Abstract
Background:
The optimal adjuvant therapy for oral squamous cell carcinoma (SCC) patients achieving pathological complete response (pCR) after neoadjuvant immunochemotherapy (NAIC) remains uncertain. While radiotherapy (RT) and chemoradiotherapy (CRT) improve locoregional control, their comparative efficacy and toxicity profiles in this setting are poorly defined.
Methods:
Oral SCC patients with pCR post-NAIC were retrospectively enrolled and stratified into RT and CRT groups. Propensity score matching balanced baseline characteristics. Outcomes included 3-year locoregional control (LRC), overall survival (OS), and toxicity. Subgroup analyses evaluated treatment effects by radiologic extranodal extension (rENE) and tumor differentiation.
Results:
Among 116 patients analyzed (84 matched), CRT showed no significant LRC or OS benefit over RT alone in the overall cohort (LRC: HR 1.89, 95% CI 0.26–4.72, p=0.625; OS: HR 1.45, 95% CI 0.62–3.41, p=0.392). However, subgroup analyses revealed CRT improved outcomes in high-risk patients (rENE+ or poorly differentiated tumors), reducing recurrence by 50% (rENE+: HR 3.12, 95% CI 1.13–8.60, p=0.028; poor differentiation: HR 3.45, 95% CI 1.23–9.68, p=0.019) and enhancing 3-year OS (rENE+: 62.4% vs. 50.1%, p=0.036; poorly differentiated: 68.3% vs 53.8%, HR 2.88, p=0.022). CRT was associated with significantly higher acute and chronic toxicities (Grade 3–5 mucositis: 36.0% vs. 12.1%).
Conclusion:
CRT should be reserved for high-risk pCR patients (rENE+ or poorly differentiated tumors), while RT alone suffices for low-risk cases. This risk-adapted approach optimizes outcomes while minimizing toxicity.
Introduction
Oral squamous cell carcinoma (SCC) is the most common malignant tumor of the head and neck, with over half of cases presenting as locally advanced at initial diagnosis (1). Despite treatment with complete surgical resection followed by adjuvant radiotherapy (RT) or chemoradiotherapy (CRT), a substantial proportion of patients experience treatment failure (2, 3). More effective therapeutic strategies are urgently needed.
Although traditional platinum-based neoadjuvant chemotherapy regimens have not demonstrated significant survival benefits in oral SCC (4), they are associated with a nearly 50% increase in mandibular preservation rates (5). Advances in understanding immune checkpoint pathways have established immunotherapy as a superior alternative to conventional radiotherapy and chemotherapy, particularly for improving overall survival in recurrent or metastatic head and neck SCC (6, 7). The addition of immunotherapy to neoadjuvant regimens has sparked strong interest. Clinical trials show that neoadjuvant immunotherapy—with or without chemotherapy—can deliver impressive results. The objective response rate exceeds 95%. Pathological complete response (pCR) rates reach ≥30%. Major pathological response rates are around 70% (8, 9). In those achieving a pCR, adverse pathologic features typically requiring RT or CRT are no longer present, creating a dilemma in adjuvant therapy selection. Although both RT and CRT improve cancer control, their associated toxicities are substantial—approximately 50% of patients receiving RT experience grade 3/4 adverse events, and this rate rises to nearly 80% or higher with CRT (10).
Unlike other cancers (e.g., breast or rectal cancer), where pCR often permits treatment de-escalation (11, 12), oral SCC remains contentious due to its aggressive biology and high locoregional recurrence risk. While pCR may indicate favorable tumor biology, the absence of reliable biomarkers to identify patients who can safely avoid CRT complicates decision-making. Additionally, the historical precedent of CRT for high-risk features has led to cautious adoption of de-escalation, despite its potential to reduce toxicity.
Given the substantial toxicity of CRT and the uncertain benefit of chemotherapy in pCR patients, we hypothesized that CRT offers no survival advantage over RT alone in unselected pCR patients after NAIC. To test this, we compared oncologic outcomes between RT and CRT, with subgroup analyses to identify high-risk patients who may still benefit from intensified therapy.
Patients and methods
Ethical approval
This study was approved by Henan Cancer Hospital Institutional Research Committee, and written informed consent for medical research was obtained from all patients before starting the treatment. All methods were performed in accordance with the relevant guidelines and regulations.
Study design
We conducted a retrospective review of medical records from patients with primary oral SCC who received NAIC between July 2019 and December 2024. Eligible patients met the following criteria: completion of curative-intent surgery with confirmed pCR; no prior history of malignancy; complete clinical, pathological, and treatment documentation. Patients with treatment interruptions due to pandemic-related disruptions that could not be resolved per protocol were excluded. Despite these challenges, all included patients received complete NAIC and adjuvant therapy courses. We collected comprehensive data on demographics, pathological characteristics, treatment details, and follow-up outcomes, with particular attention to documenting any pandemic-related modifications to standard care pathways. During the study, COVID-19 prompted adjustments to treatment schedules and follow-up.
Variable definition
All patients were clinically staged according to the 8th edition of the AJCC staging system, incorporating findings from physical examination and imaging studies. Histologic differentiation was categorized as well, moderate, or poor. Smoking history was defined as consumption of at least 100 lifetime cigarettes or current daily use. Alcohol use was defined as regular intake of ≥1 standard drink per day (≥14 grams of pure alcohol) for ≥1 year. pCR was defined as the absence of viable tumor cells in both the primary tumor site and regional lymph nodes upon histopathologic evaluation (13). Radiologic extranodal extension (rENE) referred to radiographic evidence of tumor spread beyond the lymph node capsule into surrounding tissues, as identified on imaging (CT, MRI, or PET-CT) (14). The Combined Positive Score (CPS) measured the proportion of PD-L1-positive cells (tumor and immune cells) relative to viable tumor cells, with PD-L1 positivity defined as CPS ≥10 for therapeutic relevance in head and neck SCC.
The primary outcome was 3-year locoregional control (LRC), measured from the date of surgery to the first locoregional recurrence or last follow-up. Secondary outcomes included 3-year overall survival (OS), assessed from surgery to death or last follow-up, as well as acute and chronic toxicity related to adjuvant therapy. Acute adverse events (AEs) were defined as those occurring during or within 90 days of RT or CRT, while chronic AEs were those arising >90 days post-treatment. All toxicities were graded using the Common Terminology Criteria for Adverse Events (CTCAE v5.0) (15).
Adjuvant treatment principle
No established guidelines existed for adjuvant therapy in oral SCC patients who achieved a pCR after NAIC. At our institution, adjuvant treatment decisions were made through multidisciplinary discussion, incorporating factors such as the patient’s performance status, pre-NAIC imaging findings, and other clinical considerations. The radiation field encompassed the primary tumor site and unilateral or bilateral neck lymph nodes, delivering a total dose of 60 Gy. Adjuvant chemotherapy, when indicated, typically consisted of cisplatin-based regimens administered over 4–6 cycles.
Statistic analysis
Patients were stratified into two groups according to adjuvant therapy: RT or CRT. Clinicopathologic characteristics were compared between the cohorts using the Chi-square test, and variables with significant differences (p<0.05) were incorporated into propensity score matching (PSM) to minimize confounding. The effects of RT versus CRT on LRC and OS were assessed using univariate and multivariable Cox regression analyses in both the overall population and the PSM-matched cohort. All statistical analyses were conducted using R software (version 3.4.4), with a two-sided p-value <0.05 considered statistically significant.
Results
Baseline data
The study population’s baseline characteristics are detailed in Table 1 (overall cohort, n=116) and Table 2 (PSM cohort, n=84). Initial analysis of the overall population revealed significant imbalances between the RT (n=66) and CRT (n=50) groups in clinical stage (36.4% vs. 52.0% stage IV, p=0.020) and radiologic extranodal extension (rENE; 18.2% vs. 40.0%, p=0.008), with the CRT group containing more advanced-stage and rENE-positive cases. All other variables including age, sex, smoking, drinking, differentiation, CPS, and level IV/V metastatis showed balanced distribution (all p>0.05).
Table 1
| Variable | Total (n=116) | RT (n=66) | CRT (n=50) | P* |
|---|---|---|---|---|
| Age | ||||
| <55 | 66 | 36 | 30 | |
| ≥55 | 50 | 30 | 20 | 0.486 |
| Sex | ||||
| Male | 71 | 39 | 32 | |
| Female | 45 | 27 | 18 | 0.624 |
| Smoker | ||||
| No | 39 | 24 | 15 | |
| Yes | 77 | 42 | 35 | 0.415 |
| Drinker | ||||
| No | 64 | 39 | 25 | |
| Yes | 52 | 27 | 25 | 0.294 |
| Clinical stage | ||||
| III | 69 | 45 | 24 | |
| IV | 47 | 21 | 26 | 0.020 |
| Differentiation | ||||
| Well | 36 | 20 | 16 | |
| Moderate | 47 | 27 | 20 | |
| Poor | 33 | 19 | 14 | 0.916 |
| rENE& | ||||
| No | 84 | 54 | 30 | |
| Yes | 32 | 12 | 20 | 0.008 |
| CPS% | ||||
| <10 | 30 | 17 | 13 | |
| ≥10 | 86 | 49 | 37 | 0.885 |
| Level 4/5 metastasis | ||||
| No | 85 | 52 | 33 | |
| Yes | 31 | 14 | 17 | 0.151 |
Baseline data of the overall population.
* Comparison between RT and CRT groups using the Chi-square test;
& rENE: radiologic extranodal extension;
% CPS: Combined Positive Score.
Table 2
| Variable | Total (n=84) | RT (n=42) | CRT (n=42) | P* |
|---|---|---|---|---|
| Age | ||||
| <55 | 48 | 22 | 26 | |
| ≥55 | 36 | 20 | 16 | 0.414 |
| Sex | ||||
| Male | 56 | 29 | 27 | |
| Female | 28 | 13 | 15 | 0.655 |
| Smoker | ||||
| No | 28 | 16 | 12 | |
| Yes | 56 | 26 | 30 | 0.387 |
| Drinker | ||||
| No | 44 | 23 | 21 | |
| Yes | 40 | 19 | 21 | 0.682 |
| Clinical stage | ||||
| III | 48 | 24 | 24 | |
| IV | 36 | 18 | 18 | 1.000 |
| Differentiation | ||||
| Well | 27 | 13 | 14 | |
| Moderate | 35 | 19 | 16 | |
| Poor | 22 | 10 | 12 | 0.902 |
| rENE& | ||||
| No | 60 | 30 | 30 | |
| Yes | 24 | 12 | 12 | 1.000 |
| CPS% | ||||
| <10 | 18 | 8 | 10 | |
| ≥10 | 66 | 34 | 32 | 0.605 |
| Level 4/5 metastasis | ||||
| No | 57 | 28 | 29 | |
| Yes | 27 | 14 | 13 | 0.814 |
Baseline data of the PSM-matched population.
* Comparison between RT and CRT groups using the Chi-square test.
& rENE, radiologic extranodal extension.
% CPS, Combined Positive Score.
To address these baseline disparities, we performed 1:1 propensity score matching incorporating clinical stage and rENE status as key matching variables. The matched cohorts (RT n=42, CRT n=42) achieved excellent equilibrium across all parameters: clinical stage (p=1.000), rENE status (p=1.000), CPS distribution (p=0.605), and other baseline variables (all p>0.05). This rigorous matching approach effectively mitigated potential confounding effects, establishing a robust foundation for comparative analysis of treatment outcomes between RT and CRT groups in this pCR population.
Among 116 pCR patients followed for a median of 2.8 years, 17 (14.7%) experienced locoregional recurrence (LRR), with 80% of recurrences (14/17) occurring in rENE-positive or poorly differentiated tumors. 23 deaths (19.8%) were recorded, predominantly in rENE-positive (70%) subgroups.
LRC
The prognostic factors for locoregional control were systematically evaluated through univariate (Table 3) and multivariable analyses (Table 4) in both the overall and PSM cohorts. Univariate analysis identified clinical stage IV (overall: p<0.001; matched: p=0.009), poor differentiation (overall: p<0.001; matched: p=0.013), rENE (overall: p<0.001; matched: p=0.027), and level 4/5 metastasis (overall: p=0.008; matched: p=0.049) as significant predictors of worse locoregional control. While adjuvant CRT showed benefit in the overall cohort (p=0.035), this advantage was not maintained after matching (p=0.544). Multivariable analysis confirmed rENE as the strongest independent risk factor in both cohorts (overall: HR 3.99, 95%CI 2.02-9.65, p=0.004; matched: HR 5.12, 95%CI 2.22-12.78, p=0.009), with poor differentiation remaining significant (overall: HR 2.93, p=0.008; matched: HR 3.32, p=0.017). Notably, the protective effect of CRT diminished after adjustment (overall: HR 1.89, p=0.625; matched: HR 2.66, p=0.870), suggesting its apparent benefit in univariate analysis may have been confounded by baseline imbalances. These results underscore rENE and tumor differentiation as robust prognostic markers in our cohort, though the small subgroup sizes necessitate caution in interpretation. While hypothesis-generating, these findings highlight the need for validation in larger studies to confirm their predictive utility for treatment selection.
Table 3
| Variable | P (overall cohort) | P (matched cohort) |
|---|---|---|
| Age (≥55 vs <55) | 0.367 | 0.534 |
| Sex (Male vs female) | 0.448 | 0.679 |
| Smoker (Yes vs no) | 0.163 | 0.428 |
| Drinker (Yes vs no) | 0.209 | 0.499 |
| Clinical stage (IV vs III) | <0.001 | 0.009 |
| Differentiation (Poor vs moderate vs well) | <0.001 | 0.013 |
| rENE& (Yes vs no) | <0.001 | 0.027 |
| CPS% (≥10 vs <10) | 0.765 | 0.888 |
| Level 4/5 metastasis (Yes vs no) | 0.008 | 0.049 |
| Adjuvant therapy (CRT vs RT)^ | 0.035 | 0.544 |
Univariate analysis of predictors for locoregional control in overall and PSM-matched cohorts.
& rENE, radiologic extranodal extension.
% CPS: Combined Positive Score.
^ RT, radiotherapy; CRT, chemoradiotherapy.
Table 4
| Variable | P (overall cohort) | P (matched cohort) | ||
|---|---|---|---|---|
| HR [95%CI] | p | HR [95%CI] | p | |
| Clinical stage | ||||
| III | ref | ref | ||
| IV | 2.88 [0.65-5.89] | 0.333 | 3.13 [0.52-6.43] | 0.560 |
| Differentiation | ||||
| Well | ref | ref | ||
| Moderate | 1.87 [0.45-4.68] | 0.287 | 2.00 [0.31-5.32] | 0.342 |
| Poor | 2.93 [1.75-6.74] | 0.008 | 3.32 [1.88-9.37] | 0.017 |
| rENE& | ||||
| No | ref | ref | ||
| Yes | 3.99 [2.02-9.65] | 0.004 | 5.12 [2.22-12.78] | 0.009 |
| Level 4/5 metastasis | ||||
| No | ref | ref | ||
| Yes | 2.75 [0.43-6.35] | 0.378 | 2.89 [0.24-7.87] | 0.579 |
| Adjuvant therapy^ | ||||
| RT | ref | ref | ||
| CRT | 1.89 [0.26-4.72] | 0.625 | 2.66 [0.31-6.41] | 0.870 |
Multivariable analysis predictors for locoregional control in overall and PSM-matched cohorts.
& rENE, radiologic extranodal extension.
^ RT, radiotherapy; CRT, chemoradiotherapy.
OS
Prognostic predictors for OS were analyzed in both univariate (Table 5) and multivariable models (Table 6) for the overall and PSM cohorts. Univariate analysis identified poor differentiation (overall: p<0.001; matched: p=0.018), rENE (overall: p<0.001; matched: p=0.021), and level 4/5 metastasis (overall: p=0.005; matched: p=0.038) as significant adverse prognostic factors for OS, while adjuvant CRT showed a marginal benefit in the overall cohort (p=0.028) that dissipated after matching (p=0.291). Multivariable analysis confirmed rENE as the strongest independent predictor of worse OS in both cohorts (overall: HR 4.56, 95%CI 2.34–11.02, p<0.001; matched: HR 5.88, 95%CI 2.67–14.29, p=0.001), followed by poor differentiation (overall: HR 3.78, p<0.001; matched: HR 4.22, p=0.002). Notably, level 4/5 metastasis trended toward significance (p=0.076–0.083), while clinical stage and adjuvant therapy (CRT vs. RT) lost prognostic relevance after adjustment (p>0.1). These results suggest rENE and poor differentiation as key determinants of survival, with limited evidence supporting CRT’s impact on OS after confounding control. However, the exploratory nature of these subgroup analyses—given their limited sample size—warrants further investigation to define CRT’s role in high-risk pCR patients.
Table 5
| Variable | P (overall cohort) | P (matched cohort) |
|---|---|---|
| Age (≥55 vs <55) | 0.412 | 0.587 |
| Sex (Male vs female) | 0.325 | 0.498 |
| Smoker (Yes vs no) | 0.210 | 0.385 |
| Drinker (Yes vs no) | 0.176 | 0.452 |
| Clinical stage (IV vs III) | 0.124 | 0.234 |
| Differentiation (Poor vs moderate vs well) | <0.001 | 0.018 |
| rENE& (Yes vs no) | <0.001 | 0.021 |
| CPS% (≥10 vs <10) | 0.702 | 0.945 |
| Level 4/5 metastasis (Yes vs no) | 0.005 | 0.038 |
| Adjuvant therapy (CRT vs RT)^ | 0.028 | 0.291 |
Univariate analysis of predictors for overall survival in overall and PSM-matched cohorts.
& rENE, radiologic extranodal extension.
% CPS, Combined Positive Score.
^ RT, radiotherapy; CRT, chemoradiotherapy.
Table 6
| Variable | P (overall cohort) | P (matched cohort) | ||
|---|---|---|---|---|
| HR [95%CI] | p | HR [95%CI] | p | |
| Differentiation | ||||
| Well | ref | ref | ||
| Moderate | 1.95 [0.82-4.62] | 0.128 | 2.10 [0.75-5.91] | 0.156 |
| Poor | 3.78 [2.01-8.45] | <0.001 | 4.22 [2.15-10.33] | 0.002 |
| rENE& | ||||
| No | ref | |||
| Yes | 4.56 [2.34-11.02] | <0.001 | 5.88 [2.67-14.29] | 0.001 |
| Level 4/5 metastasis | ||||
| No | ref | |||
| Yes | 2.33 [0.91-6.11] | 0.076 | 2.67 [0.88-8.12] | 0.083 |
| Adjuvant therapy^ | ||||
| RT | ref | ref | ||
| CRT | 1.45 [0.62-3.41] | 0.392 | 1.89 [0.72-4.95] | 0.195 |
Multivariable analysis predictors for overall survival in overall and PSM-matched cohorts.
& rENE, radiologic extranodal extension.
^ RT, radiotherapy; CRT, chemoradiotherapy.
Toxicity
The comparison of toxicity profiles between RT and CRT groups revealed significant differences in both acute and chronic adverse events (Supplementary Table 1). For acute toxicities, CRT was associated with significantly higher rates of dermatitis (70.0% vs. 42.4% Grade 1/2; 24.0% vs. 7.6% Grade 3-5), mucositis (80.0% vs. 48.5% Grade 1/2; 36.0% vs. 12.1% Grade 3-5), and nausea/vomiting (56.0% vs. 22.7% Grade 1/2). Hematologic toxicities like neutropenia (24.0% vs. 6.1%) and anemia (50.0% vs. 27.3%) were also more common with CRT, though severe cases (Grade 3-5) were absent. Chronic toxicities followed a similar trend, with CRT showing elevated rates of xerostomia (60.0% vs. 33.3% Grade 1/2), fibrosis (50.0% vs. 22.7%), and dysphagia (40.0% vs. 18.2%). Severe chronic effects were rare, except for one case of osteoradionecrosis (2.0%) in the CRT group.
The median RT dose was 60 Gy, delivered as prescribed in 95.5% of RT-treated patients and 94.0% of CRT-treated patients. Treatment delays (>6 weeks post-surgery) occurred in 10.6% (RT) and 12.0% (CRT) of cases. In the CRT arm, 90.0% of patients completed at least four chemotherapy cycles, while 10.0% required dose reductions.
Subgroup analysis
In this propensity score-matched cohort of 84 oral SCC patients achieving pCR, CRT did not provided additional survival benefit of either LRC or OS than RT (Figure 1). To better clarify this question, we evaluated the differential impact of the two procedures, stratified by high-risk features (rENE and poor differentiation). For LRC, CRT demonstrated significant benefit in high-risk subgroups, reducing recurrence rates by approximately 50% compared to RT in both rENE+ patients (16.7% vs 33.3%, HR 3.12, p=0.028) and poorly differentiated tumors (16.7% vs 38.5%, HR 3.45, p=0.019), with no significant advantage in low-risk subgroups (p>0.05). Similarly, OS improvements with CRT were confined to high-risk patients, with rENE+ (3-year OS: 62.4% vs 50.1%, HR 2.45, p=0.036) and poorly differentiated subgroups (68.3% vs 53.8%, HR 2.88, p=0.022) showing clinically meaningful gains. While these results propose a tailored approach—prioritizing CRT for high-risk pCR patients (rENE+ or poor differentiation) and RT alone for low-risk cases—the small subgroups underscore the need for prospective validation. These findings should be considered hypothesis-generating for future de-escalation trials.
Figure 1
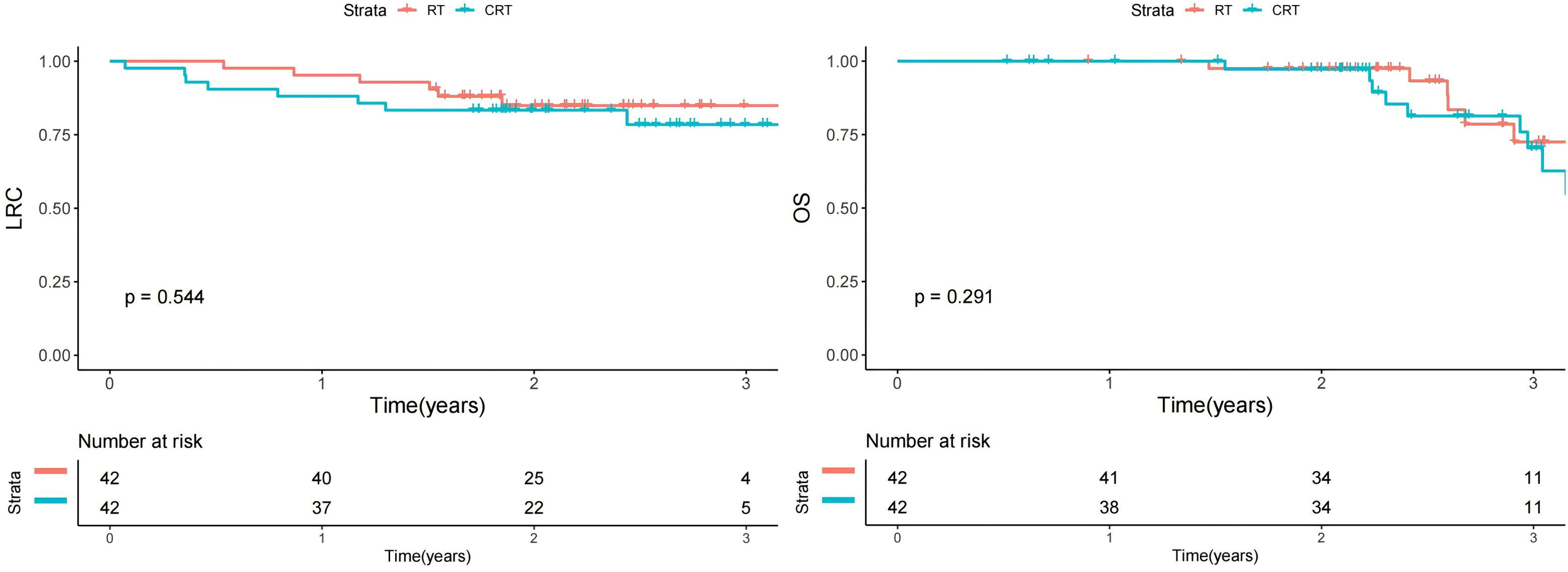
Impact of RT vs CRT on locoregional control (LRC) and overall survival (OS) in matched cohort.
Discussion
This study demonstrates that rENE and poor tumor differentiation are the strongest independent predictors of worse LRC and OS in oral SCC patients achieving pCR. While CRT initially appeared beneficial in the overall cohort, PSM analysis revealed that this advantage was primarily driven by baseline imbalances, with no significant survival benefit observed after adjustment. However, subgroup analysis identified a selective benefit of CRT in high-risk patients (rENE-positive or poorly differentiated tumors), reducing recurrence rates by approximately 50% and improving survival compared to RT. In contrast, low-risk patients (rENE-negative, well/moderately differentiated) derived no additional benefit from CRT over RT alone. Importantly, CRT was associated with significantly higher acute and chronic toxicities, including severe mucositis, dermatitis, dysphagia, xerostomia, and fibrosis. These findings underscore the importance of risk-stratified adjuvant therapy, where CRT should be prioritized for high-risk patients to maximize oncologic outcomes, while RT remains a safer and equally effective option for low-risk cases, minimizing unnecessary treatment-related morbidity. This tailored approach optimizes the balance between therapeutic efficacy and toxicity, guiding more precise clinical decision-making in pCR oral SCC management.
NAIC has emerged as a promising approach in head and neck SCC. A phase 2 trial (7) demonstrated impressive outcomes, with 30 enrolled patients showing an objective response rate of 96.7% (29/30). Among 27 patients who underwent surgery, the pCR rate reached 37.0%. With a median follow-up of 16.1 months, the 12-month disease-free survival rate was 95.8%, and no deaths occurred among pCR patients. Similarly, another study (8) reported that 17 of 27 operated patients (63.0%, 95% CI: 44.7-81.2) achieved major pathological response or pCR, with a pCR rate of 55.6%. After a median follow-up of 666 days, both 1-year overall and progression-free survival rates were 97.9%, with no adverse events observed in pCR patients. The Illuminate Trial (16) further supported these findings, demonstrating 100% completion rates for both NAIC and subsequent R0 resection in 20 patients, with major pathological response and pCR rates of 60% and 30%, respectively. At a median 23-month follow-up, disease-free and overall survival rates were 90% and 95%, with all pCR patients remaining disease-free. These consistent findings suggest that pCR may serve as a prognostic marker for favorable outcomes, regardless of whether adjuvant therapy consisted of observation, RT, or CRT. However, these studies share important limitations: small sample sizes, lack of rationale for adjuvant therapy selection, and crucially, no comparative analysis of how different adjuvant approaches impact prognosis. These knowledge gaps highlight the need for more studies to optimize post-NAIC treatment strategies in oral SCC.
While the role of adjuvant therapy following pCR achievement in oral SCC has not been systematically evaluated, valuable insights may be drawn from management approaches for other solid tumors where treatment de-escalation strategies have been successfully implemented. In breast cancer, achieving pCR following neoadjuvant therapy does not always eliminate the need for adjuvant treatment. For HER2-positive disease, HER2-targeted therapy is typically continued, while triple-negative breast cancer may still warrant adjuvant pembrolizum11ab if used neoadjuvantly. Hormone receptor-positive breast cancer, though less likely to achieve pCR, still requires standard adjuvant endocrine therapy (17). In esophageal/gastric cancer (18), observation is generally recommended after pCR, though high-risk features (e.g., residual nodal disease) may justify adjuvant immunotherapy or chemotherapy. For rectal cancer (19), a “watch-and-wait” approach is increasingly adopted to avoid surgery, and if pCR is confirmed post-resection, no further adjuvant therapy is needed. In non-small cell lung cancer (NSCLC) (20), adjuvant immunotherapy may be considered after pCR, particularly in high-risk cases, though optimal strategies are still under investigation. Bladder cancer patients with pCR after neoadjuvant chemotherapy and cystectomy typically require no further treatment, but immunotherapy may be considered in select high-risk cases (21). Similarly, soft tissue sarcoma patients with pCR after neoadjuvant therapy are usually observed unless high-risk features persist (22). Across malignancies, treatment de-escalation is increasingly favored when pCR is achieved, while some cancers allow for observation or de-escalation after pCR, oral SCC remains an exception due to its aggressive locoregional behavior, high recurrence risk, and lack of reliable biomarkers to identify low-risk patients. Adjuvant RT or CRT is still the standard unless future research identifies a subset of oral SCC patients who can safely avoid it after pCR.
In clinics, adjuvant CRT has been shown to provide a significant survival advantage over RT alone in head and neck SCC patients with ENE or positive surgical margins, as demonstrated by key clinical trials and meta-analyses. The pooled analysis of the EORTC 22931 and RTOG 9501 trials (23, 24) established that patients with ENE or positive margins derive the greatest benefit from CRT, with a 5-year OS improvement from 36% to 47% for ENE-positive cases (HR 0.72, p=0.04) and from 34% to 49% for margin-positive disease (HR 0.61, p=0.01). Subsequent meta-analyses, including a 2020 JAMA Oncology study (25), reinforced these findings, showing a ~30% reduction in mortality with CRT compared to RT alone in high-risk patients. Based on this evidence, current NCCN guidelines (26) strongly recommend adjuvant CRT (Category 1 evidence) for ENE or positive margins, with cisplatin remaining the standard systemic therapy. Debate continues over optimal regimens for HPV-associated oropharyngeal cancer or cisplatin-ineligible patients. However, the survival benefit of CRT in ENE and margin-positive head and neck SCC is well-supported. Prospective and pooled retrospective data confirm this (27).
Our study reveals important challenges in adjuvant therapy decision-making for pCR patients, as most adverse pathologic features (except histologic differentiation) were unavailable, though all patients achieved negative margins. This created significant clinical uncertainty, particularly given the substantial toxicity burden observed in both treatment groups, with CRT demonstrating more severe complications - consistent with prior reports (28). These findings underscore the critical need to identify which pCR patients truly benefit from intensified adjuvant therapy. As the first study to systematically address this question, we found that while CRT showed no overall advantage over RT alone in the matched population, it significantly reduced treatment failure and mortality risks in specific high-risk subgroups (rENE-positive or poorly differentiated tumors). This differential benefit suggests fundamental biological heterogeneity within pCR populations that conventional pathologic assessment fails to capture. The superior outcomes with CRT in these high-risk subgroups likely reflect chemotherapy’s ability to target residual micrometastatic disease that persists despite pathologic complete response, particularly in tumors with aggressive baseline features. The treatment benefit may be amplified by immunochemotherapy-induced tumor microenvironment priming, enhancing chemotherapy sensitivity. Importantly, rENE appears to identify patients with persistent aggressive biology, while poor differentiation marks intrinsically resistant phenotypes requiring multimodal therapy. These findings fundamentally challenge the conventional view of pCR as a uniform prognostic marker and instead advocate for a biologically-graded approach to pCR classification that incorporates radiographic and histologic risk features.
These results carry substantial clinical implications for personalizing adjuvant therapy in oral SCC patients achieving pCR after NAIC. They advocate for a risk-adapted approach where adjuvant treatment intensity is tailored based on residual risk features, moving beyond the current one-size-fits-all paradigm. For low-risk pCR patients (lacking rENE and with well/moderate differentiation), de-escalation to radiotherapy alone could reduce treatment-related morbidity without compromising outcomes, significantly improving quality of life. Conversely, high-risk pCR patients (with rENE or poor differentiation) should continue to receive standard CRT, as our data demonstrate clear survival benefits in this subgroup. This stratification approach parallels successful response-adaptive strategies in other malignancies, such as trastuzumab escalation in residual HER2+ breast cancer (29) or de-escalation in HPV+ oropharyngeal cancer. Future studies should validate these findings prospectively and explore integrating molecular biomarkers (e.g., ctDNA, immune profiling) with traditional risk factors to further refine patient selection. Additionally, research should investigate whether modified adjuvant approaches (e.g., immunotherapy maintenance instead of concurrent chemotherapy) could maintain efficacy while reducing toxicity in high-risk pCR patients. These findings ultimately support the development of more precise, biology-driven adjuvant strategies in the era of NAIC for head and neck cancers.
The management of advanced oral SCC presents a dual challenge: achieving oncologic control while restoring form and function through complex reconstructions. As highlighted in the scoping review by Cîrstea et al. (30), patients undergoing extensive resections often require multiple flap reconstructions—such as radial forearm, anterolateral thigh, or scapular tip free flaps—to address large defects and preserve critical functions like speech, swallowing, and cosmetics. These procedures, while achieving success rates of >95%, are fraught with complications like flap necrosis, donor-site morbidity, and prolonged recovery, underscoring the need for meticulous surgical planning and multidisciplinary collaboration. The integration of emerging techniques offers promise, yet the decision to de-escalate adjuvant therapy must account for reconstructive viability, particularly in high-risk cases with radiologic extranodal extension or poor differentiation. By bridging oncologic and reconstructive paradigms, this perspective emphasizes that optimal outcomes hinge not only on tumor biology but also on restoring quality of life, advocating for tailored strategies that balance oncologic rigor with functional rehabilitation.
Our findings may have implications beyond oral SCC, particularly for other carcinomas where treatment de-escalation is actively investigated. For example, in nasopharyngeal carcinoma (NPC), recent studies have explored reducing radiotherapy intensity or omitting chemotherapy for low-risk patients, especially those with EBV-associated early-stage disease or favorable response to induction therapy (31). Similar to our study’s risk stratification using rENE and tumor differentiation, NPC trials increasingly incorporate biomarkers to identify candidates for de-escalation (32). While direct extrapolation requires caution due to biological differences, our results underscore the importance of personalized, response-adapted strategies across head and neck malignancies. Future studies should validate whether analogous risk criteria could guide adjuvant therapy de-escalation in NPC and other carcinomas.
Limitations of this study should be acknowledged. First, as a retrospective analysis, the findings are subject to inherent selection bias, and unmeasured confounding factors may have influenced the results. Second, the relatively small sample size may have limited statistical power, particularly in subgroup analyses, potentially obscuring meaningful differences in outcomes. Third, since this was a single-institution study, the generalizability of our findings remains uncertain, and external validation in multicenter cohorts is necessary before clinical application. Future prospective studies with larger, diverse populations are needed to confirm these observations.
In conclusion, while adjuvant CRT offers no OS benefit over RT alone in unselected pCR patients, it significantly improves outcomes for high-risk subgroups with rENE or poor differentiation. A risk-stratified approach is advocated: CRT should be reserved for high-risk patients (rENE+ or poorly differentiated), while RT alone suffices for low-risk cases. This strategy optimizes therapeutic efficacy while minimizing unnecessary toxicity.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by Henan Cancer Hospital Institutional Research Committee, and written informed consent for medical research was obtained from all patients before starting the treatment. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Writing – original draft, Writing – review & editing, Data curation, Supervision, Conceptualization, Formal analysis, Project administration, Validation, Investigation, Resources, Visualization, Software. YL: Writing – original draft, Writing – review & editing. MY: Writing – original draft, Writing – review & editing. YF: Writing – original draft, Writing – review & editing. WD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1647606/full#supplementary-material
Abbreviations
AE, adverse event; CPS, combined positive score; CRT, chemoradiotherapy; HR, hazard ratio; LRC, locoregional control; LRR, locoregional recurrence; NAIC, neoadjuvant immunochemotherapy; NPC, nasopharyngeal carcinoma; OS, overall survival; pCR, pathologic complete response; PSM, propensity score matching; rENE, radiologic extranodal extension; RT, radiotherapy; SCC, squamous cell carcinoma.
References
1
Wei LY Li ZZ Xu ZY Wang GR Xiao Y Liu B et al . The ending is not the end: Lymph node metastasis in oral squamous cell carcinoma. Int Immunopharmacol. (2025) 146:113917. doi: 10.1016/j.intimp.2024.113917
2
Rao KN Sreeram MP de Bree R Mendenhall WM Strojan P Stenman G et al . The oncological outcome of postoperative radiotherapy in patients with node-negative early-stage (T1/T2/N0) oral squamous cell carcinoma and perineural invasion: A meta-analysis. Cancers (Basel). (2025) 17:862. doi: 10.3390/cancers17050862
3
Henick BS Taylor AM Nakagawa H Wong KK Diehl JA Rustgi AK . Squamous cell cancers of the aero-upper digestive tract: A unified perspective on biology, genetics, and therapy. Cancer Cell. (2025) 43:178–94. doi: 10.1016/j.ccell.2025.01.003
4
Zhong LP Zhang CP Ren GX Guo W William WN Jr Sun J et al . Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. (2013) 31:744–51. doi: 10.1200/JCO.2012.43.8820
5
Chaukar D Prabash K Rane P Patil VM Thiagarajan S Ghosh-Laskar S et al . Prospective phase II open-label randomized controlled trial to compare mandibular preservation in upfront surgery with neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. J Clin Oncol. (2022) 40:272–81. doi: 10.1200/JCO.21.00179
6
Keam B Machiels JP Kim HR Licitra L Golusinski W Gregoire V et al . Pan-Asian adaptation of the EHNS-ESMO-ESTRO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with squamous cell carcinoma of the head and neck. ESMO Open. (2021) 6:100309. doi: 10.1016/j.esmoop.2021.100309
7
Zhang Z Wu B Peng G Xiao G Huang J Ding Q et al . Neoadjuvant chemoimmunotherapy for the treatment of locally advanced head and neck squamous cell carcinoma: A single-arm phase 2 clinical trial. Clin Cancer Res. (2022) 28:3268–76. doi: 10.1158/1078-0432.CCR-22-0666
8
Wu D Li Y Xu P Fang Q Cao F Lin H et al . Neoadjuvant chemo-immunotherapy with camrelizumab plus nab-paclitaxel and cisplatin in resectable locally advanced squamous cell carcinoma of the head and neck: a pilot phase II trial. Nat Commun. (2024) 15:2177. doi: 10.1038/s41467-024-46444-z
9
Specenier P . Immunotherapy for head and neck cancer: from recurrent/metastatic disease to (neo)adjuvant treatment in surgically resectable tumors. Curr Opin Otolaryngol Head Neck Surg. (2021) 29:168–77. doi: 10.1097/MOO.0000000000000700
10
Kang JJ Yu Y Chen L Zakeri K Gelblum DY McBride SM et al . Consensuses, controversies, and future directions in treatment deintensification for human papillomavirus-associated oropharyngeal cancer. CA Cancer J Clin. (2023) 73:164–97. doi: 10.3322/caac.21758
11
Hibon J Waissi W Pasquier D Racadot S . Breast cancer tumour board: Which patients are eligible for radiotherapy de-escalation or omission? Cancer Radiother. (2025) 29:104688. doi: 10.1016/j.canrad.2025.104688
12
Ballal DS Saklani AP . Evidence-based de-escalation of radiotherapy in locally advanced rectal cancer. J Surg Oncol. (2025) 131:983–7. doi: 10.1002/jso.28071
13
Li G Wang J Fang Q Dai L Du W . Oncologic outcomes following neoadjuvant immunochemotherapy in locally advanced oral squamous cell carcinoma. Front Immunol. (2025) 16:1571285. doi: 10.3389/fimmu.2025.1571285
14
Ai QYH King AD Yuan H Vardhanabhuti V Mo FKF Hung KF et al . Radiologic extranodal extension for nodal staging in nasopharyngeal carcinoma. Radiother Oncol. (2024) 191:110050. doi: 10.1016/j.radonc.2023.110050
15
Spampinato S Tanderup K Barcellini A Burchardt E Eminowicz G Šegedin B et al . Impact of the Common Terminology Criteria for Adverse Events (CTCAE) evolution on toxicity scoring in gynaecological radiotherapy. Radiother Oncol. (2025) 207:110881. doi: 10.1016/j.radonc.2025.110881
16
Huang Y Sun J Li J Zhu D Dong M Dou S et al . Neoadjuvant immunochemotherapy for locally advanced resectable oral squamous cell carcinoma: a prospective single-arm trial (Illuminate Trial). Int J Surg. (2023) 109:2220–7. doi: 10.1097/JS9.0000000000000489
17
Cantini L Trapani D Guidi L Boscolo Bielo L Scafetta R Koziej M et al . Neoadjuvant therapy in hormone Receptor-Positive/HER2-Negative breast cancer. Cancer Treat Rev. (2024) 123:102669. doi: 10.1016/j.ctrv.2023.102669
18
Gaber CE Sarker J Abdelaziz AI Okpara E Lee TA Klempner SJ et al . Pathologic complete response in patients with esophageal cancer receiving neoadjuvant chemotherapy or chemoradiation: A systematic review and meta-analysis. Cancer Med. (2024) 13:e7076. doi: 10.1002/cam4.7076
19
Baloyiannis I Perivoliotis K Vederaki S Koukoulis G Symeonidis D Tzovaras G . Current evidence regarding the role of adjuvant chemotherapy in rectal cancer patients with pathologic complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Int J Colorectal Dis. (2021) 36:1395–406. doi: 10.1007/s00384-021-03915-9
20
Dunne EG Fick CN Isbell JM Chaft JE Altorki N Park BJ et al . The emerging role of immunotherapy in resectable non-small cell lung cancer. Ann Thorac Surg. (2024) 118:119–29. doi: 10.1016/j.athoracsur.2024.01.024
21
Joffe BI Christin JR Le Coz C Pingle SR Wei AZ Runcie KD et al . Management of patients with muscle-invasive bladder cancer achieving A clinical complete response after neoadjuvant therapy: evidence and consideration for active surveillance. Curr Urol Rep. (2025) 26:36. doi: 10.1007/s11934-025-01264-6
22
Boulouta A Kyriazoglou A Kotsantis I Economopoulou P Anastasiou M Pantazopoulos A et al . Pathologic complete response in patients with localized soft tissue sarcoma treated with neoadjuvant therapy and its correlation with clinical outcomes: A systematic review. Cancer Treat Rev. (2024) 130:102820. doi: 10.1016/j.ctrv.2024.102820
23
Bernier J Domenge C Ozsahin M Matuszewska K Lefèbvre JL Greiner RH et al . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. (2004) 350:1945–52. doi: 10.1056/NEJMoa032641
24
Cooper JS Pajak TF Forastiere AA Jacobs J Campbell BH Saxman SB et al . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. (2004) 350:1937–44. doi: 10.1056/NEJMoa032646
25
Tao Y Auperin A Sire C Martin L Khoury C Maingon P et al . Improved outcome by adding concurrent chemotherapy to cetuximab and radiotherapy for locally advanced head and neck carcinomas: results of the GORTEC 2007-01 phase III randomized trial. J Clin Oncol. (2018), JCO2017762518. doi: 10.1200/JCO.2017.76.2518
26
Colevas AD Cmelak AJ Pfister DG Spencer S Adkins D Birkeland AC et al . NCCN guidelines® Insights: head and neck cancers, version 2.2025. J Natl Compr Canc Netw. (2025) 23:2–11.
27
Mehanna H Robinson M Hartley A Kong A Foran B Fulton-Lieuw T et al . Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. (2019) 393:51–60. doi: 10.1016/S0140-6736(18)32752-1
28
Porceddu SV Bressel M Poulsen MG Stoneley A Veness MJ Kenny LM et al . Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 trial. J Clin Oncol. (2018) 36:1275–83. doi: 10.1200/JCO.2017.77.0941
29
Geyer CE Jr Untch M Huang CS Mano MS Mamounas EP Wolmark N et al . Survival with trastuzumab emtansine in residual HER2-positive breast cancer. N Engl J Med. (2025) 392:249–57. doi: 10.1056/NEJMoa2406070
30
Cîrstea AI Berteșteanu ȘVG Vrînceanu D Dumitru M Bejenaru PL Simion-Antonie CB et al . Perspectives in using multiple flaps reconstructions for advanced head and neck tumors (Scoping review). Medicina (Kaunas). (2024) 60:1340. doi: 10.3390/medicina60081340
31
Anghel I Anghel AG Dumitru M Soreanu CC . Nasopharyngeal carcinoma – analysis of risk factors and immunological markers. Chirurgia (Bucur). (2012) 107:640–5.
32
Huang BXZ Zhang X Kang MP Chua MLK . Personalising nasopharyngeal cancer: systemic therapy and radiotherapy treatment volumes. Semin Radiat Oncol. (2025) 35:173–89. doi: 10.1016/j.semradonc.2025.01.003
Summary
Keywords
chemoradiotherapy, locoregional control, oral squamous cell carcinoma, pathological complete response, radiotherapy
Citation
Yang Y, Liu Y, Yang M, Fan Y and Du W (2025) De-escalating adjuvant therapy after pathologic complete response in oral squamous cell carcinoma: Chemoradiotherapy benefits only high-risk subgroups. Front. Oncol. 15:1647606. doi: 10.3389/fonc.2025.1647606
Received
16 June 2025
Accepted
27 August 2025
Published
11 September 2025
Volume
15 - 2025
Edited by
Daniela Vrinceanu, Carol Davila University of Medicine and Pharmacy, Romania
Reviewed by
Crenguta Serboiu, Carol Davila University of Medicine and Pharmacy, Romania
Iulian-Alexandru Taciuc, Carol Davila University of Medicine and Pharmacy, Romania
Updates
Copyright
© 2025 Yang, Liu, Yang, Fan and Du.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Du, duweitj@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.