- Department of Oncology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To suggest the difference between primary and secondary testicular lymphoma, and to manifest the clinical characteristics, treatment modalities and prognostic factors of primary testicular lymphoma.
Method: This study included all lymphoma patients with testicular involvement treated at our institution between October 2012 and May 2024. We retrospectively collected data on their clinical characteristics, treatment approaches, and outcomes for further analysis.
Result: A total of 50 primary testicular lymphoma (PTL) patients and 13 secondary testicular lymphoma (STL) patients were enrolled, with diffuse large B-cell lymphoma (DLBCL) being the most common subtype. After a median follow-up of 36.0 months (range: 1.1–117.5), the median progression-free survival (PFS) was 105.9 months for PTL patients and 16.8 months for STL patients. The median overall survival (OS) was 106 months for PTL and 23.8 months for STL. Among the 46 primary DLBCL (PT-DLBCL) cases, half received central nervous system (CNS) prophylaxis, and 7 patients (15.2%) experienced CNS relapse. Patients who received maintenance therapy after orchiectomy and first-line treatment exhibited prolonged PFS. Radiotherapy was associated with improved PFS, while the double-expressor phenotype was linked to poorer OS.
Conclusion: PTL suggested distinct histopathological features, clinical responses, and survival outcomes compared to STL. A combined treatment strategy involving orchiectomy followed by chemotherapy, consolidation therapy, and radiotherapy is recommended for PT-DLBCL. As intrathecal methotrexate did not significantly reduce CNS recurrence, alternative prophylactic strategies should be explored for high-risk patients.
1 Introduction
The testes are regarded as immune-privileged sites, protected by the blood-testicular barrier and immunomodulatory mechanisms. Lymphomas arising in these immune sanctuaries represent a rare subtype of non-Hodgkin lymphoma (NHL), typically associated with aggressive clinical behavior and poor prognosis. These lymphomas can be broadly categorized as either primary testicular lymphoma (PTL) or secondary testicular lymphoma (STL).
PTL is defined by the presence of testicular masses as the initial or predominant manifestation, without obvious involvement of other extranodal organs. In contrast, STL is caused by the spread of extara-testicular lymphoma. Clinically, differentiating PTL from STL can be challenging, particularly when testicular involvement is the presenting symptom. Accurate clinical staging is also crucial for determining the appropriate treatment. PTL accounts for 1% to 2% of all NHL cases and 3% to 9% of testicular malignancies. It is the most common testicular tumor in men over 60 and is associated with a high risk of contralateral testicular and central nervous system (CNS) relapse (1). The majority of PTL cases are diffuse large B-cell lymphoma (DLBCL), while STL displays more varied histopathological types.
Although the incidence of PTL is gradually increasing in China, the overall occurrence of testicular lymphoma remains significantly lower in Asian populations compared to Western cohorts. This discrepancy has resulted in a scarcity of real-world clinical data from Chinese populations. Furthermore, despite the consensus on standard PTL treatment, optimal therapeutic approaches following orchiectomy remain a subject of debate. Novel agents, such as PD-1 and Bruton’s tyrosine kinase inhibitors (BTK-i), have shown promise. In light of these issues, we conducted this retrospective study, collecting real-world data from PTL and STL patients at our center. Our aim was to compare clinical and pathological characteristics, assess the effectiveness of various treatment regimens, and evaluate survival outcomes.
2 Materials and methods
2.1 Study design and patient selection
We retrospectively collected data from 63 lymphoma patients with testicular involvement treated at the First Affiliated Hospital of Zhengzhou University between October 2012 and May 2024. Patients were categorized into two groups: primary testicular lymphoma (PTL) and secondary testicular lymphoma (STL). Procedures of patient selection are displayed in Figure 1. The classification was based on whether the testicular mass was the primary or predominant symptom and whether there was significant involvement of other extranodal organs. PTL was confirmed through testicular pathology, while testicular involvement in STL cases was evaluated using either pathology or positron emission computed tomography (PET/CT). Disease staging was conducted according to the Ann Arbor staging system. Immunohistochemistry was performed for Bcl-2 and c-Myc expression, while fluorescence in situ hybridization (FISH) was used to identify double-expressor types as well as double-hit/triple-hit DLBCL. The cell of origin (COO) of DLBCL was determined using the HANS algorithm, based on the expression of CD10, Bcl-6, and MUM-1. Clinical characteristics such as age, Ann Arbor stage, extranodal involvement, treatment regimens, and disease response were collected for further analysis. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2022-KY-0869-001). All the processes conformed to the Declaration of Helsinki. All patients have signed written informed consents before treatment.
2.2 Treatment modalities and assessment of effectiveness
For PTL patients, orchiectomy was followed by 4 to 6 cycles of chemotherapy, CNS prophylaxis, and radiotherapy (RT). Prophylactic contralateral testicular RT or nodal RT after first-line treatment was administered with a total dose ranging from 20 to 45 grays. CNS prophylaxis was primarily carried out through intrathecal injections of methotrexate (MTX, 12–15 mg), cytarabine (AraC, 50 mg), and dexamethasone (DXM, 5 mg). Treatment response was evaluated using computed tomography (CT) or positron emission tomography/computed tomography (PET/CT). Short-term effectiveness was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The overall response rate (ORR) was defined as the percentage of patients achieving CR or PR. Overall survival (OS) was measured from the date of diagnosis to death or last observation from any cause, while progression-free survival (PFS) was defined as the time from diagnosis to the first relapse, progression, death, or last follow-up.
2.3 Statistical analysis
Descriptive statistics were expressed as percentages and medians. Treatment effectiveness was compared using the chi-squared test. Survival outcomes, including overall survival (OS) and progression-free survival (PFS), were estimated using the Kaplan–Meier method. Differences between survival curves were compared using the log-rank test. Univariate Cox proportional hazards regression models were applied to identify potential prognostic factors. Variables assessed included age at diagnosis, Ann Arbor stage, COO, laterality, IPI score, immunophenotype, first-line therapy, radiotherapy, maintenance therapy and intrathecal CNS prophylaxis. Variables with a p-value <0.05 in univariate analysis were further included in the multivariate Cox model. All statistical analyses were conducted with SPSS software version 26.0 and GraphPad Prism version 8. A p-value of <0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics, clinical response, and survival outcomes between PTL and STL patients
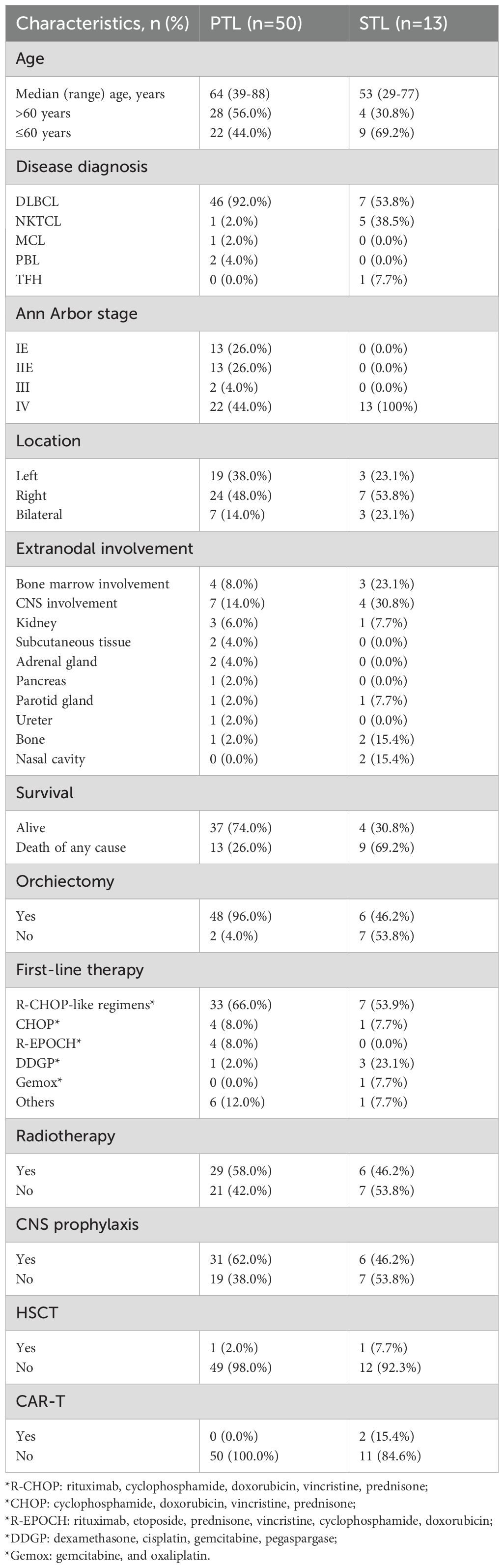
A total of 63 patients with testicular involvement were included in the study, consisting of 50 PTL and 13 STL patients. Comparisons between their clinical characteristics and their treatment options are depicted in Table 1. The median age (range) for PTL patients was 64 years (39–88), and for STL patients, it was 53 years (29–77). Among them, 56.0% of PTL patients and 30.8% of STL patients were over 60 years of age. All PTL cases and 6 STL cases were confirmed via testicular pathology, while the remaining 7 STL patients were assessed using FDG-PET/CT, with a mean testicular maximum standardized uptake value (SUVmax) of 12.3 (range: 5.0–21.1). The majority of patients were diagnosed with DLBCL, accounting for 92% of PTL and 53.8% of STL cases, with a statistically significant difference in pathology between the two groups (p < 0.001). Of the PTL patients, 52% (n = 26) presented with limited-stage lymphoma (stage I E and II E), while all STL patients were diagnosed at an advanced stage. Two PTL and one STL patient had a history of testicular injury, and four PTL and two STL patients had undergone testicular surgery, potentially linked to the development of testicular lymphoma. Most patients had unilateral disease, observed in 86% of PTL and 76.9% of STL patients. Bone marrow involvement was noted in four PTL (8%) and three STL (23.1%) patients, while CNS involvement occurred in seven PTL (14%) and four STL (30.8%) patients. During follow-up, 22 patients died, including four PTL and nine STL patients, with a significant difference in mortality between the groups (p = 0.01).
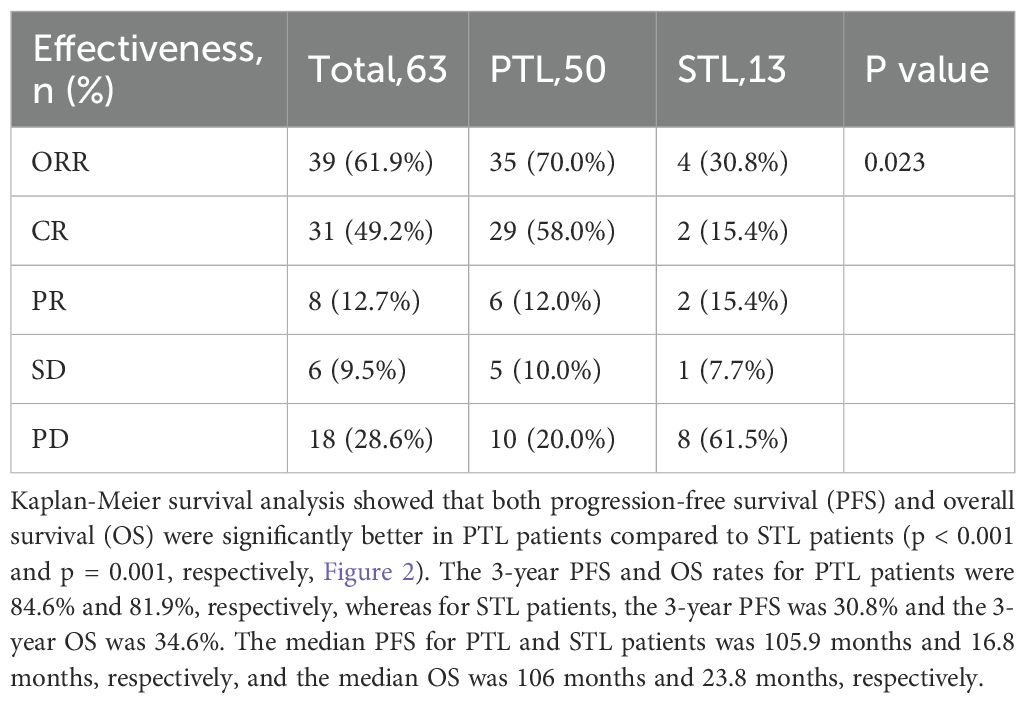
At a median follow-up of 36.0 months (range: 1.1–117.5), treatment response and survival outcomes were evaluated for all patients. The overall response rate (ORR) was 61.9%, with 31 patients achieving a complete response (CR) and eight patients achieving a partial response (PR). The total CR rate was 49.2% (Table 2). Among PTL patients, the ORR was 70.0%, with 58.0% achieving a CR, while in STL patients, the ORR was 30.8%, with 15.4% achieving a CR, representing a significant difference between the two groups (p = 0.023).
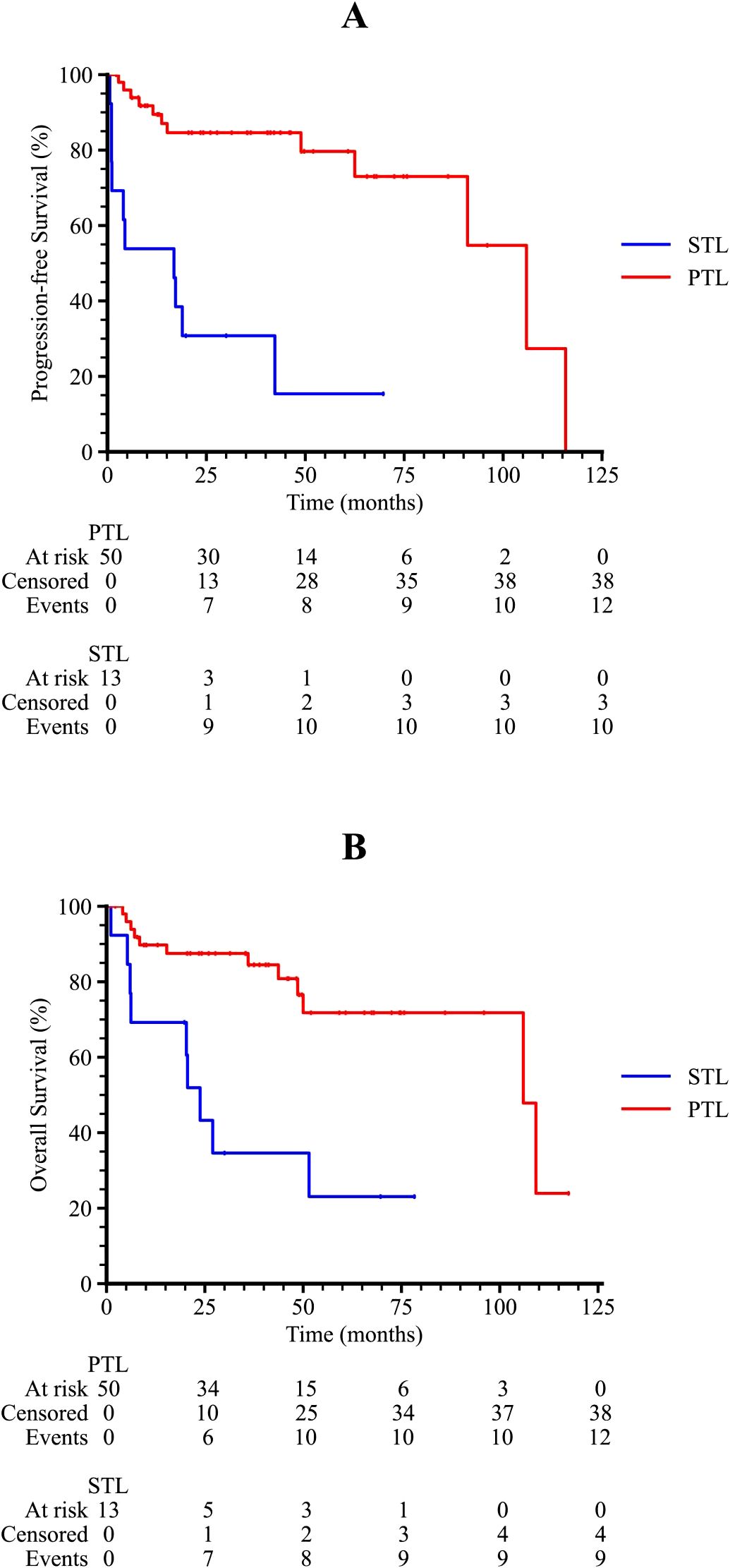
Figure 2. Kaplan-Meier survival curves of TL patients. (A) The progression-free survival curve among PTL and STL patients. (B) The overall survival curve among PTL and STL patients.
3.2 Primary testicular DLBCL
3.2.1 Clinical features and treatment regimens of primary testicular DLBCL
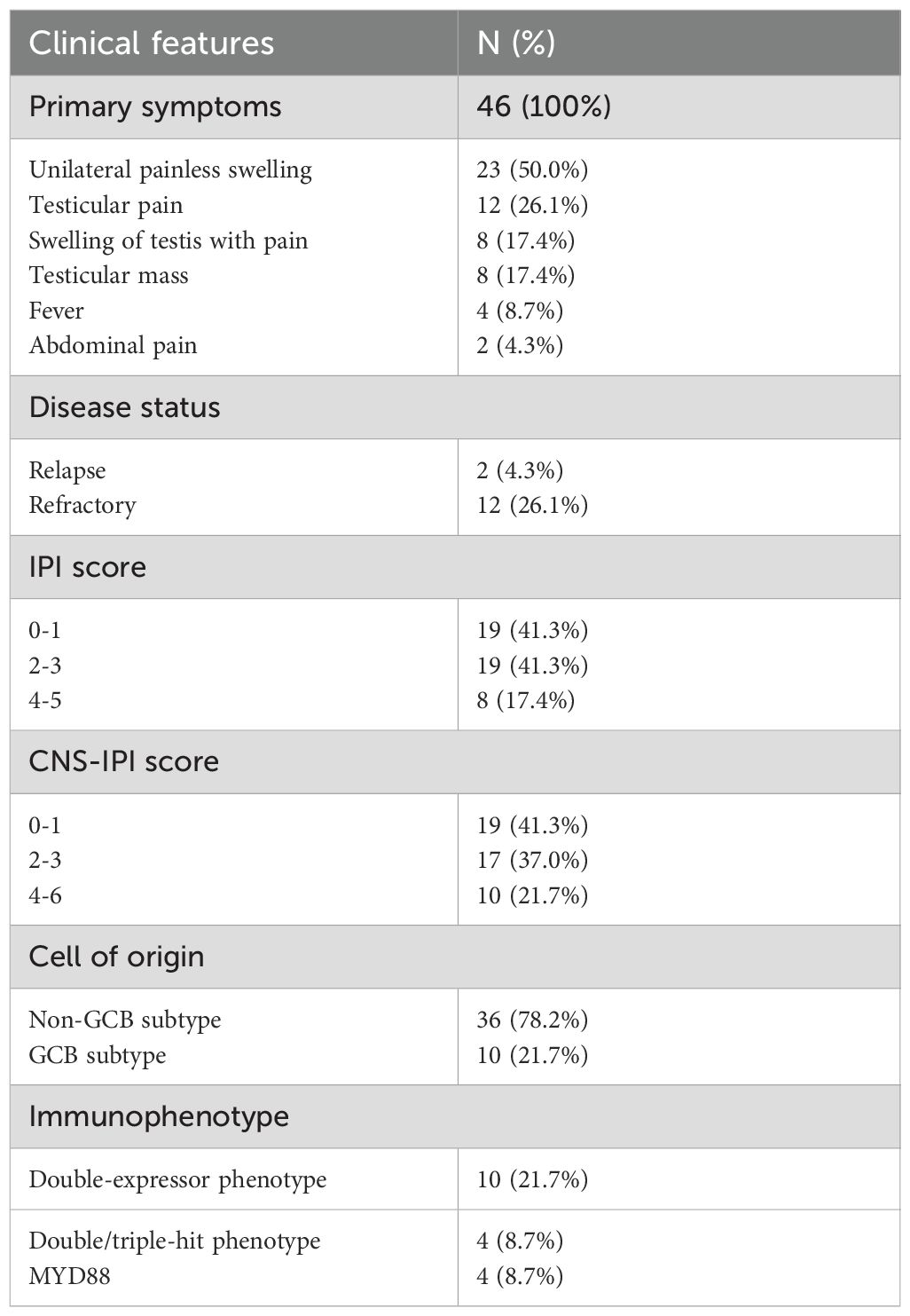
A total of 46 primary testicular DLBCL (PT-DLBCL) patients were enrolled in the study, representing the majority of PTL cases. The clinical characteristics and treatment modalities of these patients are summarized in Table 3. The most commonly reported primary symptom was unilateral painless swelling (50.0%), followed by testicular pain (26.1%), a combination of swelling with pain (17.4%), and the presence of a testicular mass (17.4%). Fourteen out of 46 patients (30.4%) had relapsed or refractory (R/R) disease, and 78.2% of cases were of the non-GCB subtype. MYD88 gene testing was performed on five patients, with four testing positive for the MYD88 mutation. All patients were diagnosed with DLBCL through pathological examination, with 44 out of 46 (95.7%) undergoing orchiectomy, while two patients were confirmed through testicular puncture biopsy without receiving any orchiectomy.
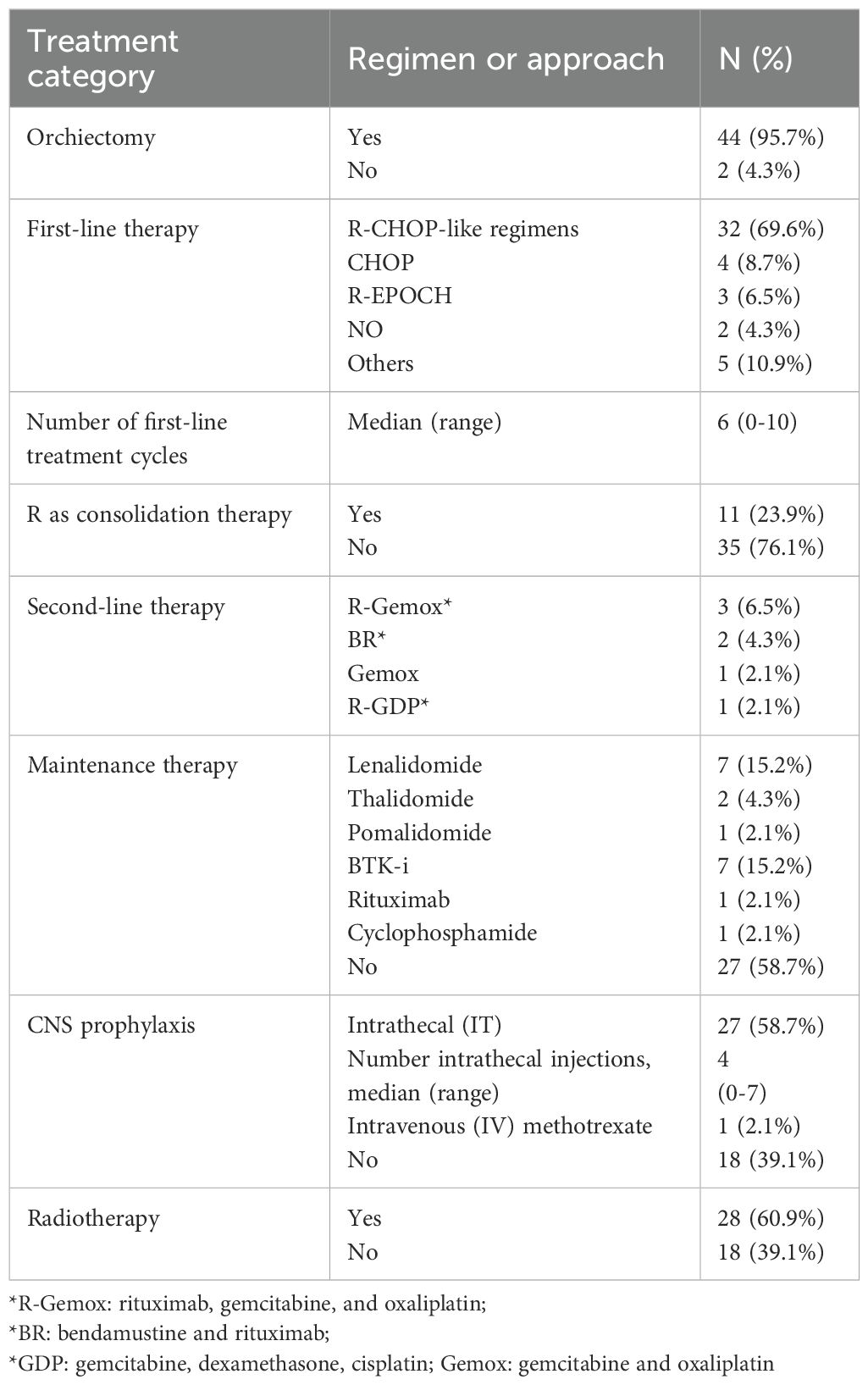
Detailed information on treatment regimens is summarized in Table 4. Among all patients, the majority received R-CHOP-like regimens as first-line treatment. These included R-CHOP, Rituximab with cyclophosphamide, pegylated liposomal doxorubicin, vincristine, and prednisone (R-CDOP); zanubrutinib with R-CHOP (ZR-CHOP); and lenalidomide with R-CHOP (R2-CHOP). Two patients did not receive chemotherapy after orchiectomy. After a median of four cycles of frontline treatment, 11 patients received Rituximab (R) monotherapy as consolidation therapy. Several second-line and maintenance regimens were used in relapsed or high-risk cases, including lenalidomide, thalidomide, and R-Gemox. Additionally, 28 out of 46 patients underwent radiotherapy following chemotherapy, and CNS prophylaxis was given to 28 patients (60.9%), including 27 patients with intrathecal methotrexate and 1 with intravenous methotrexate.
3.2.2 Contralateral testis and CNS recurrence in primary testicular DLBCL
Among the 46 PT-DLBCL patients, 3 (6.5%) experienced relapse in the contralateral testis, and 7 (15.2%) had central nervous system (CNS) recurrences. There was a trend toward lower contralateral testis relapse rates in patients who received prophylactic contralateral testicular radiotherapy (RT) compared to those who did not, although this difference did not reach statistical significance (p = 0.054). Similarly, the rate of CNS recurrence did not differ significantly between patients who received CNS prophylaxis (27 with intrathecal methotrexate and 1 with intravenous methotrexate) and those who did not (p = 0.900), nor did the cumulative incidence of CNS relapse (p = 0.54). The 6-month cumulative CNS relapse rates in the prophylaxis and non-prophylaxis groups were 9.5% and 8.5%, respectively, with the median time to CNS relapse among all PT-DLBCL patients being 47.3 months (range: 0.2–106.0).
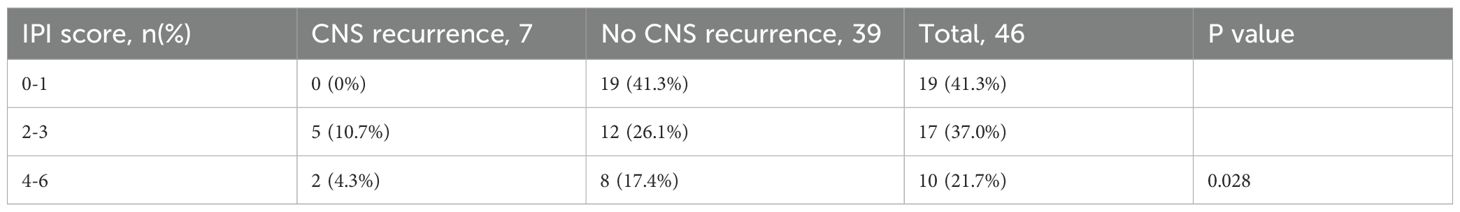
Among the 7 patients with CNS recurrence, 6 had parenchymal involvement, and 1 had leptomeningeal involvement. The most commonly affected sites were the corpus callosum, basal ganglia, frontal lobe, and parietal lobe. There was no significant difference in CNS relapse rates between patients who received Rituximab and those who did not (p = 1.000). However, patients classified as low-, intermediate-, and high-risk based on CNS-IPI scores (0–1, 2–3, and 4–6, respectively) showed a statistically significant difference in CNS recurrence rates (p = 0.028 Table 5).
3.2.3 Survival analysis by subgroup and prognostic factors for primary testicular DLBCL
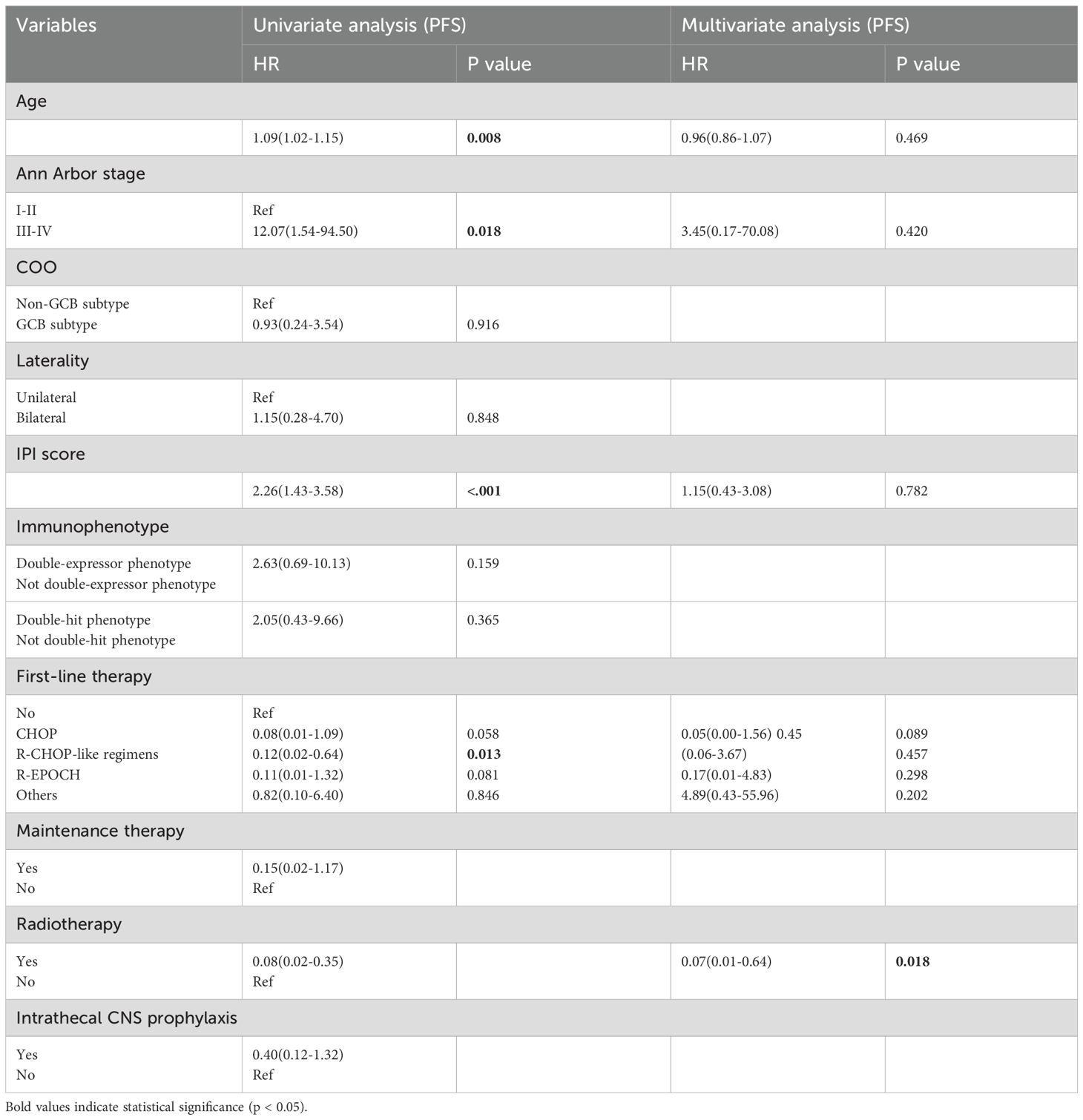
Subgroup analysis revealed that patients with an International Prognostic Index (IPI) score of 2–3 or 4–5 had significantly poorer survival outcomes compared to those with an IPI score of 0–1 (p = 0.001 and 0.011 for PFS and OS, respectively), as shown in Figure 3. Patients who received radiotherapy demonstrated improved progression-free survival (PFS) and overall survival (OS) (p = 0.009 and 0.001, respectively, Figure 4). The 3-year PFS rate was 93.3% for patients who received maintenance therapy after first-line treatment, compared to 72.4% for those who did not receive maintenance therapy (p = 0.037, Figure 5).
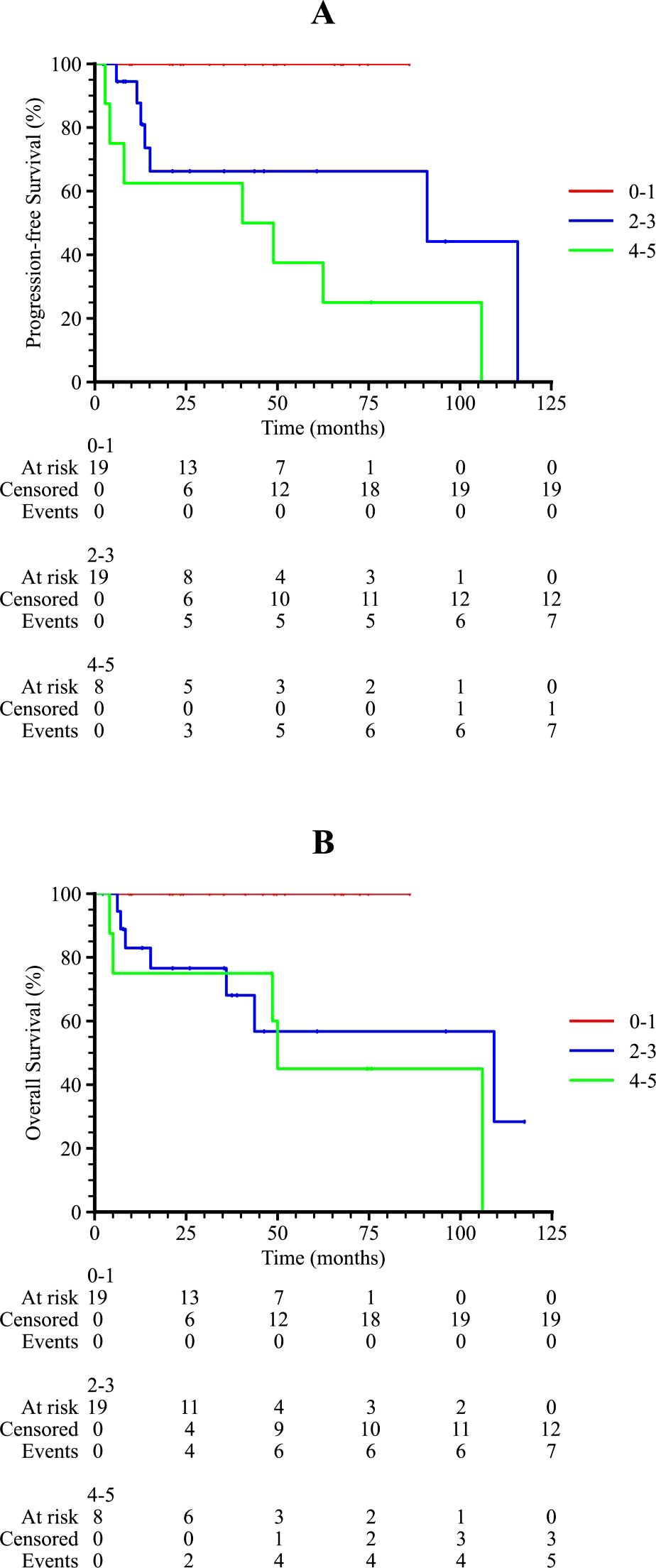
Figure 3. Kaplan-Meier survival curves of PTL patients in different IPI scores. (A) The progression-free survival curve among PTL patients in 0-1, 2–3 and 4–5 IPI score. (B) The overall survival curve among PTL patients in 0-1, 2–3 and 4–5 IPI score.
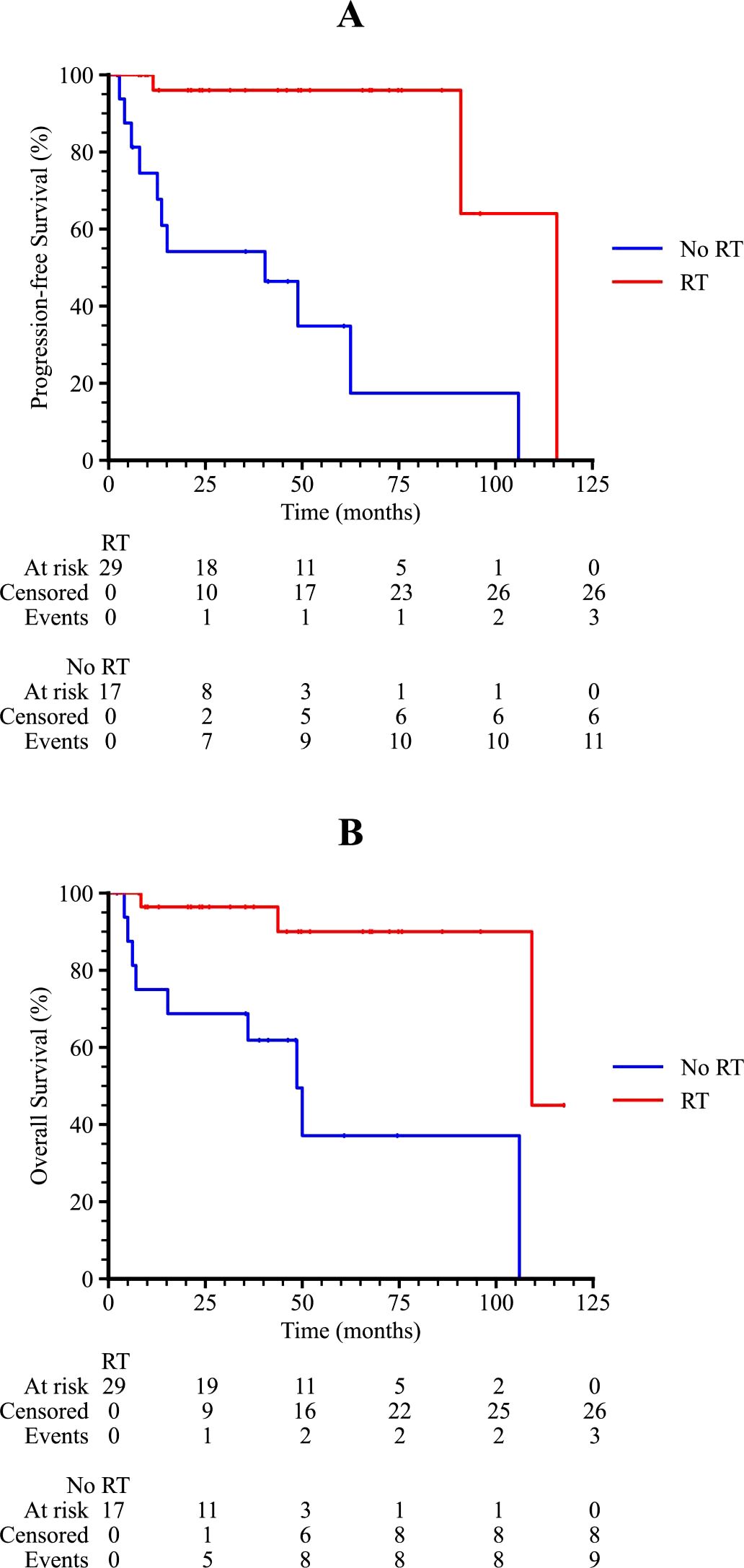
Figure 4. Kaplan-Meier survival curves of PTL patients with or without RT. (A) The progression-free survival curve among PTL patients with or without RT. (B) The overall survival curve among PTL patients with or without RT.
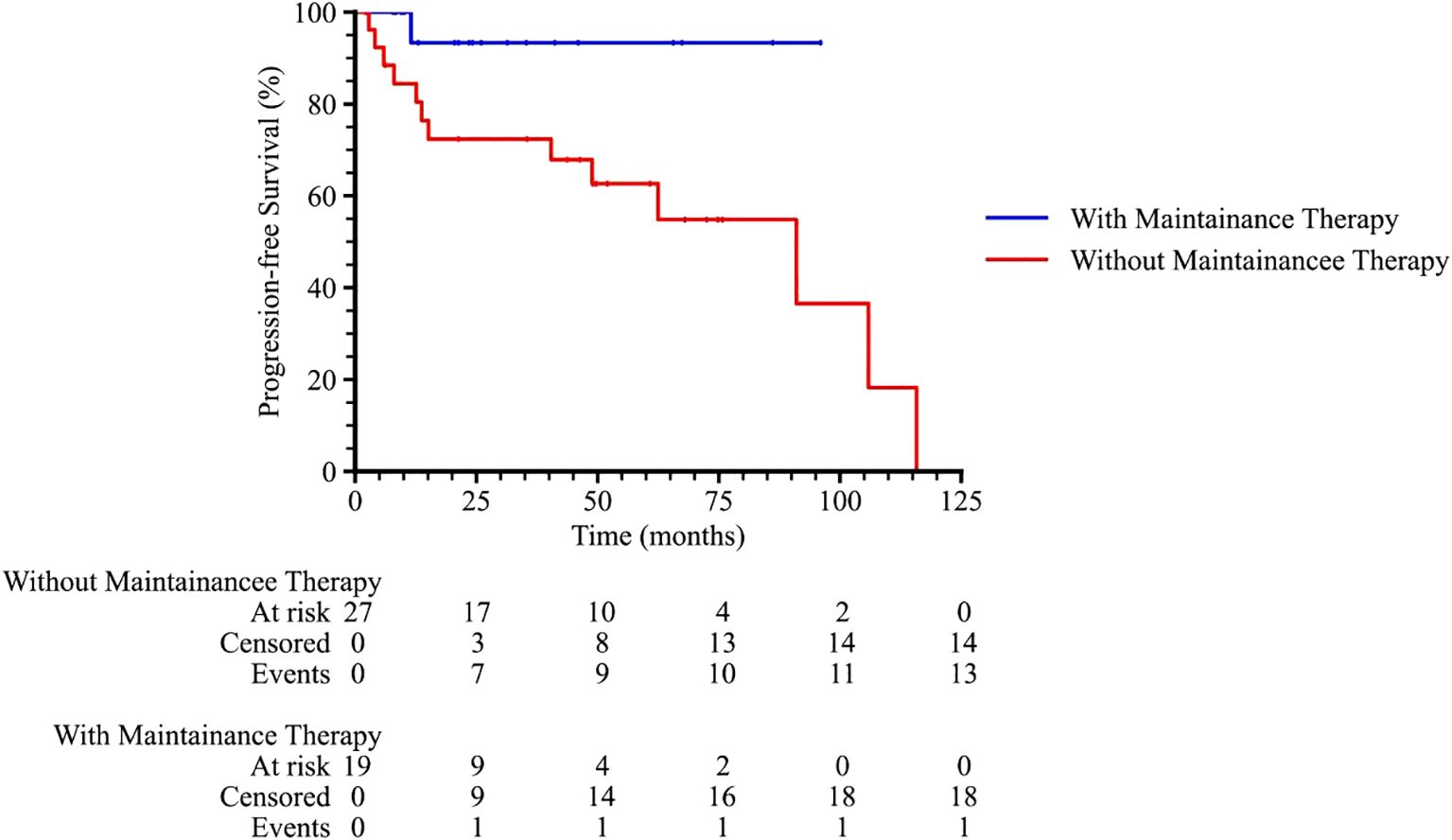
Figure 5. The progression-free survival curve among PTL patients with or without maintenance therapy.
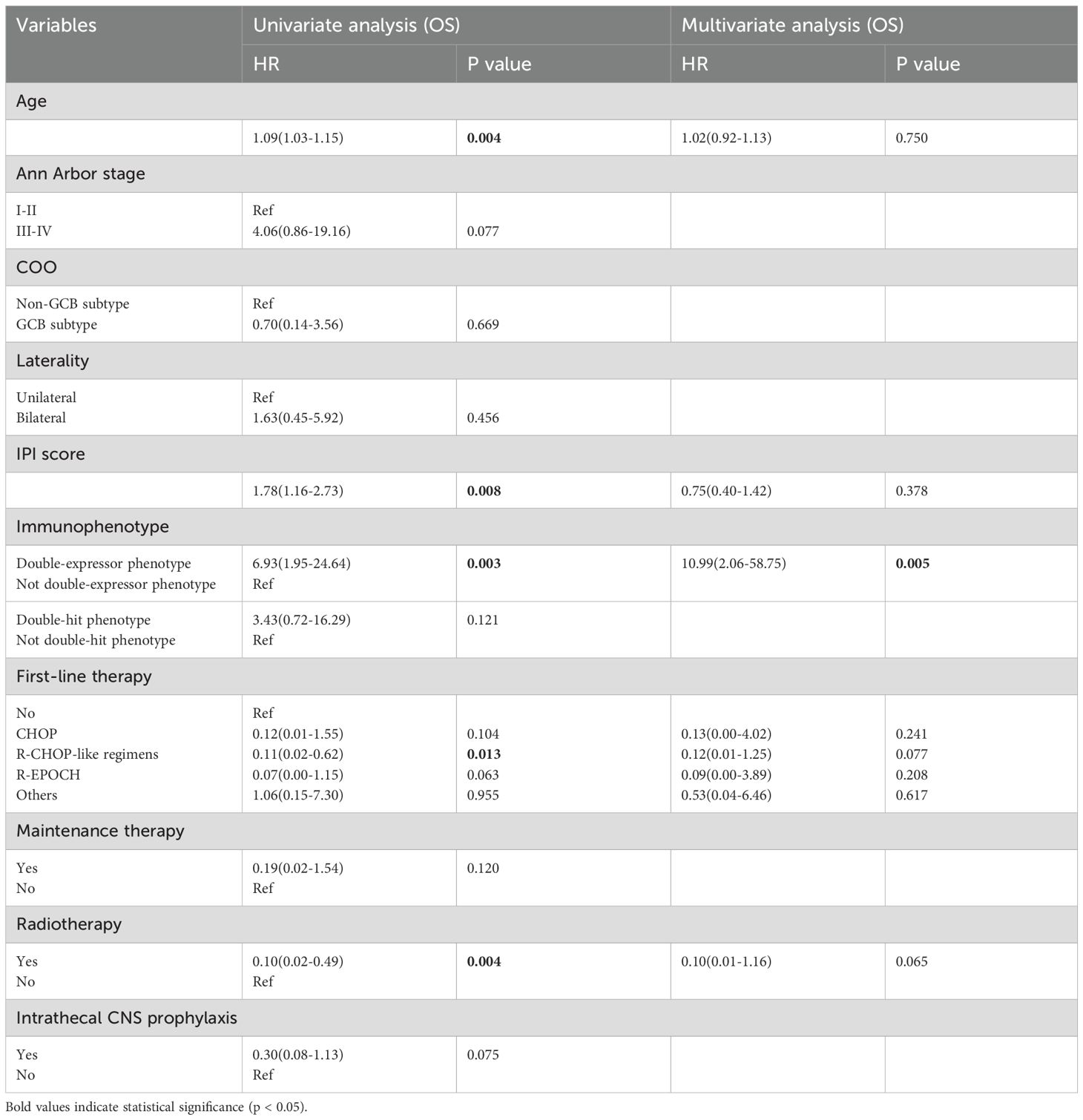
Univariate and multivariate Cox regression analyses were performed to evaluate potential prognostic factors in PT-DLBCL patients. In the univariate analysis, age, Ann Arbor stage, IPI score, first-line therapy, and radiotherapy were identified as potential prognostic factors for PFS. For OS, age, IPI score, immunophenotype, first-line therapy, and radiotherapy were identified as possible prognostic factors. Further multivariate analysis confirmed that radiotherapy was an independent prognostic factor for PFS, while the double-expressor phenotype was an independent prognostic factor for OS in PT-DLBCL patients (Tables 6, 7).
4 Discussion
Differentiating between PTL and STL remains a significant challenge in clinical practice, impacting clinical staging, treatment planning, and prognostic evaluation. Matthew et al. proposed a stricter definition of PTL, limiting it to testicular lymphomas without lymph node or bone marrow involvement (2). Moreover, Rebecca et al. suggested that phenotypic, genetic, and biological characteristics could further assist in distinguishing these two types (3). PTL, for example, is often associated with the non-GCB subtype and the common mutations of MYD88 L265P and CD79B, although this requires further clinical validation. In our study, we differentiated PTL from systemic lymphoma disease based on clinical symptoms and extranodal involvement. Our findings demonstrate that the two groups exhibited distinct pathological features, treatment responses, and survival outcomes. However, the distinction might be correlated to diverse biological features and treatment strategies, including non-GCB phenotype, MYD88/CD79B mutations, and immune-privileged site involvement between these two groups. Current guidelines recommend that PTL diagnosis be confirmed via biopsy and pathology. Combined treatment strategies, including orchiectomy followed by chemotherapy and radiotherapy, are essential. Orchiectomy not only facilitates an accurate pathological diagnosis but also disrupts the blood-testicular barrier, enhancing the efficacy of chemotherapy. In contrast, for STL patients, diagnosis primarily depends on the intensity of FDG uptake on PET/CT (4, 5). Given the difficulty in interpreting physiologic and pathologic uptake on FDG-PET/CT, we adopted a standardized uptake value (SUVmax) threshold of 5.0 to determine testicular involvement by lymphoma, referencing a previous American study (5). 14 PTLs displayed unfavorable survival outcomes in general, however, one with NKTCL received 6 cycles of DDGP regimen as front-line and then discovered testicular involvement, after 4 cycles of etoposide, PD-1 blockade and chidamide, he received chimeric antigen receptor T-cell (CAR-T) therapy and reached a PFS of 70 months. As systemic chemotherapy has shown limited efficacy in such case, novel therapies such as stem cell transplantation and CAR-T cell therapy should be considered.
Primary testicular diffuse large B-cell lymphoma (PT-DLBCL) is the most common histological subtype of PTL, accounting for 80–90% of cases. It is characterized by a high propensity for relapse in the central nervous system (CNS), contralateral testis, and other extranodal sites (3, 6). However, Chinese real-world data on this rare and aggressive disease remain limited. In our study, we analyzed a cohort of 46 patients with PT-DLBCL, focusing on their clinical features, treatments strategies, and prognosis outcomes. This analysis reflects real-world clinical practice and provides valuable insights into the management of this uncommon lymphoma subtype. The primary symptoms of PT-DLBCL were predominantly unilateral painless swelling, and the most commonly observed phenotype was the non-GCB subtype, which aligns with previous studies (7, 8). Notably, western cohorts have shown greater molecular heterogeneity. Interestingly, half of our patients were at an advanced stage at diagnosis, a higher proportion than previously reported. This may be attributed to frequent misdiagnoses as orchitis, leading to delayed treatment.
Although MYD88 gene mutation testing is not routinely conducted in Chinese clinical practice, our study found an 80% mutation rate, consistent with the 68–82% reported in other Chinese and Asian studies (9). This mutation, frequently co-occurring with CD79B mutations, is a hallmark of the activated B-cell (ABC) or non-GCB subtype, contributing to constitutive NF-κB pathway activation and poorer outcomes. BCL2 and BCL6 mutations, which have also been implicated in extranodal DLBCL prognosis in previous studies, were not assessed in our cohort due to limited access to molecular profiling (10–12). The double- or triple-hit phenotype, commonly involving rearrangements of MYC and BCL-6, was less frequent in PT-DLBCL compared to nodal DLBCL. Similarly, the double-expressor phenotype was also less prevalent in PT-DLBCL (13), while BCL6 rearrangements, double-expressor phenotype, and even double-hit lymphomas involving MYC and BCL6 or BCL2 are reported more frequently in western countries (14).
For PT-DLBCL patients, orchiectomy alone is insufficient; combined treatment modalities are essential for improving outcomes. In the Rituximab era, R-CHOP and R-CHOP-like regimens have been the most commonly used first-line therapies, demonstrating significantly better survival compared to orchiectomy alone. Additionally, Polatuzumab vedotin—a CD79b-targeted antibody–drug conjugate—has shown enhanced efficacy when combined with R-CHP in DLBCL patients, particularly in those with the ABC (non-GCB) subtype. However, its use remains limited in real-world clinical practice in China during the study period. Furthermore, the addition of radiotherapy following chemotherapy has been shown to prolong both progression-free survival (PFS) and overall survival (OS), aligning with the results from IELSG clinical trials (14). Several small molecules and targeted agents, including immunomodulatory drugs (IMiDs) such as lenalidomide and pomalidomide, BTK-i like ibrutinib, zanubrutinib, and orelabrutinib, have been confirmed to exert antitumor effects in B-cell lymphomas by inhibiting BCR signaling and reducing NF-κB pathway activation. These agents have also demonstrated efficacy in crossing the blood-testicular and blood-brain barriers (15–18). In our study, patients who received maintenance therapy with these agents showed a trend toward improved PFS. However, no statistically significant differences in survival were observed based on the specific maintenance regimens used. Interestingly, BTK inhibitors did not display a clear benefit for long-term survival in PT-DLBCL patients, which may be due to their use primarily in the relapsed/refractory (R/R) setting rather than as part of frontline therapy. Only a small number of patients received R-CHOP in combination with agents such as lenalidomide or BTK inhibitors as frontline treatment. In light of recent preclinical and clinical studies highlighting the crucial role of the PD-1/PD-L1 pathway in immune evasion in PT-DLBCL (19, 20), PD-1 blockade was explored in our cohort. One patient achieved a partial response (PR) after receiving a combination of Rituximab and a BTK inhibitor, suggesting that immunotherapy may offer additional therapeutic benefit in select cases.
PT-DLBCL is particularly characterized by a high risk of CNS relapse, occurring in 10%–25% of patients. Although the exact mechanisms underlying CNS recurrence remain unclear, they are thought to involve latent molecular features specific to these immune-privileged sites (21). The CNS-IPI score has been validated as an effective risk stratification model for predicting CNS involvement, consistent with our findings (22, 23). For high-risk patients, implementing appropriate CNS prophylaxis is critical. A phase II study demonstrated that intravenous (IV) high-dose methotrexate (HD-MTX) combined with intrathecal (IT) liposomal cytarabine and R-CHOP was effective in preventing CNS relapse (24). In comparative analyses, IV-directed CNS prophylaxis showed superior survival outcomes over IT methotrexate in high-risk patients (25).However, a real-world study similar to ours found no significant difference in CNS relapse rates between IV and IT regimens (26). In our cohort, due to concerns about the toxicity of IV HD-MTX—especially in elderly patients—only one patient received this regimen. Approximately half of the patients underwent IT MTX prophylaxis, the clinical benefit of which remains controversial. Given that CNS relapses in our patients primarily involved the parenchyma rather than the leptomeninges (6 vs. 1), the limited benefit of IT chemotherapy seems reasonable. Altogether, beyond CNS-IPI scoring, genomic biomarkers could also be explored in identifying high-risk patients for CNS recurrence, and CNS-directed chemotherapy should be considered under close health monitoring. However, considering those who are unsuitable for HD-MTX, novel treatment approaches like rational combined therapy with targeted agents such as BTK-i, PD-1 inhibitors and CAR-T cells could be the future direction (27).
Several studies, including retrospective analyses and investigations using large datasets, have explored potential prognostic factors for PT-DLBCL, leading to the development of predictive nomograms (28, 29). However, no unified conclusion has been established thus far. Factors such as age, Ann Arbor stage, laterality, IPI score, and treatment modality have been reported as significant prognostic factors for PT-DLBCL. Interestingly, no obvious difference was observed in intermediate-risk and high-risk PT-DLBCLs when classified by IPI score, perhaps owing to the small number of patients cohort and the insufficient prognostic value of IPI score in PT-DLBCL patients without considering its unique biological characteristics. In our univariate analysis, older patients with advanced-stage disease, high IPI scores, double-expressor phenotypes, orchiectomy alone, and the absence of radiotherapy were associated with poor survival outcomes, consistent with previous reports (30). In multivariate analysis, radiotherapy emerged as an independent prognostic factor for progression-free survival (PFS), while the double-expressor phenotype was an independent factor for overall survival (OS). Given the rarity of this disease and the limited number of cases, our findings warrant further validation in larger, prospective, multi-center studies, and to further optimize prognostic scoring models.
In summary, this retrospective, real-world study included a relatively large cohort of testicular lymphoma patients in China, providing valuable insights into their diagnosis and treatment. Compared with STL, PTL cases tended to demonstrate different pathological subtypes, more favorable clinical responses and survival outcomes, which may reflect earlier disease stage at diagnosis, more localized involvement, and more standardized treatment strategies, based on primary symptoms and extranodal involvement. For PT-DLBCL patients, chemotherapy following orchiectomy, combined with consolidation therapy and radiotherapy, emerged as the recommended therapeutic approach in our study. Future research should focus on optimizing CNS prophylaxis strategies, identifying genomic predictors, and exploring the potential of immunotherapy and targeted therapies for high-risk patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Clinical and Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HQ: Conceptualization, Formal analysis, Writing – original draft. SZ: Formal analysis, Writing – review & editing, Investigation. YD: Writing – original draft, Methodology, Investigation. RH: Resources, Writing – original draft, Data curation. FZ: Investigation, Writing – original draft. MZ: Funding acquisition, Writing – review & editing. XZ: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by National Natural Science Foundation of China (No.82170183) and Outstanding Youth Item of Henan Health-related Technological and Innovative Talents Project (Grant No.11459).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Twa D, Lee DG, Tan KL, Slack GW, Ben-Neriah S, Villa D, et al. Genomic predictors of central nervous system relapse in primary testicular diffuse large B-cell lymphoma. Blood. (2021) 137:1256–9. doi: 10.1182/blood.2020006338
2. Cheah CY, Wirth A, and Seymour JF. Primary testicular lymphoma. Blood. (2014) 123:486–93. doi: 10.1182/blood-2013-10-530659
3. Horne MJ and Adeniran AJ. Primary diffuse large B-cell lymphoma of the testis. Arch Pathol Lab Med. (2011) 135:1363–7. doi: 10.5858/arpa.2010-0158-RS
4. King RL, Goodlad JR, Calaminici M, Dotlic S, Montes-Moreno S, Oschlies I, et al. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Arch. (2020) 476:647–65. doi: 10.1007/s00428-019-02698-3
5. Khessib T, Itani M, Hippe D, Akaike G, Bermo M, Zare M, et al. Testicular FDG uptake on PET/CT in patients with lymphoma: correlation with age. Curr Probl Diagn Radiol. (2022) 51:474–7. doi: 10.1067/j.cpradiol.2021.07.003
6. Higgins A, Kim H, Harper L, Habermann TM, Nowakowski GS, Thompson CA, et al. Testicular FDG-PET/CT uptake threshold in aggressive lymphomas. Am J Hematol. (2021) 96:E81–81E83. doi: 10.1002/ajh.26073
7. Ollila TA and Olszewski AJ. Extranodal diffuse large B cell lymphoma: molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. (2018) 19:38. doi: 10.1007/s11864-018-0555-8
8. Lee YP, Yoon SE, Cho J, Ko YH, Oh D, Ahn YC, et al. Real-world data analysis of survival outcomes and central nervous system relapses in testicular diffuse large B cell lymphoma. Cancer Manag Res. (2023) 15:463–74. doi: 10.2147/CMAR.S407837
9. Chen B, Cao DH, Lai L, Guo JB, Chen ZY, Huang Y, et al. Adult primary testicular lymphoma: clinical features and survival in a series of patients treated at a high-volume institution in China. BMC Cancer. (2020) 20:220. doi: 10.1186/s12885-020-6711-0
10. Chen YP, Ke LF, Lu JP, Wang JC, Zhu WF, Chen FF, et al. Prevalence and clinical significance of oncogenic CD79B and MYD88 mutations in primary testicular diffuse large B-cell lymphoma: A retrospective study in China. Onco Targets Ther. (2019) 12:10165–75. doi: 10.2147/OTT.S222189
11. Shen R, Fu D, Dong L, Zhang MC, Shi Q, and Shi ZY. Simplified algorithm for genetic subtyping in diffuse large B-cell lymphoma. Signal Transduct Target Ther. (2023) 8:145. doi: 10.1038/s41392-023-01358-y
12. Twa D, Mottok A, Savage KJ, and Steidl C. The pathobiology of primary testicular diffuse large B-cell lymphoma: Implications for novel therapies. Blood Rev. (2018) 32:249–55. doi: 10.1016/j.blre.2017.12.001
13. Zhang W, Yang P, Yang Y, Liu S, Xu Y, Wu C, et al. Genomic landscape and distinct molecular subtypes of primary testicular lymphoma. J Transl Med. (2024) 22:414. doi: 10.1186/s12967-024-05140-8
14. Pollari M, Leivonen SK, and Leppä S. Testicular diffuse large B-cell lymphoma-clinical, molecular, and immunological features. Cancers (Basel). (2021) 13:4049. doi: 10.3390/cancers13164049
15. Vitolo U, Chiappella A, Ferreri AJ, Martelli M, Baldi I, Balzarotti M, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. (2011) 29:2766–72. doi: 10.1200/JCO.2010.31.4187
16. Chen N, Lau H, Choudhury S, Wang X, Assaf M, and Laskin OL. Distribution of lenalidomide into semen of healthy men after multiple oral doses. J Clin Pharmacol. (2010) 50:767–74. doi: 10.1177/0091270009355157
17. Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. (2018) 132:2240–8. doi: 10.1182/blood-2018-02-835496
18. Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. (2017) 7:1018–29. doi: 10.1158/2159-8290.CD-17-0613
19. Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. (2019) 117:121–30. doi: 10.1016/j.ejca.2019.05.024
20. Pollari M, Brück O, Pellinen T, Vähämurto P, Karjalainen-Lindsberg ML, Mannisto S, et al. PD-L1(+) tumor-associated macrophages and PD-1(+) tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica. (2018) 103:1908–14. doi: 10.3324/haematol.2018.197194
21. Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer M, Chapuy B, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. (2017) 129:3071–3. doi: 10.1182/blood-2017-01-764209
22. Schmitz N, Zeynalova S, Nickelsen M, Kansara R, Villa D, Sehn LH, et al. CNS international prognostic index: A risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. (2016) 34:3150–6. doi: 10.1200/JCO.2015.65.6520
23. Tolley ER, Nielsen TH, Hersby DS, Østergaard S, Rasmussen M, Clausen MR, et al. Incidence and characterization of secondary CNS lymphoma in 1972 patients with DLBCL: a Danish nationwide cohort study. Blood Adv. (2025) 9:893–905. doi: 10.1182/bloodadvances.2024014404
24. Conconi A, Chiappella A, Ferreri A, Stathis A, Botto B, Sassone M, et al. IELSG30 phase 2 trial: intravenous and intrathecal CNS prophylaxis in primary testicular diffuse large B-cell lymphoma. Blood Adv. (2024) 8:1541–9. doi: 10.1182/bloodadvances.2023011251
25. Mannisto S, Vähämurto P, Pollari M, Clausen MR, Jyrkkiö S, Kellokumpu-Lehtinen PL, et al. Intravenous but not intrathecal central nervous system-directed chemotherapy improves survival in patients with testicular diffuse large B-cell lymphoma. Eur J Cancer. (2019) 115:27–36. doi: 10.1016/j.ejca.2019.04.004
26. Mocikova H, Janikova A, Sykorova A, Prochazka V, Pirnos J, Duras J, et al. Outcome of patients with diffuse large B-cell lymphoma and testicular involvement - real world data. Ann Hematol. (2024). 104(1):675–84. doi: 10.1007/s00277-024-06025-y
27. Roschewski M and Hodson DJ. Diffuse large B-cell lymphoma involving the central nervous system: biologic rationale for targeted therapy. Haematologica. (2024) 109:388–400. doi: 10.3324/haematol.2021.278613
28. Yang S, Chang W, Zhang B, and Shang P. What factors are associated with the prognosis of primary testicular diffuse large B-cell lymphoma? A study based on the SEER database. J Cancer Res Clin Oncol. (2023) 149:10269–78. doi: 10.1007/s00432-023-04907-8
29. Berjaoui MB, Herrera-Caceres JO, Li T, Qaoud Y, Tiwari R, Ma D, et al. Age related differences in primary testicular lymphoma: A population based cohort study. Urol Oncol. (2023) 41:151.e1–151.e10. doi: 10.1016/j.urolonc.2022.10.032
Keywords: testicular lymphoma, diffuse large B-cell lymphoma, central nervous system, survival, real-world study
Citation: Qiao H, Zhang S, Duan Y, Hua R, Zong F, Zhang M and Zhang X (2025) Testicular lymphoma of 63 patients: a Chinese retrospective, real-world study. Front. Oncol. 15:1652944. doi: 10.3389/fonc.2025.1652944
Received: 24 June 2025; Accepted: 08 October 2025;
Published: 30 October 2025.
Edited by:
Marcelo A. Soares, National Cancer Institute (INCA), BrazilReviewed by:
Evgenia Verrou, Theageneio General Hospital, GreeceHimanshi Jain, Shree Krishna Hospital, India
Copyright © 2025 Qiao, Zhang, Duan, Hua, Zong, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Zhang, ZmNjemhhbmd4ZEB6enUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Honghan Qiao
Honghan Qiao Sijun Zhang†
Sijun Zhang† Feiyang Zong
Feiyang Zong Mingzhi Zhang
Mingzhi Zhang Xudong Zhang
Xudong Zhang