- 1Department of General Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2The Institute of Translational Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi, China
- 3Health Examination Center, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi, China
Background: Anorectal malignant melanoma (ARMM) is an exceedingly rare and highly aggressive malignancy characterized by low prevalence, high misdiagnosis rates, and frequent recurrence/metastasis.
Case report: This report details the case of a 51-year-old woman presenting with persistent bright red blood in her stool. Digital rectal examination revealed a firm, spherical mass approximately 4 cm from the anal verge. Colonoscopy identified a pedunculated polypoid lesion (~2.5 cm in diameter) near the anorectal junction. Based on clinical symptoms, physical findings, and endoscopic features, a high suspicion of rectal cancer was initially raised. However, subsequent histopathological evaluation of biopsy specimens revealed immunohistochemical positivity for MelanA, S100, and Ki-67 (~30%), suggesting a probable diagnosis of malignant melanoma. After completing preoperative contrast-enhanced abdominal CT and pelvic MRI examinations and excluding surgical contraindications, the patient underwent laparoscopic-assisted abdominoperineal resection (Miles procedure) and postoperative adjuvant therapy with toripalimab. Moreover, no signs of recurrence were found during follow-up over 3 months postoperatively.
Conclusion: This case underscores that ARMM can be clinically indistinguishable from rectal carcinoma, posing a high risk of misdiagnosis. It highlights the critical role of histopathology and immunohistochemistry (IHC) in definitive differentiation, emphasizing the necessity of accurate diagnosis through IHC. Finally, it demonstrates the evolving treatment paradigm from extensive surgery toward a multidisciplinary approach integrating radical resection with adjuvant immunotherapy, reflecting advances in molecular insights and ameliorated outcomes.
1 Introduction
Primary anorectal malignant melanoma (ARMM) is an exceptionally rare and aggressive malignancy, accounting for <0.1% of all anorectal cancers (1). It represents one of the three most common sites for mucosal melanomas, following the head/neck and urogenital regions (2, 3).
ARMM mimics benign conditions such as hemorrhoids or polyps, leading to misdiagnosis in 30%–50% of cases (4). Furthermore, due to its aggressive nature, approximately 30% of patients present with distant metastases at initial diagnosis (5). Consequently, low prevalence, frequent misdiagnosis, and rapid progression result in advanced-stage disease at diagnosis, with a dismal 5-year survival rate of <20% (5, 6). Specifically, once ARMM is misdiagnosed or diagnosed too late, the probability of liver, lung, and bone metastases for the patient significantly increases, leading to a continuous deterioration of the optimal treatment plan and severely reducing the long-term prognosis of the patient (7). In this context, we present a case that illustrates the diagnostic challenges of early-stage ARMM mimicking rectal carcinoma and discuss the surgical and adjuvant therapeutic approaches.
2 Case presentation
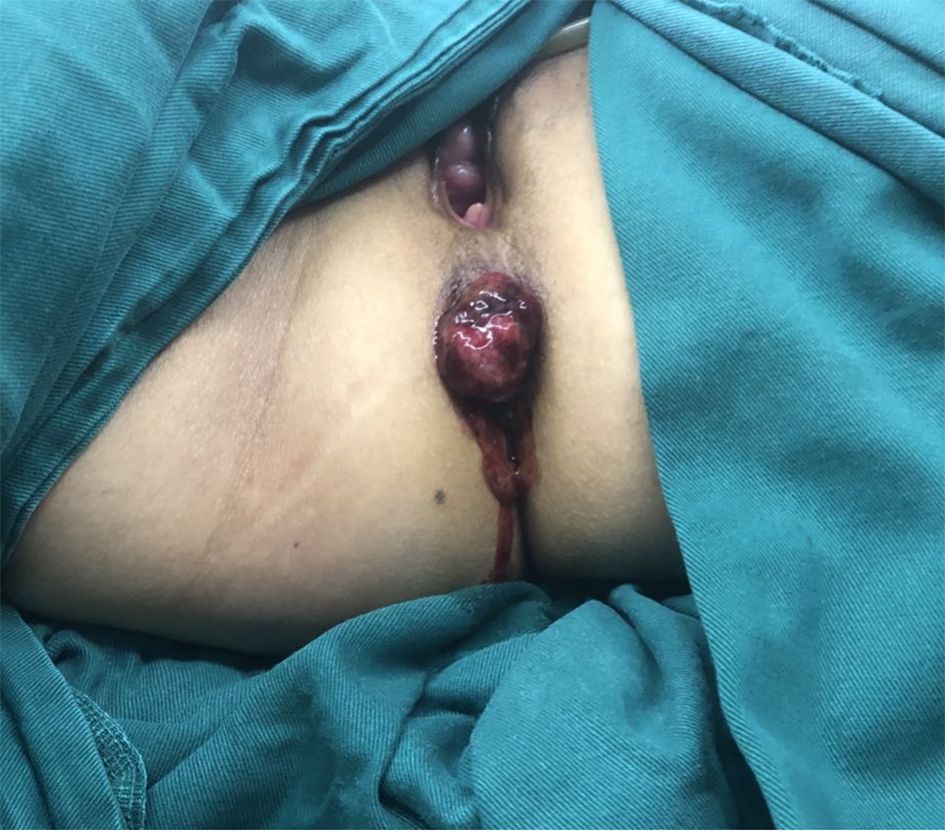
A 51-year-old woman presented to our hospital with a 1-month history of bright red blood in her stool. Hematochezia was recurrent, with blood mixed with the feces. Associated symptoms included increased bowel frequency (from 1 to 6–7 times daily) and mild abdominal distension relieved by defecation or flatulence. No fever, dizziness, palpitations, nausea, vomiting, or abdominal pain was reported. Digital rectal examination in the knee-chest position identified a firm, spherical mass ~4 cm from the anal verge, with a rough, ulcerated surface occupying half the rectal circumference; the examining glove was stained with blood upon withdrawal. Colonoscopy revealed a pedunculated polypoid lesion (~2.5 cm in diameter) near the anorectal junction, with surface erosion (Figure 1). Based on the patient’s symptoms, physical examination, and colonoscopy findings, an initial diagnosis of rectal cancer was considered. The patient denied prior gastrointestinal inflammatory or neoplastic diseases, significant personal or family medical history, or relevant travel exposures.
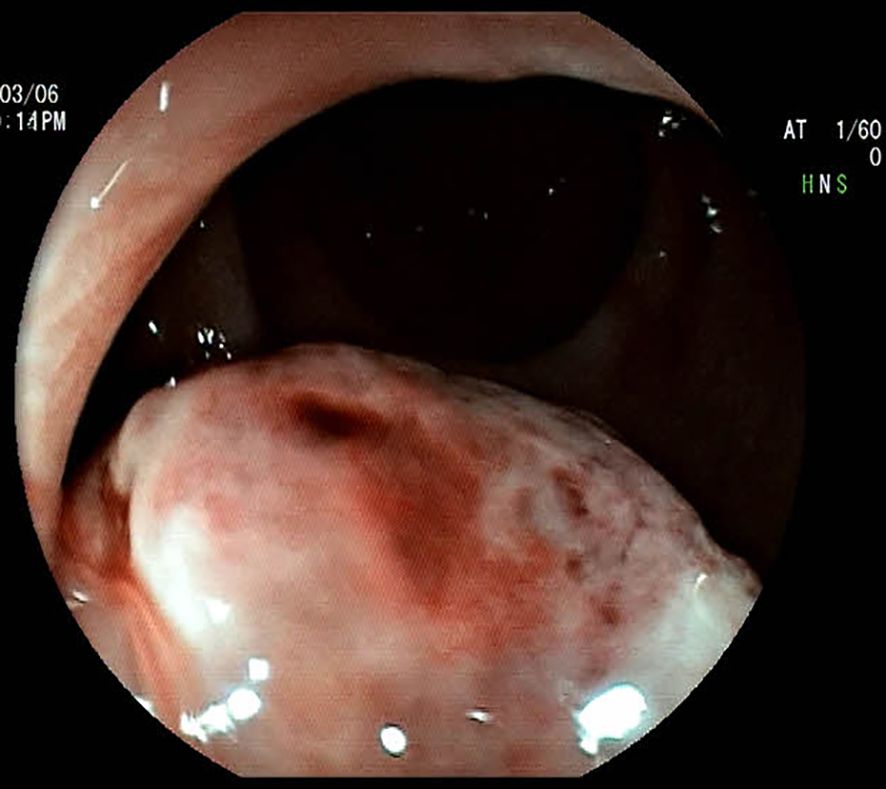
Figure 1. Colonoscopic image of the mass, demonstrating a spherical, elevated lesion with an eroded surface located approximately 3.6 cm from the anal verge.
2.1 Relevant medical laboratory examination
On admission, the patient’s vital signs were stable: body temperature was 36.7°C, blood pressure was 117/86 mmHg, and pulse rate was 75 beats per minute. Physical examination revealed no significant abnormalities in the head, neck, skin, mucous membranes, or superficial lymph nodes. The abdomen was soft without tenderness, rebound tenderness, muscle guarding, or palpable masses.
2.2 Laboratory investigations
Blood tests revealed the following: white blood cell count 5.69 × 109/L, red blood cell count 3.4 × 1012/L, and hemoglobin 97 g/L. Tumor markers included alpha-fetoprotein (AFP: 3.6 ng/mL), carcinoembryonic antigen (CEA: 0.63 ng/mL), and carbohydrate antigen 19-9 (CA19-9: 7.09 U/mL), all within normal reference ranges.
2.3 Histopathological and immunohistochemical analysis
Biopsy histopathology confirmed malignant melanoma (Figure 2). HE staining revealed disorganized tissue architecture, with neoplastic cells exhibiting enlarged, irregular nuclei, frequent mitotic figures, and uneven distribution (Figure 2A). Immunohistochemistry demonstrated MelanA positivity (Figure 2B), S100 positivity (Figure 2C), and Ki-67 expression in approximately 30% of tumor cells (Figure 2D).
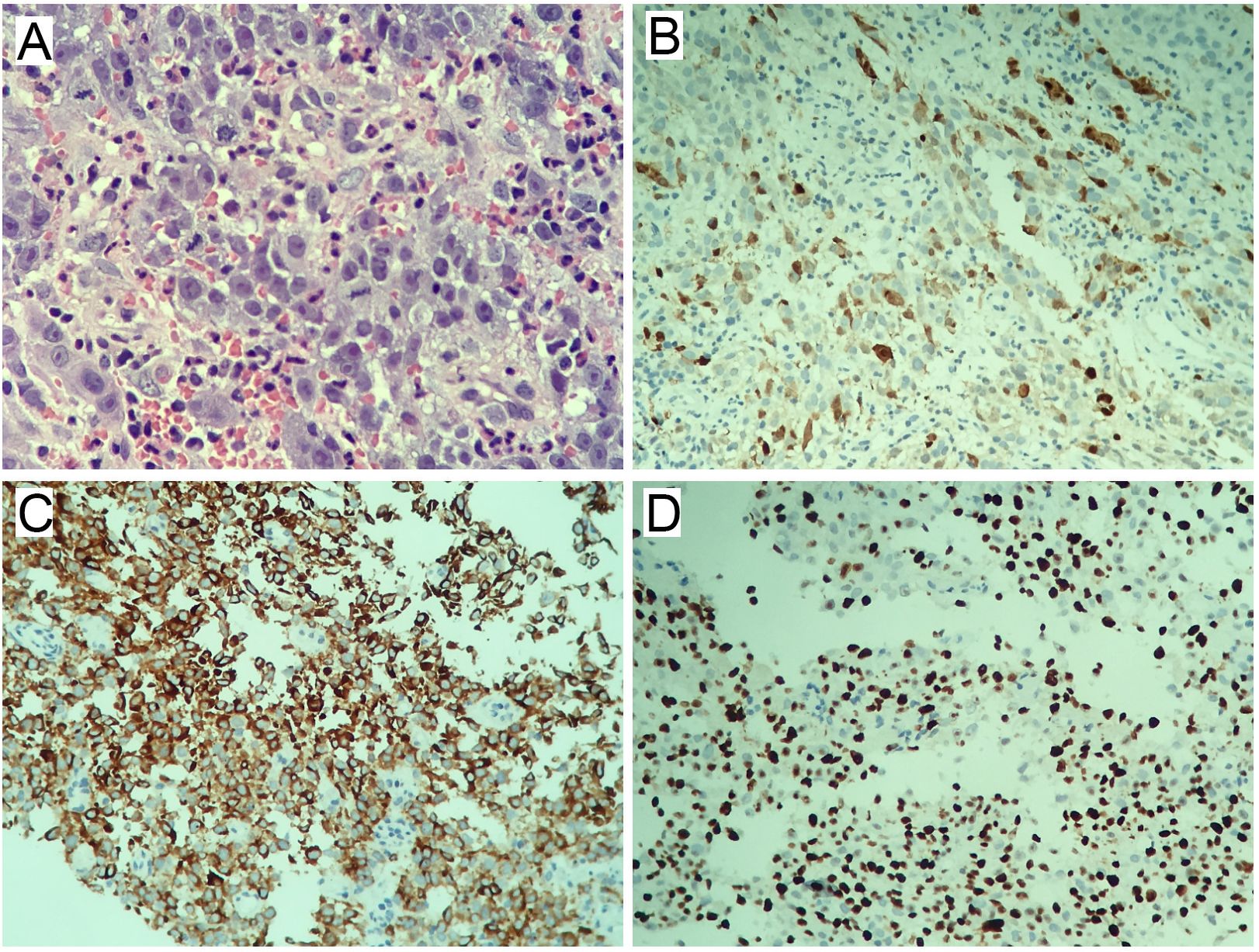
Figure 2. Histopathological and immunohistochemical staining. (A) Hematoxylin and eosin (H&E) staining (×400). (B) MelanA immunohistochemistry (IHC) (×200). (C) S100 immunohistochemistry (IHC) (×200). (D) Ki-67 immunohistochemistry (IHC) (×200).
2.4 Imaging findings
Contrast-enhanced abdominal CT revealed thickening and abnormal enhancement of the distal rectal wall (Figure 3A). Pelvic MRI demonstrated an eccentric, heterogeneous soft tissue mass ~3.6 cm from the anal verge, exhibiting slightly prolonged T1 and T2 signals, restricted diffusion on DWI (high signal intensity), and penetration through the serosal surface. The lesion measured ~5.0 cm in length, causing luminal stenosis. Several small perirectal and presacral lymph nodes were noted. Dynamic contrast-enhanced imaging showed irregular linear enhancement of the presacral fascia. The heterogeneous lateral soft tissue mass at ~4.0 cm from the anal verge exhibited continuous and marked enhancement, with a slight reduction in enhancement intensity during the delayed phase. Adjacent perirectal and presacral lymph nodes displayed moderate, heterogeneous enhancement (Figure 3B).
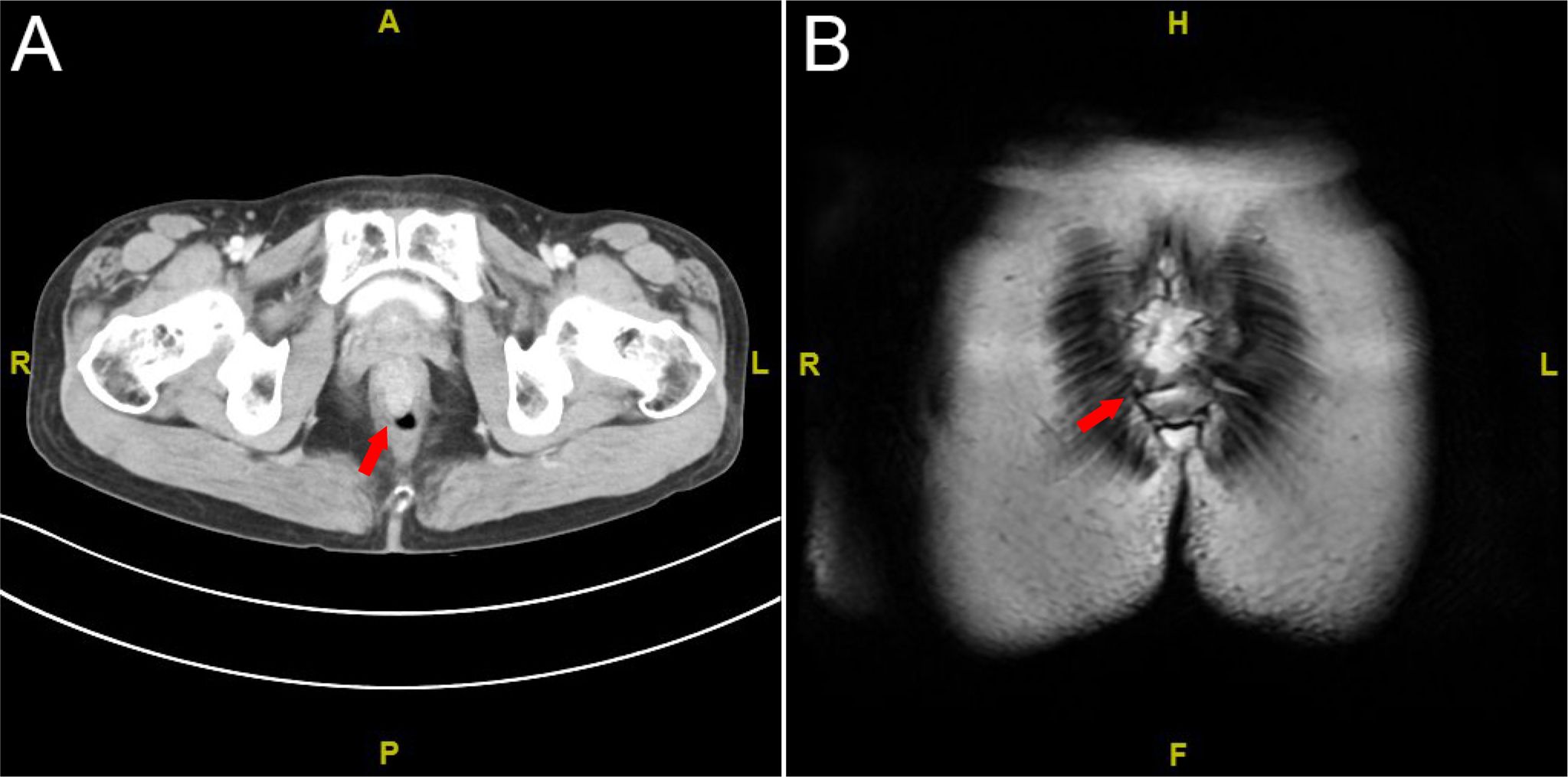
Figure 3. Imaging studies. (A) Contrast-enhanced abdominal CT scan; (B) pelvic MRI scan. The red arrow indicates the tumor.
2.5 Diagnosis and treatment
Based on clinical history and diagnostic investigations, the patient was diagnosed with ARMM. After excluding surgical contraindications, she underwent laparoscopic-assisted abdominoperineal resection (Miles procedure; Figure 4). Postoperative pathology report indicated malignant melanoma (anorectal region), measuring up to 3.0 cm in maximum diameter, with invasion into the muscular layer. No definite lymphovascular or perineural invasion was identified. The anal verge, bowel resection margins, and circumferential resection margin were free of tumor involvement. Surgical dissection revealed 13 perirectal lymph nodes, all negative for metastatic tumor. According to the AJCC Cancer Staging Manual, 8th Edition, the findings were consistent with pT2N0M0 staging. The patient was discharged in stable condition. Due to financial constraints and limited insurance coverage, the patient declined the recommended genetic testing and opted directly for adjuvant immunotherapy with toripalimab (240 mg every 2 weeks). No evidence of recurrence was observed on contrast-enhanced abdominal CT during the 3-month postoperative follow-up.
3 Discussion
Surgery is still the cornerstone of radical treatment for anorectal melanoma, a rare malignant tumor (8, 9). However, subsequent studies have shown comparable postoperative recurrence rates and overall survival (OS) with local and radical resection, suggesting that local resection may be a better option (10–13). Notwithstanding this, the CSCO Guidelines 2021 clearly state that for resectable anorectal melanoma, abdominoperineal resection (APR) remains the standard of care (14). In this case, given the absence of detected local or systemic metastasis and the patient’s relatively young age, where radical resection offered a potential for cure, the Miles procedure was selected. The patient responded equally positively to this suggestion.
With the growing application of adjuvant therapies, radiotherapy, chemotherapy, and immunotherapy have been increasingly integrated into ARMM management (15). Unfortunately, the long-term benefits of these modalities remain inconclusive (16–18). While adjuvant immunotherapy moderately prolongs OS in cutaneous melanoma, its efficacy in ARMM appears limited (18, 19). Current evidence only suggests that wide local excision combined with adjuvant therapy achieves survival outcomes comparable to radical resection while preserving sphincter function (15, 18). Nevertheless, we emphasize that adjuvant immunotherapy remains a rapidly evolving field. Studies indicate that PD-1 inhibitors (e.g., pucotenlimab) may still benefit certain patients with unresectable locally advanced or distantly metastatic mucosal melanoma (20).
Emerging evidence highlights the promise of neoadjuvant therapy combined with local or radical surgery. Compared to standard adjuvant regimens, neoadjuvant immunotherapy achieves 2-year recurrence-free survival rates of 70%–80% (21). A National Cancer Database retrospective study reported a 3-year OS of 61% with neoadjuvant therapy followed by local excision (11). In advanced-stage disease, PD-1 inhibitors combined with BRAF/MEK-targeted therapy significantly extend OS, particularly with the ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) combination, which achieves a 5-year OS rate of 52% (22–24). Data from the International Neoadjuvant Melanoma Consortium (INMC) underscore the superiority of neoadjuvant immunotherapy over targeted therapy, yielding higher pathologic and radiologic response rates, as well as improved RFS and OS (25). These studies suggest that neoadjuvant therapy, especially neoadjuvant immunotherapy combined with surgery, may be a new direction to improve the prognosis of patients. Regrettably, the patient in this case declined preoperative neoadjuvant therapy due to financial constraints as well as fear of living with the tumor.
CAR-T therapy emerges as a novel mucosal melanoma treatment following molecular-targeted immunotherapies. Preclinical studies show CAR-T cells targeting Muc18/Tyrp1 effectively eradicate tumors and inhibit recurrence (26, 27). In vitro/in vivo studies confirm significant antitumor efficacy across diverse targets (28–31). Consequently, multiple clinical trials evaluate CAR-T efficacy (31). Despite challenges—off-target toxicity, antigen loss resistance, and relapse—innovative strategies (e.g., targeting TAAs, engineering chemokine receptors) show potential to overcome limitations (31, 32). However, currently, no specific CAR targets have been developed for ARMM, and related research remains in the early stages.
In conclusion, early detection, accurate diagnosis, and multidisciplinary individualized treatment are essential to improving the prognosis of patients with ARMM.
4 Conclusion
This case highlights hematochezia as the initial manifestation of ARMM and underscores the importance of early digital rectal examination and colonoscopy in elderly patients with gastrointestinal symptoms to mitigate diagnostic delays.
Despite the persistent challenges posed by ARMM’s rarity, diagnostic complexity, and limited therapeutic options—all of which hinder prognostic improvement—this case report offers valuable insight into the evolving management paradigm by meticulously documenting the complete diagnostic and therapeutic journey.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YCZ: Writing – original draft. YJZ: Data curation, Writing – original draft. LP: Investigation, Validation, Writing – original draft. XYH: Supervision, Writing – review & editing. WX: Funding acquisition, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was supported by the National Natural Science Foundation of China - Youth Science Foundation Project (Project approval number: 82400653).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cagir B, Whiteford MH, Topham A, Rakinic J, and Fry RD. Changing epidemiology of anorectal melanoma. Dis Colon Rectum. (1999) 42:1203–8. doi: 10.1007/BF02238576
2. Kharroubi H, Osman B, Kakati RT, Korman R, and Khalife MJ. Metastatic melanoma to the small bowel causing intussusception: A case report. Int J Surg Case Rep. (2022) 93:106916. doi: 10.1016/j.ijscr.2022.106916
3. Fastner S, Hieken TJ, McWilliams RR, and Hyngstrom J. Anorectal melanoma. J Surg Oncol. (2023) 128:635–44. doi: 10.1002/jso.27381
4. Alvarez J and Smith JJ. Anorectal mucosal melanoma. Semin Colon Rectal Surg. (2023) 34:1–7. doi: 10.1016/j.scrs.2023.100990
5. El Achchi A, Majdoubi A, El Hammouti M, Bouhout T, and Serji B. Anorectal melanoma: Report of two cases. Int J Surg Case Rep. (2025) 126:110621. doi: 10.1016/j.ijscr.2024.110621
6. Pasch JA, Liu W, Kabir S, and Pennington T. Approaches to Surgical Management of Anorectal Melanoma in the Pre- and Post-Immunotherapy Eras. Dis Colon Rectum. (2025) 68(6):746–52. doi: 10.1097/DCR.0000000000003690
7. Saffaf Y, Arab H, Aldolly A, Terkaoui R, and Shikh Saeed M. Early diagnosis and delayed treatment of anorectal melanoma in a 19-year-old female: A case report. Int J Surg Case Rep. (2025) 132:111518. doi: 10.1016/j.ijscr.2025.111518
8. Ogata D, Tsutsui K, Namikawa K, Moritani K, Nakama K, Jinnai S, et al. Treatment outcomes and prognostic factors in 47 patients with primary anorectal malignant melanoma in the immune therapy era. J Cancer Res Clin Oncol. (2023) 149:749–55. doi: 10.1007/s00432-022-03933-2
9. Tariq MU, Ud Din N, Ud Din NF, Fatima S, and Ahmad Z. Malignant melanoma of anorectal region: a clinicopathologic study of 61 cases. Ann Diagn Pathol. (2014) 18:275–81. doi: 10.1016/j.anndiagpath.2014.08.002
10. Kottakota V, Warikoo V, Yadav AK, Salunke A, Jain A, Sharma M, et al. Clinical and oncological outcomes of surgery in Anorectal melanoma in Asian population: A 15 year analysis at a tertiary cancer institute. Cancer Treat Res Commun. (2021) 28:100415. doi: 10.1016/j.ctarc.2021.100415
11. Sassun R, Sileo A, Ng JC, Ferrari D, Block MS, Perry WRG, et al. Multidisciplinary Management of Anorectal Melanoma: a Retrospective Analysis of Surgical and Systemic Therapies from the National Cancer Database. J Gastrointest Cancer. (2025) 56:108. doi: 10.1007/s12029-025-01234-8
12. Fadel MG, Mohamed HS, Weir J, Hayes AJ, Larkin J, and Smith MJ. Surgical Management of Primary Anorectal Melanoma: Is Less More? J Gastrointest Cancer. (2024) 55:714–22. doi: 10.1007/s12029-023-01009-z
13. Liu C, Tang C, Zhang J, and Zhu P. Extensive resection improves overall and disease-specific survival in localized anorectal melanoma: A SEER-based study. Front Surg. (2022) 9:997169. doi: 10.3389/fsurg.2022.997169
14. P. Chinese Society of and Dermatopathology Group of Chinese Society of. Chinese guidelines on standardized pathological diagnosis of melanoma(2021 version). Zhonghua bing li xue za zhi = Chin J Pathol. (2021) 50:572–82. doi: 10.3760/cma.j.cn112151-20210417-00299
15. Menon H, Patel RR, Cushman TR, Amini A, Seyedin SN, Adams AC, et al. Management and outcomes of primary anorectal melanoma in the United States. Future Oncol. (2020) 16:329–38. doi: 10.2217/fon-2019-0715
16. Tchelebi L, Guirguis A, and Ashamalla H. Rectal melanoma: epidemiology, prognosis, and role of adjuvant radiation therapy. J Cancer Res Clin Oncol. (2016) 142:2569–75. doi: 10.1007/s00432-016-2245-x
17. Sahu A, Ramaswamy A, Singhal N, Doshi V, Mirani J, Desouza A, et al. Metastatic anorectal melanomas - An exploratory retrospective analysis on the benefits of systemic therapy versus best supportive care in a resource-limited setting from India. South Asian J Cancer. (2017) 6:147–50. doi: 10.4103/sajc.sajc_276_16
18. Wong DL, Glazer ES, Tsao M, Deneve JL, Fleming MD, and Shibata D. Impact of adjuvant therapies following surgery for anal melanoma. Am J Surg. (2022) 223:1132–43. doi: 10.1016/j.amjsurg.2021.10.041
19. Adileh M, Yuval JB, Huang S, Shoushtari AN, Quezada-Diaz F, Pappou EP, et al. Anorectal Mucosal Melanoma in the Era of Immune Checkpoint Inhibition: Should We Change Our Surgical Management Paradigm? Dis Colon Rectum. (2021) 64:555–62. doi: 10.1097/DCR.0000000000001872
20. Cui CL, Chen Y, Luo ZG, Zou ZY, Jiang Y, Pan HM, et al. Safety and efficacy of Pucotenlimab (HX008)-a humanized immunoglobulin G4 monoclonal antibody in patients with locally advanced or metastatic melanoma: a single-arm, multicenter, phase II study. BMC Cancer. (2023) 23:121–32. doi: 10.1186/s12885-022-10473-y
21. Kakish H, Xu K, Ahmed FA, Loftus AW, Elshami M, Hoehn RS, et al. Preoperative therapy in melanoma: Evolving perspectives in clinical trials. Crit Rev Oncol Hematol. (2024) 193:104193. doi: 10.1016/j.critrevonc.2023.104193
22. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
23. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
24. Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2018) 19:603–15. doi: 10.1016/S1470-2045(18)30142-6
25. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. (2021) 27:301–9. doi: 10.1038/s41591-020-01188-3
26. Zhang F, Du H, Liu K, Guo Q, Liang M, Shi J, et al. MUC18-Directed chimeric antigen receptor T cells for the treatment of mucosal melanoma. J Transl Med. (2025) 23:473. doi: 10.1186/s12967-025-06365-x
27. Jilani S, Saco JD, Mugarza E, Pujol-Morcillo A, Chokry J, Ng C, et al. CAR-T cell therapy targeting surface expression of TYRP1 to treat cutaneous and rare melanoma subtypes. Nat Commun. (2024) 15:1244. doi: 10.1038/s41467-024-45221-2
28. Mishra AK, Kemler I, and Dingli D. Preclinical development of CD126 CAR-T cells with broad antitumor activity. Blood Cancer J. (2021) 11:3. doi: 10.1038/s41408-020-00405-z
29. Yang M, Tang X, Zhang Z, Gu L, Wei H, Zhao S, et al. Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics. (2020) 10:7622–34. doi: 10.7150/thno.43991
30. Simon B, Harrer DC, Schuler-Thurner B, Schuler G, and Uslu U. Arming T Cells with a gp100-Specific TCR and a CSPG4-Specific CAR Using Combined DNA- and RNA-Based Receptor Transfer. Cancers (Basel). (2019) 11:696–709. doi: 10.3390/cancers11050696
31. Soltantoyeh T, Akbari B, Karimi A, Mahmoodi Chalbatani G, Ghahri-Saremi N, Hadjati J, et al. Chimeric Antigen Receptor (CAR) T Cell Therapy for Metastatic Melanoma: Challenges and Road Ahead. Cells. (2021) 10:1450–79. doi: 10.3390/cells10061450
Keywords: case report, primary anorectal malignant melanoma, hematochezia, laparoscopic-assisted abdominoperineal resection, treatment strategies
Citation: Zhong Y, Zou Y, Peng L, Hu X and Xu W (2025) Rare primary anorectal malignant melanoma presenting with painless hematochezia: a case report. Front. Oncol. 15:1640063. doi: 10.3389/fonc.2025.1640063
Received: 03 June 2025; Accepted: 31 July 2025;
Published: 20 August 2025.
Edited by:
Xiangjiu Ding, Shandong University, ChinaReviewed by:
Xiang Zhang, Shandong University, ChinaHaijie Che, Yantai Yuhuangding Hospital, China
Fenghe Li, The First Affiliated Hospital of Chongqinge Medical University, China
Copyright © 2025 Zhong, Zou, Peng, Hu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyun Hu, MjY4MzcyMTIyOEBxcS5jb20=; Wei Xu, eHVfd2VpMTExQDEyNi5jb20=
†These authors have contributed equally to this work
 Yuchun Zhong
Yuchun Zhong Yueji Zou3†
Yueji Zou3† Wei Xu
Wei Xu