- 1Department of Radiation Oncology, Shanghai Cancer Center, Fudan University, Shanghai, China
- 2Department of Biochemistry and Molecular Biology, College of Basic Medical Sciences, Naval Medical University, Shanghai, China
- 3Department of Medical Oncology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 4Medical Oncology, Shanghai Cancer Center, Fudan University, Shanghai, China
Nephroblastoma (Wilms tumor, WT) is an extremely rare and aggressive malignancy in adults with nonspecific clinical and imaging features. There is no standard therapy for patients with progressive disease despite surgery and chemotherapy. Here, we report a unique case of a 27-year-old male patient with recurrent metastatic nephroblastoma who developed resistance to PD-1 inhibitor and targeted therapy. Radiofrequency ablation (RFA) was performed on the largest porta pulmonic lesion. Notably, 3 months post-ablation, a non-ablated pleural lesion exhibited a partial response. Follow-up confirmed PR of the pleural lesion and total disappearance of pleura-irritative symptoms. This case demonstrates a potential abscopal effect induced by RFA, in which local treatment of one tumor site coincided with systemic regression of distant, untreated lesions and reversal of prior PD-1 inhibitor resistance.
1 Introduction
Nephroblastoma (Wilms tumor, WT) is commonly diagnosed in children (1). It is an extremely rare tumor in adults, with an incidence of fewer than 0.2 per million per year (2). WT usually presents with abdominal or flank pain and is clinically indistinguishable from more common adult malignant renal neoplasms such as renal cell carcinoma (3). As a result, most cases are identified at advanced stages, frequently with local invasion or metastatic spread. Due to the rarity of this tumor in adults, there are no firmly established treatment regimens (4). Although histological differences between children and adults are insignificant, the prognosis of adult WT compared with pediatric WT is much poorer (5). Current treatment for adult patients largely follows children’s WT treatment protocols with some changes (2, 5). Recently, immune checkpoint inhibitor (ICI) therapy and targeted therapy have shown antitumor activity in various cancers, including renal cell carcinoma (6, 7), but little is known about their effect in WT. Here, we report an adult patient with WT who relapsed after achieving partial response (PR) to combined immunotherapy and targeted therapy. Radiofrequency ablation (RFA) of a single progressive lesion not only overcame resistance to immunotherapy but also induced abscopal regression of an additional metastatic lesion.
2 Case description
A 27-year-old male patient was diagnosed with a left renal mass 7 years earlier. His treatment history is shown in Figure 1. After a confirmed diagnosis of nephroblastoma, he underwent a left radical nephrectomy. Postoperatively, the patient received 14 cycles of DD4A chemotherapy (vincristine, actinomycin-D, and doxorubicin [VAD]) according to the Children’s Oncology Group (COG) DD4A protocol. One month after the completion of chemotherapy, routine imaging surveillance revealed metastatic lesions in both lungs. Over the following 2 years, the patient was treated with multiple chemotherapy regimens, but the pulmonary lesions eventually progressed, leading to a persistent cough. In September 2020, the patient started treatment with camrelizumab (a PD-1 inhibitor) in combination with apatinib, achieving a partial response (PR) that lasted for 9 months.
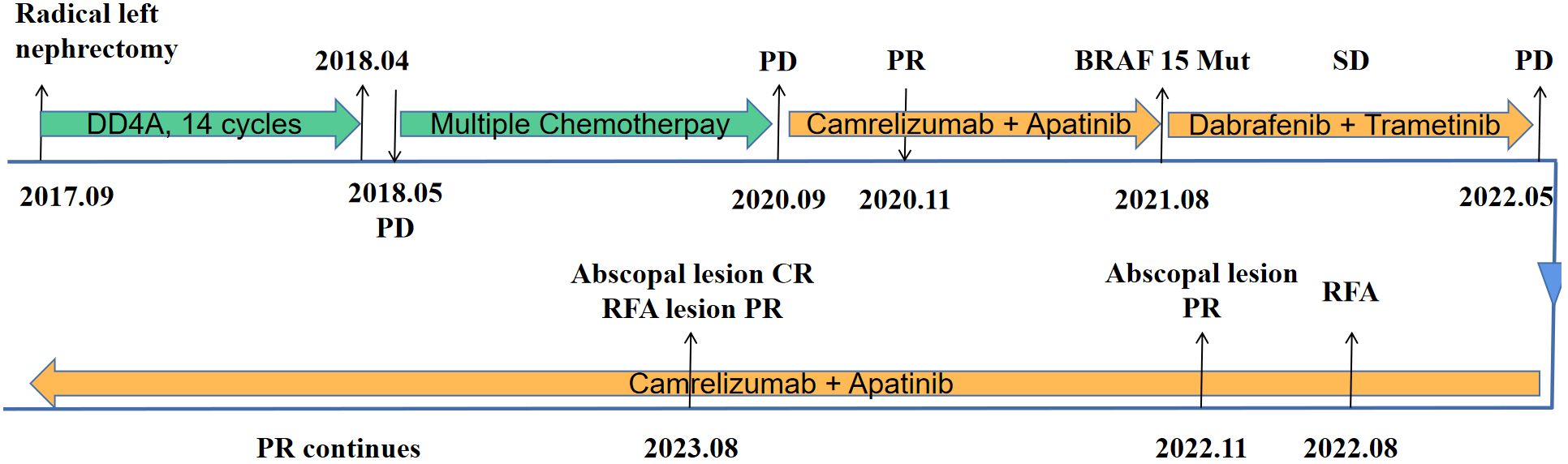
Figure 1. Timeline of the patient’s disease course. DD4A, vincristine, actinomycin-D, and doxorubicin (VAD) chemotherapy according to the COG DD4A protocol. CR, complete response. PR, partial response. SD, stable disease. PD, progressive disease. RFA, radiofrequency ablation.
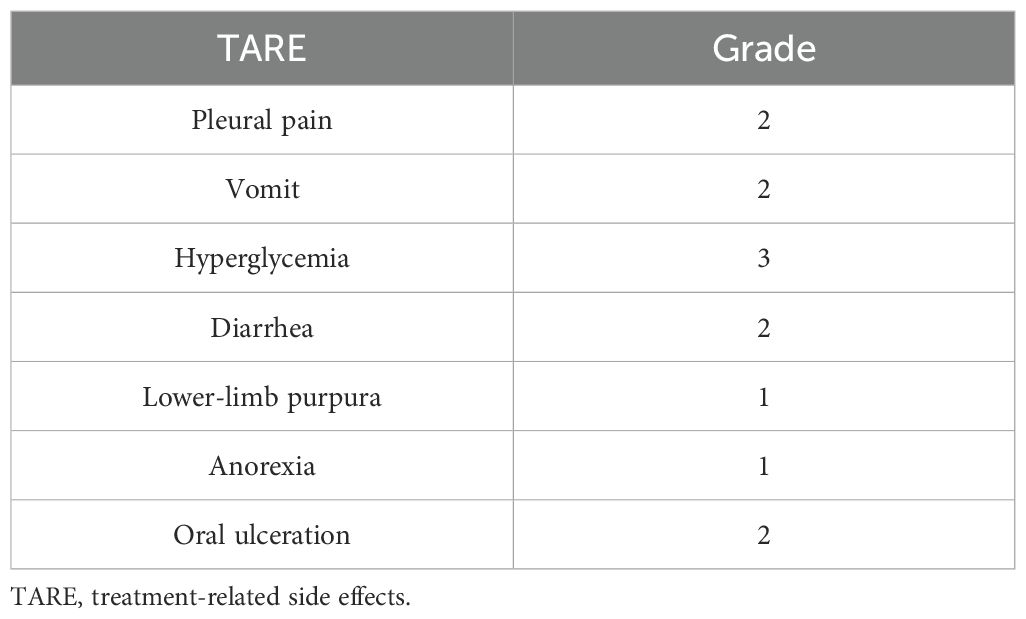
After resistance to the PD-1 inhibitor developed, genetic testing of peripheral blood and tumor tissue identified a vrafmurine sarcoma viral oncegene homolog B (BRAF) mutation in exon 15. Based on this finding, in August 2021, the patient commenced treatment with dabrafenib and trametinib. Computed tomography (CT) assessment indicated stable disease (SD), and his cough improved. Nine months later, he reported worsening cough and persistent right-sided chest pain. CT revealed a 2-cm pleural lesion (Figure 2A). The patient stopped BRAF-targeted therapy and was rechallenged with camrelizumab (200 mg, every 21 days) and apatinib (250 mg, once daily). Three months later, the pulmonary lesions remained stable. He then underwent RFA of the largest lesion in the hilar region of the right lung. The longest diameter of the target lesion was approximately 4.39 cm. Only partial ablation was achieved, as the current criterion for complete RFA applies to lesions 3 cm or smaller. Three months post-RFA, CT evaluation confirmed a PR in another pleural lesion that had not undergone RFA (Figure 2B). The patient continued combined treatment, and at 12 months post-RFA, CT evaluation demonstrated complete response of the pleural lesion and regression of the hilar lesion that had undergone RFA (Figure 2C). His chest pain resolved, and his cough diminished. Serial follow-up documented a sustained partial response. Camrelizumab was discontinued in July 2025, and the patient is currently maintained on oral apatinib therapy (250 mg once daily). Treatment-related toxicities have remained generally tolerable (Table 1).
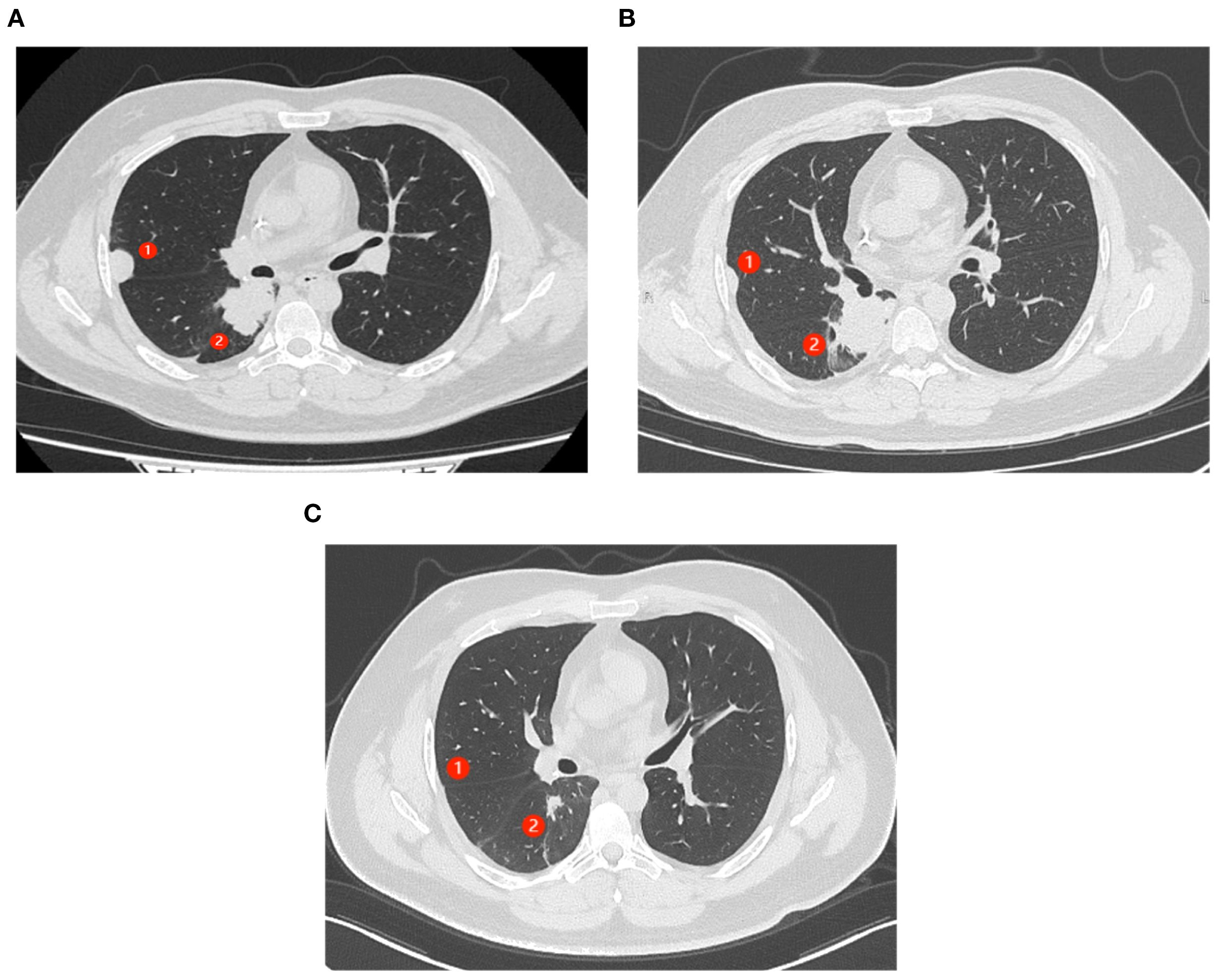
Figure 2. Abscopal response in a patient with nephroblastoma following radiofrequency ablation (RFA). ① represents the lesion near the chest wall that did not undergo RFA. ② represents the lesion in the hilar region where RFA had been performed. (A) CT scans of the patient’s chest show progressing lung lesions before the initiation of RFA. (B) CT scan shows the resolution of ① after 3 months of therapy (C). (C) CT scans (axial views) show complete response (CR) of ① and partial response (PR) of ② after 12 months of therapy.
3 Discussion
WT is a rare malignant renal neoplasm in adults (8). Diagnosis of nephroblastoma in adults is challenging because its clinical presentation overlaps with other renal masses and no pathognomonic imaging findings exist (9). The most frequent manifestations are flank pain, gross hematuria, and abdominal mass. Cross-sectional imaging (CT or MRI) readily detects a renal mass but cannot reliably distinguish it from renal cell carcinoma; thus, definitive diagnosis rests on postsurgical histopathology. Compared with childhood Wilms tumor, the adult counterpart carries a markedly worse prognosis, presumably due to advanced stage at detection, lower chemosensitivity, and frequent comorbidities (5). Because of the rarity of adult cases, therapeutic strategies remain investigational and warrant prospective study.
Evidence suggests that PD-1/PD-L1 expression in nephroblastoma is associated with poor prognosis, but the role of PD-1 inhibitors in its treatment remains unclear (10). Previous studies have indicated that one cause of resistance to PD-1/PD-L1 therapy is the lack of highly immunogenic tumor-specific antigens, which prevents tumor cells from being recognized by T cells (11). Furthermore, tumor cells can induce the surrounding microenvironment to suppress antitumor immunity, with immunosuppressive cells, cytokines, and tumor metabolites acting as extrinsic factors that contribute to resistance (12). By triggering the release of tumor-specific antigens and reshaping the tumor microenvironment, RFA is emerging as a promising approach to overcome immunotherapy resistance (13).
In the present case, RFA was performed primarily to relieve symptoms. Several studies have demonstrated that RFA is a feasible and safe treatment for various cancers, such as thyroid nodules (14), hepatocellular carcinoma (15), and hilar cholangiocarcinoma (16). RFA is a minimally invasive modality that induces local coagulative necrosis while concurrently releasing danger-associated molecular patterns (DAMPs) capable of activating both innate and adaptive immunity (17). Nevertheless, the immune response elicited by RFA alone is typically subtherapeutic. Consequently, RFA combined with immunotherapy has emerged as a strategy to eradicate residual tumor cells and prevent post-ablative recurrence (18).
Interestingly, the lesion on the right chest wall, which did not undergo RFA, regressed and achieved a complete response to camrelizumab and apatinib. This may represent a possible abscopal effect of RFA, given the patient’s prior progression on these drugs. The role of RFA in releasing tumor-specific antigens and activating systemic immunity has garnered increasing attention (19): RFA can cause elevation in proinflammatory cytokines, which appear within hours after ablation and persist for several days. It can also reduce the number of Treg (CD4+CD25+Foxp3+) cells, diminish inhibitory immune responses, and promote antitumor immunity (20). Furthermore, studies have demonstrated that thermal ablation paired with ICI can synergistically amplify antitumor immunity (21, 22), and RFA is expected to produce comparable benefits.
In conclusion, this case highlights the potential of RFA to induce abscopal effects and reverse resistance to PD-1 inhibitors. Further clinical trials are warranted to validate this strategy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Fudan University Shanghai Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Data curation, Formal Analysis, Writing – original draft. MJ: Methodology, Writing – original draft. MS: Investigation, Writing – review & editing. WL: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Balis F, Green DM, Armstrong A, Aye J, Benedetti D, Brown B, et al. Wilms tumor, version 2.2025, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2025) 23:319–42. doi: 10.6004/jnccn.2025.0037
2. Madan A and Sonpavde G. Adult wilms’ Tumor. In: Rare genitourinary tumors Switzerland: Springer International Publishing (2016). p. 79–93.
3. Alijani B, Abbaspour E, Karimzadhagh S, Reihanian Z, Haghani Dogahe M, Jafari M, et al. First incidence of extrarenal wilms tumor within the spinal canal in the adult population: a novel case report and literature review. BMC Urol. (2024) 24(1):119. doi: 10.1186/s12894-024-01508-6
4. Howell J, Maughan B, Fluchel M, and Poppe M. Pediatric regimens in the treatment of adult-onset, metastatic Wilms tumor: A case report. Urol Case Rep. (2024) 56:102799. doi: 10.1016/j.eucr.2024.102799
5. Sakthivel V, Ismail ZA, and Vijayabalan D. Recent improvements in adult wilms tumor diagnosis and management: review of literature. J Kidney Cancer VHL. (2023) 10:32–6. doi: 10.15586/jkcvhl.v10i3.281
6. Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. (2020) 31:1030–9. doi: 10.1016/j.annonc.2020.04.010
7. Guo J, Jin J, Oya M, Uemura H, Takahashi S, Tatsugami K, et al. Safety of pazopanib and sunitinib in treatment-naive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. J Hematol Oncol. (2018) 11:69. doi: 10.1186/s13045-018-0617-1
8. Chan GJ, Stohr BA, Osunkoya AO, Croom NA, Cho S-J, Balassanian R, et al. Wilms tumor: an unexpected diagnosis in adult patients. Arch Pathol Lab Med. (2024) 148:722–7. doi: 10.5858/arpa.2023-0127-OA
9. Škarda J, Grepl M, Skopelidou V, Židlík V, Hurník P, Skanderová D, et al. Blastemal predominant WT1 negative Wilms tumour of the young adult: a unique case report and review of the literature. Front Med (Lausanne). (2025) 12:1507011. doi: 10.3389/fmed.2025.1507011
10. Valind A and Gisselsson D. Immune checkpoint inhibitors in Wilms’ tumor and Neuroblastoma: What now? Cancer Rep (Hoboken). (2021) 4:e1397. doi: 10.1002/cnr2.1397
11. Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. (2023) 22:55. doi: 10.1186/s12943-023-01759-1
12. Jin Y, Cai S, Zhou Y, Guo D, Zeng Y, Xu W, et al. Targeting SLC7A11/xCT improves radiofrequency ablation efficacy of HCC by dendritic cells mediated anti-tumor immune response. Imeta. (2024) 3:e248. doi: 10.1002/imt2.248
13. Wu J, Zhou Z, Huang Y, Deng X, Zheng S, He S, et al. Radiofrequency ablation: mechanisms and clinical applications. MedComm (2020). (2024) 5:e746. doi: 10.1002/mco2.746
14. Kaltoft M, Todsen T, and Holst Hahn C. Treatment of thyroid nodules with radiofrequency ablation. Ultraschall der Med - Eur J Ultrasound. (2023) 44:220–1. doi: 10.1055/a-2015-5535
15. Xi M, Yang Z, Hu L, Fu Y, Hu D, Zhou Z, et al. Radiofrequency ablation versus stereotactic body radiotherapy for recurrent small hepatocellular carcinoma: A randomized, open-label, controlled trial. J Clin Oncol. (2025) 43:1073–82. doi: 10.1200/JCO-24-01532
16. Zhou H, Khizar H, Wang J, and Yang J. Assessing the impact of radiofrequency ablation on hilar cholangiocarcinoma: a systematic review and meta-analysis. Int J Surg. (2025). doi: 10.1097/JS9.0000000000003242
17. Chen W, Wu Y, Luo J, Wei W, Hu Q, Xu Y, et al. A synergistic 3D-printed collar transforms radiofrequency ablation into potent thermal immunotherapy for lung cancer. Adv Mater. (2025):e02375. doi: 10.1002/adma.202502375
18. Seligmann J, Koessler T, Mauer M, Evrard S, Freedman J, Gootjes EC, et al. Durvalumab and tremelimumab plus local partial tumour ablation (radiofrequency ablation or stereotactic radiotherapy) in patients with unresectable liver metastases from metastatic colorectal cancer: results of the EORTC-1560-GITCG multicentre, single-arm phase II study (ILOC). ESMO Open. (2025) 10(8):105508.
19. Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. (2016) 22:1173–84. doi: 10.1158/1078-0432.CCR-15-1352
20. Liao C, Zhang G, Huang R, Zeng L, Chen B, Dai H, et al. Inducing the abscopal effect in liver cancer treatment: the impact of microwave ablation power levels and PD-1 antibody therapy. Pharm (Basel). (2023) 16(12):1672. doi: 10.3390/ph16121672
21. Wu S, Jiang H, Fang Z, Wu Y, Jiao J, Fang W, et al. Enhanced abscopal anti-tumor response via a triple combination of thermal ablation, IL-21, and PD-1 inhibition therapy. Cancer Immunol Immunother. (2024) 73:138. doi: 10.1007/s00262-024-03718-1
Keywords: apatinib, camrelizumab, radiofrequency ablation, nephroblastoma, PD-1 resistance, reversal
Citation: Ju M, Sun M, Li W and Zhang S (2025) Case report: Abscopal response and reversal of PD-1 resistance in a patient with nephroblastoma following radiofrequency ablation. Front. Oncol. 15:1640409. doi: 10.3389/fonc.2025.1640409
Received: 03 June 2025; Accepted: 15 September 2025;
Published: 08 October 2025.
Edited by:
Jeffrey Dome, Children’s National Hospital, United StatesReviewed by:
AeRang Kim, Children’s National Hospital, United StatesMarie Nelson, William Beaumont Hospital, United States
Copyright © 2025 Ju, Sun, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Zhang, emhhbmdfc0BmdWRhbi5lZHUuY24=
†These authors share first authorship
 Mengyang Ju
Mengyang Ju Mingjuan Sun2†
Mingjuan Sun2† Wenfeng Li
Wenfeng Li Sheng Zhang
Sheng Zhang