- 1Department of Pharmacy, the Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 3School of Pharmaceutical Science, Sun Yat-Sen University, Guangzhou, China
Background: Colorectal cancer (CRC) patients with cancer-associated venous thromboembolism (VTE) face high risks of recurrence and anticoagulant-related bleeding.
Objectives: Our aim was to assess risk factors associated with recurrence and bleeding and analyze the impact of these outcomes on survival during one-year follow up.
Design: Retrospective study.
Methods: This analysis included consecutive VTE patients treated with anticoagulants from January 2019 to January 2023. The incidence of recurrent VTE, major bleeding (MB), and clinically relevant non-major bleeding (CRNMB) was evaluated and their associated risk factors were identified using univariate and multivariate models. Furthermore, the impact of anticoagulant treatment outcomes on all-cause mortality was analyzed by Cox proportional hazards model and Kaplan-Meier method.
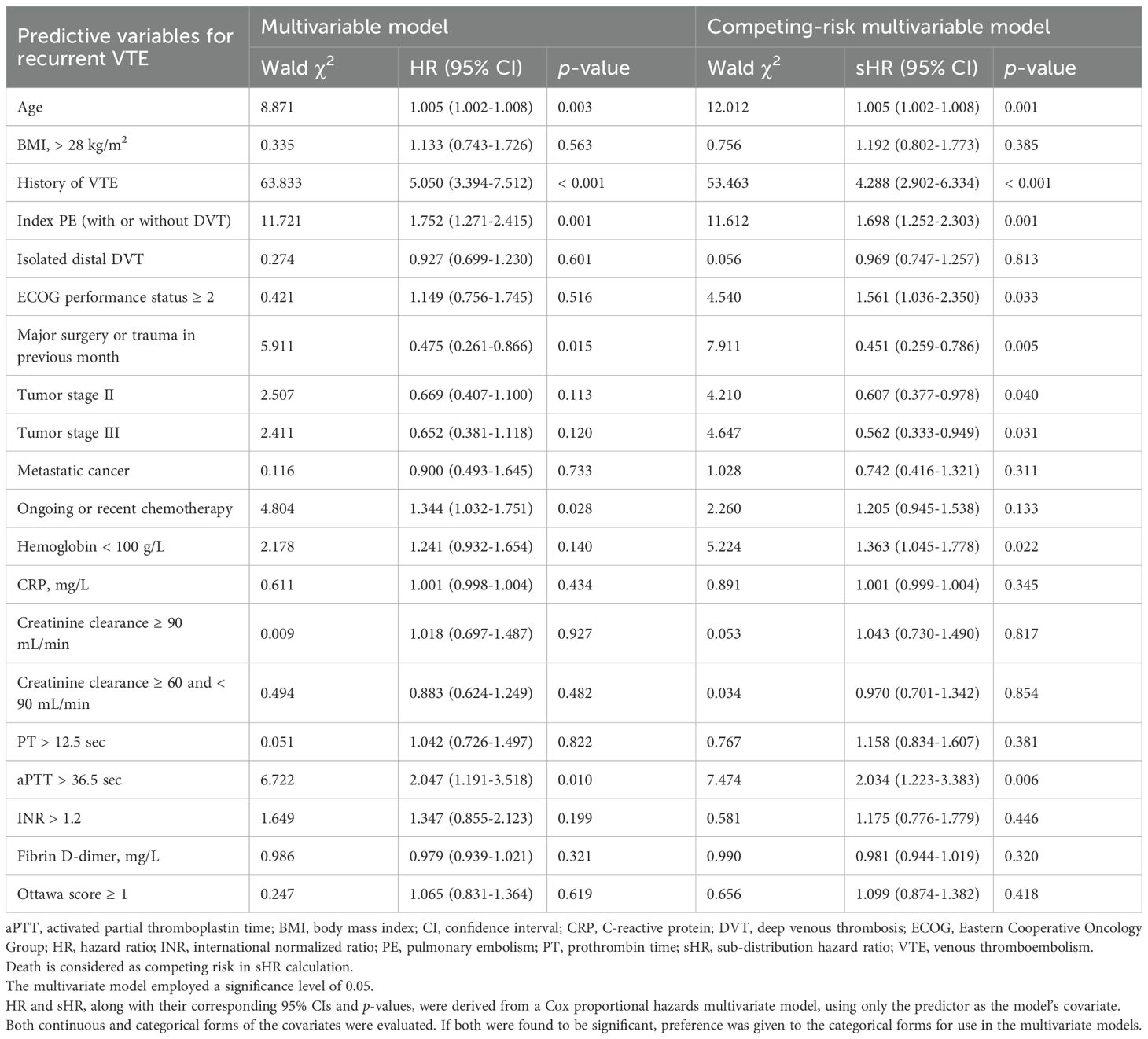
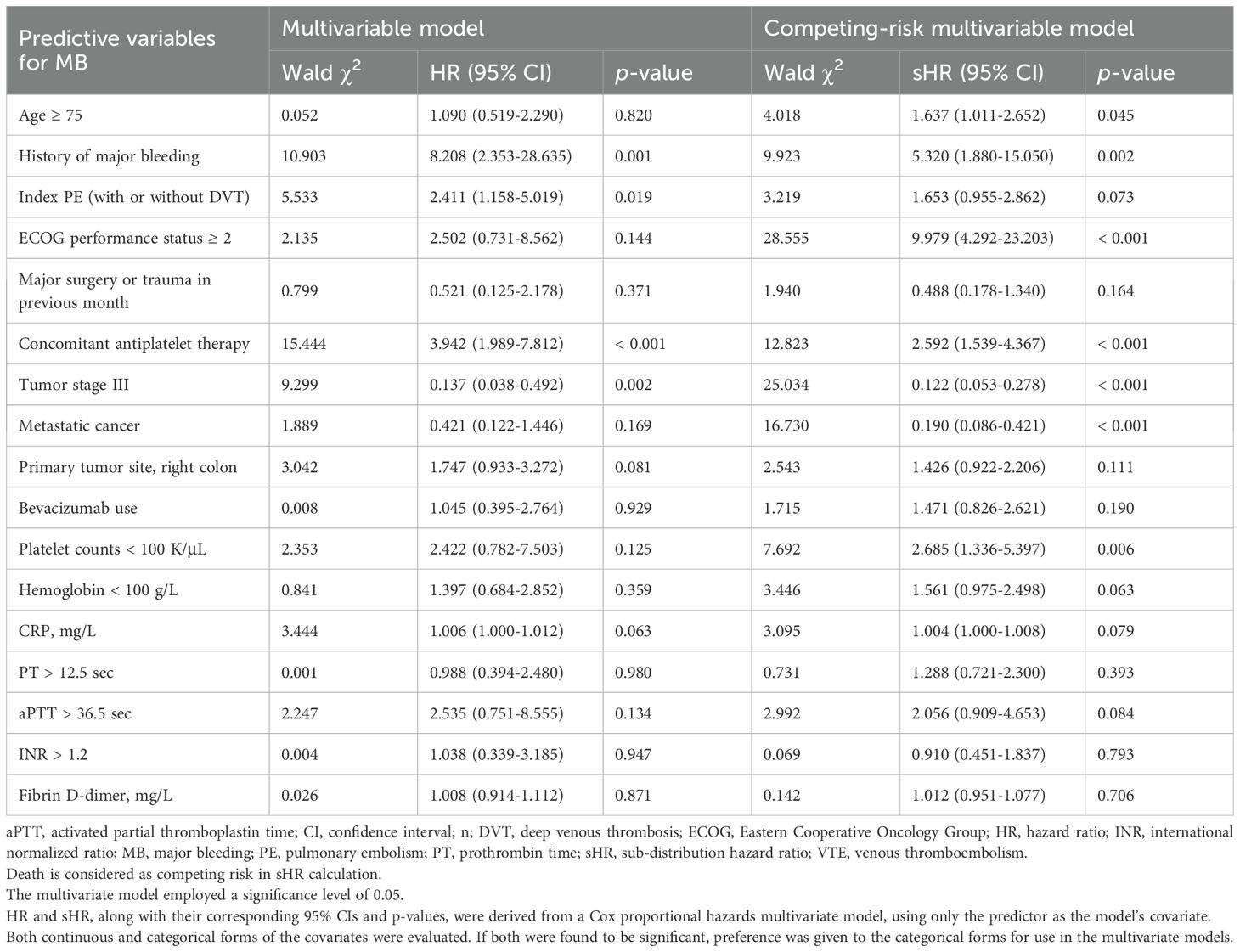
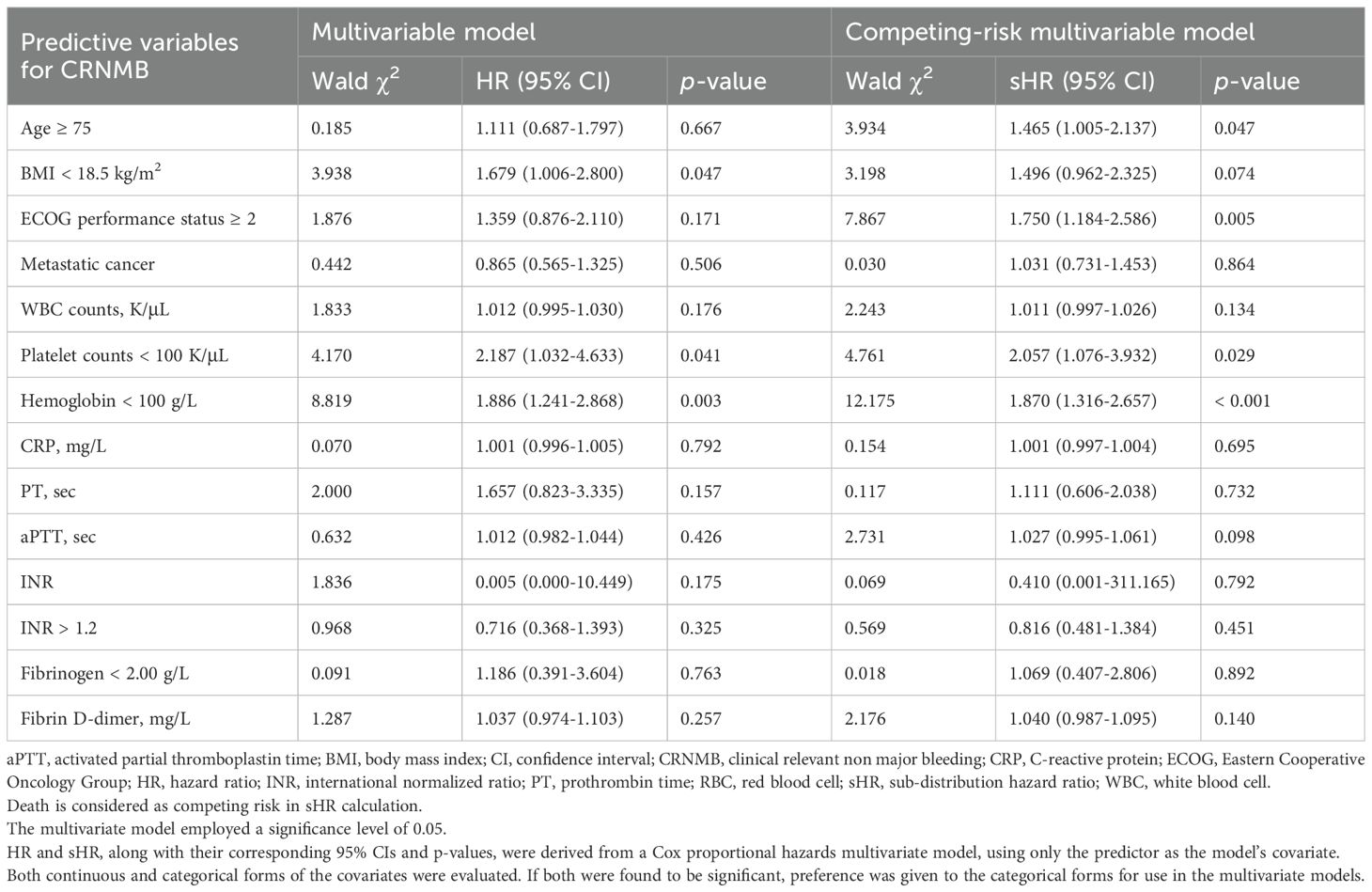
Results: This study included 1,792 CRC patients with cancer-associated VTE. In competing-risk multivariate analysis, independent predictors of recurrent VTE included age (HR with 95%CI: 1.005 [1.002-1.008] per year), history of VTE (4.288 [2.902-6.334]), index pulmonary embolism (PE) (1.698 [1.252-2.303]), ECOG ≥ 2 (1.561 [1.036-2.350]), hemoglobin < 100 g/L (1.363 [1.045-1.778]), and aPTT > 36.5 s (2.034 [1.223-3.383]); whereas recent major surgery or trauma within 1 month (0.451 [0.259-0.786]) and tumor stage II (0.607 [0.377-0.978]) or III (0.562 [0.333-0.949]) were associated with lower recurrence risk. Independent predictors of MB included age ≥ 75 (1.637 [1.011-2.652]), history of MB (5.320 [1.880-15.050]), ECOG ≥ 2 (9.979 [4.292-23.203]), antiplatelet therapy (2.592 [1.539-4.367]), and platelet count < 100×109/L (2.685 [1.336-5.397]); whereas tumor stage III (0.122 [0.053-0.278]) and metastatic cancer (0.190 [0.086-0.421]) predicted lower bleeding risk. Similarly, independent predictors of CRNMB included age ≥ 75 (1.465 [1.005-2.137]), ECOG ≥ 2 (1.750 [1.184-2.586]), hemoglobin < 100 g/L (1.870 [1.316-2.657]), and platelet count < 100×109/L (2.057 [1.076-3.932]). Recurrent VTE, MB, and CRNMB each adversely impacted one-year survival.
Conclusions: The independent risk factors identified in this study may serve as a reference for improving risk stratification in CRC patients receiving anticoagulant treatment. Additionally, adverse outcomes such as VTE recurrence, MB, and CRNMB significantly increase the one-year all-cause mortality risk in CRC patients.
Introduction
Cancer and venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), are closely linked by a bidirectional relationship (1). Cancer is responsible for approximately 18% of all VTE cases (2), and cancer-associated VTE accounts for about 20% of the total VTE disease burden (3). Managing VTE in cancer patients is particularly challenging due to the increased risk of thrombus recurrence and bleeding events associated with anticoagulant therapy (4).
The development of cancer-associated VTE involves a complex interplay of multiple overlapping mechanisms. The risk factors contributing to cancer-associated VTE can be classified into categories such as tumor-specific factors, patient-specific characteristics, treatment-related factors, the site of the index VTE, and laboratory findings. The combination of these risk factors increases the chances of recurrent thrombosis and bleeding events, both of which have detrimental effects on survival. It is well-documented that VTE negatively impacts the survival of cancer patients, with those developing PE experiencing a significantly higher mortality rate compared to those without PE (24.8% vs 6.5%, p-value < 0.0001) (5, 6). Prior research has also demonstrated that anticoagulant treatment outcomes, including VTE recurrence, major bleeding (MB), and clinically relevant non-major bleeding (CRNMB), can adversely affect patient prognosis (7).
Patients with colorectal cancer (CRC) face an elevated risk of both VTE and MB (8). A subsequent analysis of the Hokusai study indicated that the increased MB risk was particularly significant in patients with gastrointestinal cancers (9). Data from the RIETE registry revealed that patients with gastrointestinal or genitourinary cancers had higher incidences of bleeding, whereas those with brain or lung cancers were more prone to thrombotic events (10). A recent meta-analysis of four studies comparing direct oral anticoagulants (DOACs) with low molecular weight heparin (LMWH) reported a higher bleeding risk associated with DOACs, especially in patients with gastrointestinal cancers (11). However, a subgroup analysis of the Caravaggio trial showed that the incidence of major gastrointestinal bleeding in CRC patients was similar for those treated with apixaban and those treated with LMWH, regardless of cancer type (12). Despite concerns about the bleeding risk posed by DOACs in CRC patients (13), these anticoagulants, including apixaban and rivaroxaban, are commonly used in clinical practice (14, 15). Major trials examining the treatment of cancer-associated VTE with anticoagulants often underrepresent CRC patients. Balancing the risks of thrombosis recurrence and bleeding is challenging and necessitates a nuanced, individualized approach to optimize decision-making for anticoagulant therapy in this population.
To the best of our knowledge, clinical risk factors influencing anticoagulant outcomes in CRC patients have been sparsely systematically investigated in large cohorts. Data on the rates of recurrent thrombosis, MB, and CRNMB in this population remain scarce. Therefore, we conducted a comprehensive retrospective cohort study of CRC-associated VTE patients who received standard anticoagulant treatment according to local guidelines. Our study aimed to evaluate the incidence of recurrent VTE, MB, and CRNMB, and to identify their associated risk factors. Furthermore, we analyzed the impact of anticoagulant treatment outcomes on the all-cause mortality.
Materials and methods
Study design and patients
We conducted a single-center retrospective chart review of patients with histologically confirmed CRC and symptomatic or incidental VTE, who received anticoagulant treatment at The Sixth Affiliated Hospital, Sun Yat-sen University from January 2019 to January 2023 (Figure 1). Initially, a query was conducted on a prospectively maintained gastrointestinal cancer database to identify all patients with CRC and VTE. Subsequently, each patient’s electronic record was reviewed to determine whether they met the inclusion criteria. This study was registered (NCT06440044) and approved by the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University, with a waiver of informed consent granted because of the retrospective nature of the research (approval NO. 2024ZSLYEC-226). The study was conducted in accordance with the Helsinki Declaration (43).
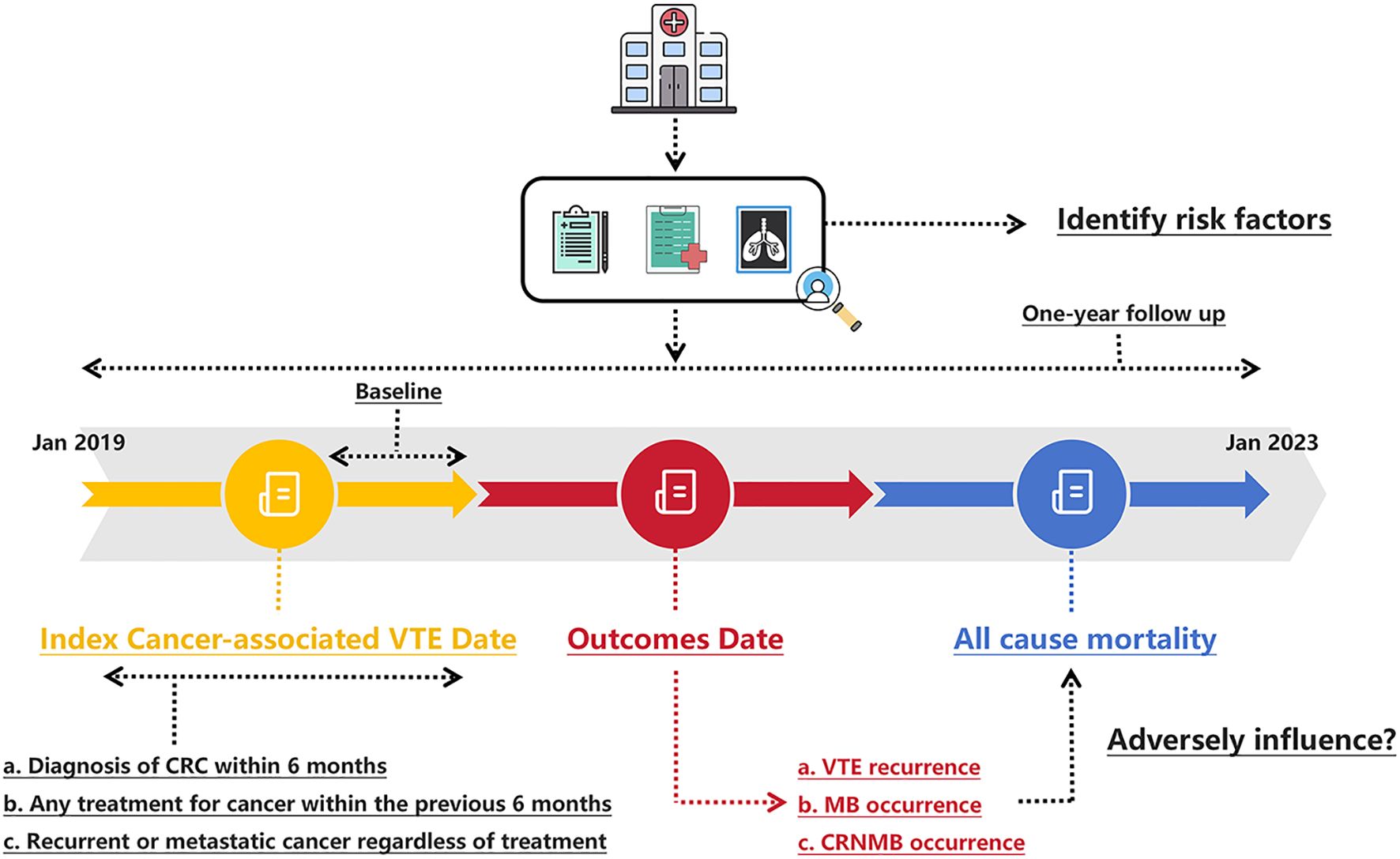
Figure 1. Schematic of the study design. CRC, colorectal cancer; CRNMB, clinical relevant non major bleeding; MB, major bleeding; VTE, venous thromboembolism.
Briefly, CRC patients with VTE who were treated with an anticoagulant (rivaroxaban or LMWH) for at least six months were identified. Patients diagnosed with PE and/or DVT using radiologic imaging techniques, such as CT or ultrasound, were classified as having VTE. There was no standardized protocol or prospective screening plan in place for detecting occult VTE. Both symptomatic individuals diagnosed based on imaging prompted by symptoms, and asymptomatic individuals identified through imaging performed for other medical purposes (such as cancer restaging), were included. VTE was considered cancer-related if the patient had a diagnosis of CRC within six months before or after the VTE diagnosis, any cancer treatment within the previous six months, or recurrent/metastatic cancer. Participation in this study required active anticoagulant treatment. Beyond this, no specific exclusion criteria were applied. Prescription adherence and persistence were verified by examining prescription refill records and provider notes. Cancer treatment at the time of anticoagulant initiation, tumor status, baseline renal function, hemoglobin concentration, global coagulation tests, D-dimer level, and results of other laboratory tests were assessed when available.
Primary and secondary outcomes
The primary outcome was objectively confirmed recurrent VTE, including DVT and PE. Recurrent DVT was verified using duplex ultrasonography, venography, CT, or MRI. Recurrent PE was confirmed through methods such as CT, MRI, conventional pulmonary angiography, or VQ (ventilation/perfusion) imaging. Incidental VTE recurrences were detected via surveillance imaging. To be classified as a recurrent event, a new filling defect had to be evident on the second study and not appreciated on the original images, or an interval study clearly showing thrombus resolution.
The safety outcome was bleeding events including MB and CRNMB. MB was characterized by overt bleeding accompanied by a hemoglobin drop of ≥ 2 g/dL, the need for transfusion of ≥ 2 units of packed red blood cells, or bleeding in critical areas such as intracranial, intraspinal, intraocular, pericardial, retroperitoneal, or intramuscular regions causing compartment syndrome, or fatal bleeding (16). The secondary safety outcome, CRNMB, was defined as overt bleeding that did not meet the criteria for MB yet required medical intervention, led to unscheduled healthcare visits, caused temporary discontinuation of treatment, or impaired daily activities (17). The objective of this analysis was to determine the risk factors associated with recurrence and bleeding in CRC patients with VTE. All-cause mortality included deaths from any cause, irrespective of the underlying mechanism.
Statistical analysis
Continuous variables were described as means and standard deviations, while discrete variables were summarized by frequencies and percentages. Univariate analysis was performed to assess the association between each potential predictor and the outcomes: recurrent VTE, MB, and CRNMB. Hazard ratios (HRs) along with their 95% confidence intervals (CIs) were presented. Variables with potential predictive value (univariate p-value ≤ 0.05) were further assessed using Cox proportional hazards multivariable analysis, considering recurrent VTE, MB, and CRNMB as the dependent variables. Variables excluded during the model selection process were not reintroduced. Due to the high expected mortality rate among the study patients, Fine and Gray risk-adjusted models were employed to evaluate recurrent VTE and bleeding, with death treated as a competing event. The results were expressed as sub-distribution hazard ratios (sHR) with corresponding 95% CIs and p-values.
Actual rates of outcomes, including VTE recurrence, MB, and CRNMB, were estimated using life-table methods. Predicted rates were visualized using cumulative incidence plots, with death considered as a competing event. One-year survival was estimated using the Kaplan-Meier method. The relationship between recurrence, bleeding, and mortality was assessed by incorporating these factors as time-dependent covariates in a Cox proportional hazards model, treating death as the outcome. Study data were collected and managed using Epidata 3.1 (EpiData for Windows; EpiData Association, Odense, Denmark). Data analysis was conducted using SPSS version 20.0 (IBM, Armonk, NY, USA) and the R statistical package (https://cran.r-project.org). All reported p-values in this study are two-tailed, with p-values < 0.05 considered statistically significant.
Results
Patient characteristics at baseline
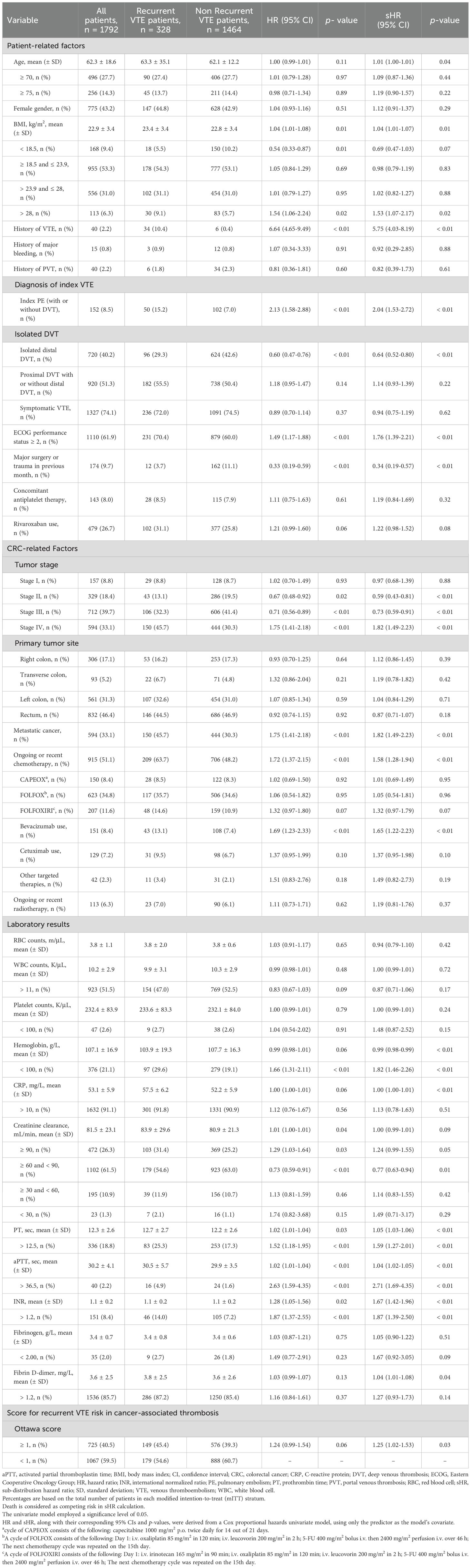
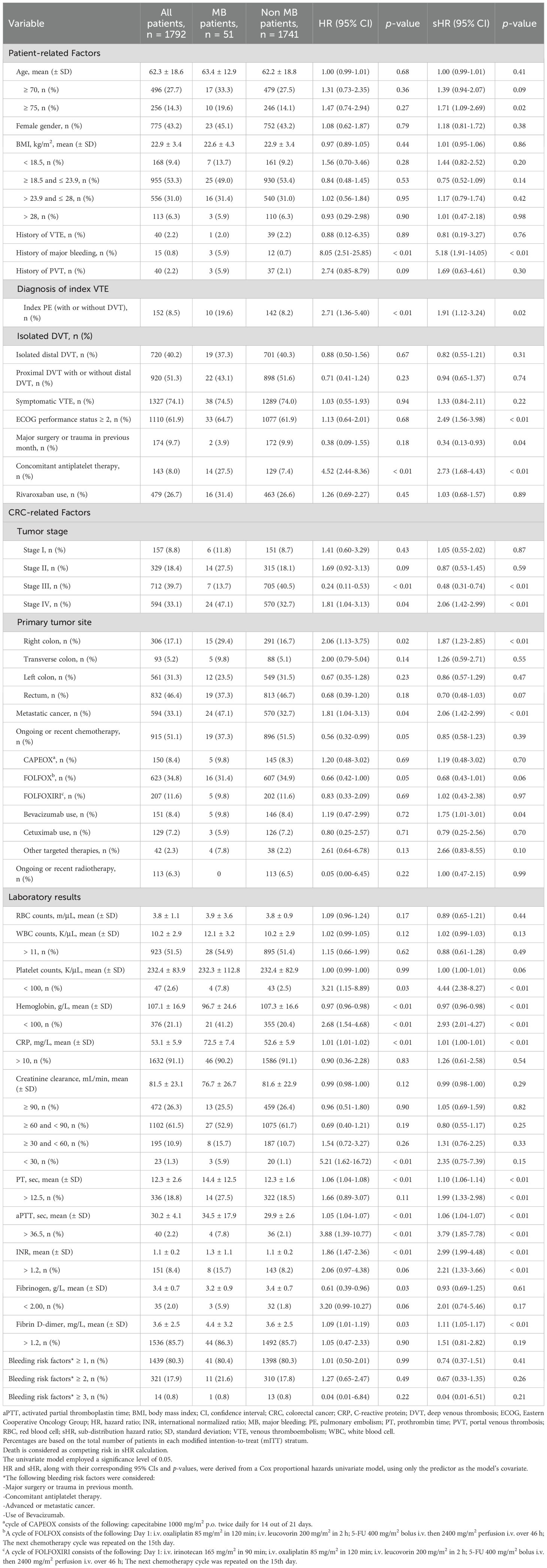
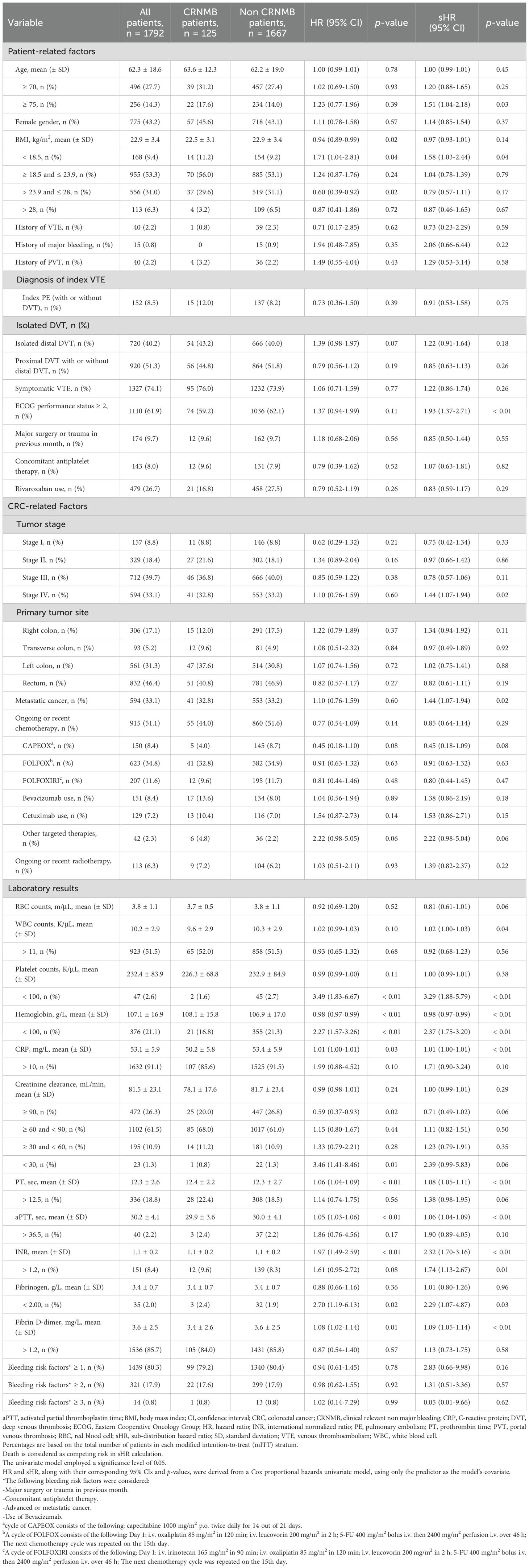
Over the study period, 1,792 CRC patients with VTE were enrolled (Supplementary Figure S1). Among these, 328 (18.3%) experienced recurrent VTE despite anticoagulant therapy, classifying them as anticoagulant failures. Additionally, 51 patients (2.8%) experienced MB associated with the use of anticoagulants, and 125 patients (6.9%) experienced CRNMB. The clinical demographics, including patient-related factors, cancer-related factors, and baseline laboratory results, were stratified based on VTE recurrence (Table 1), MB (Table 2), and CRNMB (Table 3) outcomes. There were no significant differences in these outcomes between patients treated with LMWH and those treated with rivaroxaban.
Predictors for recurrent VTE
In the competing-risk univariate analysis, 20 potential predictors of VTE recurrence were identified (Table 1). These variables were further evaluated in a multivariable analysis. Independent predictors of recurrence included age, history of VTE, index PE (with or without DVT), ECOG performance status ≥ 2, major surgery or trauma in the previous month, tumor stages II and III, hemoglobin < 100 g/L, and aPTT > 36.5 seconds (Table 4). The results of the univariate and multivariate models solely focusing on recurrence are also presented in Table 4. The proportions of thrombotic recurrences were stratified by different primary tumor sites, initial VTE events, and tumor stages, as shown in Supplementary Tables S1–S3. Predicted recurrence rates within these risk strata, accounting for death as a competing event, were estimated and visualized using cumulative incidence plots (Supplementary Figures S2A–D, S3A–C, S4A–D).
Predictors for bleeding outcomes
In the competing-risk univariate Cox model, 17 potential predictors of MB were identified (Table 2), while 14 potential predictors of CRNMB were identified (Table 3). These variables were then evaluated in a multivariable analysis. Independent predictors for MB included age ≥ 75, history of MB, ECOG performance status ≥ 2, concomitant antiplatelet therapy, tumor stage III, metastatic cancer, and platelet counts < 100 K/μL (Table 5). Among these, three predictors (age ≥ 75, ECOG performance status ≥ 2, and platelet counts < 100 K/μL) were common risk factors for CRNMB, with hemoglobin < 100 g/L identified as an additional predictor for CRNMB (Table 6). The results of univariate and multivariate models considering only bleeding outcomes are shown in Tables 2 and 3. The proportions of bleeding events stratified by primary tumor site, initial VTE events, and tumor stages are detailed in Supplementary Tables S1–S3. Predicted rates of bleeding outcomes within these risk strata, accounting for death as a competing event, were estimated and visualized using cumulative incidence plots (Supplementary Figures S2E–L, S3D–I, S4E–L).
Anticoagulant outcomes and mortality
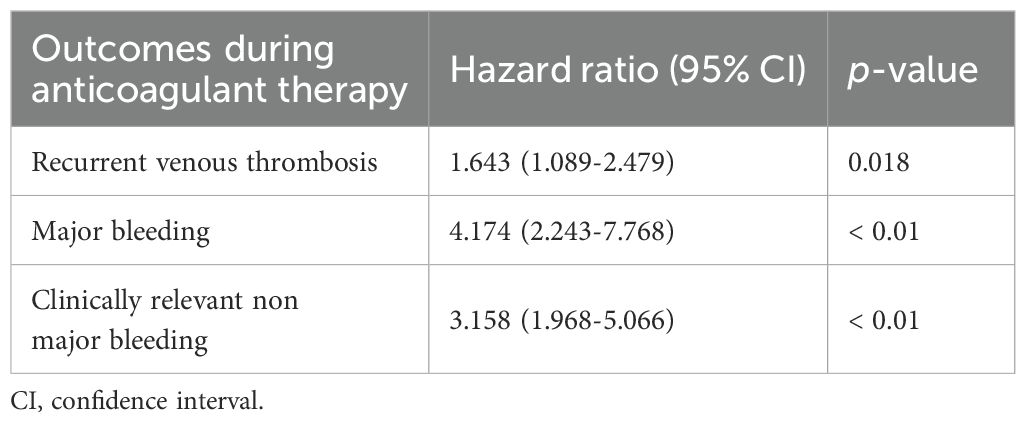
The influence of VTE recurrence and bleeding on mortality is shown in Table 7. In cancer patients undergoing anticoagulant therapy, recurrent VTE was associated with an over 60% increase in one-year mortality rates, with a HR of 1.643 (95% CI 1.089-2.479). Likewise, bleeding events significantly increased mortality, with HRs of 4.174 (95% CI 2.243-7.768) for MB and 3.158 (95% CI 1.968-5.066) for CRNMB. The results from the Kaplan-Meier survival curves are shown in Figure 2.
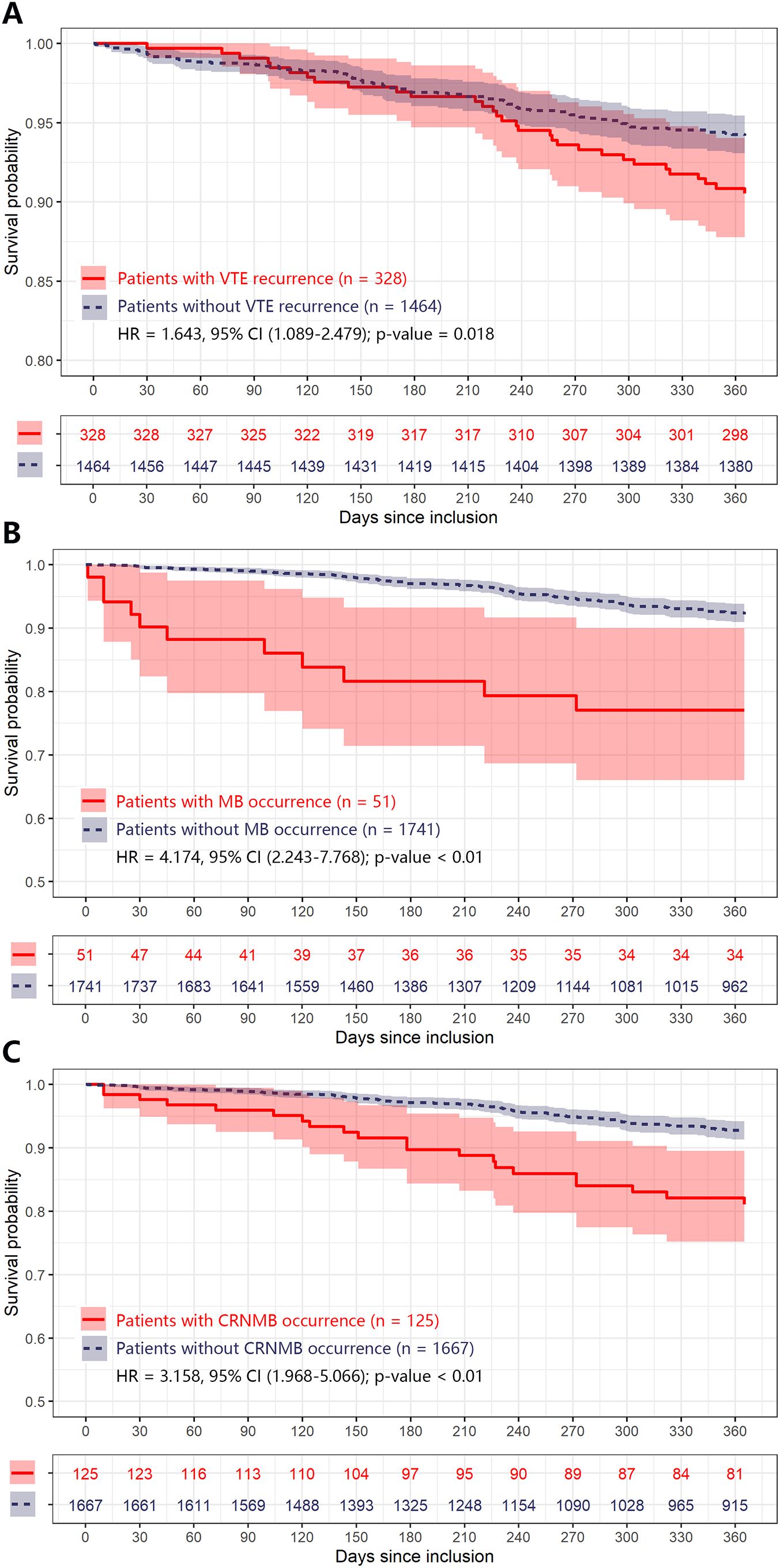
Figure 2. Kaplan-Meier plot of estimated one-year mortality according to different adverse anticoagulant outcomes in CRC patients. Any differences in the incidence were evaluated with a log-rank test. Plot (A–C) were grouped by patients with different adverse anticoagulant outcomes (including VTE recurrence, MB, and CRNMB) during one-year follow, respectively. CI, confidence interval; CRC, colorectal cancer; CRNMB, clinical relevant non major bleeding; HR, hazard ratio; MB, major bleeding; VTE, venous thromboembolism.
Discussion
This analysis of clinical and laboratory data in CRC patients with cancer-associated VTE, who received standardized anticoagulant treatment in a real-world setting, identified several independent predictors through competing-risk multivariate analysis. In this study, the incidence of VTE recurrence and total bleeding (including MB and CRNMB) was approximately 18% and 10%, respectively. A recent multicenter cohort study of cancer patients reported a cumulative VTE recurrence incidence of 11.4% at six months during anticoagulant treatment (18). Another prospective cohort study observed a high bleeding rate of 11.4% in cancer patients receiving anticoagulants (7). However, in rigorous clinical trials of anticoagulants, VTE recurrence rates ranged from 3.5% to 9.6%, and MB rates varied between 0.7% and 5.4% (13, 19–22). The higher recurrence and bleeding rates observed in this study may be attributed to the real-world nature of the cohort, including spontaneous discontinuation of anticoagulant therapy due to bleeding concerns and the lack of tolerability in CRC patients.
Our results showed that each of the outcomes (VTE recurrence, MB, and CRNMB) negatively impacts patient survival (Figure 2, Table 7). Such effects on VTE patients with various cancer types have been evaluated by other researchers. Lecumberri et al. used the RIETE registry to investigate mortality rates among VTE patients during anticoagulant treatment (23). The overall mortality rate for recurrent VTE patients was 12.1%, with recurrent PE patients having a higher rate (18.5%) compared to recurrent DVT patients (6.3%). The overall mortality rate for MB patients was 19.7%, rising to 24.4% in the subgroup of cancer patients, which is in line with our results (approximately 22%). However, a previous systematic review reported identical mortality rates of 11.3% for both recurrent VTE and MB patients within the first three months of anticoagulant therapy (24). More recently, a prospective cohort study indicated that episodes of VTE recurrence, MB, and CRNMB increased the overall mortality rate in cancer patients by more than 50%, 80%, and 30%, respectively (7). This finding is also consistent with our data. Given the negative impact of these adverse events on prognosis, it is crucial to identify cancer patients at the highest risk of such outcomes, especially those with cancer types like CRC that have high rates of VTE and bleeding.
A history of VTE and bleeding are well-established predictors of recurrence and bleeding in cancer-associated VTE patients (8–10). In our multivariate model, index PE was identified as a significant risk factor for VTE recurrence. However, similar tendencies were not observed in patients with index proximal DVT or isolated distal DVT. Previous studies have shown that residual pulmonary artery obstructions after anticoagulant treatment for PE or DVT can predict recurrent VTE (25–27). In the Vienna prediction model for non-cancer patients, index proximal DVT and index PE were identified as risk factors for VTE recurrence, with HRs of 2.08 (95% CI 1.16-3.74) and 2.60 (95% CI 1.49-4.53), respectively, compared to index distal DVT (28). A recent study including both cancer and non-cancer patients reported a similar HR direction for index PE (1.02, 95% CI 0.89-1.18), though it was not statistically significant (29).
Surgery is recognized as a significant but temporary risk factor for VTE and is typically associated with a low likelihood of recurrence (30). In our study, it was found that surgery was associated with a lack of recurrence even in the presence of active cancer. This finding aligns with a multicenter cohort study that found cancer patients who underwent surgery within the previous two months had a reduced risk of recurrence, with a HR of 0.60 (95% CI 0.40-0.92) (31). Another cohort study investigated surgery at the time of incident VTE as a predictor of recurrence but did not find statistically significant results (32). On the other hand, the association between an ECOG performance status of 1 or higher and an increased rate of recurrent VTE is clinically plausible, reflecting more advanced cancer disease.
In clinical practice, the potential benefits of anticoagulant therapy are typically compromised by the increased bleeding risk in cancer patients, particularly those with CRC. This bleeding risk is further exacerbated by malignancy-related conditions and treatments (33–35). To complicate matters, some predictors for recurrence are also correlated with an increased risk of bleeding (10, 36). Our analysis revealed limited overlap between the risks of VTE recurrence and bleeding in CRC patients. Notably, age and ECOG status were the only two common risk factors for both VTE recurrence and bleeding. Specifically, age over 75 years was a significant risk factor for both MB and CRNMB, while age was a continuous variable risk factor for VTE recurrence. Hemoglobin levels less than 100 g/L emerged as a common risk factor for VTE recurrence and CRNMB in the multivariate analysis. A similar trend was observed for MB, although this did not reach statistical significance (sHR = 1.561, 95% CI 0.975-2.498).
It is generally believed that the incidence of VTE is variable between different cancer types (8–10). Due to the apparent differences in blood supply and environmental carcinogens among different primary tumor locations in CRC (37, 38), it is presumed that VTE recurrence and bleeding risks might also differ by anatomical subsites. However, our multivariate analysis did not reveal any statistically significant correlations. Only patients with primary tumors in the right colon showed a higher risk of MB in the univariate analysis (sHR = 1.87, 95% CI 1.23-2.85). Similarly, we found no evidence linking portal venous thrombosis (PVT) with anticoagulant outcomes, despite previous studies indicating that patients with PVT are at risk for bleeding, and thrombosis recurrence in both splanchnic veins and deep veins of the lower extremities, as well as pulmonary artery (39, 40).
Risk factors for VTE recurrence and bleeding have been used to develop various risk scores. In our cohort of CRC patients, we evaluated the Ottawa score (41), which was specifically designed to identify cancer patients at risk for VTE recurrence (Supplementary Table S4). Although the 1067 low-risk patients (score < 1) exhibited a numerically lower cumulative incidence of recurrent VTE compared to high-risk patients (16.7% vs. 20.5%, respectively), the C-index value was still relatively low (0.60). This finding aligns with a previous prospective multicenter study involving patients with various cancer types, which also reported the insufficient accuracy of the Ottawa score in predicting recurrent VTE (42). Previous studies have demonstrated that a diagnosis of gastrointestinal cancer is an independent risk factor for both MB and CRNMB in cancer patients (8, 10, 11). For cancer-associated VTE patients, due to the absence of prospectively validated bleeding scores, we selected four well-recognized bleeding risk factors for analysis. However, no meaningful correlations were found (Tables 2, 3). Further investigation into bleeding outcome prediction in cancer-associated VTE patients is warranted.
This study has several limitations. First, the retrospective design does not allow for the inclusion over treatment-related parameters typical of a randomized controlled trial. While the univariate analysis identified the thrombogenic effects of concurrent anticancer treatments, the diverse range of anticancer agents and their varying administration among patients prevented the correlating of recurrence and bleeding risk with any specific agent. Importantly, our study focused specifically on a large real-world cohort of CRC patients with VTE, who are underrepresented in prior research. Furthermore, we found that the rates of VTE recurrence and bleeding in CRC patients under routine clinical care were higher than those reported in major trials, highlighting important real-world challenges and the clinical significance of our findings. Another limitation of this study is that only rivaroxaban and LMWH were analyzed, as other DOACs were not accessible at our center during the study period. Third, the use of DOACs is often discouraged in patients with gastrointestinal or genitourinary cancers, which may introduce bias in future studies that compare outcomes in cancer-associated VTE patients treated with these anticoagulants.
Conclusions
This study identified risk factors for VTE recurrence and bleeding in CRC patients undergoing anticoagulant therapy. Our analysis demonstrated the negative impact of these outcomes on survival, highlighting the need for improved stratification methods for CRC patients. Furthermore, the limited overlap between risk factors for recurrence and bleeding observed in our analysis may aid in the development of more effective risk prediction models in CRC patients, thereby enhancing the overall risk-benefit assessment of anticoagulant treatment in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University (2024ZSLYEC-226). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the retrospective nature of the research.
Author contributions
ZL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JX: Project administration, Validation, Writing – original draft, Writing – review & editing. XL: Project administration, Validation, Writing – original draft, Writing – review & editing. LQ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1648003/full#supplementary-material
References
1. Kakkar AK and Williamson RC. Prevention of venous thromboembolism in cancer patients. Semin Thromb Hemost. (1999) 25:239–43. doi: 10.1055/s-2007-994925
2. Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. (2002) 162:1245–8. doi: 10.1001/archinte.162.11.1245
3. Key NS, Khorana AA, Mackman N, McCarty OJ, White GC, Francis CW, et al. Thrombosis in cancer: research priorities identified by a national cancer institute/national heart, lung, and blood institute strategic working group. Cancer Res. (2016) 76:3671–5. doi: 10.1158/0008-5472.CAN-15-3100
4. Sørensen HT, Mellemkjaer L, Olsen JH, and Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. (2000) 343:1846–50. doi: 10.1056/NEJM200012213432504
5. Khorana AA, Francis CW, Culakova E, Kuderer NM, and Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. (2007) 5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x
6. Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. (2010) 125:490–3. doi: 10.1016/j.thromres.2009.12.023
7. McBane RD II, Vlazny DT, Houghton D, Casanegra AI, Froehling D, Daniels P, et al. Survival implications of thrombus recurrence or bleeding in cancer patients receiving anticoagulation for venous thromboembolism treatment. Thromb Haemost. (2023) 123:535–44. doi: 10.1055/s-0042-1758835
8. Kyrle PA. Predicting recurrent venous thromboembolism in cancer: is it possible? Thromb Res. (2014) 133:S17–22. doi: 10.1016/S0049-3848(14)50003-5
9. Kraaijpoel N, Di Nisio M, Mulder FI, van Es N, Beyer-Westendorf J, Carrier M, et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: results from the Hokusai VTE Cancer Study. Thromb Haemost. (2018) 118:1439–49. doi: 10.1055/s-0038-1667001
10. Trujillo-Santos J, Nieto JA, Tiberio G, Piccioli A, Di Micco P, Prandoni P, et al. Predicting recurrences or major bleeding in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. (2008) 100:435–9. doi: 10.1160/TH08-02-0125
11. Moik F, Posch F, Zielinski C, Pabinger I, and Ay C. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res Pract Thromb Haemost. (2020) 4:550–61. doi: 10.1002/rth2.12359
12. Agnelli G, Muñoz A, Franco L, Mahé I, Brenner B, Connors JM, et al. Apixaban and dalteparin for the treatment of venous thromboembolism in patients with different sites of cancer. Thromb Haemost. (2022) 122:796–807. doi: 10.1055/s-0041-1735194
13. Angelini DE, Attia D, Wei W, Wilks ML, Tripp B, D’Andrea C, et al. Bleeding outcomes of gastrointestinal cancer patients treated with direct oral anticoagulants vs. low molecular weight heparin. Blood. (2020) 136:27. doi: 10.1182/blood-2020-136757
14. Tsoukalas N, Tsapakidis K, Galanopoulos M, Karamitrousis E, Kamposioras K, and Tolia M. Real world data regarding the management of cancer-associated thrombosis. Curr Opin Oncol. (2020) 32:289–94. doi: 10.1097/CCO.0000000000000646
15. Guo JD, Hlavacek P, Poretta T, Wygant G, Lane D, Gorritz M, et al. Inpatient and outpatient treatment patterns of cancer-associated thrombosis in the United States. J Thromb Thrombolysis. (2020) 50:386–94. doi: 10.1007/s11239-019-02032-3
16. Schulman S and Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
17. Kaatz S, Ahmad D, Spyropoulos AC, and Schulman S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. (2015) 13:2119–26. doi: 10.1111/jth.13140
18. Muñoz AJ, Souto JC, Lecumberri R, Obispo B, Sanchez A, Aparicio J, et al. Development of a predictive model of venous thromboembolism recurrence in anticoagulated cancer patients using machine learning. Thromb Res. (2023) 228:181–8. doi: 10.1016/j.thromres.2023.06.015
19. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. (2018) 378:615–24. doi: 10.1056/NEJMoa1711948
20. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. (2018) 36:2017–23. doi: 10.1200/JCO.2018.78.8034
21. McBane RD II, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, et al. Apixaban and dalteparin in active Malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. (2020) 18:411–21. doi: 10.1111/jth.14662
22. Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. (2020) 382:1599–607. doi: 10.1056/NEJMoa1915103
23. Lecumberri R, Alfonso A, Jiménez D, Fernández Capitán C, Prandoni P, Wells PS, et al. Dynamics of case-fatalilty rates of recurrent thromboembolism and major bleeding in patients treated for venous thromboembolism. Thromb Haemost. (2013) 110:834–43. doi: 10.1160/TH13-02-0132
24. Carrier M, Le Gal G, Wells PS, and Rodger MA. Systematic review: casefatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. (2010) 152:578–89. doi: 10.7326/0003-4819-152-9-201005040-00008
25. Bikdeli B, Sharif-Kashani B, Bikdeli B, Valle R, Falga C, Riera-Mestre A, et al. Impact of thrombus sidedness on presentation and outcomes of patients with proximal lower extremity deep vein thrombosis. Semin Thromb Hemost. (2018) 44:341–7. doi: 10.1055/s-0037-1621716
26. Becattini C, Giustozzi M, Cerdà P, Cimini LA, Riera-Mestre A, and Agnelli G. Risk of recurrent venous thromboembolism after acute pulmonary embolism: Role of residual pulmonary obstruction and persistent right ventricular dysfunction. A meta-analysis. J Thromb Haemost. (2019) 17:1217–28. doi: 10.1111/jth.14477
27. Orione C, Tromeur C, Le Mao R, Le Floch PY, Robin P, Hoffmann C, et al. The impact of pulmonary vascular obstruction on the risk of recurrence of pulmonary embolism: a French prospective cohort. Thromb Haemost. (2021) 121:955–63. doi: 10.1055/s-0040-1722190
28. Eichinger S, Heinze G, Jandeck LM, and Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. (2010) 121:1630–6. doi: 10.1161/CIRCULATIONAHA.109.925214
29. de Winter MA, Büller HR, Carrier M, Cohen AT, Hansen JB, Kaasjager KAH, et al. Recurrent venous thromboembolism and bleeding with extended anticoagulation: the VTE-PREDICT risk score. Eur Heart J. (2023) 44:1231–44. doi: 10.1093/eurheartj/ehac776
30. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, and Kyrle PA. Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. (2016) 14:1480–3. doi: 10.1111/jth.13336
31. Lapébie FX, Bura-Rivière A, Espitia O, Bongard V, Ciammaichella MM, Martínez JG, et al. Predictors of recurrence of cancer-associated venous thromboembolism after discontinuation of anticoagulant therapy: a multicenrter cohort study. J Thromb Haemost. (2023) 21:2189–201. doi: 10.1016/j.jtha.2023.04.010
32. Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ 3rd, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. (2014) 123:3972–8. doi: 10.1182/blood-2014-01-549733
33. Becattini C, Bauersachs R, Maraziti G, Bertoletti L, Cohen A, Connors JM, et al. Renal function and clinical outcome of patients with cancer-associated venous thromboembolism randomized to receive apixaban or dalteparin. Results from the Caravaggio trial. Haematologica. (2022) 107:1567–76. doi: 10.3324/haematol.2021.279072
34. Sanfilippo KM, Wang TF, Gage BF, Luo S, Riedell P, and Carson KR. Incidence of venous thromboembolism in patients with non-Hodgkin lymphoma. Thromb Res. (2016) 143:86–90. doi: 10.1016/j.thromres.2016.05.008
35. Caruso V, Di Castelnuovo A, Meschengieser S, Lazzari MA, de Gaetano G, Storti S, et al. Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18–018 patients and 1149 events. Blood. (2010) 115:5322–8. doi: 10.1182/blood-2010-01-258624
36. Angelini DE, Radivoyevitch T, McCrae KR, and Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. (2019) 94:780–5. doi: 10.1002/ajh.25494
37. Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault MC, Carbonnel F, et al. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European countries: a multinational cohort study. Clin Gastroenterol Hepatol. (2019) 17:1323–31.e6. doi: 10.1016/j.cgh.2018.07.030
38. Feng Z, Shi X, Zhang Q, Zhang X, Li X, Chen Z, et al. Analysis of clinicopathological features and prognosis of 1315 cases in colorectal cancer located at different anatomical subsites. Pathol Res Pract. (2019) 215:152560. doi: 10.1016/j.prp.2019.152560
39. Ageno W, Riva N, Schulman S, Beyer-Westendorf J, Bang SM, Senzolo M, et al. Long-term clinical outcomes of splanchnic vein thrombosis: results of an international registry. JAMA Intern Med. (2015) 175:1474–80. doi: 10.1001/jamainternmed.2015.3184
40. Riva N, Ageno W, Schulman S, Beyer-Westendorf J, Duce R, Malato A, et al. Clinical history and antithrombotic treatment of incidentally detected splanchnic vein thrombosis: a multicentre, international prospective registry. Lancet Haematol. (2016) 3:e267–75. doi: 10.1016/S2352-3026(16)30020-5
41. Louzada ML, Carrier M, Lazo-Langner A, Dao V, Kovacs MJ, Ramsay TO, et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. (2012) 126:448–54. doi: 10.1161/CIRCULATIONAHA.111.051920
42. Girard P, Laporte S, Chapelle C, Falvo N, Falchero L, Cloarec N, et al. Failure of the Ottawa score to predict the risk of recurrent venous thromboembolism in cancer patients: the prospective PREDICARE cohort study. Thromb Haemost. (2022) 122:151–7. doi: 10.1055/a-1486-7497
43. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. (2013). Available online at: https://www.wma.net/wp-content/uploads/2024/10/DoH-Oct2013.pdf
Keywords: colorectal cancer, cancer-associated venous thromboembolism, recurrent thrombosis, bleeding events, patient survival
Citation: Liang Z, Mao J, Xie J, Li X and Qin L (2025) Risk factors for recurrence and bleeding in colorectal cancer patients with cancer-associated venous thrombembolism. Front. Oncol. 15:1648003. doi: 10.3389/fonc.2025.1648003
Received: 04 July 2025; Accepted: 07 August 2025;
Published: 27 August 2025.
Edited by:
Paola Patrignani, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Stefania Tacconelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyNataliia Dovganych, National Scientific Center “M.D. Strazhesko Institute of Cardiology, Ukraine
Copyright © 2025 Liang, Mao, Xie, Li and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Li, bGl4eWFuNUBtYWlsLnN5c3UuZWR1LmNu; Li Qin, cWlubGkzQG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
 Zhikun Liang
Zhikun Liang Jieling Mao
Jieling Mao Jingwen Xie1,2
Jingwen Xie1,2 Xiaoyan Li
Xiaoyan Li Li Qin
Li Qin