- 1Department of Abdominal Radiation Oncology, Zhongshan People’s Hospital, Zhongshan, China
- 2College of Nursing and Health, Henan University, Kaifeng, China
- 3Headquarters of Cancer Branch, Zhongshan People’s Hospital, Zhongshan, China
Objective: This study aims to systematically retrieve, evaluate, and summarize the best evidence regarding the prevention and management of radiation dermatitis in patients undergoing endoluminal radiotherapy for gynecologic neoplasms. The goal is to provide an evidence-based foundation for developing personalized skin management programs.
Methods: We conducted a comprehensive search of domestic and international databases, guideline networks, and the websites of relevant professional associations to identify all evidence related to the prevention and management of radiation dermatitis in patients receiving endoluminal radiotherapy for gynecologic neoplasms. The types of literature include guidelines, evidence summaries, systematic reviews, expert consensus, clinical decision-making, and randomized controlled trials. The search period spanned from database inception to September 2024. In addition, we performed hand-searching of key journals and tracked gray literature (including conference abstracts and unpublishe doi:
Results: The review included 14 articles, including 1 clinical decision-making paper, 4 guideline articles, 3 systematic reviews, 3 evidence summary papers, 1 expert consensus document, and 2 randomized controlled trials. We summarized 27 pieces of evidence categorized into 5 themes: assessment and evaluation, health education, general nursing measures, symptom management, and continuity of care.
Conclusions: This study consolidates the most effective evidence for managing radiation dermatitis in patients receiving three-dimensional brachytherapy for gynecologic tumors. The findings can serve as a valuable resource for minimizing skin damage and enhancing patients’ quality of life.
Clinical Trial Registration: http://ebn.nursing.fudan.edu.cn/home, identifier ES20256976.
1 Introduction
In recent years, the incidence of gynecologic neoplasms in China has been increasing annually, with approximately 290,000 new cases of gynecologic cancer and about 100,000 deaths in 2022 (1). Among these, the three most commonly diagnosed gynecologic neoplasms are cervical cancer, endometrial cancer, and ovarian cancer (2). Most patients are diagnosed at middle to advanced stages, and chemoradiotherapy is predominantly used in clinical practice (3).
The traditional standard treatment strategy for locally advanced gynecologic tumors involves external beam radiotherapy (EBRT) combined with concurrent chemotherapy, followed by intracavitary brachytherapy (ICBT) (4). Endovascular brachytherapy, also referred to as intraluminal post-loading radiotherapy, is typically administered at a total dose of 24 Gy over six sessions, once a week (5). According to the radiotherapy protocol, patients are usually admitted to the hospital the day before each treatment and can be discharged without discomfort on the same day as the procedure. This approach has become a mainstream method in radiotherapy technology and is widely utilized in clinical settings (6). However, due to the combination of these two radiotherapy modalities and the location of treatment—primarily the perineum, which has a high density of nerve endings—radiation dermatitis (RD) is a common complication (7). RD typically presents as exudation and edema in the early stages, progressing to erosion, secondary infection, hemorrhage, and the eventual formation of ulcers in later stages (8). The study indicates that grade 1 and 2 radiation dermatitis are relatively common among gynecologic cancer patients undergoing radiotherapy, with incidence rates ranging from 10% to 50% in cervical and endometrial cancer patients. However, severe radiation (grade 3 or higher) dermatitis is relatively rare, generally occurring in 1% to 5% of cases (9–12). It significantly impacts patients’ quality of life and treatment adherence. However, current research mainly provides general skin care advice and lacks a systematic review of nursing strategies specific to this group. Because gynecological tumors involve unique anatomy and radiation sites, their dermatitis has different characteristics that are not well understood or managed with existing protocols. Furthermore, there are no widely accepted, evidence-based nursing guidelines tailored to these patients, including the best topical medications, dressings, and nursing interventions. Most current practices are based on limited or isolated evidence, which makes it harder to improve care and reduce skin problems effectively.
This study aims to fill these gaps by reviewing the existing evidence to identify effective prevention and treatment strategies for radiation dermatitis in gynecological patients. The ultimate goal is to establish standardized nursing protocols to reduce the incidence of radiation dermatitis, improve patient outcomes, and enhance quality of life. This study has been registered on the website of the Center for Evidence-Based Nursing at Fudan University (registration number: ES20256976).
2 Information and methods
2.1 Establishment of evidence-based issues
Based on the “PIPOST” problem model developed by the Center for Evidence-Based Nursing at Fudan University, we constructed the following evidence-based question (13). Population (P): Patients with gynecologic tumors receiving intracavitary brachytherapy radiation therapy. Intervention (I): Strategies for the prevention and management of radiation dermatitis. Professional (P): Medical staff, patients, and their families. Outcome (O): The occurrence of radiation dermatitis. Setting (S): Gynecologic oncology ward, outpatient clinic, and home care settings. Type of Evidence (T): Guidelines, evidence summaries, expert consensus, clinical decision-making resources, clinical practice documents, systematic reviews, and high-quality randomized controlled trials.
2.2 Literature search strategy
Following the top-down search principle of the evidence-based “6S” pyramid model, we conducted a comprehensive search using various resources (14). We searched BMJ Best Practice and UpToDate as key evidence-based resources. The evidence-based databases included the Cochrane Library and the Joanna Briggs Institute (JBI), while comprehensive databases comprised CINAHL, Web of Science (WOS), PubMed, Wiley Online, and Embase. Additionally, we explored websites such as the Guidelines International Network (GIN), the American Guideline Network, the Scottish Intercollegiate Guidelines Network, and professional association sites like the Registered Nurses Association of Ontario (RNAO). We also reviewed several Chinese databases, including the Wanfang Database, China National Knowledge Infrastructure (CNKI), the Chinese Biology Medicine (CBM) Database, and the VIP Chinese Science and Technology Journal Database (VIP). The search utilized the following subject terms and free-text keywords: “genital neoplasms, female/female genital neoplasms/gynecologic neoplasms/neoplasms, gynecologic/neoplasm, female genital”, “Endometrial Neoplasms/Uterine Cervical Neoplasms/Ovarian Neoplasms/Vaginal Neoplasms”, “radiodermatitis/radiation dermatitis/radioactive dermatitis/radiation-induced skin toxicity/radiation injury/radiation recall dermatitis/radiation-induced dermatitis/radiation-induced skin injury/RD” and “caregivers/family caregivers/spouse caregivers”. The search timeframe extended from the establishment of each database until September 2024. In addition, we performed hand-searching of key journals and tracked gray literature (including conference abstracts and unpublished reports). The PubMed search strategy, shown in Figure 1, was systematically constructed using the following approach:
1. Population component (#1-#3): Combines MeSH terms and free-text terms for gynecological cancers (e.g., cervical, ovarian, endometrial, vaginal neoplasms).
2. Outcome component (#4-#6): Incorporated both controlled vocabulary (‘Radiodermatitis’ MeSH) and comprehensive free-text terms for radiation-induced skin toxicity.
3. Contextual component (#7-#9): Included caregiver-related terms to capture psychosocial aspects.
4. Final combination (#10): Intersected all concept groups using Boolean AND operator.
The strategy was optimized through iterative testing to balance sensitivity (recall) and specificity (precision).
2.3 Literature inclusion and exclusion criteria
Inclusion Criteria: The study must focus on management and prevention strategies for radiation dermatitis. Eligible study types include: Guidelines, Expert consensus, Evidence summaries, Clinical decision-making resources, Clinical practice documents, Systematic Reviews, and randomized controlled trials, Publications must be available in both Chinese and English. Exclusion Criteria: Original studies that focus solely on pharmacological interventions, Articles lacking complete information, Publications without full-text access, Duplicate publications, Studies that failed the literature quality assessment, low correlation studies (e.g., those with study populations differing from the target population in PIPOST or failing to report prespecified outcome measures), investigate articles (e.g., uncontrolled observational designs, small-scale cross-sectional studies, or non-standardized case series).
2.4 Literature quality evaluation criteria
The guideline evaluation process was carried out independently by four experts using the Appraisal of Guidelines for Research and Evaluation (AGREE II) system (15). The assessment included 23 items across six domains: scope and purpose, participants, rigor of formulation, clarity, applicability, and editorial independence. Each item was rated on a 7-point scale, where 7 signifies full compliance and 1 indicates non-compliance.
Two researchers with expertise in evidence-based knowledge evaluated other types of literature. The literature that met the inclusion criteria was compiled, and its quality was assessed independently. The quality of included systematic reviews and expert consensus documents was evaluated using the JBI Evaluation Criteria for Evidence-Based Health Care Centers (2016 edition) (13). Randomized controlled trials were assessed using the Cochrane Risk of Bias Tool (16). The quality assessment of evidence summarization and clinical decision-making traces back to the original literature, followed by evaluating its quality using appropriate assessment standards according to the type of literature.
2.5 Evidence extraction, synthesis, and evaluation
In cases where contradictory conclusions arise from different pieces of literature, this study will adhere to the principles of evidence-based practice, prioritizing high-quality evidence, the most recently published authoritative literature, and domestic guidelines. The integration of results will be reviewed by a third researcher.
The JBI Evidence Pre-Grading and Evidence Recommendation Level System (2014 Edition) was employed to classify the original literature identified in the final included evidence (17). This classification system ranks evidence from levels 1 to 5, with level 1 being the highest quality and level 5 the lowest. The recommendation grades were determined based on the FAME attributes of the evidence (Feasibility, Appropriateness, Meaningfulness, and Effectiveness), with Grade A indicating strong recommendation and Grade B indicating weak recommendation.
3 Results
3.1 General characteristics of the included literature
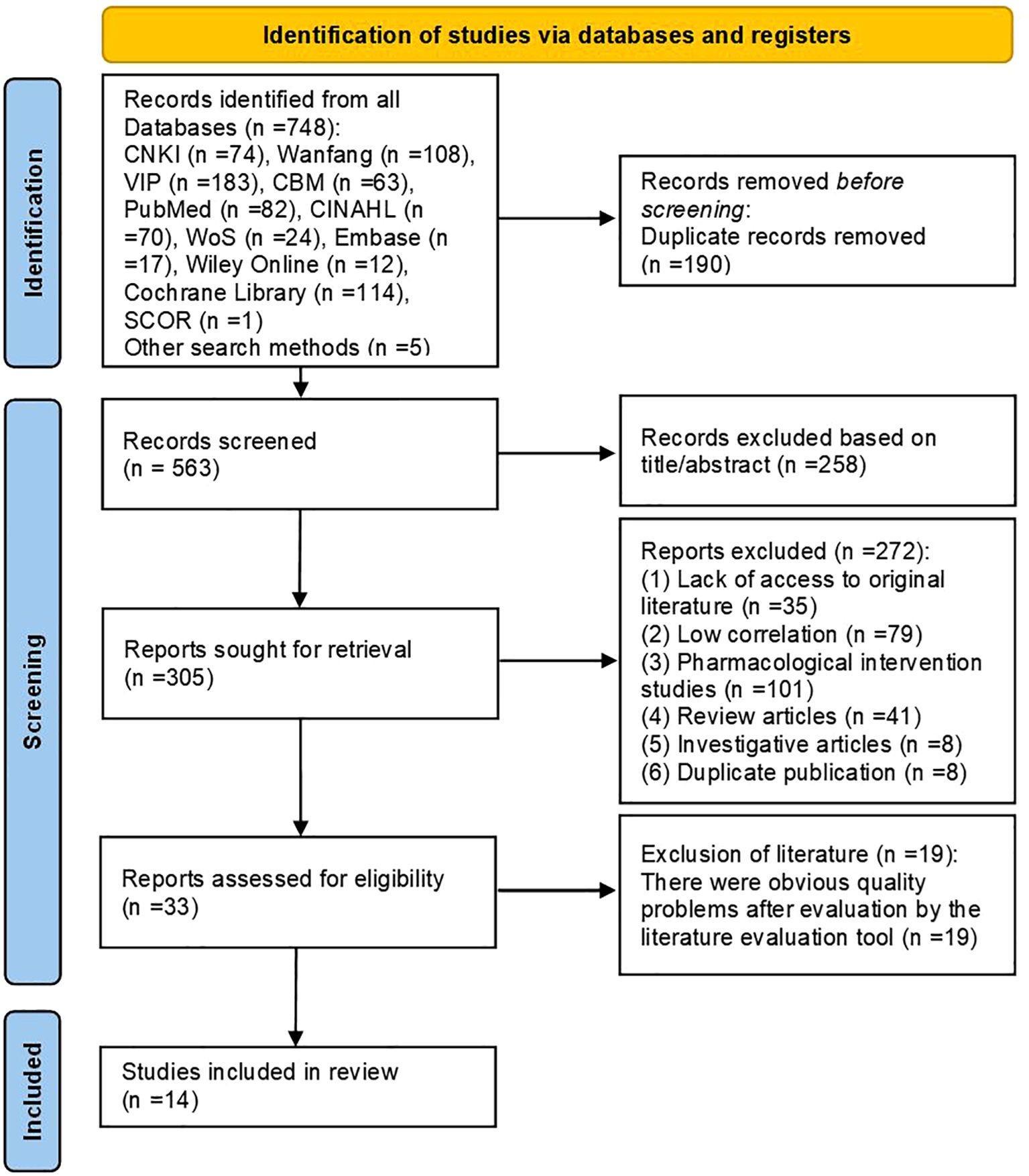
This study retrieved 748 relevant articles from database searches. Additionally, by reviewing related meta-analyses, reviews, and the reference lists of included articles, relevant studies were identified through hand-searching. Finally, five more articles were obtained through manual searching in the library, bringing the total number of articles to 753. After excluding duplicates, articles that did not meet the topic criteria, and other irrelevant literature, an initial total of 33 eligible articles were identified. Following quality assessment of these articles, 26 articles with poor quality were excluded, resulting in a final inclusion of 14 articles.
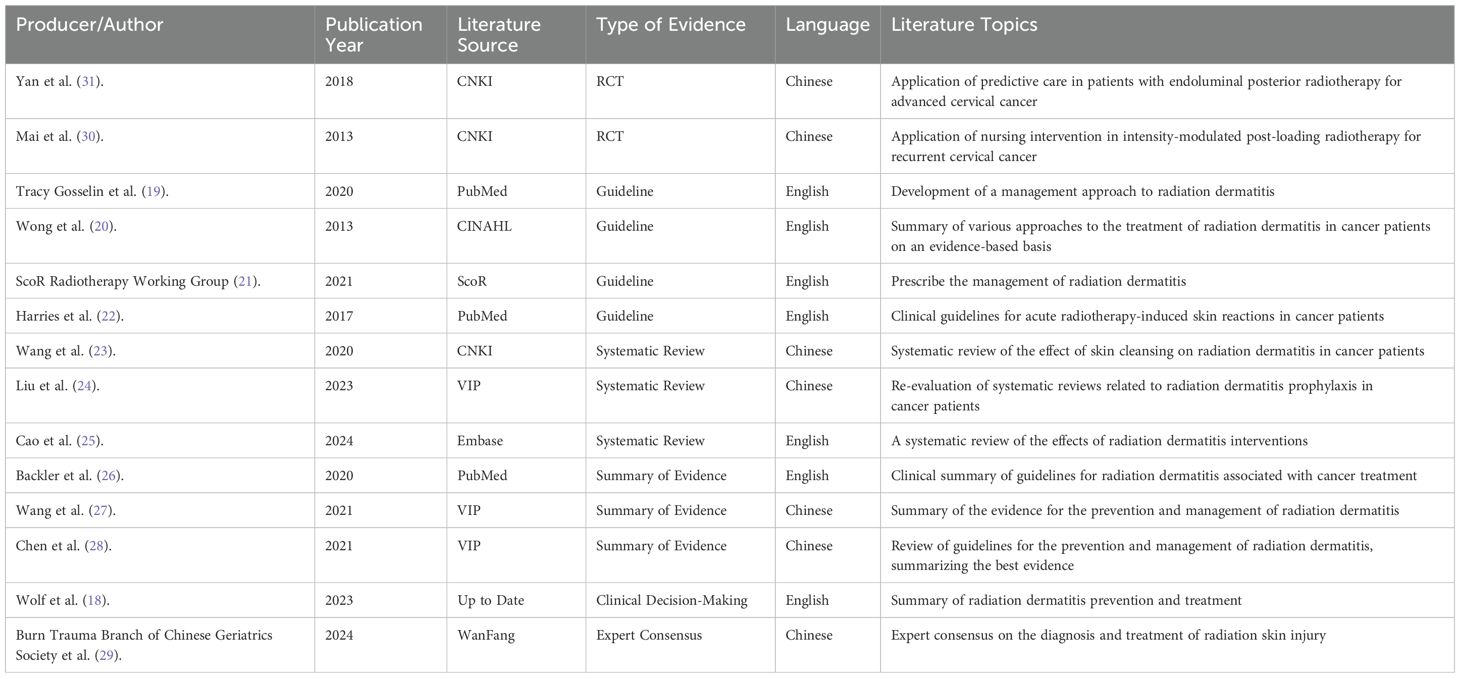
This included: 1 clinical decision (18), 4 guideline articles (19–22), 3 systematic reviews (23–25), 3 evidence summaries (26–28), 1 expert consensus (29) and 2 randomized controlled studies (30, 31). The flow chart of the literature retrieval process is presented in Figure 2, accompanied by a map (Figure 3) illustrating the global distribution. Additionally, the overall characteristics of the included literature are summarized in Table 1.
3.2 Quality evaluation results of the included literature
3.2.1 Quality evaluation results of the guidelines
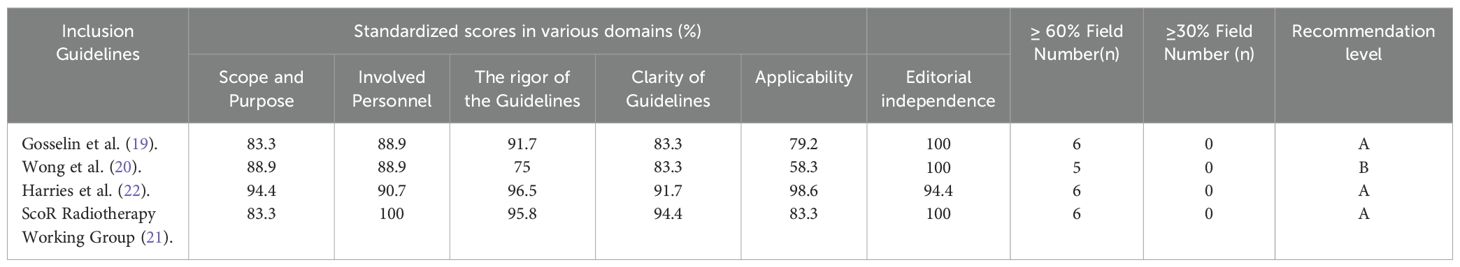
A total of four guidelines were included in this study (19–22), of which, three guidelines received standardized scores of ≥ 60 percent across six domains and were rated as A, while the remaining guideline was rated as B, as detailed in Table 2.
3.2.2 Quality evaluation results of the systematic review
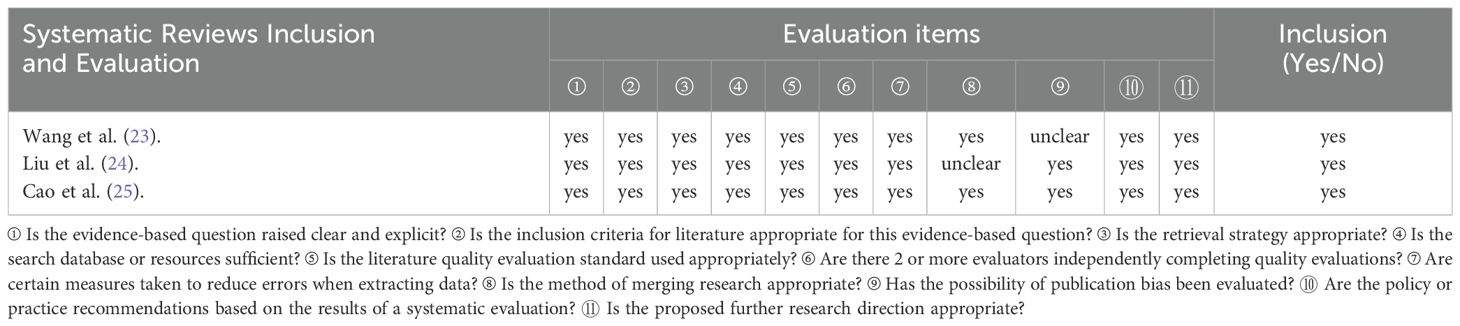
A total of three relevant systematic reviews were included in this study (23–25). The evaluation results were as follows:
Review (23): Item 9 regarding the assessment of publication bias is “unclear,” while all other items are “yes”.
Review (24): The evaluation result for “whether error minimization was sufficiently attempted during data extraction (Item 8)” was “unclear”.
Review (25): All items were rated “yes”.
The full evaluation results are presented in Table 3.
3.2.3 Quality evaluation results of clinical decision-making and evidence summarization
In this study, one clinical decision and three evidence summaries were included (18, 26–28). The original literature that retrospectively aligned with this study included two guidelines (20, 21), both of which were accepted following the quality evaluation of the aforementioned literature. In one systematic review (32), the results were “unclear” in the “appropriateness of the quality standards used”, “no” in the “whether measures were used to reduce errors when extracting data” and “whether possible publication bias was assessed”, and “yes” for all other items, not included; A review was evaluated according to expert opinions (33), and the evaluation results were “unclear” in the items “whether the views are derived from influential experts in the field” and “whether there are any inconsistencies between the opinions presented and previous literature”, and “yes” in all other items, not included.
3.2.4 Quality evaluation results of expert consensus
In this study, one expert consensus was included (29). The evaluation results for all items were rated as “yes”.
3.2.5 Quality evaluation results of randomized controlled studies
In this review, two randomized controlled trials were included (30, 31). Two studies were included in the “yes” with the exception of “allocation concealment”, “blinding of investigators and participants”, and “blinding of outcome assessors” as “unclear”.
3.3 Summary of evidence
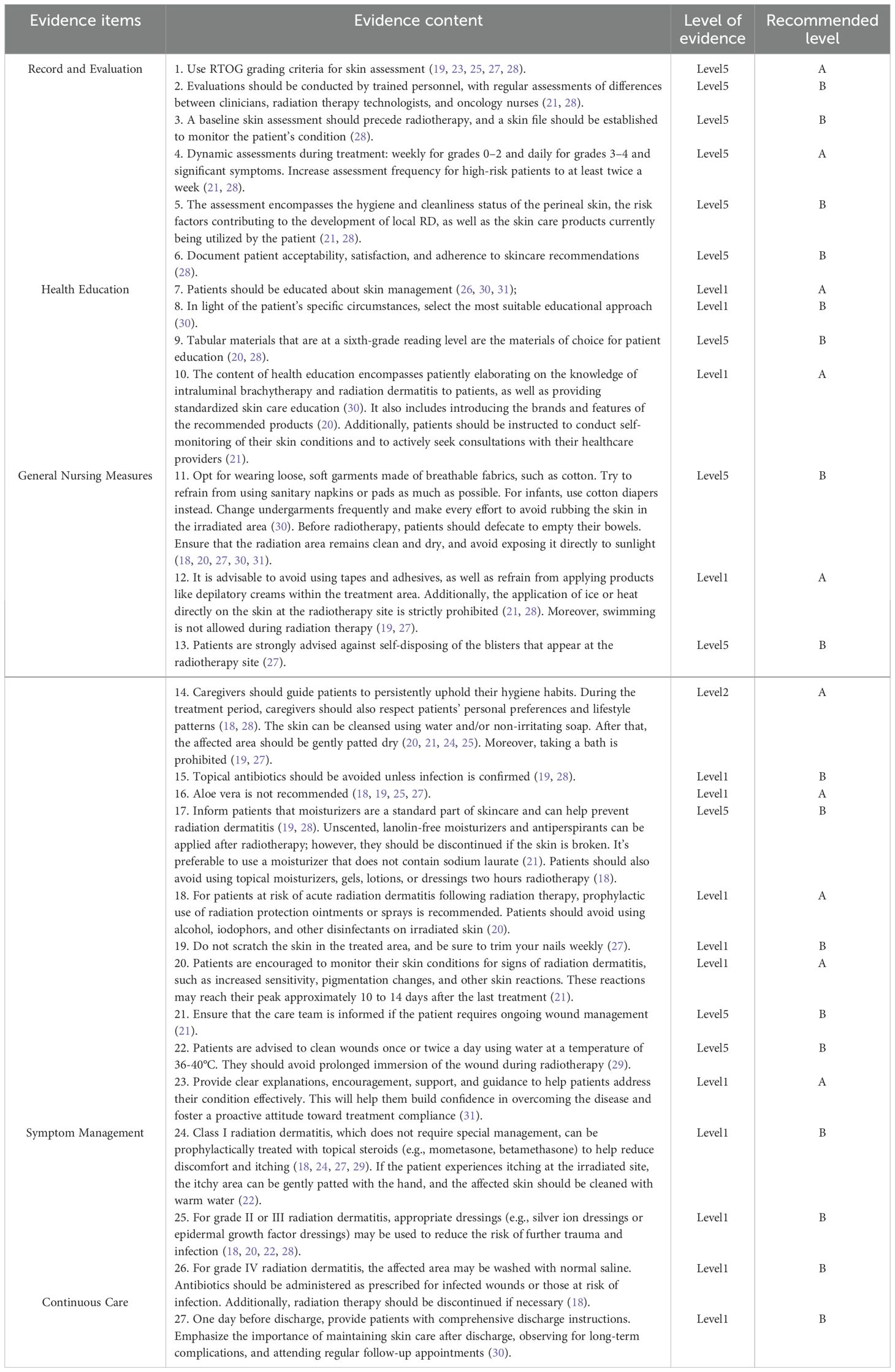
A total of twenty-seven pieces of relevant evidence were preliminarily extracted. The contents related to the same topics were classified and summarized through group analysis, comparison, and discussion. This process resulted in the formation of five main themes:
Record and Evaluation.
Health Education.
General Nursing Measures.
Symptom Management.
Continuous Care.
These themes are presented in Table 4.
4 Discussion
4.1 Skin assessment and its documentation serve as the cornerstone for the prevention of radiation dermatitis
Currently, the most commonly used tool for clinical assessment of radiation dermatitis is the RTOG (Radiation Therapy Oncology Group) grading system (32). When using this tool to assess patients, the evaluation should be conducted by personnel who have received training in its use. Additionally, regular assessments should be performed to compare the differences in evaluations among clinicians, radiotherapy technicians, and radiotherapy nurses, ensuring the accuracy and consistency of radiation dermatitis assessments (21, 28). And it is necessary to dynamically assess and record the patient’s skin condition and symptoms that occur during radiotherapy (21, 22, 28). This allows for timely intervention. The assessment should include the condition of the perineal skin, hygiene and cleanliness, risk factors for the development of local radiation dermatitis, and the skin products currently used by the patient (21, 28). The results indicate that these measures can detect skin issues at an early stage and effectively reduce the incidence of radiation dermatitis (33, 34).
4.2 The importance of health education for patients undergoing postoperative intracavitary radiotherapy
Implantation-based intracavitary radiotherapy is usually initiated when external beam radiation therapy is completed or nearly completed, and the entire radiotherapy process lasts about two months (35). Unlike external beam radiation therapy, patients usually undergo only 1–2 sessions of radiotherapy per week, and they can be discharged after each session if they experience no unusual discomfort (36). Before the patient’s next admission for radiotherapy, medical staff are unable to monitor the patient’s skin changes during this period. Therefore, skin management and health education should be provided to the patient after the first intracavitary radiotherapy and before discharge (26, 30, 31). The content of health education includes patiently explaining relevant knowledge about radiotherapy and radiation dermatitis to the patient, providing standardized skin care education (30); recommending specific brands and images of products (20); guiding patients to self-monitor their skin and actively consult and discuss with healthcare professionals (21). The main approaches include oral guidance, written materials, and multimedia promotion, which significantly improve patients’ self-care ability and awareness of skin care, thereby reducing the occurrence of radiation dermatitis (30, 31). During the literature review, it was found that there is currently a lack of research on education and support strategies targeting patients’ family members. This gap limits the potential of family involvement in patient care and also suggests that future research should focus on developing and evaluating educational and support programs for family members to strengthen their role in preventing radiation dermatitis and to improve patient compliance and care outcomes.
4.3 Skin protection during radiotherapy is key to preventing radiation dermatitis
Daily skin care is crucial for preventing radiation dermatitis in patients undergoing radiotherapy as well as for promoting healing after its occurrence. Studies have shown that providing anticipatory guidance and care during the patient’s radiotherapy can effectively reduce the incidence of radiation dermatitis (37, 38). 20 pointed out that patients undergoing radiotherapy can use plain water and/or non-irritating, non-alkaline soap to wash their skin, which should become a routine clinical care measure (20). Several randomized trials involving breast cancer and head and neck cancer patients, as well as a meta-analysis, have evaluated the benefits of routine cleansing during treatment in preventing severe radiation dermatitis (39–42), but bath soaking should be avoided. In addition, non-irritating and highly moisturizing care products can be used, while avoiding irritant medications such as alcohol and povidone-iodine (19, 20). These measures help alleviate adverse reactions such as skin dryness and swelling. However, it is recommended that patients do not apply topical moisturizers, gels, lotions, or dressings within two hours before radiotherapy to prevent a bolus effect, which can increase the radiation dose to the epidermis (18, 43). At the same time, professional nursing guidance should avoid any measures that could cause local pressure or friction, thereby effectively controlling skin damage caused by radiation (30). In summary, reasonably preventing the bolus effect through scientific skin care measures is of great significance for improving treatment compliance and the quality of life in patients undergoing intracavitary postoperative radiotherapy.
4.4 The use of medications during radiotherapy is a key focus in managing radiation dermatitis
Compared to radiotherapy for head and neck cancer and breast cancer, cervical cancer radiation dermatitis receives less attention, and there is even a lack of relevant research on radiation dermatitis in patients undergoing three-dimensional brachytherapy (44). The irradiated area is located in the perineal region, where the skin is moist, wrinkled, and involves patient privacy. The characteristic features of radiation dermatitis in this area include the formation of papules and rashes, making treatment much more challenging than other forms of radiation dermatitis (7). Previous studies have used treatments such as Bao-Shi-Jie, Kangfu-Xin liquid, Fufang Tongye Burn Oil, and Cu Yu-Ling ointment for cervical cancer radiation dermatitis (45–47), with some reports indicating moderate effectiveness. However, these treatments have not yet been widely adopted. A clinical decision guideline recommends the use of corticosteroids (18), which have been shown to effectively reduce itching caused by radiation dermatitis. Additionally, a meta-analysis indicated that multiple randomized controlled trials confirmed that topical corticosteroids can decrease the risk of radiation dermatitis in breast cancer patients (48). In a randomized trial for the treatment of radiation dermatitis in breast cancer, sulfonamide silver demonstrated good anti-inflammatory effects and is often used to treat radiation dermatitis (49). Moreover, certain dressings, such as silicone gel dressings and silver-nylon dressings, have also been shown to be effective in treating Grade II or Grade III radiation dermatitis (50, 51). However, these medications and dressings are primarily used in breast cancer and head and neck cancer patients, and their applicability to cervical cancer radiotherapy patients has not yet been established. Therefore, regarding radiation dermatitis in patients with intracavitary postoperative radiotherapy for cervical cancer, more clinical research and trials are needed to validate the efficacy and safety of existing treatment options. Additionally, it is important to explore preventive and therapeutic methods that are more suitable for this specific anatomical site and its pathological characteristics.
4.5 The necessity of ongoing care after patient discharge
Acute radiation dermatitis is typically defined as skin changes that occur within 90 days after radiotherapy, while changes that occur after more than 90 days are considered chronic radiation dermatitis (52). For patients undergoing three-dimensional intracavitary postoperative radiotherapy, the treatment course lasts about a month, making it highly likely that they may develop radiation dermatitis after discharge. Therefore, ongoing care and continuous monitoring of their skin condition after discharge are especially important. Through telephone follow-up and home care guidance, the effectiveness of clinical care can be extended, and patients can be advised to undergo regular follow-up visits after discharge. This approach helps to effectively reduce the incidence of radiation dermatitis and improves patients’ quality of life and satisfaction (30, 37).
5 Conclusions
This study summarizes the evaluation and documentation of radiation dermatitis, health education, general nursing measures, radiation dermatitis management, and continuity care for patients with gynecologic tumor intracavitary postoperative radiotherapy. It provides a basis for developing clinical skin management protocols. However, after conducting the literature search, we found that the relevant articles are primarily concentrated on cervical cancer. This also reflects that the current research focus on radiation dermatitis related to radiotherapy for gynecologic tumors is mainly centered on cervical cancer. The two RCTs we included also fall within the cervical cancer category. Nonetheless, because the mechanisms of radiation dermatitis are similar across different gynecologic tumors—especially since the affected areas are mainly concentrated in the vulva, perineum, and inguinal regions—there is a certain degree of commonality in the available data across different cancer types.
In the future, further randomized controlled trials (RCTs) should be encouraged to investigate radiation dermatitis specifically in endometrial and ovarian cancers, aiming to enhance the evidence for targeted management of radiation dermatitis in these cancer categories.
Additionally, we have summarized the similarities and differences in the occurrence and management of radiation dermatitis during brachytherapy for cervical, ovarian, vaginal, and endometrial cancers in Table 5.
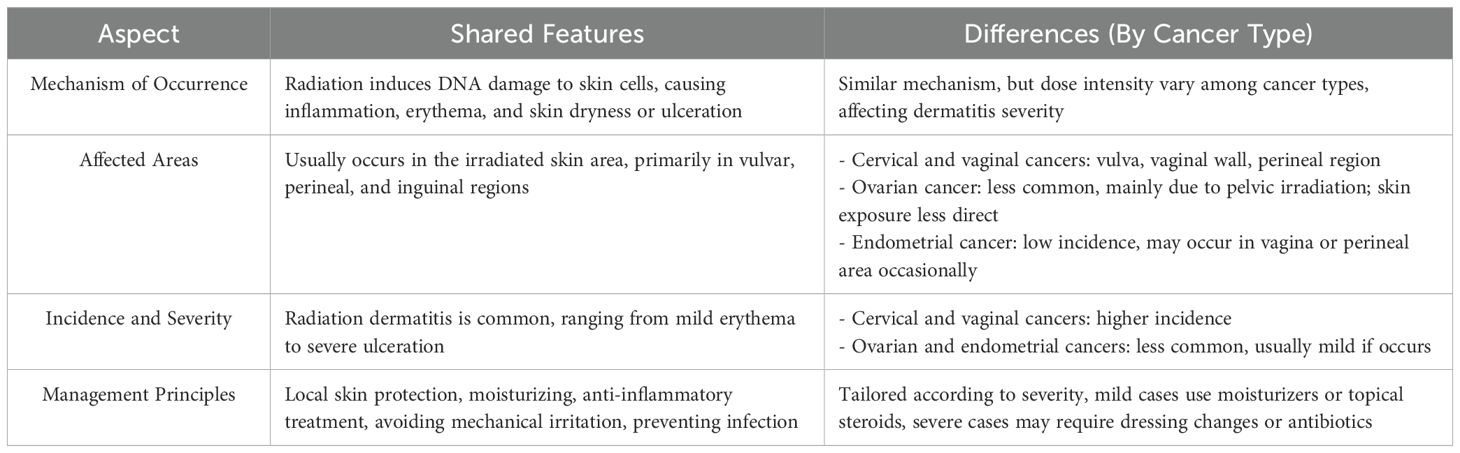
Table 5. Comparative analysis of radiation dermatitis related to radiotherapy in gynecologic cancers.
Clinicians should determine the recommended levels of evidence based on expert opinions and perform evidence translation according to the FAME (Findings, Appraisal, Management, Evidence) framework. This approach will facilitate the formulation and validation of prevention and management strategies for radiation dermatitis in patients undergoing intracavitary postoperative radiotherapy for gynecologic tumors. Ultimately, it aims to reduce the incidence of radiation dermatitis in these patients, alleviate skin injuries, and improve their quality of life and prognosis.
Author contributions
ZF: Data curation, Methodology, Conceptualization, Writing – original draft. LW: Conceptualization, Project administration, Writing – review & editing. HL: Writing – original draft, Formal analysis, Data curation. XW: Methodology, Writing – original draft. BC: Writing – original draft, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
I would like to express my heartfelt gratitude to my mentor, Ms. Wang Lin, for her kindness, illuminating guidance, and profound knowledge. Through her insightful and engaging lectures, I gained a deeper understanding of translation theories and developed a lasting interest in the field. Despite her busy schedule, she meticulously reviewed my paper and provided detailed feedback. Without her keen insights and unwavering encouragement, this work would not have been possible. I also extend my sincere appreciation to all the teachers who have taught me. Their enlightening lectures greatly contributed to my academic and research growth.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Hanna TP, Delaney GP, and Barton MB. The population benefit of radiotherapy for gynecological cancer: Local control and survival estimates. Radiother Oncol. (2016) 120:370–7. doi: 10.1016/j.radonc.2016.04.008
4. Zhang B, Zhang S, Sun L, Wu Y, and Yang Y. Characteristics of preplan-based three-dimensional individual template-guided brachytherapy compared to freehand implantation. J Appl Clin Med Phys. (2023) 24:e13840. doi: 10.1002/acm2.13840
5. Ha IB, Jeong BK, Kang KM, Jeong H, Lee YH, Choi HS, et al. Who really benefits from 3D-based planning of brachytherapy for cervical cancer? J Korean Med Sci. (2018) 33:e135. doi: 10.3346/jkms.2018.33.e135
6. Ling BZ, Chen L, Zhang J, Cao X, Ye W, Ouyang Y, et al. Dosimetric analysis of different optimization algorithms for three-dimensional brachytherapy for gynecologic tumors. Nan Fang Yi Ke Da Xue Xue Bao= J South Med Univ. (2024) 44:773–9. doi: 10.12122/j.issn.1673-4254.2024.04.20
7. Hu F and Zhu M. Analysis of influencing factors of perineal radiation dermatitis in patients with cervical cancer after posterior radiotherapy and observation of humanistic care on its nursing effect. Jilin Med J. (2023) 44:277–80. doi: 10.3969/j.issn.1004-0412.2023.01.089
8. Jia AY and Viswanathan AN. Vaginal necrosis: A rare late toxicity after radiation therapy. Gynecol Oncol. (2021) 160:602–9. doi: 10.1016/j.ygyno.2020.11.025
9. Jin Y, Cui Y, Xin LL, Liu Y, and Yan MZ. Analysis of influencing factors of perineal radiation dermatitis in patients with cervical cancer undergoing radiotherapy. Nurs Pract Res. (2021) 18:2089–91. doi: 10.3969/j.issn.1672-9676.2021.14.009
10. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. (2004) 92:744–51. doi: 10.1016/j.ygyno.2003.11.048
11. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. (1999) 340:1144–53. doi: 10.1056/nejm199904153401502
12. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. (1999) 340:1137–43. doi: 10.1056/nejm199904153401501
13. Xing WJ, Hu Y, Zhou YF, Zhu Z, Gu Y, Zhang X, et al. Promoting the transformation of evidence into clinical practice: Making and writing evidence summary. J Nurses Training. (2020) 35:1129–32. doi: 10.16821/j.cnki.hsjx.2020.12.016
14. DiCenso A, Bayley L, and Haynes RB. ACP Journal Club. Editorial: Accessing preappraised evidence: fine-tuning the 5S model into a 6S model. Ann Intern Med. (2009) 151:Jc3–2. doi: 10.7326/0003-4819-151-6-200909150-02002
15. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Cmaj. (2010) 182:E839–842. doi: 10.1503/cmaj.090449
16. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
17. Wang CQ and Hu Y. JBI evidence pre-grading and evidence recommendation level system (2014 Edition). J Nurses Training. (2015) 30:964–7. doi: 10.16821/j.cnki.hsjx.2015.11.002
18. Ryan Wolf J, Gewandter JS, Bautista J, Heckler CE, Strasser J, Dyk P, et al. Utility of topical agents for radiation dermatitis and pain: a randomized clinical trial. Support Care Cancer. (2020) 28:3303–11. doi: 10.1007/s00520-019-05166-5
19. Gosselin T, Ginex PK, Backler C, Bruce SD, Hutton A, Marquez CM, et al. ONS guidelines™ for cancer treatment-related radiodermatitis. Oncol Nurs Forum. (2020) 47:654–70. doi: 10.1188/20.Onf.654-670
20. Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. (2013) 21:2933–48. doi: 10.1007/s00520-013-1896-2
21. Bennet C, Burke G, Davies R, Faithfull S, Harris R, and Probst H. The Society and College of Radiographers practice guideline document radiation dermatitis guidelines for radiotherapy healthcare professionals [EB/OL]. (2020). Available online at: http://www.sor.org/ learning/ document-library/The Society and College of Radiographers Practice Guideline Document (Accessed February 20, 2025).
22. Harris R. Summary of interventions for acute radiotherapy-induced skin reactions in cancer patients: a clinical guideline recommended for use by the Society and College of Radiographers [EB/OL]. (2011). Available online at: https://www.sor.org/learning/document-library/summary-interventions-acute-radiotherapy-induced-skin-reactions-cancer-patients-clinical-guideline (Accessed March 3, 2025).
23. Wang Y, Qiang WM, Li J, and Li GY. Skin cleanness for radiation-induced skin reaction in cancer patients: A systematic review. J Nurses Training. (2020) 35:426–32. doi: 10.16821/j.cnki.hsjx.2020.05.009
24. Liu M, Liu YY, Xu Y, Fang TT, and XU DD. Reevaluation of systematic evaluation of preventive measures for radiation dermatitis in cancer patients. Military Nursing. (2023) 40:93–7. doi: 10.3969/j.issn.2097-1826.2023.04.023
25. Cao H, Li W, and Cai H. The effect of various interventions on the prevention of radiation dermatitis: a network meta-analysis. Am J Transl Res. (2024) 16:1859–79. doi: 10.62347/xlgt5405
26. Backler C, Bruce SD, Suarez L, and Ginex PK. Radiodermatitis: clinical summary of the ONS guidelines™ for cancer treatment-related radiodermatitis. Clin J Oncol Nurs. (2020) 24:681–4. doi: 10.1188/20.Cjon.681-684
27. Wang Q, Li Z, Zhang Y, Li GQ, and Yan R. Evidence summary for prevention and management of radiation dermatitis. J Nurs Sci. (2020) 35:83–6. doi: 10.3870/j.issn.1001-4152.2020.01.083
28. Chen ZH, Zhong Q, and Chen YH. Quality appraisal and content analysis of clinical practice guidelines on prevention and management of radiodermatitis. Chin Evidence-Based Nursing. (2021) 7:151–156 + 173. doi: 10.12102/j.issn.2095-8668.2021.02.002
29. Burn and Trauma Branch of Chinese Geriatric Society, Tissue Repair and Regeneration Branch of Chinese Medical Association, Regenerative Medicine and Rehabilitation Professional Committee of Chinese Rehabilitation Medical Association, Tumor Plastic Surgery Professional Committee of Chinese Anti-Cancer Association, Editorial Committee of Chinese Journal of Burns and Wounds, Shi CM, et al. Expert consensus on diagnosis and treatment of radiation-induced skin injury (2024 version). Chin J Burns Wounds. (2024) 40(8):701–12. doi: 10.3760/cma.j.cn501225-20240126-00033
30. Mai MQ, Feng GR, and Chen PF. Application of nursing intervention in intensity-modulated post-loading radiotherapy for recurrent cervical cancer. Guangdong Med J. (2013) 34:3215–7. doi: 10.13820/j.cnki.gdyx.2013.20.024
31. Yan JL, Qian CY, and Chen TT. Application of predictive nursing in patients with endovascular radiotherapy for advanced cervical cancer. Clin Med Engi. (2018) 25:1401–2. doi: 10.3969/j.issn.1674-4659.2018.10.1401
32. Lu XM, Liu FX, Geng WH, Gao Y, and Han L. Research progress on assessment tools for acute radiation dermatitis. Military Nursing. (2024) 41:86–9. doi: 10.3969/j.issn.2097-1826.2024.07.020
33. Zhang SZ, Yu WR, Zhang JF, Jiang JY, Luo M, Lin BY, et al. Evidence-based nursing practice in prevention and management of radiation dermatitis in cancer patients undergoing radiotherapy. J Nurs (China). (2024) 31:62–7. doi: 10.16460/j.issn1008-9969.2024.16.062
34. Liu L, Gan JW, Jiang TT, and Shi TY. Construction and preliminary application of a skin care program in breast cancer patients with radiodermatitis. Chin J Nurs. (2021) 58:2575–82. doi: 10.3761/j.issn.0254-1769.2023.21.002
35. Liu Z and Dai L. Radiotherapy strategies for geriatric gynecologic Malignancies. J Pract Obstet Gynecol. (2019) 35:573–6.
36. Li Y, Lu L, Li Y, Luo S, Chen L, Liu QL, et al. Application value analysis of the 3D intra-cavitary brachytherapy of different phases of cervical cancer with TAUS. J Imaging Res Med Applications. (2024) 8:37–39 + 43. doi: 10.3969/j.issn.2096-3807.2024.10.011
37. Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. (2010) 17:94–112. doi: 10.3747/co.v17i4.493
38. McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. (2011) 27:e1–17. doi: 10.1016/j.soncn.2011.02.009
39. Zhang Q, Wang Y, Yang S, Wu Q, and Qiang W. What is the appropriate skin cleaning method for nasopharyngeal cancer radiotherapy patients? A randomized controlled trial. Support Care Cancer. (2022) 30:3875–83. doi: 10.1007/s00520-022-06835-8
40. Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, and Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. (2014) 14:53. doi: 10.1186/1471-2407-14-53
41. Campbell IR and Illingworth MH. Can patients wash during radiotherapy to the breast or chest wall? A randomized controlled trial. Clin Oncol (R Coll Radiol). (1992) 4:78–82. doi: 10.1016/s0936-6555(05)80971-9
42. Baumann BC, Verginadis II, Zeng C, Bell B, Koduri S, Vachani C, et al. Assessing the validity of clinician advice that patients avoid use of topical agents before daily radiotherapy treatments. JAMA Oncol. (2018) 4:1742–8. doi: 10.1001/jamaoncol.2018.4292
43. Bernier J, Bonner J, Vermorken JB, Bensadoun RJ, Dummer R, Giralt J, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. (2008) 19:142–9. doi: 10.1093/annonc/mdm400
44. Hamada K, Fujibuchi T, Arakawa H, Yokoyama Y, Yoshida N, Ohura H, et al. A novel approach to predict acute radiation dermatitis in patients with head and neck cancer using a model based on Bayesian probability. Phys Med. (2023) 116:103181. doi: 10.1016/j.ejmp.2023.103181
45. Xiong Y, Xu XY, and Xia WS. Observation of application effect of oxygen therapy combined with compound tongue burns oil in cervical radiation dermatitis. Contemp Med. (2021) 26:58–61. doi: 10.3969/j.issn.1009-4393.2020.36.021
46. Zhang ST and Yang X. Application of bao shi jie combined with kang fu xin ye in the treatment of acute radiation dermatitis of cervical cancer. J Jinzhou Med Univ. (2023) 44:88–91. doi: 10.13847/j.cnki.lnmu.2023.04.020
47. Zhang XY, Yue CH, Zhang W, Zhang CL, Lu YQ, Zhou JH, et al. Effect of Cu Yu ling Liniment combined with oxygen therapy in radiation dermatitis in patients undergoing radiotherapy for cervical cancer. China Modern Med. (2024) 31:154–7. doi: 10.3969/j.issn.1674-4721.2024.34.034
48. Ferreira EB, Vasques CI, Gadia R, Chan RJ, Guerra EN, Mezzomo LA, et al. Topical interventions to prevent acute radiation dermatitis in head and neck cancer patients: a systematic review. Support Care Cancer. (2017) 25:1001–11. doi: 10.1007/s00520-016-3521-7
49. Hemati S, Asnaashari O, Sarvizadeh M, Motlagh BN, Akbari M, Tajvidi M, et al. Topical silver sulfadiazine for the prevention of acute dermatitis during irradiation for breast cancer. Support Care Cancer. (2012) 20:1613–8. doi: 10.1007/s00520-011-1250-5
50. Chan RJ, Blades R, Jones L, Downer TR, Peet SC, Button E, et al. A single-blind, randomized controlled trial of Strata XRT® - A silicone-based film-forming gel dressing for prophylaxis and management of radiation dermatitis in patients with head and neck cancer. Radiother Oncol. (2019) 139:72–8. doi: 10.1016/j.radonc.2019.07.014
51. Niazi TM, Vuong T, Azoulay L, Marijnen C, Bujko K, Nasr E, et al. Silver clear nylon dressing is effective in preventing radiation-induced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys. (2012) 84:e305–310. doi: 10.1016/j.ijrobp.2012.03.062
Keywords: gynecologic neoplasms, brachytherapy, radiodermatitis, evidence summary, evidence-based nursing
Citation: Fan Z, Liang H, Wei X, Cui B and Wang L (2025) Prevention and management of radiodermatitis in patients with brachytherapy for gynecologic neoplasms summary of the evidence. Front. Oncol. 15:1645101. doi: 10.3389/fonc.2025.1645101
Received: 11 June 2025; Accepted: 23 July 2025;
Published: 08 August 2025.
Edited by:
Dorothy Lombe, Cancer Screening, Treatment and Support Services MidCentral, New ZealandReviewed by:
Paula Elaine Diniz Dos Reis, University of Brasilia, BrazilCheng-Hsien Hung, Chang Bing Show Chwan Memorial Hospital, Taiwan
Copyright © 2025 Fan, Liang, Wei, Cui and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Wang, ODA3ODMxMzc0QHFxLmNvbQ==
 Zhanxin Fan
Zhanxin Fan Hongxia Liang2
Hongxia Liang2