- 1Department of Plastic and Burn Surgery, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Pathology, West China Hospital of Sichuan University, Chengdu, China
Adolescent breast hypertrophy is a rare and persistent condition that affects peripubertal females both physiologically and psychologically. We present a case of a 14-year-old patient with bilateral giant breast enlargement who underwent a successful reduction mammoplasty of 16.5% Body Weight. The right breast exhibited focal interstitial proliferation myofibroblasts, suggesting a potential risk for phyllodes tumor development. This finding represents a novel histopathological association, unreported in the literature and first documented in China.
1 Introduction
Adolescent breast hypertrophy, also known as juvenile macromastia or virgin breast hypertrophy, is a benign condition characterized by rapid, disproportionate unilateral or bilateral breast enlargement during puberty (1). Its precise etiology remains unclear (2). Hormonal assays are typically unremarkable (3–5). Early intervention may be beneficial for normal social activities and mental health in adolescence (6, 7). This report describes a case of a 14-year-old Chinese female with adolescent breast hypertrophy who underwent surgical intervention. Notably, postoperative pathology suggested features indicative of phyllodes tumor transformation.
2 Case report
A 14-year-old female patient presented to our Plastic and Reconstructive Surgery Department with excessive breast volume and ptosis. The patient’s height was 155 cm, preoperative weight was 59 kg, and body mass index (BMI) was 24 kg/m². Progressive bilateral breast enlargement over two postpubertal years was accompanied by prominent superficial venous engorgement. The patient’s breasts were basically symmetrical on both sides, with the right breast sagging 10 cm below the rib cage and the left sagging 9 cm below the rib cage (Figure 1). Excessive breast volume precluded accurate volumetric MRI assessment. Preoperatively she underwent a puncture biopsy of the right breast mass, which indicated mesenchymal hyperplasia accompanied by normal-type ductal epithelial hyperplasia. No contributory family history, pharmacotherapy, or gonadotropin axis abnormalities were identified. Breast ultrasonography demonstrated multiple bilateral solid breast lesions suggestive of fibrocystic hyperplasia. Therefore, we can temporarily rule out abnormal breast enlargement caused by endocrine disorders or local breast lesions such as tumors or inflammations.
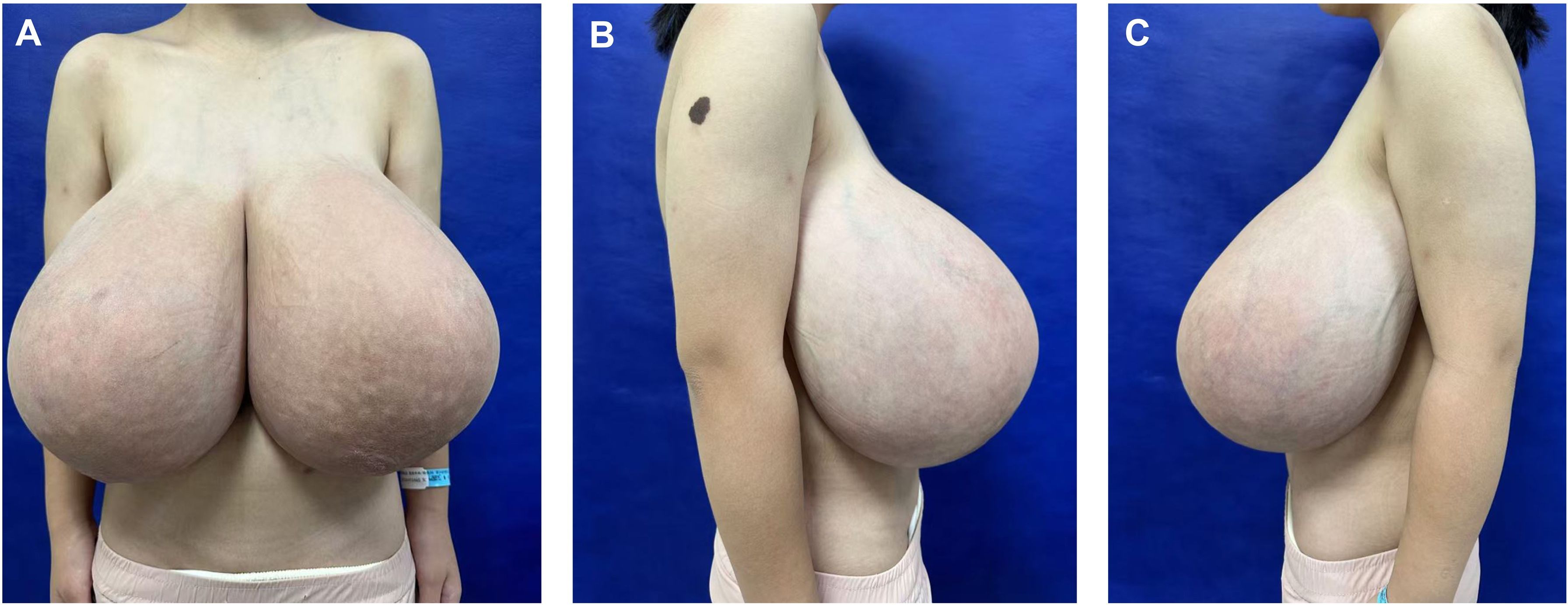
Figure 1. Preoperative frontal (A) and lateral (B, C) views of a patient with adolescent macromastia.
Given the rapid increase in breast volume and the patient’s strong desire for surgical intervention, medical therapy was no longer considered the preferred approach. Furthermore, evidence suggests that in cases involving long pedicles (>10 cm mobilization), large reductions (>2000 g), or severe ptosis, consideration should be given to free nipple–areola complex grafting (8). After thorough preoperative communication with the patient and her family members, the inverted-T incision with inferior pedicle technique and free nipple-areola complex (NAC) graft were ultimately chosen as the operative approach. The distances from the right and left NACs to the inframammary fold (IMF) were 16 cm and 15 cm, respectively. The neo-nipple areolar complex (neo-NAC) on both breasts was positioned 26 cm from the sternal notch. The total amount of breast tissue removed during the operation was 9.6 kg, with 4.7 kg excised from the left side and 4.9 kg from the right (Figure 2). Histopathological analysis revealed bilateral hyperplasia of interstitial fibrous tissue and myofibroblasts, accompanied by collagenization. The right breast additionally exhibited nuclear fission and pronounced focal hyperplasia of interstitial myofibroblasts, indicating a tendency toward phyllodes formation (Figure 3). Immunohistochemical staining of the right breast lesion showed: Desmin (–), SMA (+), CD10 (+), CD34 (+), S-100 (–), SOX10 (–), EMA (–), and Ki67 positivity (10%-15%). Postoperative pathological examination revealed prominent focal interstitial proliferation with mitotic figures. The immunophenotype supported a stromal origin, excluding epithelial or neurogenic lesions. The stroma lacked a typical leaf-like architecture, and based on these findings, the diagnosis was bilateral mammary hypertrophy with a phyllodes-like tendency in the right breast. Given these findings suggesting malignant potential, we recommended close follow-up with biannual postoperative breast imaging (ultrasound and MRI) to monitor recurrence or malignancy.
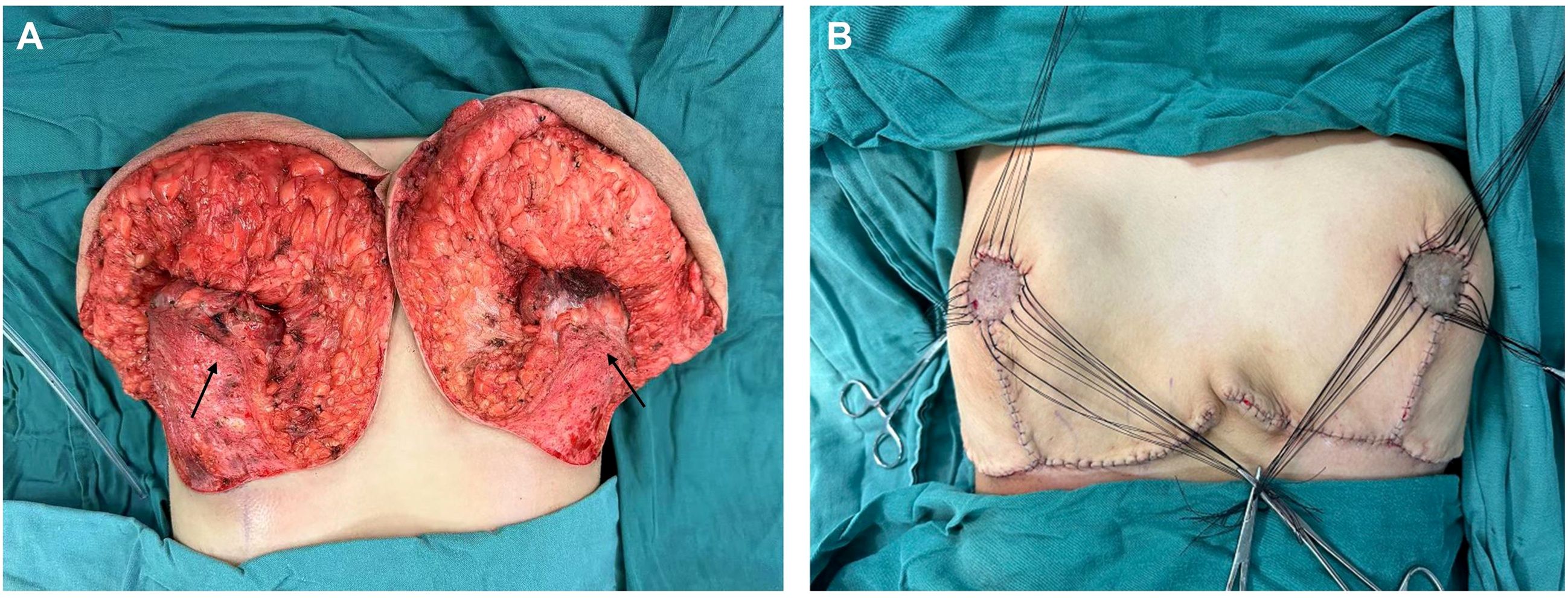
Figure 2. The illustrations show the breast gland that was preserved during the surgery. (The arrow in the figure indicates the pedicle preserved during surgery).
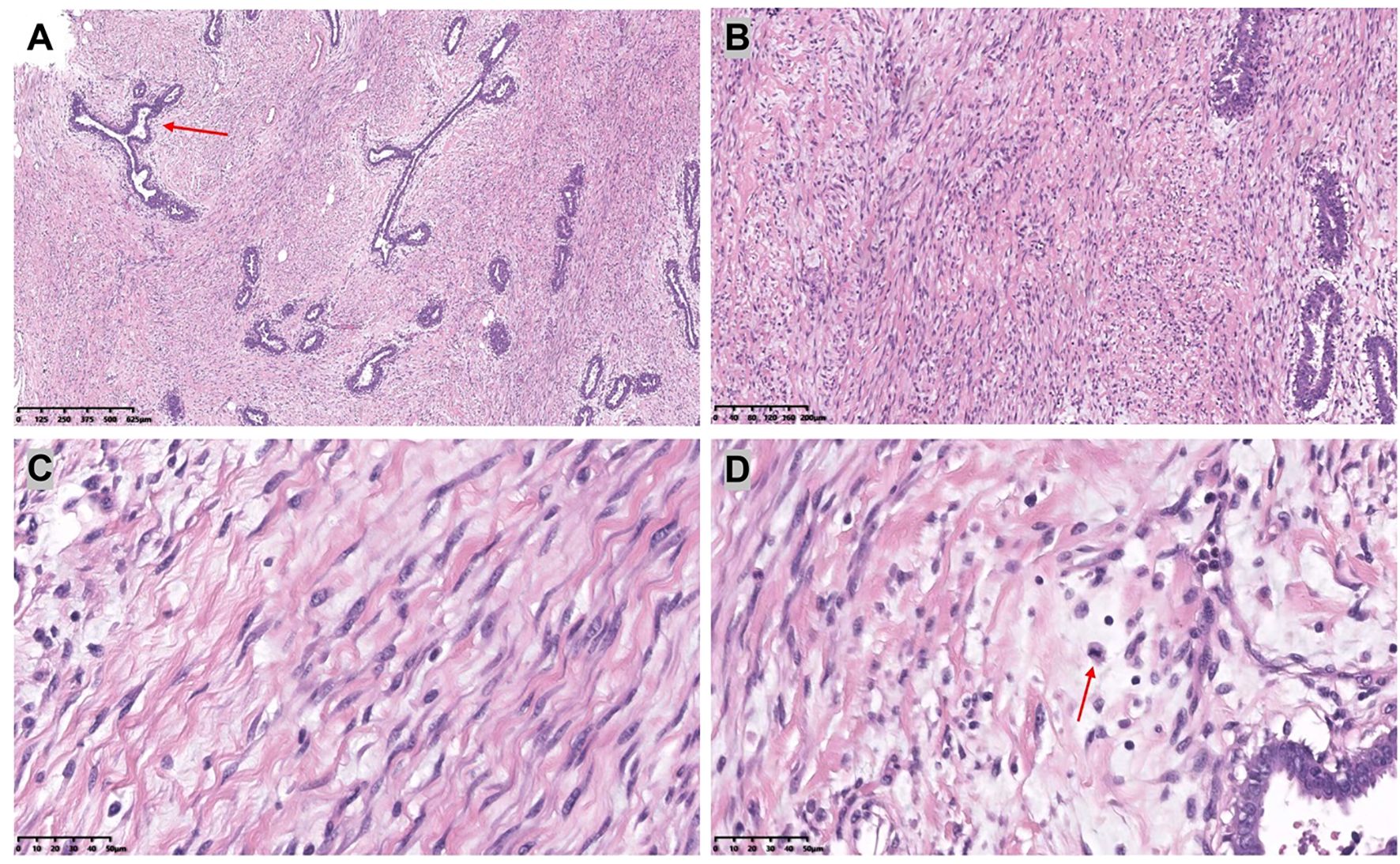
Figure 3. [(A): H&E,×4, (B): H&E,×10] The bilateral mammary glands were composed of dilated ducts and hyperplastic epithelium. There was a proliferation of interstitial fibrous tissue and myofibroblasts.[(C): H&E,×40] Myofibroblasts are blazingly proliferative. [(D): H&E,×40] The interstitium showed prominent mitotic activity.
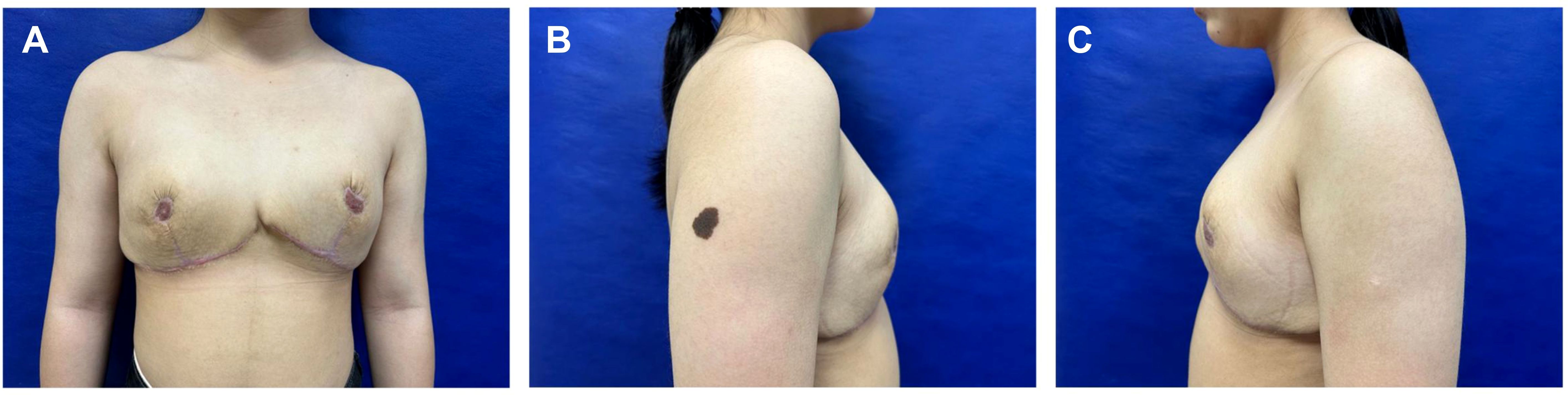
The patient was discharged on postoperative day 7 without medication. At 6-month follow-up, she reported satisfaction with breast aesthetics, and there were no signs of recurrence. (Figure 4). Postoperative magnetic resonance imaging (MRI) revealed left and right breast volumes of 632 cm³ and 605 cm³, respectively. Bilateral breast ultrasonography revealed no significant abnormalities (Figure 5). Her emotional state and social participation improved significantly—she actively joined the school badminton club, no longer experienced anxiety when choosing clothing, and reported resolution of the sleep disturbance associated with supine positioning prior to surgery. These subjective improvements were consistent with the objective therapeutic outcomes, further supporting the value of breast reduction surgery in adolescent patients with macromastia. Given the patient’s adolescent stage, long-term surveillance is recommended every 6–12 months thereafter.
3 Discussion
Adolescent breast hypertrophy is characterized by the excessive development of breast tissue in adolescent females, resulting in disproportionate breast volume relative to torso size (1). This rapid breast growth can lead to skin stretching, loss of areolar definition, and even ulceration (9, 10). Besides the symptoms like back and neck pain, the patient’s primary grievances stemmed from social awkwardness and her avoidance of social engagements, which were caused by her exceptionally large breast size. The etiology and pathogenesis of this condition are complex, involving various factors such as hormone levels, genetic predispositions, receptor sensitivity, and environmental influences (2, 11–14). Notably, significant unilateral or bilateral enlargement can occur despite normal gonadal hormone levels, suggesting idiopathic end-organ hypersensitivity— manifested as elevated estrogen sensitivity or progesterone receptor upregulation (2, 15, 16). This patient’s endocrinology profile were normal, and there was no history of related medication use. The condition may be associated with changes at the genetic or molecular level. Currently, there is no published data clarifying the prevalence of adolescent breast hypertrophy. With the rising health awareness and environmental shifts, more individuals are seeking specialized medical advice and treatment for adolescent breast hypertrophy in hospitals (15). Therapeutic strategies comprise medical and surgical interventions. Pharmacotherapy (e.g., tamoxifen, bromocriptine) demonstrates limited efficacy in established macromastia but may adjunctively prevent postoperative recurrence (4, 5, 17). For this specific patient, given the limited efficacy of pharmacotherapy and the risks associated with adverse drug reactions, surgical treatment alone was sufficient to achieve the therapeutic goals, eliminating the need for additional drug assistance. Since the patient’s breast volume was still increasing, the inverted-T incision with inferior pedicle technique and free nipple-areola complex (NAC) graft was ultimately selected to optimize the removal of glandular tissue and minimize the risk of recurrence after surgery, while also considering the shape and volume of the breast. Compared with pedicled reductions, staged procedures, or skin-sparing resections, this technique enables more effective and complete management of enlarged glands within a shorter treatment course, while simultaneously resolving the issues of skin excess and residual hyperplasia (18, 19). Besides, Fiumara et al. provided statistical evidence suggesting that the use of free nipple–areola complex grafting is associated with a lower recurrence rate compared with the pedicled technique (20).
Based on the H&E staining and the immunohistochemical results provided, the pathological examination of this case revealed phyllodes tumor formation predisposition in the right breast. This pathological type has not been reported previously and expands the clinicopathological spectrum of the disease. This type of macromastia indicates that we should consider early assessment, intervention, or surgical treatment. Phyllodes tumors are known for their high recurrence rate. Phyllodes tumors can be classified into three categories: benign, borderline, and malignant. Nuclear division count is one of the core criteria for grading of nodular tumors. Its combined assessment with the Ki-67 index can significantly improve the diagnostic accuracy (21, 22). Benign phyllodes tumors are the most common and exhibit histological and clinical features similar to those of fibroadenomas. Higher-grade phyllodes tumors correlate with increased risk of recurrence or metastasis. The similar histologic features of phyllodes tumors and fibroadenomas warrant careful evaluation for the potential progression of fibroadenomas (23). Currently, the standard treatment for phyllodes tumors involves surgical excision with clear margins, often in conjunction with breast-conserving surgery and is closely monitored during postoperative follow-up (24).
Adolescent patients face recurrence risks from ongoing breast development or pregnancy-related hormonal shifts (4, 13, 25, 26). Consequently, we prioritized reduction mammoplasty as initial therapy, reserving mastectomy for recurrence. There were no signs of recurrence during 6-month follow-up period. The girl was pleased although the aesthetic result was only acceptable from an objective point of view. However, this case also has some limitations, including a relatively short follow-up period and potential diagnostic uncertainties.
4 Conclusion
Surgical intervention remains the primary treatment option for adolescent breast hypertrophy. Medical therapy demonstrates promising potential for disease control and recurrence risk reduction. However, further research is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
RL: Writing – original draft, Writing – review & editing. YX: Writing – review & editing. YC: Writing – review & editing. ZZ: Data curation, Writing – review & editing. YZ: Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
We are also very thankful for the editors’ and reviewers’ valuable suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Samuelov R and Siplovich L. Juvenile gigantomastia. J Pediatr Surg. (1988) 23:1014–5. doi: 10.1016/S0022-3468(88)80010-1
2. Kikuchi DS, Mustin DE, Ghanouni A, and Walsh MD. A review of pediatric macromastia etiology and indications for reduction mammaplasty. J Plast Reconstr Aesthet Surg. (2023) 77:209–17. doi: 10.1016/j.bjps.2022.12.003
3. Kupfer D, Dingman D, and Broadbent R. Juvenile breast hypertrophy: report of a familial pattern and review of the literature. Plast Reconstr Surg. (1992) 90:303–9. doi: 10.1097/00006534-199290020-00023
4. Baker SB, Burkey BA, Thornton P, and LaRossa D. Juvenile gigantomastia: presentation of four cases and review of the literature. Ann Plast Surg. (2001) 46:517–25; discussion 25-6. doi: 10.1097/00000637-200105000-00011
5. Karagüzel G, Bilen S, Karaçal N, Yıldız K, and Livaoğlu M. Virginal breast hypertrophy: different presentations of 2 cases and the role of tamoxifen as an adjuvant therapy. J Pediatr Adolesc Gynecol. (2016) 29:e71–e4. doi: 10.1016/j.jpag.2016.03.008
6. Cerrato F, Webb ML, Rosen H, Nuzzi L, McCarty ER, DiVasta AD, et al. The impact of macromastia on adolescents: a cross-sectional study. Pediatrics. (2012) 130:e339–46. doi: 10.1542/peds.2011-3869
7. Nuzzi LC, Firriolo JM, Pike CM, Cerrato FE, Webb ML, Faulkner HR, et al. The effect of reduction mammaplasty on quality of life in adolescents with macromastia. Pediatrics. (2017) 140. doi: 10.1542/peds.2017-1103
8. Rancati A, Irigo M, and Angrigiani C. Management of the ischemic nipple-areola complex after breast reduction. Clinics Plast Surg. (2016) 43:403–14. doi: 10.1016/j.cps.2015.12.011
9. Gentimi F, Loupatatzi A, Euthimoglou KP, Michailidou EG, Tzovaras AA, Kaja AD, et al. Juvenile gigantomastia in a 12-year-old girl: a case report. Aesthetic Plast Surg. (2011) 35:414–7. doi: 10.1007/s00266-010-9597-4
10. Pokhrel B, Gautam S, Sharma S, Pokhrel NB, Bhatta NC, Rayamajhi S, et al. Virginal breast hypertrophy in a 14-year-old girl: A case report. Clin Case Rep. (2021) 9:198–202. doi: 10.1002/ccr3.3498
11. Lozito A, Gemelli M, Micello D, Vinci V, Pelosi G, and Ricotta R. Immune-related gigantomastia: a case study. Immunotherapy. (2023) 15:1001–7. doi: 10.2217/imt-2023-0056
12. Mori T, Yokogawa N, Higuchi R, Tsujino M, Shimada K, and Sugii S. Bucillamine-induced gigantomastia with galactorrhea and hyperprolactinaemia. Mod Rheumatol Case Rep. (2020) 4:122–5. doi: 10.1080/24725625.2019.1673939
13. Govrin-Yehudain J, Kogan L, Cohen HI, and Falik-Zaccai TC. Familial juvenile hypertrophy of the breast. J Adolesc Health. (2004) 35:151–5. doi: 10.1016/j.jadohealth.2003.09.017
14. Chen J, Sun J, Wang Q, Du Y, Cheng J, Yi J, et al. Systemic deficiency of PTEN accelerates breast cancer growth and metastasis. Front Oncol. (2022) 12:825484. doi: 10.3389/fonc.2022.825484
15. Kasielska-Trojan A, Danilewicz M, Strużyna J, Bugaj M, and Antoszewski B. The role of oestrogen and progesterone receptors in gigantomastia. Arch Med Sci. (2019) 18:1016–20. doi: 10.5114/aoms.2019.88280
16. Santen RJ, Karaguzel G, Livaoglu M, Yue W, Cline JM, Ratan A, et al. Role of ERα and aromatase in juvenile gigantomastia. J Clin Endocrinol Metab. (2024) 109:1765–72. doi: 10.1210/clinem/dgae019
17. Alhindi N, Mortada H, Alzaid W, Al Qurashi AA, and Awan B. A systematic literature review of the clinical presentation, management, and outcome of gestational gigantomastia in the 21st century. Aesthetic Plast Surg. (2023) 47:10–29. doi: 10.1007/s00266-022-03003-5
18. Bianchi de Aguiar B, Santos Silva R, Costa C, Castro-Correia C, and Fontoura M. Juvenile breast hypertrophy. Endokrynol Polska. (2020) 71:202–3. doi: 10.5603/EP.a2019.0063
19. Mareti E, Vatopoulou A, Spyropoulou GA, Papanastasiou A, Pratilas GC, Liberis A, et al. Breast disorders in adolescence: A review of the literature. Breast Care (Basel Switzerland). (2021) 16:149–55. doi: 10.1159/000511924
20. Fiumara L, Gault DT, Nel MR, Lucas DN, and Courtauld E. Massive bilateral breast reduction in an 11-year-old girl: 24% ablation of body weight. J Plastic Reconstruct Aesthetic Surg: JPRAS. (2009) 62:e263–6. doi: 10.1016/j.bjps.2007.10.053
21. Li X, Nguyen TTA, Zhang J, Nayak A, Liu Y, Duckworth LA, et al. Validation study of the newly proposed refined diagnostic criteria for Malignant phyllodes tumor with 136 borderline and Malignant phyllodes tumor cases. Am J Surg Pathol. (2024) 48:1146–53. doi: 10.1097/PAS.0000000000002264
22. Yu CY, Huang TW, and Tam KW. Management of phyllodes tumor: A systematic review and meta-analysis of real-world evidence. Int J Surg (London England). (2022) 107:106969. doi: 10.1016/j.ijsu.2022.106969
23. Tan J, Ong CK, Lim WK, Ng CC, Thike AA, Ng LM, et al. Genomic landscapes of breast fibroepithelial tumors. Nat Genet. (2015) 47:1341–5. doi: 10.1038/ng.3409
24. Lissidini G, Mulè A, Santoro A, Papa G, Nicosia L, Cassano E, et al. Malignant phyllodes tumor of the breast: a systematic review. Pathologica. (2022) 114:111–20. doi: 10.32074/1591-951X-754
25. Hudson AS, Morzycki AD, and Guilfoyle R. Reduction mammaplasty for macromastia in adolescents: A systematic review and pooled analysis. Plast Reconstr Surg. (2021) 148:31–43. doi: 10.1097/PRS.0000000000008102
Keywords: adolescent breast hypertrophy, reduction mammoplasty, phyllodes tumor, pathology, breast tumor
Citation: Liao R, Xiao Y, Chang Y, Zhang Z and Zhang Y (2025) Case report: Adolescent breast hypertrophy with a tendency toward phyllodes tumor formation: first reported case in China. Front. Oncol. 15:1652446. doi: 10.3389/fonc.2025.1652446
Received: 23 June 2025; Accepted: 09 October 2025;
Published: 15 October 2025.
Edited by:
Shuhei Suzuki, Yamagata Prefectural Shinjo Hospital, JapanReviewed by:
Andi Muh. Maulana, Universitas Muhammadiyah Purwokerto, IndonesiaYosuke Saito, Yamagata University, Japan
Copyright © 2025 Liao, Xiao, Chang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yange Zhang, Wmhhbmd5YW5nZTc4MDFAMTYzLmNvbQ==
 Ruiheng Liao
Ruiheng Liao Yang Xiao1
Yang Xiao1 Zhang Zhang
Zhang Zhang