- Department of Breast Surgery, Huzhou Maternity & Child Health Care Hospital, Huzhou, Zhejiang, China
Background: Fertility preservation is a critical aspect of care for young breast cancer (BC) patients undergoing gonadotoxic treatments. BRCA mutation and hormone receptor (HR) status influence tumor biology and treatment outcomes. This study evaluated the impact of BRCA mutation and HR status on fertility preservation outcomes in BC patients.
Methods: PubMed, Embase, Scopus, and Web of Science databases were searched for publications from inception to March 31, 2025 that report on fertility preservation outcomes stratified by BRCA mutation or HR status. Primary outcomes included the number of retrieved oocytes, maturation rates, and ovarian reserve indices such as anti-Müllerian hormone (AMH) levels and antral follicular count (AFC). Random-effects meta-analyses were performed.
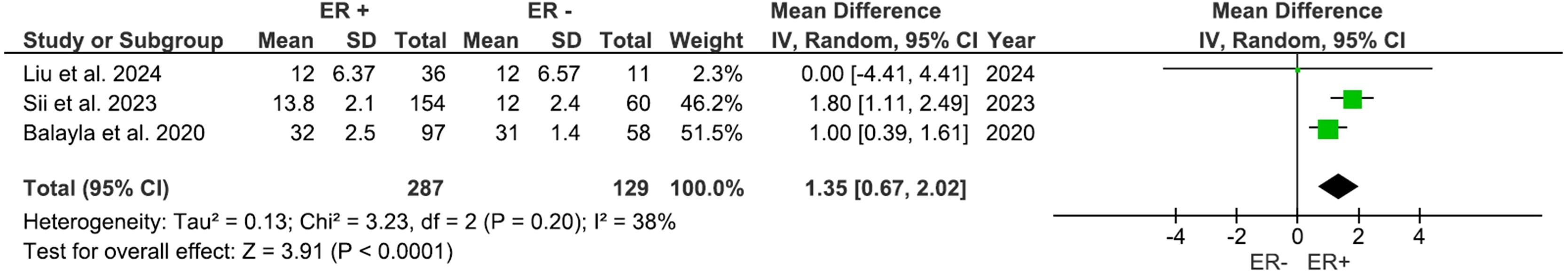
Results: Thirteen studies involving approximately 1,654 participants were included in the meta-analysis. Patients with no BRCA mutations reported significantly higher mature oocytes (MD: -1.48, 95% CI: -2.63 to -0.34) compared to those with BRCA mutations and non-significant total oocyte yield (MD: -1.37, 95% CI: -3.13 to 0.40). AFC and AMH levels showed no significant intergroup differences. Additionally, estrogen receptor (ER)-positive patients exhibited better ovarian response, with higher AFC (MD: 1.37, 95% CI: 0.48 to 2.26) and greater oocyte yield (MD: 1.35, 95% CI: 0.67 to 2.02).
Conclusion: Our results show that BRCA mutations may be associated with significantly diminished mature oocyte production during fertility preservation in BC patients. On the contrary, ER-positive status seems to be associated with high AFC and oocyte yield indicating a more advantageous ovarian response. The present findings are from a limited number of heterogenous studies and hence must be interpreted with caution.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42025641361.
Introduction
Breast cancer (BC) is the most prevalent form of malignancy in women of reproductive age (1). The hormonal receptor (HR) profile and mutation state of BC, in addition to tumor grade and stage, may provide critical information about the aggressiveness and evolution of the disease (2, 3). Estrogen receptors (ER), progesterone receptors (PR), or human epidermal growth factor receptor 2 (HER2) expression has significant implications for prognosis and the selection of therapeutic modalities and is routinely used for tumor classification (4, 5). ER-positive (ER+) tumors are responsive to anti-hormonal therapies like selective estrogen receptor modulators or aromatase inhibitors, while ER-negative (ER−) tumors are usually more aggressive and respond less to hormonal therapies (6, 7). Triple-negative breast cancer, lacking ER, PR, and HER2 expression, carries a poorer prognosis compared to ER+ or HER2-enriched tumors (8).
In many countries, the evaluation of ER, PR, and HER2 status is routinely incorporated into diagnostic workflows, serving not only as key biomarkers for guiding therapy but also as important prognostic and monitoring tools. Specific mutations and expression patterns of these receptors have been shown to correlate with mammographic findings, thereby enhancing diagnostic accuracy and disease surveillance. On mammograms, ER/PR-positive tumors typically appear as spiculated, low-density masses, whereas HER2-positive and triple-negative malignancies are more commonly associated with pleomorphic calcifications, irregular high-density masses, or without distinguishing features despite aggressive behavior. Integrating imaging characteristics and receptor profiling has been shown to improve diagnosis accuracy and prognosis in BC care (9, 10).
Other than these receptors, genetic mutations, such as those in the BRCA1 and BRCA2 genes, also play a pivotal role in BC pathogenesis (11). BRCA genes belong to the family of ATM-mediated DNA double-strand break repair genes, essential for maintaining genomic stability and telomere integrity. BRCA1 and BRCA2 mutations in females are linked to a significantly increased risk of developing breast and ovarian cancers, often at a younger age and before menopause (12). Moreover, BRCA mutations not only influence BC prognosis but may also affect reproductive outcomes and ovarian reserve, further complicating treatment planning in young patients (13).
With current advances in oncology, the long-term survival rates of young women with BC have considerably improved, reaching as high as 85–90% (14). As survival rates improve, the ability to bear children post-treatment has become a critical consideration in therapeutic planning, shifting the focus towards fertility preservation (15). Consequently, BC patients represent the majority of individuals seeking oocyte and embryo cryopreservation today (16). Fertility preservation strategies, including cryopreservation of oocytes or embryos, are essential for mitigating the gonadotoxic effects of chemotherapy and radiation (17).
However, the impact of the HR status and BRCA mutation status on the fertility preservation outcomes remains unclear. ER and PR status were shown to directly impact tumor biology and treatment, which may, in turn, affect the ovarian response to stimulation during fertility preservation procedures (18). Similarly, BRCA mutations, which may alter ovarian reserve and function, could influence the number and quality of retrieved oocytes (19). According to the NCCN (National Comprehensive Cancer Network) guidelines, fertility preservation should be discussed with all reproductive-aged women at diagnosis, ideally before initiation of systemic therapy (20). The recommendations emphasize early referral to reproductive specialists and the use of ovarian stimulation protocols adapted to HR status, such as letrozole-based regimens for ER-positive patients, to balance oncologic safety with fertility outcomes. However, there remains a deficiency in literature quantifying the impact of BRCA mutation and HR status on fertility outcomes. There have been a prior review examining fertility outcomes in BRCA carriers (12) but with limited data on BC patients. A recent updated review has also summarized evidence on the impact of BRCA mutations on fertility outcomes but without a quantitative analysis (18). Given this deficiency in literature, we conducted this present systematic review and meta-analysis to evaluate whether HR status (ER+, PR+) and BRCA mutation status affect fertility preservation outcomes in BC patients.
Materials and methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (21). The protocol for performing this review was framed a priori and was registered in PROSPERO (CRD42025641361).
Research question
This review addressed the research question: Do HR status and BRCA mutation status influence fertility preservation outcomes in BC patients?
To frame this question, the PICO model was applied:
Population (P): Women diagnosed with BC who underwent fertility preservation procedures.
Exposure (E): BRCA+ or HR+ (ER or PR) status.
Comparison (C): BRCA- or HR- (ER or PR) status.
Outcome (O): Fertility metrics including the number of retrieved oocytes, oocyte maturation rates, anti-Müllerian hormone (AMH) levels and antral follicular count (AFC).
Search strategy
Digital searches were conducted across PubMed, Embase, Web of Science, and Scopus databases for studies published up to March 31, 2025. The following search string was developed and applied, using keywords and Medical Subject Headings (MeSH): (“fertility preservation” OR “oocyte cryopreservation” OR “embryo cryopreservation”) AND (“BRCA mutation” OR “BRCA1” OR “BRCA2”) AND (“hormone receptor status” OR “ER positive” OR “PR positive” OR “triple-negative breast cancer” OR “TNBC”) AND (“breast cancer”). The search strategies for individual databases are provided in Table 1. Additionally, reference lists of eligible studies were reviewed manually to ensure no relevant articles were overlooked.
The search results were imported into a citation management tool to organize references systematically. Citation manager’s automated tools were used for deduplication, followed by manual verification to ensure accuracy.
Study selection
In the first stage, the two independent authors screened the titles and abstracts of all identified studies for eligibility. Studies meeting the requirements were advanced to the second stage of full-text assessment for eligibility.
Inclusion criteria
● Studies involving women diagnosed with BC who underwent fertility preservation procedures such as oocyte or embryo cryopreservation were included.
● Studies reporting outcomes of fertility preservation techniques, including ovarian stimulation, oocyte retrieval, and embryo cryopreservation.
● Studies reporting at least one of the following fertility-related outcomes: Number of oocytes retrieved, Oocyte maturation rates, AMH levels, AFC, etc.
● Prospective or retrospective cohort studies, case-control studies, or randomized controlled trials (RCTs).
● Articles published in English.
Exclusion criteria
● Studies lacking data on fertility-related outcomes (e.g., oocyte yield, maturation rates, fertilization rates).
● Case reports, case series with fewer than 10 participants, or review articles.
● Studies with duplicate data published in multiple articles.
All disagreements were resolved by discussion between authors or with a third reviewer. All included studies were required to report on fertility preservation outcomes stratified by HR status or BRCA mutation status in BC patients.
Data extraction
A standardized data extraction form was generated to collect study characteristics (e.g., authors, publication year, study design, sample size), patient data (e.g., age, BRCA mutation status, HR status), and fertility preservation outcomes (e.g., number of retrieved oocytes, oocyte maturation rates, rates of fertilization and pregnancy).
Two independent reviewers extracted data and resolved all discrepancies by discussion. A third reviewer verified the data extraction to ensure accuracy.
Data synthesis and quality assessment
The quality of the studies was assessed by the Newcastle-Ottawa Scale (NOS) that is scoring the selection of study groups, comparability, and ascertainment of outcomes, with a maximum score of 9, and higher scores indicating better study quality (22).
Quantitative data were synthesized using meta-analytical techniques when possible. The meta-analysis used RevMan (version 5.4, The Cochrane Collaboration, UK). Pooled analyses were conducted using random-effects models to account for heterogeneity between studies. The continuous data was expressed as mean and standard deviation (SD). Data expressed as median and range were converted into mean and SD, after considering the sample size according to the provided equation (23). Statistical heterogeneity was evaluated using the I² statistic. I2 of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively. Where meta-analysis was not feasible, a narrative synthesis of findings was performed.
Publication bias
Visual inspection of Funnel plots was carried out to assess publication bias.
Results
Search results
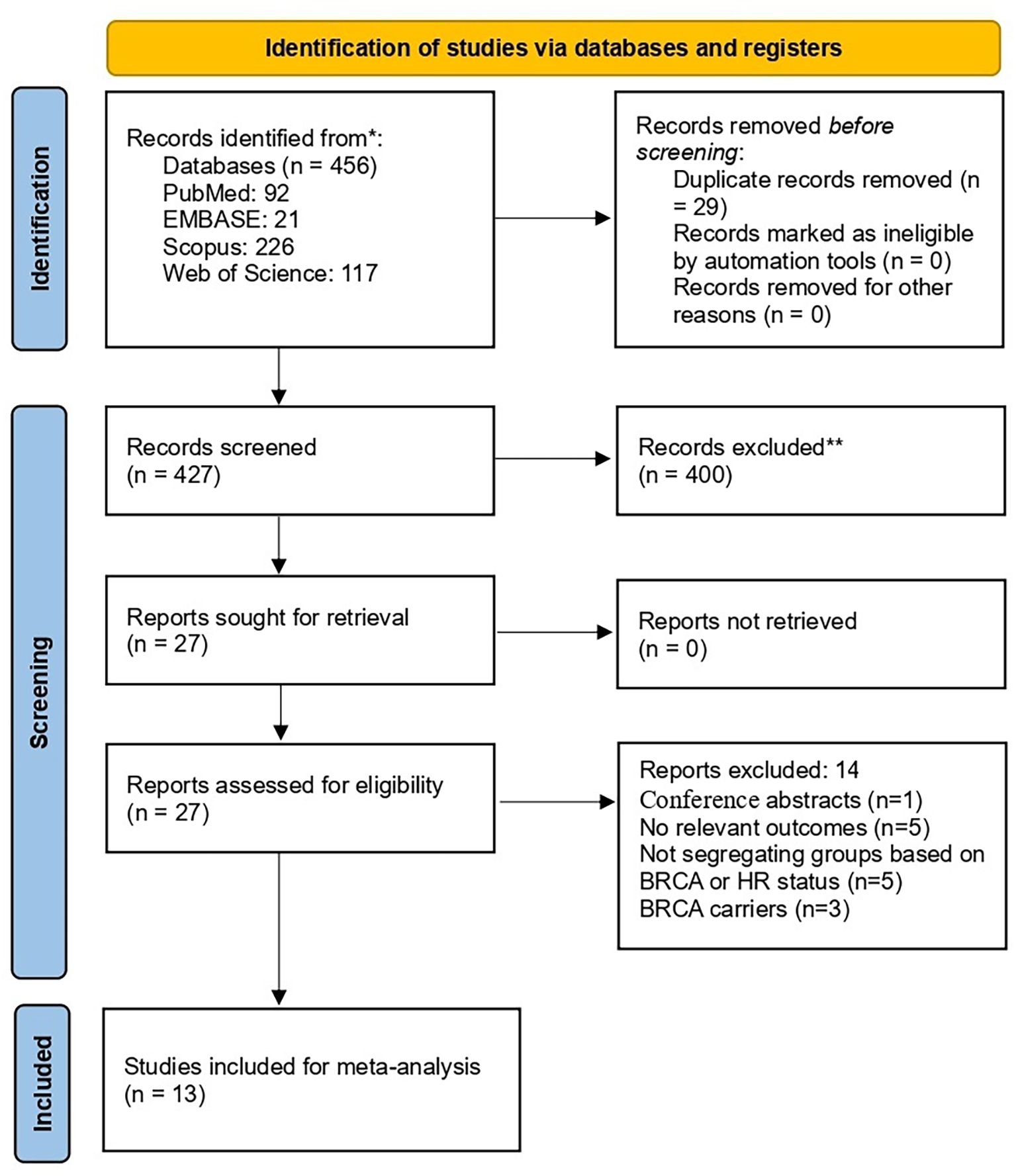
The initial search retrieved 456 records. After removing the duplicates, 427 underwent title and abstract screening. Subsequently, full-text evaluation of eligibility was carried out for 27 articles. Finally, thirteen articles (24–36) were included (Figure 1).
Baseline details
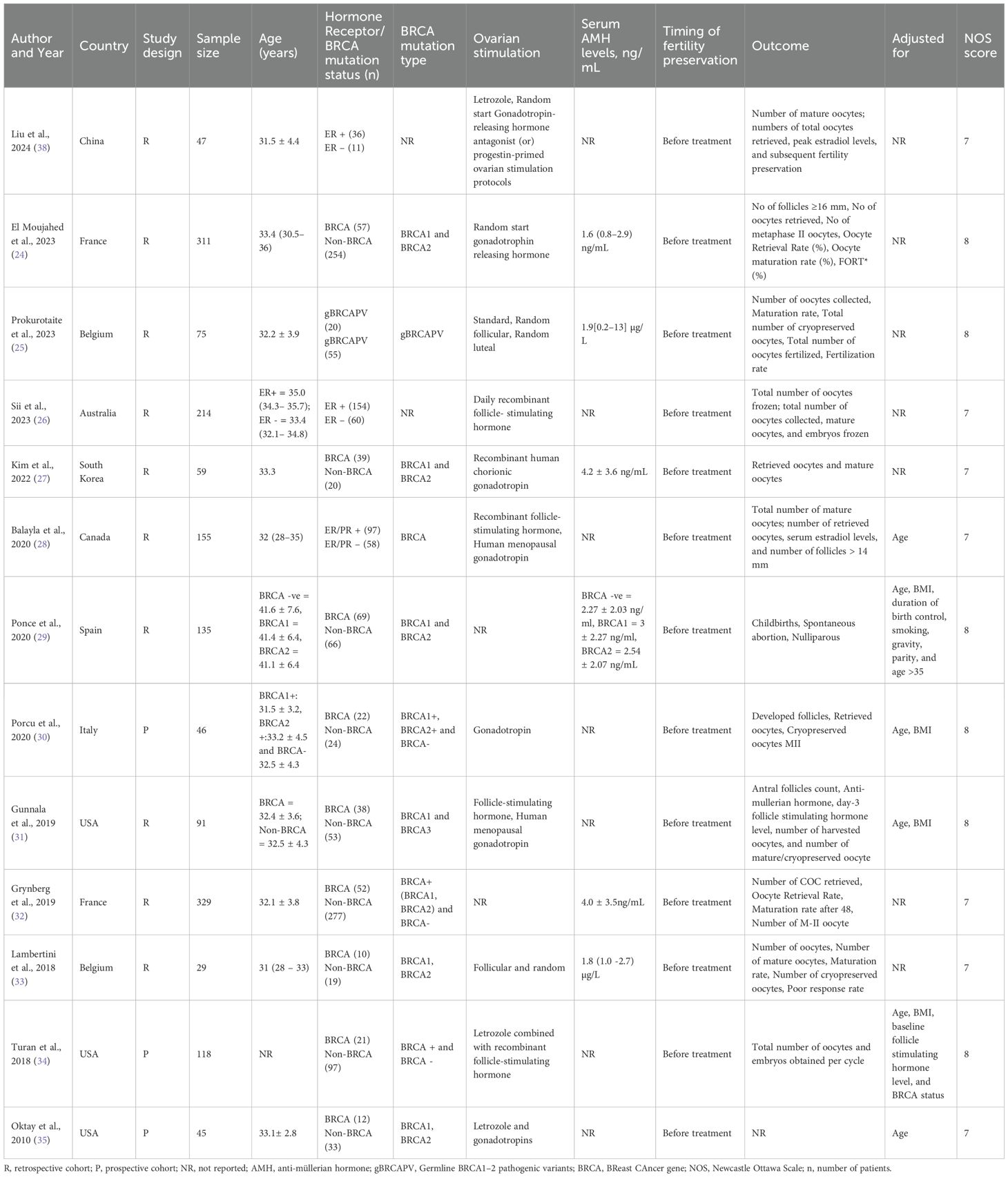
A total of thirteen studies published between 2010 and 2024 were included, comprising both retrospective (n=10) and prospective (n=3) cohort designs, with sample sizes ranging from 29 to 329 participants. The total sample size of all studies was 1,654. The majority of studies were conducted in Europe and North America, with few studies from Asia and Australia. The mean age of participants was approximately 31–35 years across studies. Majority studies evaluated women with BC carrying BRCA1/2 mutations and only three studies stratified patients based on ER status. Fertility preservation was consistently performed prior to initiation of systemic therapy in all studies. The ovarian stimulation protocols varied among studies. Random-start gonadotropin-releasing hormone (GnRH) was the most frequently used stimulation protocol (24, 36, 37). Letrozole combined with GnRH antagonists was also commonly employed (34, 35, 38). Recombinant follicle-stimulating hormone (FSH) with or without human menopausal gonadotropin (hMG), was widely used (26, 28, 31). The outcomes reported by the studies also showed wide variation. The NOS quality scores ranged from 7 to 8, indicating generally moderate-to-high methodological quality. Table 2 provides the characteristics and quality of the individual studies.
BRCA mutation
A total of 10 studies with 1,579 participants compared the number of retrieved oocytes in women with and without BRCA mutations (Figure 2). The pooled analysis showed no statistically significance (p=0.13), with an MD of -1.37 (95% CI: -3.13 to 0.40), in the number of oocytes in the Non-BRCA group (I2 = 68%).
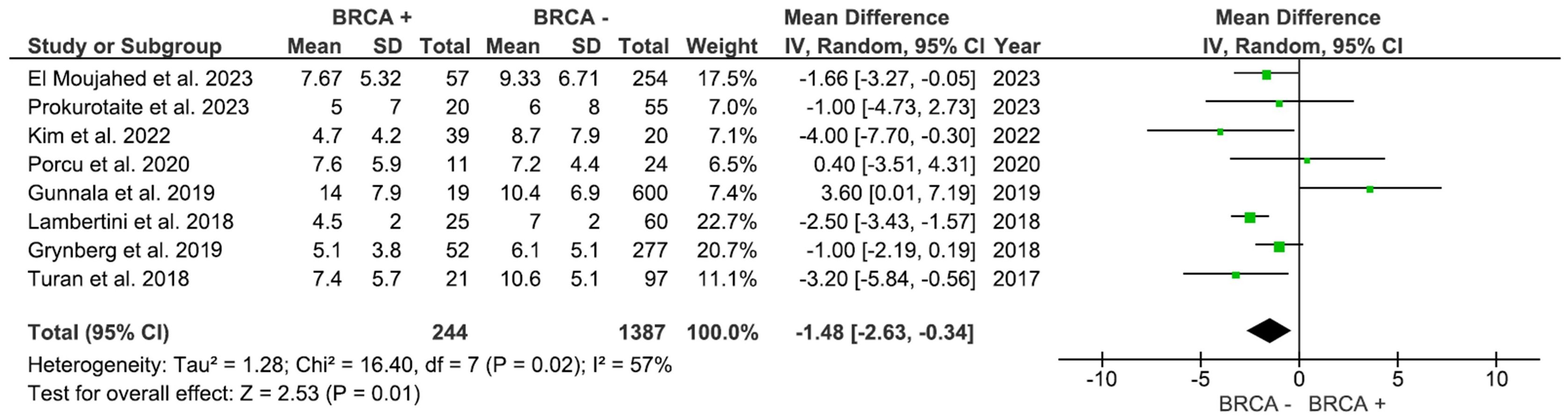
The number of retrieved mature oocytes was reported in 8 studies comprising 1,631 participants. As shown in Figure 3, the number of mature oocytes was markedly higher in the non-BRCA group (MD: -1.48, 95% CI: -2.63 to -0.34), p=0.01 (I2 = 57%).
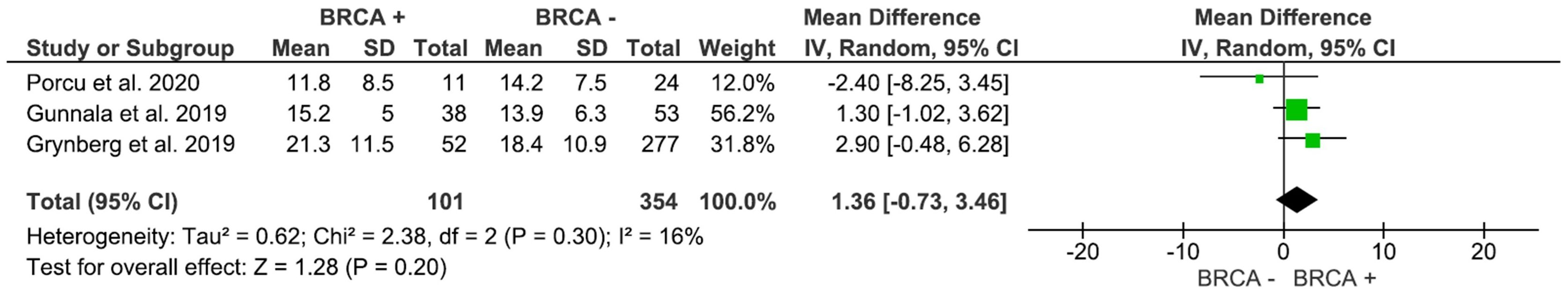
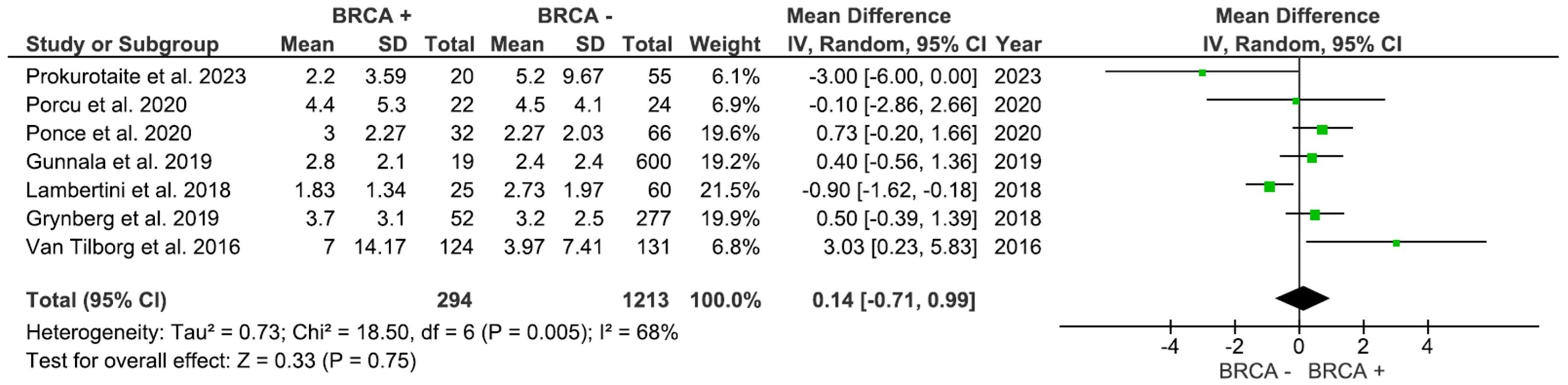
AFC, assessed in 3 studies with 455 participants, was higher in individuals with BRCA mutations compared to patients with no mutations (Figure 4); however, the result was not significant (MD: 1.36, 95% CI: -0.73 to 3.46), p=0.20 (I2 = 16%). As shown in Figure 5, AMH levels, pooled from 7 studies including 1,507 participants, was not significantly different between BRCA and non-BRCA BC patients (MD: 0.14, 95% CI: -0.71 to 0.99), p=0.75 (I2 = 68%).
ER status
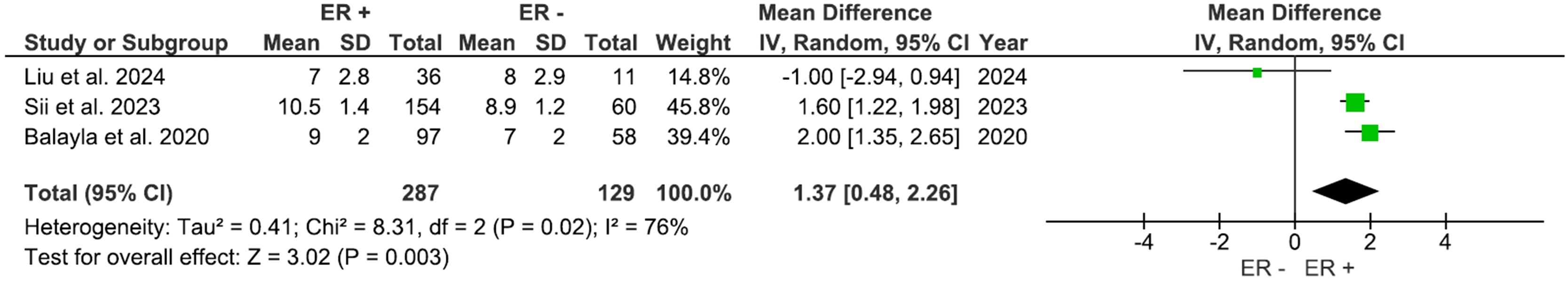
Three studies, including 416 participants, evaluated outcomes based on the ER status. AFC was significantly higher (Figure 6) in the ER-positive group compared to other groups, with a mean difference of 1.37 (95% CI: 0.48 to 2.26), p=0.003 (I2 = 76%). The number of retrieved oocytes was also considerably higher in the ER-positive group (Figure 7), with a mean difference of 1.35 (95% CI: 0.67 to 2.02), p<0.0001 (I2 = 38%).
Discussion
This systematic review and meta-analysis analyzed data from 13 studies involving over 1,654 BC patients undergoing fertility preservation to assess the impact of BRCA mutation status and HR status, specifically ER expression, on ovarian response and fertility outcomes. Our data show that BRCA mutation carriers have considerably fewer mature oocytes and a tendency of lower total oocytes as compared to non-carriers. The meta-analysis also showed that ER-positive patients have a superior ovarian response, as measured by both AFC and oocyte yield.
Our findings augment and expand upon previous reviews on this subject. Hong et al. (17) have previously presented a comprehensive narrative overview of fertility preservation in young women with BC, emphasizing the challenges associated with tumor biology, gonadotoxic therapies, and the necessity for personalized stimulation protocols, yet refraining from quantitatively assessing the influence of BRCA mutations or HR status. Dias Nunes et al. (18) specifically examined BRCA mutations and concluded that BRCA carriers, especially those with BRCA1, may have reduced ovarian reserve and potentially poorer oocyte yield. Nevertheless, their conclusions mostly relied on AMH and AFC data instead of aggregated fertility preservation outcomes. Our results are also consistent with the earlier meta-analysis by Gasparri et al. (12), which examined ovarian reserve markers in women with and without BRCA pathogenic variants. Their study reported lower AMH levels among BRCA1 mutation carriers, suggesting an accelerated decline in ovarian reserve. However, their review could include just two studies specific to BC patients. Our results support these findings by indicating that carriers of BRCA mutations among BC patients have impaired ovarian function. However, we also add to the body of data by demonstrating substantial reductions in retrieved and mature oocytes, which are more clinically significant fertility preservation outcomes. Furthermore, in contrast to previous studies (12, 17, 18), our study distinctly includes ER status, providing a more comprehensive overview of evidence on impact of both BRCA mutation and receptor status on fertility outcomes of BC patients.
The pooled analysis indicated that carriers of the BRCA mutation exhibited a tendency of diminished oocyte yield and significantly lower maturation rates. In contrast, AMH levels and AFC did not exhibit significant differences between the groups. It is crucial to remember that AMH and AFC may understate the qualitative effect of BRCA mutations on oocyte competence and are not perfect indicators of reproductive capacity (39). On the pathophysiological perspective, it seems plausible for BRCA mutations to affect oocyte production and maturation rates due to the pivotal function of BRCA1 and BRCA2 in the homologous recombination repair of DNA double-strand breaks (DSBs). Loss-of-function mutations hinder the identification and repair of double-strand breaks (DSBs) in oocytes, hence expediting follicular atresia and ovarian aging (35, 40). At the cellular level, BRCA-deficient oocytes exhibit impaired RAD51 loading and accumulate unrepaired DSBs, which are indicated by elevated γ-H2AX foci. This results in checkpoint failure and increased primordial follicle apoptosis (41, 42). In addition to repairing DSBs, BRCA proteins preserve telomere integrity and regulate replication forks and therefore their absence can fosters genomic instability, meiotic errors, and aneuploidy during oogenesis (43, 44). Our findings extend these molecular insights, illustrating that DNA repair failure in BRCA carriers significantly influences clinically relevant fertility preservation outcomes, including oocyte yield and maturation.
Our meta-analysis also demonstrated that ER-positive patients exhibit higher AFC and greater oocyte retrieval compared with ER-negative patients. These results may be explained by several mechanisms. By primarily regulating granulosa cell proliferation, differentiation, and gonadotropin sensitivity, estrogen signaling plays a crucial and permissive role in folliculogenesis. Estrogens synthesized by granulosa cells bind with ERs (ERα and ERβ) in granulosa and theca cells, enhancing FSH-induced follicle growth, antral follicle survival, and oocyte maturation (45, 46). Mechanistically, ER activation upregulates genes implicated in granulosa cell proliferation. It also enhances FSH receptor expression and downstream cAMP signaling, thereby increasing the population of follicles responsive to exogenous gonadotropin stimulation during controlled ovarian stimulation (45, 46). As a result, patients with ER-positive tumors probably have a systemic endocrine environment (or preserved intragonadal estrogen signaling) that is better for recruiting and maturing antral follicles. This is in line with the higher AFC and oocyte yields we found in our meta-analysis. In addition to direct impacts on follicles, clinical and logistical considerations associated with ER positive status can enhance this biological advantage. ER-positive BC are frequently identified at earlier stages, facilitating the implementation of planned, letrozole-enhanced stimulation protocols that decrease peak circulating estradiol levels while preserving follicular response. Studies have shown that letrozole co-treatment sustains oocyte yield while minimizing estrogen exposure (47), a methodology widely adopted and endorsed in modern fertility-preservation practices. In contrast, ER-negative and triple-negative cancers require more urgent systemic therapy, which can narrow the window for optimum stimulation and force shorter or changed protocols that diminish oocyte yield (45, 47, 48). These biological (ER-mediated folliculogenesis) and pragmatic (timing and protocol choice) factors likely elucidate the superior ovarian response observed in ER-positive individuals compared to their ER-negative counterparts in our pooled analysis.
While the aim of this review was to assess the impact of all types of HR status and fertility outcomes, our results were limited to ER only due to paucity of data on PR or HER2 expression. BC with HER2 is often seen in young women and is aggressive in nature with poor patient survival (49). Literature suggests that HER2 expression may be associated with reduced oocyte maturation rate but data remains limited (50). Likewise, therapies specifically targeting HER2 like trastuzumab are being widely used but with limited data on their impact on fertility. Animal studies have shown that trastuzumab effectively mitigated vascular damage and apoptosis induced by cyclophosphamide and paclitaxel, leading to an increased ovarian reserve post-treatment and indicating a potential protective effect (51). However, how these therapies affect human fertility outcomes remain to be studied.
The timing of fertility preservation in young BC patients continues to be a persistent issue. The effects of cancer treatment on ovarian reserve and fertility outcomes contrast with concerns over the influence of controlled ovarian stimulation and assisted reproductive technologies on disease outcomes. In all included studies, fertility preservation was conducted prior to the commencement of anti-cancer therapy due to the established toxicity of BC treatment. Chemotherapy is highly deleterious, directly harming oocytes, diminishing antral follicle count and anti-AMH levels, and causing treatment-related amenorrhea or premature ovarian insufficiency (52, 53). Hormonal medications, notably long-term tamoxifen, may not directly impair ovarian reserve, but they do delay childbirth and lead to transitory amenorrhea, limiting the reproductive window (54, 55). Novel targeted agents, including PARP and CDK4/6 inhibitors, pose further issues by compromising follicular integrity and granulosa cell functionality (56, 57), whereas immunotherapies and checkpoint inhibitors may induce primary or secondary hypogonadism (58, 59). Significantly, findings from an extensive meta-analysis demonstrate that fertility preservation methods—such as controlled ovarian stimulation, oocyte and embryo cryopreservation, and assisted reproductive technologies—are oncologically safe, exhibiting no heightened recurrence risk and even a tendency toward enhanced outcomes, including diminished recurrence and mortality rates (60). Additionally, this advantageous trend was noted in HR–positive subgroups and among patients undergoing neoadjuvant chemotherapy. Collectively, these data emphasize the necessity of including fertility preservation into treatment planning at an early stage, while ensuring both patients and physicians of its safety in both pre- and post-treatment contexts.
This study has some limitations. There was a heterogeneity in study design, participant demographics, ovarian stimulation protocols, and fertility outcomes. This heterogeneity limited the ability to draw definitive conclusions and underscores the need for standardization in future research. Despite this limitation, the comprehensive approach and adherence to PRISMA guidelines enhance the reliability of the review. Many studies did not adequately adjust for patient age, a key determinant of ovarian reserve and fertility outcomes, which may have confounded the findings. There was also a substantial heterogeneity in study designs and ovarian stimulation protocols, including variations in the use of random-start regimens, letrozole-based approaches, and gonadotropins, limiting comparability across studies. And, most included studies focused on surrogate markers such as oocyte yield, AMH levels, and antral follicle counts, without reporting long-term reproductive outcomes like pregnancies or live births, thereby restricting the clinical applicability of the results. Additional limitations of the study include reliance on retrospective studies from different geographic regions, which carry a risk of selection bias, and the absence of data on long-term reproductive outcomes (pregnancies, live births).
Future research must rectify these deficiencies by including more homogenous patient cohort and examining long-term reproductive outcomes especially pregnancy rates and live-birth rates. Studies are also needed to identify the molecular and clinical processes by which BRCA mutations and HR status affect fertility. Furthermore, there is a need for establishing consistent protocols for ovarian stimulation in BC patients with varying receptor and mutation profiles. Research should focus in developing the best ovarian stimulation protocol for optimal fertility outcomes in these patients.
Conclusions
This systematic review and meta-analysis reveals that BRCA mutations seems to be associated with considerably diminished mature oocyte production during fertility preservation in BC patients. On the contrary, ER-positive status was associated with high AFC and oocyte yield indicating a more advantageous ovarian response. The present findings are from a limited number of heterogenous studies and hence must be interpreted with caution.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: PubMed, Embase, Scopus, and Web of Science databases.
Author contributions
LY: Conceptualization, Writing – original draft, Writing – review & editing. WY: Data curation, Formal Analysis, Methodology, Writing – original draft, Software. HG: Data curation, Formal Analysis, Methodology, Writing – review & editing, Project administration, Software, Validation.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, and Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. (2021) 13:4287. doi: 10.3390/cancers13174287
2. Clusan L, Le Goff P, Flouriot G, and Pakdel F. A closer look at estrogen receptor mutations in breast cancer and their implications for estrogen and antiestrogen responses. Int J Mol Sci. (2021) 22:756. doi: 10.3390/ijms22020756
3. Wei S. Hormone receptors in breast cancer: An update on the uncommon subtypes. Pathol Res Pract. (2023) 250:154791. doi: 10.1016/j.prp.2023.154791
4. Walter V, Fischer C, Deutsch TM, Ersing C, Nees J, Schütz F, et al. Estrogen, progesterone, and human epidermal growth factor receptor 2 discordance between primary and metastatic breast cancer. Breast Cancer Res Treat. (2020) 183:137–44. doi: 10.1007/s10549-020-05746-8
5. Ameli F, Entezarian M, Masir N, and Chin TG. Expression of estrogen receptor (ER), progesterone receptor (PR), her2/neu in various types of epithelial ovarian tumors. J Obstet Gynecol Cancer Res. (2024) 9:7–13. doi: 10.30699/jogcr.9.1.7
6. Patel HK and Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. (2018) 186:1–24. doi: 10.1016/j.pharmthera.2017.12.012
7. Yin L, Duan J-J, Bian X-W, and Yu S-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res BCR. (2020) 22:61. doi: 10.1186/s13058-020-01296-5
8. Radenkovic S, Konjevic G, Isakovic A, Stevanovic P, Gopcevic K, and Jurisic V. HER2-positive breast cancer patients: correlation between mammographic and pathological findings. Radiat Prot Dosimetry. (2014) 162:125–8. doi: 10.1093/rpd/ncu243
9. Radenkovic S, Milosevic Z, Konjevic G, Karadzic K, Rovcanin B, Buta M, et al. Lactate dehydrogenase, catalase, and superoxide dismutase in tumor tissue of breast cancer patients in respect to mammographic findings. Cell Biochem Biophys. (2013) 66:287–95. doi: 10.1007/s12013-012-9482-7
10. Shiovitz S and Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol Off J Eur Soc Med Oncol. (2015) 26:1291–9. doi: 10.1093/annonc/mdv022
11. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. (1998) 62:676–89. doi: 10.1086/301749
12. Gasparri ML, Di Micco R, Zuber V, Taghavi K, Bianchini G, Bellaminutti S, et al. Ovarian reserve of women with and without BRCA pathogenic variants: A systematic review and meta-analysis. Breast Edinb Scotl. (2021) 60:155–62. doi: 10.1016/j.breast.2021.09.006
13. El Saghir NS, Khalil LE, El Dick J, Atwani RW, Safi N, Charafeddine M, et al. Improved survival of young patients with breast cancer 40 years and younger at diagnosis. JCO Glob Oncol. (2023) 9:e2200354. doi: 10.1200/GO.22.00354
14. Benedict C, Thom B, and Kelvin JF. Fertility preservation and cancer: challenges for adolescent and young adult patients. Curr Opin Support Palliat Care. (2016) 10:87–94. doi: 10.1097/SPC.0000000000000185
15. Boutas I, Kontogeorgi A, Koufopoulos N, Dimas DT, Sitara K, Kalantaridou SN, et al. Breast cancer and fertility preservation in young female patients: A systematic review of the literature. Clin Pract. (2023) 13:1413–26. doi: 10.3390/clinpract13060127
16. Roberts J, Ronn R, Tallon N, and Holzer H. Fertility preservation in reproductive-age women facing gonadotoxic treatments. Curr Oncol Tor Ont. (2015) 22:e294–304. doi: 10.3747/co.22.2334
17. Hong YH, Park C, Paik H, Lee K-H, Lee JR, Han W, et al. Fertility preservation in young women with breast cancer: A review. J Breast Cancer. (2023) 26:221–42. doi: 10.4048/jbc.2023.26.e28
18. Dias Nunes J, Demeestere I, and Devos M. BRCA mutations and fertility preservation. Int J Mol Sci. (2023) 25:204. doi: 10.3390/ijms25010204
19. Mahajan N. Fertility preservation in female cancer patients: An overview. J Hum Reprod Sci. (2015) 8:3–13. doi: 10.4103/0974-1208.153119
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. Ottawa Hospital Research Institute. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 12, 2025).
22. Wan X, Wang W, Liu J, and Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Liu S-M, Huang S-Y, Wu H-M, Chang C-L, and Huang H-Y. Ovarian stimulation response and fertility outcomes in patients with breast cancer across different stages, grades, and hormone receptor status for fertility preservation. J Formos Med Assoc Taiwan Yi Zhi. (2025) 124:241–5. doi: 10.1016/j.jfma.2024.08.031
24. El Moujahed L, Philis R, Grynberg M, Laot L, Mur P, Amsellem N, et al. Response to ovarian stimulation for urgent fertility preservation before gonadotoxic treatment in BRCA-pathogenic-variant-positive breast cancer patients. Cancers. (2023) 15:895. doi: 10.3390/cancers15030895
25. Prokurotaite E, Condorelli M, Dechene J, Bouziotis J, Lambertini M, and Demeestere I. Impact of breast cancer and germline BRCA pathogenic variants on fertility preservation in young women. Life Basel Switz. (2023) 13:930. doi: 10.3390/life13040930
26. Sii S, Polyakov A, Rozen G, Agresta F, and Stern K. Controlled ovarian hyperstimulation in breast cancer patients: Does oestrogen receptor status make a difference? Aust N Z J Obstet Gynaecol. (2023) 63:774–9. doi: 10.1111/ajo.13721
27. Kim Y-R. Mediating effect of self-cognitive oral health status on the effect of obstructive sleep apnea risk factors on quality of life (HINT-8) in middle-aged korean women: the korea national health and nutrition examination survey. Life Basel Switz. (2022) 12:1569. doi: 10.3390/life12101569
28. Balayla J, Tulandi T, Buckett W, Holzer H, Steiner N, Shrem G, et al. Outcomes of ovarian stimulation and fertility preservation in breast cancer patients with different hormonal receptor profiles. J Assist Reprod Genet. (2020) 37:913–21. doi: 10.1007/s10815-020-01730-9
29. Ponce J, Fernandez-Gonzalez S, Calvo I, Climent M, Peñafiel J, Feliubadaló L, et al. Assessment of ovarian reserve and reproductive outcomes in BRCA1 or BRCA2 mutation carriers. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. (2020) 30:83–8. doi: 10.1136/ijgc-2019-000626
30. Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, et al. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet. (2020) 37:709–15. doi: 10.1007/s10815-019-01658-9
31. Gunnala V, Fields J, Irani M, D’Angelo D, Xu K, Schattman G, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. (2019) 111:363–71. doi: 10.1016/j.fertnstert.2018.10.014
32. Grynberg M, Dagher Hayeck B, Papanikolaou EG, Sifer C, Sermondade N, and Sonigo C. BRCA1/2 gene mutations do not affect the capacity of oocytes from breast cancer candidates for fertility preservation to mature in vitro. Hum Reprod Oxf Engl. (2019) 34:374–9. doi: 10.1093/humrep/dey358
33. Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol Off J Eur Soc Med Oncol. (2018) 29:237–43. doi: 10.1093/annonc/mdx639
34. Turan V, Bedoschi G, Emirdar V, Moy F, and Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci Thousand Oaks Calif. (2018) 25:26–32. doi: 10.1177/1933719117728800
35. Oktay K, Kim JY, Barad D, and Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol Off J Am Soc Clin Oncol. (2010) 28:240–4. doi: 10.1200/JCO.2009.24.2057
36. Fabiani C, Guarino A, Meneghini C, Licata E, Paciotti G, Miriello D, et al. Oocyte quality assessment in breast cancer: implications for fertility preservation. Cancers. (2022) 14:5718. doi: 10.3390/cancers14225718
37. Grynberg M, Zeghari F, Peigné M, Benoit A, Rakrouki S, Sifer C, et al. Effect of breast cancer prognostic factors on ovarian reserve and response in fertility preservation. Reprod BioMed Online. (2024) 49:104109. doi: 10.1016/j.rbmo.2024.104109
38. Liu S-M, Huang S-Y, Wu H-M, Chang C-L, and Huang H-Y. Ovarian stimulation response and fertility outcomes in patients with breast cancer across different stages, grades, and hormone receptor status for fertility preservation. J Formos Med Assoc. (2024) 124(3):241–5. doi: 10.1016/j.jfma.2024.08.031
39. Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, et al. Antral follicle count determines poor ovarian response better than anti-Müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. (2013) 30:657–65. doi: 10.1007/s10815-013-9975-3
40. Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. (2013) 5:172ra21. doi: 10.1126/scitranslmed.3004925
41. Pathania S, Bade S, Le Guillou M, Burke K, Reed R, Bowman-Colin C, et al. BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat Commun. (2014) 5:5496. doi: 10.1038/ncomms6496
42. Lin W, Titus S, Moy F, Ginsburg ES, and Oktay K. Ovarian aging in women with BRCA germline mutations. J Clin Endocrinol Metab. (2017) 102:3839–47. doi: 10.1210/jc.2017-00765
43. Oktay K, Turan V, Titus S, Stobezki R, and Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod. (2015) 93:67. doi: 10.1095/biolreprod.115.132290
44. Xu X-L, Huang Z-Y, Yu K, Li J, Fu X-W, and Deng S-L. Estrogen biosynthesis and signal transduction in ovarian disease. Front Endocrinol. (2022) 13:827032. doi: 10.3389/fendo.2022.827032
45. Chauvin S, Cohen-Tannoudji J, and Guigon CJ. Estradiol signaling at the heart of folliculogenesis: its potential deregulation in human ovarian pathologies. Int J Mol Sci. (2022) 23:512. doi: 10.3390/ijms23010512
46. Lee EB, Chakravarthi VP, Wolfe MW, and Rumi MAK. ERβ Regulation of gonadotropin responses during folliculogenesis. Int J Mol Sci. (2021) 22:10348. doi: 10.3390/ijms221910348
47. Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. (2006) 91:3885–90. doi: 10.1210/jc.2006-0962
48. Benvenuti C, Laot L, Grinda T, Lambertini M, Pistilli B, and Grynberg M. Is controlled ovarian stimulation safe in patients with hormone receptor-positive breast cancer receiving neoadjuvant chemotherapy? ESMO Open. (2024) 9:102228. doi: 10.1016/j.esmoop.2023.102228
49. Ruiz-Saenz A and Moasser MM. Targeting HER2 by combination therapies. J Clin Oncol Off J Am Soc Clin Oncol. (2018) 36:808–11. doi: 10.1200/JCO.2017.77.1899
50. Raad J, Sonigo C, Benoit A, Cedrin-Durnerin I, Sifer C, Sermondade N, et al. Influence of breast cancer prognostic factors on oocyte in vitro maturation outcomes performed for urgent fertility preservation. Hum Reprod Oxf Engl. (2022) 37:1480–8. doi: 10.1093/humrep/deac109
51. Rosario R, Cui W, and Anderson RA. Potential ovarian toxicity and infertility risk following targeted anti-cancer therapies. Reprod Fertil. (2022) 3:R147–62. doi: 10.1530/RAF-22-0020
52. Çelebi F, Ordu Ç, Ilgün S, Oztürk A, Erdoğan Iyigün Z, Alço G, et al. The effect of systemic chemotherapy on ovarian function: A prospective clinical trial. Eur J Breast Health. (2020) 16:177–82. doi: 10.5152/ejbh.2020.5114
53. Mauri D, Gazouli I, Zarkavelis G, Papadaki A, Mavroeidis L, Gkoura S, et al. Chemotherapy associated ovarian failure. Front Endocrinol. (2020) 11:572388. doi: 10.3389/fendo.2020.572388
54. Llarena NC, Estevez SL, Tucker SL, and Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. (2015) 107:djv202. doi: 10.1093/jnci/djv202
55. Kim HJ, Noh WC, Nam SJ, Park B-W, Lee ES, Im SA, et al. Five-year changes in ovarian function restoration in premenopausal patients with breast cancer taking tamoxifen after chemotherapy: An ASTRRA study report. Eur J Cancer Oxf Engl. (2021) 151:190–200. doi: 10.1016/j.ejca.2021.03.017
56. Li J, Li Q, Zhang L, Zhang S, and Dai Y. Poly-ADP-ribose polymerase (PARP) inhibitors and ovarian function. BioMed Pharmacother Biomed Pharmacother. (2023) 157:114028. doi: 10.1016/j.biopha.2022.114028
57. Scavone G, Ottonello S, Blondeaux E, Arecco L, Scaruffi P, Stigliani S, et al. The role of cyclin-dependent kinases (CDK) 4/6 in the ovarian tissue and the possible effects of their exogenous inhibition. Cancers. (2023) 15:4923. doi: 10.3390/cancers15204923
58. Garutti M, Lambertini M, and Puglisi F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open. (2021) 6:100276. doi: 10.1016/j.esmoop.2021.100276
59. Helgadottir H, Matikas A, Fernebro J, Frödin J-E, Ekman S, and Rodriguez-Wallberg KA. Fertility and reproductive concerns related to the new generation of cancer drugs and the clinical implication for young individuals undergoing treatments for solid tumors. Eur J Cancer Oxf Engl. (2024) 202:114010. doi: 10.1016/j.ejca.2024.114010
60. Arecco L, Blondeaux E, Bruzzone M, Ceppi M, Latocca MM, Marrocco C, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod Oxf Engl. (2022) 37:954–68. doi: 10.1093/humrep/deac035
Keywords: fertility preservation, breast cancer, brca mutation, hormone receptor status, ovarian stimulation
Citation: Ye L, Yang W and Guan H (2025) The impact of BRCA mutation and hormone receptor status on the outcomes of fertility preservation in breast cancer patients: a systematic review and meta-analysis. Front. Oncol. 15:1639420. doi: 10.3389/fonc.2025.1639420
Received: 02 June 2025; Accepted: 03 November 2025;
Published: 28 November 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Vladimir Jurisic, University of Kragujevac, SerbiaSaber A Amin, University of Nebraska Medical Center, United States
Copyright © 2025 Ye, Yang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiyuan Guan, R3VhbkdoeTE2MjhAMTYzLmNvbQ==
 Liyu Ye
Liyu Ye Huiyuan Guan
Huiyuan Guan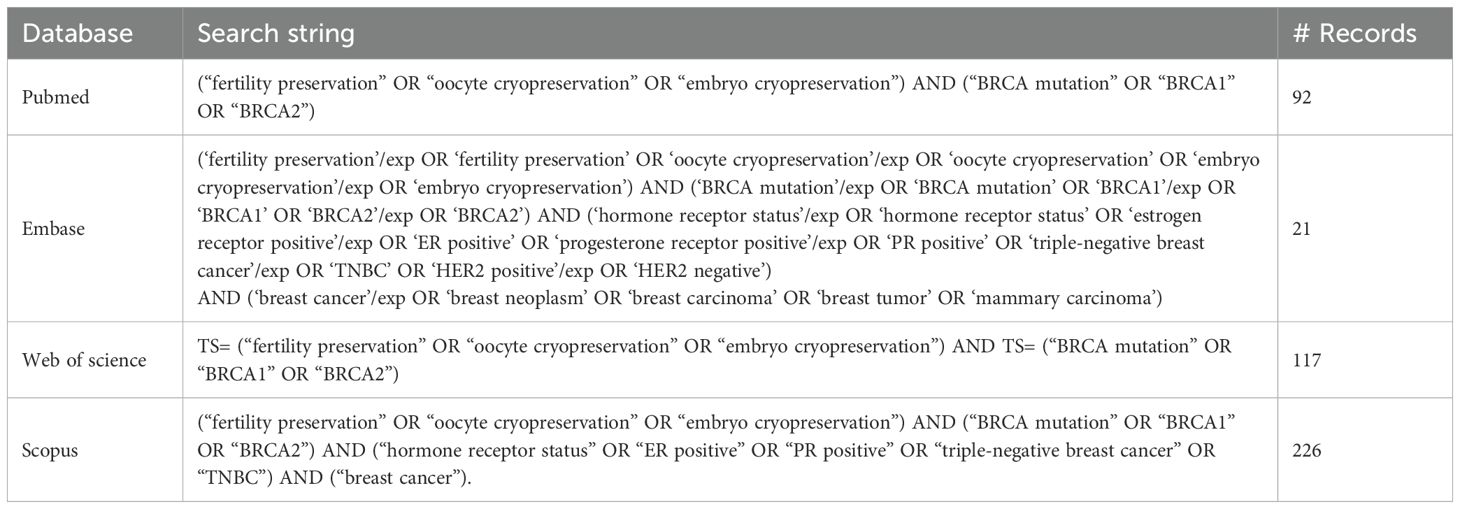
![A forest plot comparing BRCA positive and negative groups across ten studies. Each study shows mean, standard deviation, total sample size, and weight percentage. The mean differences are plotted with confidence intervals, depicted as green squares and horizontal lines on the graph. The overall effect size is represented by a diamond. Statistical details include heterogeneity measures (Tau² = 5.28; Chi² = 27.80, df = 9, P = 0.001; I² = 68%) and an overall effect of -1.37 [-3.13, 0.40].](https://www.frontiersin.org/files/Articles/1639420/fonc-15-1639420-HTML/image_m/fonc-15-1639420-g002.jpg)