Abstract
Background:
The health-related quality of life (HRQoL) of patients with locally advanced head and neck squamous cell carcinoma (LA HNSCC) is impacted by both disease- and treatment-related factors. Treatments that preserve and maximize HRQoL in this setting represent a substantial unmet need.
Methods:
KEYNOTE-412 (NCT03040999) was a randomized, double-blind, placebo-controlled phase 3 study of pembrolizumab plus chemoradiotherapy (CRT) versus placebo plus CRT for maintenance therapy in participants with treatment-naïve LA HNSCC. Patient-reported outcomes (PROs) assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and EORTC QLQ Head and Neck 35 (H&N35) were pre-specified secondary endpoints and administered at baseline and throughout the study. Least squares mean (LSM) change from baseline was assessed using a constrained longitudinal data analysis model. No formal statistical significance testing was performed.
Results:
The PRO analysis population included 395 participants randomized to receive pembrolizumab plus CRT and 397 to receive placebo plus CRT. Completion rates for all assessed PROs were >95% at baseline and >66% at week 45. LSM change from baseline to week 45 was similar between groups across EORTC QLQ-C30 and QLQ-H&N35 subscale scores. There were no notable differences in empirical mean change or the proportion of participants with improvement, stability, or deterioration from baseline to week 45 between treatment groups.
Conclusion:
The addition of pembrolizumab to CRT did not meaningfully impact HRQoL in participants with LA HNSCC.
Introduction
Head and neck squamous cell carcinomas (HNSCCs) often arise in the oral cavity, pharynx, and larynx (1). HNSCCs can cause significant morbidity because these sites are central to basic physiological (breathing and swallowing), sensory (taste and smell), and personal characteristics (appearance and speech) (2). Treatments with curative intent for locally advanced (LA) HNSCCs, including surgery and concurrent chemoradiotherapy (CRT), have improved disease control, but also risk further impairing functional and cosmetic outcomes and profoundly impact patient quality of life (QoL) (2–6).
Health-related QoL (HRQoL) has emerged as a critical end point in clinical studies of patients with head and neck cancers (6, 7), offering insights into the impact of cancer and its treatment from the patient’s perspective. By directly assessing the patient’s perspective of treatment, patient-reported outcomes (PROs) can provide a more complete picture of treatment outcomes in clinical studies (8, 9). Difficulty with swallowing, difficulty with speech, and pain have been identified as the most clinically meaningful symptoms for patients with HNSCC (10), highlighting the unmet need for treatment options that preserve and maximize function and HRQoL.
In the phase 3 KEYNOTE-412 study of participants with LA HNSCC, pembrolizumab plus CRT followed by pembrolizumab maintenance therapy did not significantly improve event-free survival (EFS) compared with placebo plus CRT (hazard ratio [95% confidence interval]: 0.83 [0.68–1.03]; P = 0.0429), and no new safety signals were observed (11). This paper reports the pre-specified secondary and exploratory PRO end points of KEYNOTE-412 and evaluates the impact of adding pembrolizumab to CRT as maintenance therapy on HRQoL.
Methods
KEYNOTE-412 (NCT03040999) was a double-blind, multicenter, randomized, placebo-controlled phase 3 study designed to evaluate the efficacy and safety of pembrolizumab plus CRT compared with placebo plus CRT as maintenance therapy in participants with treatment-naïve LA HNSCC.
The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. All participants provided written informed consent.
Study population
Participants were eligible for enrollment if they were at least 18 years old and had a pathologically proven new diagnosis of LA HNSCC (either T3-T4 [N0-N3] M0 or any N2-3 [T1-T4] M0 larynx/hypopharynx/oral cavity/p16-negative oropharynx cancers, or either T4 [N0-N3] M0 or N3 [T1-T4] M0 p16-positive oropharynx cancer). Other key eligibility criteria included no prior treatment for the HNSCC under investigation, an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–1, a tumor burden that was evaluable by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, and being a candidate for definitive high-dose cisplatin-based CRT.
Study design
Eligible participants were randomly assigned 1:1 to receive intravenous pembrolizumab 200 mg or placebo every 3 weeks plus CRT for either 17 cycles of pembrolizumab/placebo or until disease progression, intolerable toxicity, or physician or participant decision to withdraw from the study. CRT included intravenous cisplatin 100 mg/m2 every 3 weeks plus accelerated fractionation radiotherapy (70 Gy, 6 fractions/week for 6 weeks [5 fractions in the final week]; 35 fractions in total) or standard fractionation radiotherapy (70 Gy, 5 fractions/week for 7 weeks; 35 fractions in total). Pembrolizumab and placebo were first administered as a priming dose 1 week prior to CRT, followed by 2 doses during CRT, and 14 doses as maintenance therapy after CRT.
Randomization was stratified by radiotherapy regimen (accelerated vs standard fractionation), p16 status (p16-positive oropharyngeal tumors vs p16-negative oropharyngeal or laryngeal/hypopharyngeal/oral cavity tumors), and tumor stage (III vs IV).
Study outcomes
The primary study end point was event-free survival (defined as the time from randomization to radiographically or pathologically confirmed progressive disease, surgery, or death due to any cause, whichever occurred first), which has been reported elsewhere (11).
Pre-specified secondary end points included change from baseline in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) global health status/quality of life (GHS/QoL) score, EORTC QLQ-C30 physical functioning score, EORTC QLQ-Head and Neck 35 (H&N35) swallowing symptom score, EORTC QLQ-H&N35 speech symptom score, and EORTC QLQ-H&N35 pain symptom score. HRQoL utilities were also assessed as an exploratory end point using the EuroQoL five dimensions visual analog scale (EQ-5D VAS).
Study assessments and procedures
EORTC questionnaires are widely used to assess the QoL of patients with cancer. The QLQ-C30 contains 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, nausea and vomiting, and pain), 6 single-symptom items (dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact), and a GHS/QoL dimension (12). The QLQ-H&N35 contains 7 multi-item scales (pain in the mouth, problems with swallowing, senses, speech, social eating, social contact, and sexuality) and 11 single-item scales (problems with teeth, mouth opening, dry mouth, sticky saliva, coughing, feeling ill, use of analgesics, use of nutritional supplements, use of feeding tube, weight gain, and weight loss) (13). The EORTC QLQ-C30 and QLQ-H&N35 are scored from 0–100, with higher scores for functioning scales and GHS indicating better functioning and higher scores for symptom and single-item scales indicating worsening symptoms. These instruments have been psychometrically and clinically validated in patients with head and neck cancers, and a ≥10-point difference on the QLQ-C30 and QLQ-H&N35 scales (either from baseline or between treatment groups) is considered clinically relevant (14, 15).
The EQ-5D is a standardized instrument that has been used extensively in oncology studies to measure health outcomes. The health state dimensions in the EQ-5D include mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, as well as a visual analog scale graded from 0–100 for general state of health at the time of the assessment (with higher scores indicating higher HRQoL).
PRO questionnaires were administered electronically at the study site at baseline, week 6, and week 9, then every 12 weeks during the maintenance phase. PROs were also completed at treatment discontinuation and 30 days after the last dose of treatment at the safety follow-up visit, then every 3 months during year 2 and once a year until year 5. PRO questionnaires were completed prior to other study procedures, including the administration of study treatment; the EQ-5D was administered first, followed by the EORTC QLQ-C30, then the EORTC QLQ-H&N35.
Statistical analysis
PRO analyses were assessed in all randomly assigned participants who completed at least one PRO and received at least one dose of study treatment. PRO analyses were conducted at prespecified questionnaire completion rates of at least 60% and compliance rates of at least 80%. Completion was defined as the proportion of participants who completed at least one questionnaire at each time point among those in the PRO analysis population. Compliance was defined as the proportion of participants who completed at least one questionnaire at each time point among those who were expected to complete the instruments at that time point, excluding those missing by design (such as death, discontinuation, and translations not available).
A constrained longitudinal data analysis model with PRO scores as the response variable and treatment-by-time interaction and stratification factors as covariates was used to estimate the LSM change from baseline and between-group difference in EORTC QLQ-C30 and QLQ-H&N35 PROs. EORTC QLQ-C30 and QLQ-H&N35 PROs were also assessed by rates of overall improvement (≥10-point increase [in the positive direction] from baseline at any time, confirmed by a ≥10-point increase from baseline at the next consecutive visit), stability (when criteria for improvement are not met, a change in score of <10 points from baseline at any time that is confirmed at the next consecutive visit), and deterioration (≥10-point decrease from baseline at any time when none of the criteria for improvement or stability are met).
Results
Overall, 804 participants were randomly assigned to receive pembrolizumab plus CRT (n=402) or placebo plus CRT (n=402). Baseline characteristics were well balanced between treatment groups (Supplementary Table S1). The most common tumor site for both groups was oropharynx (n=200, pembrolizumab plus CRT; n=204, placebo plus CRT) followed by larynx (n=92, pembrolizumab plus CRT; n=86, placebo plus CRT), hypopharynx (n=71, pembrolizumab plus CRT; n=73, placebo plus CRT), and oral cavity (n=39, pembrolizumab plus CRT; n=39, placebo plus CRT). At baseline, most participants were stage T3-T4b (n=335, pembrolizumab plus CRT; n=347, placebo plus CRT) and overall stage IVa-IVb (n=262, pembrolizumab plus CRT; n=265, placebo plus CRT) (Supplementary Table S1). Most participants in both groups had a PD-L1 combined positive score of at least 1 (CPS ≥1; n=339, pembrolizumab plus CRT; n=346, placebo plus CRT). The median time from randomization to the database cutoff date (31 May 2022) was 47.7 months (IQR, 42.1–52.3). The PRO population included 792 participants (n=395 in the pembrolizumab plus CRT group and n=397 in the placebo plus CRT group).
Completion and compliance rates were >95% at baseline for all PRO questionnaires across treatment groups (Supplementary Table S2). At week 45, completion rates for the EORTC QLQ-C30 were 67.8% in the pembrolizumab plus CRT group and 67.0% in the placebo plus CRT group, and compliance rates were 97.1% and 97.4%, respectively. For the EORTC QLQ-H&N35, completion rates were 67.8% in the pembrolizumab plus CRT group and 66.9% in the placebo plus CRT group, and compliance rates were 97.1% and 97.4%, respectively. For the EQ-5D, completion rates were 67.8% in the pembrolizumab plus CRT group and 67.0% in the placebo plus CRT group, and compliance rates were 90.5% and 93.3%, respectively.
Baseline scores for all PRO instruments were similar between treatment groups (Table 1). Baseline scores for EORTC QLQ-C30 and EORTC QLQ-H&N35 were similar between groups based on tumor location (Supplementary Table S3) and by T-stage and overall tumor staging (Supplementary Table S4). Treatment with pembrolizumab plus CRT resulted in LSM changes from baseline to week 45 ranging from -10.6 to +2.0 across EORTC QLQ-C30 and QLQ-H&N35 subscale scores (Table 1). An improvement from baseline in GHS/QoL scores was observed in the placebo plus CRT group compared with the pembrolizumab plus CRT group. Patient-reported outcomes remained generally stable over time in both treatment groups for measures of physical functioning or for disease-related symptom scores; however, an improvement from baseline in the EORTC QLQ-H&N35 pain symptom score was observed in both treatment groups (-10.6 [95% CI: -12.9 to -8.3] for participants treated with pembrolizumab plus CRT and -12.1 [95% CI: -14.4 to -9.8] for participants treated with placebo plus CRT). Results were generally similar to the total PRO population when analyzed by primary tumor site (Figures 1A-E) and by PD-L1 CPS ≥1 (Figures 2A, B), except for a decline in swallowing in participants in the pembrolizumab plus CRT group with hypopharynx as the primary tumor site. Results were also similar when analyzed by T-stage and by overall tumor staging, except for a decline in swallowing for T1-T2 stage and stage II-III in both treatment groups and a decline in speech for T1-T2 stage in both treatment groups (Figures 3A-E). There were no meaningful differences within or between treatment groups in EQ-5D VAS score.
Table 1
| Assessment | Baseline | Week 45 | Change from baseline to week 45 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| na | Mean (SD) | na | Mean (SD) | Nb | LSM (95% CI) | Difference in LSM (95% CI) | |||
| EORTC QLQ | C30 GHS/QoL | Pembrolizumab + CRT | 378 | 69.6 (20.7) | 268 | 73.0 (18.1) | 395 | 2.0 (-0.1 to 4.0) | -4.1 (-6.7 to -1.5) |
| Placebo + CRT | 382 | 67.8 (20.4) | 266 | 76.3 (16.8) | 397 | 6.1 (4.0 to 8.1) | |||
| C30 PF | Pembrolizumab + CRT | 378 | 88.8 (15.8) | 268 | 84.8 (17.0) | 395 | -5.5 (-7.3 to -3.7) | -2.1 (-4.5 to 0.4) |
|
| Placebo + CRT | 382 | 88.2 (16.0) | 266 | 86.8 (15.1) | 397 | -3.5 (-5.3 to -1.7) | |||
| H&N35 pain | Pembrolizumab + CRT | 378 | 25.8 (24.3) | 268 | 15.0 (18.1) | 395 | -10.6 (-12.9 to -8.3) | 1.44 (-1.3 to 4.2) |
|
| Placebo + CRT | 378 | 27.6 (25.6) | 265 | 13.0 (16.1) | 396 | -12.1 (-14.4 to -9.8) | |||
| H&N35 swallowing | Pembrolizumab + CRT | 378 | 22.5 (24.8) | 268 | 18.3 (22.3) | 395 | -3.9 (-6.6 to -1.2) | -0.3 (-3.8 to 3.1) |
|
| Placebo + CRT | 378 | 25.1 (26.3) | 265 | 17.7 (22.1) | 396 | -3.6 (-6.3 to -0.9) | |||
| H&N35 speech | Pembrolizumab + CRT | 378 | 23.7 (27.2) | 268 | 17.3 (21.6) | 395 | -6.2 (-8.9 to -3.6) | -1.3 (-4.6 to 2.1) |
|
| Placebo + CRT | 378 | 26.2 (27.7) | 265 | 17.5 (22.2) | 396 | -5.0 (-7.6 to -2.3) | |||
| EQ-5D | VAS | Pembrolizumab + CRT | 384 | 75.6 (18.6) | 268 | 78.8 (16.1) | 395 | 3.5 (1.6 to 5.3) | -1.4 (-3.7 to 0.9) |
| Placebo + CRT | 386 | 72.1 (21.2) | 266 | 79.9 (15.3) | 397 | 4.9 (3.1 to 6.8) | |||
Mean change from baseline to week 45 in EORTC QLQ-C30, EORTC QLQ-H&N35, and EQ-5D subscale scores.
an is the number of patients in each treatment group with non-missing assessments at the specific time point. bN is the number of participants in the PRO analysis population in each treatment group.
C30, Core 30; CI, confidence interval; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; EQ-5D, EuroQol-5 Dimensions; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; LSM, least squares mean; PF, physical functioning; PRO, patient-reported outcome; SD, standard deviation; VAS, visual analog scale.
Figure 1
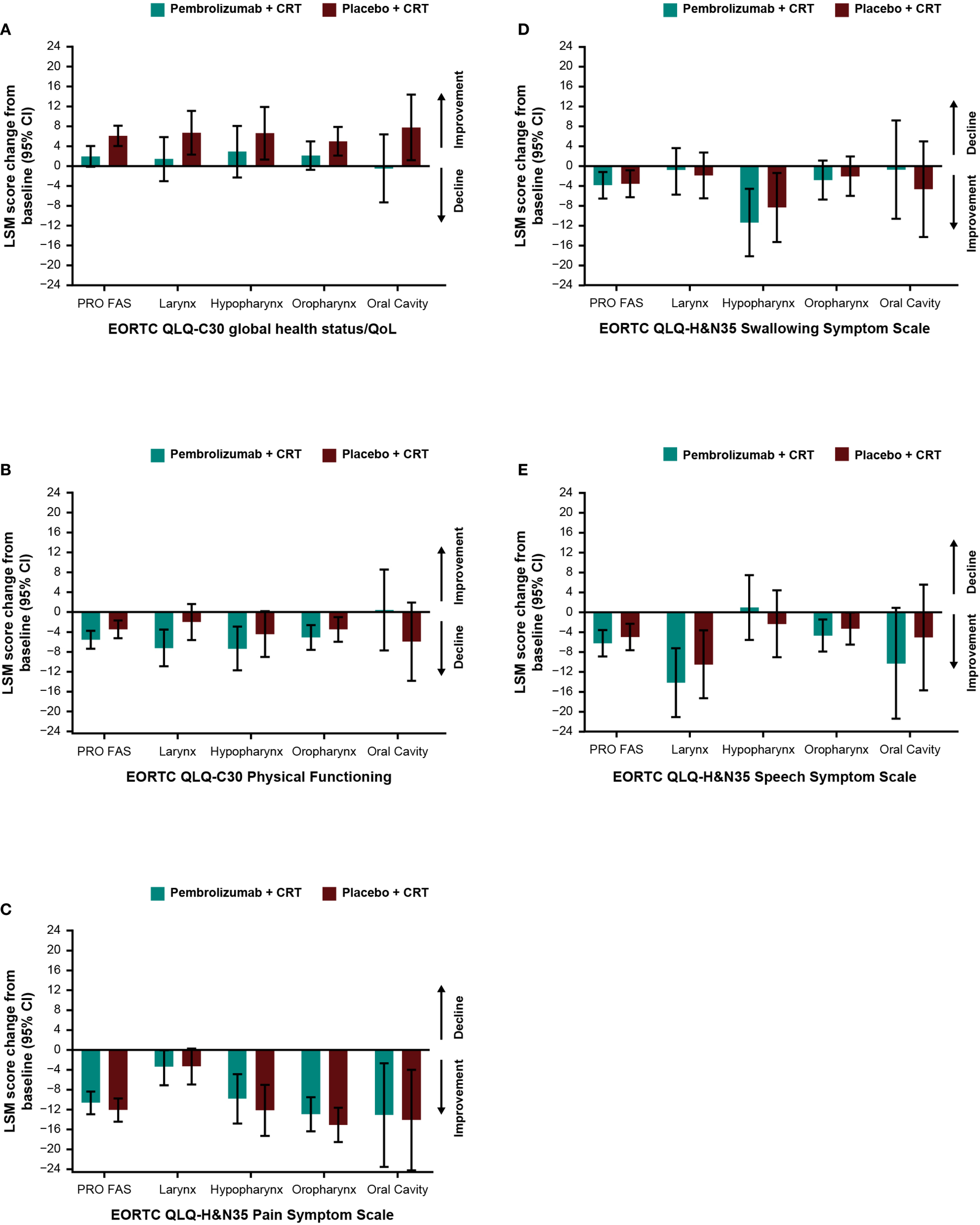
Difference in least squares mean from baseline to week 45 by primary tumor site location in (A) EORTC QLQ-C30 GHS/QoL, (B) EORTC QLQ-C30 PF, (C) EORTC QLQ-H&N35 pain, (D) EORTC QLQ-H&N35 swallowing, and (E) EORTC QLQ-H&N35 speech scores. C30, Core 30; CI, confidence interval; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; PF, physical functioning, PRO FAS, patient-reported outcomes full analysis set.
Figure 2
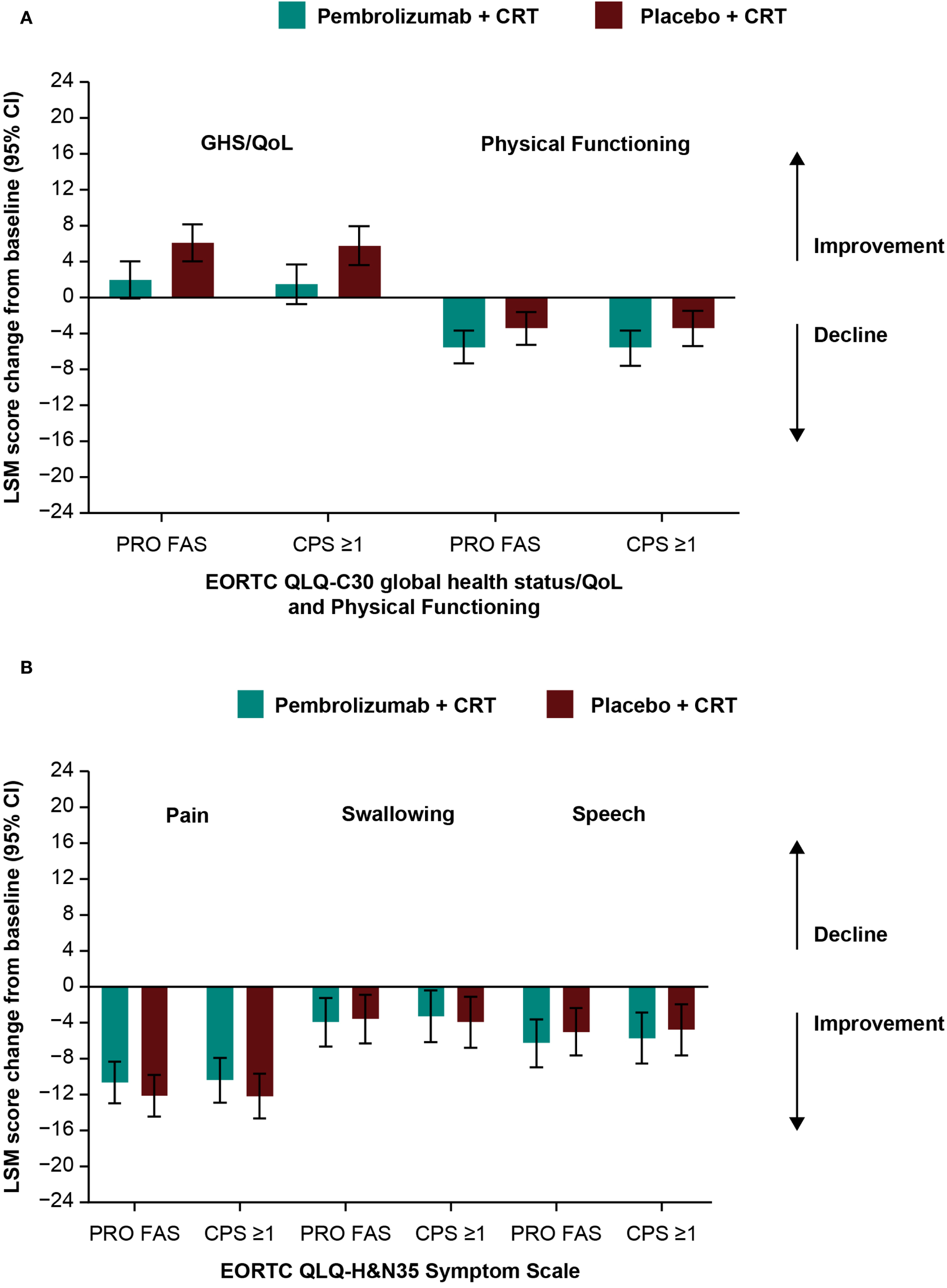
Difference in least squares mean from baseline to week 45 by PD-L1 CPS ≥1 in (A) EORTC QLQ-C30 GHS/QoL and PF and (B) EORTC QLQ-H&N35 pain, swallowing, and speech scores. C30, Core 30; CI, confidence interval; CPS, combined positive score; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; LSM, least squares mean; PD-L1, programmed cell death ligand 1; PF, physical functioning, PRO FAS, patient-reported outcomes full analysis set.
Figure 3
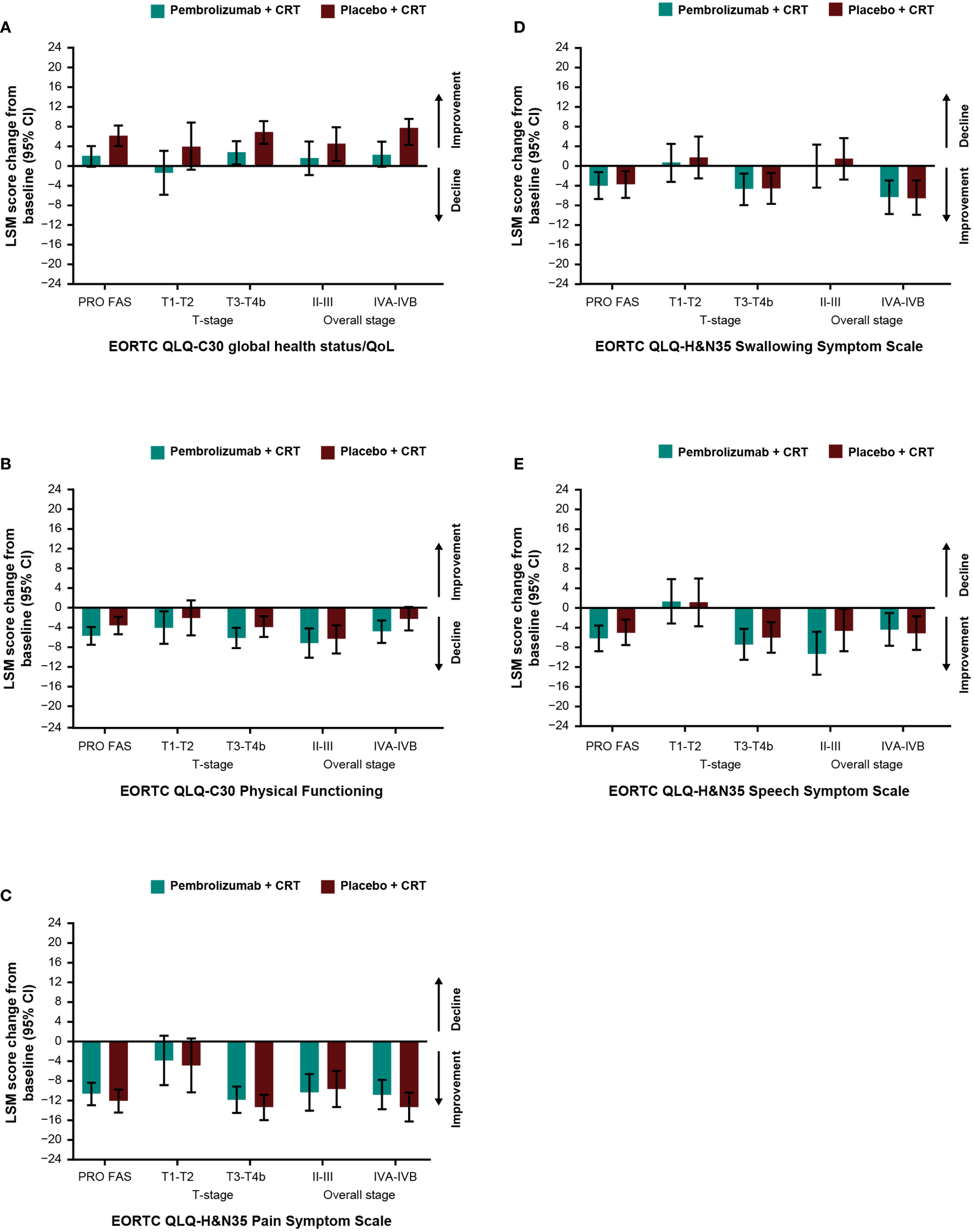
Difference in least squares mean from baseline to week 45 by cancer stage in (A) EORTC QLQ-C30 GHS/QoL (B) EORTC QLQ-C30 PF (C) EORTC QLQ-H&N35 pain (D) EORTC QLQ-H&N35 swallowing and (E) EORTC QLQ-H&N35 speech. C30, Core 30; CI, confidence interval; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; LSM, least squares mean; PF, physical functioning, PRO FAS, patient-reported outcomes full analysis set.
Empirical mean change from baseline to week 45 for EORTC QLQ-C30 GHS/QoL was generally stable, with a decline at the start of treatment and recovery to baseline by week 45 (Figure 4A). This pattern was evident for all assessed PRO scores (Figures 4A–E). There were no notable differences in empirical mean change from baseline to week 45 between treatment groups (Figures 4A–E). The proportion of participants with improvement, stability, or deterioration from baseline to week 45 was comparable between treatment groups for both EORTC QLQ-C30 (Figure 5A) and EORTC QLQ-H&N35 scores (Figure 5B).
Figure 4
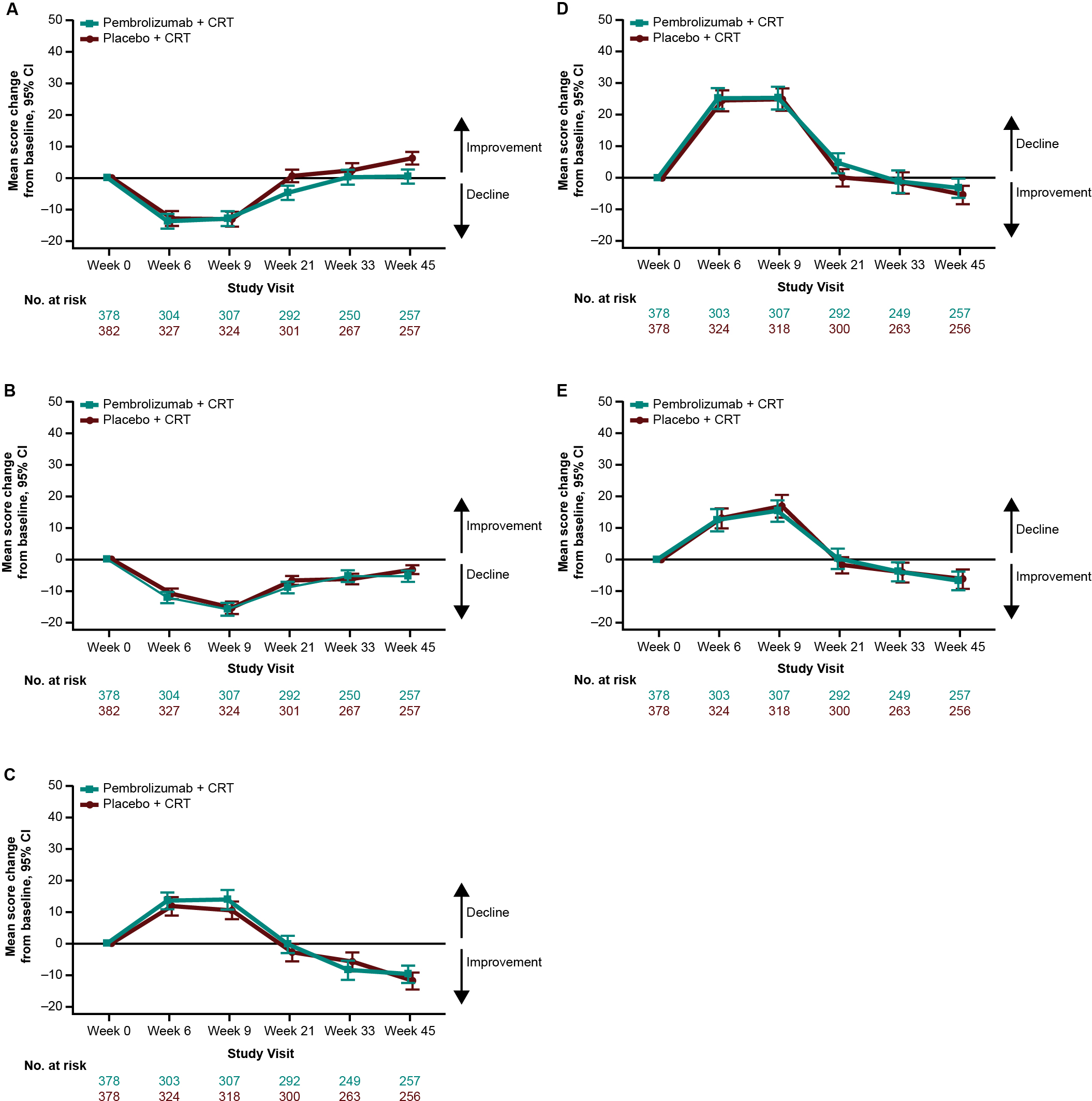
Empirical mean change from baseline to week 45 in (A) EORTC QLQ-C30 GHS/QoL, (B) EORTC QLQ-C30 PF, (C) EORTC QLQ-H&N35 pain, (D) EORTC QLQ-H&N35 swallowing, and (E) EORTC QLQ-H&N35 speech scores. C30, Core 30; CI, confidence interval; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; PF, physical functioning.
Figure 5
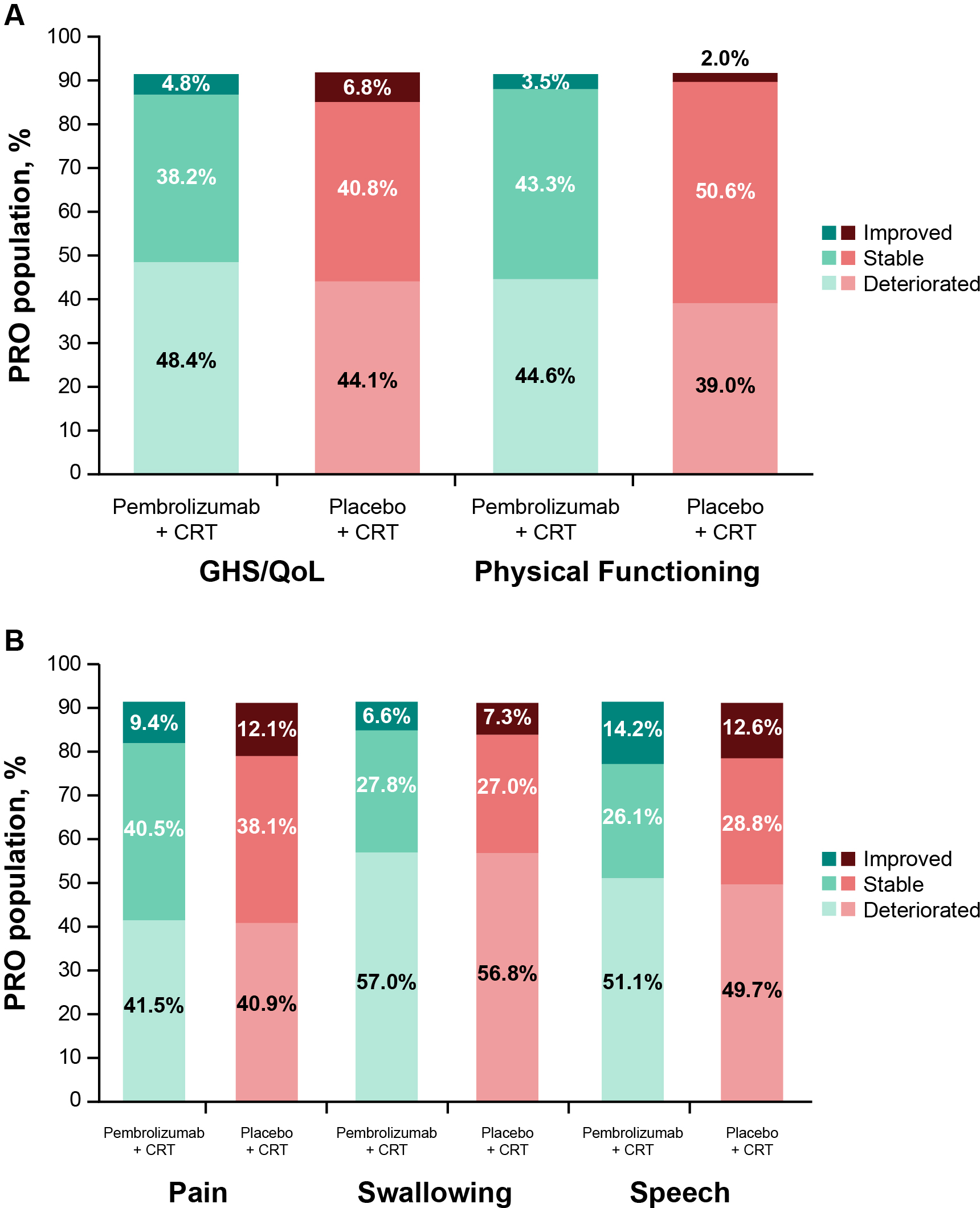
Proportion of participants with improved, stable, or deteriorated scores from baseline to week 45 in (A) EORTC QLQ-C30 GHS/QoL and PF, and (B) EORTC QLQ-H&N35 pain, swallowing, and speech. C30, Core 30; CRT, chemoradiotherapy; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life questionnaire; GHS/QoL, Global Health Score/quality of life; H&N35, Head and Neck 35; PF, physical functioning; PRO, patient-reported outcome.
Discussion
In the KEYNOTE-412 study, participants with LA HNSCC treated with pembrolizumab plus CRT reported generally similar HRQoL outcomes as participants treated with placebo plus CRT. Results were generally similar by tumor site location, PD-L1 CPS ≥1, and cancer stage, and no major differences were observed in these subgroups. Declines were observed in both treatment groups for participants with T1–T2 stage (speech and swallowing) and stage II–III (swallowing) disease. There was also a decline in swallowing in the pembrolizumab plus CRT group with hypopharynx as the primary tumor site. Notably, an improvement in the EORTC QLQ-H&N35 pain symptom score was observed in both pembrolizumab plus CRT and placebo plus CRT groups. To the best of our knowledge, this is the first detailed report of PROs in participants with LA HNSCC treated with an immune checkpoint inhibitor.
Previous analyses of PROs in participants with HNSCC treated with pembrolizumab in phase 3 studies have shown comparable results. In KEYNOTE-040, participants with recurrent and/or metastatic (R/M) HNSCC treated with pembrolizumab had stable GHS/QoL scores (LSM [95% CI]: 0.38 [-3.00 to 3.78]) compared with participants treated with the standard of care (LSM [95% CI]: -5.86 [-9.68 to -2.04]) (16). Participants in both treatment groups had stable functioning and symptom scores. In KEYNOTE-048, participants with treatment-naïve R/M HNSCC were treated with pembrolizumab, pembrolizumab–chemotherapy, or cetuximab–chemotherapy; PRO scores remained stable, and GHS/QoL scores were similar between groups (17).
These results also largely align with the limited studies of PROs in participants with head and neck cancers treated with other therapies targeting the PD-(L)1 axis in the literature (18, 19). In the phase 3 CheckMate 141 and phase 4 VOLUME-PRO studies, participants with R/M HNSCC treated with nivolumab reported mostly stable symptoms and functioning, with few differences observed from baseline in EORTC QLQ-C30, QLQ-H&N35, and EQ-5D VAS scores (18, 20). Participants with R/M HNSCC participating in the phase 2 HAWK study who received durvalumab reported an improvement in EORTC QLQ-C30 GHS/QoL, physical functioning, and fatigue, as well as in EORTC QLQ-H&N35 mouth pain, swallowing, taste and smell, and speech symptom scores (21). An exploratory study evaluating PROs in participants with HNSCC starting treatment with immune checkpoint inhibitor monotherapy or combination therapy with cetuximab also found that QoL stabilized over time (19).
The participants in these prior studies had more advanced disease and had received previous treatment; given that these participants often face shorter survival times, changes in PROs from baseline were evaluated at earlier time points than the results presented in this analysis (week 15 in KEYNOTE-040 and KEYNOTE-048, weeks 9–15 in CheckMate 141, weeks 6–8 in VOLUME-PRO, and weeks 16–24 in HAWK) (17, 18, 20–22). This highlights the value of the extended follow-up period in this study, which can yield insights into the longer-term effect of treatment on the QoL of patients with head and neck cancer.
A common challenge in HRQoL studies is the decrease over time in the number of participants completing PRO assessments, which is a limitation of this analysis. Some subgroups had relatively small numbers of participants and KEYNOTE-412 was not powered to determine the statistical significance of PROs and lacked multiplicity control, so the results should be interpreted with caution.
In conclusion, PROs were similar between treatment with pembrolizumab plus CRT and placebo plus CRT in the first-line setting for participants with LA HNSCC, suggesting that the addition of pembrolizumab to CRT did not meaningfully impact HRQoL.
Statements
Data availability statement
The datasets presented in this article are not readily available because Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: https://externaldatasharing-msd.com) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses. Requests to access the datasets should be directed to https://externaldatasharing-msd.com).
Ethics statement
The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
J-PM: Investigation, Writing – review & editing, Conceptualization. YT: Writing – review & editing, Conceptualization, Investigation. LL: Investigation, Writing – review & editing. BBu: Conceptualization, Writing – review & editing, Investigation. MTah: Investigation, Conceptualization, Writing – review & editing. DR: Conceptualization, Investigation, Writing – review & editing. GA: Writing – review & editing, Investigation. IL: Investigation, Writing – review & editing. BH: Writing – review & editing, Investigation. YP: Conceptualization, Writing – review & editing, Investigation. SA: Writing – review & editing, Investigation. SL: Investigation, Writing – review & editing. RG: Investigation, Writing – review & editing. MB: Writing – review & editing, Investigation. MH: Investigation, Writing – review & editing. J-PD: Investigation, Writing – review & editing. RM: Investigation, Writing – review & editing. MTab: Investigation, Writing – review & editing. JW: Investigation, Writing – review & editing, Conceptualization. CS: Writing – review & editing, Investigation. VG: Writing – review & editing, Conceptualization, Investigation. KH: Investigation, Writing – review & editing, Conceptualization. CB: Writing – review & editing, Investigation. JN: Writing – review & editing, Conceptualization, Investigation. AW: Investigation, Writing – review & editing. BG: Writing – review & editing, Conceptualization, Investigation. BBi: Writing – review & editing, Conceptualization, Investigation. LS: Writing – review & editing, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Acknowledgments
The authors wish to acknowledge the contribution of the study participants and investigators. Medical writing assistance was provided by Robert Steger, PhD, of ApotheCom (Yardley, PA, USA), which was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Conflict of interest
Authors CB, JN, AW, BG, and BB were employed by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time of the study. J-PM has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; support for attending meetings and/or travel from Amgen, Bristol Myers Squibb, Pfizer, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Gilead, and Sanofi. YT has received research funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; consulting fees and travel support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and Merck KGaA, Darmstadt, Germany; honoraria from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Seagen. LL has received research funding paid to institution from Adlai Nortye, AstraZeneca, Bristol Myers Squibb, Debiopharm International SA, Eisai, Eli Lilly and Company, Exelixis, Hoffman-La Roche Ltd, Isa Therapeutics, Kura Oncology, Merck Serono, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Nektar Therapeutics, Novartis, Regeneron, Roche, Sanofi, Syneos, Sun Pharmaceutica, Incyte Biosciences International Sàrl, Gilead Sciences, Inc., Genmab, and Merck Healthcare KGaA; and consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck Serono Spa, Merck KGaA, GSK, F. Hoffman-La Roche Ltd, EMD Serono Research & Development Institute, Inc., Boehringer Ingelheim International GmbH, Simon-Kucher & Partners Strategy & Marketing Consultants, Rgenta Therapeutics, Inc, Alentis Therapeutics AG, MedImmune Limited, Simon-Kucher & Partners Italia Srl; honoraria from Merck Serono Spa, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck KGaA, Bristol Mayers Squibb, and ALTIS Omnia Pharma Service Srl; travel support from TAE Life Science; personal fees from advisory or data safety monitoring boards from Merck KGaA, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, EMD Serono Research & Development Institute, Inc., F. Hoffman-La Roche Ltd, Seagen International GmbH, Genmab US, Inc., AbbVie Srl, Simon-Kucher & Partners Strategy & Marketing Consultants, Purple Biotech, Ltd, Aveo Pharmaceuticals, Inc, Alx Oncology Inc., Medscape LLC, and Bicara Therapeutics Inc. BBu has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; research funding paid to institution from GlaxoSmithKline, Cue BioPharma, IO Biotech, Johnson and Johnson, and EMD Serono; royalties or licenses from Up-to-Date; consulting fees from GlaxoSmithKline, Astellas, Pfizer, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck KgAm Takeda, Johnson and Johnson, IO Biotech Vaccinex, Cue BioPharma, Coherus, AstraZeneca, and Genmab; honoraria from Coherus; payment for expert testimony from Cell Sci; travel support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; data monitoring or advisory board participation from ALX Oncology, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Kura; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Cardiff Oncology and Vitrac Pharmaceuticals. MTah has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; consulting fees from Boehringer Ingelheim, Astellas, Janssen Pharmaceutical, Genmab, and AbbVie; honoraria from Ono Pharmaceutical, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Bayer, Rakuten Medical, Novartis, Bristol Myers Squibb, Merck Biopharma, Eisai, and Lilly; and participated on data monitoring or advisory boards for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Bayer, Bristol Myers Squibb, Eisai, AstraZeneca, Merus, Merck Biopharma, Pfizer, Lilly, Boehringer Ingelheim, and GSK. DR has participated on advisory boards for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Regeneron, Eisai, GSK, and Bicara Therapeutics. GA has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; research funding paid to institution from Roche, AstraZeneca, BMS, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck-Serono, Pfizer, Beigene, and Ipsen; consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and AstraZeneca; honoraria from GSK, AstraZeneca, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. IL has received honoraria for Janssen; and travel support from Adium. BH has participated on a data safety monitoring or advisory board for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Bristol Myers Squibb, AstraZeneca, Pfizer, Roche, Eisai, Sanofi, and Regeneron. YP reports personal financial interests for an advisory board with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Bristol Myers Squibb, and Merck Serono. SA has received honoraria AstraZeneca, Eli Lilly, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Novartis, Pfizer, Bristol Myers Squibb, Roche, Daiichi Sankyo, Menarini Türkiye, Astellas Pharma, Eczacıbaşı, Pierre Fabre, and Baxter. SL has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, GlaxoSmithKline, and Böhringer Ingelheim; honoraria from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; travel support from Merck Healthcare; has a pending patent for tumor-exclusive peptides for vaccine development; participation on a data safety monitoring or advisory board for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Bristol Myers Squibb. RG has received grants or contracts from Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AstraZeneca, Agmen, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Sandoz, and Gilead; consulting fees from Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead, Daiichi Sankyo; honoraria from Celgene, Roche, Merck, BMS, Takeda, AbbVie, AstraZeneca, Agmen, MSD, Sandoz, Merck, Gilead, Daiichi Sankyo, and Pfizer; and owns stock in Novo Nordisk, Lilly, Regeneron, and Vertex. MB has participated on advisory boards for and received travel support from MSD Austria. RM has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; honoraria from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Merck KGaA. KH has received grants or contracts from Boehringer-Ingelheim and RepImmune. CB is an employee of and owns stock in Merck & Co., Inc., Rahway, NJ, USA. JN is an employee of and owns stock in Merck & Co., Inc., Rahway, NJ, USA. AW is an employee of and owns stock in Merck & Co., Inc., Rahway, NJ, USA. BG is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, owns stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. BBi is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, owns stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. LS has received support for the present manuscript from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; research grants paid to institution from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Bristol Myers Squibb, Roche/Genentech, GlaxoSmithKline, Novartis, Pfizer, AstraZeneca, Boehringer Ingelheim, Bayer, Amgen, Daiichi Sankyo, EMD Serono, Astellas, Gilead, Incyte, Legochem Biosciences, Loxo/Lilly, Takara Bio, Incyte, Marengo; consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, AstraZeneca, Bristol Myers Squibb, Roche/Genentech, Voronoi, GlaxoSmithKline, Arvinas, Navire, Relay Therapeutics, Daiichi Sankyo, Tubulis, LTZ Therapeutics, Marengo, Nerviano, Amgen, Pangea, Incyte, Gilead and Velivago; and owns stock in Marengo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funders contributed to study design, data collection, data analysis, data interpretation, and writing of the report in collaboration with the authors. Investigators and site personnel collected data, which were housed on the Merck & Co database.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1645509/full#supplementary-material
References
1
Chow LQM . Head and neck cancer. N Engl J Med. (2020) 382:60–72. doi: 10.1056/NEJMra1715715
2
Saddawi-Konefka R Simon AB Sumner W Sharabi A Mell LK Cohen EEW . Defining the role of immunotherapy in the curative treatment of locoregionally advanced head and neck cancer: Promises, challenges, and opportunities. Front Oncol. (2021) 11:738626. doi: 10.3389/fonc.2021.738626
3
Johnson DE Burtness B Leemans CR Lui VWY Bauman JE Grandis JR . Head and neck squamous cell carcinoma. Nat Rev Dis Primers. (2020) 6:92. doi: 10.1038/s41572-020-00224-3
4
Okamoto I Okada T Tokashiki K Tsukahara K . Quality-of-life evaluation of patients with unresectable locally advanced or locally recurrent head and neck carcinoma treated with head and neck photoimmunotherapy. Cancers (Basel). (2022) 14:4413. doi: 10.3390/cancers14184413
5
Rathod S Livergant J Klein J Witterick I Ringash J . A systematic review of quality of life in head and neck cancer treated with surgery with or without adjuvant treatment. Oral Oncol. (2015) 51:888–900. doi: 10.1016/j.oraloncology.2015.07.002
6
So WK Chan RJ Chan DN Hughes BG Chair SY Choi KC et al . Quality-of-life among head and neck cancer survivors at one year after treatment–a systematic review. Eur J Cancer. (2012) 48:2391–408. doi: 10.1016/j.ejca.2012.04.005
7
Heutte N Plisson L Lange M Prevost V Babin E . Quality of life tools in head and neck oncology. Eur Ann Otorhinolaryngol Head Neck Dis. (2014) 131:33–47. doi: 10.1016/j.anorl.2013.05.002
8
Ossowski S Kammerer A Stram D Piazza-DeLap L Basch E Katzel JA . Patient-reported outcomes integrated within an electronic medical record in patients with head and neck cancer. JCO Clin Cancer Inform. (2021) 5:842–8. doi: 10.1200/CCI.21.00058
9
Peach MS Trifiletti DM Vachani C Arnold-Korzeniowski K Bach C Hampshire M et al . Patient-reported outcomes in head and neck cancer: prospective multi-institutional patient-reported toxicity. Patient Relat Outcome Meas. (2018) 9:245–52. doi: 10.2147/PROM.S153919
10
Mendez AI Wihlidal JGJ Eurich DT Nichols AC MacNeil SD Seikaly HR . Validity of functional patient-reported outcomes in head and neck oncology: A systematic review. Oral Oncol. (2022) 125:105701. doi: 10.1016/j.oraloncology.2021.105701
11
Machiels J Tao Y Burtness B Tahara M Rischin D Alves G et al . Primary results of the phase III KEYNOTE-412 study: Pembrolizumab (pembro) with chemoradiation therapy (CRT) vs placebo plus CRT for locally advanced (LA) head and neck squamous cell carcinoma (HNSCC). Ann Oncol. (2022) 33:S808–S69. doi: 10.1016/j.annonc.2022.08.029
12
Aaronson NK Ahmedzai S Bergman B Bullinger M Cull A Duez NJ et al . EORTC QLQ-C30 scoring manual. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
13
Bjordal K Ahlner-Elmqvist M Tollesson E Jensen AB Razavi D Maher EJ et al . Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol. (1994) 33:879–85. doi: 10.3109/02841869409098450
14
Bjordal K de Graeff A Fayers PM Hammerlid E van Pottelsberghe C Curran D et al . A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer. (2000) 36:1796–807. doi: 10.1016/S0959-8049(00)00186-6
15
Musoro JZ Coens C Singer S Tribius S Oosting SF Groenvold M et al . Minimally important differences for interpreting European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 scores in patients with head and neck cancer. Head Neck. (2020) 42:3141–52. doi: 10.1002/hed.26363
16
Harrington KJ Soulières D Le Tourneau C Dinis J Licitra LF Ahn MJ et al . Quality of life with pembrolizumab for recurrent and/or metastatic head and neck squamous cell carcinoma: KEYNOTE-040. J Natl Cancer Inst. (2021) 113:171–81. doi: 10.1093/jnci/djaa063
17
Rischin D Harrington KJ Greil R Soulières D Tahara M de Castro G Jr. et al . Pembrolizumab alone or with chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: Health-related quality-of-life results from KEYNOTE-048. Oral Oncol. (2022) 128:105815. doi: 10.1016/j.oraloncology.2022.105815
18
Harrington KJ Ferris RL Blumenschein G Jr. Colevas AD Fayette J Licitra L et al . Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. (2017) 18:1104–15. doi: 10.1016/S1470-2045(17)30421-7
19
Kirtane K Hoogland AI Li X Rodriguez Y Scheel K Small BJ et al . Patient-reported outcomes in immunotherapy for head and neck cancer. Head Neck. (2023) 45:1761–71. doi: 10.1002/hed.27388
20
Gogate A Bennett B Poonja Z Stewart G Medina Colmenero A Szturz P et al . Phase 4 multinational multicenter retrospective and prospective real-world study of nivolumab in recurrent and metastatic squamous cell carcinoma of the head and neck. Cancers (Basel). (2023) 15:3552. doi: 10.3390/cancers15143552
21
Zandberg DP Algazi AP Jimeno A Good JS Fayette J Bouganim N et al . Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. (2019) 107:142–52. doi: 10.1016/j.ejca.2018.11.015
22
Harrington KJ Burtness B Greil R Soulières D Tahara M de Castro G Jr. et al . Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: Updated results of the phase III KEYNOTE-048 study. J Clin Oncol. (2023) 41:790–802. doi: 10.1200/JCO.21.02508
Summary
Keywords
head and neck cancer, health-related quality of life, immunotherapy, patient reported outcomes, pembrolizumab, chemoradiotherapy
Citation
Machiels J-P, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, Alves GV, Lima IPF, Hughes BGM, Pointreau Y, Aksoy S, Laban S, Greil R, Burian M, Hetnal M, Delord J-P, Mesia R, Taberna M, Waldron J, Simon C, Gregoire V, Harrington K, Black CM, Norquist JM, Wang A, Gumuscu B, Bidadi B and Siu LL (2025) Health-related quality of life outcomes from KEYNOTE-412: chemoradiotherapy with or without pembrolizumab in participants with head and neck squamous cell carcinoma. Front. Oncol. 15:1645509. doi: 10.3389/fonc.2025.1645509
Received
11 June 2025
Accepted
04 September 2025
Published
06 October 2025
Volume
15 - 2025
Edited by
Ashish V. Chintakuntlawar, Mayo Clinic, United States
Reviewed by
Chia-Jung Busch, University of Greifswald, Germany
Michihisa Kono, Dana–Farber Cancer Institute, United States
Updates
Copyright
© 2025 Machiels, Tao, Licitra, Burtness, Tahara, Rischin, Alves, Lima, Hughes, Pointreau, Aksoy, Laban, Greil, Burian, Hetnal, Delord, Mesia, Taberna, Waldron, Simon, Gregoire, Harrington, Black, Norquist, Wang, Gumuscu, Bidadi and Siu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J.-P. Machiels, jean-pascal.machiels@saintluc.uclouvain.be
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.