- Department of Pathology, Institute of Clinical Pathology, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Despite the generally favorable prognosis of differentiated thyroid carcinoma (DTC) following surgery and radioactive iodine (RAI) therapy, approximately 10% of cases eventually develop resistance to RAI. This condition, known as radioiodine-refractory differentiated thyroid carcinoma (RAIR-DTC), is associated with a poor prognosis, with a 10-year survival rate of only 10% from the time of metastasis detection. The limited availability of safe and effective alternative treatments poses a significant challenge to clinical management. However, early identification and intervention targeting high-risk factors are critical for preventing disease progression. Integrating current insights into DTC pathogenesis with established clinical strategies offers valuable opportunities to inform the development of novel therapies and improve patient outcomes. Hence, in this review, we first examine high-risk predictors of RAIR, including demographic factors (e.g., age, sex), gene mutations (e.g., RAS, BRAF, TERT), high-risk histopathological subtypes (e.g., extrathyroidal extension and the tall cell variant), and serum biomarkers (e.g., thyroglobulin and Cyfra 21.1), all of which are widely recognized for monitoring and risk stratification. Notably, we also emphasize that inappropriate pharmacological management of comorbidities—such as diabetes, myeloid leukemia, and hypertension—may suppress sodium-iodide symporter (NIS) expression and RAI uptake, thereby contributing to RAIR development. We then summarize the molecular mechanisms underlying impaired NIS expression and function in RAIR-DTC, followed by a discussion of recent advances in clinical treatment, focusing on the efficacy and safety of both approved and investigational therapeutic agents.
1 Introduction
Thyroid carcinoma (TC) is the most common malignancy of the endocrine system and is categorized into three main histological subtypes: differentiated thyroid carcinoma (DTC), which includes both well-differentiated and poorly differentiated forms (PDTC); undifferentiated carcinoma, known as anaplastic thyroid carcinoma (ATC); and medullary thyroid carcinoma, which arises from calcitonin-producing C-cells. Among these, DTC is the most prevalent, accounting for over 90% of all TC cases (1, 2), and comprises papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and Hürthle cell carcinoma (3). Epidemiological data indicate a sharp increase in TC incidence in China, with more than 460,000 new cases reported annually, approximately 120,000 in males and 340,000 in females, making it the third most common cancer in the country and responsible for around 11,500 deaths each year (4). Following initial treatment, which typically includes thyroidectomy, thyroid-stimulating hormone (TSH) suppression, and radioactive iodine (RAI) therapy (5), DTC generally carries a favorable prognosis (6). Nonetheless, distant metastases develop in approximately 7–23% of DTC patients (7), and nearly two-thirds of these cases undergo dedifferentiation, leading to reduced iodine uptake and the development of RAI resistance (8), known as radioactive iodine-refractory differentiated thyroid carcinoma (RAIR-DTC) (9). RAIR-DTC is defined by the following criteria (1): no RAI uptake in known DTC lesions; or (2) disease progression within one year following RAI therapy despite iodine exposure. Disease progression may manifest as:① increased serum thyroglobulin (TG) or TG antibodies, lesion enlargement, or the appearance of new lesions; ②worsening symptoms related to the original disease, emergence of new symptoms, or death (10). Clinically, RAIR-DTC is typically classified into two phenotypes: asymptomatic indolent and symptomatic progressive. The asymptomatic form is characterized by low tumor burden, absence of significant symptoms, and stable or slowly progressing disease. The symptomatic form can be further divided into oligometastatic and advanced or widely metastatic subtypes (11).
Conventional therapies for RAIR-DTC, such as cytotoxic agents like doxorubicin, used alone or in combination with cisplatin or interferon alpha-2b, have demonstrated low response rates and are often accompanied by severe adverse effects (AEs) (12, 13). Advances in understanding the molecular mechanisms of RAIR-DTC have led to the development of targeted therapies, which have significantly extended progression-free survival (PFS). Nevertheless, only Apatinib and Lenvatinib (specifically in patients over 65 years of age) have shown a survival benefit in terms of overall survival (OS) (14, 15). Despite these benefits, targeted therapies are associated with a range of AEs, including hypocalcemia (16), salivary gland dysfunction (17), and metastatic diseases (lung, bone, liver and brain metastases) (18–20), as well as thyroid hormone imbalance (21) and cardiovascular diseases (hypertension, diabetes, obesity, dyslipidemia, heart rhythm abnormalities and heart failure) (22). Currently, there is also no consensus on the optimal timing for initiating multi-kinase inhibitors (MKIs) therapy in asymptomatic RAIR-DTC patients. Delaying treatment risks disease progression and loss of control, while premature initiation may negatively impact quality of life (23). Given that RAIR-DTC often results from thyroid dedifferentiation, redifferentiation therapy aimed at restoring RAI uptake offers a potential treatment avenue. However, most redifferentiation agents remain in experimental stages or have shown limited clinical efficacy (24, 25). Notably, whether through emerging targeted therapies or redifferentiation strategies, tumor cells frequently develop resistance via new genetic mutations, compensatory activation of bypass pathways, or alterations in drug targets (26, 27). Due to the lack of safe, effective and specifical therapies for RAIR-DTCs, the prognosis remains poor, with a 5-year disease-specific survival rate of 60–70% and a 10-year overall survival rate of merely 10% following metastatic progression (28).
Given the limited availability of specialized therapies for RAIR-DTC, numerous studies have aimed to identify predictors of its development. In this review, we examine common high-risk factors, including demographic character (e.g., age, ethnicity), genetic mutations (e.g., RAS, BRAF, TERT promoter, and RET), clinicopathological features (e.g., hobnail variant PTC, and lymph node metastasis), and early monitoring markers (Thyroglobulin (TG), Cyfra 21.1), that contribute to RAIR-DTC risk stratification. We also highlight potential inducing factors that may promote RAIR-DTC onset, such as comorbidities (e.g., diabetes and myeloid leukemia) and inappropriate use of certain medications (e.g., metformin, nilotinib, and etilefrine), which may impair RAI uptake and facilitate disease progression. Additionally, we summarize the molecular basis of RAIR-DTC, primarily characterized by reduced expression and impaired function of the sodium-iodide symporter (NIS). This impairment is often driven by genetic alterations (e.g., RAS, RAF, PIK3CA) that activate signaling pathways such as MAPK, PI3K/Akt, and AMPK. Finally, we discuss recent advances in clinical management, including approved therapies and agents currently under investigation in clinical trials. Moving forward, research should prioritize early identification of high-risk factors to prevent disease progression. At the same time, studies on molecular pathogenesis and treatment strategies should evaluate their influence on NIS expression and function, with the goal of minimizing AEs and improving patient outcomes.
2 High-risk factors for RAIR-DTCs
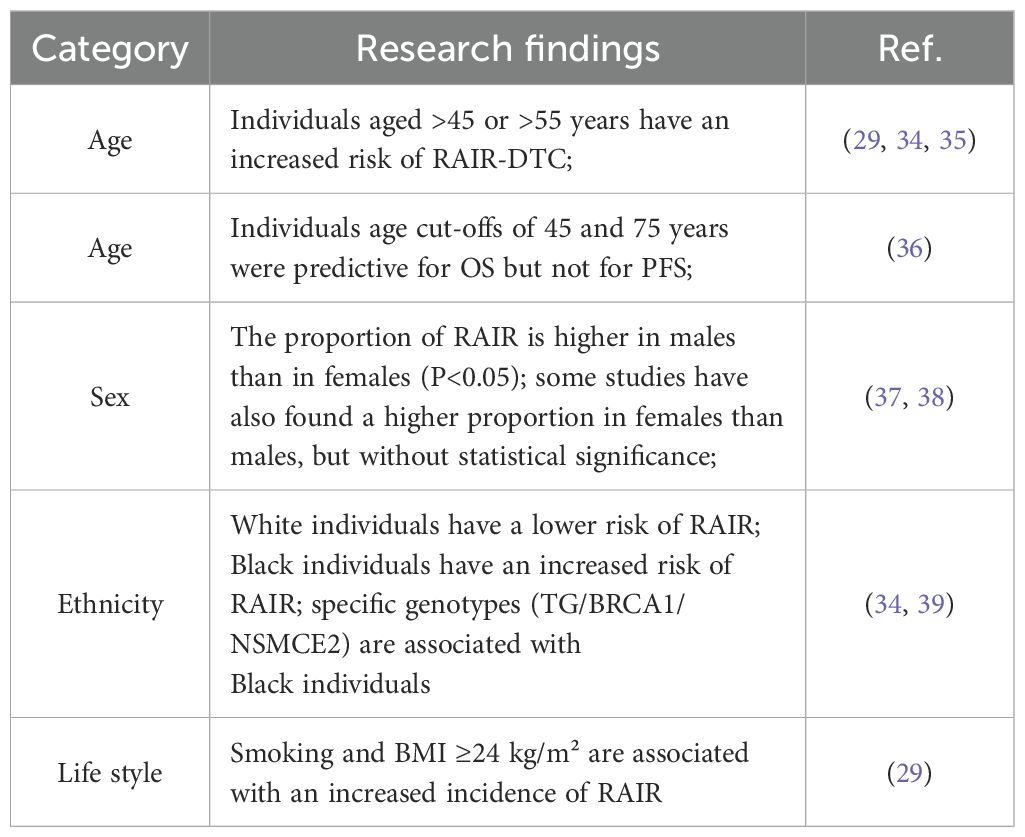
Accurate identification of high-risk factors for RAIR-DTC is essential for guiding personalized prevention and treatment strategies. In this context, we conducted a retrospective literature review to synthesize key risk factors associated with RAIR-DTC development. This comprehensive analysis includes demographic characteristics, genetic mutations, clinicopathological features, early monitoring markers, and promoting factors related to comorbidities and their associated medications (29–32) (Figure 1).
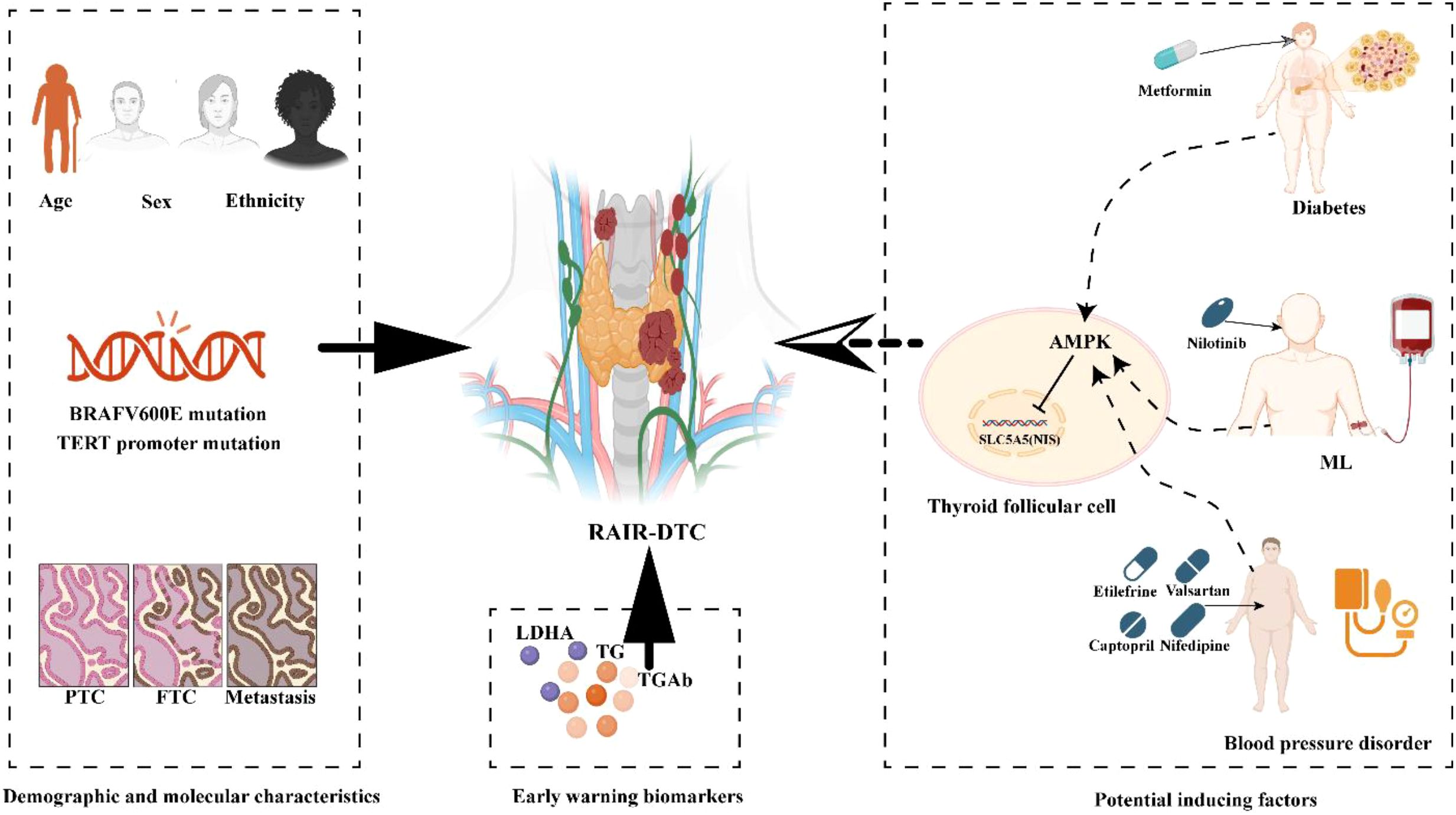
Figure 1. Schematics showing active preventive strategies of RAIR-DTC. Common high-risk factors include demographic characteristics, genetic mutations, clinicopathological features, early warning markers, and promoting factors related to comorbidities and their associated medications. Demographic characteristics include patient age, gender, and ethnicity. Genetic mutations include common BRAF V600E and TERT promoter mutations. Clinicopathological features include Tall cell variant and Lymph node metastasis. Common monitor biomarkers include TG and TGAb. Potential inducing factors in unsuitable combination led by related comorbidity. TG, Thyroglobulin; TGAb, Thyroglobulin Antibodies; LDHA, Lactate Dehydrogenase A; ML, Myeloid leukemia. The diagram was created with BioRender.com.
2.1 Demographic characteristics
Common demographic factors include age, ethnicity, sex, geographic distribution, and lifestyle (33). In this section, we review the relevant literature and summarize the associations between age, gender, ethnicity, and lifestyle and the development of RAIR-DTC (Table 1).
2.1.1 Age and sex
Numerous studies have identified age threshold as a predictive factor for RAIR-DTC. Retrospective analyses by Shobab et al. and Chen et al. found that patients over 45 years old had a higher odds ratio (OR) for developing RAIR-DTC (34, 35). Similarly, Li et al. reported an increased incidence of RAIR in patients over 55 years of age (29). Interestingly, Saie et al. noted that age thresholds of 45 and 75 years were predictive of OS, but not PFS, in RAIR-DTC patients (36). Regarding sex, Wang et al. observed a significantly higher proportion of male RAIR patients compared to females (P < 0.05), with logistic regression confirming male sex as a predictive factor (37). In contrast, Liu et al. found a higher proportion of female RAIR patients, although the difference was not statistically significant (38).
2.1.2 Ethnicity and life style
Shobab et al. analyzed the relationship between ethnicity and RAIR-DTC, finding that individuals of white ethnicity had a lower OR for RAIR, whereas those of black ethnicity had a higher OR (34). In a germline genetic analysis comparing African American and Caucasian patients, Hurst et al. identified specific haplotypes—TG, BRCA1, and NSMCE2—that were associated with RAIR occurrence in African American patients with over 80% African ancestry (39). Regarding lifestyle factors, Li et al. reported that smoking and a body mass index (BMI) of 24 kg/m² or higher were associated with an increased risk of RAIR development (29).
2.2 Gene alterations
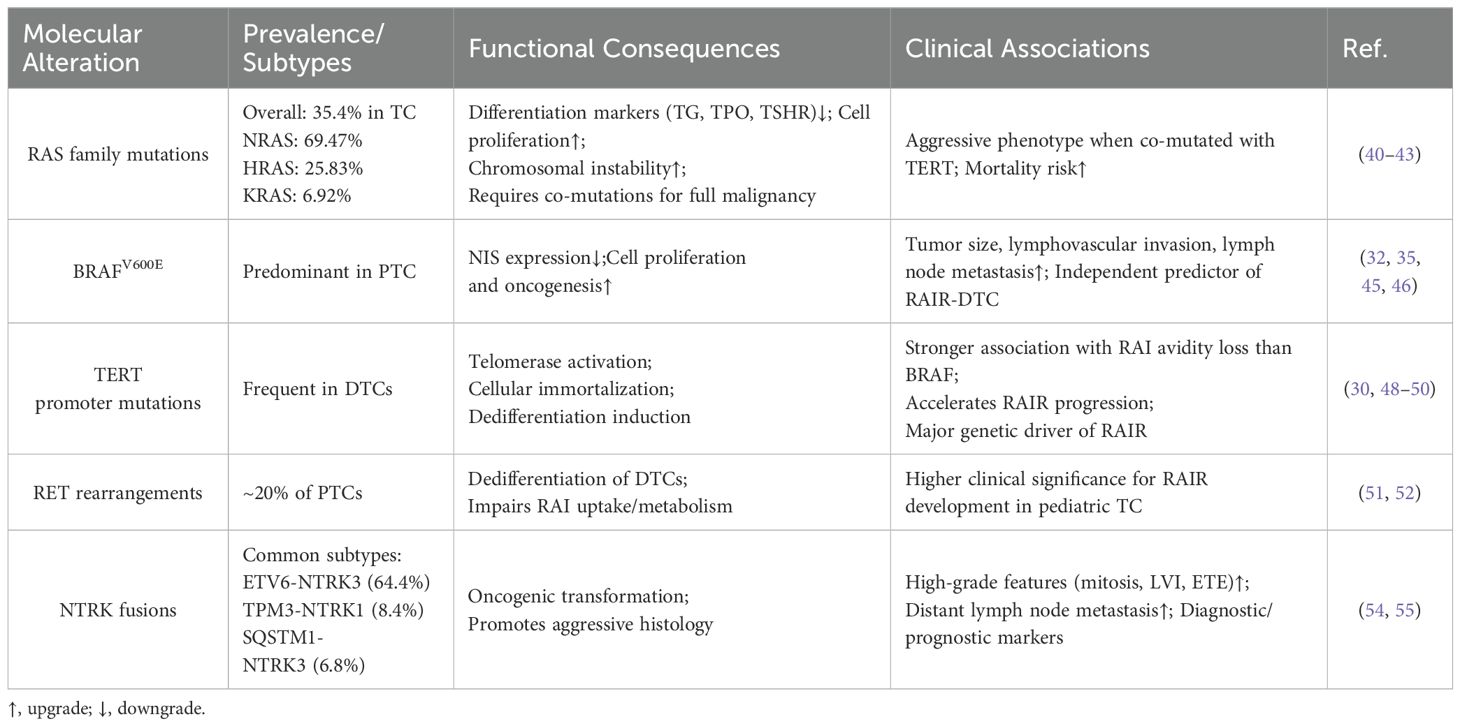
The Fifth Edition of the World Health Organization (WHO) Classification of Endocrine and Neuroendocrine Tumors highlights genetic mutations—particularly BRAF—as key early drivers in the development of RAIR-DTC and valuable predictors of its occurrence (3). For instance, in a molecular analysis of 220 cases of distantly metastatic DTC, Mu et al. compared non-RAIR (N = 81) and RAIR (N = 139) cohorts. Their results showed significantly higher mutation frequencies in the RAIR group for BRAFV600E (59.7% vs. 17.3%), TERT promoter (43.9% vs. 7.4%), and TP53 (43.9% vs. 7.4%). In contrast, rearranged during transfection (RET) mutations were less common in RAIR-DTCs than in non-RAIRs (15.8% vs. 39.5%) (32). In this section, we emphasize the associations between RAS, BRAFV600E, TERT, RET, and NTRK mutations and RAIR prediction, underscoring their relevance for prognosis and therapeutic decision-making in RAIR-DTC (Table 2).
2.2.1 RAS
The RAS gene family (NRAS, HRAS, KRAS) encodes GTP-binding proteins, and activating mutations in these genes are frequently observed in TCs, with an overall prevalence of approximately 35.4%—NRAS accounting for 69.47%, HRAS for 25.83%, and KRAS for 6.92% (40). Studies by Bond et al., Monaco et al., and Portella et al. have shown that RAS mutations reduce the expression of thyroid differentiation markers such as thyroglobulin (TG), thyroid peroxidase (TPO), and the thyrotropin receptor (TSHR), while promoting increased thyroid cell proliferation. Although RAS mutations alone are insufficient to drive full malignant transformation, the presence of co-occurring mutations, particularly TERT promoter mutations, has been linked to more aggressive tumor phenotypes and increased mortality in RAS-mutant DTCs (41, 42). Furthermore, Saavedra et al. demonstrated that RAS mutations promote chromosomal instability in PCCL3 cells, potentially facilitating malignant progression by increasing susceptibility to additional genetic alterations (43).
2.2.2 BRAFV600E
BRAF, a member of the RAF kinase family, plays a key role in cell signaling pathways that regulate proliferation and survival. The BRAFV600E mutation, which is more prevalent in PTCs than RAS mutations, drives oncogenesis by promoting uncontrolled cell proliferation (44). Dong et al. demonstrated that BRAFV600E significantly reduced sodium-iodide symporter (NIS) expression in a subset of PTC patients without Hashimoto’s thyroiditis (P = 0.046) (45). Additionally, Makboul et al., in an analysis of clinical data from 78 PTC patients, found that BRAFV600E was significantly associated with increased tumor size, lymphovascular invasion, and lymph node metastasis (46). Furthermore, both Mu et al. and Wang et al. identified BRAFV600E as an independent predictor of RAIR-DTC (32, 35).
2.2.3 TERT promoter
The TERT gene encodes the catalytic subunit of telomerase, an enzyme critical for maintaining telomere length and promoting cellular immortality. Mutations in the TERT promoter are commonly observed in DTC and are strongly associated with tumor dedifferentiation (47). Yang et al. conducted a retrospective analysis of 66 DTC cases with distant metastases and found that TERT promoter mutations were more strongly associated with reduced RAI avidity and negative RAI uptake compared to BRAFV600E mutations (48). Similarly, Tan et al., in a cohort of 243 patients (non-RAIR: N = 212; RAIR: N = 31), reported that TERT promoter mutations accelerated progression to RAIR (49). Parvathareddy et al. also demonstrated a significant association between TERT promoter mutations and RAIR-DTC through multivariate analysis of 268 RAIR-DTC cases (30). Furthermore, Ju et al., using next-generation sequencing on 278 RAIR-DTC tumor samples, confirmed that TERT promoter mutations were the predominant genetic drivers of RAIR in adults (50).
2.2.4 RET
The RET proto-oncogene, located at chromosome 10q11.2, encodes a receptor tyrosine kinase involved in several TC subtypes. RET rearrangements, present in approximately 20% of PTCs, promote dedifferentiation of DTCs and significantly impair RAI uptake and metabolism (51). Notably, thyroid cells in children are more vulnerable to ionizing radiation and have reduced DNA repair capacity, making them more susceptible to RET rearrangements and subsequent malignant transformation (52). These rearrangements play a critical role in compromising RAI responsiveness and contribute to the development of RAIR-DTC. Therefore, detecting RET mutations and fusions in pediatric TC cases holds important clinical significance.
2.2.5 NTRK
The TRK family of transmembrane receptorsranes. TRKB, and TRKC,to encoded by the NTRK1, NTRK2, and NTRK3 genes, respectively. NTRK gene fusions have oncogenic potential in both neural and non-neural tissues (53). In DTCs, the most common fusion types include ETV6udeEN. (64.4%), TPM34%),N. (8.4%), and SQSTM1,N.CI (6.8%) (54). Notably, non-secretory TCs characterized by multinodular growth of eosinophilic cells harboring NTRK fusions are often associated with more aggressive clinical behavior. Chu et al. reported that NTRK fusion–positive. PTCs frequently exhibit high-grade features such as elevated mitotic activity, extensive lymphatic invasion, and extrathyroidal extension (ETE) (55). Similarly, Pekova et al. found that thyroid cancers with NTRK1 fusions show increased lymphatic invasion and distant lymph node metastases (54). Both NTRK1 and NTRK3 fusions have emerged as valuable diagnostic and prognostic biomarkers in TC.
2.3 Clinical-pathological features
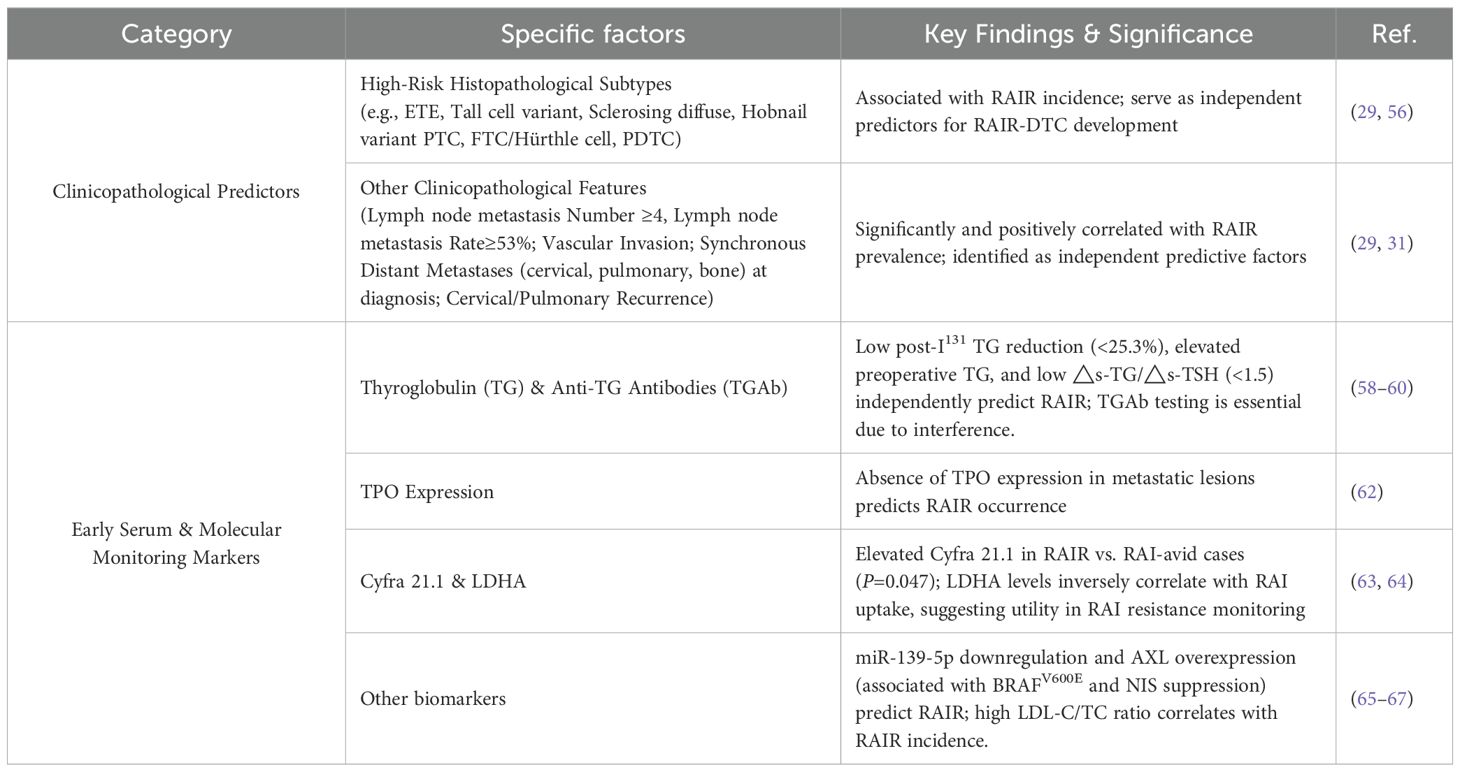
Although no definitive consensus exists regarding the histopathological features that define RAIR-DTC, several clinicopathological characteristics commonly observed in RAIR-DTC have been identified as independent predictors of its development (56). This section examines the role of high-risk histopathological subtypes and other clinicopathological factors in predicting RAIR-DTC. These insights are critical for advancing personalized precision medicine and improving prognostic assessment (Table 3).
2.3.1 High-risk histopathological subtypes
Li et al. and Luo et al. conducted retrospective analyses involving approximately 6,594 cases and found that several histopathological subtypes, such as extrathyroidal extension (ETE), tall cell variant, diffuse sclerosing and hobnail variants of PTC, follicular thyroid carcinoma (FTC, including Hürthle cell), and poorly differentiated thyroid carcinoma (PDTC), are significantly associated with RAIR-DTC. These features serve as independent predictors for the development of RAIR-DTC (29, 56).
2.3.2 Other clinical-pathological features
Li et al. found that the number of lymph node metastases (≥4), lymph node metastasis rate (≥53%), and pN stage (N1) were significantly and positively associated with the prevalence of RAIR-DTC (29). In a comparative analysis of 159 RAIR cases and 759 non-RAIR cases, Schubert et al. identified vascular invasion, synchronous cervical, pulmonary, and bone metastases at initial diagnosis, as well as cervical and pulmonary recurrence during follow-up, as independent predictive factors for RAIR development (31).
2.4 Early monitoring markers for RAIR-DTCs
Currently, only a limited number of early monitoring markers for RAIR-DTC have been well established (57). Clinically significant biomarkers include thyroglobulin (TG), anti-thyroglobulin antibodies (TGAb), thyroid-stimulating hormone (TSH), as well as molecular markers such as microRNAs (miRNAs) and tyrosine kinase receptors. Notably, TG and TGAb have been incorporated into the 2025 edition of the China Guidelines for Integrated Treatment of Tumors as recommended serum markers for RAIR-DTC follow-up (10). In this section, we summarize several commonly used biomarkers for predicting RAIR occurrence (Table 3).
2.4.1 Thyroglobulin
Thyroglobulin (TG) is an effective biomarker for monitoring clinical outcomes in patients with distant metastatic DTC. Sa et al. reported that a post-I¹³¹ TG reduction of less than 25.3% in these patients predicted a poor response to subsequent I¹³¹ therapy (58). In a retrospective analysis of 876 DTC cases (786 non-RAIR vs. 90 RAIR), Cheng et al. found that elevated preoperative TG (pre-TG) levels were significantly associated with RAIR development and served as an independent predictor (59). Meng et al. developed a nomogram model using a cutoff value of △s-TG/△s-TSH < 1.5(where △s-TG represents the difference between pre-treatment stimulated and suppressed TG, and △s-TSH denotes the difference in TSH levels before and after treatment) to predict RAIR-DTC progression (60). Importantly, serum TG measurements should be interpreted alongside anti-thyroglobulin antibody (TGAb) levels, as TGAb can interfere with TG accuracy.
2.4.2 Thyroid peroxidase
Nilsson et al. demonstrated a positive correlation between thyroid peroxidase (TPO) expression and RAI avidity by analyzing surgical specimens, including primary tumors and lymph node metastases, from 28 DTC patients (61). Similarly, Zelinskaya et al., through immunocytochemical analysis of 104 metastatic DTC lesions, confirmed that the absence of TPO expression is a predictive marker for RAIR development (62).
2.4.3 Cyfra 21.1 and lactate dehydrogenase A
Jeong et al. reported that significantly higher serum Cyfra 21.1 levels in patients with metastatic TCs compared to those with non-metastatic disease and healthy controls (distant metastasis: N = 51; non-distant metastasis: N = 76; P = 0.012). Among the metastatic group, RAIR patients (N = 13) exhibited significantly higher Cyfra 21.1 levels than RAI-avid patients (N = 12; P = 0.047), suggesting its potential as a biomarker for RAIR-DTC, particularly in cases where TG is undetectable (63). Additionally, Tian et al. demonstrated an inverse correlation between lactate dehydrogenase A (LDHA) expression and RAI uptake in a cohort of 69 DTC patients, indicating a potential role of LDHA in mediating RAI resistance (64).
2.4.4 Others
Pecce et al. reported that miR-139-5p was significantly downregulated in RAIR-DTC patients (N = 14) compared to non-RAIR-DTC patients (N = 12). Overexpression of miR-139-5p enhanced NIS expression and RAI uptake, suggesting its potential as a predictive biomarker for RAIR-DTC (65). Additionally, Collina et al. found that a strong association between high expression of anexelekto (AXL), BRAFV600E mutations, and RAIR occurrence (P < 0.0001) in a cohort of 110 PTC patients and 5 controls. Their findings indicate that elevated AXL expression may contribute to RAIR by suppressing NIS expression, thus serving as a potential predictive marker (66). Liu et al. also identified that a significant correlation between the low density lipoprotein-cholesterol-total cholesterol ratio (LDL-Ch/TCh) ratio and RAIR incidence in TC patients (RAIR: N = 12; controls: N = 24) (67).
2.5 Promoting factors from comorbidities and its medication
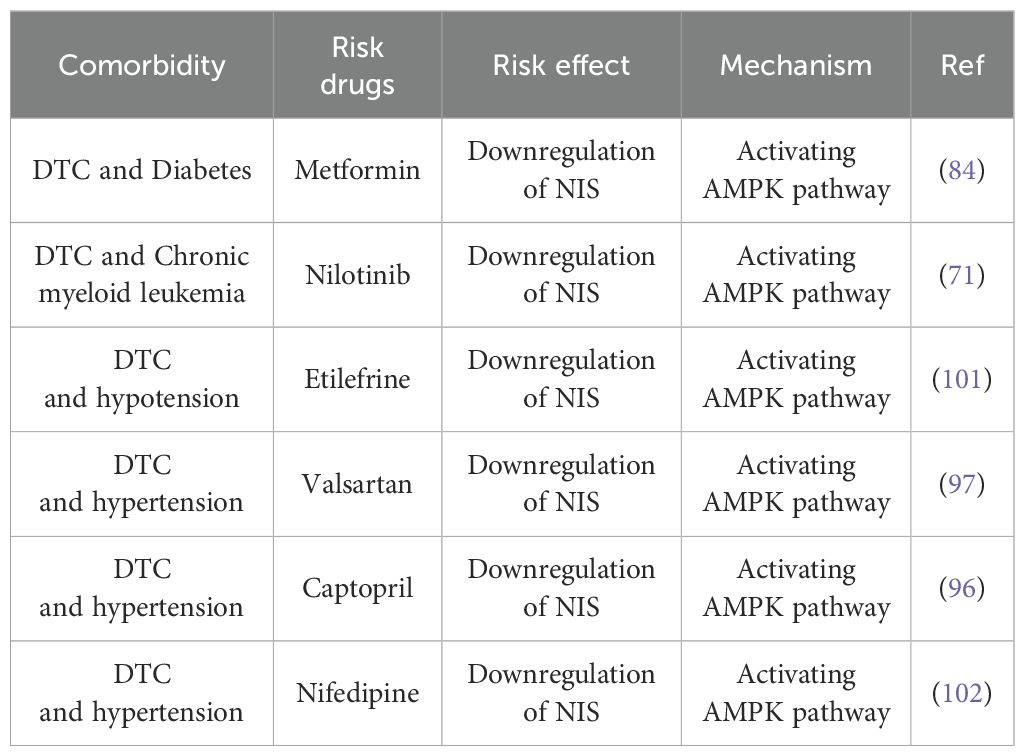
Research suggests that environmental factors, dietary habits, and metabolic syndrome, including central obesity and insulin resistance, may contribute to the onset and progression of RAIR-DTC (68–70). DTC is also commonly comorbid with conditions such as diabetes, myeloid leukemia (ML), and blood pressure disorders, which may influence disease progression, potentially through activation of the AMPK signaling pathway (71–75). Although limited studies have examined the impact of comorbidities and associated medications on RAIR development, our retrospective literature review highlights several medications linked to DTC related comorbidities that may act as potential inducers of RAIR, as summarized in Table 4.
2.5.1 Diabetes and RAIR-DTC
Studies have established a connection between diabetes mellitus and thyroid disorders, indicating that diabetes related traits such as elevated insulin levels, insulin resistance, metabolic dysregulation, and oxidative stress may exacerbate the severity of thyroid conditions (76–78). Zhang et al. utilized the TyG index, a marker of insulin resistance, to analyze a cohort of 47,710 subjects, revealing a significant increase in the prevalence of thyroid disorders and the risk of subclinical hypothyroidism associated with higher TyG index values (79). Moreover, impaired sensitivity to TSH or reduced TSH levels in individuals with diabetes contribute to metabolic dysregulation, resulting in decreased RAI sensitivity and diminished expression of NIS (80, 81). The oxidative stress induced by diabetes, characterized by elevated lipid peroxides and downregulated TSH levels, disrupts thyroide redox balance of the thyroid gland (81, 82). Azouzi et al. demonstrated that the upregulation of reactive oxygen species (ROS) can lead to the downregulation of NIS in DTC cells (78), suggesting that diabetes may promote the RAIR-DTC development.
In addition to diabetes mellitus, anti-diabetic medications such as Metformin, an AMPK agonist approved by the U.S. Food and Drug Administration (FDA), may have varying effects patients. While some studies indicate that metformin reduces tumor volume in diabetic patients with RAIR-DTC (83), others report a decreases in NIS expression and iodine uptake in vitro (84). Consequently, the use of Metformin in diabetic patients with RAIR-DTC may yield antagonistic effects through AMPK activation.
2.5.2 Myeloid leukemia and RAIR-DTC
Evidence suggests that prolonged RAI therapy exceeding 100 mCi may increase the risk of acute or chronic myeloid leukemia (AML or CML) in DTC patients, particularly males. This risk is potentially linked to ROS induced oxidative stress and subsequent DNA damage (71, 74, 85). Supporting this mechanism, Rajeshwari et al. observed elevated lipid peroxidation and protein oxidation in AML (N=30) and CML (N=30) patients compared to controls (N=13) (86). Similarly, Zhou et al. and Pascu et al. emphasized oxidative stress’s role in the etiology and treatment of AML and CML (87, 88). Furthermore, Cazarin et al. noted that ROS can alter the expression and function of the NIS in thyrocytes and TC cells (89).
Nilotinib, an FDA-approved BCR-ABL inhibitor for CML, enhances TC sensitivity to RAI by inducing autophagy through PI3K/Akt pathway inhibition (90, 91). However, it also activates AMPK, which promotes NIS degradation. This reduces RAI uptake and can induce RAIR (92, 93).
2.5.3 Blood pressure disorder and RAIR-DTC
Yu et al. found that altered thyroid hormone (TH) sensitivity—specifically reduced central sensitivity and increased peripheral sensitivity—is associated with a heightened risk of hypertension in patients with coronary heart disease (N=34,310). Logistic regression analysis revealed an inverse relationship between central thyroid resistance (reflected by the TSH index) and hypertension risk, alongside a positive correlation between the FT3/FT4 ratio (indicating peripheral sensitivity) and hypertension risk (94). Separately, MKIs like sorafenib and Lenvatinib, which inhibit the VEGF axis, are associated with hypertension in advanced DTC (72, 95). Furthermore, although less common, Kitamura et al. observed that sternal or mediastinal lymph node metastases in metastatic TC can compress the vena cava, causing hypotension and sudden death (75). Collectively, these findings suggest that blood pressure dysregulation may influence the incidence or progression of RAIR-DTC.
In the management of blood pressure disorders in patients with DTC, anti-hypertensive medications such as Valsartan, Captopril, and Nifedipine, along with anti-hypotensive drug Etilefrine, which is known for its activation of the AMPK pathway, are used (96–99). Consequently, these medications may influence the expression of the NIS and RAI uptake through AMPK activation, potentially impacting the development and progression of RAIR-DTC.
2.6 Clinical implication
We recommend that patients with DTC undergo regular monitoring of RAIR-DTC related gene mutations and serum biomarker expression levels following initial treatment to prevent disease progression. Although clinical evidence has not confirmed that diabetes, myeloid leukemia (ML), blood pressure disorders, or treatments such as metformin, nilotinib, etilefrine, and valsartan directly promote RAIR-DTC or worsen its prognosis, research suggests a potential link via AMPK pathway activation, which may contribute to adverse outcomes in DTC (80, 84, 93, 96, 97, 100). Given the retrospective nature of available studies and uncertain causality, caution is advised when prescribing these medications to DTC patients with relevant comorbidities.
3 Molecular features in RAIR-DTC
Existing evidence indicates that the core molecular pathogenesis of RAIR-DTC involves downregulation or functional impairment of the sodium-iodide symporter (NIS), which prevents effective RAI uptake. This NIS dysfunction results from dysregulated signaling pathways driven by genetic alterations, including RAS mutations, BRAFV600E mutations, TERT promoter mutations, RET/NTRK fusions, and PIK3CA mutations) (103). Here, we elaborate on how these abnormally activated signaling pathways impact NIS expression or activity and consequently reduce RAI efficacy (46, 48, 52) (Figure 2).
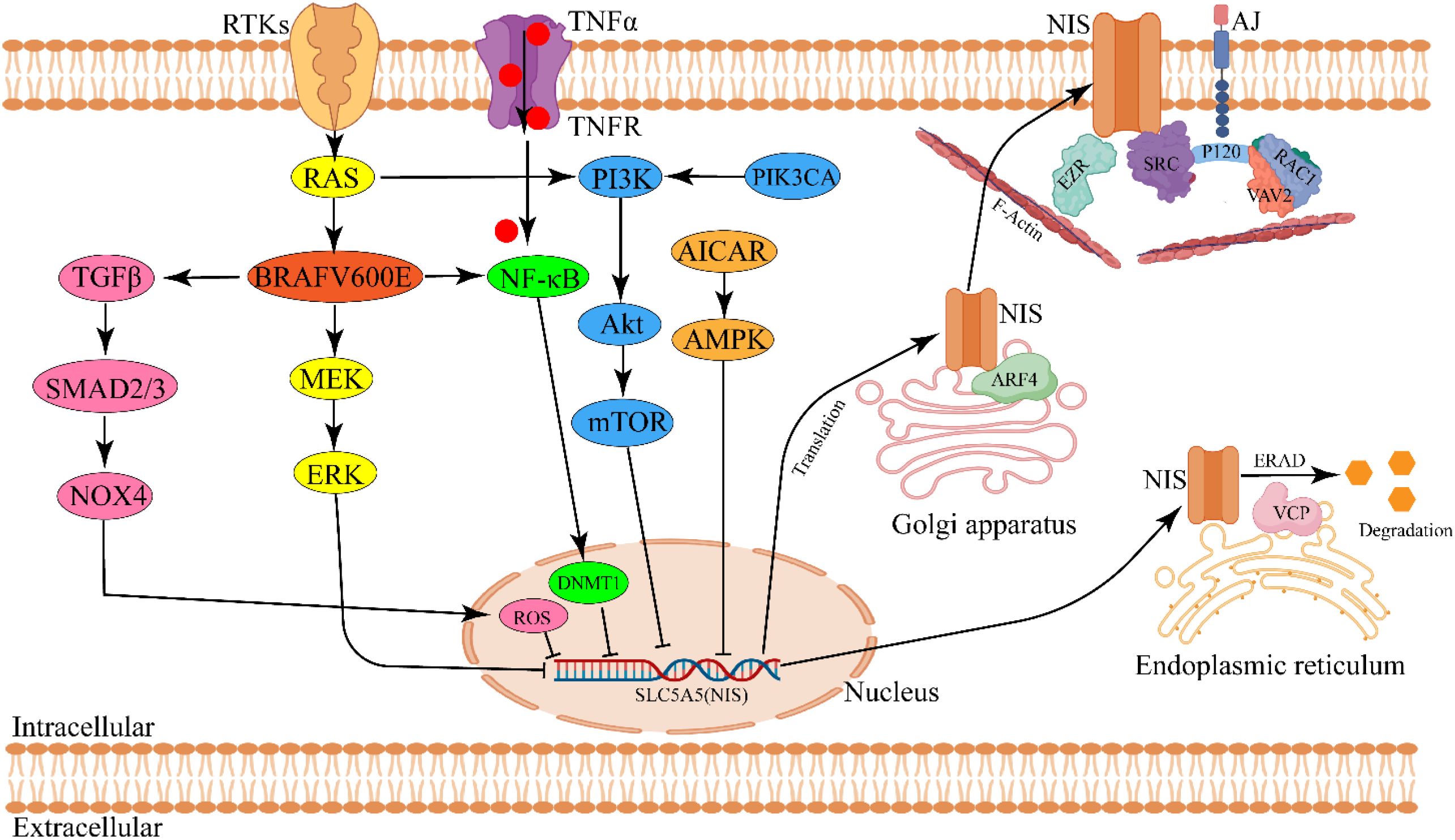
Figure 2. Schematic diagram of molecular pathogenesis in modulating NIS expression and activity in RAIR-DTC. Aberrant activation of signaling pathway derived by gene mutation (RAS/BRAF) is responsible for regulating NIS expression. NIS membrane targeting and glycosylation modulate NIS activity. ARF4, ADP-ribosylation factor 4; AICAR, AMPK agonist 5-aminoimidazole-4-carboxamide-ribonucleoside; DNMT1, DNA methyltransferase1; ERAD, Endoplasmic-reticulum-associated protein degradation; EZR, EZRIN; VCP, Valosin-containing protein. The image was created with BioRender.com.
3.1 Decrease in NIS expression
3.1.1 MAPK signaling pathway
Mitogen-activated protein kinase (MAPK) cascades in mammalian cells consist of three major families: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38/stress-activated protein kinases (SAPKs) (106). The MAPK pathway, a downstream branch of BRAF signaling, significantly influences the expression of key genes involved in thyroid hormone biosynthesis, including NIS, TPO, and TG, and plays a crucial role in the progression of RAIR-DTC (107, 108). Liu et al. demonstrated that inhibiting MEK or silencing the BRAFV600E mutation in PCCL3 cells (a rat thyroid cell line) restored the expression of iodide-metabolizing genes (109). Furthermore, BRAFV600E induced deacetylation of nucleotides −297/−107 in the rat NIS promoter and −692/−370 in the human NIS promoter contributes to NIS gene silencing (110). Overexpression of miR-20b has been shown to reduce cell viability and migration in human TC cells and to suppress tumor growth in xenografts by directly targeting SOS1 and ERK2, thereby inhibiting the MAPK/ERK pathway (111). Similarly, Shen et al. reported that miR-106a overexpression decreased NIS expression and RAI uptake in TC cells by directly targeting retinoic acid receptor beta (RARB) and activating the MAPK pathway in vitro (112). Overall, BRAFV600E mediated hyperactivation of the MAPK pathway plays a key role in suppressing NIS expression.
3.1.2 PI3K/Akt signaling pathway
Besides regulating cell growth, transcription, and translation, the phosphoinositide 3-kinase (PI3K) pathway plays a significant role in the pathogenesis of RAIR-DTC (113). Bibian et al. demonstrated that IGF1-mediated activation of PI3K markedly inhibited TSH-dependent NIS transcription in FRTL5 cells, a rat thyroid cell line (114). Similarly, Kogai et al. showed that the PI3K inhibitor LY294002 significantly increased iodide uptake and enhanced PAX8 expression in PCCL3 cells by stimulating NIS post-translational regulation (115). Pharmacological inhibition of PI3K led to approximately a 3.5-fold increase in exogenous NIS expression and iodine uptake in BHP 2–7 cells, which harbor the RET/PTC1 rearrangement (115). Liu et al. found that inhibition of Akt using Akti-1/2 in PCCL3 cells did not increase NIS protein expression but significantly reduced iodide efflux and enhanced the affinity between iodine and NIS, thereby promoting RAI uptake (116). Rapamycin, an mTOR inhibitor, improved iodine uptake and reduced cell viability in PCCL3 cells, despite not elevating NIS protein levels. Its synthetic analog, everolimus, similarly enhanced thyroid iodine uptake in vivo (117). Moreover, Plantinga et al. reported that rapamycin increased both mRNA and protein levels of human NIS (hNIS) and RAI uptake capacity in TC cells by inhibiting mTOR via transcriptional upregulation of TTF1 expression (118). Additionally, Chen et al. found that overexpression of small nucleolar RNA host gene 7 upregulated dipeptidyl peptidase 4 (DPP4), promoting TC cell proliferation and I131 resistance through activation of the PI3K/Akt pathway (119). In summary, these findings support the conclusion that aberrant activation of the PI3K/Akt/mTOR pathway reduces NIS mediated radioiodine accumulation in the thyroid gland.
3.1.3 AMPK signaling pathway
The AMP-activated protein kinase (AMPK) signaling pathway is activated in response to cellular energy depletion and is notably upregulated in PTCs, contributing to TC progression (92). The AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) significantly reduced NIS mRNA and protein expression in PCCL3 cells. However, this effect was reversed by TSH-induced activation of the cyclic adenosine monophosphate (cAMP)/protein kinase A pathway or treatment with bafilomycin A1, both of which inhibited AMPK signaling (92, 93). Gonçalves et al. demonstrated that rutin increased NIS mRNA and protein expression and reduced RAI efflux both in vitro and in vivo by inhibiting the AMPK pathway and ROS generation (120, 121). Additionally, Compound C, an AMPK inhibitor, enhanced NIS expression by promoting activation of the cyclic AMP response element (CRE) in the NIS promoter, through suppression of AMPK signaling (84). In summary, aberrant activation of the AMPK pathway can downregulate RAI uptake in both non-neoplastic thyroid follicular cells and TC cells.
3.1.4 Other signaling pathway
Activation of the transforming growth factor-beta (TGF-β) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways also negatively regulates NIS expression and RAI uptake in the thyroid gland. Further details are available in reference (104).
3.2 Impairment of NIS function
In addition to adequate overall NIS expression, proper localization, dimerization, and glycosylation of NIS are also essential for enhancing the efficacy of RAI therapy (105, 122). Faria et al. demonstrated that P120 catenin, a component of adherens junctions (AJs), recruits SRC and RAC1 kinases. SRC phosphorylates VAV2, which in turn activates RAC1, promoting NIS retention on the plasma membrane (PM) (105, 122). Fletcher et al. further showed that ADP-ribosylation factor 4 (ARF4) increases NIS presence on the PM by facilitating its transport from the Golgi apparatus, while valosin-containing protein (VCP) mediates NIS degradation (123). Additionally, Thompson et al. reported that point mutations in NIS residues (e.g., Y242 and T243) impair RAI uptake by disrupting NIS dimerization (124). Regarding glycosylation, Chung et al. found that cAMP enhances hNIS expression, membrane localization, and RAI uptake by promoting NIS glycosylation in HeLa cells and xenograft models. These effects were reversed by tunicamycin, which inhibited NIS glycosylation in vitro (125). Collectively, disruptions in PM localization, dimerization, or glycosylation impair NIS function and reduce RAI uptake (Figure 2).
4 Current clinical treatments and clinical trials
Given the heterogeneity of RAIR-DTC, a range of treatment strategies is employed in clinical practice. Asymptomatic and indolent cases are typically managed with suppressive levothyroxine therapy, while symptomatic, progressive oligometastatic RAIR-DTC may require surgical intervention, external beam radiotherapy, or thermal ablation (10). In contrast, symptomatic, rapidly progressive, inoperable locally advanced or widely metastatic RAIR-DTC presents significant clinical challenges and is often primarily treated with multi-kinase inhibitors (MKIs), selective kinase inhibitors (SKIs), or redifferentiation therapy (126, 127). Due to its complexity, this aggressive subtype has become a primary focus of our clinical management efforts. Unlike cytotoxic chemotherapy, these newer targeted therapies have significantly prolonged PFS in patients with RAIR-DTC (Table 5). However, improvements in OS have been limited, with only Apatinib and Lenvatinib (in patients over 65) showing OS benefits. In this review, we retrospectively analyze and synthesize the efficacy and safety profiles of targeted and redifferentiation therapies, both approved agents and those under clinical investigation, used as monotherapy or in combination, as summarized below (Figure 3).
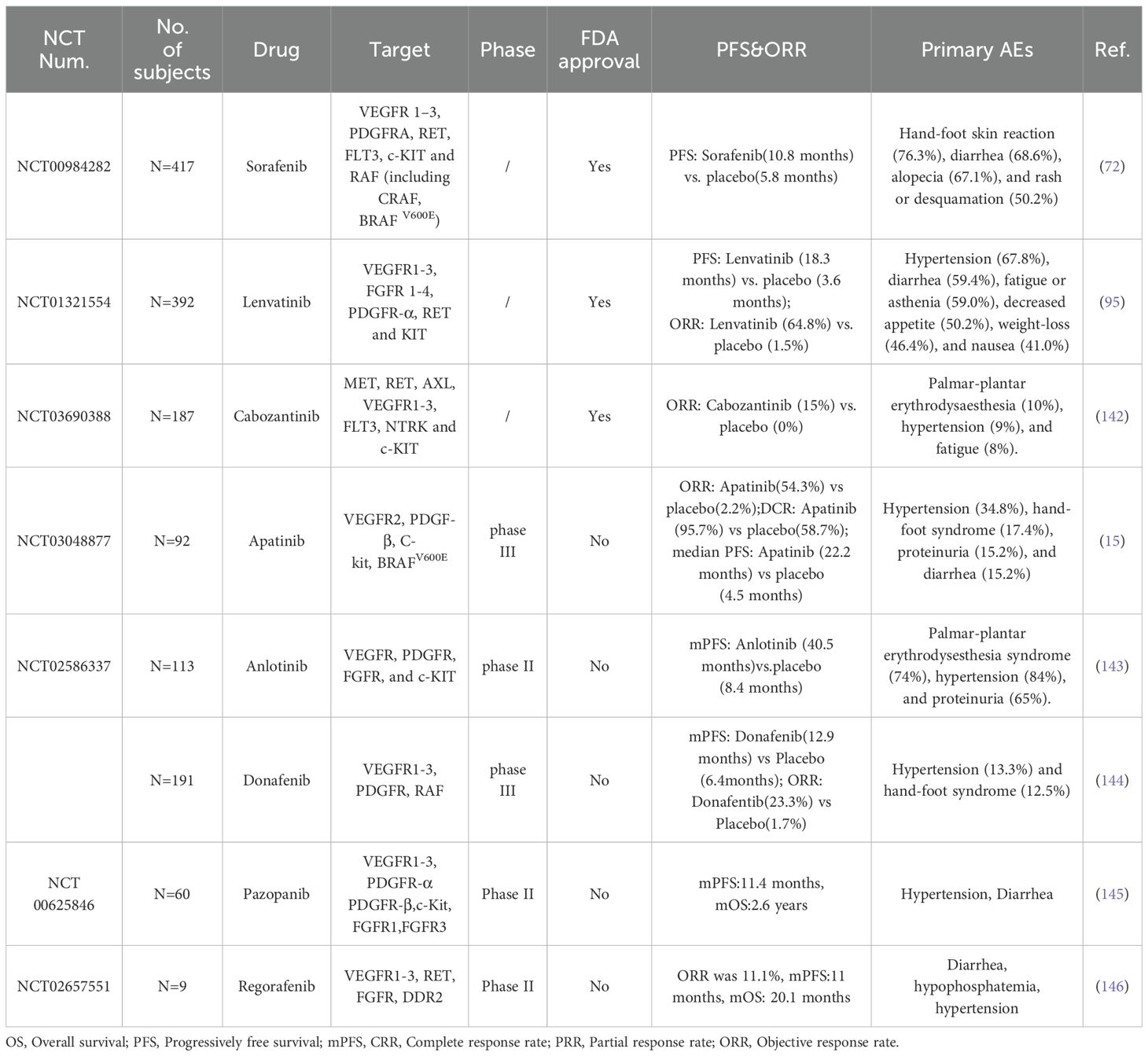
Table 5. Summary of different MKIs in RAIR-DTCs that are either approved or undergoing clinical trials.
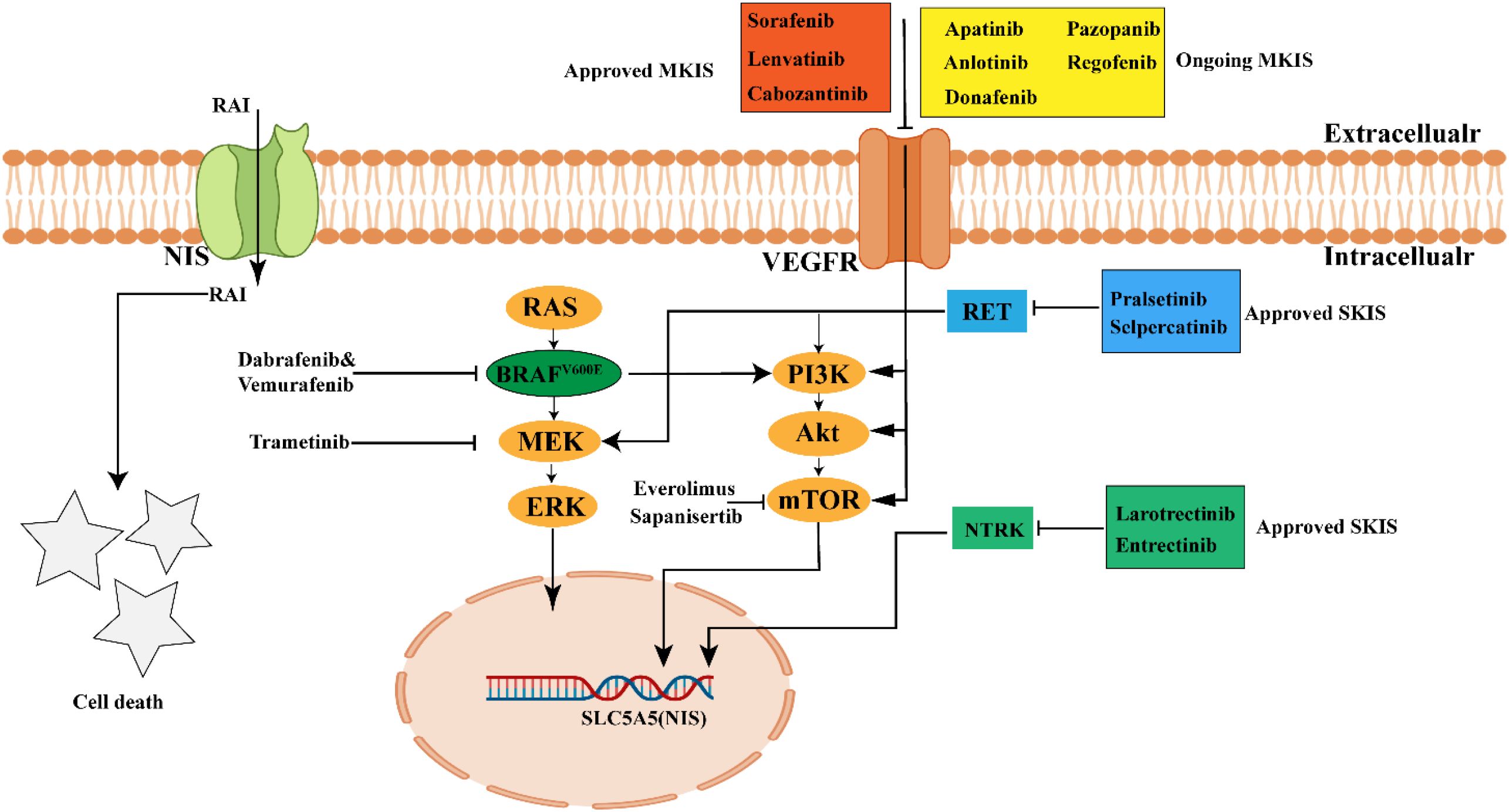
Figure 3. Current treatment drugs in RAIR-DTCs. MKIs (such as Lenvatinib, Sorafenib, Cabozantinib) and SKIs (such as Pralsetinib, Selpercatinib, Larotrectinib and Entrectinib) have been approved by FDA to treat RAIR-DTCs. Other kinase inhibitors are ongoing in Clinical trial. MKIs, muliti-kinase inhibitors; SKIs, selective kinase inhibitors; The picture was created with BioRender.com.
4.1 Targeted therapy
Targeted therapy for RAIR-DTC primarily relies on the use of MKIs and SKIs to achieve therapeutic efficacy (126). The MKIs function by inhibiting a range of receptors and kinases, including the vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and fibroblast growth factor receptor (FGFR), thereby suppressing tumor cell proliferation and growth (128). Notably, Sorafenib, Lenvatinib, and Cabozantinib have been approved by the FDA for the treatment of RAIR-DTC (129–131), while other MKIs are still under clinical investigation. Regarding SKIs, the FDA has approved RET inhibitors (Selpercatinib and Pralsetinib) and NTRK fusion inhibitors (Larotrectinib and Entrectinib) for RAIR-DTC patients with RET or NTRK alterations (132–135). In this section, we highlight the clinical progress of FDA approved MKIs and SKIs in improving RAIR-DTC prognosis and summarize other MKIs currently in clinical trials (Tables 5, 6).
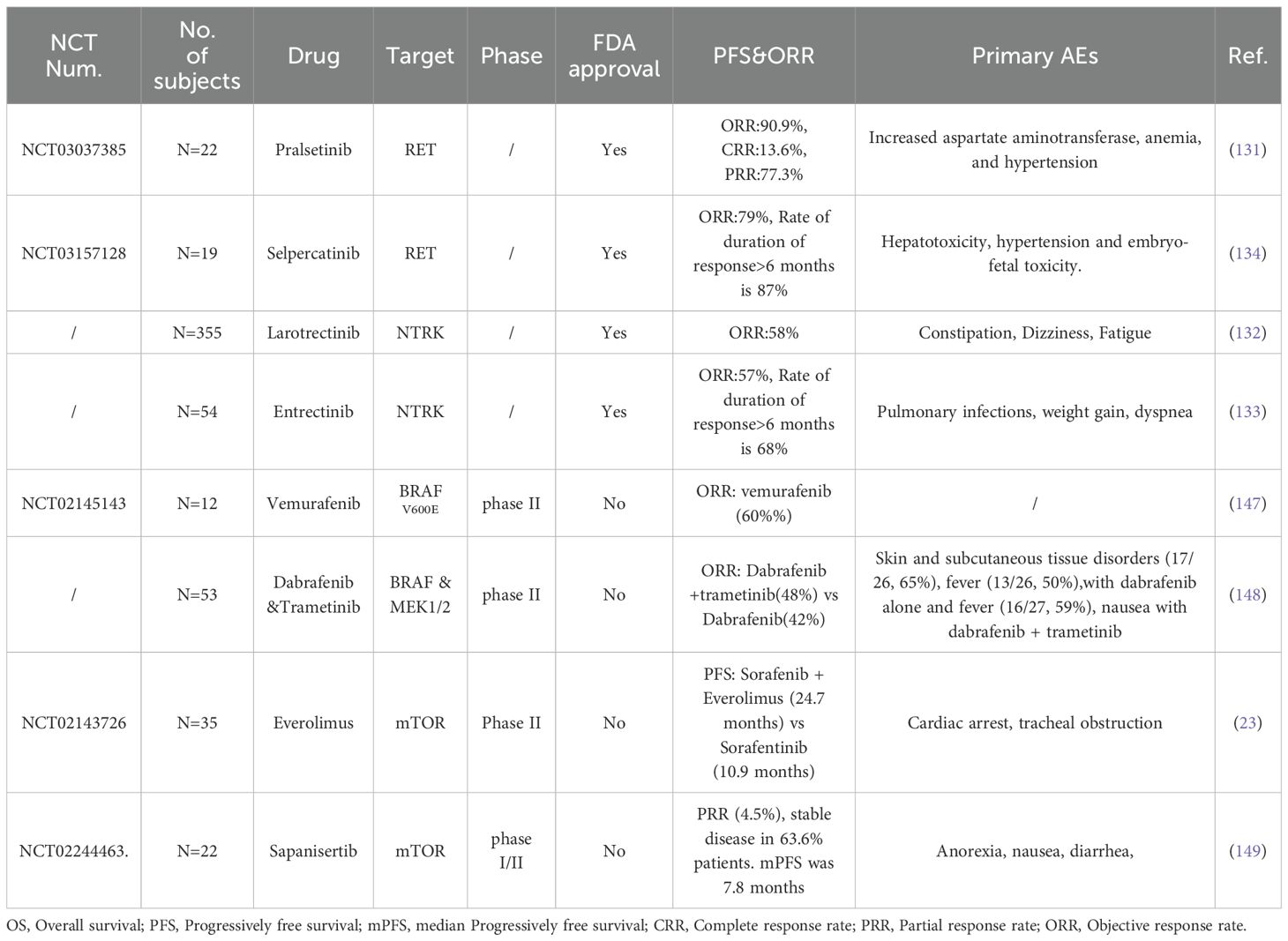
Table 6. Summary of different selective kinase inhibitors (SKIs) in RAIR-DTC that are either approved or undergoing clinical trials.
4.1.1 Approved MKIs
4.1.1.1 Sorafenib and Lenvatinib
Sorafenib, a multi-targeted kinase inhibitor, exhibits activity against RET, CRAF, BRAF (including both wild-type and V600E mutant), VEGFR1-3, FLT3, and c-KIT. In a multicenter, randomized, double-blind, placebo-controlled, phase 3 clinical trial (N=417, NCT00984282), Sorafenib significantly prolonged the PFS of RAIR-DTCs than placebo (10.8 vs. 5.8 months) (72). Consequently, Sorafenib became the first MKI approved by the FDA for the treatment of RAIR-DTC in both first-line and second-line settings (136). Similarly, Lenvatinib, another oral MKI targeting VEGFR1-3, FGFR1-4, PDGFRα, and RET, markedly extended the PFS in RAIR-DTCs than placebo (18.3 vs. 3.6 months) in a randomized, double-blind, multicenter phase 3 clinical trial(NCT01321554) (95). Subsequently, Lenvatinib also received FDA approval for use as a first- or second-line therapy in the RAIR-DTC treatment (129). Notably, a sub-analysis evaluating age-related efficacy and safety of Lenvatinib indicated a significant OS advantage over placebo in patients aged over 65 years old(P=0.02) (14). As for it, a phase 3 clinical trial conducted in the Chinese population demonstrated that Lenvatinib significantly improved PFS (23.9 months vs. 3.7 months) and objective response rate(ORR)(59% vs.24%) in RAIR-DTC compared to placebo, accompanied with several AEs similar to those observed in Schlumberger’s clinical studies (N=151, NCT02966093) (137).
4.1.1.2 Cabozantinib
Cabozantinib was developed to overcome resistance to VEGFR inhibitors by targeting the MET pathway (138), and it significantly prolonged PFS compared with control (11.0 months vs. 1.9 months) in a randomized, double-blind, placebo-controlled phase 3 trial (NCT03690388). At present, Cabozantinib is approved for the treatment of adult patients with locally advanced or metastatic DTC who have progressed after receiving VEGFR targeted therapy. It is also indicated a second-line treatment option for RAIR-DTCs after the failure of first-line treatment, such as Lenvatinib.
4.1.2 Unapproved MKIs
4.1.2.1 Apatinib
Apatinib is an oral inhibitor that targets VEGFR2, PDGFR-β, C-kit, and BRAFV600E. In a phase 3 randomized clinical trial involving 92 patients with RAIR-DTC, Apatinib significantly prolonged PFS compared to placebo (22.2 vs. 4.5 months). The ORR in the Apatinib group was 54.3%, with a disease control rate (DCR) of 95.7%, compared to an ORR of 2.2% and a DCR of 58.7% in the placebo group. The most common grade ≥3 AE was hypertension, occurring in 34.8% of participants (15).
4.1.2.2 Anlotinib
As a MKIs, Anlotinib targets VEGFR, PDGFR, FGFR, and c-KIT. In a randomized, double-blind, multicenter phase 2 trial of Anlotinib in locally advanced or metastatic RAIR-DTC (NCT02586337), the median PFS was 40.54 months in Anlotinib and 8.38 months in placebo. The ORR was 59.21% in Anlotinib, in addition, significant DCR benefit was observed in Anlotinib treatment (97.37% vs. 78.38%). The most common AEs were hypertension (84.21%) and hypertriglyceridemia (68.42%) (139). Although the Anlotinib is not approved by the FDA, but it is approved by the China National Medical Products Administration (NMPA) for the indication of RAIR-DTC, based on its promising efficacy.
4.1.2.3 Donafenib
Donafenib is a novel oral MKI that targets VEGFR1-3, PDGFR, and RAF. In a multicenter, randomized, double-blind phase 3 trial, Donafenib demonstrated a prolonged median PFS compared to placebo (12.9 vs. 6.4 months) in Chinese RAIR-DTCs. Additionally, the Donafenib group exhibited improved ORR of 23.3% compared to 1.7% for placebo (P = 0.0002) and DCR of 93.3% versus 79.3% for placebo (P = 0.0044). The most common grade≥3 treatment-related AEs associated with Donafenib included hypertension (13.3%) and hand-foot syndrome (12.5%). 42.2% of patients required dose reduction or interruption, while 6.3% experienced treatment discontinuation (140). Based on the safety and efficacy data of Donafenib in clinical treatments, it has been approved by the NMPA for the treatment of RAIR-DTC.
4.1.2.4 Pazopanib
Pazopanib is an oral inhibitor of VEGFR1-3, PDGFR-α/β, c-Kit, and FGFR1/3. In an international phase 2 study of progressive and metastatic thyroglobulin antibody negative RAIR-DTCs (NCT00625846), the median PFS and OS of Pazopanib group were estimated to be 11.4 months and 2.6 years from start of study therapy initiation, respectively. Common AEs included one death (thromboembolic) deemed possibly associated with pazopanib (141).
4.1.2.5 Regorafenib
Regorafenib is an oral inhibitor of VEGFR1-3, RET, FGFR and DDR2. In a phase 2 trial of RAIR-DTCs (NCT02657551), the ORR of Regorafenib was 11.1%, median PFS and OS were 11.0 and 20.1 months respectively. As for AEs, it mainly includes diarrhea, hypophosphatemia and hypertension and there were no treatment-related deaths (142). Overall, the clinical efficacy of Regorafenib is not ideal.
4.1.3 Approved selective kinase inhibitors
4.1.3.1 RET inhibitors
Currently, the FDA has approved RET inhibitors (Selpercatinib and Pralsetinib) for adult and pediatric patients aged 12 years and older with advanced or metastatic RET fusion-positive TC who require systemic therapy and are classified as RAIR. Subbiah et al. conducted a study demonstrating that the ORR of Pralsetinib in patients with RET fusion-positive thyroid cancer who had received prior systemic treatment was 90.9% (N=22), with several AEs reported, including increased aspartate aminotransferase, anemia, and hypertension (132). The efficacy of Selpercatinib was evaluated in 27 RAIR-DTCs with RET fusion-positive and ORR was 85%, common AEs include diarrhea, hypertension and fatigue (135).
4.1.3.2 NTRK fusion inhibitors
Moreover, NTRK inhibitors, namely Larotrectinib and Entrectinib, have been approved for patients harboring NTRK1–3 fusion mutations in RAIR-DTC. Waguespack et al. analyzed the ORR of Larotrectinib, which was found to be 71%(N=28) in NTRK fusion positive TCs (143). Notably, the most common AEs associated with Larotrectinib include fatigue, cough, and constipation. The ORR of Entrectinib in TCs with NTRK fusion positivity was reported at 20% (N=5), with a duration of response (DOR) of 7.9 months (134). The most frequently observed AEs for Entrectinib included pulmonary infections, weight gain, and dyspnea.
4.2 Redifferentiation therapy
Due to dedifferentiation, the functional expression of the NIS is diminished in RAIR-DTC, consequently reducing the uptake and efficacy of RAI. Redifferentiation therapy aims to restore RAI uptake capacity by enhancing NIS expression. Current, clinical research has demonstrated the potential of BRAF inhibitors (Dabrafenib and Vemurafenib) (145, 148), MAPK kinase (MEK) inhibitors (Trametinib) (149) and mTOR inhibitors (Everolimus and Sapanisertib) (24, 147) to enhance NIS expression and improve iodine uptake. Notably, Leboulleux et al. conducted a phase 2 clinical trial, showing that the combination of Dabrafenib and Trametinib for redifferentiation therapy significantly improved the efficacy of I131 uptake in BRAFV600E mutant RAIR-DTCs, with 38% of patients achieving partial remission and 52% presenting with stable disease (25). Moreover, Sherman et al. conducted a phase 2 clinical trial, demonstrating that the combination of Sorafenib and Everolimus for redifferentiation therapy significantly prolonged the PFS in RAIR-DTCs than Sorafenib alone(24.7 vs 10.7 months) (24). Although these inhibitors have demonstrated promising efficacy in clinical trials, they have not yet been approved by the FDA and require further clinical trial evaluation.
These clinical investigations demonstrate that target and redifferentiation therapies can improve the living conditions of patients in RAIR-DTCs, but the high cost and serious AEs have not yet reached the expectations of patients. The future drug development should be closely combined with the molecular pathogenesis, namely the development of specific and effective drugs against RAIR-DTC should be a mainstream direction.
5 Conclusions
In recent decades, there has been a significant improvement in treatment outcomes and prognoses for DTCs. Nonetheless, approximately 10% of patients with DTC still experience recurrence, metastasis, and resistance to RAI, leading to a markedly poor prognosis. Despite considerable advancements in elucidating the molecular mechanisms and developing targeted therapies for RAIR-DTC, SKIs or MKIs including those approved or currently undergoing clinical trial have demonstrated improvement in PFS. However, most targeted therapies have not demonstrated substantial enhancements in in OS, with the notable exceptions of Lenvatinib and Apatinib, which are associated with numerous AEs.
To reduce the incidence of RAIR-DTC and improve its treatment prognosis, we first summarized high-risk factors of RAIR-DTC in this review. Through detecting genetic mutations (e.g., RAS/BRAF/TERT promoter), clinical pathological features (e.g., ETE, and distant metastasis), and early monitoring biomarkers(e.g., Cyfra21.1 and TG) could aids in the early detection or prediction of RAIR and timely clinical intervention (34, 47, 56, 58, 63). Importantly, we have discussed several potential inducing factors stemming from comorbidities (e.g., diabetes, ML, and blood pressure disorders) and unsuitable medications (e.g., Metformin, Nilotinib, Etilefrine, etc.) that could activate the AMPK pathway, thereby inducing RAIR (84, 96, 97, 100–102). Subsequent, we reviewed the molecular mechanisms by which the abnormal activation of signaling pathways, driven by genetic mutations, leads to impaired expression and function of NIS in RAIR-DTC. Concurrently, we summarized the safety and efficacy of KIs that are currently approved by the FDA for RAIR-DTC, as well as those undergoing clinical trials. Based on the results of ongoing clinical trials, although existing drugs demonstrate certain efficacy, they are frequently associated with severe AEs. Future efforts should focus on further exploring the molecular mechanisms, optimizing existing drugs, and developing new RAIR-DTC treatments that are safe, effective, and specific.
Author contributions
TM: Writing – original draft, Writing – review & editing, Visualization. YX: Investigation, Writing – review & editing. XL: Investigation, Writing – review & editing. FY: Funding acquisition, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82172850, 82472697), the Sichuan Provincial Department of Science and Technology (2023YFS0120, 23NSFSC1623) and the China Society of Clinical Oncology (Y-HR2019-0310).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Avram AM, Zukotynski K, Nadel HR, and Giovanella L. Management of differentiated thyroid cancer: the standard of care. J Nucl Med. (2022) 63:189–95. doi: 10.2967/jnumed.121.262402
2. Li M, Dal Maso L, and Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. (2020) 8:468–70. doi: 10.1016/S2213-8587(20)30115-7
3. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
4. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4(1):47–53. doi: 10.1016/j.jncc.2024.01.006
5. Valerio L, Maino F, Castagna MG, and Pacini F. Radioiodine therapy in the different stages of differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. (2023) 37:101703. doi: 10.1016/j.beem.2022.101703
6. Zhang S, Zhu M, Zhang H, Liu H, Fan X, Zhang J, et al. The effect of radioiodine therapy on the prognosis of differentiated thyroid cancer with lung metastases. Biomedicines. (2024) 12:532. doi: 10.3390/biomedicines12030532
7. Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. (2003) 197:191–7. doi: 10.1016/S1072-7515(03)00332-6
8. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
9. Voinea I-A, Petrova E, Dumitru N, Cocoloş A, Ioachim D, Goldstein AL, et al. Pathogenesis and management strategies in radioiodine-refractory differentiated thyroid cancer: from molecular mechanisms toward therapeutic approaches: A comprehensive review. J Clin Med. (2024) 13:7161. doi: 10.3390/jcm13237161
10. Lin Y-S, Wang R-F, Huang R, Wen Q, Cao W, Chen L-B, et al. Chinese management guidelines for radioactive iodine-refractory differentiated thyroid cancer (2025 edition). Eur J Nucl Med Mol Imaging. (2025), 1–18. doi: 10.1007/s00259-025-07222-1
11. Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. (2018) 28:1083–93. doi: 10.1089/thy.2018.0048
12. Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, and DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. (1985) 56:2155–60. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e
13. Argiris A, Agarwala SS, Karamouzis MV, Burmeister LA, and Carty SE. A phase II trial of doxorubicin and interferon alpha 2b in advanced, non-medullary thyroid cancer. Invest New Drugs. (2008) 26:183–8. doi: 10.1007/s10637-007-9091-2
14. Brose MS, Worden FP, Newbold KL, Guo M, and Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. (2017) 35:2692–9. doi: 10.1200/JCO.2016.71.6472
15. Lin Y, Qin S, Li Z, Yang H, Fu W, Li S, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY randomized clinical trial. JAMA Oncol. (2022) 8:242–50. doi: 10.1001/jamaoncol.2021.6268
16. Steinmüller T, Klupp J, Wenking S, and Neuhaus P. Complications associated with different surgical approaches to differentiated thyroid carcinoma. Langenbecks Arch Surg. (1999) 384:50–3. doi: 10.1007/s004230050173
17. Auttara-Atthakorn A, Sungmala J, Anothaisintawee T, Reutrakul S, and Sriphrapradang C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials. Front Endocrinol (Lausanne). (2022) 13:960265. doi: 10.3389/fendo.2022.960265
18. Zhang Y, Li Y, Lin Z, and Chen W. Can 99 Tc m -3PRGD 2 (α ν β 3) and 18 F-FDG dual-tracer molecular imaging change the therapeutic strategy for progressive refractory differentiated thyroid cancer: Case report. Med (Baltimore). (2023) 102:e32751. doi: 10.1097/MD.0000000000032751
19. Malik N, Nikitski AV, Klam E, Hunt J, Witt B, Chadwick B, et al. MOLECULAR PROFILE AND CLINICAL OUTCOMES IN DIFFERENTIATED THYROID CANCER PATIENTS PRESENTING WITH BONE METASTASIS. Endocr Pract. (2019) 25:1255–62. doi: 10.4158/EP-2019-0265
20. Yoon JH, Jeon MJ, Kim M, Hong AR, Kim HK, Shin DY, et al. Unusual metastases from differentiated thyroid cancers: A multicenter study in Korea. PloS One. (2020) 15:e0238207. doi: 10.1371/journal.pone.0238207
21. Samimi H and Haghpanah V. Molecular evidence reveals thyrotropin intervention enhances the risk of developing radioiodine-refractory differentiated thyroid carcinoma. Cancer Cell Int. (2022) 22:61. doi: 10.1186/s12935-022-02484-3
22. Jimenez-Fonseca P. Use of multikinase inhibitors/lenvatinib in patients with high cardiovascular risk/vasculopathy and radioiodine refractory-differentiated thyroid cancer. Cancer Med. (2022) 11 Suppl 1:17–25. doi: 10.1002/cam4.5127
23. Díaz Vico T, Martínez-Amores Martínez B, Mihic Góngora L, Jiménez-Fonseca P, Peinado Martín P, Grao Torrente I, et al. Systemic therapeutic options in radioiodine-refractory differentiated thyroid cancer: current indications and optimal timing. Cancers (Basel). (2025) 17:1800. doi: 10.3390/cancers17111800
24. Sherman EJ, Foster NR, Su YB, Shergill A, Ho AL, Konda B, et al. Randomized phase II study of sorafenib with or without everolimus in patients with radioactive iodine refractory Hürthle cell thyroid cancer (HCC)(Alliance A091302/ITOG 1706). J Clin Oncol. (2021):6076. doi: 10.1200/JCO.2021.39.15_suppl.6076
25. Leboulleux S, Do Cao C, Zerdoud S, Attard M, Bournaud C, Lacroix L, et al. A phase II redifferentiation trial with dabrafenib-trametinib and 131I in metastatic radioactive iodine refractory BRAF p. V600E-mutated differentiated thyroid cancer. Clin Cancer Res. (2023) 29:2401–9. doi: 10.1158/1078-0432.CCR-23-0046
26. Cabanillas ME, Dadu R, Iyer P, Wanland KB, Busaidy NL, Ying A, et al. Acquired secondary RAS mutation in BRAFV600E-mutated thyroid cancer patients treated with BRAF inhibitors. Thyroid. (2020) 30:1288–96. doi: 10.1089/thy.2019.0514
27. Ohno K, Shibata T, and Ito K-I. Epidermal growth factor receptor activation confers resistance to lenvatinib in thyroid cancer cells. Cancer Sci. (2022) 113:3193–210. doi: 10.1111/cas.15465
28. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. (2014) 2:356–8. doi: 10.1016/S2213-8587(13)70215-8
29. Li G, Lei J, Song L, Jiang K, Wei T, Li Z, et al. Radioiodine refractoriness score: A multivariable prediction model for postoperative radioiodine-refractory differentiated thyroid carcinomas. Cancer Med. (2018) 7:5448–56. doi: 10.1002/cam4.1794
30. Parvathareddy SK, Siraj AK, Siraj N, Ahmed SO, Al-Rasheed M, Qadri Z, et al. Radioactive iodine refractoriness in Middle Eastern differentiated thyroid cancer: clinical outcome and risk factor analysis. Front Endocrinol (Lausanne). (2024) 15:1326976. doi: 10.3389/fendo.2024.1326976
31. Schubert L, Mbekwe-Yepnang AM, Wassermann J, Braik-Djellas Y, Jaffrelot L, Pani F, et al. Clinico-pathological factors associated with radioiodine refractory differentiated thyroid carcinoma status. J Endocrinol Invest. (2024) 47:1573–81. doi: 10.1007/s40618-024-02352-z
32. Mu Z, Zhang X, Liang D, Fang J, Chen G, Guo W, et al. Risk stratification for radioactive iodine refractoriness using molecular alterations in distant metastatic differentiated thyroid cancer. Chin J Cancer Res. (2024) 36:25–35. doi: 10.21147/j.issn.1000-9604.2024.01.03
33. Chai J, Zhang R, Zheng W, Zhang G, Jia Q, Tan J, et al. Predictive value of clinical and pathological characteristics for metastatic radioactive iodine-refractory differentiated thyroid carcinoma: A 16-year retrospective study. Front Endocrinol (Lausanne). (2022) 13:930180. doi: 10.3389/fendo.2022.930180
34. Shobab L, Gomes-Lima C, Zeymo A, Feldman R, Jonklaas J, Wartofsky L, et al. Clinical, pathological, and molecular profiling of radioactive iodine refractory differentiated thyroid cancer. Thyroid. (2019) 29:1262–8. doi: 10.1089/thy.2019.0075
35. Wang C, Zhang X, Li H, Li X, and Lin Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PloS One. (2017) 12:e0179664. doi: 10.1371/journal.pone.0179664
36. Saïe C, Wassermann J, Mathy E, Chereau N, Leenhardt L, Tezenas du Montcel S, et al. Impact of age on survival in radioiodine refractory differentiated thyroid cancer patients. Eur J Endocrinol. (2021) 184:667–76. doi: 10.1530/EJE-20-1073
37. Wang R, Zhang Y, Tan J, Zhang G, Zhang R, Zheng W, et al. Analysis of radioiodine therapy and prognostic factors of differentiated thyroid cancer patients with pulmonary metastasis: An 8-year retrospective study. Med (Baltimore). (2017) 96:e6809. doi: 10.1097/MD.0000000000006809
38. Liu W, Jiang B, Xue J, Liu R, Wei Y, and Li P. Clinicopathological features of differentiated thyroid carcinoma as predictors of the effects of radioactive iodine therapy. Ann Diagn Pathol. (2024) 69:152243. doi: 10.1016/j.anndiagpath.2023.152243
39. Hurst Z, Liyanarachchi S, He H, Brock P, Sipos J, Nabhan F, et al. Risk haplotypes uniquely associated with radioiodine-refractory thyroid cancer patients of high african ancestry. Thyroid. (2019) 29:530–9. doi: 10.1089/thy.2018.0687
40. Riccio I, Laforteza A, Landau MB, Hussein MH, Linhuber J, Staav J, et al. Decoding RAS mutations in thyroid cancer: A meta-analysis unveils specific links to distant metastasis and increased mortality. Am J Otolaryngol. (2024) 46:104570. doi: 10.1016/j.amjoto.2024.104570
41. Bikas A, Ahmadi S, Pappa T, Marqusee E, Wong K, Nehs MA, et al. Additional oncogenic alterations in RAS-driven differentiated thyroid cancers associate with worse clinicopathologic outcomes. Clin Cancer Res. (2023) 29:2678–85. doi: 10.1158/1078-0432.CCR-23-0278
42. Portella G, Vitagliano D, Borselli C, Melillo RM, Salvatore D, Rothstein JL, et al. Human N-ras, TRK-T1, and RET/PTC3 oncogenes, driven by a thyroglobulin promoter, differently affect the expression of differentiation markers and the proliferation of thyroid epithelial cells. Oncol Res. (1999) 11:421–7.
43. Saavedra HI, Knauf JA, Shirokawa JM, Wang J, Ouyang B, Elisei R, et al. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. (2000) 19:3948–54. doi: 10.1038/sj.onc.1203723
44. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, and Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. (2003) 63:1454–7.
45. Dong H, Shen W-Z, Yan Y-J, Yi J-L, and Zhang L. Effects of BRAF(V600E) mutation on Na(+)/I(-) symporter expression in papillary thyroid carcinoma. J Huazhong Univ Sci Technolog Med Sci. (2016) 36:77–81. doi: 10.1007/s11596-016-1545-3
46. Makboul R, Mostafa NM, El-Deek HEM, Aboulhagag NA, Shehata MR, and Abdelhafez YG. Do BRAFV600E mutation and sodium-iodide symporter expression affect the response to radioactive iodine therapy in patients with papillary thyroid carcinoma? Nucl Med Commun. (2020) 41:416–25. doi: 10.1097/MNM.0000000000001171
47. Yu P, Qu N, Zhu R, Hu J, Han P, Wu J, et al. TERT accelerates BRAF mutant-induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci Adv. (2023) 9:eadg7125. doi: 10.1126/sciadv.adg7125
48. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
49. Tan G, Jin B, Qian X, Wang Y, Zhang G, Agyekum EA, et al. TERT promoter mutations contribute to adverse clinical outcomes and poor prognosis in radioiodine refractory differentiated thyroid cancer. Sci Rep. (2024) 14:23719. doi: 10.1038/s41598-024-75087-9
50. Ju G, Sun Y, Wang H, Zhang X, Mu Z, Sun D, et al. Fusion oncogenes in patients with locally advanced or distant metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. (2024) 109:505–15. doi: 10.1210/clinem/dgad500
51. Subbiah V, Yang D, Velcheti V, Drilon A, and Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. (2020) 38:1209–21. doi: 10.1200/JCO.19.02551
52. Lee YA, Lee H, Im S-W, Song YS, Oh D-Y, Kang HJ, et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. (2021) 131(18). doi: 10.1172/JCI144847
53. Manea CA, Badiu DC, Ploscaru IC, Zgura A, Bacinschi X, Smarandache CG, et al. A review of NTRK fusions in cancer. Ann Med Surg (Lond). (2022) 79:103893. doi: 10.1016/j.amsu.2022.103893
54. Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, et al. NTRK fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers (Basel). (2021) 13(8):1932. doi: 10.3390/cancers13081932
55. Chu Y-H, Dias-Santagata D, Farahani AA, Boyraz B, Faquin WC, Nosé V, et al. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol. (2020) 33:2186–97. doi: 10.1038/s41379-020-0574-4
56. Luo Y, Jiang H, Xu W, Wang X, Ma B, Liao T, et al. Clinical, pathological, and molecular characteristics correlating to the occurrence of radioiodine refractory differentiated thyroid carcinoma: A systematic review and meta-analysis. Front Oncol. (2020) 10:549882. doi: 10.3389/fonc.2020.549882
57. Capdevila J, Matos I, Mancuso FM, Iglesias C, Nuciforo P, Zafon C, et al. Identification of expression profiles defining distinct prognostic subsets of radioactive-iodine refractory differentiated thyroid cancer from the DECISION trial. Mol Cancer Ther. (2020) 19:312–7. doi: 10.1158/1535-7163.MCT-19-0211
58. Sa R, Cheng L, Jin Y, Fu H, Shen Y, and Chen L. Distinguishing patients with distant metastatic differentiated thyroid cancer who biochemically benefit from next radioiodine treatment. Front Endocrinol (Lausanne). (2020) 11:587315. doi: 10.3389/fendo.2020.587315
59. Cheng X, Xu S, Zhu Y, Wu J, Bao J, Yu H, et al. Markedly elevated serum preoperative thyroglobulin predicts radioiodine-refractory thyroid cancer. Eur J Clin Invest. (2022) 52:e13721. doi: 10.1111/eci.13721
60. Meng C, Song J, Long W, Mu Z, Sun Y, Liang J, et al. A user-friendly nomogram for predicting radioiodine refractory differentiated thyroid cancer. Front Endocrinol (Lausanne). (2023) 14:1109439. doi: 10.3389/fendo.2023.1109439
61. Nilsson JN, Siikanen J, Condello V, Jatta K, Saini R, Hedman C, et al. Iodine avidity in papillary and poorly differentiated thyroid cancer is predicted by immunohistochemical and molecular work-up. Eur Thyroid J. (2023) 12:4. doi: 10.1530/ETJ-23-0099
62. Zelinskaya A. Immunocytochemical characteristics of thyrocytes in radioiodine refractory metastases of papillary thyroid cancer. Exp Oncol. (2019) 41:342–5. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-4.13705
63. Jeong C, Lee J, Yoon H, Ha J, Kim M-H, Bae J-S, et al. Serum CYFRA 21.1 level predicts disease course in thyroid cancer with distant metastasis. Cancers (Basel). (2021) 13:811. doi: 10.3390/cancers13040811
64. Tian T, Dai H, Zhang M, Su M, Chen X, and Huang R. Lactate dehydrogenase A is associated with elevated FDG metabolism, radioiodine non-avidity, and poor prognosis in differentiated thyroid cancer. Acad Radiol. (2024) 31:4011–20. doi: 10.1016/j.acra.2024.04.033
65. Pecce V, Sponziello M, Verrienti A, Grani G, Abballe L, Bini S, et al. The role of miR-139-5p in radioiodine-resistant thyroid cancer. J Endocrinol Invest. (2023) 46:2079–93. doi: 10.1007/s40618-023-02059-7
66. Collina F, La Sala L, Liotti F, Prevete N, La Mantia E, Chiofalo MG, et al. AXL is a novel predictive factor and therapeutic target for radioactive iodine refractory thyroid cancer. Cancers (Basel). (2019) 11:785. doi: 10.3390/cancers11060785
67. Liu H, Chen Q, Liu B, Wang J, Chen C, and Sun S. Blood profiles in the prediction of radioiodine refractory papillary thyroid cancer: A case-control study. J Multidiscip Healthc. (2023) 16:535–46. doi: 10.2147/JMDH.S403045
68. Coperchini F, Croce L, Denegri M, Pignatti P, Agozzino M, Netti GS, et al. Adverse effects of in vitro GenX exposure on rat thyroid cell viability, DNA integrity and thyroid-related genes expression. Environ pollut. (2020) 264:114778. doi: 10.1016/j.envpol.2020.114778
69. Li L-R, Song J-L, Liu H-Q, and Chen C. Metabolic syndrome and thyroid Cancer: risk, prognosis, and mechanism. Discov Oncol. (2023) 14:23. doi: 10.1007/s12672-022-00599-7
70. Agate L, Minaldi E, Basolo A, Angeli V, Jaccheri R, Santini F, et al. Nutrition in advanced thyroid cancer patients. Nutrients. (2022) 14:1298. doi: 10.3390/nu14061298
71. Yousef Hailan MY. SECONDARY CHRONIC MYELOID LEUKEMIA FOLLOWING RADIOACTIVE IODINE (I131). Hematology Transfusion Cell Therapy,. (2021) 43::S38. doi: 10.1016/j.htct.2021.10.1024
72. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
73. Shih S-R, Chiu W-Y, Chang T-C, and Tseng C-H. Diabetes and thyroid cancer risk: literature review. Exp Diabetes Res. (2012) 2012:578285. doi: 10.1155/2012/578285
74. Oluwasanjo A, Pathak R, Ukaigwe A, and Alese O. Therapy-related acute myeloid leukemia following radioactive iodine treatment for thyroid cancer. Cancer Causes Control. (2016) 27:143–6. doi: 10.1007/s10552-015-0682-5
75. Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. (1999) 84:4043–9. doi: 10.1210/jcem.84.11.6115
76. Nieto H and Boelaert K. WOMEN IN CANCER THEMATIC REVIEW: Thyroid-stimulating hormone in thyroid cancer: does it matter? Endocr Relat Cancer. (2016) 23:T109–T21. doi: 10.1530/ERC-16-0328
77. Akbar DH, Ahmed MM, and Al-Mughales J. Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Acta Diabetol. (2006) 43:14–8. doi: 10.1007/s00592-006-0204-8
78. Azouzi N, Cailloux J, Cazarin JM, Knauf JA, Cracchiolo J, Al Ghuzlan A, et al. NADPH oxidase NOX4 is a critical mediator of BRAFV600E-induced downregulation of the sodium/iodide symporter in papillary thyroid carcinomas. Antioxid Redox Signal. (2017) 26:864–77. doi: 10.1089/ars.2015.6616
79. Zhang C, Wang H, Li Y, Wang X, Han Y, Gao X, et al. Association between the triglyceride-glucose index and thyroid disorders: a cross-sectional survey and Mendelian randomization analysis. Endocrine. (2024) 86:173–85. doi: 10.1007/s12020-024-03858-5
80. Guo Y, Zhang Y, Chen Z, and Xin Z. Effects of recombinant human thyroid stimulating hormone on 131I therapy for the treatment of differentiated thyroid cancer. Exp Ther Med. (2015) 9:1847–50. doi: 10.3892/etm.2015.2330
81. Pasupathi P, Chandrasekar V, and Kumar US. Evaluation of oxidative stress, antioxidant and thyroid hormone status in patients with diabetes mellitus. J Med. (2009) 10:60. doi: 10.3329/jom.v10i2.2816
82. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. (2016) 2016:6757154. doi: 10.1155/2016/6757154
83. Klubo-Gwiezdzinska J, Costello J, Patel A, Bauer A, Jensen K, Mete M, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. (2013) 98:3269–79. doi: 10.1210/jc.2012-3799
84. Abdulrahman RM, Boon MR, Sips HCM, Guigas B, Rensen PCN, Smit JWA, et al. Impact of Metformin and compound C on NIS expression and iodine uptake in vitro and in vivo: a role for CRE in AMPK modulation of thyroid function. Thyroid. (2014) 24:78–87. doi: 10.1089/thy.2013.0041
85. Holt C. RAI following thyroid cancer surgery increases risk for AML & CML. Oncol Times. (2018):18–19. doi: 10.1097/01.COT.0000532223.98742.88
86. Rajeshwari U, Shobha I, Raghunatha R, and Andallu B. Oxidative stress and antioxidant status in acute and chronic myeloid Leukemia patients. Open J Blood Dis. (2013) 3:17–22. doi: 10.4236/ojbd.2013.33A004
87. Pascu EG, Găman M-A, Moisă C, Assani AD, and Găman AM. The involvement of oxidative stress in chronic myeloid leukemia. Rom Biotechnol Lett. (2020) 25:1267–74. doi: 10.25083/rbl/25.1/1267.1274
88. Zhou F-L, Zhang W-G, Wei Y-C, Meng S, Bai G-G, Wang B-Y, et al. Involvement of oxidative stress in the relapse of acute myeloid leukemia. J Biol Chem. (2010) 285:15010–5. doi: 10.1074/jbc.M110.103713
89. Cazarin J, Dupuy C, and Pires de Carvalho D. Redox homeostasis in thyroid cancer: implications in na+/I– symporter (NIS) regulation. Int J Mol Sci. (2022) 23:6129. doi: 10.3390/ijms23116129
90. Masarova L, Cortes JE, Patel KP, O’Brien S, Nogueras-Gonzalez GM, Konopleva M, et al. Long-term results of a phase 2 trial of nilotinib 400 mg twice daily in newly diagnosed patients with chronic-phase chronic myeloid leukemia. Cancer. (2020) 126:1448–59. doi: 10.1002/cncr.32623
91. Meng L, Zhao P, Hu Z, Ma W, Niu Y, Su J, et al. Nilotinib, a tyrosine kinase inhibitor, suppresses the cell growth and triggers autophagy in papillary thyroid cancer. Anticancer Agents Med Chem. (2022) 22:596–602. doi: 10.2174/1871520621666210402110331
92. Andrade BM, Araujo RL, Perry RLS, Souza ECL, Cazarin JM, Carvalho DP, et al. A novel role for AMP-kinase in the regulation of the Na+/I–symporter and iodide uptake in the rat thyroid gland. Am J Physiol Cell Physiol. (2011) 300:C1291–C7. doi: 10.1152/ajpcell.00136.2010
93. Cazarin JM, Andrade BM, and Carvalho DP. AMP-activated protein kinase activation leads to lysome-mediated NA(+)/I(-)-symporter protein degradation in rat thyroid cells. Horm Metab Res. (2014) 46:313–7. doi: 10.1055/s-0034-1371803
94. Yu L, Pan G, Li Z, Li L, Gao S, Liu F, et al. Impaired sensitivity to thyroid hormones is associated with different grades of hypertension: A multicenter cross-sectional study. Nutr Metab Cardiovasc Dis. (2024) 34:1581–9. doi: 10.1016/j.numecd.2023.12.019
95. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
96. Moon J-H, Jeong J-K, Hong J-M, Seol J-W, and Park S-Y. Inhibition of autophagy by captopril attenuates prion peptide-mediated neuronal apoptosis via AMPK activation. Mol Neurobiol. (2019) 56:4192–202. doi: 10.1007/s12035-018-1370-8
97. Zhao Y, Shang F, Shi W, Zhang J, Zhang J, Liu X, et al. Angiotensin II receptor type 1 antagonists modulate vascular smooth muscle cell proliferation and migration via AMPK/mTOR. Cardiology. (2019) 143:1–10. doi: 10.1159/000500038
98. Kim SG, Sung JY, Kim J-R, and Choi HC. Nifedipine-induced AMPK activation alleviates senescence by increasing autophagy and suppressing of Ca2+ levels in vascular smooth muscle cells. Mech Ageing Dev. (2020) 190:111314. doi: 10.1016/j.mad.2020.111314
99. Xu C, Ai D, Shi D, Suo S, Chen X, Yan Y, et al. Accurate drug repositioning through non-tissue-specific core signatures from cancer transcriptomes. Cell Rep. (2019) 29:1055. doi: 10.1016/j.celrep.2019.10.023
100. Yu H-C, Lin C-S, Tai W-T, Liu C-Y, Shiau C-W, and Chen K-F. Nilotinib induces autophagy in hepatocellular carcinoma through AMPK activation. J Biol Chem. (2013) 288:18249–59. doi: 10.1074/jbc.M112.446385
101. Xu C, Ai D, Shi D, Suo S, Chen X, Yan Y, et al. Accurate drug repositioning through non-tissue-specific core signatures from cancer transcriptomes. Cell Rep. (2018) 25:523–35. e5. doi: 10.1016/j.celrep.2018.09.031
102. Kim SG, Sung JY, Kim J-R, and Choi HC. Development. Nifedipine-induced AMPK activation alleviates senescence by increasing autophagy and suppressing of Ca2+ levels in vascular smooth muscle cells. Mech Ageing Dev. (2020) 190:111314. doi: 10.1016/j.mad.2020.111314
103. Singh A, Ham J, Po JW, Niles N, Roberts T, and Lee CS. The genomic landscape of thyroid cancer tumourigenesis and implications for immunotherapy. Cells. (2021) 10:1082. doi: 10.3390/cells10051082
104. Oh JM and Ahn B-C. Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. (2021) 11:6251–77. doi: 10.7150/thno.57689
105. Faria M, Vareda J, Miranda M, Bugalho MJ, Silva AL, and Matos P. Adherens junction integrity is a critical determinant of sodium iodide symporter residency at the plasma membrane of thyroid cells. Cancers (Basel). (2022) 14:5362. doi: 10.3390/cancers14215362
106. Zhang W and Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. (2002) 12:9–18. doi: 10.1038/sj.cr.7290105
107. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. (2007) 92:2840–3. doi: 10.1210/jc.2006-2707
108. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao X-H, West BL, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. (2011) 121:4700–11. doi: 10.1172/JCI46382
109. Liu D, Hu S, Hou P, Jiang D, Condouris S, and Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. (2007) 13:1341–9. doi: 10.1158/1078-0432.CCR-06-1753
110. Zhang Z, Liu D, Murugan AK, Liu Z, and Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. (2014) 21:161–73. doi: 10.1530/ERC-13-0399
111. Hong S, Yu S, Li J, Yin Y, Liu Y, Zhang Q, et al. MiR-20b displays tumor-suppressor functions in papillary thyroid carcinoma by regulating the MAPK/ERK signaling pathway. Thyroid. (2016) 26:1733–43. doi: 10.1089/thy.2015.0578
112. Shen C-T, Qiu Z-L, Song H-J, Wei W-J, and Luo Q-Y. miRNA-106a directly targeting RARB associates with the expression of Na(+)/I(-) symporter in thyroid cancer by regulating MAPK signaling pathway. J Exp Clin Cancer Res. (2016) 35:101. doi: 10.1186/s13046-016-0377-0
113. Nozhat Z and Hedayati M. PI3K/AKT pathway and its mediators in thyroid carcinomas. Mol Diagn Ther. (2016) 20:13–26. doi: 10.1007/s40291-015-0175-y
114. García B and Santisteban P. PI3K is involved in the IGF-I inhibition of TSH-induced sodium/iodide symporter gene expression. Mol Endocrinol. (2002) 16:342–52. doi: 10.1210/mend.16.2.0774
115. Kogai T, Sajid-Crockett S, Newmarch LS, Liu Y-Y, and Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol. (2008) 199:243–52. doi: 10.1677/JOE-08-0333
116. Liu Y-Y, Zhang X, Ringel MD, and Jhiang SM. Modulation of sodium iodide symporter expression and function by LY294002, Akti-1/2 and Rapamycin in thyroid cells. Endocr Relat Cancer. (2012) 19:291–304. doi: 10.1530/ERC-11-0288
117. de Souza ECL, Padrón AS, Braga WMO, de Andrade BM, Vaisman M, Nasciutti LE, et al. MTOR downregulates iodide uptake in thyrocytes. J Endocrinol. (2010) 206:113–20. doi: 10.1677/JOE-09-0436
118. Plantinga TS, Heinhuis B, Gerrits D, Netea MG, Joosten LAB, Hermus ARMM, et al. mTOR Inhibition promotes TTF1-dependent redifferentiation and restores iodine uptake in thyroid carcinoma cell lines. J Clin Endocrinol Metab. (2014) 99:E1368–E75. doi: 10.1210/jc.2014-1171
119. Chen W, Yu J, Xie R, Zhou T, Xiong C, Zhang S, et al. Roles of the SNHG7/microRNA−9−5p/DPP4 ceRNA network in the growth and 131I resistance of thyroid carcinoma cells through PI3K/Akt activation. Oncol Rep. (2021) 45:1–1. doi: 10.3892/or.2021.7954
120. Gonçalves CFL, dos Santos M, Ginabreda MG, Fortunato RS, de Carvalho DP, and Freitas Ferreira AC. Flavonoid rutin increases thyroid iodide uptake in rats. PloS One. (2013) 8:e73908. doi: 10.1371/journal.pone.0073908
121. Gonçalves CFL, de Freitas ML, Fortunato RS, Miranda-Alves L, Carvalho DP, and Ferreira ACF. Rutin scavenges reactive oxygen species, inactivates 5’-adenosine monophosphate-activated protein kinase, and increases sodium-iodide symporter expression in thyroid PCCL3 cells. Thyroid. (2018) 28:265–75. doi: 10.1089/thy.2016.0585
122. Faria M, Domingues R, Bugalho MJ, Silva AL, and Matos P. Analysis of NIS plasma membrane interactors discloses key regulation by a SRC/RAC1/PAK1/PIP5K/EZRIN pathway with potential implications for radioiodine re-sensitization therapy in thyroid cancer. Cancers (Basel). (2021) 13:5460. doi: 10.3390/cancers13215460
123. Fletcher A, Read ML, Thornton CEM, Larner DP, Poole VL, Brookes K, et al. Targeting novel sodium iodide symporter interactors ADP-ribosylation factor 4 and valosin-containing protein enhances radioiodine uptake. Cancer Res. (2020) 80:102–15. doi: 10.1158/0008-5472.CAN-19-1957
124. Thompson RJ, Fletcher A, Brookes K, Nieto H, Alshahrani MM, Mueller JW, et al. Dimerization of the sodium/iodide symporter. Thyroid. (2019) 29:1485–98. doi: 10.1089/thy.2019.0034
125. Chung T, Youn H, Yeom CJ, Kang KW, and Chung J-K. Glycosylation of sodium/iodide symporter (NIS) regulates its membrane translocation and radioiodine uptake. PloS One. (2015) 10:e0142984. doi: 10.1371/journal.pone.0142984
126. Iyer PC and Cubb TD. Radioactive iodine-refractory differentiated thyroid cancer: real-world treatment. Clin Thyroidol. (2024) 36:329–32. doi: 10.1089/ct.2024;36.329-332
127. Liu J, Zhu Z, Shi D, and Zheng F. Molecular mechanism of dedifferentiation and molecular targeted drugs for redifferentiation therapy of radioiodine refractory differentiated thyroid cancer. Int J Radiat Med Nucl Med. (2023) 47:123–6. doi: 10.3760/cma.j.cn121381-202205003-00267
128. Gild ML, Tsang VHM, Clifton-Bligh RJ, and Robinson BG. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol. (2021) 17:225–34. doi: 10.1038/s41574-020-00465-y
129. Nair A, Lemery SJ, Yang J, Marathe A, Zhao L, Zhao H, et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin Cancer Res. (2015) 21:5205–8. doi: 10.1158/1078-0432.CCR-15-1377
130. Cabanillas ME, Ryder M, and Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. (2019) 40:1573–604. doi: 10.1210/er.2019-00007
131. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
132. Subbiah V, Hu MI, Mansfield AS, Taylor MH, Schuler M, Zhu VW, et al. Pralsetinib in patients with advanced/metastatic rearranged during transfection (RET)-altered thyroid cancer: updated efficacy and safety data from the ARROW study. Thyroid. (2024) 34:26–40. doi: 10.1089/thy.2023.0363
133. Kummar S, Shen L, Hong DS, McDermott R, Keedy VL, Casanova M, et al. Larotrectinib efficacy and safety in adult patients with tropomyosin receptor kinase fusion sarcomas. Cancer. (2023) 129:3772–82. doi: 10.1002/cncr.35036
134. Marcus L, Donoghue M, Aungst S, Myers CE, Helms WS, Shen G, et al. FDA approval summary: entrectinib for the treatment of NTRK gene fusion solid tumors. Clin Cancer Res. (2021) 27:928–32. doi: 10.1158/1078-0432.CCR-20-2771
135. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. (2021) 27:2130–5. doi: 10.1158/1078-0432.CCR-20-3558
136. Cortas C and Charalambous H. Tyrosine kinase inhibitors for radioactive iodine refractory differentiated thyroid cancer. Life (Basel). (2023) 14:22. doi: 10.3390/life14010022
137. Zheng X, Xu Z, Ji Q, Ge M, Shi F, Qin J, et al. A randomized, phase III study of lenvatinib in chinese patients with radioiodine-refractory differentiated thyroid cancer. Clin Cancer Res. (2021) 27:5502–9. doi: 10.1158/1078-0432.CCR-21-0761
138. Duke ES, Barone AK, Chatterjee S, Mishra-Kalyani PS, Shen Y-L, Isikwei E, et al. FDA approval summary: cabozantinib for differentiated thyroid cancer. Clin Cancer Res. (2022) 28:4173–7. doi: 10.1158/1078-0432.CCR-22-0873
139. Chi Y, Zheng X, Zhang Y, Shi F, Cheng Y, Guo Z, et al. Anlotinib in locally advanced or metastatic radioiodine-refractory differentiated thyroid carcinoma: A randomized, double-blind, multicenter phase II trial. Clin Cancer Res. (2023) 29:4047–56. doi: 10.1158/1078-0432.CCR-22-3406
140. Lin Y, Qin S, Yang H, Shi F, Yang A, Han X, et al. Multicenter randomized double-blind phase III trial of donafenib in progressive radioactive iodine-refractory differentiated thyroid cancer. Clin Cancer Res. (2023) 29:2791–9. doi: 10.1158/1078-0432.CCR-22-3613
141. Bible KC, Menefee ME, Lin C-CJ, Millward MJ, Maples WJ, Goh BC, et al. An international phase 2 study of pazopanib in progressive and metastatic thyroglobulin antibody negative radioactive iodine refractory differentiated thyroid cancer. Thyroid. (2020) 30:1254–62. doi: 10.1089/thy.2019.0269
142. Sehgal K, Shi R, Pappa T, Min JK, Oakley LB, Oneill A, et al. Phase II trial of regorafenib in metastatic medullary thyroid carcinoma (MTC) and radioactive iodine refractory differentiated thyroid carcinoma (RAIR DTC). J Clin Oncol. (2024) 42:6109. doi: 10.1200/JCO.2024.42.16_suppl.6109
143. Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur J Endocrinol. (2022) 186:631–43. doi: 10.1530/EJE-21-1259
144. Brose MS, Robinson B, Sherman SI, Krajewska J, Lin C-C, Vaisman F, et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2021) 22:1126–38. doi: 10.1016/S1470-2045(21)00332-6
145. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab. (2019) 104:1417–28. doi: 10.1210/jc.2018-01478
146. Busaidy NL, Konda B, Wei L, Wirth LJ, Devine C, Daniels GA, et al. Dabrafenib versus dabrafenib + Trametinib in BRAF-mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial. Thyroid. (2022) 32:1184–92. doi: 10.1089/thy.2022.0115
147. Sehgal K, Serritella A, Liu M, Oneill A, Nangia C, Pappa T, et al. A phase I/II trial of sapanisertib in advanced anaplastic and radioiodine refractory differentiated thyroid carcinoma. J Clin Endocrinol Metab. (2024) 110(5):1315–23. doi: 10.1210/clinem/dgae443
148. Niu J, Milas K, Edmond A, Camou M, Tomeh C, Shellenberger T, et al. 1930P Resensitization of BRAF-mutated radioactive iodine refractory differentiated thyroid cancer with longer duration of dabrafenib and trametinib. Ann Oncol. (2024) 35:S1122. doi: 10.1016/j.annonc.2024.08.2010
Keywords: radioactive iodine-refractory differentiated thyroid carcinoma (RAIR-DTC), sodium-iodide symporter (NIS), high-risk factors, molecular pathogenesis, clinical management
Citation: Ma T, Xie Y, Long X and Ye F (2025) High risk factors, molecular features and clinical management for radioactive iodine-refractory differentiated thyroid carcinoma. Front. Oncol. 15:1644562. doi: 10.3389/fonc.2025.1644562
Received: 10 June 2025; Accepted: 04 August 2025;
Published: 25 August 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Hengyi Xu, The University of Texas at Austin, United StatesAyman Alameen, Al Jouf University, Saudi Arabia
Copyright © 2025 Ma, Xie, Long and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ye, ZmVuZ3llQHNjdS5lZHUuY24=
 Tengyun Ma
Tengyun Ma Yiting Xie
Yiting Xie Xinyi Long
Xinyi Long Feng Ye
Feng Ye