- 1Dipartimento di Scienze, Università di Roma Tre, Roma, Italy
- 2Department of Epidemiology, Preclinical Research and Advanced Diagnostics, National Institute for Infectious Diseases Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) ‘L. Spallanzani’, Rome, Italy
- 3Dipartimento di Fisica e Astronomia, Università degli Studi di Firenze, Sesto Fiorentino, Italy
- 4Istituto Nazionale di Fisica Nucleare-Istituto Nazionale di Fisica Nucleare (INFN), Sezione di Firenze, Sesto Fiorentino, Italy
Therapeutic agents into the brain are a major challenge for treatment of brain cancer due to the blood-brain barrier (BBB) that prevents many drugs from reaching the brain. The deadliest form of brain cancer is glioblastoma (GBM), and its current standard treatment involves surgical removal of the tumor, followed by chemotherapy and radiotherapy. The main limitations of chemotherapy for brain tumors are BBB permeability, lack of specificity, and potential damage to healthy tissue. Enhanced molecular understanding of the underlying glioblastoma pathogenesis doesn’t lead to better therapeutic options. The emergence of nanotechnologies offers a promising solution, as controlled drug delivery using nanoparticles to bypass the BBB. Nanoparticles embrace a wide range of synthetic and natural biological materials effective in enhancing diagnostic and therapeutic efforts, alone or in combination with immunological, genetic, or cellular therapies. Lipid-based, inorganic, and polymeric nanoparticles are on the cutting edge of precision medicine for cancer as both therapeutic and diagnostic tools. Currently, there is no consensus on the most effective nanoparticle formulation for treating brain tumors, including their size, composition, targeting, and drug delivery mechanisms. Nanoparticles also have some drawbacks, including uncertain toxicity, reproducibility, and high cost. This short review provides a selection of primary research on nanoparticles as delivery chemotherapeutic systems, with a highlight on Photodynamic therapy (PDT) and radiotherapy (RT) combinatorial modalities. Here we critically examine the most significant research findings in the field of nanomedicine as applied to glioblastoma therapy, with a particular emphasis on chemotherapeutic nanoparticle (NP)-based drug delivery. In parallel, we provide an overview of the physicochemical properties of nanoparticles, informed by recent advances in their engineering, with a special focus on combinatorial strategies involving photodynamic therapy (PDT) and radiotherapy (RT). Our analysis focuses on highly potent anticancer drugs that are well characterized in terms of their pharmacokinetics and pharmacodynamics. The latest developments in immunotherapy and molecular-targeted treatments are intentionally excluded. Our viewpoint is grounded in the conventional yet highly effective chemotherapy-based delivery approach, which remains widely used against many of the most lethal human cancers. Despite being underrepresented in current literature, this strategy holds strong potential for clinical translation and competitiveness.
1 Introduction
Improved treatments for brain cancer remain an urgent unmet need. Mostly, glioblastoma current therapy includes surgical resection followed by radiation and/or chemotherapy with temozolomide (TMZ) (as a first line treatment) with overall survival times among the worst of any cancer (1–3). Chemotherapy of brain tumors has been mainly limited by a lack of effective methods of drug delivery, due to the blood-brain barrier (BBB) that prevents many drugs from reaching the cancer mass (4, 5). Intratumoral heterogeneity is a hallmark of the disease, showing multiple driving mutations within a single tumor (6) which is reflected in morphological, transcriptional, genetic, epigenetic, functional diversity. This pronounced molecular heterogeneity of glioblastoma hampers advances in the development of chemotherapeutic drugs in comparison with other cancer types. Common glioblastoma driver mutations are PTEN loss, mutant activated epidermal growth factor receptor variant III (EGFR vIII), p53 loss, and overexpression of platelet-derived growth factor receptor A (PDGFR), and, more rarely, activating mutations in B-RAF (7). Despite the deep molecular and histological characterization of glioblastoma based on the current WHO 2021 classification (3), the failure of novel targeted therapies mirrors the complexity of the regulatory network (8, 9), and lastly glioblastoma remains largely elusive to current immunotherapies (10).
Nanotechnology-based therapies involve delivering therapeutic cargo directly to tumor cells. The limited progress made in treating brain tumors is partly due to the inaccuracy of preclinical models. Many types of NPs have been developed such lipid, inorganic and polymeric NPs and many types of therapeutic cargoes ranging from classical alkylating agent such as temozolomide (TMZ), doxorubicin (DOX), cisplatin (CisPt), paclitaxel (PTX) to newest molecular target. Nanoparticle transport across the blood–brain barrier is achieved through two mechanisms: passive accumulation of plain nanocarriers or active targeting of the BBB via ligands (such as protein, peptide, aptamer, folate carbohydrates) detectable by receptors located on the BBB and/or glioma membranes. The shortcomings of using nanoparticles relies on poor stability, poor biocompatibility, low tumor retention, and suboptimal drug release control. Moreover, the structural complexity of nanoparticles and the limitations of current methods for nanoparticle physico-chemical characterization are challenging due to parameters such as size, morphology, charge, purity, drug encapsulation and coating efficiency, and density of conjugated ligands. A detailed discussion of the various novel diagnostic and therapeutic approaches to glioblastoma currently being investigated by NPs is beyond the scope of this article. Moreover, we neglect liposomal encapsulation technology showing limited physico-chemical stability due to fragile phospholipid membranes and their peroxidation (11). Herein, we highlight the current state and emerging research directions for pre-clinical studies in nanoparticle approaching clinical applications in glioblastoma chemotherapy, with a special focus on classical alkylating agent cargoes, considering of state-of-art mechanisms and stimuli-responsive strategies enhancing drug delivery.
2 Nanotechnologies for glioblastoma
2.1 Generation of nanoparticles for glioblastoma
In the brain cancer context, challenges remain in the clinical translation of engineered nanoparticles (NPs) able to cross the BBB. These nanoparticles possess specific intrinsic—such as electronic, optical, and magnetic features—and extrinsic properties —like size, surface-to-volume ratio, or surface energy—to enhance delivery efficiency, minimize off-target effects, and optimize drug kinetics. Acting as “Trojan horses,” they facilitate the delivery of both classical alkylating agents or new biological targeted molecules (i.e. VEGF, antibodies, RNA, and peptides) straight to cancer cells.
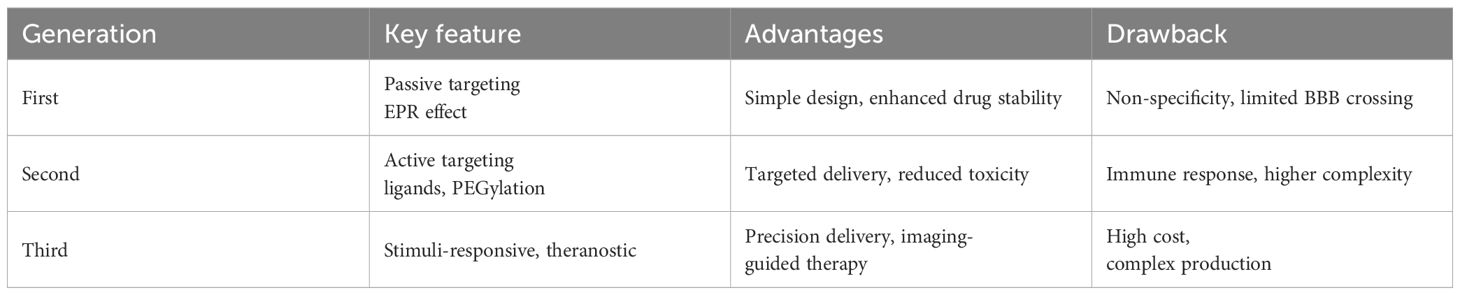
Nanoparticle surfaces can be functionalized with targeted ligands capable of selectively binding to receptors expressed on brain endothelial cells, thereby promoting their translocation across the blood–brain barrier via mechanisms such as receptor-mediated transcytosis. This is the case of transferrin receptor (TfR) and low-density lipoprotein receptor (LRP1) which serve as common molecular targets on brain endothelial cells exploited by nanoparticles to enable transcytosis (12, 13). Some authors employed glycosylated micelles to deliver bioactive compounds via glucose transporter-1 (GLUT1), a key mediator of cerebral glucose uptake. By precisely controlling the glucose density on the nanoparticle surface, they were able to modulate its biodistribution within the brain, thereby significantly enhancing nanocarrier accumulation in cerebral tissue (14). Furthermore, PEGylation lengthened their circulation time in the bloodstream, reducing protein interactions and enhancing their therapeutic efficacy (15). The evolution of nanotechnological systems for glioblastoma therapy is generally classified into different generations, as summarized in Table 1.
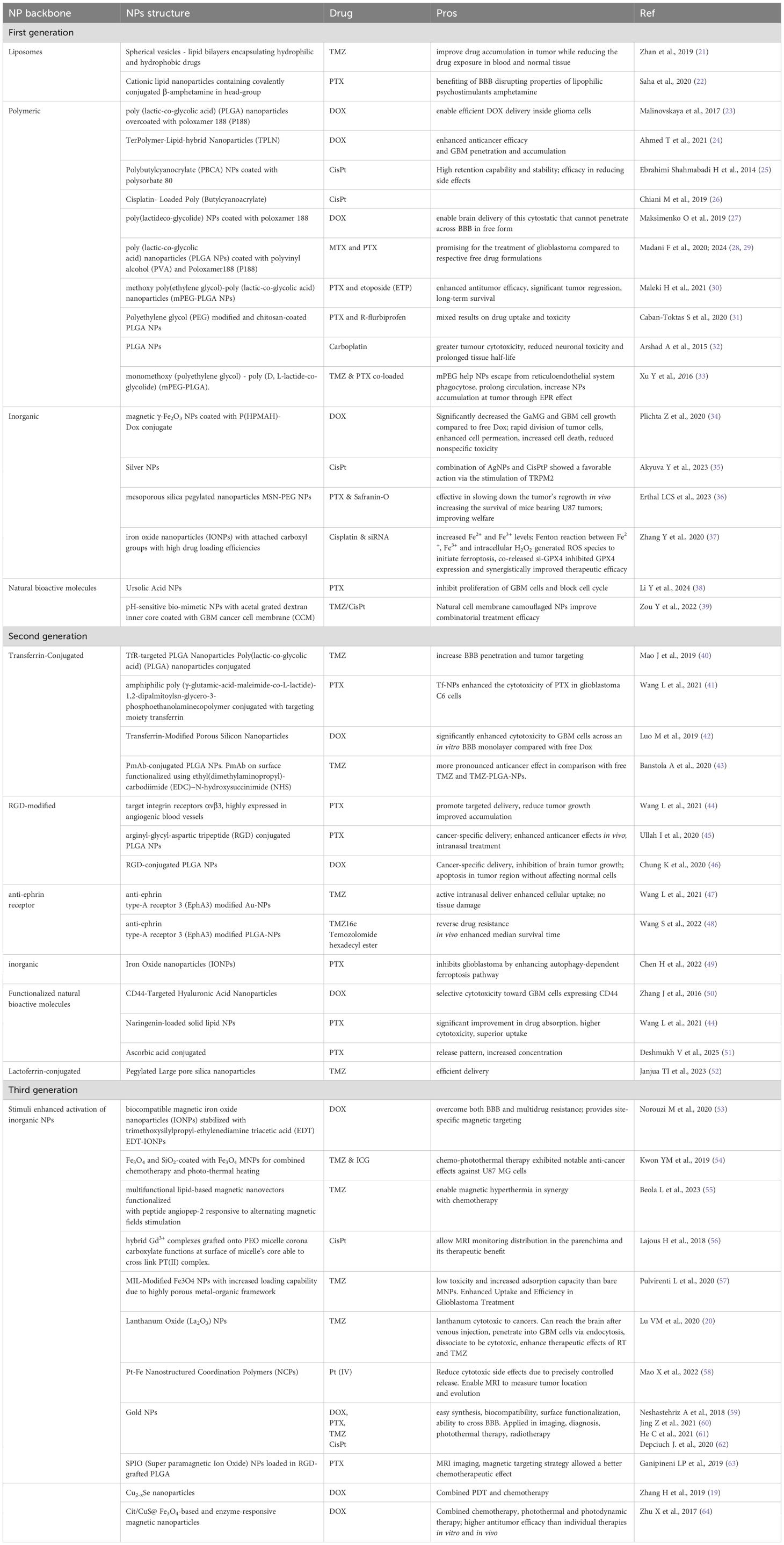
In brief, first-generation NPs nonspecifically targets tumor cells, second-generation focuses on active targeting through incorporation of ligands and specific antigens at NPs surfaces and third generation provides a multi-stage strategy best matching therapy and diagnostic (theranostic) purposes. Whereas first-generation nanotechnologies are clinical approved, second-generation platforms are currently being evaluated in clinical trials for combinatorial drug delivery strategies. Third generation instead have only recently emerged, primarily aimed at modulating immune responses and facilitating self-recognition mechanisms (16). First generation NPs mainly relies on Enhanced Permeability and Retention (EPR) effect due to leaky tumor vasculature and poor lymphatic drainage. These nano-systems are principally designed for improve the solubility and bioavailability of hydrophobic drugs and to protect encapsulated drugs from premature degradation, providing sustained release over time. Applications in GBM treatment concern the delivery of TMZ, DOX and PTX; main advantages are improved drug stability, enhanced solubility, prolonged drug action, simple design and manufacturing processes. Drawbacks of these nanotechnology are non-specific targeting and limited BBB penetration. In fact, although the BBB is disrupted at the tumor core, it remains largely intact in infiltrated brain regions, limiting systemic drug access to invasive GBM cells that drive recurrence after surgical resection (17). Second-generation NPs, retaining benefits of first-generation, add the ability to actively target glioblastoma cells. These NPs are designed to incorporate features as active targeting ligands and they rely on biocompatible surface modifications to improve specificity, reduce off-target effects, and enhance drug delivery to tumor cells. Finally, third-generation NPs are stimuli-responsive systems releasing payloads upon internal triggers (pH, redox, enzymes) or external stimuli (magnetic fields, light, ultrasound), combining therapeutic and diagnostic (theranostic) capabilities for real-time monitoring and treatment. They rely on techniques like magnetic guidance, receptor-mediated transport, ultrasound, as well as computed tomography (CT), Near InfraRed (NIR) and Magnetic Resonance (MR) imaging (18), photodynamic therapy (PDT) (19) and radiosensitization (20). The therapeutic protocol in PDT involves delivery of a photosensitizer (PS), followed by illumination of the target tissue with wavelength-specific light whereas radio-sensitization involves the use of X-rays. Ligands, antibodies, or peptides are used for receptor-specific targeting. As a resume, Figure 1 presents a schematic view of the main action mechanisms of anti-GBM actively targeted and stimuli-responsive nano-drugs. The different ways of NPs-based intervention are depicted. Beyond strategies as antiangiogenic, immune mechanisms and gene-therapy, chemotherapy maintain a central role. Examples of NPs platforms along the three generations, as well as their applications and advantages in GBM treatment are resumed in Table 2.
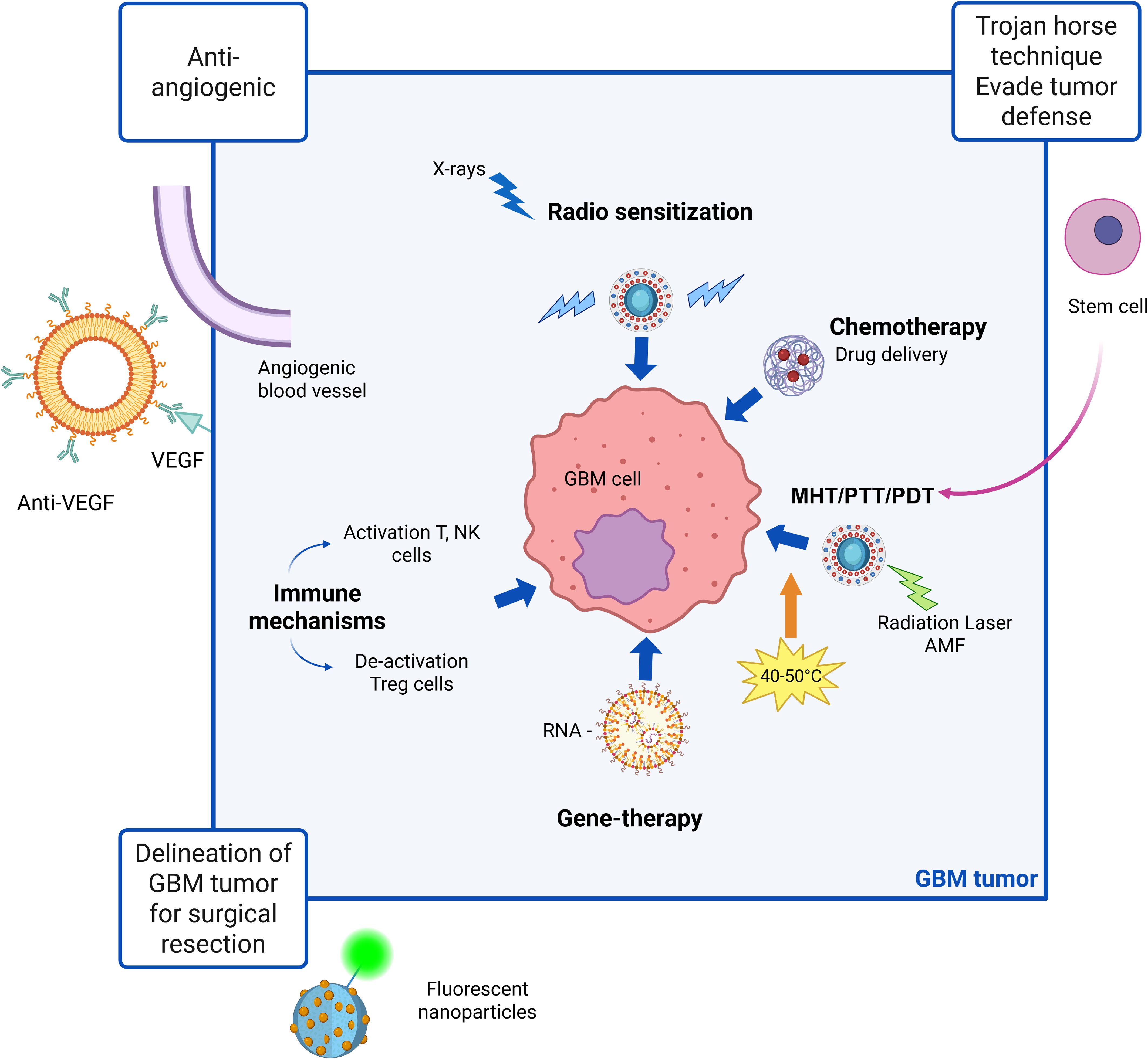
Figure 1. Schematic view of main action mechanisms of anti-GBM actively targeted and stimuli-responsive nano-drugs. Magnetic heating treatment (MHT), photothermal treatment (PTT) and photodynamic therapy (PDT) are specific strategies involving application of magnetic or radiation stimuli to the nanoparticles which may be synergistic in increasing drug delivery efficacy. Created in https://BioRender.com.
Polyethylene glycol (PEG) or poly(lactide-co-glycolide) (PLGA) are a commonly used coating agent in nanomedicine (23, 27, 28, 30–33). These polymers FDA-approved for human medical applications show biodegradability, biocompatibility and non-toxicity properties. NPs derived from PLGA can be prepared by well-assessed methodologies, as nano-precipitation methods, and they may be coated or grafted with a variety of moieties. Polyethylene glycol (PEG) conjugation is particularly advantageous as it mitigates nanoparticle hydrophobicity by imparting a hydrophilic steric barrier on the surface (30, 33). PEG-PLGA nanoparticles have demonstrated significant efficacy in the co-delivery of two or more therapeutic agents (30). Metal-based nanoparticles are promising cancer therapies, and recent studies have focused on their applications yielding the following types: (i) gold (59); (ii) silver (35); (iii) iron oxide (37, 49, 54); (iv) Magnetic-nanoparticles (20, 53, 63) to profit of MHT effect; (v) mesoporous silica nanoparticles (MSN) (36), where porous surface functionalization enables strategic pore closure to regulate drug release and achieve targeted delivery to specific sites (vi) nanocarriers acting as photosensitizers as Cu2-xSe (19) to combine PDT with chemotherapy; (vii) NPs cytotoxic to cancer sensitizing glioblastoma cells to radiation therapy and temozolomide (20).
2.2 Morphological issues in nanoparticle systems for GBM
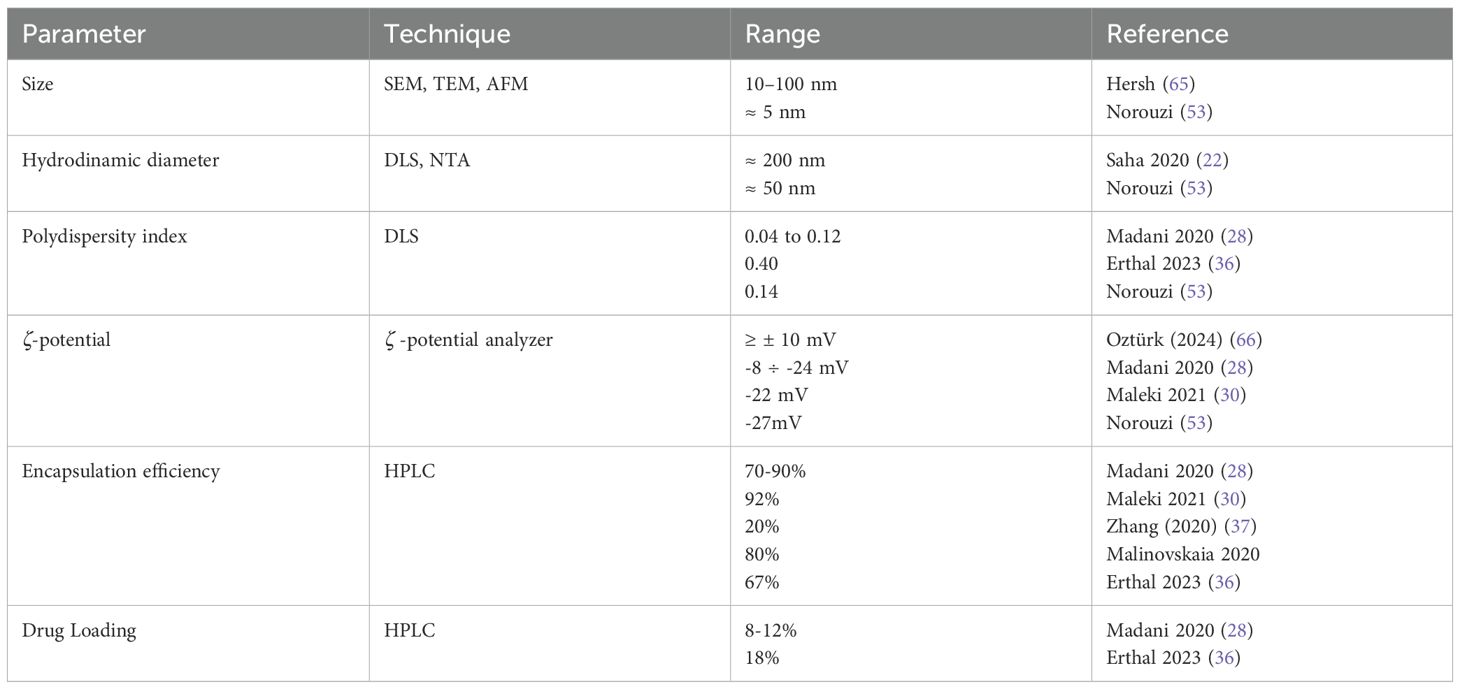
Size and surface characteristics as size, shape, porosity, charge are critical factors influencing the NPs drug delivery effectiveness. To investigate these parameters, nanoparticles are usually characterized by standard methods, using transmission and scanning electron microscopy (TEM and SEM) before and after surface functionalization, along with powder X-ray diffraction (PXRD) and infrared (IR) spectra. For SEM analysis, non-metallic NPs are coated with a gold/palladium thin layer under vacuum. Dynamic light scattering (DLS) and Nanoparticle Tracking Analysis (NTA) are commonly used to determine the mean particle size and size distribution of nanoparticles, and the zeta potential of suspended particles using a zeta potential analyzer. The technique involves using electrophoretic light scattering, also known as laser Doppler electrophoresis. Surface area and pore size distribution are analyzed by nitrogen adsorption–desorption porosimetry techniques. A sketch of typical NPs relative size versus other cells of the body are reported in Figure 2.
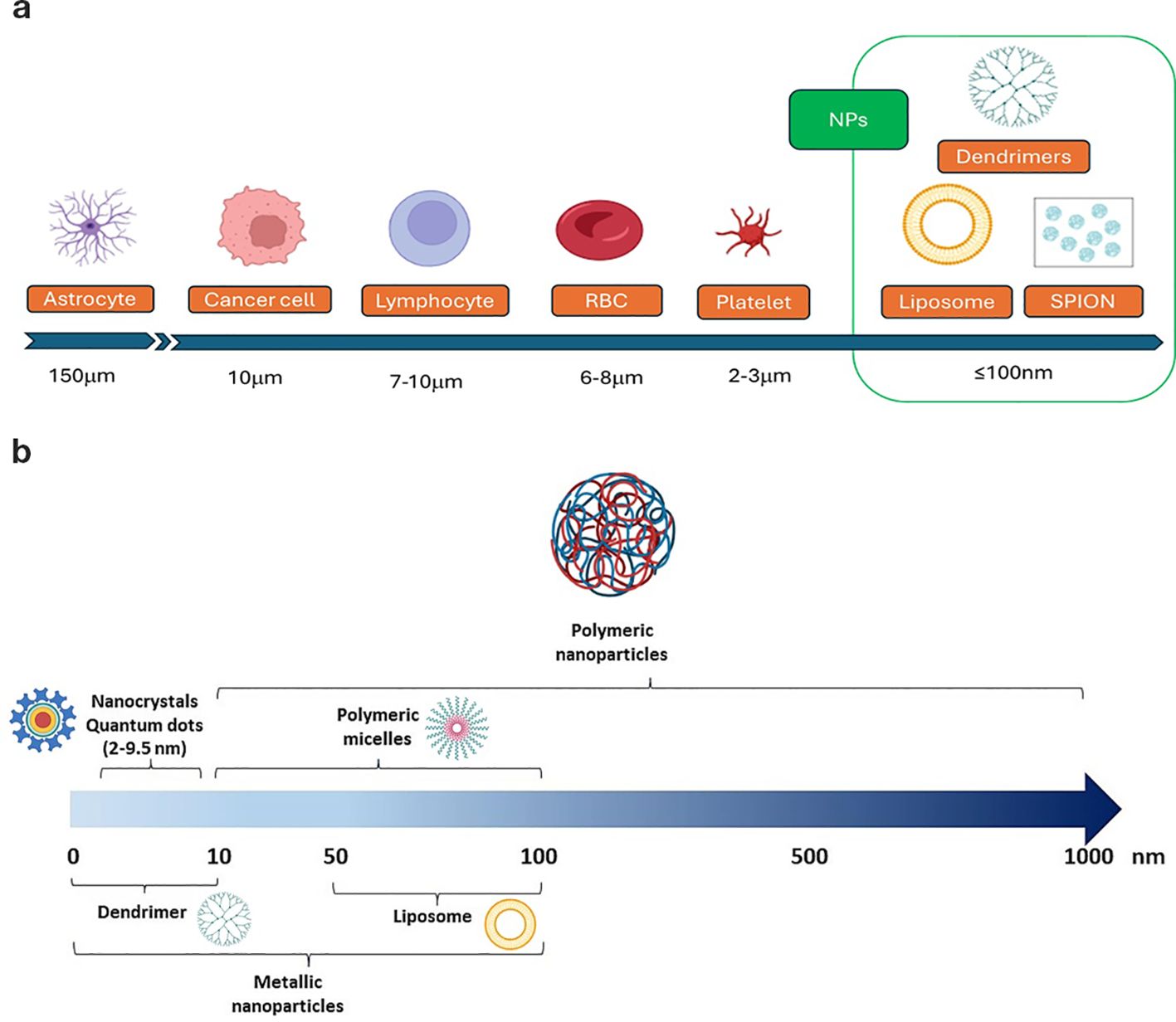
Figure 2. Typical size of (a) body cells and of (b) some of the nanoparticle systems involved in GBM therapy.
The small size of nanoparticles (NPs) is an advantage for crossing the blood-brain barrier (BBB). Studies have shown that the BBB permeability through gaps increases as NP size decreases, with minimal permeability for particles larger than 200 nm, nonetheless, renal filtration rapidly clears NPs < 5 nm, so typical sizes are within 10 to 100 nm (65). Diameters ranging between 10 nm and 40 nm are ideal for prolonged circulation in the bloodstream, increasing their efficiency in targeting the tumor microenvironment and crossing the blood-brain barrier. Smaller sizes ensure better diffusion and deeper penetration into the glioblastoma tissue. Nanoparticles may be characterized by a tiny core surrounded by structures of higher size. As an example, in (22) the diameter of the PLGA NPs without any coating, about 50 nm, increases to about 84 nm with PVA/P188 coating and a further increase is observed after drug co-loading (about 200 nm). A smaller core size is a general characteristic of iron oxide nanoparticles used in biomedical applications, in the range 5 nm to 60 nm, and a hydrodynamic size diameter up to 100 nm. Due to their small size, these cores cannot form stable magnetic domains, leading to the phenomenon of superparamagnetism. Single-domain nanoparticles exhibit uniform magnetization, and when exposed to a magnetic field, their magnetic moments align with the field direction. Superparamagnetic nanoparticles therefore do not exhibit remanence or coercivity, and their magnetic moments disappear when the magnetic field is switched off. The behavior of such super-paramagnetic ions (SPIONS) is like paramagnetic substances, but with much larger magnitude. This property allows to stabilize iron oxide magnetic nanoparticles in the target area using an external magnetic field, enhancing targeted delivery while minimizing systemic toxicity (16). For instance, in (37), a system based on porous IONPs functionalized with carboxyl groups was developed to naturally bind iron, initiating the Fenton reaction, while si-GPX4 loading amplified ferroptosis-mediated antitumor activity.
Directly correlated with the size, polydispersity index (PDI), defined as the square of the nanoparticle population standard deviation over the nanoparticle mean diameter, characterizes the spread width of the nanoparticle size distribution profile. For a most uniform population, PD should be as close as possible to 0, while FDA’s guidelines suggest that PDI should remain below 0.3. As an example, excellent uniformity is reported for co-loaded Paclitaxel/methotrexate PLGA nanoparticles, with PDI 0.04 to 0.12 (28) while a significantly high PDI (0.4) was reported for mesoporous silica nanoparticles in (36).
Zeta potential is the electrical potential difference between a nanoparticle’s surface and the surrounding liquid. It is closely related to surface charge and significantly impacts drug delivery. Nanoparticle’s net surface charge is surrounded in an ionic solution by a tightly bound layer of oppositely charged ions, as well as a loosely associated electrical double layer. As the nanoparticle moves, some ions within the diffuse layer travel with it, while others remain behind. A high zeta potential, indicating stronger electrostatic repulsion, can enhance nanoparticle stability by preventing aggregation. In contrast, neutral or weakly charged nanoparticles typically exhibit reduced stability.
The surface charge is in general modified by surfactant or active molecule adsorption, PEGylation, by coating the surface and conjugating targeting ligands. For instance, mPEG-PLGA NPs in (30) characterized by a -22mV zeta-potential, are the range of excellent colloidal stability. Moreover, cationic molecules can traverse the barrier via adsorption-mediated transcytosis, enabled by electrostatic attraction to the negatively charged endothelial cell plasma membrane. With this aim, cationic lipid nanoparticles have been considered in (22). Zeta-potential may change after drug loading. As a good example, EDT-coated IONPs in (53) uses EDT as a biocompatible coating providing many negatively charged sites on the surface of the NP that can interact with the positively charged DOX molecules. In fact, DOX-EDT-IONPs resulted in a zeta potential of 0.0 mV compared to – 20 mV for EDT-IONPs. This shift in surface charge following drug loading is due to the electrostatic interactions between the amine groups of DOX and carboxylic acid groups of EDT coating.
In general, nanoparticles with a high zeta potential (ζ ≥ ± 10 mV), due to their strong electrostatic repulsion, are more stable and less prone to aggregation (66). Other important parameters are the encapsulation efficiency, EE%, namely the ability to encapsulate the drug within the NP system, and drug loading, amount of total drug loaded over the total nanporaticle weight: , , parameters estimated using High-Performance Liquid Chromatography (HPLC), see e.g. (36). Typical physicochemical ranges for nanocarriers in glioblastoma drug delivery are reported in Table 3.
3 Nanoparticle-loaded with cytotoxic chemotherapeutic drugs
Despite TMZ being the sole chemotherapeutic agent currently used in glioblastoma treatment (as first line) there are a lot of other promising therapeutic cargoes for nanoparticles. Several classic antitumor agents (such as TMZ, PTX, CisPt, DOX) have been delivered to GBM using many types of nanosystem. First-generation nanocarriers use passive targeting based on the EPR effect, but recent research focuses on adding active targeting to better direct drugs to tumors. Solid lipid nanoparticles, polymeric nanoparticles, micelles, gold nanoparticles, super-paramagnetic iron oxide nanoparticles (IONs) and mesoporous silica nanoparticles are the most widely used at this scope. Herein a short overview of the recent findings on improvement in delivery classical chemotherapeutic drugs and their shortcomings and progress.
3.1 Temozolomide
Temozolomide is an oral alkylating prodrug of 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (67). In 2005, a landmark phase III trial described its efficacy and, since then, temozolomide has become the first-line chemotherapeutic agent for treating malignant glioma (68, 69). Nevertheless, TMZ faces pharmacokinetic challenges, including a short plasma half-life (~2 hours), susceptibility to P-glycoprotein (P-gp) efflux, and limited penetration across the blood-brain barrier (~20%) (70). Organic and inorganic nanoparticles and passive vs active targeting has been used to improve TMZ pharmacokinetics and pharmacodynamics properties. Although TMZ can partially cross the blood–brain barrier to exert its therapeutic effect, the high systemic doses required often lead to severe side effects (70). At first, temozolomide-loaded PLGA nanoparticles have failed to improve cytotoxicity in U87MG cultures (TMZ alone vs TMZ-PLGA NPs) (71). Then, PLGA-nanoparticles active targeting by both overexpression of Epidermal Growth Factor Receptor (EGFR) and transferrin-conjugated (TfR) enhanced cellular uptake of TMZ alone and in combination with Bortezomib (BTZ) (43, 72). Notably, unmodified nanoparticles were more effective at inhibiting tumor cell growth than transferrin-conjugated (TfR) nanoparticles. This is likely due to their faster drug release rate, which promotes increased intracellular drug accumulation. Next, a powerful synergistic approach, based on intertumoral chemo-hyperthermia induced a potent TMZ anti-glioma effect. In this study, lipid-based magnetic nano-vectors functionalized with angiopep-2 peptide (Ang-TMZ-LMNVs) enhanced the specificity in an orthotopic human GBM mouse model. They accumulate in tumors without affecting normal tissue, inhibiting tumor growth and extending life (median survival time doubled) when combined with an alternating magnetic field (55). Inorganic nanoparticles were efficiently delivered through ultrasmall, large-pore silica nanoparticles (USLP), which are PEGylated silica nanoparticles with lactoferrin as a tumor-targeting moiety. In vitro assays demonstrated increased cytotoxicity against human and mouse cell lines, and a 3D spheroidal model revealed decreased TMZ efflux across the blood-brain barrier (BBB) measured by trans-endothelial electrical resistance. In vivo studies confirmed that the USLP delivery system accumulates in the brain (52). Metallic iron oxide IONPs Fe3O4 TMZ-loaded obtained an excellent photothermal effect by synergistic combination of a chemo-phototherapy approach, increasing the anticancer effects in human glioblastoma cells by enhanced ROS generation (54). Similarly, magnetic Fe3O4 hybrid nano-system enhanced uptake and efficiency of TMZ treatment by a co-precipitation method (57). A proof-of-concept study using lanthanum oxide (La2O3)-based nanoparticles was performed exclusively on patient-derived glioblastoma cell lines, successfully overcoming the unavailability of experimental data for non-immortalized cells. Lanthanum oxide (La2O3) nanoparticles offer therapeutic benefits by virtue of lanthanum’s unique chemical properties. This study presents in vitro evidence that cytotoxic La2O3 nanoparticles sensitize glioblastoma cells to both radiation therapy and temozolomide (20). Inorganic gold nanoparticles have been used for the active TMZ delivery in glioblastoma by intranasal delivery. The direct neural connections (olfactory and trigeminal pathways) permit to bypass the blood-brain barrier, enabling efficient delivery of nanoparticles directly to the brain. In central nervous system diseases, including brain cancer, it can improve drug bioavailability, reduce systemic side effects, and allow non-invasive administration. In vitro studies showed that anti-EphA3-TMZ@GNPs significantly enhanced cellular uptake and toxicity toward both human and rat cell lines. To evaluate intranasal administration, an orthotopic glioma-bearing rat model was employed to perform a comprehensive in vivo safety assessment (47). More, intranasal administration was used in another study with TMZ-hexadecyl ester (TMZ16e). Anti-ephrin type-A receptor 3 (EphA3) modified TMZ16e loaded nanoparticles (NPs) were found to improve brain targeting efficiency and anti-glioma activity, as well as reverse TMZ resistance (48).
3.2 Paclitaxel
Paclitaxel (PTX) is a widely recognized and potent chemotherapeutic agent used to treat peripheral solid tumors (73). PTX suffers from poor BBB penetration and early trials failed. Recently, optical blood-brain-tumor barrier modulation expands treatment options with vascular-targeted gold nanoparticles, significantly enhancing the PTX delivery in GEMM (genetically engineered mouse model) (74). Many PLGA-based NPs has been designed to overcome the limitations of the systemic delivery of PTX. Combination of PTX and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration, by in vitro and in vivo assays. This work is the only pre-clinical study that use a nonsteroidal anti-inflammatory drug, R-Flurbiprofen, in association with PTX. The experiments on rat model RG2 and in brain-tumorized rats obtained mixed results on drug uptake and toxicity, but remarkably this combination reduced inflammation in the peri-tumoral area (31). Organic PLGA nanoparticles were co-loaded with methotrexate or etoposide and PTX in two similar studies. In vivo orthotopic glioblastoma-bearing rats, treatment with PTX/ETP co-loaded mPEG-PLGA NPs resulted in enhanced antitumor efficacy with significant tumor regression and a long-term survival in 40% of the animals. Noteworthy, hemocompatibility assays confirmed the blood safety of this approach (30). Further, surface-modified (with poloxamer 188) nanoparticles PLGA co-loaded with paclitaxel/methotrexate enhanced survival rates, showed better neurological outcomes, and favorable histopathological profiles of major organs (28, 29). Recent studies have shown that both ferroptosis and autophagy are key mechanisms in cancer therapy. An example, iron oxide nanoparticles (IONP@PTX) PTX-loaded inhibited glioblastoma by enhanced autophagy-dependent ferroptosis. IONP@PTX inhibited cell viability, migration and invasion in vitro and decreased the tumor volume of GBM xenografts in vivo (49). Efflux transporters (i.e. ABC superfamily) include also P-glycoprotein (P-gp) as key contributors to chemotherapy failure through multidrug resistance mechanisms. P-gp/ABCB1 is found to be highly expressed in cerebral vascular endothelial cells and brain tumors, and, notably, PTX is mainly eliminated through this efflux pump. An interesting study describes Ursolic Acid Nanoparticles (UA NPs) as effective inhibitors of P-gp transporters that enhance the delivery and efficacy of PTX in glioblastoma, showing an excellent biocompatibility in vivo (38). Another type of organic-based NPs (cationic lipid nanoparticle), amphetamine-functionalized PTX-loaded efficiently crossed the BBB and combined with PD-L1 siRNA synergistically improved survival in glioblastoma-bearing mice (22). Similarly, peptide-modified [arginyl-glycyl-aspartic tripeptide (RGD)] PTX-loaded NPs showed cancer-specific intranasal delivery, enhanced in vitro anticancer effects and reduced tumor growth in vivo (45).
Leveraging the active targeting of αvβ3 integrin, overexpressed in both neoangiogenic vasculature and glioblastoma cells, magnetic targeting PLGA-based NPs improved accumulation in the tumors. PTX-loaded RGD-multifunctional nanoparticles showed in vivo safety and anti-tumor efficacy on U87MG cultures (63). A novel local chemotherapy formulation, GlioGel—comprising free temozolomide (TMZ) and paclitaxel (PTX)-loaded PEGylated mesoporous silica nanoparticles (MSNs)—was developed and tested both in vitro and in a preclinical GBM mouse model following tumor resection. In vivo, MSNs effectively delayed tumor regrowth, prolonged survival in U87 tumor-bearing mice, and improved overall welfare (measured by the pattern of body weight change over time) (36). Combined delivery of paclitaxel (PTX) and naringenin, a bioactive phytocompound with anticancer properties, were obtained by solid lipid NPs. RGD-conjugated formulations (cRGD; Gly-Asp-Arg) significantly improved the release rate and drug absorption performance, as shown by in vitro and in vivo detailed pharmacokinetic studies (44). Transferrin-conjugated improved tumor targeting of PTX-loaded NPs and showed synergistic effects through transferrin-mediated endocytosis and high biocompatibility in a rat glioma model (41). More recently, intranasal delivery of surface-modified (AA Ascorbic Acid) PTX-loaded polymeric nanoparticles (AA-PTX-PNPs) increased PTX concentration. This study reports an accurate characterization by in vitro and in vivo pharmacokinetic assays showing a biphasic release pattern and a minimal alteration by histopathological results (51).
3.3 Cisplatin
Cisplatin (CisPt) is one of the most potent antitumor agents known, displaying clinical activity against a wide variety of solid tumors outside the central nervous system. Systemically delivered CisPt penetrates poorly into normal brain tissue due to the BBB. Although cisplatin is cytotoxic on human glioblastoma cells in vitro, the response in clinical treatment is weak and has not improved the overall survival of patients with brain tumors. Moreover, its efficacy is often limited by the development of resistance (75).
Hence, cisplatin-loaded nanoparticles are poorly represented in the current literature. Some examples of cisplatin carrying nanoparticles are listed below. Iron oxide nanoparticles (IONPs) were used for codelivery of CisPt and GPx in immortalized cultures and patient-derived glioblastoma. They enhanced therapeutic efficacy synergistically via a ferroptosis-related mechanism (37). Another study demonstrated that inorganic hybrid gadolinium nanoparticles exhibited up to a 50-fold increase in accumulation within human glioblastoma cells, along with a 32-fold enhancement in Pt-DNA adduct formation compared to free cisplatin. Additionally, non-invasive MRI tracking of nanoparticle biodistribution confirmed the potential of this innovative bimodal platform for applications in nuclear medicine (56). A similar approach has been developed by Mao et al. for intranasal administration of Catechol-Based Pt (IV) coordination polymer. MRI monitoring demonstrated enhanced tumor accumulation, resulting in complete remission and extended survival outcomes as measured by Kaplan–Meier curves (58). Some efficacies of CisPt-loaded poly-Butyl cyanoacrylate NPs were reported both in vitro and in vivo rat model of glioblastoma (25, 26). More recently, brain co-delivery of TMZ and CisPt has been proposed as a combinatorial glioblastoma chemotherapy. These biomimetic nanoparticles MNPs@TMZ+CisPt exhibited a potent anti-GBM activity in a mice orthotopic model (39). Carboplatin, a less toxic analogue of cisplatin, has been used in a study using convection-enhanced delivery with PLGA nanoparticles (32). Alginate nanogel CisPt co-loaded with gold nanoparticles and silver nanoparticles has been used to potentiate the oxidant actions of CisPt via the stimulation of TRPM2 channel in glioblastoma cells (35, 59).
3.4 Doxorubicin
Doxorubicin (DOX) is an anthracycline antitumor drug discovered in 1969. DOX was among the first chemotherapies encapsulated in a cell membrane-cloaked polymeric nanoparticle (76). One of most used chemotherapeutic agents for treating both solid and hematologic malignancies. Covalent linkage of DOX to three different types of NPs-metallic, silica/organo-silica and polymeric has been shown to overcome its cardiotoxicity (77).
Intranasal delivery of DOX-loaded PLGA NPs arrests growth in rat model of glioblastoma. In this study, PLGA nanoparticles NPs were modified with the RGD arginyl-glycyl-aspartic tripeptide (RGD) ligand to enable active targeting of αvβ3 integrin. Moreover, its intranasal administration enhanced apoptosis in the tumor area, without harming normal brain tissue (46). Two examples of organic DOX-loaded PLGA confirmed the nanoparticles’ anti-tumor efficacy: (i) a pilot-scale manufacturing process yielded strong anti-tumor efficacy in in vivo orthotopic model, with negligible blood toxicity at therapeutic concentrations (27) and (ii) a detailed confocal characterization of intracellular trafficking of DOX-loaded PLGA NPs in human U87MG describing NPs internalization by clathrin-mediated endocytosis (23).
Many examples of second-generation nanostructure reports DOX−loaded inorganic metal nanoparticles for glioblastoma therapy. Nourouzi et al. report a combinational approach for enhanced delivery of iron oxide nanoparticles (IONPs). A cadherin binding peptide with trimethoxy silyl propyl-ethylenediamine triacetic acid (EDT) and an external magnetic field enhanced the NPs penetration and increased therapeutic response and apoptosis in human U251 cells (53). Plichta et al. moved one step further using five patient-derived primary glioblastoma cultures for cellular assays. Poly[N-(2-hydroxypropyl) methacrylamide]-modified magnetic γ-Fe2O3 nanoparticles Dox-conjugated were proposed as a blood plasma substitute instead of PEG for glioblastoma treatment (34). A detailed study reports terpolymer-lipid hybrid nanoparticle (TPLN) developed for DOX delivery to GBM, demonstrating enhanced in vitro and in vivo efficacy measured by cellular uptake, cytotoxicity, 3D spheroid penetration, and biodistribution in a murine orthotopic GBM model (24). A study exclusively performed on brain microvascular cells (instead of an orthotopic glioma model), reports active targeting by transferrin-modified porous silicon nanoparticles Tf@pSiNPs in in vitro monoculture U87MG and coculture BBB model describing clathrin- and caveolae-endocytic pathways (42). Again, CD44-targeted and redox-responsive drug delivery system was based on mesoporous silica nanoparticles (MSNs) exhibited higher cellular uptake efficacy via CD44-mediated endocytosis and higher cytotoxicity (50).
4 Combining PDT/RT and chemotherapeutic NPs in GBM treatment
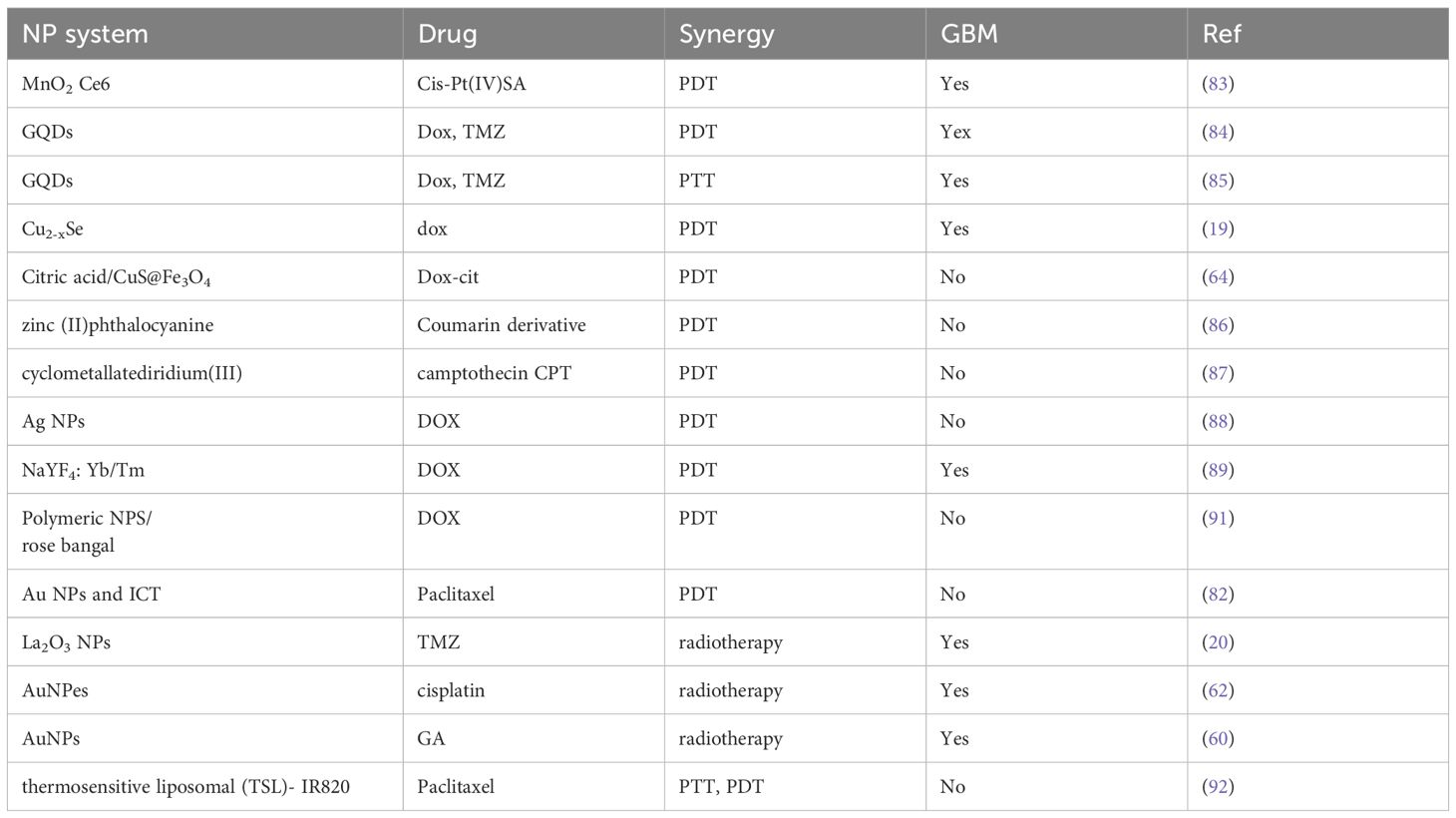
Nanoparticles for photodynamic therapy (PDT) are approved by the US Food and Drug Administration (FDA) for many cancers (78). 5-ALA is the only fluorescence-guided glioma surgery agent approved by the FDA (79). Integration of Photodynamic therapy (PDT) and Radioterapy (RT) to chemotherapeutic NPs represents a novel strategy to enhance GBM treatment outcomes, to mitigate toxicities associated with individual agents and substantially enhance overall therapeutic efficacy (80). Photodynamic therapy (PDT) involves the administration of a photosensitizer (PS), either topically or systemically, followed by irradiation of the target tissue with a light source matched to the absorption wavelength of the PS. Photosensitizers that absorb in the visible or near-infrared spectrum are preferred due to their lower phototoxicity compared to ultraviolet light. Photodynamic therapy (PDT) may be utilized as a monotherapy or integrated with conventional treatment modalities, administered either prior to or following their application.
Several NP systems may serve a dual role as radio- or photosensitizers and drug delivery carriers. Polymersomes (or polymer vescicles) functionalized with angiopeptide-2 (Ang-2), loaded with gold nanoparticles (AuNPs), and conjugated with doxorubicin (DOX) (Au-DOX@PO-ANG) have demonstrated enhanced permeability across the blood–brain barrier (BBB) and selective accumulation in malignant brain tissue (61). Targeted delivery of this therapeutic platform to tumor sites significantly enhanced radiosensitization, resulting in a 40% decrease in cell viability post-radiotherapy, indicating a substantial cytotoxic effect. Near-infrared (NIR) imaging analysis revealed that rats receiving combined treatment with Au-DOX@PO-ANG and radiotherapy exhibited significantly reduced tumor growth and prolonged survival compared to controls. Additionally, the delivery system demonstrated high stability and no observable toxicity in major organs.
Besides classical drugs, AuNPs may also deliver other compounds that display antitumor effects. Gallic acid (GA) has been investigated as a potential anti-cancer agent in glioblastoma tumors (60). GA-AuNPs reduced U251 GBM cell viability by up to 31.25% particularly by day 3, and increased apoptosis. Treated cells showed S and G2/M phase arrest, with 150–200 µg/mL GA-AuNPs enhancing radiosensitivity across 2–12 Gy doses improving the efficiency of radiotherapy. Finally, gold nanopeanuts (AuNPes), owing to their unique shape and high surface area, exhibit enhanced drug-loading capacity (e.g., for cisplatin), making them promising candidates for combined chemo- and radiotherapy applications (62).
Some studies have recently investigated the efficacy of combining chemotherapy and PDT for glioblastoma treatment. Zhang and colleagues developed a Cu2-xSe-based nanoplatform for treating malignant glioblastoma through a combination of near-infrared photodynamic therapy and chemotherapy using doxorubicin as the therapeutic agent (19). Cu2-xSe nanoparticles exhibited strong infrared absorption at approximately 1064 nm, enabling deep tissue penetration. Additionally, they effectively catalyzed the degradation of H2O2 and intratumoral oxygen, generating substantial levels of reactive oxygen species (ROS) (81). A long-term issue for the limitation of PDT is represented by hypoxia. A promising tumor microenvironment-triggered oxygen nanogenerator for self-enhanced PDT primed antitumor immunotherapy has been designed in (82), using indocyanine green (ICG) PS and gold nanoshells in photothermal therapy (PTT) to promote the catalysis of hydrogen peroxide and self-enhance PDT. The relief of tumor hypoxia broke the chemoresistance and promoted the polarization of tumor-associated macrophages from M2 to M1 type, increasing the efficacy of chemotherapy and immunotherapy. In (83) MnO2-Ce6 nanoparticles have been applied for an effective combination of photodynamic and chemotherapy. They are designed to react with H2O2 in tumor microenvironment so to produce oxygen and thus overcome hypoxia-associated photodynamic resistance. Meanwhile, gradual decomposition into individual therapeutic albumin complexes improve intratumoral diffusion. As a result, effective in-vivo antitumor therapeutic outcome is obtained by a single treatment at a rather low dose. More recently, surface functionalized graphene quantum dots (GQDs) have been shown the capability to cross the blood–brain barrier and exert synergistic photodynamic and photothermal effects in combination with chemotherapeutic doxorubicin and temozolomide (84, 85). In particular, the capability of GQDs to absorb and convert near-infrared light into heat in PhotoThermal Therapy (PTT) enhanced membrane permeability, increasing the release of reactive oxygen species and ultimately the efficacy of antitumor drugs at subtherapeutic doses against glioblastoma.
Many examples exist that report nanoparticles as both nanocarriers and photosensitizers such as citric acid/CuS@Fe3O4 (64), zinc (II)phthalocyanine (86), cyclometallatediridium (III) (87), silver nanoparticles (88) and NaYF4: Yb/Tm (89). All nano-platforms demonstrated good in vitro and in vivo therapeutic efficacy. Nonetheless, they have not been applied to GBM.
Nanoparticle-enhanced radiotherapy represents an emerging frontier in the treatment of brain tumors (90). In general, NPs enhance the efficacy of RT by boosting the production of ROS, increasing oxidative stress and binding to DNA in terms of chemical interactions. High atomic number (Z) metal nanoparticles can enhance radiotherapy efficacy by targeting specific biological pathways. Upon irradiation, these nanoparticles emit secondary X-rays, photoelectrons, and Auger electrons, thereby amplifying the local radiation dose delivered to tumor tissues. Conversely, elevated biological responses in tumor tissues—such as oxidative stress and DNA damage—can potentiate the therapeutic effects of radiotherapy.
Lanthanum-based nanoparticles (La2O3 NPs) offer therapeutic advantages in glioblastoma treatment (20) due to their preferential accumulation in tumor cells over astrocytes and their ability to cross the BBB. When combined with radiotherapy or temozolomide, La2O3 NPs enhance apoptosis, DNA double-strand breaks, and autophagy by molecular mechanisms involving ROS/γ-H2AX signaling and Bcl-2 expression. (See Table 4 application of nanocarriers in PDT/PTT/RT).
5 Current clinical trials of drug-loaded nanoparticles in glioblastoma
Although extensive research explores nanoparticles as potential brain cancer therapies, only a few have gained approval from the FDA and EMA (93). This stems from an incomplete understanding of glioblastoma biology and a gap between preclinical drug development and clinical evaluation (94). Preclinical testing based on in vitro IC50 evaluation of chemotherapeutic drugs on glioblastoma cultures rely on sketchy and mixed results (unpublished observations). Many early clinical trials of chemotherapies and molecularly targeted treatments in patients with primary and metastatic brain tumors failed to produce patient benefits (1–3). Liposomal encapsulation technology showed limited physico-chemical stability due to fragile phospholipid membranes and their peroxidation and clinical trial using pegylated liposomal doxorubicin (Caelyx™, PEG-Dox) failed to produce a significant improvement in patient’s (95). In the era of the cancer nanomedicine, many formulations, examples such as Abraxane, NanoTherm, and Combidex—comprising both organic and inorganic nanoparticles—have received clinical approval or are currently in clinical trials for solid tumors, but very few for glioblastoma (96, Table 5).
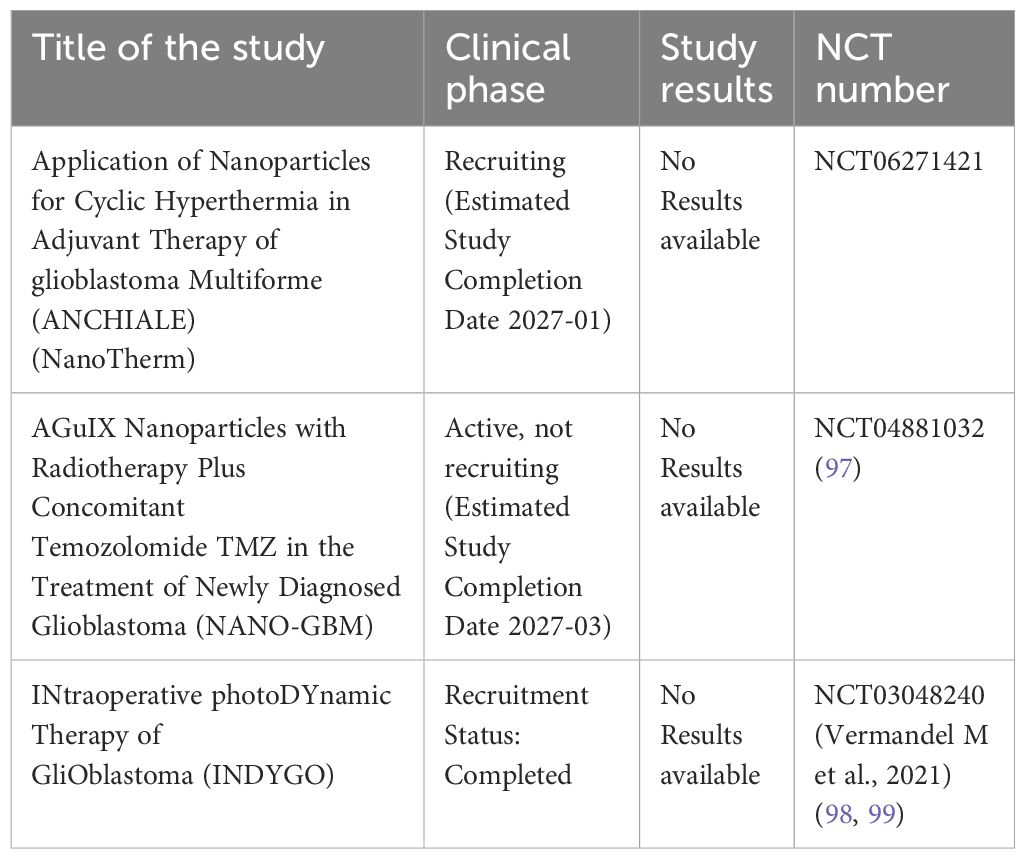
Table 5. Summary table of major phase I-III ongoing clinical trials with drug-loaded nanoparticles in Glioblastoma.
Here we report only those based on classical delivery of chemotherapeutic drugs, not molecularly targeted (i.e. EGFR or other not-validated target gene). A phase I/II clinical trial (NANO-GBM) is currently ongoing to evaluate AGuIX nanoparticles combined with radiotherapy and concomitant TMZ for newly diagnosed glioblastoma, with the primary objective to establish the recommended dose of AGuIX combined with radiotherapy and TMZ during concomitant chemoradiotherapy, and to assess the efficacy of this combination by evaluating the 6-month progression-free survival (PFS) rate (phase II) (NCT04881032; 97, 100). The only EMA-approved brain cancer drug therapies based on nanotechnology is NanoTherm (MagForce). An iron oxide aminosilane-coated nanoparticles, in form of nanocrystal which has been registered in Europe (EMA) as a method of treating glioblastoma multiforme recurrence. This is a magnetic hyperthermia device (NanoTherm® Therapy, NTT) approved as an adjunct therapy for patients with recurrent glioblastoma who are also receiving radiotherapy (101, 102) (ANCHIALE, NCT06271421). Nonetheless, nanoparticle-based delivery remains in the early pilot stage, requiring further research and documentation prior to approval. The limited availability of nano-delivery treatments stems from lengthy testing requirements, lack of standardized nanotoxicology assays, and high manufacturing costs (93). Moreover, in glioblastoma PDT is still at the stage of preclinical in vitro experimental phase (103). One example is the INDYGO trial; a prospective, non-randomized, single-center, open-label, phase I study (NCT 03048240) that reports safety and efficacy after intraoperative treatment of glioma with photodynamic therapy (PDT) after administration of 5-ALA acid (5 aminolevulinic acid hydrochloride) (98, 99). Another ongoing study in Germany is evaluating stereotactic biopsy followed by 5-ALA-based stereotactic PDT and the feasibility of 5-ALA in stereotactic interstitial PDT in a subset of adult glioma patients (103). 5-ALA is the only fluorescence-guided glioma surgery agent approved by the FDA (79).
6 Conclusions
This brief review presents a comparative analysis of various chemotherapeutic strategies for GBM treatment based on nanotechnology, providing insights into the relative effectiveness and potential of different NP systems. Indeed, recent advancements in NPs development are promising, given the complexity of the BBB microenvironment, and enabling a more efficient targeted drug delivery. Chemotherapeutic multifunctional NPs that combine imaging, targeting, and therapeutic capabilities hold significant promise in improving GBM outcomes. Nonetheless, clinical adoption of chemotherapeutic NPs for glioblastoma treatment is still in its early stages. Both research challenges and processing standardization issues are to be overcome to proceed toward a clinical practice. In the future, collaborative efforts among material science researchers and clinicians will be crucial to fully exploit the potential of chemotherapeutic NPs for glioblastoma.
Author contributions
SM: Writing – original draft, Writing – review & editing. CZ: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The Grant of Excellence Departments, MUR (ARTICOLO 1, COMMI 314 – 337 LEGGE 232/2016) to the Department of Science, Roma Tre University. INFN (Istituto Nazionale di Fisica Nucleare) CSN5 COMMON FUND - DOTA5 Firenze.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stupp R. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. (2017) 318:2306–16. doi: 10.1001/jama.2017.18718
2. Weller M. Challenges in the diagnoses and treatment of CNS tumors. Neurooncol Pract. (2019) 6:329. doi: 10.1093/nop/npz044
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella- Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
4. Steeg PS. The blood-tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. (2021) 18:696–714. doi: 10.1038/s41571-021-00529-6
5. Noorani I and de la Rosa J. Breaking barriers for glioblastoma with a path to enhanced drug delivery. Nat Commun. (2023) 14:5909. doi: 10.1038/s41467-023-41694-9
6. Mathur R, Wang Q, Schupp PG, Nikolic A, Hilz S, Hong C, et al. Glioblastoma evolution and heterogeneity from a 3D whole-tumor perspective. Cell. (2024) 187:446–63.e16. doi: 10.1016/j.cell.2023.12.013
7. Verdugo E, Puerto I, and Medina MÁ. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun (Lond). (2022) 42:1083–111. doi: 10.1002/cac2.12361
8. Dewdney B, Jenkins MR, Best SA, Freytag S, Prasad K, Holst J, et al. From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress. Sig Transduct Target Ther. (2023) 8:400. doi: 10.1038/s41392-023-01637-8
9. Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang Z, et al. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. (2022) 21:39. doi: 10.1186/s12943-022-01513-z
10. Chan HY, Choi J, Jackson C, and Lim M. Combination immunotherapy strategies for glioblastoma. J Neurooncol. (2021) 151:375–91. doi: 10.1007/s11060-020-03481-0
11. Hasan I, Roy S, Ehexige E, Wu R, Chen Y, Gao Z, et al. A state-of-the-art liposome technology for glioblastoma treatment. Nanoscale. (2023) 15:18108–38. doi: 10.1039/d3nr04241c
12. Wiley DT, Webster P, Gale A, and Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U.S.A. (2013) 110:8662–7. doi: 10.1073/pnas.1307152110
13. Tian X, Leite DM, Scarpa E, Nyberg S, Fullstone G, Forth J, et al. On the shuttling across the blood-brain barrier via tubule formation: mechanism and cargo avidity bias. Sci Adv. (2020) 6:eabc4397. doi: 10.1126/sciadv.abc4397
14. Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. (2017) 8:1001. doi: 10.1038/s41467-017-00952-3
15. Roberts MJ, Bentley MD, and Harris JM. Chemistry for peptide and protein pegylation. Adv Drug Deliv Rev. (2002) 54:459–76. doi: 10.1016/s0169-409x(02)00022-4
16. Ghaznavi H, Afzalipour R, Khoei S, Sargazi S, Shirvalilou S, and Sheervalilou R. New insights into targeted therapy of glioblastoma using smart nanoparticles. Cancer Cell Int. (2024) 24:160. doi: 10.1186/s12935-024-03331-3
17. Wadajkar AS, Dancy JG, Hersh DS, Anastasiadis P, Tran NL, Woodworth GF, et al. Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2017) 9:10.1002/wnan.1439. doi: 10.1002/wnan.1439
18. Gusmão LA, Matsuo FS, Barbosa HF, and Tedesco AC. Advances in nano-based materials for glioblastoma multiforme diagnosis: A mini-review. Front Nanotechnol. (2022) 4:836802. doi: 10.3389/fnano.2022.836802
19. Zhang H, Wang T, Liu H, Ren F, Qiu W, Sun Q, et al. Second near-infrared photodynamic therapy and chemotherapy of orthotopic Malignant glioblastoma with ultra-small Cu2-xSe nanoparticles. Nanoscale. (2019) 11:7600–8. doi: 10.1039/c9nr01789e
20. Lu VM, Jue TR, and McDonald KL. Cytotoxic lanthanum oxide nanoparticles sensitize glioblastoma cells to radiation therapy and temozolomide: an in vitro rationale for translational studies. Sci Rep. (2020) 10:18156. doi: 10.1038/s41598-020-75372-3
21. Zhan W. Delivery of liposome encapsulated temozolomide to brain tumour: Understanding the drug transport for optimisation. Int J Pharm. (2019) 557:280–92. doi: 10.1016/j.ijpharm.2018.12.065
22. Saha S, Yakati V, Shankar G, Jaggarapu MMCS, Moku G, MadhuSudana K, et al. Amphetamine decorated cationic lipid nanoparticles cross the blood-brain barrier: therapeutic promise for combating glioblastoma. J Mater Chem B. (2020) 8:4318–30. doi: 10.1039/c9tb02700a
23. Malinovskaya Y, Melnikov P, Baklaushev V, Gabashvili A, Osipova N, Mantrov S, et al. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int J Pharm. (2017) 524:77–90. doi: 10.1016/j.ijpharm.2017.03.049
24. Ahmed T, Liu FF, He C, Abbasi AZ, Cai P, Rauth AM, et al. Optimizing the design of blood-brain barrier-penetrating polymer-lipid-hybrid nanoparticles for delivering anticancer drugs to glioblastoma. Pharm Res. (2021) 38:1897–914. doi: 10.1007/s11095-021-03122-9
25. Ebrahimi Shahmabadi H, Movahedi F, Koohi Moftakhari Esfahani M, Alavi SE, Eslamifar A, Mohammadi Anaraki G, et al. Efficacy of Cisplatin-loaded polybutyl cyanoacrylate nanoparticles on the glioblastoma. Tumour Biol. (2014) 35:4799–806. doi: 10.1007/s13277-014-1630-9
26. Chiani M, Toofani Milani A, Nemati M, Rezaeidian J, Ehsanbakhsh H, Ahmadi Z, et al. Anticancer effect of cisplatin- loaded poly (Butylcyanoacrylate) nanoparticles on A172 brain cancer cells line. Asian Pac J Cancer Prev. (2019) 20:303–9. doi: 10.31557/APJCP.2019.20.1.303
27. Maksimenko O, Malinovskaya J, Shipulo E, Osipova N, Razzhivina V, Arantseva D, et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int J Pharm. (2019) 572:118733. doi: 10.1016/j.ijpharm.2019.118733
28. Madani F, Esnaashari SS, Bergonzi MC, Webster TJ, Younes HM, Khosravani M, et al. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life Sci. (2020) 256:117943. doi: 10.1016/j.lfs.2020.117943
29. Madani F, Morovvati H, Webster TJ, Najaf Asaadi S, Rezayat SM, Hadjighassem M, et al. Combination chemotherapy via poloxamer 188 surface-modified PLGA nanoparticles that traverse the blood-brain-barrier in a glioblastoma model. Sci Rep. (2024) 14:19516. doi: 10.1038/s41598-024-69888-1
30. Maleki H, Hosseini Najafabadi MR, Webster TJ, Hadjighassem MR, Sadroddiny E, et al. Effect of Paclitaxel/etoposide co-loaded polymeric nanoparticles on tumor size and survival rate in a rat model of glioblastoma. Int J Pharm. (2021) 604:120722. doi: 10.1016/j.ijpharm.2021.120722
31. Caban-Toktas S, Sahin A, Lule S, Esendagli G, Vural I, Karlı Oguz K, et al. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int J Pharm. (2020) 578:119076. doi: 10.1016/j.ijpharm.2020.119076
32. Arshad A, Yang B, Bienemann AS, Barua NU, Wyatt MJ, Woolley M, et al. Convection-enhanced delivery of carboplatin PLGA nanoparticles for the treatment of glioblastoma. PloS One. (2015) 10:e0132266. doi: 10.1371/journal.pone.0132266
33. Xu Y, Shen M, Li Y, Sun Y, Teng Y, Wang Y, et al. The synergic antitumor effects of paclitaxel and temozolomide co-loaded in mPEG-PLGA nanoparticles on glioblastoma cells. Oncotarget. (2016) 7:20890–901. doi: 10.18632/oncotarget.7896
34. Plichta Z, Horák D, Mareková D, Turnovcová K, Kaiser R, and Jendelová P. Poly[N-(2-hydroxy propyl) methacrylamide]-Modified Magnetic γ-F2 O3 Nanoparticles Conjugated with Doxorubicin for Glioblastoma Treatment. ChemMedChem. (2020) 15:96–104. doi: 10.1002/cmdc.201900564
35. Akyuva Y and Nazıroğlu M. Silver nanoparticles potentiate antitumor and oxidant actions of cisplatin via the stimulation of TRPM2 channel in glioblastoma tumor cells. Chem Biol Interact. (2023) 369:110261. doi: 10.1016/j.cbi.2022.110261
36. Erthal LCS, Shi Y, Sweeney KJ, Gobbo OL, and Ruiz-Hernandez E. Nanocomposite formulation for a sustained release of free drug and drug-loaded responsive nanoparticles: an approach for a local therapy of glioblastoma multiforme. Sci Rep. (2023) 13:5094. doi: 10.1038/s41598-023-32257-5
37. Zhang Y, Fu X, Jia J, Wikerholmen T, Xi K, Kong Y, et al. Glioblastoma therapy using codelivery of cisplatin and glutathione peroxidase targeting siRNA from iron oxide nanoparticles. ACS Appl Mater Interfaces. (2020) 12:43408–21. doi: 10.1021/acsami.0c12042
38. Li Y, Zhao Q, Zhu X, Zhou L, Song P, Liu B, et al. Self-Assembled nanoparticles of natural bioactive molecules enhance the delivery and efficacy of paclitaxel in glioblastoma. CNS Neurosci Ther. (2024) 30:e14528. doi: 10.1111/cns.14528
39. Zou Y, Wang Y, Xu S, Liu Y, Yin J, Lovejoy DB, et al. Brain co-delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Adv Mater. (2022) 34:e2203958. doi: 10.1002/adma.202203958
40. Mao J, Meng X, Zhao C, Yang Y, and Liu G. Development of transferrin-modified poly(lactic-co-glycolic acid) nanoparticles for glioma therapy. Anticancer Drugs. (2019) 30:604–10. doi: 10.1097/CAD.0000000000000754
41. Wang L, Liu C, Qiao F, Li M, Xin H, Chen N, et al. Analysis of the cytotoxic effects, cellular uptake and cellular distribution of paclitaxel-loaded nanoparticles in glioblastoma cells in vitro. Exp Ther Med. (2021) 21:292. doi: 10.3892/etm.2021.9723
42. Luo M, Lewik G, Ratcliffe JC, Choi CHJ, Mäkilä E, Tong WY, et al. Systematic evaluation of transferrin-modified porous silicon nanoparticles for targeted delivery of doxorubicin to glioblastoma. ACS Appl Mater Interfaces. (2019) 11:33637–49. doi: 10.1021/acsami.9b10787
43. Banstola A, Duwa R, Emami F, Jeong JH, and Yook S. Enhanced caspase-mediated abrogation of autophagy by temozolomide-loaded and panitumumab-conjugated poly (lactic-co-glycolic acid) nanoparticles in epidermal growth factor receptor overexpressing glioblastoma cells. Mol Pharm. (2020) 17:4386–400. doi: 10.1021/acs.molpharmaceut.0c00856
44. Wang L, Wang X, Shen L, Alrobaian M, Panda SK, Almasmoum HA, et al. Paclitaxel and naringenin-loaded solid lipid nanoparticles surface modified with cyclic peptides with improved tumor targeting ability in glioblastoma multiforme. BioMed Pharmacother. (2021) 138:111461. doi: 10.1016/j.biopha.2021.111461
45. Ullah I, Chung K, Bae S, Li Y, Kim C, Choi B, et al. Nose-to-brain delivery of cancer-targeting paclitaxel- loaded nanoparticles potentiates antitumor effects in Malignant glioblastoma. Mol Pharm. (2020) 17:1193–204. doi: 10.1021/acs.molpharmaceut.9b01215
46. Chung K, Ullah I, Kim N, Lim J, Shin J, Lee SC, et al. Intranasal delivery of cancer-targeting doxorubicin-loaded PLGA nanoparticles arrests glioblastoma growth. J Drug Target. (2020) 28:617–26. doi: 10.1080/1061186X.2019.1706095
47. Wang L, Tang S, Yu Y, Lv Y, Wang A, Yan X, et al. Intranasal delivery of temozolomide-conjugated gold nanoparticles functionalized with anti-ephA3 for glioblastoma targeting. Mol Pharm. (2021) 18:915–27. doi: 10.1021/acs.molpharmaceut.0c00911
48. Wang S, Yu Y, Wang A, Duan X, Sun Y, Wang L, et al. Temozolomide hexadecyl ester targeted PLGA nanoparticles for drug-resistant glioblastoma therapy via intranasal administration. Front Pharmacol. (2022) 13:965789. doi: 10.3389/fphar.2022.965789
49. Chen H and Wen J. Iron oxide nanoparticles loaded with paclitaxel inhibits glioblastoma by enhancing autophagy-dependent ferroptosis pathway. Eur J Pharmacol. (2022) 921:174860. doi: 10.1016/j.ejphar.2022.174860
50. Zhang J, Sun Y, Tian B, Li K, Wang L, Liang Y, et al. Multifunctional mesoporous silica nanoparticles modified with tumor-shedable hyaluronic acid as carriers for doxorubicin. Colloids Surf B Biointerfaces. (2016) 144:293–302. doi: 10.1016/j.colsurfb.2016.04.015
51. Deshmukh V, Narwade M, and Gajbhiye KR. Intranasal delivery of paclitaxel-loaded ligand conjugated polymeric nanoparticles for targeted brain delivery. AAPS PharmSciTech. (2025) 26:49. doi: 10.1208/s12249-025-03046-2
52. Janjua TI, Cao Y, Ahmed-Cox A, Raza A, Moniruzzaman M, Akhter DT, et al. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. J Control Release. (2023) 357:161–74. doi: 10.1016/j.jconrel.2023.03.040
53. Norouzi M, Yathindranath V, Thliveris JA, Kopec BM, Siahaan TJ, and Miller DW. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: a combinational approach for enhanced delivery of nanoparticles. Sci Rep. (2020) 10:11292. doi: 10.1038/s41598-020-68017-y
54. Kwon YM, Je JY, Cha SH, Oh Y, and Cho WH. Synergistic combination of chemo−phototherapy based on temozolomide/ICG−loaded iron oxide nanoparticles for brain cancer treatment. Oncol Rep. (2019) 42:1709–24. doi: 10.3892/or.2019.7289
55. Beola L, Iturrioz-Rodríguez N, Pucci C, Bertorelli R, and Ciofani G. Drug-loaded lipid magnetic nanoparticles for combined local hyperthermia and chemotherapy against glioblastoma multiforme. ACS Nano. (2023) 17:18441–55. doi: 10.1021/acsnano.3c06085
56. Lajous H, Riva R, Lelièvre B, Tétaud C, Avril S, Hindré F, et al. Hybrid Gd3+/cisplatin cross-linked polymer nanoparticles enhance platinum accumulation and formation of DNA adducts in glioblastoma cell lines. Biomater Sci. (2018) 6:2386–409. doi: 10.1039/c8bm00346g
57. Pulvirenti L, Monforte F, Lo Presti F, Li Volti G, Carota G, Sinatra F, et al. Synthesis of MIL-modified fe3O4 magnetic nanoparticles for enhancing uptake and efficiency of temozolomide in glioblastoma treatment. Int J Mol Sci. (2022) 23:2874. doi: 10.3390/ijms23052874
58. Mao X, Calero-Pérez P, Montpeyó D, Bruna J, Yuste VJ, Candiota AP, et al. Intranasal administration of catechol-based pt(IV) coordination polymer nanoparticles for glioblastoma therapy. Nanomater (Basel). (2022) 12:1221. doi: 10.3390/nano12071221
59. Neshastehriz A, Khateri M, Ghaznavi H, and Shakeri-Zadeh A. Investigating the therapeutic effects of alginate nanogel co-loaded with gold nanoparticles and cisplatin on U87-MG human glioblastoma cells. Anticancer Agents Med Chem. (2018) 18:882–90. doi: 10.2174/1871520618666180131112914
60. Jing Z, Li M, Wang H, Yang Z, Zhou S, Ma J, et al. Gallic acid-gold nanoparticles enhance radiation-induced cell death of human glioma U251 cells. IUBMB Life. (2021) 73:398–407. doi: 10.1002/iub.2436
61. He C, Zhang Z, Ding Y, Xue K, Wang X, Yang R, et al. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. J Nanobiotechnol. (2021) 19:29. doi: 10.1186/s12951-020-00751-x
62. Depciuch J, Miszczyk J, Maximenko A, Zielinski PM, Rawoj´c K, Panek A, et al. Gold nanopeanuts as prospective support for cisplatin in glioblastoma nano-chemo-radiotherapy. Int J Mol Sci. (2020) 21:9082. doi: 10.3390/ijms21239082
63. Ganipineni LP, Ucakar B, Joudiou N, Riva R, Jérôme C, Gallez B, et al. Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J Drug Target. (2019) 27:614–23. doi: 10.1080/1061186X.2019.1567738
64. Zhu X, Huang H, Zhang Y, Zhang H, Hou L, and Zhang Z. Cit/CuS@Fe3O4-based and enzyme-responsive magnetic nanoparticles for tumor chemotherapy, photothermal, and photodynamic therapy. J Biomater Appl. (2017) 31:1010–25. doi: 10.1177/0885328216676159
65. Hersh AM, Alomari S, and Tyler BM. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mol Sci. (2022) 23:4153. doi: 10.3390/ijms23084153
66. Öztürk K, Kaplan M, and Çalış S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int J Pharm. (2024) 666:124799. doi: 10.1016/j.ijpharm.2024.124799
67. Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. (1987) 47:5846–52.
68. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/nejmoa043330
69. van den Bent MJ, Hegi ME, and Stupp R. Recent developments in the use of chemotherapy in brain tumours. Eur J Cancer. (2006) 42:582–8. doi: 10.1016/j.ejca.2005.06.031
70. Dutra JAP, Luiz MT, Tavares Junior AG, Di Filippo LD, Carvalho SG, and Chorilli M. Temozolomide: an overview of biological properties, drug delivery nanosystems, and analytical methods. Curr Pharm Des. (2022) 28:2073–88. doi: 10.2174/1381612828666220603152918
71. Ananta JS, Paulmurugan R, and Massoud TF. Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: a biophysical and cell culture evaluation. Neurol Res. (2016) 38:51–9. doi: 10.1080/01616412.2015.1133025
72. Ramalho MJ, Torres ID, Loureiro JA, Lima J, and Pereira MC. Transferrin-conjugated PLGA nanoparticles for co-delivery of temozolomide and bortezomib to glioblastoma cells. ACS Appl Nano Mater. (2023) 6:14191–203. doi: 10.1021/acsanm.3c02122
73. Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. (2021) 2021:3687700. doi: 10.1155/2021/3687700
74. Cai Q, Li X, Xiong H, Fan H, Gao X, Vemireddy V, et al. Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment. Nat Commun. (2023) 14:4934. doi: 10.1038/s41467-023-40579-1
75. Rottenberg S, Disler C, and Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. (2021) 21:37–50. doi: 10.1038/s41568-020-00308-y
76. Aryal S, Hu CM, Fang RH, Dehaini D, Carpenter C, Zhang DE, et al. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomed (Lond). (2013) 8:1271–80. doi: 10.2217/nnm.12.153
77. Lin X, Ma X, Zhao S, Yao J, Han L, Jing Y, et al. Cardiovascular toxicity in antitumor therapy: biological and therapeutic insights. Trends Cancer. (2024) 10:920–34. doi: 10.1016/j.trecan.2024.07.004
78. Li G, Wang C, Jin B, Sun T, Sun K, Wang S, et al. Advances in smart nanotechnology-supported photodynamic therapy for cancer. Cell Death Discov. (2024) 10:466. doi: 10.1038/s41420-024-02236-4
79. Liu Z, Mela A, Argenziano MG, Banu MA, Furnari J, Kotidis C, et al. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J Neurosurg. (2023) 140:968–78. doi: 10.3171/2023.7.JNS23122
80. Liu D, Dai X, Ye L, Wang H, Qian H, Cheng H, et al. Nanotechnology meets glioblastoma multiforme: Emerging therapeutic strategies. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2023) 15:e1838. doi: 10.1002/wnan.1838
81. Liu Z, Wang J, Qiu K, Liao X, Rees TW, Ji L, et al. Fabrication of Red blood cell membrane-camouflaged Cu2-xSe nanoparticles for phototherapy in the second near-infrared window. Chem Commun. (2019) 55:6523–6. doi: 10.1039/c9cc03148k
82. He Y, Cong C, He Y, Hao Y, Li C, Wang S, et al. Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy. Chem Eng J. (2019) 375:122079. doi: 10.1016/j.cej.2019.122079
83. Chen Q, Feng L, Liu J, Zhu W, Dong Z, Wu Y, et al. Intelligent albumin-mnO2 nanoparticles as pH-/H2 O2 -responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater. (2016) 28:7129–36. doi: 10.1002/adma.201601902
84. Perini G, Palmieri V, Ciasca G, D’Ascenzo M, Gervasoni J, Primiano A, et al. Graphene quantum dots’ Surface chemistry modulates the sensitivity of glioblastoma cells to chemotherapeutics. Int J Mol Sci. (2020) 21:6301. doi: 10.3390/ijms21176301
85. Perini G, Palmieri V, Friggeri G, Augello A, De Spirito M, and Papi M. Carboxylated graphene quantum dots-mediated photothermal therapy enhances drug-membrane permeability, ROS production, and the immune system recruitment on 3D glioblastoma models. Cancer Nano. (2023) 14:13. doi: 10.1186/s12645-023-00168-9
86. Zhou XQ, Meng LB, Huang Q, Li J, Zheng K, Zhang FL, et al. Synthesis and in vitro anticancer activity of Zinc (II) phthalocyanines conjugated with coumarin derivatives for dual photodynamic and chemotherapy. ChemMedChem. (2015) 10:304–11. doi: 10.1002/cmdc.201402401
87. Xiang H, Chen H, Tham HP, Phua SZF, Liu JG, and Zhao Y. Cyclometalated iridium (III)-complex-based micelles for glutathione-responsive targeted chemotherapy and photodynamic therapy. ACS Appl Mater Interfaces. (2017) 9:27553–62. doi: 10.1021/acsami.7b09506
88. Srinivasan S, Bhardwaj V, Nagasetti A, Fernandez-Fernandez A, and McGoron AJ. Multifunctional surface-enhanced raman spectroscopy-detectable silver nanoparticles for combined photodynamic therapy and PH-triggered chemotherapy. J Biomed Nanotechnol. (2016) 12:2202–19. doi: 10.1166/jbn.2016.2312
89. Yuan Y, Min Y, Hu Q, Xing B, and Liu B. NIR photoregulated chemo-and photodynamic cancer therapy based on conjugated polyelectrolyte–drug conjugate encapsulated upconversion nanoparticles. Nanoscale. (2014) 6:11259–72. doi: 10.1039/c4nr03302g
90. Liu Sf, Li MJ, Liang B, Sun W, Shao Y, Hu X, et al. Breaking the barrier: Nanoparticle-enhanced radiotherapy as the new vanguard in brain tumor treatment. Front Pharmacol. (2024) 15:1394816. doi: 10.3389/fphar.2024.1394816
91. Chen K, Chang C, Liu Z, Zhou Y, Xu Q, Li C, et al. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf B Biointerfaces. (2020) 194:111166. doi: 10.1016/j.colsurfb.2020.111166
92. Meng X, Wang K, Lv L, Zhao Y, Sun C, Ma L, et al. Photothermal/photodynamic therapy with immune-adjuvant liposomal complexes for effective gastric cancer therapy. Part Part Syst Charact. (2019) 36:1900015. doi: 10.1002/ppsc.201900015
93. Reddy S, Tatiparti K, Sau S, and Iyer AK. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov Today. (2021) 26:1944– 52. doi: 10.1016/j.drudis.2021.04.008
94. Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. (2019) 16:509–20. doi: 10.1038/s41571-019-0177-5
95. Beier CP, Schmid C, Gorlia T, Kleinletzenberger C, Beier D, Grauer O, et al. RNOP-09: pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma–a phase II study. BMC Cancer. (2009) 9:308. doi: 10.1186/1471-2407-9-308
96. Huang H, Feng W, Chen Y, and Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. (2020) 35:100972. doi: 10.1016/j.nantod.2020.100972
97. Thivat E, Casile M, Moreau J, Molnar I, Dufort S, Seddik K, et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer. (2023) 23:344. doi: 10.1186/s12885-023-10829-y
98. Vermandel M, Dupont C, Lecomte F, Leroy HA, Tuleasca C, Mordon S, et al. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: a preliminary analysis of the INDYGO clinical trial. J Neurooncol. (2021) 152:501–14. doi: 10.1007/s11060-021-03718-6
99. Peciu-Florianu I, Vannod-Michel Q, Vauleon E, Bonneterre ME, and Reyns N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: an update from the INDYGO trial (NCT03048240). J Neurooncol. (2024) 168:495–505. doi: 10.1007/s11060-024-04693-4
100. Biau J, Durando X, Boux F, Molnar I, Moreau J, Leyrat B, et al. NANO-GBM trial of AGuIX nanoparticles with radiotherapy and temozolomide in the treatment of newly diagnosed Glioblastoma: Phase 1b outcomes and MRI-based biodistribution. Clin Transl Radiat Oncol. (2024) 48:100833. doi: 10.1016/j.ctro.2024.100833
101. Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. (2011) 103:317–24. doi: 10.1007/s11060-010-0389-0
102. Shirvalilou S, Khoei S, Esfahani AJ, Kamali M, Shirvaliloo M, Sheervalilou R, et al. Magnetic Hyperthermia as an adjuvant cancer therapy in combination with radiotherapy versus radiotherapy alone for recurrent/progressive glioblastoma: a systematic review. J Neurooncol. (2021) 152:419–28. doi: 10.1007/s11060-021-03729-3
Keywords: nanoparticles, delivery, glioblastoma, chemotherapy, radiotherapy, PDT
Citation: Messina S, Zuchegna C and Bruzzi M (2025) Chemotherapeutic nanoparticles for glioblastoma. Front. Oncol. 15:1641752. doi: 10.3389/fonc.2025.1641752
Received: 05 June 2025; Accepted: 22 July 2025;
Published: 11 August 2025.
Edited by:
Kolawole Olofinsan, University of the Free State, South AfricaReviewed by:
Jayaprakash N Kolla, Institute of Molecular Genetics (ASCR), CzechiaStabak Das, Brainware University, India
Copyright © 2025 Messina, Zuchegna and Bruzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samantha Messina, c2FtYW50aGEubWVzc2luYUB1bmlyb21hMy5pdA==
 Samantha Messina
Samantha Messina Candida Zuchegna
Candida Zuchegna Mara Bruzzi
Mara Bruzzi