- 1Department of Surgical Oncology, Gansu Provincial Hospital, Lanzhou, China
- 2The First School of Clinical Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou, China
- 3Department of General Surgery, Chengxian People’s Hospital, Longnan, China
- 4Department of Medical Oncology, Liangzhou Hospital, Wuwei, China
- 5Department of Interventional Oncology, Gansu Provincial Hospital, Lanzhou, China
Background: Hepatoid adenocarcinoma of the stomach (HAS) is a rare subtype of gastric cancer (GC) characterized by alpha-fetoprotein (AFP) production and invasive liver and lymph node metastases, typically associated with a poor prognosis. Although immuno-chemotherapy has made significant achievements in the conversion therapy of advanced GC in recent years, the management of HER2-negative, proficient mismatch repair (pMMR), and a programmed cell death ligand-1 (PD-L1) combined positive score (CPS)<5 cases, particularly in the context of synchronous multiple liver metastases and lymph node involvement, poses significant challenges. This is attributable not only to its rapid progression but also to its poor prognosis. We retrospectively report a case of HAS with concurrent multiple liver and lymph node metastases. Following six cycles of immuno-chemotherapy, R0 resection was achieved, and postoperative pathological examination confirmed a pathological complete response (pCR). No recurrence or metastasis was observed at the 32-month postoperative follow-up (last follow-up: April 26, 2025). To our knowledge, no previous reports have documented pCR in HER2-negative, pMMR, and PD-L1 CPS<5 patients with advanced HAS following conversion therapy with combined immuno-chemotherapy. This report aims to provide further clinical reference for the treatment of advanced HAS.
Case summary: A 51-year-old male patient was diagnosed with HAS accompanied by multiple liver and lymph node metastases. Following six cycles of immunotherapy (sintilimab) combined with chemotherapy (Nab-paclitaxel, oxaliplatin, and S-1), the primary tumor exhibited significant reduction. Multiple liver metastases showed partial shrinkage or disappearance (the target lesion diameter must be less than 10 mm), and retroperitoneal lymph nodes were no longer detectable. After thorough evaluation, R0 resection was deemed achievable. Therefore, radical distal gastrectomy with D2 lymphadenectomy and liver metastasectomy were performed. Postoperative pathology confirmed pCR. The patient has remained progression-free survival (PFS) for 32 months and overall survival (OS) for 38 months, with no evidence of recurrence or metastasis.
Conclusion: HAS is a highly invasive malignant tumor of the stomach. The dynamic changes in AFP serve as a reliable indicator for detecting HAS, evaluating treatment efficacy, and predicting recurrence. In advanced HER-2-negative, PD-L1 CPS<5, pMMR-type HAS, employing a conversion therapy regimen combining sintilimab with Nab-paclitaxel, oxaliplatin, and S-1 may reduce tumor staging, enhance conversion therapy success rates, and prolong survival.
Introduction
GC is the fifth leading cause of cancer-related mortality worldwide, with approximately one million new cases reported annually (1). Epidemiological data indicate that China accounts for approximately 40% of global GC incidence, with 30%-40% of patients presenting with stage IV at initial diagnosis, and the 5-year survival rate is below 10% due to limited therapeutic options (2, 3). HAS is a rare and highly aggressive subtype of GC, characterized by a strong tendency to metastasize to the liver and lymph nodes. The 5-year survival rate is approximately 9% (4, 5). Conversion surgery is a therapeutic approach that enables successful tumor resection after aggressive conversion therapy in patients with initially unresectable tumors. In recent years, the combination of chemotherapy and immunotherapy has shown significant efficacy in the treatment of advanced GC, extending OS to 13.1–15.2 months in the general population and to 18.4–19.1 months in patients with a PD-L1 CPS ≥5 (6, 7). Since Yoshida et al. (8) introduced the surgical use of conversion therapy in GC, it has become a key strategy in the management of advanced GC. Accumulating evidence indicates that conversion therapy promotes significant tumor regression, improves the R0 resection rate, and extends OS in patients with advanced GC (9, 10). Nowadays, immuno-chemotherapy has become the first-line treatment option for advanced GC, resulting in a substantially higher probability of tumor regression (6, 11, 12). Shen et al. (13) and Sun et al. (14) each reported a case of HER2-positive advanced HAS successfully converted by chemotherapy. However, follow-up periods were only 2 and 7 months, respectively, and key biomarkers and TRG were not available (NA). Fakhruddin et al. (15) reported a case of HER2-positive advanced HAS achieving 18 months of OS following trastuzumab combined with chemotherapy. Castria et al. (16) described a case of advanced HAS with over 19 months of OS after combined treatment with trastuzumab, ramucirumab, and chemotherapy. Tang et al. (17) reported a case of advanced HAS with a PD-L1 CPS = 20 achieving clinical complete remission after immuno-chemotherapy, with OS exceeding 9 months. Li et al. (18) documented a dMMR advanced HAS patient who achieved pCR with PFS exceeding 6 months after successful conversion therapy with immuno-chemotherapy. Lu et al. (19) reported an advanced HER2-negative, pMMR HAS case with OS exceeding 15 months, although PD-L1 CPS was NA. Although Zhou et al. (20) reported an unresectable cT4aN3aMx HER2-negative, pMMR HAS case with PD-L1 CPS<1 achieving conversion and pCR after three cycles of SOX plus toripalimab conversion therapy, with OS exceeding 12 months, effective treatment options remain elusive for patients with advanced HAS who are HER2-negative, pMMR, programmed cell death protein-1 (PD-1) (note: although we measured PD-1 expression by IHC, it is non-standardized and has not been clinically validated) negative, and PD-L1 CPS<5, and present with both multiple liver and lymph node metastases (Table 1).
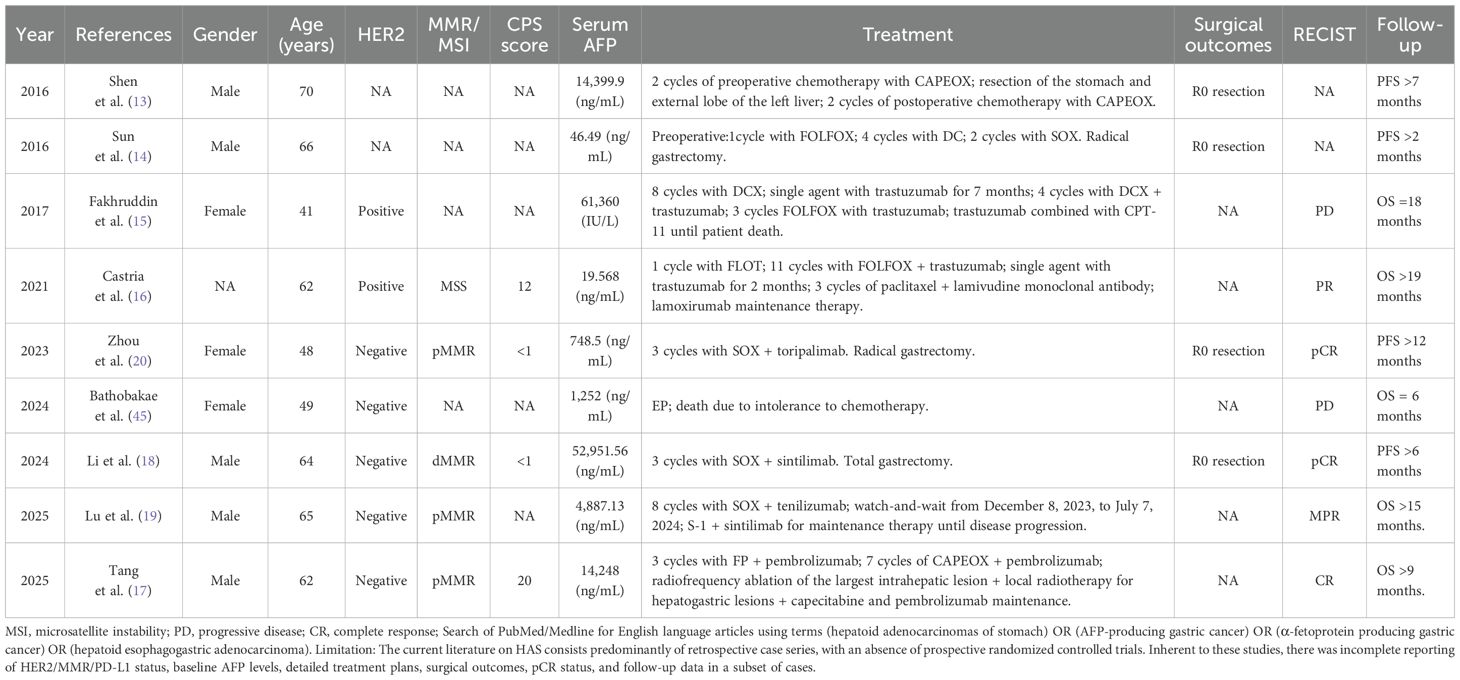
Table 1. Case reports on the conversion therapy and outcomes of all unresectable HAS/AFP-producing gastric cancers from February 2016 to April 2025.
This study presents a retrospective analysis of a case of HAS with synchronous multiple liver and lymph node metastases. The patient underwent R0 resection following six cycles of conversion therapy, which incorporated immunotherapy in combination with chemotherapy. Postoperative pathological examination confirmed a pCR. It is important to note that this case was marked by a few things: a significantly elevated AFP, HER2-negative, the PD-L1 CPS was 3, PD-1 negative, and pMMR. The conversion regimen proved effective, enabling the patient to achieve R0 resection followed by a pCR. The patient subsequently achieved a PFS exceeding 32 months and an OS of 38 months, indicating a favorable long-term outcome. To our knowledge, this is an exceedingly rare reported case of an advanced HAS patient with HER2-negative, PD-L1 CPS of 3, PD-1-negative, and pMMR status who achieved pCR following conversion therapy with sintilimab combined with Nab-paclitaxel, oxaliplatin, and S-1. Importantly, no recurrence or metastasis has been observed during an OS period exceeding 3 years.
Case presentation
Chief complaints
A 51-year-old man was admitted to the hospital with intermittent epigastric pain with melena for 1 month.
History of present illness
One month prior, the patient experienced acid reflux and belching without an identifiable cause, along with mild epigastric distension, discomfort, and melena. The patient had no other clinical symptoms. Since symptom onset, he had experienced a weight loss of 5 kg.
History of past illness
The patient has no prior medical history. There was no family history of related conditions or hereditary diseases.
Physical examination
The patient’s vital signs were stable during the physical examination. No instances of jaundice were observed. No signs of anemia or significant enlargement of superficial lymph nodes were observed. The chest examination yielded no positive findings. The abdomen was flat, with no visible gastrointestinal peristalsis or abnormal contour. The abdomen was soft, with deep epigastric tenderness but no rebound pain. The liver and spleen were not palpably enlarged, although liver percussion tenderness was present. Murphy’s sign and shifting dullness were negative. Bowel sounds were normal, with no abdominal bruits detected. The patient’s BMI was calculated to be 19.13 kg/m², with a body surface area of 1.58 m² and an NRS2002 nutritional score of 3 points. The Eastern Cooperative Oncology Group (ECOG) score was 1.
Laboratory examinations
Laboratory tests revealed the following values: AFP was 2,359 ng/mL (Figure 1A); CA125 was 110.2 U/mL (Figure 1B); CEA was 1.92 ng/mL (Figure 1C); CA19–9 was 3.71 U/mL (Figure 1D); hemoglobin was 126.0 g/L (Figure 1G); and a positive fecal occult blood test. All other parameters were within normal limits.
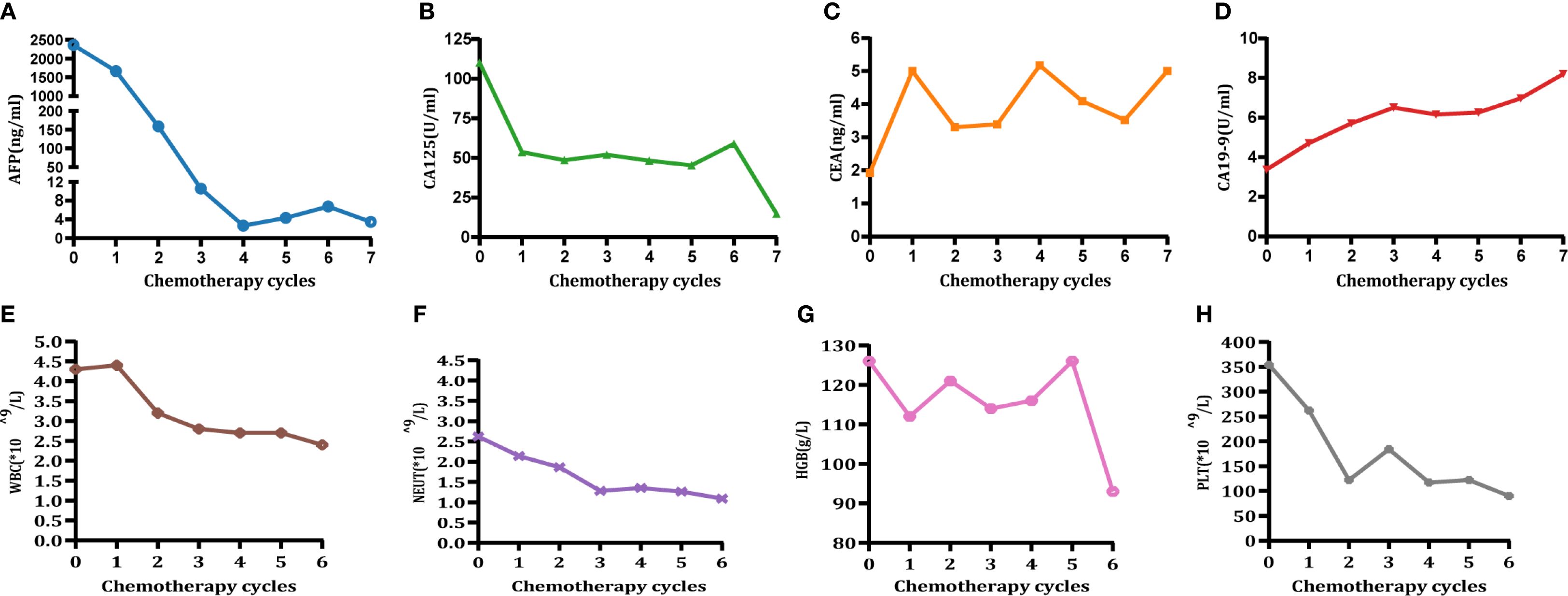
Figure 1. Changes in tumor marker levels (A-D) and blood cell count (E-H). Time points: 0 = preoperative level; 1–6 = post-chemotherapy levels for cycles 1 to 6, respectively. (A) Changes in AFP (<8.78 ng/mL). (B) Changes in CA125 (<35 U/mL). (C) Changes in CEA (<5 ng/mL). (D) Changes in CA19-9 (<37 U/mL). (E) Changes in white blood cell (WBC) count. (F) Changes in neutrophil (NEUT) count. (G) Changes in hemoglobin (HGB). (H) Changes in platelet (PLT) count (CTCAE grading adverse events refer to CTCAE - Version 5.0 (46). Grade 1 hematologic-related AEs occurred after 3-cycle treatment (assessment date: 28 April 2022) and resolved following oral therapy with Leucogen tablets (molecular formula: C14H17O4NS, molecular weight: 295.36) (Leucogen tablets: 20 mg, po, tid). Grade 2 hematological adverse events occurred after 6-cycle treatment (assessment date: 23 July 2022) and resolved following oral therapy with Leucogen tablets.).
Gastroscopy and imaging examinations
Gastroscopy identified a 2*2.5-cm ulcerative mass at the angular incisure, accompanied by peripheral mucosal erosion and nodular changes. The lesion further extended to involve the lesser curvature (Figure 2A, February 8, 2022). Abdominal enhanced CT scan demonstrated local thickening of the anterior gastric wall with ulcer formation, suggestive of ulcerative GC staging T4aN3M1. Multiple liver enhancement lesions were observed indicating metastasis along with enlargement of hepatogastric and retroperitoneal lymph nodes with nodal fusion (Figure 2C, February 9, 2022). Abdominal enhanced MRI exhibited multiple abnormal signal shadows in the liver, consistent with metastatic tumors. It also revealed multiple enlarged lymph nodes in both the hepatogastric space and retroperitoneum, indicative of lymph node metastasis (Figure 2G, February 9, 2022).
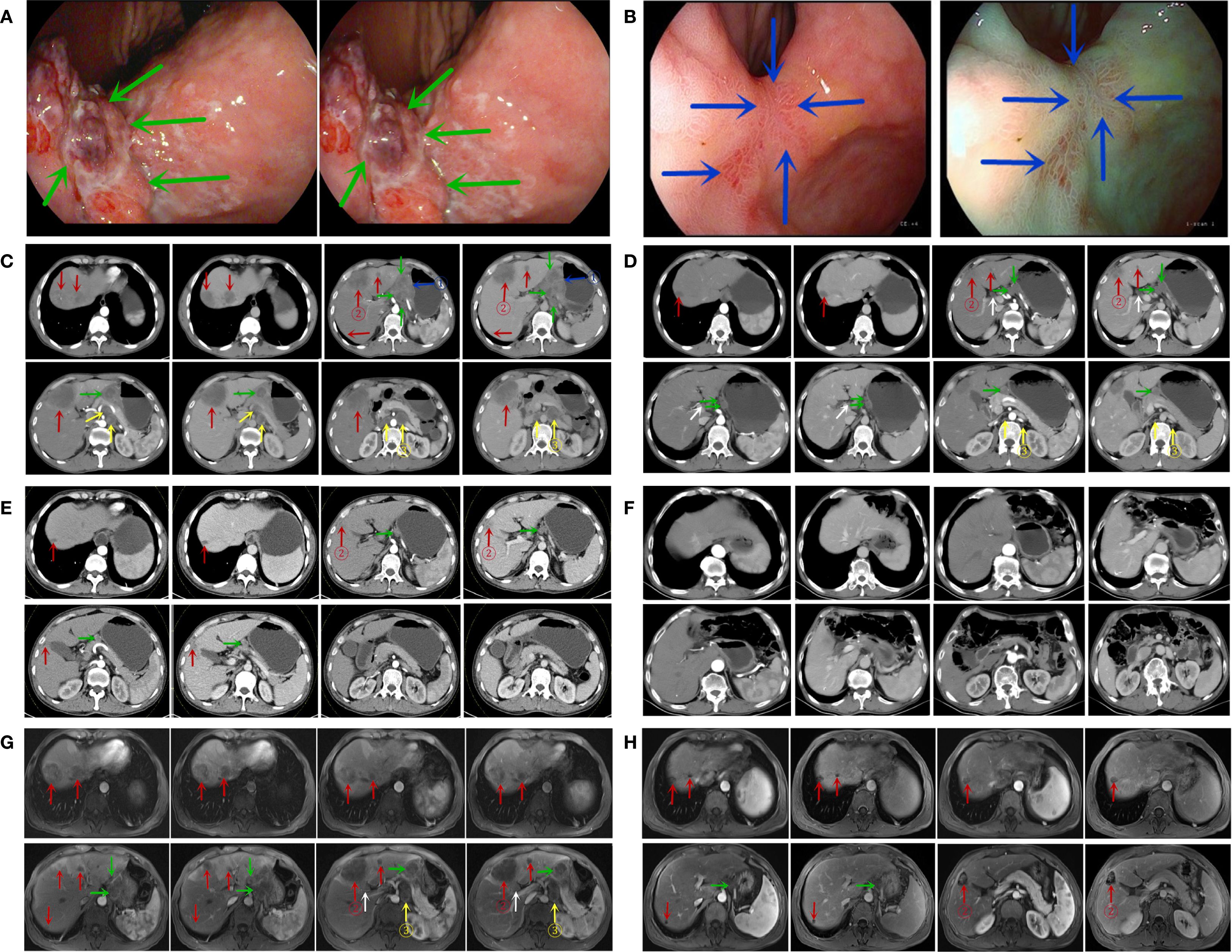
Figure 2. Gastroscopic findings (A, B). (A) Gastroscopy at the time of initial diagnosis; (B) gastroscopy at the conclusion of six cycles of treatment. (Notes: The green arrow indicates the lesion in the stomach prior to treatment, whereas the blue arrow indicates the lesion in the stomach at the conclusion of the 6-cycle treatment.) CT findings (C-F). (C) CT at the initial diagnosis (February 9, 2022); (D) CT at the conclusion of three treatment cycles (May 1, 2022); (E) CT at the end of six treatment cycles (July 27, 2022); (F) CT scan results from the most recent follow-up visit (April 26, 2025). MRI findings (G, H). (G) initial MRI at the time of diagnosis (February 9, 2022); (H) follow-up MRI after completion of six treatment cycles (July 27, 2022). (The blue arrows indicate the primary gastric lesion, the red arrows indicate liver metastases, the green arrows indicate perigastric lymph nodes, the white arrows indicate hilar lymph nodes, and the yellow arrows indicate retroperitoneal lymph nodes; ① represents the target lesion of the primary gastric lesion; ② represents the target lesion of the liver metastasis; ③ represents the target lesion of the retroperitoneal metastatic lymph node.).
Final diagnosis
The final diagnosis of this patient was HAS combined with liver metastases staged as cT4aN3M1, which corresponds to stage IVB. The endoscopic biopsy confirmed poorly differentiated gastric adenocarcinoma (Figure 3A). Further immunohistochemical staining confirmed it to be HAS (Figure 4), whereas liver fine-needle aspiration (FNA) biopsy showed hepatic metastases originating from gastric adenocarcinoma (Figure 3B). Immunohistochemistry (IHC) revealed HER2-negative (Figure 3E), pMMR (Figure 3F), PD-1 negative (Figure 3G), and PD-L1 CPS = 3 (Figure 3H).
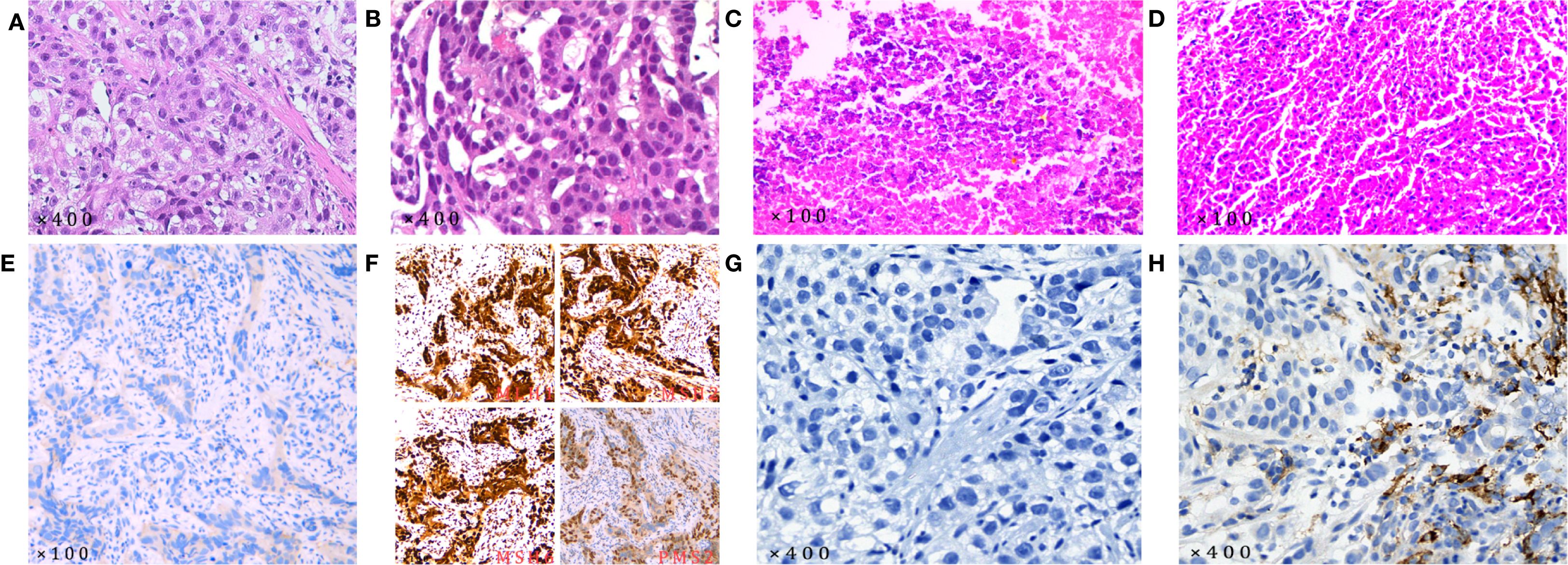
Figure 3. Pathological examination results (all pathological slide examinations were conducted independently, and each slide underwent a secondary review). (A) The initial diagnosis revealed gastric adenocarcinoma based on the gastric biopsy results; (B) the initial diagnosis was metastatic adenocarcinoma based on the results of the liver FNA biopsy; (C) postoperative gastric pathology examination revealed chronic mucosal inflammation; (D) no cancer cells were detected in the postoperative liver lesion pathology examination. IHC results. (E) HER2-negative; (F) pMMR; (G) PD-1-negative; (H) PD-L1 CPS = 3. (HER-2 antibody: 4B5, with IHC0 and IHC1+ results classified as HER2-negative. The antibodies used for MLH1, MSH2, MSH6, and PMS2 were MX063, MX061, MX056, and MXR019, respectively. Tumors with positive nuclear staining for all four markers are classified as pMMR. PD-1 antibody: MX033. PD-L1 antibody: SP263, with classification based on the CPS: .).
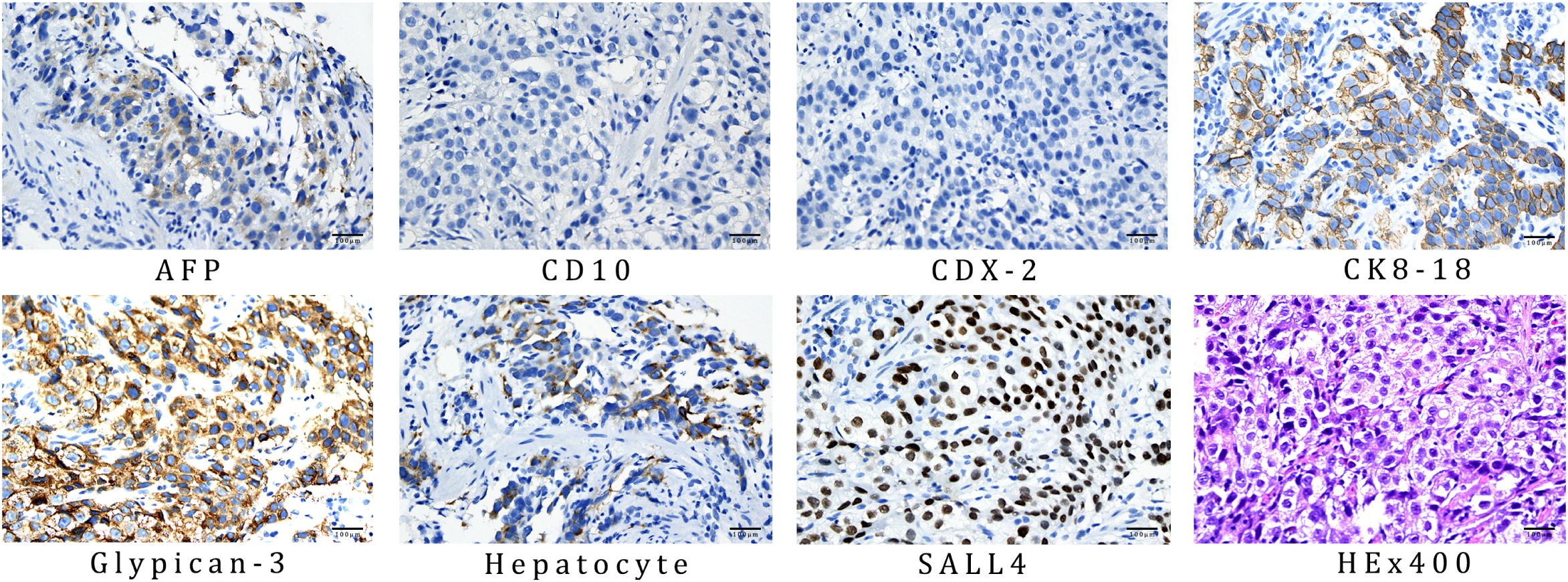
Figure 4. IHC results of gastric biopsy specimens: AFP positive, CD10 negative, CDX-2 negative, CK8–18 positive, glypican-3 positive, hepatocyte positive, and SALL4 positive. The antibodies used for AFP, CD10, CDX-2, CK8-18, glypican-3, hepatocyte, and SALL4 was OTI5D2, MX002, EPR2764Y, 5D3, MAXIM001, OCH1E5, and 6E3, respectively.
Treatment
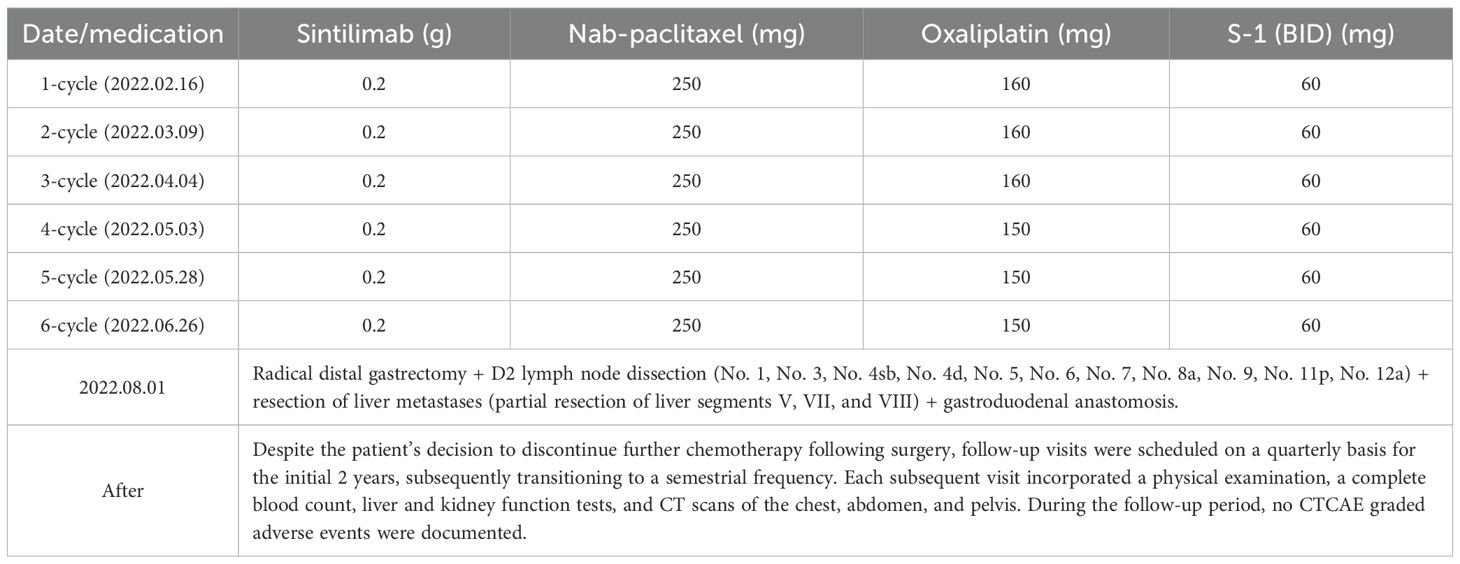
The patient received six cycles of conversion therapy involving the administration of sintilimab in combination with Nab-paclitaxel, oxaliplatin, and S-1 (sintilimab: 0.2 g, ivgtt, day 1, q3w; Nab-paclitaxel: 160 mg/m², ivgtt, day 1, q3w; Oxaliplatin: 100 mg/m², ivgtt, day 1, q3w; S-1: 40 mg/m², po, bid, d1–14) (Table 2). Throughout the course of treatment, the patient tolerated the chemotherapeutic agents well; Grade 1 hematologic-related AEs occurred after Cycle 3 treatment (assessment date: 28 April 2022) and resolved following oral treatment with Leucogen tablets (molecular formula: C14H17O4NS, molecular weight: 295.36) (Leucogen tablets: 20 mg, po, tid). Grade 2 hematologic-related AEs occurred after Cycle 6 treatment (assessment date: 23 July 2022) and resolved following oral treatment with Leucogen tablets (Figures 1E-H), with no non-hematologic AEs. The patient strictly adhered to the treatment protocol and completed each cycle of therapy according to the treatment schedule and oxaliplatin dose adjusted to 150 mg following Grade 1 hematologic-related AEs.
Outcome and follow-up
After three cycles of conversion treatment, review of enhanced CT (Figure 2D, May 1, 2022) compared with pretreatment CT (Figure 2C, February 9, 2022), the results showed significant reduction in gastric wall thickening. Liver metastases partially regressed or disappeared, and lymph nodes in the perigastric and retroperitoneal regions showed similar patterns of regression or disappearance. The target lesions tumor regression rates (TRR) for gastric lesions, liver metastases, and retroperitoneal lymph nodes were 42.67%, 79.80%, and 82.53%, respectively (Table 3). Serum tumor marker tests suggested decreases in AFP to 10.56 ng/mL (Figure 1A), CA125 to 52.0 U/mL (Figure 1B), CEA (Figure 1C), and CA19-9 (Figure 1D) which were stable in the normal range. The disease was evaluated as partial response (PR) [RECIST 1.1 protocol (21)]. However, multidisciplinary team (MDT) discussions concluded that the tumor was still at risk of vascular infiltration and R0 resection was difficult. To maximize the potential for radical surgery and considering the possibility that continued treatment after PR could further reduce tumor burden or achieve pCR, the original conversion therapy regimen was extended for an additional three cycles.
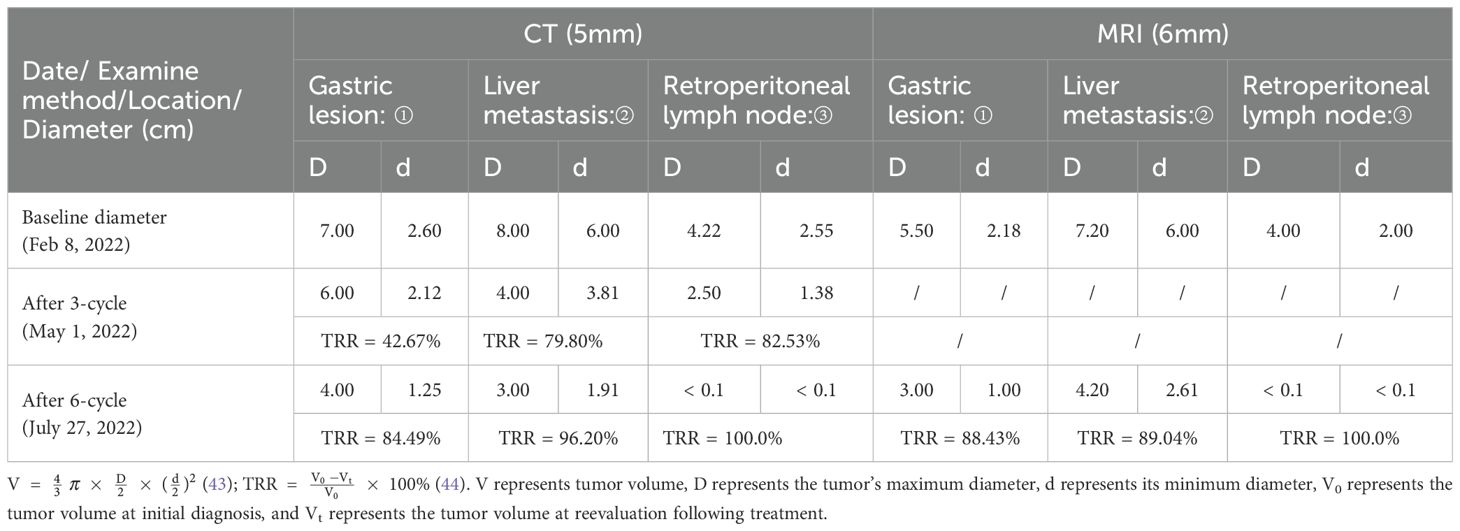
Table 3. TRR and diameter of target lesions following treatment, assessed by RECIST 1.1 (“/” indicates absence of MRI data).
Gastroscopy after six cycles of treatment showed an angular incisure ulcer with scarring changes and mucosal congestion (Figure 2B, July 26, 2022). Enhanced CT scan (Figure 2E, July 26, 2022) showed further shrinkage of gastric lesions. Liver metastases had either partially regressed or disappeared. Similarly, some perigastric lymph nodes had regressed or disappeared, whereas peritoneal lymph nodes were no longer detectable, compared with the previous scan (Figure 2D, May 1, 2022). The target lesion TRR for gastric lesions, liver metastases, and retroperitoneal lymph nodes reached 84.49%, 96.20%, and 100%, respectively (Table 3). Enhanced MRI scans (Figure 2H, July 27, 2022) confirmed that compared with pretreatment (Figure 2G, February 9, 2022), the primary gastric tumor had significantly decreased in size. Liver metastases showed partial resolution and partial reduction. Some perigastric lymph nodes decreased in size, whereas others disappeared completely. Retroperitoneal lymph nodes were no longer detectable. The maximum tumor contraction rates were 88.43% for gastric lesions, 89.04% for liver metastases, and 100% for retroperitoneal lymph nodes (Table 3). These decreases met the PR criteria of the RECIST 1.1 protocol (21).
Following a consensus by the MDT, the patient underwent a radical distal gastrectomy + D2 lymph node dissection (No. 1, No. 3, No. 4sb, No. 4d, No. 5, No. 6, No. 7, No. 8a, No. 9, No. 11p, No. 12a) + resection of liver metastases (partial resection of liver segments V, VII, and VIII) + gastroduodenal anastomosis on August 1, 2022. Pathology of the postoperative specimen revealed chronic inflammation of the gastric mucosa and reactive hyperplasia of the perigastric lymph nodes (Figure 3C). No cancerous cells were detected in the hepatic lesion (Figure 3D). The surgical margins of both the stomach and liver were found to be negative. Primary gastric lesions, metastatic liver lesions, and lymph nodes all demonstrated TRG grade 0, thus confirming a pCR (22). After the operation, the patient declined further treatment but continued with regular follow-up. At the 32-month postoperative follow-up, an enhanced CT (Figure 2F, April 26, 2025) showed no evidence of tumor recurrence. Serum AFP was 3.49 ng/mL (Figure 1A) and CA125 was 14.8 U/mL (Figure 1B), and CEA (Figure 1C) and CA19-9 (Figure 1D) were within the normal range.
Patient perspective and ethical enrichment
Throughout the treatment, patients and their families were informed about all treatment measures and agreed and signed informed consent forms. The patient had a strong willingness for treatment but was less compliant, especially since no further treatment was administered after surgery. The case report and its accompanying images were published after obtaining written informed consent from the patient.
Discussion
HAS, a rare GC subtype with histological features mimicking hepatocellular carcinoma (HCC), is the predominant contributor to alpha-fetoprotein-producing GC (AFPGC). Although the incidence of HAS has been reported in the relevant literature to be limited, possibly accounting for only 0.3%-1.0% of all GC (23, 24), with an estimated rate of 0.58-0.83 cases per million people per year (25), it has received increasing attention due to its aggressiveness, especially its susceptibility to hepatic metastasis and poor prognosis (26). Compared with classic GC, HAS had different clinicopathologic features and prognosis than non-HAS. Since the rates of vascular invasion, lymph node metastasis, and liver metastasis were significantly higher in HAS than in non-HAS, the prognosis of HAS was worse than that of non-HAS (P<0.05), and the 5-year survival rate was only 9% (27). A clinical study (28) demonstrated that HAS has unique molecular and diagnostic features, including pMMR, positivity for AFP, salt-like transcription factor 4 (SALL4), overexpression of HER2, PD-L1 CPS, and c-MET, and Epstein–Barr virus-encoded RNA (EBER) negativity. While pMMR and EBER-negative reflect key molecular features of HAS, positivity for AFP and/or positivity for SALL4 are key diagnostic markers for HAS (28). Among these characteristic molecules, patients with HER2-positive or dMMR have a favorable prognosis. AFP-positive GCs show greater aggressiveness and poorer prognosis than AFP-negative ones (29). Moreover, HAS with a high AFP expression (AFP-high HAS) demonstrates significantly worse OS compared with AFP-low HAS (P = 0.046) (30). A survival analysis study showed that preoperative serum AFP levels ≥500 ng/mL were significantly associated with worse OS (p = 0.007) and tended to be associated with worse DFS (p = 0.05) (31). Therefore, an elevated AFP level is an independent prognostic marker for HAS, regardless of liver metastatic status (32). Real-time AFP monitoring holds significant clinical utility, not only in guiding management of advanced GC with elevated AFPGC (33) but also in reinforcing the prognostic relevance of AFP expression across GC subtypes, including HAS (29, 30, 32). Similarly, AFP is an ideal marker for predicting treatment efficacy and determining HAS recurrence. Since AFP expression correlates with tumor cell stemness and immune evasion pathways in HAS, it can be used as a surrogate marker of tumor load and immune response (8, 34). In addition, HAS responds poorly to conventional chemotherapy, leading to a significant shortening of OS and posing a serious threat to patient survival (27). In this case, the patient had a high AFP of 2,359 ng/mL at initial diagnosis and multiple liver metastases and abdominal lymph node metastases, as well as HER2-negative and pMMR, indicating an extremely high degree of malignancy, and the limited effectiveness of conventional treatment to improve the patient’s prognosis. However, the patient exhibited symptoms such as upper abdominal pain and black stools, indicative of a strong desire for treatment and excellent compliance. The patient strictly adhered to the treatment regimen, and his family provided full support and encouragement. AFP rapidly decreased from 2,359 ng/mL to normal levels after four cycles of immuno-chemotherapy and has been maintained within normal levels thereafter. This suggests that the treatment regimen effectively induced tumor regression and reduced tumor viability without evidence of recurrence. These findings are consistent with previous studies, indicating that AFP kinetics correlates with AFPGC and HAS (33, 35).
In recent years, ICIs combined with chemotherapy have shown promise in therapeutic effects in the neoadjuvant treatment of locally advanced CC. This combination can not only improve the R0 resection rate but also increase the pCR rate, thereby improving OS. In the conversion therapy of advanced GC, this strategy improves both the success rate of conversion and the R0 resection and pCR rates, demonstrating a favorable therapeutic effect. A single-arm, phase II clinical study (36) evaluating sintilimab combined with FLOT in patients with HER2-negative, locally advanced adenocarcinoma of the stomach or gastroesophageal junction reported the objective regression rate (ORR) of 84.4% (95% CI, 68.3%-93.1%), the disease control rate (DCR) of 96.9% (95% CI, 84.3%-99.5%), the pCR rate of 17.2% (95% CI, 5.8%-35.8%), the major pathological response (MPR) rate of 55.2%, and the favorable safety profile. A meta-analysis (37) including 21 prospective phase I/II studies with a total of 687 patients reported that immuno-chemotherapy in locally advanced GC resulted in a pCR of 21% (95% CI 0.18-0.24), an MPR rate of 41% (95% CI 0.31-0.52), and an R0 resection rate of 94% (95% CI 0.92-0.96). The incidence of grade 3 or higher AEs was 0.23 (95% CI 0.13-0.38), indicating an overall favorable safety profile (37). Furthermore, Nab-paclitaxel combined with Oxaliplatin and fluorouracil analogs has shown improved therapeutic efficacy and a favorable safety profile in the treatment of GC. FOXAGAST study (38) reported that the combination of Nab-paclitaxel, oxaliplatin, and 5-fluorouracil (FOLFOX) achieved an MPR rate of 38.8% among resectable GC patients, with 16.3% achieving pCR as classified by the Mandard system. The primary toxicities observed were neutropenia, peripheral neuropathy, and nausea; however, most were manageable. These findings suggest that our protocol provided a robust chemotherapeutic foundation for treating this case of HAS. Xia et al. (39) reported a case of advanced GC that received pCR after conversion therapy with Nab-paclitaxel combined with oxaliplatin and an S-1 regimen. Another study, including 147 patients in the final analysis, demonstrated that the DOS (docetaxel, oxaliplatin, and S-1) regimen improved the MPR rate to 25.4% and the R0 resection rate to 78.9% (40).
Shen et al. (13) and Sun et al. (14) each reported one case of advanced HAS that was successfully converted through chemotherapy. However, the follow-up periods were only 2 and 7 months, respectively, and key biomarkers and TRG were NA. A relevant study (28) has shown that HAS patients treated with anti-HER2 therapy or with dMMR have a favorable prognosis. For HER2-positive advanced HAS, trastuzumab combined with chemotherapy may be employed. Fakhruddin et al. (15) documented a case of HAS where the patient achieved an OS of 18 months after treatment with trastuzumab combined with chemotherapy. Castria et al. (16) documented a case of advanced HAS where the patient achieved pCR with successful conversion therapy using trastuzumab, ramucirumab, and chemotherapy, with an OS exceeding 19 months. For HER2-negative advanced HAS, although no ideal targeted therapy exists, immuno-chemotherapy may be considered for dMMR or CPS >5 cases. Tang et al. (17) reported a case of advanced HAS with a CPS of 20 that achieved a clinical complete response and an OS exceeding 9 months following immuno-chemotherapy. Li et al. (18) documented a case of advanced HAS with dMMR that achieved pCR and PFS exceeding 6 months following successful conversion therapy with immuno-chemotherapy. However, there is currently no ideal treatment for HER2-negative, pMMR, CPS<5 cases. Zhou et al. (20) reported a case of advanced HER2-negative, pMMR-type HAS with an undetermined CPS that achieved pCR and PFS exceeding 1 year following successful conversion therapy with immuno-chemotherapy. Lu et al. (19) reported a case of advanced, HER2-negative, pMMR-type HAS with an undetermined CPS that achieved MPR without surgery, with an OS exceeding 15 months. Although an ORR of 58.8% was achieved in a small cohort of AFPGC treated with immuno-chemotherapy (10, 41). However, HAS patients with hepatic metastases face a poor prognosis, with a 5-year survival rate of only 9% (4), with a median OS of only 6–14 months when treated with chemotherapy alone (28, 42). Notably, for patients with advanced HAS with HER-2-negative expression and concurrent multiple liver and lymph node metastases, no effective treatment currently exists. Consequently, these patients face poor survival outcomes, which significantly threaten their prognosis. In our report, the patient declined postoperative treatment but insisted on regular follow-up. The patient, despite being HER2-negative, pMMR, PD-1-negative, and PD-L1 CPS = 3, received immuno-chemotherapeutic conversion therapy. Remarkably, the patient achieved a pCR and survived 32 months postoperatively, with no evidence of recurrence or residual lesions at the last follow-up. In this particular instance, the dosage of Nab-paclitaxel was modified to 160 mg/m², and that of oxaliplatin to 100 mg/m². This regimen was found to maintain therapeutic efficacy while concomitantly reducing the occurrence of dose-dependent AEs associated with the combination therapy. It is worthy to note that the treatment remained effective even subsequent to a reduction in the oxaliplatin dose, on account of Grade 1 hematologic-related AEs. Notwithstanding the presence of pMMR and PD-L1 CPS = 3, sintilimab elicited an immune response, yielding synergistic effects when administered concurrently with chemotherapy. This case suggests that, even in HER2-negative, pMMR, PD-1-negative, and PD-L1 CPS<5 advanced HAS, the combined immuno-chemotherapy offers therapeutic advantages. The following mechanisms may be postulated: firstly, the administration of chemotherapy has been demonstrated to induce immunogenic cell death; secondly, the use of ICIs has been shown to enhance the tumor microenvironment, increase T-cell infiltration, and reverse immune exhaustion by blocking PD-1-PD-L1 binding and its subsequent effects. This results in significant tumor regression and the attainment of substantial therapeutic outcomes.
Summary
In summary, our patient presented with advanced HAS characterized by extremely high AFP expression, HER2 negativity, PD-1 negativity, pMMR, and PD-L1 CPS of 3, indicating poor response to conventional chemotherapy and a dismal prognosis. However, AFP levels rapidly normalized after six cycles of immuno-chemotherapy conversion therapy. Post-conversion surgical resection confirmed pCR in the primary gastric tumor, hepatic metastases, and lymph nodes, with an OS exceeding 38 months—a finding unprecedented in existing literature. These results suggest that immuno-chemotherapy-based conversion therapy may be an effective strategy for advanced HAS, significantly prolonging survival and potentially achieving curative outcomes.
Limitations
While this study suggests the potential efficacy of combined immuno-chemotherapy with conversion therapy for advanced HAS, several limitations must be acknowledged. Firstly, as a retrospective case report, it is not possible for it to fully reflect the heterogeneity of the disease. Furthermore, the formulations and dosages of the drugs used cannot yield generalizable conclusions about treatment response patterns. Second, the existing literature on HAS, primarily based on case reports from East Asia (as retrieved from PubMed/Web of Science/Medline for English language articles), may introduce geographical selection bias, making the generalizability of our findings to broader populations uncertain. Furthermore, inherent selection biases and potential confounding factors in treatment selection and disease assessment cannot be ruled out. Therefore, the execution of large-scale prospective cohort studies is imperative to validate our observations, particularly to evaluate the predictive value of biomarkers such as MMR and PD-1/PD-L1. Concurrently, the alternative biomarkers capable of effectively predicting treatment outcomes in cases of pMMR, PD-1-negative, and PD-L1<5 remain to be identified.
Conclusion
HAS is a highly invasive malignant tumor of the stomach. The dynamic changes in AFP serve as a reliable indicator for detecting HAS, evaluating treatment efficacy, and predicting recurrence. In advanced HER-2-negative, PD-L1 CPS<5, pMMR-type HAS, employing a conversion therapy regimen combining sintilimab with Nab-paclitaxel, oxaliplatin, and S-1 may reduce tumor staging, enhance conversion therapy success rates, and prolong survival.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Medical Ethics Committee of Gansu Provincial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Writing – review & editing, Supervision, Writing – original draft, Conceptualization, Data curation, Resources, Methodology. YW: Writing – review & editing, Writing – original draft, Visualization, Methodology. TY: Writing – original draft, Writing – review & editing. DW: Writing – review & editing, Writing – original draft. HM: Data curation, Writing – original draft. JM: Data curation, Writing – original draft. MD: Visualization, Methodology, Conceptualization, Data curation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Gansu Province (grant number 25JRRA871).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Jin G, Lv J, Yang M, Wang M, Zhu M, Wang T, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. (2020) 21(10):1378–86. doi: 10.1016/s1470-2045(20)30460-5
3. Li Y, Wang Y, Shen R, Liu W, and Zhu C. Hyperprogression disease induced by Sintilimab combined with Oxaliplatin and S-1 after surgery: a case report and literature review. Front Oncol. (2025) 15:1494007. doi: 10.3389/fonc.2025.1494007
4. Jiang J, Ding Y, Lu J, Chen Y, Chen Y, Zhao W, et al. Integrative analysis reveals a clinicogenomic landscape associated with liver metastasis and poor prognosis in hepatoid adenocarcinoma of the stomach. Int J Biol Sci. (2022) 18:5554–74. doi: 10.7150/ijbs.71449
5. Liu X, Sheng W, and Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. (2012) 106:299–303. doi: 10.1002/jso.23073
6. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
7. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. Jama. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
8. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, and Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z
9. Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, et al. International retrospective cohort study of conversion therapy for stage IV gastric cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. (2022) 6:227–40. doi: 10.1002/ags3.12515
10. Liang H, Yan X, Li Z, Chen X, Qiu Y, Li F, et al. Clinical outcomes of conversion surgery following immune checkpoint inhibitors and chemotherapy in stage IV gastric cancer. Int J Surg. (2023) 109:4162–72. doi: 10.1097/JS9.0000000000000738
11. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
12. Chong X, Madeti Y, Cai J, Li W, Cong L, Lu J, et al. Recent developments in immunotherapy for gastrointestinal tract cancers. J Hematol Oncol. (2024) 17:65. doi: 10.1186/s13045-024-01578-x
13. Shen Z, Liu X, Lu B, and Ye M. Hepatoid adenocarcinoma of the stomach: A case report of a rare type of gastric cancer. Oncol Lett. (2016) 11:1077–80. doi: 10.3892/ol.2015.4023
14. Sun N, Sun Q, Liu Q, Zhang T, Zhu Q, Wang W, et al. α-fetoprotein-producing gastric carcinoma: A case report of a rare subtype and literature review. Oncol Lett. (2016) 11:3101–4. doi: 10.3892/ol.2016.4372
15. Fakhruddin N, Bahmad HF, Aridi T, Yammine Y, Mahfouz R, Boulos F, et al. Hepatoid adenocarcinoma of the stomach: A challenging diagnostic and therapeutic disease through a case report and review of the literature. Front Med (Lausanne). (2017) 4:164. doi: 10.3389/fmed.2017.00164
16. de Castria TB, Tang L, Queiroz MM, Awni BM, Paroder V, Shamseddine A, et al. Hepatoid esophagogastric adenocarcinoma and tumoral heterogeneity: a case report. J Gastrointest Oncol. (2021) 12:3123–32. doi: 10.21037/jgo-21-287
17. Tang Y, Li X, and Yang Y. Case Report: Second-line favourable effect of pembrolizumab plus chemotherapy in a patient with metastatic hepatoid adenocarcinoma of the stomach. Front Pharmacol. (2025) 16:1387661. doi: 10.3389/fphar.2025.1387661
18. Li L, Zhang D, Zhu J, and Zhang G. Hepatoid adenocarcinoma of the stomach with ideal response to neoadjuvant chemo-immunotherapy: a case report. Front Immunol. (2024) 15:1496342. doi: 10.3389/fimmu.2024.1496342
19. Lu G, Tu J, Tu J, and Jiang R. Case report: Chemotherapy plus sintilimab for the treatment of gastroesophageal junction hepatoid adenocarcinoma with liver metastasis: a case study with literature review. Front Immunol. (2025) 16:1513604. doi: 10.3389/fimmu.2025.1513604
20. Zhou Y, Dong L, Dai L, Hu S, Sun Y, Wu Y, et al. Pathologic complete response of hepatoid adenocarcinoma of the stomach after chemo-immunotherapy: A rare case report and literature review. Front Surg. (2023) 10:1133335. doi: 10.3389/fsurg.2023.1133335
21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
22. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
23. Zhu M, Chen E, Yu S, Xu C, Yu Y, Cao X, et al. Genomic profiling and the impact of MUC19 mutation in hepatoid adenocarcinoma of the stomach. Cancer Commun (Lond). (2022) 42:1032–5. doi: 10.1002/cac2.12336
24. Lin JX, Wang ZK, Hong QQ, Zhang P, Zhang ZZ, He L, et al. Assessment of clinicopathological characteristics and development of an individualized prognostic model for patients with hepatoid adenocarcinoma of the stomach. JAMA Netw Open. (2021) 4:e2128217. doi: 10.1001/jamanetworkopen.2021.28217
25. Xia R, Zhou Y, Wang Y, Yuan J, and Ma X. Hepatoid adenocarcinoma of the stomach: current perspectives and new developments. Front Oncol. (2021) 11:633916. doi: 10.3389/fonc.2021.633916
26. Huang YQ, Huang ZN, Hong QQ, Zhang P, Zhang ZZ, He L, et al. Development of a staging system for hepatoid adenocarcinoma of the stomach based on multicenter data: a retrospective cohort study. Int J Surg. (2025) 111:718–27. doi: 10.1097/JS9.0000000000001768
27. Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, et al. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. (2010) 34:1465–71. doi: 10.1097/PAS.0b013e3181f0a873
28. Yang X, Wu Y, Wang A, Ma X, Zhou K, Ji K, et al. Immunohistochemical characteristics and potential therapeutic regimens of hepatoid adenocarcinoma of the stomach: a study of 139 cases. J Pathol Clin Res. (2024) 10:e343. doi: 10.1002/cjp2.343
29. Wang D, Li C, Xu Y, Xing Y, Qu L, Guo Y, et al. Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp Pathol. (2015) 8:6345–55.
30. Chen EB, Wei YC, Liu HN, Tang C, Liu ML, Peng K, et al. Hepatoid adenocarcinoma of stomach: emphasis on the clinical relationship with alpha-fetoprotein-positive gastric cancer. BioMed Res Int. (2019) 2019:6710428. doi: 10.1155/2019/6710428
31. Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. (2019) 22:1183–92. doi: 10.1007/s10120-019-00965-5
32. Chen Y, Qu H, Jian M, Sun G, and He Q. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Markers. (2015) 30:e387–93. doi: 10.5301/jbm.5000167
33. Wang YK, Shen L, Jiao X, and Zhang XT. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterol. (2018) 24:266–73. doi: 10.3748/wjg.v24.i2.266
34. Taniguchi Y, Kiyozawa D, Kohashi K, Kawatoko S, Yamamoto T, Torisu T, et al. Volume of hepatoid component and intratumor M2 macrophages predict prognosis in patients with hepatoid adenocarcinoma of the stomach. Gastric Cancer. (2025) 28:41–50. doi: 10.1007/s10120-024-01562-x
35. Wang R, Zeng H, Shi M, Wu Y, Liu Y, and Zhang T. Dynamic variations in peripheral blood indices and their association with efficacy and adverse reactions of pd- 1 inhibitor combined chemotherapy in patients with advanced gastric cancer. BMC Gastroenterol. (2025) 25:264. doi: 10.1186/s12876-025-03883-2
36. Li N, Li Z, Fu Q, Zhang B, Zhang J, Wan XB, et al. Efficacy and safety of neoadjuvant sintilimab in combination with FLOT chemotherapy in patients with HER2-negative locally advanced gastric or gastroesophageal junction adenocarcinoma: an investigator-initiated, single-arm, open-label, phase II study. Int J Surg. (2024) 110:2071–84. doi: 10.1097/JS9.0000000000001119
37. Li S, Xu Q, Dai X, Zhang X, Huang M, Huang K, et al. Neoadjuvant therapy with immune checkpoint inhibitors in gastric cancer: A systematic review and meta-analysis. Ann Surg Oncol. (2023) 30:3594–602. doi: 10.1245/s10434-023-13143-w
38. Watson S, de la Fouchardière C, Kim S, Cohen R, Bachet JB, Tournigand C, et al. Oxaliplatin, 5-Fluorouracil and Nab-paclitaxel as perioperative regimen in patients with resectable gastric adenocarcinoma: A GERCOR phase II study (FOXAGAST). Eur J Cancer. (2019) 107:46–52. doi: 10.1016/j.ejca.2018.11.006
39. Xia Y, Zhu C, Xu L, Yao J, and Da M. S-1 and oxaliplatin combined with nanoparticle albumin-bound paclitaxel adjuvant chemotherapy for advanced gastric adenocarcinoma. Chin Med J (Engl). (2022) 135:2261–3. doi: 10.1097/CM9.0000000000002003
40. Jiang Z, Xie Y, Zhang W, Du C, Zhong Y, Zhu Y, et al. Perioperative chemotherapy with docetaxel plus oxaliplatin and S-1 (DOS) versus oxaliplatin plus S-1 (SOX) for the treatment of locally advanced gastric or gastro-esophageal junction adenocarcinoma (MATCH): an open-label, randomized, phase 2 clinical trial. Gastric Cancer. (2024) 27:571–9. doi: 10.1007/s10120-024-01471-z
41. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
42. Li W, Li Q, Yu Y, Wang Y, Chen E, Chen L, et al. Effect of immune checkpoint inhibitors plus chemotherapy on advanced gastric cancer patients with elevated serum AFP or hepatoid adenocarcinoma. Cancer Manag Res. (2020) 12:11113–9. doi: 10.2147/CMAR.S276969
43. Le Fèvre C, Sun R, Cebula H, Thiery A, Antoni D, Schott R, et al. Ellipsoid calculations versus manual tumor delineations for glioblastoma tumor volume evaluation. Sci Rep. (2022) 12:10502. doi: 10.1038/s41598-022-13739-4
44. Fang FM, Tsai WL, Go SF, Ho MW, Wu JM, Wang CJ, et al. Implications of quantitative tumor and nodal regression rates for nasopharyngeal carcinomas after 45 Gy of radiotherapy. Int J Radiat Oncol Biol Phys. (2001) 50:961–9. doi: 10.1016/S0360-3016(01)01531-0
45. Bathobakae L, Elagami M, Mahmoud A, Kesrani J, Yuridullah R, Melki G, et al. Alpha-fetoprotein-producing hepatoid adenocarcinoma of the stomach. J Med Cases. (2024) 15:304–9. doi: 10.14740/jmc4263
Keywords: HAS, pCR, immunotherapy, chemotherapy, conversion surgery
Citation: Li Y, Wang Y, Yu T, Wang D, Ma H, Ma J and Da M (2025) Pathologic complete response of advanced hepatoid adenocarcinoma of the stomach following immuno-chemotherapy and conversion surgery: a rare case report and review of the literature. Front. Oncol. 15:1648766. doi: 10.3389/fonc.2025.1648766
Received: 17 June 2025; Accepted: 19 September 2025;
Published: 02 October 2025.
Edited by:
Chang Li, University of Washington, United StatesReviewed by:
Chenbing Sun, Shanghai University of Traditional Chinese Medicine, ChinaHsi-Chang Shih, Johns Hopkins University, United States
Copyright © 2025 Li, Wang, Yu, Wang, Ma, Ma and Da. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxu Da, bGR5eV9kYW14QGx6dS5lZHUuY24=
†These authors have contributed equally to this work
 Yaoqi Li
Yaoqi Li You Wang
You Wang Tao Yu3†
Tao Yu3† Jichun Ma
Jichun Ma Mingxu Da
Mingxu Da