- 1Department of Radiation Oncology, University of Nebraska Medical Center, Omaha, NE, United States
- 2Department of Surgery, Mayo Hospital, Lahore, Pakistan
- 3Department of Heart and Cardiovascular Diseases, Nebraska Medicine, Omaha, NE, United States
Background: The use of proton beam radiation therapy (PBT) has increased in patients diagnosed with sarcoma. However, there is a lack of information about its survival benefit in these patients.
Objective: We want to investigate the association of PBT with the overall survival (OS) of sarcoma patients.
Methods: We used the National Cancer Database and assessed the OS using multivariable Cox regression analysis adjusted for age, sex, race, education, income, insurance status, histology, comorbidity score, radiation therapy dose, chemotherapy, surgery, and year of diagnosis.
Results: Among the 117,694 patients, 3,573 (3%) received PBT. Patients receiving PBT had longer OS than those receiving photon radiation therapy (PRT) (HR: 0.73, CI: 0.68 -0.79). PBT was also associated with improved OS compared to PRT in chordoma (HR: 0.57, CI: 0.44-0.74), rhabdomyosarcoma (HR: 0.58, 95% CI: 0.47- 0.72), or chondrosarcoma (HR: 0.35, CI: 0.24-0.51) patients. Among patients who received surgery, PBT was associated with improved OS compared to PRT in chordoma (HR: 0.37, CI: 0.22-0.60), chondrosarcoma (HR: 0.52, CI: 0.28-0.97), or osteosarcoma (HR: 0.32, CI: 0.12-0.89) patients. Among patients with no surgery, PBT was associated with improved OS compared to PRT in chordoma (HR: 0.57, CI: 0.44-0.74), rhabdomyosarcoma (HR:0.56, CI: 0.44-0.70), chondrosarcoma (HR:0.24, CI: 0.15-0.37), osteosarcoma (HR: 0.51, CI: 0.30- 0.87), or other histology type (HR:0.76, CI: 0.66-0.86) patients.
Conclusion: The use of PBT was associated with improved OS compared to photon RT in sarcoma patients. PBT was associated with improved OS in patients diagnosed with chordoma, rhabdomyosarcoma, or chondrosarcoma.
Background
Sarcoma is a rare tumor, which represents only 0.7% of all cancer cases in the United States (1). Surgery is the mainstay of treatment (2, 3) for adult-type sarcomas, and chemotherapy is the mainstay of treatment, with surgery or radiation therapy (RT) serving for local control, for pediatric-type sarcomas such as rhabdomyosarcoma and Ewing sarcoma. The ability of sarcoma to arise from any anatomic site and the proximity to critical organs at risk make the local treatment options challenging (2, 3). In some patients, it is impossible to have a complete resection without major impairments. Surgery could be complemented or replaced by high-precision radiation therapy in these patients (3). Proton beam therapy (PBT) might be a viable and effective treatment option for some patients due to its superior dosimetry and sparring normal tissues, especially if the tumor is near the radiosensitive critical organs (4, 5).
Some evidence exists to support the clinical use of PBT in cancer patients. However, clinical evidence is still scarce. The early clinical use of PBT was mostly in pediatric cancers, including sarcomas of the skull base and spine (6–8). The strongest evidence has been in pediatric CNS tumors (9, 10). Most findings are from non-randomized, early-stage, and usually small studies with a retrospective character (11–15). However, the upcomg long-term results of prospective studies will help in understanding the role of PBT in various cancer patients (16–21).
With the increase in PBT facilities from only two in 2004 to 42 in 2022 in the U.S., the number of cancer patients who received PBT also increased from 1,206 in 20004 to 6,291 in 2022 (22). In sarcoma patients who receive surgery, RT could be used to improve local control (23). Studies also suggest that a high dose of RT could improve local control if there is a positive surgical margin, tumor recurrence, or tumor in the trunk, head, or neck (23–25). Radiation therapy is a reliable treatment option in young sarcoma patients among whom reducing toxicities such as the risk of second malignancy, fertility problems, and cognitive issues is of a great interest (26). Given that there is a lack of information about the use of PBT in sarcoma patients and its impact on overall survival (OS), we aim to investigate the association of PBT with the OS and identify factors associated with the use of PBT.
Materials and method
Data source
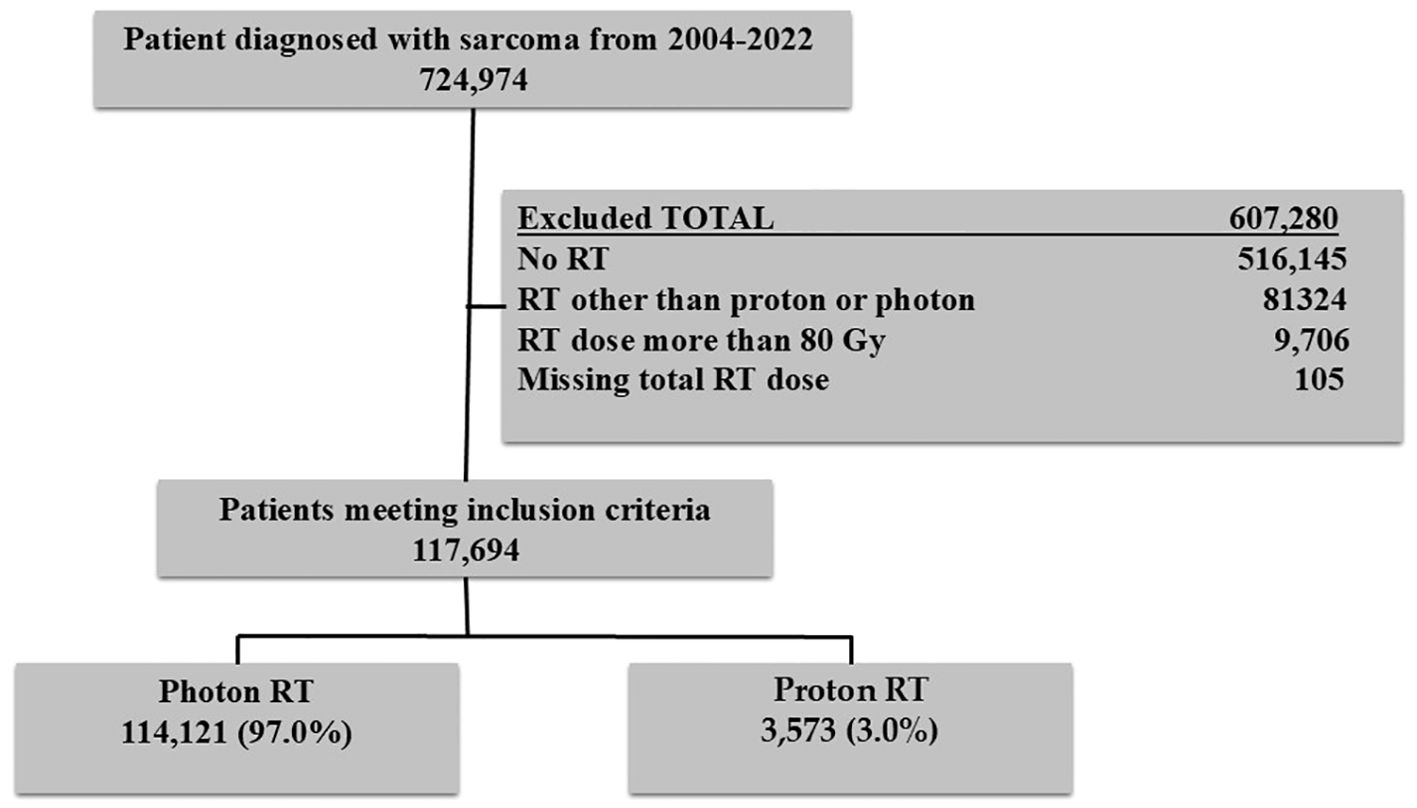
Data were extracted from the National Cancer Database (NCDB). The NCDB is the largest hospital-based cancer registry in the U.S., representing more than 70% of the cancer cases diagnosed annually. The NCDB is a consortium of more than 1500 accredited cancer hospitals that the American College of Surgeons administers. The analysis included patients diagnosed with sarcoma between 2004 and 2022. Other variables included age at diagnosis (years), insurance types (private, Medicare, Medicaid, other governmental, and no insurance), race, sex, Charlson-Deyo Comorbidity Index (0, 1, ≥2), year of diagnosis (2004–2013 or 2014–2022), neighborhood education level, median household income, and histology type. International Classification of Disease for Oncology, Third Edition (ICD-O-3) was used to determine sarcoma patients. Major histology types included Chordoma (9370,9371,9372), Rhabdomyosarcoma (8900,8901,8902,8910,8912,8920,8921), Ewing sarcoma ((9260), Chondrosarcoma (9220,9221,9231,9240,9242,9243), and other types that included the remaining histology codes. Patients who received RT dose >80Gy or who were missing RT dose were excluded. The study was exempt from review by the institutional review board as it analyzes de-identified data. Informed consent was also not needed. The flowchart of the study participants is provided in Figure 1.
Statistical analysis
We reported the frequency and proportion of all the variables by PBT and photon RT. Time trends in the use of PBT were also reported by year of diagnosis. The association of the factors associated with the use of PBT was investigated by performing multivariable logistic regression analysis. The odds ratio was reported as the measure of association with the probability of receiving PBT compared to photon RT. The Kaplan–Meier survival curves were used to estimate the median survival time and compute the log-rank test to compare survival time across the PBT and photon RT groups. We used Cox proportional hazard regression models to estimate the variable’s HR and 95% CI with a focus on PBT vs. photon RT. SAS, version 9.4 (SAS Institute Inc), was used to perform analyses. All tests were 2-sided with a significance level being set at P = 0.5.
Results
Patient characteristics
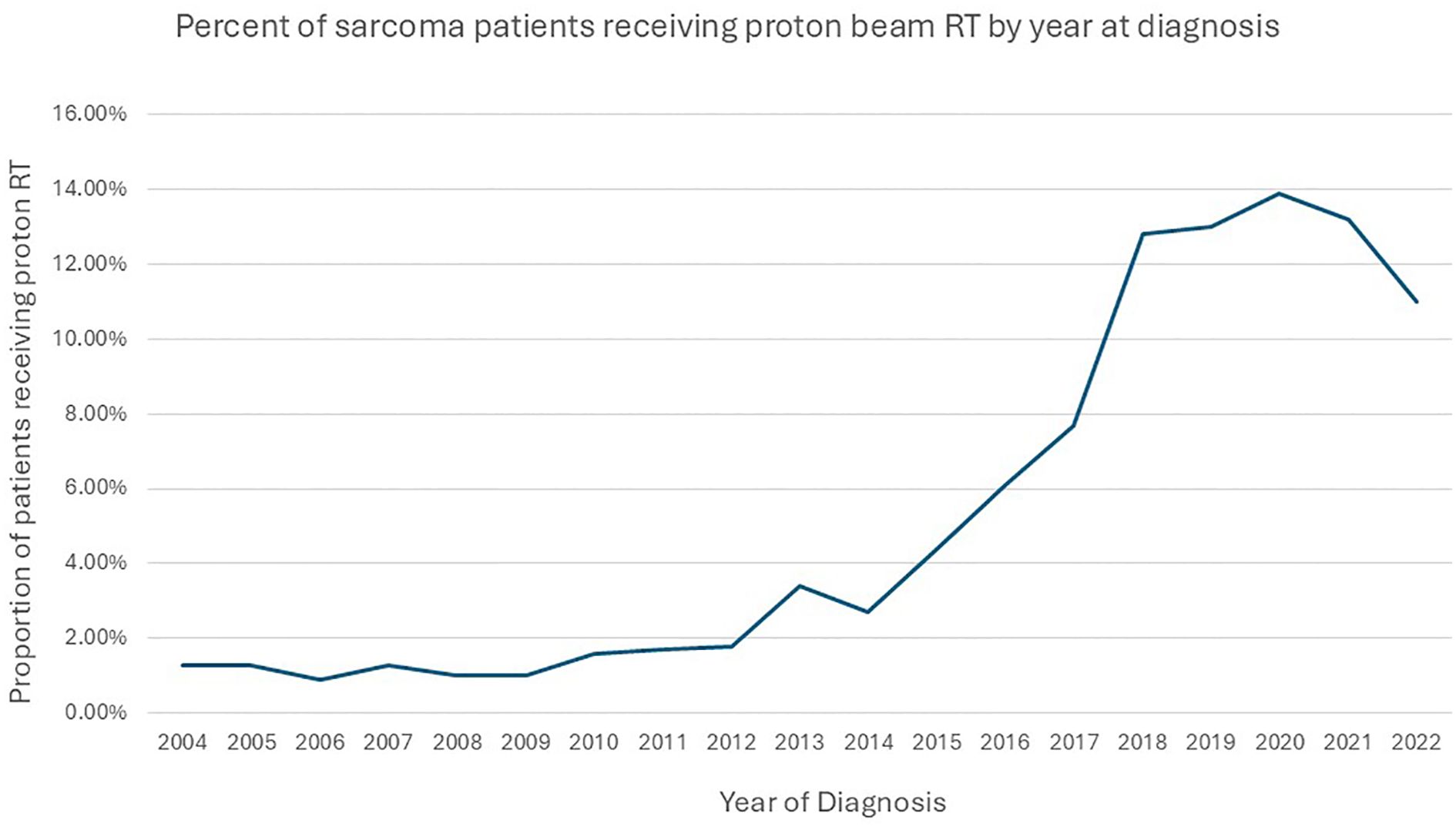
Of the 117,694 study participants, 3,573 (3%) patients received PBT. Among those who received PBT, 391(10.9%) received <45 Gy,1512 (42.3%) 45–60 Gy, and 1670 (46.8%) 60–80 Gy RT dose. The use of PBT increased from 1.3% in 2004 to 11% in 2022. The use of PBT was <1% in patients younger than 18 years and 1.7% in adult patients in 2004 (Figure 2). In the multivariable logistic regression analysis, patients aged <18 years old, belonging to non-white non-black racial groups, and those who received 60–80 Gy were more likely to receive PBT compared to their counterparts. Patients with Medicaid, Medicare, other governmental insurance, or with no health insurance compared to (private insurance), patients with rhabdomyosarcoma, ewing sarcoma, chondrosarcoma, osteosarcoma, and other histology types compared to (chordoma) were less likely to receive PBT. In addition, patients living an area with income ≤$50353, having a comorbidity score of 1 or ≥2 compared to (zero score), a diagnosis between 2004-2013, and not receiving chemotherapy were all less likely to receive PBT. The details of the factors associated with the use of PBT are provided in Table 1.
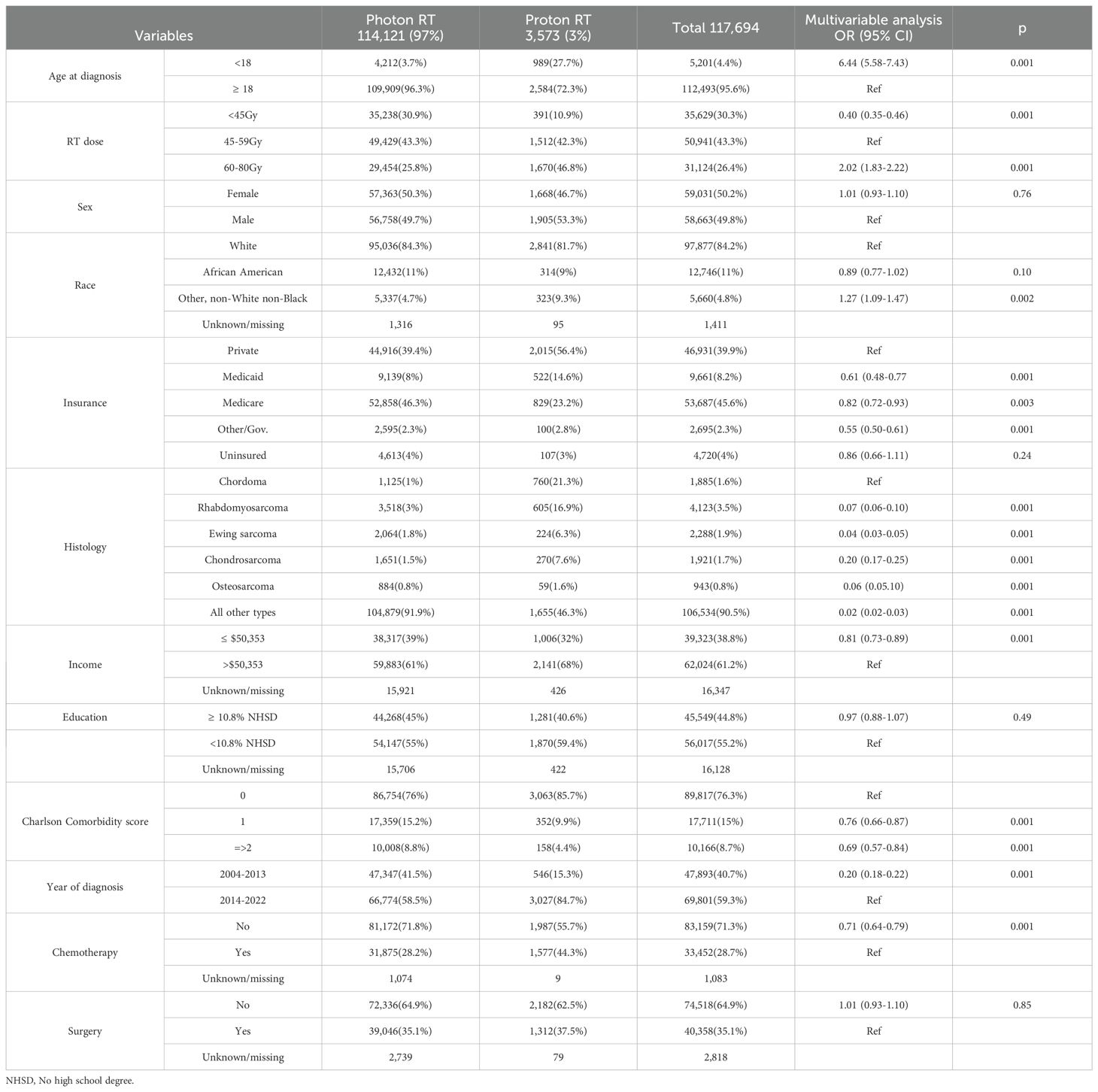
Table 1. Baseline characteristics and factors associated with the use of PBT among patients diagnosed with sarcoma between 2004 and 2022 and were reported to the National Cancer Database (N = 117,694).
Survival outcomes
Patients who received PBT had better median OS compared to those who received photon RT. The three and five- year survival rates were 79% (95% CI: 77.4%-80.6%) and 70% (95% CI: 68%-72%) for patients who received PBT, while 61% (95% CI: 60.7%-61.3%) and 52% (95% CI: 51.7%-52.3%) for patients who received photon RT.
Patients who received PBT had better OS than those who received photon RT in the univariable Cox regression analysis (HR: 0.51, 95% CI: 0.48-0.55; p<0.001) (Table 2). Other factors associated with improved OS included age<18 years old, female sex, other racial groups, receiving RT dose of 60–80 Gy compared to 45–59 Gy, and not receiving chemotherapy. Black race compared to White, insurance type of Medicaid, Medicare, other governmental, and no insurance compared to private insurance, rhabdomyosarcoma, Ewing sarcoma, chondrosarcoma, osteosarcoma, and other histology types compared with chordoma, low neighborhood income level, low neighborhood education level, comorbidity score of 1 or ≥2 compared to zero comorbidity score, and no surgery compared to definitive surgery were associated with worse OS (Table 2). Since all variables except the year of diagnosis were significant in the univariable analyses, all except the year of diagnosis were included in the multivariable analysis. The multivariable analysis was adjusted for age at diagnosis, race, sex, insurance type, histology type, neighborhood income level, neighborhood education level, comorbidity score, use of chemotherapy, and receipt of surgery.
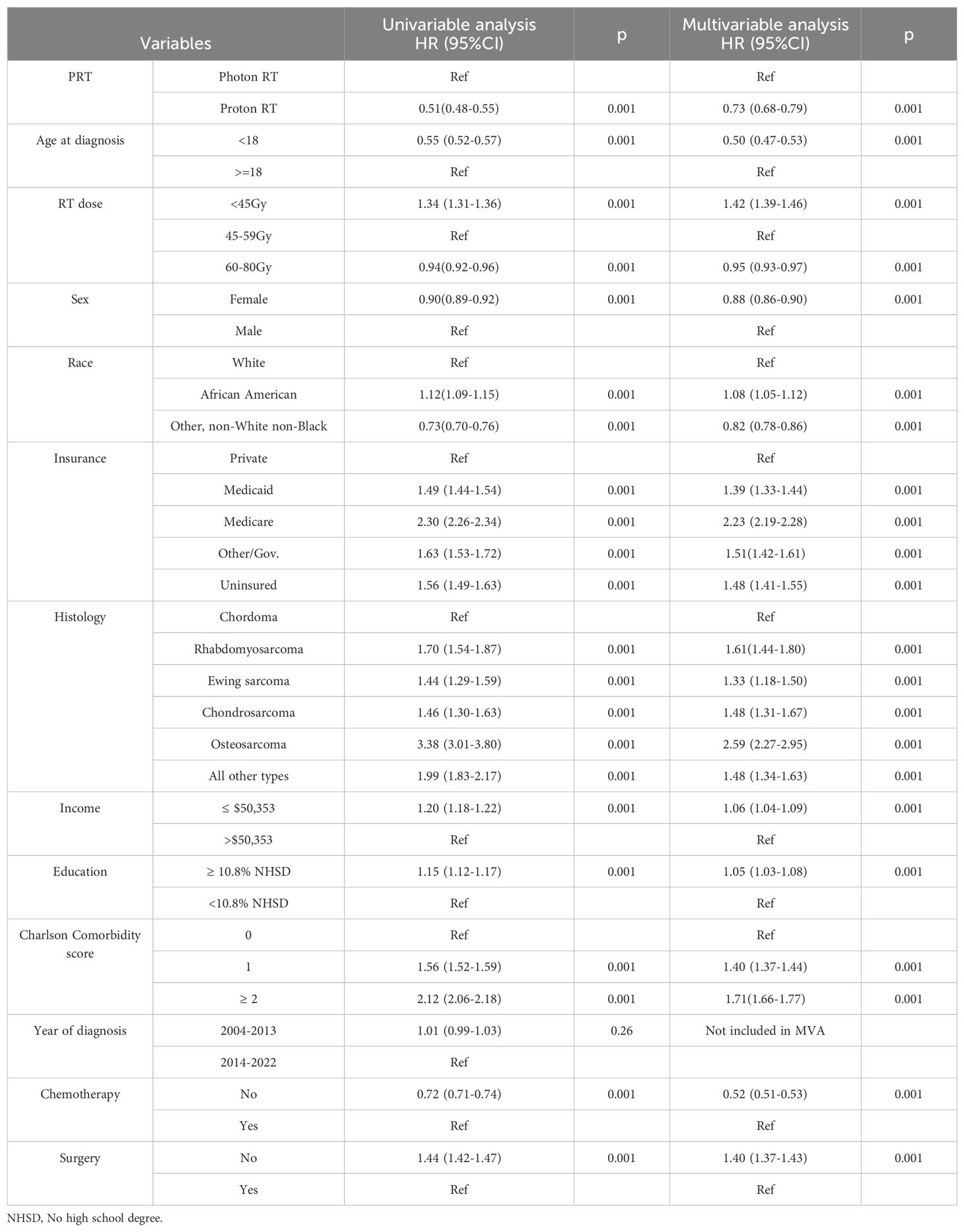
Table 2. Univariate and multivariable Cox regression analysis of the factors associated with the OS of patients diagnosed with sarcoma between 2004 and 2022.
Patients who received PBT had longer OS than patients who received photon RT In the multivariable analysis (HR: 0.73, 95% CI: 0.68 -0.79; p<0.001) (Table 2). Age <18 years old compared to ≥18 years old, female sex, and other race groups compared to White, RT dose of 60–80 Gy compared to 45–59 Gy, and no chemotherapy compared to receiving chemotherapy were other variables that were associated with improved OS in the multivariable analysis. Black race compared to White, Medicaid, Medicare, other governmental insurance, having no health insurance compared to private insurance, rhabdomyosarcoma, Ewing sarcoma, chondrosarcoma, osteosarcoma, and other histology types compared chordoma, low neighborhood income level, low neighborhood education level, comorbidity score of 1 or ≥2, compared to comorbidity score of zero, diagnosis year between 2003–2013 compared to 2014-2021, and no surgery compared to definitive surgical resection of the tumor were all associated with worse OS (Table 2). To reduce the imbalance noticed in Table 1 between PBT and photon, we performed 1:1 propensity score matched analysis in which, PBT was still associated with improved OS than photon RT (HR: 0.70, 95% CI: 0.64 -0.77; p<0.001) (Supplementary Table 1).
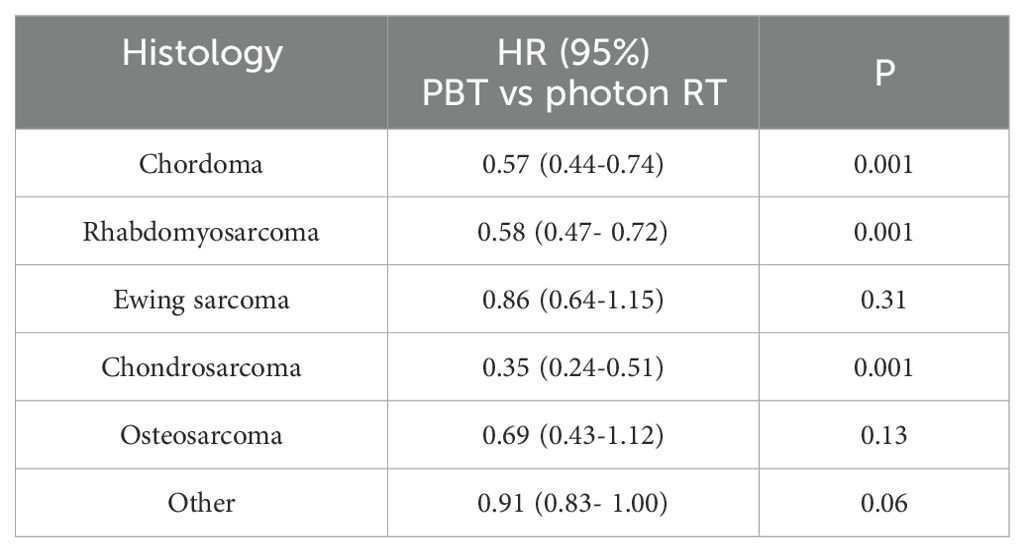
In the subset analysis stratified by histology, PBT was associated with improved OS compared to photon RT in patients diagnosed with chordoma (HR: 0.57, 95% CI: 0.44-0.74; p<0.001), rhabdomyosarcoma (HR: 0.58, 95% CI: 0.47- 0.72; p<0.001), or chondrosarcoma (HR: 0.35, 95% CI: 0.24-0.51; p<0.001) (Table 3).
In the analysis stratified by surgery, PBT was associated with improved OS compared to photon RT in patients diagnosed with chordoma (HR: 0.37, 95% CI: 0.22-0.60; p<0.001), chondrosarcoma (HR: 0.52, 95% CI: 0.28-0.97; p<0.04), or osteosarcoma (HR: 0.32, 95% CI: 0.12-0.89; p<0.001) in patients who received definitive surgery of the tumor (Table 4). Among patients who did not receive surgery, PBT was associated with improved OS in patients diagnosed with chordoma (HR: 0.57, 95% CI: 0.44-0.74; p<0.001), rhabdomyosarcoma (HR:0.56, 95% CI: 0.44-0.70; p<0.001), chondrosarcoma (HR:0.24, 95% CI: 0.15-0.37; p<0.001), osteosarcoma (HR: 0.51, 95% CI: 0.30- 0.87; p<0.001), or other histology type (HR:0.76, 95% CI: 0.66-0.86; p<0.001) (Table 4).
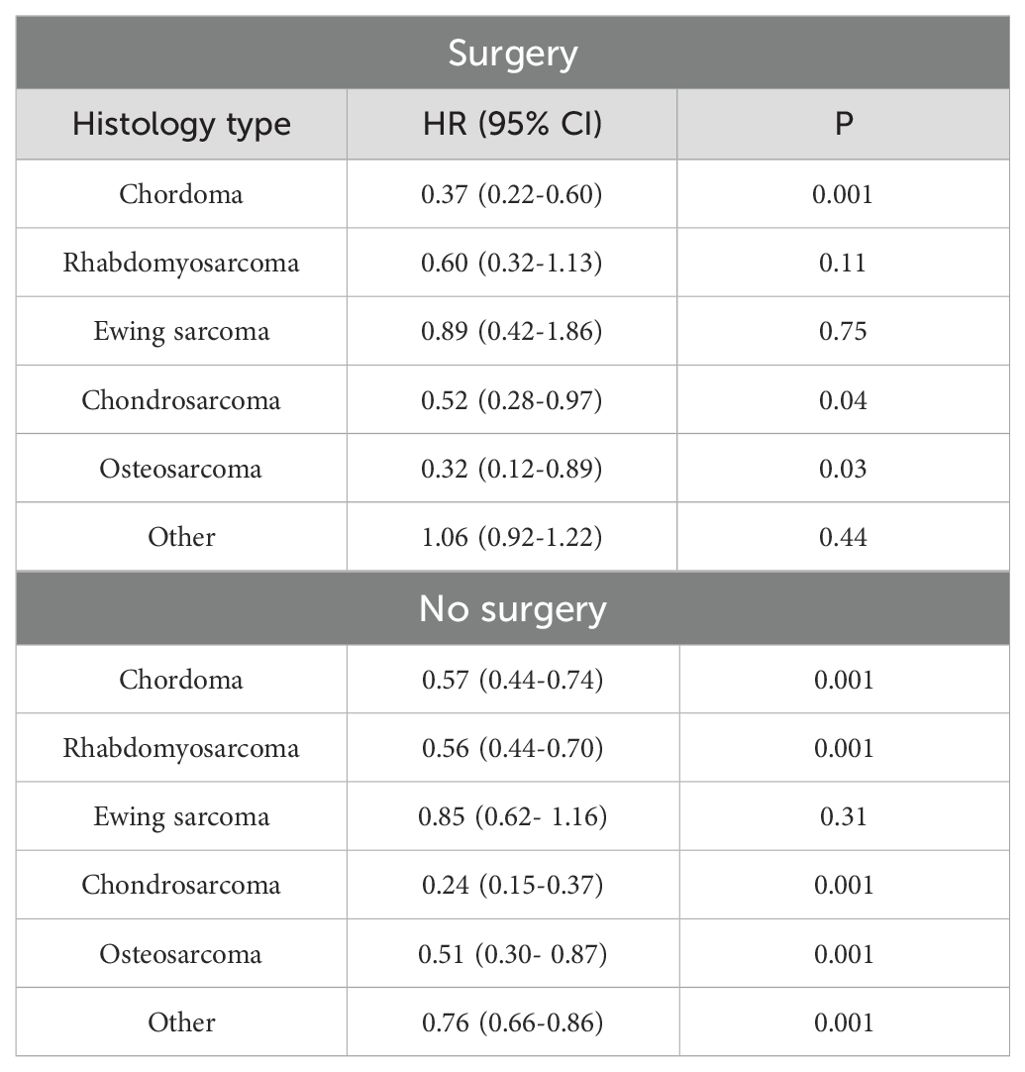
Table 4. Multivariable Cox regression analysis of PBT vs. photon RT stratified by surgery and histology.
When the analysis was restricted to RT dose 60–80 Gy and stratified by surgery, PBT was associated with improved OS compared to photon RT only in patients diagnosed with chordoma (HR: 0.36, 95% CI: 0.20-0.68; p<0.002) among those who received definitive surgery (Table 5). Among those who did not receive surgery, PBT was associated with improved OS compared to photon RT in patients diagnosed with chordoma (HR: 0.56 (0.41- 0.78) or chondrosarcoma (HR: 0.26, 95% CI: 0.15-0.44; p<0.001) (Table 5). The sample size for other dose categories and histology types was small and no analysis was performed.
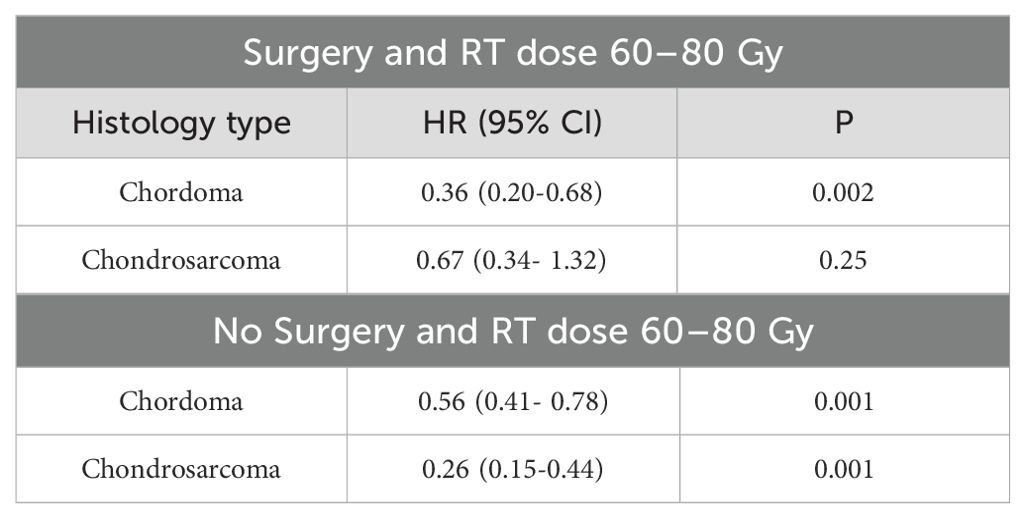
Table 5. Multivariable Cox regression analysis stratified by surgery and histology for RT dose 60-80.
When the analysis was stratified by RT dose and histology, we had enough sample size only for rhabdomayosarcoma and other histology types among patients who received RT dose <45 Gy. Proton RT was associated with improved OS than photon RT in patients diagnosed with rhabdomayosarcoma (HR:0.45, 95% CI: 0.27-0.72; P<0.001) but not in other histology type (HR:0.91. 95% CI: 0.72-1.16; p=0.45). Among patients who received RT dose 45–59 Gy, PBT was associated with improved OS in rhabdomayosarcoma patients (HR: 0.62, 95% CI: 0.48-0.79; p<0.001) and other histology type (HR: 0.75, 95% CI: 0.64-0.87; p<0.001) but not in Ewing sarcoma patients (HR: 0.95, 95% CI: 0.68-1.32; p=0.75). There was not enough sample size for the remaining histology types. Among patients who received RT dose 60–80 Gy, PBT was associated with improved OS in chordoma (HR:0.53, 95% CI: 0.40-0.70; p<0.001) and chondrosarcoma (HR: 0.34, 95% CI: 0.22-0.52; p<0.001) but not in all other types (HR: 1.11, 95% CI: 0.96-1.28; p=0.15) (Supplementary Table 2).
In a subset analysis of the subtypes of rhabdomayosarcoma (NOS, embryonal, and alveolar), PBT was associated with improved OS compared to photon RT in NOS (HR: 0.41, 95% CI: 0.23-0.73; p<0.001), embryonal (HR: 0.48, 95% CI: 0.33-0.71; p<0.001), and alveolar (HR: 0.75, 95% CI: 0.55-1.00; p=0.05).
While a higher proportion of patients received chemotherapy in the proton RT group than photon RT 1,577 (44.3%) vs. 31875 (28.2%), the overall results of proton RT vs. photon RT did not change when we stratified by chemotherapy. Proton RT was associated with improved OS than photon RT in patients who received chemotherapy (HR:0.73, 95% CI: 0.66-0.81; p<0.001) and those who did not receive chemotherapy (HR:0.72, 95% CI: 0.64-0.80; p<0.001).
We wanted to perform an analysis stratify by histology and neoadjuvant and adjuvant RT. However, there was not enough sample size for the neoadjuvant group. Among the adjuvant RT, the OS results did not change from the general Cox regression model.
Discussion
The current study is the largest to evaluate PBT’s association with sarcoma patients’ OS in real-world settings. It is the first study to report that sarcoma patients who received PBT had longer OS than those who received photon RT. In the current study, more than 50% of the patients who received PBT were younger than 40 years old. This is important since PBT has been reported to improve normal tissue sparing and to reduce cognitive issues (26).
In the analysis stratified by histology, the use of PBT was associated with improved OS compared to photon RT only in patients diagnosed with chordoma, rhabdomyosarcoma, or chondrosarcoma. Among patients who received surgery, PBT was associated with improved OS in patients diagnosed with chordoma, chondrosarcoma, or osteosarcoma. Among patients who did not receive surgery, the use of PBT was associated with improved OS in patients diagnosed with chordoma, rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and other histology types. When the analysis was restricted to an RT dose of 60–80 Gy, among patients who received surgery, PBT was associated with improved OS compared to photon RT only in patients diagnosed with chordoma. Among those who did not receive surgery, PBT was associated with improved OS compared to photon RT in patients diagnosed with chordoma or chondrosarcoma.
The survival findings reported in our study are similar to some other studies of PBT in sarcoma patients. The five-year survival rate of 85% in chordoma patients in the current study, is comparable to the five-year survival rate reported in some published case series (27–30). The five-year survival rates in these series were,86%, 74.6%, 66.7%, and 80.5% (27–30). The five-year and two-year survival rates of 85% and 95% of chondrosarcoma patients in our study are similar to the five-year survival rate of 83.5%, and a two-year survival rate of 93.5% reported by previous studies (27, 31). The four-year survival rate of 89% for chondrosarcoma patients in our study is better than the four-year survival rate of 72% reported in a case series of patients treated with PBT who had either chordoma or chondrosarcoma (32).
The three and five-year survival rates of 78% and 71% for rhabdomyosarcoma patients in our study are comparable to 81% and 77% reported in clinical trials (33, 34). The five-year survival rate of 71% in our study is slightly higher than the 64% (35) reported by a previous study. These studies were case series and did not have a comparison group and included only pediatric patients (34, 35). The one, two, three, four, and five-year survival rates of 95%, 85%, 78%, 73%, and 71% of rhabdomyosarcoma patients in our study are also comparable with 93%, 85%, 80%, 71%, and 82% reported by a systematic review and meta-analysis that investigated the efficacy and safety of PBT in rhabdomyosarcoma patients (36).
The improved OS associated with the use of PBT compared to photon RT in chordoma and chondrosarcoma patients might be due to a higher dose of RT being delivered safely when PBT was used in these patients. Most chordoma and chondrosarcoma patients who received 60–80 Gy were treated with PBT. In chordoma specifically, 90% of PBT-treated patients received 60–80 Gy versus 44.5% in the photon RT cohort. Thus, the apparent overall survival advantage with PBT could reflect dose escalation, as these tumors are relatively radioresistant. Nevertheless, in our study PBT remained associated with improved OS even when analyses were restricted to patients receiving 60–80 Gy. The survival benefit of PBT in chordoma and chondrosarcoma patients was also not driven by the benefit of surgery as PBT was associated with better OS compared to photon RT when the analysis was stratified by surgery.
Our study found that, among patients who did not undergo surgery, proton beam therapy (PBT) was associated with improved overall survival across chordoma, chondrosarcoma, rhabdomyosarcoma, osteosarcoma, and pooled “other” sarcoma histologies. Ewing sarcoma was the only histology in which PBT was not associated with improved OS, irrespective of surgical status. These results suggest that PBT may confer a survival advantage in the presence of gross disease, including tumors traditionally considered radioresistant. The signal in the non-surgical subgroup is notable given longstanding skepticism about radiotherapy for radioresistant histologies such as osteosarcoma. Although osteosarcoma is generally less responsive to radiation and surgery is preferred when feasible, radiotherapy can improve local control—and potentially survival—for unresectable axial disease or after resections with positive margins (4). Consistent with this, the ongoing Children’s Oncology Group trial (AOST2032; NCT05691478) includes radiotherapy for unresectable disease, postoperative positive margins, and selected metastatic lesions.
An argument could be made that the improved OS associated with the use of PBT may be due to the imbalance between PBT and photon RT reported in Table 1. However, PBT was associated with improved OS compared to photon in the propensity matched analysis. PBT was also associated with improved OS after stratifying by important factors such as surgery, histology, and age at diagnosis. These results provide indication that the survival benefit associated with the use of PBT is not due to the difference in other factors between the two groups of RT.
The use of PBT increased from 1.4% in 2004 to 12% in 2022. The increase in the use of PBT is due to many reasons, the most important being the increase in the number of PBT facilities in operation (37–39). In 2004, only two facilities were operating, while in 2022, 42 facilities offered treatment with PBT. The proportion of sarcoma patients receiving PBT increased by more than 800% from 45 in 2004 to 394 in 2022, coinciding with an almost 20 times increase in facilities from 2004 to 2022. More than 85% of the patients who received PBT were diagnosed between 2014 and 2022. The acceptance of PBT use by oncologists and the growing number of prospective clinical trials are some additional contributing factors (38, 39).
Pediatric patients were more likely to receive PBT compared to adult sarcoma patients. The higher likelihood could be due to the stronger clinical evidence and a stronger belief in the benefit of the use of PBT in pediatric cancers, including CNS tumors and sarcomas of the skull base and spine (6, 40, 41).
The higher odds of using PBT in patients with private insurance compared to Medicare, Medicaid, and no insurance is an indication of the challenge and barriers in accessing advanced treatment techniques in patients without private insurance. Patients with Medicare had the lowest odds of receiving PBT, which may be due to the strict requirements for the approval of PBT. An alternative payment model has been introduced for Medicare recipients, which focuses on reducing Medicare expenditure and overuse of treatment with unproven benefits while preserving the quality of care (42). It has to be noted that access to proton therapy is extremely difficult worldwide, with significant healthcare costs for society.
Patients living in an area with ≤$50,353 neighborhood income level were also less likely to receive PBT. Previous studies of non-small cell lung cancer and other cancers have reported similar findings (43–45). The finding, together with racial disparity, is an indication of the presence of structural racism and disparity in access to modern treatment across the U.S. It is also an elaboration that access to innovative cancer treatments is not uniform. Much work is needed to overcome socioeconomic barriers in access to modern treatments such as PBT. These issues must be addressed at the healthcare policy level. Patients with a comorbidity score of 1 or ≥2 were less likely to receive PBT compared to a score of zero, a probable indication that PBT has been used in patients who are healthier, live longer, and are more likely to benefit from it.
Despite being the first and largest study of PBT use in sarcoma patients, it has several limitations. Important limitations include the retrospective nature of NCDB, which makes the database prone to errors. Some additional limitations include the lack of information about acute and late toxicity, recurrence, reirradiation, performance status, cause of death, and disease progression. Only 3% of the patients received PBT, which makes this population unique with different characteristics, such as the ability to have access to new and modern treatments at a high cost. Due the different characteristics of the PBT group, the role of different time periods, different age groups, and different dose levels can not be ingored when assessing the impact of PBT on the OS of these patients.
Nevertheless, this comprehensive study of the NCDB is the first study to report the survival benefit associated with the receipt of PBT in children and adult sarcoma patients using a large database. Despite a sharp increase in the number of PBT facilities, the use of PBT in sarcoma patients remains low compared to the number of patients who receive photon RT. In conclusion, the use of PBT was associated with improved OS compared to photon RT. In the stratified analysis by histology, the use of PBT was associated with improved OS in patients diagnosed with chordoma, rhabdomyosarcoma, or chondrosarcoma. In the subset analysis among patients who received surgery on the tumor, the use of PBT was associated with improved OS in patients diagnosed with Chordoma, chondrosarcoma, or osteosarcoma. In the no-surgery group, only in Ewing sarcoma patients, PBT was not associated with improved OS. When the analysis was stratified by surgery and restricted only to 60–80 Gy, PBT use was associated with improved OS only in chordoma patients who received surgery, while its use was associated with improved OS in chordoma and chondrosarcoma patients who did not receive surgery. Finally, our study revealed that the use of PBT among sarcoma patients has increased, but there is still a large gap between the number of patients who should receive PBT and patients who are receiving PBT. The increase in the number of PBT facilities is a step in the right direction, but much more is needed to adopt a broader use of PBT.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data will be provided upon a reasonable request from the corresponding author. The NCBD is not a publicly available database. Requests to access these datasets should be directed to Y2xpbkB1bm1jLmVkdQ==.
Ethics statement
Ethical approval was not required for the studies involving humans because we used the National Cancer Database, which is a de-identified data. No IRB, and no informed consent is required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because The data is de-identified data, and no informed consent is required.
Author contributions
SA: Formal Analysis, Writing – review & editing, Methodology, Investigation, Writing – original draft. ZM: Formal Analysis, Writing – review & editing. PA: Formal Analysis, Writing – review & editing, Methodology. CL: Supervision, Conceptualization, Data curation, Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1644829/full#supplementary-material
References
1. National Cancer Institute. The surveillance E, and end results program, in: Cancer Stat Facts: Soft Tissue including Heart Cancer (2024).
2. Frisch S and Timmermann B. The evolving role of proton beam therapy for sarcomas. Clin Oncol. (2017) 29:500–6. doi: 10.1016/j.clon.2017.04.034
3. Lee A, Kang JJ, Bernstein H, Marqueen KE, Neal B, Kelly CM, et al. Proton radiotherapy for recurrent or metastatic sarcoma with palliative quad shot. Cancer Med. (2021) 10:4221–7. doi: 10.1002/cam4.3646
4. Keole S, Ashman JB, and Daniels TB. Proton therapy for sarcomas. Cancer J. (2014) 20:409–14. doi: 10.1097/PPO.0000000000000084
5. Rombi B and Timmermann B. Proton beam therapy for pediatric chordomas: state of the art. Int J Particle Ther. (2014) 1:368–85. doi: 10.14338/IJPT.13.00008.1
6. Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. (2009) 75:1111–8. doi: 10.1016/j.ijrobp.2008.12.055
7. Bernstein KDE, Sethi R, Trofimov A, Zeng C, Fullerton B, Yeap BY, et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. Int J Radiat Oncol Biol Phys. (2013) 86:114–20. doi: 10.1016/j.ijrobp.2012.12.004
8. DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. (2009) 74:732–9. doi: 10.1016/j.ijrobp.2008.08.058
9. Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, and Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. (2015) 93:400–7. doi: 10.1016/j.ijrobp.2015.06.012
10. Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. (2016) 17:287–98. doi: 10.1016/S1470-2045(15)00167-9
11. Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. (2014) 90:354–61. doi: 10.1016/j.ijrobp.2014.05.051
12. Hauswald H, Rieken S, Ecker SW, Kessel KA, Herfarth K, Debus J, et al. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol. (2012) 7:1–7. doi: 10.1186/1748-717X-7-189
13. Nakayama H, Sugahara S, Tokita M, Satoh H, Tsuboi K, Ishikawa S, et al. Proton beam therapy for patients with medically inoperable stage I non–small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys. (2010) 78:467–71. doi: 10.1016/j.ijrobp.2009.07.1707
14. Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. (2016) 121:395–401. doi: 10.1016/j.radonc.2016.11.001
15. Xi M, Xu C, Liao Z, Chang JY, Gomez DR, Jeter MJ, et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: a retrospective, single-institutional analysis. Int J Radiat Oncol Biol Phys. (2017) 99:667–76. doi: 10.1016/j.ijrobp.2017.06.2450
16. Al Feghali KA, Randall JW, Liu DD, Wefel JS, Brown PD, et al. Phase II trial of proton therapy versus photon IMRT for GBM: secondary analysis comparison of progression-free survival between RANO versus clinical assessment. Neuro-Oncol Adv. (2021) 3:vdab073. doi: 10.1093/noajnl/vdab073
17. Brown PD, Chung C, Liu DD, McAvoy S, Grosshans D, Al Feghali KA, et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. (2021) 23:1337–47. doi: 10.1093/neuonc/noab040
18. Cella L, Monti S, Xu T, Liuzzi R, Stanzione A, Durante M, et al. Probing thoracic dose patterns associated to pericardial effusion and mortality in patients treated with photons and protons for locally advanced non-small-cell lung cancer. Radiother Oncol. (2021) 160:148–58. doi: 10.1016/j.radonc.2021.04.025
19. Gjyshi O, Xu T, Elhammali A, Boyce-Fappiano DB, Chun SG, Gandhi S, et al. Toxicity and survival after intensity-modulated proton therapy versus passive scattering proton therapy for NSCLC. J Thorac Oncol. (2021) 16:269–77. doi: 10.1016/j.jtho.2020.10.013
20. Hoffmann L, Mortensen H, Shamshad M, Berbee M, Bizzocchi N, Bütof R, et al. Treatment planning comparison in the PROTECT-trial randomising proton versus photon beam therapy in oesophageal cancer: Results from eight European centres. Radiother Oncol. (2022) 172:32–41. doi: 10.1016/j.radonc.2022.04.029
21. Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. (2021) 74:603–12. doi: 10.1016/j.jhep.2020.09.026
22. Hartsell WF, Simone CB II, Godes D, Maggiore J, Mehta MP, et al. Temporal evolution and diagnostic diversification of patients receiving proton therapy in the United States: A ten-year trend analysis (2012 to 2021) from the national association for proton therapy. Int J Radiat Oncol Biol Phys. (2024) 119:1069–77. doi: 10.1016/j.ijrobp.2023.12.041
23. Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. (1998) 16:197–203. doi: 10.1200/JCO.1998.16.1.197
24. Wells S, Ager B, Hitchcock YJ, and Poppe MM. The radiation dose-response of non-retroperitoneal soft tissue sarcoma with positive margins: An NCDB analysis. J Surg Oncol. (2019) 120:1476–85. doi: 10.1002/jso.25748
25. Zagars GK and Ballo MT. Significance of dose in postoperative radiotherapy for soft tissue sarcoma. Int J Radiat Oncol Biol Phys. (2003) 56:473–81. doi: 10.1016/S0360-3016(02)04573-X
26. Doyen J, Aloi D, Groulier A, Vidal M, Lesueur P, Calugaru V, et al. Role of proton therapy in reirradiation and in the treatment of sarcomas. Cancer/Radiothérapie. (2021) 25:550–3. doi: 10.1016/j.canrad.2021.06.024
27. Demizu Y, Mizumoto M, Onoe T, Nakamura N, Kikuchi Y, Shibata T, et al. Proton beam therapy for bone sarcomas of the skull base and spine: a retrospective nationwide multicenter study in Japan. Cancer Sci. (2017) 108:972–7. doi: 10.1111/cas.13192
28. Igaki H, Tokuuye K, Okumura T, Sugahara S, Kagei K, Hata M, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. (2004) 60:1120–6. doi: 10.1016/j.ijrobp.2004.05.064
29. Noël G, Feuvret L, Dhermain F, Mammar H, Haie-Méder C, Ponvert D, et al. Les chordomes de la base du crâne et du rachis cervical haut. À propos d’une série de 100 patients irradiés selon une technique conformationnelle 3D par une association de faisceaux de photons et de protons. Cancer/Radiothérapie. (2005) 9:161–74. doi: 10.1016/j.canrad.2005.04.005
30. Takagi M, Demizu Y, Nagano F, Terashima K, Fujii O, Jin D, et al. Treatment outcomes of proton or carbon ion therapy for skull base chordoma: a retrospective study. Radiat Oncol. (2018) 13:1–9. doi: 10.1186/s13014-018-1173-0
31. Holliday EB, Mitra HS, Somerson JS, Rhines LD, Mahajan A, Brown PD, et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: adjuvant versus salvage radiation therapy. Spine (Phila Pa 1976). (2015) 40:544–9. doi: 10.1097/BRS.0000000000000804
32. Indelicato DJ, Rotondo RL, Begosh-Mayne D, Scarborough MT, Gibbs CP, Morris CG, et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys. (2016) 95:297–303. doi: 10.1016/j.ijrobp.2016.01.057
33. Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. (2001) 19:3091–102. doi: 10.1200/JCO.2001.19.12.3091
34. Ladra M, Szymonifka J, MacDonald S, Yeap BY, Friedmann AM, Kasper H, et al. Proton radiation therapy for rhabdomyosarcoma: Preliminary results from a multicenter prospective study. Int J Radiat Oncol Biol Phys. (2013) 87:S597–8. doi: 10.1016/j.ijrobp.2013.06.1582
35. Childs SK, Kozak KR, Friedmann AM, Yeap BY, Adams J, MacDonald SM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. (2012) 82:635–42. doi: 10.1016/j.ijrobp.2010.11.048
36. Dong M, Wu J, Wu R, Wang D, Liu R, Luo H, et al. Efficacy and safety of proton beam therapy for rhabdomyosarcoma: a systematic review and meta-analysis. Radiat Oncol. (2023) 18:31. doi: 10.1186/s13014-023-02223-6
37. Corkum MT, Liu W, Palma DA, et al. Online advertising and marketing claims by providers of proton beam therapy: are they guideline-based? Radiat Oncol. (2018) 13:1–8. doi: 10.1186/s13014-018-0988-z
38. Nogueira LM, Jemal A, Yabroff KR, and Efstathiou JA. Assessment of proton beam therapy use among patients with newly diagnosed cancer in the US, 2004-2018. JAMA Netw Open. (2022) 5:e229025–e229025. doi: 10.1001/jamanetworkopen.2022.9025
39. Steinberg ML and Konski A. Proton beam therapy and the convoluted pathway to incorporating emerging technology into routine medical care in the United States. Cancer J. (2009) 15:333–8. doi: 10.1097/PPO.0b013e3181af5b5c
40. Brada M, Pijls-Johannesma M, and De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. (2009) 15:319–24. doi: 10.1097/PPO.0b013e3181b6127c
41. Odei BC, Boothe D, Keole SR, Vargas CE, Foote RL, et al. A 20-year analysis of clinical trials involving proton beam therapy. Int J Particle Ther. (2016) 3:398–406. doi: 10.14338/IJPT-D-16-00030.1
42. DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. (2014) 110:115–22. doi: 10.1002/jso.23617
43. Higgins KA, O’Connell K, Liu Y, Gillespie TW, McDonald MW, Pillai RN, et al. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. (2017) 97:128–37. doi: 10.1016/j.ijrobp.2016.10.001
44. Parikh-Patel A, Morris CR, Maguire FB, Daly ME, and Kizer KW. A population-based assessment of proton beam therapy utilization in California. Am J Manag Care. (2020) 26:e28–35. doi: 10.37765/ajmc.2020.42398
Keywords: proton beam radiation therapy, sarcoma, chordoma, rhabdomyosarcoma, chondrosarcoma, Ewing sarcoma, osteosarcoma, national cancer database
Citation: Amin SA, Mateen ZA, Amin P and Lin C (2025) Proton beam radiation therapy vs. photon radiation therapy and the overall survival of adult and pediatric patients diagnosed with sarcoma. Front. Oncol. 15:1644829. doi: 10.3389/fonc.2025.1644829
Received: 10 June 2025; Accepted: 26 August 2025;
Published: 18 September 2025.
Edited by:
James C. L. Chow, University of Toronto, CanadaReviewed by:
Menglin Nie, Capital Medical University, ChinaKubicek Pierre, Institut de Cancérologie de l’Ouest (ICO), France
Copyright © 2025 Amin, Mateen, Amin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi Lin, Y2xpbkB1bm1jLmVkdQ==
 Saber A. Amin
Saber A. Amin Zubair A. Mateen
Zubair A. Mateen Palwasha Amin3
Palwasha Amin3 Chi Lin
Chi Lin