- 1McGill University, Montreal, QC, Canada
- 2Astellas Pharma Europe, Addlestone, United Kingdom
- 3Global Health Economics & Outcomes Research (HEOR), Astellas Pharma, IL, United States
- 4Global Medical Affairs, Astellas Pharma, IL, United States
- 5IQVIA Ltd, Athens, Greece
- 6IQVIA Commercial GmbH & Co OHG, Munich, Germany
- 7Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 8Durham Veterans Affairs (VA) Medical Center, Durham, NC, United States
Introduction: Enzalutamide is the only androgen receptor pathway inhibitor approved by the United States Food and Drug Administration and the European Medicines Agency to treat high-risk biochemically recurrent non-metastatic hormone-sensitive prostate cancer. The objective of this network meta-analysis was to provide indirect evidence of the efficacy of enzalutamide relative to other therapies for biochemical recurrence after definitive therapy.
Materials and Methods: We conducted a systematic literature review to identify trials that assessed the efficacy and safety of current and emerging interventions. Outcomes of interest were metastasis-free survival, overall survival, time to prostate-specific antigen progression, time to castration resistance, proportion of patients with prostate-specific antigen <0.2 ng/ml at 36 (± 4) weeks of treatment, and grade ≥3 treatment-related adverse events. Fixed- and random-effects models were run under the Bayesian framework.
Results: Enzalutamide with androgen-deprivation therapy (i.e., combination therapy) demonstrated superiority over most comparators for overall survival (except androgen-deprivation therapy + docetaxel, which was similar), and over all comparators for metastasis-free survival, time to prostate-specific antigen progression, and time to castration resistance. Enzalutamide combination therapy demonstrated superiority over enzalutamide monotherapy for all efficacy outcomes, and similar performance for safety. Enzalutamide monotherapy demonstrated superiority over androgen-deprivation therapy alone and androgen-deprivation therapy + docetaxel for metastasis-free survival and time to prostate-specific antigen progression. Treatment-related adverse events were least common for androgen-deprivation therapy alone.
Discussion: This network meta-analysis provides evidence that enzalutamide combination therapy provides considerable oncological benefit in high-risk biochemically recurrent non-metastatic hormone-sensitive prostate cancer, albeit with a higher risk of treatment-related adverse events.
1 Introduction
Approximately one-third of patients who receive primary localized treatment for prostate cancer experience biochemical recurrence within 10 years (1, 2). This recurrence is characterized by a rise in prostate-specific antigen (PSA) levels and is associated with a higher risk of worse clinical outcomes (2). To determine the best treatment approach for patients with biochemical recurrence, risk classification systems have been developed, as described in the American Society of Clinical Oncology Clinical Practice Guidelines (2021) (3) and prostate cancer guidelines from the European Association of Urology-European Association of Nuclear Medicine-European Society for Radiotherapy and Oncology-European Society of Urogenital Radiology-International Society of Geriatric Oncology (EAU-EANM-ESTRO-ESUR-SIOG) (4). According to these guidelines, patients are classified as having high-risk biochemical recurrence after radical prostatectomy if they have a PSA doubling time of <10–12 months or a Gleason score of 8–10, whereas high-risk biochemical recurrence after radiotherapy (RT) requires an interval from primary therapy to biochemical failure of <18 months or an initial biopsy Gleason score of 8–10 (3, 4). By contrast, guidelines from the American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology (AUA/ASTRO/SUO) (2024) define patients at high risk of developing metastasis with biochemical recurrence after local treatment as those with a PSA doubling time of <12 months, a shorter time to biochemical recurrence, or a higher grade or stage of disease (5, 6).
Before the EMBARK trial, specific treatment guidelines for patients with high-risk biochemically recurrent (BCR) non-metastatic hormone-sensitive prostate cancer (nmHSPC, also known as non-metastatic castration-sensitive prostate cancer [nmCSPC]) were limited and variable (1, 3, 7). Patients with nmHSPC have no detectable metastases according to conventional imaging, but if left untreated, many will eventually develop metastatic disease that can be seen on conventional imaging (8). While some guidelines recommend observation, others recommend continuous androgen-deprivation therapy (ADT), and yet others recommend intermittent ADT (i.e., an ADT regimen that includes periods of treatment suspension between ADT cycles in the absence of signs of biochemical or clinical progression) (9, 10). Although previous studies had demonstrated that enzalutamide in combination with ADT improved survival in patients with mHSPC (11, 12), little research had been conducted into the effects of this combination therapy in patients with nmHSPC. To address this gap, EMBARK—a phase 3, three-arm randomized trial that included 1068 patients—was conducted to evaluate the use of enzalutamide for high-risk BCR nmHSPC (13–15). The results of EMBARK demonstrated that enzalutamide with or without ADT was associated with improved oncological outcomes, with no new observed safety signals or decrease in quality of life (13, 16).
Based on the findings of EMBARK, enzalutamide is now the only androgen receptor pathway inhibitor approved by the United States Food and Drug Administration (FDA) (17) and European Medicines Agency (18) for the treatment of high-risk BCR nmHSPC, and it is specifically named as a preferred systemic treatment option for high-risk BCR nmHSPC by the National Comprehensive Cancer Network® (NCCN) (10) and the European Association of Urology (9). However, other novel therapies may also have potential benefits in the treatment of BCR nmHSPC. Therefore, the objective of this network meta-analysis (NMA) was to provide indirect evidence of the relative efficacy of enzalutamide versus current and emerging systemic therapies for the management of patients with high-risk BCR nmHSPC whose disease has progressed after definitive therapy.
2 Materials and methods
We conducted a systematic literature review (SLR) to form the evidence base for the NMA. Searches were conducted on April 13, 2022, and October 3, 2023. Articles and conference abstracts were obtained by searching the Embase®, MEDLINE®, and Cochrane® databases. Search strategies are presented in Supplementary Tables 1A–E. We also conducted manual searches on October 3, 2023, of conference records, trial registries, and reference lists of existing systematic literature reviews. A protocol was not prepared for this review. This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
2.1 Eligibility
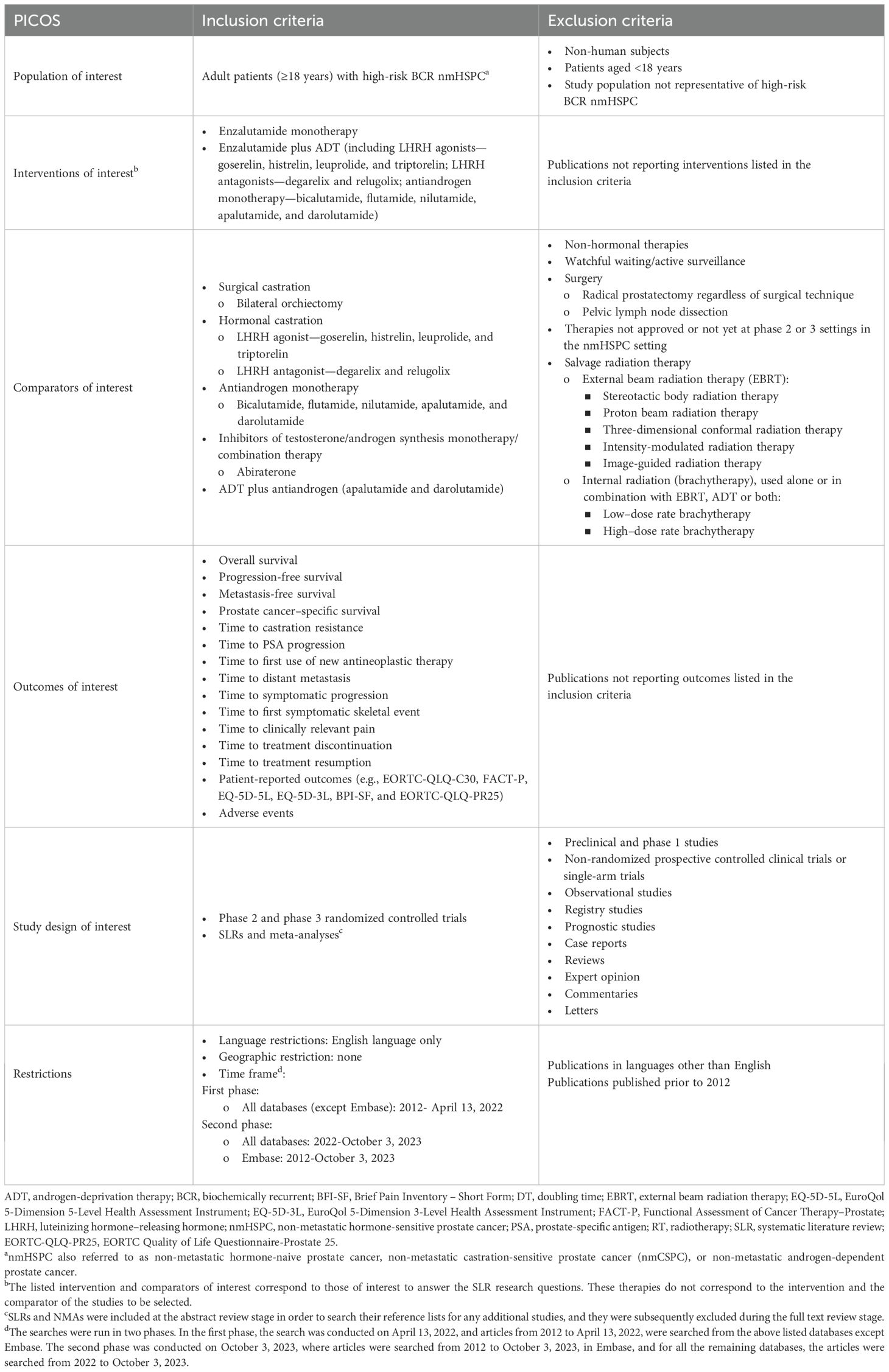
Eligible trials assessed the efficacy and safety of systemic drug interventions in adult patients with high-risk BCR nmHSPC (Table 1). Eligible patients were adults who (a) had been diagnosed with histologically or cytologically confirmed adenocarcinoma of the prostate at initial biopsy, (b) had previously received definitive therapy, (c) had non-metastatic disease according to bone scan or computed tomography/magnetic resonance imaging assessment, (d) were ineligible for salvage RT (SRT), and (e) had increasing PSA levels with features of high-risk disease. Eligibility criteria for patients were intentionally broad to account for variability in how patients were selected across trials. Eligible interventions were those suggested for high-risk BCR nmHSPC following definitive therapy, which therefore included regimens of hormonal therapies with or without chemotherapy, and could include either continuous or intermittent ADT, as well as other pharmacological agents assessed for use in high-risk BCR nmHSPC. Interventions using SRT were excluded as SRT is generally recommended for patients with relatively low PSA levels (5), and some patients with biochemical recurrence may therefore be unsuitable for it.3Interventions that focused on supplements or lifestyle changes were also excluded.
2.2 Screening and data extraction
Records were screened for eligibility by two independent researchers. Disagreements were resolved by a third independent reviewer. Data were extracted by one researcher into a data extraction form, and a quality check was performed by a second independent reviewer.
2.3 Feasibility assessment
A feasibility assessment was conducted for each outcome of interest to determine whether an NMA was possible for the outcome. Efficacy outcomes of interest were overall survival (OS), metastasis-free survival (MFS), time to PSA progression, time to castration resistance, and the proportion of patients who achieved PSA <0.2 ng/ml at 36 (± 4) weeks of treatment. To compare time to PSA progression across studies, studies that evaluated time to PSA progression and those that covered PSA progression-free survival were both included for this outcome, as these terms are often used interchangeably. The safety endpoint of interest was the occurrence of any type of grade ≥3 treatment-related adverse events (TRAEs). The feasibility assessment included an evaluation of heterogeneity across the trials identified in the SLR (e.g., study design, population, comparators, outcome definitions, and patient baseline characteristics) and an appraisal of whether sufficient consistent data were available for each outcome of interest. Risk of bias was assessed by one researcher using the Cochrane Risk of Bias 2 (RoB-2) tool, and a quality check was performed by a second independent reviewer.
2.4 Statistical analyses
Statistical analyses were conducted under the Bayesian framework (19) and run with R (version 4.2.1 or higher) (20) using the “multinma” package (21). Four Markov chain Monte Carlo chains with different starting values were used for all models, with a burn-in of at least 2000 iterations and a further sample of at least 10,000 iterations. Fixed- and random-effects models were run, with the latter accounting for heterogeneity among studies. Given the small number of studies informing each treatment comparison in the networks of evidence, fixed-effects models were used for inference.
The proportional hazards assumption was evaluated for the EMBARK trial using visual inspection of log-cumulative hazard plots, the Grambsch–Therneau statistical test for the scaled Schoenfeld residuals, and visual inspection of Schoenfeld residuals. As all other trials in the NMA reported constant hazard ratios based on Cox proportional hazards models, the proportional hazards assumption was assumed to be met.
Where appropriate, sensitivity analyses were conducted to evaluate the influence of studies with a high risk of bias according to the RoB-2 tool or where the feasibility assessment identified potential sources of clinical or methodological heterogeneity. The feasibility of conducting subgroup analyses was explored but was ultimately not deemed viable due to the low number of studies reporting subgroup results.
2.5 Ethics statement
Ethics clearance was not required for this study as all analyses were conducted on previously published or presented data.
3 Results
A total of 3560 citations were identified in the SLR, from which 16 publications or presentations based on 10 eligible trials were included in the NMA (Figure 1). The characteristics of the 10 trials are presented in Table 2, and trial design details are presented in Supplementary Table 2.
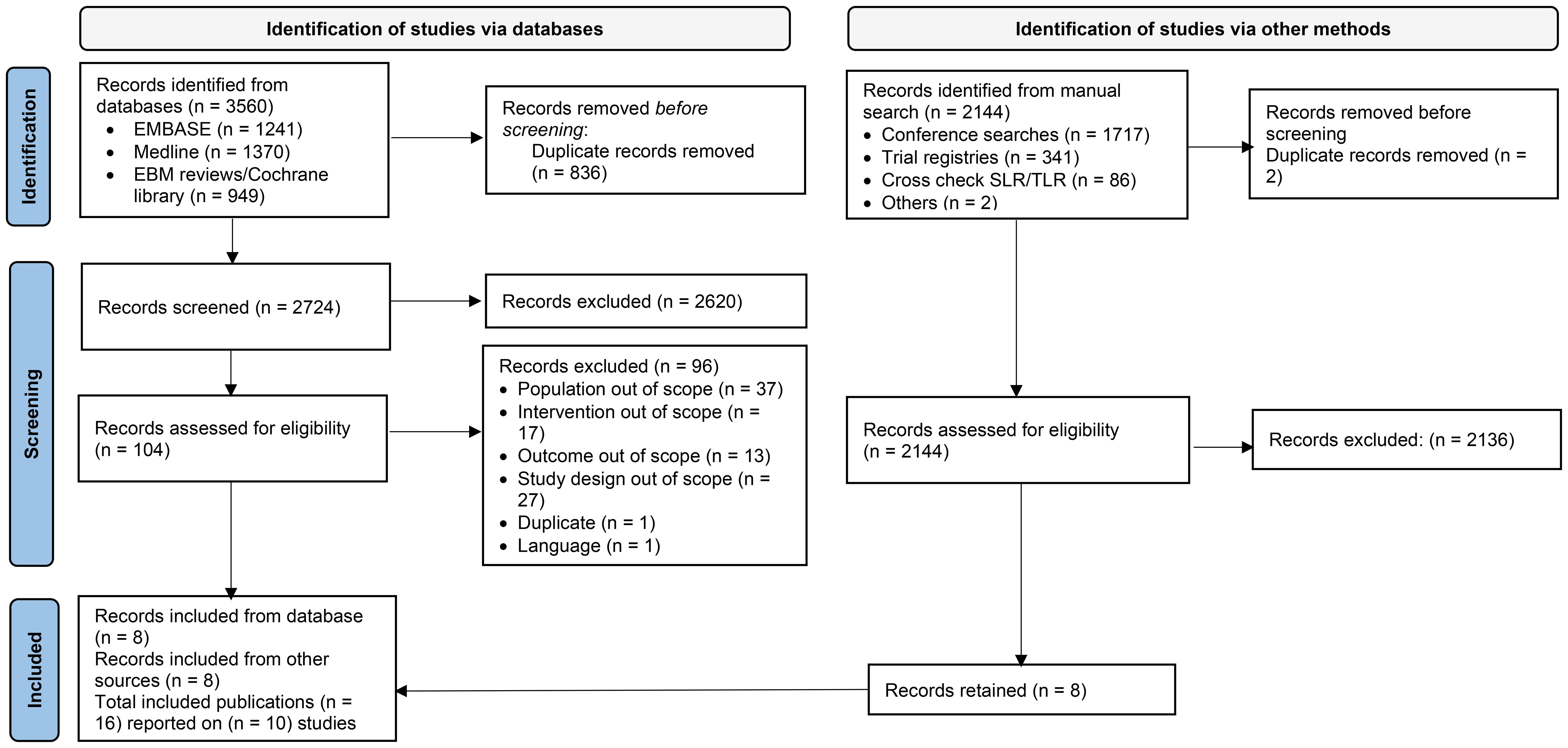
Figure 1. PRISMA flow diagram of study selection process. EBM, evidence-based medicine; SLR, systematic literature review; TLR, targeted literature review.
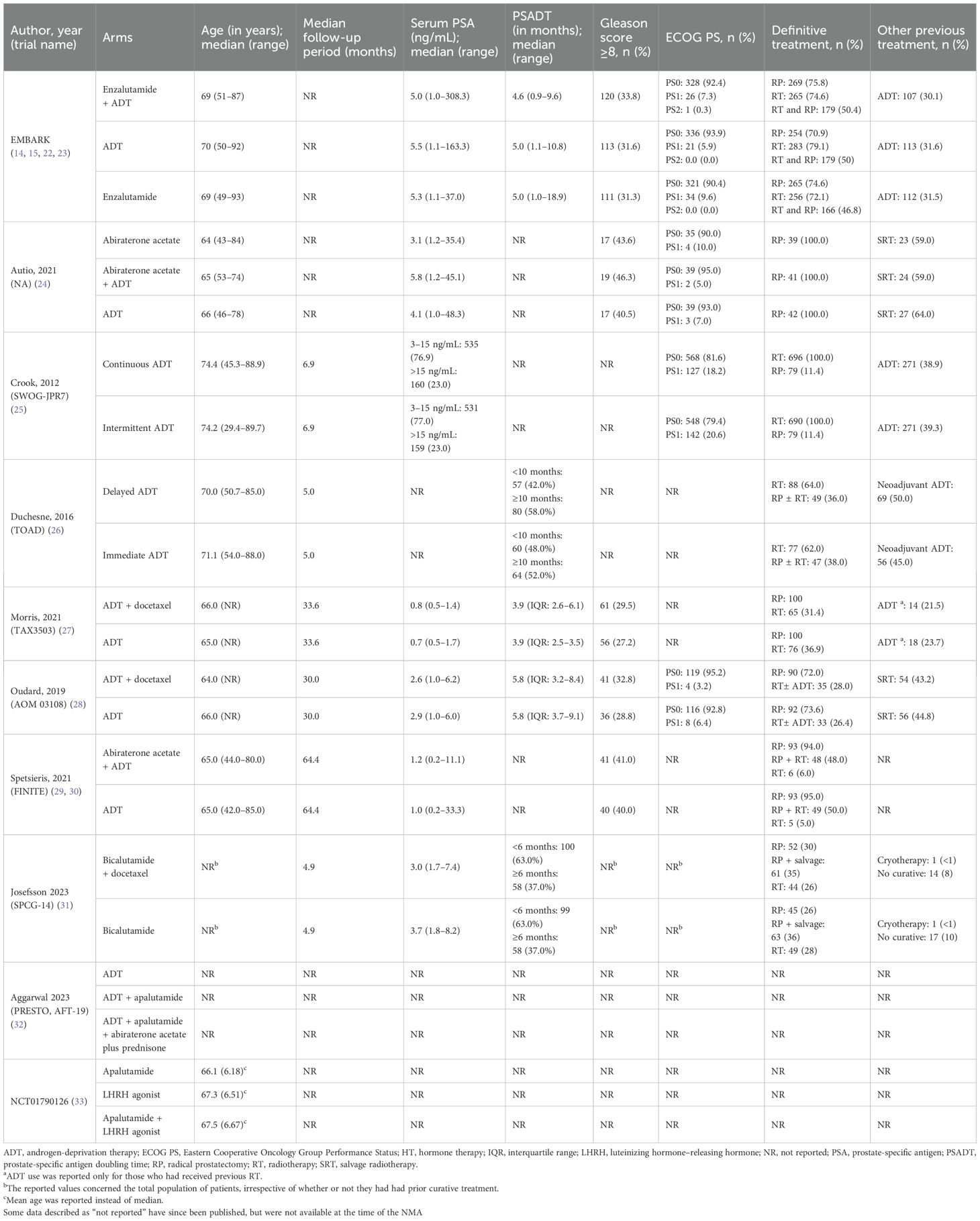
In terms of heterogeneity, baseline patient characteristics and duration of follow-up were generally comparable between studies (Table 2). Patients’ median age ranged from 64–74 years. Median serum PSA was lowest among patients in the Morris 2021 study (ADT alone: 0.7; ADT + docetaxel: 0.8). Follow-up periods ranged from 4.9 months to 64.4 months. The availability of each outcome is presented in Supplementary Table 3, and networks of evidence are presented in Supplementary Figures 1A–F. Outcome definitions were generally consistent across trials. In all trials, OS was defined as the time from randomization to death from any cause. For time to PSA progression, variations were noted in PSA progression thresholds across the included trials. Time to castration resistance was defined as “three increases in the PSA level at least 1 month apart or evidence of new clinical disease while the patient was receiving ADT and the testosterone was at castrate levels” in two studies (14, 25) and as “a rise in PSA while on ADT” in one study (26). For the proportion of patients with PSA < 0.2 ng/ml, EMBARK assessed serum PSA at 36 weeks (14, 15, 22, 23), while Autio et al. (2021) assessed serum PSA at 32 weeks (24). Finally, safety events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) versions 3.0 (34), 4.0 (35), or 4.03, with the exception of the SWOG-JPR7 study, where the methods for assessing adverse events (AEs) were not specified. For the purposes of this NMA, AEs in the trial by Morris et al. (27) that were not specified as “treatment emergent” but were presented by treatment arm were assumed to be treatment related, as no “unexpected” grade ≥3 AEs were reported.
In the evaluation of the proportional hazards assumption for the EMBARK trial, no pattern was identified in the Schoenfeld residual plot over time, indicating no evidence against the null hypothesis of proportional hazards for OS and MFS. Similarly, no violations of the proportional hazards assumption were identified for time to PSA progression or time to castration resistance. The results of the proportional hazards evaluation are presented in Supplementary Figure 2.
The results of the risk-of-bias assessment are presented in Supplementary Table 4. Most studies were deemed to be low risk or to have some concerns.
3.1 Efficacy results
Overall results for the relative efficacy of enzalutamide compared with other treatments using the fixed-effects model are presented in Tables 3A and 3B. Results of the random-effects model are presented in Supplementary Tables 5 and 6.
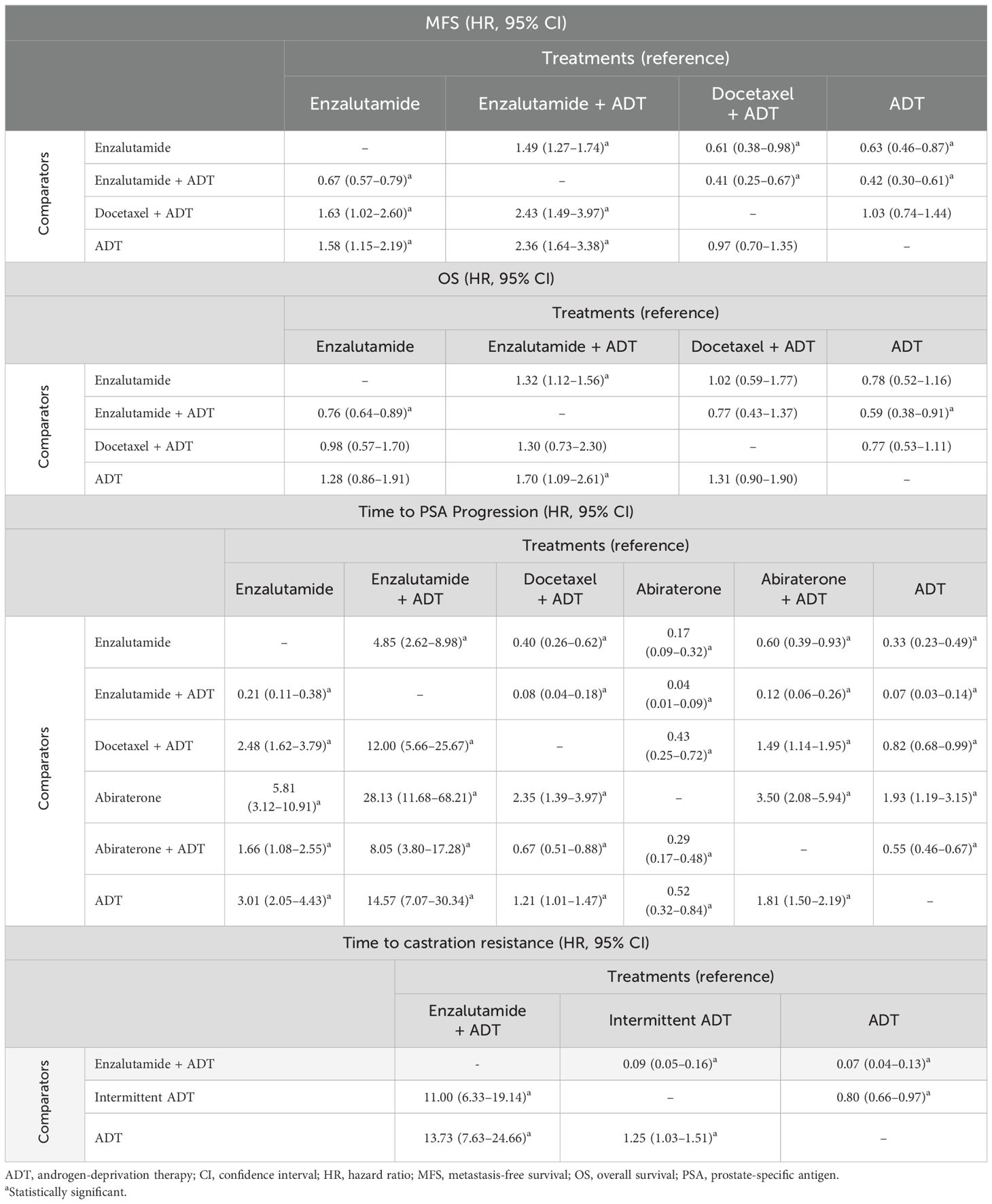
Table 3A. League table for outcomes of interest presenting the relative efficacy of enzalutamide (monotherapy and combination therapy) versus comparator treatments.
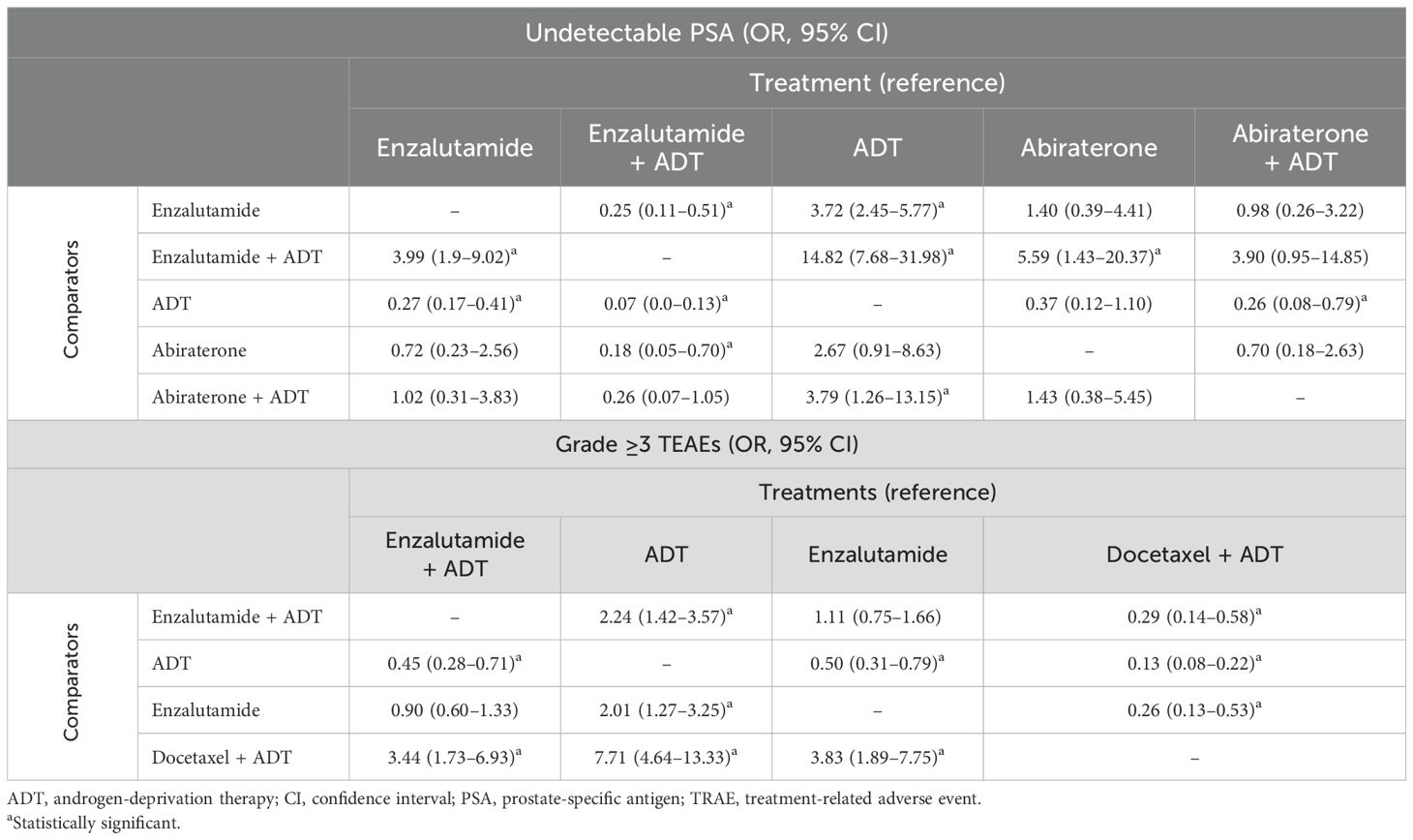
Table 3B. League table for outcomes of interest presenting the relative efficacy of enzalutamide (monotherapy and combination therapy) versus comparator treatments.
3.1.1 Overall survival
For OS, enzalutamide combination therapy (i.e., enzalutamide with ADT) demonstrated superiority over ADT alone and enzalutamide monotherapy (i.e., ADT alone) but similar performance to ADT + docetaxel (Figure 2A). Enzalutamide monotherapy demonstrated similar performance to ADT + docetaxel and ADT alone (Figure 2B).
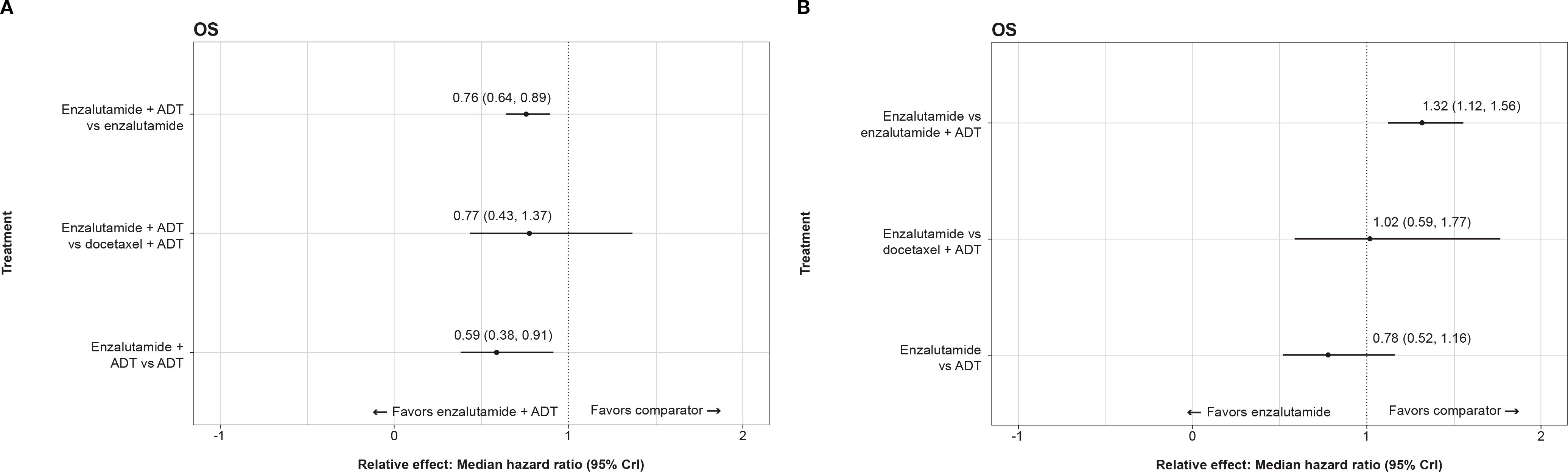
Figure 2. (A) Forest plot representing the relative efficacy of enzalutamide combined with ADT against other active treatments in the analysis of overall survival. (B). Forest plot representing the relative efficacy of enzalutamide monotherapy against other active treatments in the analysis of overall survival. ADT, androgen-deprivation therapy; CrI, credible interval; OS, overall survival.
3.1.2 Metastasis-free survival
For MFS, enzalutamide combination therapy demonstrated superiority over all comparators. Specifically, both enzalutamide combination therapy and enzalutamide monotherapy demonstrated superiority over ADT + docetaxel and ADT alone (Supplementary Figures 3A, B). Enzalutamide combination therapy demonstrated superiority over enzalutamide monotherapy.
3.1.3 Time to prostate-specific antigen progression
For PSA progression, enzalutamide combination therapy demonstrated superiority over all comparators (Supplementary Figure 3C). Enzalutamide monotherapy also demonstrated superiority over most comparators (ADT + docetaxel, ADT + abiraterone, abiraterone monotherapy, and ADT alone), but was inferior to enzalutamide combination therapy (Supplementary Figure 3D).
3.1.4 Time to castration resistance
For time to castration resistance, enzalutamide combination therapy demonstrated superiority over ADT alone (Supplementary Figure 3E).
3.1.5 Prostate-specific antigen < 0.2 ng/ml at 36 (± 4) weeks of treatment
For the proportion of patients with PSA < 0.2 ng/ml at 36 (± 4) weeks of treatment, enzalutamide combination therapy demonstrated superiority over abiraterone monotherapy and ADT alone (Supplementary Figure 3F). Although the results for enzalutamide combination therapy were numerically favorable compared with ADT + abiraterone, they did not indicate superiority. Enzalutamide monotherapy demonstrated inferiority compared to enzalutamide combination therapy; however, it demonstrated superiority over ADT alone and similar performance to ADT + abiraterone and abiraterone monotherapy (Supplementary Figure 3G).
3.2 Safety (grade ≥3 TRAEs)
In terms of safety, enzalutamide combination therapy demonstrated superiority over ADT + docetaxel and inferiority compared with ADT alone (Supplementary Figure 3H). Enzalutamide monotherapy demonstrated superiority over ADT + docetaxel and inferiority to ADT alone (Supplementary Figure 3I). Enzalutamide combination therapy demonstrated similar performance to enzalutamide monotherapy.
3.3 Sensitivity analyses
Sensitivity analyses were conducted to assess the impact of studies that had a high risk of bias or heterogeneity, which could affect the results of the base case analysis. Four such studies were identified: first, in Duchesne et al. (2016) (TOAD) (26), more than 50% of patients had PSA doubling time ≥10 months, and only around 40% of patients in each arm were classified as having high-risk disease. Second, Morris et al. (2021) (TAX3503) (27) included patients with relatively low serum PSA levels at baseline, suggesting a much earlier stage of biochemical recurrence compared with patients in other trials. Third, Spetsieris et al. (2021) (FINITE) (29) provided limited information regarding baseline patient characteristics, and median serum PSA levels in their study were relatively lower than those reported in other trials. Finally, Aggarwal 2023 (PRESTO, AFT-19) (32) was an ongoing study with only preliminary results available. The results of three sensitivity analyses conducted to evaluate the impact of excluding Duchesne et al. (2016) (26), Morris et al. (2021) (27), and Spetsieris et al. (2021) (29), as well as one conducted on the impact of including Aggarwal et al. (2023) (32), did not differ from the results of the base case analysis. Details are presented in Supplementary Tables 7A, B.
4 Discussion
Patients with high-risk BCR have several different treatment options. To systematically compare treatments, we conducted, to our knowledge, the first NMA focusing on the effectiveness of enzalutamide for the treatment of high-risk BCR nmHSPC. Our analysis included 16 citations based on 10 phase 2/3 trials. We assessed the efficacy of multiple treatments on several clinically important endpoints, including OS, MFS, and time to PSA progression. Finally, to explore the impact of trial heterogeneity on our conclusions, we conducted a wide range of sensitivity analyses and found consistent results across all scenarios. We found that enzalutamide combination therapy demonstrated superiority over ADT alone and enzalutamide monotherapy for OS and over all comparators for MFS (vs enzalutamide monotherapy, ADT alone, and ADT + docetaxel) and time to castration resistance (vs ADT alone). It also demonstrated superiority to all comparators for time to PSA progression (vs enzalutamide monotherapy, abiraterone monotherapy, ADT + abiraterone, ADT + docetaxel, and ADT alone, as well as ADT + apalutamide and ADT + abiraterone + apalutamide in sensitivity analysis). Importantly, for oncological outcomes, no treatment was superior to enzalutamide combination therapy. Likewise, enzalutamide monotherapy demonstrated superiority to ADT alone and ADT + docetaxel in terms of MFS and time to PSA progression. These benefits must be balanced against the higher rates of grade ≥3 TRAEs with enzalutamide (with or without ADT) relative to other options except ADT + docetaxel, which was associated with more grade ≥3 TRAEs than enzalutamide. Together, the findings support the use of enzalutamide with or without ADT as a new standard of care for patients with high-risk BCR.
The benefits identified for enzalutamide in this analysis are aligned with treatment recommendations already included in current clinical guidelines. Specifically, the NCCN guidelines list enzalutamide as a preferred treatment option for patients with high-risk BCR nmHSPC (10). Similarly, the 2024 EAU guidelines contain a “strong” recommendation for providers to offer enzalutamide with or without ADT to patients with high-risk BCR nmHSPC (9). Further, our analysis is consistent with previous safety signals (36). In terms of the rate of grade ≥3 TRAEs, enzalutamide with and without ADT demonstrated superiority over ADT + docetaxel. The greater toxicity of docetaxel identified in our analysis, combined with a lack of greater clinical benefit, supports current recommendations for the use of enzalutamide in this patient population. Moreover, recent evidence from a Canadian study suggests that, compared with ADT alone, ADT + enzalutamide is cost-effective at established willingness to pay thresholds, making it a preferred treatment option for patients with high-risk BCR nmHSPC (37).
This study has some limitations. Survival data from several of the trials included in the NMA, including the EMBARK trial, are relatively immature, as the median survival time had not been reached for all survival outcomes at the time of data cut-off for each study; this could potentially lead to implausible estimates of survival benefit. However, previous trials that reported immature OS data for non-metastatic castration-resistant prostate cancer (nmCRPC) (PROSPER, SPARTAN, and ARAMIS) and metastatic hormone-sensitive prostate cancer (mHSPC) (ARCHES and TITAN) found that data maturity strengthened the results, and eventually, statistically significant and clinically meaningful benefits were achieved (38–42). In other indications for the use of enzalutamide in prostate cancer (nmCRPC, mHSPC, and mCRPC), a consistent effect was observed for enzalutamide at all evaluated disease stages, lending credibility to our findings (11, 12, 43–45). Within the trials included in this NMA, EMBARK had the longest follow-up period of all eligible trials that reported OS and included data from over 1000 patients. Of 271 OS events required across the treatment groups in EMBARK to achieve the protocol-defined power needed for this outcome, nearly half (n = 130, 48%) had occurred by the cut-off date, which supports these potential survival benefits.
Although both fixed- and random-effects models were run, the latter were limited by the small number of studies informing each treatment comparison in our analysis, resulting in less precise estimates. Thus, the fixed-effects models were used for inference, which does not account for between-study heterogeneity, and may, therefore, underestimate uncertainty in effect estimates As potential sources of clinical heterogeneity were identified in the evidence base, a series of sensitivity analyses were conducted to explore the impact of removing studies that deviated from the other studies in terms of baseline characteristics. After assessing the impact of excluding these studies, the overall results remained consistent, which lends credibility to our findings. In addition, the sparse evidence networks in this NMA mean that credible intervals were wide, leading to high uncertainty, particularly for the proportion of patients with undetectable PSA at 36 weeks and the rate of grade ≥3 TRAEs. Specifically, treatment-emergent and unspecified grade ≥3 AEs in the Morris study (27) were assumed to be TRAEs, which may have resulted in overestimation. However, as no unexpected grade ≥3 AEs were reported in the included trial, we do not expect this assumption to have substantially impacted the results. Additionally, treatment comparisons for TRAEs were not adjusted for time on treatment, which may have impacted our findings. Finally, we did not assess the exact make-up of TRAEs. For example, although enzalutamide combination showed superiority over enzalutamide monotherapy in multiple oncological outcomes with similar safety, there are differences in the side effect profiles of the two that may favor use of one regimen over another (i.e., better preserved sexual function with monotherapy). As such, the choice to use a particular regimen should be based on shared decision-making after evaluating efficacy, grade ≥3 TRAEs, and any specific side effects.
We evaluated the feasibility of conducting subgroup analyses, but the small number of studies reporting subgroup results meant that this was not viable. However, based on the results of the studies that conducted and reported the results of subgroup analyses (e.g., EMBARK), no groups were identified that would not be expected to benefit from treatment with enzalutamide (13).
Finally, a limitation of meta-analyses in general is that the results of more recent studies that were published or analyzed after the initial literature review was conducted will not be captured. In this study, available data on apalutamide was preliminary, or included only in sensitivity analyses, and was only available for time to PSA progression. Although enzalutamide with and without ADT demonstrated superiority over apalutamide in a sensitivity analysis, future analyses should incorporate more recently available apalutamide data to confirm this finding. Similarly, although the SLR search strategy followed best practice guidelines, there remains a risk that not all relevant studies were captured.
5 Conclusion
Overall, this novel NMA provides up-to-date evidence on the relative efficacy and safety profile of interventions for the treatment of high-risk BCR nmHSPC, demonstrating that enzalutamide with or without ADT provides considerable oncological benefit in high-risk BCR nmHSPC, albeit with a higher risk of TRAEs compared to ADT alone. Future research should prioritize updating this NMA to incorporate more mature OS data, as well as more recent data from the treatments evaluated in the NMA. Further research is also needed to identify predictive biomarkers that may help to identify tumors that are more sensitive to treatment with ARPIs compared with other mechanisms of action (e.g., chemotherapy).
Data availability statement
All data generated or analyzed during this study, which support the findings of this study, are included within this article and its supplementary information files. Researchers may access analysis not present in the manuscript from the corresponding author upon reasonable request. For the Astellas criteria on data sharing, see: https://www.clinicaltrials.astellas.com/transparency/.
Author contributions
AA: Methodology, Conceptualization, Writing – review & editing. AC: Data curation, Formal analysis, Validation, Conceptualization, Writing – review & editing, Supervision, Investigation, Methodology. TM: Formal analysis, Methodology, Supervision, Validation, Data curation, Conceptualization, Writing – review & editing, Investigation. AN: Conceptualization, Validation, Methodology, Formal analysis, Supervision, Writing – review & editing, Data curation, Investigation. AG: Writing – review & editing, Conceptualization, Methodology. AS: Formal analysis, Supervision, Writing – original draft, Methodology, Investigation, Data curation, Validation, Conceptualization. FG: Investigation, Formal analysis, Writing – review & editing, Validation, Data curation, Conceptualization, Methodology, Supervision. IB: Writing – review & editing, Supervision, Investigation, Formal analysis, Validation, Methodology, Data curation, Conceptualization. MZ: Writing – review & editing, Validation, Investigation, Supervision, Formal analysis, Methodology, Data curation, Conceptualization. SF: Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. This study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide. Authors AC, TM, AN, AG, AS, and FG are employees of Astellas Pharma, and were involved in the study design, as well as the acquisition, analysis, and interpretation of the data. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Support for medical writing, editorial, and graphic design was provided by Lindsay Wilson (MSc), Jay Patel (PharmD), Rucha Kurtkoti (MSc), and Samila Sakhabuth (BA [Hons]) from IQVIA, funded by the study sponsors.
Conflict of interest
AA: Payment or honoraria for lectures, presentations, speaker bureaus, publication writing, or educational events: AbbVie, Astellas Pharma, Bayer, Tersera, Tolmar; Support for attending meetings and/ or travel: Tersera, Tolmar; Leadership or fiduciary role in other board, society, committee or advocacy group (unpaid) Canadian Urology Association, Cedars Cancer Foundation. IB: Employment: IQVIA Ltd; Grants (past 36 months): IQVIA Commercial GmbH & Co. AC: Employment: Astellas Pharma; Support for meetings and/or travel: Astellas Pharma; Other financial or non-financial interests: Astellas Pharma. SF: Study support: Astellas Pharma, Pfizer; Consulting: Astellas Pharma, AstraZeneca, Bayer, Eli Lilly, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi; Payment or honoraria for speakers bureaus: AstraZeneca, Astellas Pharma, Pfizer, Sanofi; Participation on Data Safety Monitoring Board or Advisory Board: Astellas Pharma, Pfizer, Janssen; Receipt of Equipment, materials, drugs, medical writing, gifts, other: Astellas Pharma, Pfizer. AG: Employment: Astellas Global; Stock or Other ownership: AbbVie. FG: Employment: Astellas Pharma. TM: Employment: Astellas Pharma. AN: Employment (past 36 months): Astellas Pharma; Travel support: Astellas Pharma. AS: Employment: Astellas Pharma. MZ: Contract employment: IQVIA Commercial GmbH & Co.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1638405/full#supplementary-material
References
1. Shore ND, Moul JW, Pienta KJ, Czernin J, King MT, and Freedland SJ. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: treatment based on risk stratification. Prostate Cancer Prostatic Dis. (2024) 27:192–201. doi: 10.1038/s41391-023-00712-z, PMID: 37679602
2. Simon NI, Parker C, Hope TA, and Paller CJ. Best approaches and updates for prostate cancer biochemical recurrence. Am Soc Clin Oncol Educ Book. (2022) 42:1–8. doi: 10.1200/EDBK_351033, PMID: 35503984
3. Virgo KS, Rumble RB, de Wit R, Mendelson DS, Smith TJ, Taplin ME, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. (2021) 39:1274–305. doi: 10.1200/JCO.20.03256, PMID: 33497248
4. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. (2021) 79:243–62. doi: 10.1016/j.eururo.2020.09.042, PMID: 33172724
5. Morgan TM, Boorjian SA, Buyyounouski MK, Chapin BF, Chen DYT, Cheng HH, et al. Salvage therapy for prostate cancer: AUA/ASTRO/SUO guideline part I: Introduction and treatment decision-making at the time of suspected biochemical recurrence after radical prostatectomy. J Urol. (2024) 211:509–17. doi: 10.1097/JU.0000000000003892, PMID: 38421253
6. Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. (2023) 209:1082–90. doi: 10.1097/JU.0000000000003452, PMID: 37096583
7. Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2020) 31:1119–34. doi: 10.1016/j.annonc.2020.06.011, PMID: 32593798
8. Saad F, Bogemann M, Suzuki K, and Shore N. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. (2021) 24:323–34. doi: 10.1038/s41391-020-00310-3, PMID: 33558665
9. Cornford P, Tilki D, van den Bergh R, et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer (2024). EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2024_2024-04-09-132035_ypmy_2024-04-16-122605_lqpk.pdf.
10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.2.2025. © National Comprehensive Cancer Network, Inc. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way (202).
11. Armstrong AJ, Azad AA, Iguchi T, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. (2022) 40:1616–22. doi: 10.1200/JCO.22.00193, PMID: 35420921
12. Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. (2019) 381:121–31. doi: 10.1056/NEJMoa1903835, PMID: 31157964
13. Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. (2023) 389:1453–65. doi: 10.1056/NEJMoa2303974, PMID: 37851874
14. Freedland SJ, Gleave ME, De Giorgi U, Rannikko A, Pieczonka C, Sridharan S, et al. 1778P Treatment (tx) of high-risk biochemically recurrent prostate cancer with enzalutamide (enza) in combination with leuprolide acetate (LA): Secondary endpoints from EMBARK. Annal Oncol. (2023) 34:S961. doi: 10.1016/j.annonc.2023.09.2728
15. De Giorgi U, Freedland SJ, Gleave ME, Tutrone R, Bailen JL, Roos E, et al. 1777P Enzalutamide (enza) monotherapy for the treatment (tx) of prostate cancer with high-risk biochemical recurrence (BCR): EMBARK secondary endpoints. Annal Oncol. (2023) 34:S960–1. doi: 10.1016/j.annonc.2023.09.2727
16. Freedland SJ, Gleave M, De Giorgi U, Rannikko A, Pieczonka CM, Tutrone RF, et al. Enzalutamide and quality of life in biochemically recurrent prostate cancer. NEJM Evid. (2023) 2:EVIDoa2300251. doi: 10.1056/EVIDoa2300251, PMID: 38320501
17. United States Food and Drug Administration. FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence (2023). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enzalutamide-non-metastatic-castration-sensitive-prostate-cancer-biochemical-recurrence (Accessed 14 January 2025).
18. European Medicines Agency. Xtandi (enzalutamide) (2024). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/xtandiproduct-info (Accessed 14 January 2025).
19. Dias S, Welton NJ, Sutton AJ, and Ades AE. NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: NICE Decision Support Unit Technical Support Documents (2014).
20. R Core Team. R: A language and environment for statistical computing. MSOR Connections (2014). p. 1.
21. Phillippo DM. multinma: Bayesian network meta-analysis of individual and aggregate data (2020). Available online at: https://dmphillippo.github.io/multinma/ (Accessed 14 January 2025).
22. Freedland S, Gleave M, De Giorgi U, Rannikko A, Pieczonka C, Tutrone R, et al. 1766MO Health-related quality of life (HRQoL) in nonmetastatic hormone-sensitive prostate cancer (nmHSPC) patients (pts) with high-risk biochemical recurrence (BCR) from the EMBARK study. Annal Oncol. (2023) 34:S955. doi: 10.1016/j.annonc.2023.09.2716
23. Shore ND, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Haas GP, et al. LBA02–09 EMBARK: A phase 3 randomized study of enzalutamide or placebo plus leuprolide acetate and enzalutamide monotherapy in high-risk biochemically recurrent prostate cancer. J Urol. (2023) 209:e1190. doi: 10.1097/JU.0000000000003361.09
24. Autio KA, Antonarakis ES, Mayer TM, Shevrin DH, Stein MN, Vaishampayan UN, et al. Randomized phase 2 trial of abiraterone acetate plus prednisone, degarelix, or the combination in men with biochemically recurrent prostate cancer after radical prostatectomy. Eur Urol Open Sci. (2021) 34:70–8. doi: 10.1016/j.euros.2021.09.015, PMID: 34934969
25. Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. (2012) 367:895–903. doi: 10.1056/NEJMoa1201546, PMID: 22931259
26. Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. (2016) 17:727–37. doi: 10.1016/S1470-2045(16)00107-8, PMID: 27155740
27. Morris MJ, Mota JM, Lacuna K, Hilden P, Gleave M, Carducci MA, et al. Phase 3 randomized controlled trial of androgen deprivation therapy with or without docetaxel in high-risk biochemically recurrent prostate cancer after surgery (TAX3503). Eur Urol Oncol. (2021) 4:543–52. doi: 10.1016/j.euo.2021.04.008, PMID: 34020931
28. Oudard S, Latorzeff I, Caty A, Migliancio L, Sevin E, Hardy-Bessard AC, et al. Effect of adding docetaxel to androgen-deprivation therapy in patients with high-risk prostate cancer with rising prostate-specific antigen levels after primary local therapy: A randomized clinical trial. JAMA Oncol. (2019) 5:623–32. doi: 10.1001/jamaoncol.2018.6607, PMID: 30703190
29. Spetsieris N, Boukovala M, Alafis I, Davis J, Zurita A, Wang X, Tu SM, et al. Abiraterone acetate plus prednisone in non-metastatic biochemically recurrent castration-naïve prostate cancer. Eur J Cancer. (2021) 157:259–67. doi: 10.1016/j.ejca.2021.06.017, PMID: 34536949
30. Hahn AW, Wang X, Efstathiou E, Hwang H, Zurita AJ, Spetsieris N, et al. Third analysis of a randomized trial of finite abiraterone acetate (AA) plus LHRH agonist (LHRHa) versus LHRHa in biochemically recurrent, non-metastatic hormone-naïve prostate cancer (M0HNPC). J Clin Oncol. (2022) 40:135. doi: 10.1200/JCO.2022.40.6_suppl.135
31. Josefsson A, Jellvert Å, Holmberg E, Brasso K, Meidahl Petersen P, Aaltomaa S, et al. Effect of docetaxel added to bicalutamide in Hormone-Naïve non-metastatic prostate cancer with rising PSA, a randomized clinical trial (SPCG-14). Acta Oncologica. (2023) 62:372–80. doi: 10.1080/0284186X.2023.2199940, PMID: 37073813
32. Aggarwal RR, Heller G, Hillman DW, Xiao H, Picus J, Taplin ME, et al. Baseline characteristics associated with PSA progression-free survival in patients (pts) with high-risk biochemically relapsed prostate cancer: Results from the phase 3 PRESTO study (AFT-19). J Clin Oncol. (2023) 41:208. doi: 10.1200/JCO.2023.41.6_suppl.208
33. ClinicalTrials.gov. NCT01790126. The role of highly selective androgen receptor (AR) targeted therapy in men with biochemically relapsed hormone sensitive prostate cancer (2020). Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT01790126 (Accessed 14 January 2025).
34. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3. 0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. (2003) 13:176–81. doi: 10.1016/S1053-4296(03)00031-6, PMID: 12903007
35. National Cancer Institute. Common Terminology Criteria for Adverse Events. CTCAE (2009). Available online at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
36. Saad F, Hamilou Z, and Lattouf JB. A drug safety evaluation of enzalutamide to treat advanced prostate cancer. Expert Opin Drug Saf. (2021) 20:741–9. doi: 10.1080/14740338.2021.1919620, PMID: 34114527
37. Aprikian A, Saad F, Wywial E, McLean T, Johnston K, Li Y, et al. Cost-effectiveness of enzalutamide with androgen-deprivation therapy (ADT) versus ADT alone for the treatment of high-risk biochemically recurrent non-metastatic castration-sensitive prostate cancer in Canada. J Med Econ. (2025) 28:766–77. doi: 10.1080/13696998.2025.2503660, PMID: 40395149
38. Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. (2018) 378:2465–74. doi: 10.1056/NEJMoa1800536, PMID: 29949494
39. Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A randomized, phase iii study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. (2019) 37:2974–86. doi: 10.1200/JCO.19.00799, PMID: 31329516
40. Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. (2018) 19:1404–16. doi: 10.1016/S1470-2045(18)30456-X, PMID: 30213449
41. Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. (2020) 383:1040–9. doi: 10.1056/NEJMoa2001342, PMID: 32905676
42. Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. (2019) 381:13–24. doi: 10.1056/NEJMoa1903307, PMID: 31150574
43. Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. (2020) 382:2197–206. doi: 10.1056/NEJMoa2003892, PMID: 32469184
44. Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: Extended analysis of the phase 3 PREVAIL study. Eur Urol. (2017) 71:151–4. doi: 10.1016/j.eururo.2016.07.032, PMID: 27477525
Keywords: biochemical recurrence, enzalutamide, hormone-sensitive, network meta-analysis, prostatic neoplasms, systematic review
Citation: Aprikian A, Chilelli A, McLean T, Nasr A, Ganguli A, Serikoff A, Guzman F, Barouma I, Zacharioudaki M and Freedland SJ (2025) Comparing the safety and efficacy of systemic therapies for high-risk biochemically recurrent hormone-sensitive prostate cancer: a network meta-analysis. Front. Oncol. 15:1638405. doi: 10.3389/fonc.2025.1638405
Received: 30 May 2025; Accepted: 31 July 2025;
Published: 29 August 2025.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Georgios Ioannis Papageorgiou, IASO General Hospital, GreeceJingyuan Ning, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2025 Aprikian, Chilelli, McLean, Nasr, Ganguli, Serikoff, Guzman, Barouma, Zacharioudaki and Freedland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen J. Freedland, U3RlcGhlbi5GcmVlZGxhbmRAY3Nocy5vcmc=
 Armen Aprikian
Armen Aprikian Andrew Chilelli
Andrew Chilelli Thomas McLean
Thomas McLean Anchen Nasr3
Anchen Nasr3 Alexis Serikoff
Alexis Serikoff Stephen J. Freedland
Stephen J. Freedland