- 1Department of Oncology, Changzhi People’s Hospital, The Affiliated Hospital of Changzhi Medical College, Changzhi, China
- 2Department of Ophthalmology, Changzhi People’s Hospital, The Affiliated Hospital of Changzhi Medical College, Changzhi, China
Background: Isolated ocular metastasis from esophagogastric junction (EGJ) cancer is extremely rare, and no standardized therapeutic approach has been established to date. Reporting such cases may help clarify optimal management strategies for uncommon metastatic patterns.
Case presentation: We describe a 44-year-old male patient who developed isolated ocular oligometastasis 10 months after radical EGJ cancer resection. Comprehensive evaluation confirmed left eye involvement. Following multidisciplinary team (MDT) discussion, a combined treatment plan was implemented, starting with systemic therapy consisting of docetaxel, tegafur/gimeracil/oteracil (S-1), and tislelizumab. After achieving disease stabilization, the patient underwent left eye enucleation with ocular prosthesis implantation, followed by adjuvant radiotherapy and maintenance S-1 chemotherapy. At a 55-month follow-up, he remains disease-free with an excellent performance status (PS 0).
Conclusion: This case highlights that integrated multimodal therapy—including systemic and local interventions—can result in long-term survival for patients with isolated ocular metastasis from EGJ cancer. MDT-based, individualized treatment planning is essential for optimizing outcomes in rare metastatic scenarios and may inform future precision oncology approaches.
Introduction
Gastric cancer ranks fifth worldwide in both incidence and mortality among malignant tumors (1). The esophagogastric junction (EGJ) is a frequent site of origin, and the tumor commonly spreads through direct invasion, lymphatic dissemination, or hematogenous metastasis. The peritoneum, distant lymph nodes, and liver are the most frequent metastatic sites (2), whereas ocular metastasis is exceedingly rare, with a reported incidence of less than 0.1% (3). Most lesions arise via hematogenous spread to the choroid, leading to visual impairment or blindness.
Because of its rarity, ocular metastasis from EGJ cancer lacks standardized diagnostic and therapeutic guidelines. Diagnosis relies on correlation with prior malignancy, imaging findings, and histopathology, and differentiation from primary ocular tumors such as uveal melanoma or inflammatory pseudotumor is essential. The prognosis is generally poor, and treatment is often palliative. Recent studies, however, suggest that multimodal management—including systemic therapy combined with local surgery or radiotherapy—may improve survival in patients with oligometastatic disease (4).
Here, we report a rare case of isolated ocularoligometastasis after radical surgery for EGJ cancer, successfully managed with immunochemotherapy and local therapy. This case highlights the potential benefit of combining systemic and local treatment for rare ocular metastases and provides insight into individualized strategies for EGJ cancer oligometastasis.
Case report
A 44-year-old male patient presented in December 2019 with upper abdominal pain and dysphagia. Gastroscopy revealed carcinoma of the EGJ, and histopathology confirmed moderately differentiated adenocarcinoma. Serum CEA and CA72–4 levels were within normal ranges. The patient underwent combined thoracoscopic–laparoscopic radical resection, and postoperative staging was pT3N1M0, stage IIB, with proficient mismatch repair (pMMR) and negative HER-2 expression. From February to July 2020, he completed six cycles of SOX adjuvant chemotherapy [oxaliplatin 130 mg/m2 i.v. on day 1; tegafur/gimeracil/oteracil (S-1) 40 mg/m2 orally twice daily on days 1–14, every 3 weeks], with good tolerance and no complications.
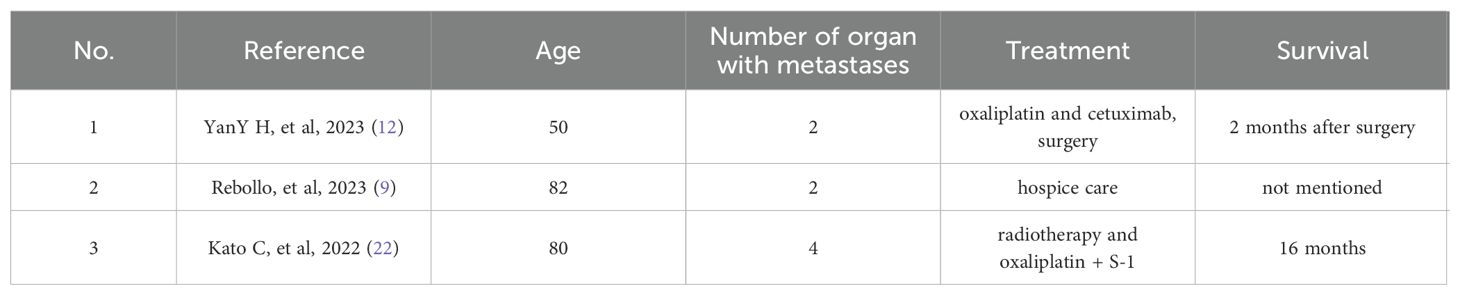
In October 2020, the patient experienced progressive visual impairment in the left eye, leading to complete blindness within several weeks. Physical examination: blindness in the left eye. Ocular ultrasonography and fundus color Doppler imaging revealed a posterior intraocular mass and retinal detachment (Figures 1, 2). Orbital magnetic resonance imaging (MRI) showed thickening of the posterior wall of the left eyeball (Figure 3), and PET-CT demonstrated increased FDG uptake confined to the ocular lesion without evidence of systemic metastasis. Serum CEA and CA72-4 remained within normal limits. Considering the patient’s medical history and distinctive imaging findings, a multidisciplinary team (MDT) consultation suggested the presence of distant metastasis. It was therefore recommended that systemic therapy be initiated first to achieve tumor control.
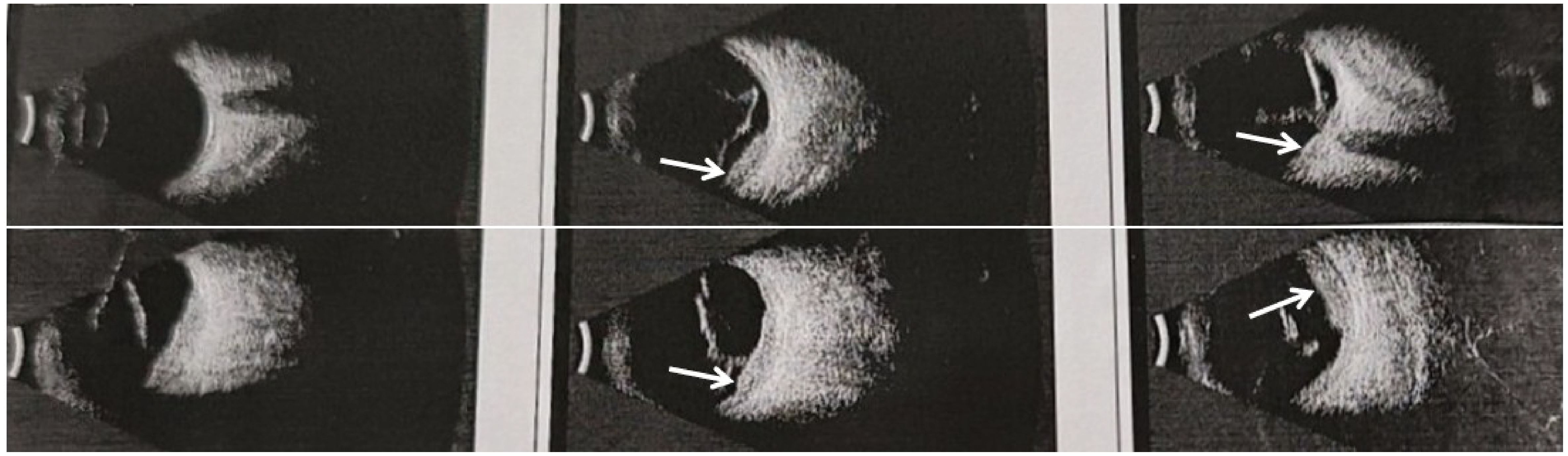
Figure 1. Ultrasonography of the left eyeball showing thickening of the choroid at the posterior pole, suggestive of an intraocular space-occupying lesion.
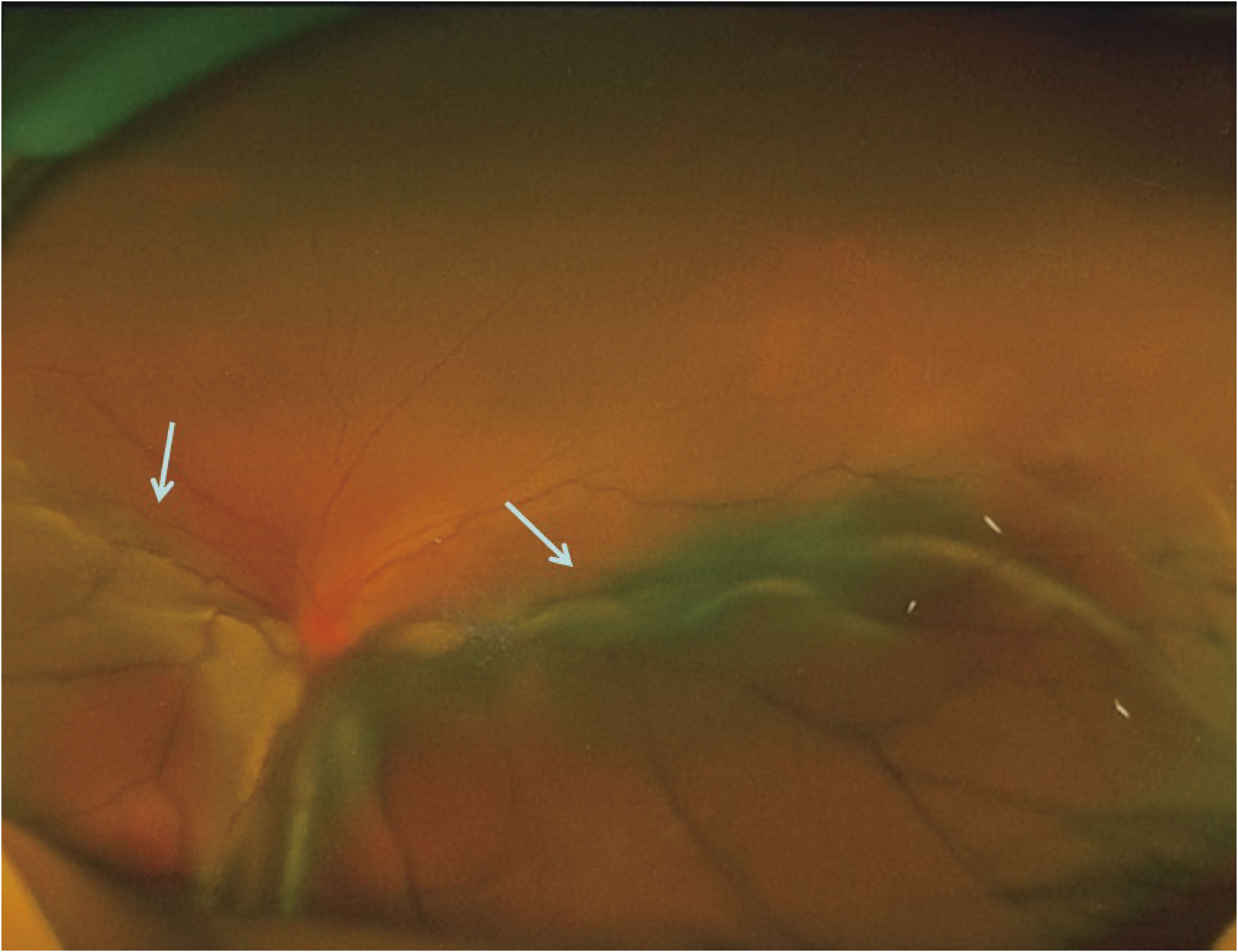
Figure 2. Fundus color photograph of the left eye showing elevation of the inferior retina involving the posterior pole.
Figure 3. Timeline summarizing the patient’s diagnostic and therapeutic course for ocular metastasis (target lesion diameter, TLD).
From November 2020 to March 2021, the patient received combination systemic therapy consisting of docetaxel (75 mg/m2, day 1), S-1 (40 mg/m2, days 1–14), and tislelizumab (200 mg, day 1) every 3 weeks for four cycles. Follow-up orbital MRI was performed, and based on the immune-related Response Evaluation Criteria in Solid Tumors (irRECIST), the ocular lesion was assessed as stable disease (SD). The MDT concluded that the patient met the criteria for oligometastatic disease and that the tumor was under adequate systemic control; surgical resection was therefore recommended. In May 2021, the patient underwent left eye enucleation with orbital implant placement. Postoperative histopathology (Figure 4) confirmed metastatic gastric adenocarcinoma involving the eyeball, with a positive optic nerve margin. Immunohistochemistry showed PD-L1 combined positive score (CPS) = 3, HER2 negative, and proficient mismatch repair (pMMR). From 9 July to 17 August 2021, adjuvant local radiotherapy was administered to the orbital region (total dose 50 Gy in 25 fractions), followed by adjuvant chemotherapy with S-1 for 3 months. As of the end of April 2025, after 55 months of follow-up, the patient’s serum CEA and CA72-4 levels remain within normal ranges, and imaging studies show no evidence of recurrence or new metastases. His performance status (PS) score is 0, with good quality of life, no signs of anxiety or depression, and full ability to work. The patient was satisfied with the entire diagnostic and treatment process. The patient continues to be followed up regularly (treatment course shown in Figure 3).
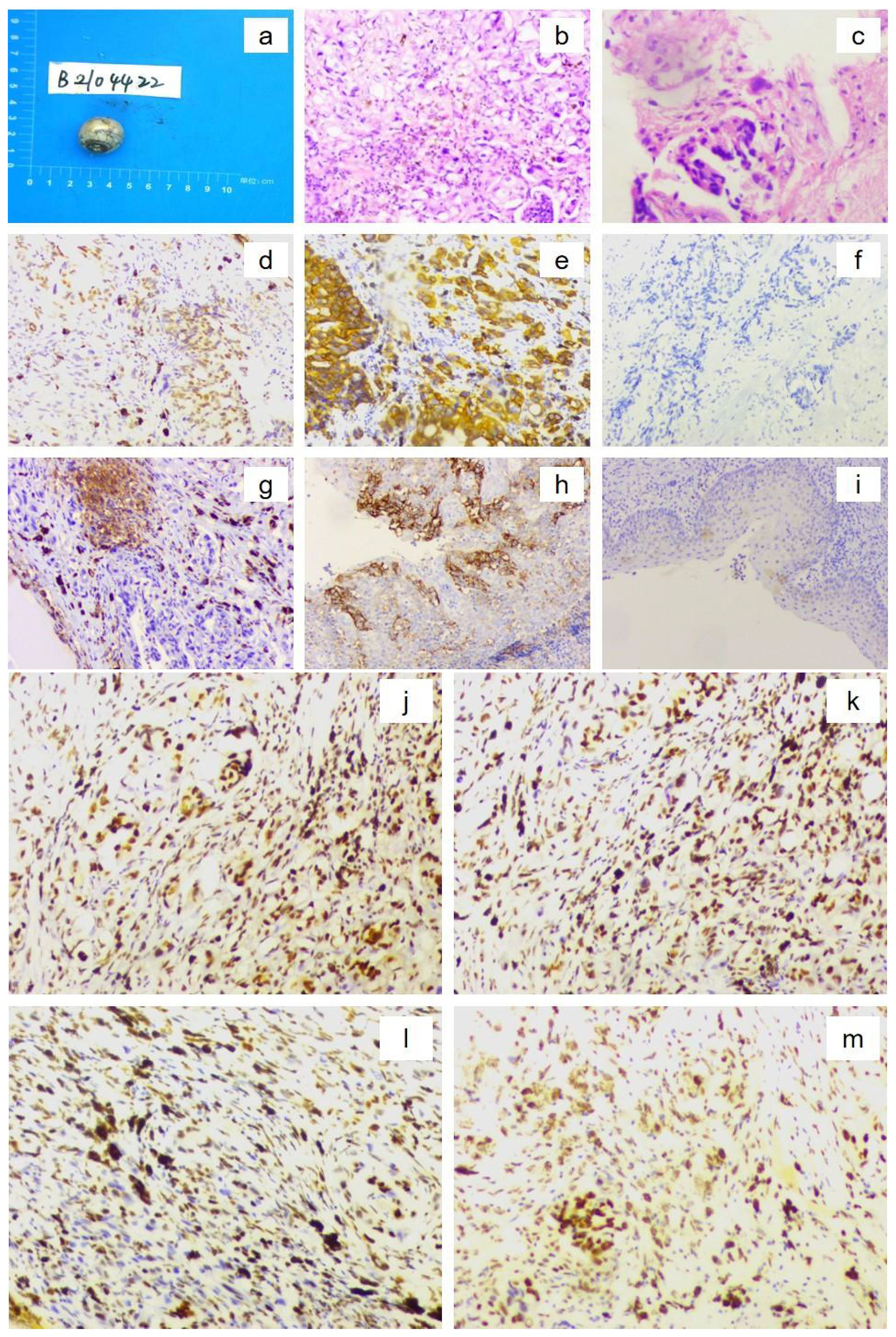
Figure 4. Postoperative histopathological findings of the left eyeball confirming metastatic gastric adenocarcinoma. [(a) Gross specimen of the resected eyeball; (b) Hematoxylin–eosin (H&E) staining showing numerous atypical cells (×10 objective); (c) H&E staining demonstrating tumor cell invasion into the optic nerve (×10 objective). Immunohistochemistry showing (×10 magnification): (d) CDX2 positive; (e) CK18 positive; (f) HER2 score 0; (g) PD-L1 expression, combined positive score (CPS) = 3; (h) PD-L1-positive control; (i) PD-L1-negative control; (j) MLH1 positive; (k) MSH2 positive; (l) MSH6 positive; and (m) PMS2 positive.].
Discussion
Ocular metastasis from gastric cancer is extremely rare and generally associated with poor prognosis, often accompanied by multi-organ dissemination (3, 5). Its incidence is closely related to age, occurring predominantly in patients aged 80 years and older (6). The choroid, orbit, and extraocular muscles are among the most commonly affected sites (7–10). Because of the choroid’s rich vascular supply and slow blood flow, circulating tumor cells can easily lodge there and form metastatic deposits. Patients with ocular metastasis from gastric cancer often present initially with ocular symptoms (11, 12). Choroidal metastases typically manifest as decreased visual acuity or visual field defects (12, 13), while orbital metastases may present with proptosis, diplopia, or ptosis (7, 8). Diagnosis of ocular metastasis still relies on imaging modalities, such as MRI or CT, and definitive confirmation by histopathology (5, 6). Choroidal metastasis must be distinguished from primary choroidal melanoma and inflammatory lesions (12). Furthermore, many reported cases show concurrent distant metastases at the time of ocular involvement (3, 5), adding to the diagnostic complexity. In the present case, the patient developed a solitary ocular metastasis without evidence of other distant lesions, consistent with the biological characteristics of oligometastasis, thereby providing an opportunity for potentially curative multidisciplinary treatment.
For patients with gastric cancer, MDT discussion prior to treatment initiation is strongly recommended, as it allows for comprehensive assessment of the patient’s overall condition, better coordination of treatment sequence and modalities, and improvement in both quality of life and overall survival (14). According to the European OMEC-4 guidelines, for patients with metachronous oligometastatic gastric cancer and a disease-free interval (DFI) ≤ 2 years, systemic therapy should be administered first, followed by reassessment of local resectability (15). Systemic treatment options include surgery, radiotherapy, chemotherapy, targeted therapy, and immune checkpoint inhibitors (ICIs) (6, 16). Programmed death ligand-1 (PD-L1) combined positive score (CPS) is one of the key biomarkers for assessing potential benefit from ICI therapy; a higher CPS is generally associated with improved response and prognosis (17, 18). In the present case, the tumor exhibited PD-L1 expression with CPS > 1, suggesting a potential benefit from immunotherapy. Tislelizumab has demonstrated favorable efficacy both in the perioperative setting and as second-line therapy for gastric cancer (19, 20); therefore, a combination regimen of tislelizumab plus chemotherapy was selected for this patient. Following completion of two and four cycles of systemic therapy, timely MDT discussions were conducted, enabling appropriate evaluation of treatment response and facilitating surgical intervention at the optimal time.
Patients with ocular metastasis from gastric cancer generally have a poor prognosis and short median survival (3, 5). Studies have shown that once ocular metastasis occurs, the mean interval from diagnosis of the primary gastric tumor to the onset of ocular symptoms is approximately 25.4 months, while the average time from ocular symptom onset to death is only 3.3 months (21). A review of reports published over the past 5 years (Table 1) identified only three documented cases of ocular metastasis from gastric cancer, all accompanied by metastases to other organs. Treatment approaches in these cases included chemotherapy, surgery, and radiotherapy. Kato et al. reported a rare case of choroidal metastasis after surgery for EGJ carcinoma in an elderly male; despite active treatment, the survival time was only 16 months (22). Huang et al. described a middle-aged man with advanced gastric cancer and multiple metastases to the choroid and bone, who underwent systemic therapy followed by eye enucleation but died 2 months postoperatively (12). In contrast, the present patient developed solitary ocular oligometastasis. Initial chemo-immunotherapy effectively controlled disease progression and provided a therapeutic window for surgery. Subsequent postoperative radiotherapy eliminated residual tumor cells, and adjuvant chemotherapy suppressed potential micrometastases. This integrated multimodal approach ultimately led to long-term disease-free survival.
This case provides several important insights: (1) Early recognition of intraocular symptoms and timely completion of comprehensive imaging studies can improve the diagnostic accuracy of ocular metastasis from gastric cancer. (2) For patients with oligometastatic disease, striving to achieve a no-evidence-of-disease (NED) status is essential; MDT deciding the treatment sequence is the key to improve the efficacy of treatment. (3) Although solitary ocular metastasis after EGJ carcinoma resection is extremely rare, a comprehensive treatment strategy combining systemic therapy with local surgery and radiotherapy can achieve long-term disease-free survival. This case highlights the crucial role of MDT collaboration in developing individualized therapeutic strategies for EGJ carcinoma and provides a valuable reference for managing similar cases. However, as a single case report, it has inherent limitations and lacks molecular-level mechanistic investigation. Further studies with larger cohorts are warranted to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by China Clinical Trial Registration and Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YG: Writing – review & editing, Writing – original draft, Supervision, Validation, Investigation. YZ: Writing – review & editing. NM: Data curation, Writing – review & editing. XZ: Formal Analysis, Writing – review & editing. YD: Investigation, Writing – review & editing. JZ: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Clinical Specialty Talent Professional Competence Innovation and Application Research Project (RCLX2315091).
Acknowledgments
We thank Dr. Bin Qiao for editing this manuscript. We thank the patient for permitting us to use his data to complete this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PS, Performance status; MDT, Multidisciplinary team; SD, Stable disease; MRI, Magnetic resonance imaging; CT, Computed tomography; DFI, Disease-free interval; ICIs, Immune checkpoint inhibitors; PD-L1, Programmed cell death ligand 1; TLD, Target lesion diameter; irRECIST, Immune-related Response Evaluation Criteria in Solid Tumors.
References
1. Zhang X, Yang L, Liu S, Cao L, Wang N, Li H, et al. Interpretation of the global Malignant tumor statistics report 2022. Chin J Oncol. (2024) 46:710–21. doi: 10.3760/cma.j.cn112152-20240416-00152
2. Kwee RM and Kwee TC. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J Gastroenterol. (2015) 21:10502–9. doi: 10.3748/wjg.v21.i37.10502
3. Chen Y, Yang YC, Tang LY, Ge QM, Shi WQ, Su T, et al. Risk factors and their diagnostic values for ocular metastases in gastric adenocarcinoma. Cancer Manag Res. (2021) 13:5835–43. doi: 10.2147/CMAR.S311474
4. Narita YK, Muro K, and Takahari D. Practical management of oligometastatic gastric cancer. ESMO Gastrointest Oncol. (2024) 6:1–12. doi: 10.1016/j.esmogo.2024.100108
5. Shields CL, Kalafatis NE, Gad M, Sen M, Laiton A, Silva AMV, et al. Metastatic tumours to the eye. Review of metastasis to the iris, ciliary body, choroid, retina, optic disc, vitreous, and/or lens capsule. Eye (Lond). (2023) 37:809–14. doi: 10.1038/s41433-022-02015-4
6. Tsuruta Y, Maeda Y, Kitaguchi Y, Hayama M, Nojima S, Tsuda T, et al. A case of endonasal endoscopic surgery for intraorbital metastasis of gastric ring cell carcinoma. Ear Nose Throat J. (2022) 101:NP24–7. doi: 10.1177/0145561320943372
7. Kim J, Kim J, and Baek S. Orbital metastasis from gastric cancer presenting as orbital cellulitis with ptosis. J Craniofac Surg. (2022) 33:e133–5. doi: 10.1097/SCS.0000000000008031
8. Sellami M, Ayadi S, Abbes A, Mnejja M, Hammami B, Boudaouara T, et al. Diplopia secondary to gastric adenocarcinoma metastasis to the superior oblique muscle. Ear Nose Throat J. (2022) p:1455613221145277. doi: 10.1177/01455613221145277
9. Rebollo NP, Yeaney GA, Hwang CJ, and Perry JD. Metastatic gastric carcinoma to the eyelids masquerading as a chalazion: A case report. Am J Ophthalmol Case Rep. (2023) 29:101814. doi: 10.1016/j.ajoc.2023.101814
10. Roohe SL, Gan IM, Weerd KV, Lopuhaa B, Verdijk RM, and Paridaens D. Diplopia as the first sign of gastric carcinoma. Case Rep Ophthalmol. (2021) 12:870–4. doi: 10.1159/000519953
11. Chong YJ, Azzopardi M, Ng B, Salvi SM, and Sreekantam S. Ocular metastasis as first presentation of large-cell neuroendocrine carcinoma. Case Rep Ophthalmol. (2023) 14:684–91. doi: 10.1159/000535233
12. Huang YY, Zhu LY, and Li ZD. Choroidal metastasis from gastric cancer: A case report and review of the literature. J Int Med Res. (2023) 51:3000605231187943. doi: 10.1177/03000605231187943
13. Zou J, Shen YK, Wu SN, Wei H, Li QJ, Xu SH, et al. Prediction model of ocular metastases in gastric adenocarcinoma: machine learning-based development and interpretation study. Technol Cancer Res Treat. (2024) 23:15330338231219352. doi: 10.1177/15330338231219352
14. Rivera F, Longo F, Martin Richard M, Richart P, Alsina M, Carmona A, et al. SEOM-GEMCAD-TTD clinical guideline for the diagnosis and treatment of gastric cancer (2023). Clin Transl Oncol. (2024) 26:2826–40. doi: 10.1007/s12094-024-03600-7
15. Kroese TE, Bronzwaer S, van Rossum PSN, Schoppman SF, Deseyne PRAJ, van Cutsem E, et al. European clinical practice guidelines for the definition, diagnosis, and treatment of oligometastatic esophagogastric cancer (OMEC-4). Eur J Cancer. (2024) 204:114062. doi: 10.1016/j.ejca.2024.114062
16. Tsutsumi WD, Rattanasuwan A, and Aryasit O. Clinical features and treatment outcomes of intraocular and ocular adnexal metastasis. Sci Rep. (2024) 14:15258. doi: 10.1038/s41598-024-64464-z
17. Kono K, Nakajima S, and Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. (2020) 23:565–78. doi: 10.1007/s10120-020-01090-4
18. Hou WY, Zhao, and Zhu H. Predictive biomarkers for immunotherapy in gastric cancer: current status and emerging prospects. Int J Mol Sci. (2023) 24:15321. doi: 10.3390/ijms242015321
19. Nie RC, Yuan SQ, Ding Y, Chen YM, Li YF, Liang CC, et al. Perioperative tislelizumab plus chemotherapy for locally advanced gastroesophageal junction adenocarcinoma (NEOSUMMIT-03): a prospective, nonrandomized, open-label, phase 2 trial. Signal Transduct Target Ther. (2025) 10:60. doi: 10.1038/s41392-025-02160-8
20. Li J, Xu Y, Zang AM, Gao Y, Gao Q, Zhang Y, et al. Tislelizumab in previously treated, locally advanced unresectable/metastatic microsatellite instability-high/mismatch repair-deficient solid tumors. Chin J Cancer Res. (2024) 36:257–69. doi: 10.21147/j.issn.1000-9604.2024.03.03
21. Amemiya T, Hayashida H, and Dake H. Metastatic orbital tumors in Japan: a review of the literature. Ophthalmic Epidemiol. (2002) 9:35–47. doi: 10.1076/opep.9.1.35.1718
Keywords: esophagogastric junction carcinoma, ocular metastasis, oligometastasis, multimodal treatment, long survival
Citation: Gao Y, Zhai Y, Ma N, Zhang X, Du Y and Zhao J (2025) Long-term survival after solitary ocular metastasis following radical resection of esophagogastric junction carcinoma: a rare case report. Front. Oncol. 15:1650111. doi: 10.3389/fonc.2025.1650111
Received: 19 June 2025; Accepted: 10 November 2025; Revised: 06 November 2025;
Published: 20 November 2025.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaCopyright © 2025 Gao, Zhai, Ma, Zhang, Du and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhao, emhhb2p1bjM4MEBvdXRsb29rLmNvbQ==
 Yangjun Gao
Yangjun Gao Yuru Zhai2
Yuru Zhai2 Xiaoling Zhang
Xiaoling Zhang Yunyi Du
Yunyi Du Jun Zhao
Jun Zhao