- Laser Research Centre, University of Johannesburg, Johannesburg, South Africa
Melanoma is a highly aggressive cancer with poor prognosis and resistance to many treatments, especially after metastasis. Developing new preventive and adjuvant therapies is critical for improving melanoma outcomes. Photodynamic therapy (PDT) has shown potential in selectively targeting malignant cells while minimizing damage to healthy tissue. However, improving the delivery of photosensitizers (PS) to melanoma cells while reducing systemic toxicity remains a challenge. Microneedles, a transcutaneous drug delivery method, offer advantages such as better patient compliance and easier management compared to traditional methods like intramuscular or intravenous injection. Despite these benefits, manufacturing precise microneedles remains a hurdle. Recent research has focused on 3D printing techniques for creating transdermal drug delivery devices, including microneedles. This review summarizes recent advantages in 3D printed biopolymer-based drug delivery systems using microneedles, evaluates their potential, and discusses the challenges and future prospects of 3D printing in transdermal therapy.
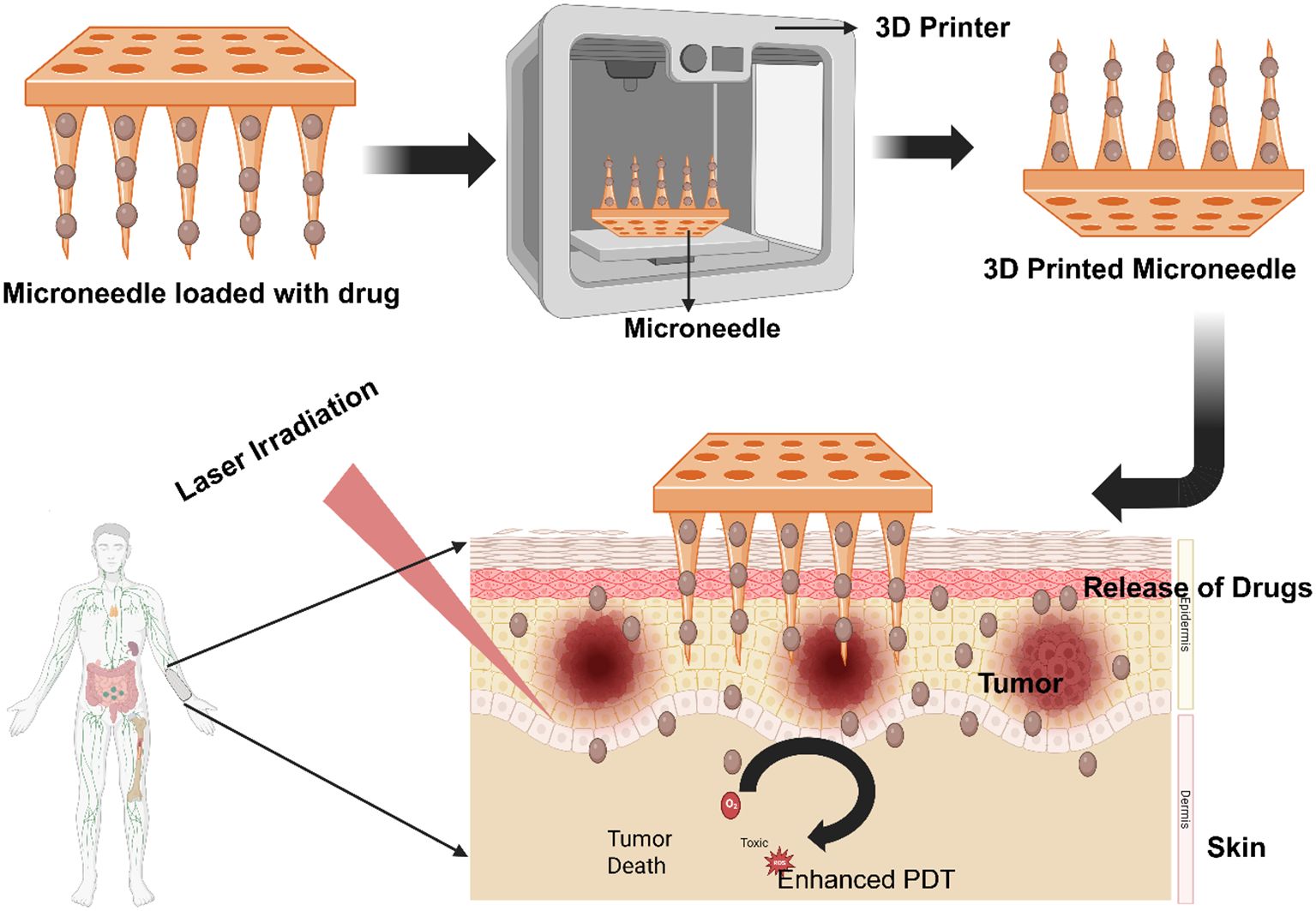
Graphical Abstract. 3D-Printed Biopolymer Microneedles for Enhanced PDT in Melanoma Treatment- Created in BioRender. Dhilip kumar, S. (2025) https://BioRender.com/fear27s
1 Introduction
Skin cancer has become a major global health concern, with its incidence steadily increasing, which may significantly affect both the global workforce and economy. The skin has two primary strata: the dermis and the epidermis. The outermost layer of skin, known as the epidermis, comprises Langerhans, Merkel, melanocyte, and keratinocyte cells (1). Cancer is one kind of skin injury that may result from any aberration in this layer. There are two main categories of skin cancer: non-melanoma, which originates from cells originating from the skin’s surface, and melanoma skin cancers, which result from malfunctioning melanocytes (2). Nearly 95% of skin cancer cases are non-melanoma skin cancer (NMSC) (3, 4). Compared to other skin injuries, melanoma represents a negligible fraction of skin malignant tumors—just 1%. Even with recent improvements in treatment methods, melanoma remains the most dangerous kind of skin cancer, with just 15-20% of cases surviving for five years (5).
Oral, intravenous, and intraperitoneal routes are among the traditional techniques for delivering cancer-based medicinal medicines; nevertheless, these routes often have several significant drawbacks (6). Transdermal drug delivery systems (TDDS) offer a non-intrusive, painless method of distribution as opposed to oral and intravenous methods. It is well known to increase the bioavailability of medications with limited permeability and solubility (7, 8). One of the main obstacles to TDDS is the stratum corneum layer, which only allows a few indications to penetrate the skin (9).
To overcome this limitation, scientists have recently developed Microneedle (MN)-based matrices that detect cancer and enable transdermal administration of medications inside the tumor area (10), Although MN-based drug delivery systems were proposed over thirty years ago, their clinical use has recently gained popularity (11). Notwithstanding these unique qualities, the difficulty in accurately producing such micro-scaled devices has significantly impeded their actual industrial applications (12). Remarkably, the advent of three-dimensional Printing (3D printing) technology has effectively addressed the constraints associated with microneedles; this has allowed for the continuous production of one-step products and the development of a desirable microneedle for individualized customization (13, 14).
Additive manufacturing, or 3D printing, is a procedure where a computer-aided design (CAD) module positions materials in layers and selectively creates things with any desired geometric complexity (15, 16). Various 3D printing innovations have been put out in the last ten years to provide adaptable medication doses for a range of uses accurately. These include implants for targeted drug delivery (17, 18) and printlets for oral administration (13). These technologies have great potential in manufacturing pharmaceutical items, especially those with the potential to be individualized treatments (19).
2 Principles of photodynamic therapy and its application in melanoma
The most common skin cancer that may be caused by both internal and external sources is melanoma (20). Melanocytes give rise to this kind of cancer. The most common type, cutaneous melanoma, accounts for almost 90–95% of all melanoma occurrences (21) and typically spreads to the brain, eyes, anus, liver, and bone. The degree of involvement and spread to lymph nodes and other nearby healthy tissues determines the stage of melanoma (22). Age, gender, immunodeficiency, family history, and prolonged exposure to ultraviolet radiation (UV) are the most frequent risk factors for melanoma (23). The development of better treatment methods in recent decades has not slowed the sharp increase in melanoma cancer incidence (20). As a result, the concerning increase in MM-related morbidity and death continues to pose a significant obstacle to global healthcare (24). Treatment options for MM include surgery, chemotherapy, radiation, immunotherapy, and molecularly targeted therapy, depending on the patient’s location, stage, and genetics (25). Nevertheless, these therapies often result in unfavorable side effects. While radiation is strongly advised for the treatment of bone, skin, and brain metastases (25), surgery is the primary treatment for early stage melanoma in order to prevent metastasis and improve survival chances (26). Chemotherapeutic medications such dacarbazine (DTIC), temozolomide (TMZ), and fotemustine have been used to treat MM successfully for many years (22). Chemotherapy is still essential for the palliative treatment of tumors that are resistant, progressing, and recurrent, despite the fact that it may have unfavorable effects on nearby normal cells (27). Neutralizing antibodies having a high affinity for immunological blockades, such as programed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), have improved patient survival rates among immunotherapies (21). Additionally, MM has been significantly impacted by therapies that have target specificity for the oncogenic serine/threonine-protein kinase B-Raf (BRAF) proteins, which are invariably expressed in melanoma patients (21). However, severe patient immunological responses and medication resistance also pose a challenge to these therapy (27). Within eight months of therapy, the majority of patients who have a first and significant tumor recurrence may see a progression in their illness (21). In order to overcome medication resistance and increase the alternatives accessible to MM patients, additional effective medicines are desperately needed (21). In recent years, photodynamic therapy (PDT) has become one of the best cancer treatment options for overcoming the difficulties associated with melanoma (20).
Photodynamic therapy (PDT) is a type of phototherapy used to treat cancer (28). It relies on a Photosensitiser (PS), visible light, and the molecular oxygen around the target tissue that coincides with the PS’s absorbance range to cause cellular damage (29). One of thexmain ideas behind PDT is the selective accumulation of photosensitizers in tumor tissues. This happens a lot because of the increased permeability and retention (EPR) effect or receptor-mediated uptake. When photosensitizing agents (PSs) are photoactivated, PDT initiates photochemical processes that destroy a localized tumor in the target area (30). The reactive oxygen species (ROS) produced during the PDT action method cause cell death by necrotic, autophagic, or apoptotic mechanisms (28) (Figure 1).
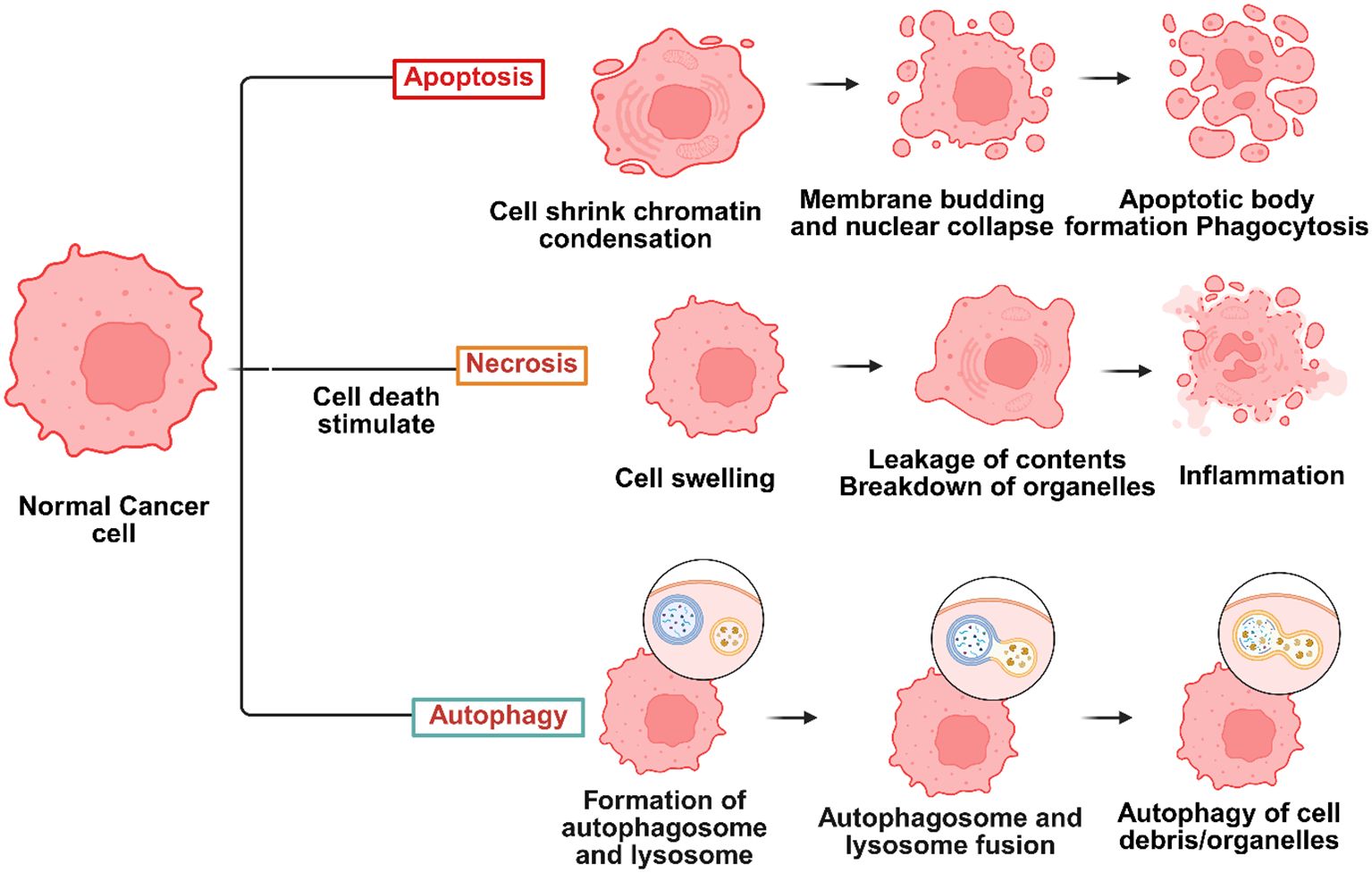
Figure 1. Several types of cell death, including necrosis, autophagy, and apoptosis, may be triggered during photodynamic therapy (PDT) cancer therapies – Created in BioRender. Dhilip kumar, S. (2025) https://BioRender.com/yrtk89u.
Cell death, known as apoptosis, is regulated and often distinguished by nuclear and membrane deterioration (31). This kind of cell death is the most frequent associated mechanism of cell death in PDT, and the PSs typically localize in cellular mitochondria when it happens (32). Certain signals cause target cells to undergo apoptosis, which sets off several suicide pathways in response to the signals (33). Protein caspases are triggered to break down cellular components, including nucleic and polypeptide material, when the pathways break down (34). As a result, apoptosis is an initiated, controlled process (35). When an external stimulus, such as an infection or trauma, triggers an inflammatory response, necrosis is an unprogramed form of cell death (36). The PS that causes necrosis often localizes inside the target cell’s plasma membrane (37). Membrane permeability and calcium ion transport across the endoplasmic reticulum are components of necrotic cell death pathways (38). Recent research by Dewaele and colleagues (37) has shown that another method of cell death called autophagy may be produced after PDT irradiation of certain PSs. When a cell attempts self-healing to recover from photodamage, it is signaled for programed apoptosis; if this response is unsuccessful, the cell undergoes photodynamic therapy-induced autophagy (39). According to research by Valli and associates, photoactivated zinc phthalocyanine (ZnPcS) showed a higher degree of ROS production, which caused MM cells to undergo necrosis and apoptosis (40). The phototoxicity of Chlorin e6 (Ce6) conjugated to PAMAM dendrimer (generation 7.0) functionalized with RGD peptide was examined in A375 tumor spheroids by Yuan et al. Twelve hours after irradiation, RGD-P-Ce6 produced a noteworthy 25.7% of early apoptotic cells and 25.2% of dead cells (41). A mesoporous nanocarrier containing dabrafenib, phthalocyanine (Pc), was created by Tham et al. In spheroids with 8% cell viability, the nanocomposite demonstrated a much higher cell-killing effectiveness, according to the research (42). Furthermore, PcNP-dug effectively targeted BRAF-positive cancer cells in vivo while preserving normal cells that did not express BRAF, achieving 76% tumor shrinkage (42). Some clinical results have been reported, despite ongoing controversy about the therapeutic use of PDT in the treatment of MM. Barbazetto et al. examined four individuals with choroidal melanoma to determine the phototoxicity of verteporfin. According to the findings, melanomas remained resistant and required surgical removal in the remaining instances; however, PDT caused tumor regression in two of them (43). In a similar vein, research conducted by Donaldson et al. examined the effects of verteporfin and laser irradiation in a patient with choroidal amelanotic melanoma and observed a full tumor suppression (44).
2.1 Photosensitizers in the treatment of metastatic melanoma
Many PS classes have been studied for PDT therapy of metastatic melanomas Table 1 (60). There are many things to consider when deciding the kind of PS to use with a certain PDT intervention, including its properties, method of action, localization, and kind of cellular demise it causes. PDT-utilized PSs are divided into three generations according to their photochemical and photophysical characteristics in relation to their cellular mode of action (61). First-generation PSs have significant side effects and often cause vascular tissue damage as their localization, suggesting that their exact location inside the intended cells is constrained (62). Second-generation PS often only has cytotoxic effects on tumor cells (63). To enhance the specific cellular drug absorption and uptake of photosynthetic medicines, third-generation PSs have been supplemented with additional targeted biomolecules (64).
3 Transdermal drug delivery system
A collection of physical-chemical innovations that have the potential to control how pharmacologically active substances are released and transported into tissues, cells, and organs such that these active substances can have the greatest possible effects are collectively referred to as drug delivery systems (DDS) (65, 66). Stated differently, DDS include drug delivery methods and formulations that effectively distribute the medicine to enhance therapeutic effectiveness while reducing adverse effects (11).
One of the most researched non-invasive cutaneous drug delivery methods is TDDS. The delivery of many medications has been significantly impacted by TDDS, particularly in the areas of hormone therapy, pain management, and the treatment of conditions affecting the cardiovascular and central nervous systems (67–69). Since TDDS eliminates the need for gastrointestinal tract passage, medications may be administered without pH, enzymes, or intestinal flora interference. It also eliminates loss from first-pass metabolism. Furthermore, TDDS may regulate medication release by consumption limitations, which adds to the method’s high persistence. Most significantly, medications may be safely and conveniently administered to children or the elderly using TDDS, a non-invasive method of delivery that typically causes minimal discomfort to the individual (70, 71).
3.1 Patches
When the scopolamine patch was introduced in the 1970s, marking the switch from patchless systems to the TDD patch to achieve a more continuous, regulated, and safe form of drug administration, transdermal drug treatment garnered international interest. The multi-layered drug-storing patch creates a constant medication flow to the skin, enabling continuous administration over an extended length of time. Two different approaches are used to develop patches: the first, known as the “reservoir patch,” has a section where the medication is kept in the proper formulation, and a membrane controls how much is delivered to the skin’s surface. Although Shaw and Theeuwes (1985) found that this design produces release rates that are typically steady, overdose events have been documented because of malfunctioning membranes (72). However, the “matrix patch,” which incorporates the medication evenly into a matrix from which it is delivered to the skin, was created to circumvent the shortcomings of the reservoir-type patch (73). This design may be divided into two primary subcategories: the first uses a polymer that contains a medication, and the second adds an adhesive material to provide a secure fit against the skin. As an alternative, the adhesive—where the medication is contained—is the sole part of the design. At first, TDD patches were quite popular, and many systems based on them were even sold commercially. Commercial patches are available today for a variety of drug administration purposes, including antidepressants, contraceptives, and Alzheimer’s disease treatment (74). However, various impediments prevented the transdermal patch from being widely adopted. According to Hadgraft and Lane (2016), there might be problems with efficacy if a medication crystallizes on the skin before diffusing or if the composition becomes unstable when stored (75).
Consequently, the two patch designs that maximize the percentage of deliveries that can be achieved are the same as the maximum rate that the stratum corneum’s physiology naturally permits. The drawbacks highlight how inappropriate such technologies are for quick, bolus-style medication delivery. Another significant drawback is that these systems depend on the kind of medicine. This restriction has significantly limited the drug palette in the past. It presents substantial obstacles to the transdermal distribution of various medications, including peptides and macromolecules, with greater molecular weights or hydrophilic characteristics (76). Several permeability-enhancing techniques have been developed to overcome these restrictions (77). These techniques use both passive and active ways to increase drug transportation by altering the permeability of the skin. It is noteworthy, however, that the bulk of these methods has been linked to exorbitant expenses, skin irritation, or even discomfort (76, 78). Table 2 shows different studies on topical patch systems using 3D printing.
3.2 Microneedles
Microneedles (MN) are arrays of tiny needles arranged in a matrix on its surface. The height of the MNs varies from 25 to 2000 µm, which guarantees their ability to enter the skin’s network of capillaries. Their diameter ensures the device stays away from cutaneous nerve endings and blood arteries. As a result, there is a decreased chance of tissue injury, painless delivery, and infection with microorganisms (19, 91). To maintain the honor of the skin after device removal, the form, size, and material of the MNs must be carefully chosen. Taller MNs have a higher medication loading capacity because of their increased volume. However, patients may experience discomfort from very tall MNs. The ability of the medication to load an array is enhanced by increasing the number of needles in it, but this also results in a proportionate increase in the force needed to insert the MNs. These trade-offs make designing MNs difficult, especially when creating effective, high-quality MNs (92). Based on in-plane and out-of-plane architectures, several varieties of MNs have been created (93). These systems are very patient-friendly because of features like painlessness and self-applying (19).
Furthermore, the shape of MNs allows for more efficient delivery of macromolecules through the skin (94, 95). The lack of resolution, the expensive cost of manufacturing, and localized irritation at the point of application have all been noted as drawbacks of this system despite its popularity as an alternative to standard hypodermic and subcutaneous injections (10). These issues can make it challenging to produce effective MNs on a large scale (93). As previously indicated for patches, changing the mix of the carriers (polymeric substance and medication) may reduce the risk of skin irritation and sensitization.
3.2.1 Types of microneedles
Microneedles are categorized based on their structure, material, and intended use. Each microneedle form has distinct qualities, benefits, drawbacks, uses, and material types. These are a few typical varieties of microneedles Figure 2.
![Illustration showing various microneedle patch designs for enhancing photodynamic therapy (PDT) in tumor treatment. Panels [a] to [d] depict different types of microneedles penetrating the skin and delivering PDT agents to enhance tumor cell death. The left side shows a diagram of a human body indicating treatment areas.](https://www.frontiersin.org/files/Articles/1642448/fonc-15-1642448-HTML/image_m/fonc-15-1642448-g002.jpg)
Figure 2. Different types of microneedles. (a) Solid removable microneedle, (b) Coated microneedle, (c) Dissolving microneedle, (d) Hollow microneedle, and (e) Hydrogel-forming microneedle. Created in BioRender. Dhilip kumar, S. (2025) https://BioRender.com/dg77ew4.
3.2.1.1 Solid microneedles
Solid microneedles are mostly used to produce pores on the skin to treat it. The spitzer tips of the needles puncture the skin when a drug patch is placed, creating micron-sized channels that enable the medication to directly penetrate the skin layers and improve penetration; the medication has a systemic impact after being absorbed by the capillaries (96), the medication is applied to epidermal layers via solid microneedles via passive diffusion (97). Biodegradable polymer solid microneedles may improve medication absorption and have enough mechanical strength to penetrate the stratum corneum, according to research on polylactic acid microneedles by Li et al. (96).
3.2.1.2 Coated microneedles
Microneedles with coatings serve two primary purposes. One involves piercing the skin, while the other applies the required medication on the micro needle’s surface. Regrettably, less than 1 milligram is the maximum medication dosage, which explains why coated microneedle development has been restricted (98). Coated microneedles come in a variety of forms that are designed to facilitate drug loading and penetration. Pere et al. created cone and pyramid MN designs for insulin administration using the 3DP approach (99).
3.2.1.3 Hollow microneedles
A hollow chamber that may be utilized for injecting or storing medicine makes up the hollow microneedle (10). Using a non-pressurized drug reservoir as the basis, A route for drug diffusion into the dermis is created using hollow microneedles, an active drug delivery technique. The material composition and manufacturing characteristics of hollow microneedles may be combined to allow for adjustable release kinetics. Depending on the purpose of the application, pharmaceuticals at higher concentrations may provide pharmacological profiles with burst release. In contrast, medications put into a matrix may allow for a medication release that occurs steadily over many days or weeks (100). The hollow microneedle has been used effectively for several vaccinations and vaccines. However, since hollow microneedles are comparatively weaker than solid microneedles and need special attention regarding needle design and insertion technique, they have garnered less attention than solid microneedles. In addition, there are technical issues with the hollow microneedle, such as leaks and blockage while injecting (101).
Long, sharp microneedles that could enter blood arteries for analysis were produced by optimizing the hollow microneedle production process, as described by Li et al. (102). In addition, hollow microneedles exhibit excellent control over drug dosage and release timing despite their challenging manufacturing process that carries hazards of needle breakage and lumen obstruction (10).
3.2.1.4 Biodegradable microneedles
Soluble/biodegradable microneedles offer high loading capacities and complete dissolution upon skin insertion. They also have great biocompatibility. In the first three minutes after applying soluble microneedles to mouse ears, the length of the subcutaneous needles rapidly decreased, After that, the microneedles dissolved steadily but slowly over the next ten minutes. However, this characteristic implies that patients must hold off until the skin’s microneedles disintegrate. It can also be employed for prolonged medication administration (103).
3.2.1.5 Hydrogel microneedles
Hydrogel-based MN drug delivery functions by putting the needle inside the skin, letting the medicine out, and then throwing away the needle. Due to their hydrophilous properties, materials with appropriate swelling characteristics and biocompatibility should be used for hydrogel-based microneedles (97). Aung and colleagues examined the swelling of hydrogel-based microneedles. Even with their little distortion, the microneedles maintained their mechanical strength (104). Table 3 illustrates current investigations on 3D-printed transdermal microneedle systems.
3.3 Gel
European Pharmacopoeia defines gels as semisolid preparations for topical application of liquids gelled using an appropriate gelling agent. The gel may be categorized according to a wide range of factors; physical gels, covalently cross-linked gels, and entanglement network gels are the three primary varieties that may be categorized according to the method of cross-linking (113).
Physical hydrogels are dosage forms where the gelling agent is physically cross-linked by hydrogen bonds, electrostatic interaction, or other mechanisms (84). The medium used in physical hydrogels can include water alone or in combination with other polar liquids. These gels are most often characterized by their temperature-dependent sol-gel transition and reversibility (82). Physical gel application may potentially be beneficial for photodynamic treatment (PDT). Photosensitizers are nontoxic substances that may be used topically or orally. When exposed to the right wavelength of light, they can cause visible fluorescence (114). Porphyrins and 5-aminolevulinic acid (5-ALA), its precursor, are well-known examples of photodynamic therapy (PDT) medication. Despite 5-ALA’s tiny size, its hydrophilic nature prevents it from passing through the stratum corneum. The problem may be solved chemically by changing it to a methyl or hexyl ester. Using penetration enhancers like DMSO as an alternative is a possibility (115).
Chemically cross-linked gels make up the second category. Chemical bonds join the cross-linker and the macromolecules in their structure, and these ties are only broken by heat. Because of the higher solvent volume and the flexible polymer chain, these gels have a high elasticity. Three-dimensional swelling networks, covalently cross-linked hydrogels, are created from hydrophilic polymers with different functional groups; these groups can absorb and hold on to enormous volumes of biological fluids and water since they are either grafted or implanted in their structure. Instead of dissolving in water, they inflate and stabilize the medium. The structure may be adjusted by adjusting the hydrogel’s affinity for the aqueous medium and the degree of chemical cross-linking (113, 116).
Polymeric chains interact topologically in a melt or solution to generate or produce polymer entanglement network gels. This is often seen in polymeric gels that comprise one or more higher-molecular-weight polymers, especially elastomers. At frequencies higher than the lifespan of the topological entanglements, they behave like “pseudogels” (117).
3.4 Transdermal spray
TS is a topical liquid preparation used as a solution composed of a volatile and non-volatile vehicle containing the fully dissolved medication in solution. Its use allows for better drug penetration through the skin and reaches a sustained level. The potential benefits of TS include improved delivery potential without causing skin irritation due to its nonocclusive nature, increased acceptability, dose flexibility, and ease of manufacture (118). TS is made up of a volatile solvent solution that creates a film that dries quickly when sprayed on the skin (119, 120). The metered dosage transdermal spray (MDTS) system makes sure that the dose is delivered in the right amount from its main packing material. The volatile solvent will deliver the medicine into the top layers of the skin when it is applied, and then it will evaporate. This process leaves a lot of the medication in the skin, which functions as a reservoir to slowly and steadily release the drug into the blood. Once the volatile solvent evaporates from the SC layers, it leaves behind axthin, even coating of the medication that has a lot of thermodynamic activity and quickly gets into the skin (121, 122).
3.5 Iontophoresis
A technique known as iontophoresis uses a small electrical current to push a charged drug molecule through the skin (123). The drug molecule’s polarity, valency, mobility, affect iontophoresis effectiveness. Specifically, in contrast to most other drug delivery methods, the reliance on current renders medication absorption via iontophoresis less reliant on biological characteristics (124). This strategy can also include electronic reminders for patients to change their prescriptions as required in order to increase patient compliance (125). Its main advantages are the system’s regulated medication distribution and the ability to be switched on and off as needed. Irritation and discomfort are the system’s limitations, which restrict the drug’s dosage. Now, it is used to provide lidocaine for local anesthesia quickly (126). It’s important to remember that a current of 0.5 mA/cm2 or less is safe for the body and might be used (127). When you apply an iontophoretic patch, the drug molecule or the current may sometimes be too strong, which can produce mild to moderate skin redness or irritation (128). But if the current density and exposure duration get up, these little responses might happen more often. There have been complaints in the past of people getting burns or scars on their skin from using the iontophoretic sumatriptan patch. The FDA has looked at these incidents, even if they don’t happen very often. One technique to deal with this issue is to not put a patch over skin that is fractured or injured (127).
3.6 Laser radiation
Laser radiation entails exposing the skin to a laser beam to ablate the stratum corneum without harming the epidermis that is still in touch with it. This method of removing the stratum cornea is thought to enhance the administration of lipophilic and hydrophilic medications (129). According to reports, transdermal therapy with laser treatment has benefits, including well-controlled tissue removal, a brief treatment duration, a painless administration route, and minimal side effects. Thus, Norwood Abbey Ltd. has created a handheld portable laser device. In a human volunteer trial, the Norwood Abbey laser device shortened the duration of lidocaine’s start of action to 3–5 minutes, while the control group needed 60 minutes to have a comparable effect (130).
3.7 Electroporation
By applying brief, high-voltage electrical pulses to the skin, this novel technique improves medication dispersion by raising permeability. The electrical pulses create microscopic holes in the stratum corneum (SC) used for drug transit. Electrodes placed closely apart introduce electrical pulses to reserve the electric field inside the SC for a painless and safe administration (118). Using hairless mice, it has also been possible to achieve improved transport of bare DNA to the skin in vivo; compared to intradermal injection, gene expression was stimulated 100 times more (131).
3.8 Ultrasound/sonophoresis
Using a gel, cream, or ointment as a coupling agent, the medicinal component is mixed to transport ultrasonic energy traveling to the skin from the system. To achieve this, the lipids in the stratum cornea must be broken down, allowing the drug to cross the biological barrier (129). Percutaneous absorption is known to be influenced by ultrasound parameters, the most significant of which are frequency, treatment duration, intensity, and intensity (132). In the Katz et al. investigation, dermal anesthesia was achieved after 5 minutes of ultrasonic therapy for skin treated for an average of 9 seconds. This was similar to the 60 minutes needed for skin that had not been treated (133). Smith et al. have also reported on using additional tiny, light new ultrasonic transducers to improve insulin skin transfer in vitro (134).
4 3D printing
3D printing technology originated with building three-dimensional (3D) structures directly from computer-aided design (CAD) models, layer by layer (135). The technology of 3D Printing is very inventive and has become a flexible technological platform. 3D printing technology is being utilized increasingly in the automotive, aerospace, healthcare, and agricultural sectors to produce open-source designs and mass customization (136). Figures 3, 4 show the in vitro and in vivo application of 3D printing technology.
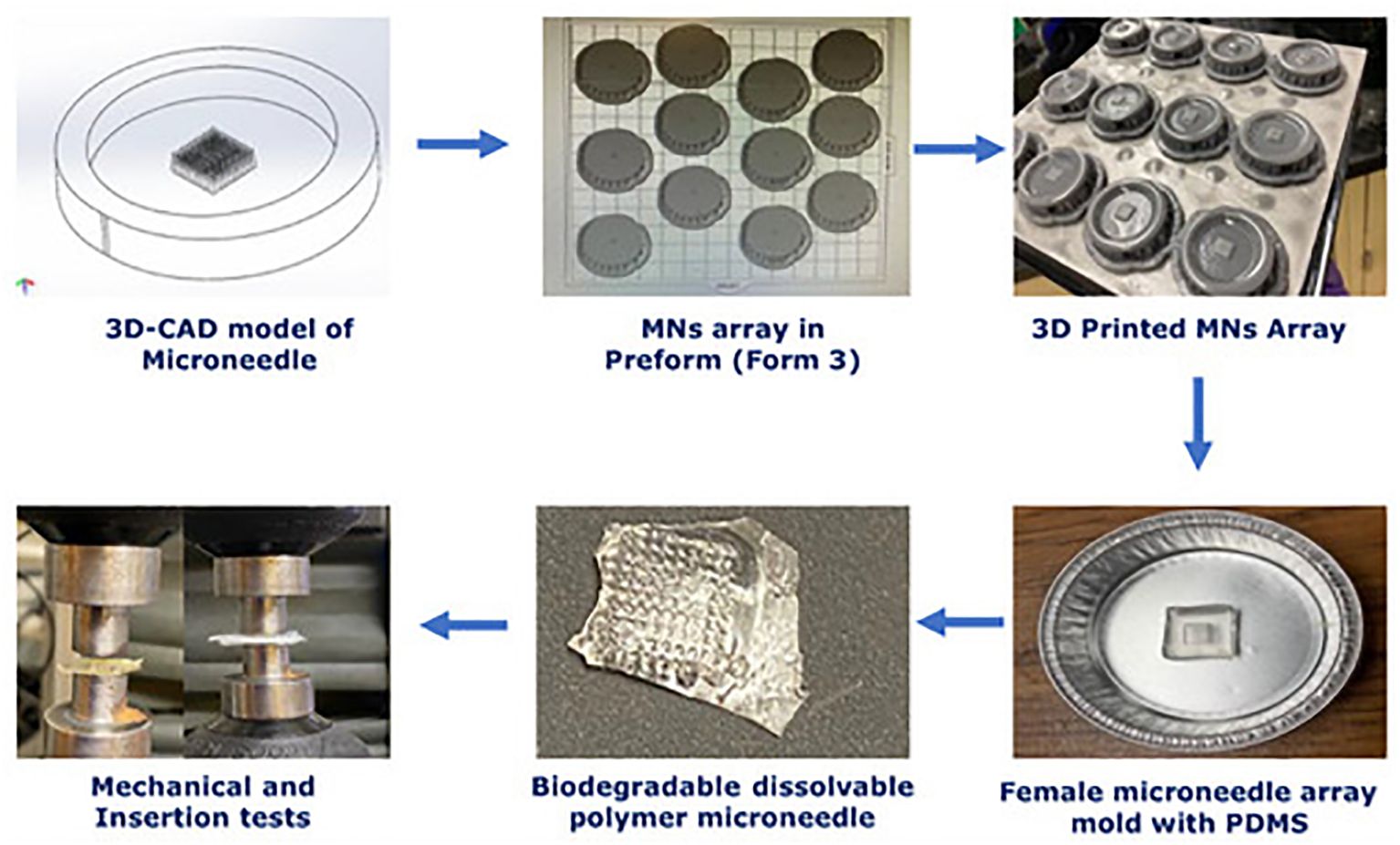
Figure 3. In vitro analysis of 3D-printed biopolymer-based microneedle – Adapted from reference (137) under the terms and conditions of the Creative Commons Attribution (CC BY) license.
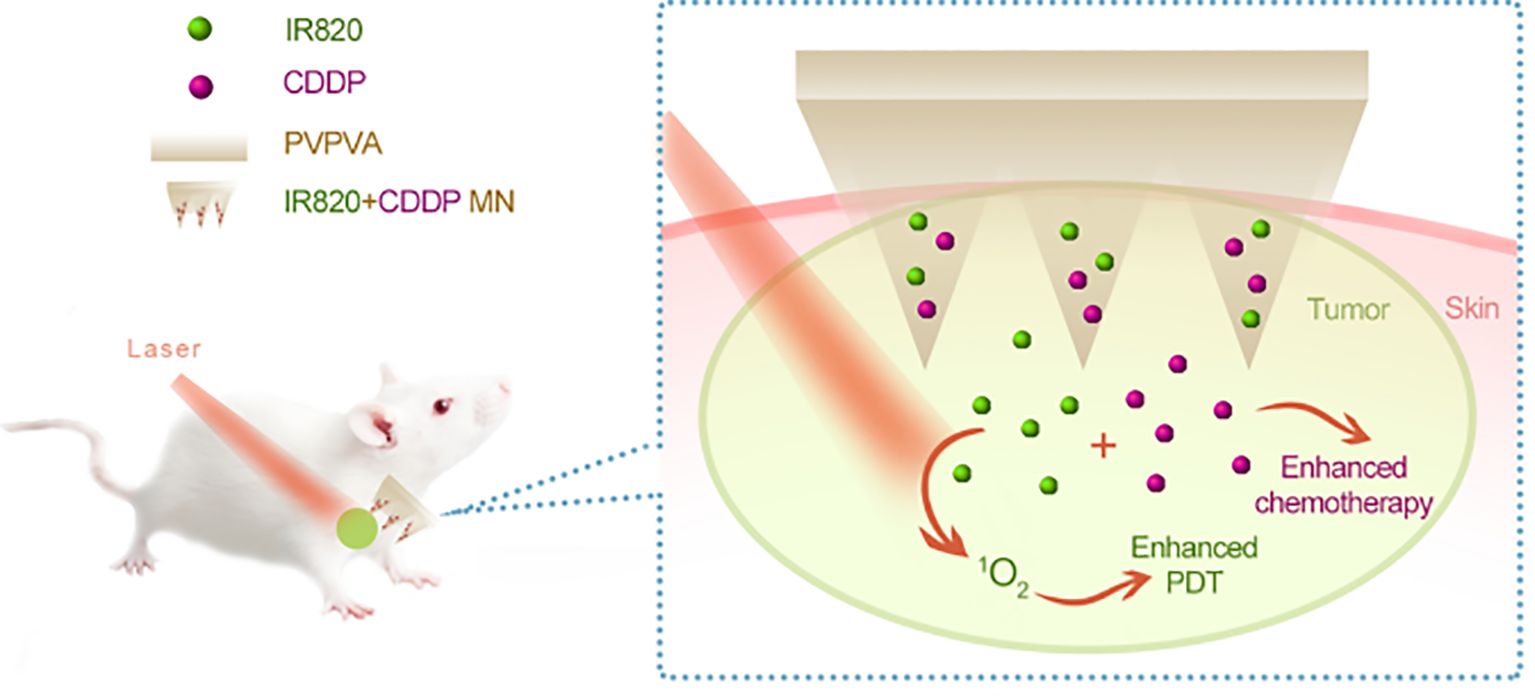
Figure 4. In vivo analysis of 3D-printed biopolymer-based microneedle. Adapted from the reference (138) under a Creative Commons Attribution 4.0 International License.
4.1 Categories of 3D printing
The ASTM categorized 3D printing methods into seven classes, Since every technology and machine has a particular set of uses, there are no arguments about which one is superior. These days, 3D printing technologies are increasingly used to produce various goods, not only prototypes (139).
4.1.1 Binder jetting
Powder particles are fused to create a three-dimensional structure via “binder jetting,” where a liquid bonding agent is selectively deposited on the powder particles. The binder jetting technology has many benefits, including huge build volume, fast print speed, cheap cost, free support, and design flexibility. ExOne production printers are a few machines that use the BJ method. The binder jetting method may print on diverse substances, such as polymers, metal, glass, etc (140). A recent optimization study of binder-jetting focused on surface finish and dimensional accuracy. The most significant effects on component shrinkage were caused by layer thickness and binder saturation (141). Another recent work examined the mechanical characteristics of 316 SS lattice structures made by binder jetting and discovered that the elastic modulus was much lower than that of similar AM techniques (142).
4.1.2 Directed energy deposition
Directed energy deposition (DED), a printing technology that is becoming more sophisticated, is often used to improve or fix pre-existing components. High-quality objects may be produced via directed energy deposition, providing excellent control over grain structure. Two examples of this technique are laser deposition and laser-engineered net shaping (LENS) (135). Laser deposition is a scalable technology that may be used to create or repair objects with dimensions ranging from millimeters to meters due to its versatility in a single machine. The oil and gas, aerospace, machining, and transportation sectors are seeing an increase in the usage of this technology. LENS uses thermal energy to melt objects during and after casting (143). Rooyen et al. adopted the DED procedure to fix the fractures in the stainless steel; they discovered that underwater pressure crack sealing of 4.5 and 6.0 mm plate thickness could be effectively fixed with a relatively high power efficiency (144). A marine diesel crankshaft was repaired by Koehler et al. via the DED technique. Based on microstructural analysis, they observed extremely excellent interfacial bonding between the deposited layer and the base metal (145).
4.1.3 Material extrusion
One additive manufacturing method is material extrusion, in which continuous pressure forces the material through a nozzle. When the extruded material exits the nozzle, it will drop onto the substrate steadily and harden completely. To enable the formation of a solid portion and its maintenance in that structure throughout the process, the material also must bond with earlier materials. A few machines based on the ME method include the Fortus Production Series (380, 450, and 900 mc) from Stratasys (146). Periard et al. (7) evaluated many recipes to print sugars via extrusion and printed cake frostings and processed cheeses using Fab@homeTM (147). Co-extrusion was used by Vancauwenberghe et al. (33) to ease worries about the length of time needed for gelling incubation. They created a printer head with an outside layer of CaCl2 (a cross-linking solution) and an interior layer of food stimulant based on pectin. Pectin was gelled using this method right during the printing process (148).
4.1.4 Material jetting
For three-dimensional materials, material jetting entails moving the print head and platform in the x, y, and z directions. When the content is sent, for it to print correctly, cross-linking is required. Crosslinking reactions include UV, thermal, ionic, and pH-dependent effects, much as semisolid extrusion. One of this technique’s most significant benefits is the ability to print numerous materials simultaneously, even with distinct characteristics. Bioprinting and small molecules are two applications for material jetting. The droplet size affects the ultimate resolution of material-jet printed prints’ features. Print feature resolution is also strongly influenced by the fluid’s rheological characteristics and print speed, which need precise parameterization. Droplets spread out before they are completely crosslinked as they land on the print, reducing the inkjet’s resolution (149). Coppi et al. used thermal ink jetting to embed human amniotic fluid-derived stem (AFS) cells in an alginate/collagen scaffold. Before being implanted into immunodeficient mice, the printed construct was cultured in vitro in an osteogenic medium (150). Michael et al. developed a completely cellularized skin replacement using LIFT. After being put into mice, this construct developed into tissue that resembled basic skin (151). Demirci and Montesano revealed how to use acoustic droplet ejection to encapsulate a single or a small number of cells that were expelled from an open pool. They also demonstrated the potential of this technique to print cells in a variety of biological fluids and hydrogels (152).
4.1.5 Powder bed fusion
In this process, an electron or laser beam is utilized to melt or fuse the material powder. The most common example of this technique is selective laser sintering (SLS). SLS is a quick, high-accuracy 3D printing technique with adjustable surface finishes. SLS uses a powerful laser to sinter powdered polymers to generate a 3D product (153). Irrinki et al.’s study examined the effects of processing settings and powder properties on the corrosion behavior of steel items made using the PBF technology. They discovered that the PBF process components had a higher density (154). With the addition of HA, Tan et al. tested several biocompatible polymers. They proved that such polymers might be used to create TE scaffolds for tissue regeneration (155). Chua et al. created scaffolds for craniofacial and joint abnormalities utilizing SLS and a bio-composite mixture of PVA and HA as a powder material. PVA was chosen because it has strong adhesion, can create complicated forms, and has tensile strength comparable to human articular cartilage. A bioactive substance with osteoconductivity characteristics is HA. HA has the ability to increase the creation of collagen and form links to bones that are as strong as those of a 3- to 6-month-old baby. Examinations of HA’s bioactivity revealed that it remained bioactive in the environment and that the laser sintering procedure had no effect. Therefore, a combination of PVA and HA may create appropriate scaffolds (156).
4.1.6 Sheet lamination
The two primary types of this method are laminated object manufacturing (LOM) and ultrasonic additive manufacturing (UAM), where material sheets are either joined by ultrasound or sliced using a laser. Every sheet of material may be seen as one of the solid object’s cross-sectional layers. The materials used in the SL process are paper, plastic, and metal sheets (157). Wimpenny et al. brazed laser-cut steel sheets to create laminated steel tools with conformal cooling channels (158). In a similar vein, Shuping et al. used a new LOM process with diffusion welding for the rapid manufacturing of metal parts (159).
4.1.7 VAT photopolymerization
The main 3D printing process often used is called photopolymerization, commonly defined as curing photo-reactive polymers using a laser, light, or ultraviolet (UV). Two instances of photopolymerization are digital light processing (DLP) and stereolithography (SLA). Photopolymerization works well for producing luxury goods with exquisite attributes and a smooth surface (160). A biocompatible aliphatic for the DLP process was created by Tzeng et al. After 15 minutes of post-curing, the produced photopolymer had enhanced mechanical characteristics and showed no signs of cytotoxicity (161). Schwartz et al. studied printed polymer constructs resembling anisotropic human hand and multi-material vat photopolymerization. They employed a stiff polymer using dual-wavelength photopolymerization. A soft acrylate network was created by mixing acrylate and epoxy resin and curing it orthogonally with visible light, whereas cationic polymerization (UV light) was used to create the rigid epoxy-acrylate IPN (162).
4.2 Materials used for 3D printing
Materials suitable for 3D Printing can be utilized to create desired objects by applying 3D printing technology. Like other production processes, 3D Printing requires improved material properties that adhere to set requirements to generate reliably higher-grade products. This section greatly details the many materials utilized in 3D printing processes (153).
4.2.1 Metals
Due to its strong physical properties, metal may be used in complex manufacturing processes, including printing human organs or aeronautical components. Many industries are showing great interest in metal 3D printing technology because of its advantages (163). These materials include, among others, Cobalt-based alloys that are suitable for use in dental applications that are 3D printed because of their elevated levels of elongation, particular stiffness, resilience, and heat-treated conditions, nickel-base alloys can be used to create aeronautical components as they can tolerate high temperatures and have considerable resistance to corrosion and titanium alloys could also be used by 3D printing technology due to their low density, excellent corrosion, oxidation resistance, and ductility (164, 165).
4.2.2 Polymers
This material is a big molecule of structural units that repeat; natural and manmade polymers fall into this broad category. Due to its superior laser melting and binding properties, nylon is one of the most used and researched polymers. In industry, polymer-based components are often produced “indirectly” by injection molding when produced in medium- and large-scale numbers. Rapid tooling, or AM techniques, may create these molds (166). The work by Nematollahi et al. investigated how the inclusion of polypropylene fiber affected the properties of geopolymers created via 3D printing for use in digital buildings. The researchers’ inclusion of fibers improved pure polypropylene’s ductility, compressive strength, and shape-holding properties (167).
4.2.3 Ceramics
These materials are arduous, brittle, resistant to heat, and erosive. By modifying process settings, 3D printing methods may generate objects with ceramic coatings with no visible holes or cracks, leading to superior mechanical qualities. Because of their fluid condition before curing, ceramics may be utilized to create items with complicated geometry and form; this makes them perfect for buildings and structures, Aeronautical and dental applications can benefit from ceramic materials (168).
3D printing methods may be used to process alumina powder, and this resilient ceramic oxide finds applications in microelectronics, chemicals, adsorbents, and other high-tech fields. Solid bulk ceramics with superior bending characteristics and outstanding compressive strength may be produced via SLCM (Stereolithographic Ceramic Manufacturing) (169)., Li et al. created a porous alumina ceramic that demonstrated an extremely strong flexural elasticity (170). Maurath and Willenbacher showed that a honeycomb structure with a high specific strength and superior dimensional stability may be created by optimizing the ink-printing process (171).
4.2.4 Composites
Composites are materials that comprise many components with various chemical and physical properties. It provides better chemical, physical, and mechanical properties, making them more and more frequent in additive manufacturing due to their accessibility, improved features, and capacity to customize products precisely and economically (172). Currently, composites are used in many Fields, including manufacturing, aerospace, biomedicine, structural, and automotive (173). A bioinspired composite has been published by Leon et al. to enhance the products’ mechanical properties that are 3D printed (174). Digital projection printing was used by Kim et al. to develop an effective piezoelectric nanoparticle-polymer composite material that can be printed into a 3D microstructure (175).
4.2.5 Biomaterials
Biomaterials are the materials most commonly used in the medical field to provide customized solutions. The present research indicates that because of developments in the industry, including enhanced printing accuracy, the ability to print complicated geometry at an inexpensive cost, and reduced material waste, the use of biomaterials in 3D Printing is expanding. Advances in 3D printing technology have enabled noteworthy advancements in health technology, such as implant materials, cell printing, and medical equipment. Tissue engineering, dentistry, and organ model Printing are only a few medical uses for 3D printing technology (176). An integrated tissue-organ printer (ITOP) device that can create human-scale tissue constructions with high mechanical stability in any form was described by Kang et al. (177). Using multi-material 3D bioprinting, Lind et al. presented the creation of a novel class of instrumented cardiac microphysiological devices (178).
4.3 Microneedle and PDT applications in melanoma
Researchers have been searching for novel treatments to combat melanoma for years. Chemotherapy, immunotherapy, and surgical excision are the mainstays of conventional treatment. However, the traditional methods of administration oral and subcutaneous in these treatments often lead to low levels of drug accumulation. Additionally, systemic exposure to anti-melanoma medications often results in severe side effects that are unpleasant and reduce the effectiveness of treatment. Regarding the management of melanoma, MN is a very novel approach that may increase the transdermal permeability of medications, boost their curative efficacy, and lessen their negative effects. The utilization of MNs as a novel medication delivery mechanism to treat malignant melanoma has drawn interest from several researchers. Melanoma mostly affects the skin. Because the MNs can immediately permeate the skin’s dermis layer, the medication has high permeability, which makes local targeted treatment a reality (179). Figures 5, 6 show the in vitro and in vivo analysis of the PDT Microneedle.
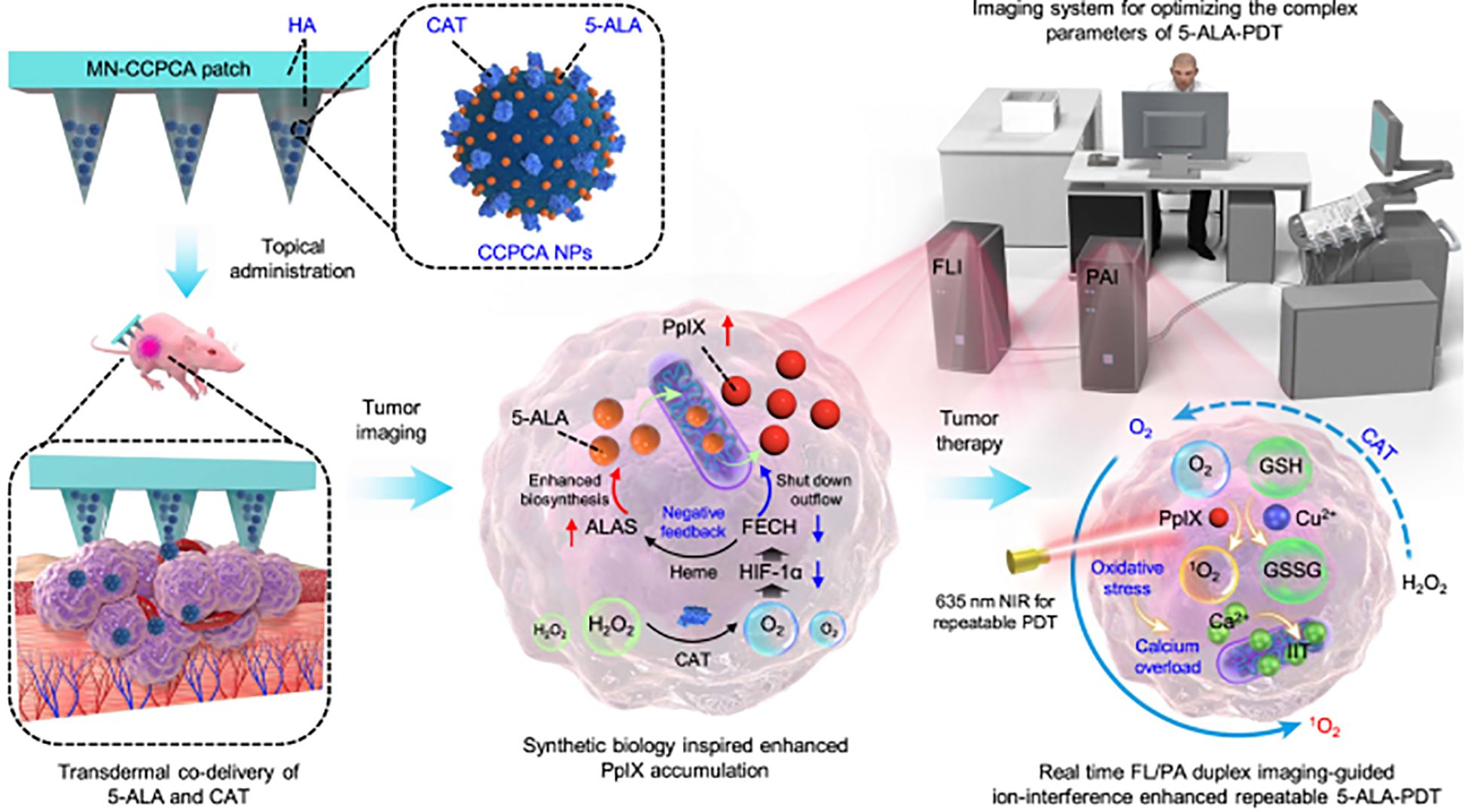
Figure 5. In vitro analysis of PDT microneedle – adapted from the reference (180) under a Creative Commons Attribution 4.0 International License.
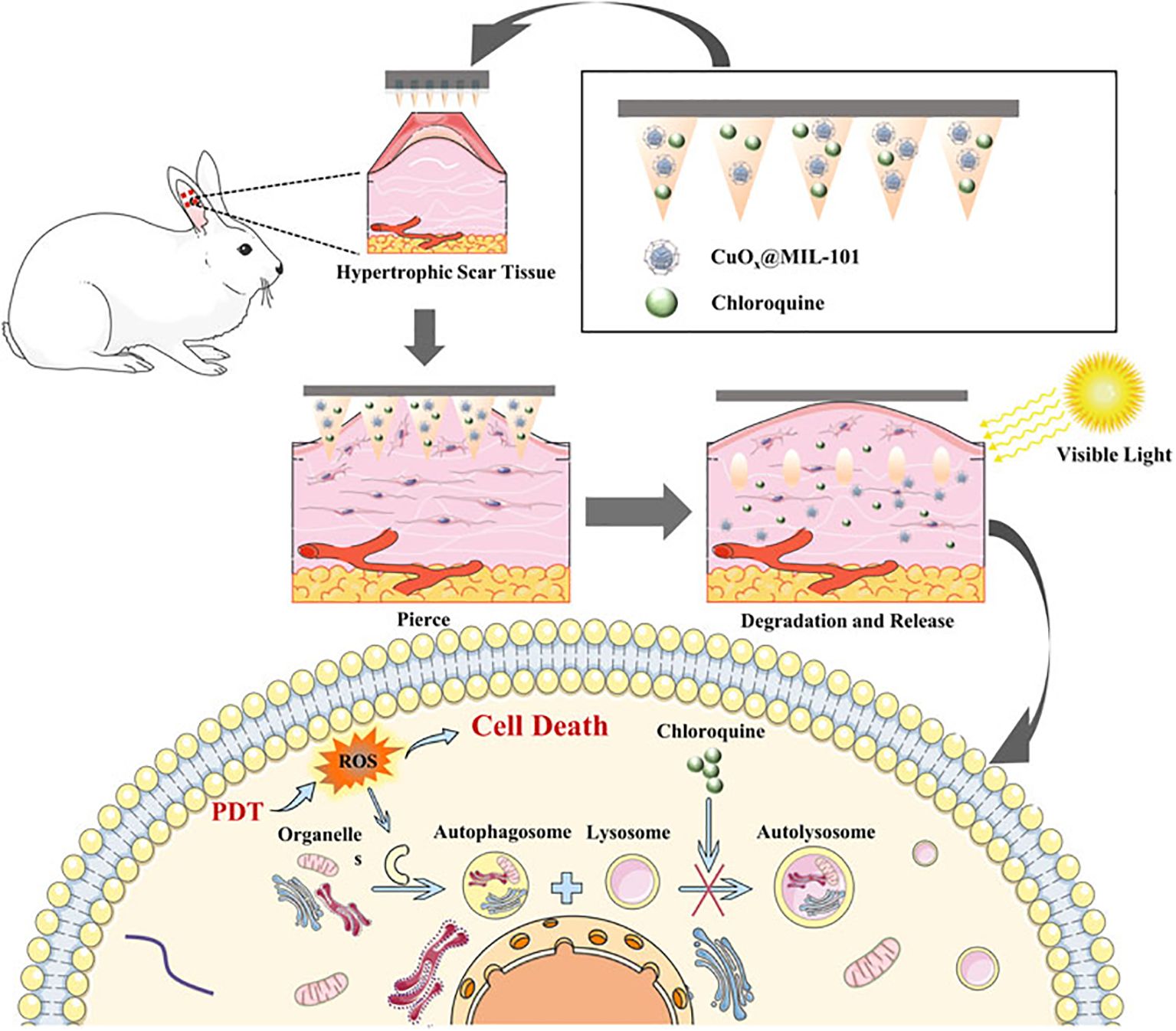
Figure 6. In vivo analysis of PDT microneedle – adapted from reference (181) under the terms of the Creative Commons Attribution License (CC BY).
Surgical excision is the mainstay of conventional melanoma therapy; nevertheless, the procedure severely damages the skin. Patients have a poor prognosis, a high risk of recurrence, and a high cost (182). As a result, a novel approach to treating melanoma that is minimally invasive and causes no serious side effects in patients is desperately needed. To accomplish local targeted treatment, MNs provide special benefits when used to treat melanoma as compared to other drug delivery methods.
MNs may directly deliver the medications to the lesion via the skin’s dermis layer. MNs may treat melanoma with minimally invasive procedures and do less harm on the skin. MNs are user-friendly, and their clinical uses will increase patients’ adherence to their prescription regimens (183). Drugs with larger molecular weights and hydrophilicity might be delivered by microneedles to the skin or deeper to the underlying tissues, where they could accumulate locally and have an impact or be released into the systemic circulation (184). The microneedle technology is easy to use, minimally invasive, and economically viable. microneedle arrays are often applied manually or with electric force using a patch, roller, applicator, or injection device in the case of the hollow variety. The process of creating pores or holes in SC either before or in conjunction with applying a medicine to the skin’s surface is the basis for the solid disposable microneedles’ improved drug delivery mechanism. These pores/holes boost the drug’s delivery flow and raise SC’s conductivity. Microneedles come in a variety of shapes and sizes (ranging from 25 to 2000 μm) and are composed of a wide range of materials, including steel, silicon, ceramics, and biodegradable polymers (185). The approach has a wide range of applications due to the described diversity of properties and various drug delivery enhancement processes, which also make microneedles a research focus in the area of transdermal drug administration. Additionally, because of their tiny size, microneedles provide a way to get beyond the SC barrier without hurting or harming the reticular dermal blood vessels (94, 184). Microneedles (MNs) have been explored for treating various skin cancers, including squamous cell carcinoma and basal cell carcinoma (BCC), in addition to malignant melanoma. For instance, Sabri et al. utilized MNs to deliver imiquimod for BCC treatment and compared its efficacy with a commercial topical ointment. Although the MN formulation had lower drug loading, in vitro studies showed comparable intradermal penetration to the commercial product (186).
PDT has shown promise for clinical use in melanoma therapy. It may cause cancer cell necrosis by producing a large quantity of reactive oxygen species (ROS) via photosensitizers when exposed to laser light (187). Like chemotherapy medications, photosensitizers are often administered intravenously, which frequently results in systemic damage. Accurate drug release, reduced risk of systemic harm and improved local immune responses are all possible when MNs and PDT are used together (188).
Microneedle technology provides a unique way to administer drugs topically. The stratum corneum may be penetrated by means of hollow microneedles, drug-coated microneedles, drug-encapsulating microneedles, or drug administration on skin perforated with microneedles (189). The application site experiences little discomfort due to the carefully regulated micron-size range of microneedles, and the ability to self-administer for some applications may significantly improve patient compliance (190). Also, to enable precise delivery, the size and form of the microneedle patch may be flexibly modified to meet the uneven shapes of superficial skin melanoma lesions. This is especially important when treating diffuse-type skin melanoma. The possibility of producing systemic toxicity will also be decreased concurrently. (ii) By stimulating the production of proimmune cytokines from epidermal tissues, the MNs may be used as “mechanical adjuvants” to enhance local immune responses (191, 192). Previously, the microneedle-based poke-and-patch method was used to increase dermal penetration of 5-ALA. This method included utilizing microneedles to pierce the skin surface and produce micro-pores before applying a drug-containing patch or formulation (193, 194). A wide range of medications and biomolecules have been delivered to the skin using coated microneedles in recent years, These include medications that are insoluble in water, which are delivered via molten coatings (188, 195).
An oligopeptide hyaluronic acid (oligo-HA) MN patch was created by Bian et al., supplemented with chlorin e6 (Ce6). The MN’s adequate mechanical strength to penetrate the skin barrier facilitated the transport of Ce6 into the deep layer of the skin. The MN made full use of PDT’s effectiveness in preventing the growth of primary and metastatic melanoma under 660 nm laser irradiation. In addition to lowering the expense and danger of systemic toxicity, our Ce6 MN patch improved anti-melanoma immune activity without the need for additional chemotherapeutics or immunological medications, offering a straightforward and useful approach to clinical change (196).
In order to obtain a high killing efficiency of remaining tumor cells after surgery and to increase antitumor immunity in order to avoid tumor recurrence and metastasis, organic PS nanoparticles (N3–4F NPs) were additionally loaded onto the soluble MNs. In in vivo breast cancer mice models, it was shown that NPs in the gelatin MNs exhibit superior photothermal conversion efficiency and increased reactive oxygen generation capacity, which results in tumor suppression (197). Li et al. created MNs loaded with catalase and Cu2+ for improved PDT of melanoma by concurrently depleting GSH and producing O2. Fluorescence imaging made it possible to trace the photosensitizers released by the ZIF, allowing for image-guided repeatable PDT for a stronger antitumor impact (198). In order to load dihydroartemisinin (DHA) into the hydrogel network, researchers used acylhydrazone cross-linking to form a connection between the molecule HA-adipic dihydrazide (ADH)-protoporphyrin IX (PpIX) and the “iron reservoir” protocatechualdehyde (PA)-Fe3+ complex in the needle tip. Because of its increased water solubility, HA-ADH-PpIX keeps PpIX from aggregating too much, ensuring effective PDT (199). A study from song et al. showed that both in vitro and in vivo experiments demonstrate that the CPSA NPs have effective tumor cell toxicity, cholesterol regulation effect, and immunity system evoking ability, which brings a new strategy toward immunosuppression from TME, and paves a new way for adjuvant immunotherapy (200).
For melanoma PDT, ALA-loaded dissolving MNs were also developed, and they showed a stronger anti-tumor effect than ALA injection (201). Silicon MNs-mediated administration of 5-aminolevulinic acid (ALA) was carried out by Donnelly et al.; this method may effectively conduct PDT of skin tumors. ALA released from a bioadhesive patch was considerably enhanced by pretreating the skin with silicon MNs (202). For the photodynamic treatment of melanoma, Tham et al. created mesoporous nanovehicles co-loaded with trametinib, dabrafenib, and phthalocyanine. Additionally, they promoted the transport of nanovehicles into deep tumors for improved antitumor effects using MN technology (42).
5 Clinical trial status of microneedle patches for skin cancer treatments
Microneedle-based patches are rapidly advancing in skin cancer research, and the current clinical trial landscape is summarized in Table 4.
6 Challenges associated with 3D printing
Innovations in 3D Printing provide various advantages throughout medication development and clinical settings. Although it is a powerful technology, its application requires significant adjustments to operations and regulations. Before 3D printing technologies are widely used in clinical settings, several regulatory constraints must be fulfilled, primarily regarding product safety and quality (203).
Material science, life sciences, and clinical science are all closely integrated in the rapidly burgeoning area of 3D printing innovation for health purposes, which will effectively address the organ transplant scarcity problem. Even though cells can already be printed directly, more work must be done before in vitro tissue engineering can be accomplished (204). Due to its complexity and many constituent parts, the ECM is challenging to replicate in vitro in terms of structure and biological function. Current methods cannot address the problems of oxygen supply and cellular feeding since they mainly stack cell-seeded hydrogels. There are currently insufficient cells available for bigger scaffolds. Compared to cells that adhere to scaffold surfaces, preprophase cells do not get enough nutrition. In other words, the cells exist in 3D space in disequilibrium (204). For printed scaffolds, tissues, and organs to go further, additional constraints about the challenges of cell fusion, differentiation, survival, and development must be met. Material limitations are another issue.
Furthermore, as there are presently no worldwide guidelines for the selection of medicinal materials for 3D printing, assessments based on structure, function, clinical consequences, and other criteria may only be made artificially rather than utilizing trustworthy indicators and enough experimental data. Thus, it will take time and effort to use 3D printing for medical purposes (205, 206).
However, due to 3D Printing, construction automation offers labor-free building. However, further research is needed to make it competitive with more antiquated technology in the mass production of daily items. Studying various preprocessing and postprocessing techniques has shown how crucial surface quality is for 3D-printed parts. Thus, achieving a high-quality surface finish for every size of 3D-printed product is crucial (207).
Adoption in the aviation sector is hampered by factors such as high prices, few resources, and uneven quality of 3D printed parts. Compared to conventional techniques, the main disadvantages of 3D printing in the building sector are its high cost and the possibility of mechanical risk (207).
7 Future perspectives and conclusions
Transdermal microneedles have been gaining popularity for various biomedical applications, including medication administration, due to their unique features of little invasion, painlessness, and ease of usage. More remarkably, recent developments in electronic mechanical and intellectual engineering and advancements in photopolymerization have stimulated advances in 3D printing techniques that have made it possible to manufacture artificial and bio-inspired microneedles and patches with complex, functional structures accurately.
Furthermore, the idea of using 3D printing techniques to create microneedles offers exciting possibilities for the administration of certain drugs. More lately, much work has been done to create multifunctional microneedles using various 3D printing methods. The two primary approaches that were the focus of those efforts were photopolymerization and fused deposition modeling. These cutting-edge methods enabled many artificial and naturally inspired printed microneedle designs with ever-more complex structures, much higher printing resolution, and manufacturing accuracy. Another advantage among the technological developments in 3D printing is the growing availability of printing materials and printable pharmaceutical activities. The advancements guarantee that 3D printing technology will soon be able to be essential to the actual manufacture of transdermal microneedles.
Notwithstanding their rapid advancement, the following constraints have a major role in the continued clinical translation of 3D-printed microneedles. Producing precise microneedles requires combining operator experience, material selection, and printing parameter optimization. Even with the steadily increasing availability of printing materials over the last ten years, it makes sense that there are still not enough appropriate formulations, particularly for 3D-printed microneedles, given their mechanical and biological requirements, printing requirements, and possible material toxicity. Furthermore, current technology does not allow for the mass manufacture of 3D-printed goods, particularly for the small-scale manufacture of microneedles and patches. While precision manufacturing and fast prototyping promise to create customized medication with tiny dosages, conventional techniques are still needed for large-scale mass production.
Author contributions
AO: Data curation, Formal Analysis, Methodology, Visualization, Investigation, Software, Writing – original draft. HA: Resources, Supervision, Funding acquisition, Writing – review & editing. SD: Supervision, Project administration, Conceptualization, Writing – review & editing, Funding acquisition, Software, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98337), as well as grants received from the University of Johannesburg (URC), the National Research Foundation (NRF), and the CSIR (Council for Scientific and Industrial Research) – NLC (National Laser Centre) Laser Rental Pool Programme. The research reported in this publication was supported by the South African Medical Research Council under a Self-Initiated Research Grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed are those of the author(s) and do not necessarily represent the official views of the SA MRC.
References
1. Losquadro WD. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast Surg Clinics North America. (2017) 25:283–9. doi: 10.1016/j.fsc.2017.03.001
2. Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. (2017) 542:115–8. doi: 10.1038/nature21056
3. Didona D, Paolino G, Bottoni U, and Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. (2018) 6:6. doi: 10.3390/biomedicines6010006
4. Barton V, Armeson K, Hampras S, Ferris LK, Visvanathan K, Rollison D, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. (2017) 309:243–51. doi: 10.1007/s00403-017-1724-5
5. Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA: A Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
6. Dang Y and Guan J. Nanoparticle-based drug delivery systems for cancer therapy. In: Smart Materials in Medicine. (2020) 1:10–9.
7. Singh P, Carrier A, Chen Y, Lin S, Wang J, Cui S, et al. Polymeric microneedles for controlled transdermal drug delivery. J Controlled Release. (2019) 315:97–113. doi: 10.1016/j.jconrel.2019.10.022
8. Azmana M, Mahmood S, Hilles AR, Mandal UK, Saeed Al-Japairai KA, and Raman S. Transdermal drug delivery system through polymeric microneedle: A recent update. J Drug Delivery Sci Technol. (2020) 60:101877. doi: 10.1016/j.jddst.2020.101877
9. Yang D, Chen M, Sun Y, Jin Y, Lu C, Pan X, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomaterialia. (2021) 121:119–33. doi: 10.1016/j.actbio.2020.12.004
10. Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomedicine Pharmacotherapy. (2019) 109:1249–58. doi: 10.1016/j.biopha.2018.10.078
11. Li S, Gao D, Song C, Tan C, and Jiang Y. Isotope labeling strategies for acylcarnitines profile in biological samples by liquid chromatography-mass spectrometry. Analytical Chem. (2019) 91:1701–5. doi: 10.1021/acs.analchem.8b05120
12. Bilal M, Mehmood S, Raza A, Hayat U, Rasheed T, and Iqbal HMN. Microneedles in smart drug delivery. Adv Wound Care. (2021) 10:204–19. doi: 10.1089/wound.2019.1122
13. Ligon SC, Liska R, Stampfl J, Gurr M, and Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. (2017) 117:10212–90. doi: 10.1021/acs.chemrev.7b00074
14. George E, Liacouras P, Rybicki FJ, and Mitsouras D. Measuring and establishing the accuracy and reproducibility of 3D printed medical models. Radiographics. (2017) 37:1424–50. doi: 10.1148/rg.2017160165
15. Tay YW, Panda B, Paul SC, Tan MJ, Qian SZ, Leong KF, et al. Processing and properties of construction materials for 3D printing. Materials Sci Forum. (2016) 861:177–81. doi: 10.4028/www.scientific.net/MSF
16. Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Adib Kadri N, et al. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Science and Technology of Advanced Materials. (2015) 16(3):033502.
17. Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. (2019) 25:263–9. doi: 10.1038/s41591-018-0296-z
18. Cidonio G, Glinka M, Kim YH, Kanczler JM, Lanham SA, Ahlfeld T, et al. Nanoclay-based 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication. (2020) 12:035010. doi: 10.1088/1758-5090/ab8753
19. Kjar A and Huang Y. Application of micro-scale 3D printing in pharmaceutics. Pharmaceutics. (2019) 11:390. doi: 10.3390/pharmaceutics11080390
20. Monge-Fuentes V, Muehlmann LA, and de Azevedo RB. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. (2014) 5:24381–1. doi: 10.3402/nano.v5.24381
21. Dhillon SK, Porter SL, Rizk N, Sheng Y, McKaig T, Burnett K, et al. Rose bengal–amphiphilic peptide conjugate for enhanced photodynamic therapy of Malignant melanoma. J Medicinal Chem. (2020) 63:1328–36. doi: 10.1021/acs.jmedchem.9b01802
22. Nguyen K, Hignett E, and Khachemoune A. Current and emerging treatment options for metastatic melanoma: a focused review. Dermatol Online J. (2020) 26. doi: 10.5070/D3267049551
23. Madamsetty VS, Paul MK, Mukherjee A, and Mukherjee S. Functionalization of nanomaterials and their application in melanoma cancer theranostics. ACS Biomaterials Sci Eng. (2019) 6:167–81. doi: 10.1021/acsbiomaterials.9b01426
24. Li X-Y, Tan L-C, Dong L-W, Zhang W-Q, Shen X-X, Lu X, et al. Susceptibility and resistance mechanisms during photodynamic therapy of melanoma. Front Oncol. (2020) 10:597. doi: 10.3389/fonc.2020.00597
25. Domingues B, Lopes JM, Soares P, and Pópulo H. Melanoma treatment in review. ImmunoTargets Ther. (2018) 7:35–49. doi: 10.2147/ITT.S134842
26. Tambunlertchai S, Geary SM, and Salem AK. Skin penetration enhancement strategies used in the development of melanoma topical treatments. AAPS J. (2021) 23:19. doi: 10.1208/s12248-020-00544-y
27. Tang JQ, Hou XY, Yang CS, Li YX, Xin Y, Guo WW, et al. Recent developments in nanomedicine for melanoma treatment. Int J Cancer. (2017) 141:646–53. doi: 10.1002/ijc.30708
28. Maiuri MC, Zalckvar E, Kimchi A, and Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. (2007) 8:741–52. doi: 10.1038/nrm2239
29. Nkune NW and Abrahamse H. Nanoparticle-based drug delivery systems for photodynamic therapy of metastatic melanoma: A review. Int J Mol Sci. (2021) 22:12549. doi: 10.3390/ijms222212549
30. Kumar SSD and Abrahamse H. Biocompatible nanocarriers for enhanced cancer photodynamic therapy applications. Pharmaceutics. (2021) 13:1933. doi: 10.3390/pharmaceutics13111933
31. Kamal A, Faazil S, and Malik MS. Apoptosis-inducing agents: A patent review (2010-2013). Expert Opin Ther Patents. (2014) 24:339–54. doi: 10.1517/13543776.2014.877445
32. Mroz P, Yaroslavsky A, Kharkwal GB, and Hamblin MR. Cell death pathways in photodynamic therapy of cancer. Cancers. (2011) 3:2516–39. doi: 10.3390/cancers3022516
33. Melo-Lima S, Gajate C, and Mollinedo F. Triggers and signaling cross-talk controlling cell death commitment. Cell Cycle. (2015) 14:465–6. doi: 10.1080/15384101.2015.1006540
34. Redza-Dutordoir M and Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta - Mol Cell Res. (2016) 1863:2977–92. doi: 10.1016/j.bbamcr.2016.09.012
35. Holohan C, Van Schaeybroeck S, Longley DB, and Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. (2013), 714–26. doi: 10.1038/nrc3599
36. Kono H, Kimura Y, and Latz E. Inflammasome activation in response to dead cells and their metabolites. Curr Opin Immunol. (2014) 30:91–8. doi: 10.1016/j.coi.2014.09.001
37. Dewaele M, Martinet W, Rubio N, Verfaillie T, de Witte PA, Piette J, et al. Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. J Cell Mol Med. (2011) 15:1402–14. doi: 10.1111/j.1582-4934.2010.01118.x
38. Belizário J, Vieira-Cordeiro L, and Enns S. Necroptotic cell death signaling and execution pathway: Lessons from knockout mice. Mediators Inflammation. (2015) 2015:128076. doi: 10.1155/2015/128076
39. Castano AP, Demidova TN, and Hamblin MR. Mechanisms in photodynamic therapy: Part two - Cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther. (2005) 2:1–23. doi: 10.1016/S1572-1000(05)00030-X
40. Valli F, Garcia Vior MC, Roguin LP, and Marino J. Oxidative stress generated by irradiation of a zinc (II) phthalocyanine induces a dual apoptotic and necrotic response in melanoma cells. Apoptosis. (2019) 24:119–34. doi: 10.1007/s10495-018-01512-w
41. Yuan A, Yang B, Wu J, Hu Y, and Ming X. Dendritic nanoconjugates of photosensitizer for targeted photodynamic therapy. Acta biomaterialia. (2015) 21:63–73. doi: 10.1016/j.actbio.2015.04.014
42. Tham HP, Xu K, Lim WQ, Chen H, Zheng M, Thng TGS, et al. Microneedle-assisted topical delivery of photodynamically active mesoporous formulation for combination therapy of deep-seated melanoma. ACS nano. (2018) 12:11936–48. doi: 10.1021/acsnano.8b03007
43. Barbazetto IA, Lee TC, Rollins IS, Chang S, and Abramson DH. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. (2003) 135:898–9. doi: 10.1016/S0002-9394(02)02222-5
44. Donaldson MJ, Lim L, Harper CA, Mackenzie J, and Campbell WG. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Exp Ophthalmol. (2005) 33:548–9. doi: 10.1111/j.1442-9071.2005.01083.x
45. Swavey S and Tran M. Porphyrin and phthalocyanine photosensitizers as PDT agents: a new modality for the treatment of melanoma. Recent Adv biology Ther Manage melanoma. (2013) 11:253–82. doi: 10.5772/54940
46. Nowak-Sliwinska P, Karocki A, Elas M, Pawlak A, Stochel G, and Urbanska K. Verteporfin, photofrin II, and merocyanine 540 as PDT photosensitizers against melanoma cells. Biochem Biophys Res Commun. (2006) 349:549–55. doi: 10.1016/j.bbrc.2006.08.060
47. Yue J, Liang L, Shen Y, Guan X, Zhang J, Li Z, et al. Investigating dynamic molecular events in melanoma cell nucleus during photodynamic therapy by SERS. Front Chem. (2018) 6:665. doi: 10.3389/fchem.2018.00665
48. Kleemann B, Loos B, Scriba TJ, Lang D, and Davids LM. St John’s Wort (Hypericum perforatum L.) photomedicine: hypericin-photodynamic therapy induces metastatic melanoma cell death. PLoS One. (2014) 9:e103762. doi: 10.1371/journal.pone.0103762
49. Mohammadi Z, Sazgarnia A, Rajabi O, Soudmand S, Esmaily H, and Sadeghi HR. An in vitro study on the photosensitivity of 5-aminolevulinic acid conjugated gold nanoparticles. Photodiagnosis Photodyn Ther. (2013) 10:382–8. doi: 10.1016/j.pdpdt.2013.03.010
50. da Silva DB, da Silva CL, Davanzo NN, da Silva Souza R, Correa RJ, Tedesco AC, et al. (PpIX) loaded PLGA nanoparticles for topical Photodynamic Therapy of melanoma cells. Photodiagnosis Photodyn Ther. (2021) 35:102317. doi: 10.1016/j.pdpdt.2021.102317
51. Vadarevu H, Juneja R, Lyles Z, and Vivero-Escoto JL. Light-activated protoporphyrin IX-based polysilsesquioxane nanoparticles induce ferroptosis in melanoma cells. Nanomaterials. (2021) 11:2324. doi: 10.3390/nano11092324
52. Rizzi M, Tonello S, Estevão BM, Gianotti E, Marchese L, and Renò F. Verteporfin based silica nanoparticle for in vitro selective inhibition of human highly invasive melanoma cell proliferation. J Photochem Photobiol B: Biol. (2017) 167:1–6. doi: 10.1016/j.jphotobiol.2016.12.021
53. Nistorescu S, Udrea A-M, Badea MA, Lungu I, Boni M, Tozar T, et al. Low blue dose photodynamic therapy with porphyrin-iron oxide nanoparticles complexes: in vitro study on human melanoma cells. Pharmaceutics. (2021) 13:2130. doi: 10.3390/pharmaceutics13122130
54. Ogawara K-i, Shiraishi T, Araki T, Watanabe T-i, Ono T, and Higaki K. Efficient anti-tumor effect of photodynamic treatment with polymeric nanoparticles composed of polyethylene glycol and polylactic acid block copolymer encapsulating hydrophobic porphyrin derivative. Eur J Pharm Sci. (2016) 82:154–60. doi: 10.1016/j.ejps.2015.11.016
55. Lee KL, Carpenter BL, Wen AM, Ghiladi RA, and Steinmetz NF. High aspect ratio nanotubes formed by tobacco mosaic virus for delivery of photodynamic agents targeting melanoma. ACS biomaterials Sci Eng. (2016) 2:838–44. doi: 10.1021/acsbiomaterials.6b00061
56. Song C, Ran J, Wei Z, Wang Y, Chen S, Lin L, et al. Organic near-infrared-II nanophotosensitizer for safe cancer phototheranostics and improving immune microenvironment against metastatic tumor. ACS Appl Materials Interfaces. (2021) 13:3547–58. doi: 10.1021/acsami.0c18841
57. Bolfarini GC, Siqueira-Moura MP, Demets GJ, Morais PC, and Tedesco AC. In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbituril zinc phthalocyanine complex on melanoma. J Photochem Photobiol B: Biol. (2012) 115:1–4. doi: 10.1016/j.jphotobiol.2012.05.009
58. Song C, Xu W, Wei Z, Ou C, Wu J, Tong J, et al. Anti-LDLR modified TPZ@ Ce6-PEG complexes for tumor hypoxia-targeting chemo-/radio-/photodynamic/photothermal therapy. J Materials Chem B. (2020) 8:648–54. doi: 10.1039/C9TB02248A
59. Mello VC, Araújo VHS, de Paiva KLR, Simões MM, Marques DC, da Silva Costa NR, et al. Development of new natural lipid-based nanoparticles loaded with aluminum-phthalocyanine for photodynamic therapy against melanoma. Nanomaterials. (2022) 12:3547. doi: 10.3390/nano12203547
60. Vera RE, Lamberti MJ, Rivarola VA, and Rumie Vittar NB. Developing strategies to predict photodynamic therapy outcome: the role of melanoma microenvironment. Tumor Biol. (2015) 36:9127–36. doi: 10.1007/s13277-015-4059-x
61. Josefsen LB and Boyle RW. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br J Pharmacol. (2008) 154:1–3. doi: 10.1038/bjp.2008.98
62. Yoon I, Li JZ, and Shim YK. Advance in photosensitizers and light delivery for photodynamic therapy. Clin Endoscopy. (2013) 46:7–23. doi: 10.5946/ce.2013.46.1.7
63. Abrahamse H and Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. (2016) 473:347–64. doi: 10.1042/BJ20150942
64. Kataoka H, Nishie H, Hayashi N, Tanaka M, Nomoto A, Yano S, et al. New photodynamic therapy with next-generation photosensitizers. Ann Trans Med. (2017) 5:. doi: 10.21037/atm.2017.03.59
65. Vega-Vasquez P, Mosier NS, and Irudayaraj J. Nanoscale drug delivery systems: from medicine to agriculture. Front Bioeng Biotechnol. (2020) 8:79. doi: 10.3389/fbioe.2020.00079
66. Vargason AM, Anselmo AC, and Mitragotri S. The evolution of commercial drug delivery technologies. Nat BioMed Eng. (2021) 5:951–67. doi: 10.1038/s41551-021-00698-w
67. Roohnikan M, Laszlo E, Babity S, and Brambilla D. A snapshot of transdermal and topical drug delivery research in Canada. Pharmaceutics. (2019) 11:256. doi: 10.3390/pharmaceutics11060256
68. Pena-Juarez MC, Guadarrama-Escobar OR, and Escobar-Chavez JJ. Transdermal delivery systems for biomolecules. J Pharm Innov. (2022) 17:319–32. doi: 10.1007/s12247-020-09525-2
69. Leppert W, Malec-Milewska M, Zajaczkowska R, and Wordliczek J. Transdermal and topical drug administration in the treatment of pain. Molecules. (2018) 23:681. doi: 10.3390/molecules23030681
70. Akhtar N, Singh V, Yusuf M, and Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices, techniques and biomedical applications. BioMed Tech (Berl). (2020) 65:243–72. doi: 10.1515/bmt-2019-0019
71. Pires LR, Vinayakumar KB, Turos M, Miguel V, and Gaspar J. A perspective on microneedle-based drug delivery and diagnostics in paediatrics. J Pers Med. (2019) 9:49. doi: 10.3390/jpm9040049
72. Mansfield AS and Jatoi A. Asphyxiation with a fentanyl patch. Case Rep Oncol. (2013) 6:242–4. doi: 10.1159/000351220
73. Margetts L and Sawyer R. Transdermal drug delivery: principles and opioid therapy. Continuing Educ Anaesthesia Crit Care Pain. (2007) 7:171–6. doi: 10.1093/bjaceaccp/mkm033
74. Krestyn E, Kolarova H, Bajgar R, and Tomankova K. Photodynamic properties of ZnTPPS(4), ClAlPcS(2) and ALA in human melanoma G361 cells. Toxicol In Vitro. (2010) 24:286–91. doi: 10.1016/j.tiv.2009.08.015
75. Hadgraft J and Lane ME. Drug crystallization - implications for topical and transdermal delivery. Expert Opin Drug Delivery. (2016) 13:817–30. doi: 10.1517/17425247.2016.1140146
76. Alkilani AZ, McCrudden MT, and Donnelly RF. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics. (2015) 7:438–70. doi: 10.3390/pharmaceutics7040438
77. Prausnitz MR and Langer R. Transdermal drug delivery. Nat Biotechnol. (2008) 26:1261–8. doi: 10.1038/nbt.1504
78. Roustit M, Blaise S, and Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol. (2014) 77:63–71. doi: 10.1111/bcp.12128
79. Olmos-Juste R, Guaresti O, Calvo-Correas T, Gabilondo N, and Eceiza A. Design of drug-loaded 3D printing biomaterial inks and tailor-made pharmaceutical forms for controlled release. Int J Pharm. (2021) 609:121124. doi: 10.1016/j.ijpharm.2021.121124
80. Falcone G, Saviano M, Aquino RP, Del Gaudio P, and Russo P. Coaxial semi-solid extrusion and ionotropic alginate gelation: A successful duo for personalized floating formulations via 3D printing. Carbohydr Polym. (2021) 260:117791. doi: 10.1016/j.carbpol.2021.117791
81. Bergonzi C, Bianchera A, Remaggi G, Ossiprandi MC, Bettini R, and Elviri L. 3D printed chitosan/alginate hydrogels for the controlled release of silver sulfadiazine in wound healing applications: design, characterization and antimicrobial activity. Micromachines (Basel). (2023) 14:137. doi: 10.3390/mi14010137
82. Bom S, Santos C, Barros R, Martins AM, Paradiso P, Claudio R, et al. Effects of starch incorporation on the physicochemical properties and release kinetics of alginate-based 3D hydrogel patches for topical delivery. Pharmaceutics. (2020) 12:719. doi: 10.3390/pharmaceutics12080719
83. Teoh JH, Tay SM, Fuh J, and Wang CH. Fabricating scalable, personalized wound dressings with customizable drug loadings via 3D printing. J Control Release. (2022) 341:80–94. doi: 10.1016/j.jconrel.2021.11.017
84. Wang J-C, Chang M-W, Ahmad Z, and Li J-S. Fabrication of patterned polymer-antibiotic composite fibers via electrohydrodynamic (EHD) printing. J Drug Delivery Sci Technol. (2016) 35:114–23. doi: 10.1016/j.jddst.2016.06.009
85. Yao ZC, Wang JC, Ahmad Z, Li JS, and Chang MW. Fabrication of patterned three-dimensional micron scaled core-sheath architectures for drug patches. Mater Sci Eng C Mater Biol Appl. (2019) 97:776–83. doi: 10.1016/j.msec.2018.12.110
86. Wu S, Ahmad Z, Li JS, and Chang MW. Fabrication of flexible composite drug films via foldable linkages using electrohydrodynamic printing. Mater Sci Eng C Mater Biol Appl. (2020) 108:110393. doi: 10.1016/j.msec.2019.110393
87. Andriotis EG, Eleftheriadis GK, Karavasili C, and Fatouros DG. Development of bio-active patches based on pectin for the treatment of ulcers and wounds using 3D-bioprinting technology. Pharmaceutics. (2020) 12:56. doi: 10.3390/pharmaceutics12010056
88. Muwaffak Z, Goyanes A, Clark V, Basit AW, Hilton ST, and Gaisford S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm. (2017) 527:161–70. doi: 10.1016/j.ijpharm.2017.04.077
89. Liu J, Tagami T, and Ozeki T. Fabrication of 3D-printed fish-gelatin-based polymer hydrogel patches for local delivery of pegylated liposomal doxorubicin. Mar Drugs. (2020) 18:325. doi: 10.3390/md18060325
90. Yi H-G, Choi Y-J, Kang KS, Hong JM, Pati RG, Park MN, et al. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J Controlled release. (2016) 238:231–41. doi: 10.1016/j.jconrel.2016.06.015
91. Economidou SN, Lamprou DA, and Douroumis D. 3D printing applications for transdermal drug delivery. Int J Pharmaceutics. (2018) 544:415–24. doi: 10.1016/j.ijpharm.2018.01.031
92. Johnson AR, Caudill CL, Tumbleston JR, Bloomquist CJ, Moga KA, Ermoshkin A, et al. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PloS One. (2016) 11:e0162518. doi: 10.1371/journal.pone.0162518
93. Bird D and Ravindra NM. Transdermal drug delivery and patches—An overview. Med Devices Sensors. (2020) 3:E10069. doi: 10.1002/mds3.10069
94. Bariya SH, Gohel MC, Mehta TA, and Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. (2012) 64:11–29. doi: 10.1111/j.2042-7158.2011.01369.x
95. Park HG, Yoon SY, Choi JY, Lee GS, Choi JH, Shin CY, et al. Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur J Pharmacol. (2007) 574:112–9. doi: 10.1016/j.ejphar.2007.07.011
96. Li J, Zeng M, Shan H, and Tong C. Microneedle patches as drug and vaccine delivery platform. Curr Med Chem. (2017) 24:2413–22. doi: 10.2174/0929867324666170526124053
97. Ita K. Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics. (2015) 7:90–105. doi: 10.3390/pharmaceutics7030090
98. Li QY, Zhang JN, Chen BZ, Wang QL, and Guo XD. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. (2017) 7:15408–15. doi: 10.1039/C6RA26759A
99. Pere CPP, Economidou SN, Lall G, Ziraud C, Boateng JS, Alexander BD, et al. 3D printed microneedles for insulin skin delivery. Int J pharmaceutics. (2018) 544:425–32. doi: 10.1016/j.ijpharm.2018.03.031
101. Zhang P, Dalton C, and Jullien GA. Design and fabrication of MEMS-based microneedle arrays for medical applications. Microsystem Technol. (2009) 15:1073–82. doi: 10.1007/s00542-009-0883-5
102. Li CG, Lee CY, Lee K, and Jung H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed microdevices. (2013) 15:17–25. doi: 10.1007/s10544-012-9683-2
103. Zhu DD, Zhang XP, Zhang BL, Hao YY, and Guo XD. Safety assessment of microneedle technology for transdermal drug delivery: a review. Advanced Ther. (2020) 3:2000033. doi: 10.1002/adtp.202000033
104. Jina A, Tierney MJ, Tamada JA, McGill S, Desai S, Chua B, et al. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol. (2014) 8:483–7. doi: 10.1177/1932296814526191
105. Xenikakis I, Tsongas K, Tzimtzimis EK, Zacharis CK, Theodoroula N, Kalogianni EP, et al. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int J Pharm. (2021) 597:120303. doi: 10.1016/j.ijpharm.2021.120303
106. Yadav V, Sharma PK, Murty US, Mohan NH, Thomas R, Dwivedy SK, et al. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int J Pharm. (2021) 605:120815. doi: 10.1016/j.ijpharm.2021.120815
107. Xenikakis I, Tzimtzimis M, Tsongas K, Andreadis D, Demiri E, Tzetzis D, et al. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro. Eur J Pharm Sci. (2019) 137:104976. doi: 10.1016/j.ejps.2019.104976
108. Economidou SN, Pere CPP, Reid A, Uddin MJ, Windmill JFC, Lamprou DA, et al. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater Sci Eng C Mater Biol Appl. (2019) 102:743–55. doi: 10.1016/j.msec.2019.04.063
109. Caudill CL, Perry JL, Tian S, Luft JC, and DeSimone JM. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J Control Release. (2018) 284:122–32. doi: 10.1016/j.jconrel.2018.05.042
110. Uddin MJ, Scoutaris N, Economidou SN, Giraud C, Chowdhry BZ, Donnelly RF, et al. 3D printed microneedles for anticancer therapy of skin tumours. Mater Sci Eng C Mater Biol Appl. (2020) 107:110248. doi: 10.1016/j.msec.2019.110248
111. Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, and Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. (2018) 18:1223–30. doi: 10.1039/C8LC00098K
112. Amer RI, El-Osaily GH, Bakr RO, El Dine RS, and Fayez AM. Characterization and pharmacological evaluation of anti-cellulite herbal product(s) encapsulated in 3D-fabricated polymeric microneedles. Sci Rep. (2020) 10:6316. doi: 10.1038/s41598-020-63271-6
113. Nayak AK and Das B. Introduction to polymeric gels. Polymeric gels. (2018), 3–27. doi: 10.1016/B978-0-08-102179-8.00001-6
114. Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. (2017) 8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514
115. De Rosa FS and Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharmaceutical Research. (2000) 17(12):1447–55. doi: 10.1023/A:1007612905378
116. Capanema NS, Mansur AA, Carvalho SM, Carvalho IC, Chagas P, de Oliveira LCA, et al. Bioengineered carboxymethyl cellulose-doxorubicin prodrug hydrogels for topical chemotherapy of melanoma skin cancer. Carbohydr polymers. (2018) 195:401–12. doi: 10.1016/j.carbpol.2018.04.105
117. Kavanagh GM and Ross-Murphy SB. Rheological characterisation of polymer gels. Prog Polymer Sci. (1998) 23:533–62. doi: 10.1016/S0079-6700(97)00047-6
118. Bala P, Jathar S, Kale S, and Pal K. Transdermal drug delivery system (TDDS)-a multifaceted approach for drug delivery. J Pharm Res. (2014) 8:1805–35.
119. Ibrahim SA. Spray-on transdermal drug delivery systems. Expert Opin Drug delivery. (2015) 12:195–205. doi: 10.1517/17425247.2015.961419
120. Bakshi A, Bajaj A, Malhotra G, Madan M, and Amrutiya N. A novel metered dose transdermal spray formulation for oxybutynin. Indian J Pharm Sci. (2008) 70:733. doi: 10.4103/0250-474X.49094
122. Patel D, Kumar P, and Thakkar HP. Lopinavir metered-dose transdermal spray through microporated skin: Permeation enhancement to achieve therapeutic needs. J Drug Delivery Sci Technol. (2015) 29:173–80. doi: 10.1016/j.jddst.2015.07.004
123. Karamkar PG, Agrawal A, and Chatap VK. A review article: Formulation of topical gel by QbD approach. Adv Pharmacol Pharm. (2023) 11:90–101. doi: 10.13189/app.2023.110202
124. Jeong WY, Kwon M, Choi HE, and Kim KS. Recent advances in transdermal drug delivery systems: a review. Biomater Res. (2021) 25:24. doi: 10.1186/s40824-021-00226-6
125. Moarefian M, Davalos RV, Tafti DK, Achenie LE, and Jones CN. Modeling iontophoretic drug delivery in a microfluidic device. Lab Chip. (2020) 20:3310–21. doi: 10.1039/D0LC00602E
126. Kesarwani A, Yadav AK, Singh S, Gautam H, Singh HN, Sharma A, et al. Theoretical aspects of transdermal drug delivery system. Bull Pharm Res. (2013) 3:78–89.
127. Bakshi P, Vora D, Hemmady K, and Banga AK. Iontophoretic skin delivery systems: Success and failures. Int J Pharm. (2020) 586:119584. doi: 10.1016/j.ijpharm.2020.119584
128. Curdy C, Kalia YN, and Guy RH. Non-invasive assessment of the effects of iontophoresis on human skin in-vivo. J Pharm Pharmacol. (2001) 53:769–77. doi: 10.1211/0022357011776117
129. Shingade GM. Review on: recent trend on transdermal drug delivery system. J Drug delivery Ther. (2012) 2:66–75. doi: 10.22270/jddt.v2i1.74
130. Baron ED, Harris L, Redpath WS, Shapiro H, Hetzel F, Morley G, et al. Laser-assisted penetration of topical anesthetic in adults. Arch Dermatol. (2003) 139:1288–90. doi: 10.1001/archderm.139.10.1288
131. Zhang L, Nolan E, Kreitschitz S, and Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta (BBA)-General Subj. (2002) 1572:1–9. doi: 10.1016/S0304-4165(02)00270-2
132. Mitragotri S and Kost J. Low-frequency sonophoresis: a review. Advanced Drug delivery Rev. (2004) 56:589–601. doi: 10.1016/j.addr.2003.10.024
133. Katz N, Shapiro D, and Herrmann T. Ultrasound pretreatment of skin enhances the speed of EMLA cream. Anesth Analg. (2004) 98:371–6. doi: 10.1213/01.ANE.0000099716.02783.C4
134. Smith NB, Lee S, Maione E, Roy RB, McElligott S, and Shung KK. Ultrasound-mediated transdermal transport of insulin in vitro through human skin using novel transducer designs. Ultrasound Med Biol. (2003) 29:311–7. doi: 10.1016/S0301-5629(02)00706-8
135. Tofail SAM, Koumoulos EP, Bandyopadhyay A, Bose S, O’Donoghue L, and Charitidis C. Additive manufacturing: scientific and technological challenges, market uptake and opportunities. Materials Today. (2018) 21:22–37. doi: 10.1016/j.mattod.2017.07.001
136. Keleş Ö, Blevins CW, and Bowman KJ. Effect of build orientation on the mechanical reliability of 3D printed ABS. Rapid Prototyping J. (2017) 23:320–8. doi: 10.1108/RPJ-09-2015-0122
137. Aldawood FK, Parupelli SK, Andar A, and Desai S. 3D printing of biodegradable polymeric microneedles for transdermal drug delivery applications. Pharmaceutics. (2024) 16:237. doi: 10.3390/pharmaceutics16020237
138. Fu J-j, Li C-w, Liu Y, Chen M-y, Zhang Q, Yu X-y, et al. The microneedles carrying cisplatin and IR820 to perform synergistic chemo-photodynamic therapy against breast cancer. J nanobiotechnology. (2020) 18:146. doi: 10.1186/s12951-020-00697-0
139. Wang Y, Blache R, and Xu X. Selection of additive manufacturing processes. Rapid Prototyping J. (2017) 23:434–47. doi: 10.1108/RPJ-09-2015-0123
140. Bhushan B and Caspers M. An overview of additive manufacturing (3D printing) for microfabrication. Microsystem Technol. (2017) 23:1117–24. doi: 10.1007/s00542-017-3342-8
141. Chen H and Zhao YF. Process parameters optimization for improving surface quality and manufacturing accuracy of binder jetting additive manufacturing process. Rapid Prototyping J. (2016) 22:527–38. doi: 10.1108/RPJ-11-2014-0149
142. Tang Y, Zhou Y, Hoff T, Garon M, and Zhao Y. Elastic modulus of 316 stainless steel lattice structure fabricated via binder jetting process. Materials Sci Technol. (2016) 32:648–56. doi: 10.1179/1743284715Y.0000000084
143. Dilberoglu UM, Gharehpapagh B, Yaman U, and Dolen M. The role of additive manufacturing in the era of industry 4.0. Proc Manufacturing. (2017) 11:545–54. doi: 10.1016/j.promfg.2017.07.148
144. Van Rooyen C, Burger H, Theron M, and Doubell P. In-situ crack repair by laser cladding. (2010).
145. Koehler H, Partes K, Seefeld T, and Vollertsen F. Laser reconditioning of crankshafts: From lab to application. Phys Proc. (2010) 5:387–97. doi: 10.1016/j.phpro.2010.08.160
146. Vithani K, Goyanes A, Jannin V, Basit AW, Gaisford S, and Boyd BJ. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm Res. (2019) 36:1–20. doi: 10.1007/s11095-018-2531-1
147. Pereira T, Barroso S, and Gil MM. Food texture design by 3D printing: A review. Foods. (2021) 10:320. doi: 10.3390/foods10020320
148. Vancauwenberghe V, Verboven P, Lammertyn J, and Nicolaï B. Development of a coaxial extrusion deposition for 3D printing of customizable pectin-based food simulant. J Food Eng. (2018) 225:42–52. doi: 10.1016/j.jfoodeng.2018.01.008
149. Bhattacharjee N, Urrios A, Kang S, and Folch A. The upcoming 3D-printing revolution in microfluidics. Lab Chip. (2016) 16:1720–42. doi: 10.1039/C6LC00163G
150. De Coppi P, Bartsch G Jr., Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. (2007) 25:100–6. doi: 10.1038/nbt1274
151. Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, et al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PloS One. (2013) 8:e57741. doi: 10.1371/journal.pone.0057741
152. Demirci U and Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip. (2007) 7:1139–45. doi: 10.1039/b704965j
153. Shahrubudin N, Lee TC, and Ramlan R. An overview on 3D printing technology: Technological, materials, and applications. Proc manufacturing. (2019) 35:1286–96. doi: 10.1016/j.promfg.2019.06.089
154. Kibira D, Morris KC, and Kumaraguru S. Methods and tools for performance assurance of smart manufacturing systems. J Res Natl Institute Standards Technol. (2016) 121:282. doi: 10.6028/jres.121.013
155. Tan K, Chua C, Leong K, Cheah C, Gui W, Tan W, et al. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio-medical materials Eng. (2005) 15:113–24. doi: 10.1177/0959298920050151-2012
156. Chua C, Leong K, Tan K, Wiria F, and Cheah C. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J Materials Science: Materials Med. (2004) 15:1113–21. doi: 10.1023/B:JMSM.0000046393.81449.a5
157. Gibson I, Rosen D, Stucker B, and Khorasani M. Additive Manufacturing Technologies. (Vol. 17, pp. 160–86.). Cham, Switzerland: Springer (2021).
158. Wimpenny DI, Bryden B, and Pashby IR. Rapid laminated tooling. J Materials Process Technol. (2003) 138:214–8. doi: 10.1016/S0924-0136(03)00074-8
159. Yi S, Liu F, Zhang J, and Xiong S. Study of the key technologies of LOM for functional metal parts. Journal of Materials Processing Technology. (2004) 150:175–81.
160. Low Z-X, Chua YT, Ray BM, Mattia D, Metcalfe IS, and Patterson DA. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J Membrane Sci. (2017) 523:596–613. doi: 10.1016/j.memsci.2016.10.006
161. Tzeng J-J, Yang T-S, Lee W-F, Chen H, and Chang H-M. Mechanical properties and biocompatibility of urethane acrylate-based 3D-printed denture base resin. Polymers. (2021) 13:822. doi: 10.3390/polym13050822
162. Schwartz J and Boydston A. Multimaterial actinic spatial control 3D and 4D printing. Nat Commun. (2019) 10:791. doi: 10.1038/s41467-019-08639-7
163. Martin JH, Yahata BD, Hundley JM, Mayer JA, Schaedler TA, and Pollock TM. 3D printing of high-strength aluminium alloys. Nature. (2017) 549:365–9. doi: 10.1038/nature23894
164. Hitzler L, Alifui-Segbaya F, Williams P, Heine B, Heitzmann M, Hall W, et al. Additive manufacturing of cobalt-based dental alloys: analysis of microstructure and physicomechanical properties. Adv materials Sci Eng. (2018) 2018:8213023. doi: 10.1155/2018/8213023
165. Murr LE. Frontiers of 3D printing/additive manufacturing: from human organs to aircraft fabrication. J Materials Sci Technol. (2016) 32:987–95. doi: 10.1016/j.jmst.2016.08.011
166. Zarringhalam H, Majewski C, and Hopkinson N. Degree of particle melt in Nylon-12 selective laser-sintered parts. Rapid Prototyping J. (2009) 15:126–32. doi: 10.1108/13552540910943423
167. Nematollahi B, Vijay P, Sanjayan J, Nazari A, Xia M, Naidu Nerella V, et al. Effect of polypropylene fibre addition on properties of geopolymers made by 3D printing for digital construction. Materials. (2018) 11:2352. doi: 10.3390/ma11122352
168. Owen D, Hickey J, Cusson A, Ayeni OI, Rhoades J, Deng Y, et al. 3D printing of ceramic components using a customized 3D ceramic printer. Prog additive manufacturing. (2018) 3:3–9. doi: 10.1007/s40964-018-0037-3
169. Tang X and Yu Y. Electrospinning preparation and characterization of alumina nanofibers with high aspect ratio. Ceramics Int. (2015) 41:9232–8. doi: 10.1016/j.ceramint.2015.04.157
170. Li X, Gao M, and Jiang Y. Microstructure and mechanical properties of porous alumina ceramic prepared by a combination of 3–D printing and sintering. Ceramics Int. (2016) 42:12531–5. doi: 10.1016/j.ceramint.2016.05.027
171. Maurath J and Willenbacher N. 3D printing of open-porous cellular ceramics with high specific strength. J Eur Ceramic Soc. (2017) 37:4833–42. doi: 10.1016/j.jeurceramsoc.2017.06.001
172. Pervaiz S, Qureshi TA, Kashwani G, and Kannan S. 3D printing of fiber-reinforced plastic composites using fused deposition modeling: A status review. Materials (Basel). (2021) 14:4520. doi: 10.3390/ma14164520
173. Singh S, Ramakrishna S, and Berto F. 3D Printing of polymer composites: A short review. Material Design Process Commun. (2019) 2:E97. doi: 10.1002/mdp2.97
174. Dimas LS, Bratzel GH, Eylon I, and Buehler MJ. Tough composites inspired by mineralized natural materials: computation, 3D printing, and testing. Advanced Funct Materials. (2013) 23:4629–38. doi: 10.1002/adfm.201300215
175. Kim K, Zhu W, Qu X, Aaronson C, McCall WR, Chen S, et al. 3D optical printing of piezoelectric nanoparticle–polymer composite materials. ACS nano. (2014) 8:9799–806. doi: 10.1021/nn503268f
176. Nadagouda MN, Rastogi V, and Ginn M. A review on 3D printing techniques for medical applications. Curr Opin Chem Eng. (2020) 28:152–7. doi: 10.1016/j.coche.2020.05.007
177. Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, and Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. (2016) 34:312–9. doi: 10.1038/nbt.3413
178. Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat materials. (2017) 16:303–8. doi: 10.1038/nmat4782
179. Li X, Zhao Z, Zhang M, Ling G, and Zhang P. Research progress of microneedles in the treatment of melanoma. J Controlled release. (2022) 348:631–47. doi: 10.1016/j.jconrel.2022.06.021
180. He G, Li Y, Younis MR, Fu L-H, He T, Lei S, et al. Synthetic biology-instructed transdermal microneedle patch for traceable photodynamic therapy. Nat Commun. (2022) 13:6238. doi: 10.1038/s41467-022-33837-1
181. Chen D, Zhang Y, Long W, Chai L, Myint TP, Zhou W, et al. Visible light-driven photodynamic therapy for hypertrophic scars with MOF armored microneedles patch. Front Chem. (2023) 11:1128255. doi: 10.3389/fchem.2023.1128255
182. Sondak VK and Gibney GT. Surgical management of melanoma. Hematology/Oncology Clinics. (2014) 28:455–70. doi: 10.1016/j.hoc.2014.02.009
183. Etzkorn JR, Sharkey JM, Grunyk JW, Shin TM, Sobanko JF, and Miller CJ. Frequency of and risk factors for tumor upstaging after wide local excision of primary cutaneous melanoma. J Am Acad Dermatol. (2017) 77:341–8. doi: 10.1016/j.jaad.2017.03.018
184. Rzhevskiy AS, Singh TRR, Donnelly RF, and Anissimov YG. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. (2018) 270:184–202. doi: 10.1016/j.jconrel.2017.11.048
185. Indermun S, Luttge R, Choonara YE, Kumar P, du Toit LC, Modi G, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. (2014) 185:130–8. doi: 10.1016/j.jconrel.2014.04.052
186. Sabri AH, Cater Z, Gurnani P, Ogilvie J, Segal J, Scurr DJ, et al. Intradermal delivery of imiquimod using polymeric microneedles for basal cell carcinoma. Int J pharmaceutics. (2020) 589:119808. doi: 10.1016/j.ijpharm.2020.119808
187. Li X, Lovell JF, Yoon J, and Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. (2020) 17:657–74. doi: 10.1038/s41571-020-0410-2
188. Jain AK, Lee CH, and Gill HS. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Controlled Release. (2016) 239:72–81. doi: 10.1016/j.jconrel.2016.08.015
189. Kim YC, Park JH, and Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Delivery Rev. (2012) 64:1547–68. doi: 10.1016/j.addr.2012.04.005
190. Birchall JC, Clemo R, Anstey A, and John DN. Microneedles in clinical practice–an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. (2011) 28:95–106. doi: 10.1007/s11095-010-0101-2
191. Vassilieva EV, Wang S, Li S, Prausnitz MR, and Compans RW. Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine. Sci Rep. (2017) 7:17855. doi: 10.1038/s41598-017-18140-0
192. Esser ES, Romanyuk A, Vassilieva EV, Jacob J, Prausnitz MR, Compans RW, et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J Control Release. (2016) 236:47–56. doi: 10.1016/j.jconrel.2016.06.026
193. Mikolajewska P, Donnelly RF, Garland MJ, Morrow DI, Singh TR, Iani V, et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. (2010) 27:2213–20. doi: 10.1007/s11095-010-0227-2
194. Donnelly RF, Raj Singh TR, and Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Delivery. (2010) 17:187–207. doi: 10.3109/10717541003667798
195. Ma Y and Gill HS. Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J Pharm Sci. (2014) 103:3621–30. doi: 10.1002/jps.24159
196. Bian Q, Huang L, Xu Y, Wang R, Gu Y, Yuan A, et al. A facile low-dose photosensitizer-incorporated dissolving microneedles-based composite system for eliciting antitumor immunity and the abscopal effect. ACS Nano. (2021) 15:19468–79. doi: 10.1021/acsnano.1c06225
197. Song C, Wu X, Wang J, Liu R, and Zhao Y. Photosensitizer-immunotherapy integrated microneedles for preventing tumor recurrence and metastasis. Nano Today. (2023) 51:101913. doi: 10.1016/j.nantod.2023.101913
198. Li Y, He G, Fu L-H, Younis MR, He T, Chen Y, et al. A microneedle patch with self-oxygenation and glutathione depletion for repeatable photodynamic therapy. ACS nano. (2022) 16:17298–312. doi: 10.1021/acsnano.2c08098
199. Huang Y, Lai H, Jiang J, Xu X, Zeng Z, Ren L, et al. pH-activatable oxidative stress amplifying dissolving microneedles for combined chemo-photodynamic therapy of melanoma. Asian J Pharm Sci. (2022) 17:679–96. doi: 10.1016/j.ajps.2022.08.003
200. Song C, Zhang X, Cao Z, Wei Z, Zhou M, Wang Y, et al. Regulating tumor cholesterol microenvironment to enhance photoimmunotherapy in oral squamous cell carcinoma. Chem Eng J. (2023) 462:142160. doi: 10.1016/j.cej.2023.142160
201. Zhao X, Li X, Zhang P, Du J, and Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J Controlled release. (2018) 286:201–9. doi: 10.1016/j.jconrel.2018.07.038
202. Donnelly RF, Morrow DI, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: potential for enhanced topical photodynamic therapy. J Controlled Release. (2008) 129:154–62. doi: 10.1016/j.jconrel.2008.05.002
203. Diment LE, Thompson MS, and Bergmann JH. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open. (2017) 7:e016891. doi: 10.1136/bmjopen-2017-016891
204. Yan Q, Dong H, Su J, Han J, Song B, Wei Q, et al. A review of 3D printing technology for medical applications. Engineering. (2018) 4:729–42. doi: 10.1016/j.eng.2018.07.021
205. McGuigan AP and Sefton MV. Design criteria for a modular tissue-engineered construct. Tissue Eng. (2007) 13:1079–89. doi: 10.1089/ten.2006.0245
206. Heisler DB, Johnson KA, Ma DH, Ohlson MB, Zhang L, Tran M, et al. A concerted mechanism involving ACAT and SREBPs by which oxysterols deplete accessible cholesterol to restrict microbial infection. Elife. (2023) 12:e83534. doi: 10.7554/eLife.83534.sa2
Keywords: 3D printing, microneedles, melanoma, biopolymers, photodynamic therapy
Citation: Obalola AA, Abrahamse H and Dhilip Kumar SS (2025) 3D-printed biopolymer-based microneedle for enhanced photodynamic therapy in melanoma treatment. Front. Oncol. 15:1642448. doi: 10.3389/fonc.2025.1642448
Received: 06 June 2025; Accepted: 27 August 2025;
Published: 17 September 2025.
Edited by:
Jennifer Yunyan Zhang, Duke University, United StatesCopyright © 2025 Obalola, Abrahamse and Dhilip Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sathish Sundar Dhilip Kumar, c2F0aGlzaGRAdWouYWMuemE=
†ORCID: Sathish Sundar Dhilip Kumar, orcid.org/0000-0001-6964-1138
Aishat Adejoke Obalola, orcid.org/0009-0004-3399-0693
Heidi Abrahamse, orcid.org/0000-0001-5002-827X
 Aishat Adejoke Obalola
Aishat Adejoke Obalola Heidi Abrahamse
Heidi Abrahamse Sathish Sundar Dhilip Kumar
Sathish Sundar Dhilip Kumar