Abstract
Background:
Systemic inflammatory markers, particularly pretreatment red cell distribution width (RDW) and hemoglobin to red cell distribution width ratio (HRR), have been associated with prognosis in several cancers. This study aimed to investigate the relationship between the preoperative RDW, HRR and clinicopathologic characteristics of patients undergoing total laryngectomy and their correlation with prognosis.
Methods:
The optimal cut-off values of RDW and HRR to the overall survival (OS) of patients were determined by the receiver operating characteristic (ROC) curves, which in turn divided the patients into high-value and low-value groups for further stratified analyses. Patient survival was analyzed using Kaplan-Meier survival curves. Additionally, univariate and multivariate Cox regression analyses were conducted to evaluate the predictive roles of RDW and HRR on the prognosis of patients following total laryngectomy.
Results:
The high RDW group demonstrated statistically significant associations with TNM clinical stage, cervical lymph node metastasis, and vascular infiltration (P < 0.05). Similarly, the low HRR group exhibited significant associations with gender, histologic grade, TNM clinical stage, cervical lymph node metastasis, and vascular infiltration (P < 0.05).The optimal cut-off values for predicting overall survival (OS) for patients, as determined by ROC curves, were 13.75 for RDW and 10.79 for HRR. Additionally, RDW emerged as an independent prognostic factor for OS in this population (HR = 3.060, 95% CI 2.222–4.215, P < 0.001).
Conclusion:
Preoperative RDW and HRR are prognostic risk factors for OS in patients undergoing total laryngectomy, with RDW serving as an independent predictor of prognosis.
Highlights
-
A single-center retrospective study found that the preoperative RDW parameter and the hemoglobin-to-RDW ratio (Hb-to-RDW ratio, HRR) were prognostic risk factors for survival in laryngeal cancer patients undergoing total laryngectomy.
-
RDW and HRR are simple, easy to measure and inexpensive indicators that can provide a valuable diagnostic basis for the prognosis of laryngeal cancer patients.
1 Introduction
Laryngeal cancer presents a significant public health challenge in China, with rising incidence and mortality rates over recent decades.As one of the most common malignant tumors of the head and neck, it accounts for nearly one-third of all head and neck cancers (1) and has a significant impact on global health. In 2022, an estimated 103,216 individuals are projected to die from laryngeal cancer worldwide, with age-standardized incidence and mortality rates of 3.9 and 2.1 per 100,000, respectively (2). Total laryngectomy is the primary treatment for patients with locally advanced laryngeal cancer; however, it inevitably impacts laryngeal functions, including phonation, swallowing, and respiration. To enhance the quality of life for patients, it is crucial to investigate prognostic factors for individuals with middle and late stage laryngeal cancer and to implement appropriate preventive and therapeutic measures.
Biomarkers are defined as physiological characteristics that can be objectively assessed and serve as indicators of pathological processes, risk, and pharmacological responses to therapy (3, 4). Recent studies have shown that the red blood cell distribution width (RDW) parameter in routine blood counts is strongly associated with the prognosis of various tumors. Red blood cell distribution width (RDW) is a marker of changes in red blood cell size. RDW contributes to cancer development through inflammatory responses in the microenvironment and has diagnostic and prognostic value for various tumor types, including lung, liver, prostate, esophageal cancers, and chronic lymphocytic leukemia (5–10). It is also considered a biomarker potentially involved in endothelial dysfunction (11), oxidative stress, and inflammatory processes in vascular diseases (3, 12).Hemoglobin (Hb) is one of the most important indicators in routine blood tests, reflecting the human body’s ability to produce red blood cells while also assisting in the diagnosis and treatment of various diseases. Studies have shown that hemoglobin levels correlate with prognosis in the treatment of various tumors. Hb serves as a diagnostic indicator of anemia and is also utilized for prognostic assessment in patients with lung cancer (13). Low Hb levels are recognized as a prognostic marker in patients with oral squamous cell carcinoma (14). Additionally, combining Hb with RDW has been shown to correlate with prognosis in various tumors (6, 7). In this paper, we analyze the clinicopathological characteristics and prognostic data of patients who underwent total laryngectomy to explore the potential connection between RDW, the Hb/RDW ratio (HRR), clinicopathological characteristics, and survival, aiming to identify a more convenient, reliable, and cost-effective index for the diagnosis and treatment of laryngeal cancer.
2 Materials and methods
2.1 Patients and data
In this study, we collected cases of patients who underwent total laryngectomy at the Department of Otorhinolaryngology and Head and Neck Surgery at the First Hospital of Jilin University, from January 2015 to December 2021. The collected data included age, gender, smoking history, drinking history, history of hypertension and diabetes mellitus, tumor site, T-stage, clinical stage of TNM, degree of histological differentiation, vascular infiltration, Perineural infiltration, and metastasis to the cervical lymph nodes. Based on pathological and imaging findings, clinicopathological staging was performed according to the eighth edition of the TNM staging criteria for laryngeal cancer published by the American Joint Committee on Cancer (AJCC).Additionally, the red blood cell distribution width (RDW) and hemoglobin (Hb) levels were obtained from the patients’ routine peripheral blood test results within one week prior to surgery. The hemoglobin-to-RDW ratio (HRR) was calculated as the ratio of peripheral blood Hb to RDW. All included patients were newly treated individuals with complete clinicopathological data and comprehensive peripheral blood hematology parameters, and the final number of patients included in the study was 117.
Inclusion criteria: 1) Patients with primary laryngeal squamous cell carcinoma treated by total laryngectomy; 2) No distant metastasis detected in the preoperative examination; 3) Good general health without serious systemic diseases; 4) Complete results of clinicopathological data and preoperative hematology tests.
Exclusion Criteria: 1) Patients with previous malignant tumors in the head and neck region; 2) Patients who have undergone prior treatments such as radiotherapy or chemotherapy before the operation; 3) Patients with preoperative conditions related to blood transfusion, hepatic or renal insufficiency, or any blood or immune system disorders that may affect inflammatory index levels; 4) Patients who have taken anticoagulants, antiplatelet agents, hormones, or other medications that could influence blood test results within one month; 5) Patients with infectious diseases, immune system disorders, or hematological diseases; 6) Patients with incomplete follow-up data. The primary endpoint of this study was overall survival (OS), defined as the duration from the day of surgery to the date of death from any cause or the last follow-up visit. As this was a retrospective study, informed consent from patients was waived. This study was approved by the Ethics Committee of the First Hospital of Jilin University, under Ethics Approval No. (2025) and Pro-review No. (2025-076).
Methods Peripheral blood was collected from the fasting antecubital vein of all patients in the morning. Hematological analysis was conducted using the SYSMEX XN9000 with routine blood testing, calibration and quality control strictly performed according to the manufacturer’s instructions.
2.2 Statistical analysis
Statistical analyses were performed using IBM SPSS version 27.0 (IBM, Chicago, Illinois, USA). Count data were expressed as numbers and percentages. The chi-square test was employed for comparisons between groups. Kaplan-Meier survival curves were generated for survival analysis, and Log-rank tests were utilized to detect group differences. Cox proportional hazards models were employed for both univariate and multivariate regression analyses related to prognosis. A p-value < 0.05 was considered statistically significant. Survival curves and forest plots were created using R software (version 4.4.1). The area under the curve (AUC) was calculated using Receiver Operating Characteristic (ROC) analysis, where an AUC > 0.5 indicated predictive value. Confidence intervals for all p-values were two-sided, and a p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of study population
A total of 117 patients met the inclusion criteria for this study. We stratified the patients by age (median age 62 years), gender, smoking history, drinking history, hypertension, diabetes mellitus, tumor site, tumor T-stage, TNM clinicopathological stage, degree of histological differentiation, vascular infiltration, perineural infiltration, and metastasis to the cervical lymph nodes (see Table 1).
Table 1
| Variable | Count(Percentage %) |
|---|---|
| Gender | |
| Male | 97 (82.9) |
| Female | 20 (17.1) |
| Age group (years) | |
| ≤62 | 55 (47.0) |
| >62 | 62 (53.0) |
| Smoking history | |
| No | 42 (35.9) |
| Yes | 75 (64.1) |
| Drinking history | |
| No | 68 (58.1) |
| Yes | 49 (41.9) |
| Hypertension | |
| No | 94 (80.3) |
| Yes | 23 (19.7) |
| Diabetes mellitus | |
| No | 110 (94.0) |
| Yes | 7 (6.0) |
| TNM clinical stage | |
| I-II | 26 (22.2) |
| III-IV | 91(77.8) |
| Tumor T-stage | |
| T1-T2 | 30(25.6) |
| T3-T4 | 87(74.4) |
| Tumor site | |
| Supraglottic | 33 (28.2) |
| Glottic | 79(67.5) |
| Subglottic | 5 (4.3) |
| Histological grade | |
| Poorly differentiated | 26 (22.2) |
| Moderately differentiated | 83 (70.9) |
| Well differentiated | 8 (6.9) |
| Cervical lymph node metastasis | |
| No | 68 (58.1) |
| Yes | 49 (41.9) |
| Vascular infiltration | |
| No | 67 (57.3) |
| Yes | 50 (42.7) |
| Perineural infiltration | |
| No | 104 (88.9) |
| Yes | 13 (11.1) |
Baseline characteristics of study population.
3.2 Determination of optimal cutoff values for RDW and HRR using ROC curve analysis
In this study, we assessed the predictive efficacy of RDW and HRR for the prognosis of patients undergoing total laryngectomy by comparing RDW and HRR values with their survival status, using the area under the curve (AUC) from ROC analysis. The optimal cutoff values were determined using the maximum Youden index (sensitivity + specificity - 1), and the ROC curves for RDW and HRR were generated (see Figure 1A). The AUC for RDW was 0.825 (95% CI: 0.748-0.902, P < 0.001), with a maximum Youden index of 0.596. The sensitivity and specificity were 66.7% and 92.9%, respectively, corresponding to an RDW value of 13.75. The AUC for HRR was 0.718 (95% CI: 0.625-0.812, P < 0.001), with a maximum Youden index of 0.415. The sensitivity and specificity were 78.3% and 63.2%, respectively, corresponding to an HRR value of 10.79 (see Table 2). The optimal cutoff values for stratification analysis were 13.75 for RDW and 10.79 for HRR. Patients were categorized into a low RDW group (RDW < 13.75) and a high RDW group (RDW ≥ 13.75), as well as a low HRR group (HRR < 10.79) and a high HRR group (HRR ≥ 10.79). We evaluated the predictive efficacy of RDW and HRR for the prognosis of patients undergoing total laryngectomy. This was accomplished by comparing RDW and HRR values with survival status using the area under the curve (AUC) from ROC analysis. The optimal cutoff values were determined using the maximum Youden index (sensitivity + specificity - 1), and the ROC curves for both RDW and HRR were generated (Figure 1A). The AUC for RDW was 0.825 (95% CI: 0.748-0.902, P < 0.001), with a maximum Youden index of 0.596. The sensitivity and specificity were 66.7% and 92.9%, respectively, corresponding to a cutoff value of 13.75 for RDW. For HRR, the AUC was 0.718 (95% CI: 0.625-0.812, P < 0.001), with a maximum Youden index of 0.415. The sensitivity and specificity were 78.3% and 63.2%, respectively, corresponding to a cutoff value of 10.79 for HRR (Table 2). In this study, optimal cutoff values of RDW (13.75) and HRR (10.79) were established for stratification analysis. Patients were classified into four groups: low RDW (RDW < 13.75), high RDW (RDW ≥ 13.75), low HRR (HRR < 10.79), and high HRR (HRR ≥ 10.79) (Table 3).
Figure 1
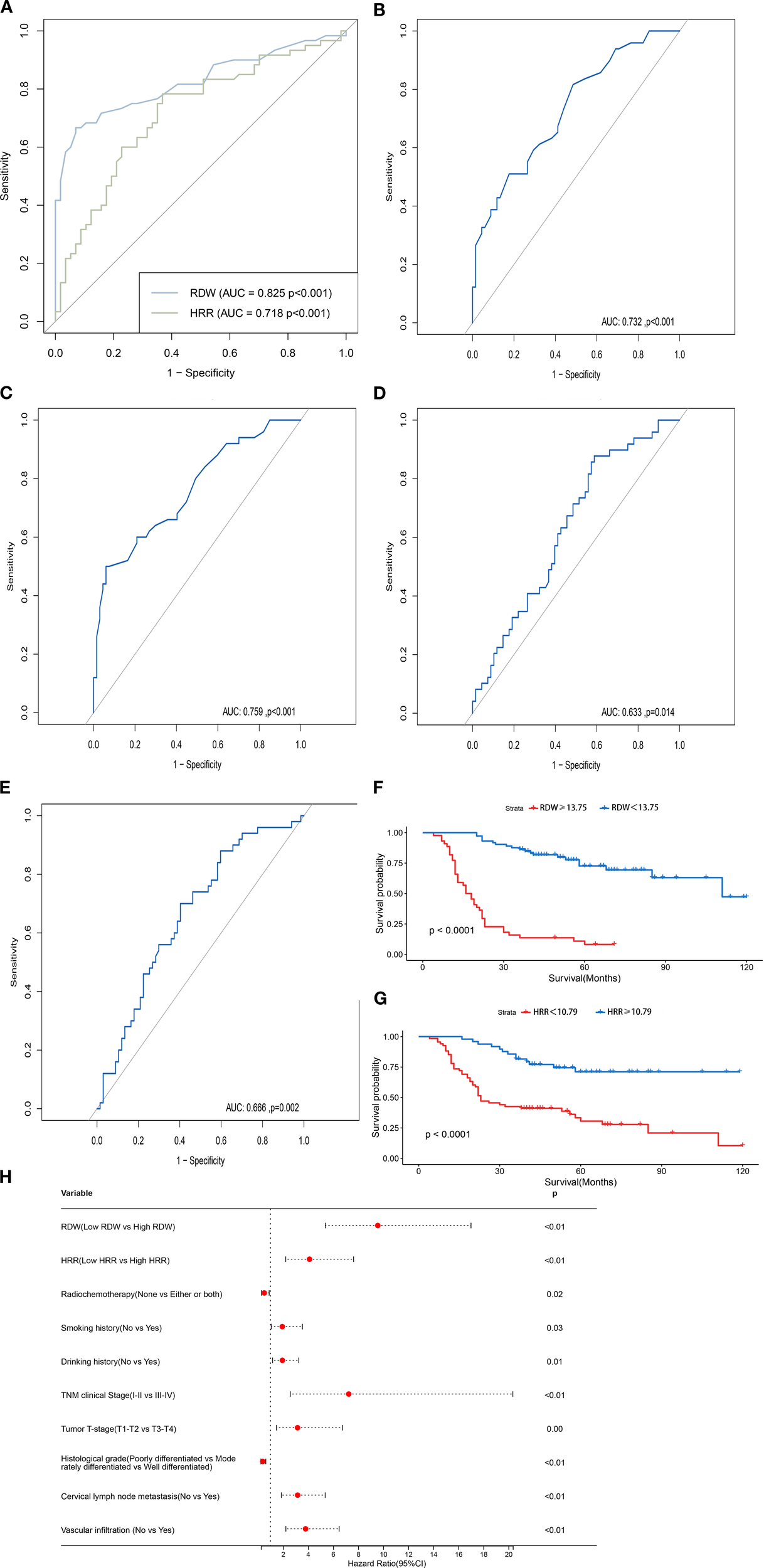
(A) The ROC curves of preoperative RDW and HRR cutoff values. (B) The ROC curve for RDW concerning cervical lymph node. metastasis. (C) The ROC curve for RDW concerning vascular infiltration. (D) The ROC curve for HRR concerning cervical lymph. node metastasis. (E) The ROC curve for HRR concerning vascular infiltration. (F) Survival curves for patients in high and low RDW. groups. (G) Survival curves for patients in high and low HRR groups. (H) HR Forest Plot for the Univariate Cox Proportional. Hazards Model of OS.
Table 2
| Index | AUC (95%CI) | Cutoff value | Youden index | Sensitivity (%) | Specificity (%) | P-value |
|---|---|---|---|---|---|---|
| RDW | 0.825(0.748-0.902) | 13.75 | 0.596 | 66.7% | 92.9% | <0.001 |
| HRR | 0.718(0.625-0.218) | 10.79 | 0.415 | 78.3% | 63.2% | <0.001 |
Preoperative ROC curve analysis of RDW and HRR.
Table 3
| Variable | RDW | X2 | p | HRR | X2 | p | ||
|---|---|---|---|---|---|---|---|---|
| Low RDW | High RDW | Low HRR | High HRR | |||||
| (N=73) | (N=44) | (N=68) | (N=49) | |||||
| Gender | 0.562 | 0.453 | 4.745 | 0.029 | ||||
| Male | 62(84.9%) | 35(79.5%) | 52(76.5%) | 45(91.8%) | ||||
| Female | 11(15.1%) | 9(20.5%) | 16(23.5%) | 4(8.2%) | ||||
| Age (years) | 1.608 | 0.205 | 0.131 | 0.717 | ||||
| ≤62 | 31(42.5%) | 24(54.5%) | 31(45.6%) | 24(49.0%) | ||||
| >62 | 42(57.5%) | 20(45.5%) | 37(54.4%) | 25(51.0%) | ||||
| Smoking History | 2.280 | 0.131 | 0.886 | 0.346 | ||||
| No | 30(41.1%) | 12(27.3%) | 22(32.4%) | 20(40.8%) | ||||
| Yes | 43(58.9%) | 32(72.7%) | 46(67.6%) | 29(59.2%) | ||||
| Drinking History | 0.990 | 0.320 | 0.039 | 0.843 | ||||
| No | 45(61.6%) | 23(52.3%) | 39(57.4%) | 29(59.2%) | ||||
| Yes | 28(38.4%) | 21(47.7%) | 29(42.6%) | 20(40.8%) | ||||
| Hypertension | 0.097 | 0.755 | 0.089 | 0.766 | ||||
| No | 58(79.5%) | 36(81.8%) | 54(79.4%) | 40(81.6%) | ||||
| Yes | 15(20.5%) | 8(18.2%) | 14(20.6%) | 9(18.4%) | ||||
| Diabetes Mellitus | 0.259 | 0.611 | 0.713 | 0.399 | ||||
| No | 68(93.2%) | 42(95.5%) | 65(95.6%) | 45(91.8%) | ||||
| Yes | 5(6.8%) | 2(4.5%) | 3(4.4%) | 4(8.2%) | ||||
| TNM Clinical Stage | 4.811 | 0.028 | 7.587 | 0.006 | ||||
| I-II | 21(28.8%) | 5(11.4%) | 9(13.2%) | 17(34.7%) | ||||
| III-IV | 52(71.2%) | 39(88.6%) | 59(86.8%) | 32(65.3%) | ||||
| Tumor T- Stage | 2.058 | 0.151 | 2.174 | 0.140 | ||||
| T1-T2 | 22(30.1%) | 8(18.2%) | 14(20.6%) | 16(32.7%) | ||||
| T3-T4 | 51(69.9%) | 36(81.8%) | 54(79.4%) | 33(67.3%) | ||||
| Tumor Site | 1.117 | 0.572 | 1.761 | 0.415 | ||||
| Supraglottic | 21(28.8%) | 12(27.3%) | 17(25.0%) | 16(32.7%) | ||||
| Glottic | 50(68.5%) | 29(65.9%) | 49(72.1%) | 30(61.2%) | ||||
| Subglottic | 2(2.7%) | 3(6.8%) | 2(2.9%) | 3(6.1%) | ||||
| Histological Grade | 5.866 | 0.053 | 7.154 | 0.028 | ||||
| Poorly | 11(15.1%) | 15(34.1%) | 19(27.9%) | 7(14.3%) | ||||
| Moderately | 57(78.1%) | 26(59.1%) | 42(61.8%) | 41(83.7%) | ||||
| Well | 5(6.8%) | 3(6.8%) | 7(10.3%) | 1(2.0%) | ||||
| Cervical Lymph Node Metastasis | 8.582 | 0.003 | 6.135 | 0.013 | ||||
| No | 50(68.5%) | 18(40.9%) | 33(48.5%) | 35(71.4%) | ||||
| Yes | 23(31.5%) | 26(59.1%) | 35(51.5%) | 14(28.6%) | ||||
| Vascular Infiltration | 18.660 | <0.001 | 9.046 | 0.003 | ||||
| No | 53(72.6%) | 14(31.8%) | 31(45.6%) | 36(73.5%) | ||||
| Yes | 20(27.4%) | 30(68.2%) | 37(54.4%) | 13(26.5%) | ||||
| Perineural Infiltration | 0.005 | 0.946 | 0.742 | 0.389 | ||||
| No | 65(89.0%) | 39(88.6%) | 59(86.8%) | 45(91.8%) | ||||
| Yes | 8(11.0%) | 5(11.4%) | 9(13.2%) | 4(8.2%) | ||||
Relationship between RDW, HRR, and clinicopathological characteristics of laryngeal cancer.
3.3 Relationship between RDW, HRR, and clinical indicators of laryngeal cancer
Based on the optimal cutoff values for RDW and HRR, patients were categorized into two groups: low RDW (73 patients) and high RDW (44 patients), as well as low HRR (68 patients) and high HRR (49 patients).
The chi-square test indicated a significant correlation between the high RDW group and TNM clinical stage, cervical lymph node metastasis, and vascular infiltration. Additionally, there were statistically significant differences between the low HRR group and factors such as gender, histologic grade, TNM clinical stage, cervical lymph node metastasis, and vascular infiltration (P < 0.05) (Table 4).
Table 4
| Variable | Category | HR | 95%CI | P |
|---|---|---|---|---|
| Gender | Male | ref. | ||
| Female | 0.80 | 0.39-1.62 | 0.535 | |
| Age(years) | ≤62 | ref. | ||
| >62 | 0.85 | 0.51-1.42 | 0.554 | |
| Smoking History | No | ref. | ||
| Yes | 1.94 | 1.06-3.55 | 0.031 | |
| Drinking History | No | ref. | ||
| Yes | 1.95 | 1.17-3.26 | 0.010 | |
| Hypertension | No | ref. | ||
| Yes | 0.61 | 0.30-1.25 | 0.173 | |
| Diabetes Mellitus | No | ref. | ||
| Yes | 0.42 | 0.10-1.73 | 0.231 | |
| TNM Clinical Stage | I-II | ref. | ||
| III-IV | 7.25 | 2.58-20.35 | <0.001 | |
| Tumor T-Stage | T1-T2 | ref. | ||
| T3-T4 | 3.16 | 1.48-6.75 | 0.003 | |
| Radiochemotherapy | None | ref. | ||
| Either or both | 0.51 | 0.29-0.90 | 0.020 | |
| Tumor Site | 0.250 | |||
| Supraglottic | ref. | |||
| Glottic | 1.063 | 0.59-1.93 | 0.109 | |
| Subglottic | 2.474 | 0.82-7.49 | 0.840 | |
| Histological Grade | 0.001 | |||
| Poorly differentiated | ref. | |||
| Moderately differentiated | 4.94 | 0.68-35.99 | 0.115 | |
| Well differentiated | 11.80 | 1.58-88.33 | 0.016 | |
| Cervical Lymph Node Metastasis | No | ref. | ||
| Yes | 3.2 | 1.87-5.37 | <0.001 | |
| Vascular Infiltration | No | ref. | ||
| Yes | 3.81 | 2.23-6.48 | <0.001 | |
| Perineural Infiltration | No | ref. | ||
| Yes | 1.22 | 0.55-2.69 | 0.626 |
Univariate cox regression analysis of clinicopathological indicators.
Given the significant correlation between the high RDW and low HRR groups with cervical lymph node metastasis and vascular infiltration, the ROC curves for RDW and HRR concerning cervical lymph node metastasis and vascular infiltration were plotted, with cervical lymph node metastasis and vascular infiltration as the endpoints (Figures 1B–E). The AUC for the ROC curve calculated for cervical lymph node metastasis was 0.732 (95% CI, 0.641-0.823, P < 0.001), while the AUC for the ROC curve for vascular infiltration was 0.759 (95% CI, 0.671-0.848, P < 0.001), which indicate that RDW holds diagnostic value in predicting the presence or absence of cervical lymph node metastasis and vascular infiltration in patients undergoing total laryngectomy. The AUC for the ROC curve calculated for cervical lymph node metastasis was 0.633 (95% CI, 0.533-0.734, P = 0.014), while the AUC for the ROC curve calculated for vascular infiltration was 0.666 (95% CI, 0.567-0.764, P = 0.002),which indicate that HRR is equally valuable in predicting the presence of cervical lymph node metastasis and vascular infiltration in patients undergoing total laryngectomy.
3.4 Relationship between RDW, HRR, and prognosis of laryngeal cancer
As of the cut-off date for follow-up (December 31, 2024), patients who underwent total laryngectomy had a 1-year overall survival (OS) rate of 87.2% and a 3-year OS rate of 59.0%.
3.4.1 RDW survival analysis
Patients in the low RDW group had a 1-year OS rate of 100.0%, significantly higher than the 3-year OS rate of 65.9% in the high RDW group. Additionally, the 3-year OS rate for the low RDW group was 84.9%, compared to 66.7% for the high RDW group. Survival curves for RDW were plotted using the Kaplan-Meier method (Figure 1F). The differences between the groups were compared using the Log-rank test. The results indicated that the OS in the low RDW group was significantly better than in the high RDW group (P < 0.001).
3.4.2 HRR survival analysis
Patients in the high HRR group had a 1-year OS rate of 100.0%, significantly higher than the 77.9% rate in the low HRR group. The 3-year OS rate for the high HRR group was 81.6%, compared to 42.6% for the low HRR group. Survival curves for HRR were plotted using the Kaplan-Meier method (Figure 1G). The differences between the groups were compared using the Log-rank test. The results indicated that the OS in the high HRR group was significantly better than in the low HRR group (P < 0.001).
3.5 Univariate survival analysis of clinicopathological data
The univariate analysis incorporated clinicopathological indicators, revealing that RDW, HRR, smoking history, drinking history, tumor T-stage, TNM clinical stage, degree of histological differentiation, cervical lymph node metastasis, vascular infiltration, and postoperative radiochemotherapy status all significantly influenced OS in patients undergoing total laryngectomy (P < 0.05; Figure 1H).
3.6 Analysis of prognostic factors
The prognostic factors affecting survival in patients who underwent total laryngectomy included RDW, HRR, smoking history, drinking history, tumor T-stage, TNM clinical stage, degree of histological differentiation, cervical lymph node metastasis, vascular infiltration, and administration of postoperative radiochemotherapy.The positive results were incorporated into a multifactorial Cox proportional hazards model, which indicated that only RDW, histologic grade, smoking history, TNM clinical stage, and vascular infiltration were significantly associated with OS in patients who underwent total laryngectomy. RDW emerged as an independent prognostic factor for OS in patients undergoing total laryngectomy (HR = 3.060, 95% CI: 2.222-4.215, P < 0.001). Consequently, preoperative RDW was identified as an independent prognostic risk factor for patients undergoing total laryngectomy (P < 0.05) (Table 5).
Table 5
| Variable | B | SE | Wald | P | HR | 95%CI |
|---|---|---|---|---|---|---|
| RDW | 1.118 | 0.163 | 46.883 | <0.001 | 3.060 | 2.222-4.215 |
| Histological Grade | -1.081 | 0.290 | 13.535 | <0.001 | 0.339 | 0.191-0.603 |
| TNM Clinical Stage | 1.498 | 0.725 | 4.273 | 0.039 | 4.472 | 1.081-18.508 |
| Smoking History | 0.932 | 0.392 | 4.273 | 0.017 | 2.540 | 1.178-5.447 |
| Vascular Infiltration | 0.727 | 0.357 | 4.139 | 0.042 | 2.069 | 1.027-4.170 |
Multivariate survival analysis of clinicopathological indicators.
4 Discussion
The prevalence of laryngeal cancer is 1-2% among all cancer types (15). It is the second most prevalent malignant neoplasm of the upper respiratory system, following lung carcinoma (16). Every stage of tumor development is accompanied by an inflammatory response, in addition to raising the risk of cancer (17), chronic inflammation is closely linked to the onset of cancer. Inflammation and immune system activation are pivotal in tumor development, growth, and progression (18). RDW and HRR are measurable indices of systemic inflammatory response employed in the prognosis and diagnosis of diverse malignancies. Currently, no standardized criteria exist for their ideal cut-off levels. The principal techniques for ascertaining these values involve computations derived from ROC curves and crucial values defined in prior research.
In this study, the predicted values of RDW and HRR for OS in patients who underwent total laryngectomy for laryngeal cancer, as shown by our ROC curve, are 13.75 and 10.79, respectively. Medine Kara et al. discovered that elevated RDW increased postoperative mortality by 4.6 times in laryngeal cancer patients, with an acceptable cutoff value of 14.05 for death prediction based on ROC analysis (18). Other investigations revealed an appropriate cutoff value of 13.5% for tongue cancer (4). The found HRR value for nasopharyngeal cancer was 0.97 (19), which closely corresponds with the threshold value determined in our investigation. Factors like tumor kind, case quantity, treatment variability, and selection bias impede the standardization of a precise ideal cutoff value. Nonetheless, credible studies can provide a legitimate spectrum of alternatives for establishing acceptable RDW and HRR criteria.
In this study, we analyzed the clinical characteristics of two subgroups: the low RDW group (RDW < 13.75) and the high RDW group (RDW ≥ 13.75), as well as the low HRR group (HRR < 10.79) and the high HRR group (HRR ≥ 10.79). Our findings indicated that preoperative RDW was associated with TNM clinical stage, cervical lymph node metastasis, and vascular infiltration. Additionally, the preoperative HRR was significantly correlated with gender, histologic grade, TNM clinical stage, cervical lymph node metastasis, and vascular infiltration. Additionally, Medine Kara’s study indicated that RDW served as a predictor of postoperative mortality in laryngeal cancer, with elevated RDW increasing postoperative mortality by 4.6 times in these patients (18). Marcus K’s study revealed that elevated RDW was associated with nonspecific complications after laryngectomy, including deep vein thrombosis, pneumonia, cardiac events, and difficulties in extubation (20). Marcin Miszczyk’s research indicated that patients with RDW ≥ 13.5% had markedly reduced overall survival in comparison to those with RDW < 13.5%. The study determined that a pretreatment RDW of > 13.5% is a significant predictor of reduced overall survival in individuals with squamous cell carcinoma of the tongue (4).Yakup Bozkaya’s study demonstrated that low HRR was an independent variable affecting OS and disease-free survival in patients with locally advanced nasopharyngeal carcinoma (19).
These findings highlight the important role of RDW and HRR in evaluating the prognosis of patients with head and neck tumors, as well as their potential in predicting disease progression and overall patient outcomes. In this study, the RDW group showed a substantial correlation with TNM clinical stage, cervical lymph node metastasis, and vascular infiltration, with statistically significant differences noted. Statistically significant differences were identified between the HRR group and gender, histologic grade, TNM clinical stage, cervical lymph node metastasis, and vascular infiltration. The favorable outcomes from the univariate analysis were incorporated into the multivariate COX regression model. This investigation showed that RDW, histologic grade, smoking status, TNM clinical stage, and vascular infiltration were significantly associated with OS in patients undergoing total laryngectomy, with RDW identified as an independent prognostic factor for OS in this cohort. Consequently, this study concludes that RDW and HRR are straightforward, cost-effective, and readily identifiable markers. Additionally, this study is the inaugural publication that explicitly investigates HRR as a biomarker in the peripheral blood of patients undergoing total laryngectomy. This study indicates potential markers of laryngeal cancer progression, offering a valuable diagnostic basis for clinical practice; however, the prognostic predictive value of HRR is inferior to that of RDW.
Despite the association of increased RDW and low HRR with adverse prognostic outcomes, their precise mechanisms remain ambiguous. The correlation between erythrocytes, tumor prognosis, and immunological function is intricate and multifarious. In the tumor microenvironment, erythrocyte precursors can develop into myeloid cells that inhibit T-cell immunity (21). Concurrently, erythrocytes may potentially associate with cytokines that facilitate tumor proliferation and angiogenesis (22). Metabolic byproducts, such as lactic acid, might modify the pH of the tumor microenvironment, so indirectly affecting tumor growth (23). Moreover, direct interaction between erythrocytes and tumor cells might augment the invasive and migratory properties of malignancies (21). HRR, as a hematological indicator, denotes the ratio of HB to RDW. Low HRR values generally signify diminished hemoglobin levels and elevated RDW. This may be linked to chronic inflammation, which not only inhibits erythropoietin synthesis (24) but also modifies erythrocyte production and metabolism, hence reducing erythrocyte longevity (25, 26). Moreover, malnutrition, especially deficits in iron, folate, and vitamin B12, in conjunction with oxidative stress—which compromises erythrocytes and heightens their fragility—are substantial factors contributing to increased RDW. Hb, indicative of indicative of erythrocyte production capacity, frequently signifies a worse prognosis when levels are diminished. This process may arise from anemia leading to tumor hypoxia, which induces resistance to radiation and accelerates tumor angiogenesis.
However, this study has several limitations. Firstly, it is a retrospective case study that covers a restricted number of cases from a single center, hence impacting the generalizability of the findings. Secondly, HPV infection has been identified as a considerable risk factor for laryngeal cancer; however, HPV status was not assessed in this investigation due to absent data. In addition, the follow-up duration was somewhat short, and solely the OS metric was incorporated;disease-free survival, progression-free survival, and other critical prognostic markers in oncology patients were not considered. Consequently, additional prospective research with bigger sample numbers across other centers and geographies is essential to corroborate these findings in the future.
5 Conclusion
In conclusion, preoperative RDW and HRR are prognostic risk factors for overall survival (OS) in patients undergoing total laryngectomy, with RDW serving as an independent predictor of prognosis. Preoperative RDW showed significant associations with TNM clinical stage, cervical lymph node metastasis, and vascular infiltration, while preoperative HRR was significantly associated with gender, histologic grade, TNM clinical stage, cervical lymph node metastasis, and vascular infiltration. RDW and HRR, as simple and effective biomarkers, are closely associated with the occurrence and progression of laryngeal cancer, thereby providing clinicians with robust support in patient management and the development of treatment strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZF: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. KN: Conceptualization, Formal Analysis, Project administration, Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RDW, red blood cell distribution width; Hb, Hemoglobin; HRR, Hb-to-RDW ratio; AJCC, The American Joint Committee on Cancer; OS, overall survival; ROC, Receiver operating characteristic; AUC, Area under curve.
References
1
Nocini R Sanchis-Gomar F Lippi G Mattiuzzi C . Red blood cell distribution width (RDW) is a significant predictor of survival in laryngeal cancer patients: Systematic literature review and meta-analysis. J Med Biochem. (2023) 42:557–64. doi: 10.5937/jomb0-42947
2
Chen B Zhan Z Fang W Zheng Y Yu S Huang J et al . Long-term trends and future projections of larynx cancer burden in China: a comprehensive analysis from 1990 to 2030 using GBD data. Sci Rep. (2024) 14:26523. doi: 10.1038/s41598-024-77797-6
3
Danese E Lippi G Montagnana M . Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. (2015) 7:E402–11. doi: 10.3978/j.issn.2072-1439.2015.10.04
4
Miszczyk M Jabłońska I Magrowski Ł Masri O Rajwa P . The association between RDW and survival of patients with squamous cell carcinoma of the tongue. Simple, cheap and convenient? Rep Pract Oncol Radiother. (2020) 25:494–9. doi: 10.1016/j.rpor.2020.03.026
5
Li N Zhou H Tang Q . Red blood cell distribution width: A novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. (2017) 2017:7089493. doi: 10.1155/2017/7089493
6
Lin Z Zhang X Luo Y Chen Y Yuan Y . The value of hemoglobin-to-red blood cell distribution width ratio (Hb/RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) for the diagnosis of nasopharyngeal cancer. Med (Baltimore). (2021) 100:e26537. doi: 10.1097/MD.0000000000026537
7
Tham T Olson C Wotman M Teegala S Khaymovich J Coury J et al . Evaluation of the prognostic utility of the hemoglobin-to-red cell distribution width ratio in head and neck cancer. Eur Arch Otorhinolaryngol. (2018) 275:2869–78. doi: 10.1007/s00405-018-5144-8
8
Wan GX Chen P Cai XJ Li LJ Yu XJ Pan DF et al . Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta. (2016) 452:199–203. doi: 10.1016/j.cca.2015.11.025
9
Smirne C Grossi G Pinato DJ Burlone ME Mauri FA Januszewski A et al . Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. (2015) 47:488–94. doi: 10.1016/j.dld.2015.03.011
10
Koma Y Onishi A Matsuoka H Oda N Yokota N Matsumoto Y et al . Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PloS One. (2013) 8:e80240. doi: 10.1371/journal.pone.0080240
11
Shrivastava R Gupta A Mehta N Das D Goyal A . Dietary patterns and risk of oral and oropharyngeal cancers: A systematic review and meta-analysis. Cancer Epidemiol. (2024) 93:102650. doi: 10.1016/j.canep.2024.102650
12
Eren A Giray S . Value of the red blood cell distribution width (RDW) and neutrophil lymphocyte ratio (NLR) in the prediction of functional recovery and 3-month mortality following endovascular treatment for acute anterior circulation ischemic stroke. Heliyon. (2024) 10:e38030. doi: 10.1016/j.heliyon.2024.e38030
13
Qin Y Wang P Huang Z Huang G Tang J Guo Y et al . The value of red cell distribution width in patients with ovarian cancer. Med (Baltimore). (2017) 96:e6752. doi: 10.1097/MD.0000000000006752
14
Cordella C Luebbers HT Rivelli V Grätz KW Kruse AL . An evaluation of the preoperative hemoglobin level as a prognostic factor for oral squamous cell carcinoma. Head Neck Oncol. (2011) 3:35. doi: 10.1186/1758-3284-3-35
15
Kara A Guven M Demir D Yilmaz MS Gundogan ME Genc S . Are calculated ratios and red blood cell and platelet distribution width really important for the laryngeal cancer and precancerous larynx lesions. Niger J Clin Pract. (2019) 22:701–6. doi: 10.4103/njcp.njcp_478_18
16
Cavaliere M Bisogno A Scarpa A D’Urso A Marra P Colacurcio V et al . Biomarkers of laryngeal squamous cell carcinoma: a review. Ann Diagn Pathol. (2021) 54:151787. doi: 10.1016/j.anndiagpath.2021.151787
17
Chi G Lee JJ Montazerin SM Marszalek J . Prognostic value of hemoglobin-to-red cell distribution width ratio in cancer: a systematic review and meta-analysis. biomark Med. (2022) 16:473–82. doi: 10.2217/bmm-2021-0577
18
Kara M Uysal S Altinişik U Cevizci S Güçlü O Dereköy FS . The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. (2017) 274:535–42. doi: 10.1007/s00405-016-4250-8
19
Bozkaya Y Dilber M Bilgili AM Aktaş C . A new prognostic parameter associated with recurrence in patients with nasopharyngeal cancer treated with chemoradiotherapy: the ratio of the hemoglobin-to-red cell distribution width. Cureus. (2023) 15:e39907. doi: 10.7759/cureus.39907
20
Marcus K Sullivan CB Al-Qurayshi Z Buchakjian MR . Can red blood cell distribution width predict laryngectomy complications or survival outcomes? Ann Otol Rhinol Laryngol. (2022) 131:1102–8. doi: 10.1177/00034894211056117
21
Zhang H Wan GZ Wang YY Chen W Guan JZ . The role of erythrocytes and erythroid progenitor cells in tumors. Open Life Sci. (2022) 17:1641–56. doi: 10.1515/biol-2022-0102
22
Karsten E Breen E McCracken SA Clarke S Herbert BR . Red blood cells exposed to cancer cells in culture have altered cytokine profiles and immune function. Sci Rep. (2020) 10:7727. doi: 10.1038/s41598-020-64319-3
23
Shevchenko JA Nazarov KV Alshevskaya AA Sennikov SV . Erythroid cells as full participants in the tumor microenvironment. Int J Mol Sci. (2023) 24:15141. doi: 10.3390/ijms242015141
24
Jelkmann I Jelkmann W . Impact of erythropoietin on intensive care unit patients. Transfus Med Hemother. (2013) 40:310–8. doi: 10.1159/000354128
25
Cho JK Hyun SH Choi JY Choi N Kim MJ Lee SH et al . Prognostic significance of clinical and (18) F-FDG PET/CT parameters for post-distant metastasis survival in head and neck squamous cell carcinoma patients. J Surg Oncol. (2016) 114:888–94. doi: 10.1002/jso.24412
26
Salvagno GL Sanchis-Gomar F Picanza A Lippi G . Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. doi: 10.3109/10408363.2014.992064
Summary
Keywords
laryngeal cancer, red cell distribution width, hemoglobin-to-red blood cell distribution width, prognosis, survival
Citation
Fang Z and Niu K (2025) Analysis of the predictive value of red cell distribution width (RDW) and hemoglobin-to-RDW ratio (HRR) on the prognosis of patients undergoing total laryngectomy. Front. Oncol. 15:1651738. doi: 10.3389/fonc.2025.1651738
Received
22 June 2025
Accepted
15 September 2025
Published
01 October 2025
Volume
15 - 2025
Edited by
Gunnar Wichmann, University Hospital Leipzig, Germany
Reviewed by
Qian Song, Department of Clinical Laboratory, Zhejiang Cancer Hospital, University of Chinese Academy of Sciences, China
DOĞAN YAZILITAŞ, Dışkapı Yildirim Training and Research Hospital, Türkiye
Updates
Copyright
© 2025 Fang and Niu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Niu, niukai@jlu.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.