- 1Department of Surgery, Inner Mongolia Medical University, Hohhot, China
- 2Department of Hepatobiliary Surgery, Inner Mongolia People’s Hospital, Hohhot, China
- 3Department of Hepatobiliary Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Carcinosarcoma of the common bile duct is an exceedingly rare and highly aggressive malignancy with uncertain therapeutic outcomes. We present a case of a 70-year-old Asian female presenting with progressive jaundice. Contrast-enhanced computed tomography (CT) identified a tumor in the common bile duct. The patient underwent successful radical pancreaticoduodenectomy, and postoperative histopathological analysis confirmed carcinosarcoma with predominant sarcomatoid differentiation. This report underscores the diagnostic challenges and therapeutic considerations for this rare entity, emphasizing the critical role of pathological confirmation.
1 Introduction
Carcinosarcoma is a rare biphasic malignancy comprising both epithelial-derived carcinoma and mesenchymal sarcomatoid components. While commonly reported in the breast and female reproductive system, its occurrence in the biliary tract is exceptionally rare. Clinical manifestations, imaging findings, and serological markers of biliary carcinosarcoma lack specificity, necessitating histopathological confirmation for definitive diagnosis. Due to its aggressive nature and propensity for metastasis, prognosis remains poor, with limited evidence guiding optimal therapeutic strategies. This report details a rare case of carcinosarcoma of the common bile duct and synthesizes current literature to highlight diagnostic and therapeutic challenges.
2 Case report
A 70-year-old woman presented with a two-week history of progressive jaundice, right upper abdominal distension, fatigue, dark urine, and clay-colored stools. Her medical history was unremarkable for hepatitis, liver disease, or chronic comorbidities. Physical examination revealed scleral icterus and a weakly positive Murphy’s sign.
Liver function tests (Table 1) and Tumor markers (Table 2) showed elevated levels:
Abdominal color Doppler ultrasound revealed hypoechoic nodules in the ampulla of the duodenum behind the pancreatic head at the end of the common bile duct, along with intrahepatic and extrahepatic bile duct dilatation, hepatic cysts, and an enlarged gallbladder. Contrast-enhanced CT (Figure 1) showed uneven thickening of the wall at the pancreatic segment and junction of the common bile duct, suggesting malignant expansion of the lower biliary tract, with possible involvement of the pancreatic head. Hepatic S7 cyst was also noted.
Hepatobiliary contrast-enhanced Magnetic Resonance Imaging (MRI) with diffusion-weighted imaging (DWI) and magnetic resonance cholangiopancreatography (MRCP) (Figure 2) revealed irregular thickening at the confluence of the gallbladder duct and hepatic duct, with the thickened portion measuring approximately 8 mm. DWI showed a slightly higher signal, and the enhanced scan demonstrated significant enhancement. Hepatic S7 cyst was again noted.
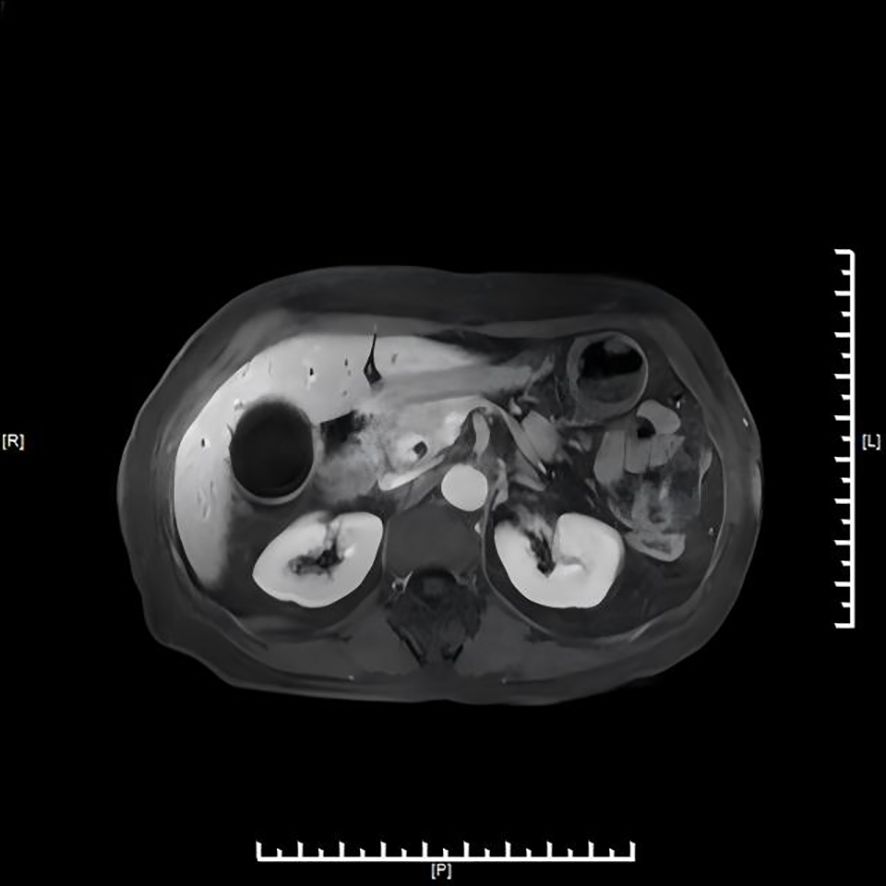
Figure 2. MRI/MRCP demonstrating low cystic duct insertion and irregular thickening at the cystic duct–common hepatic duct junction.
After preoperative evaluation and multidisciplinary discussion, the patient underwent radical pancreaticoduodenectomy with hepatic cyst incision and drainage on November 4, 2024. The surgery was successful. Postoperative pathological examination (Figures 3, 4) revealed poorly differentiated adenocarcinoma of the common bile duct with sarcomatoid and squamous differentiation, predominantly sarcomatoid components. The tumor measured approximately 2.5 cm x 1.0 cm x (0.3-1.0 cm), invading the entire wall of the common bile duct and surrounding adipose tissue, with micro-invasion of the pancreas and suspected intravascular cancer emboli. No metastasis was found in the duodenum, pancreas, or lymph nodes. Immunohistochemistry results were as follows: CK (+), CK7 (+), CK19 (+), CK20 (partially weak +), MUC-2 (-), CDX2 (partially weak +), VIM (-), SMA (-), S-100 (-), Desmin (-), CK5/6 (focal +), P63 (-), BRG-1 (+), Glypican-3 (-), P53 (individual cells +), and ki-67 (+60-70%).
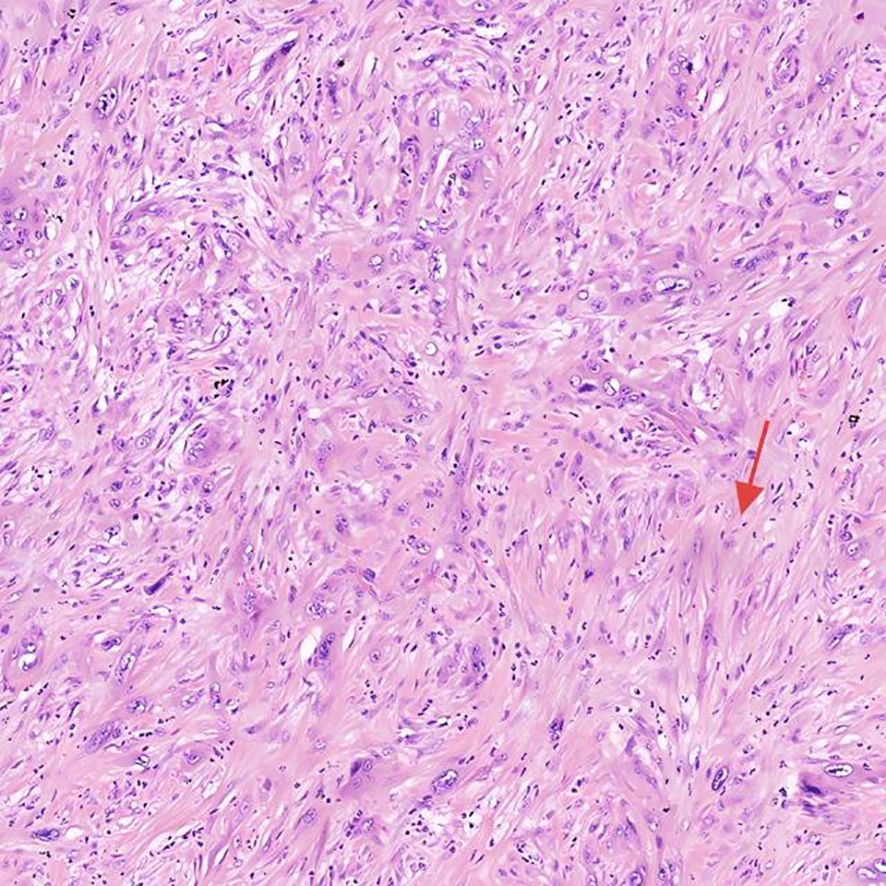
Figure 3. Histopathological and immunohistochemical features H&E of the tumor, highlighting sarcomatoid differentiation and high proliferative activity.
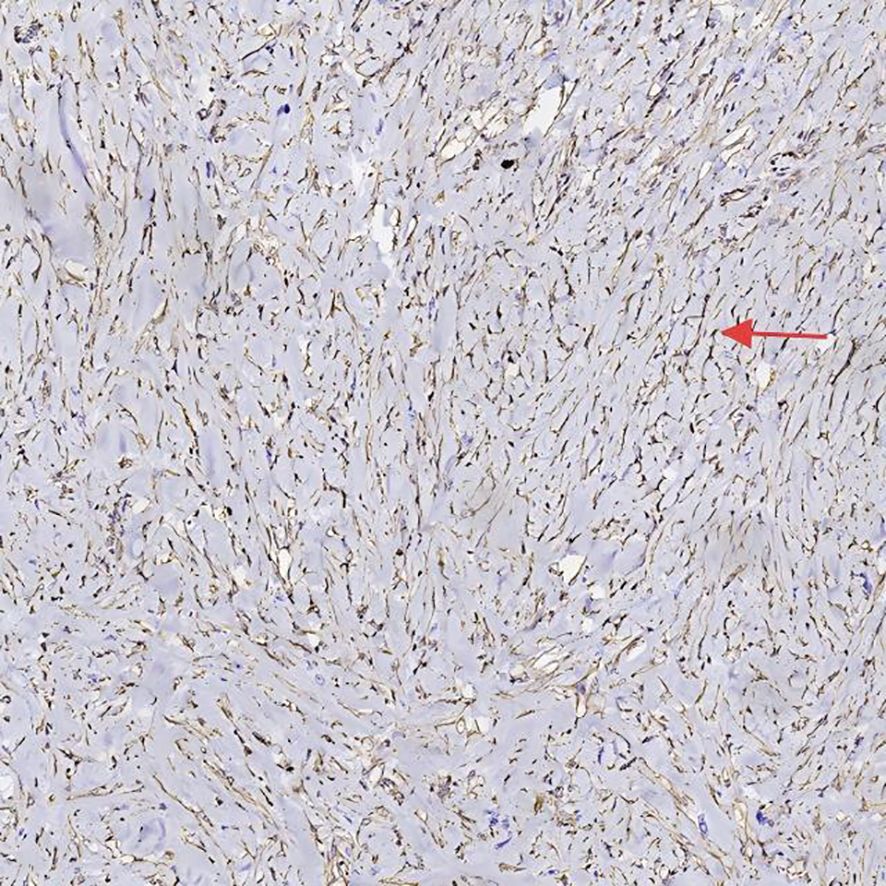
Figure 4. Histopathological and immunohistochemical features VIM of the tumor, highlighting sarcomatoid differentiation and high proliferative activity.
The patient developed a grade C pancreatic fistula postoperatively and was discharged 40 days after surgery. Follow-up contrast-enhanced CT more than five months post-surgery showed no tumor recurrence.
3 Discussion
3.1 Introduction
The concept of carcinosarcoma was first described by the German pathologist Virchow in 1864 (1).This rare and highly aggressive malignancy is characterized by the coexistence of epithelial-derived carcinoma and mesenchymal-derived sarcomatoid tissue, with the sarcomatoid component often predominating. Car cinosarcomas can arise in various organs, including the breast, pancreas, lung, uterus, ovary, esophagus, stomach, and kidney (2–9). According to Sasamoto et al., breast cancer (approximately 39.8% of cases) and uterine cancer (24.9%) represent the most common sites of carcinosarcoma (10). Recent epidemiological studies indicate a rising incidence of carcinosarcoma, particularly in the female reproductive and respiratory systems. Notably, carcinosarcoma of the digestive system is associated with the poorest prognosis, with a median survival period of approximately six months (11).
3.2 Etiology and pathogenesis
The origin of carcinosarcoma remains a subject of ongoing debate, with three primary hypotheses proposed in the literature. The first, proposed by Wiebren van den Berg et al., suggests that carcinosarcoma arises from phenotypic diversity within monoclonal tumors. This hypothesis is supported by molecular studies of mucinous cystic tumors of the pancreas, which demonstrated nearly identical genetic alterations between sarcomatous and epithelial components in two out of three cases. In the third case, allele loss and retention were identical in five of six chromosomal loci (12). The second hypothesis, termed the “metaplasia theory,” posits that sarcomatoid components result from the metaplastic transformation of epithelioid cancer tissues (13). The third hypothesis, the “collision theory,” proposes that carcinosarcoma arises from the independent development and subsequent fusion of carcinoma and sarcoma tissues, which originate from distinct cell lineages (14).
3.3 Clinical presentation and diagnostic challenges
The clinical manifestations of cholangiocarcinoma sarcoma are nonspecific and closely resemble those of conventional cholangiocarcinoma. Okabayashi et al. reported that the mean age of patients with extrahepatic cholangiocarcinoma sarcoma is approximately 68 years, with obstructive jaundice being the most common presenting symptom (15). Imaging studies typically reveal polypoid masses within the bile duct, a finding also observed in carcinosarcomas of other hollow organs, such as the esophagus and bladder (16). Common imaging features include bile duct dilation and biliary obstruction, as demonstrated by cholangiography and computed tomography (CT). However, these findings are not pathognomonic for carcinosarcoma and may also be observed in conditions such as polypoid cholangiocarcinoma, gallbladder cancer with bile duct involvement, pseudotumors, or metastatic disease. Consequently, definitive diagnosis relies on histopathological examination.
3.4 Therapeutic approaches and prognosis
Carcinosarcoma is characterized by its high malignant potential, aggressive local invasion, and propensity for metastasis. Lee et al. have suggested that the sarcomatoid component is primarily responsible for the tumor’s invasiveness, as it exhibits a greater tendency to metastasize to lymph nodes and distant organs (17). Radical surgical resection remains the cornerstone of treatment. For intrahepatic cholangiocarcinosarcoma, surgical options include partial liver resection (e.g., hemihepatectomy, lobectomy, or segmentectomy) combined with lymph node dissection. For extrahepatic cholangiocarcinosarcoma, pancreaticoduodenectomy or tumor resection with choledochojejunostomy is performed, depending on the tumor’s anatomical location (18). The choice of surgical approach is guided by the tumor’s location, extent, and progression, with operative time, blood loss, and transfusion requirements varying accordingly. In the case presented by the authors, the tumor was located in the common bile duct, and radical pancreaticoduodenectomy was performed following multidisciplinary discussion.
3.5 Survival outcomes and adjuvant therapy
Okabayashi et al. conducted a comprehensive review of clinicopathological data from 131 patients who underwent surgical resection for hepatobiliary carcinosarcoma between 1970 and 2012. The overall survival rates at 1, 3, and 5 years post-surgery were 44.0%, 29.3%, and 27.0%, respectively. Among these, 24 patients had extrahepatic cholangiocarcinoma sarcoma, with 1-year, 3-year, and 5-year survival rates of 43.3%, 28.9%, and 28.9%, respectively (15). Several case studies highlight the challenges associated with treating choledochal carcinosarcoma. For instance, Suguru Sasamoto et al. reported a patient who developed liver metastasis nine months post-pancreaticoduodenectomy and succumbed to the disease 26 months after surgery (10). Similarly, Kadono et al. described a patient who underwent pylorus-preserving pancreaticoduodenectomy but experienced local recurrence and died two years postoperatively (19). Tanaka et al. reported a patient who died ten months after radical pancreaticoduodenectomy (20). In contrast, Zhang Shuisheng et al. documented a patient who received adjuvant chemotherapy (cisplatin and gemcitabine) two months post-surgery and remained disease-free at three years of follow-up (21). Emerging evidence suggests that chemotherapy regimens incorporating docetaxel and gemcitabine may improve response rates and survival outcomes in sarcomatoid carcinoma (22). These findings underscore the potential benefit of combining radical surgery with tailored adjuvant chemotherapy to enhance patient prognosis.
4 Conclusion
This case highlights the diagnostic complexity and aggressive nature of carcinosarcoma of the common bile duct. Preoperative differentiation from other malignancies is challenging, underscoring the indispensability of histopathology. Radical surgery remains the primary therapeutic option, but multimodal approaches incorporating chemotherapy may improve outcomes. Future research should focus on standardized adjuvant protocols and molecular profiling to refine management strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Ethics Committee Approval of Inner Mongolia People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
J-QL: Writing – review & editing, Writing – original draft. Z-WH: Writing – review & editing. KL: Writing – review & editing. R-LA: Writing – review & editing. H-YF: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Doctoral Research Start-up Fund of the Inner Mongolia People’s Hospital (Grant No. 2024BS03).
Acknowledgments
We are deeply grateful to the research team studying the Effects and Mechanisms of LHPP on Pancreatic Cancer Cell Proliferation, Apoptosis, Migration, and Invasion for their support and collaboration. We particularly thank Professor Zhiwei Hu for his guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1642467/full#supplementary-material
References
1. Ansari-Lari MA, Hoque MO, Califano J, and Westra WH. Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol. (2002) 26:1024–31. doi: 10.1097/00000478-200208000-00007
2. Neelamraju Lakshmi H, Saini D, Om P, and Verma N. A case of carcinosarcoma of the breast presenting as inflammatory carcinoma and review of the literature. Cureus. (2020) 12:e10104. doi: 10.7759/cureus.10104
3. Li P, Zhang L, Liang J, and Tuo HF. A sarcomatoid carcinoma in the pancreas tail: A case report with literature review. Asian J Surg. (2024) 47:2349–51. doi: 10.1016/j.asjsur.2024.01.183
4. Rainosek DE, Ro JY, Ordonez NG, Kulaga AD, and Ayala AG. Sarcomatoid carcinoma of the lung. A case with atypical carcinoid and rhabdomyosarcomatous components. Am J Clin Pathol. (1994) 102:360–4. doi: 10.1093/ajcp/102.3.360
5. Cui T, Jin Y, Li B, Li J, and Yue Y. Dynamic contrast-enhanced MR and PET/CT findings of uterine sarcomatoid carcinoma: a case report. BMC Womens Health. (2020) 20:223. doi: 10.1186/s12905-020-01084-5
6. Brustmann H. Ovarian carcinosarcoma associated with bilateral tubal intraepithelial carcinoma: a case report. Int J Gynecol Pathol. (2013) 32:384–9. doi: 10.1097/PGP.0b013e318264aece
7. Raza MA and Mazzara PF. Sarcomatoid carcinoma of esophagus. Arch Pathol Lab Med. (2011) 135:945–8. doi: 10.5858/2010-0074-RSR.1
8. Zhang A, He X, and Fan D. Gastric sarcomatoid carcinoma: Report of a rare case. Asian J Surg. (2024) 47:4937–8. doi: 10.1016/j.asjsur.2024.05.204
9. Bian L, Duan J, Wang X, Yang Y, Zhang X, and Xiao S. Sarcomatoid chromophobe renal cell carcinoma: A case report and review of the literature. Am J Case Rep. (2019) 20:1225–30. doi: 10.12659/AJCR.916651
10. Suguru S, Takeshi A, Yoshihiko T, Kazuhiro M, Tomotake K, Tomokazu K, et al. Experience of the pancreas duodenectomy for so-called carcinosarcoma of the common bile duct:a case report and review of literature. Int Cancer Conf J. (2021) 10:134–8. doi: 10.1007/s13691-020-00462-y
11. Xu Z, Wang L, Tu L, Liu Y, Xie X, Tang X, et al. Epidemiology of and prognostic factors for patients with sarcomatoid carcinoma: a large population-based study. Am J Cancer Res. (2020) 10:3801–14.
12. van den Berg W, Tascilar M, Offerhaus GJ, Albores-Saavedra J, Wenig BM, Hruban RH, et al. Pancreatic mucinous cystic neoplasms with sarcomatous stroma: molecular evidence for monoclonal origin with subsequent divergence of the epithelial and sarcomatous components. Mod Pathol. (2000) 13:86–91. doi: 10.1038/modpathol.3880013
13. Kench JG and Frommer DJ. Sarcomatoid carcinoma of the ampulla of Vater. Pathology. (1997) 29:89–91. doi: 10.1080/00313029700169634
14. Kozłowski M, Nowak K, Kordek A, and Cymbaluk-Płoska A. Therapeutic management of rare primary ovarian neoplasms: carcinosarcoma, leiomyosarcoma, melanoma and carcinoid. Int J Environ Res Public Health. (2021) 18:7819. doi: 10.3390/ijerph18157819
15. Okabayashi T, Shima Y, Iwata J, Iiyama T, Sumiyoshi T, Kozuki A, et al. Surgical outcomes for 131 cases of carcinosarcoma of the hepatobiliary tract. J Gastroenterol. (2014) 49:982–91. doi: 10.1007/s00535-013-0882-2
16. Loud PA, Warshauer DM, Woosley JT, and Hartmann TM. Carcinosarcoma of the extrahepatic bile ducts: cholangiographic and CT appearance. Abdom Imaging. (1997) 22:85–6. doi: 10.1007/s002619900146
17. Lee SY, Shia J, Kingham TP, and Jarnagin WR. Carcinosarcoma of the bile duct: a case report and review of literature. Hepatobiliary Surg Nutr. (2016) 5:72–8. doi: 10.3978/j.issn.2304-3881.2015.06.09
18. Zhang N, Li Y, Zhao M, Chang X, Qu Q, and He X. Sarcomatous carcinoma in biliary system: A retrospective study. Med (Baltimore). (2019) 98:e14585. doi: 10.1097/MD.0000000000014585
19. Kadono J, Hamada N, Higashi M, Ishizaki N, Nakamura N, and Sakata R. Carcinosarcoma of the extrahepatic bile duct. J Hepatobiliary Pancreat Surg. (2005) 12:328–31. doi: 10.1007/s00534-005-0988-x
20. Tanaka M, Ajiki T, Matsumoto I, Asari S, Fukumoto T, Masuda A, et al. Duodenal protrusion by carcinosarcoma of the extrahepatic bile duct. Dig Endosc. (2012) 24:484. doi: 10.1111/j.1443-1661.2012.01341.x
21. Zhang S, Jia J, Bi X, Jiang Q, Zhao Y, Chen Y, et al. Sarcomatoid carcinoma of the common bile duct: A case report. Med (Baltimore). (2017) 96:e5751. doi: 10.1097/MD.0000000000005751
Keywords: carcinosarcoma, common bile duct, pancreaticoduodenectomy, sarcomatoid differentiation, prognosis
Citation: Lou J-q, Hu Z-W, Liu K, A R-L and Fang H-Y (2025) Case Report: Carcinosarcoma of the common bile duct: a rare case report with literature review. Front. Oncol. 15:1642467. doi: 10.3389/fonc.2025.1642467
Received: 06 June 2025; Accepted: 31 July 2025;
Published: 18 August 2025.
Edited by:
Zhen Liu, Zhejiang University, ChinaReviewed by:
Nikolaos Benetatos, University of Patras, GreeceZhaohui Tang, Shanghai Jiao Tong University, China
Jin-Ming Zhang, University of Texas Health Science Center at Houston, United States
Copyright © 2025 Lou, Hu, Liu, A and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Wei Hu, ODUyMTM2NzZAcXEuY29t
 Jia-qi Lou
Jia-qi Lou Zhi-Wei Hu2*
Zhi-Wei Hu2* Kun Liu
Kun Liu

![CT scan of the abdominal region showing cross-sectional view of internal organs. The image includes the spine, kidneys, and surrounding soft tissues, with indicators denoting anatomical directions labeled [R], [L], and [A].](https://www.frontiersin.org/files/Articles/1642467/fonc-15-1642467-HTML/image_m/fonc-15-1642467-g001.jpg)