- Department of Gastroenterology, Affiliated Puren Hospital of Wuhan University of Science and Technology, Wuhan, Hubei, China
Background: Cases of early gastrointestinal cancers (EGC) are often identified as advanced stages due to the lack of typical clinical manifestations, leading to missed or delayed diagnoses. Confocal laser endomicroscopy (CLE), a novel microscopic imaging technique, enables real-time in vivo histological examination during endoscopy, providing a valuable tool for the early detection of EGC.
Intervention: A 62-year-old woman with a gastric antral lesion, considered a polyp or early cancer, underwent CLE during gastroscopy to refine and confirm the diagnosis.
Findings: CLE imaging showed mild irregularities in the glandular architecture, thickening of the mucosal margins, the presence of pleated structures, dilated glandular openings, increased vascularity, and mild fluorescein leakage, all consistent with mild to moderate inflammatory changes. No features suggestive of malignant lesions were revealed.
Conclusion: CLE may play a pivotal role in the early differentiation of benign and malignant gastrointestinal lesions. While not yet a replacement for histopathology, CLE demonstrates significant clinical utility and potential for improving the accuracy of early cancer detection and long-term monitoring.
1 Introduction
Recently, gastric cancer (GC) has emerged as a major global health concern, with rising incidence and mortality rates, now ranking the fourth among all cancers (1). The pathogenesis of GC involves a multistep progression from chronic gastritis, chronic atrophic gastritis (AG), gastrointestinal metaplasia (GIM), and gastric intraepithelial neoplasia (GIN), representing key precancerous stages, to early gastric cancer (EGC) (2). Early detection of GC and its precursors is critical for improving treatment outcomes and prognosis. Traditional white-light endoscopy (WLE) is limited in detecting subtle lesions, as it primarily visualizes mucosal and submucosal changes, such as atrophy or intestinal metaplasia (3). WLE’s tumor detection miss rate remains high, ranging from 4.6% to 25.8% (4). In contrast, confocal laser endomicroscopy (CLE), a novel imaging technique, enables real-time in vivo histological assessment during endoscopy, termed “optical biopsy” (5). Its principle relies on blue laser illumination and fluorescence detection, with only focal plane light passing through a pinhole to enhance resolution and contrast (6). CLE also provides 1000× magnification, allowing cellular or subcellular visualization of mucosal structures.The Miami Classification system is widely adopted in clinical practice to standardize the interpretation of CLE imaging and establish diagnostic criteria for gastrointestinal mucosal alterations. This framework categorizes mucosal changes into four distinct classes based on three key microstructural features: (1) glandular architecture, (2) cellular morphology, and (3) microvascular patterns. Within this classification, early gastric cancer (EGC) is defined by a diagnostic triad: completely disorganized epithelium, heterogeneous fluorescein leakage, and dark irregular epithelial cells with loss of polarity (7) Here, CLE was employed for intraoperative diagnosis in a female with gastrointestinal symptoms, and demonstrated its clinical use for real-time lesion characterization and improved diagnostic precision.
2 Case presentation
The diagnostic trajectory is summarized in Table 1.
2.1 Clinical history
A 62-year-old female was admitted on December 25, 2024, with a six-month history of a suspected gastric space-occupying lesion. Six months earlier, the patient underwent a painless gastroscopy and endoscopic ultrasound, which revealed antral protrusion along with mildly hyperechoic mucosal lesions suggestive of polyps or early-stage cancer. Symptoms were temporarily attenuated after self-administered medication. However, 4 weeks later, progressive epigastric pain, postprandial exacerbation, nocturnal discomfort, nausea, occasional vomiting, dysphagia, acid reflux, and fatigue with a 5 kg weight loss were reported.
2.2 Physical examination and past medical history
Physical examination revealed no significant abnormalities. The patient had a history of Helicobacter pylori (Hp) infection, erosive gastritis, stable angina, coronary artery disease, and tricuspid insufficiency.
2.3 Diagnostic and therapeutic course
All gastroscopy and confocal examinations of the patient were conducted with informed consent, in compliance with ethical requirements. During the CLE examination, the patient did not show any discomfort. The initial gastroscopy on June 4, 2024 revealed a 1.2 × 0.8 cm reddish, smooth-surfaced bulge on the lesser curvature of the gastric antrum (Figure 1).
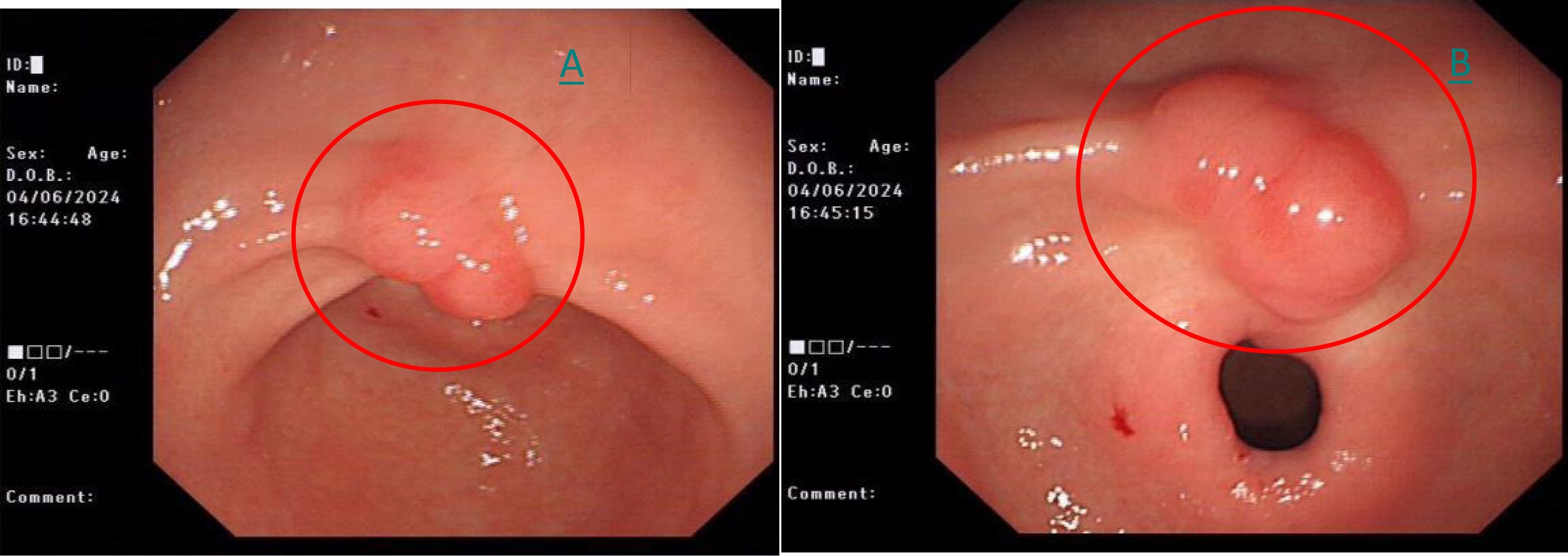
Figure 1. (A) White-light endoscopy shows a 1.2*0.8cm red and elevated lesion on the lesser curvature of stomach (red circle). (B) Close-up picture of the same lesion in white light (red circle).
A follow-up gastroscopy on December 26, 2024 showed the same lesion, which is consistent with the initial examination (Figure 2).
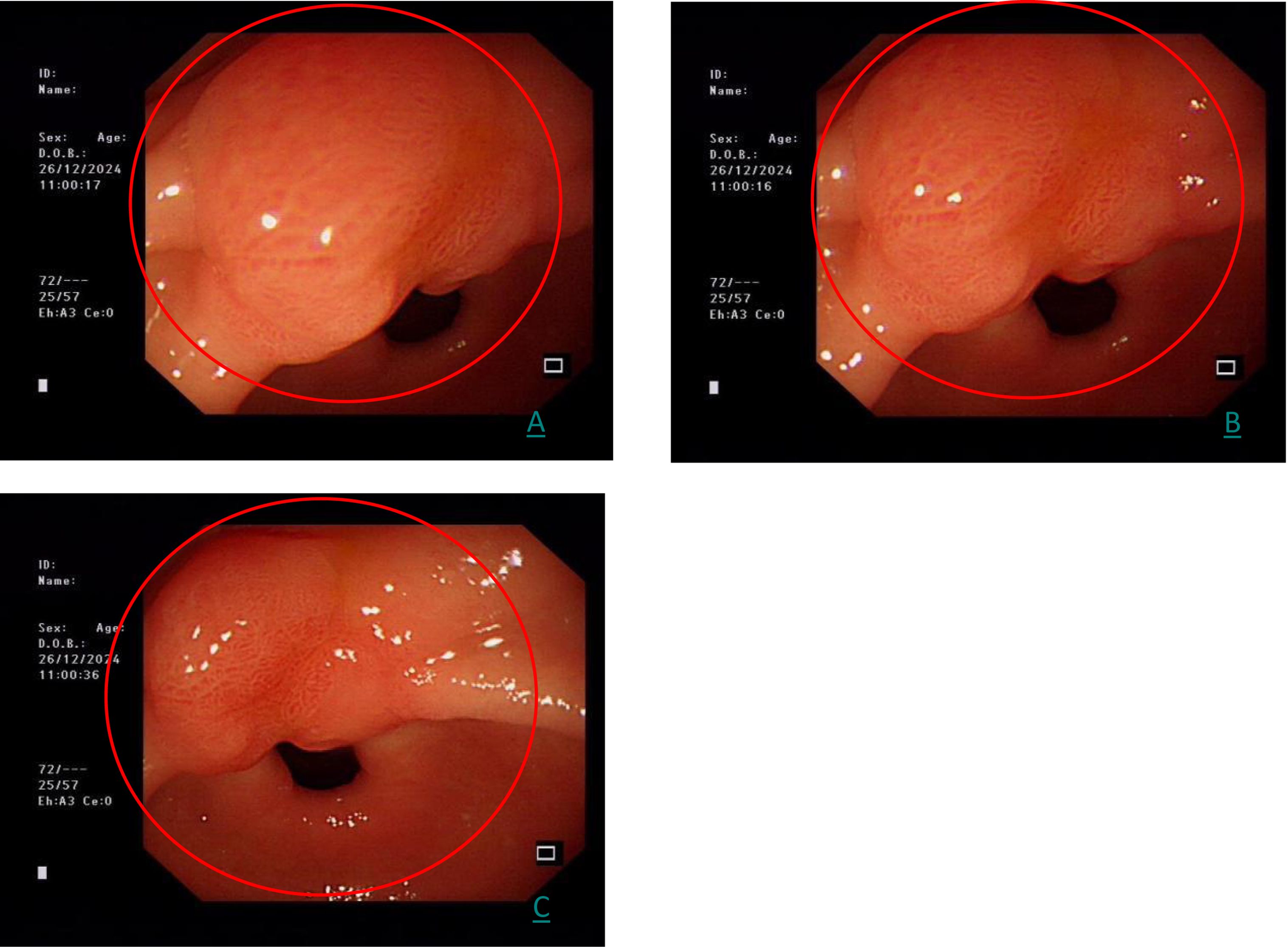
Figure 2. (A–C) represents the three images of the same gastric antrum lesion at different angles by white light endoscopy (red circle).
A CLE examination was performed on December 28, 2024. After a negative fluorescein sodium allergy test, intravenous fluorescein sodium was administered. CLE imaging using a BIOPSEE® probe revealed mildly irregular glandular architecture, thickened mucosal margins, folded structures, dilated glandular openings, increased vascularity, and minimal fluorescein leakage, consistent with mild-to-moderate inflammatory changes (Figure 3).
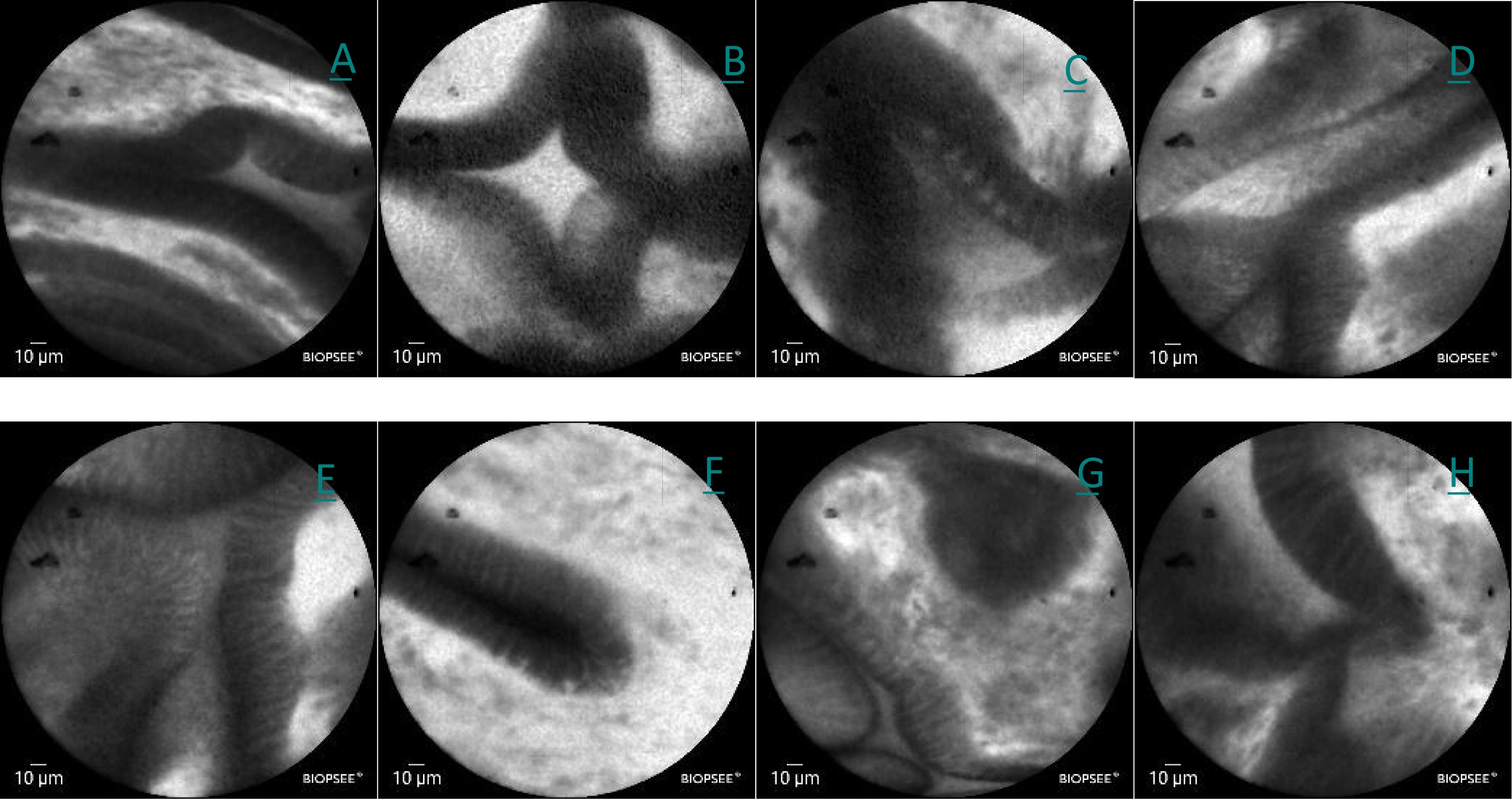
Figure 3. (A–H) Confocal images of lesions in the lesser curvature of the stomach in white light, showing a mild irregularity in the arrangement the glands, slight thickening of the edges, folding, dilation of the orifices, a slight increase in the number of vessels, and a small amount of flucein sodium leakage.
A histopathological analysis was conducted using the biopsy samples when the CLE was performed. The examination confirmed moderate mucosal inflammation (activity+++) with hyperplastic polypoid changes and reactive glandular alterations (Figure 4).
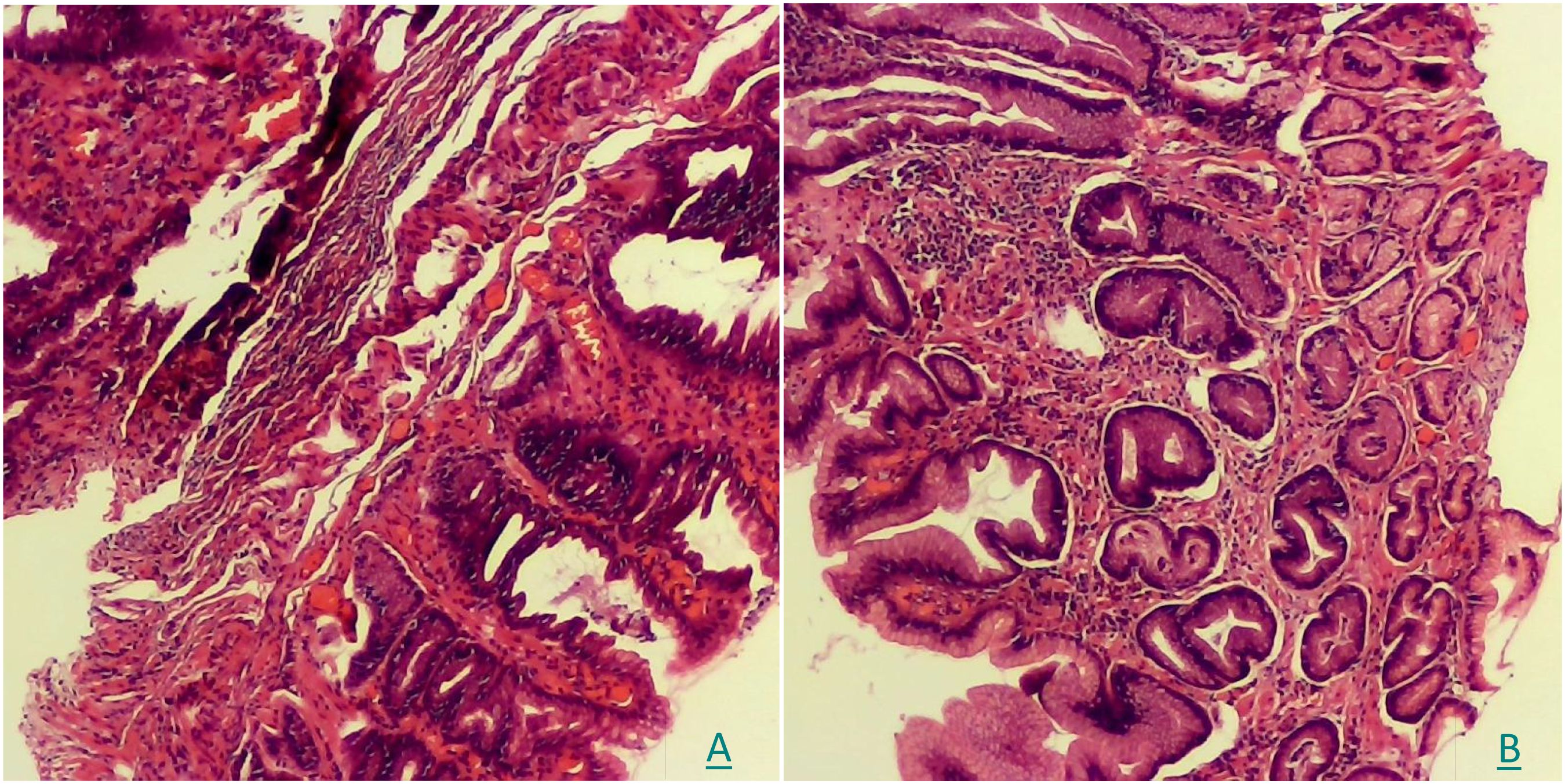
Figure 4. (A, B) The pathological examination of HE staining showed moderate mucosal inflammation (activity+++) with hyperplastic polypoid changes and reactive glandular alterations.
2.4 Final diagnosis
Integrated findings from gastroscopy, CLE, and histopathology confirmed gastric antral inflammatory lesions without evidence of malignancy.
2.5 Follow-up
At one-month post-discharge, telephone follow-up revealed resolution of gastrointestinal symptoms with no reported epigastric discomfort. The patient exhibited favorable recovery indicators: normal mental status, adequate nutritional intake, and stable sleep patterns. Notably, she expressed strong preference for CLE over repeated biopsies, citing “significantly reduced post-procedural discomfort”. Furthermore, she reported high satisfaction with the diagnostic efficiency (“The real-time diagnosis effectively alleviated my anxiety during the waiting period for histopathology results”). Written informed consent for experience publication was reconfirmed.
3 Discussion
Chronic gastritis, characterized by persistent inflammation of the gastric mucosa, is frequently associated with Hp infection, one of its most common etiological factors. Patients typically present with epigastric discomfort or a burning sensation. Left untreated, chronic gastritis carries a risk of malignant transformation, emphasizing the importance of early and accurate diagnosis, as well as the timely pharmacological intervention to control disease progression and improve patient prognosis. CLE significantly altered clinical management in this case. Initial gastroscopic and endosonographic findings identified a slightly hyperechoic mucosal lesion in the gastric antrum, yet failed to definitively characterize its nature. This diagnostic uncertainty necessitated plans for invasive procedures. Critically, CLE provided real-time intraoperative diagnosis of benign inflammation (later corroborated by histopathology), thereby enabling a shift to conservative pharmacotherapy and avoiding unnecessary surgery. Notably, CLE delivers immediate pathological insights days before conventional histopathology, offering clinicians accelerated therapeutic decision-making. Additionally, CLE enables Hp visualization using acriflavine staining, demonstrating 93% sensitivity and 86% specificity for diagnosing Hp-associated gastritis (8).
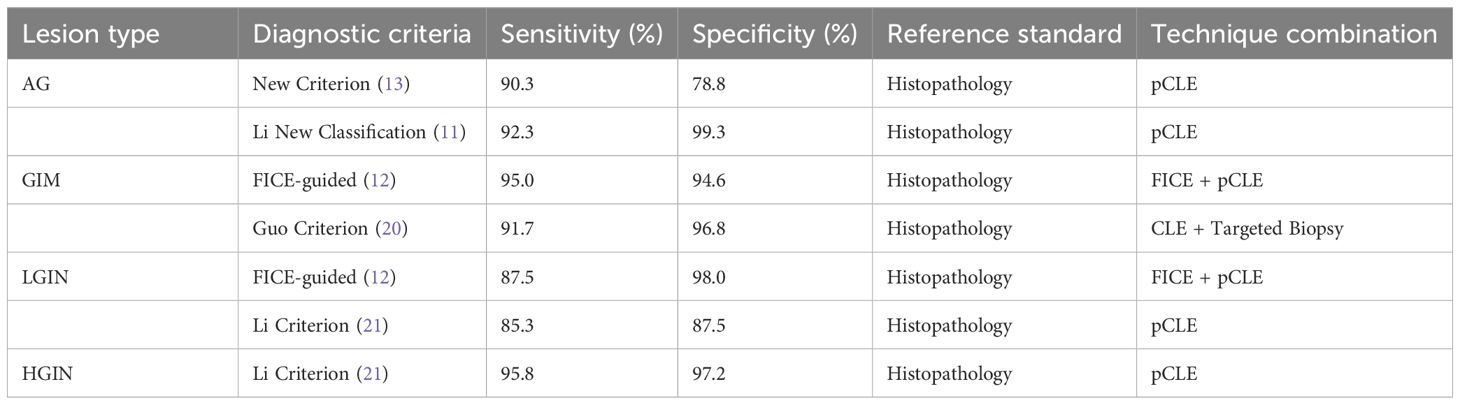
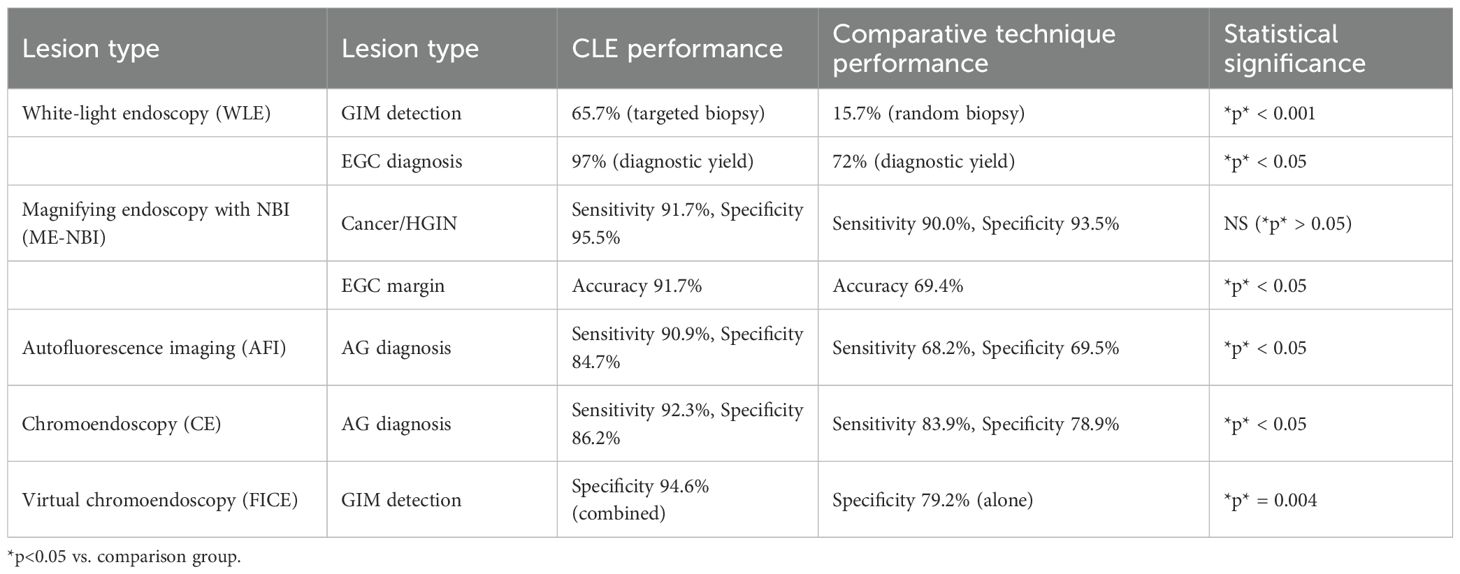
CLE requires fluorescent agents, typically administered intravenously, with fluorescein sodium being the most common. This agent highlights cellular and subcellular details without nuclear staining. CLE systems are categorized into three types: endoscope-based CLE (eCLE), probe-based CLE (pCLE), and needle-based CLE (nCLE). eCLE integrates a confocal microscope into the endoscope’s distal end but is rarely used due to poor maneuverability. pCLE employs a microprobe inserted through the biopsy channel, though its scanning depth is fixed. nCLE, a miniaturized version of pCLE, allows microscopic imaging of deeper mucosal or visceral tissues during endoscopic ultrasound (EUS) via a 19G needle, overcoming pCLE’s limitation to superficial lesions. Currently, nCLE is the most widely used in clinical practice (9).Compared with conventional biopsy, the combination of conventional biopsy or in vivo pCLE demonstrated significantly higher sensitivity (96.9% vs. 75.0%) (10).This supports pCLE as a valuable adjunct to traditional biopsy, enabling targeted sampling or biopsy replacement in endoscopically suspicious lesions to mitigate tissue damage from repeated procedures.As summarized in Table 2, CLE exhibits excellent diagnostic performance for gastric precancerous lesions:For high-grade intraepithelial neoplasia (HGIN), CLE achieved a sensitivity of 95.8% and specificity of 97.2% using the Li criterion (11);FICE-guided pCLE demonstrated 95.0% sensitivity and 94.6% specificity in detecting GIM (12).Critically, CLE combined with targeted biopsy (e.g., the Guo criterion (13)) optimizes diagnostic workflows by reducing sampling requirements.Further comparisons with endoscopic techniques (Table 3) reveal:CLE significantly outperformed WLE in early gastric cancer (EGC) diagnosis (97% vs. 72%, *p* < 0.05) (14);For AG, CLE provided higher specificity than autofluorescence imaging (AFI) (84.7% vs. 69.5%, *p* < 0.05) (15).Thus, CLE allows for biopsy reduction while maintaining diagnostic accuracy, demonstrating its clinical utility in risk stratification.CLE offers significant advantages: it reduces biopsy requirements, enhances diagnostic sensitivity, and minimizes complications such as mucosal injury, infection, and bleeding. These attributes make it ideal for long-term monitoring of early gastric cancer (6). It also enables rapid intraoperative assessment of epithelial cells, glandular morphology, and microvascular structures, shortening diagnostic timelines and improving tumor margin evaluation (16, 17).However, CLE has limitations: its narrow field of view restricts examination of the entire gastric cavity, respiratory movements can cause instability, and fluorescein’s inability to stain nuclei limits diagnosis to structural atypia (18). Additionally, its shallow tissue penetration hinders visualization of deeper structures, and high costs and specialized training requirements limit its widespread adoption (19). Despite these limitations, CLE remains a promising imaging technology for detecting gastrointestinal malignancies.
4 Conclusion
CLE represents a transformative advancement in endoscopic imaging, offering real-time histological evaluation for early detection of precancerous and malignant gastric lesions. While complementary to traditional histopathology, its integration into clinical practice holds promise for improving diagnostic accuracy and patient outcomes. Future research should focus on overcoming technical limitations and expanding its applications in gastrointestinal oncology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Puren Hospital Affiliated to Wuhan University of Science and Technology(2025 Annual Review No.005). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XP: Supervision, Writing – review & editing, Formal Analysis, Writing – original draft, Conceptualization. Y-PZ: Writing – original draft, Supervision, Investigation. YZ: Validation, Formal Analysis, Writing – review & editing, Conceptualization, Resources.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. The Scientific Research Project of Wuhan Municipal Health Commission (Grant No. WX23A67).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Machlowska J, Baj J, Sitarz M, Maciejewski R, and Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. (2020) 21:4012. doi: 10.3390/ijms21114012
3. Yu X, Chen J, Zheng L, Song J, Lin R, and Hou X. Quantitative diagnosis of atrophic gastritis by probe-based confocal laser endomicroscopy. BioMed Res Int. (2020) 2020:9847591. doi: 10.1155/2020/9847591
4. Liu J, Song Y, Luo J, Yang P, Shi X, and Zhao Q. Confocal laser endomicroscopy for the diagnosis of gastric adenocarcinoma of the fundic gland type. Gastrointestinal Endoscopy. (2024) 100:948–9. doi: 10.1016/j.gie.2024.06.025
5. Chauhan SS, Abu Dayyeh BK, Bhat YM, Gottlieb KT, Hwang JH, Komanduri S, et al. Confocal laser endomicroscopy. Gastrointestinal Endoscopy. (2014) 80:928–38. doi: 10.1016/j.gie.2014.06.021
6. Evola G, Vacante M, and Evola FR. Confocal laser endomicroscopy as a new diagnostic tool for poorly differentiated gastric adenocarcinoma. World J Clin cases. (2024) 12:5845–9. doi: 10.12998/wjcc.v12.i26.5845
7. Wallace M, Lauwers G, Chen Y, Dekker E, Fockens P, Sharma P, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. (2011) 43:882–91. doi: 10.1055/s-0030-1256632
8. Ueyama H, Hirasawa T, Yano T, Doyama H, Isomoto H, Yagi K, et al. Advanced diagnostic endoscopy in the upper gastrointestinal tract: review of the Japan gastroenterological endoscopic society core sessions. DEN Open. (2024) 4:e359. doi: 10.1002/deo2.359
9. Iacucci M, Santacroce G, Majumder S, Morael J, Zammarchi I, Maeda Y, et al. Opening the doors of precision medicine: novel tools to assess intestinal barrier in inflammatory bowel disease and colitis-associated neoplasia. Gut. (2024) 73:e331579. doi: 10.1136/gutjnl-2023-331579
10. Bok GH, Jeon SR, Cho JY, Cho JH, Lee WC, Jin SY, et al. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointestinal Endoscopy. (2013) 77:899–908. doi: 10.1016/j.gie.2013.01.018
11. Li Z, Zuo XL, Li CQ, Liu ZY, Ji R, Liu J, et al. New classification of gastric pit patterns and vessel architecture using probe-based confocal laser endomicroscopy. J Clin Gastroenterol. (2016) 50:23. doi: 10.1097/MCG.0000000000000298
12. Zuo XL, Li Z, Li CQ, Zheng YY, Xu LD, Chen J, et al. Probe-based endomicroscopy for in vivo detection of gastric intestinal metaplasia and neoplasia: A multicenter randomized controlled trial. Endoscopy. (2017) 49:1033–42. Georg Thieme Verlag KG. doi: 10.1055/s-0043-115382
13. Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, et al. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: A prospective study. Endoscopy. (2008) 40:547–53. Georg Thieme Verlag KG Stuttgart New York. doi: 10.1055/s-2007-995633
14. Horiguchi N, Tahara T, Yamada H, Yoshida D, Okubo M, Nagasaka M, et al. In vivo diagnosis of early-stage gastric cancer found after helicobacter pylori eradication using probe-based confocal laser endomicroscopy. Digestive Endoscopy: Off J Japan Gastroenterological Endoscopy Soc. (2018) 30:219–27. doi: 10.1111/den.12926
15. Lim LG, Yeoh KG, Srivastava S, Chan YH, Teh M, and Ho KY. Comparison of probe-based confocal endomicroscopy with virtual chromoendoscopy and white-light endoscopy for diagnosis of gastric intestinal metaplasia. Surg Endoscopy. (2013) 27:4649–55. doi: 10.1007/s00464-013-3098-x
16. Popa P, Matei M, Streba L, Florescu MM, and Streba CT. Probe - based confocal endomicroscopy and endoscopy in NBI module - the role in clinical decision - case reports. Curr Health Sci J. (2023) 49:423–8. doi: 10.12865/CHSJ.49.03.16
17. Zhang HP, Yang S, Chen WH, Chen WH, Hu TT, and Lin J. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among asian population: A system review and meta-analysis. Scandinavian J Gastroenterol. (2017) 52:382–8. doi: 10.1080/00365521.2016.1275770
18. Persaud AB and Diehl DL. Training endosonographers in needle-based confocal laser endomicroscopy: is there still a need? Gastrointestinal Endoscopy. (2023) 98:965–7. doi: 10.1016/j.gie.2023.09.029
19. Pilonis ND, Januszewicz W, and Di Pietro M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: technical aspects and clinical applications. Trans Gastroenterol Hepatol. (2022) 7:7. doi: 10.21037/tgh.2020.04.02
20. Li Z, Zuo XL, Yu T, Gu XM, Zhou CJ, Li CQ, et al. Confocal laser endomicroscopy for in vivo detection of gastric intestinal metaplasia: A randomized controlled trial. Endoscopy. (2014) 46:282–90. doi: 10.1055/s-0033-1359215
Keywords: confocal laser endomicroscopy, early diagnosis, gastrointestinal cancer, chronic gastritis, optical biopsy
Citation: Peng X, Zheng Y-P and Zhang Y (2025) The benefits of using confocal laser endomicroscopy in the diagnosis of gastric cancer and precancerous lesions: a case report. Front. Oncol. 15:1649985. doi: 10.3389/fonc.2025.1649985
Received: 20 June 2025; Accepted: 15 September 2025;
Published: 06 October 2025.
Edited by:
Jonathan Soldera, University of Caxias do Sul, BrazilReviewed by:
Monica Rusticeanu, Asklepios Klinikum Schwalmstadt, GermanyClara Lavita Angelina, Politeknik Manufaktur Negeri Bangka Belitung, Indonesia
Copyright © 2025 Peng, Zheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, eWFuazEyMjNAMTYzLmNvbQ==
 Xiaoting Peng
Xiaoting Peng Yan Zhang
Yan Zhang