- 1Department of Radiology, Shandong Provincial Hospital, Jinan, China
- 2Department of Radiology, Linyi People’s Hospital, Linyi, China
A 40-year-old man with two accidentally discovered subcapsular liver tumors was admitted to our hospital for further treatment. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed two hypervascular lesions, which showed marked enhancement in arterial phase and prolonged enhancement in portal venous phase. The patient had not received any prior treatment, and the two lesions were pathologically confirmed after partial hepatectomy. Hematoxylin-eosin staining revealed a partially cystic, highly vascular, and well-encapsulated neoplasm. Immunohistochemistry findings demonstrated that the epithelioid tumor cells were positive for vimentin, calretinin, WT-1, cytokeratin, CD 31, CD 34, and D2-40, which supported their mesothelial origin. Immunohistochemistry for a mesothelial marker should be performed for determining the presence of an adenomatoid tumor when benign epithelioid cells are seen.
Introduction
Adenomatoid tumors (AT) are benign and typically well-circumscribed neoplasms of mesothelial origin (1), which were first described by Golden and Ash (2). They mostly occur in the male and female genital tracts during a patient’s reproductive age. Extragenital adenomatoid tumors in adrenal gland (3), heart (4), mediastinum (5), liver (6–10), pancreas (11), peritoneum (12, 13), and pleura (14) have also been rarely reported. The tumors are usually discovered accidentally and are easy to be misdiagnosed by clinical and imaging examination.
We report a case of surgically confirmed liver adenomatoid tumors. To our knowledge, this is the first report of a case with two concurrent adenomatoid tumors in the liver.
Case description
A 40-year-old man was admitted to the hospital for the accidentally discovered tumors of the liver. No obvious discomfort and remarkable previous medical history were reported. Physical examination was normal. Tumor marker levels, including carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 125 and α-fetoprotein levels were within the normal range. The patient was negative for both the hepatitis B virus surface antigen and the hepatitis C virus antibody. No previous treatment was received. The patient denied any genetic basis, relevant family history or relationship to any other disease or syndrome. Contrast material-enhanced abdominal computed tomography (CT) revealed two relatively well-circumscribed oval lesions, both of which were on the segment VI. Magnetic resonance imaging (MRI) was then performed for further evaluation.
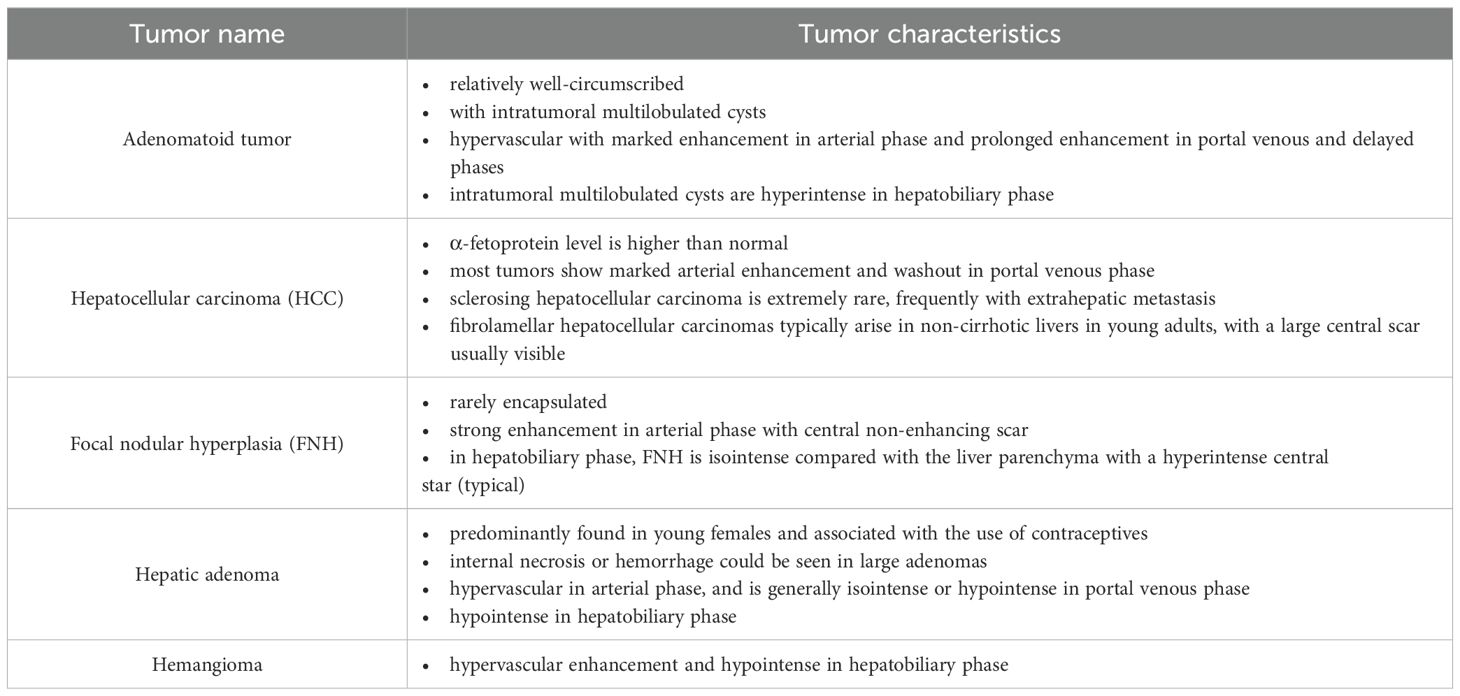
On abdominal CT images, the larger mass was heterogeneous with some intratumoral multilobulated cysts, and the smaller nodule was homogeneous, measuring 4.5 cm and 1.5 cm correspondingly, both of which were closely related to the peritoneum. The tumors were hypervascular, demonstrating marked enhancement in arterial phase and prolonged enhancement in portal venous and delayed phases after intravenous injection of contrast material (Figure 1).
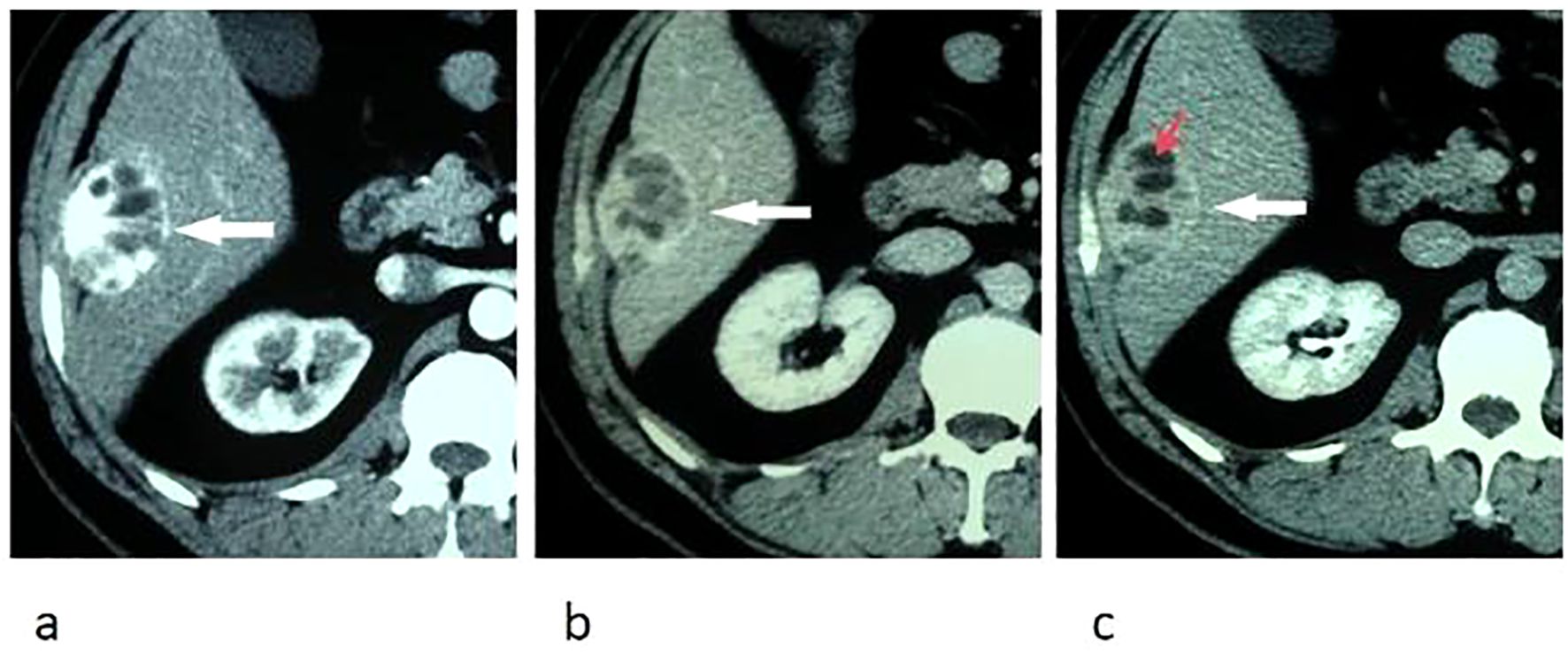
Figure 1. Transverse CT images of arterial phase (a), portal venous phase (b), and delay phase (c) show marked and prolonged enhancement of an oval liver mass, with unenhanced intratumoral cystic areas (red arrow) and enhanced capsule (white arrow).
Upper abdominal MRI (3.0T, Magnetom Verio, Siemens, Germany) was subsequently performed. The large liver mass was heterogeneous on MR images, with hypointense on T1-weighted images and hyperintense on T2-weighted images. There were multi-lobulated, cystic areas within the mass. After injection of gadoxetic acid (Gd-BOPTA, MultiHance, Shanghai Bracco Sine Pharmaceutical, China), the mass showed inhomogeneous hyperintense in arterial phase and revealed prolonged enhancement in the portal venous phase and delayed phase (3 min). In hepatobiliary phase, the mass was hypointense with the cystic areas hyperintense. In addition, the liver capsule and the adjacent peritoneum also showed obvious enhancement (Figure 2). The nodule was hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging. It revealed marked enhancement in arterial phase, and showed prolonged enhancement in portal venous phase and delayed phase (Figure 3).
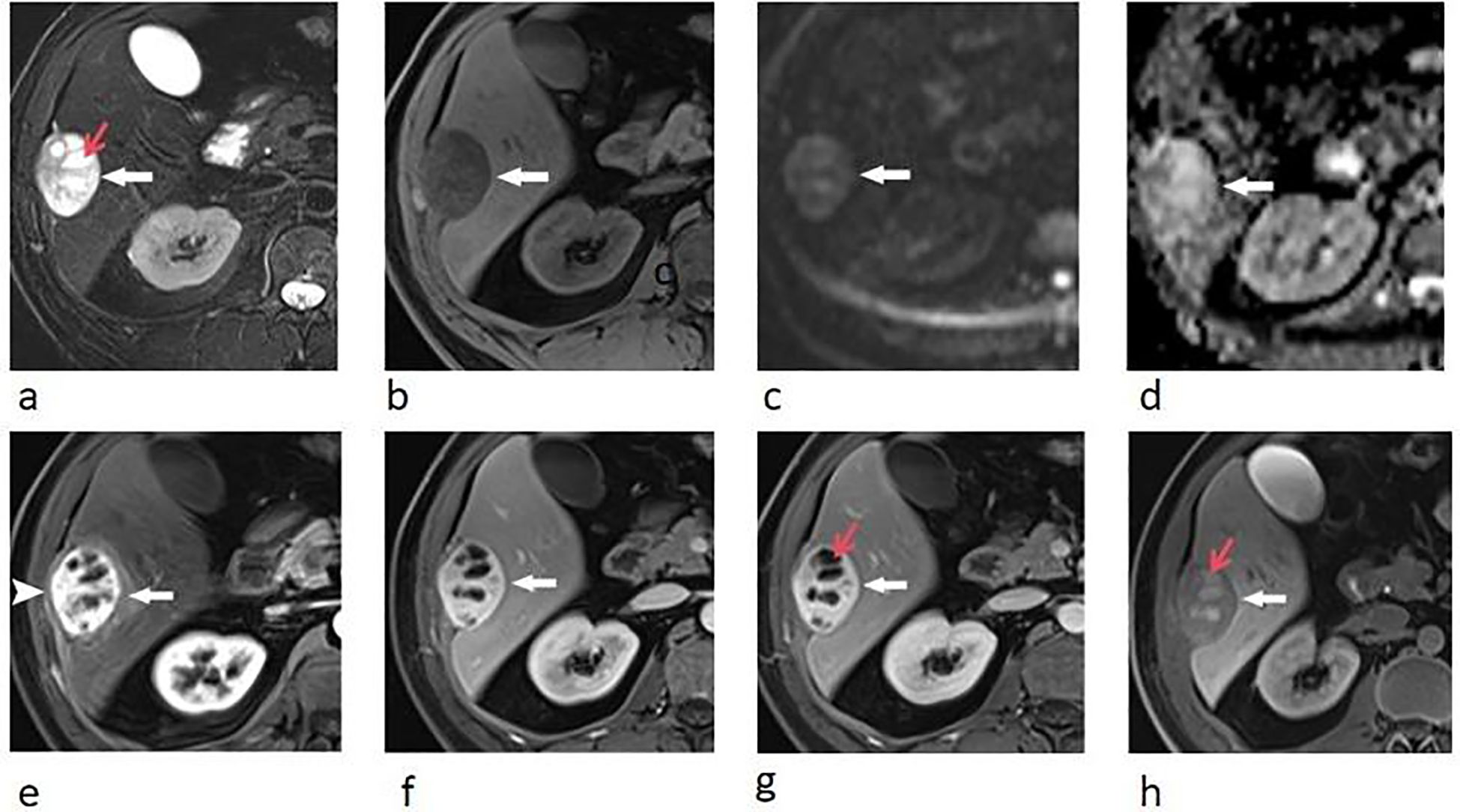
Figure 2. Transverse breath-hold turbo spin-echo T2-weighted image (repetition time msec/echo time msec, 3000/104) (a) and fat-suppressed T1-weighted volume interpolated body examination (VIBE) image (repetition time msec/echo time msec, 3.92/1.39) (b) shows a well-circumscribed liver mass with intratumoral cystic areas (red arrow). Diffusion-weighted image (repetition time msec/echo time msec, 4000/73, b = 800 s/mm2) (c) and apparent diffusion coefficient (ADC) (d) map show that the water mobility of the mass was slightly restricted. The water mobility of intratumoral cystic areas was not restricted. Transverse enhanced VIBE images of arterial phase (e), portal venous phase (f), and delay phase (g) show marked and prolonged enhancement of the liver mass (arrows), with unenhanced intratumoral cystic areas (red arrow). The liver capsule also showed enhancement (arrow head). In hepatobiliary phase (h), the mass was hypointense compared with the liver parenchyma, with these cystic areas hyperintense (red arrow).
Figure 3. Transverse breath-hold turbo spin-echo T2-weighted image (repetition time msec/echo time msec, 3000/104) (a) and fat-suppressed T1-weighted volume interpolated body examination (VIBE) image (repetition time msec/echo time msec, 3.92/1.39) (b) shows a well-circumscribed liver nodule. Transverse arterial phase (c) and portal venous phase (d) show marked enhancement of the nodule.
Given the uncertainty of the diagnosis and the patient’s anxiety about the tumors, the patient chose to undergo surgical treatment. The two liver lesions were resected in our hospital through partial hepatectomy of segment VI. After the surgery, the patient recovered well and expressed satisfaction about the treatment.
Grossly, the tumors showed a hemorrhagic cut surface and was encapsulated with a complete capsule, the larger mass also showed obvious cystic structures (Figure 4). Hematoxylin-eosin staining revealed a partially cystic, highly vascular, and well-encapsulated neoplasm. Immunohistochemistry findings demonstrated that the epithelioid tumor cells were positive for vimentin, calretinin, WT-1, cytokeratin, CD 31, CD 34, and D2-40, which supported their mesothelial origin. The Ki-67 proliferation index was less than 5% (Figure 4).
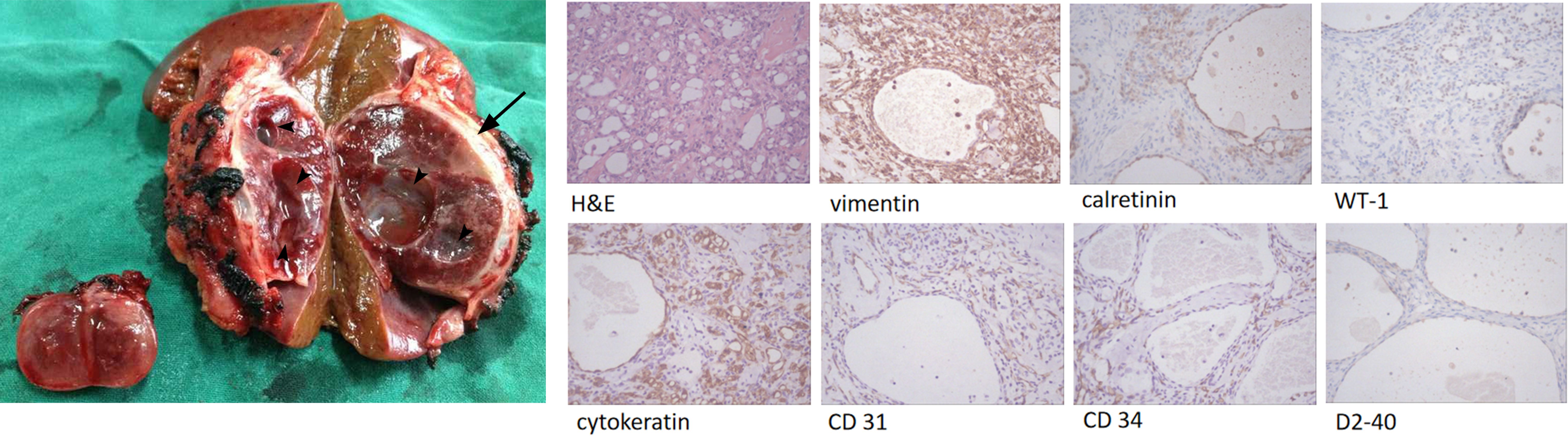
Figure 4. Gross pathologic specimen shows a heterogeneous well-circumscribed and encapsulated tumor (arrow) with multiple cystic areas (arrow heads), and a small well-circumscribed nodule. High-power-field view of the resected tumor specimen show a lesion with various-sized capillary anastomosis and fibrotic component (H&E). Immunohistochemistry shows positive for vimentin, calretinin, WT-1, cytokeratin, CD 31, CD 34, and D2-40.
Postoperative follow-up was conducted annually, and till the last follow-up, no recurrence or metastasis has been detected (Figure 5).
Discussion
Adenomatoid tumors are benign uncommon neoplasms of mesothelial origin. Only five cases of hepatic adenomatoid tumor have been described in the medical literature (6–10). We report a case of two concurrent liver adenomatoid tumors, which has not been reported before.
Due to the different content of cystic spaces, smooth muscle, and fibrous tissue, adenomatoid tumors could show various image features, leading them difficult to be differentiated from other benign or malignant tumors on clinical CT or MRI examination (8). In this case, the preoperative imaging differential diagnosis of the liver mass included hepatocellular carcinoma (HCC), focal nodular hyperplasia (FNH), hepatic adenoma and hemangioma because of the hypervascular feature of the tumor in this patient (Table 1).
HCC is the most common primary malignant tumor of the liver. It typically appears as a liver lesion with marked arterial enhancement and washout in portal venous phase. Although some variants of HCC may not show washout in portal venous phase, such as sclerosing and fibrolamellar hepatocellular carcinomas, which have abundant fibrous stroma and exhibit prolonged enhancement (15), they still should not taken into consideration because of the normal α-fetoprotein level. In addition, sclerosing hepatocellular carcinoma is extremely rare, frequently with extrahepatic metastasis in most cases. Fibrolamellar hepatocellular carcinomas typically arise in non-cirrhotic livers in young adults, with a large central scar usually visible (16). These findings were not present in this patient.
Focal nodular hyperplasia (FNH) is a proliferation of non-neoplastic hepatocytes that are abnormally arranged. It is rarely encapsulated. FNH often shows strong enhancement in arterial phase with central non-enhancing scar. In hepatobiliary phase, the tumor is isointense compared with the liver parenchyma with a hyperintense central star, which is typical on MRI. The central scar is a helpful distinguishing feature of FNH (although not specific) that can be seen in 78% of lesions (17, 18).
Hepatic adenoma is a rare monoclonal benign liver tumour, predominantly found in young females and associated with the use of contraceptives (19). It is formed by large plats or cord cells that are similar to hepatocytes (20). Internal necrosis or hemorrhage could be seen in large adenomas (17). Lesions often appear hypervascular in arterial phase, and are generally isointense or hypointense to the surrounding liver in portal venous phase. In hepatobiliary phase, it is hypointense because of the lack of normal hepatic cells which can uptake Gd-BOPTA. In addition, some hepatic adenomas containing fatty tissue will show signal attenuation on out-phase image, which was not seen in this case.
Atypical hemangiomas should carefully be differentiated. Liang C et al. has reported that some atypical hemangiomas could have cystic changes and even fluid-fluid level (21). Its hypervascular enhancement and hypointense in hepatobiliary phase could mimic the right diagnosis of this patient.
Hepatocyte specific contrast agents including gadoxetic acid (Gd-EOB-DTPA, Primovist, Bayer, Germany) and Gd-BOPTA were introduced in recent years. The hepatocytes with normal function which have the cloned organic anion transporting polypeptides (OATPs) specifically take up these agents, and excreted them by multidrug resistance-associated proteins (MRPs) to bile canaliculi (MRP2 = apical transporter) or sinusoidal space (MRP3, MRP4 = basolateral transporters) (22). Hepatobiliary phase (about 20 to 90 minutes later after intravenous administration of these agents) helps to differentiate malignant from benign lesions. In hepatobiliary phase, benign lesions with normally functioning hepatocytes will show iso- or hyperintense compared with liver parenchyma, and vice-versa.
Five cases of hepatic adenomatoid tumor have been described in the medical literature, and among them, only Kim JB et al. (8) mentioned the use of hepatocyte specific contrast agent. In his study, he used Gd-EOB-DTPA as the contrast agent. The tumor in their case had some similar multi-lobulated, cystic areas as seen in our case, but in our case, the cystic areas within the larger mass showed marked hyperintense in hepatobiliary phase, which was not reported in their study.
Gd-BOPTA has both the function of hepatocyte specific contrast agent and tissue interstitial contrast agent. Therefore, in tissues with abundant fibrous structures, Gd-BOPTA can remain in the tissue interstitial space for a longer period of time. It is a well-established phenomenon that the tumor with abundant fibrous stroma could reveal persistent enhancement up to 4h after administration of gadolinium chelates (23). Hematoxylin-eosin staining revealed that the lesion in our case has a large fibrotic component. So we hypothesized that the delayed enhancement of these cystic areas may be because that the interstitial space could contain contrast agent for a long time.
In summary, the diagnosis of liver adenomatoid tumor is challenging due to its rarity and the similar manifestation of other neoplasms. Immunohistochemistry analysis enabled us to identify tumor cells of mesothelial origin. Hypervascular and multi-lobulated, cystic areas are clues to the imaging diagnosis of them. In addition, in hepatobiliary phase, these cystic areas could show hyperintense when the lesion contains a large fibrotic component. Since this is the first case of two concurrent liver adenomatoid tumors, the etiology of dual tumor occurrence is still unclear. More researches are needed to explain this issue.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shandong Provincial Hospital Affiliated to Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LB: Writing – review & editing, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Medical and Health Science and Technology Development Plans of Shandong Province (grant number 202209011102).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karpathiou G, Hiroshima K, and Peoc’H M. Adenomatoid tumor: A review of pathology with focus on unusual presentations and sites, histogenesis, differential diagnosis, and molecular and clinical aspects with a historic overview of its description. Adv Anat Pathol. (2020) 27:394–407. doi: 10.1097/PAP.0000000000000278
3. Guan J, Zhao C, Li H, Zhang W, Lin W, Tang L, et al. Adenomatoid tumor of the adrenal gland: report of two cases and review of the literature. Front Endocrinol (Lausanne). (2021) 12:692553. doi: 10.3389/fendo.2021.692553
4. Natarajan S, Luthringer DJ, and Fishbein MC. Adenomatoid tumor of the heart: report of a case. Am J Surg Pathol. (1997) 21:1378–80. doi: 10.1097/00000478-199711000-00014
5. Plaza JA, Dominguez F, and Suster S. Cystic adenomatoid tumor of the mediastinum. Am J Surg Pathol. (2004) 28:132–8. doi: 10.1097/00000478-200401000-00016
6. Zhou R and Wang HL. Adenomatoid tumor of the liver with prominent admixed cavernous hemangioma-like vessels: an exceptional occurrence. Int J Surg Pathol. (2025) 33:1505–12. doi: 10.1177/10668969251323930
7. Adachi S, Yanagawa T, Furumoto A, Fujino S, Doi R, Dono K, et al. Adenomatoid tumor of the liver. Pathol Int. (2012) 62:153–4. doi: 10.1111/j.1440-1827.2011.02767.x
8. Kim JB, Yu E, Shim JH, Song GW, Kim GU, Jin YJ, et al. Concurrent hepatic adenomatoid tumor and hepatic hemangioma: a case report. Clin Mol Hepatol. (2012) 18:229–34. doi: 10.3350/cmh.2012.18.2.229
9. Nagata S, Aishima S, Fukuzawa K, Takagi H, Yonemasu H, Iwashita Y, et al. Adenomatoid tumour of the liver. J Clin Pathol. (2008) 61:777–80. doi: 10.1136/jcp.2007.054684
10. Hayes SJ, Clark P, Mathias R, Formela L, Vickers J, and Armstrong GR. Multiple adenomatoid tumours in the liver and peritoneum. J Clin Pathol. (2007) 60:722–4. doi: 10.1136/jcp.2005.035386
11. Overstreet K, Wixom C, Shabaik A, Bouvet M, and Herndier B. Adenomatoid tumor of the pancreas: a case report with comparison of histology and aspiration cytology. Mod Pathol. (2003) 16:613–7. doi: 10.1097/01.MP.0000072803.37527.C8
12. Wang LY, Zhong YQ, Li HG, Zeng YJ, Zhang SN, Chen WX, et al. Adenomatoid tumor of peritoneum–a case report. Zhonghua Yi Xue Za Zhi. (2005) 85:495–7.
13. Hatano Y, Hirose Y, Matsunaga K, Kito Y, Yasuda I, Moriwaki H, et al. Combined adenomatoid tumor and well differentiated papillary mesothelioma of the omentum. Pathol Int. (2011) 61:681–5. doi: 10.1111/j.1440-1827.2011.02720.x
14. Minato H, Nojima T, Kurose N, and Kinoshita E. Adenomatoid tumor of the pleura. Pathol Int. (2009) 59:567–71. doi: 10.1111/j.1440-1827.2009.02407.x
15. Mittal PK, Moreno CC, Kalb B, Mittal A, Camacho JC, Maddu K, et al. Primary biliary tract Malignancies: MRI spectrum and mimics with histopathological correlation. Abdom Imaging. (2015) 40:1520–57. doi: 10.1007/s00261-014-0300-0
16. Soussan M, Felden A, Cyrta J, Morere JF, Douard R, and Wind P. Case 198: solitary fibrous tumor of the liver. Radiology. (2013) 269:304–8. doi: 10.1148/radiol.13121315
17. Jang H-J, Yu H, and Kim TK. Imaging of focal liver lesions. Semin Roentgenology. (2009) 44:266–82. doi: 10.1053/j.ro.2009.05.008
18. Kamaya A, Maturen KE, Tye GA, Liu YI, Parti NN, and Desser TS. Hypervascular liver lesions. Semin Ultrasound CT MRI. (2009) 30:387–407. doi: 10.1053/j.sult.2009.06.001
19. Maillette de Buy Wenniger L, Terpstra V, and Beuers U. Focal nodular hyperplasia and hepatic adenoma: epidemiology and pathology. Dig Surg. (2010) 27:24–31. doi: 10.1159/000268404
20. Palmucci S. Focal liver lesions detection and characterization: The advantages of gadoxetic acid-enhanced liver MRI. World J Hepatol. (2014) 6:477–85. doi: 10.4254/wjh.v6.i7.477
21. Liang C, Yu R, Wang L, and Chen Y. Case series multilocular cystic hemangioma of the liver: Three cases and literature review. Med (Baltimore). (2024) 103:e39287. doi: 10.1097/MD.0000000000039287
22. Jeong WK, Kim YK, Song KD, Choi D, and Lim HK. The MR imaging diagnosis of liver diseases using gadoxetic acid: emphasis on hepatobiliary phase. Clin Mol Hepatol. (2013) 19:360–6. doi: 10.3350/cmh.2013.19.4.360
Keywords: adenomatoid tumor, liver, computed tomography, magnetic resonance imaging, case report
Citation: Bi L (2025) Case Report: Concurrent two adenomatoid tumors of liver. Front. Oncol. 15:1640018. doi: 10.3389/fonc.2025.1640018
Received: 03 June 2025; Accepted: 13 August 2025;
Published: 28 August 2025.
Edited by:
Theodoros Androutsakos, National and Kapodistrian University of Athens, GreeceReviewed by:
Kahan Mehta, GMERS Medical College, Gotri, IndiaNaganath Babu Lakshmanamoorthy, SRM Medical College Hospital and Research Centre, India
Copyright © 2025 Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Bi, bTUxMDQwNkAxMjYuY29t
 Lei Bi
Lei Bi