- 1Department of Radiation Oncology, University of Tsukuba, Tsukuba, Japan
- 2Department of Pediatrics, University of Tsukuba Hospital, Tsukuba, Japan
- 3Department of Child Health, Institute of Medicine, University of Tsukuba, Tsukuba, Japan
- 4Department of Radiation Oncology, Tsukuba Medical Center Hospital, Tsukuba, Japan
- 5Department of Neurosurgery, Institute of Medicine, University of Tsukuba, Tsukuba, Japan
- 6Department of Radiology, University of Tsukuba Hospital, Tsukuba, Japan
Background: Proton beam therapy (PBT) is increasingly used for pediatric intracranial tumors due to lower long-term radiation-associated toxicities. However, data on late adverse effects, particularly brain necrosis and intracranial secondary cancer, remain limited. The aim of this study is to evaluate the incidence of these events following PBT in pediatric patients treated at a single center.
Procedure: We retrospectively reviewed the medical records of 189 patients under 20 years of age who received PBT for intracranial tumors between 1991 and 2023. Clinical information, irradiation parameters, concurrent chemotherapy, and follow-up outcomes were collected. Brain necrosis and intracranial secondary cancers were assessed based on events presenting with grade ≥2 clinical symptoms.
Results: Among 151 patients with sufficient follow-up data (median follow-up: 41.7 months), two cases of brain necrosis (1.3%) and two cases of intracranial secondary cancer (1.3%) were identified. The 5-year cumulative incidence was 2.3% (95% CI: 0-5.4%) for brain necrosis and 2.7% (95% CI: 0-6.4%) for intracranial secondary cancer. These respective incidence rates were similar for patients followed for more than two years (n=94), and slightly higher at 2.7% and 3.1% for those receiving a total dose >50 Gy (n=134). Among patients treated with PBT alone (n=125), the incidence was 1.7% for brain necrosis and 3.6% for secondary malignancy.
Conclusions: This single-center retrospective study shows a low incidence of brain necrosis and secondary malignancy following PBT for pediatric patients with intracranial tumors. These findings indicate a favorable long-term safety profile of PBT in this population.
Introduction
Multidisciplinary treatment strategies, including surgery, chemotherapy, and radiotherapy (RT), have markedly improved outcomes for pediatric tumors (1, 2). As a result, the 5-year survival rate for children with cancer has reached approximately 80% (3). However, late adverse events, particularly those associated with RT, have become an important clinical issue. In pediatric intracranial tumors, such late complications include brain necrosis, secondary malignancies, hypopituitarism, and neurocognitive dysfunction (4–6). Proton beam therapy (PBT) has the potential to reduce the risk of these toxicities by minimizing radiation exposure to adjacent normal tissues, compared to conventional photon RT (7, 8). Although PBT has shown comparable efficacy to photon therapy when used with the same treatment protocols, clinical data on long-term adverse effects in pediatric patients remain limited. In particular, the incidence and nature of late adverse events such as intracranial secondary cancers and brain necrosis following PBT are not yet fully understood. To address this gap in knowledge, we conducted a single-center retrospective study to evaluate the frequency of these complications in pediatric patients with brain tumors treated with PBT.
Methods
Patients
From 1991 to 2023, a total of 189 patients under 20 years of age underwent PBT for intracranial tumors at our center. One radiation oncologist and one pediatric oncologist evaluated the following parameters in these cases: age, gender, total PBT dose, history of prior photon RT, combination with photon RT, irradiation method (e.g., local, whole-ventricle, craniospinal irradiation [CSI]), irradiation site, treatment outcome (alive or deceased), presence of brain necrosis, presence of intracranial secondary cancer, concurrent chemotherapy during irradiation, high-dose chemotherapy, intrathecal therapy, and concurrent methotrexate administration. The evaluation of all cases was independently conducted by one radiation oncologist and one pediatrician. When findings suggestive of secondary cancer or brain necrosis were identified, both physicians reviewed the case together. Brain necrosis was evaluated according to the CTCAE v5.0 criteria, and secondary intracranial cancers were identified based on pathological diagnosis or clinical diagnosis by radiologists. Symptoms of Grade 2 or higher were assessed using information obtained through written correspondence or telephone communication from referring centers or the patients themselves.
The typical treatment protocols were local irradiation of approximately 50–60 Gy (the total dose is expressed in GyE, which is calculated by multiplying the physical dose by an RBE of 1.1) for glioma and ependymoma, approximately 50–55 Gy following CSI of about 23.4-30.6 Gy for medulloblastoma, and approximately 50 Gy following whole-ventricle irradiation or CSI of about 30 Gy for embryonal brain tumors. Chemotherapy regimens and irradiation techniques and doses were generally consistent with those used in conventional photon RT.
Statistical analysis
Intracranial secondary cancer incidence and brain necrosis incidence were calculated using SPSS v.29 (IBM, Armonk, NY, USA). The incidence rate was calculated using the start date of irradiation, the last follow-up date, and the date of death as the censoring date.
Results
Of the 189 patients initially considered, 151 were included in the analysis. Thirty eight patients were excluded due to lack of availability of post-irradiation follow-up data, irradiation dates that were too remote to ensure data integrity, or treatment protocols that did not meet the study standards. The characteristics of the 151 patients are summarized in Table 1. The median age was 8.3 years (range, 0–19 years) and 90 patients (59.6%) were male. Twenty-six patients received a combination of PBT and photon RT. Proton beam therapy was performed using local (63.6%) or whole ventricle irradiation (20.5%). Craniospinal irradiation was administered in 11.3% of cases, and whole-brain irradiation in 4.6%. Patient characteristics and treatment details, including age distribution, tumor types, irradiation methods (e.g., local, whole-ventricle, craniospinal irradiation), and chemotherapy regimens, are summarized in Table 1. Photon therapy was combined in cases requiring craniospinal or whole-brain irradiation. The tumor types was germ cell tumor (n=41), ependymoma (n=31), brainstem tumor (n=22), glioma (n=19), medulloblastoma (n=11), atypical teratoid/rhabdoid tumor (AT/RT; n=10), primitive neuroectodermal tumor (PNET; n=4), pineal tumor (n=3), meningioma (n=3), craniopharyngioma (n=2), and other tumors including pathologically unclassified brain tumors (n=5).
Concurrent chemotherapy was administered in 74 patients. In addition, high-dose chemotherapy was given in 16 patients, intrathecal chemotherapy in 30, and high-dose methotrexate in 12. These treatments may have overlapped among patients. The median interval between high-dose chemotherapy and proton therapy was 52 days (range, 28–957 days). This interval was defined as the number of days from autologous hematopoietic stem cell transplantation to the initiation of the nearest proton therapy session.
The chemotherapy regimens were documented in most cases, with the most common being CARE-based protocols (n=23, 87.0% with concurrent chemotherapy), ICE-based regimens (n=14, 92.9%), and TMZ-based regimens (n=16, 87.5%). Other regimens (n=73) included a variety of institutional or tumor-specific combinations. The regimens were selected based on tumor histology, clinical risk stratification, and institutional preferences. RT strategies included local irradiation (n=96), CSI (n=17), whole-brain irradiation (n=7), and whole-ventricular irradiation (n=31). The median total dose was 54.0 Gy (range, 23.4-73.5 Gy), with 134 patients receiving a dose >50 Gy. Details of the treatment methods for each patient are provided in the supplement, along with the corresponding references documenting the established treatment protocols (9–23).
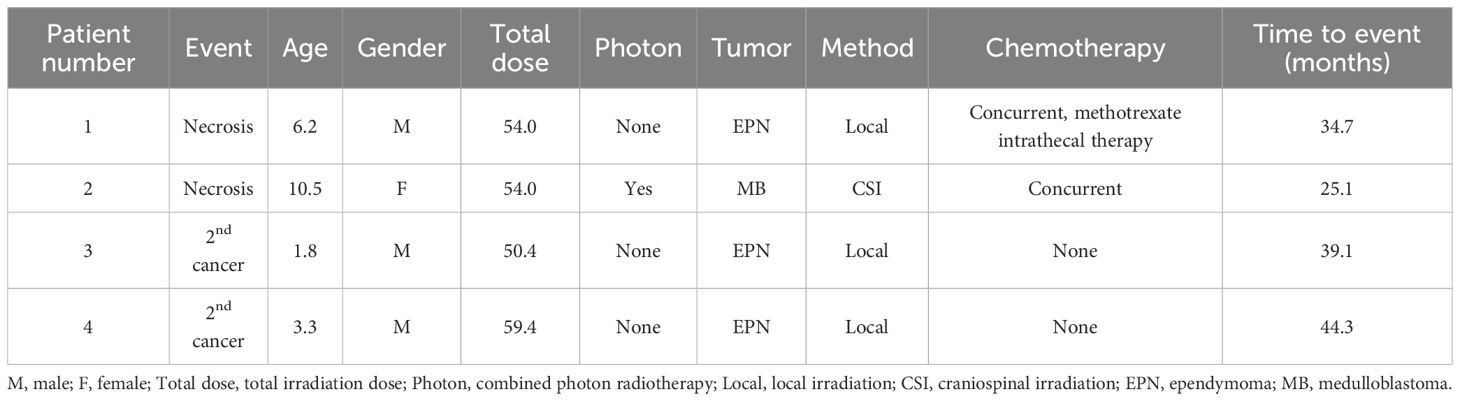
The median follow-up period for all patients was 41.7 months (range, 0-168.2 months). Brain necrosis was observed in two patients (Table 2). Patient 1 underwent reirradiation with PBT for local recurrence (cumulative dose: 113 Gy) and developed mild symptomatic brain necrosis 34.7 months after the initial irradiation. Patient 2 developed a small, asymptomatic contrast-enhancing lesion within the irradiated area 25.1 months after PBT. Although minor symptoms occasionally occurred thereafter, these were manageable with medical treatment. Secondary malignancies occurred in two patients. Patient 4 developed glioblastoma, while Patient 3 presented with a brainstem tumor of unconfirmed pathology. Both patients died of these secondary malignancies.
The 5-year cumulative incidence rates of brain necrosis and secondary malignancy for all patients (n=151) were 2.3% (95% CI, 0-5.4%) and 2.7% (95% CI, 0-6.4%), respectively (Figure 1). These rates were 2.3% (95% CI, 0-5.4%) and 2.7% (95% CI, 0-6.4%) in patients (n=94) followed for more than two years (Figure 2); 2.7% (95% CI, 0-6.4%) and 3.1% (95% CI, 0-7.6%) in patients (n=134) with a total dose >50 Gy (Figure 3); and 1.7% (95% CI, 0-5.0%) and 3.6% (95% CI, 0-8.5%) in patients (n=125) treated with PBT alone (Figure 4). Radiation necrosis was observed in one patient who underwent reirradiation, suggesting a potential increased risk in this subgroup. In contrast, RN occurred in only one patient among those who received initial irradiation. Additionally, patients who received a total dose exceeding 50 Gy showed slightly higher incidence rates of RN and secondary malignancy (2.7% and 3.1%, respectively) compared to those receiving ≤50 Gy. Given the limited number of events, no definitive statistical analysis was conducted; however, these trends suggest that reirradiation and higher total dose may be associated with an increased risk of late adverse events.
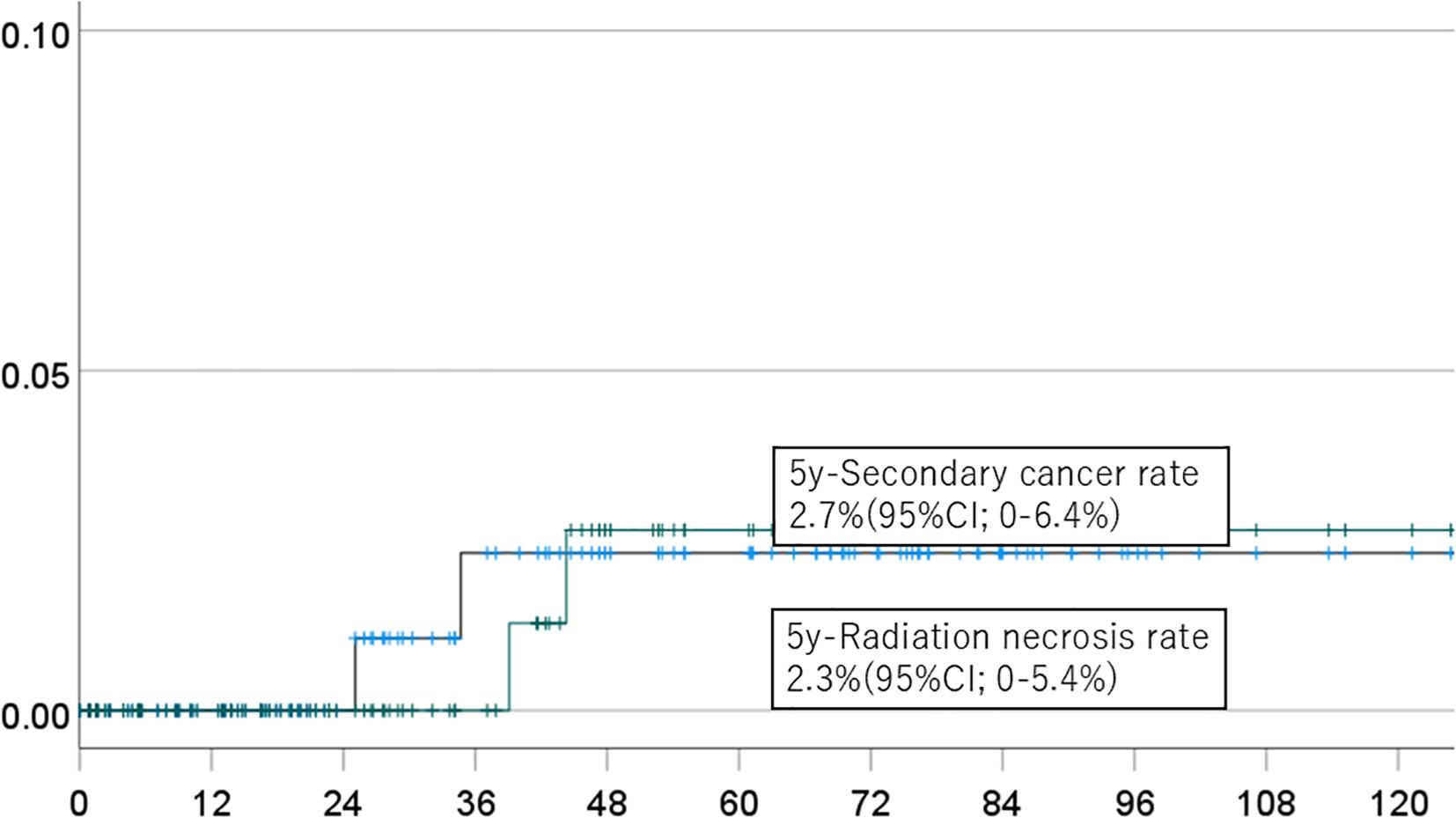
Figure 1. Incidence of brain necrosis (solid line) and intracranial secondary cancer (dotted line) in all patients.

Figure 2. Incidence of brain necrosis (solid line) and intracranial secondary cancer (dotted line) in patients followed for more than 2 years.
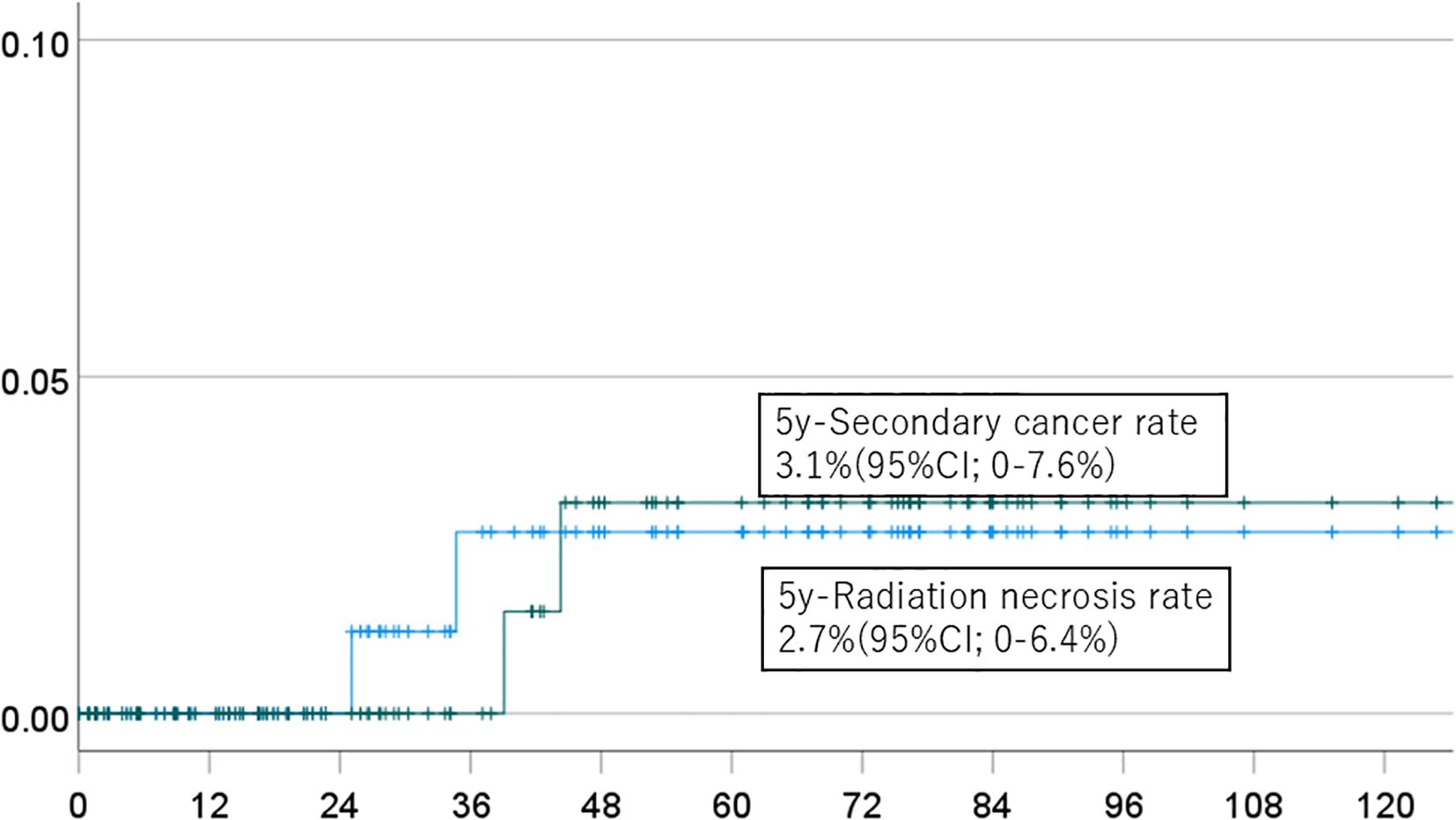
Figure 3. Incidence of brain necrosis (solid line) and intracranial secondary cancer (dotted line) in patients who received a dose of 50 Gy or more.
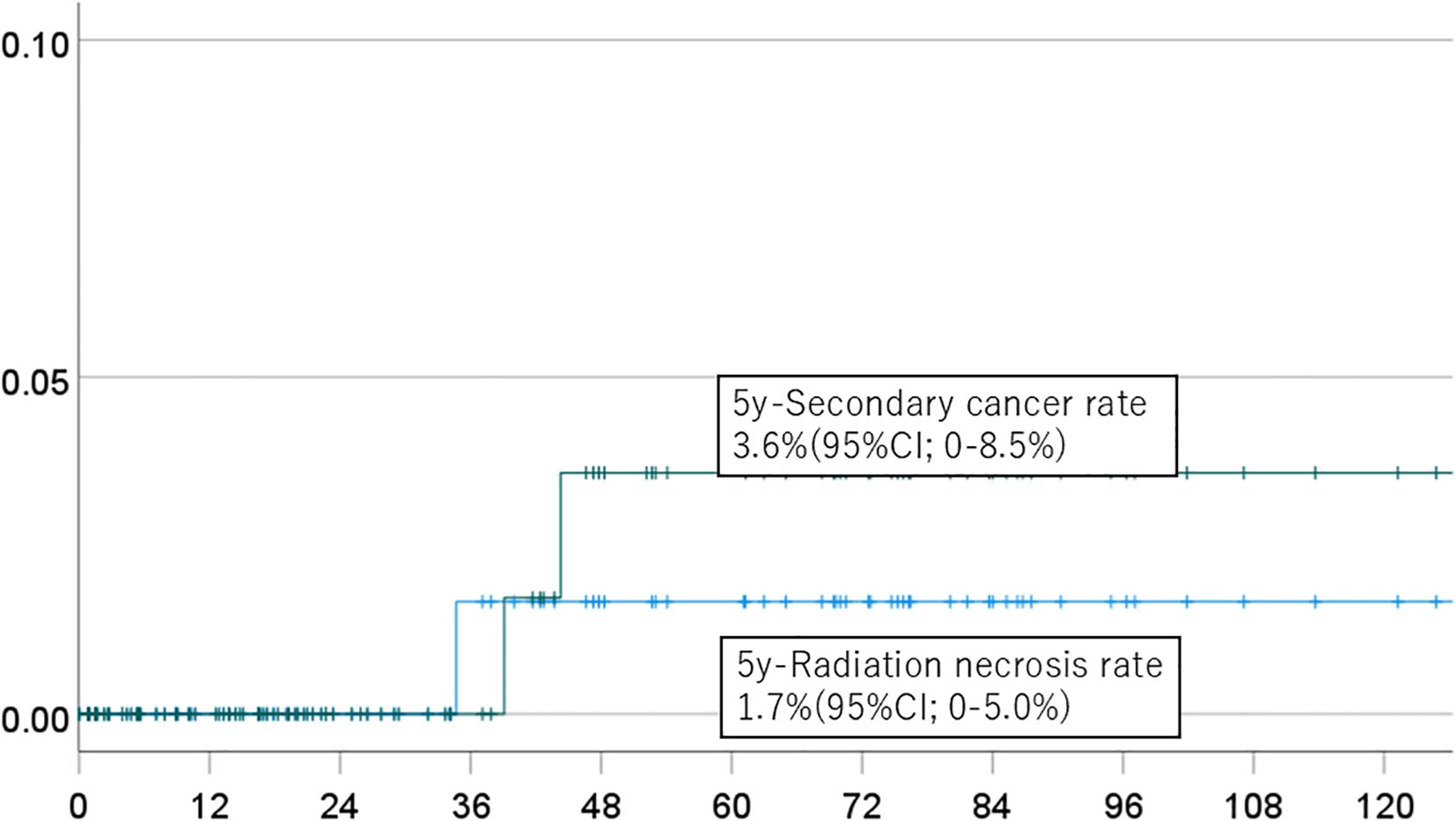
Figure 4. Incidence of brain necrosis (solid line) and intracranial secondary cancer (dotted line) in patients who received proton beam therapy alone.
Discussion
Since both proton and photon beams are categorized as low linear energy transfer (low-LET) radiation, their therapeutic effects are considered equivalent when the same area is irradiated with comparable dose fractions (7). Indeed, Eaton et al. found no significant difference in overall survival or disease control between photon and proton therapies in pediatric patients with medulloblastoma (24). However, due to the presence of the Bragg peak, proton beams allow for superior dose localization compared to photon beams. This enables a reduction in the volume of normal tissue exposed to low-dose radiation, and multiple dosimetric studies have shown the superiority of PBT in this regard (25–28). This is particularly relevant in pediatric patients, in whom cognitive impairment is a major concern and the mean dose to the brain may be associated with long-term neurocognitive outcomes (6). The dose to tissues adjacent to the high-dose target volume in PBT can be comparable to that of photon RT, and the associated risk of local toxicities such as brain necrosis remains. However, few studies have examined the incidence of brain necrosis following RT in pediatric patients.
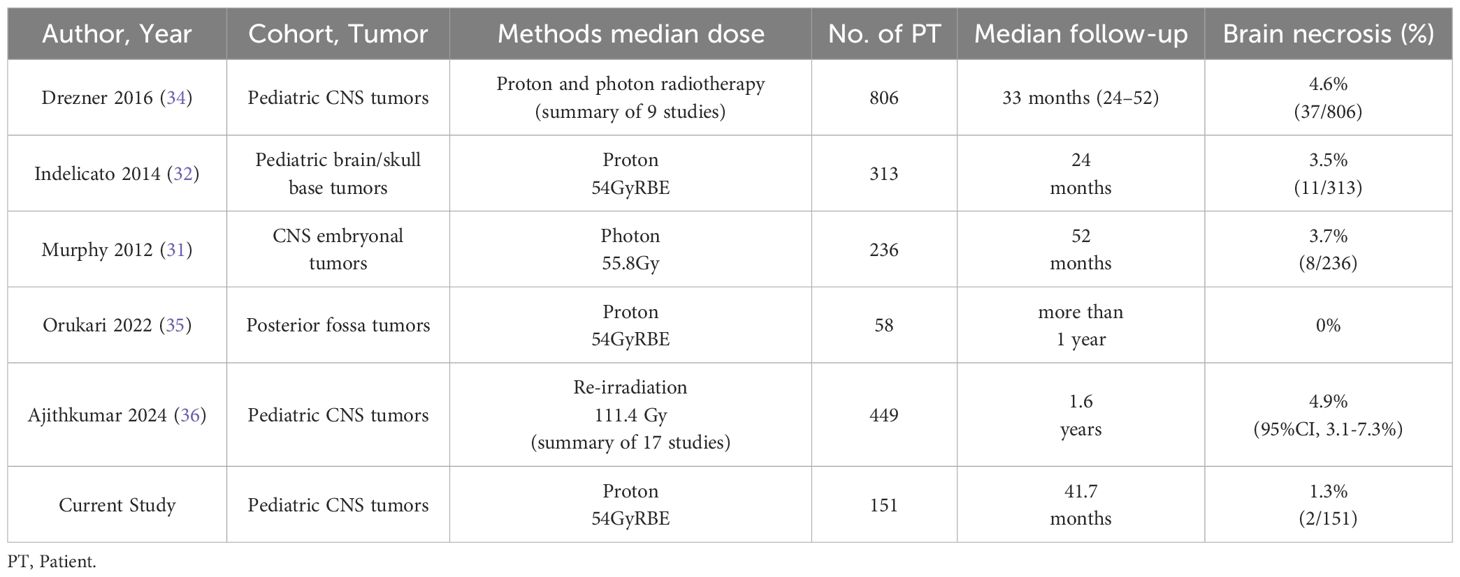
In a study at St. Jude Children’s Research Hospital, brainstem necrosis occurred in 3.7% of patients within five years after photon RT, and incidence rates in other trials have ranged from 2.2% to 8.6% (29–31). For PBT, Indelicato et al. analyzed 313 patients who received ≥50.4 Gy to the brainstem and found a 2-year incidence of late adverse events of 3.8%, including a 2.1% rate of Grade ≥3 brainstem injury (32). A meta-analysis by Alrasheed et al. found a 1.5% incidence of Grade 2 brainstem toxicity following PBT for pediatric brain tumors (33). To further contextualize the incidence of brain necrosis observed in our cohort, we summarized representative studies reporting brain necrosis rates in pediatric brain tumor patients treated with proton or photon radiotherapy (Table 3). The risk of brain necrosis is influenced by multiple factors, including radiation dose, irradiated volume, age at the time of treatment, tumor location in the infratentorial region, prior surgical interventions, and use of high-dose chemotherapy (32, 37). Additionally, pediatric brain tumors are rare and treatment protocols vary, making it challenging to establish high-level evidence. In the present study, we investigated the incidence of brain necrosis following PBT in pediatric brain tumor cases. Brain necrosis occurred in two patients, one of whom had undergone reirradiation before the onset of necrosis. Given that reirradiation is a known significant risk factor for radiation necrosis (36), exclusion of these cases from the analysis could have potentially reduced the observed incidence. However, we deliberately chose not to exclude patients who underwent reirradiation with proton therapy in order to avoid selection bias and to present an unbiased representation of real-world clinical practice. Excluding such cases might have been interpreted as selectively removing unfavorable data, which could compromise the transparency and generalizability of our findings. Therefore, we included all patients except those who had undergone prior photon radiotherapy before proton beam therapy, ensuring that the reported incidence rates reflect the actual clinical outcomes of our institution’s PBT practice. In this study, the number of events for both secondary malignancies and brain necrosis was limited, precluding a multivariate analysis to identify patient-specific risk factors. To address this limitation, we are currently collaborating with the two largest pediatric proton therapy centers in Japan and the leading pediatric proton facility in China to conduct an expanded analysis with a larger patient cohort. Additionally, we recognize that the relatively short median follow-up period limits the ability to assess very late-onset adverse events such as secondary malignancies. Continued long-term follow-up and additional analyses will be essential to provide a more comprehensive understanding of these risks.
The estimated 5-year incidence of Grade ≥2 brain necrosis ranged from 1.7% to 2.7%, suggesting that the risk of brain necrosis in our cohort was relatively low. This study has several limitations. First, the number of events was small, which may limit the statistical power to detect rare adverse outcomes. Second, the median follow-up duration of 41.7 months may not be sufficient to fully capture very late adverse effects such as secondary malignancy, which can develop over a longer time frame. Third, in some cases, long-term follow-up and imaging were conducted at referring institutions, and we could not centrally confirm all radiological findings. Therefore, the true incidence of late toxicities might be underestimated. Thus, PBT for pediatric intracranial tumors delivered using protocols comparable to those for conventional photon RT appears to have no increased risk of secondary malignancy or radiation-induced brain necrosis.
Secondary malignancy remains a significant concern following cranial irradiation in pediatric patients. Sethi et al. reported 10-year incidence rates of secondary malignancies in the irradiated field of 14% for photon RT and 0% for PBT in a cohort of 84 patients with retinoblastoma (38). Zhang et al. used in silico modeling of 17 CSI cases of medulloblastoma to estimate a lifetime risk ratio of 0.10 to 0.22 for secondary malignancy associated with PBT vs. photon RT (39). Similarly, Yoon et al. analyzed 10 CSI cases and found that the risk of secondary malignancy with photon RT was at least five times greater than that associated with PBT (40). In a comparison of 558 patients (including 44 children) treated with PBT to a matched cohort treated with photon RT selected from the U.S. SEER database, Chung et al. found a secondary malignancy incidence of 5.2% after PBT and 7.5% after photon RT, with a 10-year cumulative incidence of 5.4% vs. 8.6% and a hazard ratio of 0.52 (p = 0.009) (41). In our previous nationwide survey in Japan, the 10-year incidence of secondary malignancy following pediatric PBT was 5% (42).
In the current study, secondary malignancy occurred in 2 of 151 patients, giving a 5-year cumulative incidence of 2.7 to 3.6%. The follow-up period was relatively short and the number of secondary malignancy cases was low, which prevents a definitive conclusion, but our findings do not suggest a higher incidence compared with previous reports. Thus, PBT for pediatric intracranial tumors delivered using protocols comparable to those for conventional photon RT appears to have no increased risk of secondary malignancy or radiation-induced brain necrosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the University of Tsukuba Hospital, T-CREIDO (IRB No. H30-099). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MM: Writing – review & editing, Conceptualization, Writing – original draft. HF: Writing – review & editing, Writing – original draft, Data curation. YO: Writing – original draft, Conceptualization. TSa: Data curation, Writing – review & editing. AM: Writing – review & editing. YY: Writing – review & editing, Data curation. SH: Data curation, Writing – review & editing. MI: Writing – review & editing, Data curation. TIg: Writing – review & editing. MH: Writing – review & editing. HiN: Writing – review & editing. TId: Writing – review & editing, Data curation. TSu: Writing – review & editing. KB: Writing – review & editing. MN: Writing – review & editing. HaN: Writing – review & editing. KN: Writing – review & editing. HS: Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1644839/full#supplementary-material
Abbreviations
PBT, Proton beam therapy; RT, Radiotherapy; CSI, Craniospinal irradiation.
References
1. Gajjar A, Mahajan A, Bale T, Bowers DC, Canan L, Chi S, et al. Pediatric central nervous system cancers, version 2.2025, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2025) 23:113–30. doi: 10.6004/jnccn.2025.0012
2. Bagatell R, Park JR, Acharya S, Aldrink J, Allison J, Alva E, et al. Neuroblastoma, version 2.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2024) 22:413–33. doi: 10.6004/jnccn.2024.0040
3. Sultan I, Alfaar AS, Sultan Y, Salman Z, and Qaddoumi I. Trends in childhood cancer: Incidence and survival analysis over 45 years of SEER data. PloS One. (2025) 20:e0314592. doi: 10.1371/journal.pone.0314592
4. Merchant TE, Edmonston DY, Wu S, Li Y, Boop FA, and Lustig RH. Endocrine outcomes after limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: Long-term results from the RT1 protocol. Neuro Oncol. (2022) 24:2210–20. doi: 10.1093/neuonc/noac115
5. Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, and Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. (2011) 29:4776–80. doi: 10.1200/JCO.2011.37.9453
6. Merchant TE, Kiehna EN, Li C, Shukla H, Sengupta S, Xiong X, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. (2006) 65:210–21. doi: 10.1016/j.ijrobp.2005.10.038
7. Mizumoto M, Fuji H, Miyachi M, Soejima T, Yamamoto T, Aibe N, et al. Proton beam therapy for children and adolescents and young adults (AYAs): JASTRO and JSPHO Guidelines. Cancer Treat Rev. (2021) 98:102209. doi: 10.1016/j.ctrv.2021.102209
8. Mizumoto M, Hosaka S, Nakai K, Li Y, Oshiro Y, Iizumi T, et al. Systematic review and meta-analysis of photon radiotherapy versus proton beam therapy for pediatric intracranial ependymoma: TRP-ependymoma 2024. Heliyon. (2024) 10:e40372. doi: 10.1016/j.heliyon.2024.e40372
9. Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. (1997) 86:747–54. doi: 10.3171/jns.1997.86.5.0747
10. Matsutani M, Sano K, Takakura K, Fujimaki T, and Nakamura O. Combined treatment with chemotherapy and radiation therapy for intracranial germ cell tumors. Childs Nerv Syst. (1998) 14:59–62. doi: 10.1007/s003810050176
11. Garvin JH Jr, Selch MT, Holmes E, Berger MS, Finlay JL, Flannery A, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma: Children’s Cancer Group Protocol 9942. Pediatr Blood Cancer. (2012) 59:1183–9. doi: 10.1002/pbc.24274
12. Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. (2009) 27:385–9. doi: 10.1200/JCO.2008.18.7724
13. Jennings MT, Gelman R, and Hochberg F. Intracranial germ cell tumors: natural history and pathogenesis. J Neurosurg. (1985) 63:155–67. doi: 10.3171/jns.1985.63.2.0155
14. Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. (2005) 352:978–86. doi: 10.1056/NEJMoa042176
15. Mynarek M, von Hoff K, Pietsch T, Ottensmeier H, Warmuth-Metz M, Bison B, et al. Nonmetastatic medulloblastoma of early childhood: results from the prospective clinical trial HIT-2000 and an extended validation cohort. J Clin Oncol. (2020) 38:2028–40. doi: 10.1200/JCO.19.03057
16. von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the HIT 2000 trial. Neuro Oncol. (2011) 13:669–79. doi: 10.1093/neuonc/nor025
17. Yamasaki K, Okada K, Soejima T, Sakamoto H, and Hara J. Strategy to minimize radiation burden in infants and high-risk medulloblastoma using intrathecal methotrexate and high-dose chemotherapy: a prospective registry study in Japan. Pediatr Blood Cancer. (2020) 67:e28012. doi: 10.1002/pbc.28012
18. Okada K, Soejima T, Sakamoto H, Hirato J, and Hara J. Phase II study of reduced-dose craniospinal irradiation and combination chemotherapy for children with newly diagnosed medulloblastoma: a report from the Japanese Pediatric Brain Tumor Consortium. Pediatr Blood Cancer. (2020) 67:e28572. doi: 10.1002/pbc.28572
19. Slavc I, Chocholous M, Leiss U, Haberler C, Peyrl A, Azizi AA, et al. Atypical teratoid rhabdoid tumor: improved long-term survival with intensive multimodal therapy and delayed radiotherapy. Cancer Med. (2014) 3:91–100. doi: 10.1002/cam4.161
20. Grundy RG, Wilne SA, Weston CL, Robinson K, Lashford LS, Ironside J, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. (2007) 8:696–705. doi: 10.1016/S1470-2045(07)70208-5
21. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (SJMB-96): long-term results from a prospective, multicentre trial. Lancet Oncol. (2006) 7:813–20. doi: 10.1016/S1470-2045(06)70867-1
22. Odagiri K, Omura M, Hata M, Aida N, Niwa T, Goto H, et al. Treatment outcomes and late toxicities in patients with embryonal central nervous system tumors. Radiat Oncol. (2014) 9:201. doi: 10.1186/1748-717X-9-201
23. Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium study. J Clin Oncol. (2016) 34:3537–43. doi: 10.1200/JCO.2016.68.1585
24. Eaton BR, Esiashvili N, Kim S, Weyman EA, Thornton LT, Mazewski C, et al. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: A comparison of disease control and overall survival. Int J Radiat Oncol Biol Phys. (2016) 94:133–8. doi: 10.1016/j.ijrobp.2015.09.014
25. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, and Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. (2008) 51:110–7. doi: 10.1002/pbc.21530
26. Boehling NS, Grosshans DR, Bluett JB, Palmer MT, Song X, Amos RA, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. (2012) 82:643–52. doi: 10.1016/j.ijrobp.2010.11.027
27. Brodin NP, Munck af Rosenschöld P, Blomstrand M, Kiil-Berthlesen A, Hollensen C, Vogelius IR, et al. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. (2014) 16:594–602. doi: 10.1093/neuonc/not225
28. Park J, Park Y, Lee SU, Kim T, Choi YK, and Kim JY. Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors. Radiat Oncol. (2015) 10:135. doi: 10.1186/s13014-015-0441-5
29. Gunther JR, Sato M, Chintagumpala M, Ketonen L, Jones JY, Allen PK, et al. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J Radiat. Oncol Biol Phys. (2015) 93:54–63. doi: 10.1016/j.ijrobp.2015.05.018
30. McGovern SL, Okcu MF, Munsell MF, Kumbalasseriyil N, Grosshans D, McAleer MF, et al. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat. Oncol Biol Phys. (2014) 90:1143–52. doi: 10.1016/j.ijrobp.2014.08.354
31. Murphy ES, Merchant TE, Wu S, Xiong X, Lukose R, Wright KD, et al. Necrosis after craniospinal irradiation: Results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat. Oncol Biol Phys. (2012) 83:e655–60. doi: 10.1016/j.ijrobp.2012.01.061
32. Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. (2014) 53:1298–304. doi: 10.3109/0284186X.2014.957414
33. Alrasheed AS, Aleid AM, Alharbi RA, Alhodibi MH, Alhussain AA, Alessa AA, et al. Brainstem toxicity following proton beam radiation therapy in pediatric brain tumors: A systematic review and meta-analysis. Cancers (Basel). (2024) 16:3655. doi: 10.3390/cancers16213655
34. Drezner N, Hardy KK, Wells E, Vezina G, Ho CY, Packer RJ, et al. Treatment of pediatric cerebral radiation necrosis: a systematic review. J Neurooncol. (2016) 130:141–8. doi: 10.1007/s11060-016-2219-5
35. Orukari I, Perkins S, Zhao T, Huang J, Caruthers DF, and Duriseti S. Brainstem toxicity in pediatric patients treated with protons using a single-vault synchrocyclotron system. Int J Part Ther. (2022) 9:12–7. doi: 10.14338/IJPT-22-00008.1
36. Ajithkumar T, Avanzo M, Yorke E, Tsang DS, Milano MT, Olch AJ, et al. Brain and brain stem necrosis after reirradiation for recurrent childhood primary central nervous system tumors: A PENTEC comprehensive review. Int J Radiat Oncol Biol Phys. (2024) 119:655–68. doi: 10.1016/j.ijrobp.2023.12.043
37. Spreafico F, Gandola L, Marchianò A, Simonetti F, Poggi G, Adduci A, et al. Brain magnetic resonance imaging after high-dose chemotherapy and radiotherapy for childhood brain tumors. Int J Radiat. Oncol Biol Phys. (2008) 70:1011–9. doi: 10.1016/j.ijrobp.2007.07.2377
38. Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. (2014) 120:126–33. doi: 10.1002/cncr.28387
39. Zhang R, Howell RM, Taddei PJ, Giebeler A, Mahajan A, and Newhauser WD. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother Oncol. (2014) 113:84–8. doi: 10.1016/j.radonc.2014.07.003
40. Yoon M, Shin DH, Kim J, Kim JW, Kim DW, Park SY, et al. Craniospinal irradiation techniques: a dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys. (2011) 81:637–46. doi: 10.1016/j.ijrobp.2010.06.039
41. Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, and Tarbell NJ. Incidence of second Malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. (2013) 87:46–52. doi: 10.1016/j.ijrobp.2013.04.030
Keywords: brain, brain necrosis, secondary cancer, proton beam therapy, particle therapy pediatric, radiation toxicity
Citation: Mizumoto M, Fukushima H, Oshiro Y, Saito T, Muroi A, Yamaki Y, Hosaka S, Inaba M, Ishiguro T, Harada M, Niitsu H, Ishida T, Sumiya T, Baba K, Nakamura M, Numajiri H, Nakai K and Sakurai H (2025) Analysis of brain necrosis and secondary cancers after proton beam therapy for pediatric intracranial tumors: a single-center retrospective study. Front. Oncol. 15:1644839. doi: 10.3389/fonc.2025.1644839
Received: 10 June 2025; Accepted: 26 August 2025;
Published: 12 September 2025.
Edited by:
Eleanor Kane, University of York, United KingdomReviewed by:
Hamid Mammar, Institut Curie, FranceMarco Cianchetti, Azienda Provinciale per i Servizi Sanitari (APSS), Italy
Copyright © 2025 Mizumoto, Fukushima, Oshiro, Saito, Muroi, Yamaki, Hosaka, Inaba, Ishiguro, Harada, Niitsu, Ishida, Sumiya, Baba, Nakamura, Numajiri, Nakai and Sakurai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masashi Mizumoto, bWl6dW1vdG9AcG1yYy50c3VrdWJhLmFjLmpw
 Masashi Mizumoto
Masashi Mizumoto Hiroko Fukushima2,3
Hiroko Fukushima2,3 Yoshiko Oshiro
Yoshiko Oshiro Takashi Saito
Takashi Saito Ai Muroi
Ai Muroi Masatoshi Nakamura
Masatoshi Nakamura