- 1Department of Oncology, The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, Guangdong, China
- 2Department of Radiology, The Sixth Affiliated Hospital, School of Medicine, South China University of Technology, Foshan, Guangdong, China
Purpose: To evaluate the efficacy and safety of radiotherapy combined with immunotherapy and targeted therapy (RT+IO+T) versus immunotherapy plus targeted therapy alone (IO+T) in patients with unresectable hepatocellular carcinoma (HCC). Given the limited prospective evidence supporting the integration of radiotherapy into systemic regimens, particularly in real-world populations with advanced disease, this study aims to clarify the clinical value of this multimodal approach.
Methods: This retrospective study analyzed 71 patients with unresectable HCC treated between 2020 and 2025. Patients received either IO+T (n=42) or RT+IO+T (n=29), including immune checkpoint inhibitors (ICIs) (e.g., camrelizumab), targeted agents (e.g., lenvatinib), and RT. Outcomes were assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria. Survival analysis was performed using Kaplan–Meier and Cox regression models.
Results: Compared with the IO+T group, the RT+IO+T group demonstrated superior short-term efficacy, as indicated by the objective response (69.0% vs. 35.7%, p=0.006) and disease control (89.7% vs. 57.1%, p=0.003) rates. Additionally, the median progression-free survival (PFS) and overall survival (OS) were significantly prolonged in the RT+IO+T group compared with the IO+T group (PFS: 12.6 vs. 4.6 months, p<0.001; OS: 17.8 vs. 10.9 months, p=0.009). Subgroup analyses confirmed consistent survival benefits across patient characteristics. However, the RT+IO+T group showed increased hematologic toxicity (grade ≥3 lymphopenia: 62.1% vs. 19.0%, p<0.001) and hepatic enzyme elevation (aspartate aminotransferase: 75.9% vs. 35.7%, p<0.001).
Conclusion: Adding RT to IO+T significantly improved tumor response and survival in unresectable HCC, despite higher manageable hematologic and hepatic toxicities.
Clinical significance: The results of this study support RT+IO+T as a promising strategy for advanced HCC, particularly in patients with high tumor burden or portal vein invasion. The synergistic effect of RT, immunotherapy, and target therapy highlights its potential to redefine treatment paradigms, although toxicity monitoring remains critical.
1 Introduction
Liver cancer, predominantly hepatocellular carcinoma (HCC), is the third most common cause of cancer-related death worldwide (1). In China, HCC accounts for approximately 75–85% of primary liver cancers, making it the fourth most frequently diagnosed malignancy and the second leading cause of cancer-related deaths (2). Most advanced cases are unsuitable for surgical resection and rely on non-surgical local or systemic therapies. Despite advances in early detection and treatment, a significant proportion of patients present with advanced-stage disease at diagnosis, where curative options are limited. Systemic therapies, particularly combinations of immune checkpoint inhibitors (ICIs) and targeted agents, have become the cornerstone of treatment for advanced HCC (3).
Improvements in techniques such as intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy have increased the use of RT for treating tumors in HCC (4). In patients with HCC complicated by venous tumor thrombus, who traditionally have a poor prognosis, with a median untreated survival of only 3–4 months, RT significantly improved both survival and quality of life (5–7).
Combining RT with immunotherapy and/or targeted therapy can have synergistic effects in treating unresectable or metastatic HCC (4, 8–10). RT enhances the efficacy of immunotherapy by triggering local inflammation and releasing tumor antigens, which boost immune responses. This combination can also induce an “abscopal effect,” in which tumor shrinkage is observed even in non-irradiated areas (11). Additionally, targeted therapy improves tumor vascular normalization, enhancing radiation sensitivity while promoting RT-induced vascular damage and apoptosis through the ceramide pathway (10, 12, 13).
However, the optimal combination of these three modalities in clinical practice remains unclear. Therefore, the present study evaluated the efficacy and safety of RT combined with immunotherapy and target therapy in patients with unresectable HCC to explore the optimal strategy for this triple therapy approach.
2 Materials and methods
2.1 Patients
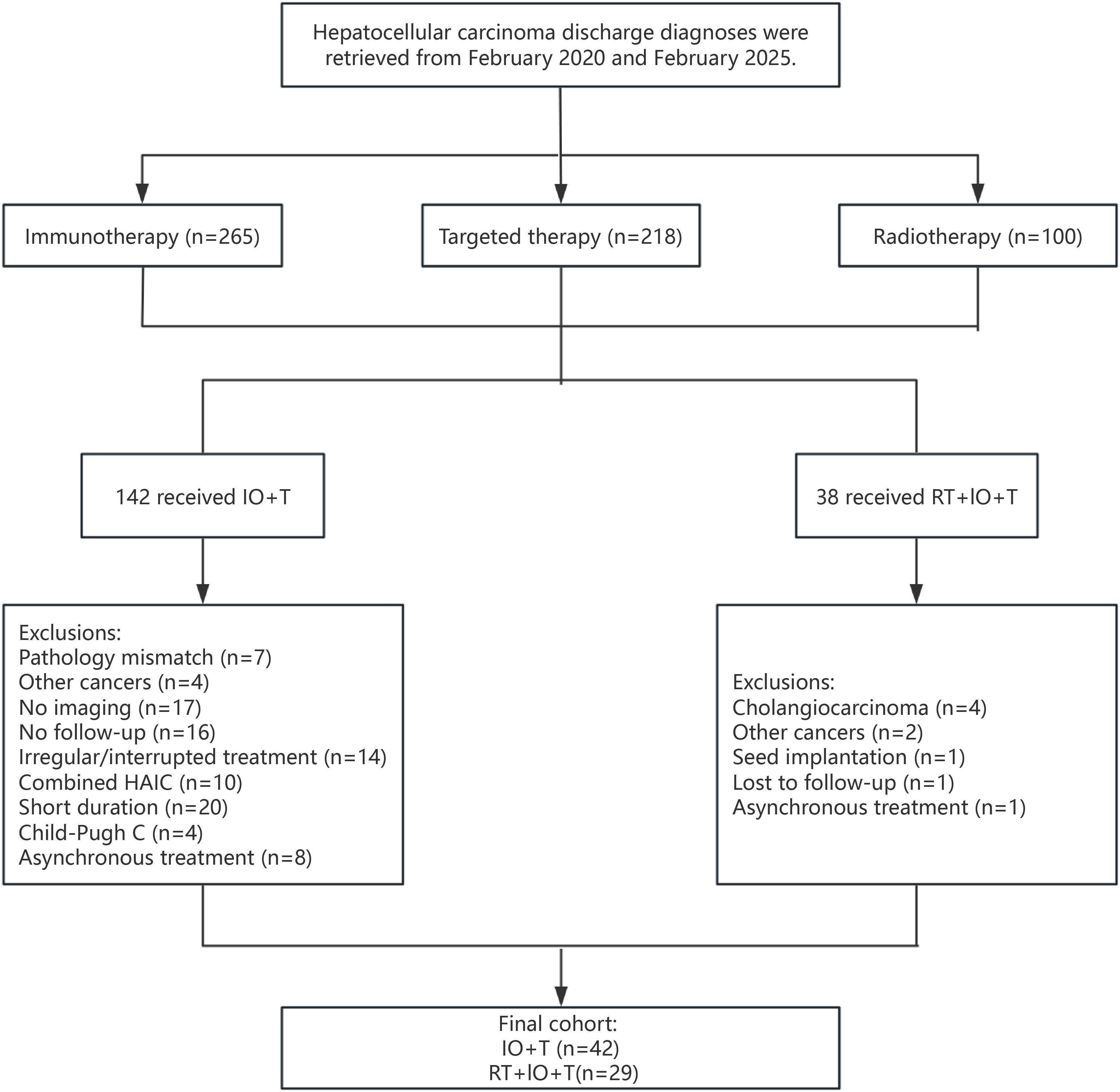
This retrospective study reviewed patients with unresectable HCC treated between February 2020 and February 2025 at the Cancer Center of the Sixth Affiliated Hospital of South China University of Technology. Patients received either ICIs with targeted therapy (IO+T) or a triple-modality regimen of RT, ICIs, and targeted therapy (RT+IO+T). All patients were diagnosed with HCC according to clinical or histopathological criteria (3). The inclusion criteria included age >18 years, Child–Pugh class A or B liver function, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, unresectable HCC determined by surgical assessment or patient refusal of surgery, and at least one measurable intrahepatic lesion according to the modified Response Evaluation Criteria in Solid Tumors mRECIST. The exclusion criteria were other malignancies; other concurrent locoregional therapies, such as transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), radiofrequency ablation (RFA), or surgical resection, during the study period; incomplete medical records, Child–Pugh class C liver function, or lack of follow-up data (Figure 1).
This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of South China University of Technology (Approval No. 2022242) and registered in the Medical Research Information Registration System of the National Health Insurance Information platform (https://www.medicalresearch.org.cn/) (Registration Number: MR-44-23-028102). Given the retrospective nature of this study, the requirement for informed consent was waived in accordance with institutional guidelines. All procedures adhered to the ethical principles of the Declaration of Helsinki (as revised in 2013), ensuring the protection of patient privacy and the confidentiality of medical data.
2.2 Treatments
The study participants were administered either IO+T or a RT+IO+T triple-modality regimen. The ICIs included camrelizumab (200 mg), tislelizumab (200 mg), sintilimab (200 mg), or atezolizumab (1200 mg), administered intravenously every three weeks. The targeted agents included lenvatinib, anlotinib, donafenib, bevacizumab, regorafenib, or apatinib, selected at the treating physician’s discretion based on disease characteristics and patient tolerance.
In the RT+IO+T group, RT was delivered using a 6-MV linear accelerator (Varian) via stereotactic body RT (SBRT; 36–60 Gy in 3–10 fractions) or IMRT (IMRT; 50–60 Gy in 20–30 fractions), depending on the tumor location, size, and hepatic reserve. Gross tumor volume (GTV) was defined using contrast-enhanced computed tomography (CT) and, when available, magnetic resonance imaging (MRI). A 5-mm margin was added to the GTV to generate the planning target volume (PTV), accounting for setup variability and respiratory motion. RT was generally initiated 2–3 weeks after the start of immunotherapy and targeted therapy.
The biologically effective dose (BED) was calculated using the linear-quadratic model according to the following equation: BED = nd × [1 + d/(α/β)], where n is the number of fractions, d is the dose per fraction (Gy), and an α/β ratio of 10 Gy was used for tumor tissues (14).
For patients with Barcelona Clinic Liver Cancer (BCLC) stage C disease, the RT primarily targeted intrahepatic tumors. In patients with extrahepatic metastases, radiation was selectively applied to symptomatic or clinically significant lesions, guided by tumor burden, anatomical accessibility, and palliative intent. For example, retroperitoneal lymph node metastases were treated concurrently in select patients, while lesions in the lung or spine were typically managed with sequential RT. These decisions were made to enhance local control, alleviate symptoms, and mitigate the risk of disease progression.
The dose constraints for organs-at-risk (OARs) adhered to standard recommendations (Quantitative Analyses of Normal Tissue Effects in the Clinic/Radiation Therapy Oncology Group [QUANTEC/RTOG[), including a mean liver dose <28 Gy, liver V30 <60%, gastrointestinal Dmax <54 Gy, spinal cord Dmax <45 Gy, and kidney mean dose <18 Gy or V20 <30%, depending on proximity and function. RT plans were individualized to balance optimal tumor coverage with toxicity mitigation, especially in patients with cirrhosis. All patients undergoing RT were monitored closely for treatment-related adverse events, and dose adjustments were made when necessary to preserve safety.
2.3 Follow-up
Treatment safety was continuously monitored, and tumor response was evaluated using the mRECIST every two treatment cycles or earlier if clinically warranted (15). The objective response rate (ORR) was defined as the proportion of patients achieving either a complete response (CR) or partial response (PR), while the disease control rate (DCR) encompassed CR, PR, and stable disease (SD). OS was calculated from the initiation of immunotherapy combined with target agents to the date of death or the latest available follow-up. Progression-free survival (PFS) was defined as the time from treatment onset to radiologically confirmed progression or last documented follow-up. The most recent follow-up date was determined by the latest patient contact, including radiologic exams, outpatient notes, telephone communication, or death data sourced from the Guangdong Provincial Medical Death Registry System. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0, with grade 3 or 4 adverse events classified as severe.
2.4 Statistical analysis
All statistical analyses were conducted using R software and IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables are summarized as medians with interquartile ranges, whereas categorical variables are reported as counts and percentages. Between-group comparisons were performed using the Mann–Whitney U test and the chi-square test for continuous and categorical data, respectively. Survival outcomes, including OS and PFS, were estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Associations between clinical variables and survival outcomes were further explored through univariate and multivariate analyses using Cox proportional hazard ratio (HR) models. Subgroup analyses were also carried out where appropriate. Two-sided p<0.05 was considered statistically significant.
3 Results
3.1 Patient baseline characteristics
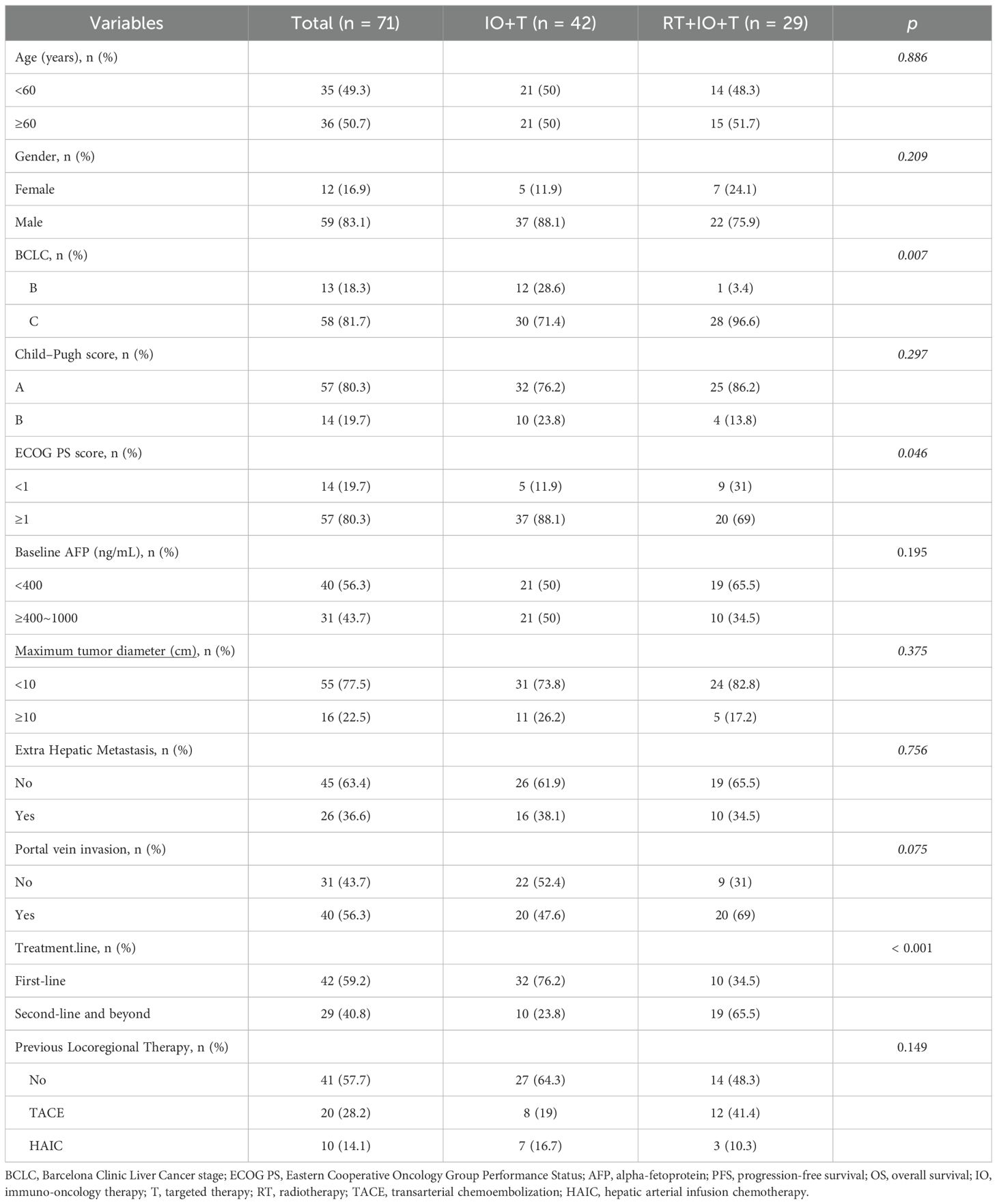
The analysis included a total of 71 patients with unresectable HCC, with the IO+T and RT+IO+T groups comprising 42 and 29 patients, respectively (Table 1). The baseline characteristics were generally comparable between the two groups, with no significant differences in age, sex, Child–Pugh score, alpha-fetoprotein (AFP) levels, maximum tumor diameter, or presence of extrahepatic metastasis. However, the RT+IO+T group had a significantly higher proportion of patients with advanced disease (BCLC stage C: 96.6% vs. 71.4%, p=0.007) and a trend toward more patients showing portal vein invasion (69.0% vs. 47.6%, p=0.075). Although the RT+l0+T had a higher proportion of patients with BCLC stage C disease (96.6% vs.71.4%), they paradoxically exhibited better performance status (ECOG 0: 31.0% vs.11.9%, p=0.046) and were more often treated in the recurrent setting (i.e., second-line or later systemic therapy: 65.5% vs. 23.8%, p<0.001). This apparent discrepancy may be attributed to disease heterogeneity within BCLC stage C. Specifically, patients with macrovascular invasion (e.g., portal vein tumor thrombus [PVTT]) may retain preserved liver function and performance status compared with patients with diffuse intrahepatic tumors or extrahepatic spread, who often present with more severe clinical symptoms.
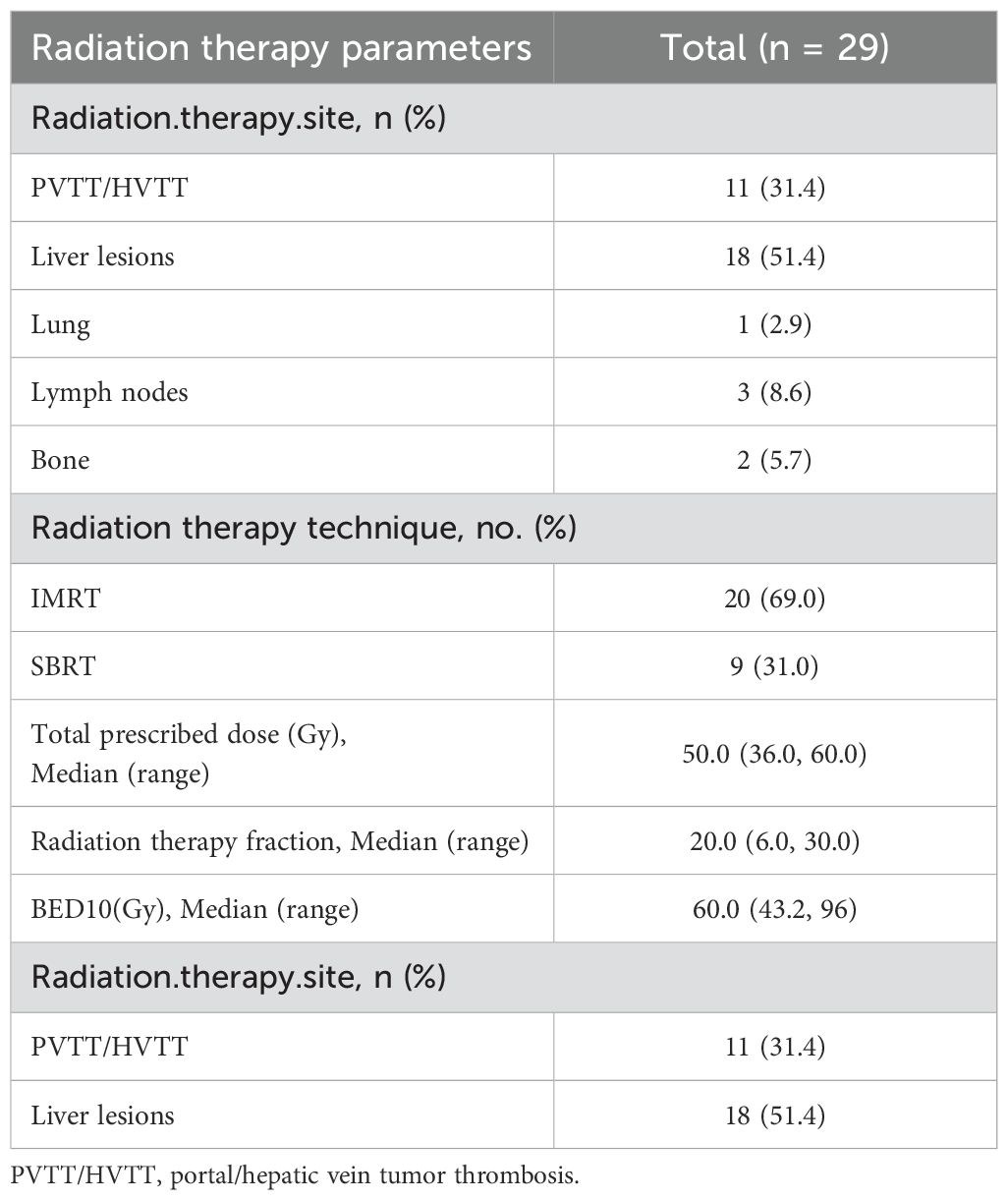
Collectively, while most baseline characteristics were well balanced, the RT+IO+T cohort presented with both more aggressive disease features and better functional status, which could influence treatment selection and clinical outcomes. RT was administered based on anatomical site and clinical indications. The detailed parameters of RT administered to the combination therapy cohort are presented in Table 2. Among 29 patients receiving RT, intrahepatic lesions (51.4%) and PVTT/hepatic vein tumor thrombus (HVTT) (31.4%) were the most common targets. Of the seven patients with extrahepatic metastases, six received RT targeting metastatic sites. Retroperitoneal lymph nodes (8.6%) and lung (2.9%) were treated concurrently, while spinal bone lesions (5.7%) received sequential RT. IMRT and SBRT were employed in 69.0% and 31.0% of patients, respectively. For hepatic lesions, the median total dose was 50.0 Gy (range, 36.0–60.0 Gy) over a median of 20 fractions (range, 6–30). The median BED10 was 60.0 Gy (range, 43.2–96.0 Gy). Data on RT parameters were limited to liver-directed treatments and excluded extracranial metastatic sites.
3.2 Short-term efficacy
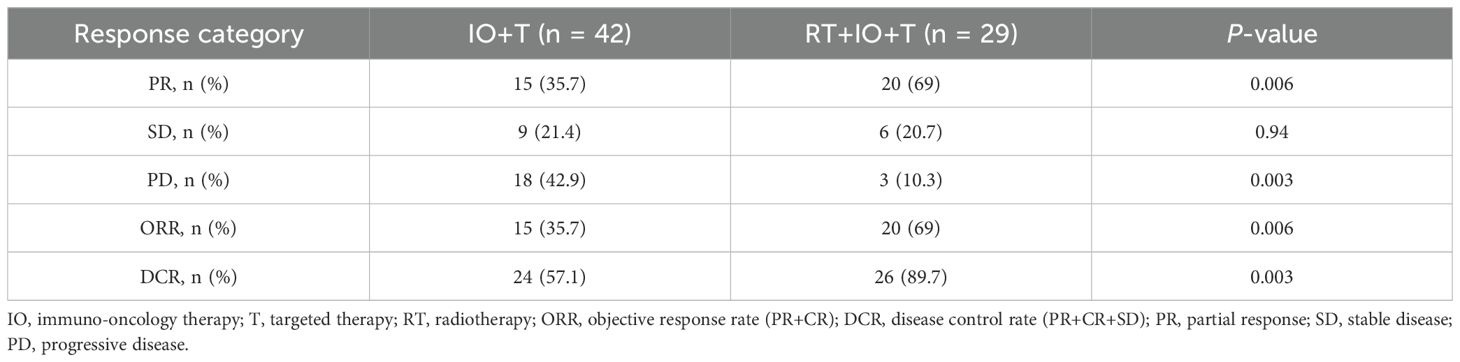
The combination therapy cohort (RT+IO+T) demonstrated significantly enhanced short-term efficacy compared with the IO+T group at 3 months (Table 3). The ORR was double in the RT+IO+T arm compared with the IO+T arm (69.0% vs. 35.7%, p=0.006), with PR constituting most of the achieved responses (69.0% vs. 35.7%), no CR were observed. The DCR was also markedly higher in the combination group (89.7% vs. 57.1%, p=0.003), paralleled by a substantial reduction in disease progression (10.3% vs. 42.9%, p=0.003). Finally, the SD rates remained comparable between cohorts (20.7% vs. 21.4%, p=0.940). These findings indicate synergistic antitumor activity when RT is integrated into therapeutic regimens.
3.3 Safety
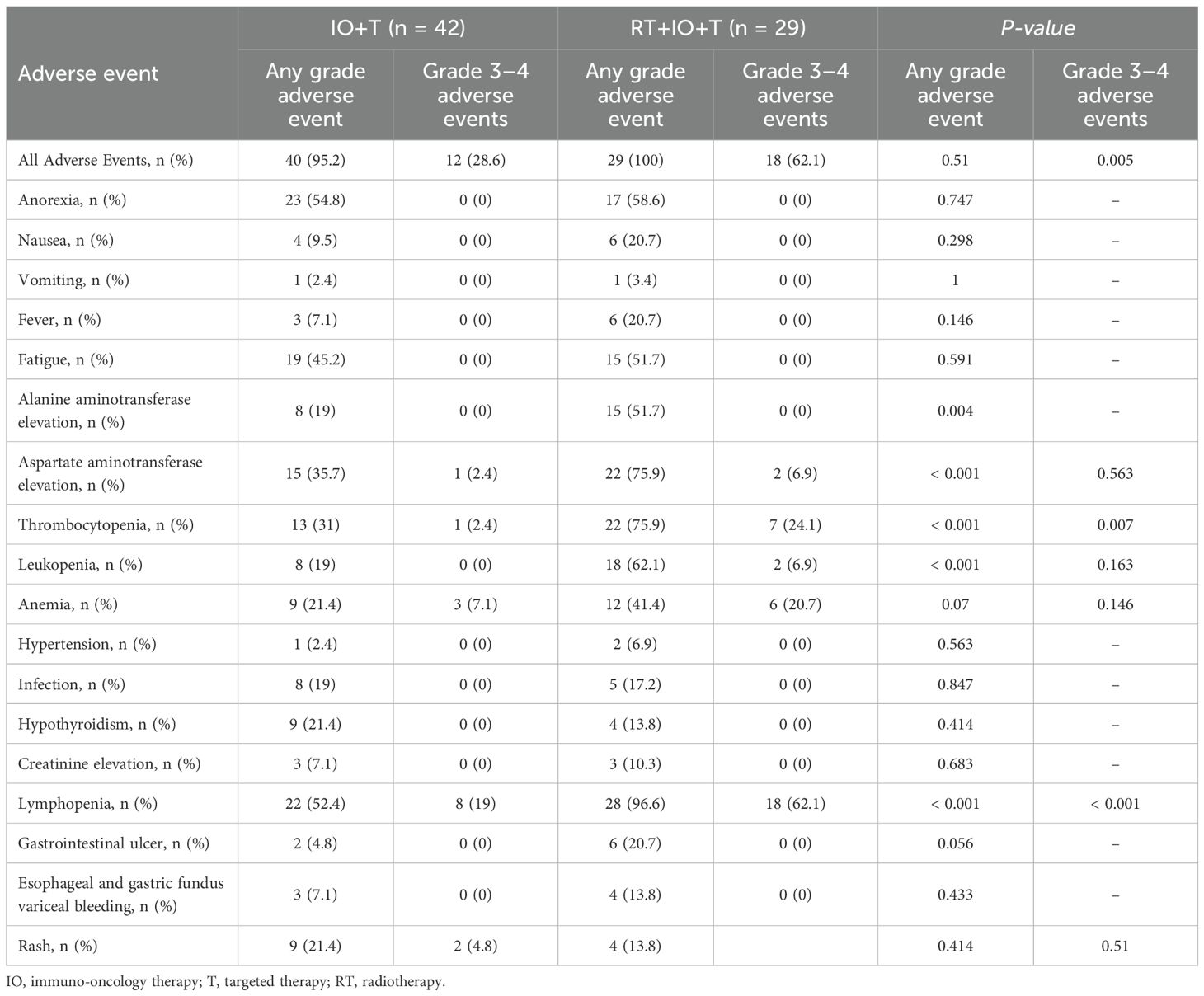
Treatment-related adverse events occurred commonly in both groups (Table 4), with comparable frequencies of low-grade symptoms including anorexia (58.6% vs. 54.8%, p=0.747), fatigue (51.7% vs. 45.2%, p=0.591), and mild gastrointestinal events including nausea, vomiting, and fever (all p>0.2). These symptoms were generally well tolerated and manageable. However, patients in the RT+IO+T group experienced significantly higher rates of hematologic and hepatic toxicities, including lymphopenia (96.6% vs. 52.4%, p<0.001), thrombocytopenia (75.9% vs. 31.0%, p<0.001), and leukopenia (62.1% vs. 19.0%, p<0.001), among which grade 3–4 lymphopenia (62.1% vs. 19.0%, p<0.001) and thrombocytopenia (24.1% vs. 2.4%, p=0.007) represented key dose-limiting toxicities. Additionally, hepatic enzyme elevations were more common in the RT+IO+T group, with AST increased in 75.9% of patients compared with 35.7% of patients in the IO+T group (p<0.001) and ALT in 51.7% vs. 19.0% of patients (p=0.004). Although gastrointestinal ulceration was observed more frequently in the RT+IO+T group (20.7% vs. 4.8%, p=0.056), this difference was not statistically significant. Despite the higher incidence of select grade ≥3 adverse events, all toxicities were manageable with standard supportive care, with no treatment-related mortality. These findings suggest that while the RT+IO+T regimen is associated with an increased hematologic and hepatic toxicity profile, it remains clinically tolerable and feasible in patients with advanced HCC.
3.4 Cox regression analysis
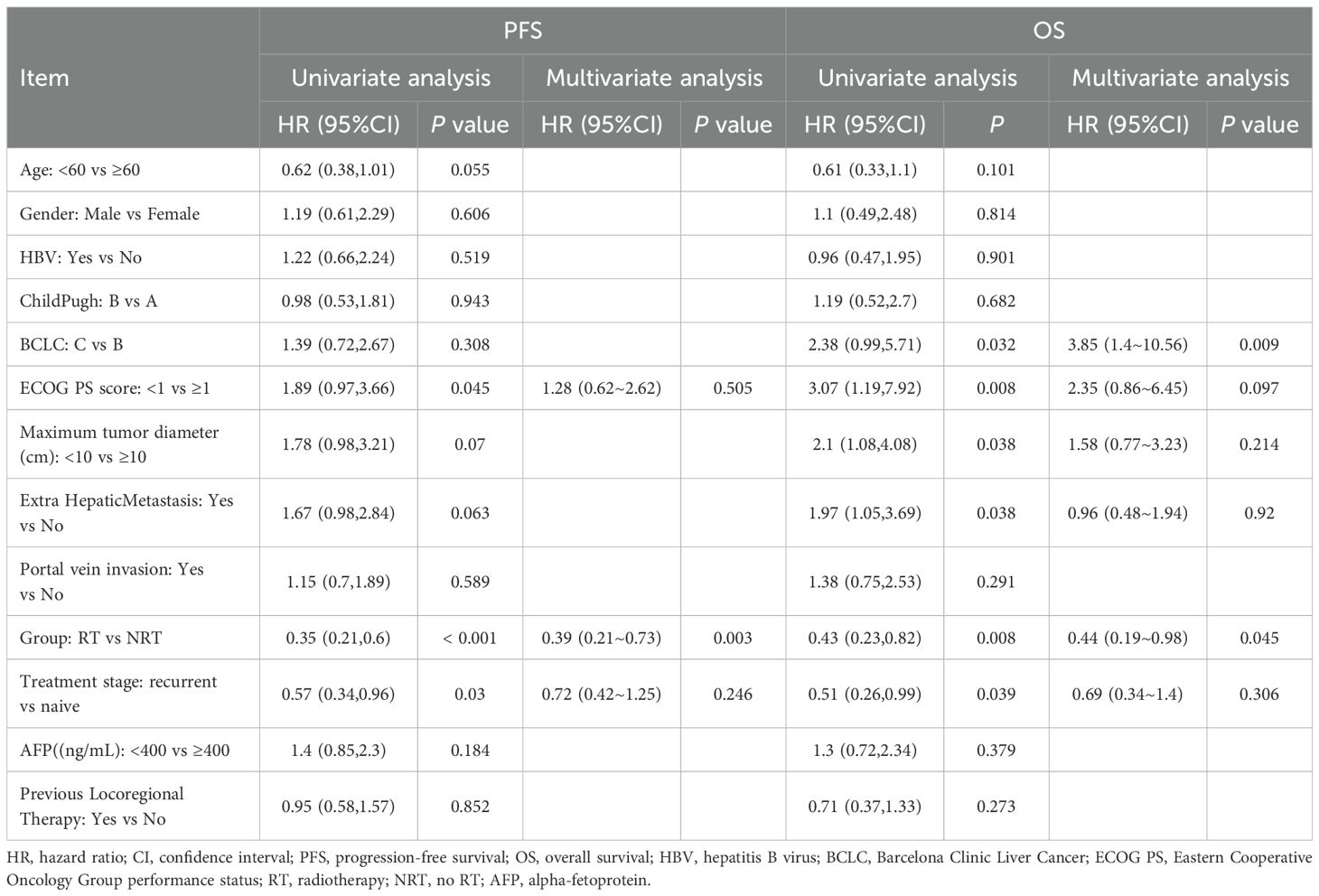
Multivariate Cox regression analysis revealed that the RT+IO+T regimen was independently associated with improved survival outcomes, reinforcing the clinical benefit of adding RT to IO+T. Specifically, RT+IO+T significantly prolonged PFS (HR=0.39, 95% confidence interval [CI]: 0.21–0.73, p=0.003) and OS (HR=0.44, 95% CI: 0.19–0.98, p=0.045). Among clinical variables, BCLC stage C was an independent risk factor for worse OS (HR=3.85, 95% CI: 1.40–10.56, p=0.009) (Table 5). While ECOG performance status, tumor diameter, and extrahepatic metastasis were associated with poor prognosis in univariate analysis, these associations did not remain significant after multivariate adjustment. Other factors, including age, sex, hepatitis B virus (HBV) infection status, and prior locoregional therapy, did not significantly correlate with PFS or OS. Collectively, these results highlighted the added therapeutic value of RT in the systemic management of advanced HCC.
3.5 Subgroup analysis
Subgroup analyses for PFS (Figure 2) and OS (Figure 3) consistently demonstrated the survival benefit of the RT+IO+T regimen across various patient subgroups.
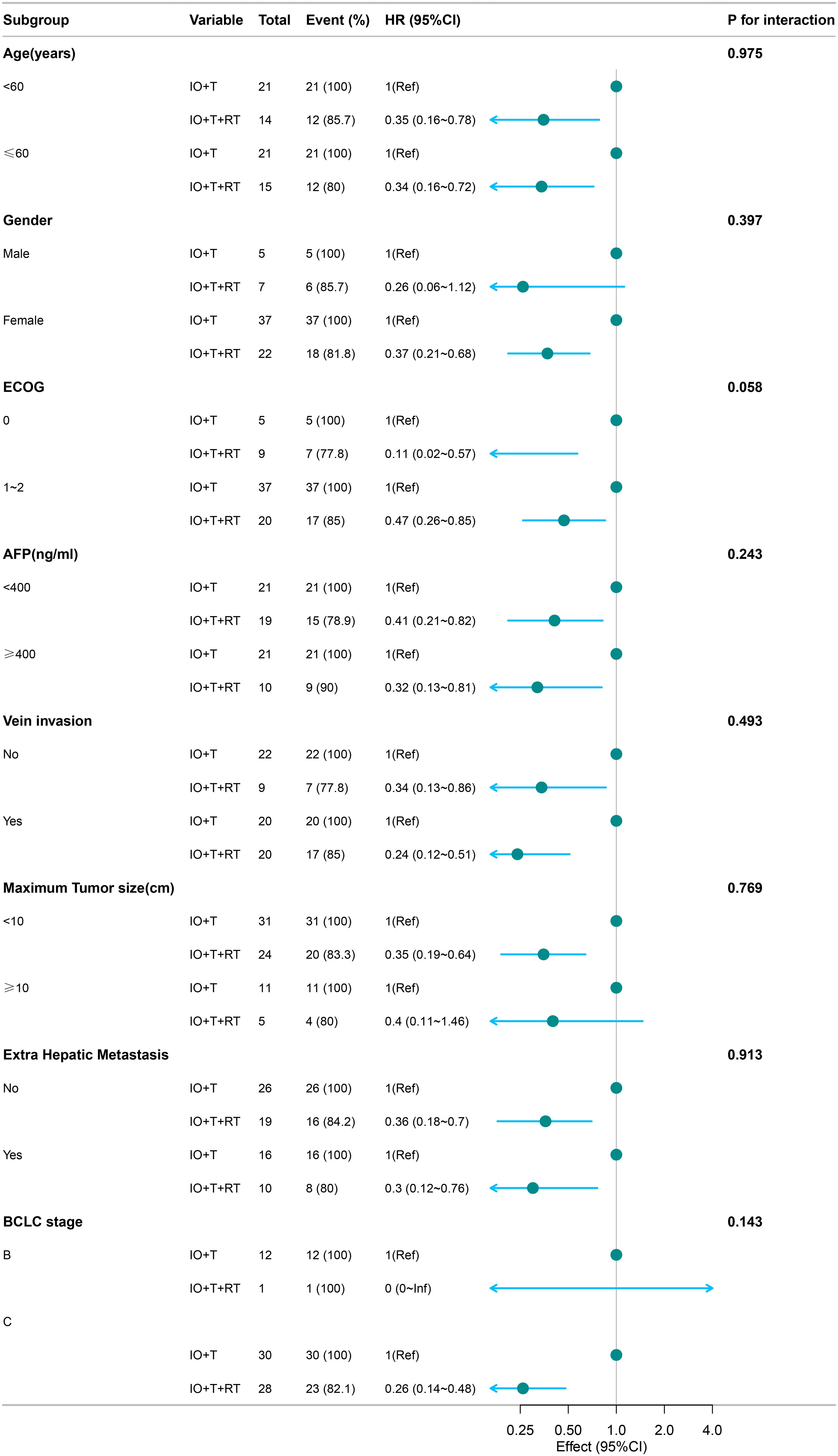
Figure 2. Subgroup analysis of PFS in patients receiving RT+IO+T versus IO+T. The RT+IO+T regimen showed consistently improved PFS across all strata, including both age groups (<60 and ≥60 years), AFP levels, tumor size, portal vein invasion status, and presence of extrahepatic metastasis. No statistically significant interaction was observed across subgroups (all p-interaction >0.05). PFS, progression-free survival; RT, radiotherapy; IO, immunotherapy; T, targeted therapy; AFP, alpha-fetoprotein.
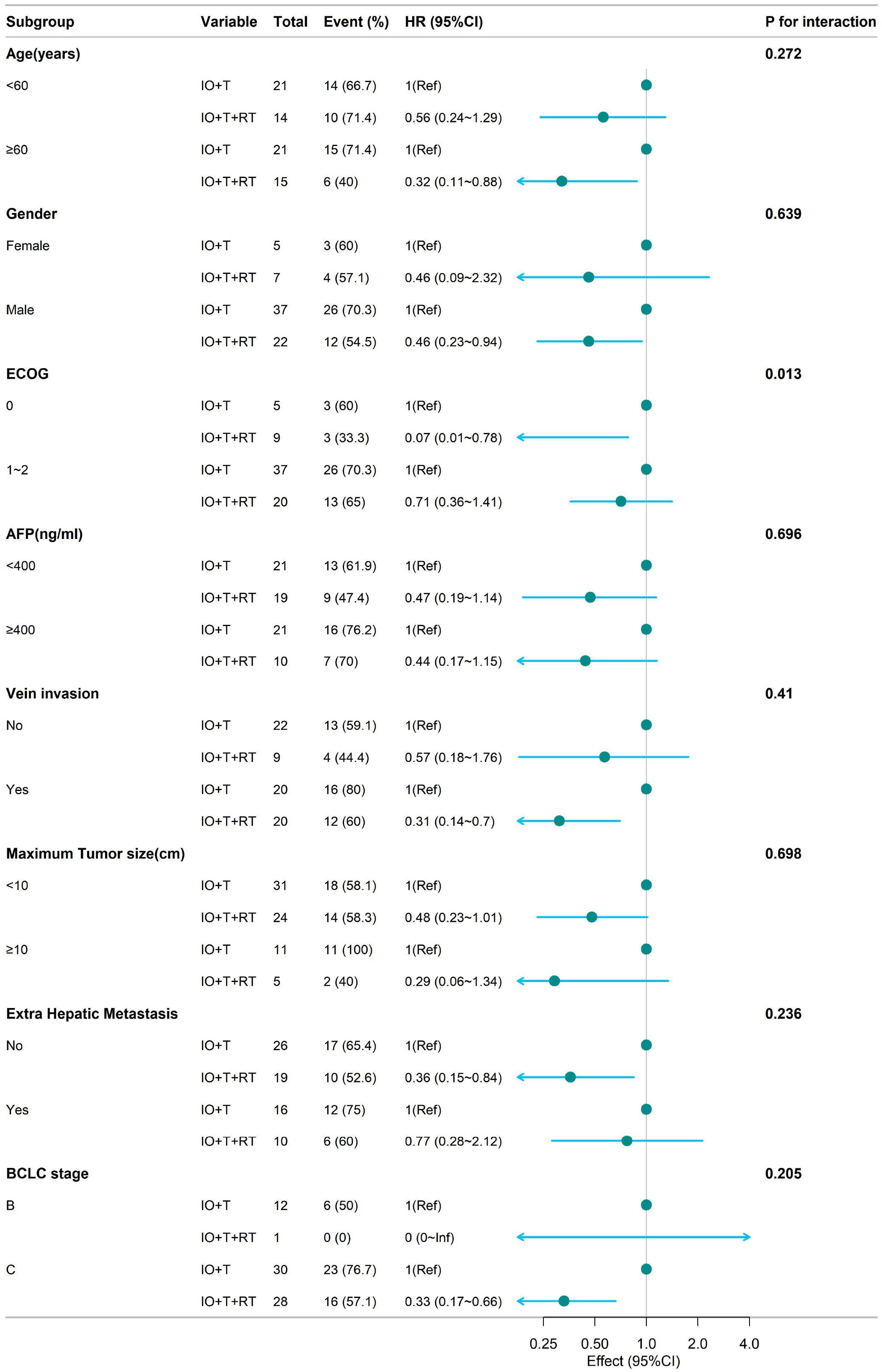
Figure 3. Subgroup analysis of OS in patients receiving RT+IO+T versus IO+T. The RT+IO+T regimen demonstrated a consistent survival benefit across age groups (<60 years: HR = 0.56; ≥60 years: HR = 0.32), sex, AFP levels, tumor size, portal vein invasion, and presence of extrahepatic metastasis. OS, overall survival; RT, radiotherapy; IO, immunotherapy; T, targeted therapy; HR, hazard ratio; AFP, alpha-fetoprotein.
RT+IO+T significantly improved PFS in both age groups (<60 years: HR=0.35, 95% CI: 0.16–0.78; ≥60 years: HR=0.34, 95% CI: 0.16–0.72) without significant interaction by age (p=0.975). Pronounced benefits were also observed in patients with ECOG 0 (HR=0.11, 95% CI: 0.02–0.57) and ECOG 1–2 (HR=0.47, 95% CI: 0.26–0.85), with an interaction trend (p=0.058). The advantage remained consistent regardless of AFP level, portal vein invasion, tumor diameter, or extrahepatic metastasis (Figure 2).
Analysis of OS (Figure 3) demonstrated a survival benefit of RT+IO+T regardless of age (<60 years: HR=0.56, 95% CI: 0.24–1.29; ≥60 years: HR=0.32, 95% CI: 0.11–0.88), with no significant interaction (p=0.272). Marked benefits were observed in patients with ECOG 0 (HR=0.07, 95% CI: 0.01–0.78), as well as those with ECOG 1–2 (HR=0.71, 95% CI: 0.36–1.41), with a significant interaction (p=0.013). RT+IO+T improved OS in patients with or without portal vein invasion, those with tumors <10 cm, and those with or without extrahepatic metastasis.
The subgroup of patients with BCLC stage C disease who were administered RT+IO+T showed significantly improved PFS (HR=0.33, 95% CI: 0.17–0.66) compared with the IO+T group. Similarly, the RT+IO+T group also showed improved OS in patients with BCLC stage C disease (HR=0.33, 95% CI: 0.17–0.66). In contrast, among patients with BCLC stage B disease, only one patient received RT+IO+T, limiting statistical interpretation. This lack of events in the RT+IO+T subgroup resulted in an HR value of 0 (0–Inf) for OS and precluded reliable comparative analysis. These results highlight the consistent survival benefit of RT+IO+T, especially in patients with better performance status.
3.6 Survival analysis
The survival data was censored on April 30, 2025. Kaplan–Meier survival analysis demonstrated a significant survival benefit for both PFS and OS in the RT+IO+T group compared with the IO+T group (Figure 4). Specifically, the median PFS in the RT+IO+T group (12.6 months, range: 5.4–17.3 months), significantly longer than that in the IO+T group (4.6 months, range: 2.8–8.7 months) in the IO+T group (p<0.001). The 6-and 12-month PFS values were better in the RT+IO+T group (72.4% vs. 40.5%, 51.7% vs. 19%, respectively, p<0.001).
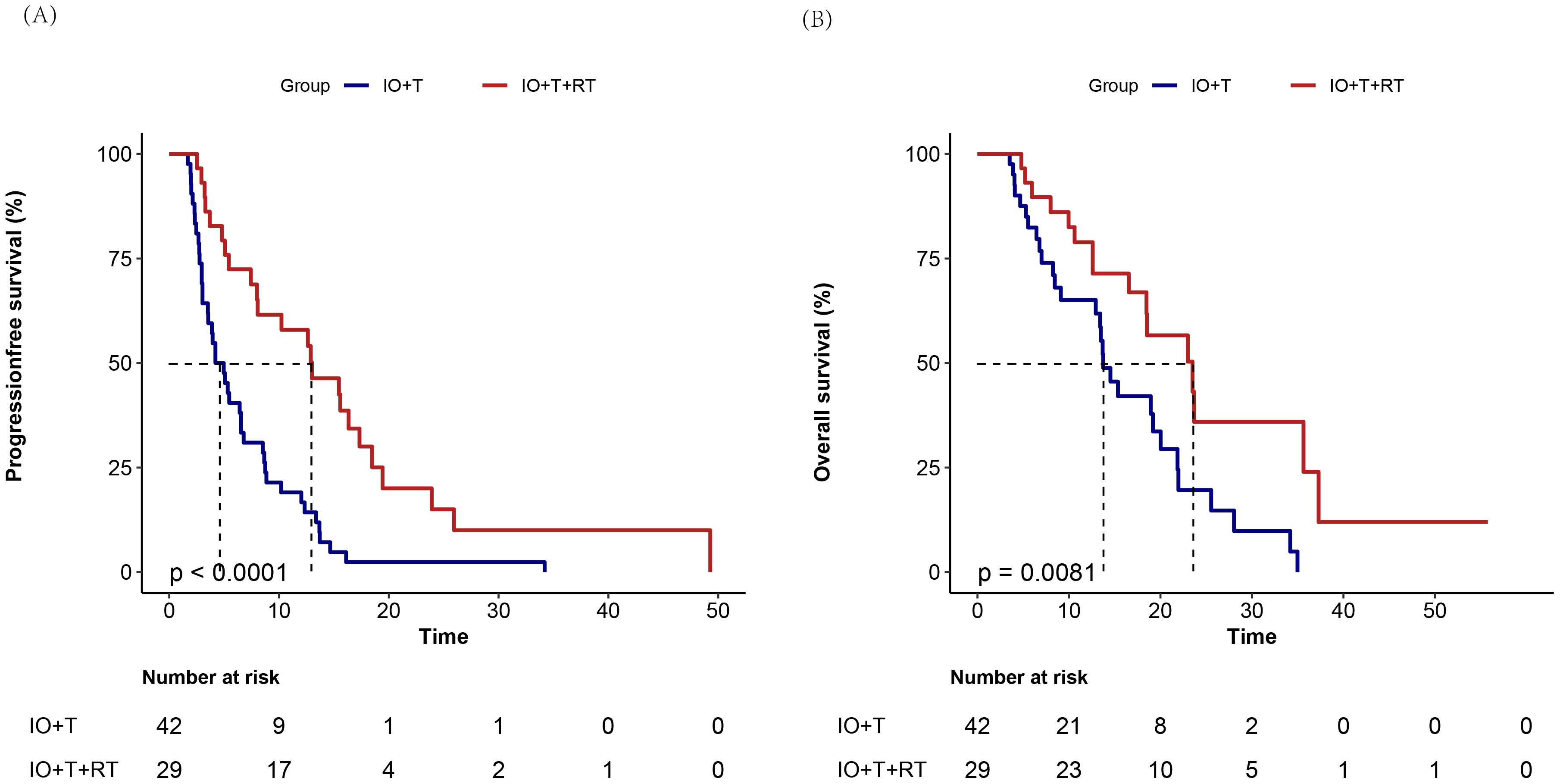
Figure 4. Kaplan–Meier survival curves comparing treatment outcomes between RT+IO+T and IO+T in hepatocellular carcinoma. Compared with the IO+T group, the RT+IO+T group shows superior (A) significantly improved progression-free survival (median 12.6 months [95% CI: 5.4–17.3] vs. 4.6 months [95% CI: 2.8–8.7]; p<0.001) and (B) OS (median 17.8 months [95% CI: 11.9-23.1] vs. 10.9 months [95% CI: 6.1–17.4]; p=0.009). RT, radiotherapy; IO, immunotherapy; T, targeted therapy; HCC, hepatocellular carcinoma; PFS, progression-free survival; CI, confidence interval; OS, overall survival.
Similarly, the median OS in the RT+IO+T group was 17.8 months (range: 11.9–23.1 months), compared with 10.9 months (range: 6.1–17.4 months) in the IO+T group (p=0.009). The 6-, 12-, and 24-month OS values were also better in the RT+IO+T group (89.7% vs. 76.2%, 72.48% vs. 50%, and 17.2% vs. 9.5%, respectively, all p>0.05). Notably, the median OS in the RT+RO+T group was 17.8 months, with nine patients still under follow-up. These findings indicate that the addition of RT to the standard IO+T regimen substantially prolonged disease control and OS in patients with unresectable HCC.
Subgroup analysis stratified by RT target site to investigate the potential effects of treatment location on the survival benefit of RT showed a numerically longer PFS among patients receiving liver-directed RT compared with those treated for PVTT (median PFS: 11.4 vs. 7.4 months), although the difference was not statistically significant (p=0.11) (Figure 5A). This trend suggests that RT targeting intrahepatic lesions may provide improved local disease control and better synergize with systemic therapies. The absence of statistical significance in the present study may be attributable to limited sample size; therefore, these findings warrant further validation in larger studies.
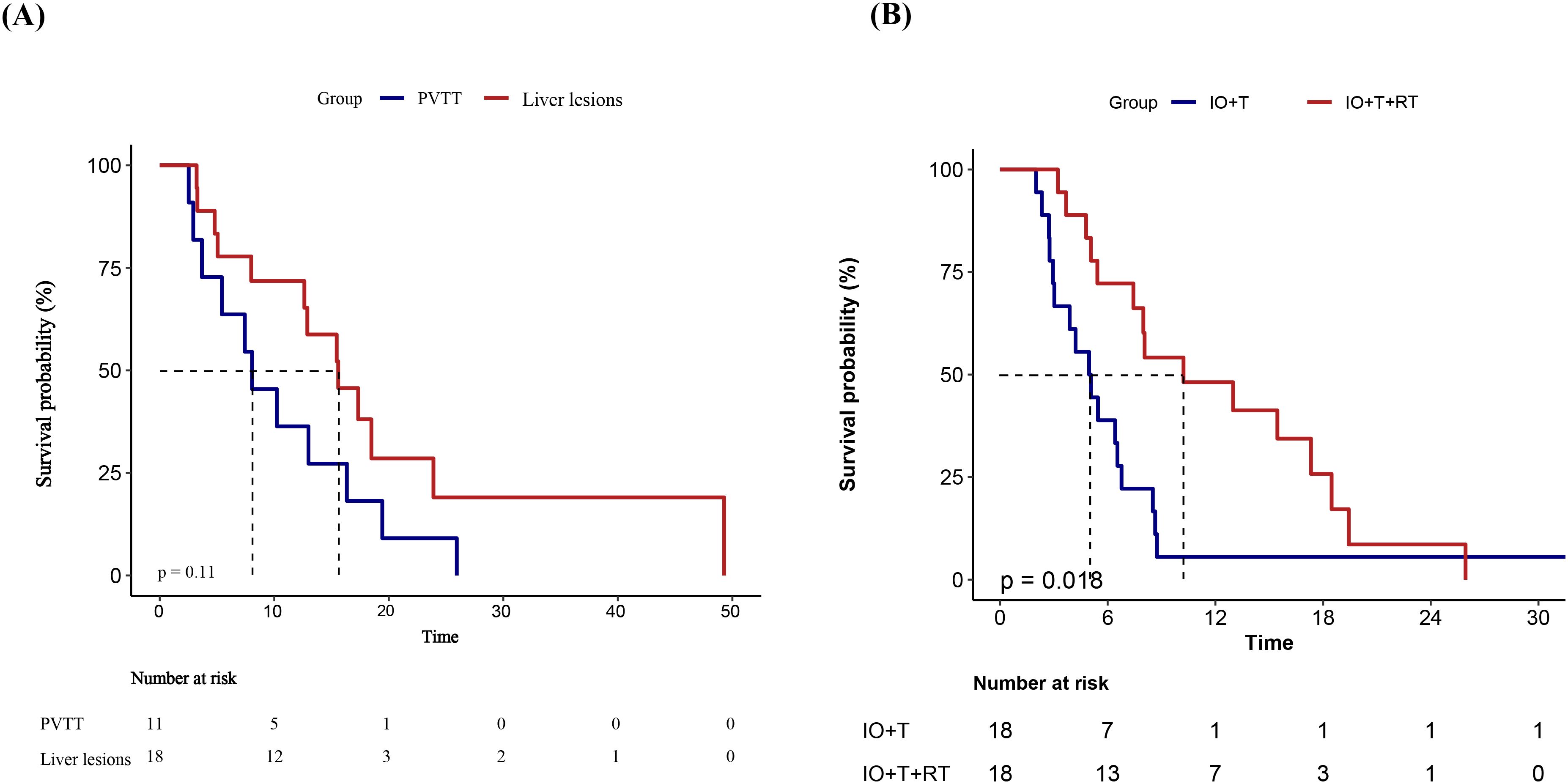
Figure 5. Kaplan–Meier survival curves of PFS in different subgroups.(A) PFS comparison between patients receiving RT targeting PVTT versus intrahepatic liver lesions in the RT+IO+T group. Although patients with liver lesion irradiation demonstrated a longer median PFS, the difference was not statistically significant (p = 0.11). (B) PFS comparison after 1:1 PSM between the IO+T and RT+IO+T groups. Patients receiving the RT+IO+T regimen showed a significantly prolonged PFS compared with those receiving IO+T alone (p=0.018). PFS, progression-free survival; RT, radiotherapy; PVTT, portal vein tumor thrombus; IO, immunotherapy; T, targeted therapy; PSM, propensity score matching.
3.7 PSM results
To reduce potential confounding, PSM was performed based on variables associated with PFS in the univariate analysis (p<0.1), including age, ECOG performance status, maximum tumor diameter, extrahepatic metastasis, and treatment line. A 1:1 nearest-neighbor matching algorithm without replacement was applied using a caliper width of 0.2, yielding 18 matched pairs. The baseline characteristics after matching are summarized in Supplementary Table 1. Kaplan–Meier analysis of the matched cohort demonstrated a significant improvement in PFS for the RT+IO+T group compared with the IO+T group (median PFS: 9.1 vs. 5.0 months; p=0.018, Figure 5B).
Furthermore, after PSM, the RT+IO+T group maintained a statistically significant PFS benefit compared with the IO+T group, with an HR of 0.43 (95% CI: 0.21–0.88, p=0.021) (Table 6). These consistent findings suggest that the addition of RT to immunotherapy and targeted therapy significantly prolongs PFS, even after adjusting for baseline differences. These PSM results strengthened the internal validity of the observed treatment effect and supported the robustness of the survival advantage conferred by the triple-modality regimen.
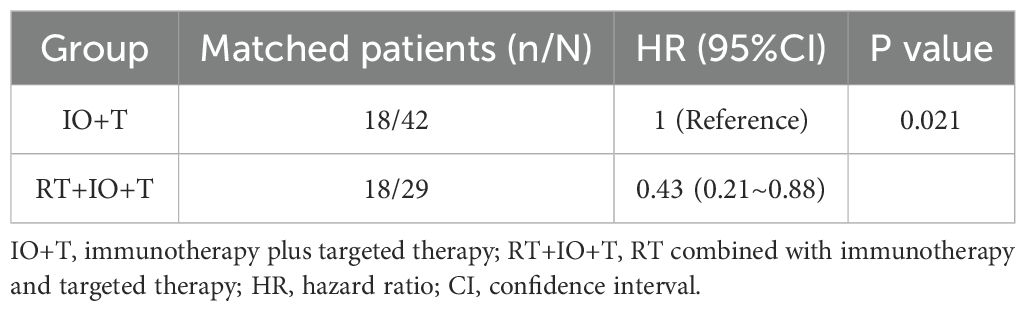
Table 6. Propensity score-matched analysis comparing progression-free survival between IO+T and RT+IO+T groups.
4 Discussion
The results of this study offer novel evidence of the significant clinical benefits of the addition of RT to IO+T regimens in patients with unresectable HCC. Compared with the IO+T regimen alone, RT+IO+T provided a markedly improved median PFS of 12.6 months vs. 4.6 months (p<0.001), and a median OS of 17.8 months vs. 10.9 months (p=0.009). These survival benefits remained robust in PSM analyses of 18 patient pairs matched for key clinical covariates. RT+IO+T also demonstrated superior short-term efficacy, with higher ORR (69.0% vs. 35.7%, p=0.006) and DCR (89.7% vs. 57.1%, p=0.003). The safety profile was generally acceptable, although higher incidences of grade ≥3 hepatotoxicity and lymphopenia were observed in the RT+IO+T group. However, no treatment-related deaths occurred, and most adverse events were manageable with supportive care. These findings underscore the therapeutic potential and tolerability of integrating RT into the standard IO+T treatment paradigm for advanced HCC.
Previous small-scale studies have suggested that triple-modality approaches incorporating the addition of SBRT or proton beam therapy to atezolizumab and bevacizumab may still confer survival benefits even in patients with highly advanced HCC (16, 17). Previous studies have explored the combination of immunotherapy, targeted therapy, and RT in unresectable HCC, focusing primarily on patients with BCLC stage C and PVTT (4, 14, 16, 18–20). These retrospective, single-center studies assessed efficacy endpoints including PFS, OS, and ORR, with some incorporating PSM and reporting BED parameters (4, 14, 16). In contrast, the present study included a more heterogeneous real-world population treated with diverse ICIs and targeted agents, reported detailed RT parameters and target sites (e.g., PVTT, intrahepatic, and extrahepatic lesions), and conducted site-specific and multi-method PSM analyses, thereby enhancing the robustness and clinical relevance of the findings. A recent matched cohort study in advanced HCC showed that adding RT to ICIs and targeted therapy significantly improved outcomes, with a median PFS of 8.3 vs. 4.2 months, ORR of 75.9% vs. 24.1%, and DCR of 100% vs. 75.9% compared with the non-RT group. The median OS was not reached in the RT group but was significantly prolonged (p=0.002) (21). In comparison, our study demonstrated even longer PFS (12.6 months), high ORR (69.0%), and a median OS of 17.8 months, despite a higher proportion of patients with advanced-stage disease. These results reinforce the added value of RT in combination with immunotherapy and targeted therapy, supporting its broader integration into treatment strategies for advanced HCC.
Compared with patient populations in other studies evaluating the combination of ICIs and antiangiogenic agents, such as IMbrave150, ORIENT-32, RESCUE, CARES-310, and APOLLO (22–26), the cohort in the present study exhibited a higher prevalence of poor prognostic factors, including greater baseline tumor burden, a higher incidence of PVTT, and more frequent occurrence of distant metastases. These high-risk features may partially explain the relatively shorter PFS of 4.6 months and OS of 10.9 months observed in the IO+T group compared with previous studies. The addition of RT to the IO+T regimen yielded significantly improved outcomes across multiple efficacy parameters. While our cohort achieved a median OS of 17.8 months, comparable to 19.2 months reported for the IMbrave150, it demonstrated superior PFS (12.6 vs. 6.8 months) and remarkable improvements in tumor response rates (ORR 69.0% vs. 27.3%, DCR 89.7% vs. 73.6%) (22). This comprehensive advantage was consistently observed in cross-trial comparisons; compared with ORIENT-32 (PFS 4.6 months, ORR 20.3%, DCR 66.7%), the RT+IO+T regimen in the present study showed a 2.7-fold longer PFS with substantially higher ORR and DCR (23). Compared with the RESCUE trial, which reported a PFS of 5.5–5.7 months (ORR 34.3–46.0%, DCR 77.2–84.5%), we observed superior disease control across all efficacy measures (24). Our results also exceeded the outcomes reported in the CARES-310 trial for all evaluated endpoints (PFS 5.6 months, ORR 25.4%, DCR 78.3%) (25). Finally, compared with the APOLLO outcomes (PFS 6.9 months, ORR 13.6%, DCR 73.8%), the regimen in the present study also demonstrated significant advantages in both survival and response metrics (26).
Compared with other locoregional strategies combined with systemic therapy, the RT+IO+T regimen demonstrated favorable efficacy and safety in patients with advanced HCC. TACE combined with tyrosine kinase inhibitors and ICIs has demonstrated an ORR of 50.9%, PFS of 9.1 months, and OS of 19.1 months (27), while TACE combined with molecular-targeted agents plus ICIs achieved an ORR of 65.1%, PFS of 15.4 months, and OS of 27.2 months in selected high-burden cases (28). Tang et al. reported that HAIC combined with lenvatinib and tislelizumab yielded an ORR of 77.1%, a median PFS of 6.6 months, and an OS of 23.2 months in patients with Vp4 portal vein invasion (29). Li et al. demonstrated that HAIC plus camrelizumab and rivoceranib achieved a median PFS of 10.0 months, OS of 19.6 months, ORR of 55.5%, and DCR of 89.0% in patients with PVTT (30). Compared with these locoregional combinations, the RT+IO+T regimen in the present study showed strong tumor control efficacy. While TACE- or HAIC-based strategies may offer comparable or even higher OS in select populations, RT+IO+T provides a non-invasive and highly tolerable alternative, potentially offering broad applicability and favorable safety profiles for patients with advanced-stage HCC.
Although the RT+IO+T regimen was associated with a higher rate of treatment-related adverse events, including significantly more grade ≥3 toxicities than the IO+T group, these events were generally manageable, and no treatment-related deaths occurred. The most common severe toxicities were thrombocytopenia (24.1% vs. 2.4%, p<0.001) and lymphopenia (62.1% vs. 18.6%, p<0.001), likely related to radiation exposure and cumulative hematologic effects (31, 32). Given that many patients had advanced liver disease and portal hypertension, baseline hypersplenism may have contributed to cytopenias (33). Increased rates of variceal bleeding and gastrointestinal ulcers in the RT+IO+T group highlight the need for pre-treatment endoscopic evaluation in high-risk patients. Compared with TACE- or HAIC-based regimens, RT+IO+T demonstrated a comparable or lower incidence of severe adverse events (29, 34–36) while offering a non-invasive approach. These results support RT+IO+T as a well-tolerated and practical treatment option for patients with advanced HCC.
This notable survival benefit may be attributed to the synergistic and immunomodulatory interactions among RT, targeted therapy, and programmed cell death protein 1/programmed cell death-ligand 1 pathway blockade (13, 30, 37). Targeted agents help normalize the tumor vasculature and improve perfusion, thereby enhancing the delivery and efficacy of both RT and immunotherapy (12, 38, 39). Additionally, RT may trigger systemic immune responses, including the abscopal effect, which is further amplified when combined with ICIs (10, 11). The results of our subgroup analysis stratified by radiotherapy target revealed the association of liver-directed radiotherapy with longer PFS compared to RT targeting PVTT. This disparity may be attributed to enhanced radiosensitization and immune modulation within the hepatic parenchyma. Unlike PVTT lesions, which are often poorly perfused, hypoxic, and exhibit a suppressive immune microenvironment, irradiation of intrahepatic tumors benefits from transient vascular normalization induced by antiangiogenic therapy, thereby improving perfusion and oxygen delivery, enhancing radiosensitivity, and mitigating hypoxia-driven resistance (40–43). Well-oxygenated liver tumors also respond more favorably to radiation due to increased DNA damage and reduced hypoxia-mediated radioresistance (44). Furthermore, hepatic irradiation promotes immunogenic cell death, upregulation of major histocompatibility complex I (MHC-I), and increased infiltration of cytotoxic T lymphocytes. These effects are further amplified when combined with ICIs and targeted therapeutic agents (45, 46). Lastly, the liver’s unique immunologic architecture, with spatial proximity between hepatic dendritic cells and sinusoidal antigen-presenting cells, may facilitate stronger immune priming in response to liver-targeted radiation (41). Collectively, these mechanisms offer a compelling explanation for the superior PFS observed for liver-targeted radiotherapy compared to PVTT-directed treatment. The integration of RT, ICI, and targeted therapy exerts synergistic effects to enhance tumor antigen presentation, improve vascular normalization and oxygenation, and amplify cytotoxic immune responses, thereby overcoming the individual limitations of each modality and maximizing therapeutic efficacy in advanced HCC.
Despite its promising findings, this study has some limitations. First, owing to the single-center retrospective cohort design, methodological constraints are unavoidable, including selection bias, information bias, and residual confounding. Additionally, the patients were not randomized, and the decision to administer RT was based on clinical judgment. Hence, patients in the IO+T group may have been excluded from receiving RT due to contraindications such as tumor location or poor performance status, introducing potential baseline differences that could influence PFS outcomes. Second, although baseline characteristics were generally well balanced between the two groups—except for BCLC stage and ECOG performance status—unmeasured confounding cannot be entirely ruled out. In addition, post-progression treatment strategies, such as the use of second- or third-line therapies, may have differed between groups, potentially impacting OS comparisons. Third, the relatively small sample size, particularly the number of events, limited the statistical power of the multivariable adjustment and subgroup analysis. The wide confidence intervals observed in Cox models further highlight potential instability in the survival estimates, highlighting the need for cautious interpretation. Nevertheless, the observed improvements in key clinical endpoints, including PFS, OS, ORR, and DCR, suggest adding RT to immunotherapy and targeted therapy may provide a meaningful therapeutic advantage. Finally, owing to the retrospective study design, quality-of-life data were not systematically collected, which limited assessment of the patient-reported impact of adding RT. Given the potential for increased toxicity, this remains a relevant concern. These findings highlight the need for prospective, multicenter randomized trials incorporating standardized quality of life measures to validate survival benefits and define the optimal role of RT in advanced HCC combination strategies.
5 Conclusion
The incorporation of RT into systemic regimens combining ICIs with targeted therapy showed promising tolerability in patients with unresectable HCC. This multimodal strategy appears to enhance therapeutic efficacy, offering higher objective response rates and clinically meaningful gains in both PFS and OS compared with dual-agent approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Sixth Affiliated Hospital of South China University of Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin. Given the retrospective nature of this study, the requirement for informed consent was waived in accordance with institutional guidelines. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YY: Methodology, Investigation, Writing – review & editing, Software, Writing – original draft, Project administration, Data curation. YC: Data curation, Writing – review & editing, Investigation, Writing – original draft. CH: Writing – review & editing, Investigation, Writing – original draft, Validation. ML: Data curation, Writing – review & editing, Writing – original draft. LT: Data curation, Writing – review & editing, Writing – original draft. WT: Writing – original draft, Project administration, Writing – review & editing, Conceptualization, Supervision. WY: Methodology, Writing – review & editing, Writing – original draft, Funding acquisition, Resources, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Foshan Fourteenth Five-Year Key Specialty Construction Project (2022); the Foshan Self-Funded Scientific and Technological Innovation Project (2220001004231) and the Nanhai District of Foshan City (WY), a well-known Doctor Studio fund.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1643304/full#supplementary-material
Abbreviations
HCC, Hepatocellular carcinoma; RT, Radiotherapy; ICI, Immune checkpoint inhibitor; RT+IO+T, Radiotherapy plus immunotherapy and targeted therapy; IO+T, Immunotherapy plus targeted therapy; ORR, Objective response rate; DCR, Disease control rate; CR, Complete response; PR, Partial response; SD, Stable disease; OS, Overall survival; PFS, Progression-free survival; HR, Hazard ratio; CI, Confidence interval; TACE, Transarterial chemoembolization; HAIC, Hepatic artery infusion chemotherapy.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Cao W, Chen H-D, Yu Y-W, Li N, and Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
3. Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Liver Cancer. (2023) 12:405–44. doi: 10.1159/000530495
4. Li Z, Zhai Y, Wu F, Cao D, Ye F, Song Y, et al. Radiotherapy with targeted therapy or immune checkpoint inhibitors for hepatocellular carcinoma with hepatic vein and/or inferior vena cava tumor thrombi. J Hepatocell Carcinoma. (2024) 11:1481–93. doi: 10.2147/JHC.S464140
5. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol: Off J Am Soc Clin Oncol. (2019) 37:2141–51. doi: 10.1200/JCO.18.02184
6. Yoon SM, Ryoo B-Y, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. (2018) 4:661–9. doi: 10.1001/jamaoncol.2017.5847
7. Rim CH, Kim CY, Yang DS, and Yoon WS. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: a meta-analysis and systemic review. Radiother Oncol: J Eur Soc Ther Radiol Oncol. (2018) 129:123–9. doi: 10.1016/j.radonc.2018.02.030
8. Lee YH, Tai D, Yip C, Choo SP, and Chew V. Combinational immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. (2020) 11:568759. doi: 10.3389/fimmu.2020.568759
9. Qiu H, Ke S, Cai G, Wu Y, Wang J, Shi W, et al. An exploratory clinical trial of apatinib combined with intensity-modulated radiation therapy for patients with unresectable hepatocellular carcinoma. Cancer Med. (2023) 12:213–22. doi: 10.1002/cam4.4900
10. Sheng H, Luo Y, Zhong L, Wang Z, Sun Z, Gao X, et al. Antiangiogenic treatment facilitates the abscopal effect of radiation therapy combined with anti-PD-1 in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2025) 121:534–46. doi: 10.1016/j.ijrobp.2024.09.024
11. Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. (2014) 2:831–8. doi: 10.1158/2326-6066.CIR-14-0069
12. Wu J-B, Tang Y-L, and Liang X-H. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. OncoTargets Ther. (2018) 11:6901–9. doi: 10.2147/OTT.S172042
13. Kanthou C and Tozer G. Targeting the vasculature of tumours: combining VEGF pathway inhibitors with radiotherapy. Br J Radiol. (2019) 92:20180405. doi: 10.1259/bjr.20180405
14. Wu G, Liu Y, Fan H, Rao M, Zhang J, and Zhang J. Tislelizumab plus anlotinib with or without radiotherapy as first-line therapy in advanced hepatocellular carcinoma: a single center, non-randomized retrospective case-control study. Discov Oncol. (2025) 16:387. doi: 10.1007/s12672-025-02171-5
15. Llovet JM and Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. (2020) 72:288–306. doi: 10.1016/j.jhep.2019.09.026
16. Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. (2021) 11:686621. doi: 10.3389/fonc.2021.686621
17. Su C-W, Teng W, Shen EY-L, Huang B-S, Lin P-T, Hou M-M, et al. Concurrent atezolizumab plus bevacizumab and high-dose external beam radiotherapy for highly advanced hepatocellular carcinoma. Oncologist. (2024) 29:e922–31. doi: 10.1093/oncolo/oyae048
18. Liu C, Jiang W, Sun J, Cui J, He D, Cheng S, et al. Sintilimab plus lenvatinib with or without radiotherapy for advanced hepatocellular carcinoma with pulmonary metastasis. J Hepatocell Carcinoma. (2024) 11:2283–92. doi: 10.2147/JHC.S491733
19. Ma J, Zhang H, Zheng R, Wang S, and Ding L. Radiotherapy with targeted and immunotherapy improved overall survival and progression-free survival for hepatocellular carcinoma with portal vein tumor thrombosis. Oncologist. (2024) 30:oyae209. doi: 10.1093/oncolo/oyae209
20. Liang X, Jiang Y, Yao W, Deng Y, Yang S, and Liu Q. Liver-directed moderately hypo-fractionated radiotherapy combined with pembrolizumab and bevacizumab for advanced hepatocellular carcinoma: a retrospective observational study of 23 cases. Transl Cancer Res. (2024) 13:1508–18. doi: 10.21037/tcr-23-1333
21. Ning C, Zhang X, Wang Y, Yang X, Yang X, Chao J, et al. Radiation therapy with combination therapy of immune checkpoint inhibitors and antiangiogenic therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2024) 118:1461–71. doi: 10.1016/j.ijrobp.2023.07.001
22. Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
23. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
24. Mei K, Qin S, Chen Z, Liu Y, Wang L, and Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort a report in a multicenter phase ib/II trial. J Immunother Cancer. (2021) 9:e002191. doi: 10.1136/jitc-2020-002191
25. Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet (lond Engl). (2023) 402:1133–46. doi: 10.1016/S0140-6736(23)00961-3
26. Zhou J, Bai L, Luo J, Bai Y, Pan Y, Yang X, et al. Anlotinib plus penpulimab versus sorafenib in the first-line treatment of unresectable hepatocellular carcinoma (APOLLO): a randomised, controlled, phase 3 trial. Lancet Oncol. (2025) S1470-2045:190–1. doi: 10.1016/S1470-2045(25)00190-1
27. Zhang J-X, Cheng Y, Wei J, Fan W-L, Liu J, Zhou C-G, et al. Transarterial chemoembolization combined with tyrosine kinase inhibitors plus immune checkpoint inhibitors versus tyrosine kinase inhibitors plus immune checkpoint inhibitors in unresectable hepatocellular carcinoma with first- or lower-order portal vein tumor thrombosis. Cardiovasc Intervent Radiol. (2024) 47:751–61. doi: 10.1007/s00270-024-03724-x
28. Chen W, Yan H-T, Zhang J-X, Zhou C-G, Liu J, Liu S, et al. Transarterial chemoembolization combined with molecular targeted agents plus immune checkpoint inhibitors for unresectable hepatocellular carcinoma beyond the up-to-seven criteria: a propensity score-matching analysis. Ann Med. (2024) 56:2419993. doi: 10.1080/07853890.2024.2419993
29. Tang S, Shi F, Xiao Y, Cai H, Ma P, Zhou Y, et al. HAIC plus lenvatinib and tislelizumab for advanced hepatocellular carcinoma with Vp4 portal vein invasion. Hepatol Int. (2025) 19:106–17. doi: 10.1007/s12072-024-10762-7
30. Li Y, Guo J, Liu W, Pang H, Song Y, Wu S, et al. Hepatic artery infusion chemotherapy combined with camrelizumab plus rivoceranib for hepatocellular carcinoma with portal vein tumor thrombosis: a multicenter propensity score-matching analysis. Hepatol Int. (2024) 18(4):1286–98. doi: 10.1007/s12072-024-10672-8
31. Byun HK, Kim N, Park S, and Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol: Organ Dtsch Rontgenges. (2019) 195(11):1007–17. doi: 10.1007/s00066-019-01462-5
32. De B, Ng SP, Liu AY, Avila S, Tao R, Holliday EB, et al. Radiation-associated lymphopenia and outcomes of patients with unresectable hepatocellular carcinoma treated with radiotherapy. J Hepatocell Carcinoma. (2021) 8:57–69. doi: 10.2147/JHC.S282062
33. Kim BH, Park HC, Kim TH, Koh Y-H, Hong JY, Cho Y, et al. Concurrent nivolumab and external beam radiation therapy for hepatocellular carcinoma with macrovascular invasion: a phase II study. JHEP Rep. (2023) 6:100991. doi: 10.1016/j.jhepr.2023.100991
34. Chiang C-L, Chiu KW-H, Lee FA-S, Kong F-MS, and Chan AC-Y. Combined stereotactic body radiotherapy and immunotherapy versus transarterial chemoembolization in locally advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. (2021) 11:798832. doi: 10.3389/fonc.2021.798832
35. Yu J, Yan D, Wei S, Yang L, and Yi P. Efficacy and safety of TACE combined with tyrosine kinase inhibitors and camrelizumab for unresectable hepatocellular carcinoma: a systematic review and meta−analysis. Oncol Lett. (2024) 28:401. doi: 10.3892/ol.2024.14534
36. Meng L, Li H, Ji Y, Yu P, Wang Z, Cao L, et al. Efficacy, safety, and biomarker analysis of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a real-world study. Cancer Immunol Immunother: CII. (2024) 74:13. doi: 10.1007/s00262-024-03857-5
37. Pierini S, Mishra A, Perales-Linares R, Uribe-Herranz M, Beghi S, Giglio A, et al. Combination of vasculature targeting, hypofractionated radiotherapy, and immune checkpoint inhibitor elicits potent antitumor immune response and blocks tumor progression. J Immunother Cancer. (2021) 9:e001636. doi: 10.1136/jitc-2020-001636
38. Georganaki M, van Hooren L, and Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. (2018) 9:3081. doi: 10.3389/fimmu.2018.03081
39. Yi M, Jiao D, Qin S, Chu Q, Wu K, and Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. (2019) 18:60. doi: 10.1186/s12943-019-0974-6
40. Lee SM, Choi JH, Yoon J-H, Kim YJ, Yu SJ, Lee J-H, et al. Efficacy and safety of image-guided hypofractionated radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis: a retrospective, multicenter study. BMC Cancer. (2025) 25:736. doi: 10.1186/s12885-025-13739-3
41. Goedegebuure RSA, de Klerk LK, Bass AJ, Derks S, and Thijssen VLJL. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; a therapeutic triad for cancer? Front Immunol. (2018) 9:3107. doi: 10.3389/fimmu.2018.03107
42. Hu Y, Qin T, Li S, Zhang T, and Xue J. Efficacy and safety of SBRT combined with camrelizumab and apatinib in HCC patients with PVTT: study protocol of a randomized controlled trial. Front Oncol. (2020) 10:1589. doi: 10.3389/fonc.2020.01589
43. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. (2017) 9:eaak9679. doi: 10.1126/scitranslmed.aak9679
44. Gray LH, Conger AD, Ebert M, Hornsey S, and Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. (1953) 26:638–48. doi: 10.1259/0007-1285-26-312-638
45. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, K.Wansley E, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. (2006) 203:1259–71. doi: 10.1084/jem.20052494
Keywords: hepatocellular carcinoma, immunotherapy, radiotherapy, targeted therapy, combination therapy, survival outcomes, toxicity profile
Citation: Yuan Y, Chen Y, Huang C, Liu M, Tong L, Tang W and Yang W (2025) Efficacy and safety of radiotherapy combined with immunotherapy and targeted therapy versus immunotherapy plus targeted therapy alone in unresectable hepatocellular carcinoma: a retrospective study. Front. Oncol. 15:1643304. doi: 10.3389/fonc.2025.1643304
Received: 08 June 2025; Accepted: 29 July 2025;
Published: 20 August 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Puja Sahai, The Institute of Liver and Biliary Sciences (ILBS), IndiaTsai Kun Feng, An Nan Hospital, Taiwan
Haohua Wang, Shandong Tumor Hospital, China
Copyright © 2025 Yuan, Chen, Huang, Liu, Tong, Tang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Yang, ZnNuaHlhbmd3ZW5AMTI2LmNvbQ==; Wubing Tang, bHl0YW5nd2JAc2N1dC5lZHUuY24=
†These authors have contributed equally to this work
 Yanling Yuan
Yanling Yuan Yongsheng Chen
Yongsheng Chen Chumin Huang
Chumin Huang Mindong Liu
Mindong Liu Lihua Tong
Lihua Tong Wubing Tang
Wubing Tang Wen Yang
Wen Yang