- 1San Matteo Hospital Foundation (IRCCS), Pavia, Italy
- 2Department of Radiation Oncology, Centro Nacional de Radioterapia, San Salvador, El Salvador
- 3Department of Radiation Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 4Department of Medical Research, Instituo Nacional de Salud, San Salvador, El Salvador
- 5Unit of Respiratory Diseases, Cardiothoracic and Vascular Department, IRCCS Policlinico San Matteo, Pavia, Italy
- 6Department of Internal Medicine and Medical Therapeutics, University of Pavia Medical School, Pavia, Italy
- 7Department of Medical Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 8Unit of Thoracic Surgery, Cardiothoracic and Vascular Department, IRCCS Policlinico San Matteo, Pavia, Italy
- 9Diagnostic Imaging and Radiotherapy Unit, Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy
- 10Radiology Institute, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 11Pathology Unit, Department of Diagnostical Services and Imaging, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 12Department of Medical Physics, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Background: Radiation therapy is used in the clinical scenario of oligo-metastatic lung cancer as a weapon to delay the subsequent line of systemic therapy, particularly in the case of oligo-progressive disease. In this setting, the integration of immunotherapy and radiotherapy plays an important role to achieve local control and improve progression-free survival (PFS).
Case presentation: We reported the case of an elderly fragile patient affected by advanced non-small cell lung cancer treated with pembrolizumab as first systemic line and immuno-modulant radiation therapy at oligo-progression. More specifically, he underwent stereotactic body radiation therapy using non-ablative regimen (24 Gy in 3 fractions) achieving partial response with abscopal effect and without drug interruption. After one year, during immunotherapy mediastinal and parenchymal progression occurred and he received another radiation treatment using conventional non-ablative regimen (40 Gy in 20 fractions). Complete response was observed without severe side effects (his poor respiratory function did not change during both treatments).
Conclusion: In this case report we showed that the association of immunotherapy and non-ablative radiation regimens may represent a safe and effective strategy to achieve complete response also in fragile patients, in whom the burden of side effects should be prioritized.
Introduction
Immunomodulatory radiotherapy (iRT) refers to the use of ionizing radiation with the goal of positively modulating immune system activation, promoting the recognition of tumor cells through a mechanism similar of that usually triggered by vaccines (1–3), inducing a loco-regional and systemic immune-mediated response (abscopal effect) and consequently achieving a synergistic effect when combining with immunotherapeutic agents.
Patients with metastatic non-oncogene addicted non-small cell lung cancer (NSCLC) are generally treated with systemic therapy (immune check-point inhibitors alone or in combination with chemotherapy according to anti programmed death-ligand 1 status -anti-PD-L1-) as first-line treatment approach (4).Pembrolizumab (anti-PD-L1) received approval in 2016 in the first line setting as a single agent for patients whose tumors have high PD-L1 expression (tumor proportion score of >50%). The efficacy of pembrolizumab in combination with platinum-based chemotherapy was also demonstrated in several large phase III randomized trials in patients with metastatic NSCLC regardless of PD-L1 expression level (5, 6). As reported in registrative clinical trials, the median PFS of pembrolizumab as monotherapy in PD-L1 overexpressed patients is 10.3 months (7–9) and 8.8 months (5, 10) when used in combination with chemotherapy for non-squamous NSCLC.
In cases of oligo-progression, radiotherapy (RT) is often added to systemic therapy and plays an important role in disease control. In clinical practice RT in terms of hypo-fractionated regimen or stereotactic ablative therapy (SABR) is common used and well tolerated with concomitant immunotherapy (IO).
Recently, a systematic review focused on reporting the distant radiobiological effects (abscopal or bystander effect) of stereotactic body radiation therapy (SBRT) (11); most of these responses are reported for melanoma (24%) and NSCLC (13%) patients and occurred more frequently when using sub-ablative hypo-fractionated doses and concomitant IO.
At the moment, there are no clear recommendations on the use of sub-ablative doses in a radical treatment setting for patients with oligo-progression undergoing IO and SABR is generally used in this setting. However, in patients with pulmonary comorbidities such as severe obstructive pulmonary disease (COPD) or interstitial lung diseases, where treatment-related radiotoxicity may be a significant concern, sub-ablative dosing may represent an opportunity for systemic disease control.
This case report presents a patient affected by advanced NSCLC and concomitant severe COPD/emphysema who started pembrolizumab as first-line systemic therapy and then developed oligoprogression at two different time points. In both instances, iRT treatment with sub-ablative doses was administered, achieving complete response (CR) and continuation of the same therapy for up to 45 months.
Case report
In 2021 a former smoker (40 pack-year) 76-year-old male patient, with a performance status of 1, according to eastern cooperative oncology group (ECOG), with very severe COPD (GOLD 4), emphysema (Goddard 18 points) and cardio-vascular comorbidities (arterial hypertension, history of transient ischemic attack and paroxysmal atrial fibrillation in treatment with oral anticoagulant), received diagnosis of bilateral lung adenocarcinoma in the upper lobes and positive mediastinal lymph nodes (cT1b cN2 M1a; stage IV according to AJCC VIII edition, Figure 1) based on tomography scan (CT scan); positron emission tomography (PET) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). The immunohistochemical analysis revealed a PD-L1 tumor proportion score (TPS) of more than 50%, and the genetic analysis with next-generation sequencing (NGS) of EGFR, KRAS, BRAF, LKB1, ERBB2 and MET did not reveal any targetable mutations.
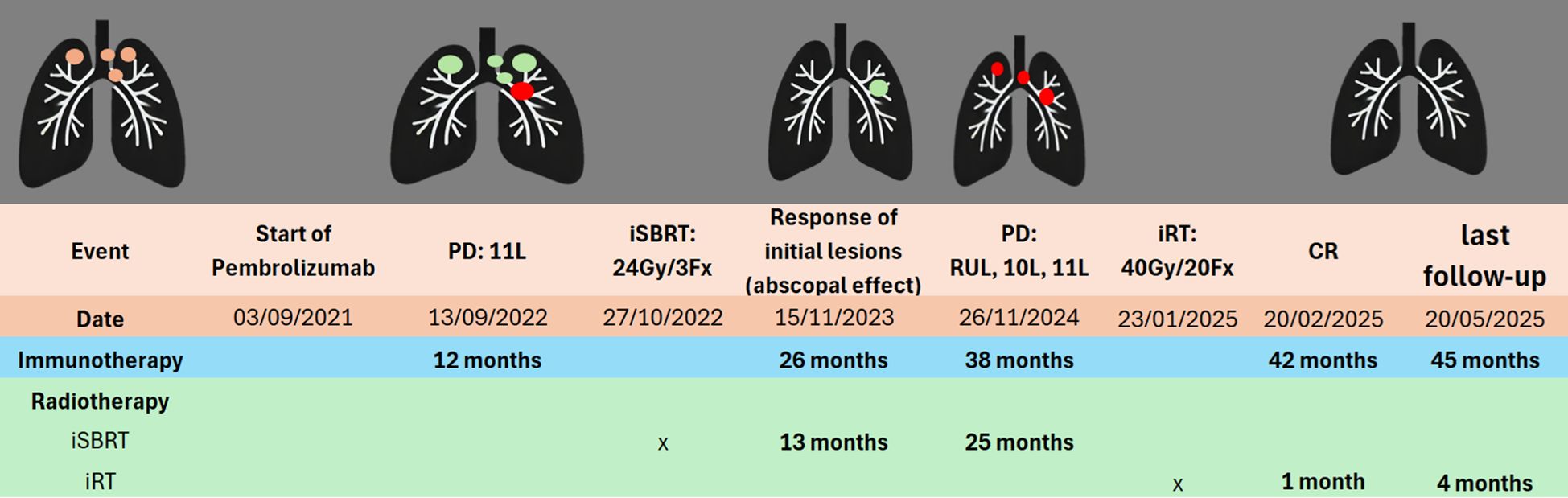
Figure 1. Timeline of treatments. Initial lesions  SD
SD  PD
PD  . SD, Stable disease; PD, Progressive disease; CR, Complete response; iSBRT, immune-modulating stereotactic body radiotherapy; iRT, immune-modulating radiotherapy; 11L, Left lymph node station 11; 10L, Left lymph node station 10; RUL, Right upper lobe.
. SD, Stable disease; PD, Progressive disease; CR, Complete response; iSBRT, immune-modulating stereotactic body radiotherapy; iRT, immune-modulating radiotherapy; 11L, Left lymph node station 11; 10L, Left lymph node station 10; RUL, Right upper lobe.
Following multidisciplinary discussion, the patient was considered as oligometastatic setting but not suitable for radical local treatment due to the mediastinal lymph nodes involvement. Thus he started pembrolizumab, as first-line systemic treatment, 200 mg each three weeks (q3w), reporting partial response which was maintained for one year.
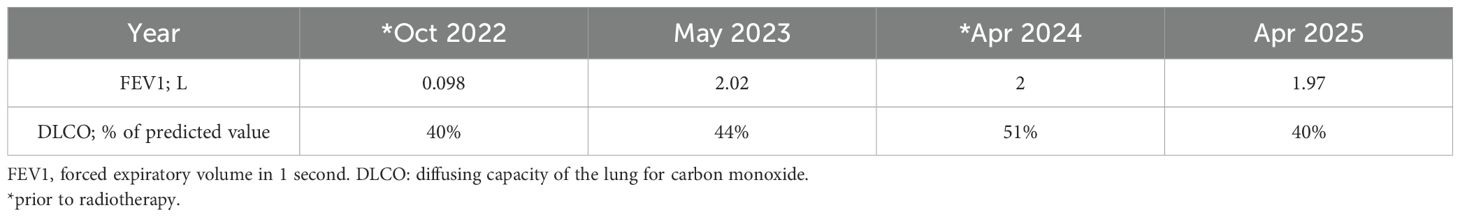
After 12 months, the patient experienced oligo-progression at lymph node station 11L, confirmed trough computed tomography (CT). Considering the pulmonary status and the ongoing IO treatment, an immune-modulating stereotactic body radiotherapy (iSBRT) treatment was planned to target only the site of progression, with a sub-ablative dose of 24 Gy in three fractions to the 80% isodose to the planning target volume (PTV), using volumetric modulated arc therapy (VMAT) without drug interruption. Prior to treatment, the patient underwent pulmonary spirometry showing permissive results for treatment as shown below (Table 1).
A CR was documented 13 months from iSBRT in the untreated lesions, while the irradiated lymph node station was stable; this response was maintained for other 12 months.
In November 2024, 25 months after iSBRT, mediastinal and lung oligo-progression was detected at stations 10L, 11L (pre-treated site) and right upper lobe (RUL), confirmed by PET-CT.
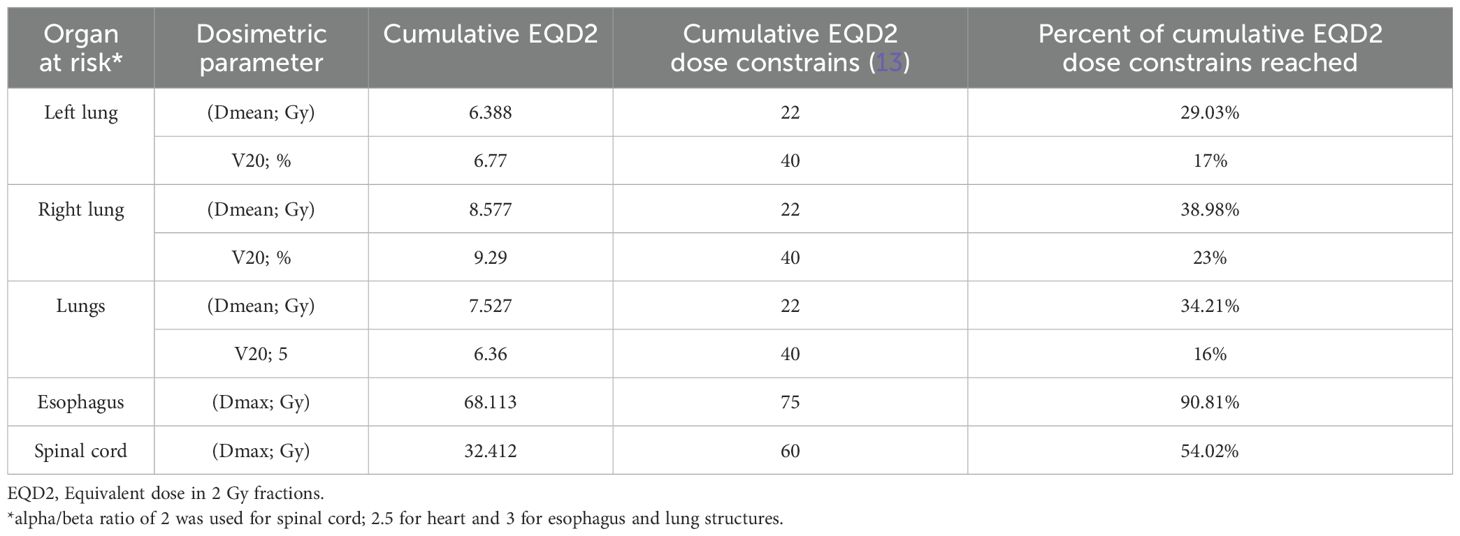
After a multidisciplinary discussion and respiratory function assessment (Table 1) it was decided to proceed with a new iRT treatment, but outside the context of SBRT. An equivalent dose in 2 Gy fractions (EQD2) with an alpha/beta of 10 (12) was used to reduce the risk of radiotoxicity, prescribing 40 Gy in 20 fractions to achieve volume coverage with VMAT technique, targeting the three sites of oligoprogression. The evaluation of the treatment plan was performed using rigid registration for dose accumulation (Figure 2) and biological summation; the data are presented in Table 2.
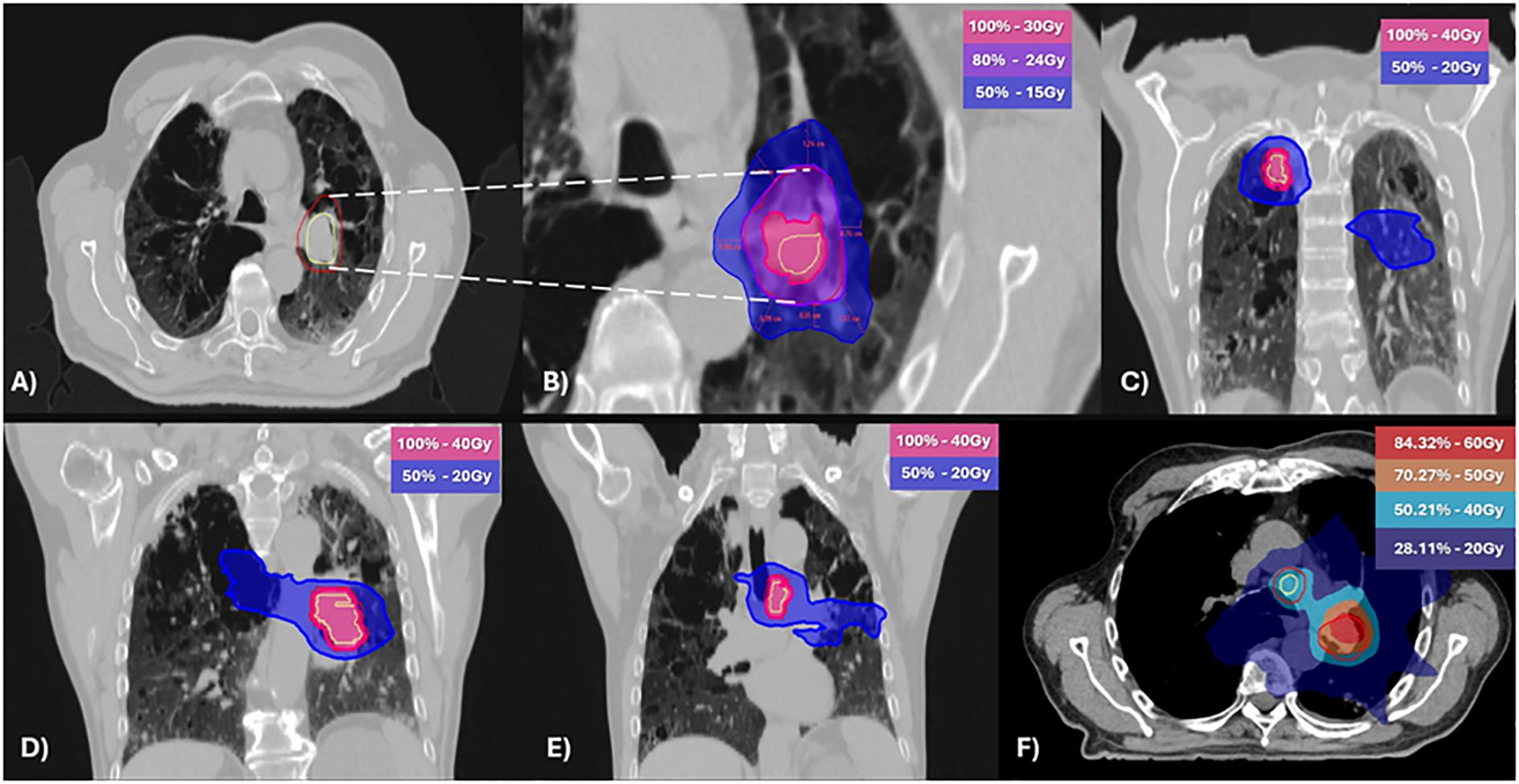
Figure 2. Stereotactic body radiotherapy [SBRT; (A, B)] and normo-fractionated radiotherapy (C-E) plans with geometrical dose accumulation (F). (A) Clinical target volume (CTV) in yellow and planning target volume (PTV) in red. (B) Dose at 2 cm from the PTV (D2cm) not exceeding 50% of the prescribed PTV dose. (C–E) Isodose distributions of the normo-fractionated radiotherapy plan (F) The accumulative dose of 60Gy and 50Gy was limited to the pretreated area. Light blue (40Gy) and blue (20Gy) correspond to regions treated with normo-fractionated radiotherapy.
During treatment, the patient developed Grade 2 pneumonitis (mild cough), according to common terminology criteria for adverse events version 5 (CTCAEv5), and steroid therapy was prescribed.
CR was obtained one month after treatment and it has been maintained until the last follow-up (4 months after treatment, Figure 1).
Throughout the treatment period, since 2021, no decline in respiratory function was observed (Table 1).
Discussion
This case report shows how the role of immune-modulant radiotherapy can be harnessed and integrated with IO to achieve prolonged locoregional control of metastatic NSCLC. Here the initial response to pembrolizumab was 12 months, in line with literature reports of PFS (7–9, 14–16).
The median PFS2 (defined as time from randomization to subsequent disease progression after initiation of new anticancer therapy or death from any cause) has been reported in the 5-year analyses of KEYNOTE-024 (15) and KEYNOTE-042 (14) trials as 24.1 months (CI95%: 15 to 31.4 months) and 15 months (CI95%: 11.6 to 19.2 months) respectively. In these trials palliative RT was consented with suspension of IO during RT and continuation afterward (17, 18), but it has not been specified how many patients received RT in this context. KEYNOTE-024 (15) reports that 6.5% of patients received RT as “subsequent therapy” (9.1% in the pembrolizumab group and 6% in the chemotherapy group) without specifying the intent of the treatment, radiation dose, continuation of IO or specific PFS2.
In our report, iSBRT treatment was delivered with a sub-ablative dose (24Gy in 3 fractions; 8Gy/fraction) (1), with the intention not being direct tumor control through RT, but rather to induce an “in situ vaccination” (2, 3) that could sustain the effectiveness of IO and achieve tumor control trough pembrolizumab. Recently Dan Duda and colleagues (19) described prospectively that using SBRT with doses <10Gy per fraction increased the proportion of proliferating CD8+ T-cells after the radiotherapy treatment in patients with oligo-metastatic and oligo-progressive pulmonary lesions. In this case, the using of this fractionation may have contributed to observe the abscopal effect that allowed the continuation of pembrolizumab as first-line treatment for up to 45 months (52 cycles).
Considering iSBRT a new anticancer therapy, the PFS2 could be reported as 39 months, longer than the median and confidence interval reported in KEYNOTE-024, and more than twice as long as observed in KEYNOTE-042.
The use of sub-ablative doses has certain advantages: the immunomodulatory effect, already highlighted and exemplified in this case report; the safety of the treatment, especially in patients with pulmonary frailty; and the possibility of re-treatment with an adequate safety profile.
The biological sum of both treatments in the re-irradiated lesion (station 11L) was EQD2: 76 Gy and biologically effective dose (BED): 91.2 Gy (12) with an adequate dose limitation to the organs at risk (OARs) (20), as showed in Table 2.
Furthermore, both treatments were delivered without changing respiratory function as presented in Table 1.
Finally, it is important to recognize the shift in trend in reported cases of distant immunological effects of RT in NSCLC before and after the use of IO as part of routine clinical practice. Before the introduction of IO, reported cases in NSCLC accounted for only 6% (13), and consequently did not represent a pathology of interest in this area. However, in the IO era, a 116% increase in reported cases has been reported (11, 13), second only to melanoma.
These findings in literature, along with the present case report, suggest that the iRT with sub-ablative doses in oligo-progressive settings may enhance the outcomes already achieved in metastatic NSCLC patients receiving IO.
Conclusions
We report the induction of a complete response with iRT treatment in combination with pembrolizumab in a patient with metastatic NSCLC. Given its potential impact on survival and prolonged benefit from IO, this strategy warrants further investigation and validation in larger cohorts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Methodology, Writing – original draft, Data curation, Conceptualization, Writing – review & editing. DS: Writing – review & editing, Project administration, Visualization, Investigation, Data curation, Methodology, Writing – original draft, Conceptualization. GS: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. GG: Validation, Writing – review & editing, Supervision. SB: Writing – review & editing, Validation. EB: Writing – review & editing, Validation. AL: Supervision, Writing – review & editing, Validation. SL: Validation, Writing – review & editing. SC: Validation, Writing – review & editing. LS: Writing – review & editing, Validation. GB: Validation, Writing – review & editing. CB: Writing – review & editing, Validation. GD: Writing – review & editing, Validation. LM: Software, Writing – review & editing, Conceptualization. PP: Supervision, Writing – review & editing, Validation. FA: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Demaria S, Guha C, Schoenfeld J, Morris Z, Monjazeb A, Sikora A, et al. Radiation dose and fraction in immunotherapy: one-size regimen does not fit all settings, so how does one choose? J Immunother Cancer. (2021) 9:e002038. doi: 10.1136/jitc-2020-002038
2. Daguenet E, Louati S, Wozny AS, Vial N, Gras M, Guy JB, et al. Radiation-induced bystander and abscopal effects: important lessons from preclinical models. Br J Cancer. (2020) 123:339–48. doi: 10.1038/s41416-020-0942-3
3. Farias V de A, Tovar I, del Moral R, O’Valle F, Expósito J, Oliver FJ, et al. Enhancing the bystander and abscopal effects to improve radiotherapy outcomes. Front Oncol. (2020) 9. doi: 10.3389/fonc.2019.01381
4. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:358–76. doi: 10.1016/j.annonc.2022.12.013
5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
6. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. New Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
7. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
8. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
9. Amrane K, Geier M, Corre R, Léna H, Léveiller G, Gadby F, et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med. (2020) 9:2309–16. doi: 10.1002/cam4.2806
10. Leonetti A, Perrone F, Puntoni M, Maglietta G, Bordi P, Bria E, et al. Real-world outcomes of Italian patients with advanced non-squamous lung cancer treated with first-line pembrolizumab plus platinum-pemetrexed. Eur J Cancer. (2024) 202:114006. doi: 10.1016/j.ejca.2024.114006
11. Barillaro A, Caroprese M, Feoli C, Chioccola E, Goodyear CA, Oliviero C, et al. Non-targeted effects ofstereotactic radiotherapy: a review of the evidence coming from the clinical field. Explor Target Antitumor Ther. (2025) 6:1002290. doi: 10.37349/etat
12. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. (1989) 62:679–94. doi: 10.1259/0007-1285-62-740-679
13. Abuodeh Y, Venkat P, and Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. (2016) 40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001
14. de Castro G, Kudaba I, Wu YL, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non–small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol. (2023) 41:1986–91. doi: 10.1200/JCO.21.02885
15. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
16. Tamayo-Bermejo R, del Rio-Valencia JC, Mora-Rodriguez B, and Muñoz-Castillo I. Effectiveness and safety of pembrolizumab monotherapy in patients with locally advanced or metastatic non-small-cell lung cancer. J Oncol Pharm Pract. (2023) 29:138–44. doi: 10.1177/10781552211061117
17. Study of pembrolizumab (MK-3475) versus platinum-based chemotherapy for participants with programmed cell death-ligand 1 (PD-L1)-positive advanced or metastatic non-small cell lung cancer (MK-3475-042/KEYNOTE-042). Available online at: https://clinicaltrials.gov/study/NCT02220894 (Accessed March 30, 2025).
18. Study of Pembrolizumab (MK-3475) Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non-Small Cell Lung Cancer (MK-3475-024/KEYNOTE-024). [cited 2025 Jul 23]; Available online at: https://cdn.clinicaltrials.gov/large-docs/38/NCT02142738/Prot_SAP_000.pdf.
19. Gkika E, Firat E, Adebahr S, Graf E, Eichhorst A, et al. A prospective study of immune responses in patients with lung metastases treated with stereotactic body radiotherapy with or without concurrent systemic treatment. Radiother Oncol. (2025) 207:110889. doi: 10.1016/j.radonc.2025.110889
Keywords: NSCLC, immunotherapy, radiotherapy, non-ablative SBRT, SBRT
Citation: Saddi J, Santos Hernandez DA, Stella GM, Galli G, Borgetto S, Bonzano E, Lancia A, La Mattina S, Colombo S, Squillace L, Baietto G, Bortolotto C, D’Ambrosio G, Mantovani L, Pedrazzoli P and Agustoni F (2025) A non-small cell lung cancer fragile elderly patient treated with immunotherapy and non-ablative radiation therapy: a case report of a winning combination. Front. Oncol. 15:1642564. doi: 10.3389/fonc.2025.1642564
Received: 06 June 2025; Accepted: 16 July 2025;
Published: 15 August 2025.
Edited by:
Reza Farjam, Johns Hopkins University, United StatesReviewed by:
Francesca Di Pressa, ASST Lecco, ItalyDavid Miller, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2025 Saddi, Santos Hernandez, Stella, Galli, Borgetto, Bonzano, Lancia, La Mattina, Colombo, Squillace, Baietto, Bortolotto, D’Ambrosio, Mantovani, Pedrazzoli and Agustoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Agustoni, Zi5hZ3VzdG9uaUBzbWF0dGVvLnB2Lml0
 Jessica Saddi
Jessica Saddi David Alberto Santos Hernandez
David Alberto Santos Hernandez Giulia Maria Stella
Giulia Maria Stella Giulia Galli
Giulia Galli Sabrina Borgetto7
Sabrina Borgetto7 Elisabetta Bonzano
Elisabetta Bonzano Andrea Lancia
Andrea Lancia Salvatore La Mattina
Salvatore La Mattina Paolo Pedrazzoli
Paolo Pedrazzoli Francesco Agustoni
Francesco Agustoni