- 1Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Department of Neurosurgery, Tengzhou Central People’s Hospital, Tengzhou, Shandong, China
Purpose: The aim of this study is to investigate whether the therapeutic efficacy of bevacizumab (BEV) in the treatment of high-grade glioma (HGG) is associated with the methylation status of O6-methylguanine-DNA methyltransferase (MGMT) on the basis of excluding the interference from chemotherapy drugs.
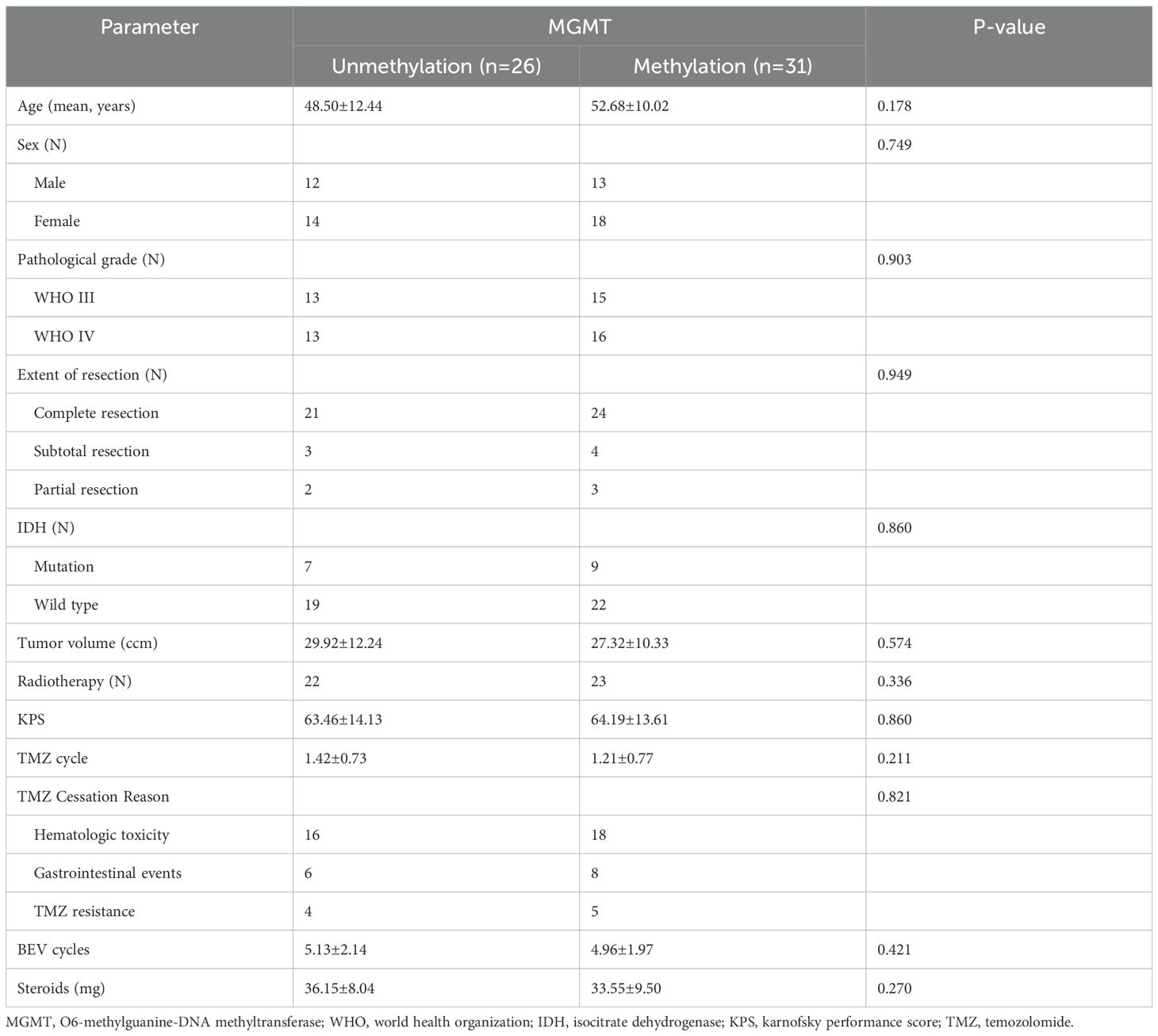
Methods: This study included 57 patients with histologically confirmed HGG who, due to various reasons, were unable to complete the standard chemotherapy protocol and thus received BEV treatment. Patients enrolled in the study were divided into two groups based on their MGMT status: the unmethylated MGMT group and the methylated MGMT group. Depending on whether the numerical variables of the patients satisfied a normal distribution, either the T-test or the rank-sum test was employed. For the comparison of categorical variables, the chi-square test was used.
Results: After BEV treatment, both PFS and OS were higher in the methylated MGMT group compared to the unmethylated group. Additionally, the tumor control rate was also higher in the methylated group. Furthermore, in both patient groups, a decrease in steroid dosage was observed following BEV treatment, accompanied by an increase in KPS. Multivariate COX regression analysis revealed that radiotherapy and complete tumor resection were significant factors influencing PFS and OS in HGG patients. Additionally, pathological grade was found to influence PFS in HGG patients.
Conclusion: Based on the exclusion of the interference from chemotherapy drugs, our study is the first to confirm the correlation between the methylation status of MGMT and the therapeutic effect of BEV on HGG.
1 Introduction
High-grade gliomas (HGG) are a common intracranial malignancy. The standard treatment typically involves surgical resection followed by synchronous radiotherapy and chemotherapy, with continued adjuvant chemotherapy thereafter (1–3). Temozolomide (TMZ) remains the most frequently employed chemotherapeutic agent in the management of these aggressive tumors (4, 5). A pivotal factor influencing the therapeutic response to TMZ is the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) gene (6). However, TMZ use is frequently associated with treatment discontinuation due to thrombocytopenia and other adverse effects (7). Bevacizumab (BEV), a monoclonal antibody targeting vascular endothelial growth factor (VEGF), has emerged as a promising agent in the treatment paradigm of HGG, demonstrating potential in prolonging progression-free survival, albeit with equivocal impacts on overall survival (8–10). From a biological perspective, MGMT promoter methylation leading to gene silencing is not only associated with alkylating agent resistance but may also influence anti-angiogenic therapy by altering tumor angiogenesis characteristics (11). Studies suggest that MGMT methylation status may be potentially linked to aberrant activation of the VEGF pathway in the tumor microenvironment, with methylated tumors potentially exhibiting greater dependence on angiogenesis, thereby increasing sensitivity to VEGF inhibitors like BEV (12). Previous studies involving the relationship between MGMT methylation and the efficacy of BEV have been confounded by various chemotherapy agents. Consequently, the correlation between BEV’s therapeutic effect and MGMT methylation remains elusive. In this retrospective study, we aimed to investigate whether the methylation status of MGMT influences the therapeutic response of HGG to BEV, after excluding the interference of chemotherapy drugs. Our ultimate goal is to inform personalized treatment strategies for these patients.
2 Materials and methods
2.1 Patients
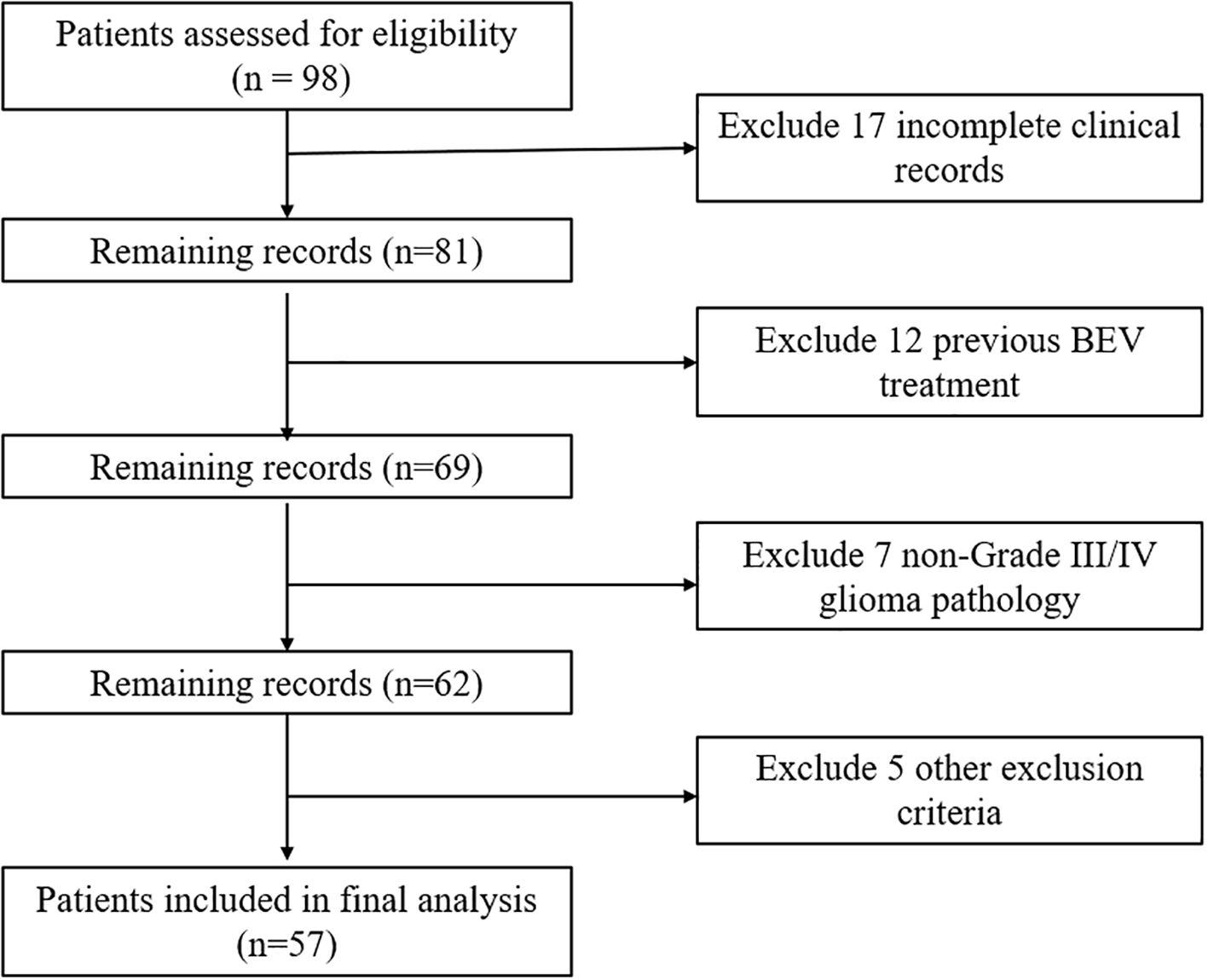
Inclusion Criteria: (1) Patients must have a confirmed pathological diagnosis of grade III or IV glioma. (2) Only adult patients aged 18 years or older will be included. (3) Patients must have received standard treatment for glioma but must not have undergone treatment with BEV. (4) Patients must possess comprehensive clinical data. Exclusion Criteria: (1) Patients with a recent history of abnormal bleeding will be excluded due to potential contraindications with study treatments. (2) Patients with concurrent malignancies other than the primary glioma diagnosis will be excluded. (3) The presence of severe systemic diseases that could significantly impact patient outcomes or interfere with the study assessments will result in exclusion from the study. Figure 1 shows the CONSORT flow diagram of patient screening and enrollment. A total of 57 patients were included in this study. All these patients had received surgery but started to receive BEV treatment after stopping TMZ treatment due to various reasons. Table 1 summarizes the detailed demographic characteristics of the enrolled patients. This study adhered to the principles of the Declaration of Helsinki and followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). All data utilized in this study were de-identified to ensure the privacy and confidentiality of human subjects. The study protocol was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (Approval No. ZQ-3194). Written informed consent was obtained from all participants and/or their legal guardian(s) prior to the study.
2.2 Therapeutic process
All enrolled HGG patients underwent surgical resection followed by TMZ chemotherapy. Of these, 45 patients received postoperative radiotherapy. All participants completed the standard 5-day TMZ regimen (28-day cycles) (13). The maximum number of cycles for TMZ treatment in this study was 3. Due to hematologic toxicity, drug resistance, and other reasons, these patients discontinued TMZ treatment after receiving various cycles of the drug and began BEV therapy. The recommended BEV dosage in previous studies was 5-15 mg/kg (14–16). In this study, all patients received a BEV dosage of 5 mg/kg, administered every two weeks.
2.3 Efficacy evaluation
Imaging studies were completed within three days post-surgery, utilizing t1-weighted enhancement sequences to assess tumor status (17). Complete resection was defined as postoperative absence of tumor enhancement on imaging. Subtotal resection indicated residual enhancement ≤5% of preoperative tumor volume, while partial resection represented residual enhancement >5%. Follow-up imaging was performed at 1- to 2-month intervals (18). Therapeutic response was evaluated according to the Response Assessment in Neuro-Oncology (RANO) criteria (19). Complete response (CR) was defined as the disappearance of tumor signals, partial response (PR) as a reduction in tumor area by at least 50%, stable disease (SD) as a reduction in tumor size by less than 50% or an increase by less than 25%, and disease progression (PD) as an increase in tumor size by at least 25% (20). The overall response rate (OR) encompassed both CR and PR. Progression-free survival (PFS) and overall survival (OS) were calculated from the beginning of BEV treatment.
2.4 Statistical analysis
Continuous variables were expressed as mean ± standard deviation (mean ± SD). All categorical variables were described using the number of patients or percentages (%). The data of all continuous variables were first tested for normal distribution. If the data followed a normal distribution, the t-test was used for comparison. If the data did not conform to a normal distribution, the rank-sum test was employed for comparison. The chi-square test was used for the comparison of categorical variables. Univariate and multifactorial COX regression analyses were used to explore independent risk factors for PFS and OS in HGG patients. The survival curves for PFS and OS were plotted using the Kaplan-Meier method. All data in this study were analyzed using SPSS (version 27.0, IBM). A P value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
All patients received TMZ therapy postoperatively, and 45 patients also underwent radiotherapy. During the course of treatment, all patients stopped receiving TMZ treatment and began BEV treatment due to severe adverse reactions such as hematologic toxicity, vomiting, and other reasons. Prior to BEV treatment, the number of TMZ cycles in the MGMT unmethylated group and the methylated group were 1.42 ± 0.73 and 1.21 ± 0.77 cycles, respectively (P > 0.05). The mean number of BEV cycles administered was comparable between groups, with 4.96 ± 1.97 cycles in the methylated cohort versus 5.13 ± 2.14 cycles in the unmethylated cohort (P > 0.05), confirming equivalent treatment exposure. Among the 26 patients in the MGMT unmethylated group, 2 (7.7%) experienced tumor recurrence. In the 31 patients of the methylated group, 3 (9.7%) had tumor recurrence. The results showed no significant differences in baseline characteristics between the two groups prior to BEV treatment.
3.2 Cox regression analysis
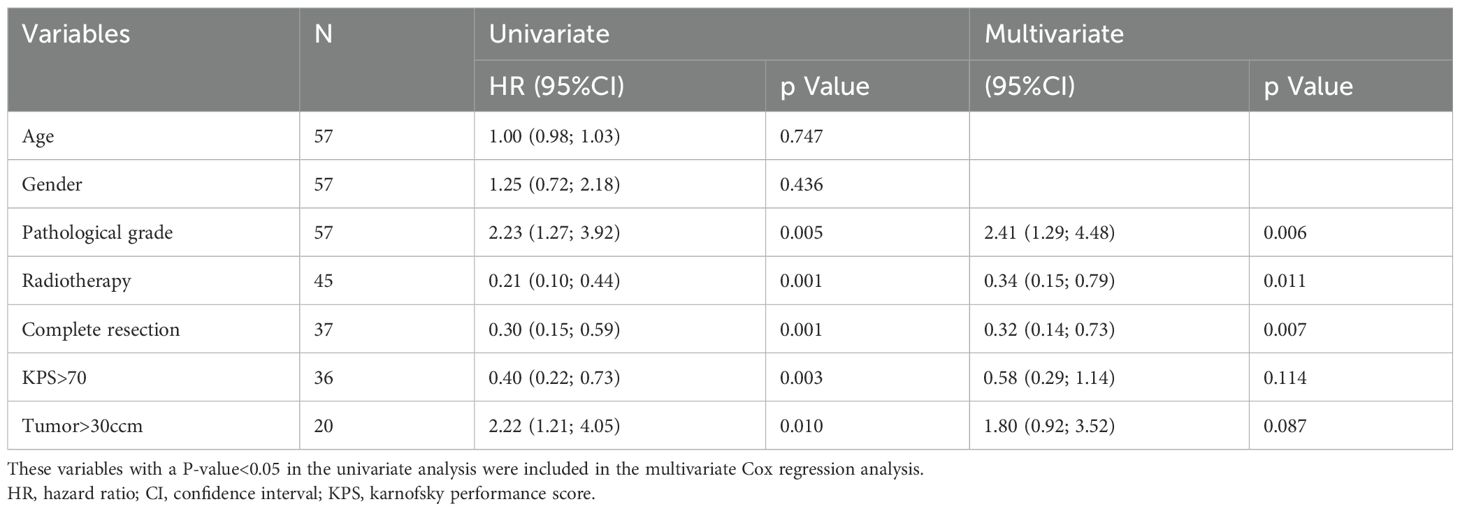
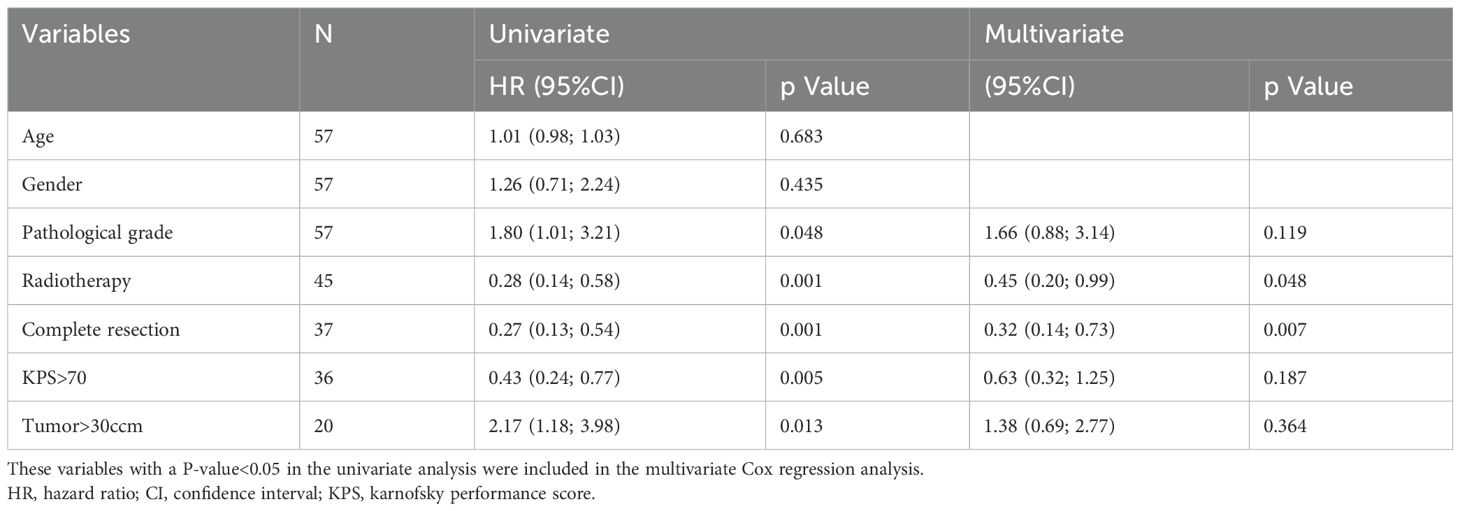
This study employed COX regression analysis to investigate the risk factors for PFS and OS in patients with HGG. Univariate analysis revealed that high pathological grade and large tumor volume were risk factors for both PFS and OS, while radiotherapy, complete tumor resection, and preoperative KPS score > 70 were protective factors for PFS and OS in patients with HGG. Multivariate analysis further indicated that pathological grade (HR 2.41, 95% CI 1.29-4.48, p=0.006), radiotherapy (HR 0.34, 95% CI 0.15-0.79, p=0.011), and complete resection (HR 0.32, 95% CI 0.14-0.73, p=0.007) were significant predictor of PFS. Additionally, multivariate analysis demonstrated that radiotherapy (HR 0.45, 95% CI 0.20-0.99, p=0.048) and complete resection (HR 0.32, 95% CI 0.14-0.73, p=0.007) were significant predictor of OS. Table 2 summarizes the results of both univariate and multivariate COX regression analyses for PFS. Table 3 presents the results of both univariate and multivariate COX regression analysis for OS.
3.3 Therapeutic effect
After initiating BEV treatment, the PFS for the unmethylated and methylated MGMT groups were 5.77 ± 4.75 months and 9.81 ± 6.67 months, respectively, while the OS were 7.73 ± 4.31 months and 11.42 ± 6.36 months, respectively (P<0.05). At 6 months of BEV treatment, 19 patients in the MGMT unmethylated group and 14 patients in the methylated group experienced tumor progression, with PFS of 26.9% and 54.8%, respectively (P<0.05). Figure 2 displays the PFS and OS curves for patients with different MGMT statuses. Regarding the control rates, after 6 months of BEV treatment, 26.9% and 7.7% of patients in the MGMT unmethylated group achieved CR and PR, respectively. Twelve patients achieved SD, while the remaining patients exhibited PD. In the MGMT methylated group, 54.8% and 19.4% of patients achieved CR and PR, respectively. Five patients exhibited SD, and the remaining three patients exhibited PD. In summary, the OR for the MGMT unmethylated group was 34.6%, while the OR for the methylated group was 74.2% (P<0.05).
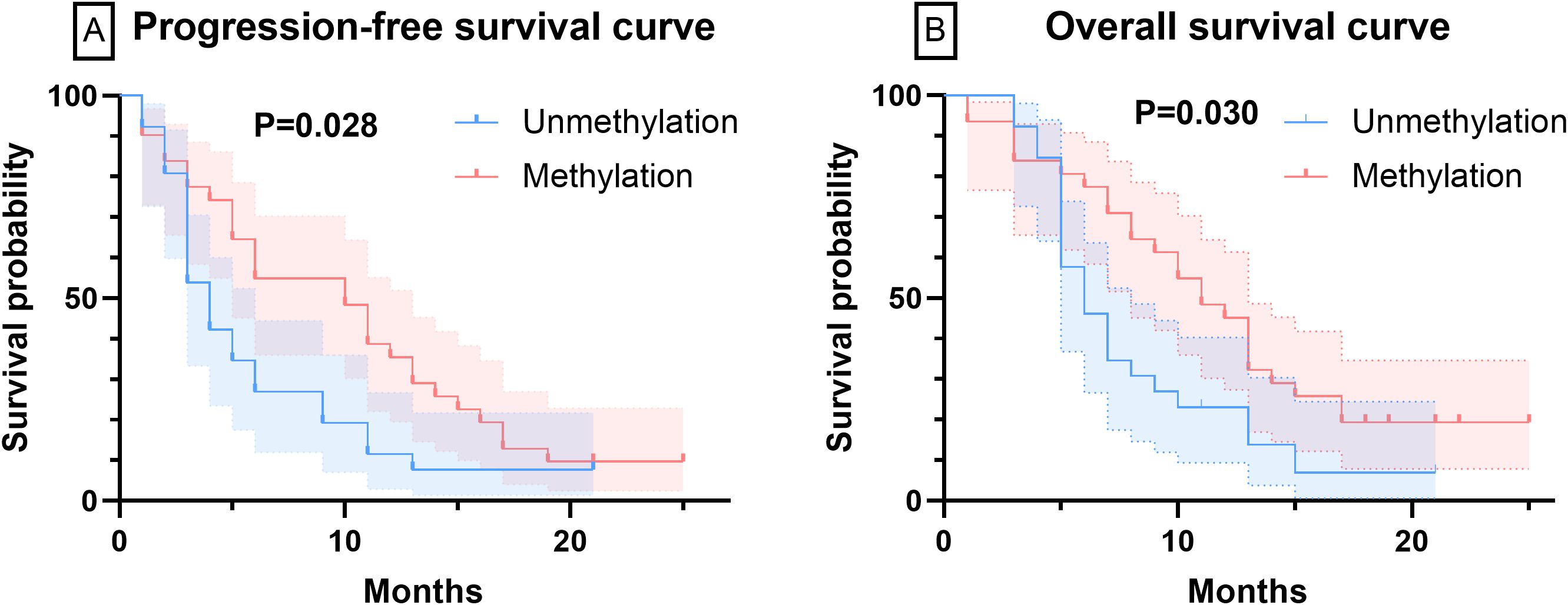
Figure 2. The curves of PFS and OS were plotted by Kaplan-Meier method. (A) The PFS curves of patients with varying MGMT methylation statuses. (B) The OS curves of patients with varying MGMT methylation statuses.
3.4 Clinical symptoms
Prior to BEV treatment, all patients received steroidal hormones (methylprednisolone) to manage clinical symptoms. Specifically, patients in the MGMT unmethylated group received a steroidal dose of 36.15 ± 8.04 mg, while those in the methylated group received 33.55 ± 9.50 mg. After BEV treatment, the steroidal dose decreased significantly in both groups, with the unmethylated group receiving 21.54 ± 13.77 mg and the methylated group receiving 18.71 ± 15.44 mg (P > 0.05). Prior to BEV treatment, the KPS score in the unmethylated group was 63.46 ± 14.13, while that in the methylated group was 64.19 ± 13.61. Following BEV treatment, the KPS scores increased in both groups, with the unmethylated group scoring 71.15 ± 8.64 and the methylated group scoring 73.23 ± 13.51 (P > 0.05).
3.5 Adverse drug reactions
BEV-associated adverse effects included various types of bleeding, headaches, hypertension, blood toxicity, thrombosis, proteinuria, gastrointestinal perforation, delayed wound healing, congestive heart failure, sepsis, and nephrotic syndrome (21, 22). In this study, hypertension was the most common adverse reaction, occurring in 11 patients. Additionally, 5 patients exhibited headache, 2 patients developed hematological toxicity, and 1 patient presented with proteinuria. No adverse reactions occurred that were severe enough to necessitate discontinuation of BEV treatment.
4 Discussion
After receiving BEV treatment, patients in both groups exhibited a significant decrease in steroid dosage. Concurrently, there was varying degrees of improvement in clinical symptoms, with the majority of patients maintaining a high KPS level similar to the level before treatment or even achieving further improvements. Previous studies have also demonstrated that BEV can effectively alleviate peritumoral edema in patients with HGG, thereby improving their clinical symptoms (23–25). No significant differences were observed between the two groups in terms of steroid dosage and KPS. To explore the factors influencing PFS and OS in HGG patients, we employed COX regression analysis. Univariate analysis revealed that pathological grade, tumor volume, radiotherapy, complete tumor resection, and KPS were significant factors affecting PFS and OS in HGG patients. Multivariate analysis further indicated that radiotherapy and complete tumor resection were the main influencing factors for both PFS and OS. These findings are consistent with previous studies (26). Additionally, pathological grade emerged as a factor influencing PFS but not OS.
Analysis of our cohort demonstrated markedly reduced progression-free and overall survival durations in WHO CNS grade 4 glioma patients relative to grade 3 cases, consistent with established prognostic correlations in current neuro-oncology practice (27). The clinical relevance of histopathological grading emerges through multiple mechanisms: foremost, advanced-grade neoplasms demonstrate enhanced microvascular proliferation and necrosis patterns- morphological hallmarks of tumor aggressiveness (28, 29). Additionally, progressive histological malignancy shows strong associations with amplified cellular proliferation indices and chromosomal instability. Importantly, our adjusted regression models established pathological grading as maintaining independent predictive value for disease progression even when accounting for MGMT epigenetic status.
Standard treatment for HGG typically involves maximal surgical resection followed by six weeks of concurrent radiotherapy and TMZ, followed by six months of adjuvant TMZ chemotherapy (30). It is well-established that MGMT methylation is a crucial prognostic predictor for HGG patients receiving TMZ treatment (31). However, in our study, the patients received a much lower dose of TMZ than the standard TMZ protocol, therefore, we believe that the interference of TMZ on the study results was limited. In this study, a single-center retrospective analysis was conducted on 57 patients with HGG who underwent BEV treatment. The results indicated that the PFS of the non-methylated group was 5.77 months, whereas the PFS of the methylated group was 9.81 months. Additionally, the OS of the non-methylated and methylated groups were 7.73 months and 11.4 months, respectively. Significant differences in PFS and OS were observed between the two groups, suggesting that HGG patients with MGMT methylation respond better to BEV treatment than those without methylation. Furthermore, notable disparities were also observed in tumor control rates between the two groups. Specifically, the OR of the unmethylated MGMT group was 34.6%, while the OR of the methylated group was 74.2%. Therefore, we propose that MGMT methylation serves as a predictor for the therapeutic response of BEV in patients with HGG.
In a study involving 191 patients, the authors analyzed the predictive value of MGMT status on tumor prognosis in patients with recurrent HGG treated with BEV (32). Their research demonstrated that MGMT status was not associated with patient prognosis. Although the number of cases was substantial, it is noteworthy that the MGMT status of 63% of the patients in their study was unknown. A separate multicenter retrospective study evaluated the effectiveness of BEV in treating patients with recurrent glioblastoma. In this study, the rates of IDH and MGMT status were 74% and 83%, respectively. However, the authors did not analyze the predictive effect of MGMT status on prognosis (33). In a study involving 437 patients with recurrent glioblastoma, the authors demonstrated that the progression-free survival (PFS) was prolonged with the combination of lomustine and BEV compared to lomustine alone. However, no survival advantage was observed. Notably, in this study, patients with MGMT-methylated recurrent glioblastoma had a PFS twice as high as those without methylation (34). Nevertheless, it remains uncertain whether this difference was exaggerated by the effect of lomustine. In another study encompassing 92 patients with recurrent HGG, the authors reported a higher incidence of MGMT methylation among long-term responders (PFS ≥ 6 months) (35). However, it is worth mentioning that some patients in this study received concurrent treatment with TMZ or other chemotherapy agents such as fotemustine. The prognostic and predictive role of TMZ in HGG with MGMT methylation is well-established (36, 37). Therefore, we believe that the results of this study may have been influenced by the chemotherapy agents.
In our study, all patients discontinued treatment with TMZ at far lower doses than the standard protocol due to various reasons. Consequently, the impact of chemotherapy agents on PFS and OS in HGG patients was minimal in our study. To the best of our knowledge, our study is the first to investigate the correlation between MGMT methylation and the prognosis of HGG patients treated with BEV, while excluding the interference of chemotherapy drugs. Our findings suggest that BEV is more effective in patients with MGMT-methylated HGG compared to those without methylation, indicating that MGMT can serve as a prognostic predictor for BEV treatment in HGG. However, it is important to acknowledge some limitations of this study. Firstly, as a single-center study, the results may not be fully representative. Furthermore, due to the limited number of patients who discontinued TMZ treatment, the case numbers in this study are restricted. Future research will aim to include a larger sample size to obtain more accurate results. Secondly, due to its retrospective nature, our study had limitations in evidence collection. Therefore, larger, multicenter, prospective studies with a greater sample size are needed to comprehensively evaluate the efficacy of BEV.
5 Conclusion
Based on the exclusion of the interference from chemotherapy drugs, our study is the first to confirm the correlation between the methylation status of MGMT and the therapeutic effect of BEV on HGG. Our results indicate that BEV is more effective in patients with MGMT-methylated HGG compared to unmethylation, suggesting that MGMT can serve as a prognostic predictor for BEV treatment in HGG.
Data availability statement
The data presented in the study are deposited in the Zenodo repository, accession number 10.5281/zenodo.16903955 (URL: https://zenodo.org/records/16903955).
Ethics statement
This study adhered to the principles of the Declaration of Helsinki and followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). All data utilized in this study were de-identified to ensure the privacy and confidentiality of human subjects. The study protocol was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (Approval No. ZQ-3194). Written informed consent was obtained from all participants and/or their legal guardian(s) prior to the study.
Author contributions
CY: Data curation, Supervision, Methodology, Writing – review & editing, Writing – original draft, Project administration, Resources. XB: Writing – review & editing, Formal Analysis, Conceptualization, Supervision, Investigation, Data curation. CW: Formal Analysis, Funding acquisition, Validation, Visualization, Writing – review & editing. WM: Visualization, Formal Analysis, Validation, Funding acquisition, Writing – review & editing. MF: Project administration, Supervision, Methodology, Formal Analysis, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors acknowledge the following financial support: the National Science and Technology Major Project (2022ZD0116002); CAMS Innovation Fund for Medical Sciences (2023-I2M-C&T-B-008); the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-113); the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2021-I2M-1-014); the CAMS Innovation Fund for Medical Sciences (CIFMS) (2024-I2M-C&T-B-021); the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2024-JKCS-23); and Peking Union Medical College Hospital Talent Cultivation Program (Category C) (UBJ10254).
Acknowledgments
The authors are grateful to all patients included in this study for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Krystal J, Hanson D, Donnelly D, and Atlas M. A phase 1 study of mebendazole with bevacizumab and irinotecan in high-grade gliomas. Pediatr Blood Cancer. (2024) 71:e30874. doi: 10.1002/pbc.30874
2. Lin H, Liu C, Hu A, Zhang D, Yang H, and Mao Y. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J Hematol Oncol. (2024) 17:31. doi: 10.1186/s13045-024-01544-7
3. Shah S. Novel therapies in glioblastoma treatment: review of glioblastoma; current treatment options; and novel oncolytic viral therapies. Med Sci (Basel). (2023) 12(1):1. doi: 10.3390/medsci12010001
4. Dhungel L, Rowsey ME, Harris C, and Raucher D. Synergistic effects of temozolomide and doxorubicin in the treatment of glioblastoma multiforme: enhancing efficacy through combination therapy. Molecules. (2024) 29(4):840. doi: 10.3390/molecules29040840
5. Singh R, Lehrer EJ, Wang M, Perlow HK, Zaorsky NG, Trifiletti DM, et al. Dose escalated radiation therapy for glioblastoma multiforme: an international systematic review and meta-analysis of 22 prospective trials. Int J Radiat Oncol Biol Phys. (2021) 111:371–84. doi: 10.1016/j.ijrobp.2021.05.001
6. Hao Z, Wang J, Lv Y, Wu W, Zhang S, Hao S, et al. Identification of MGMT promoter methylation as a specific lipid metabolism biomarker, reveals the feasibility of atorvastatin application in glioblastoma. Metabolism. (2024) 153:155794. doi: 10.1016/j.metabol.2024.155794
7. Gately L, Mesía C, Sepúlveda JM, Del Barco S, Pineda E, Gironés R, et al. A combined analysis of two prospective randomised studies exploring the impact of extended post-radiation temozolomide on survival outcomes in newly diagnosed glioblastoma. J Neurooncol. (2024) 166:407–15. doi: 10.1007/s11060-023-04513-1
8. Cho SJ, Kim HS, Suh CH, and Park JE. Radiological recurrence patterns after bevacizumab treatment of recurrent high-grade glioma: A systematic review and meta-analysis. Korean J Radiol. (2020) 21:908–18. doi: 10.3348/kjr.2019.0898
9. Moon HH, Park JE, Kim YH, Kim JH, and Kim HS. Contrast enhancing pattern on pre-treatment MRI predicts response to anti-angiogenic treatment in recurrent glioblastoma: comparison of bevacizumab and temozolomide treatment. J Neurooncol. (2022) 157:405–15. doi: 10.1007/s11060-022-03980-2
10. Obrador E, Moreno-Murciano P, Oriol-Caballo M, López-Blanch R, Pineda B, Gutiérrez-Arroyo JL, et al. Glioblastoma therapy: past, present and future. Int J Mol Sci. (2024) 25(5):2529. doi: 10.3390/ijms25052529
11. Tamura R, Morimoto Y, Kosugi K, Sato M, Oishi Y, Ueda R, et al. Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series. BMC Cancer. (2020) 20:196. doi: 10.1186/s12885-020-6589-x
12. Kessler T, Schrimpf D, Doerner L, Hai L, Kaulen LD, Ito J, et al. Prognostic markers of DNA methylation and next-generation sequencing in progressive glioblastoma from the EORTC-26101 trial. Clin Cancer Res. (2023) 29:3892–900. doi: 10.1158/1078-0432.Ccr-23-0926
13. Yuan J, Liu J, Fan R, and Liu Z. Effect of temozolomide combined with intensity modulated radiation therapy on serum factor, immune function and clinical efficacy in postoperative glioma patients. Radiat Res. (2023) 200:289–95. doi: 10.1667/rade-22-00198
14. Dasgupta P, Ou A, Lin H, Gregory T, Alfaro-Munoz KD, Yuan Y, et al. The risk and burden of thromboembolic and hemorrhagic events in patients with Malignant gliomas receiving bevacizumab. J Neurooncol. (2024) 167:181–88. doi: 10.1007/s11060-023-04551-9
15. Detti B, Scoccianti S, Teriaca MA, Maragna V, Lorenzetti V, Lucidi S, et al. Bevacizumab in recurrent high-grade glioma: a single institution retrospective analysis on 92 patients. Radiol Med. (2021) 126:1249–54. doi: 10.1007/s11547-021-01381-5
16. Xu Y, Guan H, Yu K, Ji N, and Zhao Z. Efficacy and safety of pharmacotherapy for recurrent high-grade glioma: a systematic review and network meta-analysis. Front Pharmacol. (2023) 14:1191480. doi: 10.3389/fphar.2023.1191480
17. Baris MM, Celik AO, Gezer NS, and Ada E. Role of mass effect, tumor volume and peritumoral edema volume in the differential diagnosis of primary brain tumor and metastasis. Clin Neurol Neurosurg. (2016) 148:67–71. doi: 10.1016/j.clineuro.2016.07.008
18. Falk AT, Barrière J, François E, and Follana P. Bevacizumab: A dose review. Crit Rev Oncol Hematol. (2015) 94:311–22. doi: 10.1016/j.critrevonc.2015.01.012
19. Imber BS, Lin AL, Zhang Z, Keshavamurthy KN, Deipolyi AR, Beal K, et al. Comparison of radiographic approaches to assess treatment response in pituitary adenomas: is RECIST or RANO good enough? J Endocr Soc. (2019) 3:1693–706. doi: 10.1210/js.2019-00130
20. Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. (2011) 13:1143–50. doi: 10.1093/neuonc/nor091
21. Chen J, Lu Y, and Zheng Y. Incidence and risk of hypertension with bevacizumab in non-small-cell lung cancer patients: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. (2015) 9:4751–60. doi: 10.2147/dddt.S87258
22. Matikas A, Kentepozidis Ν, Ardavanis A, Vaslamatzis M, Polyzos A, Emmanouilides CH, et al. Efficacy and tolerance of frontline bevacizumab-based chemotherapy for advanced non-small cell lung cancer patients: a multicenter, phase IV study of the Hellenic Oncology Research Group (HORG). Cancer Chemother Pharmacol. (2016) 78:369–76. doi: 10.1007/s00280-016-3094-7
23. Wang X, Chen D, Qiu J, Li S, and Zheng X. The relationship between the degree of brain edema regression and changes in cognitive function in patients with recurrent glioma treated with bevacizumab and temozolomide. Quant Imaging Med Surg. (2021) 11:4556–68. doi: 10.21037/qims-20-1084
24. Bai X, Feng M, Ma W, and Wang S. Predicting the efficacy of bevacizumab on peritumoral edema based on imaging features and machine learning. Sci Rep. (2025) 15:15990. doi: 10.1038/s41598-025-00758-0
25. Liao Y, Bai X, Cao Y, and Zhang M. Effect of low-dose bevacizumab on health-related quality of life in patients with recurrent high-grade glioma: A retrospective clinical study. J Clin Neurosci. (2024) 120:196–203. doi: 10.1016/j.jocn.2024.01.018
26. Lara-Velazquez M, Al-Kharboosh R, Jeanneret S, Vazquez-Ramos C, Mahato D, Tavanaiepour D, et al. Advances in brain tumor surgery for glioblastoma in adults. Brain Sci. (2017) 7(12):166. doi: 10.3390/brainsci7120166
27. Gritsch S, Batchelor TT, and Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. (2022) 128:47–58. doi: 10.1002/cncr.33918
28. Yoda RA and Cimino PJ. Classification and grading of central nervous system tumors according to the world health organization 5th edition. Semin Neurol. (2023) 43:833–44. doi: 10.1055/s-0043-1776793
29. Jeck J, Kassubek R, Coburger J, Edenhofer S, Schönsteiner SS, Ludolph AC, et al. Bevacizumab in temozolomide refractory high-grade gliomas: single-centre experience and review of the literature. Ther Adv Neurol Disord. (2018) 11:1756285617753597. doi: 10.1177/1756285617753597
30. Fisher JP and Adamson DC. Current FDA-approved therapies for high-grade Malignant gliomas. Biomedicines. (2021) 9(3):324. doi: 10.3390/biomedicines9030324
31. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. (2005) 352:997–1003. doi: 10.1056/NEJMoa043331
32. Chen C, Huang R, MacLean A, Muzikansky A, Mukundan S, Wen PY, et al. Recurrent high-grade glioma treated with bevacizumab: prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neurooncol. (2013) 115:267–76. doi: 10.1007/s11060-013-1225-0
33. Morisse MC, Etienne-Selloum N, Bello-Roufai D, Blonski M, Taillandier L, Lorgis V, et al. Long-term survival in patients with recurrent glioblastoma treated with bevacizumab: a multicentric retrospective study. J Neurooncol. (2019) 144:419–26. doi: 10.1007/s11060-019-03245-5
34. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
35. Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y, Naz-Khan H, Stubbs A, van der Spek P, et al. Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: A report from the BELOB trial. Cancer Res. (2016) 76:525–34. doi: 10.1158/0008-5472.Can-15-0776
36. Wong ET, Gautam S, Malchow C, Lun M, Pan E, and Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. (2011) 9:403–7. doi: 10.6004/jnccn.2011.0037
Keywords: bevacizumab, high-grade glioma, temozolomide, methylation, predictor
Citation: Yan C, Bai X, Wu C, Ma W and Feng M (2025) Correlation between MGMT methylation and the efficacy of bevacizumab in high-grade glioma. Front. Oncol. 15:1644934. doi: 10.3389/fonc.2025.1644934
Received: 11 June 2025; Accepted: 08 August 2025;
Published: 02 September 2025.
Edited by:
Supriya Mallick, All India Institute of Medical Sciences, IndiaReviewed by:
Deepam Pushpam, All India Institute of Medical Sciences, IndiaRony Benson, Marsleeva Medicty, India
Soumyajit Roy, Indian Institute of Technology Ropar, India
Copyright © 2025 Yan, Bai, Wu, Ma and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Ma, bWF3YjIwMDFAaG90bWFpbC5jb20=; Ming Feng, cHVtY2hmbWluZ0AxMjYuY29t
†These authors have contributed equally to this work
 Chengrui Yan
Chengrui Yan Xuexue Bai1†
Xuexue Bai1† Wenbin Ma
Wenbin Ma