, irisin, and anti-inflammatory mediators that cross the BBB to modulate tumor cell signaling (e.g., PI3K/AKT, JAK/STAT), leading to reduced proliferation and inflammation. In preclinical GBM models, exercise decreased reactive oxygen species and genomic instability, further supporting its antitumor potential (76). Moreover, exercise synergizes with chemotherapy and immunotherapy, enhancing treatment efficacy and reducing side effects, highlighting its role as a potential adjunct therapy in GBM treatment (77). Beyond tumor control, exercise induces beneficial neurobiological and epigenetic effects, upregulating neurotrophic factors (BDNF, VEGF) to support neuronal health adjacent to GBM, and eliciting epigenetic modifications that can reactivate tumor suppressor genes while suppressing oncogenic pathways (77). Collectively, these mechanisms of immune modulation, BBB normalization, microenvironmental remodeling, metabolic reprogramming, synergism with standard interventions, and neuroprotective/epigenetic adaptations form a cohesive framework supporting the integration of exercise as a targeted adjunct therapy in GBM management (78).
3.1 Exercise and immune modulation
It has long been known that physical activity affects both innate and adaptive immunity (79). However, there is still discussion and research surrounding the specific underlying mechanisms underpinning these immunomodulatory benefits of physical exercise (80). Through processes including exercise-induced immune cell migration and redistribution, immunostimulatory myokine release, exercise-induced immune cell metabolic reprogramming, and immunosenescence mitigation, physical activity has been demonstrated to impact immunological regulation (81). The main immunological benefits of exercise are highlighted in this synopsis, especially those linked to boosting anti-tumor immune responses.
3.2 Exercise-induced immune cell mobilization and redistribution
It is commonly known that physical activity causes a brief rise in the number of leukocytes in circulation (82–85). Exercise-induced leukocytosis (EIL) is observed following both resistance and endurance training; however, comparative examinations of different exercise modalities reveal that the leukocytic response is often more pronounced following aerobic exercise than strength training (86, 87). The main mechanisms behind EIL include catecholamine-induced endothelium adhesion molecule downregulation, increased shear stress, and raised arterial blood pressure, all of which contribute to leukocyte detachment and mobilization into the bloodstream (82–85), Cellular populations in the immune system’s innate and adaptive branches are impacted by this phenomenon (79, 88). A summary of key immune cell subsets mobilized in response to acute exercise and their relevance to GBM and other cancer immunosurveillance is presented in Table 2.
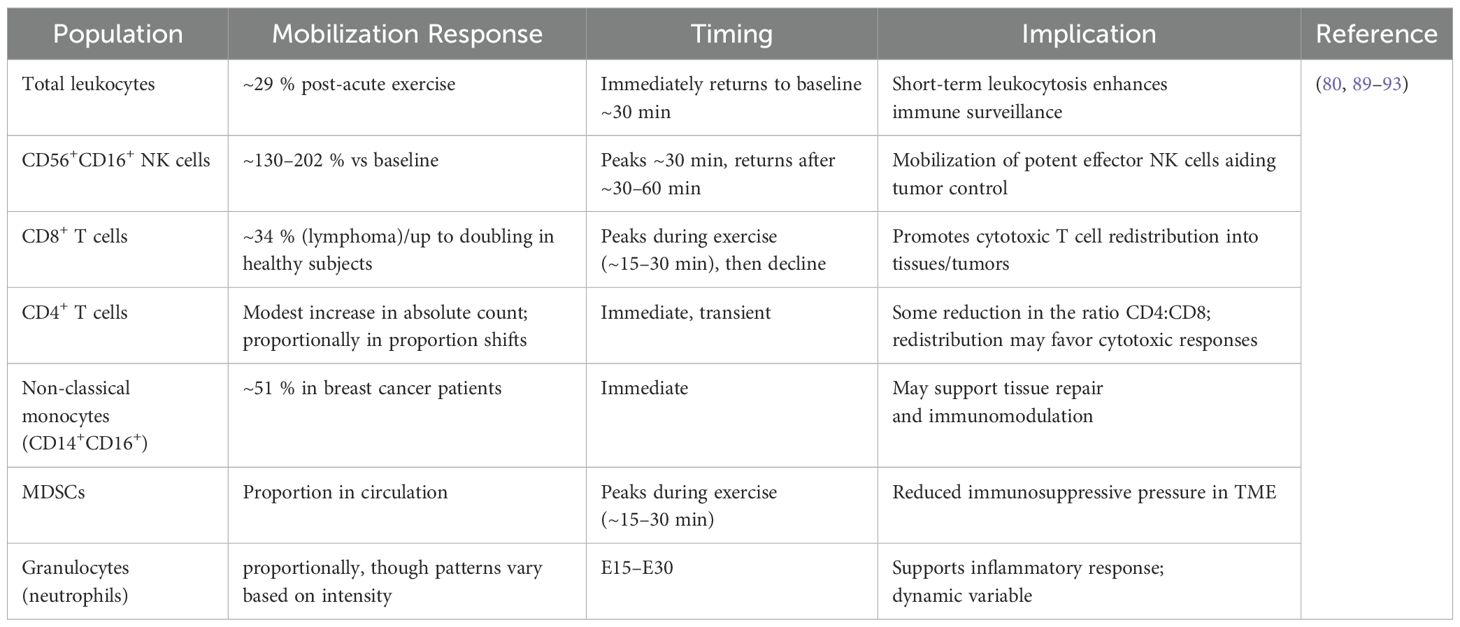
Table 2. Immune cell mobilization and redistribution induced by acute exercise in cancer and healthy subjects.
As a result, the degree of leukocyte mobilization seems to be correlated with the expression of β2-adrenergic receptors on the surface, which mostly results in the recruitment of CD8 T lymphocytes and natural killer (NK) cells (Figure 2) (94–96). For example, a 20-minute high-intensity cycling exercise at 85% of peak power output (Wattmax) has been demonstrated to boost peripheral CD8+ T cell counts by up to 2.5 times and peripheral NK cell counts by five to ten times (89). Notably, fractions with enhanced effector capabilities and tissue-migratory capacity are preferentially recruited within the NK and CD8+ T cell compartments, improving the immune system’s potential to eradicate malignant, damaged, or pathogen-infected cells (89, 97–102). The pattern of exercise-induced leukocytosis is usually biphasic, peaking 45 to 60 minutes after exercise and then experiencing a brief drop in leukocyte counts 1 to 2 hours later (103–105). Within 24 to 48 hours after exercise, immune cell counts usually revert to baseline, indicating that the changes in immune cell populations brought on by exercise are temporary (81, 106).
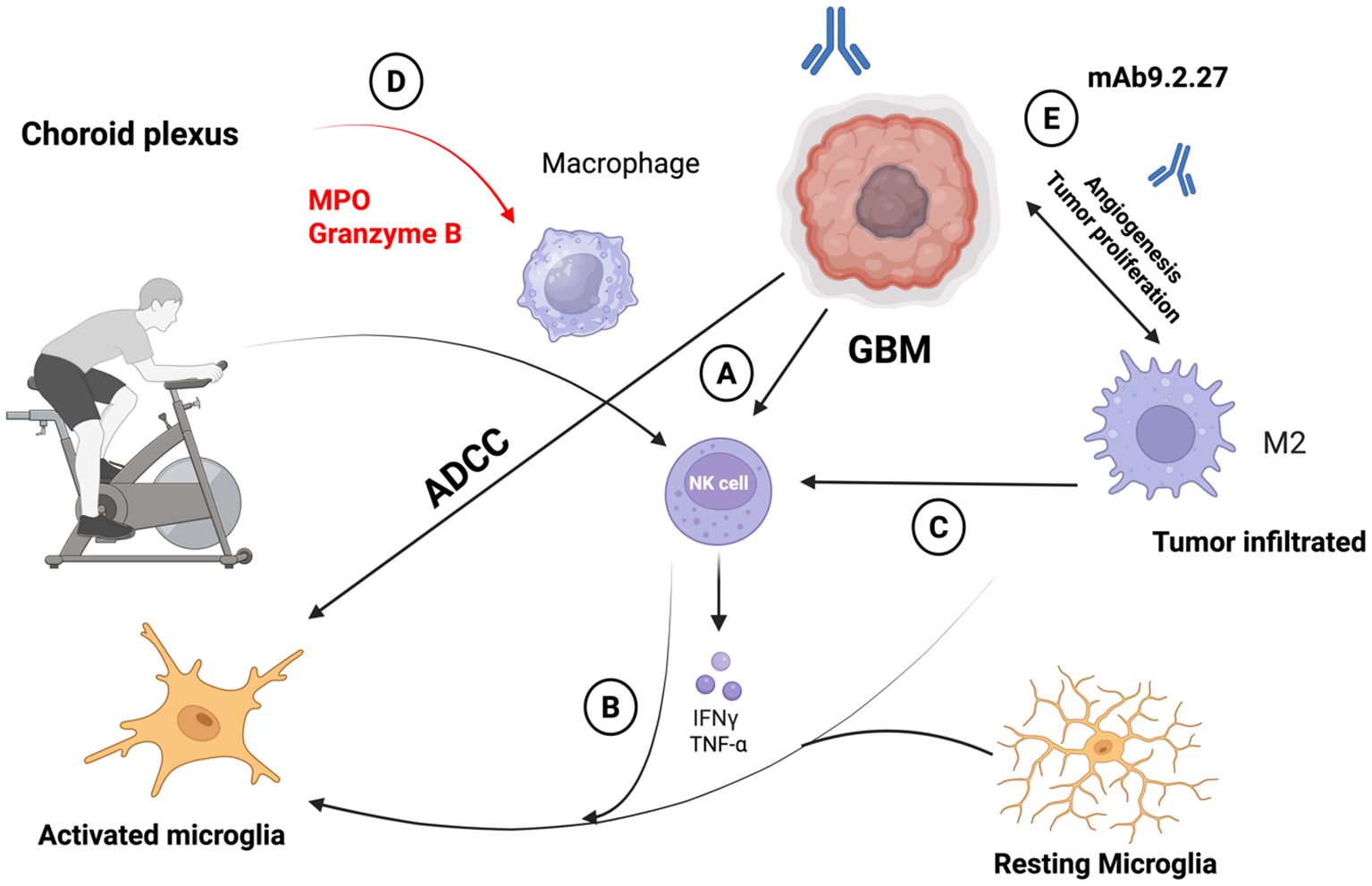
Figure 2. (A) Adoptive transfer of activated natural killer (NK) cells represents a promising therapeutic strategy that physical activity can stimulate for inducing regression of glioblastoma (GBM). When delivered directly into the brain, these NK cells exert targeted cytotoxic effects against GBM cells through cell-mediated mechanisms. (B) Upon engaging with GBM cells, NK cells release key pro-inflammatory cytokines interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) both in laboratory models and in living systems. (C) These cytokines play a pivotal role in shifting the tumor microenvironment: they can awaken otherwise dormant microglia and reprogram tumor-associated macrophages (TAMs) that typically support tumor growth into a pro-inflammatory, anti-tumor phenotype. NK cells further contribute to sustaining this inflammatory milieu by selectively eliminating immunosuppressive microglial populations. (D) Once activated, microglia and macrophages contribute to tumor clearance via both innate cytotoxic mechanisms and antibody-dependent responses. For instance, the monoclonal antibody mAb9.2.27 targets the NG2/CSPG4 antigen expressed on GBM cells, enhancing immune-mediated cell killing. (E) Beyond enhancing immune responses, mAb9.2.27 also directly impairs GBM progression by inhibiting tumor growth and angiogenesis. This effect is particularly evident in rat glioma models, where the antibody disrupts the tumor-promoting activities of microglia and TAMs.
The brief decrease in circulating immune cells that occurs after exercise has long been referred to as an “immunosuppressive window,” implying a brief lapse in immunological monitoring (80, 107). Because the leukocytes mobilized during physical activity have strong effector phenotypes and actively redistribute to peripheral tissues and sites of potential immune challenge, a process known as exercise-induced redistribution (EIR), new evidence, however, supports a revised interpretation that emphasizes the immunoenhancing effects of exercise (89). Therefore, exercise-induced leukocytosis’s biphasic character indicates a selective redistribution of highly functioning immune cells to peripheral organs, including the gastrointestinal tract, lungs, bone marrow, and mucosal surfaces, therefore enhancing immunosurveillance (80, 108).
Apart from its impact on the adaptive immune system, exercise also causes significant alterations in innate immunity, such as increased numbers of neutrophils, monocytes, and NK cells in the blood (88). Additionally, exercise has a tissue-specific effect on macrophage polarization and activity, which modifies local immune responses and aids in tissue homeostasis and repair (109). Numerous investigations have shown that, as shown in mouse models, exercise causes macrophages to change from a pro-inflammatory M1-like condition to an anti-inflammatory M2-like phenotype, especially in situations like obesity or tissue damage (110–112). Exercise-induced macrophage responses are context-dependent and influenced by the physiological or pathological state of the tissue, according to other murine studies that have shown that exercise can suppress M2-like macrophage populations and instead promote polarization toward a pro-inflammatory M1-like phenotype (113–115). According to recent research, macrophages, including tumor-associated macrophages (TAMs), often display activation states that defy the conventional M1/M2 dichotomy, reflecting a wider range of functional phenotypes influenced by the local microenvironment. This should be acknowledged in this context (116). In order to completely clarify the underlying processes and functional consequences, future research should examine the impact of exercise on macrophage differentiation in a variety of physiological and pathological situations (109, 117).
3.3 Exercise-derived immunomodulatory myokines
Apart from the systemic immune response, which is typified by the mobilization and redistribution of immune cells brought on by exercise, skeletal muscle is becoming more and more understood as a secretory and immunoregulatory organ that functions by releasing myokines, which are cytokines generated from muscles (118). Skeletal muscle secretes myokines, including hormones, proteins, nucleic acids, and metabolites, in response to physical exercise. These myokines mediate the crosstalk between muscle tissue and other distant organs and facilitate inter-organ communication (119). Too far, several myokines have been found, including important mediators like interleukins IL-7 and IL-15, tumor necrosis factor (TNF), and interferon-gamma (IFN-γ) that help create an anti-TME (120, 121). TNF and IFN-γ play essential roles in the control of T cell development and functional activation, hence crucially defining adaptive immune responses (122, 123). Interleukin-7 (IL-7) and interleukin-15 (IL-15) are cytokines essential for the activation, survival, and maintenance of NK cells and T lymphocytes, contributing significantly to the development and persistence of effective immune responses (124–127). These can thereby enhance immunological responses. The first described myokine (128, 129) was interleukin-6 (IL-6), which, depending on the target tissue, had pleiotropic effects (130). IL-6 is typically regarded as a pro-inflammatory cytokine in cancer patients, encouraging the growth and spread of tumor cells (131). Tumor-induced secretion of IL-6 fosters skeletal muscle wasting, leading to cancer cachexia (131). Increased levels of interleukin-6 in the blood have been found to be negative prognostic indicators, which are associated with worse clinical outcomes and decreased effectiveness of immune checkpoint inhibitor treatment (132, 133). Nevertheless, new data indicate that interleukin-6 may play a more intricate and situation-specific function during exercise as a myokine, possibly exhibiting counterintuitive effects such as anti-tumoral qualities (131). Exercise-induced production of muscle-derived interleukin-6 has been linked to anti-inflammatory benefits, such as improved immune cell absorption of glucose, greater leukocyte mobilization, and reduction of tumor-induced muscle atrophy (cachexia) (130, 134). As a result, the effects of IL-6 vary depending on whether it is released as an exercise-derived myokine or an interleukin generated by tumors.
3.4 Exercise-induced alterations in immune cell metabolism
The functional phenotype of immune cells is closely linked to their metabolic activity, which is controlled by both inherent cellular characteristics and the availability of nutrients. There is mounting evidence that exercise affects the transport of nutrients throughout the body and directly modifies important immunometabolic signaling pathways, which in turn affect immune cell activity (81, 135, 136).
Exercise has been demonstrated to modify the systemic metabolic profile by changing the plasma availability of essential nutrients such as glucose, fatty acids, and glutamine. Skeletal muscle is a vital store of energy substrates (137–139). Therefore, exercise affects the nutrition that circulating immune cells like lymphocytes receive (139). Exercise triggers glucose and glutamine consumption in lymphocytes (140). The metabolic reprogramming brought on by exercise affects immune cell activity by encouraging the production of more interleukin-2 (IL-2) and less interleukin-4 (IL-4). Since skeletal muscle is the main source of glutamine, a metabolite essential for T cell activation and proliferation, glutamine availability may become severely restricted in cancer patients who are cachectic, which would compromise immunological competence (137, 141, 142). Additionally, exercise may enhance immune cells’ glutamine supply (143).
Additionally, it has been demonstrated that exercise affects lymphocyte mitochondrial mass, making them more resistant to TME, and also enhances mitochondrial biogenesis in muscles (144–146). Additionally, exercise improves insulin sensitivity and glucose homeostasis, which helps to reduce chronic inflammatory diseases like diabetes, which are linked to a higher risk of developing cancer (137, 147, 148). Exercise helps to create a more balanced and less inflammatory immunological milieu by lowering total body fat content, which in turn lowers circulating free fatty acids and adipokine production, which are known to boost pro-inflammatory immune cell morphologies (149). On the other hand, physical activity can also result in increased muscle-derived lactic acid release, known to blunt immunosurveillance (150). But unlike the TME, the rise in plasma lactic acid brought on by exercise is quickly neutralized (150, 151), muscle-derived lactate does not contribute to considerable plasma acidification. It doesn’t negatively affect circulating immune cells (152).
Exercise directly affects intracellular signaling pathways and transcriptional regulators that are essential for maintaining metabolic homeostasis, such as AMPK, mTOR, and hypoxia-inducible factor 1-alpha (HIF1α), in addition to its effects on metabolite availability and nutrient supply (153–160). Moreover, it is known that myokines released during contraction might alter the metabolism of immune cells, especially macrophages (81, 161). It has been demonstrated that exercise-induced IL-6 and interleukin-10 (IL-10) production improve oxidative metabolic pathways in macrophages, which in turn affects their immunoregulatory ability and functional polarization (161–164). The activation and polarization states of macrophages are inherently linked to metabolic reprogramming; a change toward increased oxidative metabolism is often linked to the development of an anti-inflammatory, tissue-repairing macrophage phenotype (136). It is still unknown and being studied whether exercise-induced changes in macrophage polarization can also affect TAMs in the TME and cause pro- or anti-tumoral effects (109, 165).
3.5 Exercise-mediated effects on immunosenescence
Furthermore, new research has shown that exercise can prevent immunosenescence, a term used to describe the age-related deterioration of immune function (166). Thymic involution, decreased naïve T cell production, and dramatic remodeling of T cell-mediated immunity are among the major effects of aging on immune cell populations and lymphoid organs. These changes lead to the development of senescent and worn-out immune cell phenotypes, which are typified by decreased cytokine production, downregulated co-stimulatory molecules, and compromised mitochondrial function (167–169). Infections, autoimmune illnesses, a decreased response to vaccinations, and an increased frequency of tumors in the elderly are all caused by immunosenescence (170, 171).
This age-related loss in immunological competence can be stopped and reversed with regular exercise (166). The first evidence came from vaccination research showing that those who regularly exercise had stronger vaccine-induced immune responses than people who don’t exercise (172, 173). It was later demonstrated that exercise increases thymopoietic production, most likely due to IL-7 released by muscles (174, 175). Exercise has also been demonstrated to decrease the percentage of senescent and tired CD8+ T cells, which encourages the development of T cell populations that are more immunologically sensitive and functionally competent (176).
Together, exercise-induced myokine secretion, leukocyte trafficking, and immunosenescence reversal highlight how physical activity may be used in conjunction with immunotherapeutic treatments to treat cancer (80, 81, 121, 166).
3.6 Influence on immune checkpoint molecules
Immune checkpoint blockade has transformed the therapeutic landscape for solid tumors; however, its efficacy remains limited across certain cancer types. Emerging evidence suggests that exercise-induced modulation of intra-tumoral immune cell composition may enhance the responsiveness to checkpoint inhibitor therapy, positioning physical activity as a promising adjunct to immunotherapeutic strategies (81). A summary of current evidence on the impact of exercise on immune checkpoint molecules in glioblastoma and other cancer models is provided in Table 3.
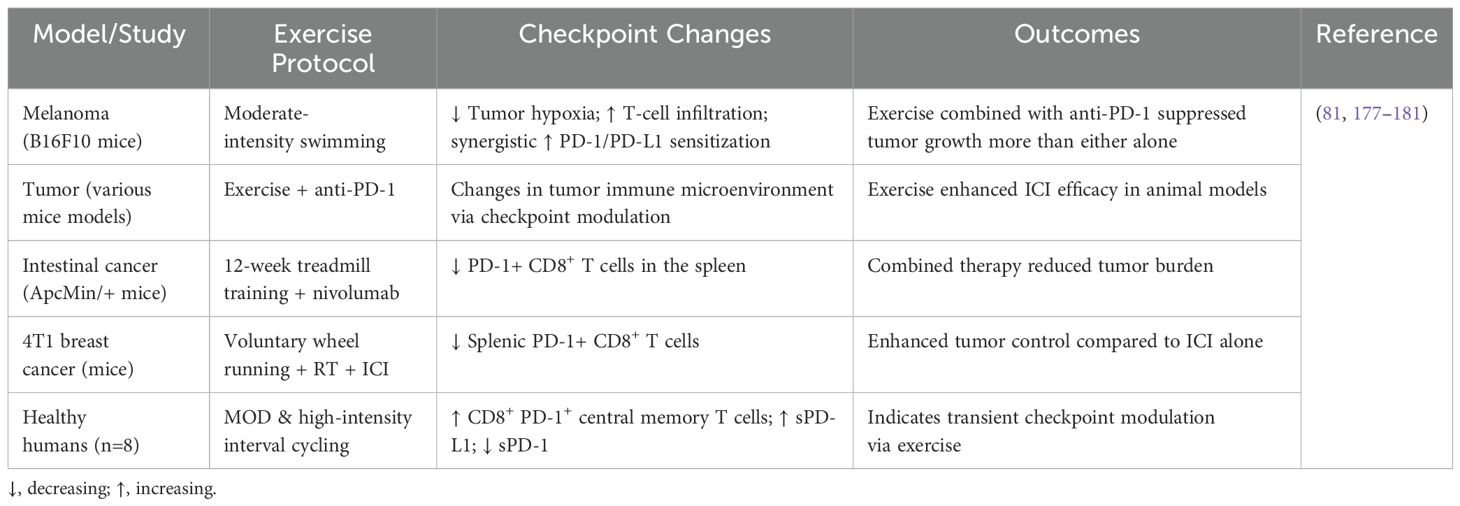
Table 3. Evidence of exercise-induced modulation of immune checkpoint molecules in glioblastoma and other cancer models.
3.6.1 Preclinical evidence
The TME of B16 melanoma-bearing mice that were given voluntary wheel running for four weeks before tumor inoculation showed a significant change from a pro-tumoral to an anti-tumoral profile, along with the upregulation of immune checkpoint molecules such as PD-1, PD-L1, PD-L2, CD28, B7.1, and B7.2. Tumor growth was significantly reduced by voluntary exercise monotherapy, but no additional tumor-suppressive effect was seen when exercise was combined with anti-PD-1 or anti-PD-L1 therapy, possibly because exercise alone had already significantly inhibited tumor growth (−72%) (182). In a murine model of pancreatic adenocarcinoma, post-transplant programmed exercise sensitized tumors to immune checkpoint inhibition. While anti-PD-1 monotherapy alone conferred no significant therapeutic benefit, its combination with exercise led to a marked increase in cytotoxic T cell infiltration and significantly enhanced tumor growth suppression (182). These results imply that by changing the TME toward a more immunologically active, anti-tumoral state, exercise may be able to overcome the resistance of normally insensitive pancreatic tumors to PD-1 inhibition.
In a murine model of unresectable hepatocellular carcinoma (HCC), post-transplant exercise enhanced the therapeutic efficacy of combined treatment with anti-PD-1 and the tyrosine kinase inhibitor Lenvatinib. Notably, while long-term combination therapy alone led to the development of an immunosuppressive TME characterized by increased infiltration of regulatory T cells (Tregs) and upregulation of inhibitory immune checkpoints, this immunosuppressive reprogramming was absent in the exercise group, indicating a protective role of physical activity against therapy-induced immune exhaustion (183). Furthermore, Gomes-Santos et al. showed that starting moderate-intensity running after tumor inoculation might sensitize MCa-M3C breast cancer-bearing mice, which were resistant to immune checkpoint suppression. The synergistic potential of physical activity in overcoming resistance to immune checkpoint inhibition was highlighted by the considerable delay in tumor advancement that occurred when exercise was added to this immunotherapeutic regimen, even though therapy with anti-PD-1 and anti-CTLA-4 alone was unable to reduce tumor growth (184). In another breast cancer mouse model (4T1), which is unresponsive to immune checkpoint inhibition monotherapy, the addition of exercise to a combination of immune checkpoint inhibitors (ICI) and radiation therapy (RT) resulted in significantly slower tumor progression compared to the dual treatment with RT and anti-PD-1 alone, suggesting a synergistic effect of exercise in enhancing the efficacy of multimodal cancer therapies (185). Conversely, Buss et al. reported no significant enhancement of checkpoint inhibitor efficacy through exercise in B16-F10 melanoma and E0771 breast cancer mouse models. Crucially, the exercise intervention was voluntary in this study, highlighting the potential dose-dependency of exercise-induced immunomodulatory effects and indicating that exercise intensity, duration, and modality may critically influence therapeutic outcomes. However, the interpretability of these findings is limited by the lack of a dedicated intervention group receiving only exercise combined with immune checkpoint inhibition for direct comparison (186).
3.6.2 Clinical evidence
In addition to preclinical research, increasing clinical efforts are being made to assess how exercise affects cancer patients’ immune checkpoint inhibition. Exercise has been shown to improve the effectiveness of first-line combination treatment with Lenvatinib and anti-PD-1 checkpoint inhibitors in patients with incurable hepatocellular cancer. This improvement led to better overall survival (OS), progression-free survival (PFS), and overall response rate (ORR) and was linked to a change in the TME toward a more anti-tumoral character (183). In addition to preclinical research, increasing clinical efforts are being made to assess how exercise affects cancer patients’ immune checkpoint inhibition. Exercise has been shown to improve the effectiveness of first-line combination treatment with Lenvatinib and anti-PD-1 checkpoint inhibitors in patients with incurable hepatocellular cancer. This improvement led to better OS, PFS, and ORR and was linked to a change in the TME toward a more anti-tumoral character (187). Retrospective surveys were used to gauge training intensity.
Several studies, with an emphasis on psychological and physical benefits rather than molecular processes (188–190), the Sportivumab research (NCT03171064) investigated the effects of exercise in cancer patients receiving immune checkpoint inhibitor medication. In this study, individuals with melanoma underwent a 12-week, supervised resistance and endurance exercise regimen that lasted 60 minutes twice a week. Pain, muscular strength, cardiovascular fitness, physical activity behavior, depression, sleep quality, exhaustion, quality of life, and intervention feasibility were among the primary objectives. However, examinations of tumor or blood specimens to assess immune-related biomarkers were not included in the research. The experiment is over, but the findings haven’t been released yet.
The ERICA trial (NCT04676009) is a forward-looking, single-center, open-label, randomized controlled study designed to evaluate how a single session of exercise performed for one hour before treatment impacts the immediate response to a combination of immune checkpoint blockade (pembrolizumab) and platinum-based doublet chemotherapy in patients with NSCLC. A total of 30 participants are being enrolled to assess these acute effects (Table 4) (191). As part of the study protocol, patients participated in a three-month structured exercise program comprising two components: a home-based walking routine monitored via activity trackers, and a supervised interval training session, lasting 35 minutes at submaximal intensity conducted one hour prior to administration of immune-chemotherapy. Throughout the study, peripheral blood samples were collected to assess immune and inflammatory biomarkers, alongside evaluations of clinical status, physical performance, biochemical indicators, and psychological well-being. The outcome data remain pending. In parallel, the HI AIM trial (NCT04263467), a randomized controlled study involving 70 individuals with non-small cell lung cancer (NSCLC), aims to investigate the immunological impact of exercise. This study compares immune cell responses in patients undergoing checkpoint inhibitor therapy, combined chemo-immunotherapy, or standard oncological surveillance. Data are gathered through analyses of peripheral blood and ultrasound-guided tumor biopsies to explore how physical activity modulates immune dynamics in the context of cancer treatment (192). Patients in the treatment arm performed a supervised group-based exercise training consisting of intermediate to high-intensity interval training thrice weekly for six weeks. Results are not published yet.
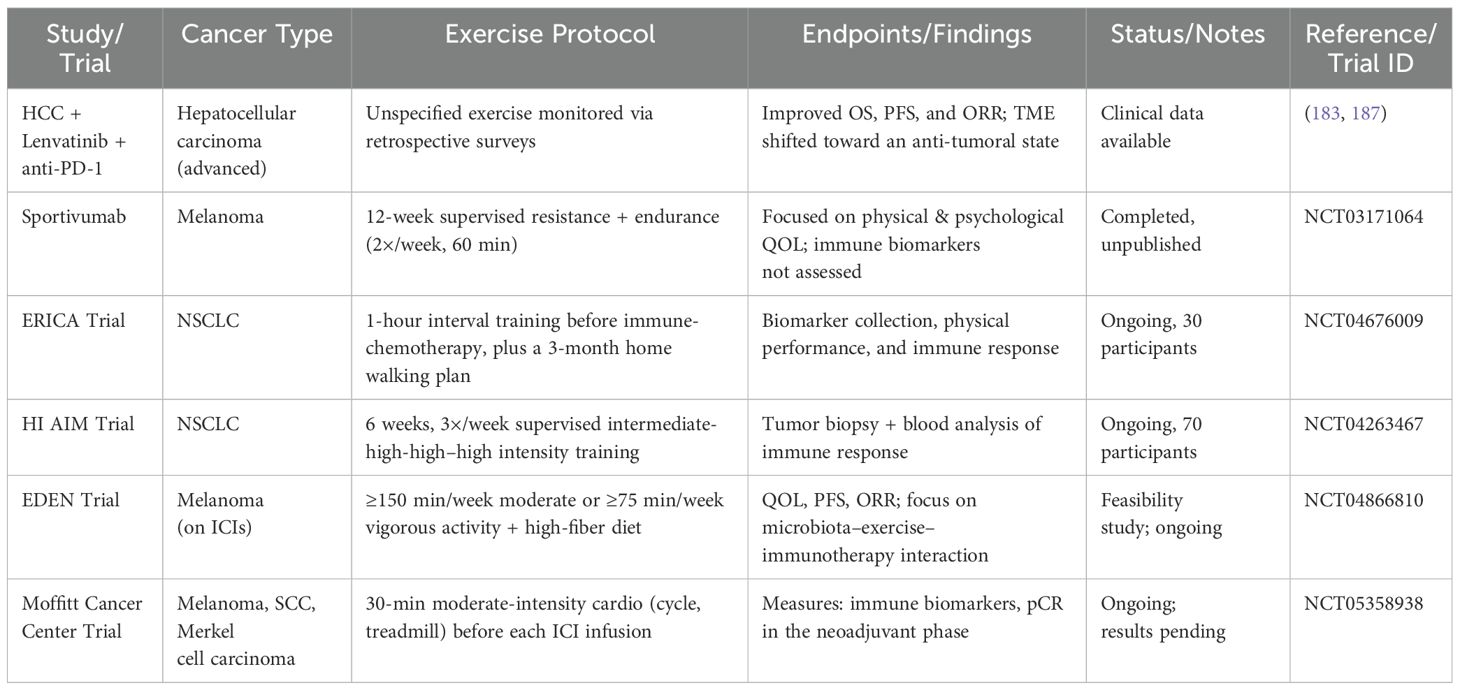
Table 4. Clinical trials investigating exercise and immune checkpoint inhibition in cancer patients.
Using a fitness tracker app, participants in the intervention group of the EDEN trial (NCT04866810) consume a plant-based, high-fiber diet and engage in at least 150 minutes of moderate or 75 minutes of high-intensity activity each week. PFS, QOL, and ORR are secondary objectives, whereas feasibility is the study’s main aim. In melanoma patients undergoing immune checkpoint inhibitor treatment, the study looks at how food and exercise interact to affect immunotherapy response and microbiota composition. The Moffitt Cancer Center has also started a randomized interventional trial (NCT05358938) to assess the effect of exercise on neoadjuvant and adjuvant immunotherapy in patients with melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma, furthering the clinical study of exercise as an adjunct to immune checkpoint blockade. Blood samples are obtained at baseline, after exercise, and after infusion during the first, third, midpoint, and final infusion sessions. The interventional arm of the trial entails patients engaging in moderate-intensity exercise for 30 minutes on an arm ergometer, cycle ergometer, or treadmill immediately before each ICI infusion throughout all treatment cycles. Exercise’s effects on tumor immunological biomarkers in the adjuvant environment and pathological full response rates in the neoadjuvant phase, as well as its potential integration into immunotherapy regimens, are the main outcome measures of the study. The results of the experiment have not yet been made public.
4 Therapeutic mechanism of exercise in cancer
4.1 Exercise and epigenetic modification
A variety of physiological adaptations are brought about by structured exercise training, which is typified by the increased activation of molecular signaling pathways that control transcription, DNA replication, and protein synthesis (1, 193). Through epigenetic control, exercise is also linked to slowing the progression of cancer. According to earlier research, anaerobic exercise increased the expression of the tumor suppressors PTEN and p53 while decreasing that of MDM2, which together suppressed the IGF-1 signaling pathway in skin cancer (194). According to recent studies, in mouse models of breast cancer, a four-week high-intensity interval training (HIIT) program increased p53 mRNA expression and decreased tumor growth (195). These results imply that by increasing the expression of tumor-suppressor genes like p53 and PTEN, exercise may have therapeutic benefits. Furthermore, one of the main causes of the development and spread of cancer is aberrant hypermethylation of these genes (196). According to a review paper, exercise reduces promoter hypermethylation in nonmalignant breast cancer via altering the methylation patterns of tumor-suppressor genes (197). Additionally, six months of moderate-intensity exercise was shown to decrease methylation of the tumor-suppressor gene L3MBTL1 in breast cancer, a change associated with a decreased risk of death and recurrence (198). According to a preclinical investigation, mice who engaged in regular physical exercise had lower levels of circulating microRNA, especially miR-21 (199). Increased expression of miR-21 is known to stimulate the production of VEGF and HIF-1α in prostate cancer and has been connected to human estrogen receptor (ER)α-positive breast cancer (200). When combined, these findings suggest that exercise may help prevent cancer and enhance the effectiveness of targeted treatments by modifying epigenetic processes.
Exercise affects synaptic plasticity and activates epigenetic pathways, according to several studies. A single acute exercise session was found to have a favorable impact on histone post-translational changes in the rat hippocampal region by increasing histone acetyltransferase activity and decreasing histone deacetylase activity (201). Additionally, a number of studies have shown that exercise stimulates the activation of genes involved in synaptic plasticity in rats and affects epigenetic regulators of brain-derived neurotrophic factor (BDNF) production (202). Prior studies showed that a one-week wheel-running intervention increased global histone 3 acetylation in the mouse hippocampal region, which in turn increased BDNF transcriptional activity (203). According to recent research, individuals with GBM have lower levels of BDNF in their cerebrospinal fluid and plasma, which may be related to the disease’s cognitive impairment (204, 205). More study is required to completely explain the underlying processes and their clinical importance, even though several studies have suggested that epigenetic alterations regulate the adjuvant therapeutic effects of exercise in cancer patients (197), To the best of our knowledge, no research has looked particularly at how exercise might help patients with GBM with their epigenetic changes. Therefore, more studies are necessary to clarify the molecular impact of exercise within the TME in the setting of GBM and to replicate findings from animal models in human populations. An overview of the major therapeutic mechanisms by which exercise exerts its anticancer effects is provided in Table 5.
4.2 Exercise and the RTK signaling pathway
Previous research has demonstrated that in triple-negative breast cancer, a subtype distinguished by the lack of EGFR/HER2/neu expression, progesterone receptor, and estrogen receptor, regular physical exercise inhibits the PI3K/Akt/mTOR signaling pathway and slows tumor development (210). Exercise seems to alter several systemic signaling pathways, which leads to physiological changes in the TME of breast cancer and helps to inhibit mTOR signaling (165). By downregulating the PI3K/Akt/mTOR signaling cascade and simultaneously promoting apoptosis through the overexpression of pro-apoptotic markers like caspase-3 and Bax, physical activity inhibited tumor development in breast cancer models, according to a preclinical study (211). Exercise has been shown to decrease the PI3K/Akt/mTOR signaling pathway and improve the TME in several malignancies (1), but its effects on this system in the setting of GBM have not yet been thoroughly investigated.
4.3 Exercise and angiogenesis
Some tumors can be treated using anti-angiogenic treatments that target VEGF or its receptors (212). Nevertheless, even though these treatments aim to limit tumor vascularization, they could unintentionally create hypoxic conditions in the TME, which might accelerate tumor growth and resistance to treatment (1). On the other hand, exercise’s ability to modulate angiogenesis and vascular remodeling inside the TME is one of its most advantageous impacts on tumors (213). Exercise has been shown to increase intertumoral VEGF levels and promote angiogenic processes (213). In a mouse model of breast cancer, exercise training has been shown to increase VEGF expression, encourage tumor angiogenesis, and lessen tumor burden (214). Exercise-induced increased tumor vascularization and perfusion may reduce intertumoral hypoxia, enhance therapeutic drug delivery, and increase the tumor’s radiation treatment responsiveness (213, 215, 216). In a preclinical investigation, exercise combined with tamoxifen and letrozole therapy resulted in lower expression of ERα, HIF-1α, VEGF, and miR-21. This was linked to improved vascularization and decreased tumor development in mice models of breast cancer (217). According to a different preclinical investigation, miR-21 increased tumor vascularization in prostate cancer cells by targeting the tumor suppressor PTEN, which then triggered the ERK1/2 and AKT signaling pathways and increased the production of VEGF and HIF-1 (200). Experimental data from voluntary wheel running in mice models of breast and prostate cancer have shown an unanticipated inhibitory impact on metastasis, despite the hypothesis that exercise-induced stabilization of HIF-1 would promote metastatic spread (218, 219). However, a recent meta-analysis found that regular exercise had no statistically meaningful impact on the total risk of metastasis or the quantity of metastatic lesions in preclinical cancer models (220). To fully understand how exercise affects hypoxia, angiogenesis, and cancer metastases, further research is required, with a focus on how exercise affects GBM.
4.4 Exercise and AMPK
The AMPK pathway, a crucial regulator of glucose uptake, glycogen synthesis, and insulin sensitivity in skeletal muscle tissue, has been demonstrated to be activated by regular physical exercise (221). By controlling aerobic glycolysis, imposing metabolic checkpoints, and preventing cellular proliferation, AMPK activation may also aid in tumor suppression (222). Furthermore, because of its regulatory effects on cellular metabolism, growth, and survival pathways, AMPK activation has been linked to a number of cancer types and has been suggested as a key factor in their prevention and therapy (223). For example, Lee et al. showed that wogonin, an AMPK activator, increased the expression of p53 and p21 in GBM cells, promoting apoptosis and inhibiting cell growth (40). Furthermore, a different study showed that regular exercise decreased the size and quantity of hepatocellular tumors by downregulating mTOR expression and raising AMPK phosphorylation (224). AMPK may, however, change from being a tumor suppressor to a tumor promoter in advanced stages of colorectal and breast malignancies, allowing cancer cells to survive by reducing oxidative, genotoxic, and metabolic stress, according to several studies (225–228). Consequently, it appears that the AMPK signaling pathway plays a crucial role in early-stage targeted cancer treatment. To fully understand how exercise affects AMPK regulation in different cancer types, with a focus on GBM, more research is required.
4.5 Exercise and lactate metabolism
According to studies, seven weeks of aerobic exercise can decrease the development of tumors, the amount of lactate that accumulates in the TME, and the expression of monocarboxylate transporters in tumors. This may be achieved via altering the activity of the estrogen receptor alpha (ERα) (229). Bacurau et al. reported that aerobic exercise decreased lactate buildup and glucose absorption in cancer tissues (230). It is well established that elevated lactate levels in the TME stimulate angiogenesis and may inhibit cytotoxic immunological T cell function (207). Additionally, regular exercise improves ALDH, which is linked to the metabolic dysfunction of GBM (231). It is still unclear how exercise affects ALDH activity and lactate metabolism in cancer, especially GBM. Therefore, in order to clarify this association and its possible therapeutic consequences, further study is required.
4.6 Exercise and immune system function
Recent research emphasizes how exercise helps cancer patients retain a strong immune system (232). Frequent exercise fortifies immune surveillance systems, allowing pre the early identification and destruction of aberrant or altered cells before they develop into cancerous tumors (80, 233). In addition to encouraging the mobilization and circulation of important immune components, such as immunoglobulins, anti-inflammatory cytokines, neutrophils, natural killer NK cells, cytotoxic T lymphocytes, and immature B cells elements crucial for immune defense and metabolic homeostasis acute exercise sessions also stimulate tissue macrophages to increase their antipathogenic activity (80, 233–235). The movement of innate immune cells and associated components between lymphoid organs and the circulation is facilitated by acute exercise (1). Despite the brief duration of these immunological changes, their recurrent occurrence over time helps to reduce systemic inflammation and improve immunosurveillance against infections and cancerous cells (80, 233). By encouraging tumor vascularization, lowering hypoxia, decreasing glucose uptake, and lowering lactate production, all of which improve immune cell infiltration and function within the TME, regular exercise also indirectly boosts the immune response in cancer (232). Moreover, cytotoxic immune cell infiltration into the TME is a favorable prognostic indicator for cancer outcome and death (236). Increased NK cell recruitment and infiltration is one possible way that exercise enhances immune system activity in solid tumors (92). Acute intermittent exercise causes NK cells to be mobilized into the circulation of breast cancer patients to a level similar to that seen in age-matched healthy persons, according to a clinical trial (237). According to a different study, in breast cancer models, HIIT increased the quantity of NK cells, decreased tumor volume, and enhanced metabolic health (238). Pedersen et al. reported that voluntary wheel running in mice was shown to reduce tumor development, which was linked to higher amounts of adrenaline from the adrenal glands, IL-6 produced by exercising skeletal muscle, and an increase in the infiltration of NK cells (239). According to this study, NK cell recruitment into the TME may be aided by muscle-derived IL-6. Furthermore, a different preclinical study shown that exercise in conjunction with PD-L1 immune checkpoint suppression lowered tumor burden, slowed tumor growth, decreased myeloid-derived suppressor cell (MDSC) presence, and increased NK cell activity (185), By producing programmed death-ligand 1 (PD-L1), which interacts with cytotoxic immune cells’ PD-1 to prevent their activation and activity, MDSCs have immunosuppressive effects inside the TME. The immune responses against tumors are suppressed in part by this mechanism (240). It is still unclear how immunological dysfunction brought on by tumors and the TME affects immune cell activity. The intricate relationships between cancer, immune modulation, and exercise’s modulatory function in preserving or regaining immunological competence require more investigation.
5 Metabolic reprogramming by exercise
5.1 Tumor energy metabolism
Early in the 20th century, Otto Warburg discovered that cancer cells preferentially engage in a metabolic process called “aerobic glycolysis,” which turns glucose into lactate even when there is enough oxygen present. This finding is now referred to as the Warburg effect (241, 242). Warburg also postulated that compromised mitochondrial respiration was “the origin of the cancer cell,” suggesting that malignant transformation’s metabolic reprogramming is caused by mitochondrial failure (242–244). Initially, tumor aggressiveness was linked to increased dependence on aerobic glycolysis and decreased mitochondrial respiratory performance, a process called the Warburg Effect. On the other hand, it has been demonstrated that increasing oxidative phosphorylation may inhibit tumor development, indicating a connection between tumor advancement and mitochondrial metabolism (Figure 3) (242, 245, 246).
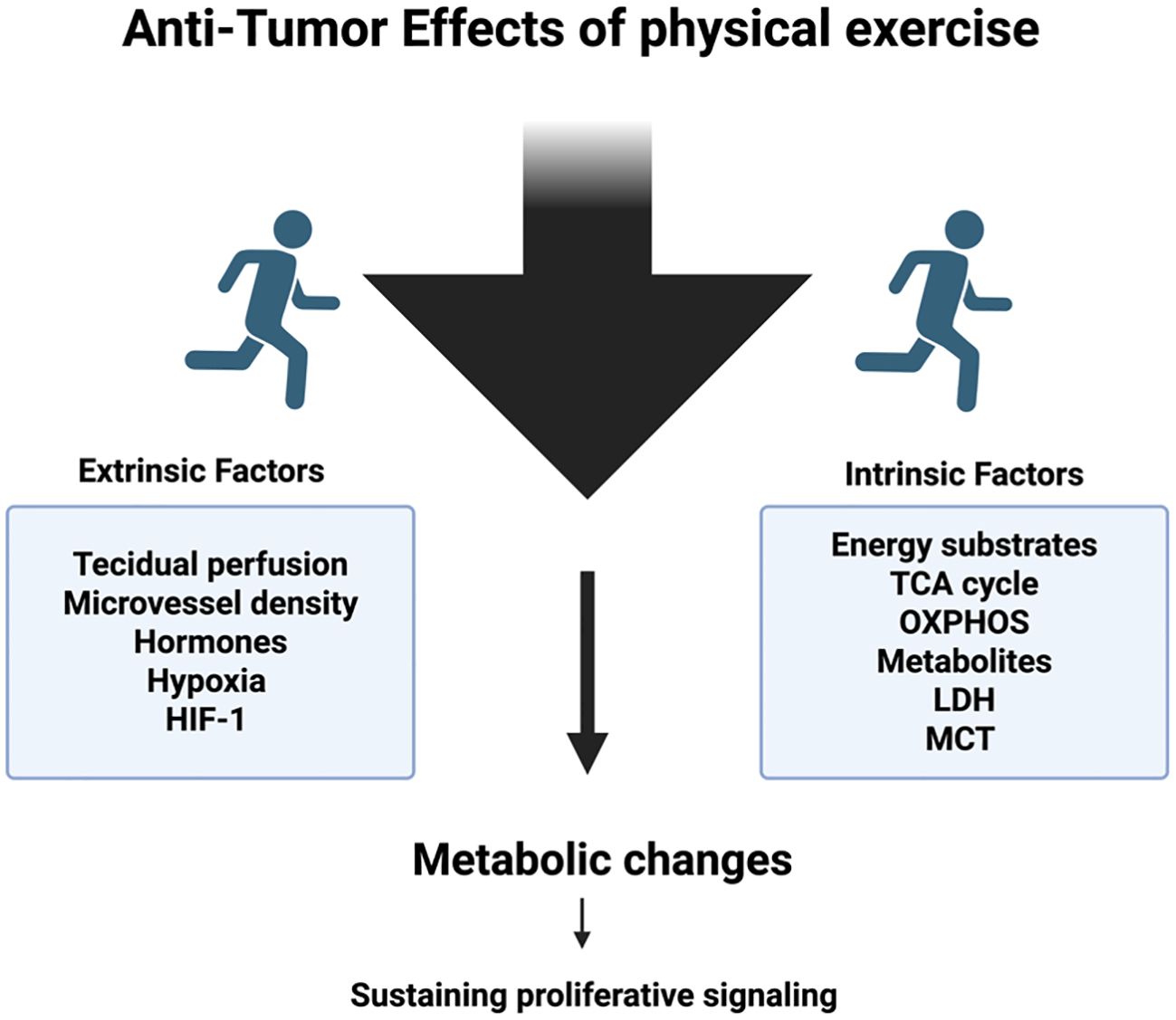
Figure 3. Regular exercise induces systemic and cellular adaptations that can reshape the energy metabolism of tumors. These adaptations include altered nutrient availability, improved oxygen delivery, and modulation of metabolic pathways such as glycolysis, oxidative phosphorylation, and lipid utilization within cancer cells. By influencing these processes, physical activity may disrupt the metabolic flexibility that tumors rely on for growth and survival, thereby impairing their progression and responsiveness to therapy.
Though some tumors that primarily rely on glycolysis still maintain functional mitochondrial respiration and metabolic versatility, other studies have questioned the universality of the Warburg Effect by showing that some tumors have elevated oxidative phosphorylation despite being malignant (247–249). As a result, the metabolic phenotypes of tumors vary according to their origin and kind. Glycolysis, for example, is more frequently used as the main metabolic pathway by brain malignancies such as C6 glioma, medulloblastoma, and meningioma, as well as certain colon cancers (like CT-26) and hepatic adenocarcinomas (like Novikoff) (249). On the other hand, malignancies that primarily show increased oxidative metabolism include melanoma, lung cancer, and several subtypes of breast cancer. Furthermore, glioblastoma, Ehrlich carcinoma, Walker-256 carcinoma, and MCF-7 breast cancer cells are among the cancers that exhibit a hybrid metabolic profile, which is defined by the simultaneous use of both glycolytic and oxidative pathways (249). The crucial reliance of tumor cells on functioning mitochondria for maintaining growth and metastatic potential is highlighted by evidence showing that cancer cells missing mitochondrial DNA (mtDNA) have decreased colonization capacity and decreased proliferation (250–252). According to Tan and associates’ studies on breast cancers, conducted in vivo experiments that cells devoid of mtDNA cannot develop lung metastases (252). The complete restoration of mitochondrial respiratory activity was demonstrated by cells isolated from established lung metastases, on the other hand, underscoring the critical role that functioning mitochondria play in metastatic competence (252).
Because of their metabolic flexibility, cancer cells can change how they produce energy in response to changing microenvironmental circumstances. They frequently increase ATP synthesis by cytosolic glycolysis (241). Reactive oxygen species (ROS), mutations in important mitochondrial tricarboxylic acid cycle (TCA) enzymes, and the activity of hypoxia-inducible factor 1α (HIF-1α), especially in hypoxic environments, are all closely related to this metabolic plasticity and help rewire tumor metabolism (242, 253). The stability and translocation of HIF1α to tenhanceus enhances the glycolytic pathway and modulates cellular energy metabolism (242). By upregulating pyruvate dehydrogenase kinase (PDK), HIF-1α enhances the phosphorylation and inactivation of pyruvate dehydrogenase (PDH), preventing pyruvate from being converted to acetyl-CoA. HIF-1α promotes the production of important glycolytic enzymes at the same time, which accelerates the metabolic transition to glycolysis (254). Furthermore, the loss of tumor suppressors like PTEN or p53, as well as oncogenic mutations in genes like K-ras, c-Myc, and phosphatidylinositol-3 (PI3) kinase, lead to mitochondrial changes that inhibit oxidative phosphorylation (OXPHOS) and promote a metabolic shift toward glycolysis, which supports tumor growth and survival (255). Furthermore, by providing energy, preserving redox equilibrium, managing apoptotic mechanisms, and regulating oncogenic signaling pathways all of which are essential for tumor cell survival and proliferation mitochondria play a key role in promoting the progression of cancer (242, 248, 253, 254, 256, 257), In contrast to glycolysis associated with mitochondrial OXPHOS, which is more efficient in terms of ATP yield, cytosolic glycolysis proceeds at a much faster rate, allowing cancer cells to quickly meet their energy demands under a variety of microenvironmental conditions. This highlights the crucial role that mitochondria play in preserving cellular homeostasis and promoting tumor development (258). Furthermore, the diversion of glucose into the pentose phosphate pathway is made easier by the downregulation of OXPHOS, which boosts the synthesis of ribose sugars required for nucleic acid synthesis and supports the biosynthetic and proliferative needs of tumor cells (242, 249). Citrate is thus converted into acetyl-CoA, a crucial precursor for the de novo synthesis of fatty acids and cholesterol, processes necessary for membrane biogenesis and fast cell proliferation in tumor development by the accumulation and export of citrate from the mitochondrial matrix to the cytosol (249, 259).
Additionally, the TME, a complex and diverse environment made up of malignant cells, stromal fibroblasts, endothelial cells, different immune and bone marrow-derived inflammatory cells, as well as a variety of signaling molecules and extracellular matrix (ECM) components like collagen, fibronectin, hyaluronan, and laminin, interacts dynamically with cancer cells. These interactions collectively affect tumor progression, immune evasion, and resistance to treatment (260–262). TME and cancer cells have a strong relationship and are always interacting (263), the TME is significantly influenced by changes in the tumor’s energy metabolism. In particular, the excess lactate produced by tumor cells is exported into the extracellular environment, which lowers pH and causes the TME to become acidic. The hostile barrier produced by this acidic environment hinders immune effector cell infiltration and function, which promotes immune evasion and tumor growth (264). On the other hand, a process called the “Reverse Warburg Effect,” in which stromal cells engage in aerobic glycolysis and transfer energy-rich metabolites to boost tumor growth and survival, may allow tumor cells to get metabolic substrates from cancer-associated fibroblasts (242, 260, 265). Monocarboxylate transporters (MCTs) carry lactate into tumor cells during this process, where it acts as a substrate to power mitochondrial oxidative metabolism, boosting cellular energy generation and promoting tumor growth (260, 265). Furthermore, the TME becomes hypoxic and acidic due to the decrease in vascularization and blood perfusion (264). Under cancer cells, glutamine serves as a vital anaplerotic substrate that supports ATP synthesis and tumor development by restoring tricarboxylic acid cycle intermediates and maintaining oxidative phosphorylation, even under hypoxic conditions (265).
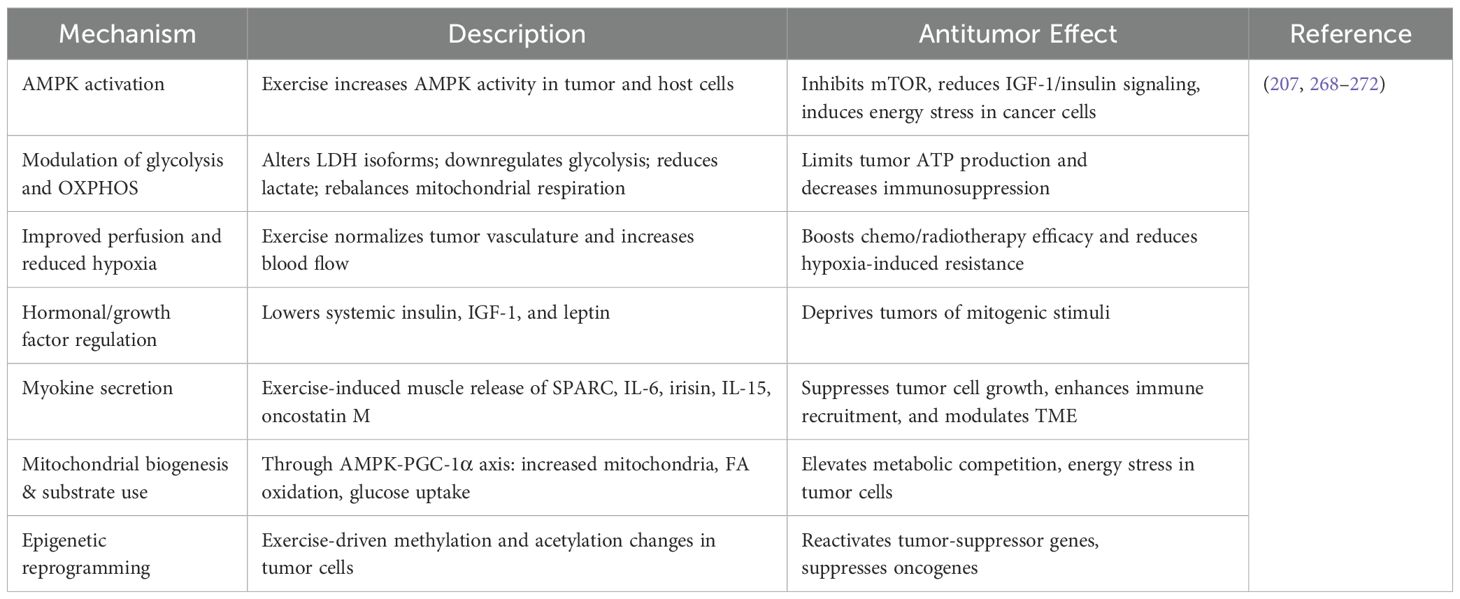
Therefore, tumors can alter their surrounding milieu to promote their growth, and elements of the TME in turn affect the behavior of cancer cells, including metabolic changes. Consequently, cancer cells modify their metabolic processes to promote the growth of tumors. Growing research indicates that extrinsic modulators inside the TME have a role in causing these metabolic changes, even though metabolic reprogramming has historically been thought of as an inherent characteristic of cancer cells (260, 263, 266, 267). A summary of exercise-induced metabolic reprogramming effects on tumor biology is presented in Table 6.
5.2 Physical exercise and tumoral energy metabolism
Exercise is a powerful modulator of systemic and tumor-specific responses because of its impact on tumor biology and ability to interfere with a variety of physiological processes controlled by intricate, linked homeostatic regulatory networks (241, 273, 274).
Exercise can change the energy metabolism of tumors by influencing extrinsic variables in the extracellular environment surrounding cancer cells. Exercise has been demonstrated to change the metabolic processes of cancer cells by influencing the amounts and activity of hormones, signaling molecules, and vascular structures, such as blood vessels and capillaries (241). In this context, it has been shown that voluntary aerobic exercise, such as wheel running, inhibits the growth of tumors in murine models of breast cancer (4T1 cells). This effect is linked to increased microvascular density and perfusion, enhanced apoptosis, and a corresponding decrease in intertumoral hypoxia (275). Compared to a sedentary state with chemotherapy, exercise plus chemotherapy increased the delay in tumor development (275). Voluntary aerobic exercise for 44 days did not change the overall tumor volume in a xenograft experimental model in which human breast cancer MDA-MB-231 cells were implanted into the mammary glands of female mice. Despite the lack of notable alterations in PGC1-α and AMPK protein expression, the trained group did show enhanced tumor vascularization and raised levels of HIF-1 protein expression (219), thought of as metabolic sensors (276–278).
On the other hand, aerobic exercise was linked to lower VEGF concentrations, lower HIF-1α expression in tumor tissue, and lower levels of 17β-estradiol in the bloodstream in the MC4-L2 experimental model. Alongside these molecular alterations, angiogenesis was suppressed, which led to a decrease in tumor growth (217). The combined effects of exercise and chemotherapy were further examined in this study by Isanejad and colleagues, who found that concurrent physical activity application increased tumor size reduction beyond what chemotherapy alone could do (217). Exercise’s impact on tumor growth, however, can vary depending on the kind of cancer cell. Accordingly, Schadler et al. showed that physical activity considerably accelerated the growth of B16F10 melanoma tumors; however, it did not generate a noteworthy effect on the growth of pancreatic ductal adenocarcinoma cells (PDAC-4662) (275). Exercise, however, increased the delay in tumor growth in both the B16F10 and PDAC-4662 models when paired with treatment, surpassing the effects of chemotherapy alone. The normalization of tumor vasculature brought on by exercise was credited with this synergistic benefit. A more organized and functioning vascular network that closely matches normal tissue vasculature is the outcome of vascular normalization, which restores the balance of angiogenic regulators by lowering pro-angiogenic factors (279). Consequently, the intertumoral transport and effectiveness of chemotherapeutic drugs may be improved by the normalization of the tumor vasculature (275, 279). However, intertumoral levels of lactate, glutamate, glutamine, and glucose did not significantly change, according to metabolic studies (275). Remarkably, McCullough and associates on conscious rats, in vivo studies at rest and exercise, showed a significant decrease in tumor arteriole vasoconstriction in prostate cancer models (280), along with a roughly 200% increase in tumor blood flow and a subsequent decrease in hypoxic areas in comparison to resting conditions (280).
Therefore, in addition to reducing the hypoxic TME, exercise may improve the administration of tumor-targeting medications, and exercise-induced vascular remodeling may increase the infiltration of immune cells in the tumor environment (165). It is unclear how long exercise-induced changes in tumor perfusion last, and it is also unclear how much physical activity and for how long it takes to provide the most clinically meaningful therapeutic effects (281). Exercise is regularly shown to be a crucial modulator in enhancing blood circulation inside tumor tissues, even in the face of variations in experimental techniques (165, 281).
Tumor cellular energy metabolism may change as a result of intrinsic cancer cell components that are sensitive to exercise. In particular, it has been shown that exercise affects important proteins, enzymes, receptors, and metabolites that are essential to the metabolic pathways of cancer cells (241). Aerobic training led to an isoform shift in lactate dehydrogenase (LDH), with increased expression of LDH-B (isoforms LDH 1 and 2) and decreased expression of LDH-A (isoform LDH 5) in comparison to controls, and decreased expression of monocarboxylate transporter type 1 (MCT1) in an experimental model of breast cancer using the MC4-L2 cell line (229). These metabolic changes were associated with decreased lactate levels in tumor lysates and the systemic circulation, as well as a corresponding decrease in tumor mass in those who exercised (229). These changes are crucial because the hypoxic TME usually promotes LDH-A expression upregulation, which promotes tumor growth; on the other hand, LDH-A silencing has been demonstrated to reduce the tumorigenic capacity of breast cancer cells (282). Moreover, the down-regulation of LDH-B is linked to the rise in lactate production (283).
Aerobic voluntary exercise has been shown to reduce the incidence of breast cancer and alter hormonal and growth factor profiles in an animal model that uses chemically induced breast carcinogenesis. This is demonstrated by decreases in levels of leptin, corticosterone, insulin-like growth factor I (IGF-I), and circulating insulin (284). Engagement in physical activity led to a noticeable decline in both the overall frequency of cancer development (dropping from 98.1% to 84.6%) and the tumor burden, as reflected by a lower average number of tumors per rat (decreasing from 3.72 to 2.67) (284). Structured exercise programs have been widely recognized for their ability to lower the risk of breast cancer across different life stages, including in both premenopausal and postmenopausal women (285). Large-scale epidemiological research consistently demonstrates a negative correlation between physical activity levels and BC incidence, particularly in postmenopausal populations. For instance, Howard et al. reported that regular physical activity may reduce BC risk by as much as 20% to 80% (286). Notably, the protective influence of exercise appears to be stronger in women diagnosed with hormone-sensitive tumors after menopause, as opposed to those with hormone-insensitive subtypes typically seen before menopause (285). Alongside decreased signaling through important cell growth pathways, such as decreased phosphorylation of Akt, mTOR, p70S6 kinase (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), there was also an increase in AMPK activation (284), This implies that the mechanisms controlling glucose homeostasis within cancer cells are modulated by physical activity. As a result, exercise-induced metabolic changes impact tumor energy metabolism and may modify important metabolic pathways necessary for tumor growth.
Additionally, both during active exercise and during rest, aerobic activity is essential for the total oxidation of macronutrients (287, 288). Furthermore, other studies have shown that after tumor implantation, animals with tumors show changes in substrate oxidation. While trained animals go from lipid oxidation toward higher carbohydrate oxidation, sedentary animals change from mostly carbohydrate oxidation to a mixed lipid-carbohydrate oxidation (289, 290). Therefore, the change in macronutrient oxidation seen in trained mice seems to be consistent with tumor metabolism, which mainly depends on mitochondrial function and the availability of carbohydrates for energy, even while tumor development impairs overall metabolism. Although the precise effects of exercise and macronutrient oxidation on tumor growth are yet unknown, it is important to understand that cell cycle regulation can be impacted by the availability of energy substrates (291). For instance, cellular division may be inhibited by low glucose availability (292). The activation of molecular pathways that propel cell growth is triggered by a sufficient supply of nutrients and cellular energy (293, 294). Researchers employed a co-culture system in a study looking at the effect of aerobic exercise on breast cancer, supplementing the medium with serum from exercised women. They discovered that MCF-7 breast cancer cells showed growth inhibition, but not MDA-MB-231 cells. This result was attributed to the suppression of the Hippo signaling pathway caused by YAP phosphorylation (295). The study supported these conclusions by showing that decreased expression of the gene Lats-2 and increased expression of the tumor suppressor gene p53 were associated with the reduction in tumor development observed in trained rats, which was connected to compromised mitochondrial function (290). A positive feedback loop is produced by the interaction of p53 and YAP/LATS2, which either promotes cell differentiation or causes cell senescence in response to replication stress. Modifying these pathways can affect the course of tumors since this process is essential for regulating aberrant cell proliferation and preserving tissue homeostasis (296). Indeed! LATS2 functions by attaching to and blocking MDM2, the protein that typically designates p53 for destruction, when cells undergo mitotic stress. LATS2 stabilizes and activates p53 by inhibiting MDM2, which results in cell cycle arrest at the G1/S checkpoint. This pause effectively stops the spread of potentially malignant cells by giving the cell time to repair damage or, if the damage cannot be repaired, to initiate senescence or death (296). It is conceivable that physical activity limits the energy sources available to tumor cells, which might be a way to prevent tumor cells from growing and proliferating. Exercise seems to have a direct impact on mitochondrial activity, which is a crucial organelle for cellular energy generation, in addition to changing the supply of substrates. When 4T1 cells were implanted in their experimental model of breast cancer, aerobic exercise led to impaired mitochondrial function in the tumor tissue. In particular, the electron transport chain’s capacity was significantly reduced, which resulted in less mitochondrial respiration. These results imply that physical exercise imposes a dual metabolic stress that may inhibit tumor growth by restricting the energy supply and reducing the tumor’s capacity to produce ATP through oxidative phosphorylation (290). In the tumor tissue, this changed metabolic state was accompanied by an upregulation of genes related to glycolytic metabolism, such as Hif1a, Pdk, and Mct1. Interestingly, tumor growth was significantly suppressed in the trained mice with impaired mitochondrial function, with tumor mass decreased by about 43% in comparison to sedentary controls. These results highlight a shift toward glycolytic reliance under conditions of mitochondrial compromise induced by exercise, which ultimately contributes to reduced tumor burden (290). In agreement, Tan and associates (252) The growth and aggressiveness of 4T1 breast cancer cells are strongly dependent on mitochondrial oxidative phosphorylation, and experimental data demonstrate that even in the presence of severe oxygen deprivation, which generally impairs mitochondrial function, the cells continue to obtain the majority of their energy from mitochondrial respiration, with up to 60% of the cell’s total ATP output coming from this pathway, despite metabolic stress (297). Exercise might therefore slow the growth of tumors by changing the metabolic profile of cancer cells, especially by affecting mitochondrial activity in cancers like the 4T1 model that mainly depend on oxidative metabolism for growth. Additionally, metabolomic analysis in colorectal cancer xenograft models (CRC282, CRC344, and CRC370) showed that aerobic exercise caused notable changes in central carbon metabolism within the tumor tissue in addition to delaying tumor development. These metabolic alterations imply that exercise may interfere with important bioenergetic pathways that cancers rely on, preventing their development and changing the course of their progression (298). Except for succinate and glutamate, exercise significantly reduces the tumor’s TCA cycle activity, suggesting a change in mitochondrial metabolism. By raising certain purine intermediates (adenine, ADP-ribose) and reducing others (IMP, hypoxanthine), it interferes with nucleotide metabolism. It also often reduces important pyrimidine precursors, except for dihydrothymine. Exercise also limits lipid-driven energy generation by lowering acylcarnitines, which are essential for fatty acid transport and β-oxidation. All things considered, exercise rewires tumor metabolism by limiting essential sources of growth-promoting energy and nucleotides. Furthermore, there was a noticeable drop in phosphocreatine levels, which hindered the quick regeneration of ATP from ADP when energy demands were high (298). Regardless of tumor size, mice that exercised voluntarily showed lower levels of HIF1α and HIF2α proteins in tumor tissue, as well as notable changes in metabolic profiles; 32 metabolites were up to 20% higher, and 10 metabolites were down (299). However, no description of any metabolite’s function in tumor energy metabolism was provided.
These results shed important light on how exercise affects the energy metabolism of cancer cells, emphasizing the vital roles of mitochondria, glycolytic intermediates, and the TCA cycle as an anabolic center that promotes tumor development. Despite being metabolic byproducts, TCA cycle metabolites are essential for the synthesis of macromolecules like as proteins, lipids, and nucleotides as well as for cell signaling pathways that aid in the growth of tumors (256, 299–301). Additionally, metabolites such as fumarate, succinate, α-ketoglutarate, and acetyl-CoA might change the immunological response (302), lymphangiogenesis, and the preservation of stem cell pluripotency (303, 304). Additionally, glutamine metabolism generates glutamate, which supports the synthesis of fatty acids by converting into α-ketoglutarate, an alternate carbon donor to the TCA cycle. In order to produce citrate, isocitrate dehydrogenase uses a non-canonical glutaminolysis route to use NADPH and reductively carboxylate α-ketoglutarate (305). The intricacy of the tumor energy metabolism is illustrated by the reductive carboxylation and metabolism regulation by TCA cycle intermediates.
Exercise typically inhibits the growth of tumors relative to sedentary settings, despite the intricacy of tumor metabolism. Although the degree of tumor size reduction varies depending on the particular tumor subtype’s susceptibility to exercise, the effect of aerobic exercise on tumor growth has been consistently demonstrated in animal models (295, 298, 306). Therefore, it is essential to assess the effects of physical activity according to the stage of advancement and tumor subtype. Data interpretation is made more difficult by the large number of training regimens. Even while current research shows that exercise can lower the incidence of cancer and decrease the growth of tumors (241, 307), further study is needed to determine the exact molecular processes behind these anticancer benefits.
6 Exercise as a complementary therapy in glioblastoma treatment and other neurological effects
For people with brain tumors, physical activity can greatly improve their cognitive function, motor coordination, and general well-being, which will improve their quality of life (Figure 4) (1, 36, 308). According to the European Association of Neuro-Oncology, individuals with brain tumors should exercise as part of a planned, well-monitored rehabilitation program (309). However, there are only a few evidence-based programs created especially for rehabilitative exercise that are meant to help people with GBM improve their functional abilities and quality of life while also reducing cognitive deterioration (310). The lack of studies specifically focused on GBM poses a significant challenge to successfully incorporating exercise into oncology care for these patients, given the wealth of data demonstrating the advantages of physical activity in treating a variety of malignancies (311) (312) (313). Physical exercise in mice has been shown to significantly reduce tumor cell growth, limit the onset of motor deterioration, and support the maintenance of self-care abilities in glioblastoma models (75, 314). Exercise increases the tumor size decrease in mice when combined with temozolomide, surpassing the effects of either temozolomide alone or no therapy (315). Comparing glioma patients who participate in an organized exercise program to those who do not, it has been demonstrated that the former improve motor skills and cognitive function while also reducing symptoms such as anxiety, sadness, headaches, exhaustion, and communication problems (316–318). Patients with grade III and IV gliomas who exercised more than nine metabolic-equivalent hours per week survived a median of 7.8 months longer than those who exercised fewer than nine metabolic-equivalent hours per week, according to one study (319). A 12-week exercise program increased cortical thickness primarily in sensorimotor areas and increased white matter volume in pediatric brain tumor patients who had undergone at least one surgery and cranial radiation. These improvements were linked to improvements in cognitive function, behavior, and physical abilities (320). According to a related research, the exercise group’s bilateral coordination improved, and these gains lasted for 12 weeks after the exercise intervention ended (321). A clinical study (NCT03390569) is now being conducted to see if exercise regimens might enhance glioblastoma patients’ quality of life, overall survival, and progression-free survival. A different experiment (NCT03775369) is evaluating the effects of physical exercise on the general well-being and quality of life of patients with high-grade gliomas. An overview of the multifaceted neurological and therapeutic benefits of exercise in glioblastoma treatment is presented in Table 7.
Figure 4. Cancer is frequently linked to a range of systemic metabolic disturbances, including diminished insulin sensitivity, elevated blood lipid levels, mitochondrial dysfunction, persistent low-grade inflammation, and the progressive loss of muscle mass and strength known as cancer cachexia. In healthy individuals, regular physical activity has been shown to significantly improve these metabolic and physiological impairments. This evidence supports the notion that exercise may serve as an effective therapeutic approach to mitigate cancer-related metabolic dysfunctions and restore systemic homeostasis.
Table 7. Summary of exercise benefits as a complementary therapy in glioblastoma and other neurological effects.
6.1 Feasibility of exercise in GBM
Research investigating exercise as a non-pharmacological treatment for brain tumors has shown promising findings. For example, inpatient rehabilitation programs have been shown to enhance daily living activities, physical ability, and functional performance in patients with brain tumors, including GBM (1, 322, 323). Patients with neuro and head and neck cancers were the focus of the ENHANCE program, which showed that a 12-week aerobic and strength training regimen was feasible to implement and significantly improved the psychological well-being, physical function, and general quality of life of those with brain tumors (316). 12 weeks of aerobic exercise is a safe, cost-effective, and successful strategy for boosting brain repair in children with brain tumors, according to a randomized clinical trial. This is demonstrated by higher white matter and hippocampus sizes as well as quicker response times (324). A home-based fitness program was also proven to be both feasible and safe for patients with grade II and III gliomas in a preliminary randomized controlled study (325). HIIT was demonstrated to be possible for a GBM patient undergoing multimodal therapy in a previous clinical case study (326). Last but not least, a qualitative investigation verified that combination exercise regimens are safe and practical for GBM patients receiving chemoradiotherapy (327). All things considered, these findings point to the safety, viability, and prospective adjuvant treatment of exercise for GBM patients.
6.2 Exercise improves the performance and quality of life of patients with GBM
Rehabilitation therapies can improve the quality of life and functional results of individuals with gliomas, according to a literature review (308). Recently, Gehring et al. reported that individuals with brain tumors who received six months of at-home fitness instruction had a 7% increase in peak oxygen consumption over those who did not (325). Furthermore, a different study discovered that 12 weeks of combined exercise improved grip strength and performance in the 30-second sit-to-stand test while also decreasing waist circumference (316). A new 60-week exercise training program improved walking ability, exercise capacity, muscular strength, and quality of life in GBM patients undergoing radiation therapy, according to a previous case study (328). Intense rehabilitation improved physical function ratings and engagement in everyday activities after a brain tumor was surgically removed, according to a pilot study (329). Similarly, a 12-week inpatient or outpatient rehabilitation program improved physical competence in everyday tasks, prevented impairment, and reduced symptoms in patients with GBM and other brain tumors, according to an observational clinical study (330). These findings suggest that improving physical ability and functional performance can reduce tiredness, promote mental health, and increase general well-being, all of which will eventually improve patients’ quality of life (331). A more thorough study is required to completely understand the underlying processes and the direct influence of exercise on glioblastoma biology and development, even if existing data show that exercise improves physical functionality and quality of life in people with the disease (308). Finding the best exercise regimens, carefully assessing both motor and cognitive gains, looking into the long-term impacts of training, and comprehending how combined motor and cognitive rehabilitation results in significant improvements in patients’ daily functioning are all necessary to achieve this.
6.3 Exercise improves the cognitive function of patients with GBM
By encouraging neural plasticity, increasing BDNF levels, decreasing endogenous corticosteroids and inflammatory cytokines, reducing oxidative stress, improving blood vessel formation and cerebral blood flow, and increasing hormones that support neural health and function, regular exercise improves cognitive abilities and brain structure (332). The precise therapeutic effect of exercise on cognitive abnormalities in GBM patients is yet unknown, despite these encouraging results. Interestingly, a preclinical study showed that rats given oxaliplatin and 5-fluorouracil chemotherapy improved their memory and cognitive performance after four weeks of exercise (333). In a different preclinical investigation, five weeks of aerobic exercise reduced the fall in BDNF levels, promoted hippocampal neurogenesis, and minimized cognitive deterioration in healthy rats receiving radiation treatment (332). These findings point to the main pathways of exercise’s therapeutic effectiveness on cognitive function and imply that exercise helps reduce cognitive impairment in GBM.
There is evidence to suggest the inclusion of exercise in the treatment of GBM in people. According to one study, fitness training significantly improved a variety of cognitive abilities in people with neurological disorders (334). Another study showed that a 12-week combined exercise program increased psychological well-being, reduced dyspnea, and helped patients with brain tumors control their anxiety and depression symptoms (318). Six months of at-home fitness training improved patient-reported outcomes and cognitive test scores in glioma patients, according to a randomized controlled experiment (335). Additionally, a recent randomized controlled experiment discovered that individuals with high-grade gliomas who combined exercise with monitor-augmented reality during radiation therapy were able to avoid cognitive deterioration and muscular strength loss (336). Nevertheless, that experiment found no discernible effect of exercise on BDNF levels in spite of these beneficial effects (336). Since the majority of research has been conducted in animal models, more research is required to determine the underlying mechanisms through which exercise may mitigate cognitive impairments in humans with GBM, as well as how exercise affects cognitive function and BDNF levels in GBM patients.
6.4 Exercise and the prognosis of patients with GBM
Exercise has been shown in preclinical animal models to improve survival outcomes for patients with GBM. As an example, Lemke et al. discovered that in addition to lowering tumor size and invasiveness and avoiding severe body weight loss, exercise plus temozolomide therapy greatly increased survival in glioblastoma-bearing mice (315). In a mouse glioma model, voluntary exercise also reduced tumor cell growth, decreased motor deterioration, and preserved self-care skills, according to another recent preclinical study (314). Exercise is believed to increase BDNF levels in the blood, which may aid in preventing the growth of tumor cells (203, 332). Although the precise processes behind exercise’s protective benefits against brain tumors and GBM are yet unknown, they most likely entail physiological alterations that affect tumor development and improve the effectiveness of cancer therapies (337). According to a preclinical study, aerobic exercise in mice given injections of metastatic tumor cells altered the expression of tight junction proteins in brain microvessels, preserving the integrity of the blood-brain barrier and shielding the brain from the spread of the tumor (338). Applying these results to people, an observational research study found that patients’ performance level has predictive relevance and is associated with both disease progression and survival outcomes in GBM patients (339). According to Moore et al. study demonstrated an analysis of more than 300,000 people’s medical records, physically active teenagers had a 35% lower risk of glioma than their counterparts who were not (339). Exercise may prolong survival in GBM patients, according to another study that found patients with WHO grade III and IV malignant gliomas who exercised more than 9 MET-hours per week had a median survival that was 7.8 months longer than those who exercised less than 9 MET-hours per week (319). Additionally, information from the National Walkers’ and Runners’ Health Studies, which included more than 153,000 participants, indicated that regular physical activity, such as walking 19–37 km or running 12–25 km per week, was linked to a 43.2% lower risk of brain tumor death (340). These scientists showed that the survival rate in recurrent gliomas may be strongly predicted by general exercise habit (319). Exercise training as a strategy to enhance results in animal models of brain cancers has a solid foundation, thanks to preclinical studies. Although the data is still few and there are still many unresolved issues, recent human research also points to positive benefits of exercise on the prognosis of patients with brain tumors. More research is necessary to identify the underlying processes and the preventive potential of exercise, especially in GBM, taking into account the knowledge gathered from other malignancies (65).
6.5 Physical exercise and BBB
The endothelial cells that make up the BBB express tight junction (TJ) complexes, which form a selective vascular network. These tight junctions, which together comprise the BBB’s protective barrier, are composed of molecules that either anchor inside endothelial cells or join inside the extracellular junctional space (341, 342). Occludin, claudins, and junctional adhesion molecules are the three main transmembrane proteins that create tight junctions between brain endothelial cells. Numerous cytoplasmic proteins, including the zonula occludens family, assist these and aid in anchoring and stabilizing the junctional complex (343). Claudin-3, -5, and maybe claudin-12 are expressed by brain endothelial TJs (344, 345). It has been demonstrated that Claudin-5 actively supports the integrity of the BBB (346). Numerous investigations have demonstrated the involvement of occludin, claudin-3, and claudin-5 in BBB development (345, 347) and paracellular permeability regulation (348–351).
The activation of several receptors for vasoactive substances, including angiotensin II (Ang II) and bradykinin, as well as adhesion molecules and reactive oxygen species, might affect the control of tight junctions. Furthermore, by interacting with cytokine-activated brain endothelial cells, members of the immunoglobulin superfamily, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and platelet endothelial cell adhesion molecule 1 (PECAM-1), actively contribute to leukocyte migration and firm adhesion within the CNS (352, 353). Different substances secreted by neurovascular unit cells might affect brain endothelial tight junctions under pathological situations. While only a few variables can reverse or mitigate this disturbance, many inflammatory mediators have a tendency to exacerbate BBB permeability (354). Tight junctions and integrins (including β1, αv, and α6) may change or disappear as a result of prolonged exposure to oxidative and inflammatory stimuli. This can lead to cellular senescence and death by causing the cells to separate from the surrounding tissue (355, 356). By changing the location of junctional adhesion molecules and markedly upregulating the expression of ICAM-1 and VCAM-1, both IFN-γ and TNF-α are known to impair BBB permeability (357–359).
Occludin expression changes are linked to changes in BBB permeability (360) and endothelial barrier function (342, 348). Claudin-5 expression in brain endothelial cells is selective, indicating that it is necessary for BBB function (361), it has been demonstrated that Claudin-5 is essential for controlling the blood-brain barrier’s permeability to ions and bigger macromolecules (362). Numerous investigations have demonstrated a correlation between a decrease in claudin-5 expression and alterations in blood-brain barrier permeability (361). Recent studies conducted by Souza et al. should also be taken into consideration (363). Exercise has been demonstrated to reduce PECAM-1 expression while restoring baseline levels of tight junction protein expression in the central nervous system, including occludin and claudin-4. This was shown in a study that used a mouse model of multiple sclerosis called experimental autoimmune encephalomyelitis. According to the results, exercise preserves the integrity of the blood-brain barrier by preserving the stability of tight junctions (342). Furthermore, Schreibelt et al. reported lowering oxidative stress and reactive oxygen species generation (363, 364). Schreibelt et al. showed that physical activity in multiple sclerosis maintains claudin-4 and occludin levels in the spinal cord of mice (363, 364). Furthermore, recent research has demonstrated that, through processes unrelated to gene regulation, blocking glycogen synthase kinase-3β (GSK-3β) improves tight junction integrity in brain endothelial cells by extending the half-life of occludin and claudin-5 and raising their protein levels (365). A study by Isla et al. showed inhibition of glycogen synthase kinase-3β (GSK-3β) also has anti-inflammatory effects on brain endothelial cells (366), and voluntary exercise has been demonstrated to have a positive impact by lowering the recruitment of GSK-3β, which in turn helps protect the BBB by maintaining tight junctions (367). In addition to strengthening the BBB, GSK-3β inhibitors have potential therapeutic uses in BBB repair and protection. Most persons with brain cancer continued to be insufficiently physically active from the time of diagnosis until after treatment, according to a comprehensive analysis that included 15 relevant studies (36). Crucially, increased physical activity was consistently linked to better quality of life outcomes and less severe symptoms of brain cancer. With promising therapeutic value in reducing symptom burden, improving aerobic fitness, improving body composition, and increasing overall physical activity engagement, new research backs up the idea that structured physical exercise is a safe and realistic intervention for people with primary brain cancer (36). These advantages are highlighted by a systematic study, while participation variability is still an issue. About one-third (32%) of registered participants in an independent study left the intervention before it ended, suggesting possible obstacles to continuing participation. However, the remaining majority (68%) showed a range of compliance and adherence to recommended exercise programs, with compliance ranging from 24% to 83% for exercise doses and from 33% to 100% for complete participation. These results imply that a significant percentage of patients can participate effectively in exercise-based supportive treatment, despite difficulties with consistency and retention. The fact that no negative occurrences were documented suggests that the intervention was safe. All of the recommended workouts showed notable increases, especially in lower limb muscular strength and functional performance (368). Nevertheless, no discernible alterations were found in general physical function, body composition, tiredness, sleep quality, or general quality of life, indicating that the effects could be restricted to specific muscle outcomes in glioblastoma patients receiving adjuvant chemoradiotherapy. According to Jost et al., during cardiopulmonary testing, all 36 glioblastoma patients (median age 60; 21 male) met the predetermined peak workload criterion (369). Although they met test thresholds, they showed significant impairments in cardiorespiratory fitness, as evidenced by their mean VO2peak (1750 ± 529 ml/min), peak workload (130 ± 43 W), and physical work capacity (0.99 ± 0.38 W/kg body weight) being significantly below age- and sex-matched normative values 87%, 79%, and 90% of expected levels, respectively. Post-exercise dizziness, which did not require treatment, was the only minor and temporary adverse event (3%) that was noted. Significantly, self-reported physical activity decreased from 15.8 to 7.2 MET-hours per week after diagnosis, suggesting a substantial drop in exercise participation after diagnosis. GBM patients have significantly reduced cardiorespiratory fitness, highlighting the need for tailored exercise therapies to promote health and quality of life. In this demographic, maximal cardiopulmonary exercise testing (CPET) seems to be both safe and practical (369). It might be a useful tool for creating accurate, scientifically supported exercise recommendations. The study showed that without causing anatomical or pathological harm, physical exercise (PE) improved the behavior of trained animals. PE was linked to decreased expression of the tumor markers c-MYC, vimentin, and GFAP in mice with glioblastoma. GBM changed inflammation and oxidative stress, while PE had no discernible effect on these factors. Overall, the results show that exercise protects the GL261 mouse model from glioblastoma-induced neurotoxicity and tumor growth (370). The predefined criterion for peak physical effort was met by all participants in a different study with 36 glioblastoma patients (median age 60; 21 men). Their mean physical work capacity (0.99 ± 0.38 W/kg body weight), mean absolute VO2peak (1750 ± 529 ml/min), and mean peak workload (130 ± 43 W) were all far lower than age- and sex-matched normative values (87%, 79%, and 90%, respectively). Post-exercise dizziness was a mild, temporary adverse event that just one participant (3%) had and did not necessitate medical attention. Self-reported physical activity, which was 15.8 MET-hours/week before diagnosis, significantly decreased to 7.2 MET-hours/week after diagnosis. In glioblastoma patients who showed markedly decreased cardiorespiratory fitness, maximal CPET was shown to be both safe and practical. This underscores the need for customized exercise regimens to promote health and quality of life. When creating accurate, individualized training plans, CPET could be quite important (369). Two female patients with oligodendroglioma and glioblastoma multiforme successfully finished a 12-week exercise program under supervision, according to a case study. In addition to extra self-directed aerobic exercises, the 12-week exercise program comprised twice-weekly, one-hour supervised sessions that combined resistance and aerobic training. At the beginning, middle, and conclusion of the program, evaluations of cardiovascular fitness, physical strength, and psychological well-being (including anxiety, depression, and quality of life) were carried out. Both individuals finished every session without experiencing any negative consequences. The findings demonstrated that the intervention was both safe and effective, as evidenced by improvements in physical health indicators and decreases in psychological discomfort (318). According to the Hospital Anxiety and Sadness Scale, the study found that participants’ levels of sadness and general psychological discomfort significantly improved. Results indicate that glioblastoma patients receiving full therapy may safely and effectively engage in intense, long-term physical exercise (326). In one instance, despite tumor growth and continuous multimodal therapy, the patient continued to engage in high-intensity exercise without experiencing any negative side effects, improving their level of fitness Irisin, a myokine generated during exercise, was found in another study to be a possible molecular mediator via which physical activity prevents the spread of cancer. Surprisingly, it was shown that irisin dramatically inhibited the growth, invasion, and proliferation of glioma cells. According to these results, irisin may be used as a biomarker as well as a viable option for therapeutic intervention and molecular imaging in the treatment of gliomas (371).
6.6 Exercise and the kynurenine pathway
The BBB can be compromised by both central (brain) and peripheral inflammation, partly due to changes in tryptophan (TRP) metabolism (342). Elevated levels of kynurenine (KYN) and its downstream metabolites result from the activation of the kynurenine pathway (KP), the main mechanism for TRP degradation (342, 372), by inflammatory stimuli. Glutamatergic excitotoxicity and neurotoxicity are strongly associated with this system, which disrupts the blood-brain barrier and may exacerbate neurological symptoms in brain disorders like glioblastoma (342, 372). Tryptophan is broken down into kynurenine by the KP, which is started by the enzyme indoleamine 2,3-dioxygenase (IDO). Numerous clinical illnesses, such as cancer, diabetes mellitus, chronic inflammation, and neurological and neurodegenerative disorders, are linked to this metabolic pathway. Upregulation of IDO and increased kynurenine synthesis are linked to immune suppression, tumor growth, and disruption of the BBB integrity in the setting of cancer, especially brain tumors like glioblastoma (373, 374). A crucial tryptophan metabolite, KYN, undergoes further processing along several routes to produce either neurotoxic or neuroprotective chemicals. KYN is changed by kynurenine aminotransferases (KATs) into kynurenic acid (KYNA), a neuroprotective metabolite that controls cytokine release and promotes monocyte migration. On the other hand, KYN can also be converted into neurotoxic substances such as anthranilic acid and quinolinic acid (QUIN). QUIN is a strong neurotoxin that stimulates the generation of ROS and functions as an agonist of the NMDA receptor. Excitotoxicity and neuronal injury are associated with elevated QUIN levels. By inhibiting the growth of T cells, dendritic cells, and natural killer cells, KYN itself compromises immunological surveillance. The growth of illnesses, including cancer and neurodegeneration, immunological control, and neuroinflammation, are all significantly impacted by this equilibrium between KYNA and QUIN via the glutamatergic excitatory synapse it inhibits (375, 376). QUIN is probably one of the most significant metabolites in the kynurenine pathway in terms of toxicity and biological activity (377). It has been discovered that major depressive illnesses and schizophrenia both exhibit dysregulation of KYNA and QUIN (378). TNF-α and other pro-inflammatory mediators can increase the synthesis of QUIN (379), whereas IL-1β potentiates quinolinate-mediated excitotoxicity (372). This process is particularly important when inflammation of the CNS occurs, when macrophages and other immune cells enter the brain through a damaged BBB. In these circumstances, IDO is primarily upregulated in microglial cells, which direct tryptophan metabolism to produce QUIN, a neurotoxic NMDA receptor agonist. The most powerful inducer of IDO expression is the pro-inflammatory cytokine IFN-γ, which amplifies this pathway. This increase in QUIN leads to excitotoxicity, oxidative stress, and further disruption of the BBB, which exacerbates neuroinflammation and may facilitate tumor progression or neurodegeneration (373, 380).
This complex neuroimmune-glutamatergic system plays a key regulatory role in the body, and exercise has a particularly strong influence on its function (342). When skeletal muscle is activated by physical activity, it releases myokines like irisin and anti-inflammatory cytokines, which can help modulate immune responses and restore balance between Type 1 and Type 2 immunity (342). Additionally, exercise-induced upregulation of astrocytic activity may support glutamate clearance and buffering, which can help normalize excitatory neurotransmission (381). This muscle-brain crosstalk may counteract neuroinflammation, reduce IDO-driven tryptophan catabolism into neurotoxic metabolites like QUIN, and shield the BBB from glutamate-induced oxidative stress and permeability dysfunction (342). Therefore, regular exercise may be a non-pharmacological strategy to restore immune-metabolic homeostasis and improve CNS resilience in conditions like glioblastoma or neurodegenerative disease (342). By upregulating KATs, exercise causes tryptophan metabolism to change from producing neurotoxic metabolites like QUIN to producing neuroprotective KYNA, especially in skeletal muscle (381). This change, which is amplified during exercise training, lowers the amount of KYN in the blood that may pass across the BBB, shielding the brain from neurotoxicity brought on by inflammation. By inhibiting NMDA receptors, KYNA reduces oxidative stress and excitotoxicity, which is the main mechanism behind its neuroprotective effects (342). Additionally, András et al. emphasized that the kynurenine pathway-mediated muscle–brain axis may provide a mechanism by which exercise provides resistance against neurodegenerative illnesses, mood disorders, and neuroinflammation (382). High glutamate levels have been demonstrated to impair the integrity of the BBB by phosphorylating and disrupting occludin, a crucial tight junction protein, through the activation of NMDA receptors expressed on endothelial cells. This increases the BBB’s permeability by upsetting its structural cohesiveness (372, 383, 384). Tryptophan’s metabolite KYNA counteracts glutamate-induced excitotoxicity by blocking NMDA receptors, functioning as a neuroprotective agent. Furthermore, KYNA inhibits the α7-nicotinic acetylcholine receptor non-competitively, indicating a more extensive modulatory involvement in synaptic signaling and neuroinflammation (342). The potential therapeutic value of KYNA in maintaining BBB function and controlling brain homeostasis in diseased situations is highlighted by these dual receptor interactions (372, 383, 384) and KYNA can reduce glutamate release by this method (385). In this way, KYNA can lower abnormal glutamate levels and preserve the BBB’s integrity (382). Through a crucial inter-organ communication pathway, exercise training affects the integrity of the BBB. In particular, regular exercise promotes the conversion of neurotoxic KYN into neuroprotective KYNA via increasing the expression of KATs in skeletal muscle (342). By lowering circulating KYN levels, this metabolic change limits its transport across the blood-brain barrier and lessens its negative effects, including inflammation and excitotoxicity. Thus, exercise is an important muscle-to-brain protective axis that regulates the activation of the kynurenine pathway in muscles, acting as a peripheral regulator of central nervous system health.
6.7 Exercise and the renin-angiotensin-aldosterone pathway
BBB disruption has been closely associated with Ang II, a major effector of the renin-angiotensin-aldosterone system (RAAS), especially in hypertension situations. Endothelial dysfunction can result from increased oxidative stress and inflammatory signaling caused by elevated Ang II levels. As a result, the BBB’s tight connection integrity is compromised, increasing its permeability. Additionally, Ang II stimulates the activation of NADPH oxidase and matrix metalloproteinases (MMPs), which weaken barrier components and worsen vascular inflammation. As a result, Ang II is crucial to the BBB’s disintegration during hypertension, which leads to neurovascular and cognitive issues (386–388). Ang II has important effects outside of the cardiovascular system, while being best known as a cardiovascular regulator that keeps blood pressure and fluid balance stable. Ang II has a role in oxidative stress, neuroinflammation, and disruption of the BBB in the central nervous system. These effects are most noticeable in pathological circumstances like hypertension, when high Ang II levels weaken the integrity of the blood-brain barrier, allowing peripheral immune cells to infiltrate and encouraging damage to neurons. Therefore, Ang II serves as a modulator of neurovascular dysfunction as well as a hemodynamic regulator (389), Ang II has cardiovascular functions as well as immune system modulation. By improving vascular permeability and making it easier for immune cells to be drawn to areas of damage or illness, it might set off inflammatory reactions. By stimulating the release of cytokines and chemokines and activating pro-inflammatory signaling pathways like NF-κB, Ang II creates an environment that is favorable to inflammation, which leads to immune cell infiltration and long-term tissue damage, especially in conditions like hypertension and neurodegenerative diseases (390). Ang II may also trigger both innate and adaptive immunity (390, 391).
Through Ang II type 1 receptors (AT1) on the endothelium, either from systemic circulation or via local synthesis within the brain, Ang II directly affects both transcytotic and paracellular permeability in the BBB endothelial cells, potentially contributing significantly to the development of hypertensive encephalopathy. This interaction activates microglia and promotes vascular dysfunction, which results in increased production of ROS, decreased endothelial nitric oxide synthase (eNOS) activity, and increased secretion of pro-inflammatory cytokines. These effects are especially important in areas of the brain that control sympathetic output, such as the rostral ventrolateral medulla (RVLM) and the paraventricular nucleus (PVN) of the hypothalamus. This helps to cause and sustain neurogenic hypertension (392–394). Nevertheless, one study showed that, even in the absence of a drop in blood pressure, inhibiting AT1 receptors can stop hypertension-induced increases in BBB permeability and cerebral edema. This implies that AT1 inhibition may have protective effects on BBB integrity through mechanisms apart from its function in blood pressure control (395). This demonstrates how the renin-angiotensin-aldosterone system, and in particular Ang II, regulates the integrity of the blood-brain barrier. When the BBB is disrupted, Ang II can enter the brain more deeply, and peripheral immune cells can enter the brain parenchyma, which increases inflammation and microglial activation, particularly in autonomic regulatory centers such as the RVLM and the PVN of the hypothalamus (393, 394). Particularly under hypertension situations, these alterations impede neurovascular coupling and interfere with the control of cerebral blood flow, which increases neuronal excitability and intensifies sympathetic nervous system activity (Figure 5) (387, 396, 397).
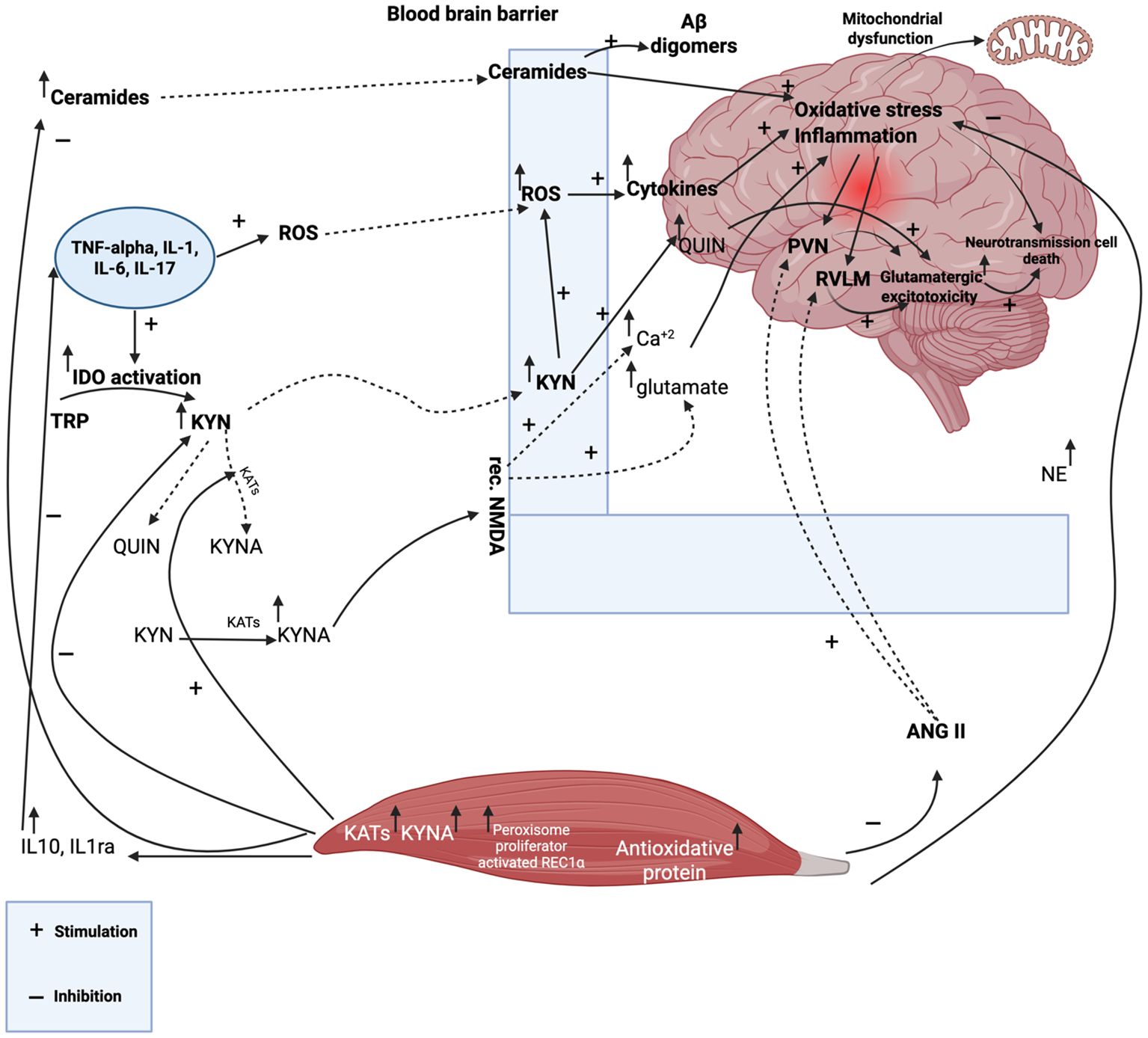
Figure 5. In states of chronic low-grade systemic inflammation, pro-inflammatory cytokines promote the generation of reactive oxygen species (ROS), which compromise the integrity of tight junctions (TJs) and lead to increased permeability of the blood-brain barrier (BBB). These cytokines can also activate the enzyme indoleamine 2,3-dioxygenase (IDO), initiating the breakdown of tryptophan (TRP) into kynurenine (KYN). KYN serves as a metabolic branching point—either being converted by kynurenine aminotransferases (KATs) into the neuroprotective metabolite kynurenic acid (KYNA), or transformed into neurotoxic byproducts, primarily quinolinic acid (QUIN). QUIN overactivates N-methyl-D-aspartate (NMDA) receptors, triggering excessive glutamate signaling, calcium influx, and further degradation of BBB integrity. In the context of insulin resistance, persistent inflammation contributes to lipid metabolism imbalances and elevated ceramide levels, which can cross a compromised BBB and exacerbate neuroinflammation. This process promotes the formation of amyloid-β (Aβ), further driving neuropathological changes. When the BBB becomes permeable, the protective barrier function of TJs is diminished, allowing pro-inflammatory molecules to infiltrate the brain more easily, compounding the damage. Within the central nervous system, elevated inflammation and oxidative stress disrupt mitochondrial and neuronal function, ultimately leading to cell death. A disrupted BBB also facilitates the entry of angiotensin II (Ang II), which can activate angiotensin II type 1 (AT1) receptors. This promotes vascular leakage, recruitment of immune cells, and ROS production—particularly affecting key autonomic centers such as the paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM). The resulting microglial activation and excitotoxicity mediated by glutamate worsen neuroinflammation. Exercise plays a protective role in this context. Physical activity upregulates KAT gene expression, enhancing the conversion of harmful KYN into KYNA, thereby supporting BBB integrity. Contracting muscles also release anti-inflammatory cytokines like IL-1 receptor antagonist (IL-1ra) and IL-10, which reduce the levels of pro-inflammatory cytokines including TNF-α, IL-1, IL-6, and IL-17. This anti-inflammatory response is further supported by the activation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in skeletal muscle. Additionally, physical exercise increases norepinephrine (NE) levels, which act through β2-adrenergic receptors on microglia, astrocytes, and lymphocytes to reduce neuroinflammation. Exercise also leads to a reduction in Ang II levels within tissues and suppresses microglial activity in the PVN and RVLM. It enhances the brain’s antioxidant defenses by stimulating the production of endogenous antioxidant molecules, reduces oxidative stress, lowers ceramide accumulation, and interrupts the destructive cycle of TJ dysfunction and BBB breakdown.
It has been shown that physical activity, especially aerobic training, can successfully lessen the disruption of the BBB caused by hypertension in the brain’s autonomic areas. Even in cases of persistent hypertension, this protective effect is strongly associated with improved regulation of both parasympathetic and sympathetic cardiovascular function. As demonstrated in models of spontaneous hypertension, a new study also shows that aerobic exercise can assist in restoring autonomic balance and reduce overactivity of the brain’s renin-angiotensin system (398, 399). It was shown that spontaneously hypertensive rats maintained normal levels of angiotensinogen expression in autonomic brain areas following only two weeks of physical activity. This normalization, which came before a little drop in arterial blood pressure, was linked to a decrease in sympathetic activity that targets the heart and blood vessels. This suggests that early central adaptations to exercise may come before systemic cardiovascular benefits (400). By controlling levels of pro- and anti-inflammatory cytokines, lowering oxidative stress, and balancing excitatory and inhibitory neurotransmitters, exercise training aids in the restoration of homeostasis in the PVN. Better cardiovascular and autonomic control results from this (401). Physical exercise quickly returns baroreceptor reflex regulation of heart rate to normal in spontaneously hypertensive rats. A notable reduction in inflammation and oxidative stress in the hypothalamic PVN is intimately associated with this impact (402). Other autonomic regions have also shown comparable training-induced benefits (398, 399). Acute physical exercise is known to cause sympathetic modulation (403). Regular exercise, on the other hand, results in a progressive decrease in sympathetic activity and an increase in parasympathetic dominance, which reflects adaptive modifications in both the central and peripheral regulatory systems (403). Autonomic dysfunction, breakdown of the BBB, and Ang II-driven neuronal activation are characteristics of hypertension. In order to reverse these Ang II-induced effects and restore autonomic control, exercise training has shown great efficacy (404). Additionally, physical exercise changes tissue levels of Ang II and decreases microglial activation, two factors that are essential for maintaining the integrity of the BBB and reestablishing autonomic cardiovascular regulation in hypertension (404). According to some studies, cerebral microvascular permeability may be broadly regulated by the cholinergic nervous system (405). Parasympathetic dominance brought on by exercise may help maintain autonomic balance and the integrity of the blood-brain barrier. Interactions among neurotransmitters, pro-inflammatory cytokines, and oxidative stress have a significant impact on sympathetic blood pressure control in the hypothalamic PVN. By regulating this interaction, exercise lessens sympathetic hyperactivity and the resulting hypertensive consequences (399). Elevated ROS in the RVLM of spontaneously hypertensive rats reduces GABAergic inhibitory input and enhances glutamatergic excitatory output. This imbalance enhances sympathetic outflow and contributes to hypertension by increasing excitatory drive from the PVN to the RVLM (406). Two important pressors that have a major impact on the control of central blood pressure are Ang II and glutamate. These chemicals function in key brain areas, including the RVLM and PVN, to regulate sympathetic nervous system activity and maintain or increase arterial pressure in both normotensive and spontaneously hypertensive rats. The onset and maintenance of hypertension are facilitated by their dysregulation (407). Vieira et al. have shown the connection between angiotensinergic and glutamatergic signaling in the brain (408). The RVLM is the primary sympathetic output channel that employs glutamate as a transmitter for the tonic and reflex regulation of blood pressure (409). Ang II increases the blood pressure response to glutamate when it is injected into the RVLM of conscious rats. It is believed that Ang II intensifies excitatory drive and sympathetic outflow by increasing glutamatergic input to the RVLM through a presynaptic mechanism. The synergistic function of glutamate and Ang II in central cardiovascular control and the pathogenesis of hypertension is highlighted by this relationship (410). In light of this, the glutamate antagonist KYNA is regarded as a hypotensive drug. Mills et al. have demonstrated that KYNA administered intrathecally reduces blood pressure, especially in anesthetized spontaneously hypertensive and stroke-prone hypertensive rats, while having little effect on normotensive animals (411). This implies that KYNA may aid in reducing high sympathetic drive in hypertensive circumstances. Through the reduction of Ang II levels, the suppression of oxidative stress and inflammation in important autonomic areas such as the PVN and RVLM, the correction of sympathetic overactivity, and the promotion of BBB integrity repair, physical exercise seems to replicate or intensify these effects (411).
6.8 Exercise and the brain noradrenergic system
Circulating catecholamines, especially epinephrine and norepinephrine (NE), activate β-adrenoceptors on vagal afferent fibers during physical activity. The locus coeruleus (LC), a brainstem nucleus that is a crucial regulator of autonomic balance and cognitive function, receives these afferents. The LC’s activation highlights the neurobiological link between physical exercise and better autonomic and cognitive outcomes by improving sympathetic-parasympathetic coordination and fostering neuroplasticity, mood management, and attentiveness (412–416). One important component of using exercise to modulate cognitive performance may be the optimum stimulation of the locus coeruleus (417). Brain NE is also reported to suppress the inflammatory gene transcription. It is clear from the findings of Hetier et al. and Frohman et al. The primary mechanism by which NE reduces inflammation during physical activity is through β2-adrenergic receptors that are expressed on microglia and astrocytes (418, 419). By lowering the synthesis of pro-inflammatory cytokines and modifying immunological responses in the brain, these glial cells react to NE signals. An anti-inflammatory environment is further enhanced by NE’s suppression of excessive immunological activation through β2-adrenergic receptors, which are primarily expressed by T and B lymphocytes in the peripheral immune system. The dual function of NE in regulating both central and peripheral immune responses is highlighted by its receptor-specific activity (420). Engagement of β2-receptors activates a cascade of signaling intermediates, including cyclic adenosine monophosphate (cAMP) and protein kinase A, which leads to the phosphorylation of cellular proteins (420). Through β2-receptor activation, NE also encourages a shift in the Th1/Th2 balance toward the Th2 response (421). By suppressing important pro-inflammatory mediators, including interleukin-1β (IL-1β), TNF-α, ICAM-1, and inducible nitric oxide synthase (iNOS), NE produces additional anti-inflammatory effects. These steps aid in protecting brain tissue and reducing neuroinflammation. NE also increases the synthesis of BDNF, which is an essential chemical for neurogenesis, synaptic plasticity, and neuronal survival. Because of its combined anti-inflammatory and neuroprotective properties, NE is crucial for preserving the homeostasis of the central nervous system, particularly during and after physical activity (422, 423). Brain-derived neurotrophic factor is modulated by β1/β2- and α2-adrenergic receptors, involving cellular signaling cascades that overlap with those that underlie the anti-inflammatory effects of norepinephrine (422–424). While glucose is still the primary energy substrate, neurons can also use lactate, particularly in situations where glucose availability is limited or energy demand is high. This reflects the brain’s metabolic adaptability (422, 423). Neurons primarily rely on the pentose phosphate pathway (PPP) to metabolize glucose, not only to generate adenosine triphosphate (ATP), but also to produce NADPH, which is essential for maintaining redox balance through glutathione regeneration (425, 426). This procedure takes place in the astrocytes, where energy is drawn from their glycogen reserves (427) is made possible by noradrenergic activation of the astrocytes’ β-adrenoceptors, which instructs them to generate lactate from glycogen (428, 429), which is subsequently sent to the neurons (430). Skeletal muscles produce more lactate in reaction to physical activity (431, 432), similarly to how the BBB expresses the lactate transporter monocarboxylate transporter 1 (433–435). Beyond its role as a metabolic substrate, lactate produced during physical exercise has been demonstrated to act as a signaling molecule, enhancing the expression of important growth factors involved in processes like angiogenesis (the formation of new blood vessels), neurogenesis (the generation of new neurons), calcium signaling, axonal myelination, synaptic plasticity, and memory formation. This link between physical activity and improved cognitive and neural health, and lactate’s important role in brain adaptability and recovery (49, 436, 437). Normal brain activity may be hampered by energy deficiencies caused by insufficient lactate availability for neurons. This lack of energy has been linked to the pathogenesis of many neurological disorders, such as attention-deficit/hyperactivity disorder (ADHD), where cognitive and behavioral symptoms may be exacerbated by disturbed metabolic support (438–440).
7 Future directions and research gaps
Despite a wealth of evidence supporting the beneficial effects of physical activity in people with GBM, there are still many obstacles to overcome before these findings can be fully applied in clinical practice (441, 442). These obstacles include a lack of personalized approaches that are tailored to the needs of each patient, a limited understanding of the underlying biological mechanisms, and difficulties in adapting exercise protocols to clinical settings. Future research must focus on the following key areas in order to make a meaningful contribution to this field:
7.1 Precision medicine approaches: personalized exercise regimens
In order to improve therapeutic outcomes, precision exercise oncology must move toward personalized regimens that are tailored to individual characteristics, including tumor molecular markers (e.g., IDH mutation, MGMT methylation), functional capacity, treatment stage, and immune-metabolic profiles (443, 444). The majority of current exercise interventions for GBM patients rely on standardized protocols, ignoring the significant biological and clinical diversity within this population. Personalization could be optimized by combining physiological metrics (e.g., heart rate variability or VO2 max) with clinical and genomic data, allowing for dynamic, real-time adjustments to exercise programs (445). Additionally, adaptive platforms that change training intensity in response to patient tolerance and symptom fluctuations are crucial for ensuring safety and efficacy throughout the course of the disease (446, 447).
7.2 Artificial intelligence and digital health in exercise oncology
By analyzing a variety of data, such as genetic profiles, activity tracker data, medical imaging, and patient-reported outcomes, machine learning models can be developed to predict how individual patients will respond to physical activity. These models can help guide risk assessment, monitor adherence, and detect complications early (448, 449). Artificial intelligence (AI) holds great promise for improving the effectiveness and personalization of exercise interventions in the care of patients with GBM. Virtual coaching systems that offer individualized and flexible training plans that adapt to the patient’s evolving health state can also be supported by AI (450). Wearable biosensors, telemedicine platforms, and electronic health records may all be integrated with AI to track patient progress over time and modify exercise regimens based on data. Exercise therapies that are more sensitive, scalable, and accurate and catered to the particular requirements of GBM patients may result from this strategy (451).
7.3 Combining exercise with novel metabolic inhibitors
GBM has a complex metabolic profile that includes increased glycolysis, lactate accumulation, and impaired mitochondrial function. Exercise has been shown to affect these metabolic pathways, but its therapeutic potential may be greatly increased when combined with specific metabolic agents (452). Future studies should look into how physical activity interacts with substances like AMPK activators, HIF-1α inhibitors, or mTOR pathway blockers to intensify tumor-specific metabolic stress. These combination strategies could simultaneously spare healthy tissue and increase systemic immune responses. However, important questions remain regarding the best time, dose, and order of exercise in conjunction with these agents’ areas that require more preclinical and clinical investigation (453).
7.4 Multi-omics analysis of exercise-induced tumor and host changes
Comprehensive mechanistic research is necessary to understand how physical activity impacts the immune response, TME, and systemic physiology in GBM. Advanced multi-omics techniques, including transcriptomics, proteomics, metabolomics, epigenomics, and single-cell sequencing, should be used to map the biological changes brought on by exercise at the cellular and molecular levels (454, 455). By combining these datasets, it may be possible to identify novel therapeutic targets, reveal biomarkers that predict patient response to exercise, elucidate the signaling pathways associated with tumor suppression, and identify new therapeutic targets. Additionally, collecting biological samples before, during, and after exercise interventions would help monitor the changing effects of physical training and provide more accurate timing and treatment plans (456).
7.5 Immune-tumor metabolism crosstalk and exercise
In GBM, where immune suppression is a hallmark, future research should examine how physical activity reshapes immune cell metabolism, particularly in reversing T cell exhaustion, boosting NK cell infiltration, and regulating the kynurenine pathway (457). Understanding these mechanisms could guide the development of combined immuno-metabolic therapies. Exercise may augment the efficacy of immune checkpoint inhibitors by improving the metabolic resilience and function of tumor-infiltrating immune cells (458).
7.6 Clinical trials with mechanistic endpoints
Large-scale, meticulously planned randomized controlled trials (RCTs) are urgently needed, even though preliminary feasibility and safety studies have shown encouraging results for exercise in patients with GBM. These trials should include mechanistic endpoints, such as circulating cytokine levels, neuroimaging biomarkers, tumor perfusion data, and BDNF concentrations, in order to better understand the biological effects of exercise. Digital phenotyping, such as wearables and mobile health data, and multi-omics profiling, such as genomics, proteomics, and metabolomics, can offer comprehensive insight into how exercise modulates disease pathways and patient physiology. Such integrated approaches will support the development of precision-based exercise prescriptions catered to individual patient profiles.
Clinical study NCT03390569 (Table 8), carried out at Princess Margaret Hospital in Canada, is a significant illustration of advancements in this field. It examines non-pharmacological treatments for glioblastoma, such as organized exercise regimens. In the field of neuro-oncology, this experiment is a major step toward evidence-based, individualized supportive treatment. The purpose of this interventional study was to evaluate the effects of a structured exercise program on clinical outcomes in patients with glioblastoma, with a particular focus on PFS and time to tumor progression over a six-month period, using the Response Assessment in Neuro-Oncology (RANO) criteria. The trial had 54 participants and represented a novel approach by incorporating physical activity into the supportive care of patients with glioblastoma, an area that has frequently been disregarded because of their generally poor functional status and limited prognosis. By assessing exercise as a non-pharmacological intervention, the study looked at its potential to improve both quality of life and disease-related outcomes.
The study’s translational potential is limited by a number of significant shortcomings, despite its novel character. Most significantly, no evaluation of the intervention’s safety or effectiveness has been possible because the data have not been made public. Additionally, the statistical power required to identify significant clinical differences is limited by the very small sample size. Since it is uncertain if any advantages observed may be directly attributed to the exercise routine, the absence of a clearly defined control group further erodes the ability to draw conclusions about causality. Furthermore, the brief six-month follow-up period might not be enough to record long-term or delayed advantages of exercise, such improved quality of life or neurocognitive function. The lack of information on functional outcomes, tiredness, or patient-reported quality of life dimensions that are extremely important in the context of GBM care is another significant restriction. Additionally, the study omits important details about the exercise regimen, such as its frequency, intensity, and degree of monitoring, all of which are necessary for practical application and repeatability. Given that patients who are able to engage in an exercise program could naturally be a healthier fraction of the glioblastoma population, potential selection bias must also be taken into consideration. In conclusion, although trial NCT03390569 shows that integrative care techniques for glioblastoma are becoming more popular, its shortcomings highlight the necessity for bigger, controlled investigations with thorough outcome reporting.
8 Implications of study
This review underscores a transformative potential in the role of physical activity as a complementary therapy in GBM management, suggesting a paradigm shift in neuro-oncology. The findings synthesize compelling evidence that exercise influences multiple dimensions of GBM biology ranging from metabolic reprogramming and angiogenesis to immune modulation and epigenetic regulation thereby offering a multi-pronged approach to tumor suppression. One of the most critical implications is the repositioning of physical activity from a supportive care adjunct to a biologically active intervention capable of modulating tumor behavior and therapeutic response. By enhancing immune surveillance, reducing treatment-induced immunosuppression, and supporting the normalization of the TME, exercise may overcome resistance to standard therapies and potentiate the efficacy of emerging strategies like immune checkpoint inhibitors. The synergistic effects observed in both preclinical and early-phase clinical studies suggest that exercise not only augments conventional treatments but also mitigates their side effects, contributing to improved survival and quality of life. Furthermore, the review highlights the mechanistic intricacies through which exercise exerts its effects, including modulation of the PI3K/Akt/mTOR and AMPK pathways, reduction in hypoxia and lactic acid buildup, and the rebalancing of immune cell populations within the TME. These findings open new avenues for integrating exercise into personalized medicine frameworks, particularly through tailoring regimens that align with tumor-specific metabolic and immunologic profiles. The study also addresses the impact of physical activity on epigenetic reprogramming, suggesting that exercise can restore tumor suppressor gene expression and silence oncogenic signaling mechanisms that may have long-term benefits in slowing GBM progression. Moreover, the neuroprotective and cognitive benefits associated with exercise are particularly significant in a cancer type where neurological decline profoundly affects patient autonomy and dignity.
However, the study also reveals important gaps and areas requiring further investigation. There is currently a lack of standardized exercise protocols tailored to GBM patients, and the precise dose, duration, and modality of exercise required to achieve optimal therapeutic outcomes remain unclear. Additionally, despite robust preclinical evidence, large-scale randomized controlled trials (RCTs) validating these benefits in human populations are scarce. The absence of mechanistic biomarker assessments in ongoing trials further limits our understanding of how exercise modifies GBM pathophysiology in vivo. Future research should therefore prioritize the incorporation of exercise into clinical trial designs, ideally with molecular profiling and multi-omics integration to elucidate patient-specific responses. In conclusion, this study provides a compelling case for redefining the role of physical activity in GBM therapy not merely as a supportive care strategy but as a biologically active intervention with the potential to reshape treatment paradigms. If validated through rigorous clinical research, exercise could become a low-cost, accessible, and scalable component of personalized oncology care, offering renewed hope for improving outcomes in one of the most lethal forms of brain cancer.
9 Conclusion
A potential area of neuro-oncology is the use of physical exercise into GBM therapy, which has been shown to have advantages for immune system performance, tumor biology, and patient well-being. Exercise improves anti-tumor immunity and lowers immunosuppression by modifying important pathways such PI3K/Akt/mTOR, angiogenesis, and lactate metabolism. Exercise has been clinically demonstrated to enhance GBM patients’ quality of life, cognitive function, and maybe survival outcomes. Personalized exercise regimens, mechanistic clarity, and bigger clinical studies with reliable objectives are still necessary, though. To maximize treatment success, future strategies should make use of artificial intelligence, precision medicine, and combination medicines with metabolic inhibitors. By filling up these gaps, exercise might become a vital component of multimodal GBM treatment, providing a transformational, affordable, and safe supplement to traditional treatments. This analysis emphasizes how urgently more research is needed to fully realize the promise of physical activity in preventing this debilitating illness.
Author contributions
LX: Writing – review & editing, Writing – original draft, Visualization, Validation. FW: Writing – review & editing, Writing – original draft, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sharif S, Harman N, Hydock D, and Olson T. Exercise intervention may play a potential therapeutic role in patients with glioblastoma multiforme (Review). World Acad Sci J. (2024) 6:41. doi: 10.3892/wasj.2024.256
2. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. (2013) 15 Suppl 2:ii1–56. doi: 10.1093/neuonc/not151
3. Kannan S, Murugan AK, Balasubramanian S, Munirajan AK, and Alzahrani AS. Gliomas: Genetic alterations, mechanisms of metastasis, recurrence, drug resistance, and recent trends in molecular therapeutic options. Biochem Pharmacol. (2022) 201:115090. doi: 10.1016/j.bcp.2022.115090
4. Pienkowski T, Kowalczyk T, Kretowski A, and Ciborowski M. A review of gliomas-related proteins. Characteristics of potential biomarkers. Am J Cancer Res. (2021) 11:3425–44.
5. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
6. Kleihues P and Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. (1999) 1:44–51. doi: 10.1093/neuonc/1.1.44
7. Ohgaki H and Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. (2013) 19:764–72. doi: 10.1158/1078-0432.CCR-12-3002
8. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
9. Chang SM, Parney IF, Huang W, Anderson FAJ, Asher AL, Bernstein M, et al. Patterns of care for adults with newly diagnosed Malignant glioma. JAMA. (2005) 293:557–64. doi: 10.1001/jama.293.5.557
10. Chandana SR, Movva S, Arora M, and Singh T. Primary brain tumors in adults. Am Fam Phys. (2008) 77:1423–30. doi: 10.1016/S0140-6736(18)30990-5
11. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
12. Hempen C, Weiss E, and Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer. (2002) 10:322–8. doi: 10.1007/s00520-001-0333-0
13. Omuro A and DeAngelis LM. Glioblastoma and other Malignant gliomas: a clinical review. JAMA. (2013) 310:1842–50. doi: 10.1001/jama.2013.280319
14. Osoba D, Brada M, Prados MD, and Yung WK. Effect of disease burden on health-related quality of life in patients with Malignant gliomas. Neuro Oncol. (2000) 2:221–8. doi: 10.1093/neuonc/2.4.221
15. Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. (2006) 76:283–91. doi: 10.1007/s11060-005-7020-9
16. Keir ST, Calhoun-Eagan RD, Swartz JJ, Saleh OA, and Friedman HS. Screening for distress in patients with brain cancer using the NCCN’s rapid screening measure. Psychooncology. (2008) 17:621–5. doi: 10.1002/pon.1271
17. Moser NJ, Phillips BA, Guthrie G, and Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. (1996) 79:100–2. doi: 10.1111/j.1600-0773.1996.tb00249.x
18. Litofsky NS and Resnick AG. The relationships between depression and brain tumors. J Neurooncol. (2009) 94:153–61. doi: 10.1007/s11060-009-9825-4
19. Cormie P, Nowak AK, Chambers SK, Galvão DA, and Newton RU. The potential role of exercise in neuro-oncology. Front Oncol. (2015) 5:85. doi: 10.3389/fonc.2015.00085
20. Sterckx W, Coolbrandt A, Dierckx de Casterlé B, Van den Heede K, Decruyenaere M, Borgenon S, et al. The impact of a high-grade glioma on everyday life: a systematic review from the patient’s and caregiver’s perspective. Eur J Oncol Nurs. (2013) 17:107–17. doi: 10.1016/j.ejon.2012.04.006
21. Daly FN and Schiff D. Supportive management of patients with brain tumors. Expert Rev Neurother. (2007) 7:1327–36. doi: 10.1586/14737175.7.10.1327
22. Budrukkar A, Jalali R, Dutta D, Sarin R, Devlekar R, Parab S, et al. Prospective assessment of quality of life in adult patients with primary brain tumors in routine neurooncology practice. J Neurooncol. (2009) 95:413–9. doi: 10.1007/s11060-009-9939-8
23. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, and Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. (2014) 17:488–504. doi: 10.1093/neuonc/nou304
24. Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. (2016) 20:S2–8. doi: 10.1188/16.CJON.S1.2-8
25. Julie DAR, Ahmed Z, Karceski SC, Pannullo SC, Schwartz TH, Parashar B, et al. An overview of anti-epileptic therapy management of patients with Malignant tumors of the brain undergoing radiation therapy. Seizure. (2019) 70:30–7. doi: 10.1016/j.seizure.2019.06.019
26. Pace A, Metro G, and Fabi A. Supportive care in neurooncology. Curr Opin Oncol. (2010) 22:621–6. doi: 10.1097/CCO.0b013e32833e078c
27. Ford E, Catt S, Chalmers A, and Fallowfield L. Systematic review of supportive care needs in patients with primary Malignant brain tumors. Neuro Oncol. (2012) 14:392–404. doi: 10.1093/neuonc/nor229
28. Halkett GKB, Lobb EA, Oldham L, and Nowak AK. The information and support needs of patients diagnosed with High Grade Glioma. Patient Educ Couns. (2010) 79:112–9. doi: 10.1016/j.pec.2009.08.013
29. Segal R, Zwaal C, Green E, Tomasone JR, Loblaw A, and Petrella T. Exercise for people with cancer: a systematic review. Curr Oncol. (2017) 24:e290–315. doi: 10.3747/co.24.3619
30. Ferioli M, Zauli G, Martelli AM, Vitale M, McCubrey JA, Ultimo S, et al. Impact of physical exercise in cancer survivors during and after antineoplastic treatments. Oncotarget. (2018) 9:14005–34. doi: 10.18632/oncotarget.24456
31. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. (2019) 51:2375–90. doi: 10.1249/MSS.0000000000002116
32. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. (2010) 42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112
33. Wu G, Chen Y, Chen C, Liu J, Wu Q, Zhang Y, et al. Role and mechanisms of exercise therapy in enhancing drug treatment for glioma: a review. Front Immunol. (2025) 16:1576283. doi: 10.3389/fimmu.2025.1576283
34. Eisenhut L, Sadeghi-Bahmani D, Gerber M, Saemann A, Staub L, Brand S, et al. Effects of two types of exercise training on psychological well-being, sleep and physical fitness in patients with high-grade glioma (WHO III and IV). J Psychiatr Res. (2022) 151:354–64. doi: 10.1016/j.jpsychires.2022.03.058
35. Basiri Z, Yang Y, Bruinsma FJ, Nowak AK, McDonald KL, Drummond KJ, et al. Physical activity and glioma: a case-control study with follow-up for survival. Cancer Causes Control. (2022) 33:749–57. doi: 10.1007/s10552-022-01559-w
36. Sandler CX, Matsuyama M, Jones TL, Bashford J, Langbecker D, and Hayes SC. Physical activity and exercise in adults diagnosed with primary brain cancer: a systematic review. J Neurooncol. (2021) 153:1–14. doi: 10.1007/s11060-021-03745-3
37. Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre P-L, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. (2004) 64:6892–9. doi: 10.1158/0008-5472.CAN-04-1337
38. Aldape K, Zadeh G, Mansouri S, Reifenberger G, and von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. (2015) 129:829–48. doi: 10.1007/s00401-015-1432-1
39. Yang L, Lin C, Wang L, Guo H, and Wang X. Hypoxia and hypoxia-inducible factors in glioblastoma multiforme progression and therapeutic implications. Exp Cell Res. (2012) 318:2417–26. doi: 10.1016/j.yexcr.2012.07.017
40. Lee D-H, Lee TH, Jung CH, and Kim Y-H. Wogonin induces apoptosis by activating the AMPK and p53 signaling pathways in human glioblastoma cells. Cell Signal. (2012) 24:2216–25. doi: 10.1016/j.cellsig.2012.07.019
41. Nagarajan RP and Costello JF. Epigenetic mechanisms in glioblastoma multiforme. Semin Cancer Biol. (2009) 19:188–97. doi: 10.1016/j.semcancer.2009.02.005
42. Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. (2014) 23:1985–96. doi: 10.1158/1055-9965.EPI-14-0275
43. Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. (2016) 7:33440–50. doi: 10.18632/oncotarget.7961
44. Huang Y, Lin D, and Taniguchi CM. Hypoxia inducible factor (HIF) in the tumor microenvironment: friend or foe? Sci China Life Sci. (2017) 60:1114–24. doi: 10.1007/s11427-017-9178-y
45. Cao W-D, Kawai N, Miyake K, Zhang X, Fei Z, and Tamiya T. Relationship of 14-3-3zeta (ζ), HIF-1α, and VEGF expression in human brain gliomas. Brain Tumor Pathol. (2014) 31:1–10. doi: 10.1007/s10014-013-0135-3
46. Mihaylova MM and Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. (2011) 13:1016–23. doi: 10.1038/ncb2329
47. Chhipa RR, Fan Q, Anderson J, Muraleedharan R, Huang Y, Ciraolo G, et al. AMP kinase promotes glioblastoma bioenergetics and tumour growth. Nat Cell Biol. (2018) 20:823–35. doi: 10.1038/s41556-018-0126-z
48. Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HHB, Elhassan GO, et al. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. (2014) 1:777–802. doi: 10.18632/oncoscience.109
49. Morland C, Andersson KA, Haugen ØP, Hadzic A, Kleppa L, Gille A, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. (2017) 8:15557. doi: 10.1038/ncomms15557
50. Mao P, Joshi K, Li J, Kim S-H, Li P, Santana-Santos L, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U.S.A. (2013) 110:8644–9. doi: 10.1073/pnas.1221478110
51. Codrici E, Enciu A-M, Popescu I-D, Mihai S, and Tanase C. Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int. (2016) 2016:5728438. doi: 10.1155/2016/5728438
52. Guichet P-O, Guelfi S, Teigell M, Hoppe L, Bakalara N, Bauchet L, et al. Notch1 stimulation induces a vascularization switch with pericyte-like cell differentiation of glioblastoma stem cells. Stem Cells. (2015) 33:21–34. doi: 10.1002/stem.1767
53. Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen A-J, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. (2008) 455:1129–33. doi: 10.1038/nature07443
54. Zhang Y, Dube C, Gibert MJ, Cruickshanks N, Wang B, Coughlan M, et al. The p53 pathway in glioblastoma. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10090297
55. Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. (2013) 155:462–77. doi: 10.1016/j.cell.2013.09.034
56. Wakimoto H, Tanaka S, Curry WT, Loebel F, Zhao D, Tateishi K, et al. Targetable signaling pathway mutations are associated with Malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. (2014) 20:2898–909. doi: 10.1158/1078-0432.CCR-13-3052
57. Chen C-Y, Chen J, He L, and Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne). (2018) 9:338. doi: 10.3389/fendo.2018.00338
58. Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. (2008) 7:1321–5. doi: 10.4161/cbt.7.9.6954
59. Han F, Hu R, Yang H, Liu J, Sui J, Xiang X, et al. PTEN gene mutations correlate to poor prognosis in glioma patients: a meta-analysis. Onco Targets Ther. (2016) 9:3485–92. doi: 10.2147/OTT.S99942
60. Pearson JRD and Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. (2017) 2:17040. doi: 10.1038/sigtrans.2017.40
61. Galvão RP and Zong H. Inflammation and gliomagenesis: bi-directional communication at early and late stages of tumor progression. Curr Pathobiol Rep. (2013) 1:19–28. doi: 10.1007/s40139-012-0006-3
62. Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. (2010) 70:5270–80. doi: 10.1158/0008-5472.CAN-10-0012
63. Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. (2004) 4:941–52. doi: 10.1038/nri1498
64. Ham SW, Jeon H-Y, Jin X, Kim E-J, Kim J-K, Shin YJ, et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ. (2019) 26:409–25. doi: 10.1038/s41418-018-0126-3
65. Moore SC, Lee I-M, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. (2016) 176:816–25. doi: 10.1001/jamainternmed.2016.1548
66. Pouyan A, Ghorbanlo M, Eslami M, Jahanshahi M, Ziaei E, Salami A, et al. Glioblastoma multiforme: insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol Cancer. (2025) 24:58. doi: 10.1186/s12943-025-02267-0
67. Mansouri A, Karamchandani J, and Das S. Molecular Genetics of Secondary Glioblastoma. De Vleeschouwer S, editor. Brisbane, AU: Codon Publications (2017). doi: 10.15586/codon.glioblastoma.2017.ch2
68. Han S, Wang P-F, Cai H-Q, Wan J-H, Li S-W, Lin Z-H, et al. Alterations in the RTK/Ras/PI3K/AKT pathway serve as potential biomarkers for immunotherapy outcome of diffuse gliomas. Aging (Albany NY). (2021) 13:15444–58. doi: 10.18632/aging.203102
69. Wang R, Zhao L, Wang S, Zhao X, Liang C, Wang P, et al. Regulatory pattern of abnormal promoter CpG island methylation in the glioblastoma multiforme classification. Front Genet. (2022) 13:989985. doi: 10.3389/fgene.2022.989985
70. An Z, Aksoy O, Zheng T, Fan Q-W, and Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. (2018) 37:1561–75. doi: 10.1038/s41388-017-0045-7
71. Ge Z, Zhang Q, Lin W, Jiang X, and Zhang Y. The role of angiogenic growth factors in the immune microenvironment of glioma. Front Oncol. (2023) 13:1254694. doi: 10.3389/fonc.2023.1254694
72. Begagić E, Bečulić H, Džidić-Krivić A, Kadić Vukas S, Hadžić S, Mekić-Abazović A, et al. Understanding the significance of hypoxia-inducible factors (HIFs) in glioblastoma: A systematic review. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16112089
73. Zanotto A, Glover RN, Zanotto T, and Boele FW. Rehabilitation in people living with glioblastoma: A narrative review of the literature. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16091699
74. Yalamarty SSK, Filipczak N, Li X, Subhan MA, Parveen F, Ataide JA, et al. Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM). Cancers (Basel). (2023) 15. doi: 10.3390/cancers15072116
75. Travers S and Litofsky NS. Daily lifestyle modifications to improve quality of life and survival in glioblastoma: A review. Brain Sci. (2021) 11. doi: 10.3390/brainsci11050533
76. Marqueze LFB, Costa AK, Pedroso GS, Vasconcellos FF, Pilger BI, Kindermann S, et al. Regulation of redox profile and genomic instability by physical exercise contributes to neuroprotection in mice with experimental glioblastoma. Antioxidants. (2023) 12. doi: 10.3390/antiox12071343
77. Keats MR, Grandy SA, Blanchard C, Fowles JR, Neyedli HF, Weeks AC, et al. The impact of resistance exercise on muscle mass in glioblastoma in survivors (RESIST): protocol for a randomized controlled trial. JMIR Res Protoc. (2022) 11:e37709. doi: 10.2196/37709
78. Fei X, Wu J, Tian H, Jiang D, Chen H, Yan K, et al. Glioma stem cells remodel immunotolerant microenvironment in GBM and are associated with therapeutic advancements. Cancer biomark. (2024) 41:1–24. doi: 10.3233/CBM-230486
79. Wang J, Liu S, Li G, and Xiao J. Exercise regulates the immune system. Adv Exp Med Biol. (2020) 1228:395–408. doi: 10.1007/978-981-15-1792-1_27
80. Campbell JP and Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. (2018) 9:648. doi: 10.3389/fimmu.2018.00648
81. Brummer C, Pukrop T, Wiskemann J, Bruss C, Ugele I, and Renner K. Can exercise enhance the efficacy of checkpoint inhibition by modulating anti-tumor immunity? Cancers (Basel). (2023) 15. doi: 10.3390/cancers15184668
82. Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. (2003) 33:261–84. doi: 10.2165/00007256-200333040-00002
83. Benschop RJ, Nijkamp FP, Ballieux RE, and Heijnen CJ. The effects of beta-adrenoceptor stimulation on adhesion of human natural killer cells to cultured endothelium. Br J Pharmacol. (1994) 113:1311–6. doi: 10.1111/j.1476-5381.1994.tb17141.x
84. Dimitrov S, Lange T, and Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. (2010) 184:503–11. doi: 10.4049/jimmunol.0902189
85. Krüger K, Lechtermann A, Fobker M, Völker K, and Mooren FC. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. (2008) 22:324–38. doi: 10.1016/j.bbi.2007.08.008
86. Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, and Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. (2003) 121:9–14. doi: 10.1590/s1516-31802003000100003
87. Nieman DC, Henson DA, Johnson R, Lebeck L, Davis JM, and Nehlsen-Cannarella SL. Effects of brief, heavy exertion on circulating lymphocyte subpopulations and proliferative response. Med Sci Sports Exerc. (1992) 24:1339–45. doi: 10.1249/00005768-199212000-00006
88. Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. (2011) 17:6–63.
89. Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJCSV, Drayson MT, et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. (2009) 23:767–75. doi: 10.1016/j.bbi.2009.02.011
90. Koivula T, Lempiäinen S, Rinne P, Hollmén M, Sundberg CJ, Rundqvist H, et al. Acute exercise mobilizes CD8(+) cytotoxic T cells and NK cells in lymphoma patients. Front Physiol. (2022) 13:1078512. doi: 10.3389/fphys.2022.1078512
91. Stampley JE, Cho E, Wang H, Theall B, Johannsen NM, Spielmann G, et al. Impact of maximal exercise on immune cell mobilization and bioenergetics. Physiol Rep. (2023) 11:e15753. doi: 10.14814/phy2.15753
92. Idorn M and Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. (2016) 22:565–77. doi: 10.1016/j.molmed.2016.05.007
93. Koivula T, Lempiäinen S, Rinne P, Rannikko JH, Hollmén M, Sundberg CJ, et al. The effect of acute exercise on circulating immune cells in newly diagnosed breast cancer patients. Sci Rep. (2023) 13:6561. doi: 10.1038/s41598-023-33432-4
94. Turner JE, Spielmann G, Wadley AJ, Aldred S, Simpson RJ, and Campbell JP. Exercise-induced B cell mobilisation: Preliminary evidence for an influx of immature cells into the bloodstream. Physiol Behav. (2016) 164:376–82. doi: 10.1016/j.physbeh.2016.06.023
95. Krüger K, Alack K, Ringseis R, Mink L, Pfeifer E, Schinle M, et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med Sci Sports Exerc. (2016) 48:2021–9. doi: 10.1249/MSS.0000000000000979
96. Clifford T, Wood MJ, Stocks P, Howatson G, Stevenson EJ, and Hilkens CMU. T-regulatory cells exhibit a biphasic response to prolonged endurance exercise in humans. Eur J Appl Physiol. (2017) 117:1727–37. doi: 10.1007/s00421-017-3667-0
97. Szlezak AM, Szlezak SL, Keane J, Tajouri L, and Minahan C. Establishing a dose-response relationship between acute resistance-exercise and the immune system: Protocol for a systematic review. Immunol Lett. (2016) 180:54–65. doi: 10.1016/j.imlet.2016.10.010
98. Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. (2004) 10:91–106.
99. Bigley AB, Rezvani K, Pistillo M, Reed J, Agha N, Kunz H, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Part II: impact of latent cytomegalovirus infection and catecholamine sensitivity. Brain Behav Immun. (2015) 49:59–65. doi: 10.1016/j.bbi.2014.12.027
100. Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. (2014) 39:160–71. doi: 10.1016/j.bbi.2013.10.030
101. Alack K, Weiss A, Krüger K, Höret M, Schermuly R, Frech T, et al. Profiling of human lymphocytes reveals a specific network of protein kinases modulated by endurance training status. Sci Rep. (2020) 10:888. doi: 10.1038/s41598-020-57676-6
102. Liu R, Fan W, Krüger K, Xiao YU, Pilat C, Seimetz M, et al. Exercise affects T-cell function by modifying intracellular calcium homeostasis. Med Sci Sports Exerc. (2017) 49:29–39. doi: 10.1249/MSS.0000000000001080
103. Bonilla FA and Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. (2010) 125:S33–40. doi: 10.1016/j.jaci.2009.09.017
104. Szlezak AM, Tajouri L, Keane J, Szlezak SL, and Minahan C. Isometric thumb exertion induces B cell and T cell lymphocytosis in trained and untrained males: physical aptitude determines response profiles. Int J Kinesiol Sport Sci. (2016) 4:55–66.
105. Mukaimoto T and Ohno M. Effects of circuit low-intensity resistance exercise with slow movement on oxygen consumption during and after exercise. J Sports Sci. (2012) 30:79–90. doi: 10.1080/02640414.2011.616950
106. Peake JM, Neubauer O, Walsh NP, and Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol. (2017) 122:1077–87. doi: 10.1152/japplphysiol.00622.2016
107. Pedersen BK and Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. (2000) 80:1055–81. doi: 10.1152/physrev.2000.80.3.1055
108. Petersen AMW and Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. (2005) 98:1154–62. doi: 10.1152/japplphysiol.00164.2004
109. Callegari IOM, Rocha GZ, and Oliveira AG. Physical exercise, health, and disease treatment: The role of macrophages. Front Physiol. (2023) 14:1061353. doi: 10.3389/fphys.2023.1061353
110. Kawanishi N, Yano H, Yokogawa Y, and Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. (2010) 16:105–18.
111. Murugathasan M, Jafari A, Amandeep A, Hassan SA, Chihata M, and Abdul-Sater AA. Moderate exercise induces trained immunity in macrophages. Am J Physiol Cell Physiol. (2023) 325:C429–42. doi: 10.1152/ajpcell.00130.2023
112. Kawanishi M, Kami K, Nishimura Y, Minami K, Senba E, Umemoto Y, et al. Exercise-induced increase in M2 macrophages accelerates wound healing in young mice. Physiol Rep. (2022) 10:e15447. doi: 10.14814/phy2.15447
113. Sugiura H, Nishida H, Sugiura H, and Mirbod SM. Immunomodulatory action of chronic exercise on macrophage and lymphocyte cytokine production in mice. Acta Physiol Scand. (2002) 174:247–56. doi: 10.1046/j.1365-201x.2002.00930.x
114. Kizaki T, Takemasa T, Sakurai T, Izawa T, Hanawa T, Kamiya S, et al. Adaptation of macrophages to exercise training improves innate immunity. Biochem Biophys Res Commun. (2008) 372:152–6. doi: 10.1016/j.bbrc.2008.05.005
115. Abdalla DR, Aleixo AAR, Murta EFC, and Michelin MA. Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol Lett. (2014) 7:886–90. doi: 10.3892/ol.2013.1774
116. Ginhoux F, Schultze JL, Murray PJ, Ochando J, and Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. (2016) 17:34–40. doi: 10.1038/ni.3324
117. Lin P-H, Yao H-Y, Huang L, Fu C-C, Yao X-L, Lian C, et al. Autoimmune astrocytopathy double negative for AQP4-IgG and GFAP-IgG: Retrospective research of clinical practice, biomarkers, and pathology. CNS Neurosci \& Ther. (2024) 30:e70042. doi: 10.1111/cns.70042
118. Pedersen BK and Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
119. Pedersen BK. Muscle as a secretory organ. Compr Physiol. (2013) 3:1337–62. doi: 10.1002/cphy.c120033
120. Alves MD de J, Silva DDS, Pereira EVM, Pereira DD, de Sousa Fernandes MS, Santos DFC, et al. Changes in cytokines concentration following long-distance running: A systematic review and meta-analysis. Front Physiol. (2022) 13:838069. doi: 10.3389/fphys.2022.838069
121. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. (2022) 18:273–89. doi: 10.1038/s41574-022-00641-2
122. Mehta AK, Gracias DT, and Croft M. TNF activity and T cells. Cytokine. (2018) 101:14–8. doi: 10.1016/j.cyto.2016.08.003
123. Bhat P, Leggatt G, Waterhouse N, and Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. (2017) 8:e2836. doi: 10.1038/cddis.2017.67
124. Brincks EL and Woodland DL. Novel roles for IL-15 in T cell survival. F1000 Biol Rep. (2010) 2:67. doi: 10.3410/B2-67
125. Lum JJ, Schnepple DJ, Nie Z, Sanchez-Dardon J, Mbisa GL, Mihowich J, et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J Virol. (2004) 78:6033–42. doi: 10.1128/JVI.78.11.6033-6042.2004
126. Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CAJ, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. (2010) 207:273–80. doi: 10.1084/jem.20092029
127. Chen D, Tang T-X, Deng H, Yang X-P, and Tang Z-H. Interleukin-7 biology and its effects on immune cells: mediator of generation, differentiation, survival, and homeostasis. Front Immunol. (2021) 12:747324. doi: 10.3389/fimmu.2021.747324
128. Ostrowski K, Rohde T, Zacho M, Asp S, and Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. (1998) 508:949–53. doi: 10.1111/j.1469-7793.1998.949bp.x
129. Starkie RL, Rolland J, Angus DJ, Anderson MJ, and Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Physiol Cell Physiol. (2001) 280:C769–74. doi: 10.1152/ajpcell.2001.280.4.C769
130. Muñoz-Cánoves P, Scheele C, Pedersen BK, and Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. (2013) 280:4131–48. doi: 10.1111/febs.12338
131. Daou HN. Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation. Am J Physiol Regul Integr Comp Physiol. (2020) 318:R296–310. doi: 10.1152/ajpregu.00147.2019
132. Blank CU, Haanen JB, Ribas A, and Schumacher TN. CANCER IMMUNOLOGY. The “cancer immunogram. Science. (2016) 352:658–60. doi: 10.1126/science.aaf2834
133. Suh S-Y, Choi YS, Yeom CH, Kwak SM, Yoon HM, Kim DG, et al. Interleukin-6 but not tumour necrosis factor-alpha predicts survival in patients with advanced cancer. Support Care Cancer. (2013) 21:3071–7. doi: 10.1007/s00520-013-1878-4
134. Hoene M, Runge H, Häring HU, Schleicher ED, and Weigert C. Interleukin-6 promotes myogenic differentiation of mouse skeletal muscle cells: role of the STAT3 pathway. Am J Physiol Cell Physiol. (2013) 304:C128–36. doi: 10.1152/ajpcell.00025.2012
135. Nieman DC and Pence BD. Exercise immunology: Future directions. J Sport Heal Sci. (2020) 9:432–45. doi: 10.1016/j.jshs.2019.12.003
136. Rosa-Neto JC, Lira FS, Little JP, Landells G, Islam H, Chazaud B, et al. Immunometabolism-fit: How exercise and training can modify T cell and macrophage metabolism in health and disease. Exerc Immunol Rev. (2022) 28:29–46.
137. Wasinski F, Gregnani MF, Ornellas FH, Bacurau AVN, Câmara NO, Araujo RC, et al. Lymphocyte glucose and glutamine metabolism as targets of the anti-inflammatory and immunomodulatory effects of exercise. Mediators Inflammation. (2014) 2014:326803. doi: 10.1155/2014/326803
138. Henderson GC, Horning MA, Lehman SL, Wolfel EE, Bergman BC, and Brooks GA. Pyruvate shuttling during rest and exercise before and after endurance training in men. J Appl Physiol. (2004) 97:317–25. doi: 10.1152/japplphysiol.01367.2003
139. Rundqvist H, Veliça P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M, et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife. (2020) 9. doi: 10.7554/eLife.59996
140. Navarro F, Bacurau AVN, Pereira GB, Araújo RC, Almeida SS, Moraes MR, et al. Moderate exercise increases the metabolism and immune function of lymphocytes in rats. Eur J Appl Physiol. (2013) 113:1343–52. doi: 10.1007/s00421-012-2554-y
141. Li F, Liu H, Zhang D, Ma Y, and Zhu B. Metabolic plasticity and regulation of T cell exhaustion. Immunology. (2022) 167:482–94. doi: 10.1111/imm.13575
142. Reina-Campos M, Scharping NE, and Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. (2021) 21:718–38. doi: 10.1038/s41577-021-00537-8
143. Rogeri PS, Gasparini SO, Martins GL, Costa LKF, Araujo CC, Lugaresi R, et al. Crosstalk between skeletal muscle and immune system: which roles do IL-6 and glutamine play? Front Physiol. (2020) 11:582258. doi: 10.3389/fphys.2020.582258
144. Freyssenet D, Berthon P, and Denis C. Mitochondrial biogenesis in skeletal muscle in response to endurance exercises. Arch Physiol Biochem. (1996) 104:129–41. doi: 10.1076/apab.104.2.129.12878
145. Buss LA, Hock B, Merry TL, Ang AD, Robinson BA, Currie MJ, et al. Effect of immune modulation on the skeletal muscle mitochondrial exercise response: An exploratory study in mice with cancer. PloS One. (2021) 16:e0258831. doi: 10.1371/journal.pone.0258831
146. Alley JR, Valentine RJ, and Kohut ML. Mitochondrial mass of naïve T cells is associated with aerobic fitness and energy expenditure of active and inactive adults. Med Sci Sports Exerc. (2022) 54:1288–99. doi: 10.1249/MSS.0000000000002914
147. Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, et al. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. (2005) 28:108–14. doi: 10.2337/diacare.28.1.108
148. Suh S-H, Paik I-Y, and Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. (2007) 23:272–9. doi: 10.1016/S1016-8478(23)10717-5
149. Mika A, Macaluso F, Barone R, Di Felice V, and Sledzinski T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front Physiol. (2019) 10:26. doi: 10.3389/fphys.2019.00026
150. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. (2016) 24:657–71. doi: 10.1016/j.cmet.2016.08.011
151. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PloS Biol. (2015) 13:e1002202. doi: 10.1371/journal.pbio.1002202
152. Péronnet F and Aguilaniu B. Lactic acid buffering, nonmetabolic CO2 and exercise hyperventilation: a critical reappraisal. Respir Physiol Neurobiol. (2006) 150:4–18. doi: 10.1016/j.resp.2005.04.005
153. Covarrubias AJ, Aksoylar HI, and Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. (2015) 27:286–96. doi: 10.1016/j.smim.2015.08.001
154. Yang Z, Kahn BB, Shi H, and Xue B-Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. (2010) 285:19051–9. doi: 10.1074/jbc.M110.123620
155. Zeng H and Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. (2013) 25:347–55. doi: 10.1016/j.coi.2013.05.002
156. Watson K and Baar K. mTOR and the health benefits of exercise. Semin Cell Dev Biol. (2014) 36:130–9. doi: 10.1016/j.semcdb.2014.08.013
157. Linke M, Fritsch SD, Sukhbaatar N, Hengstschläger M, and Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. (2017) 591:3089–103. doi: 10.1002/1873-3468.12711
158. Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. (2003) 112:645–57. doi: 10.1016/s0092-8674(03)00154-5
159. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. (2011) 208:1367–76. doi: 10.1084/jem.20110278
160. Cho SH, Raybuck AL, Blagih J, Kemboi E, Haase VH, Jones RG, et al. Hypoxia-inducible factors in CD4(+) T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc Natl Acad Sci U.S.A. (2019) 116:8975–84. doi: 10.1073/pnas.1811702116
161. Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. (2014) 15:423–30. doi: 10.1038/ni.2865
162. Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, and Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. (2000) 529 Pt 1:237–42. doi: 10.1111/j.1469-7793.2000.00237.x
163. Cabral-Santos C, de Lima Junior EA, Fernandes IM da C, Pinto RZ, Rosa-Neto JC, Bishop NC, et al. Interleukin-10 responses from acute exercise in healthy subjects: A systematic review. J Cell Physiol. (2019) 234:9956–65. doi: 10.1002/jcp.27920
164. Ip WKE, Hoshi N, Shouval DS, Snapper S, and Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. (2017) 356:513–9. doi: 10.1126/science.aal3535
165. Koelwyn GJ, Quail DF, Zhang X, White RM, and Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. (2017) 17:620–32. doi: 10.1038/nrc.2017.78
166. de Araújo AL, Silva LCR, Fernandes JR, and Benard G. Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy. (2013) 5:879–93. doi: 10.2217/imt.13.77
167. Huff WX, Kwon JH, Henriquez M, Fetcko K, and Dey M. The evolving role of CD8(+)CD28(-) immunosenescent T cells in cancer immunology. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20112810
168. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. (2003) 101:2711–20. doi: 10.1182/blood-2002-07-2103
169. Rodriguez IJ, Lalinde Ruiz N, Llano León M, Martínez Enríquez L, Montilla Velásquez MDP, Ortiz Aguirre JP, et al. Immunosenescence study of T cells: A systematic review. Front Immunol. (2020) 11:604591. doi: 10.3389/fimmu.2020.604591
170. Pawelec G. Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. (1999) 108:1–7. doi: 10.1016/s0047-6374(99)00010-x
171. Alves AS and Bueno V. Immunosenescence: participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein (Sao Paulo). (2019) 17:eRB4733. doi: 10.31744/einstein_journal/2019RB4733
172. Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon K-J, Cunnick JE, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. (2004) 22:2298–306. doi: 10.1016/j.vaccine.2003.11.023
173. de Araújo AL, Silva LCR, Fernandes JR, Matias M de ST, Boas LS, MaChado CM, et al. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age (Dordr). (2015) 37:105. doi: 10.1007/s11357-015-9843-4
174. Duggal NA, Pollock RD, Lazarus NR, Harridge S, and Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. (2018) 17. doi: 10.1111/acel.12750
175. Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, and Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. (2019) 19:563–72. doi: 10.1038/s41577-019-0177-9
176. Donovan T, Bain AL, Tu W, Pyne DB, and Rao S. Influence of exercise on exhausted and senescent T cells: A systematic review. Front Physiol. (2021) 12:668327. doi: 10.3389/fphys.2021.668327
177. Yan H, Jiang A, Huang Y, Zhang J, Yang W, Zhang W, et al. Exercise sensitizes PD-1/PD-L1 immunotherapy as a hypoxia modulator in the tumor microenvironment of melanoma. Front Immunol. (2023) 14:1265914. doi: 10.3389/fimmu.2023.1265914
178. Gouez M, Rébillard A, Thomas A, Beaumel S, Matera E-L, Gouraud E, et al. Combined effects of exercise and immuno-chemotherapy treatments on tumor growth in MC38 colorectal cancer-bearing mice. Front Immunol. (2024) 15:1368550. doi: 10.3389/fimmu.2024.1368550
179. Wadley AJ, Cullen T, Vautrinot J, Keane G, Bishop NC, and Coles SJ. High intensity interval exercise increases the frequency of peripheral PD-1+ CD8(+) central memory T-cells and soluble PD-L1 in humans. Brain Behav Immun - Heal. (2020) 3:100049. doi: 10.1016/j.bbih.2020.100049
180. Handford J, Chen M, Rai R, Moss CL, Enting D, Peat N, et al. Is there a role for exercise when treating patients with cancer with immune checkpoint inhibitors? A scoping review. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14205039
181. Liu J, Liu W, Wan Y, and Mao W. Crosstalk between exercise and immunotherapy: current understanding and future directions. Research. (2024) 7:360. doi: 10.34133/research.0360
182. Bay ML, Unterrainer N, Stagaard R, Pedersen KS, Schauer T, Staffeldt MM, et al. Voluntary wheel running can lead to modulation of immune checkpoint molecule expression. Acta Oncol. (2020) 59:1447–54. doi: 10.1080/0284186X.2020.1817550
183. Liu X-F, Zhu X-D, Feng L-H, Li X-L, Xu B, Li K-S, et al. Physical activity improves outcomes of combined lenvatinib plus anti-PD-1 therapy in unresectable hepatocellular carcinoma: a retrospective study and mouse model. Exp Hematol Oncol. (2022) 11:20. doi: 10.1186/s40164-022-00275-0
184. Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP, et al. Exercise training improves tumor control by increasing CD8(+) T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. (2021) 9:765–78. doi: 10.1158/2326-6066.CIR-20-0499
185. Wennerberg E, Lhuillier C, Rybstein MD, Dannenberg K, Rudqvist N-P, Koelwyn GJ, et al. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget. (2020) 11:452–61. doi: 10.18632/oncotarget.27464
186. Buss LA, Williams T, Hock B, Ang AD, Robinson BA, Currie MJ, et al. Effects of exercise and anti-PD-1 on the tumour microenvironment. Immunol Lett. (2021) 239:60–71. doi: 10.1016/j.imlet.2021.08.005
187. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
188. Charles C, Bardet A, Ibrahimi N, Aromatario O, Cambon L, Imbert A, et al. Delivering adapted physical activity by videoconference to patients with fatigue under immune checkpoint inhibitors: Lessons learned from the PACTIMe-FEAS feasibility study. J Telemed Telecare. (2023) 29:716–24. doi: 10.1177/1357633X211021743
189. Hyatt A, Gough K, Murnane A, Au-Yeung G, Dawson T, Pearson E, et al. i-Move, a personalised exercise intervention for patients with advanced melanoma receiving immunotherapy: a randomised feasibility trial protocol. BMJ Open. (2020) 10:e036059. doi: 10.1136/bmjopen-2019-036059
190. Lacey J, Lomax AJ, McNeil C, Marthick M, Levy D, Kao S, et al. A supportive care intervention for people with metastatic melanoma being treated with immunotherapy: a pilot study assessing feasibility, perceived benefit, and acceptability. Support Care Cancer. (2019) 27:1497–507. doi: 10.1007/s00520-018-4524-3
191. Gouez M, Pérol O, Pérol M, Caux C, Ménétrier-Caux C, Villard M, et al. Effect of acute aerobic exercise before immunotherapy and chemotherapy infusion in patients with metastatic non-small-cell lung cancer: protocol for the ERICA feasibility trial. BMJ Open. (2022) 12:e056819. doi: 10.1136/bmjopen-2021-056819
192. Holmen Olofsson G, Mikkelsen MK, Ragle A-M, Christiansen AB, Olsen AP, Heide-Ottosen L, et al. High Intensity Aerobic exercise training and Immune cell Mobilization in patients with lung cancer (HI AIM)-a randomized controlled trial. BMC Cancer. (2022) 22:246. doi: 10.1186/s12885-022-09349-y
193. Coffey VG and Hawley JA. The molecular bases of training adaptation. Sports Med. (2007) 37:737–63. doi: 10.2165/00007256-200737090-00001
194. Yu M, King B, Ewert E, Su X, Mardiyati N, Zhao Z, et al. Exercise Activates p53 and Negatively Regulates IGF-1 Pathway in Epidermis within a Skin Cancer Model. PloS One. (2016) 11:e0160939. doi: 10.1371/journal.pone.0160939
195. Nezamdoost Z, Saghebjoo M, Hoshyar R, Hedayati M, and Keska A. High-intensity training and saffron: effects on breast cancer-related gene expression. Med Sci Sports Exerc. (2020) 52:1470–6. doi: 10.1249/MSS.0000000000002274
196. Zaidi SK, Van Wijnen AJ, Lian JB, Stein JL, and Stein GS. Targeting deregulated epigenetic control in cancer. J Cell Physiol. (2013) 228:2103–8. doi: 10.1002/jcp.24387
197. Ferioli M, Zauli G, Maiorano P, Milani D, Mirandola P, and Neri LM. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J Cell Physiol. (2019) 234:14852–64. doi: 10.1002/jcp.28304
198. Zeng H, Irwin ML, Lu L, Risch H, Mayne S, Mu L, et al. Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Treat. (2012) 133:127–35. doi: 10.1007/s10549-011-1716-7
199. Khori V, Amani Shalamzari S, Isanejad A, Alizadeh AM, Alizadeh S, Khodayari S, et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. Eur J Pharmacol. (2015) 765:179–87. doi: 10.1016/j.ejphar.2015.08.031
200. Liu L-Z, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PloS One. (2011) 6:e19139. doi: 10.1371/journal.pone.0019139
201. Elsner VR, Lovatel GA, Bertoldi K, Vanzella C, Santos FM, Spindler C, et al. Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neuroscience. (2011) 192:580–7. doi: 10.1016/j.neuroscience.2011.06.066
202. Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, and Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. (2011) 33:383–90. doi: 10.1111/j.1460-9568.2010.07508.x
203. Abel JL and Rissman EF. Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci. (2013) 31:382–90. doi: 10.1016/j.ijdevneu.2012.11.002
204. Wójtowicz K, Czarzasta K, Przepiorka L, Kujawski S, Cudnoch-Jedrzejewska A, Marchel A, et al. Brain-derived neurotrophic factor (BDNF) concentration levels in cerebrospinal fluid and plasma in patients with glioblastoma: A prospective, observational, controlled study. Cureus. (2023) 15:e48237. doi: 10.7759/cureus.48237
205. Ng DQ, Cheng I, Wang C, Tan CJ, Toh YL, Koh YQ, et al. Brain-derived neurotrophic factor as a biomarker in cancer-related cognitive impairment among adolescent and young adult cancer patients. Sci Rep. (2023) 13:16298. doi: 10.1038/s41598-023-43581-1
206. Feng Y, Feng X, Wan R, Luo Z, Qu L, and Wang Q. Impact of exercise on cancer: mechanistic perspectives and new insights. Front Immunol. (2024) 15:1474770. doi: 10.3389/fimmu.2024.1474770
207. Hojman P, Gehl J, Christensen JF, and Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. (2018) 27:10–21. doi: 10.1016/j.cmet.2017.09.015
208. Luo Z, Wan R, Liu S, Feng X, Peng Z, Wang Q, et al. Mechanisms of exercise in the treatment of lung cancer – a mini-review. Front Immunol. (2023) 14:1244764. doi: 10.3389/fimmu.2023.1244764
209. Spanoudaki M, Giaginis C, Karafyllaki D, Papadopoulos K, Solovos E, Antasouras G, et al. Exercise as a promising agent against cancer: evaluating its anti-cancer molecular mechanisms. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15215135
210. Agostini D, Natalucci V, Baldelli G, De Santi M, Donati Zeppa S, Vallorani L, et al. New Insights into the Role of Exercise in Inhibiting mTOR Signaling in Triple-Negative Breast Cancer. Oxid Med Cell Longev. (2018) 2018:5896786. doi: 10.1155/2018/5896786
211. Alizadeh AM, Heydari Z, Rahimi M, Bazgir B, Shirvani H, Alipour S, et al. Oxytocin mediates the beneficial effects of the exercise training on breast cancer. Exp Physiol. (2018) 103:222–35. doi: 10.1113/EP086463
212. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. (2014) 26:605–22. doi: 10.1016/j.ccell.2014.10.006
213. Ashcraft KA, Warner AB, Jones LW, and Dewhirst MW. Exercise as adjunct therapy in cancer. Semin Radiat Oncol. (2019) 29:16–24. doi: 10.1016/j.semradonc.2018.10.001
214. Faustino-Rocha AI, Silva A, Gabriel J, Gil da Costa RM, Moutinho M, Oliveira PA, et al. Long-term exercise training as a modulator of mammary cancer vascularization. BioMed Pharmacother. (2016) 81:273–80. doi: 10.1016/j.biopha.2016.04.030
215. Wang H, Hu Y, and ZCYLXFSOYLJF. Inhibitory effect of antitumor activity extract from the celastrus orbiculatus thunb on human glioblastoma. IJP. (2023) 19:758–68. doi: 10.3923/ijp.2023.758.768
216. Firuzpour F, Saleki K, Aram C, and Rezaei N. Nanocarriers in glioblastoma treatment: a neuroimmunological perspective. Rev Neurosci. (2024). doi: 10.1515/revneuro-2024-0097
217. Isanejad A, Alizadeh AM, Amani Shalamzari S, Khodayari H, Khodayari S, Khori V, et al. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. (2016) 151:30–40. doi: 10.1016/j.lfs.2016.02.090
218. Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. (2012) 113:263–72. doi: 10.1152/japplphysiol.01575.2011
219. Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. (2010) 108:343–8. doi: 10.1152/japplphysiol.00424.2009
220. Rincón-Castanedo C, Morales JS, Martín-Ruiz A, Valenzuela PL, Ramírez M, Santos-Lozano A, et al. Physical exercise effects on metastasis: a systematic review and meta-analysis in animal cancer models. Cancer Metastasis Rev. (2020) 39:91–114. doi: 10.1007/s10555-020-09851-4
221. O’Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. (2013) 37:1–21. doi: 10.4093/dmj.2013.37.1.1
222. Li W, Saud SM, Young MR, Chen G, and Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. (2015) 6:7365–78. doi: 10.18632/oncotarget.3629
223. Kim I and He Y-Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front Oncol. (2013) 3:175. doi: 10.3389/fonc.2013.00175
224. Piguet A-C, Saran U, Simillion C, Keller I, Terracciano L, Reeves HL, et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. (2015) 62:1296–303. doi: 10.1016/j.jhep.2015.01.017
225. Vara-Ciruelos D, Russell FM, and Hardie DG. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? (†). Open Biol. (2019) 9:190099. doi: 10.1098/rsob.190099
226. Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. (2015) 33:1–7. doi: 10.1016/j.ceb.2014.09.004
227. Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. (2013) 17:113–24. doi: 10.1016/j.cmet.2012.12.001
228. Ponnusamy L, Natarajan SR, Thangaraj K, and Manoharan R. Therapeutic aspects of AMPK in breast cancer: Progress, challenges, and future directions. Biochim Biophys Acta Rev Cancer. (2020) 1874:188379. doi: 10.1016/j.bbcan.2020.188379
229. Aveseh M, Nikooie R, and Aminaie M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J Physiol. (2015) 593:2635–48. doi: 10.1113/JP270463
230. Bacurau RF, Belmonte MA, Seelaender MC, and Costa Rosa LF. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem Funct. (2000) 18:249–58. doi: 10.1002/1099-0844(200012)18:4<249::AID-CBF879>3.0.CO;2-2
231. Campos JC, Fernandes T, Bechara LRG, da Paixão NA, Brum PC, de Oliveira EM, et al. Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxid Med Cell Longev. (2015) 2015:464195. doi: 10.1155/2015/464195
232. Zhang X, Ashcraft KA, Betof Warner A, Nair SK, and Dewhirst MW. Can exercise-induced modulation of the tumor physiologic microenvironment improve antitumor immunity? Cancer Res. (2019) 79:2447–56. doi: 10.1158/0008-5472.CAN-18-2468
233. Nieman DC and Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Heal Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
234. Nieman DC, Henson DA, Austin MD, and Brown VA. Immune response to a 30-minute walk. Med Sci Sports Exerc. (2005) 37:57–62. doi: 10.1249/01.mss.0000149808.38194.21
235. Gupta P, Bigley AB, Markofski M, Laughlin M, and LaVoy EC. Autologous serum collected 1 h post-exercise enhances natural killer cell cytotoxicity. Brain Behav Immun. (2018) 71:81–92. doi: 10.1016/j.bbi.2018.04.007
236. Fridman WH, Pagès F, Sautès-Fridman C, and Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. (2012) 12:298–306. doi: 10.1038/nrc3245
237. Evans ES, Hackney AC, McMurray RG, Randell SH, Muss HB, Deal AM, et al. Impact of acute intermittent exercise on natural killer cells in breast cancer survivors. Integr Cancer Ther. (2015) 14:436–45. doi: 10.1177/1534735415580681
238. Barra NG, Fan IY, Gillen JB, Chew M, Marcinko K, Steinberg GR, et al. High intensity interval training increases natural killer cell number and function in obese breast cancer-challenged mice and obese women. J Cancer Prev. (2017) 22:260–6. doi: 10.15430/JCP.2017.22.4.260
239. Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. (2016) 23:554–62. doi: 10.1016/j.cmet.2016.01.011
240. Sharma P and Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. (2015) 161:205–14. doi: 10.1016/j.cell.2015.03.030
241. Vulczak A and Alberici LC. Physical exercise and tumor energy metabolism. Cancer Treat Res Commun. (2022) 32:100600. doi: 10.1016/j.ctarc.2022.100600
243. Warburg O. On the origin of cancer cells. Science (80-). (1956) 123:309–14. doi: 10.1126/science.123.3191.309
244. Scatena R. Mitochondria and Cancer: A Growing Role in Apoptosis, Cancer Cell Metabolism and Dedifferentiation BT - Advances in Mitochondrial Medicine. Scatena R, Bottoni P, and Giardina B, editors. Dordrecht: Springer Netherlands (2012) p. 287–308. doi: 10.1007/978-94-007-2869-1_13
245. Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, and Capaldi RA. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. (2004) 64:985–93. doi: 10.1158/0008-5472.CAN-03-1101
246. Weinberg F and Chandel NS. Mitochondrial Metabolism and cancer. Ann N Y Acad Sci. (2009) 1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x
247. Zu XL and Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. (2004) 313:459–65. doi: 10.1016/j.bbrc.2003.11.136
248. Smolková K, Plecitá-Hlavatá L, Bellance N, Benard G, Rossignol R, and Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. (2011) 43:950–68. doi: 10.1016/j.biocel.2010.05.003
249. Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, and Saavedra E. Energy metabolism in tumor cells. FEBS J. (2007) 274:1393–418. doi: 10.1111/j.1742-4658.2007.05686.x
250. Cavalli LR, Varella-Garcia M, and Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. (1997) 8:1189–98.
251. Morais R, Zinkewich-Péotti K, Parent M, Wang H, Babai F, and Zollinger M. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Res. (1994) 54:3889–96.
252. Tan AS, Baty JW, Dong L-F, Bezawork-Geleta A, Endaya B, Goodwin J, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. (2015) 21:81–94. doi: 10.1016/j.cmet.2014.12.003
253. Zong W-X, Rabinowitz JD, and White E. Mitochondria and cancer. Mol Cell. (2016) 61:667–76. doi: 10.1016/j.molcel.2016.02.011
254. Gogvadze V, Orrenius S, and Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. (2008) 18:165–73. doi: 10.1016/j.tcb.2008.01.006
255. Vander Heiden MG, Cantley LC, and Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (80-). (2009) 324:1029–33. doi: 10.1126/science.1160809
256. Vyas S, Zaganjor E, and Haigis MC. Mitochondria and cancer. Cell. (2016) 166:555–66. doi: 10.1016/j.cell.2016.07.002
257. Alirol E and Martinou JC. Mitochondria and cancer: is there a morphological connection? Oncogene. (2006) 25:4706–16. doi: 10.1038/sj.onc.1209600
258. Smolková K, Bellance N, Scandurra F, Génot E, Gnaiger E, Plecitá-Hlavatá L, et al. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J Bioenerg Biomembr. (2010) 42:55–67. doi: 10.1007/s10863-009-9267-x
259. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. (2022) 12:31–46. doi: 10.1158/2159-8290.CD-21-1059
260. Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. (2009) 8:3984–4001. doi: 10.4161/cc.8.23.10238
261. Grivennikov SI, Greten FR, and Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
262. Quail DF and Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. (2013) 19:1423–37. doi: 10.1038/nm.3394
263. Hanahan D and Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
264. Justus CR, Sanderlin EJ, and Yang LV. Molecular connections between cancer cell metabolism and the tumor microenvironment. Int J Mol Sci. (2015) 16:11055–86. doi: 10.3390/ijms160511055
265. Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients. Cell Cycle. (2012) 11:1108–17. doi: 10.4161/cc.11.6.19530
266. Mantovani A, Allavena P, Sica A, and Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
267. Mao Y, Keller ET, Garfield DH, Shen K, and Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. (2013) 32:303–15. doi: 10.1007/s10555-012-9415-3
268. Wang N, Wang B, Maswikiti EP, Yu Y, Song K, Ma C, et al. AMPK–a key factor in crosstalk between tumor cell energy metabolism and immune microenvironment? Cell Death Discov. (2024) 10:237. doi: 10.1038/s41420-024-02011-5
269. Luo Z, Zang M, and Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. (2010) 6:457–70. doi: 10.2217/fon.09.174
270. Liu S, Zhang X, Wang W, Li X, Sun X, Zhao Y, et al. Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer. Mol Cancer. (2024) 23:261. doi: 10.1186/s12943-024-02165-x
271. Schiliro C and Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. (2021) 10. doi: 10.3390/cells10051056
272. Faubert B, Solmonson A, and DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science (80-). (2020) 368:eaaw5473. doi: 10.1126/science.aaw5473
273. Hawley JA, Hargreaves M, Joyner MJ, and Zierath JR. Integrative biology of exercise. Cell. (2014) 159:738–49. doi: 10.1016/j.cell.2014.10.029
274. Pedersen L, Christensen JF, and Hojman P. Effects of exercise on tumor physiology and metabolism. Cancer J. (2015) 21:111–6. doi: 10.1097/PPO.0000000000000096
275. Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J Natl Cancer Inst. (2015) 107:djv040. doi: 10.1093/jnci/djv040
276. Liu Z, Zhao Z, Xie L, Xiao Z, Li M, Li Y, et al. Proteomic analysis reveals chromatin remodeling as a potential therapeutical target in neuroblastoma. J Transl Med. (2025) 23:234. doi: 10.1186/s12967-025-06298-5
277. Alishvandi A, Barancheshemeh M, Firuzpour F, Aram C, Kamali MJ, and Keikha M. Decoding virulence and resistance in klebsiella pneumoniae: pharmacological insights, immunological dynamics, and in silico therapeutic strategies. Microb Pathog. (2025), 107691. doi: 10.1016/j.micpath.2025.107691
278. Pourahmad R, saleki K, Zare Gholinejad M, Aram C, Soltani Farsani A, Banazadeh M, et al. Exploring the effect of gut microbiome on Alzheimer’s disease. Biochem Biophys Rep. (2024) 39:101776. doi: 10.1016/j.bbrep.2024.101776
279. SChadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. (2016) 7:65429. doi: 10.18632/oncotarget.11748
280. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science (80-). (2005) 307:58–62. doi: 10.1126/science.1104819
281. Schumacher O, Galvao DA, Taaffe DR, Chee R, Spry N, and Newton RU. Exercise modulation of tumour perfusion and hypoxia to improve radiotherapy response in prostate cancer. Prostate Cancer Prostatic Dis. (2021) 24:1–14. doi: 10.1038/s41391-020-0245-z
282. Wang Z-Y, Loo TY, Shen J-G, Wang N, Wang D-M, Yang D-P, et al. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. (2012) 131:791–800. doi: 10.1007/s10549-011-1466-6
283. Kim J-H, Kim E-L, Lee Y-K, Park C-B, Kim B-W, Wang H-J, et al. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects. Exp Cell Res. (2011) 317:1108–18. doi: 10.1016/j.yexcr.2011.02.011
284. Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, and Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev. (2008) 17:1920–9. doi: 10.1158/1055-9965.EPI-08-0175
285. Michou V, Zervoudis S, Eskitzis P, Tsamos G, Vasdeki D, Vouxinou A, et al. Exercise interventions in breast cancer: molecular mechanisms, physical benefits, and practical recommendations. Med (B Aires). (2025) 61. doi: 10.3390/medicina61071167
286. Howard RA, Leitzmann MF, Linet MS, and Freedman DM. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control. (2009) 20:323–33. doi: 10.1007/s10552-008-9246-2
287. Jamurtas AZ, Koutedakis Y, Paschalis V, Tofas T, Yfanti C, Tsiokanos A, et al. The effects of a single bout of exercise on resting energy expenditure and respiratory exchange ratio. Eur J Appl Physiol. (2004) 92:393–8. doi: 10.1007/s00421-004-1156-8
288. Nakagata T, Yamada Y, and Naito H. Energy expenditure, recovery oxygen consumption, and substrate oxidation during and after body weight resistance exercise with slow movement compared to treadmill walking. Physiol Int. (2018) 105:371–85. doi: 10.1556/2060.105.2018.4.27
289. Goh J, Endicott E, and Ladiges WC. Pre-tumor exercise decreases breast cancer in old mice in a distance-dependent manner. Am J Cancer Res. (2014) 4:378.
290. Vulczak A, Souza A de O, Ferrari GD, Azzolini AECS, Pereira-da-Silva G, and Alberici LC. Moderate exercise modulates tumor metabolism of triple-negative breast cancer. Cells. (2020) 9:628. doi: 10.3390/cells9030628
291. Moncada S, Higgs EA, and Colombo SL. Fulfilling the metabolic requirements for cell proliferation. Biochem J. (2012) 446:1–7. doi: 10.1042/BJ20120427
292. Santinon G, Pocaterra A, and Dupont S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. (2016) 26:289–99. doi: 10.1016/j.tcb.2015.11.004
293. Totaro A, Panciera T, and Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat Cell Biol. (2018) 20:888–99. doi: 10.1038/s41556-018-0142-z
294. Saxton RA and Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. (2017) 168:960–76. doi: 10.1016/j.cell.2017.02.004
295. Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, et al. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. (2017) 77:4894–904. doi: 10.1158/0008-5472.CAN-16-3125
296. Furth N, Aylon Y, and Oren M. p53 shades of hippo. Cell Death Differ. (2018) 25:81–92. doi: 10.1038/cdd.2017.163
297. Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. (2013) 9:712. doi: 10.1038/msb.2013.65
298. Lu M, Sanderson SM, Zessin A, Ashcraft KA, Jones LW, Dewhirst MW, et al. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab. (2018) 6:14. doi: 10.1186/s40170-018-0190-7
299. Wakefield ZR, Tanaka M, Pampo C, Lepler S, Rice L, Guingab-Cagmat J, et al. Normal tissue and tumor microenvironment adaptations to aerobic exercise enhance doxorubicin anti-tumor efficacy and ameliorate its cardiotoxicity in retired breeder mice. Oncotarget. (2021) 12:1737–48. doi: 10.18632/oncotarget.28057
300. Vasan K, Werner M, and Chandel NS. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. (2020) 32:341–52. doi: 10.1016/j.cmet.2020.06.019
301. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, and Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci. (1998) 95:11715–20. doi: 10.1073/pnas.95.20.11715
302. Liu X, Kim CN, Yang J, Jemmerson R, and Wang X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell. (1996) 86:147–57. doi: 10.1016/S0092-8674(00)80085-9
303. Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, et al. The role of fatty acid β-oxidation in lymphangiogenesis. Nature. (2017) 542:49–54. doi: 10.1038/nature21028
304. Carey BW, Finley LWS, Cross JR, Allis CD, and Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. (2015) 518:413–6. doi: 10.1038/nature13981
305. Altman BJ, Stine ZE, and Dang CV. Erratum: From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. (2016) 16:749. doi: 10.1038/nrc.2016.114
306. Glass OK, Bowie M, Fuller J, Darr D, sary JU, Boss K, et al. Differential response to exercise in claudin-low breast cancer. Oncotarget. (2017) 8. doi: 10.18632/oncotarget.21054
307. Kruk J, Aboul-Enein BH, Gołębiewska ME, Duchnik E, Czerniak U, and Marchlewicz M. Physical activity and cancer incidence and mortality: current evidence and biological mechanisms. Cancers (Basel). (2025) 17. doi: 10.3390/cancers17091410
308. Spina S, Facciorusso S, Cinone N, Pellegrino R, Fiore P, and Santamato A. Rehabilitation interventions for glioma patients: a mini-review. Front Surg. (2023) 10:1137516. doi: 10.3389/fsurg.2023.1137516
309. Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Rudà R, et al. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. (2017) 18:e330–40. doi: 10.1016/S1470-2045(17)30345-5
310. Piil K, Juhler M, Jakobsen J, and Jarden M. Controlled rehabilitative and supportive care intervention trials in patients with high-grade gliomas and their caregivers: a systematic review. BMJ Support Palliat Care. (2016) 6:27–34. doi: 10.1136/bmjspcare-2013-000593
311. Vargo M. Brain tumor rehabilitation. Am J Phys Med Rehabil. (2011) 90:S50–62. doi: 10.1097/PHM.0b013e31820be31f
312. Aram C, Karami L, and Ranjbar MM. Development of a candidate mRNA vaccine based on Multi-Peptide targeting VP4 of rotavirus A: an immunoinformatics and molecular dynamics approach. Sci Rep. (2025) 15:22610. doi: 10.1038/s41598-025-07433-4
313. Aram C, Firuzpour F, Barancheshmeh M, and Kamali MJ. Unveiling the translational and therapeutic potential of small interfering RNA molecules in combating SARS-CoV-2: A review. Int J Biol Macromol. (2025), 145203. doi: 10.1016/j.ijbiomac.2025.145203
314. Tantillo E, Colistra A, Baroncelli L, Costa M, Caleo M, and Vannini E. Voluntary physical exercise reduces motor dysfunction and hampers tumor cell proliferation in a mouse model of glioma. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17165667
315. Lemke D, Pledl H-W, Zorn M, Jugold M, Green E, Blaes J, et al. Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget. (2016) 7:56713–25. doi: 10.18632/oncotarget.10723
316. Capozzi LC, Boldt KR, Easaw J, Bultz B, and Culos-Reed SN. Evaluating a 12-week exercise program for brain cancer patients. Psychooncology. (2016) 25:354–8. doi: 10.1002/pon.3842
317. Hansen A, Pedersen CB, Jarden JO, Beier D, Minet LR, and Søgaard K. Effectiveness of physical therapy- and occupational therapy-based rehabilitation in people who have glioma and are undergoing active anticancer treatment: single-blind, randomized controlled trial. Phys Ther. (2020) 100:564–74. doi: 10.1093/ptj/pzz180
318. Levin GT, Greenwood KM, Singh F, Tsoi D, and Newton RU. Exercise improves physical function and mental health of brain cancer survivors: two exploratory case studies. Integr Cancer Ther. (2016) 15:190–6. doi: 10.1177/1534735415600068
319. Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, et al. Exercise behavior, functional capacity, and survival in adults with Malignant recurrent glioma. J Clin Oncol. (2011) 29:2918–23. doi: 10.1200/JCO.2011.34.9852
320. Szulc-Lerch KU, Timmons BW, Bouffet E, Laughlin S, de Medeiros CB, Skocic J, et al. Repairing the brain with physical exercise: Cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. NeuroImage Clin. (2018) 18:972–85. doi: 10.1016/j.nicl.2018.02.021
321. Piscione PJ, Bouffet E, Timmons B, Courneya KS, Tetzlaff D, Schneiderman JE, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur J Cancer. (2017) 80:63–72. doi: 10.1016/j.ejca.2017.04.020
322. Formica V, Del Monte G, Giacchetti I, Grenga I, Giaquinto S, Fini M, et al. Rehabilitation in neuro-oncology: a meta-analysis of published data and a mono-institutional experience. Integr Cancer Ther. (2011) 10:119–26. doi: 10.1177/1534735410392575
323. Roberts PS, Nuño M, Sherman D, Asher A, Wertheimer J, Riggs RV, et al. The impact of inpatient rehabilitation on function and survival of newly diagnosed patients with glioblastoma. PM R. (2014) 6:514–21. doi: 10.1016/j.pmrj.2013.12.007
324. Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. (2017) 19:440–50. doi: 10.1093/neuonc/now177
325. Gehring K, Kloek CJ, Aaronson NK, Janssen KW, Jones LW, Sitskoorn MM, et al. Feasibility of a home-based exercise intervention with remote guidance for patients with stable grade II and III gliomas: a pilot randomized controlled trial. Clin Rehabil. (2018) 32:352–66. doi: 10.1177/0269215517728326
326. Troschel FM, Brandt R, Wiewrodt R, Stummer W, and Wiewrodt D. High-intensity physical exercise in a glioblastoma patient under multimodal treatment. Med Sci Sports Exerc. (2019) 51:2429–33. doi: 10.1249/MSS.0000000000002067
327. Halkett GKB, Cormie P, McGough S, Zopf EM, Galvão DA, Newton RU, et al. Patients and carers’ perspectives of participating in a pilot tailored exercise program during chemoradiotherapy for high grade glioma: A qualitative study. Eur J Cancer Care (Engl). (2021) 30:e13453. doi: 10.1111/ecc.13453
328. Hansen A, Søgaard K, and Minet LR. Development of an exercise intervention as part of rehabilitation in a glioblastoma multiforme survivor during irradiation treatment: a case report. Disabil Rehabil. (2019) 41:1608–14. doi: 10.1080/09638288.2018.1432707
329. Yu J, Jung Y, Park J, Kim JM, Suh M, Cho KG, et al. Intensive rehabilitation therapy following brain tumor surgery: A pilot study of effectiveness and long-term satisfaction. Ann Rehabil Med. (2019) 43:129–41. doi: 10.5535/arm.2019.43.2.129
330. Hojan K and Gerreth K. Can multidisciplinary inpatient and outpatient rehabilitation provide sufficient prevention of disability in patients with a brain tumor?-A case-series report of two programs and A prospective, observational clinical trial. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17186488
331. Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
332. Ji J, Ji S, Sun R, Li K, Zhang Y, Zhang L, et al. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem Biophys Res Commun. (2014) 443:646–51. doi: 10.1016/j.bbrc.2013.12.031
333. Fardell JE, Vardy J, Shah JD, and Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacol (Berl). (2012) 220:183–93. doi: 10.1007/s00213-011-2466-2
334. Knöchel C, Oertel-Knöchel V, O’Dwyer L, Prvulovic D, Alves G, Kollmann B, et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol. (2012) 96:46–68. doi: 10.1016/j.pneurobio.2011.11.007
335. Gehring K, Stuiver MM, Visser E, Kloek C, van den Bent M, Hanse M, et al. A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: a proof of concept. Neuro Oncol. (2020) 22:103–15. doi: 10.1093/neuonc/noz178
336. Pieczyńska A, Zasadzka E, Pilarska A, Procyk D, Adamska K, and Hojan K. Rehabilitation exercises supported by monitor-augmented reality for patients with high-grade glioma undergoing radiotherapy: results of a randomized clinical trial. J Clin Med. (2023) 12. doi: 10.3390/jcm12216838
337. Betof AS, Dewhirst MW, and Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. (2013) 30 Suppl:S75–87. doi: 10.1016/j.bbi.2012.05.001
338. Wolff G, Davidson SJ, Wrobel JK, and Toborek M. Exercise maintains blood-brain barrier integrity during early stages of brain metastasis formation. Biochem Biophys Res Commun. (2015) 463:811–7. doi: 10.1016/j.bbrc.2015.04.153
339. Michaelsen SR, Christensen IJ, Grunnet K, Stockhausen M-T, Broholm H, Kosteljanetz M, et al. Clinical variables serve as prognostic factors in a model for survival from glioblastoma multiforme: an observational study of a cohort of consecutive non-selected patients from a single institution. BMC Cancer. (2013) 13:402. doi: 10.1186/1471-2407-13-402
340. Williams PT. Reduced risk of brain cancer mortality from walking and running. Med Sci Sports Exerc. (2014) 46:927–32. doi: 10.1249/MSS.0000000000000176
341. Anderson JM and Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. (2009) 1:a002584. doi: 10.1101/cshperspect.a002584
342. Małkiewicz MA, Szarmach A, Sabisz A, Cubała WJ, Szurowska E, and Winklewski PJ. Blood-brain barrier permeability and physical exercise. J Neuroinflamm. (2019) 16:15. doi: 10.1186/s12974-019-1403-x
343. Wolburg H and Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. (2002) 38:323–37. doi: 10.1016/s1537-1891(02)00200-8
344. Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. (2000) 100:323–31. doi: 10.1007/s004010000180
345. Morita K, Sasaki H, Furuse M, and Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. (1999) 147:185–94. doi: 10.1083/jcb.147.1.185
346. Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. (2003) 161:653–60. doi: 10.1083/jcb.200302070
347. Furuse M, Fujita K, Hiiragi T, Fujimoto K, and Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. (1998) 141:1539–50. doi: 10.1083/jcb.141.7.1539
348. Balda MS, Whitney JA, Flores C, González S, Cereijido M, and Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. (1996) 134:1031–49. doi: 10.1083/jcb.134.4.1031
349. Tsukita S, Furuse M, and Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. (1999) 11:628–33. doi: 10.1016/s0955-0674(99)00016-2
350. Tsukita S, Furuse M, and Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. (2001) 2:285–93. doi: 10.1038/35067088
351. Wong AST and Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. (2003) 161:1191–203. doi: 10.1083/jcb.200212033
352. Greenwood J, Amos CL, Walters CE, Couraud P-O, Lyck R, Engelhardt B, et al. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. (2003) 171:2099–108. doi: 10.4049/jimmunol.171.4.2099
353. Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, and Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. (1991) 147:2913–21. doi: 10.4049/jimmunol.147.9.2913
354. Abbott NJ, Rönnbäck L, and Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. (2006) 7:41–53. doi: 10.1038/nrn1824
355. del Zoppo GJ and Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. (2006) 26:1966–75. doi: 10.1161/01.ATV.0000232525.65682.a2
356. Baeten KM and Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. (2011) 71:1018–39. doi: 10.1002/dneu.20954
357. Alvarez JI, Cayrol R, and Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. (2011) 1812:252–64. doi: 10.1016/j.bbadis.2010.06.017
358. Larochelle C, Alvarez JI, and Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. (2011) 585:3770–80. doi: 10.1016/j.febslet.2011.04.066
359. Weiss N, Miller F, Cazaubon S, and Couraud P-O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. (2009) 1788:842–57. doi: 10.1016/j.bbamem.2008.10.022
360. Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. (2011) 193:565–82. doi: 10.1083/jcb.201010065
361. Jia JP, Meng R, Sun YX, Sun WJ, Ji XM, and Jia LF. Cerebrospinal fluid tau, Abeta1-42 and inflammatory cytokines in patients with Alzheimer’s disease and vascular dementia. Neurosci Lett. (2005) 383:12–6. doi: 10.1016/j.neulet.2005.03.051
362. Wen H, Watry DD, Marcondes MCG, and Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. (2004) 24:8408–17. doi: 10.1128/MCB.24.19.8408-8417.2004
363. Souza PS, Gonçalves ED, Pedroso GS, Farias HR, Junqueira SC, Marcon R, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol Neurobiol. (2017) 54:4723–37. doi: 10.1007/s12035-016-0014-0
364. Schreibelt G, Musters RJP, Reijerkerk A, de Groot LR, van der Pol SMA, Hendrikx EML, et al. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. (2006) 177:2630–7. doi: 10.4049/jimmunol.177.4.2630
365. Ramirez SH, Fan S, Dykstra H, Rom S, Mercer A, Reichenbach NL, et al. Inhibition of glycogen synthase kinase 3β promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. PloS One. (2013) 8:e55972. doi: 10.1371/journal.pone.0055972
366. Isla AG, Vázquez-Cuevas FG, and Peña-Ortega F. Exercise prevents amyloid-β-induced hippocampal network disruption by inhibiting GSK3β Activation. J Alzheimers Dis. (2016) 52:333–43. doi: 10.3233/JAD-150352
367. Ramirez SH, Fan S, Zhang M, Papugani A, Reichenbach N, Dykstra H, et al. Inhibition of glycogen synthase kinase 3beta (GSK3beta) decreases inflammatory responses in brain endothelial cells. Am J Pathol. (2010) 176:881–92. doi: 10.2353/ajpath.2010.090671
368. Nowak AK, Newton RU, Cruickshank T, Cormie P, Halkett GKB, Tsoi D, et al. A feasibility, safety, and efficacy evaluation of supervised aerobic and resistance exercise for patients with glioblastoma undertaking adjuvant chemoradiotherapy. Neuro-oncol Pract. (2023) 10:261–70. doi: 10.1093/nop/npad006
369. Jost J, Völker K, Brandt R, Stummer W, Urbschat S, Ketter R, et al. Maximal cardiopulmonary exercise testing in glioblastoma patients undergoing chemotherapy: assessment of feasibility, safety, and physical fitness status. J Neurooncol. (2024) 168:35–45. doi: 10.1007/s11060-024-04629-y
370. Costa AK, Marqueze LFB, Gattiboni BB, Pedroso GS, Vasconcellos FF, Cunha EBB, et al. Physical training protects against brain toxicity in mice exposed to an experimental model of glioblastoma. Neurochem Res. (2022) 47:3344–54. doi: 10.1007/s11064-022-03685-y
371. Huang C-W, Chang Y-H, Lee H-H, Wu J-Y, Huang J-X, Chung Y-H, et al. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J Off Publ Fed Am Soc Exp Biol. (2020) 34:9678–93. doi: 10.1096/fj.202000573RR
372. Stone TW, Forrest CM, Mackay GM, Stoy N, and Darlington LG. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. (2007) 22:337–52. doi: 10.1007/s11011-007-9064-3
373. Shimizu T, Nomiyama S, Hirata F, and Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. (1978) 253:4700–6. doi: 10.1016/S0021-9258(17)30447-7
374. Ball HJ, Sanchez-Perez A, Weiser S, Austin CJD, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. (2007) 396:203–13. doi: 10.1016/j.gene.2007.04.010
375. Stone TW. Kynurenic acid blocks nicotinic synaptic transmission to hippocampal interneurons in young rats. Eur J Neurosci. (2007) 25:2656–65. doi: 10.1111/j.1460-9568.2007.05540.x
376. Sas K, Robotka H, Toldi J, and Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. (2007) 257:221–39. doi: 10.1016/j.jns.2007.01.033
377. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. (2012) 279:1356–65. doi: 10.1111/j.1742-4658.2012.08485.x
378. Cervenka I, Agudelo LZ, and Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. (2017) 357. doi: 10.1126/science.aaf9794
379. Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. (2001) 78:842–53. doi: 10.1046/j.1471-4159.2001.00498.x
380. Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, and Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. (1989) 1012:140–7. doi: 10.1016/0167-4889(89)90087-6
381. Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. (2016) 310:C836–40. doi: 10.1152/ajpcell.00053.2016
382. András IE, Deli MA, Veszelka S, Hayashi K, Hennig B, and Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. (2007) 27:1431–43. doi: 10.1038/sj.jcbfm.9600445
383. Beggiato S, Antonelli T, Tomasini MC, Tanganelli S, Fuxe K, Schwarcz R, et al. Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur J Neurosci. (2013) 37:1470–7. doi: 10.1111/ejn.12160
384. Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, and Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. (2001) 21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001
385. Konradsson-Geuken A, Wu HQ, Gash CR, Alexander KS, Campbell A, Sozeri Y, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. (2010) 169:1848–59. doi: 10.1016/j.neuroscience.2010.05.052
386. Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, and Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflamm. (2015) 12:85. doi: 10.1186/s12974-015-0306-8
387. Winklewski PJ, Radkowski M, and Demkow U. Neuroinflammatory mechanisms of hypertension: potential therapeutic implications. Curr Opin Nephrol Hypertens. (2016) 25:410–6. doi: 10.1097/MNH.0000000000000250
388. Biancardi VC and Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol. (2016) 594:1591–600. doi: 10.1113/JP271584
389. Carey RM, Wang ZQ, and Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertens (Dallas Tex 1979). (2000) 35:155–63. doi: 10.1161/01.hyp.35.1.155
390. Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, and Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. (2003) 35:881–900. doi: 10.1016/s1357-2725(02)00271-6
391. Muller DN, Shagdarsuren E, Park J-K, Dechend R, Mervaala E, Hampich F, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. (2002) 161:1679–93. doi: 10.1016/S0002-9440(10)64445-8
392. Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertens (Dallas Tex 1979). (2010) 56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409
393. Waki H, Gouraud SS, Maeda M, Raizada MK, and Paton JFR. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir Physiol Neurobiol. (2011) 178:422–8. doi: 10.1016/j.resp.2011.05.004
394. Zubcevic J, Waki H, Raizada MK, and Paton JFR. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertens (Dallas Tex 1979). (2011) 57:1026–33. doi: 10.1161/HYPERTENSIONAHA.111.169748
395. Ito H, Takemori K, Kawai J, and Suzuki T. AT1 receptor antagonist prevents brain edema without lowering blood pressure. Acta Neurochir Suppl. (2000) 76:141–5. doi: 10.1007/978-3-7091-6346-7_29
396. de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, and Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. (1997) 49:143–55. doi: 10.1016/S0031-6997(24)01320-6
397. Zhang M, Mao Y, Ramirez SH, Tuma RF, and Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. (2010) 171:852–8. doi: 10.1016/j.neuroscience.2010.09.029
398. Pan Y-X, Gao L, Wang W-Z, Zheng H, Liu D, Patel KP, et al. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertens (Dallas Tex 1979). (2007) 49:519–27. doi: 10.1161/01.HYP.0000256955.74461.93
399. Agarwal D, Welsch MA, Keller JN, and Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol. (2011) 106:1069–85. doi: 10.1007/s00395-011-0231-7
400. Chaar LJ, Alves TP, Batista Junior AM, and Michelini LC. Early training-induced reduction of angiotensinogen in autonomic areas-the main effect of exercise on brain renin-angiotensin system in hypertensive rats. PloS One. (2015) 10:e0137395. doi: 10.1371/journal.pone.0137395
401. Jia L-L, Kang Y-M, Wang F-X, Li H-B, Zhang Y, Yu X-J, et al. Exercise training attenuates hypertension and cardiac hypertrophy by modulating neurotransmitters and cytokines in hypothalamic paraventricular nucleus. PloS One. (2014) 9:e85481. doi: 10.1371/journal.pone.0085481
402. Negrão CE, Moreira ED, Brum PC, Denadai ML, and Krieger EM. Vagal and sympathetic control of heart rate during exercise by sedentary and exercise-trained rats. Braz J Med Biol Res = Rev Bras Pesqui Medicas e Biol. (1992) 25:1045–52.
403. Sugawara J, Murakami H, Maeda S, Kuno S, and Matsuda M. Change in post-exercise vagal reactivation with exercise training and detraining in young men. Eur J Appl Physiol. (2001) 85:259–63. doi: 10.1007/s004210100443
404. Buttler L, Jordão MT, Fragas MG, Ruggeri A, Ceroni A, and Michelini LC. Maintenance of blood-brain barrier integrity in hypertension: A novel benefit of exercise training for autonomic control. Front Physiol. (2017) 8:1048. doi: 10.3389/fphys.2017.01048
405. Meshorer E, Biton IE, Ben-Shaul Y, Ben-Ari S, Assaf Y, Soreq H, et al. Chronic cholinergic imbalances promote brain diffusion and transport abnormalities. FASEB J. (2005) 19:910–22. doi: 10.1096/fj.04-2957com
406. Nishihara M, Hirooka Y, Matsukawa R, Kishi T, and Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. (2012) 30:97–106. doi: 10.1097/HJH.0b013e32834e1df4
407. Muratani H, Averill DB, and Ferrario CM. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am J Physiol. (1991) 260:R977–84. doi: 10.1152/ajpregu.1991.260.5.R977
408. Vieira AA, Colombari E, De Luca LAJ, Colombari DSA, De Paula PM, and Menani JV. Importance of angiotensinergic mechanisms for the pressor response to l-glutamate into the rostral ventrolateral medulla. Brain Res. (2010) 1322:72–80. doi: 10.1016/j.brainres.2010.01.066
409. Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RRJ, and Lopes OU. Role of the medulla oblongata in hypertension. Hypertens (Dallas Tex 1979). (2001) 38:549–54. doi: 10.1161/01.hyp.38.3.549
410. Kishi T, Hirooka Y, and Sunagawa K. Sympathoinhibition caused by orally administered telmisartan through inhibition of the AT1 receptor in the rostral ventrolateral medulla of hypertensive rats. Hypertens Res. (2012) 35:940–6. doi: 10.1038/hr.2012.63
411. Mills E, Minson J, Drolet G, and Chalmers J. Effect of intrathecal amino acid receptor antagonists on basal blood pressure and pressor responses to brainstem stimulation in normotensive and hypertensive rats. J Cardiovasc Pharmacol. (1990) 15:877–83. doi: 10.1097/00005344-199006000-00004
412. Schreurs J, Seelig T, and Schulman H. Beta 2-adrenergic receptors on peripheral nerves. J Neurochem. (1986) 46:294–6. doi: 10.1111/j.1471-4159.1986.tb12961.x
413. Braun V and Clarke V. What can “thematic analysis” offer health and wellbeing researchers? Int J Qual Stud Health Well-being. (2014) 9:26152. doi: 10.3402/qhw.v9.26152
414. Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, et al. Locus ceruleus norepinephrine release: A central regulator of CNS spatio-temporal activation? Front Synaptic Neurosci. (2016) 8:25. doi: 10.3389/fnsyn.2016.00025
415. Chandler DJ. Evidence for a specialized role of the locus coeruleus noradrenergic system in cortical circuitries and behavioral operations. Brain Res. (2016) 1641:197–206. doi: 10.1016/j.brainres.2015.11.022
416. Feinstein DL, Kalinin S, and Braun D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system. J Neurochem. (2016) 139 Suppl:154–78. doi: 10.1111/jnc.13447
417. O’Donnell J, Zeppenfeld D, McConnell E, Pena S, and Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. (2012) 37:2496–512. doi: 10.1007/s11064-012-0818-x
418. Hetier E, Ayala J, Bousseau A, and Prochiantz A. Modulation of interleukin-1 and tumor necrosis factor expression by beta-adrenergic agonists in mouse ameboid microglial cells. Exp Brain Res. (1991) 86:407–13. doi: 10.1007/BF00228965
419. Frohman EM, Vayuvegula B, van den Noort S, and Gupta S. Norepinephrine inhibits gamma-interferon-induced MHC class II (Ia) antigen expression on cultured brain astrocytes. J Neuroimmunol. (1988) 17:89–101. doi: 10.1016/0165-5728(88)90017-3
420. Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. (2012) 26:195–200. doi: 10.1016/j.bbi.2011.08.001
421. Huang H-W, Fang X-X, Wang X-Q, Peng Y-P, and Qiu Y-H. Regulation of differentiation and function of helper T cells by lymphocyte-derived catecholamines via α1- and β2-adrenoceptors. Neuroimmunomodulation. (2015) 22:138–51. doi: 10.1159/000360579
422. Szalewska D, Radkowski M, Demkow U, and Winklewski PJ. Exercise strategies to counteract brain aging effects. Adv Exp Med Biol. (2017) 1020:69–79. doi: 10.1007/5584_2017_3
423. Juric DM, Loncar D, and Carman-Krzan M. Noradrenergic stimulation of BDNF synthesis in astrocytes: mediation via alpha1- and beta1/beta2-adrenergic receptors. Neurochem Int. (2008) 52:297–306. doi: 10.1016/j.neuint.2007.06.035
424. Zafra F, Lindholm D, Castrén E, Hartikka J, and Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci Off J Soc Neurosci. (1992) 12:4793–9. doi: 10.1523/JNEUROSCI.12-12-04793.1992
425. Schurr A, West CA, and Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. (1988) 240:1326–8. doi: 10.1126/science.3375817
426. van Hall G, Strømstad M, Rasmussen P, Jans O, Zaar M, Gam C, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. (2009) 29:1121–9. doi: 10.1038/jcbfm.2009.35
427. Benarroch EE. Glycogen metabolism: metabolic coupling between astrocytes and neurons. Neurology. (2010) 74:919–23. doi: 10.1212/WNL.0b013e3181d3e44b
428. Fillenz M, Lowry JP, Boutelle MG, and Fray AE. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol Scand. (1999) 167:275–84. doi: 10.1046/j.1365-201x.1999.00578.x
429. Hertz L, Lovatt D, Goldman SA, and Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. (2010) 57:411–20. doi: 10.1016/j.neuint.2010.03.019
430. Pellerin L, Bouzier-Sore A-K, Aubert A, Serres S, Merle M, Costalat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. (2007) 55:1251–62. doi: 10.1002/glia.20528
431. Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. (2010) 2:33ra37. doi: 10.1126/scitranslmed.3001006
432. Delezie J and Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol. (2018) 9:698. doi: 10.3389/fneur.2018.00698
433. Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. (1998) 20:291–9. doi: 10.1159/000017324
434. Bergersen L, Rafiki A, and Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. (2002) 27:89–96. doi: 10.1023/a:1014806723147
435. Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. (2015) 35:176–85. doi: 10.1038/jcbfm.2014.206
436. Barros LF. Metabolic signaling by lactate in the brain. Trends Neurosci. (2013) 36:396–404. doi: 10.1016/j.tins.2013.04.002
437. Ruan G-X and Kazlauskas A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J Biol Chem. (2013) 288:21161–72. doi: 10.1074/jbc.M113.474619
438. Todd RD and Botteron KN. Is attention-deficit/hyperactivity disorder an energy deficiency syndrome? Biol Psychiatry. (2001) 50:151–8. doi: 10.1016/s0006-3223(01)01173-8
439. Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, et al. Response variability in Attention-Deficit/Hyperactivity Disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct. (2006) 2:30. doi: 10.1186/1744-9081-2-30
440. Medin T, Medin H, Hefte MB, Storm-Mathisen J, and Bergersen LH. Upregulation of the lactate transporter monocarboxylate transporter 1 at the blood-brain barrier in a rat model of attention-deficit/hyperactivity disorder suggests hyperactivity could be a form of self-treatment. Behav Brain Res. (2019) 360:279–85. doi: 10.1016/j.bbr.2018.12.023
441. Zhang C, Ge H, Zhang S, Liu D, Jiang Z, Lan C, et al. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol Ther. (2021) 10:1001–13. doi: 10.1007/s40120-021-00279-8
442. Feng H, Qiao Q-C, Luo Q-F, Zhou J-Y, Lei F, Chen Y, et al. Orexin neurons to sublaterodorsal tegmental nucleus pathway prevents sleep onset REM sleep-like behavior by relieving the REM sleep pressure. Res (Washington DC). (2024) 7:355. doi: 10.34133/research.0355
443. Zhang Y, Liu C, Chen X, Zhang Y, Li Y, and Hu X. Effects of web-based acceptance and commitment therapy on health-related outcomes among patients with lung cancer: A feasibility randomized controlled trial. Psychooncology. (2024) 33:e70045. doi: 10.1002/pon.70045
444. Wu X, Fu M, Ge C, Zhou H, Huang H, Zhong M, et al. m(6)A-Mediated Upregulation of lncRNA CHASERR Promotes the Progression of Glioma by Modulating the miR-6893-3p/TRIM14 Axis. Mol Neurobiol. (2024) 61:5418–40. doi: 10.1007/s12035-023-03911-w
445. Weatherwax R, Harris N, Kilding AE, and Dalleck L. Time course changes in confirmed “True” VO (2) max after individualized and standardized training. Sport Med Int Open. (2019) 3:E32–9. doi: 10.1055/a-0867-9415
446. Yang H, Zhou H, Fu M, Xu H, Huang H, Zhong M, et al. TMEM64 aggravates the Malignant phenotype of glioma by activating the Wnt/β-catenin signaling pathway. Int J Biol Macromol. (2024) 260:129332. doi: 10.1016/j.ijbiomac.2024.129332
447. Sun W, Jang M-S, Zhan S, Liu C, Sheng L, Lee JH, et al. Tumor-targeting and redox-responsive photo-cross-linked nanogel derived from multifunctional hyaluronic acid-lipoic acid conjugates for enhanced in vivo protein delivery. Int J Biol Macromol. (2025) 314:144444. doi: 10.1016/j.ijbiomac.2025.144444
448. Yang N, Lai Y, Yu G, Zhang X, Shi J, Xiang L, et al. METTL3-dependent m(6)A modification of SNAP29 induces “autophagy-mitochondrial crisis” in the ischemic microenvironment after soft tissue transplantation. Autophagy. (2025), 1–24. doi: 10.1080/15548627.2025.2493455
449. Chen Y, Liu R, Cai W, Jiang L, Chen K, Shi J, et al. Davunetide promotes structural and functional recovery of the injured spinal cord by promoting autophagy. Neural Regener Res. (2025). doi: 10.4103/NRR.NRR-D-24-00154
450. Yin M, Feng C, Yu Z, Zhang Y, Li Y, Wang X, et al. sc2GWAS: a comprehensive platform linking single cell and GWAS traits of human. Nucleic Acids Res. (2025) 53:D1151–61. doi: 10.1093/nar/gkae1008
451. Perez K, Wisniewski D, Ari A, Lee K, Lieneck C, and Ramamonjiarivelo Z. Investigation into application of AI and telemedicine in rural communities: A systematic literature review. Healthc (Basel Switzerland). (2025) 13. doi: 10.3390/healthcare13030324
452. Iranmanesh Y, Jiang B, Favour OC, Dou Z, Wu J, Li J, et al. Mitochondria’s role in the maintenance of cancer stem cells in glioblastoma. Front Oncol. (2021) 11:582694. doi: 10.3389/fonc.2021.582694
453. Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH, Kong HK, et al. Inhibition of aerobic glycolysis represses akt/mTOR/HIF-1α Axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PloS One. (2015) 10:e0132285. doi: 10.1371/journal.pone.0132285
454. Yang J, Chen Y, Jing Y, Green MR, and Han L. Advancing CAR T cell therapy through the use of multidimensional omics data. Nat Rev Clin Oncol. (2023) 20:211–28. doi: 10.1038/s41571-023-00729-2
455. Lteif C, Huang Y, Guerra LA, Gawronski BE, and Duarte JD. Using omics to identify novel therapeutic targets in heart failure. Circ Genomic Precis Med. (2024) 17:e004398. doi: 10.1161/CIRCGEN.123.004398
456. Sun J, Yu X, Li W, Jia B, Shi D, Song Y, et al. Real-time accurate detection and analysis of breath acetone using CRDS: Toward metabolic dynamic monitoring and potential application. Sensors Actuators B Chem. (2025) 433:137422. doi: 10.1016/j.snb.2025.137422
457. Firuzpour F, Barancheshmeh M, Ziarani FF, Karami L, and Aram C. The HER2 target for designing novel multi-peptide vaccine against breast cancer using immunoinformatics and molecular dynamic simulation. Biochem Biophys Rep. (2025) 43:102135. doi: 10.1016/j.bbrep.2025.102135
Keywords: glioblastoma, physical activity, neuro-oncology, brain tumor therapy, neuroplasticity, tumor microenvironment
Citation: Xie L and Wang F (2025) Physical activity and glioblastoma: a paradigm shift in neuro-oncology therapy. Front. Oncol. 15:1638060. doi: 10.3389/fonc.2025.1638060
Received: 30 May 2025; Accepted: 14 July 2025;
Published: 30 July 2025.
Edited by:
Matiullah Khan, AIMST University, MalaysiaReviewed by:
Shazia Tahira, Bahria University, Karachi, PakistanXin Wang, Guangdong Pharmaceutical University, China
Copyright © 2025 Xie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, RmVuZy53YW5nMjAwMjBAZ21haWwuY29t
 Lin Xie
Lin Xie Feng Wang
Feng Wang