- 1Italian Group for Adult Hematologic Diseases (GIMEMA), Data Center and Health Outcomes Research Unit, Rome, Italy
- 2Hematology Unit, Azienda Unità Sanitaria Locale-IRCCS, Reggio Emilia, Italy
- 3Hematology Unit, Dell'Angelo Hospital, Venezia-Mestre, Italy
- 4Hematology Unit, S. Eugenio Hospital, ASL Roma 2, Tor Vergata University, Rome, Italy
- 5Division of Hematology, Department of Translation Medicine, University of Eastern Piedmont, Novara, Italy
- 6Hematology Unit, Santa Maria Goretti Hospital, Latina, Italy
- 7Institute of Hematology "Seràgnoli" IRCCS Azienda Ospedaliero-Universitaria di Bologna, Department of Medical and Surgical Sciences University of Bologna, Bologna, Italy
- 8Hematology Unit, Businco Hospital, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 9Section of Hematology, Department of Medical and Surgical Sciences, University of Modena and Reggio Emilia, Azienda Ospedaliero-Universitaria di Modena, Modena, Italy
- 10Department of Medicine, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy
- 11Department of Engineering for Innovation Biomedicine, Section of Innovation Biomedicine, Hematology Area, University of Verona, Verona, Italy
- 12Hematology Unit, AOU Careggi, University of Florence, Florence, Italy
- 13Division of Hematology, Grande Ospedale Metropolitano 'Bianchi Melacrino Morelli', Reggio Calabria, Italy
- 14Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica Sacro Cuore, Rome, Italy
- 15Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, Netherlands
- 16Hematology Section, Department of Clinical Medicine and Surgery, University of Naples "Federico II", Naples, Italy
- 17Hematology, Department of Translational and Precision Medicine, Azienda Ospedaliera Policlinico Umberto I, Sapienza University of Rome, Rome, Italy
We aimed to explore the prognostic value of patient-reported health-related quality of life (HRQoL) data for the achievement of early molecular response (EMR) at 3 months in patients with chronic phase chronic myeloid leukemia (CP-CML). We analyzed HRQoL baseline data of 436 newly diagnosed patients with CML patients enrolled in the GIMEMA Sustrenim trial. HRQoL was assessed by the EORTC QLQ-30 and the QLQ-CML24 questionnaires. In the multivariate analysis, the following factors were found to be independently associated with achievement of EMR: Sokal risk (low vs intermediate risk p=0.046 and low vs high risk p<0.001), nilotinib treatment (p<0.001) and higher patient-reported role functioning (EORTC QLQ-C30) (p<0.001). Current findings suggest the importance of assessing HRQoL at diagnostic workup of patients with CML as it may provide valuable prognostic information.
Introduction
Tyrosine kinase inhibitors (TKIs) have improved the outcome of chronic myeloid leukemia patients (CML) with faster reduction of the burden of disease. Robust and standardized definitions of molecular responses have been introduced and are now universally accepted (1). Early molecular monitoring has become particularly useful to identify patients who might be considered for a prompt change of treatment. First evidence correlated with the early reduction of the molecular burden with the cytogenetic response (2, 3) and the lack of disease progression (3, 4). The cut-off of nearly 10% achieved within the first 3 months was first identified by Marin et al. (5) as predictive of better overall survival (OS). The same level of response, namely the early molecular response or EMR (BCR::ABL1 ratio ≤ 10% at 3 months) was then replicated in other studies as always associated with better OS, event-free survival (EFS) and subsequent increased possibility to achieve in the long-term a deep molecular response (BCR::ABL1 ratio ≤ 0.0032% or MR4.5) (6–8). The significance of EMR was then validated also with second generation TKIs, dasatinib and nilotinib, when tested as frontline treatment in two sponsored clinical trials (9, 10).
Previous work across several cancer populations (11, 12) has shown that health-related quality of life (HRQoL) data provides independent prognostic information for survival and, some evidence in the CML setting, indicated that HRQoL data may also predict achievement of a major molecular response (13). Therefore, leveraging from an international trial (14), we explored the prognostic value of baseline HRQoL data for the achievement of a BCR::ABL1 transcript level of ≤10% after 3 months of TKI treatment, being a key milestone of patients management. A Secondary objective was to describe the baseline HRQoL profile by frequently used prognostic scores: Sokal, EUTOS and ELTS.
Patients and methods
The SUSTRENIM trial (Clinical Trial number 02602314), was a prospective, interventional, randomized, two arms, study in newly diagnosed chronic phase CML (CP-CML) patients treated with a second generation TKI (Nilotinib, NIL) or with Imatinib (IM) followed by switching to NIL in absence of optimal response (14). Ethics committees of all participating sites approved this study protocol. For the purposes of this post-hoc analysis, the cohort consisted of CML patients who had a completed HRQoL assessment at study entry (i.e., before study treatment start) and were randomly assigned to receive first-line treatment with NIL or IM between November 2016 and January 2021.Treatment responses were expressed on the International Scale (IS %) and were evaluated according to ELN2020 recommendations every 3 months (15). Early molecular response (EMR) was defined by BCR::ABL1 transcript levels ≤10% on the International Scale at 3 months of TKI treatment (5). HRQoL was assessed using the cancer-generic EORTC QLQ-C30 (16) and the disease specific EORTC QLQ-CML24 questionnaires (17). The QLQ-C30 consists of five functioning scales: physical, role, emotional, cognitive, and social; three symptom scales: fatigue, nausea/vomiting, and pain; six single-item scales: dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial impact; and the global health status/QoL scale. The EORTC QLQ-CML24 consists of six scales: symptom burden, impact on worry/mood, impact on daily life, satisfaction with care and information, body image problems and satisfaction with social life). The main characteristics of newly diagnosed CML patients were summarized overall, using frequencies, proportions, means and standard deviations (SD), medians and interquartile ranges (IQR), depending on the type of variable. Given the exploratory nature of the study, all HRQoL scales were considered for their potential prognostic value for EMR. Univariate and multivariate logistic regression analyses were conducted to assess the prognostic effect of selected baseline socio-demographic and clinical characteristics along with HRQoL scales on EMR at 3 months. The starting univariate model included: sex, ECOG performance status, Sokal risk score, treatment arm, and all the HRQoL scales as continuous variables.
Results
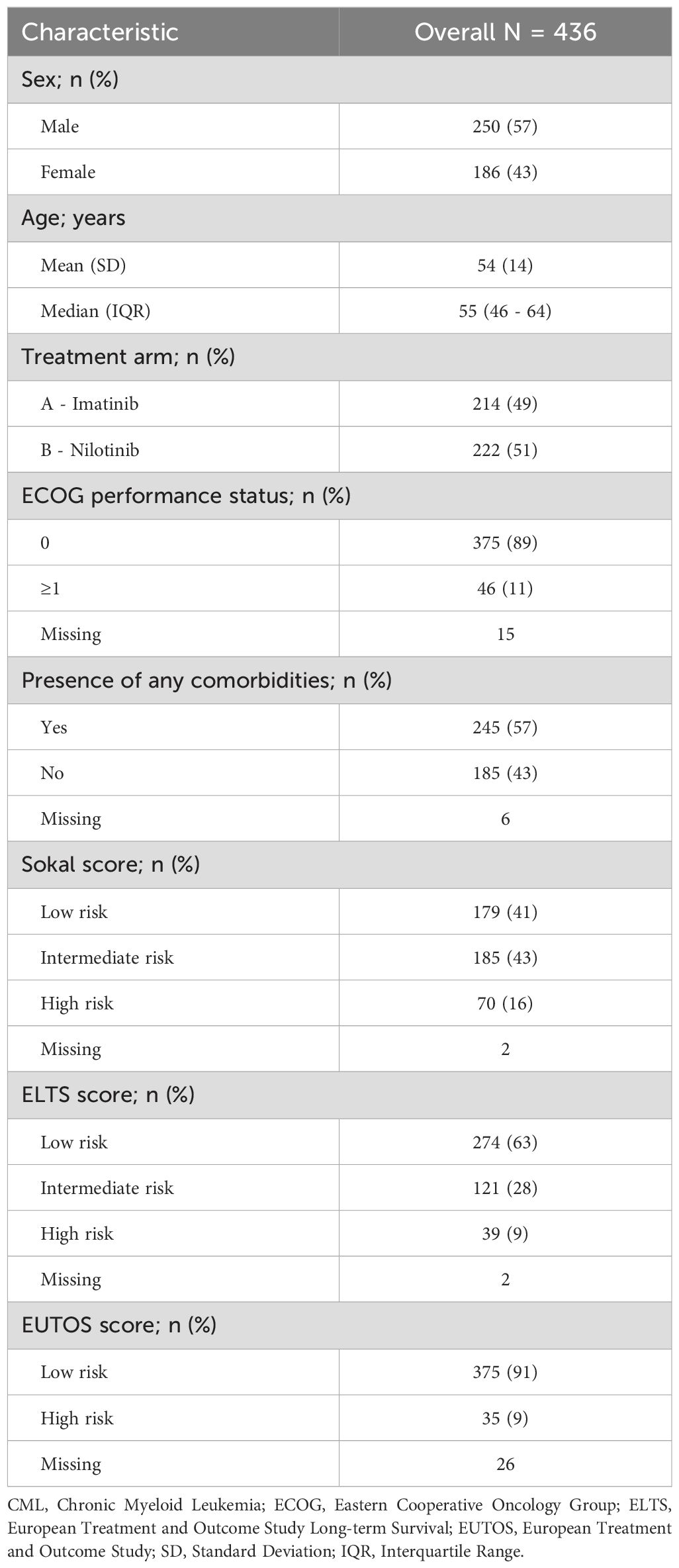
Overall, 448 newly diagnosed CML patients were enrolled and randomized to NIL and IM, 436 (97%) had a HRQoL assessment completed. Of them, 222 (51%) and 214 (49%) patients were randomly assigned to the NIL and IM arms, respectively. EMR was achieved in 190/202 (94%) and 151/194 (78%) patients, respectively (p<0.001). The median age of patients was 55 years (interquartile range, IQR 46-64) with a male predominance (57%). The Sokal score stratification identified 179 (41%), 185 (43%), and 70 (16%) as low, intermediate and high risk, respectively. According to the ELTS score, 274 (63%), 121 (28%), and 39 (9%), were classified as low, intermediate, and high risk, respectively. According to the EUTOS score, 375 (91%) were classified as low risk and 35 (9%) were classified as high risk. Details are reported in Table 1.
In the univariate logistic regression analysis, EMR was significantly associated with Sokal risk stratification, treatment arm and the following patient-reported HRQoL: physical functioning, role functioning, social functioning scales of the EORTC-QLQ-C30 and body image problems of the EORTC-QLQ-CML24. In the multivariate analysis, the following variables retained a significance: the Sokal score stratification for the high category (OR 0.13, 95% CI 0.05-0.33, p<0.001) and intermediate risk (OR 0.43, 95% CI 0.18-0.96, p=0.046), the treatment arm with nilotinib (OR 4.61, 95% CI 2.31-9.88, p<0.001), and the role functioning scale of the EORTC-QLQ-C30 (OR 1.34, 95% CI 1.10-1.63, p<0.001). This latter indicates a 34% increase in the odds of achieving an EMR with every 10-point increase (i.e., improving) in the baseline role functioning scale. Details are reported in Table 2.
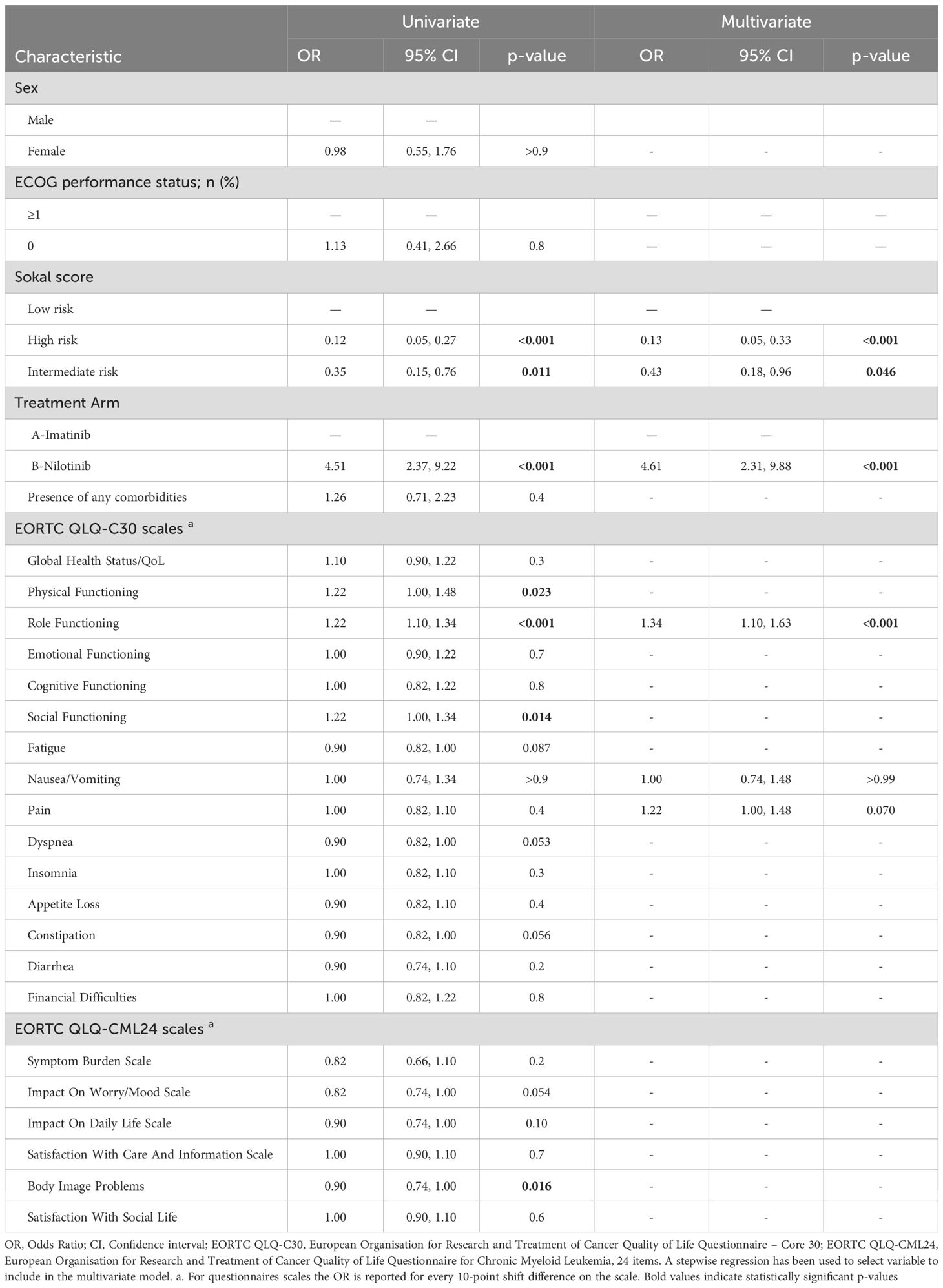
Table 2. Univariate and multivariate model on optimal vs non-optimal response achievement at 3 months from randomization.
The three CML prognostic scoring systems were able to disentangle the baseline HRQoL profiles of patients are reported in the (Supplementary Materials S1-S3).
Patients classified as high-risk by the Sokal risk score reported a significantly worse HRQoL profile than those classified as intermediate- and low-risk, as described by either worse functional impairments (i.e. lower global health status/QoL, physical and role functioning scale scores) or higher symptom severity (i.e. higher pain and dyspnea scale scores) and lower satisfaction with social life (disease-specific domains).
Discussion
We found that the role functioning scale of the EORTC QLQ-C30, which broadly reflects the ability of patients to perform daily activities, provides independent prognostic information for the achievement of EMR. This finding supports the value of a more patient-centric approach at diagnostic workup of CML as it may offer unique information to better identify those patients who are more likely to achieve an important treatment milestone.
Similar to our result, a recent longitudinal HRQoL analysis, including pooled data from the BFORE trial, showed that clinical improvement was associated with variable effect on different dimensions of HRQoL, and patients achieving deep response (i.e. MR5) reported the greatest improvement in emotional well-being and leukemia specific domains (18).
A possible explanation of our findings could be the mediating role of adherence to therapy. That is, those patients who have higher ability to perform daily activities at diagnosis may be facilitated in fitting treatment schedule into their daily life, thereby adopting a more effective medication taking behavior and ultimately achieving an EMR at 3 months. However, we note that in our cohort, achievement of MMR at 6 months was not significantly associated with any baseline HRQoL domain (data not shown). In any case, given the exploratory nature of the analysis, our findings of the prognostic value of the role functioning scale of the EORTC QLQ-C30, should be further corroborated in future prospective studies.
Of note, none of the EORTC QLQ-CML24 domains emerged in the multivariate model, possibly suggesting that the EORTC QLQ-C30 alone, well captures key important aspects of patients with CML. As expected, treatment with nilotinib was significantly associated with a higher probability of achieving EMR, and this is in line with previous evidence on the higher efficacy of second-generation TKIs in inducing faster molecular responses compared to imatinib (19). Similarly, patients with lower Sokal risk scores had higher EMR rates, consistent with its prognostic role in this disease (20).
Our descriptive analysis investigating HRQoL profile according to the commonly used prognostic scoring systems, suggests that Sokal-risk score can help to disentangle some important HRQoL parameters of newly diagnosed patients.
In conclusion, our results suggest that assessment of HRQoL at diagnostic workup may offer independent prognostic information associated with a greater likelihood to achieve EMR.
Author notes
Presented in part at the 29th European Hematology Association Congress (EHA 2024 Hybrid Congress) held in Madrid, Spain, June 13 to 16, 2024.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee "Università Federico II", reference number 121_13_ES5. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FE: Writing – original draft, Formal Analysis, Project administration, Resources, Methodology, Writing – review & editing, Conceptualization, Data curation, Investigation. LC: Writing – original draft, Data curation, Resources, Investigation. IC: Resources, Investigation, Data curation, Writing – review & editing. RS: Writing – review & editing, Data curation, Investigation, Resources. VP: Resources, Data curation, Writing – review & editing, Investigation. ML: Resources, Data curation, Writing – review & editing, Investigation. AB: Writing – review & editing, Investigation, Data curation, Resources. FC: Resources, Investigation, Data curation, Writing – review & editing. GC: Data curation, Writing – review & editing, Resources, Investigation. TB: Methodology, Data curation, Writing – review & editing, Formal Analysis. RM: Resources, Investigation, Writing – review & editing, Data curation. MBo: Writing – review & editing, Investigation, Data curation, Resources. AG: Writing – review & editing, Investigation, Data curation, Resources. BM: Writing – review & editing, Investigation, Resources, Data curation. FS: Writing – review & editing, Investigation, Data curation, Resources. PW: Investigation, Resources, Data curation, Writing – review & editing. PF: Data curation, Investigation, Resources, Writing – review & editing. MV: Resources, Investigation, Data curation, Writing – review & editing. FP: Writing – review & editing, Resources, Investigation, Data curation. MBr: Project administration, Investigation, Writing – review & editing, Formal Analysis, Data curation, Methodology, Writing – original draft, Resources, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The GIMEMA Foundation through an unrestricted grant received by Novartis Pharma covered the coordination costs of the SUSTRENIM trial.
Acknowledgments
We thank all patients for dedicating their time to this study by completing Quality of Life questionnaires, and all participating centers and local research teams.
Conflict of interest
FE: consultancy or advisory role for AbbVie, Incyte, Novartis and JAZZ Pharmaceuticals. Research funding institution from Daiichi Sankyo. All not related to this manuscript. MBr: Honoraria by Novartis, Incyte, Pfizer, GSK, BMS, AOP, Abbvie. FC: Consultancy, honoraria and research support for Novartis, Incyte, Pfizer, BMS. PW: Research support from BMS/Celgene and Novartis, consultancy fees from BMS/Celgene, Incyte, Novartis, and Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1645217/full#supplementary-material
References
1. Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. (2015) 29:999–1003. doi: 10.1038/leu.2015.29
2. Merx K, Müller MC, Kreil S, Lahaye T, Paschka P, Schoch C, et al. Early reduction of BCR-ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. (2002) 16:1579–83. doi: 10.1038/sj.leu.2402680
3. Wang L, Pearson K, Ferguson JE, and Clark RE. The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. (2003) 120:990–9. doi: 10.1046/j.1365-2141.2003.04200.x
4. Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. (2005) 11:3425–32. doi: 10.1158/1078-0432.CCR-04-2139
5. Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. (2012) 30:232–8. doi: 10.1200/JCO.2011.38.6565
6. Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. (2012) 26:2096–102. doi: 10.1038/leu.2012.85
7. Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. (2013) 121:3818–24. doi: 10.1182/blood-2012-10-462291
8. Jain P, Kantarjian H, Nazha A, O'Brien S, Jabbour E, Romo CG, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. (2013) 121:4867–74. doi: 10.1182/blood-2013-03-490128
9. Hughes TP, Saglio G, Kantarjian HM, Guilhot F, Niederwieser D, Rosti G, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. (2014) 123:1353–60. doi: 10.1182/blood-2013-06-510396
10. Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. (2014) 123:494–500. doi: 10.1182/blood-2013-06-511592
11. Efficace F, Collins GS, Cottone F, Giesinger JM, Sommer K, Anota A, et al. Patient-reported outcomes as independent prognostic factors for survival in oncology: systematic review and meta-analysis. Value Health. (2021) 24:250–67. doi: 10.1016/j.jval.2020.10.017
12. Mierzynska J, Piccinin C, Pe M, Martinelli F, Gotay C, Coens C, et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: a systematic review. Lancet Oncol. (2019) 20:e685–e98. doi: 10.1016/S1470-2045(19)30656-4
13. Efficace F, Castagnetti F, Martino B, Breccia M, D'Adda M, Angelucci E, et al. Health-related quality of life in patients with chronic myeloid leukemia receiving first-line therapy with nilotinib. Cancer. (2018) 124:2228–37. doi: 10.1002/cncr.31323
14. Breccia M, Castagnetti F, Piciocchi A, Abruzzese E, Bassan R, Binotto G, et al. Sustenim trial: sustained deep molecular response and TFR rate in the long term follow-up. HemaSphere. (2024) 8:152–3. doi: 10.1002/hem3.104
15. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34:966–84. doi: 10.1038/s41375-020-0776-2
16. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
17. Efficace F, Iurlo A, Patriarca A, Stagno F, Bee PC, Ector G, et al. Validation and reference values of the EORTC QLQ-CML24 questionnaire to assess health-related quality of life in patients with chronic myeloid leukemia. Leuk Lymphoma. (2021) 62:669–78. doi: 10.1080/10428194.2020.1838509
18. Brümmendorf TH, Gambacorti-Passerini C, Bushmakin AG, Cappelleri JC, Viqueira A, Reisman A, et al. Relationship between molecular response and quality of life with bosutinib or imatinib for chronic myeloid leukemia. Ann Hematol. (2020) 99:1241–9. doi: 10.1007/s00277-020-04018-1
19. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. (2010) 362:2251–9. doi: 10.1056/NEJMoa0912614
Keywords: quality of life, patient reported outcome (PRO), functioning, chronic myeloid leukemia (CML), early molecular response (EMR)
Citation: Efficace F, Cannella L, Capodanno I, Sancetta R, Porcu V, Lunghi M, Biagi A, Castagnetti F, Caocci G, Baldi T, Marasca R, Bonifacio M, Gozzini A, Martino B, Sora F, Westerweel PE, Fazi P, Vignetti M, Pane F and Breccia M (2025) The prognostic value of health-related quality of life for early molecular response in patients with chronic myeloid leukemia: analysis of the GIMEMA-SUSTRENIM trial. Front. Oncol. 15:1645217. doi: 10.3389/fonc.2025.1645217
Received: 11 June 2025; Accepted: 12 August 2025;
Published: 27 August 2025.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Adisak Tantiworawit, Chiang Mai University, ThailandCarlo Gambacorti, University of Milano Bicocca, Italy
Copyright © 2025 Efficace, Cannella, Capodanno, Sancetta, Porcu, Lunghi, Biagi, Castagnetti, Caocci, Baldi, Marasca, Bonifacio, Gozzini, Martino, Sora, Westerweel, Fazi, Vignetti, Pane and Breccia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Efficace, Zi5lZmZpY2FjZUBnaW1lbWEuaXQ=
 Fabio Efficace
Fabio Efficace Laura Cannella
Laura Cannella Isabella Capodanno
Isabella Capodanno Rosaria Sancetta3
Rosaria Sancetta3 Fausto Castagnetti
Fausto Castagnetti Giovanni Caocci
Giovanni Caocci Thomas Baldi
Thomas Baldi Roberto Marasca
Roberto Marasca Massimiliano Bonifacio
Massimiliano Bonifacio Antonella Gozzini
Antonella Gozzini Federica Sora
Federica Sora Peter E. Westerweel
Peter E. Westerweel Fabrizio Pane
Fabrizio Pane Massimo Breccia
Massimo Breccia